Abstract
Municipal waste treatment is inherently associated with odour emissions. The compounds characteristic of the processes used for this purpose, and at the same time causing a negative olfactory sensation, are organic and inorganic sulphur and nitrogen compounds. The tests were carried out at the waste management plant, which in the biological part, uses the methane fermentation process and is also equipped with an installation for the collection, treatment, and energetic use of biogas. The tests include measurements of the four odorant concentrations and emissions, i.e., volatile organic compounds (VOCs), ammonia (NH3), hydrogen sulphide (H2S), and methanethiol (CH3SH). Measurements were made using a MultiRae Pro portable gas detector sensor. The tests were carried out in ten series for twenty measurement points in each series. The results show a significant impact of technological processes on odorant emissions. The types of waste going to the plant are also important in shaping this emission. On the one hand, it relates to the waste collection system and, on the other hand, the season of year. In addition, it has been proved that the detector used during the research is a valuable tool enabling the control of technological processes in municipal waste processing plants.
1. Introduction
The use of fossil fuels, as well as the impact of greenhouse gases on the environment, have helped to initiate research related to the production of alternative fuels. In Europe and the world, there is an increase in greenhouse gas emissions in the atmosphere, the main source of which is carbon dioxide (CO2). Over 80% of global energy demand is covered by fossil fuels [1]. Biogas obtained as a result of biological treatment of biodegradable waste may play a key role in the energy industry in the future. Biogas as a renewable energy source can replace conventional fuels to produce heat and electricity and can also be used as gas fuel in the automotive industry. Research carried out so far indicates that biogas produced in the methane fermentation process provides significant benefits compared to other forms of bioenergy, because this technology is characterized by energy efficiency and environmental friendliness [2,3].
A modern waste management strategy should aim mainly at minimising waste generation (among others, by designing and manufacturing products that promote reuse and facilitate recycling and recovery), waste source separation and then reuse and recovery of energy and material resources from unavoidable waste [4]. The concept of “Zero Waste” is becoming more and more relevant by reducing the amount of waste, recycling, recovery, and minimisation of waste going to landfills [5,6]. However, literature data indicate that, still, unfortunately approximately 50% of municipal solid waste produced is sent to landfills [4]. Therefore, both the reduction of landfills volumes and the proper operation and reclamation of existing landfills are very important in terms of emission control (including the minimisation of uncontrolled biogas emissions). In this context, the mechanical-biological waste treatment in both aerobic and anaerobic conditions (at biogas plants) has its full justification [7]. The biological process of methane fermentation is very complex and multiphase. In the last decade, devices for controlling individual process phases, as well as analytical tools, have been developed. These changes have contributed to increasing the energy efficiency of the process. The goal of the control system is to optimize the entire biogas production process and provide early warning to prevent failure of the entire process [8]. Thanks to the development of control and analysis systems, biogas plants can operate smoothly despite these extremely complex steps [9].
Until now, mainly biogas plants from agricultural, landfill, or sewage treatment plants were subjected to testing of odorous compounds [10,11,12]. Biogas plants processing municipal waste are not yet well understood in this respect and worldwide there are fewer of them compared to the previously mentioned installations [13]. However, due to energy (biogas energy production) and environmental (waste management) benefits, as well as publicly available source material, much more will probably be created in the future.
Odorants, chemical compounds that cause an olfactory effect, are probably one of the most demanding environmental challenges for the emerging environmental policy [14,15]. Odorants that cause a negative olfactory effect generally contain nitrogen or sulphur, i.e., amines, phenolic compounds, aldehydes, thiols, ketones, and alcohols. Each of these components is produced mainly as a result of the activity of microorganisms that break down complex organic compounds present in the organic matter [16,17,18,19,20].
One of the main odorants emitted during the decomposition of biodegradable waste are volatile organic compounds (VOCs) [15,21]. The World Health Organization (WHO) has recognized volatile organic compounds as the most significant indoor air pollution. So far, about 500 volatile compounds and those present in indoor air have been identified. Only a few were considered pathogenic. Nevertheless, it is believed that many of them contribute to such health problems as: allergies, headaches, loss of concentration, drying and irritation of the nasal mucosa, throat, and eyes, etc. [21,22,23,24,25].
The term VOCs refers to a wide group of chemical compounds whose vapour pressure is at least 0.01 kPa at 20 °C [26,27]. They are also characterized by low aqueous solubility. VOCs in the atmosphere participate in photochemical reactions, producing photochemical oxidants. According to Eitzer [28], who undertook pioneering research on the exhaustive characteristics of VOCs emitted at various stages of the biodegradation process, most VOCs in composting plants are emitted at early stages of the process. For example, Delgado-Rodríguez et al. [29] found that emissions of volatile compounds are closely related to the phases of the composting process: aldehydes, alcohols, carboxylic acids, esters, ketones, sulphides, and terpenes are emitted mainly in the initial acid phase, while in the thermophilic phase ketones, organosulfur compounds, terpenes, and ammonia dominate. In the cooling phase, the main volatile compounds emitted are sulphides, terpenes, and ammonia. These authors also studied the impact of process control parameters (humidity, aeration, and C/N ratio) on the emission of volatile compounds in municipal solid waste composting. The C/N ratio had the greatest impact on VOCs emission, followed by aeration and moisture content.
There are many odorous testing methods, which include sensory, sensor, and analytical methods. Table 1 shows examples of the uses of these methods.

Table 1.
Methods of assessing odour and odorants concentration [29,30,31,32].
Sensory methods are used to determine the qualitative (method of sensory evaluation) or quantitative (olfactometry) smell. Using only sensory methods, it is not possible to obtain information on the types of compounds as well as their concentrations contained in the odorant mixture. For this purpose, analytical or sensor methods are used [33,34,35].
Portable detectors, classified as sensor methods, compared to most other methods, are characterized by uncomplicated service, as well as relatively low purchase costs [16]. The detectors use various types of sensors, which include:
- photoionization sensors—PID;
- nondispersive infrared sensors—NDIR;
- electrochemical sensors—EC;
- thermal sensors—PELLISTOR [36,37].
For the analysis of chemical compounds that cause an unpleasant olfactory effect, emitted during the treatment of municipal waste, photoionization and electrochemical sensors are most commonly used. In the case of the photoionization sensor, the operating principle is the ionization of neutral molecules of chemical compounds. When diffusing particles of VOCs encounter the UV lamp, they are ionized by photons. Then, the ions formed are directed between two polarized electrodes. The ions move towards the electrodes in the electric field generated by the electrometer. In this way, a current flow is generated, which is then converted into a voltage signal. This signal is proportional to the concentration of compounds subjected to ionization. Compounds having higher ionization energies than the maximum energies of the UV lamp photons are not detected. This type of sensor is most often used to measure the total concentration of VOCs [14,34,38,39,40].
The electrochemical sensor uses absorption of infrared radiation to identify the compounds. The principle of this type of sensor is to place the source of infrared radiation along the optical line with the detector. When the analysed gas appears in the measuring chamber, it absorbs radiation of a certain wavelength, and according to the Lambert–Beer law, there is a decrease in radiation reaching the detector which is converted into an electrical signal. This reduction in light intensity is proportional to the concentration of the gases or flammable vapours being detected [16,40].
This study undertakes intensive research aimed at analysing the impact of technological processes carried out at biogas plants (constituting waste treatment plants) on the emission of odorous compounds, using municipal waste as input material. So far, there have been few scientific studies related to the odour nuisance of this type of project or they have been carried out in a short period of time. Technological processes at municipal waste treatment plants are characterised by high variability and therefore require detailed analyses. The paper presents the results of almost a year-long research, which perfectly illustrates the complexity of the problem of odorant emissions, showing the relations between measured odorants and technological factors. The research results are potentially very valuable from the point of view of implementing new technologies in the field of both waste treatment and deodorization of process gases. Currently, there are in Poland eight biogas plants of this type but, in the future, it can be assumed that many more will be created due to the drive to obtain energy from raw materials available throughout the year.
2. Materials and Methods
2.1. Characteristic of the Analysed Plant
The plant, being the subject of research, is located in the southern part of Poland. The plant is equipped with installations for mechanical and biological treatment of municipal waste. The input material for the fermentation process is the biodegradable fraction mechanically separated from the mixed waste stream. The fermentation process is carried out in two separate chambers. Each of them is equipped with four mixers and a digestate dewatering line. The fermentation process is carried out in semi-dry, mesophilic conditions. A flow-chart of processes at the mechanical-biological waste treatment installation is shown in Figure 1.
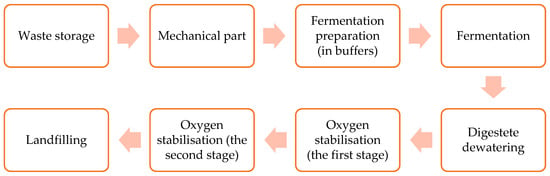
Figure 1.
Process flowchart of mechanical-biological treatment of waste at the analysed plant.
Waste storage is conducted in a closed hall. The hall is equipped with three ventilators which intakes are located: above the mixed waste and above the conveyors transporting waste to the machining hall. The processes of waste screening and sorting (mechanical treatment) are carried out in a hall equipped with a system of screens and separators connected by conveyors. The biodegradable fraction is transported to two buffers, located in a closed feedstock preparation hall for the fermentation process. The fermentation process itself takes place in two closed fermentation chambers. After the fermentation process is completed, the input material is subjected to a dewatering process (via a press and a centrifuge) in a closed dewatering hall. Dehydrated digestate is directed to special tunnels, located in a closed hall for the first stage of oxygen stabilisation (28 days) and then to the open field for the second stage of oxygen stabilization (14 days).
2.2. Study Methodology
The study reported here includes the determination of levels of ammonia, hydrogen sulphide, methanethiol, and VOCs at twenty measurement points identified during inventory and pilot tests as sources of emissions of odorous compounds (Table 2) [41]. The tests were carried out in ten measurement series, from July to December 2019 (Table 3). The sensor method was used to determine the indicated compounds—the MultiRae Pro portable multi-gas gas detector (RAE Systems, Inc.; San Jose, CA, USA). Measurements were made with five one-minute replicates at each point, and the obtained results were averaged. The characteristics of individual sensors that the detector is equipped with are presented in Table 4. At the same time, measurements (T) and relative humidity (RH) of air at a height of 1.5 m were carried out using a portable Kestrel 4500 NV weather meter. The minimum, average, and maximum values of measurements during each of the series are presented in Figure 2a,b. Emission levels were calculated according to the methodology presented in reference [42].

Table 2.
Measurement points at the examined biogas plants.

Table 3.
Dates of measurement series at a biogas plant.

Table 4.
Characteristics of the gas detector sensors.
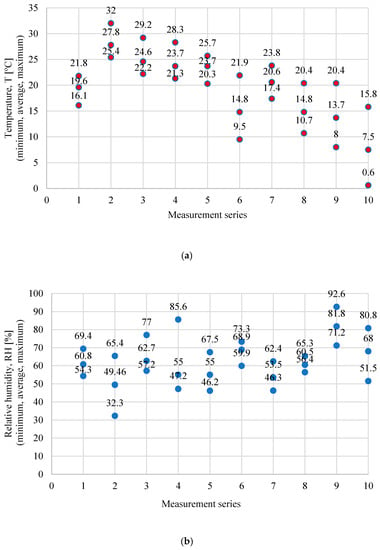
Figure 2.
Minimum, average, and maximum temperature (T) (a) and relative humidity (RH); (b) at individual measurement series.
3. Results and Discussion
The distribution of concentrations of tested odorants for the waste storage plant in individual measurement series is presented in Figure 3a,b, while the distribution of emission levels from exhaust ventilators, which are at the waste storage plant, is presented in Figure 4a–c.
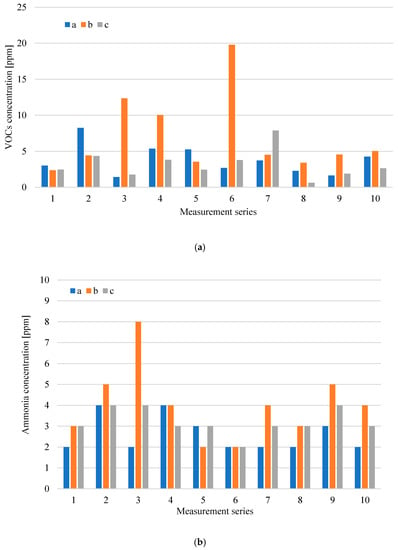
Figure 3.
Distributions of volatile organic compounds (VOCs) (a) and ammonia (NH3) concentration; (b) at the waste storage plant in particular measurement series (a-inside the hall; b-mixed waste; c-selectively collected waste).
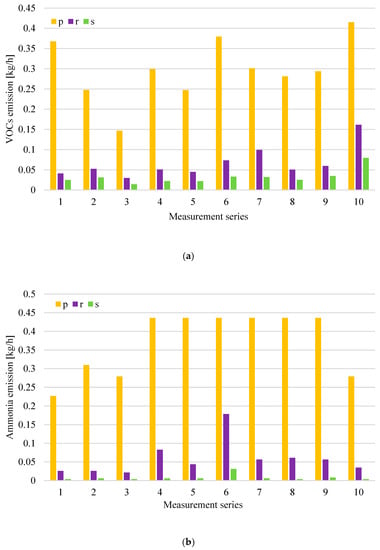
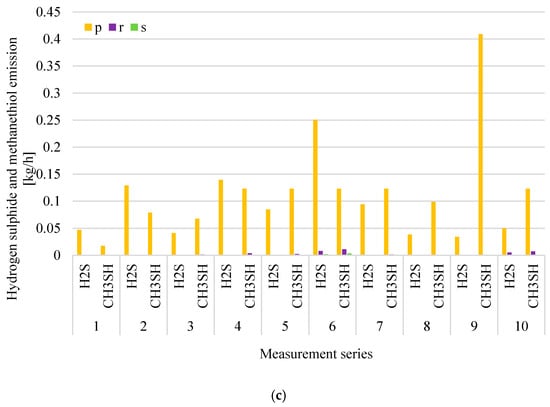
Figure 4.
Distributions of volatile organic compounds (VOCs) (a), ammonia (NH3); (b) and hydrogen sulphide (H2S) and methanethiol (CH3SH); (c) emission at the waste storage plant in particular measurement series (p-roof ventilator 1-process gases captured from over-mixed waste; r-roof ventilator 2-process gases captured from the overhead conveyor transporting waste to the sorting plant; s-roof ventilator 3-process gases captured from over selectively collected waste).
The highest level of VOCs emissions was recorded from the roof ventilator, which has its air intake located above the mixed waste stored p. This is consistent with the high concentrations of VOCs observed at the place where mixed municipal waste was stored b. Increased VOCs emissions were also recorded in the gases discharged from the ventilators, which have their air intakes located inside the hall r and above the selectively collected waste storage (10 measurement series). Periodically increased VOCs concentrations were also recorded at other measurement points—in the place of selectively collected waste storage c–measurement series 7, inside the hall a–measurement series 2. The highest levels of NH3 (ammonia) emissions were also recorded from roof ventilators discharging air from waste storage plant p and r. A similar relationship was also observed for hydrogen sulphide and methanethiol. The presence of these compounds was only noted in the gases discharging from the waste storage plant through roof ventilators p, r, and s.
The analysis of Figure 3a shows that, in the case of a mixed waste storage site, the largest increase in VOCs concentration, to a level of about 20 ppm, was observed during measurement series 6. During the same series, a significant increase in ammonia emissions from roof ventilators was also observed, the air intake of which is located inside the hall, above the conveyor transporting waste r and above the selectively collected waste storage–Figure 4b. During the tests, an odour similar to the solvent smell was perceptible in the hall. The sensing substance was probably the source of the increased VOCs emissions compared to the results obtained in the other series. In the case of waste collected selectively, increased VOCs concentration was observed only during measurement series 7–at the place of storage of this waste c (Figure 3a), and during measurement series 10–increased level of emissions–in the air discharged by the ventilator from the selectively collected waste s (Figure 4a). This probably resulted from improper and ineffective separate waste collection.
In the case of the roof ventilator p, which has its air intake located above the mixed waste, during each of the measurement series the levels of volatile compounds emission were similar; while the levels of ammonia emission increased significantly in measurement series 4–9. Analysing the results of the levels of hydrogen sulphide and methanethiol emissions in Figure 4c, a significant increase in hydrogen sulphide emissions can be observed during series 6 and 9 (p ventilator). Figure 4c shows the results only from ventilators p, r and s because, at the waste storage plant, the values of H2S (hydrogen sulphide) and CH3SH (methanethiol) concentrations were greater than 0.1 ppm only at these measurement points (the threshold level of determination of the device for the measured chemical compounds). In each of the measurement series, high levels of emissions of both hydrogen sulphide and ammonia from p and r ventilators were observed. The composition of mixed waste and storage time probably had a significant impact on the results obtained. The long storage time of waste containing biodegradable fractions contributes to their compaction and uncontrolled anaerobic processes.
Figure 5a,b shows the results of VOCs concentration and ammonia concentration for the mechanical treatment plant, storage shelter for the shredded preRDF fraction (pre refuse derived fuel), and the fermentation preparation plant.
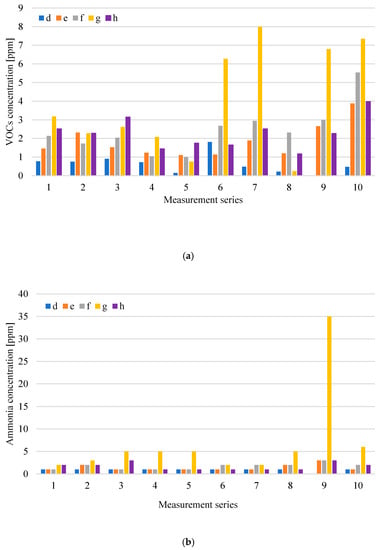
Figure 5.
Distribution of VOCs (a) and NH3; (b) concentration at measurement points related to mechanical treatment of waste and fermentation preparation in particular measurement series (d-in hall front entrance; e-inside the hall, at 1.5 m; f-inside the hall, at 4.0 m; g-shredded preRDF fraction (pre refuse derived fuel); h-inside the fermentation preparation hall).
In individual measurement series, the highest VOCs concentration accompanied the storage of fuel from preRDF g waste and mechanical waste treatment operations—inside mechanical treatment plant f and inside the fermentation preparation plant h. The preRDF fraction mechanically separated from the mixed waste stream and stored under a covered shelter was also the source of the largest ammonia emission. The results obtained at point g are characterized by a large variation in the concentration of volatile organic compounds and a relatively constant level of ammonia (2–5 ppm), not including the result obtained during series 9 (35 ppm).
Figure 6a–c shows the distribution of measuring odorant concentrations for the digestate dewatering plant, while Figure 7a,b presents the distribution of emission levels in individual measurement series from roof ventilators, which are at the digestate dewatering plant.
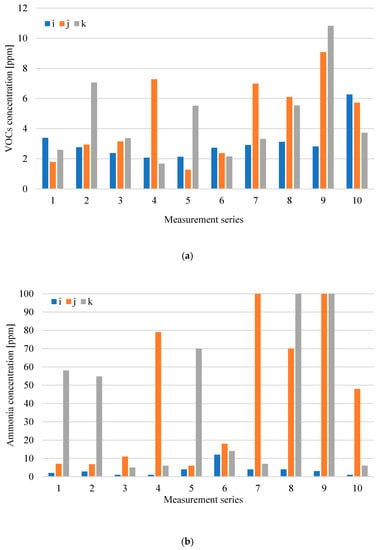
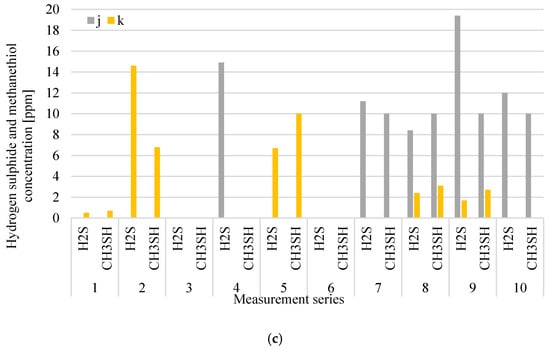
Figure 6.
Distribution of VOCs (a), NH3; (b) and H2S and CH3SH; (c) concentration at digestate dewatering plant in particular measurement series (i-inside the hall; j-over wastewater tank (after the press); k-over wastewater tank (after the centrifuge).
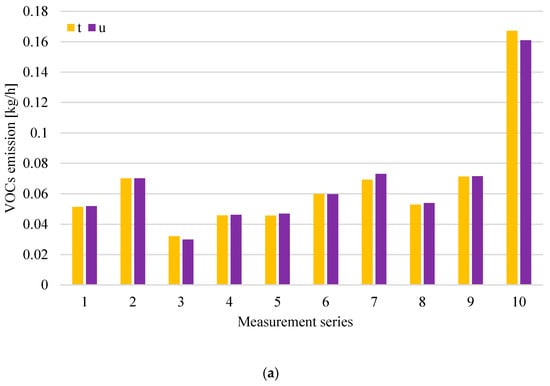
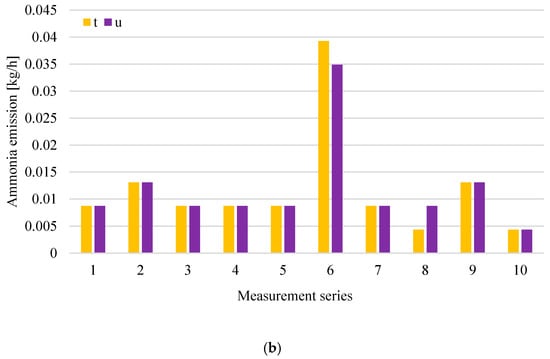
Figure 7.
Distribution of VOCs (a), NH3; (b) emission at digestate dewatering plant in particular measurement series (t-roof ventilator 4–inside the hall; u-roof ventilator 5–inside the hall).
Analysis of the results obtained for the digestate dewatering plant shows that the highest concentrations of both volatile organic compounds and ammonia occur above the process wastewater tanks after the press j and after the centrifuge k, except for series 1 and 10, where the VOCs concentration was at a similar level in all measurement points related to digestate dewatering. Figure 7b shows that, in series 6, there was a clear increase in ammonia emissions from roof exhaust ventilators, whose air intakes are located at the digestate dewatering plant. The increase in ammonia emissions was due to the failure of one of the technological lines intended for digestate dewatering. In the case of tanks (points j and k), the odorant concentration is associated to the greatest extent with operations carried out in relation to these tanks, including the maintenance of their filling levels and cleaning. The tanks are subjected to weekly cleaning, and the results of odorant concentrations above the tank after its cleaning are at a low level, compared to the results obtained before cleaning.
The concentration of hydrogen sulphide and methanethiol at the measurement points related to digestate dewatering varied during individual measurement series, reaching maximum values of 19.4 ppm (in series 9) and 10 ppm (in series 5, 9 and 10), respectively.
Analysing Figure 8 and Figure 9, it can be observed that the sources of the largest emission of the tested odorants in relation to the oxygen stabilization process are technological wastewater from this process, which is directed to the tank of the pumping station n and wastes subjected to the first-degree oxygen stabilization process. In the case of digestate subject to stabilization, the concentration levels of both VOCs and ammonia are variable, although the measurements were carried out on the same day of the technological process. This indicates the different quality of digestate sent to the oxygen stabilization process, which is most likely the result of different compliance with the technological regime during digestate drainage or the quality differentiation of waste going to the plant. On the other hand, significantly lower concentrations of VOCs and NH3 in all measurement series accompanying the 2nd phase of aerobic processing testify to a properly conducted oxygen stabilization process of the 1st degree.
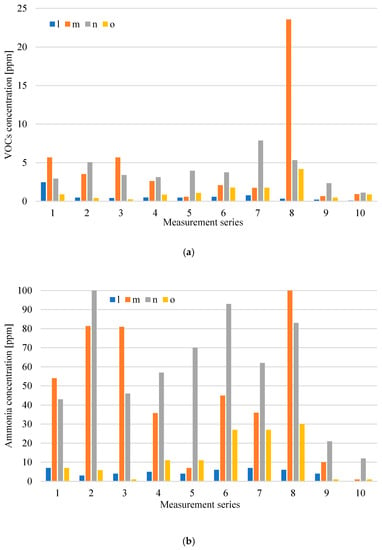
Figure 8.
Distribution of VOCs (a) and NH3; (b) concentration at measurement points related to oxygen stabilisation of digestate in particular measurement series (l-inside the hall; m-waste subjected to an oxygen stabilization process (1st stage); n-over the wastewater tank; o-waste subjected to an oxygen stabilization process (2nd stage).
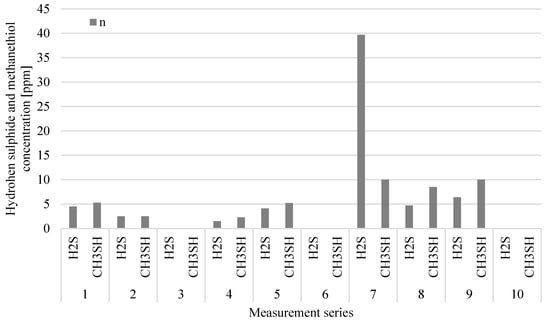
Figure 9.
Distribution of hydrogen sulphide and methanethiol related to wastewater storage from oxygen stabilisation of digestate (point n) in particular measurement series.
Figure 10a,b shows the distribution of concentrations of VOCs and ammonia for all measurement points in the ten measurement series.
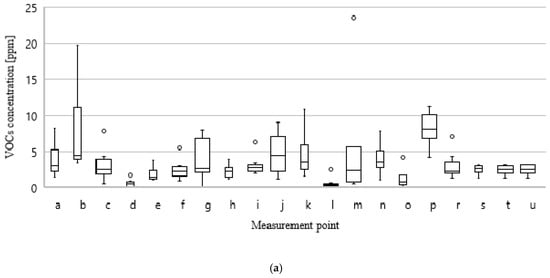
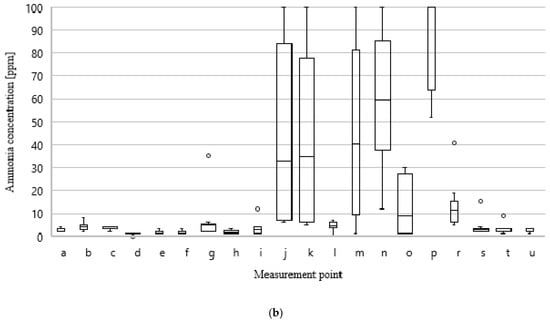
Figure 10.
Values of minimum, average, median, and maximum results of VOCs; (a) and NH3; (b) concentration at particular measurement points during all measurement series.
The above analysis indicates which places in the technological sequence are most exposed to the variability of odorant emissions as a result of changes in the quality of processed waste and implemented technological processes. The largest differences in VOCs and NH3 concentrations occur at measurement points related to the storage of mixed municipal waste (a, b, p) and preRDF (g), with the collection of process wastewater—both from the digestate dewatering process and its oxygen stabilization (j, k, n) and with waste directed to the aerobic treatment process (m, o). It is at these points in the technological sequence that the type of processed waste and the type of technological and operational measures taken have the greatest impact on odorant emissions.
Figure 11 shows the correlation matrices for the odorants tested. Along the diagonal of the matrices, there are histograms representing the distribution of values of each variable. Table 5 shows the correlation coefficients based on Figure 11.
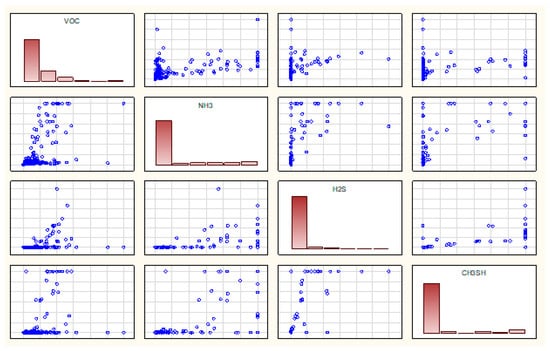
Figure 11.
Correlation matrices for the tested compounds corresponding to all measurements.

Table 5.
Correlation table of examined parameters.
Analysis of the correlations between the tested odorants (Figure 11 and Table 5) indicates the largest relationship between hydrogen sulphide and methanethiol at 0.76 and between ammonia and methanethiol at 0.73. Larger relations between the above odorants were observed at measurement points related to waste storage (a, b, c, p, r, s) and digestate dewatering (i, j, k, t, u)—at the level of 0.76–0.81. The smallest correlation was observed between hydrogen sulphide and volatile organic compounds.
Analysis of the correlation between points of subsequent stages in the technological processes showed relationships at a similar level. The differences were observed for points related to mechanical treatment and fermentation preparation—for these correlation points they were lower.
4. Conclusions
In this study, the results of several months of research conducted at a biogas plant processing municipal waste in Poland have been presented. They show the relations between particular measured odorants and between odorants and technological factors. The novelty and scientific contribution presented in this work are related to the impact of technological aspects on odorant emissions at the municipal waste biogas plant. The literature review shows that, so far, such analyses have been conducted mainly at agricultural biogas plants, biogas plants on landfills or biogas plants related to sewage treatment. The impact of technological factors was identified by measuring odorant concentration (volatile organic compounds, ammonia, hydrogen sulphide, and methanethiol) and observing their changes between individual measurement series. The main conclusions and contributions of this research can be summarised as follows:
- Odorant sources can be divided into the following five categories related to technological processes conducted at analysed biogas plant: waste storage, preRDF storage, waste mechanical treatment and fermentation preparation, digestate dewatering, and oxygen stabilization.
- The biggest odorant concentrations accompany such unit operations as: storage of mixed municipal waste, digestate dewatering, digestate oxygen stabilization of the 1st-degree, and technological wastewater storage (both from digestate dewatering and its oxygen stabilization). The largest organized emissions are related to the evacuation of gases by means of roof ventilators.
- The biggest VOCs concentrations are associated with mixed-waste storage (19.79 ppm) and aerobic stabilization of 1st-degree digestate (23.56 ppm). In turn, the highest NH3 concentrations accompany such technological processes as digestate dewatering (technological wastewater storage: 100 ppm) and 1st-stage oxygen digestate stabilization (100 ppm). The highest CH3SH concentrations also accompany the storage of mixed municipal waste, as well as digestate dewatering and 1st-stage oxygen stabilization (10 ppm). The biggest concentration of hydrogen sulphide is associated with the storage of wastewater from the digestate aerobic stabilization process (40 ppm), which indicates too long storage time and is the result of operational irregularities.
- The highest emissions of odorants tested—to 0.42 kg/h (VOCs), 0.44 kg/h (NH3), 0.41 kg/h (CH3SH), and to 0.25 kg/h (H2S)–are emissions from a roof ventilator which has its air intake located above the mixed-waste storage.
- The following factors affect the concentration of the odorants tested, and thus the volume of emissions:
- a municipal waste collection system in the service area (clearly higher odorant concentrations accompany storage and mechanical treatment of mixed municipal waste in relation to selectively collected waste);
- trouble-free and continuous work of the technological line in the waste processing plant (the sources of uncontrolled and increased odorant emissions are periodically occurring technological line failures);
- technological operations related to the unloading of transported waste and internal transport of waste in the processing plant (especially with loaders and conveyors);
- keeping equipment and storage places clean at the waste treatment plant;
- compliance with the technological regime and operational correctness.
- The largest differences in VOCs and NH3 concentrations occur at measurement points related to the storage of mixed waste and preRDF, with the collection of technological wastewater (both from digestate dewatering and its oxygen stabilization) and waste directed to the aerobic process. In this case, the type of waste processed and the type of technological and operational measures taken are of fundamental importance.
- The odour nuisance of waste management plants, including municipal waste biogas plants, should be minimised by adapting the processes carried out to the best available techniques BAT conclusions [43].
- The detector used during the research is a valuable tool enabling control of technological processes in such facilities.
- Further research should combine olfactometric and meteorological tests in addition to odorants.
Author Contributions
M.W.: data collection, investigation, writing—original draft preparation; A.K.: conceptualization, methodology, supervision; K.L.-S.: writing—review and editing, visualisation, validation. All authors have read and agreed to the published version of the manuscript.
Funding
Work was co-financed by the Dean’s grant (No. 504/04464): “The impact of technological processes and meteorological conditions on odour emission at municipal waste biogas plants”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Achinas, S.; Achinas, V.; Euverink, G.J.W. A Technological Overview of Biogas Production from Biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- van Foreest, F. Perspectives for Biogas in Europe; Oxford Institute for Energy Studies: Oxfordshire, UK, 2012. [Google Scholar]
- Nishio, N.; Nakashimada, Y. Recent development of anaerobic digestion processes for energy recovery from wastes. J. Biosci. Bioeng. 2007, 103, 105–112. [Google Scholar] [CrossRef]
- Cossu, R.; Grossule, V.; Lavagnolo, M.C. Sustainable low-cost waste management: Learning from airlines. Multidiscip. J. Waste Resour. Residues 2019, 6. [Google Scholar] [CrossRef]
- Burlakovs, J.; Jani, Y.; Kriipsalu, M.; Vincevica-Gaile, Z.; Kaczala, F.; Celma, G.; Ozola, R.; Rozina, L.; Rudovica, V.; Hogland, M.; et al. On the way to ‘zero waste’ management: Recovery potential of elements, including rare earth elements, from fine fraction of waste. J. Clean. Prod. 2018, 186, 81–90. [Google Scholar] [CrossRef]
- Burlakovs, J.; Kriipsalu, M.; Porshnov, D.; Jani, Y.; Ozols, V.; Pehme, K.M.; Rudovica, V.; Grinfelde, I.; Pilecka, J.; Vincevica-Gaile, Z.; et al. Gateway of Landfilled Plastic Waste Towards Circular Economy in Europe. Separations 2019, 6, 25. [Google Scholar] [CrossRef]
- Raga, R.; Cossu, R.; Heerenklage, J.; Pivato, A.; Ritzkowski, M. Landfill aeration for emission control before and during landfill mining. Waste Manag. 2015, 46, 420–429. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Oleskowicz-Popiel, P. Process control in biogas plants. In The Biogas Handbook; Woodhead Publishing: Sawston, UK, 2013; pp. 228–247. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, D.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Orzi, V.; Scaglia, B.; Lonati, S.; Riva, C.; Boccasile, G.; Alborali, G.L.; Adani, F. The role of biological processes in reducing both odor impact and pathogen content during mesophilic anaerobic digestion. Sci. Total Environ. 2015, 526, 116–126. [Google Scholar] [CrossRef]
- Byliński, H.; Sobecki, A.; Gębicki, J. The Use of Artificial Neural Networks and Decision Trees to Predict the Degree of Odor Nuisance of Post-Digestion Sludge in the Sewage Treatment Plant Process. Sustainability 2019, 11, 4407. [Google Scholar] [CrossRef]
- Ighravwe, D.E.; Babatunde, D.E. Evaluation of landfill gas plant siting problem: A multi-criteria approach. Environ. Health Eng. Manag. J. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. Comparative analysis of preliminary identification and characteristic of odour sources in biogas plants processing municipal waste in Poland. SN Appl. Sci. 2019, 1, 550. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. Odour Emissions of Municipal Waste Biogas Plants—Impact of Technological Factors, Air Temperature and Humidity. Appl. Sci. 2020, 10, 1093. [Google Scholar] [CrossRef]
- Delgado-Rodríguez, M.; Ruiz-Montoya, M.; Giráldez, I.; López, R.; Madejón, E.; Díaz, M.J. Effect of aeration rate and moisture content on the emissions of selected VOCs during municipal solid waste composting. J. Mater. Cycles Waste Manag. 2012, 14, 371–378. [Google Scholar] [CrossRef]
- Szulczyński, B.; Gębicki, J. Currently Commercially Available Chemical Sensors Employed for Detection of Volatile Organic Compounds in Outdoor and Indoor Air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Zabiegała, B.; Partyka, M.; Zygmunt, B.; Namieśnik, J. Determination of volatile organic compounds in indoor air in the Gdansk area using permeation passive samplers. Indoor Built Environ. 2009, 18, 492–504. [Google Scholar] [CrossRef]
- European Commission. Council directive 1999/13/EC of 11 March 1999 on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain activities and installations. Offic. J. Eur. Commun. 1999, 85, 1–24. [Google Scholar]
- Font, X.; Artola, A.; Sánchez, A. Detection, Composition and Treatment of Volatile Organic Compounds from Waste Treatment Plants. Sensors 2011, 11, 4043–4059. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R.; Céntola, P.; Rossi, A.; Austeri, C. Odour impact assessment in urban areas: Case study of the city of Terni. Procedia Environ. Sci. 2011, 4, 151–157. [Google Scholar] [CrossRef]
- De Feo, G.; De Gisi, S.; Williams, I.D. Public perception of odour and environmental pollution attributed to MSW treatment and disposal facilities: A case study. Waste Manag. 2013, 33, 974–987. [Google Scholar] [CrossRef]
- Kim, K.H.; Jo, S.H.; Song, H.C.; Pandey, S.K.; Song, H.N.; Oh, J.M.; Sunwoo, Y.; Choi, K.C. Diagnostic analysis of offensive odorants in a large municipal waste treatment plant in an urban area. Int. J. Environ. Sci. Technol. 2013, 10, 261–274. [Google Scholar] [CrossRef]
- Orzi, V.; Riva, C.; Scaglia, B.; D’Imporzano, G.; Tambone, F.; Adani, F. Anaerobic digestion coupled with digestate injection reduced odour emissions from soil during manure distribution. Sci. Total Environ. 2018, 621, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhao, Y.; Tan, H.; Liu, Y.; Lu, W. Parameter sensitivity to concentrations and transport distance of odorous compounds from solid waste facilities. Sci. Total Environ. 2019, 651, 2158–2165. [Google Scholar] [CrossRef]
- World Health Organization Publications. Air Quality Guidelines for Europe; European Series No. 91; World Health Organization: Copenhagen, Denmark, 2000. [Google Scholar]
- Bax, C.; Sironi, S.; Capelli, L. How Can Odors Be Measured? An Overview of Methods and Their Applications. Atmosphere 2020, 11, 92. [Google Scholar] [CrossRef]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Le Roux, S.; Soutrel, I.; Daumoin, M.; Benoist, J.C. Chemical and odor characterization of gas emissions released during composting of solid wastes and digestates. J. Environ. Manag. 2019, 233, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Eitzer, B.D. Emissions of volatile organic chemicals from municipal solid waste composting facilities. Environ. Sci. Technol. 1995, 29, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Rodríguez, M.; Ruiz-Montoya, M.; Giraldez, I.; Cabeza, I.O.; López, R.; Díaz, M.J. Effect of control parameters on emitted volatile compounds in municipal solid waste and pine trimmings composting. J. Environ. Sci. Health Toxic/Hazard. Subst. Environ. Eng. 2010, 45, 855–862. [Google Scholar] [CrossRef]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M. Odour Detection Methods: Olfactometry and Chemical Sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef]
- Szulczyński, B.; Wasilewski, T.; Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Namieśnik, J.; Gębicki, J. Different Ways to Apply a Measurement Instrument of E-Nose Type to Evaluate Ambient Air Quality with Respect to Odour Nuisance in a Vicinity of Municipal Processing Plants. Sensors 2017, 17, 2671. [Google Scholar] [CrossRef]
- Wiśniewska, M. Methods of Assessing Odour Emissions from Biogas Plants Processing Municipal Waste. J. Ecol. Eng. 2020, 21, 140–147. [Google Scholar] [CrossRef]
- Laor, Y.; Parker, D.; Pagé, T. Measurement, prediction, and monitoring of odors in the environment: A critical review. Rev. Chem. Eng. 2014, 30, 139–166. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of Portable and Low-Cost Sensors for the Ambient Air Monitoring of Benzene and Other Volatile Organic Compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Boeker, P. On “Electronic Nose” methodology. Sens. Actuators B Chem. 2014, 204, 2–17. [Google Scholar] [CrossRef]
- Rezende, G.C.; Le Calvé, S.; Brandner, J.J.; Newport, D. Micro photoionization detectors. Sens. Actuators B Chem. 2019, 287, 86–94. [Google Scholar] [CrossRef]
- Stetter, J.R.; Penrose, W.R. Understanding Chemical Sensors and Chemical Sensor Arrays (Electronic Noses): Past, Present, and Future. Sens. Update 2002, 10, 189–229. [Google Scholar] [CrossRef]
- Wilson, A.D. Review of Electronic-nose Technologies and Algorithms to Detect Hazardous Chemicals in the Environment. Procedia Technol. 2012, 1, 453–463. [Google Scholar] [CrossRef]
- Cao, Z.; Buttner, W.J.; Stetter, J.R. The properties and applications of amperometric gas sensors. Electroanalysis 1992, 4, 253–266. [Google Scholar] [CrossRef]
- Bontempelli, G.; Comisso, N.; Toniolo, R.; Schiavon, G. Electroanalytical sensors for nonconducting media based on electrodes supported on perfluorinated ion-exchange membranes. Electroanalysis 1997, 9, 433–443. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Kulig, A.; Lelicińska-Serafin, K. Identification and preliminary characteristics of odour sources in biogas plants processing municipal waste. SHS Web of Conf. 2018, 57, 02016. [Google Scholar] [CrossRef]
- Jia, C.; Cao, K.; Valaulikar, R.; Xianqiang Fu, X.; Sorin, A.B. Variability of Total Volatile Organic Compounds (TVOC) in the Indoor Air of Retail Stores. Int. J. Environ. Res. Public Health 2019, 16, 4622. [Google Scholar] [CrossRef]
- EUR—Lex. Commission Implementing Decision (EU) 2018/1147 of 10 August 2018 establishing best available techniques (BAT) conclusions for waste treatment, under Directive 2010/75/EU of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ:L:2018:208:TOC&uri=uriserv:OJ.L_.2018.208.01.0038.01.ENG (accessed on 17 August 2018).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).