Abstract
None of our investigations have identified stress in response to the HSP70 and HO-1 proteins in metals under stress in our study, which aimed to understand the genetic basis of the metal tolerance of Arundo donax. Thus, the present work aimed to investigate the levels of expression of two important stress-related proteins, HO-1 and HSP70, in A. donax after exposure to various metals. The plants were collected from uncontaminated sites in Abbottabad, Pakistan. Their rhizomes were grown in Hoagland solution, and upon attaining suitable biomass, the plants were used to investigate the effects of metals on protein expression. The metal treatments were carried out with synthetic wastewater containing four Cr treatments (0, 34, 66, 134, and 267 mgL−1), namely, Cd, As, Pb, Cu and Ni (0, 25, 50, 75, and 100 mgL−1), and the plants were grown for three weeks. The treatments were applied according to a randomized block design (RBD) based on hydroponics. The selected protein expression was examined after 10 days of metal exposure. For the HSP70 and HO-1 protein studies, leaves were separated following a previously reported standard procedure. The maximum level of HO-1 and HSP70 expressions was noted at 66 mgL−1 of Cr, and then it slightly declined. Significantly, high protein expression was observed at Cd exposure concentrations of 50 to 100 mgL−1. For Cu, As and Ni, significantly high HO-1 and HSP70 expressions were noted at metal exposure concentrations of 75 to 100 mgL−1. The expression levels of these two stress-related proteins showed a linear increase with increasing metal exposure in the giant reed. It is clear from the present research that HSP70 and HO-1 proteins may contribute significantly to plant tolerance to metal stress, in addition to other possible tolerance mechanisms.
1. Introduction
Environmental degradation caused by various abiotic stresses, namely, water shortages, climate change, inorganic metal pollution, and organic contamination, is a current global phenomenon [1]. Toxic heavy metals are considered pollutants and pose serious threats to many ecosystems [2]. Toxic metals have an atomic weight in the range of 63.5 to 200.6 and a density five times higher than that of water. The most commonly occurring metal pollutants are arsenic (As), chromium (Cr), copper (Cu), mercury (Hg), cadmium (Cd), cobalt (Co), lead (Pb), molybdenum (Mo), nickel (Ni), and zinc (Zn) [3,4]. Abiotic stress is caused by metals originating from the weathering of rocks, emissions from industrial toxic effluents, or surface runoff, posing serious threats to ecological systems [5,6,7,8]. Non-biodegradable, dangerous metal pollutants (Hg, Pb, As) may be found in air, soil, and water at various levels around the world [9]. Once absorbed by plants, they adversely affect plant metabolism, animal health and human health [10,11,12]. The main anthropogenic sources of Pb in soil are organic fertilizers, industrial effluent, sewage sludge and vehicle emissions [8]. High Pb concentrations cause threats to the food chain by contaminating herbal medicinal and edible plants [13].
Giant reed is a macrophyte commonly known as carrizo, Arundo, Spanish cane, wild cane, Danuban reed, giant Danube reed, etc. (3–10 m), and is a tall grass. Giant reed sprouts from rhizomes, which create thick clusters up to 1 m deep in soil [14]. Giant reed is suitable for phytoremediation purposes because of its high biomass of up to 50 Mg ha−1, with a 38 Mg DW biosolid ha−1 and high metal concentrations in its roots [15]. Giant reed is reported to be significantly tolerant and an accumulator of trace elements from contaminated sites; thus, it is useful for phytomanagement as well as wastewater treatment in CWs [16,17]. Previously, our research group screened the giant reed for the uptake of various toxic metals from aqueous and soil media [18,19] and its tolerance mechanism through gene expression [20,21]. However, the tolerance mechanism involved in metal tolerance, through the gene expression coding of various proteins, was not explored.
Plants respond to heavy metal stress by activating their stress genes and may synthesize heat shock proteins (HSPs) [22]. The HSP70 proteins have been widely studied among the HSP group of proteins, as they ensure critically protective and genetically conserved responses of plants to environmental stress [22]. Recently, it has been revealed that HSPs play important roles in plants under environmental stress and induced metal tolerance [23,24].
According to the literature review, no assessment of the application of HO-1 and HSP70 in giant reed against multi-metalliferous wastewaters has been reported. Such an analysis of protein expression in giant reed could be useful for understanding the genetic basis of plant tolerance to toxic heavy metal contamination. Therefore, the current investigation was designed to explore the expression of two widely studied proteins (HO-1 and HSP70) in giant reed, which may contribute towards metal tolerance and hyper-accumulation and would thus enrich existing knowledge about tolerance mechanisms in giant reed.
2. Materials and Methods
2.1. Plant Propagation and Metal Treatments
A small hydroponic culture was maintained to propagate the giant reed plants. Suitable, healthy cuttings containing buds were placed in Hoagland solution and allowed to germinate at room temperature for approximately one month. Upon attaining a reasonable height and biomass, the uniformly sized plants were chosen for metal treatments.
The metal treatments were chosen based on the literature survey and the prevalent pollution status of various industrial wastewaters. Different aqueous solutions of heavy metals, with their maximum and minimum concentration ranges (mgL−1), were prepared as follows: for Cr, 0, 33, 66, 134, and 268; and for each metal (As, Cd, Cu, Pb, and Ni), treatments of 0, 25, 50, 75, and 100 mgL−1.
2.2. Hydroponic Experiments
Plants with almost uniform fresh weights (200 ± 5 g) per pot were selected. A nutrient solution was also provided (Hoagland’s solution), according to Mirza et al. [18], for each respective treatment. A Randomized Block Design (RBD) was used in triplicate. The control group received no metal treatment, while the experimental group was exposed to various metal treatments, as indicated in Section 2.1. The nutrient solution was constantly monitored, and more was added to compensate for transpiration loss every three days.
2.3. Analysis of Total Proteins and SDS-PAGE
For the extraction and quantification of total proteins from giant reed plants, the following procedures were followed. The He [25] protocol was utilized for the quantitative evaluation of soluble proteins.
2.3.1. Whole Cell Protein Extraction
Frozen leaves from control and experimental plants (1 g) were crushed and homogenized by a precooled pestle and mortar with 0.1 M chilled potassium phosphate buffer (pH 7.0) ratio of 1 to 4 (w/v). Crushed samples were shifted to falcon tubes (15 mL) and stored at 4 °C for 24 h. After 24 h, the extracts were centrifuged at 10,000× g (4 °C) for 10 min to obtain residue-free plant extract. The supernatant was collected and stored at −80 °C.
2.3.2. Bradford Assay
The amounts of protein in samples were determined by their absorbance and comparison to the standard protein curve. For calibration curves (0, 2, 4, 8, 12, 16, and 20 μL) Bovine Serum Albumin (BSA) (1 mgmL−1) was taken, and the final volume (200 µL) was made with distilled water, and the addition of 200 µL of Bradford reagent proceeded the reaction. The reaction mixture was incubated in the dark at 25 °C for 5 min, and absorbance was recorded at 595 nm with a BIO-RA, iMark microplate reader. The absorbance values were corrected by subtracting the average absorbance of the blank samples and normalized by dividing by the average path length of 120 μL of water (0.6189 ± 0.002 cm). The normalized absorbance values were plotted versus the mass concentration (gL−1). Protein solutions were prepared as 50 mgL−1 from the stock solutions of crude proteins. Each well of the microplate was filled with 200 µL of Bradford reagent along with 20 µL of protein extract, with 5 replicates per treatment. After 5 min of incubation at 25 °C in the dark, the readings were noted for the control and various treatments.
2.3.3. Electrophoresis of Crude Proteins
A constant amount of protein was loaded on SDS-PAGE comprising 4% and 10% polyacrylamide slab gels, using a Bio-Rad Mini-Protean II (PowerPacTM Basic, Bio-Rad, Hercules, CA, USA) along with a molecular weight marker. Total proteins were loaded at 20–30 μg in triplicate. The samples were run for 1 h at 100 V then raised to 150 V until the proteins reached the end of the staking gel. A standard protein ladder made of proteins of known weight ranging from 14.4–116 kDa (Standard protein marker, Fermantas, Germany) was used during gel electrophoresis.
2.3.4. Transferring of Proteins from Gel to Membrane
The Polyvinylidene Difluoride (PVDF) membrane measuring 8.5 × 5.3 cm was activated with 100% methanol for 1 min and rinsed with transfer buffer with shaking at 22.8 °C and 80 R for 10 min. Staked blotting cotton pads, SDS PAGE, and membranes (PROTRAN, Shleicher & Schuell, Dassel, Germany) were run by Trans-Blot Semi-dry (Bio-Rad, Hercules, CA, USA) in transfer buffer (25 mM Tris, 192 mM glycine, and 20% (v/v) methanol, pH 8.3) and placed in an ice block tank. The time and voltage of transfer were optimized at 250 V for 2 h. After 2 h, membranes were inserted into BSA buffer and kept shaking for 2 h. After 2 h, membranes were washed with TBST three times, followed by 5 min of shaking.
2.3.5. Antibody Staining
Blocked membranes were incubated on a shaker overnight at 4 °C with appropriate dilutions (1:200) of the primary antibody in blocking buffer. The next morning membranes were processed by three washes of TBST, 5 min each. Then, these membranes were stripped and reprobed with anti-protein-specific goat anti-rabbit IgG antibody dilutions of 1:200 at room temperature for 2 h shaking. Finally, we washed the membranes three times with TBST for 5 min at room temperature.
2.3.6. Chemiluminescence Staining
For imaging, the membranes were submerged in a 1 mL mixture of illuminating solutions (super star ECL A and super star ECL B) in a dark room, (Automatic chemiluminescence image analysis system, Tenon v5200, Beijing, China) and results were captured.
2.4. Statistical Analysis
Results are expressed as means ± S.E.M. for the indicated number of separate cell preparations per experimental protocol. The data were analyzed using one-way analysis of variance followed by the LSD post hoc test. Differences between groups were considered significant at p < 0.05. Analyses were performed using the SigmaPlotTM statistical software package v10 (Systat Software, Inc., San Jose, CA, USA).
3. Results
The results related to the ability of the giant reed to uptake various toxic metals from aqueous and soil mediums have been described previously in our publications [18,19], and tolerance mechanisms through gene expression were also explored [20,21]. However, the tolerance mechanism through the production of various stress-related proteins involved in metal tolerance was not explored, and hence the present results will describe it here.
3.1. Effect of Chromium on HSP70 and HO-1
Western blot analyses for the expression of HSP70 and HO-1 with respective molecular weights of 70 kDa and 30 kDa reprobed by anti-protein-specific goat anti-rabbit IgG in giant reed plants under Cr stress were given.
It was apparent that after stress exposure, the HSP70 protein bands were intensely increased at a Cr concentration of 33 mgL−1 and further increased up to a 66 mgL−1 concentration level. Beyond the optimum concentration of Cr, the level of HSP70 expression gradually decreased to some extent at Cr concentrations of 75 mgL−1 and 100 mgL−1, but was still higher than the HSP70 contents of the control plants (Figure 1). From the expression of HO-1 proteins under Cr stress, it was shown that the various concentrations of Cr stress (25 L−1 to 75 mgL−1) did not significantly alter the HO-1 expression over control plants.
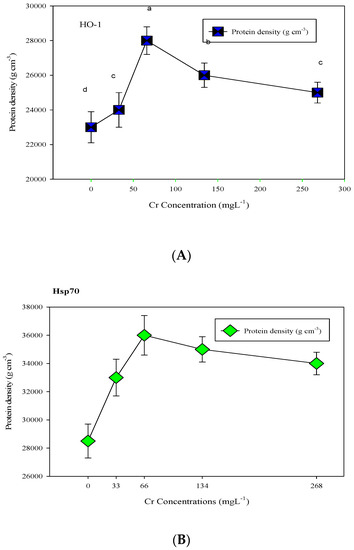
Figure 1.
Western blot analysis for HO-1 (A) and HSP70 (B) protein expression under Cr stress in giant reed plants. The membranes were stripped and reprobed with an anti-protein-specific goat anti-rabbit IgG antibody. The bars represent means ± S.E.M. (n = 3); p ≤ 0.05. Different letters along the data points represent significant differences.
But the expression of HO-1 proteins in plants under maximum stress was higher than in other control and treated plants. The expression level of HSP70 was significantly high (p ≤ 0.05) against 50 mgL−1 and HO-1 protein at 100 mgL−1 of Cr stress (Figure 1).
3.2. Effect of Cd on HSP70 and HO-1
Western blot analyses for the expression of HSP70 and HO-1 proteins against anti-goat and anti-rabbit IgG in giant reed plants under the application of various concentrations of Cd stress are given in Figure 2. It was observed that the expression of HSP70 protein bands of molecular weight 70 kDa intensely increased with increasing Cd concentrations. It was observed that HSP70 contents increased gradually with increasing Cd concentrations, and the maximum HSP70 contents were found at 75 mgL−1 and 100 mgL−1 Cd treatments. Thus, the SDS page results depicted a significant increasing trend of HSP70 proteins against various concentrations, and very light bands appeared in the control and minimum concentration (25 mgL−1) of giant reed plants.
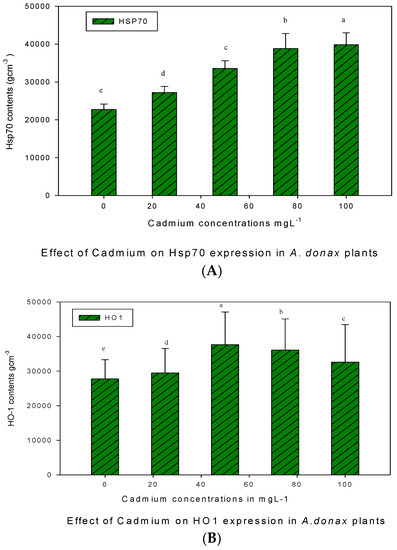
Figure 2.
Western blot analysis for HSP70 (A) and HO-1 (B) protein expression under Cd stress in giant reed plants. The membranes were stripped and reprobed with an anti-protein-specific goat anti-rabbit IgG antibody. The bars represent means ± S.E.M. (n = 3); p ≤ 0.05. Different letters along the data points represent significant differences.
The expression of HO-1 proteins of molecular weight 32 kDa in Giant reed plants against various concentrations of Cd stress is shown in Figure 2. It was observed that minimum HO-1 contents were observed in control plants, but the level of HO-1 protein was pronounced and uniform against different Cd concentrations. The expression levels of HO-1 and HSP70 were significantly high (p ≤ 0.05) against 50 mgL−1 and 100 mgL−1 concentrations of Cd stress, respectively.
3.3. Effect of Ni on HSP70 and HO-1
Under Ni treatments, the expression of HSP70 and HO-1 proteins showed significant variations in giant reed plants, as given in Figure 3. The results revealed that minimum HO-1 contents were found in control plants, which increased with metal treatment, and the level of HO-1 expression was almost equal at the first two treatment conditions (25 and 50 mgL−1). The maximum HO-1 contents were observed for the maximum concentration of Ni (100 mgL−1), which was significantly higher than the HO-1 contents found in control plants.
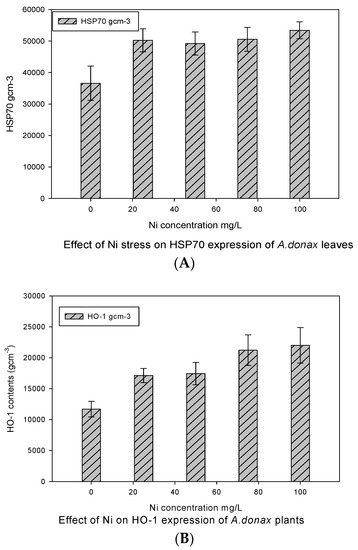
Figure 3.
Western blot analysis for HSP70 (A) and HO-1 (B) protein expression under Ni stress in giant reed plants. The membranes were stripped and reprobed with an anti-protein-specific goat and anti-rabbit IgG antibody. The bars represent means ± S.E.M. (n = 3); p ≤ 0.05.
In the case of HSP70 protein expression under various concentrations of Ni toxicity, the minimum expression was observed in control plants, but it was interesting to note that the HSP70 protein was equally expressed at all treatment levels. Therefore, the intensity of HSP70 bands was identical in all treatments up to 75 mgL−1 but it was somewhat higher against a higher concentration of Ni (100 mgL−1), which was not significantly different as suggested by the LSD post hoc test. Thus, the results of HSP70 contents were not significantly different within treatment groups but were significant in the control.
3.4. HSP70 and HO-1 Expression under Cu Exposure
Western blot analyses for the expression of HSP70 and HO-1 proteins against anti-goat and anti-rabbit IgG in A. donax plants under the application of various concentrations of Cu stress are given in Figure 4. It was observed that the HSP70 protein bands were almost equally expressed in the control and the minimum concentration of Cu (25 mgL−1). Then, the HSP70 contents progressively increased to 50 mgL−1. The maximum concentration of HSP70 protein was observed at the highest concentration of Cu (100 mgL−1).
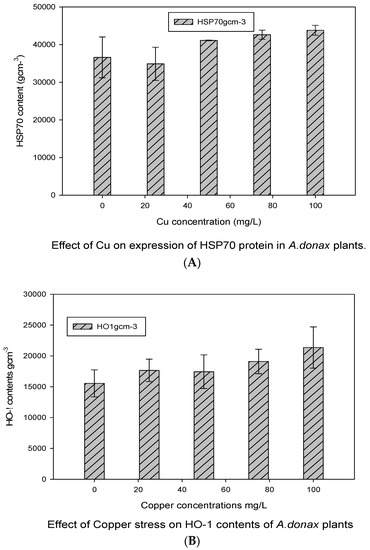
Figure 4.
Western blot analysis for HSP70 (A) and HO-1 (B) protein expression at Cu stress in A. donax plants. The membranes were stripped and reprobed with an anti-protein-specific goat and anti-rabbit IgG antibody. The bars represent means ± S.E.M. (n = 3); p ≤ 0.05.
Similarly, the expression level of HO1 proteins was also stress-dependent, and almost all concentrations of Cu stress depicted various contents of HO1 proteins. It was observed that the HO-1 contents of giant reed were the minimum in control plants, but their amount at the maximum level of treatment was significantly higher. It showed that proteins HSP70 and HO-1 were significantly (p ≤ 0.05) affected by Cu stress at the maximum concentration of Cu exposure, more prominently as compared to control giant reed plants.
3.5. HSP70 and HO-1 Expression under Pb Exposure
A Western blot analysis for the expression of HSP70 and HO-1 proteins in giant reed plants against various concentrations of Pb stress is given in Figure 5. It was observed that the expression of HSP70 proteins was stress dependent and increased with increasing concentrations of Pb. HSP70 contents were almost equal and constant at (25 and 50 mgL−1), which decreased at Pb (75 mgL−1) and then increased at 100 mgL−1 concentration of Pb. The expression level of HO1 proteins also responded against Pb toxicity, and it was noticed that the minimum HO-1 contents were found in the control and at 50 mgL−1 of Pb concentrations.
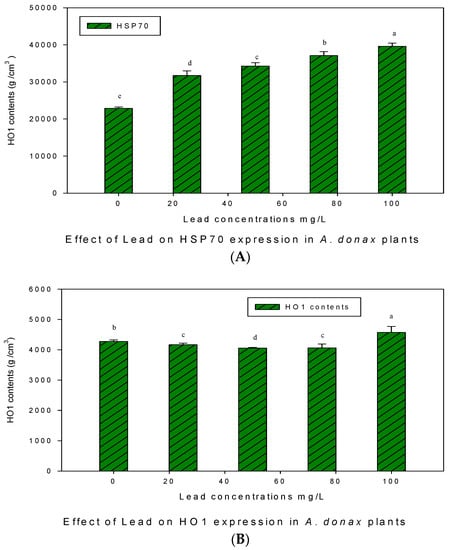
Figure 5.
Western blot analysis for HSP70 (A) and HO-1 (B) protein expression under Pb stress in giant reed plants. The membranes were stripped and reprobed with an anti-protein-specific goat and anti-rabbit IgG antibody. The bars represent means ± S.E.M. (n = 3); p ≤ 0.05. Different letters along the data points represent significant differences.
The mean HO-1 contents appeared at 75 mgL−1 exposure, while the maximum HO-1 contents were observed at 25 mgL−1 and 100 mgL−1 concentrations of Pb. The expression levels of the HO-1 and HSP70 proteins under Pb stress were significantly different for (p ≤ 0.05) giant reed plants.
3.6. The Effect of As on (HSP70 and HO-1)
The effect of As stress on the expression level of the HSP70 and HO-1 proteins in giant reed plants is given in Figure 6. The expression of the HSP70 protein was stress dependent and increased with increasing concentrations of As stress. HSP70 contents were not expressed in control plants, but their expression was high for all As treated plants. It was observed that HSP70 contents were high at the maximum treatment level (100 mgL−1). The expression level of HO1 proteins also responded to various As concentrations and it was noticed that the minimum HO-1 contents were found in the control and at 25 mgL−1.
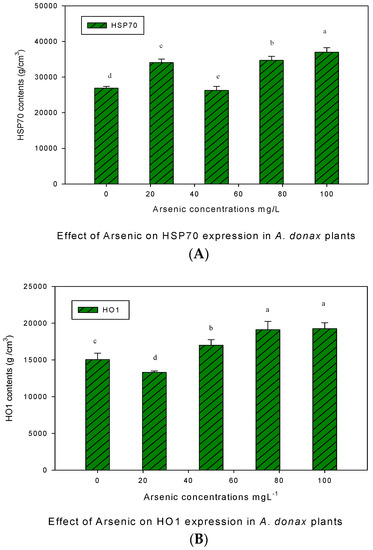
Figure 6.
Western blot analysis for HSP70 (A) and HO-1 (B) protein expression under As stress in giant reed plants. The membranes were stripped and reprobed with an anti-protein-specific goat and anti-rabbit IgG antibody. The bars represent means ± S.E.M. (n = 3); p ≤ 0.05. Different letters along the data points represent significant differences.
4. Discussion
Giant reed was evaluated for selected protein expression due to metal stress. Previous investigations have demonstrated that plants are quite capable of removing toxic metals from their growth medium [18,19,20,21,26]. It is a known fact that metal exposure results in oxidative stress [27]. Stress responsive genes may be expressed upon the exposure of plants to some toxic metals and subsequent increased ROS levels, their binding proteins, DNA damage, and lipid peroxidation [28,29,30]. Variable miRNA levels have been noted upon exposure to abiotic stress in plants under the influence of specific genes, which seemed to be an adaptation to stress [31].
It has been previously reported that plants defense responses increased through genes binding stress signaling proteins including HO-1 and HSP70, under metal stress [22,24]. Shekhawat and Verma [32] reported that HO-1 might be involved in the cellular defense against ROS mediated damage from heavy metals in combination with other defensive mechanisms. The first report on the involvement of HO-1 in plant protection was published in soybean plants upon exposure to 200 μM Cd stress for 48 h, and it was suggested to be involved in signaling against ROS (H2O2 and O2−) [33].
The present work confirmed the role of the HO-1 protein in giant reed, which was significantly expressed at various metal concentrations. The HO-1 protein in plants is expected to play an important role in various metabolic pathways like development, defense, and physiology to cope with various environmental stresses. It was also suggested that a close relationship existed between the expression of glutathione-responsive genes and HO-1 for metal detoxification in plants [34]. Additionally, the expression level of HO-1 in plants increased with antioxidant production, namely SOD, CAT, and GPOX, upon exposure to metal [35]. The HO-1 is reported as an important antioxidant defense enzyme that recently received attention in the maintenance of cell damage caused by ROS under heavy metal stress, along with other defensive mechanisms. The HO-1 was investigated for the first time in soybean plants at 200 μM Cd stress for 48 h, and it was reported that it was involved in plant protection by producing signals against ROS (H2O2 and O2−) [36]. In Eruca sativa seedlings, HO-1 proteins enhanced the Cd tolerance activity by regulating the phytoremediation efficiency using antioxidant defense machinery [37]. HO-1 has also been reported against various treatments of Cd stress for decreased GSH content in the roots of Medicago sativa [38]. A close relationship exists between the expression of HO-1 and glutathione-responsive genes for the detoxification of heavy metals in plants [39]. It was also confirmed that the expression level of HO-1 increased in plants with the production of antioxidants like SOD, CAT and GPOX under metal stress [35].
Another important group of plant proteins are housekeeping proteins (Hsp70), which are abiotic stress responding proteins that correspond to melatonin (N-acetyl-5-methoxy tryptamine) production with the help of the A1a (HsfA1a) factor that protects the plants from ROS [40]. In the roots of tomato plants, HSPs and detoxifying proteins are expressed at various concentrations of Cd (0, 10 and 100 µM) [41]. Proteomic expression of L. riparium also showed the production of HSP70 proteins when exposed to contaminated wastewater containing Cu, Cd, Cr and Pb [42]. A transcriptomic study of rice roots showed that Cd stress triggered the regulation of genes related to refolding of unfolded proteins, which activated the sulfate adjustment [43]. Similar results have also been observed in L. minor when exposed to Hg (0–30 μM for 6 days), where the expression level of HSP70 proteins was high at higher concentrations of Hg [44].
It has also been demonstrated that HSP70 expression in L. riparium was significantly elevated upon exposure of plants to various metals, namely Cd, Cu, Cr, and Pb [45]. A similar situation was observed in Lemna minor in response to Hg (0–30 μM for 6 days), resulting in high HSP70 expression for higher Hg concentrations [46]. The HSP70 protein expression has been widely accepted as a biomarker protein expressed as a signaling molecule against metal stress and high temperatures in plants [47]. Stress caused by Cr also boosts the gene expression related to antioxidants (CH, bHLH, Amidase, GR and expression of HO-1 and HSP70 at higher contamination levels) [48]. Giant reed has a hyperaccumulation ability based on its strong genetic battery, which makes it suitable for phytoremediation purposes. The role of heme oxygenase HO-1 was evaluated in Vigna radiata at 50 μM CdCl2 and 60 μM NiSO4 for changes in growth, stress parameters (LPX, H2O2 content), and non-enzymatic and enzymatic parameters ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and catalase (CAT) activity were found to be highest in leaf-induced cytotoxicity and ROS production [49]. Previously, the expression pattern of HO-1 was significantly high in transgenic B. juncea as BjHO-1 against Zn, Cd, Hg, and Pb exposure, which enhanced plant tolerance [50].
Various Cu concentrations enhanced HO-1 and HSP70 expression at all metal exposures. The highest Cu concentration in A. donax leaves could be due to the presence of copper–nicotiana amine complex and YSL16, which were observed in the phloem of rice leaves and were thought to be involved in translocation of Cu from leaves to maturing organs and seeds [51]. Copper stress has also been studied in giant reed plants for 6 weeks by Elhawat et al. [52], who found no toxicity symptoms and high biomass production. Likewise, Wang et al. [53] reported an overwhelming increase in the activities of SOD, POD, APX and CAT under Cu stress (8 µM) in roots of B. juncea seedlings after 16 days of exposure. Various Ni concentrations enhanced the expression level of HO-1 and HSP70 at all contamination levels. Previously, the similar physiological features of the giant reed plant were highlighted after heavy metal stress exposure. Papazoglou et al. [54] found no toxicity symptoms of Ni stress (5, 50 and 100 ppm) on the A. donax photosynthetic system. Various concentrations of As enhanced the expression of HO-1 and HSP70 at all contamination levels. Singh et al. [55] reported increased activity of SOD and CAT under As exposure (5 and 50 µM) in Luffa acutangula (L.). These results suggest that the cooperative action of antioxidants is required for a detoxification mechanism under heavy metal stress. Various concentrations of Pb enhanced the expression of HO-1 and HSP70 at all contamination levels. It was also reported that the exposure of fourteen varieties of rice plants to PbCl2, PbSO4, and Pb(NO3)2 (0, 100 and 200 μM) significantly (p = 0.05) decreased the growth and total chlorophyll contents [56].
5. Conclusions
The current investigation on the assessment of protein expression against metal stress in Giant reed can be concluded as below:
The expression levels of two stress-related proteins showed a linear increase with increasing metal exposure in giant reed. Overall, HO-1 and Hsp70 expression was relatively high for metals like Cd, Cu, and Ni. The maximum HO-1 and Hsp70 expressions were noted at 66 mgL−1 of Cr, then it slightly declined. Significantly high protein expression was observed at exposed Cd concentrations of 50 to 100 mgL−1. For Cu, As and Ni significantly high HO-1 and Hsp70 expressions were noted at exposed metal concentrations of 75 to 100 mgL−1. It is clear from the present research that HSP70 and HO-1 proteins might have played a significant role in plant tolerance against metal stress, in addition to other possible tolerance mechanisms.
Author Contributions
All authors contributed to the study’s conception and design. Conceptualization, Q.M.; Investigation and first draft, S.S.; Revision, Q.M. and Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are privately kept by the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adhikari, S.; Marcelo-Silva, J.; Beukes, J.P.; van Zyl, P.G.; Coetsee, Y.; Boneschans, R.B.; Siebert, S.J. Total and hexavalent chromium and other potentially toxic element contamination of useful plant leaves in a polluted mining-smelting region of south Africa and health risks. SSRN Electron. J. 2022, 9, 100260. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Velázquez, J.R.; Calleja, A.; Moreno, I.; Bautista, J.; Esteban Alonso, E. Metal profiles and health risk assessment of the most consumed rice varieties in Spain. J. Food Compos. Anal. 2023, 117, 105101. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar]
- Alloway, B.J. Sources of heavy metals and metalloids in soils. In Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Arena, E.; Fallico, B.; Maccarone, E. Evaluation of antioxidant capacity of blood orange juices as influenced by constituents, concentration process and storage. Food Chem. 2001, 74, 423–427. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Borgå, K.; McKinney, M.A.; Routti, H.; Fernie, K.J.; Giebichenstein, J.; Hallanger, I.; Muir, D.C.G. The influence of global climate change on accumulation and toxicity of persistent organic pollutants and chemicals of emerging concern in Arctic food webs. Environ. Sci. Process. Impacts 2022, 24, 1544–1576. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Zhang, Y.; Xu, Y.-P.; Qi, Z.-Y.; Li, M.-Q.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Reiter, R.J.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef]
- Csurhes, S. Invasive Weed Risk Assessment: Giant Reed (Arundo donax); Report PR09-4547; Biosecurity Queensland, Department of Agriculture and Fisheries: Brisbane, Australia, 2009; p. 17. [Google Scholar]
- Cui, W.; Fu, G.; Wu, H.; Shen, W. Cadmium-induced heme oxygenase-1 gene expression is associated with the depletion of glutathione in the roots of Medicago sativa. Biometals 2011, 24, 93–103. [Google Scholar]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 52, 675–726. [Google Scholar] [CrossRef]
- Elhawat, N.; Alshaal, T.; Domokos-Szabolcsy, É.; El-Ramady, H.; Márton, L.; Czakó, M.; Popp, J. Phytoaccumu-lation potentials of two biotechnologically propagated ecotypes of A. donax in copper-contaminated synthetic wastewater. Environ. Sci. Pollut. Res. 2014, 21, 7773–7780. [Google Scholar]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Karam, E.A.; Lentini, M.; et al. In-field and in-vitro study of the moss Leptodictyum riparium as bioindicator of toxic metal pollution in the aquatic environment: Ultrastructural damage, oxidative stress and HSP70 induction. PLoS ONE 2018, 13, e0195717. [Google Scholar] [CrossRef]
- Gao, L.; Li, R.; Liang, Z.; Wu, Q.; Yang, Z.; Li, M.; Chen, J.; Hou, L. Mobilization mechanisms and toxicity risk of sediment trace metals (Cu, Zn, Ni, and Pb) based on diffusive gradients in thin films: A case study in the Xizhi River basin, South China. J. Hazard. Mater. 2020, 410, 124590. [Google Scholar] [CrossRef]
- Guarino, F.B.K.; Castiglione, S.; Cicatelli, A.; Biondi, S. The combined effect of Cr(III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd.). Ecotoxicol. Environ. Saf. 2020, 193, 110345. [Google Scholar]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Ding, X.; Shen, W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2008, 177, 155–166. [Google Scholar] [CrossRef]
- Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Gong, Z.H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar]
- Hasan, K.M.; Cheng, Y.; Kanwar, K.M.; Chu, X.Y.; Ahammed, G.J.; Qi, Y.Z. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 14. [Google Scholar]
- Hassan, R.O.; Othman, H.O.; Ali, D.S.; Abdullah, F.O.; Darwesh, D.A. Assessment of the health risk posed by toxic metals in commonly consumed legume brands in Erbil, Iraq. J. Food Compos. Anal. 2023, 120, 105282. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; He, Y.; Cao, Q.; Zhang, T.; Lou, L.; Cai, Q. Full-Length Transcriptome Assembly of Italian Ryegrass Root Integrated with RNA-Seq to Identify Genes in Response to Plant Cadmium Stress. Int. J. Mol. Sci. 2020, 21, 1067. [Google Scholar] [CrossRef]
- Idris, S.M.; Jones, P.L.; Salzman, S.A.; Allinson, G. Performance of the Giant Reed (Arundo donax) in Experimental Wetlands Receiving Variable Loads of Industrial Stormwater. Water Air Soil Pollut. 2012, 223, 549–557. [Google Scholar] [CrossRef]
- Idris, S.M.; Jones, P.L.; Salzman, S.A.; Croatto, G.; Allinson, G. Evaluation of the giant reed (Arundo donax) in horizontal subsurface flow wetlands for the treatment of dairy processing factory wastewater. Environ. Sci. Pollut. Res. 2012, 19, 3525–3537. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Murtaza, G.; Naz, T.; Niazi, N.K.; Shakar, M.; Watto, F.M.; Farooq, O.; Ali, M.; Rehman, H.-U.; Afzal, I.; et al. Effects of Lead Salts on Growth, Chlorophyll Contents and Tissue Concentration of Rice Genotypes. Int. J. Agric. Biol. 2017, 19, 69–76. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Lamb, D.T.; Heading, S.; Bolan, N.; Naidu, R. Use of Biosolids for Phytocapping of Landfill Soil. Water Air Soil Pollut. 2012, 223, 2695–2705. [Google Scholar] [CrossRef]
- Latef, A.A. Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. J. Agric. Sci. Technol. 2018, 15, 1437–1448. [Google Scholar]
- Lecube, M.L.; Noriega, G.O.; Cruz, D.M.S.; Tomaro, M.L.; Batlle, A.; Balestrasse, K.B. Indole acetic acid is responsible for protection against oxidative stress caused by drought in soybean plants: The role of heme oxygenase induction. Redox Rep. 2014, 19, 242–250. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, P.; Shen, B.; Zhang, Y.; Shen, J. Baicalin promotes neuronal differentiation of neural stem/progenitor cells through modulating p-stat3 and bHLH family protein expression. Brain Res. 2012, 1429, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Q.; Lin, C.; He, L.; Wei, L. Histological alterations, oxidative stress, and inflammatory response in the liver of swamp eel (Monopterus albus) acutely exposed to copper. Fish Physiol. Biochem. 2021, 47, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, L.; Kumar, R.; Shekhawat, G.S. Evaluation of heme oxygenase 1 (HO 1) in Cd and Ni induced cytotoxicity and crosstalk with ROS quenching enzymes in two to four leaf stage seedlings of Vigna radiata. Protoplasma 2018, 255, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, L.; Shekhawat, G.S. Understanding the Physiological Mechanism of Heme Oxygenase for Enhanced Tolerance and Phytoremediation of Cd2+ in Eruca sativa: Co-ordinated Function of Antioxidant Defense System. J. Plant Growth Regul. 2022, 1–12. [Google Scholar] [CrossRef]
- Mirza, N.; Mahmood, Q.; Pervez, A.; Ahmad, R.; Farooq, R.; Shah, M.M.; Azim, M.R. Phytoremediation potential of Arundo donax in arsenic-contaminated synthetic wastewater. Bioresour. Technol. 2010, 101, 5815–5819. [Google Scholar] [CrossRef]
- Nawab, J.; Khan, S.; Shah, M.T.; Qamar, Z.; Din, I.; Mahmood, Q.; Gul, N.; Huang, Q. Contamination of soil, medicinal, and fodder plants with lead and cadmium present in mine-affected areas, Northern Pakistan. Environ. Monit. Assess. 2015, 187, 1–14. [Google Scholar] [CrossRef]
- Noman, A.; Fahad, S.; Aqeel, M.; Ali, U.; Amanullah; Anwar, S.; Baloch, S.K.; Zainab, M. miRNAs: Major modulators for crop growth and development under abiotic stresses. Biotechnol. Lett. 2017, 39, 685–700. [Google Scholar] [CrossRef]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef]
- Ogo, Y.; Kakei, Y.; Itai, R.N.; Kobayashi, T.; Nakanishi, H.; Nishizawa, N.K. Tissue-specific transcriptional profiling of iron-deficient and cadmium-stressed rice using laser capture microdissection. Plant Signal. Behav. 2014, 9, 781–794. [Google Scholar]
- Ohkama-Ohtsu, N.; Oikawa, A.; Zhao, P.; Xiang, C.; Saito, K.; Oliver, D.J. A γ-Glutamyl Transpeptidase-Independent Pathway of Glutathione Catabolism to Glutamate via 5-Oxoproline in Arabidopsis. Plant Physiol. 2008, 148, 1603–1613. [Google Scholar] [CrossRef]
- Papazoglou, E.G. Arundo donax L. stress tolerance under irrigation with heavy metal aqueous solutions. Desalination 2007, 211, 304–313. [Google Scholar] [CrossRef]
- Perna, S.; AL-Qallaf, Z.A.; Mahmood, Q. Evaluation of Phragmites australis for Environmental Sustainability in Bahrain: Photosynthesis Pigments, Cd, Pb, Cu, and Zn Content Grown in Urban Wastes. Urban Sci. 2023, 7, 53. [Google Scholar]
- Sabeen, M.; Mahmood, Q.; Irshad, M.; Fareed, I.; Khan, A.; Farid, U.; Hussain, J.; Hayat, Y.; Tabassum, S. Cadmium Phytoremediation by Arundo donax L. from Contaminated Soil and Water. BioMed Res. Int. 2013, 2013, 324830. [Google Scholar]
- Seleiman, M.F.; Kheir, A.M. Maize productivity, heavy metals uptake and their availability in contaminated clay and sandy alkaline soils as affected by inorganic and organic amendments. Chemosphere 2018, 204, 514–522. [Google Scholar] [CrossRef]
- Shaheen, S.; Ahmad, R.; Mahmood, Q.; Pervez, A.; Shah, M.M.; Hafeez, F. Gene expression and biochemical response of giant reed under Ni and Cu stress. Int. J. Phytore. 2019, 21, 1474–1485. [Google Scholar] [CrossRef]
- Shaheen, S.; Ahmad, R.; Jin, W.; Mahmood, Q.; Iqbal, A.; Pervez, A.; Shah, M.M. Transcriptomic responses of selected genes against chromium stress in Arundo donax L. Toxicol. Environ. Chem. 2017, 99, 900–912. [Google Scholar] [CrossRef]
- Shakoor, A.; Ashraf, F.; Shakoor, S.; Mustafa, A.; Rehman, A.; Altaf, M.M.; Mustafa, A. Biogeochemical transformation of greenhouse gas emissions from terrestrial to atmospheric environment and potential feedback to climate forcing. Environ. Sci. Pollut. Res. 2020, 27, 38513–38536. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Verma, K. Haem oxygenase (HO): An overlooked enzyme of plant metabolism and defense. J. Exp. Bot. 2010, 61, 2255–2270. [Google Scholar]
- Singh, V.P.; Srivastava, P.K.; Prasad, S.M. Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol. Biochem. 2013, 71, 155–163. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Yang, Z.M.; Yang, H.; Lu, B.; Li, S.Q.; Lu, Y.P. Copper-induced stress and antioxidative responses in roots of Brassica juncea L. Bot. Bull. Acad. Sin. 2004, 45, 203–212. [Google Scholar]
- Yang, J.; Li, G.; Bishopp, A.; Heenatigala, P.P.M.; Hu, S.; Chen, Y.; Wu, Z.; Kumar, S.; Duan, P.; Yao, L.; et al. A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front. Chem. 2018, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, W.; Yang, Y.; Shen, Z.; Ma, J.F.; Zheng, L. OsYSL16 is Required for Preferential Cu Distribution to Floral Organs in Rice. Plant Cell Physiol. 2018, 59, 2039–2051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).