Abstract
Fall armyworm (FAW) Spodoptera frugiperda is currently being considered as a serious insect pest in maize that causes significant yield losses worldwide. Silicon (Si) and plant growth regulators (PGRs) are known to induce resistance against biotic and abiotic stresses thereby enhancing the yield. This study was conducted to determine the influence of Si and PGRs on the incidence and damage of FAW on maize (Zea mays L.) under field condition. The experiment was conducted in both Kharif and Rabi seasons using a randomized complete block design with three replications and treatments. Various combinations of foliar silicic acid (FSA) and two PGRs such as gibberelic acid (GA3) and jasmonic acid (JA) were tested to study their effects on FAW incidence and maize yield. The application of FSA at 2mL/plant + GA3 at 0.5 mg/plant recorded the lowest number of larvae per plant (0.39 larva/plant) with the lowest damage score of 2.55 (Davis scale). The percent infestation was also low for the same treatment, i.e., 34.14 percent infestation with the highest percent reduction over control (56.43%). The highest yield (58.39 q/ha) and cost–benefit ratio (1:2.34) was recorded for FSA at 2 mL/plant + GA3 at 0.5 mg/plant, which was considered as the best treatment. This study demonstrated that exogenous application of Si along with PGRs has significant negative effect on field incidence of FAW and enhanced the yield of maize.
1. Introduction
Silicon (Si) is the second most abundant element in the Earth’s crust after oxygen, and it plays a significant role in plant life. Si has a range of effects on plants, including the regulation of growth and defense against various stresses [1]. The defense strategy against herbivorous insects by plants is both direct and indirect. The defense gets reinforced if plants are supplied with Si, which strengthens their physical defense [2,3]. There are increasing pieces of evidence of multiple roles of Si in the defense against pests. Silicon application in combination with plant growth regulators (PGRs) further enhances the uptake of silica and other defense-related compounds [4]. Phytohormones such as gibberellic acid (GA3), salicylic acid (SA), and jasmonic acid (JA) are the first line of defense in enhancing the plant responses to various herbivores [5,6]. These compounds regulate defenses against herbivores, particularly JA, which controls cell-content-feeding and tissue-chewing insects [7,8]. The effect of Si on both growth and yield parameters with biotic and abiotic stresses has been demonstrated in different crops such as rice (Oryza sativa L.) [9], maize (Zea mays L.) [5,10], wheat (Triticum spp.) [11], and soybean (Glycine max L.) [12]. Additionally, Si is also known to induce resistance against pathogens, insects, and non-insect pests by improving the quality parameters of the crop plants [13] and by regulating the secondary metabolites, namely phenolics, terpins, and nitrogen-containing compounds that are involved in plant protection against biotic and abiotic stresses [14].
Fall armyworm (FAW) Spodoptera frugiperda (J. E. Smith), a defoliator pest of maize, is native to America and is found in several countries such as Brazil, Argentina, and the USA [15,16], causing economic losses in a variety of crops, viz. maize, soybean, cotton, rice, and beans [17,18]. It was reported for the first time outside America in Benin and Togo, Africa [19], and in India on maize in May 2018 [20]. Studies were conducted to know its incidence; host range and management indicated very severe damage in Chikkaballapur, Hassan, Shivamogga, Davanagere, and Chitradurga in July–August 2018. The incidence ranged from 9.0 to 62.5 percent at various locations, and the maximum incidence was recorded in Hassan district followed by Chikkaballapur, Davanagere, Shivamogga, and Chitradurga [21]. However, its infestation in Northern Karnataka ranged from 6.00 to 100 percent. Thereafter, it spread to different states of India [22]. In India, following its introduction, it has become a major pest [22,23] and displaced native stem borers due to its cannibalistic nature and early occupancy behavior [24,25]; insecticides are the main mode of management strategies, and hence, the economic expenditure of maize cultivation has drastically increased [26]. On the other hand, whorl application of soil mixed insecticides is effective and advantageous over foliar spray [27]. Developing host plant resistance through Si application is one possible strategy in various crop plants to insect damage including FAW in maize [2]. Our recent study demonstrated that larvae fed on plants treated with foliar silicic acid (FSA), and JA exhibited lower larval, pre-pupal, and pupal weight, as well as larval survival fecundity and fertility [4]. These encouraging results led to the field evaluation of the exogenous application of FSA (as a source of Si), GA3, and JA (as plant growth regulators) on the Si accumulation in leaves, FAW feeding damage on plants, and yield of maize plant. Furthermore, phenols and the tannin content of the plants were analyzed to understand the relationship between the natural incidence of FAW and induced resistance against S. frugiperda due to the foliar application of silicic acid and PGRs.
2. Materials and Methods
2.1. Experimental Details
The experiment was conducted under field condition at the Agricultural and Horticultural Research Station, Bhavikere (13°42′ N latitude and 75°51′ E longitude; 667.5 mMSL), College of Agriculture, Shivamogga, during the Kharif season of 2019 and the Rabi season of 2019–2020 using randomized complete block design with eight treatments and three replications. Commercially grown maize hybrid P3550 (pioneer) offers excellent stress tolerance because of its deep root characteristics, and it also has excellent cob and grain attributes. It was sown with the spacing of 60 cm × 30 cm and a plot size of 5 m × 4 m. The experimental locations fall in the Southern Transitional Agro-climatic Zone of Karnataka located at an altitude of 650 m, latitude of 14°0′ to 14°1′ N, and longitude of 75°40′ to 75°42′ E; and the soil is of the sandy loamy type.
The treatment details were as follows: T1—foliar silicic acid* (Rexil India Pvt. Ltd., Bangalore, India) @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—GA3 (ProGibb 40% WSG; ValentBiosceinces Corp, Libertyville, IL, USA) @ 0.3 mg plant−1; T4—GA3 @ 0.5 mg plant−1; T5—jasmonic acid** (Cat: J2500, Sigma Aldrich, Burlington, MA, USA) @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + GA3 @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + GA3 @ 2 mL L−1 + 0.5 mg plant−1; and T8—untreated control. For the application of foliar silicic acid and GA3, initially, the quantity of the solution required to spray 111 plants (plot of 5 m × 4 m size with 60 cm × 30 cm spacing) was calibrated using water spray. Foliar silicic acid and PGRs such as GA3 (FSA @ 2 or 4 mL L−1 and GA @ 0.3 or 0.5 mg plant−1) and @ 2 mL L−1 (2 mM solution) of JA were sprayed three times at 15, 25, and 35 days after sowing, coinciding with the significantly high natural incidence period of fall armyworm under field condition. For the JA spray, 93.24 mg of commercial formulation was dissolved in 222 mL of water along with 1 mL ethanol and sprayed on all the parts of the plant using a fine mist sprayer (Urban King®, Bangalore, India).
2.2. Influence of Si and PGRs on the Incidence of FAW
The larval count was recorded from 20 plants per plot moving in a zigzag manner at the 25th, 35th, and 45th DAS and expressed as the mean number of larvae per plant. Damage to the plants was scored by evaluating the severity of pinholes, shot holes, lesions, and irregular leaf feeding damage. The rating scale from 1 to 9 was used for scoring the damage severity [28] modified by CIMMYT (Supplementary Table S1 and Figure S1). The percent infestation was also calculated.
2.3. Si and PGR Concentration in Leaves and the Influence of Si and PGRs on the Incidence of FAW
Samples from fully opened leaves were collected from the plants on the 10th day following the first and subsequent applications each time at the 25th, 35th, and 45th day (days at which maximum incidence is generally found in field condition and also the biology of FAW takes on an average of 15 days). Silicon, phenol, tannin, and potassium contents were analyzed by following standard procedures such as those for silicon [9], phenols [29], and tannins [30]. Since the number of larvae, damage score, and percent infestation recorded at three intervals followed the same trend, only the damage score was taken for correlation analysis.
2.4. Yield and Cost Economics
Benefit–cost analysis for all the treatments was carried out using the following relations: Cost (T8) = costs of (land preparation + fertilizer application + sowing + irrigation + weeding + harvesting + threshing); Cost (T1) = Cost (T8) + cost of (SSA) + cost of labor for SSA application; Cost (T2) = Cost (T1) + cost of (SSA) + cost of labor for SSA application; Cost (T3) = Cost (T8) + cost of (GA) + cost of labor for GA application; Cost (T4) = Cost (T8) + cost of (GA) + cost of labor for GA application; Cost (T5) = Cost (T8) + cost of (JA) + cost of labor for JA application. Net income (Rs ha−1) = (grain yield × price of grain)—costs. Benefit: cost ratio was calculated by dividing the gross income by the cost of cultivation.
2.5. Statistical Analysis
Statistical analysis was performed on the data collected during the field trial on the number of larvae and the degree of damage. All experimental data were transformed to the arc sign when the data were in percent and to the square root (x + 0.5) when the data were in counts before analysis to ensure the accuracy of the results. The data on the biochemical parameters of maize were subjected to analysis of variance (ANOVA), and treatments were compared by Tukey’s test (p = 0.05) when ANOVA was significant. Pearson’s linear correlation was performed to know the correlation of fall armyworm infestation with various biochemical parameters such as phenol, tannin, and silicon contents. Statistical analysis was carried out using IBMSPSS software (Version 16.0).
2.6. Flowchart of the Experiment
A flowchart of the experimental details is showed in Figure 1.
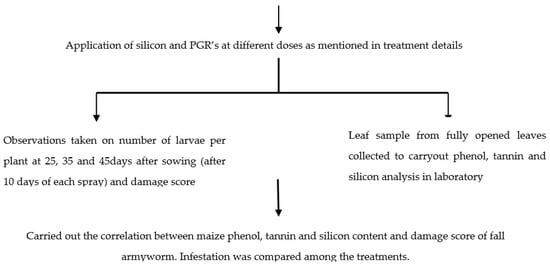
Figure 1.
The flow of the experimental details undertaken to evaluate the silicon and PGRs effects on FAW incidence and maize yield.
3. Results
3.1. Influence of Si and PGRs on the Incidence of FAW
With respect to the mean number of larvae per plant, it was the lowest for FSA at 2 mL L−1 + GA3 at 0.5 mg plant−1 (0.39 larvae/plant) with the highest percent reduction (73.36%), which was on par with FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (0.41 larvae/plant) with 71.93 percent reduction. The FSA at 4 mL L−1was recorded at 0.61 larvae plant−1 with 58.4 percent reduction over control, and this was on par with FSA at 2 mL L−1. Similarly, this was on par with JA with 1.07 larvae per plant with 27.69 percent reduction over control, GA3 at 0.3 mg plant−1 (0.95) with 35.74 percent, and GA3 at 0.5 mg plant−1 (0.89) with 39.43 percent reduction. In the untreated control, 1.48 larvae per plant were recorded and significantly differed compared with other treatments (Figure 2).
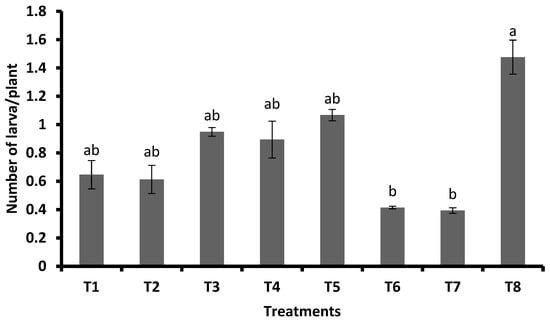
Figure 2.
Pooled means (±SE) of observations on number of larvae per plant at 25, 35, and 45 DAS (pooled data of Kharif 2019 and Rabi 2019–2020). Different letters on bars indicate significant difference by Tukey’s test (p ≤ 0.05). Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellicacid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1(2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
The mean damage rating also followed the same trend as the least damage score in FSA at 2 mL L−1 + GA3 at 0.5 mg plant−1 (2.63 damage score), which was on par with FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (2.63). This was followed by FSA at 4 mL L−1 (3.35), FSA at 2 mL L−1 (3.53), GA3 at 0.5 mg plant−1 (4.02), GA3 at 0.3 mg plant−1 (4.16), and JA (4.59). In the untreated control, the damage rating was 5.23, which significantly differed from other treatments (Figure 3).
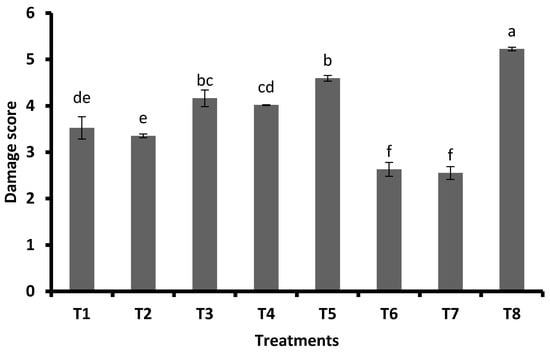
Figure 3.
Pooled means (±SE) of observations on damage score at 25, 35, and 45 DAS (pooled data of Kharif 2019 and Rabi 2019–2020). Different letters on bars indicate significant difference by Tukey’s test (p ≤ 0.05). Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellic acid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
With respect to mean percent infestation, FSA at 2 mL plant−1 + GA3 at 0.5 mg plant−1 was recorded to have the least percent infestation (37.14%) with 56.43 percent reduction over control, and it was on par with FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (38.39%) with 54.96 percent reduction in infestation over control. Furthermore, this was followed by FSA at 4 mL L−1 with 46.80 percent infestation and 45.10 percent reduction, and it was on par with FSA at 2 mL L−1 (48.10% infestation) with a 43.57 percent reduction. In the untreated control, 84.24 percent infestation was recorded and significantly differed compared with other treatments. Significantly higher percent infestation was recorded with JA (58.16%) with the least percent reduction over control (31.77%). This was on par with GA3 at 0.3 mg plant−1 (56.96%) with 33.18 percent and GA3 at 0.5 mg plant−1 (53.95%) with a 36.70 percent reduction. The untreated control recorded the 84.24 percent infestation, which is significantly higher than other treatments (Figure 4).
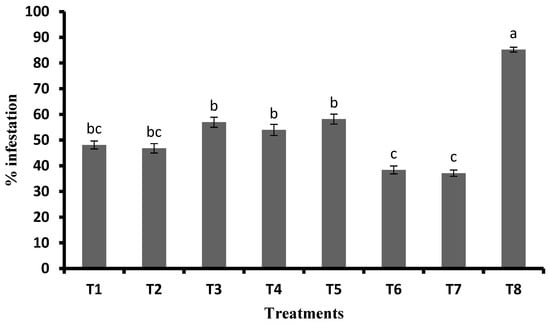
Figure 4.
Pooled means (±SE) of observations on percent infestation at 25, 35, and 45 DAS (pooled data of Kharif 2019 and Rabi 2019–2020). Different letters on bars indicate significant difference by Tukey’s test (p ≤ 0.05). Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellic acid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
3.2. Si and PGR Concentration in Leaves and the Influence of Si and PGRs on the Incidence of FAW
Plants treated with FSA at 2 mL L−1 + GA3 at 0.5 mg plant−1 and FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 had higher Si contents (2.99% and 2.98%), respectively. For FSA at 4 mL L−1, it was 2.37 percent and for FSA at 2 mL L−1 it was 2.35 percent; there was no influence of PGRs on the Si content. For the untreated control, 1.30 percent of silicon was recorded, which was significantly lower than other treatments except for PGRs (Figure 5). The highest phenol content was recorded with FSA at 2 mL plant−1 + GA3 at 0.5 mg plant−1 (4.41 mg/g), and this was followed by FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (4.31 mg/g), FSA at 4 mL L−1 (4.24 mg/g), and FSA at 2 mL L−1 (4.21 mg/g). This was followed by GA3 at 0.5 mg plant−1 (3.97 mg/g), and it was on par with GA3 at 0.3 mg plant−1 (3.88 mg/g) and JA (3.68) (Figure 6).
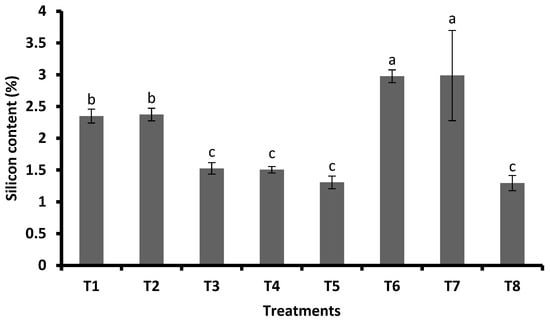
Figure 5.
Mean (±SE) of the leaf silicon content of maize treated with silicic acid and plant growth regulators. Different letters on bars indicate significant difference by Tukey’s test (p ≤ 0.05). Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellic acid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
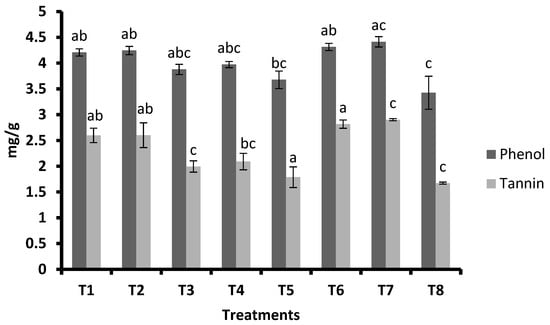
Figure 6.
Mean (±SE) of the phenol and tannin content of maize treated with silicic acid and plant growth regulators. Different letters on bars indicate significant difference by Tukey’s test (p ≤ 0.05). Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellic acid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
FSA at 2 mL plant−1 + GA3 at 0.5 mg plant−1 (2.90 mg/g) had the highest tannin content, followed by FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (2.82 mg/g), FSA at 2 mL plant−1, and FSA at 4 mL L−1 (2.60 mg/g). This was followed by GA3 at 0.5 mg plant−1 (2.09 mg/g), and it was on par with GA3 at 0.3 mg plant−1 (2.00 mg/g) and JA (1.79) (Figure 6).
Correlation analysis between Si content in plants was significantly negatively correlated with damage rating (r = −0.97**) (Figure 7a). Similarly, phenol content in plants was significantly negatively correlated with damage rating (r = −0.97**) (Figure 7b). The plant tannin content was significantly negatively correlated with damage rating (r = −0.95**) (Figure 7c).
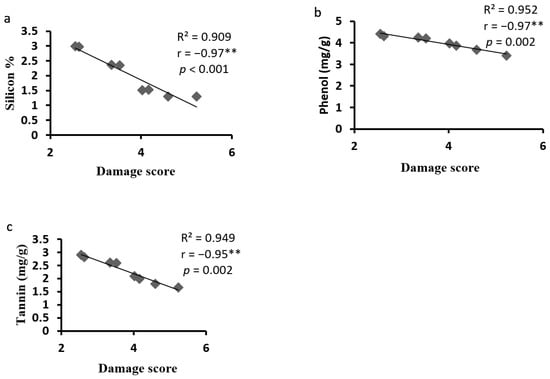
Figure 7.
Pearson’s correlation between (a) silicon content, (b) phenol, (c) tannin content of maize and damage score of Spodoptera frugiperda. Correlation coefficient (r), coefficient of determination (R2) and p-value as shown. N = 8; ** Correlation is significant at the 0.01 level; Table r value at p = 0.01 is 0.834.
3.3. Yield and Cost Economics
The highest yield was recorded for FSA at 2 mL L−1 + GA3 at 0.5 mg/plant (58.39 q/ha), FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (56.29), FSA at 4 mL L−1 (52.58 q/ha), and FSA at 2 mL L−1 (50.33 q/ha). Furthermore, this was followed by GA3 at 0.5 mg plant−1 (46.92 q/ha), GA3 at 0.3 mg plant−1 (46.67 q/ha), and JA (44.89 q/ha). The untreated control recorded the lowest yield of 33.97 q/ha, which was significantly inferior to the rest of the treatments (Figure 8).
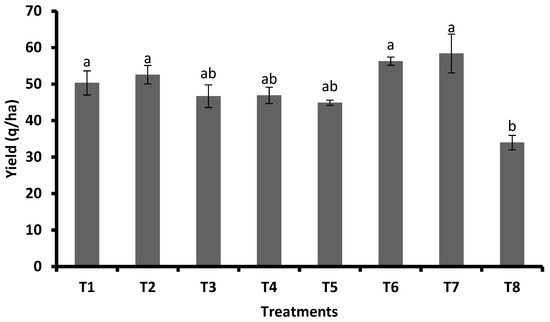
Figure 8.
Influence of FSA and PGRs on yield for the management of FAW on maize under field condition. Different letters on bars indicate significant difference by Tukey’s test (p ≤ 0.05). Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellic acid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
Similarly, for the C:B ratio, the best treatment was FSA at 2 mL L−1 + GA3 at 0.5 mg plant−1, which recorded the highest C:B ratio of 1:2.34. This was followed by FSA at 2 mL L−1 + GA3 at 0.3 mg plant−1 (1:2.31), FSA at 2 mL L−1 (1:2.16), and FSA at 4 mL L−1 (1:2.12). This was followed by GA3 at 0.3 mg plant−1 (1:2.04) and GA3 at 0.5 mg plant−1 (1:2.00). Meanwhile, JA recorded the lowest C:B ratio (1:0.02), being inferior to the rest of the treatments and even control. On the other hand, for the untreated control, the C:B ratio was 1:1.55, which was inferior to all other treatments except JA (Figure 9).
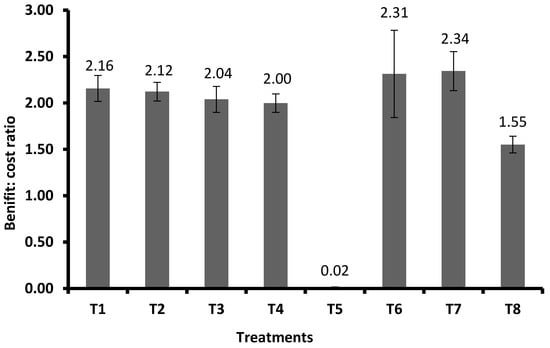
Figure 9.
Economic analysis of benefit: cost ratios of FSA and PGRs for the management of FAW on maize under field condition. Vertical bars (±) indicate SEs of the means. Note: T1—foliar silicic acid @ 2 mL L−1; T2—foliar silicic acid @ 4 mL L−1; T3—gibberellic acid @ 0.3 mg plant−1; T4—gibberellic acid @ 0.5 mg plant−1; T5—jasmonic acid @ 2 mL L−1 (2 mM solution); T6—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.3 mg plant−1; T7—foliar silicic acid + gibberellic acid @ 2 mL L−1 + 0.5 mg plant−1; and T8—control.
4. Discussion
Most Si sources are applied as foliar spray or as soil amendment. Silicon amendment to plants can induce enhanced resistance against insect pests. Silicon is known to be compatible with other amendments and PGRs. The combined effects of Si and PGRS on hindered fecundity, feeding, digestibility, and fitness of insects are well studied. A laboratory study on the influence of Si and PGRs on the biology of FAW revealed that GA3 can modify the vegetative properties of plants and Si intake of maize plants, resulting in a decrease in S. frugiperda larval consumption and female moth oviposition [4]. The mechanisms of Si-mediated resistance include one or a combination of constitutive and induced mechanical and chemical defenses for the different feeding guilds.
The foliar applied silicic acid increased the Si content in maize leaves, while PGRs had no significant influence on Si (Figure 5). Similarly, in plants treated with FSA + GA3, the Si content was higher compared with FSA alone. Increased Si in maize reduced the incidence and thus had negative correlation with the damage score (Figure 7a); it may be because of the increased accumulation of Si in leaves, which makes them harder and interfere in feeding [4,10,31]. The increased leaf Si content in rice caused a negative effect on S. frugiperda development [32], thus reducing the infestation.
In the present study, the content of phenols and tannins in maize leaves had significantly increased after the application of Si and PGRs (Figure 6). This may be due to induced gene expression of enzymes of the secondary metabolic pathway, involved in the synthesis of phenols and tannins [33]. Previously, it was proved that Si application to wheat plant increased total phenols and tannins and thus increased the resistance to aphids Sitobion avenae [34]. Similarly, JA lowered the gypsy moth growth on American chestnut by increasing the tannin content, and it was also known to increase phenols in plants [35,36]. The increased phenol and tannin contents induced resistance to rice pests [37]. Similar observations were recorded in the present study with increased phenol and tannin contents due to the application of Si and PGRs. Silicon treatment boosts the expression of defense-related genes, which activates the plant defensive enzymes, resulting in higher amounts of defensive chemicals including phenolics, phytoalexins, and momilactones [13].
It has been previously demonstrated that exogenous application of Si reduced the pink stem borer Sesamia inference (Walker) incidence and damage in wheat and finger millet [38,39], yellow stem borer Scirpophaga incertulas (Walker), and leaf folder Cnaphalocrosis medinalis (Guenée) in rice [40]. This is encouraging as similar types of results were obtained in S. frugiperda on maize in our studies indicating an opportunity to exploit Si-based induction of resistance.
Application of Si and PGRs increased the yield compared with the untreated control (Figure 8). Previous studies proved the beneficial effect of silicon applied to soil or as a foliar application on the quantity and quality of the yield of many plant species such as cereals, soybean, rapeseed, sugar beet, potato, meadows, berries, vegetables, and orchard and ornamental plants [41]. The application of FSA at 2 and 4 mL plant−1 increased the grain yield of rice [9]. Similarly, FSA increased the grain yield of wheat [38]. The application of GA3 increased the corn yield [42]. Similarly, the application of GA3 increased the grain yield of maize due to improvements in yield attributes [43]. Because silicon leaves no pesticide residues in food or the environment and is simple to combine with other pest management techniques, such as biological control, it makes up a viable component of integrated management of insect pests [44]. Combining Si and PGRs can be a powerful and long-lasting strategy for improving plant growth; hence, evaluating the combined use of Si and PGRs on plants against abiotic and biotic stresses can be potential areas of research.
5. Conclusions
It was well proven that Si and PGRs are known to induce the resistance in many crop plants against biotic and biotic stresses. Our studies also proved that application of FSA and PGRs induced the resistance in fall armyworm by reducing the infestation and damage in both seasons of the experiment. The FSA at 2 mL plant−1 + GA3 at 0.5 mg plant−1 have got the highest C:B ratio of 1:2.34, which is considered as a best treatment. The JA had a negative effect on biology; thus, it was effective in the field for the reduction of the S. frugiperda population but was costly, and hence the C:B ratio was much lower compared with other treatments and control. In order to produce pesticide-free, good-quality agricultural products with less cost of cultivation, the use of silicon and PGRs is a better pest management strategy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb14030052/s1, Figure S1: Damage rating of maize plants based on foliar damage by S. frugiperda; Table S1: Scale of leaf feeding damage used for assessing the natural incidence (Davis and Williams, 1992 modified by CIMMYT).
Author Contributions
Conceptualization, W.N. and C.M.K.; methodology, W.N., C.M.K. and N.B.P.; software, W.N.; validation, W.N., C.M.K., C.S. and M.A.H.; formal analysis, W.N. and H.B.M.; investigation, W.N., C.M.K., N.B.P. and B.C.D.; resources, W.N., B.C.D., S.S.D. and C.S.; data curation, W.N. and H.B.M.; writing—original draft preparation, W.N. and C.M.K.; writing—review and editing, W.N., C.M.K. and M.A.H.; visualization, C.M.K. and M.A.H.; supervision, C.M.K.; project administration, C.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated in this research are presented within this article.
Acknowledgments
The authors thank the Director of Research, KSNUAHS, Shivamogga-577 204, for encouragement and the facilities. The facilities generated under DST-FIST in the Department of Entomology, KSNUAHS, are also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Durgesh, K.T.; Singh, V.P.; Lux, A.; Vaculik, M. Silicon in plant biology: From past to present, and future challenges. J. Experimental Bot. 2020, 71, 6699–6702. [Google Scholar]
- Kalleshwaraswamy, C.M.; Kannan, M.; Prakash, N. Silicon as a natural plant guard against insect pests. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement: Progress and Prospectes; Etesami, H., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 219–227. [Google Scholar]
- Singh, A.; Kumar, A.; Hartley, S.; Singh, I.K. Silicon: Its ameliorative effect on plant defense against herbivory. J. Exp. Bot. 2020, 71, 6730–6743. [Google Scholar] [CrossRef] [PubMed]
- Nagaratna, W.; Kalleshwaraswamy, C.M.; Dhananjaya, B.C.; Prakash, N.B. Effect of silicon and plant growth regulators on the biology and fitness of fall armyworm, Spodoptera frugiperda, a recently invaded pest of maize in India. Silicon 2021, 14, 783–793. [Google Scholar] [CrossRef]
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal Signature and Transcriptome changes of arabidopsis during pathogen and insect attack. MPMI 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Prakash, N.B.; Chandrashekar, N.; Mahendra, C.; Patil, S.U.; Thippeshappa, G.N.; Laane, H.M. Effect of foliar spray of soluble silicic acid on growth and yield parameters of wetland rice in hilly and coastal zone soils of Karnataka, South India. J. Plant Nutr. 2011, 34, 1883–1893. [Google Scholar] [CrossRef]
- Pavani, J.; Kalleshwaraswamy, C.M.; Onkarappa, S.; Dhananjaya, B.C.; Prakash, N.B. Influence of rice husk biochar as a source of silicon on the fall armyworm, Spodoptera frugiperda incidence and growth and yield of Maize. Silicon 2023, 15, 4277–4284. [Google Scholar] [CrossRef]
- Sattar, A.; Cheema, M.A.; Sher, A.; Ijaz, M.; Wasaya, A.; Yasir, T.A.; Abbas, T.; Hussain, M. Foliar applied silicon improves water relations, stay green and enzymatic antioxidants activity in late sown wheat. Silicon 2020, 12, 223–230. [Google Scholar] [CrossRef]
- Shwethakumari, U.; Prakash, N.B. Effect of foliar application of silicic acid on soybean yield and seed quality under field conditions. J. Indian Soc. Soil Sci. 2018, 66, 406–414. [Google Scholar] [CrossRef]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Bhat, J.A.; Siddiqui, M.H.; Rinklebe, J.; Ahmad, P. Integration of silicon and secondary metabolites in plants: A significant association in stress tolerance. J. Exp. Bot. 2020, 71, 6758–6774. [Google Scholar] [CrossRef]
- Clark, P.L.; Molina-ochoa, J.; Martinelli, S.; Skoda, S.R.; Isenhour, D.J.; Lee, D.J.; Krumm, J.T.; Foster, J.E. Population variation of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. J. Insect Sci. 2007, 7, 1536–2442. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, R.N.; Silvie, P.; Meagher, R.L.; Lopez, J.; Machado, V. Identification and comparison of fall armyworm (Lepidoptera: Noctuidae) host strains in Brazil, Texas, and Florida. Ann. Entomol. Soc. Am. 2007, 100, 394–402. [Google Scholar] [CrossRef]
- De FreitasBueno, R.C.O.; De Freitas Bueno, A.; Moscardi, F.; Postali Parra, J.R.; Hoffmann-campo, C.B. Lepidopteran larva consumption of soybean foliage: Basis for developing multiple-species economic thresholds for pest management decisions. Pest Manag. Sci. 2011, 67, 170–174. [Google Scholar] [CrossRef]
- Prowell, D.C.; Mcmichael, M.; Silvain, J.F. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2004, 97, 1034–1044. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Kalleshwaraswamy, C.M.; Asokan, R.; Mahadeva Swamy, H.M.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the Fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hort. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- Shylesha, A.N.; Jalali, S.K.; Gupta, A.; Varshney, R.; Venkatesan, T.; Shetty, P.; Ojha, R.; Ganiger, P.C.; Navik, O.; Subaharan, K.; et al. Studies on new invasive pest Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control 2018, 32, 145–151. [Google Scholar] [CrossRef]
- Swamy, H.M.; Asokan, R.; Kalleshwaraswamy, C.M.; Prasad, Y.G.; Maruthi, M.S.; Shashank, P.R.; Devi, N.I.; Surakasula, A.; Adarsha, S.; Srinivas, A. Prevalence of “R” strain and molecular diversity of fall armyworm, Spodopterafrugiperda (Lepidoptera: Noctuidae) in India. Indian J. Entomol. 2018, 80, 544–553. [Google Scholar] [CrossRef]
- Mallapur, C.P.; Naik, A.K.; Hagari, S.; Prabhu, S.T.; Patil, R.K. Status of alien pest fall armyworm, Spodopterafrugiperda (JE Smith) in Northern Karnataka. J. Entomol. Zool. Stud. 2018, 6, 432–436. [Google Scholar]
- Divya, J.; Kalleshwaraswamy, C.M.; Mallikarjuna, H.B.; Deshmukh, S. Does recently invaded fall armyworm, Spodopterafrugiperda displace native lepidopteran pests of maize in India? Curr. Sci. 2021, 120, 1358–1367. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.M.; Divya, J.; Mallikarjuna, H.B.; Deshmukh, S.S.; Sunil, C. Cannibalistic nature and time of habitat occupancy of invasive maize fall armyworm, Spodoptera frugiperda are the key factors for competitive displacement of native stem borer, Sesamia inferens in India. Curr. Sci. 2023, 124, 348–354. [Google Scholar]
- Deshmukh, S.S.; Kalleshwaraswamy, C.M.; Prasanna, B.M.; Sannathimmappa, H.G.; Kavyashree, B.A.; Sharath, K.N.; Pradeep, P.; Patil, K.K.R. Economic analysis of pesticide expenditure for managing the invasive fall armyworm, Spodoptera frugiperda (JE Smith) by maize farmers in Karnataka, India. Curr. Sci. 2021, 121, 1487–1492. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.M.; Ambarish, S.; Onkarappa, S.; Deshmukh, S.S.; Sunil, C. Whorl application of soil mixed chlorantraniliprole 18.5 SC is effective against invasive fall armyworm, Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) in maize. J. Entomol. Res. 2022, 46, 738–745. [Google Scholar] [CrossRef]
- Davis, F.M.; Ng, S.S.; Williams, W.P. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Tech. Bull. (Miss. Agric. For. Exp. Stn.) 1992, 186, 1–9. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bwins, R.E. Methods for estimation of tannin in grain sorghum and other food stuff. J. Agron. 1971, 63, 511–512. [Google Scholar]
- Datnoff, L.E.; Snyder, G.H.; Korndorfer, G.H. Silicon in Agriculture; Elsevier: Amsterdam, The Netherlands, 2001; p. 424. [Google Scholar]
- Nascimento, A.M.; Assis, F.A.; Moraes, J.C.; Souza, B.H.S. Silicon application promotes rice growth and negatively affects development of Spodoptera frugiperda (JE Smith). J. Appl. Entomol. 2017, 142, 241–249. [Google Scholar] [CrossRef]
- Reymond, P.; Farmer, E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Boil. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.L.; Su, J.W.; Wang, Y.L.; Tan, J.F.; Han, Y.L. Effect of nitrogen application combined with silicon on density of Sitobionavenae and contents of biochemical materials of winter wheat at the late growth stage. J. PlantNutr. Fertil. 2013, 19, 832–839. [Google Scholar]
- Chen, J.S.; Lei, N.F.; Liu, Q. Defense signaling among interconnected ramets of a rhizomatous clonal plant, induced by jasmonic-acid application. Acta Oecol. 2011, 37, 355–360. [Google Scholar] [CrossRef]
- Malekpoor, F.; Salimi, A.; Pirbalouti, A.G. Effect of jasmonic acid on total phenolic content and antioxidant activity of extract from the green and purple landraces of sweet basil. Acta Pol. Pharm. 2016, 73, 1229–1234. [Google Scholar] [PubMed]
- Chandramani, P.; Rajendran, R.; Sivasubramanian, P.; Muthiah, C. Management of hoppers in rice through host nutrition. J. Biopestic. 2009, 2, 99–106. [Google Scholar]
- Jeer, M.; Yele, Y.; Sharma, K.C.; Prakash, N.B. Exogenous application of different silicon sources and potassium reduces pink stem borer damage and improves photosynthesis, yield and related parameters in wheat. Silicon 2021, 13, 901–910. [Google Scholar] [CrossRef]
- Jadhao, K.R.; Bansal, A.; Rout, G.R. Silicon amendment induces synergistic plant defense mechanism against pink stem borer (Sesamia inferens Walker.) in finger millet (Eleusine coracana Gaertn.). Sci. Rep. 2020, 10, 4229. [Google Scholar] [CrossRef]
- Mishra, I.O.; Panda, S.K.; Dash, A.B. Effect of organic and inorganic silicon amendments against yellow stem borer (Scirpophaga incertulas Walker) and leaf folder (Cnaphalocrocis medinalis Guenee) of rice in costal Odisha. J. Entomol. Zool. Stud. 2018, 6, 1495–1499. [Google Scholar]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality a literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef]
- Al-Shaheen, M.R.; Soh, A.; Al-Samarai, G.F. Growth response of corn (Zea maize L.) to proline and gibberellic acid spray under different irrigation levels. Int. J. Bot. Res. 2014, 4, 7–16. [Google Scholar]
- Singh, M.; Kumawat, N.; Tomar, I.S.; Dudwe, T.S.; Yadav, R.; Sahu, Y.K. Effect of gibberllic acid on growth, yield and economics of maize (Zea mays L.) under Jhabua Hills of Madhya Pradesh. J. Agric. Res. 2018, 5, 25–29. [Google Scholar]
- Laing, M.D.; Gatarayiha, M.C.; Adandonon, A. Silicon use for pest control in agriculture: A review. In Proceedings of the South African Sugar Technologists’ Association, Durban, South Africa, 18–20 July 2006; Volume 80, pp. 278–286. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).