Metabolic Profiling and In Vitro Assessment of the Immunomodulatory Effects of Hydrodistillation-Derived Extracts from the Fruticose Lichen Pseudevernia furfuracea (L.) Zopf. on Human Lymphocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Lichen Sample Collection and Extraction
2.3. Chemical Characterization
UHPLC-MS/MS Equipment and Conditions
2.4. Cell System
2.5. Cytotoxicity Evaluation
2.6. Flow Cytometry
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Putative Identification of Compounds by UHPLC-MS/MS
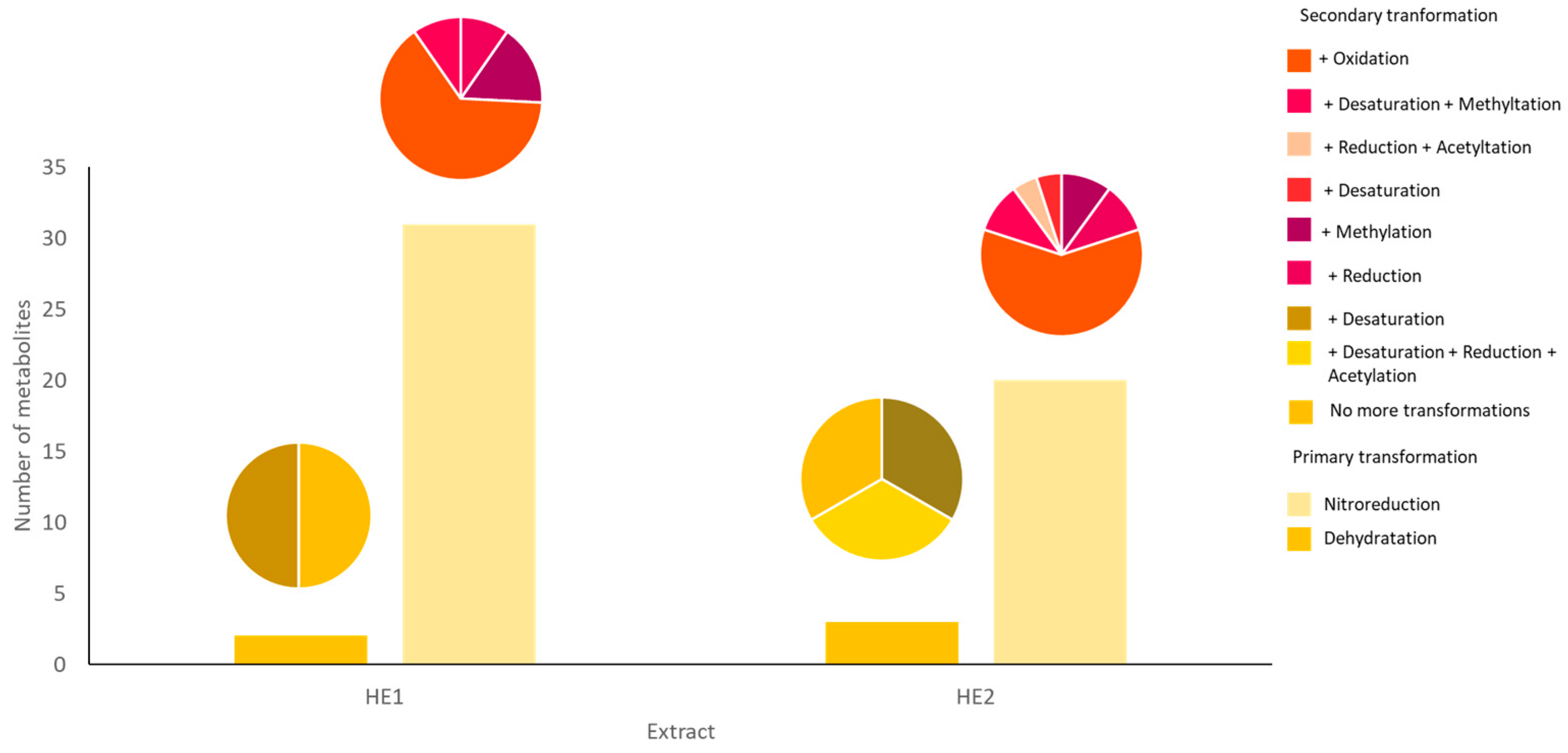
3.2. Putative Identification of the Transformation Products of Atraric Acid
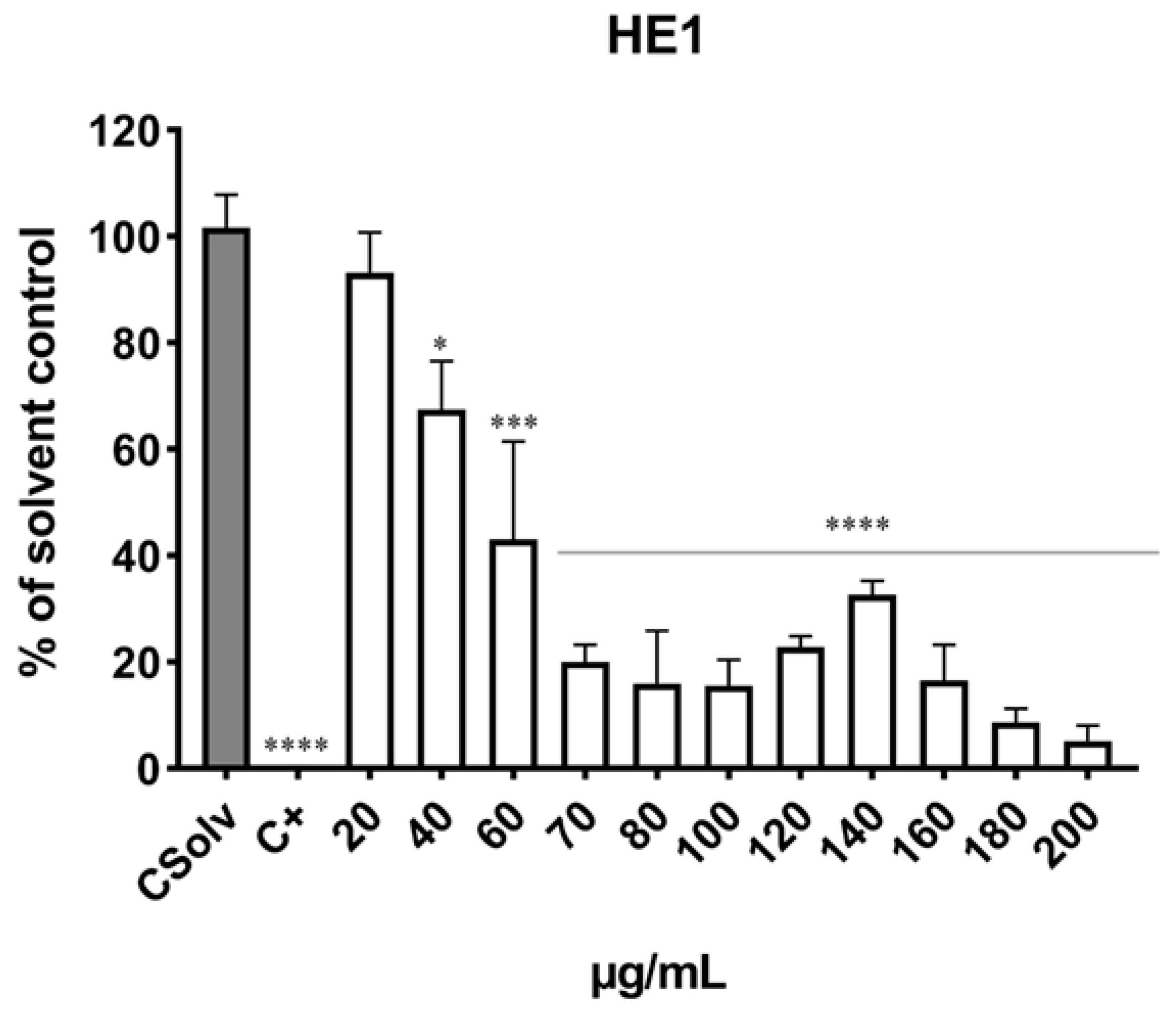
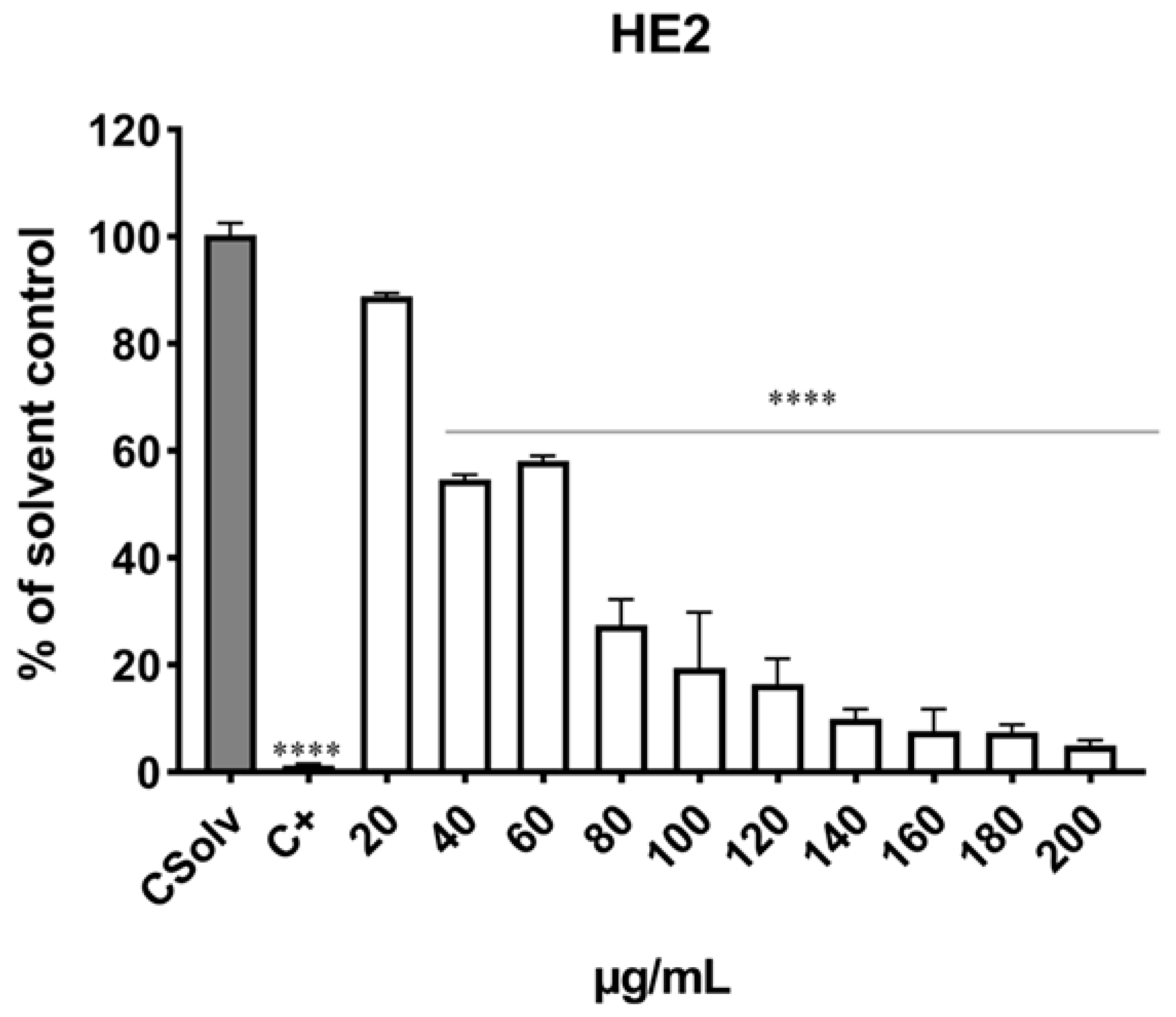
3.3. Determination of Cell Viability
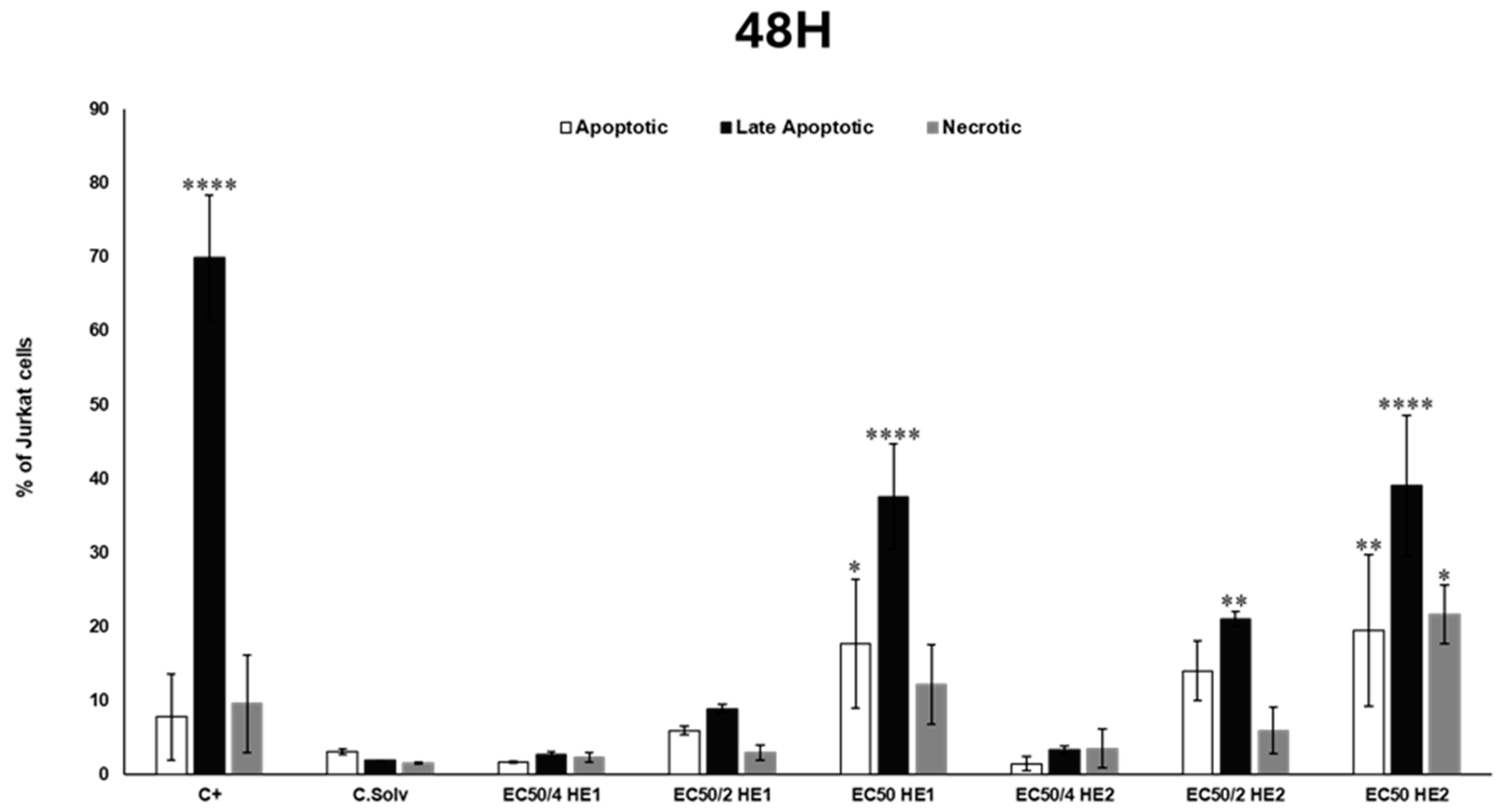
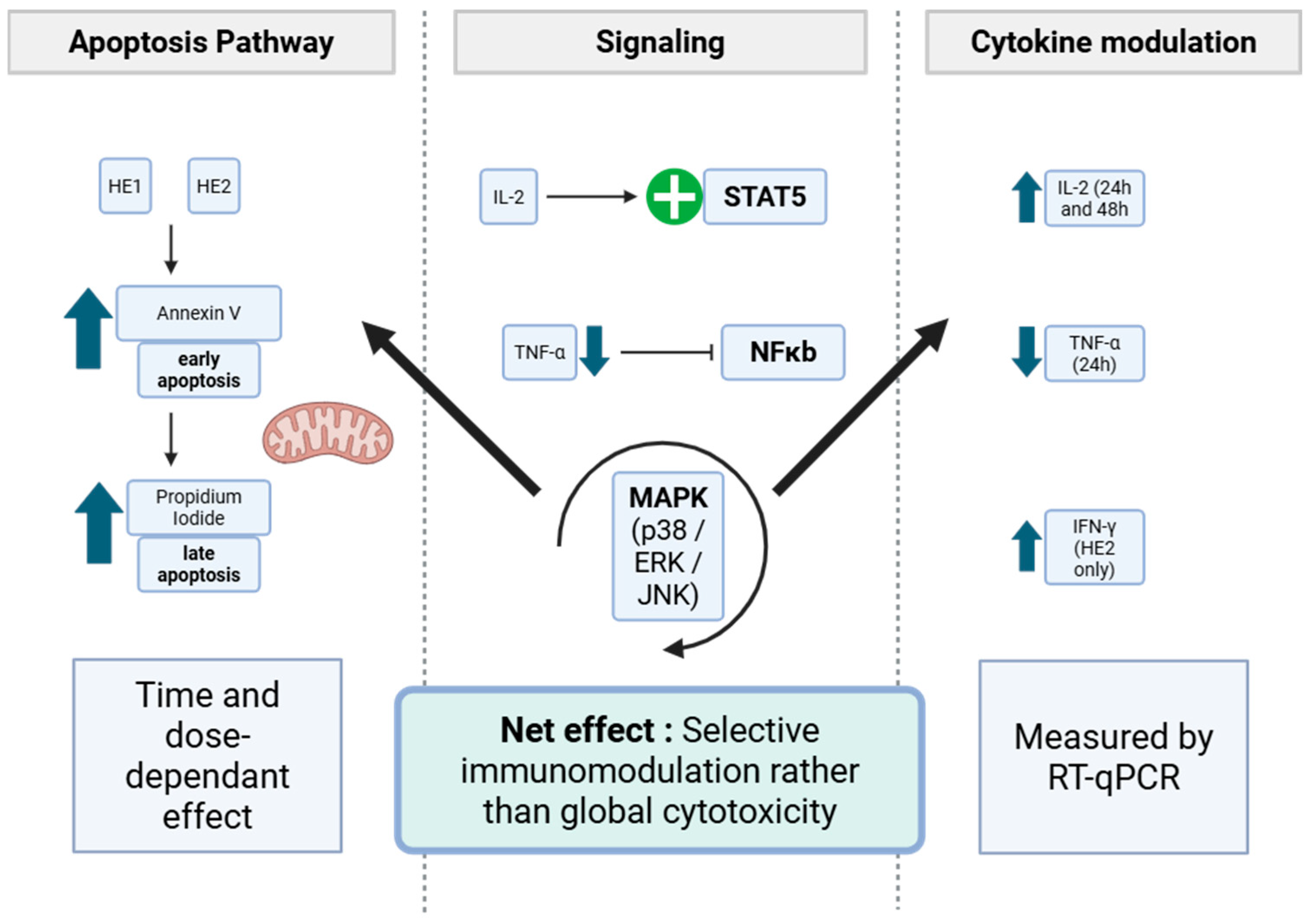
3.4. Analysis of Cell Death Mechanisms (Apoptosis/Necrosis) Through Flow Cytometry
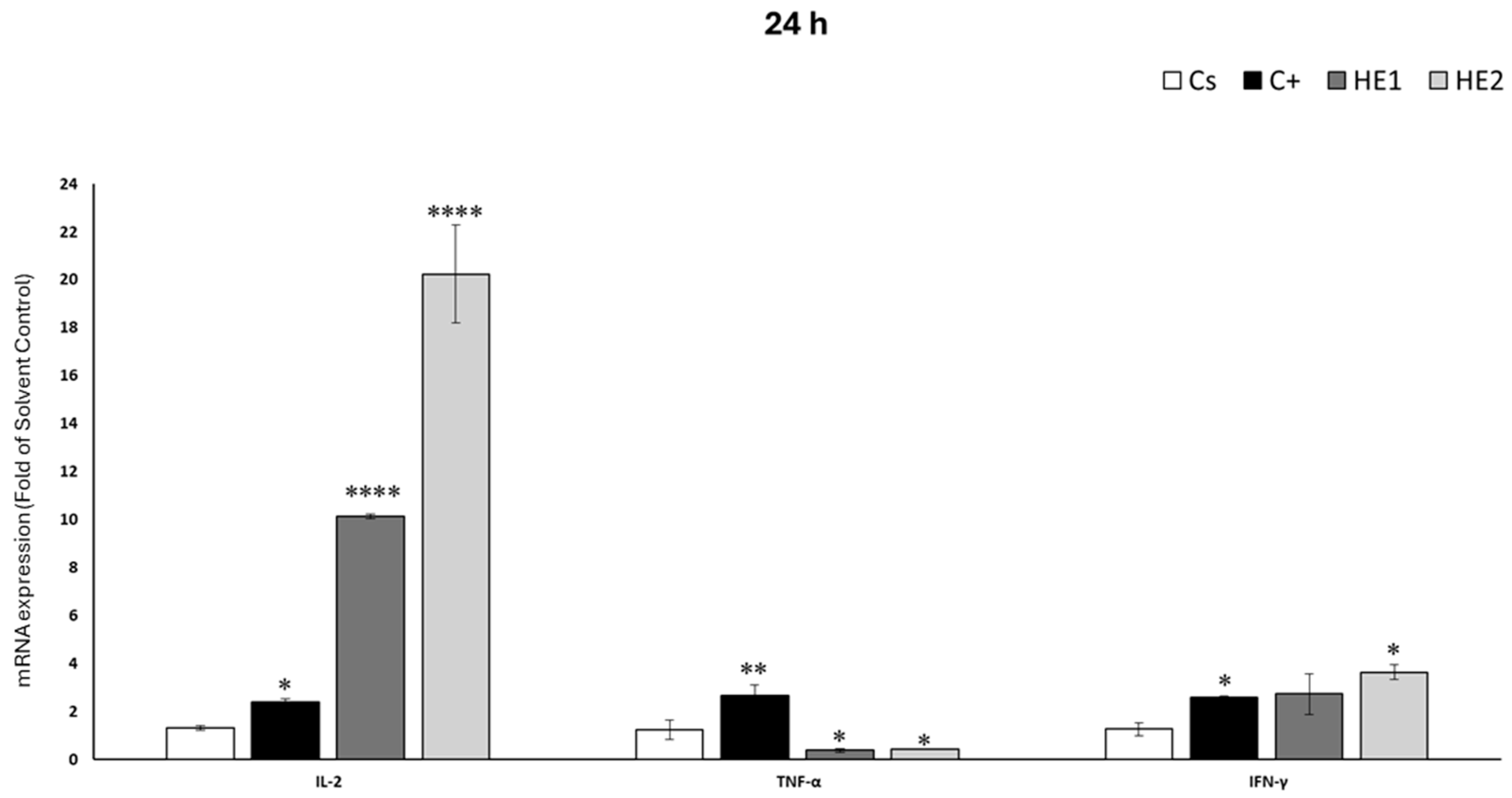
3.5. Effects of HE1 and HE2 on Cytokine mRNA Expression by RT-qPCR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UHPLC-MS/MS | ultra-high-performance liquid chromatography coupled with tandem mass spectrometry |
| RT-qPCR | quantitative real-time polymerase chain reaction |
| IL-2 | interleukin 2 |
| TNF-α | tumor necrosis factor α |
| IFN-γ | interferon γ |
| EC50 | effective concentration 50 |
| 2D | two-dimensional |
| 3D | three-dimensional |
| mRNA | messenger ribonucleic acid |
| C- | negative reaction to calcium hypochlorite |
| K- | negative reaction to potassium hydroxide |
| K+ | positive reaction to potassium hydroxide |
| GC-MS | Gas chromatography coupled to mass spectrometry |
| ATCC | American Type Culture Collection |
| DMSO | dimethylsulfoxyde |
| BB | annexin-binding buffer |
| Annexin V-FITC | annexin V conjugated with fluorescein isothiocyanate |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| LPS | lipopolysaccharide |
| RT | reverse transcription |
| cDNA | complementary DNA |
| ANOVA | analysis of variance |
| NCI | American National Cancer Institute |
| IC50 | inhibitory concentration 50 |
| MTS assay | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay |
| MTT test | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay |
| WST-1 assay | water-soluble tetrazolium-1 assay |
| phy | physodic acid |
References
- Yasmin, F.; Jabin, R. Physiology of Lichen. In Chemistry, Biology and Pharmacology of Lichen; John Wiley and Sons: Hoboken, NJ, USA, 2024; pp. 71–80. [Google Scholar]
- Ndhlovu, N.T.; Minibayeva, F.; Beckett, R.P. A Role for Secondary Metabolites in Desiccation Tolerance in Lichens. Microbiol. Res. 2024, 15, 225–235. [Google Scholar] [CrossRef]
- Shah, A.A.; Badshah, L.; Muhammad, M.; Basit, A.; Ullah, I.; Mohamed, H.I.; Khan, A. Chapter 5-Secondary metabolites of lichens and their application. In Fungal Secondary Metabolites; Abd-Elsalam, K.A., Mohamed, H.I., Eds.; Elsevier: Berlin/Heidelberg, Germany, 2024; pp. 91–115. [Google Scholar]
- Singh, G. Linking Lichen Metabolites to Genes: Emerging Concepts and Lessons from Molecular Biology and Metagenomics. J. Fungi 2023, 9, 160. [Google Scholar] [CrossRef]
- Faiz ul Rasul, H.; Afzal, F.; Khalid, W.; Ahmad, M.; Gull, S.; Faiz ul Rasool, I.; Ilyas, M.; Javed, M. Antioxidant Properties of Lichen. In Chemistry, Biology and Pharmacology of Lichen; John Wiley and Sons: Hoboken, NJ, USA, 2024; pp. 153–167. [Google Scholar]
- Ahmed, M.Z.; Rao, T.; Khan, N.A.; Aslam, M.; Pane, Y.S. Antimicrobial Activities of Lichens. In Chemistry, Biology and Pharmacology of Lichen; John Wiley and Sons: Hoboken, NJ, USA, 2024; pp. 169–191. [Google Scholar]
- Nugraha, A.S.; Lam, T.H.Y.; Wongso, H.; Firli, L.N.; Keller, P.A. Lichens. In Chemistry, Biology and Pharmacology of Lichen; John Wiley and Sons: Hoboken, NJ, USA, 2024; pp. 193–229. [Google Scholar]
- Le Pogam, P.; Herbette, G.; Boustie, J. Analysis of Lichen Metabolites, a Variety of Approaches. In Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 1, pp. 229–261. [Google Scholar]
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Sanad, H.; Belattmania, Z.; Nafis, A.; Hassouani, M.; Mazoir, N.; Reani, A.; Hassani, L.; Vasconcelos, V.; Sabour, B. Chemical Composition and In Vitro Antioxidant and Antimicrobial Activities of the Marine Cyanolichen Lichina pygmaea Volatile Compounds. Mar. Drugs 2022, 20, 169. [Google Scholar] [CrossRef]
- Güvenç, A.; Akkol, E.K.; Süntar, I.; Keles, H.; Yildiz, S.; Çalis, I. Biological activities of Pseudevernia furfuracea (L.) Zopf extracts and isolation of the active compounds. J. Ethnopharmacol. 2012, 144, 726–734. [Google Scholar] [CrossRef]
- Kosanic, M.; Manojlovic, N.; Jankovic, S.; Stanojkovic, T.; Rankovic, B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef]
- Aoussar, N.; Laasri, F.E.; Bourhia, M.; Manoljovic, N.; Mhand, R.A.; Rhallabi, N.; Ullah, R.; Shahat, A.A.; Noman, O.M.; Nasr, F.A.; et al. Phytochemical Analysis, Cytotoxic, Antioxidant, and Antibacterial Activities of Lichens. Evid.-Based Complement. Altern. Med. 2020, 2020, 8104538. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Conlan, X.A.; Areche, C.; Dilawari, R.; Goel, M. Metabolite Profiling of the Indian Food Spice Lichen, Pseudevernia furfuracea Combined with Optimised Extraction Methodology to Obtain Bioactive Phenolic Compounds. Front. Pharmacol. 2021, 12, 629695. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Goga, M.; Kotorova, K.; Sebova, D.; Frenak, R.; Tkacikova, L.; Mojzis, J. Screening Evaluation of Antiproliferative, Antimicrobial and Antioxidant Activity of Lichen Extracts and Secondary Metabolites In Vitro. Plants 2023, 12, 611. [Google Scholar] [CrossRef]
- Furmanek, Ł.; Żurek, N.; Kapusta, I.; Seaward, M.R.D.; Czarnota, P. The cytotoxic potential of polyphenols extracted from eight lichen species and their antioxidant activity against the cancer cell lines. Biocatal. Agric. Biotechnol. 2024, 62, 103424. [Google Scholar] [CrossRef]
- Essadki, Y.; Hilmi, A.; Cascajosa-Lira, A.; Girão, M.; Darrag, E.M.; Martins, R.; Romane, A.; El Amrani Zerrifi, S.; Mugani, R.; Tazart, Z.; et al. In Vitro Antimicrobial Activity of Volatile Compounds from the Lichen Pseudevernia furfuracea (L.) Zopf. Against Multidrug-Resistant Bacteria and Fish Pathogens. Microorganisms 2024, 12, 2336. [Google Scholar] [CrossRef]
- Montano, M. 2-Model systems. In Translational Biology in Medicine; Montano, M., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 9–33. [Google Scholar]
- Cebadero-Dominguez, Ó.; Casas-Rodríguez, A.; Puerto, M.; Cameán, A.M.; Jos, A. In vitro safety assessment of reduced graphene oxide in human monocytes and T cells. Environ. Res. 2023, 232, 116356. [Google Scholar] [CrossRef]
- Clauzade, G.; Roux, C. Likenoj de Okcidenta Eŭropo: Ilustrita Determinlibro; Société Botanique du Centre-Ouest: Nercillac, France, 1985. [Google Scholar]
- Nimis, P.L. ITALIC-The Information System on Italian Lichens. 2024. Available online: https://italic.units.it/ (accessed on 2 October 2025).
- Cascajosa-Lira, A.; Medrano-Padial, C.; Pichardo, S.; de la Torre, J.M.; Baños, A.; Jos, A.; Cameán, A.M. Identification of in vitro metabolites of an Allium organosulfur compound and environmental toxicity prediction as part of its risk assessment. Environ. Res. 2023, 229, 116001. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3-B. [Google Scholar] [CrossRef]
- Casas-Rodriguez, A.; Cebadero-Dominguez, O.; Puerto, M.; Camean, A.M.; Jos, A. Immunomodulatory Effects of Cylindrospermopsin in Human T Cells and Monocytes. Toxins 2023, 15, 301. [Google Scholar] [CrossRef]
- Noël, A.; Garnier, A.; Clément, M.; Rouaud, I.; Sauvager, A.; Bousarghin, L.; Vásquez-Ocmín, P.; Maciuk, A.; Tomasi, S. Lichen-associated bacteria transform antibacterial usnic acid to products of lower antibiotic activity. Phytochemistry 2021, 181, 112535. [Google Scholar] [CrossRef]
- Sadgrove, N.; Padilla-González, G.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity, 6th ed.; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Kello, M.; Kuruc, T.; Petrova, K.; Goga, M.; Michalova, Z.; Coma, M.; Rucova, D.; Mojzis, J. Pro-Apoptotic Potential of Pseudevernia furfuracea (L.) Zopf Extract and Isolated Physodic Acid in Acute Lymphoblastic Leukemia Model In Vitro. Pharmaceutics 2021, 13, 2173. [Google Scholar] [CrossRef] [PubMed]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Petrova, K.; Kello, M.; Kuruc, T.; Backorova, M.; Petrovova, E.; Vilkova, M.; Goga, M.; Rucova, D.; Backor, M.; Mojzis, J. Potential Effect of Pseudevernia furfuracea (L.) Zopf Extract and Metabolite Physodic Acid on Tumour Microenvironment Modulation in MCF-10A Cells. Biomolecules 2021, 11, 420. [Google Scholar] [CrossRef]
- Thakur, M.; Kapoor, B.; Kapoor, D.; Sharma, N.R. Lichens: A promising source of anti-cancerous activity and their molecular mechanisms. S. Afr. J. Bot. 2023, 159, 155–163. [Google Scholar] [CrossRef]
- Seklic, D.S.; Obradovic, A.D.; Stankovic, M.S.; Zivanovic, M.N.; Mitrovic, T.L.J.; Stamenkovic, S.M.; Markovic, S.D. Proapoptotic and Antimigratory Effects of Pseudevernia furfuracea and Platismatia glauca on Colon Cancer Cell Lines. Food Technol. Biotechnol. 2018, 56, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, R.; Henke, M.; Roser, L.; Ulshöfer, T.; Calchera, A.; Singh, G.; Parnham, M.J.; Geisslinger, G.; Fürst, R.; Schmitt, I.; et al. Unraveling the Pharmacological Potential of Lichen Extracts in the Context of Cancer and Inflammation with a Broad Screening Approach. Front. Pharmacol. 2020, 11, 1322. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Conlan, X.A.; Gupta, M.; Areche, C.; Bhat, M.; Goel, M. Evaluation of the anticancer potential of secondary metabolites from Pseudevernia furfuracea based on epidermal growth factor receptor inhibition. Nat. Prod. Res. 2022, 36, 6439–6442. [Google Scholar] [CrossRef] [PubMed]
- Zambare, V.P.; Christopher, L.P. Biopharmaceutical potential of lichens. Pharm. Biol. 2012, 50, 778–798. [Google Scholar] [CrossRef]
- El-Garawani, I.M.; Elkhateeb, W.A.; Zaghlol, G.M.; Almeer, R.S.; Ahmed, E.F.; Rateb, M.E.; Abdel Moneim, A.E. Candelariella vitellina extract triggers in vitro and in vivo cell death through induction of apoptosis: A novel anticancer agent. Food Chem. Toxicol. 2019, 127, 110–119. [Google Scholar] [CrossRef]
- Bačkorová, M.; Jendželovský, R.; Kello, M.; Bačkor, M.; Mikeš, J.; Fedoročko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. In Vitro 2012, 26, 462–468. [Google Scholar] [CrossRef]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell. Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yoon, S.; Yang, Y.; Lee, H.-B.; Oh, S.; Jeong, M.-H.; Kim, J.-J.; Yee, S.-T.; Crişan, F.; Moon, C.; et al. Lichen Secondary Metabolites in Flavocetraria cucullata Exhibit Anti-Cancer Effects on Human Cancer Cells through the Induction of Apoptosis and Suppression of Tumorigenic Potentials. PLoS ONE 2014, 9, e111575. [Google Scholar] [CrossRef]
- Dinçsoy, A.B.; Cansaran Duman, D. Changes in apoptosis-related gene expression profiles in cancer cell lines exposed to usnic acid lichen secondary metabolite. Turk. J. Biol. 2017, 41, 484–493. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. Apoptosis mediated cytotoxicity of citrus obacunone in human pancreatic cancer cells. Toxicol. In Vitro 2011, 25, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Zambare, V.; Suntres, Z.; Christopher, L. Isolation, Characterization, and Breast Cancer Cytotoxic Activity of Gyrophoric Acid from the Lichen Umbilicaria muhlenbergii. Processes 2022, 10, 1361. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Liu, K.D. Overview of interleukin-2 function, production and clinical applications. Cytokine 2004, 28, 109–123. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Z.; Wang, Z.; Liu, J.; Ning, K. Based on the immune system: The role of the IL-2 family in pancreatic disease. Front. Immunol. 2025, 16, 1480496. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Qian, K.; Wu, S.; Li, B.; Guo, Z.; Yin, X.; Huang, Y.; Ye, J.; Tu, X.; Fu, S. Functional characterization of TNF-α in pufferfish (Takifugu obscurus) in immune response and apoptosis against Aeromonas hydrophila. J. Fish Dis. 2021, 44, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Leonard, W.J.; Lin, J.-X.; O’Shea, J.J. The γc Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Seklic, D.S.; Jovanovic, M.M.; Virijevic, K.D.; Grujic, J.N.; Zivanovic, M.N.; Markovic, S.D. Pseudevernia furfuracea inhibits migration and invasion of colorectal carcinoma cell lines. J. Ethnopharmacol. 2022, 284, 114758. [Google Scholar] [CrossRef] [PubMed]


| Name | Molecular Formula | Molecular Weight | RT | Area Max | HE1 | HE2 |
|---|---|---|---|---|---|---|
| (-)-Menthylacetate | C12H22O2 | 198.16156 | 16.606 | 5.33 × 106 | - | + |
| [FAhydroxy(20:3)]11_12-dihydroxy-5Z_8Z_14Z-eicosatrienoicacid | C20H34O4 | 338.24515 | 15.138 | 2.65 × 107 | - | + |
| 1,2,2,6,6-Pentamethyl-4-piperidinyl acrylate | C13H23NO2 | 225.17225 | 17.992 | 1.64 × 107 | + | + |
| 1_3_8-Naphthalenetriol | C10H8O3 | 176.04704 | 10.793 | 1.48 × 107 | - | + |
| 2,4-Diethyl-thioxanthen-9-one | C17H16OS | 268.09157 | 17.101 | 4.37 × 106 | + | + |
| 2,6-Diethylaniline | C10H15N | 149.11994 | 0.595 | 4.59 × 106 | + | + |
| 2-oxa-4-azatetracyclo [6.3.1.1~6,10~.0~1,5~]tridecan-3-one | C11H15NO2 | 193.11005 | 0.562 | 1.29 × 107 | + | + |
| 3,5-di-tert-Butyl-4-hydroxybenzaldehyde | C15H22O2 | 234.16151 | 14.297 | 8.06 × 106 | - | + |
| 4-Isopropylaniline | C9H13N | 135.1044 | 0.571 | 1.98 × 107 | + | + |
| 4-O-acetyl hygrophorone A14 | C22H38O5 | 382.27127 | 15.169 | 3.36 × 107 | - | + |
| 4-Pentylaniline | C11H17N | 163.13567 | 18.945 | 1.01 × 107 | + | - |
| 5-(6-hydroxy-6-methyloctyl)-2,5-dihydrofuran-2-one | C13H22O3 | 243.18278 | 18.098 | 9.90 × 106 | + | + |
| 9,10-phenanthraquinone | C14H8O2 | 208.05211 | 11.148 | 2.70 × 107 | - | + |
| Acetone oxime | C3H7NO | 73.05259 | 0.595 | 1.27 × 107 | + | + |
| Bacillamidin G | C17H35NO | 269.27114 | 17.66 | 3.67 × 106 | + | + |
| Berkazaphilone A | C13H16O3 | 220.10947 | 14.611 | 6.35 × 106 | - | + |
| Caprolactam | C6H11NO | 113.08384 | 2.766 | 2.62 × 108 | - | + |
| Cladoacetal B | C12H12O3 | 204.07826 | 15.576 | 6.66 × 106 | - | + |
| DEET | C12H17NO | 191.13064 | 9.503 | 6.24 × 106 | - | + |
| Diaminotoluene | C7H10N2 | 122.08408 | 0.568 | 3.84 × 106 | + | - |
| Dibutyl hexanedioate | C14H26O4 | 258.18266 | 15.236 | 1.36 × 107 | - | + |
| Dibutyl phthalate | C16H22O4 | 278.15151 | 15.452 | 8.12 × 107 | + | + |
| Diethyl phthalate | C12H14O4 | 222.08883 | 10.794 | 1.02 × 107 | - | + |
| diisopropylethylamine | C8H19N | 129.15148 | 18.346 | 7.73 × 107 | - | + |
| Di-N-butylnitrosoamine | C8H18N2O | 158.14157 | 2.884 | 9.59 × 106 | - | + |
| Erucamide | C22H43NO | 337.33387 | 19.148 | 1.63 × 108 | + | + |
| Eugenol methyl ether | C11H14O2 | 178.09907 | 6.501 | 6.67 × 106 | - | + |
| F1839-B | C24H33NO6 | 431.23015 | 11.211 | 1.59 × 107 | + | + |
| FL6DDAGI0001_a | C22H26O6 | 386.1723 | 11.212 | 2.25 × 107 | + | + |
| Heptaminol | C8H19NO | 145.14635 | 0.569 | 2.33 × 106 | + | + |
| MDPBP | C15H19NO3 | 261.13602 | 15.027 | 5.62 × 106 | - | + |
| N,N-Diethylaniline | C10H15N | 149.11997 | 19.072 | 1.88 × 108 | + | + |
| N-Methylpyridinium | C6H7N | 93.05765 | 0.562 | 1.43 × 106 | + | - |
| NP-014924 | C10H12O4 | 164.04719 | 9.917 | 5.75 × 107 | + | + |
| NP-020014 | C15H26O3 | 276.17208 | 15.028 | 1.31 × 107 | - | + |
| Oleamide | C18H35NO | 281.2712 | 17.717 | 3.72 × 106 | + | - |
| Phenmetrazine | C11H15NO | 177.11504 | 0.563 | 6.46 × 106 | + | + |
| Phthalic anhydride | C8H4O3 | 148.0158 | 10.793 | 1.50 × 107 | - | + |
| Pseudocapsaicin | C17H27NO3 | 293.19865 | 14.61 | 1.54 × 107 | - | + |
| Stearoyl ethanolamide | C20H41NO2 | 309.30227 | 18.438 | 1.40 × 107 | + | + |
| Thermolide E | C28H53NO8 | 531.37608 | 15.177 | 1.36 × 107 | - | + |
| Tributyl phosphate | C12H27O4P | 266.16432 | 14.018 | 6.15 × 106 | - | + |
| Triethylene glycol | C6H14O4 | 150.08891 | 0.572 | 1.38 × 107 | - | + |
| Transformation | Composition Change | Molecular Formula | Molecular Weight | RT | Area Max | HE1 | HE2 |
|---|---|---|---|---|---|---|---|
| Dehydration, Desaturation | -(H4O) | C10H8O3 | 176.04705 | 10.794 | 1.45 × 107 | + | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07819 | 14.915 | 1.96 × 106 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07816 | 18.579 | 1.89 × 106 | - | + |
| Dehydration | -(CH4O) | C9H8O3 | 164.04704 | 9.918 | 5.87 × 106 | + | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07816 | 17.633 | 1.56 × 106 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07823 | 15.827 | 2.55 × 106 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07817 | 16.709 | 1.51 × 106 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07823 | 16.169 | 1.40 × 106 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 16.867 | 1.39 × 106 | + | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07823 | 16.015 | 3.33 × 106 | + | - |
| Dehydration, Reduction, Acetylation | +(C2H2) | C12H14O4 | 222.08885 | 10.793 | 1.03 × 107 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 19.35 | 1.18 × 106 | + | - |
| Nitro Reduction, Oxidation | -(O) + (H2) | C10H14O3 | 182.09375 | 18.056 | 6.99 × 105 | + | - |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.11452 | 18.726 | 3.98 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 16.625 | 9.97 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 16.928 | 9.96 × 105 | + | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07819 | 16.204 | 8.93 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07816 | 17.52 | 2.93 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 16.248 | 7.91 × 105 | + | - |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.1145 | 17.584 | 3.91 × 105 | - | + |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.11461 | 18.295 | 3.91 × 105 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 16.882 | 1.09 × 106 | - | + |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.11449 | 17.825 | 3.80 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07817 | 18.63 | 5.77 × 105 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07814 | 18.686 | 7.76 × 105 | + | - |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.11453 | 18.576 | 3.76 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07817 | 17.483 | 3.76 × 105 | - | + |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.1145 | 18.195 | 3.75 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07818 | 18.86 | 9.74 × 105 | + | + |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.11453 | 18.628 | 3.74 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07816 | 18.305 | 7.73 × 105 | + | + |
| Nitro Reduction, Reduction | -(CO2) + (H2) | C9H14O2 | 154.09894 | 16.789 | 4.72 × 105 | + | - |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07818 | 19.284 | 9.69 × 105 | + | - |
| Desaturation, Nitro Reduction, Methylation | -(O2) + (CH2) | C11H14O2 | 178.09886 | 17.621 | 4.64 × 105 | + | - |
| Nitro Reduction, Reduction | -(CO2) + (H2) | C9H14O2 | 154.09892 | 16.424 | 4.54 × 105 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.0782 | 17.32 | 1.05 × 106 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07823 | 17.076 | 9.48 × 105 | + | - |
| Nitro Reduction, Methylation | -(O2) + (CH4) | C11H16O2 | 180.1145 | 17.51 | 5.47 × 105 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07819 | 17.491 | 1.04 × 106 | + | - |
| Desaturation, Nitro Reduction, Methylation | -(O2) + (CH2) | C11H14O2 | 178.09885 | 18.906 | 3.35 × 105 | + | + |
| Nitro Reduction, Reduction | -(CO2) + (H2) | C9H14O2 | 154.09893 | 16.21 | 4.30 × 105 | + | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07814 | 17.505 | 3.30 × 105 | - | + |
| Desaturation, Nitro Reduction | -(O2) | C10H12O2 | 164.08324 | 18.52 | 3.24 × 105 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07817 | 17.191 | 3.23 × 105 | + | - |
| Nitro Reduction, Reduction, Acetylation | -(O) + (C2H6) | C12H18O3 | 210.12503 | 18.342 | 3.19 × 105 | - | + |
| Desaturation, Nitro Reduction, Methylation | -(O2) + (CH2) | C11H14O2 | 178.09889 | 18.742 | 4.11 × 105 | + | - |
| Desaturation, Nitro Reduction, Methylation | -(O2) + (CH2) | C11H14O2 | 178.09885 | 17.504 | 5.07 × 105 | - | + |
| Nitro Reduction, Oxidation | -(CO) | C9H12O3 | 168.07816 | 18.906 | 7.03 × 105 | - | + |
| Nitro Reduction | -(CO2) | C9H12O2 | 152.08328 | 16.93 | 5.03 × 105 | - | + |
| Nitro Reduction, Reduction | -(CO2) + (H2) | C9H14O2 | 154.09892 | 16.599 | 6.02 × 105 | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essadki, Y.; Casas-Rodríguez, A.; Cascajosa-Lira, A.; Diez-Quijada, L.; Campos, A.; Vasconcelos, V.; El Khalloufi, F.; Oudra, B.; Cameán, A.M.; Jos, A. Metabolic Profiling and In Vitro Assessment of the Immunomodulatory Effects of Hydrodistillation-Derived Extracts from the Fruticose Lichen Pseudevernia furfuracea (L.) Zopf. on Human Lymphocytes. J. Xenobiot. 2025, 15, 201. https://doi.org/10.3390/jox15060201
Essadki Y, Casas-Rodríguez A, Cascajosa-Lira A, Diez-Quijada L, Campos A, Vasconcelos V, El Khalloufi F, Oudra B, Cameán AM, Jos A. Metabolic Profiling and In Vitro Assessment of the Immunomodulatory Effects of Hydrodistillation-Derived Extracts from the Fruticose Lichen Pseudevernia furfuracea (L.) Zopf. on Human Lymphocytes. Journal of Xenobiotics. 2025; 15(6):201. https://doi.org/10.3390/jox15060201
Chicago/Turabian StyleEssadki, Yasser, Antonio Casas-Rodríguez, Antonio Cascajosa-Lira, Leticia Diez-Quijada, Alexandre Campos, Vitor Vasconcelos, Fatima El Khalloufi, Brahim Oudra, Ana M. Cameán, and Angeles Jos. 2025. "Metabolic Profiling and In Vitro Assessment of the Immunomodulatory Effects of Hydrodistillation-Derived Extracts from the Fruticose Lichen Pseudevernia furfuracea (L.) Zopf. on Human Lymphocytes" Journal of Xenobiotics 15, no. 6: 201. https://doi.org/10.3390/jox15060201
APA StyleEssadki, Y., Casas-Rodríguez, A., Cascajosa-Lira, A., Diez-Quijada, L., Campos, A., Vasconcelos, V., El Khalloufi, F., Oudra, B., Cameán, A. M., & Jos, A. (2025). Metabolic Profiling and In Vitro Assessment of the Immunomodulatory Effects of Hydrodistillation-Derived Extracts from the Fruticose Lichen Pseudevernia furfuracea (L.) Zopf. on Human Lymphocytes. Journal of Xenobiotics, 15(6), 201. https://doi.org/10.3390/jox15060201











