Ethyl Methanesulphonate (EMS)-Mediated Mutagenesis Induces Genetic and Morphological Variations in Eggplant (Solanum melongena L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Seeds
2.2. Construction of Plant ‘Kill Curve’ Analysis
2.3. Development of M1 and M2 Generation of Eggplant Libraries
2.4. Detection of Genotypic Variation among the M2 Mutant Population
2.5. Pacbio Sequence Data Processing
2.6. Morphological Screening of Unique Variations Emerging from the M2 Mutant Library
3. Results
3.1. Plant ‘Kill Curve’ Analysis
3.2. Genotypic Screening of M2 Mutants
3.3. Morphological Screening of Unique Variations in the M2 Population
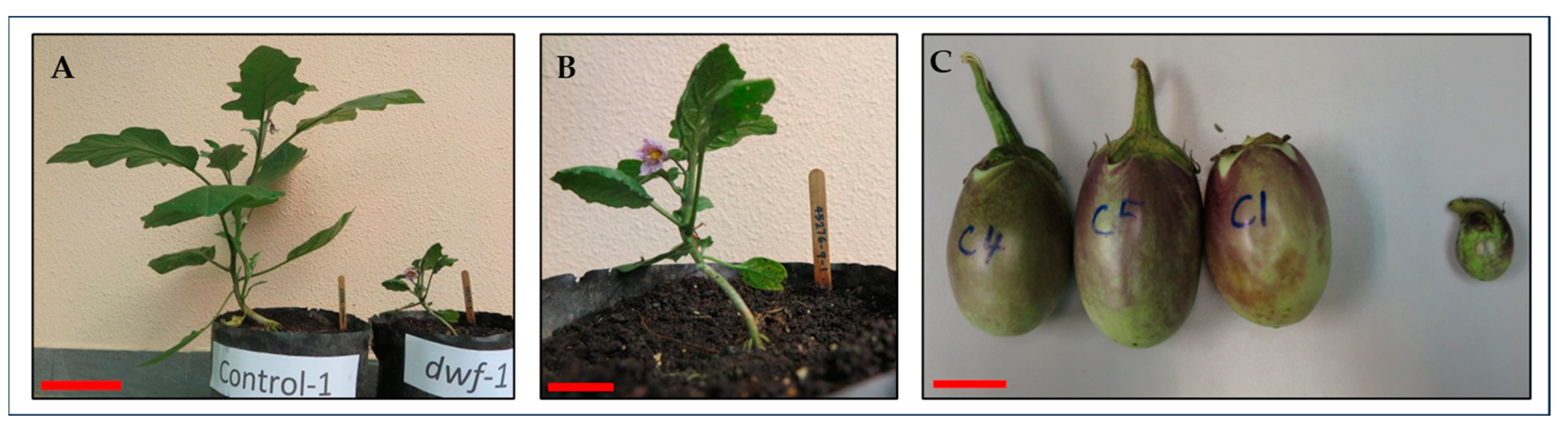
3.3.1. Plant Height
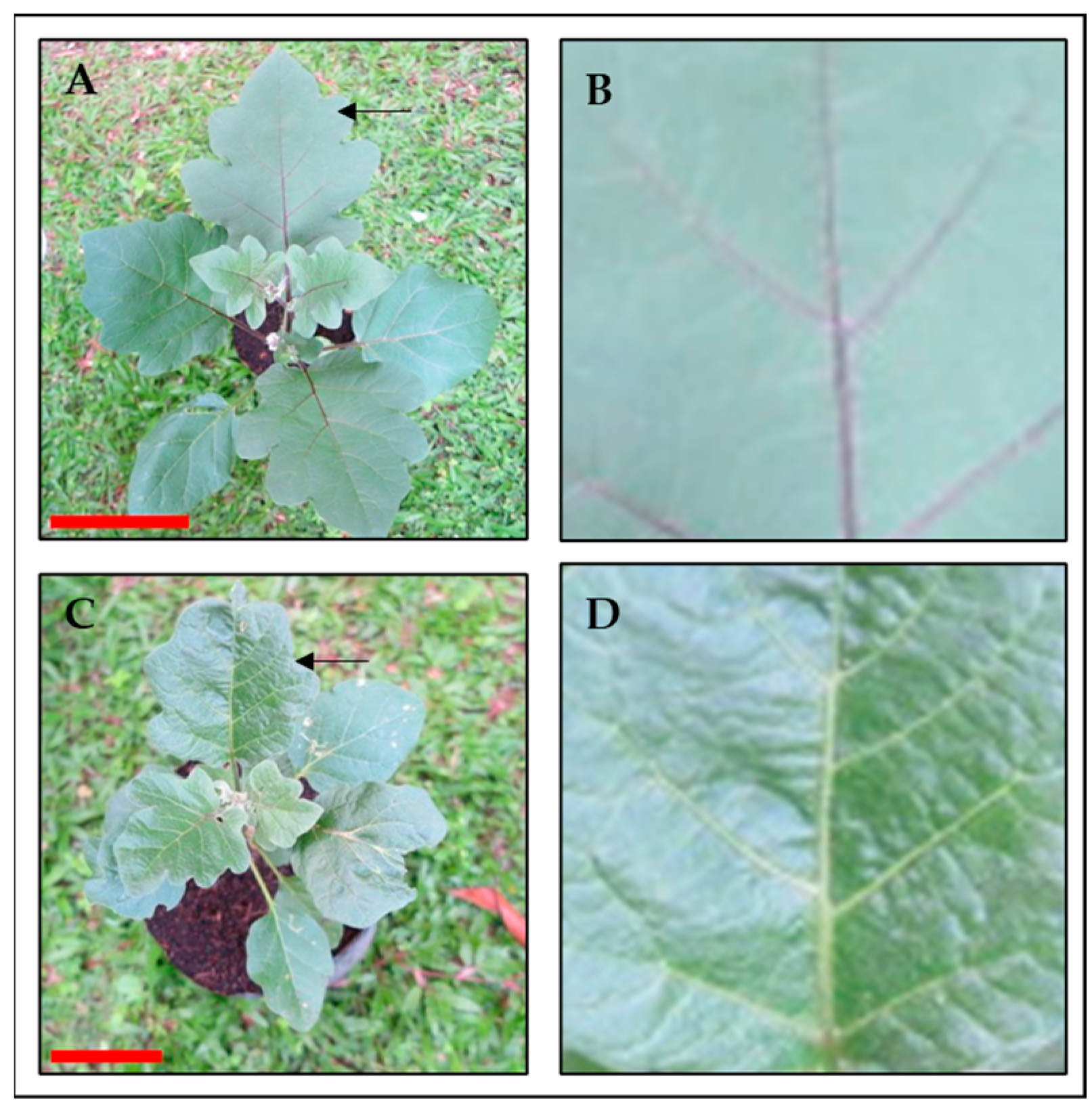
3.3.2. Leaf Structure
3.3.3. Fruit Mutants
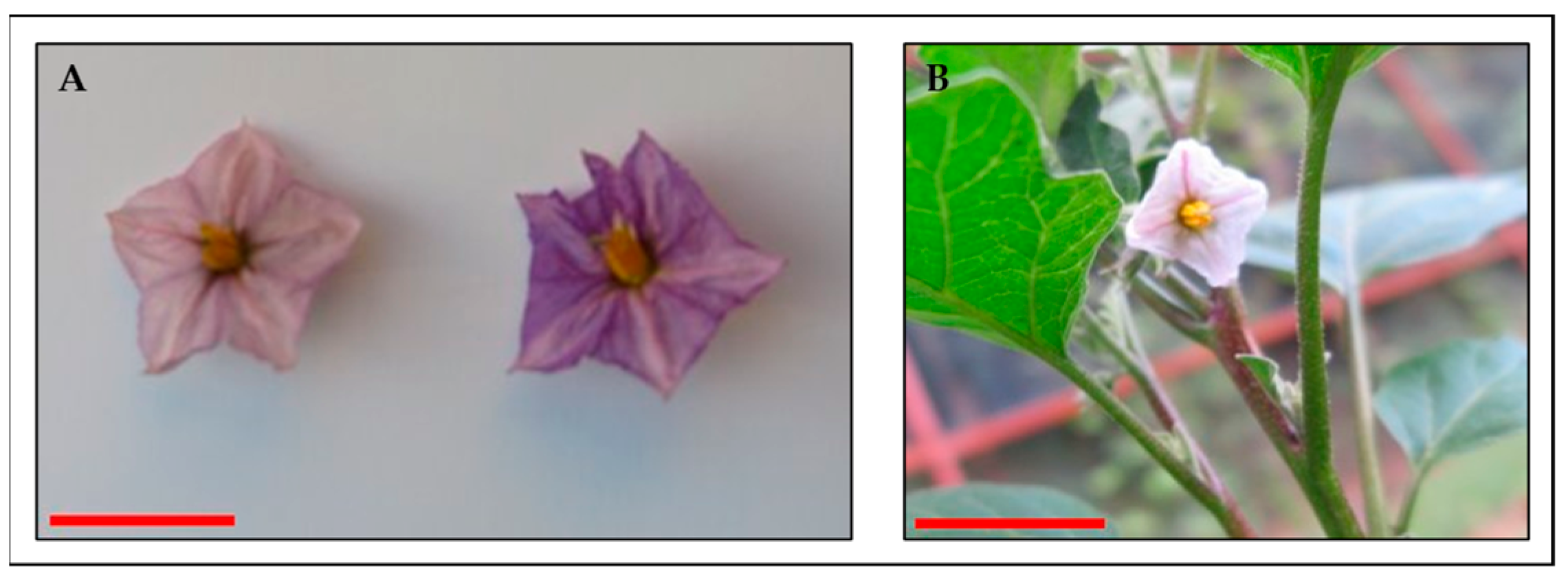
3.3.4. Flower Structures
3.3.5. Frequencies of the Observed Mutations among M2 Families
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, E.J.; Bae, M.S.; Jo, E.K.; Jo, Y.H.; Lee, S.C. Antioxidant activity of different parts of eggplant. J. Med. Plants Res. 2011, 5, 4610–4615. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 January 2023).
- Chioti, V.; Zeliou, K.; Bakogianni, A.; Papaioannou, C.; Biskinis, A.; Petropoulos, C.; Lamari, F.N.; Papasotiropoulos, V. Nutritional Value of Eggplant Cultivars and Association with Sequence Variation in Genes Coding for Major Phenolics. Plants 2022, 11, 2267. [Google Scholar] [CrossRef]
- Sreekar, K. Biotechnology and its implications in brinjal improvement: A review. J. Pharmacogn. Phytochem. 2020, 9, 1096–1102. [Google Scholar]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K.J.B.T. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Stommel, J.R.; Whitaker, B.D. Phenolic acid content and composition of eggplant fruit in a germplasm core subset. J. Am. Soc. Hortic. Sci. 2003, 128, 704–710. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Katoch, V. Biotechnological interventions in eggplant (Solanum melongena L.). J. Hortic. Sci. Biotechnol. 2020, 95, 273–285. [Google Scholar] [CrossRef]
- Kaushik, P.; Prohens, J.; Vilanova, S.; Gramazio, P.; Plazas, M. Phenotyping of eggplant wild relatives and interspecific hybrids with conventional and phenomics descriptors provides insight for their potential utilization in breeding. Front. Plant Sci. 2016, 7, 677. [Google Scholar] [CrossRef]
- Gramazio, P.; Yan, H.; Hasing, T.; Vilanova, S.; Prohens, J.; Bombarely, A. Whole-genome resequencing of seven eggplant (Solanum melongena) and one wild relative (S. incanum) accessions provides new insights and breeding tools for eggplant enhancement. Front. Plant Sci. 2019, 10, 1220. [Google Scholar]
- Jiang, S.Y.; Ramachandran, S. Natural and artificial mutants as valuable resources for functional genomics and molecular breeding. Int. J. Biol. Sci. 2010, 6, 228. [Google Scholar] [CrossRef]
- Viana, V.E.; Pegoraro, C.; Busanello, C.; Costa de Oliveira, A. Mutagenesis in rice: The basis for breeding a new super plant. Front. Plant Sci. 2019, 10, 1326. [Google Scholar] [CrossRef]
- Sikora, P.; Chawade, A.; Larsson, M.; Olsson, J.; Olsson, O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int. J. Plant Genom. 2011, 2011, 314829. [Google Scholar]
- Espina, M.J.; Ahmed, C.S.; Bernardini, A.; Adeleke, E.; Yadegari, Z.; Arelli, P.; Pantalone, V.; Taheri, A. Development and phenotypic screening of an ethyl methane sulfonate mutant population in soybean. Front. Plant Sci. 2018, 9, 394. [Google Scholar] [CrossRef]
- Greene, E.A.; Codomo, C.A.; Taylor, N.E.; Henikoff, J.G.; Till, B.J.; Reynolds, S.H.; Enns, L.C.; Burtner, C.; Johnson, J.E.; Odden, A.R.; et al. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 2003, 164, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.; Ren, W.; Yang, Q.; Chai, Z.; Chen, R.; Wang, L.; Zhao, J.; Lang, Z.; Wang, H.; et al. Gene-indexed mutations in maize. Mol. Plant. 2018, 11, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Till, B.J.; Cooper, J.; Tai, T.H.; Colowit, P.; Greene, E.A.; Henikoff, S.; Comai, L. Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol. 2007, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Deng, X.W.; Yang, C.; Tang, X. The genome-wide EMS mutagenesis bias correlates with sequence context and chromatin structure in rice. Front. Plant Sci. 2021, 12, 579675. [Google Scholar] [CrossRef]
- Dinh, T.T.; Luscher, E.; Li, S.; Liu, X.; Won, S.Y.; Chen, X. Genetic screens for floral mutants in Arabidopsis thaliana: Enhancers and suppressors. Methods Mol. Biol. 2014, 1110, 127–156. [Google Scholar]
- Xiao, X.O.; Lin, W.; Li, K.; Feng, X.; Jin, H.; Zou, H. Genome-wide analysis of artificial mutations induced by ethyl methanesulfonate in the eggplant (Solanum melongena L.). Genes 2019, 10, 595. [Google Scholar] [CrossRef]
- Xi-Ou, X.; Wenqiu, L.; Wei, L.; Xiaoming, G.; Lingling, L.; Feiyue, M.; Yuge, L. The analysis of physiological variations in M2 generation of Solanum melongena L. Mutagenized by ethyl methane sulfonate. Front. Plant Sci. 2017, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Bin Mohi Uddin, M.M.; Rasul, M.G.; Haque Swapon, M.A.; Ahmed, M.; Hasan, M. In Vitro Screening and Field Performance of EMS-Treated Eggplants for the Selection of Shoot and Fruit Borer-Resistant Plants. Agronomy 2022, 12, 1832. [Google Scholar] [CrossRef]
- Sudesh, K.S.; Anjanappa, M.; Manjunathagowda, D.C.; Shilpashree, N.; Bharathkumar, A.; Praveenkumar, N.R. Grafting in brinjal (Solanum melongena L.): A sustainable way of increasing the yield. Vegetos 2021, 34, 263–269. [Google Scholar] [CrossRef]
- Kumbar, S.; Narayanankutty, C.; Sainamole Kurian, P.; Sreelatha, U.; Barik, S. Evaluation of eggplant rootstocks for grafting eggplant to improve fruit yield and control bacterial wilt disease. Eur. J. Plant Pathol. 2021, 161, 73–90. [Google Scholar] [CrossRef]
- Sabetta, W.; Alba, V.; Blanco, A.; Montemurro, C. sunTILL: A TILLING resource for gene function analysis in sunflower. Plant Methods. 2011, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.B.; Talebi, A.B.; Shahrokhifar, B. Ethyl methane sulphonate (EMS) induced mutagenesis in Malaysian rice (cv. MR219) for lethal dose determination. Am. J. Plant Sci. 2012, 3, 1661–1665. [Google Scholar] [CrossRef]
- Subramaniam, R.; Kumar, V.S. Allele mining, amplicon sequencing and computational prediction of Solanum melongena L. FT/TFL1 gene homologs uncovers putative variants associated to seed dormancy and germination. PLoS ONE 2023, 18, e0285119. [Google Scholar]
- Webb, D.M.; Knapp, S.J. DNA extraction from a previously recalcitrant plant genus. Plant Mol. Biol. Rep. 1990, 8, 180–185. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computer platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Liu, Y.; Guo, H.; Fan, Y.; Wu, J.; Guo, H.; Jiao, C.; Tang, Z.; Zhang, L.; Fan, Y.; et al. Ethyl methanesulfonate mutant library construction in Gossypium hirsutum L. for allotetraploid functional genomics and germplasm innovation. Plant J. 2020, 103, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Kim, Y.S.; Kim, J.K. Determination of the optimal condition for ethylmethane sulfonate-mediated mutagenesis in a Korean commercial rice, Japonica cv. Dongjin. Appl. Biol. Chem. 2017, 60, 241–247. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Sang, N.; Li, Y.; Zhang, T.; Sun, J.; Huang, X. Identification of cotton MOTHER OF FT AND TFL1 homologs, GhMFT1 and GhMFT2, involved in seed germination. PLoS ONE 2019, 14, e0215771. [Google Scholar] [CrossRef]
- Chen, L.; Hao, L.; Parry, M.A.; Phillips, A.L.; Hu, Y.G. Progress in TILLING as a tool for functional genomics and improvement of crops. J. Integr. Plant Biol. 2014, 56, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Henry, I.M.; Nagalakshmi, U.; Lieberman, M.C.; Ngo, K.J.; Krasileva, K.V.; Vasquez-Gross, H.; Akhunova, A.; Akhunov, E.; Dubcovsky, J.; Tai, T.H.; et al. Efficient genome-wide detection and cataloging of EMS-induced mutations using exome capture and next-generation sequencing. Plant Cell. 2014, 26, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Wu, C.; Lei, C.; Baraoidan, M.; Bordeos, A.; Madamba, M.R.S.; Ramos-Pamplona, M.; Mauleon, R.; Portugal, A.; Ulat, V.J.; et al. Chemical-and irradiation-induced mutants of indica rice IR64 for forward and reverse genetics. Plant Mol. Biol. 2005, 59, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Serrat, X.; Esteban, R.; Guibourt, N.; Moysset, L.; Nogués, S.; Lalanne, E. EMS mutagenesis in mature seed-derived rice calli as a new method for rapidly obtaining TILLING mutant populations. Plant Methods. 2014, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Dholaniya, P.S.; Princy, K.; Madhavan, A.S.; Sreelakshmi, Y.; Sharma, R. Whole-genome profiling of ethyl Methanesulfonate mutagenesis in tomato. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kitamura, S.; Satoh, K.; Oono, Y. Detection and characterization of genome-wide mutations in M1 vegetative cells of gamma-irradiated Arabidopsis. PLoS Genet. 2022, 18, e1009979. [Google Scholar] [CrossRef]
- Saito, T.; Ariizumi, T.; Okabe, Y.; Asamizu, E.; Hiwasa-Tanase, K.; Fukuda, N.; Mizoguchi, T.; Yamazaki, Y.; Aoki, K.; Ezura, H. TOMATOMA: A novel tomato mutant database distributing Micro-Tom mutant collections. Plant Cell Physiol. 2011, 52, 283–296. [Google Scholar] [CrossRef]
- Tsuda, M.; Kaga, A.; Anai, T.; Shimizu, T.; Sayama, T.; Takagi, K.; Machita, K.; Watanabe, S.; Nishimura, M.; Yamada, N.; et al. Construction of a high-density mutant library in soybean and development of a mutant retrieval method using amplicon sequencing. BMC Genom. 2015, 16, 1014. [Google Scholar] [CrossRef]
- Chen, T.; Huang, L.; Wang, M.; Huang, Y.; Zeng, R.; Wang, X.; Wang, L.; Wan, S.; Zhang, L. Ethyl methyl sulfonate-induced mutagenesis and its effects on peanut agronomic, yield and quality traits. Agronomy 2020, 10, 655. [Google Scholar] [CrossRef]
- Wang, D.; Bosland, P.W. The genes of Capsicum. HortScience 2006, 41, 1169–1187. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, S.; Li, N.; Li, Q.; Du, W.; Zhang, W.; Yu, P.; Xuan, S.; Wang, Y.; Zhao, J.; et al. Candidate gene, smcpr1, encoding cpr1 related to plant height of the eggplant dwarf mutant dwf. Horticulturae 2021, 7, 196. [Google Scholar] [CrossRef]
- Hooda, M.; Dhillon, R.; Bangarwa, K. Albinism in jojoba (Simmondsia chinensis). Natl. J. Plant Improv. 2004, 1, 69–70. [Google Scholar]
- Chen, T.; Zhang, Y.; Zhao, L.; Zhu, Z.; Lin, J.; Zhang, S.; Wang, C. Physiological character and gene mapping in a new green-revertible albino mutant in rice. J. Genet Genomics. 2007, 34, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Pawar, N.; Pai, S.; Nimbalkar, M.; Kolar, F.; Dixit, G. Induction of chlorophyll mutants in Zingiber officinale Roscoe by gamma rays and EMS. Emir J. Food Agric. 2010, 22, 406–411. [Google Scholar] [CrossRef]
- Wani, M.R. Characterization of chlorophyll deficient mutants in mungbean (Vigna radiata (L.) Wilczek). Bangladesh J. Bot. 2020, 49, 1013–1019. [Google Scholar] [CrossRef]
- Siddique, M.I.; Back, S.; Lee, J.H.; Jo, J.; Jang, S.; Han, K.; Venkatesh, J.; Kwon, J.K.; Jo, Y.D.; Kang, B.C. Development and Characterization of an Ethyl Methane Sulfonate (EMS) Induced Mutant Population in Capsicum annuum L. Plants 2020, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Bosland, P.W. Inheritance of a novel flaccid mutant in Capsicum annuum. J. Hered. 2002, 93, 380–382. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Du, Y.; He, C.; Dietrich, C.R.; Li, J.; Ma, X.; Wang, R.; Liu, Q.; Liu, S.; Wang, G.; et al. Maize glossy6 is involved in cuticular wax deposition and drought tolerance. J. Exp. Bot. 2019, 70, 3089–3099. [Google Scholar] [CrossRef]
- Kashyap, V.; Kumar, S.V.; Collonnier, C.; Fusari, F.; Haicour, R.; Rotino, G.L.; Sihachakr, D.; Rajam, M.V. Biotechnology of eggplant. Sci. Hortic. 2003, 97, 1–25. [Google Scholar] [CrossRef]
- Xiao, X.O.; Lin, W.Q.; Li, K.; Feng, X.F.; Jin, H.; Zou, H.F. Transcriptome analyses reveal anthocyanin biosynthesis in eggplants. PeerJ Preprints 2018, 6, e27289v1. [Google Scholar]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Arisha, M.H.; Shah, S.N.; Gong, Z.H.; Jing, H.; Li, C.; Zhang, H.X. Ethyl methane sulfonate induced mutations in M2 generation and physiological variations in M1 generation of peppers (Capsicum annuum L.). Front. Plant Sci. 2015, 6, 399. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Aguado, E.; Parra, G.; Manzano, S.; Martínez, C.; Megías, Z.; Cebrián, G.; Romero, J.; Beltrán, S.; Garrido, D.; et al. Phenomic and genomic characterization of a mutant platform in Cucurbita pepo. Front. Plant Sci. 2018, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Capel, C.; Nieto-Canseco, R.; Ortiz-Atienza, A.; Bretones, S.; López-Fábregas, J.D.; Quevedo-Colmena, A.S.; Lebrón, R.; Barragán-Lozano, T.; Villalobos-Ramírez, V.; et al. A Tomato EMS-Mutagenized Population Provides New Valuable Resources for Gene Discovery and Breeding of Developmental Traits. Plants 2022, 11, 2453. [Google Scholar] [CrossRef] [PubMed]
- Martín, B.; Ramiro, M.; Martínez-Zapater, J.M.; Alonso-Blanco, C. A high-density collection of EMS-induced mutations for TILLING in Landsberg erecta genetic background of Arabidopsis. BMC Plant Biol. 2009, 9, 147. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Li, J.; Yang, X.; Ren, Z. Ethyl methanesulfonate (EMS)-mediated mutagenesis of cucumber (Cucumis sativus L.). Agric. Sci. 2014, 5, 48085. [Google Scholar]
- Yong, W.T.L.; Aswandy, A.K.; Cheong, B.E.; Rodrigues, K.F. Mutagenic effects of ethyl methanesulfonate on nine protein coding genes in tomato (Solanum lycopersicum L.). Sci. Hortic. 2021, 276, 109739. [Google Scholar] [CrossRef]



| EMS Concentration (% v/v) | Germination Percentage (%) |
|---|---|
| 0 | 58 |
| 0.2 | 53 |
| 0.4 | 51 |
| 0.6 | 34 |
| 0.8 | 30 |
| 1.0 | 6 |
| FT/TFL1 Amplicons | Output (Mutants) |
|---|---|
| SmCEN-1 | 10 |
| SmCEN-2 | 12 |
| SmCEN-4 | 13 |
| SmTFL1 | 6 |
| SmMFT-1 | 11 |
| SmMFT-2 | 14 |
| SmMFT-2 (Mutant Sample) | Amino Acid | Predicted Region | Amplicon Coverage | Predicted Accuracy (pbLAA) |
|---|---|---|---|---|
| Allele_31-3_1 | T | 5′UTR | 77 | 1 |
| Allele_31-3_2 | T > C | 5′UTR | 72 | 1 |
| First Category | Second Category | No. of Mutants | Mutant Family |
|---|---|---|---|
| Plant height (cm) | 1 Dwarf (<10 cm) | 1 | 1 |
| 2 Very short (10–19 cm) | 2 | 2 | |
| 3 Short (20–38 cm) | 48 | 15 | |
| 4 Normal (38.1–48.9) (N = 7) | 25 | 14 | |
| 4 Tall (50–60 cm) | 11 | 10 | |
| 5 Medium tall (61–70 cm) | 1 | 1 | |
| 6 Very tall (>70 cm) | 1 | 1 | |
| Size of leaves (cm2) | 1 Too small (<50 cm2) | 4 | 4 |
| 2 Medium Small (50–100 cm2) | 7 | 6 | |
| 3 Small (101–204 cm2) | 34 | 14 | |
| 4 Normal (205–300 cm2) (N = 7) | 24 | 14 | |
| 5 Big (301–400 cm2) | 3 | 3 | |
| 6 Very Big (>400 cm2) | 2 | 2 | |
| Leaf shape | Curled leaves | 1 | 1 |
| Leaf structure | Thick and shiny, reduced in size | 1 | 1 |
| Leaf color mutation | Light green | 2 | 1 |
| Color of fruits | Bright purple | 1 | 1 |
| Even coloration | 1 | 1 | |
| Shape of fruits | Elongated and/or curved | 3 | 3 |
| Flower morphology | Variation in petal formation | 2 | 2 |
| Flower color | Bright purple | 1 | 1 |
| Observed Mutations | Total No. of Plants/Family | No. of Mutant Plants | Mutant’s Frequency (%) |
|---|---|---|---|
| Dwarfism (9-1) | 6 | 1 | 16.7 |
| Leaf colour (36-5) | 7 | 2 | 28.6 |
| Curled leaf (39-6) | 7 | 1 | 14.3 |
| Glossy leaf (31-6) | 6 | 1 | 16.7 |
| Fruit shape (33-7) | 7 | 1 | 14.3 |
| Fruit shape (39-3) | 7 | 1 | 14.3 |
| Fruit color (18-2) | 6 | 1 | 16.7 |
| Flower color (31-3) | 6 | 1 | 16.7 |
| No of petals (31-1) | 6 | 1 | 16.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniam, R.; Kumar, V.S. Ethyl Methanesulphonate (EMS)-Mediated Mutagenesis Induces Genetic and Morphological Variations in Eggplant (Solanum melongena L.). Int. J. Plant Biol. 2023, 14, 714-728. https://doi.org/10.3390/ijpb14030053
Subramaniam R, Kumar VS. Ethyl Methanesulphonate (EMS)-Mediated Mutagenesis Induces Genetic and Morphological Variations in Eggplant (Solanum melongena L.). International Journal of Plant Biology. 2023; 14(3):714-728. https://doi.org/10.3390/ijpb14030053
Chicago/Turabian StyleSubramaniam, Ranjita, and Vijay Subbiah Kumar. 2023. "Ethyl Methanesulphonate (EMS)-Mediated Mutagenesis Induces Genetic and Morphological Variations in Eggplant (Solanum melongena L.)" International Journal of Plant Biology 14, no. 3: 714-728. https://doi.org/10.3390/ijpb14030053
APA StyleSubramaniam, R., & Kumar, V. S. (2023). Ethyl Methanesulphonate (EMS)-Mediated Mutagenesis Induces Genetic and Morphological Variations in Eggplant (Solanum melongena L.). International Journal of Plant Biology, 14(3), 714-728. https://doi.org/10.3390/ijpb14030053









