Beneficial Cecal Microbiome Modulation in Turkeys Exposed to Probiotics and Vaccine After Multidrug-Resistant Salmonella Heidelberg Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.1.1. Probiotic Bacteria
2.1.2. Salmonella enterica subsp. enterica Serovar Heidelberg (SH)
2.2. Experimental Design
2.3. DNA Extraction, Library Preparation, and Sequencing
2.4. Bioinformatics and Data Analysis
3. Results
3.1. DNA Sequence Read Quality
3.2. Evaluation of Changes in Cecal Microbial Taxonomic Abundance and Diversity Indices, Network Association, and Microbial Functional Profiling
3.2.1. Taxonomic Diversity Measurement
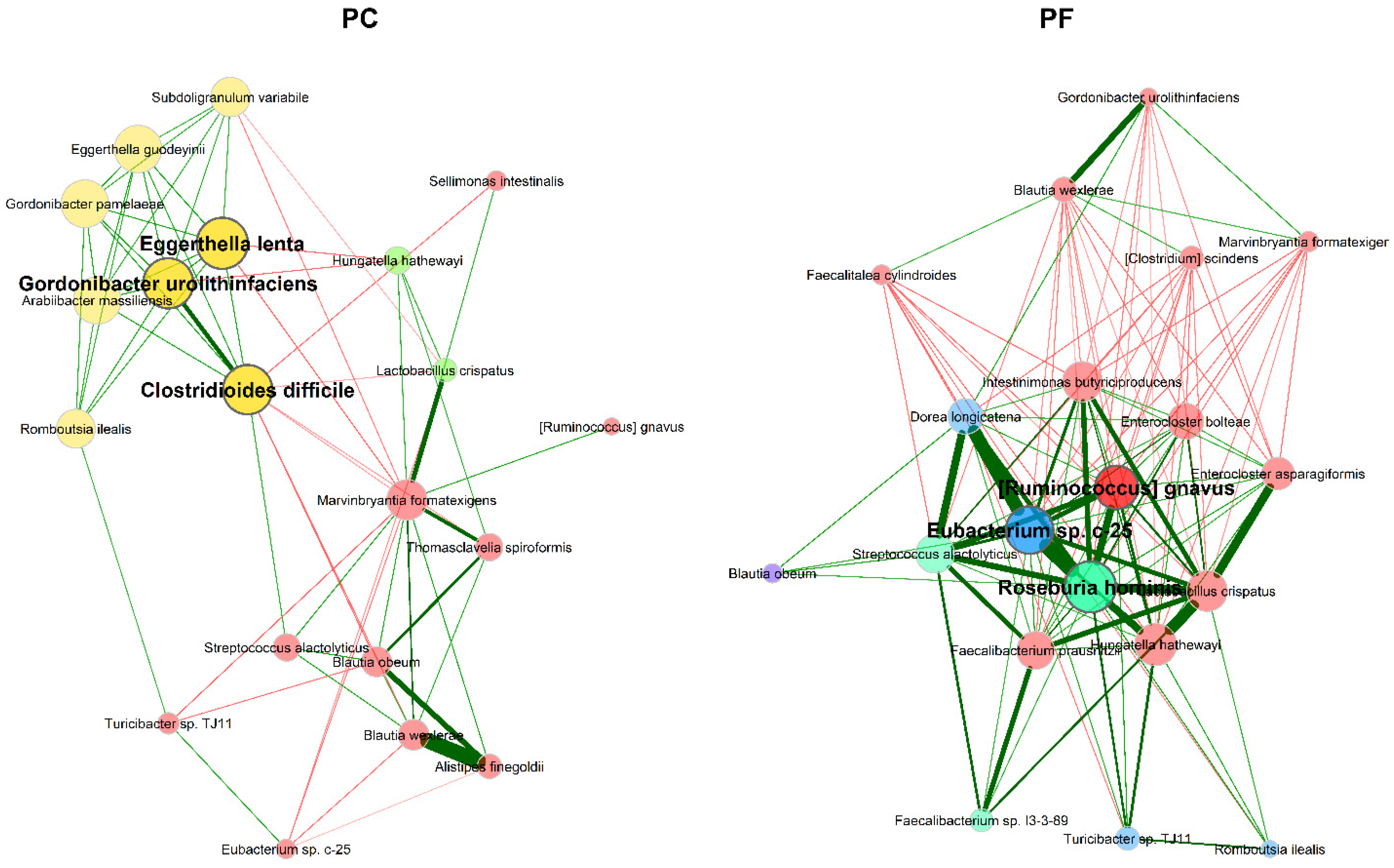
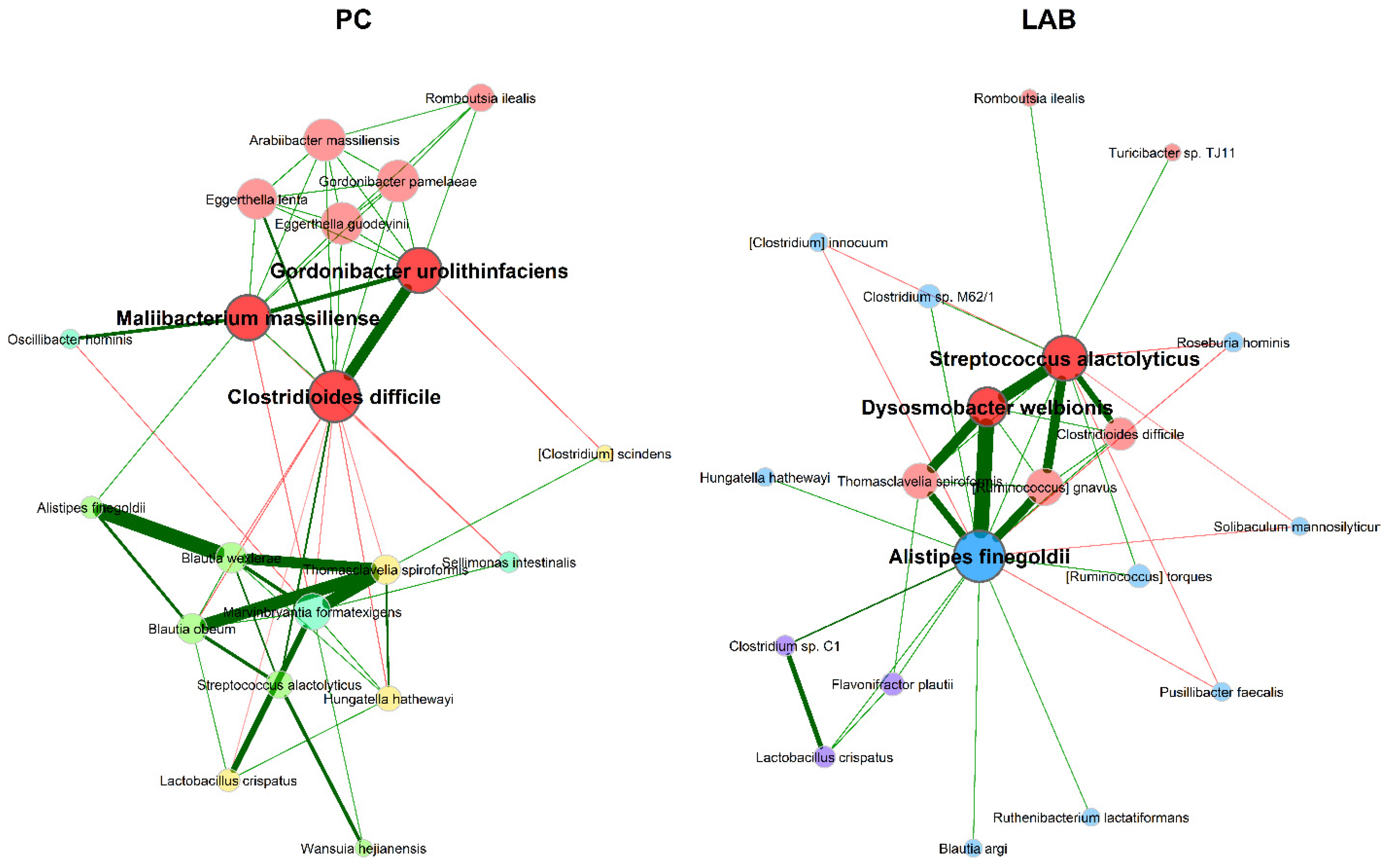
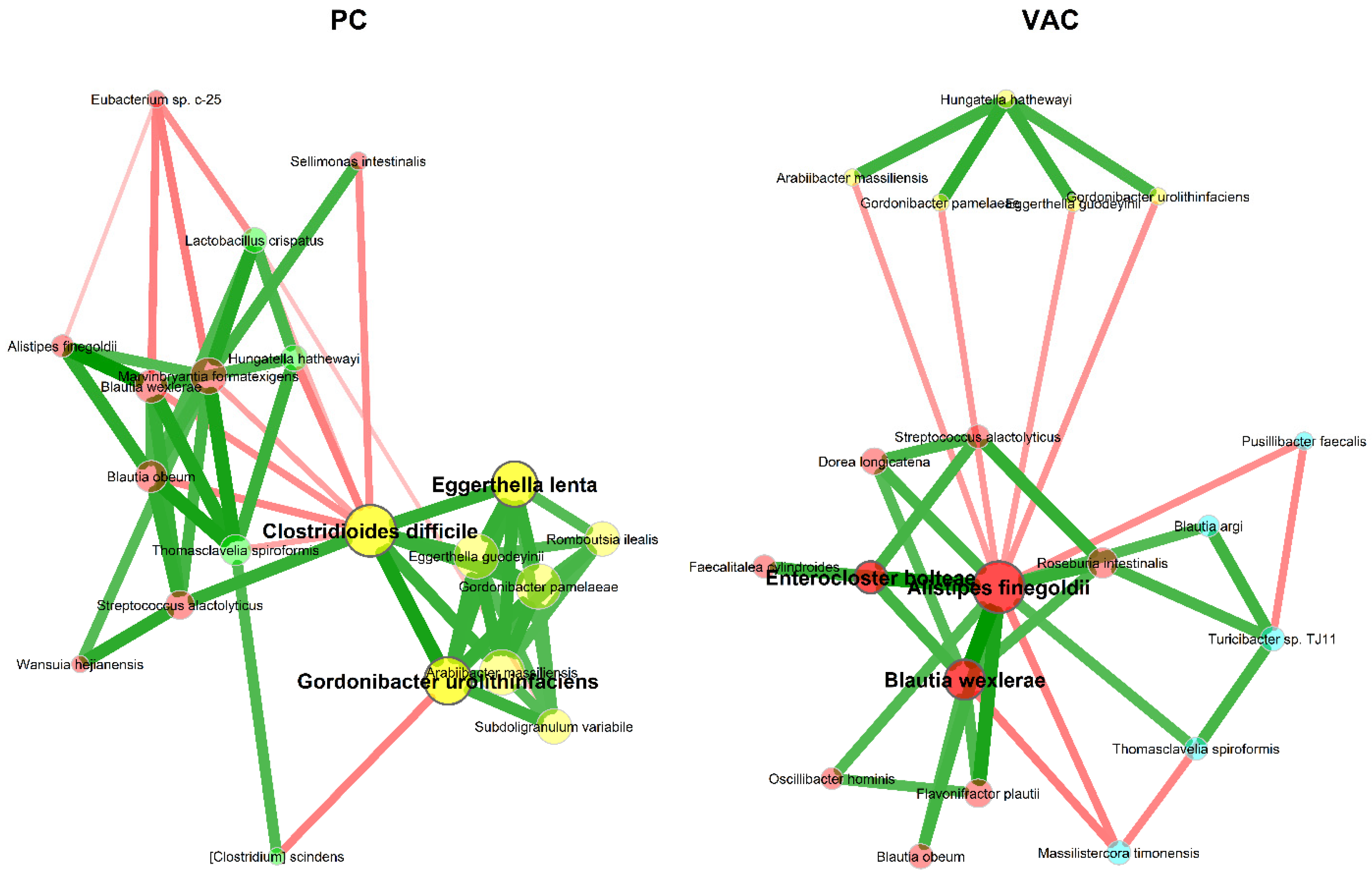
3.2.2. Microbial Association Network Analysis
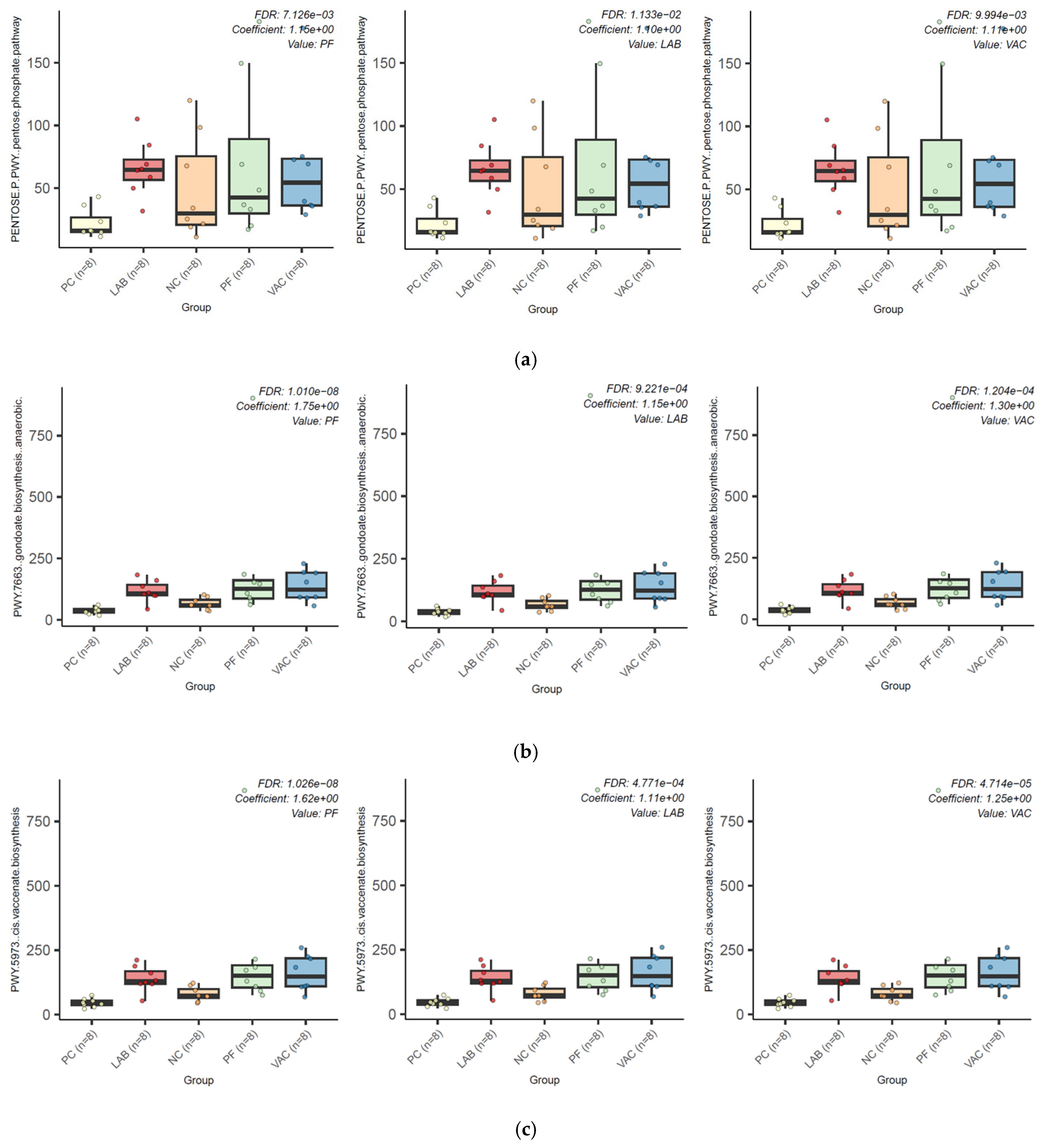
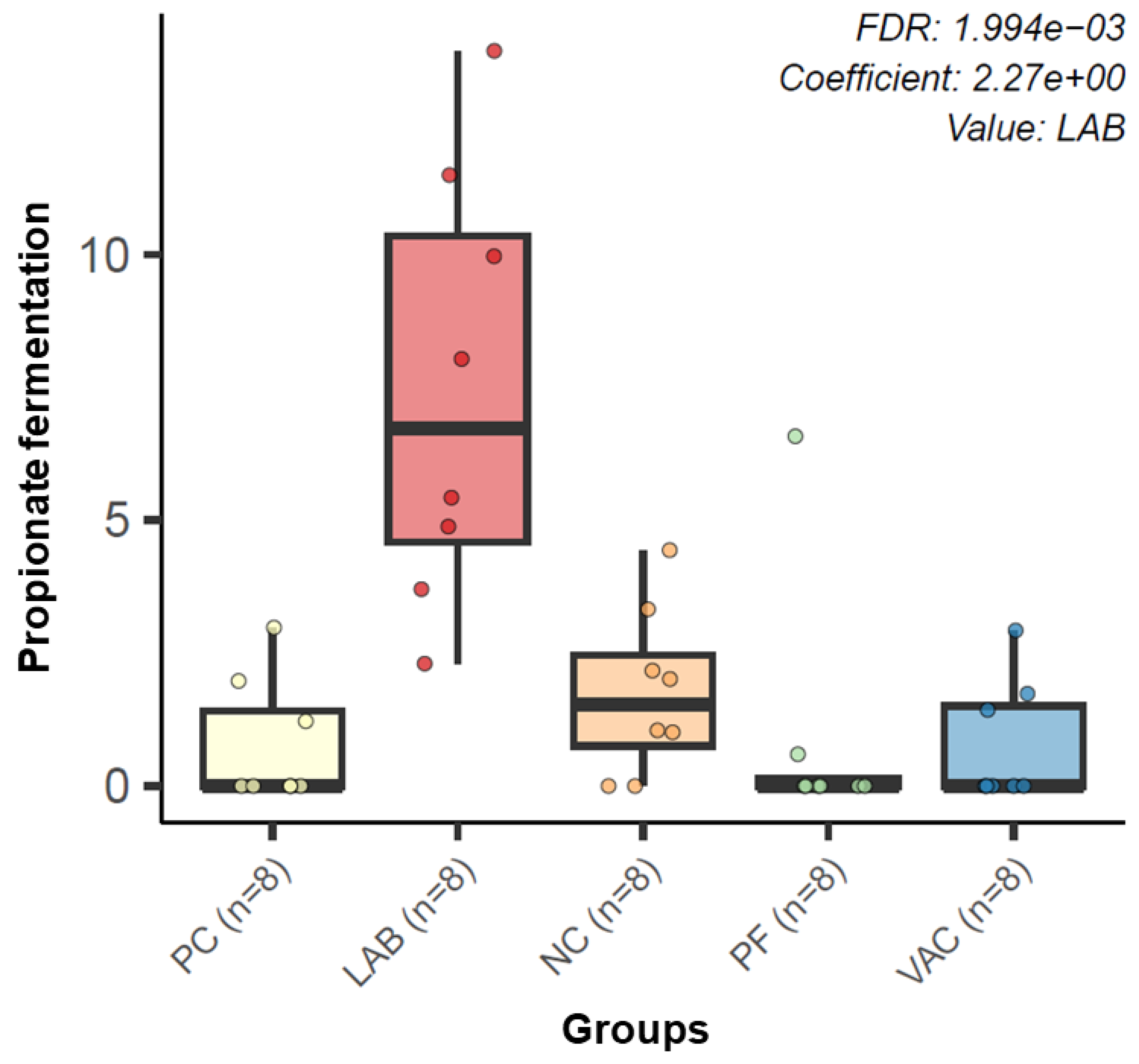
3.2.3. Microbial Functional Profiling
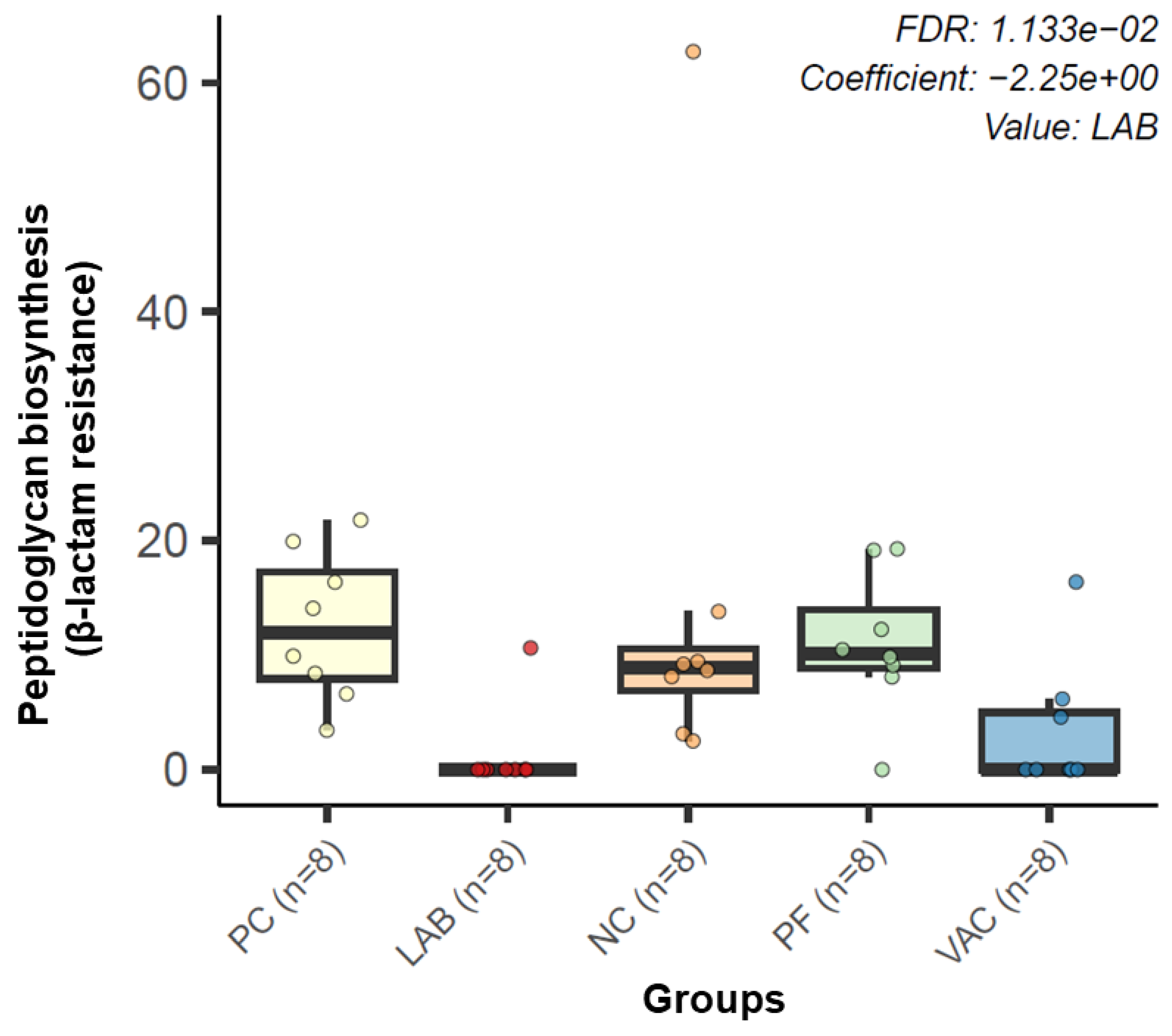
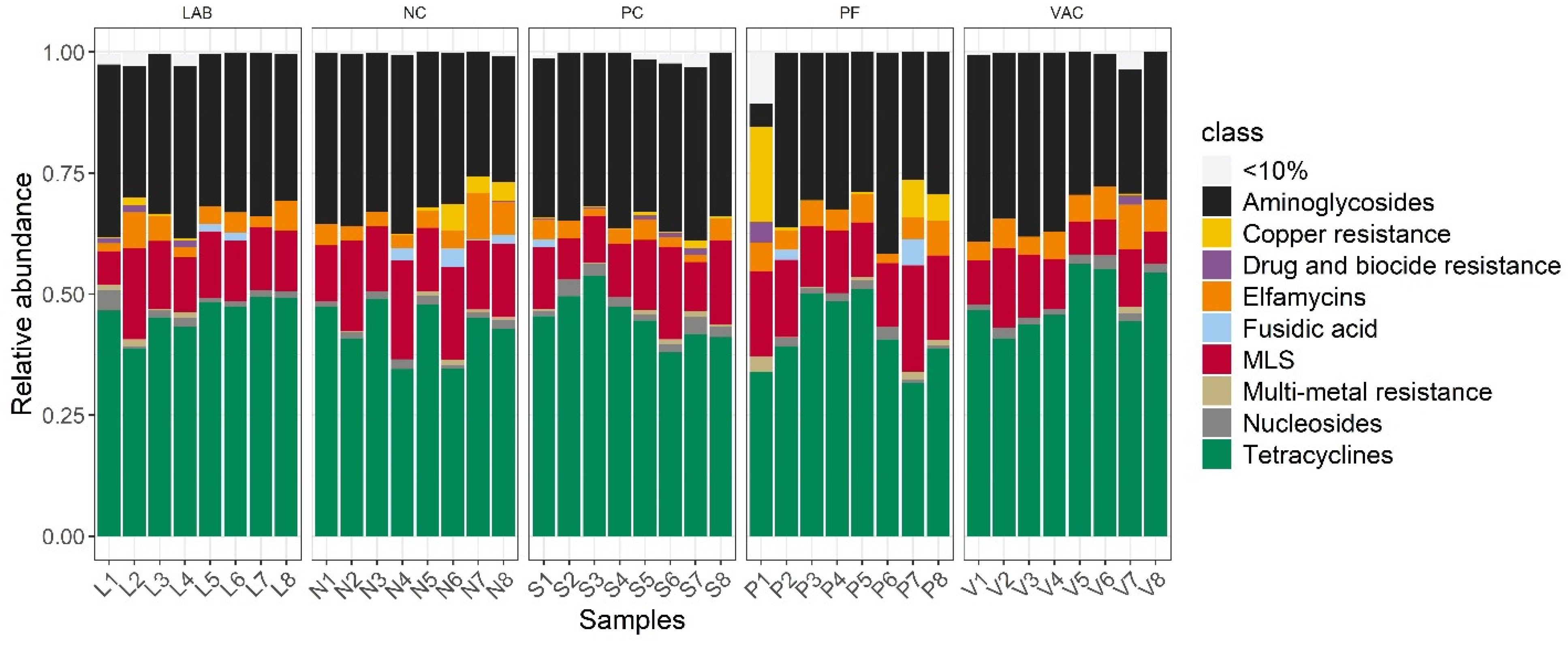
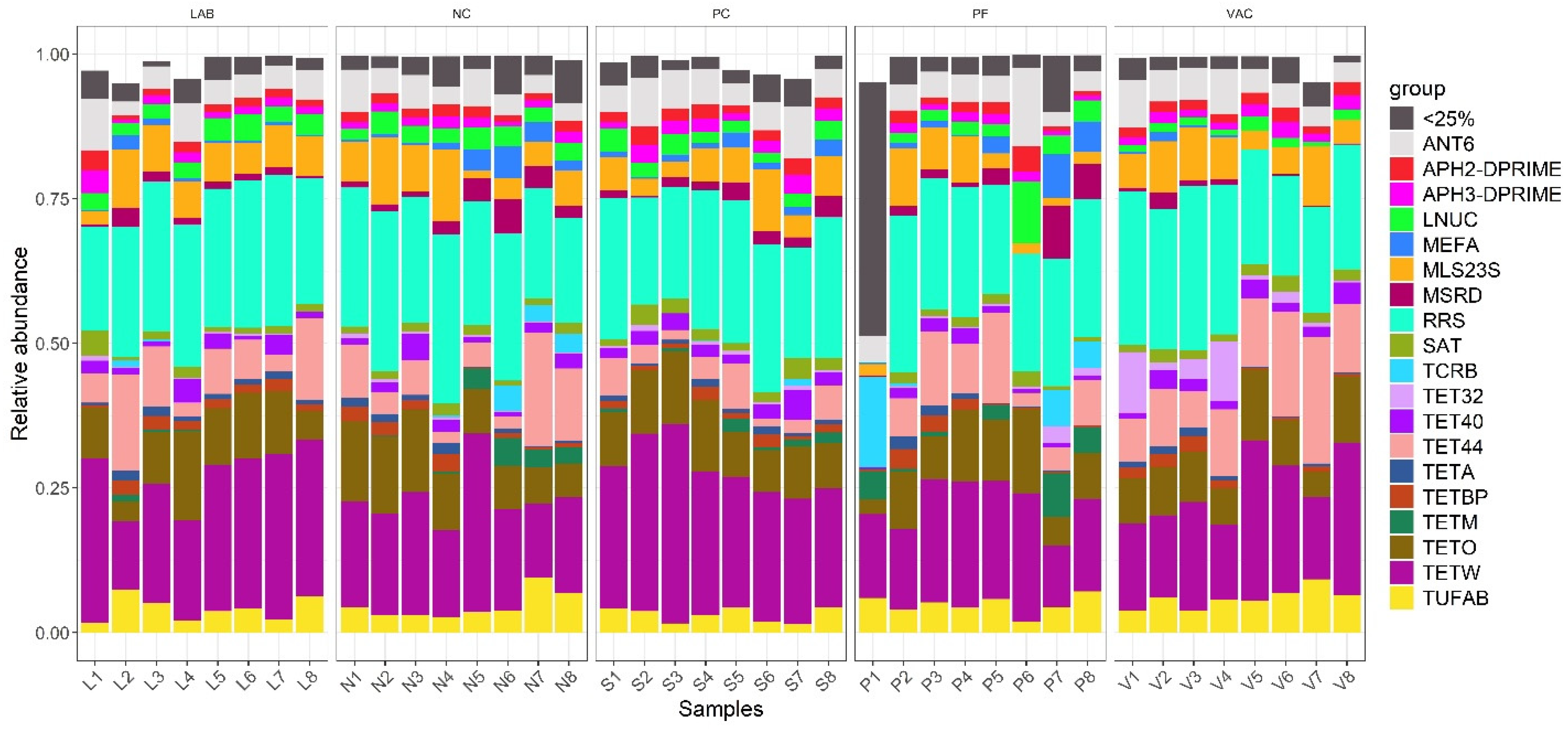
3.3. Resistance Gene Profiling
4. Discussion
4.1. Differentially Abundant Species in Treatments Compared to PC
4.2. Microbial Association Network Analysis
4.3. Metagenomic Functional Profiling
4.4. Resistance Gene Profiling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Rougière, N.; Carré, B. Comparison of Gastrointestinal Transit Times between Chickens from D+ and D− Genetic Lines Selected for Divergent Digestion Efficiency. Animal 2010, 4, 1861–1872. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal Microbiome of Poultry and Its Interaction with Host and Diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Rimet, C.-S.; Maurer, J.J.; Pickler, L.; Stabler, L.; Johnson, K.K.; Berghaus, R.D.; Villegas, A.M.; Lee, M.; França, M. Salmonella Harborage Sites in Infected Poultry That May Contribute to Contamination of Ground Meat. Front. Sustain. Food Syst. 2019, 3, 2. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic Growth Promoters in Agriculture: History and Mode of Action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry Gut Health—Microbiome Functions, Environmental Impacts, Microbiome Engineering and Advancements in Characterization Technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Cisek, A.A.; Binek, M. Chicken Intestinal Microbiota Function with a Special Emphasis on the Role of Probiotic Bacteria. Pol. J. Vet. Sci. 2014, 17, 385–394. [Google Scholar] [CrossRef]
- Yang, Y.; Iji, P.A.; Choct, M. Dietary Modulation of Gut Microflora in Broiler Chickens: A Review of the Role of Six Kinds of Alternatives to in-Feed Antibiotics. World’s Poult. Sci. J. 2009, 65, 97–114. [Google Scholar] [CrossRef]
- Mead, G.C. Prospects for ‘Competitive Exclusion’ Treatment to Control Salmonella and Other Foodborne Pathogens in Poultry. Vet. J. 2000, 159, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Van Der Wielen, P.W.J.J.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.P.; Van Knapen, F. Role of Volatile Fatty Acids in Development of the Cecal Microflora in Broiler Chickens during Growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Kollanoor Johny, A. Characterizing the Antimicrobial Function of a Dairy-Originated Probiotic, Propionibacterium freudenreichii, Against Multidrug-Resistant Salmonella enterica Serovar Heidelberg in Turkey Poults. Front. Microbiol. 2018, 9, 1475. [Google Scholar] [CrossRef]
- Thomas, J.V.; Nair, D.V.T.; Noll, S.; Johnson, T.J.; Cardona, C.; Johny, A.K. Effect of Turkey-Derived Beneficial Bacteria Lactobacillus salivarius and Lactobacillus ingluviei on a Multidrug-Resistant Salmonella Heidelberg Strain in Turkey Poults. J. Food Prot. 2019, 82, 435–440. [Google Scholar] [CrossRef]
- Manjankattil, S.; Dewi, G.; Peichel, C.; Creek, M.; Bina, P.; Lerohl, K.; Deniz, K.; Akhtar, L.; Porter, R.; Johnson, T.J.; et al. Dairy-Origin Propionibacterium freudenreichii, Turkey-Origin Lactobacillus salivarius, and a Salmonella Typhimurium Vaccine Elicit Comparable Colonization Resistance on Drug-Resistant Salmonella Serotypes (S. Reading, S. Agona, and S. Saintpaul) in Growing Turkeys After Oral Challenge. J. Appl. Poult. Res. 2024, 33, 100428. [Google Scholar] [CrossRef]
- Dewi, G.; Manjankattil, S.; Peichel, C.; Johnson, T.J.; Noll, S.; Cardona, C.; Kollanoor Johny, A. Combination of Autochthonous Lactobacillus Strains and Trans-Cinnamaldehyde in Water Reduces Salmonella Heidelberg in Turkey Poults. Front. Microbiol. 2024, 15, 1337428. [Google Scholar] [CrossRef] [PubMed]
- Desin, T.S.; Köster, W.; Potter, A.A. Salmonella Vaccines in Poultry: Past, Present and Future. Expert. Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Crouch, C.F.; Nell, T.; Reijnders, M.; Donkers, T.; Pugh, C.; Patel, A.; Davis, P.; Van Hulten, M.C.W.; De Vries, S.P.W. Safety and Efficacy of a Novel Inactivated Trivalent Salmonella enterica Vaccine in Chickens. Vaccine 2020, 38, 6741–6750. [Google Scholar] [CrossRef]
- NACMCF. Response to Questions Posed by the Food Safety and Inspection Service: Enhancing Salmonella Control in Poultry Products. J. Food Prot. 2024, 87, 100168. [Google Scholar] [CrossRef]
- Monson, M.S.; Gurung, M.; Bearson, B.L.; Whelan, S.J.; Trachsel, J.M.; Looft, T.; Sylte, M.J.; Bearson, S.M.D. Evaluating Two Live-Attenuated Vaccines against Salmonella enterica Serovar Reading in Turkeys: Reduced Tissue Colonization and Cecal Tonsil Transcriptome Responses. Front. Vet. Sci. 2024, 11, 1502303. [Google Scholar] [CrossRef]
- Crouch, C.F.; Pugh, C.; Patel, A.; Brink, H.; Wharmby, C.; Watts, A.; Van Hulten, M.C.W.; De Vries, S.P.W. Reduction in Intestinal Colonization and Invasion of Internal Organs after Challenge by Homologous and Heterologous Serovars of Salmonella enterica Following Vaccination of Chickens with a Novel Trivalent Inactivated Salmonella Vaccine. Avian Pathol. 2020, 49, 666–677. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Liu, F.; Cao, J.; Lv, N.; Zhu, B.; Zhang, G.; Gao, G.F. Integrated Metagenomic and Metatranscriptomic Profiling Reveals Differentially Expressed Resistomes in Human, Chicken, and Pig Gut Microbiomes. Environ. Int. 2020, 138, 105649. [Google Scholar] [CrossRef]
- Salyers, A.; Gupta, A.; Wang, Y. Human Intestinal Bacteria as Reservoirs for Antibiotic Resistance Genes. Trends Microbiol. 2004, 12, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Juricova, H.; Matiasovicova, J.; Kubasova, T.; Cejkova, D.; Rychlik, I. The Distribution of Antibiotic Resistance Genes in Chicken Gut Microbiota Commensals. Sci. Rep. 2021, 11, 3290. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Mi, J.; Chen, Y.; Feng, K.; Mei, L.; Liao, X.; Wu, Y.; Wang, Y. The Abundance and Diversity of Antibiotic Resistance Genes in Layer Chicken Ceca Is Associated with Farm Enviroment. Front. Microbiol. 2023, 14, 1177404. [Google Scholar] [CrossRef]
- Piccirillo, A.; Tolosi, R.; Mughini-Gras, L.; Kers, J.G.; Laconi, A. Drinking Water and Biofilm as Sources of Antimicrobial Resistance in Free-Range Organic Broiler Farms. Antibiotics 2024, 13, 808. [Google Scholar] [CrossRef]
- Vogwill, T.; MacLean, R.C. The Genetic Basis of the Fitness Costs of Antimicrobial Resistance: A Meta-Analysis Approach. Evol. Appl. 2014, 8, 284. [Google Scholar] [CrossRef]
- Nair, D.V.; Kollanoor-Johny, A. Effect of Propionibacterium freudenreichii on Salmonella Multiplication, Motility, and Association with Avian Epithelial Cells. Poult. Sci. 2017, 96, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Johny, A.K.; Baskaran, S.A.; Charles, A.S.; Amalaradjou, M.A.R.; Darre, M.J.; Khan, M.I.; Hoagland, T.A.; Schreiber, D.T.; Donoghue, A.M.; Donoghue, D.J.; et al. Prophylactic Supplementation of Caprylic Acid in Feed Reduces Salmonella Enteritidis Colonization in Commercial Broiler Chicks. J. Food Prot. 2009, 72, 722–727. [Google Scholar] [CrossRef]
- Manjankattil, S.; Nair, D.V.T.; Peichel, C.; Noll, S.; Johnson, T.J.; Cox, R.B.; Donoghue, A.M.; Kollanoor Johny, A. Effect of Caprylic Acid Alone or in Combination with Peracetic Acid against Multidrug-Resistant Salmonella Heidelberg on Chicken Drumsticks in a Soft Scalding Temperature-Time Setup. Poult. Sci. 2021, 100, 101421. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.) (Ed.) Nutrient Requirements of Poultry, 9th rev. ed.; Nutrient Requirements of Domestic Animals; National Academy Press: Washington, DC, USA, 1994; ISBN 978-0-309-04892-7. [Google Scholar]
- Martin, M. Cutadapt Removes Adapter Sequences from High-throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Bonin, N.; Doster, E.; Worley, H.; Pinnell, L.J.; Bravo, J.E.; Ferm, P.; Marini, S.; Prosperi, M.; Noyes, N.; Morley, P.S.; et al. MEGARes and AMR++, v3.0: An Updated Comprehensive Database of Antimicrobial Resistance Determinants and an Improved Software Pipeline for Classification Using High-Throughput Sequencing. Nucleic Acids Res. 2023, 51, D744–D752. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-Level Functional Profiling of Metagenomes and Metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Peschel, S.; Müller, C.L.; Von Mutius, E.; Boulesteix, A.-L.; Depner, M. NetCoMi: Network Construction and Comparison for Microbiome Data in R. Brief. Bioinform. 2021, 22, bbaa290. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. The Vegan Package Version 2.6-10, Community Ecology Package. 2025. Available online: https://cran.r-project.org/src/contrib/Archive/vegan/ (accessed on 5 March 2023).
- Lahti, L.; Shetty, S. Microbiome R Package. Bioconductor. 2017. Available online: https://research.rug.nl/en/publications/microbiome-r-package (accessed on 5 March 2023).
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. ISBN 978-1-118-44511-2. [Google Scholar]
- Wickham, H. Ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Lin, H.; Peddada, S.D. Multigroup Analysis of Compositions of Microbiomes with Covariate Adjustments and Repeated Measures. Nat. Methods 2024, 21, 83–91. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring Correlation Networks from Genomic Survey Data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef]
- Olbricht, H.; Twadell, K.; Sandel, B.; Stephens, C.; Whittall, J.B. Is There a Universal Endurance Microbiota? Microorganisms 2022, 10, 2213. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Kayani, M.R.; Huang, W.; Feng, R.; Chen, L. Genome-Resolved Metagenomics Using Environmental and Clinical Samples. Brief. Bioinform. 2021, 22, bbab030. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid Publication of the Names of Forty-Two Phyla of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Traore, S.I.; Lo, C.I.; Mossaab, M.; Durand, G.; Lagier, J.C.; Raoult, D.; Fournier, P.-E.; Fenollar, F. Maliibacterium massiliense Gen. Nov. sp. Nov., Isolated from Human Feces and Proposal of Maliibacteriaceae Fam. Nov. Curr. Microbiol. 2023, 80, 211. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Vazhakkattu Thomas, J.; Dewi, G.; Noll, S.; Brannon, J.; Kollanoor Johny, A. Reduction of Multidrug-Resistant Salmonella enterica Serovar Heidelberg Using a Dairy-Originated Probiotic Bacterium, Propionibacterium freudenreichii freudenreichii B3523, in Growing Turkeys. J. Appl. Poult. Res. 2019, 28, 356–363. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Vazhakkattu Thomas, J.; Dewi, G.; Brannon, J.; Noll, S.L.; Johnson, T.J.; Cox, R.B.; Kollanoor Johny, A. Propionibacterium freudenreichii freudenreichii B3523 Reduces Cecal Colonization and Internal Organ Dissemination of Multidrug-Resistant Salmonella Heidelberg in Finishing Turkeys. J. Appl. Poult. Res. 2021, 30, 100107. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Johnson, T.J.; Noll, S.L.; Kollanoor Johny, A. Effect of Supplementation of a Dairy-Originated Probiotic Bacterium, Propionibacterium freudenreichii subsp. freudenreichii, on the Cecal Microbiome of Turkeys Challenged with Multidrug-Resistant Salmonella Heidelberg. Poult. Sci. 2021, 100, 283–295. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Thomas, J.V.; Dewi, G.; Noll, S.; Johnson, T.; Kollanoor Johny, A. Effects of Multiple Alternatives to Antibiotic Interventions on Multidrug-Resistant Salmonella Heidelberg in Commercial Turkeys. Poult. Sci. 2019, 98 (Suppl. 1), 256. [Google Scholar]
- Zhang, T.; Xie, J.; Zhang, M.; Fu, N.; Zhang, Y. Effect of a Potential Probiotics Lactococcus garvieae B301 on the Growth Performance, Immune Parameters and Caecum Microflora of Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 413–421. [Google Scholar] [CrossRef]
- Martín, V.; Cárdenas, N.; Jiménez, E.; Maldonado, A.; Rodríguez, J.M.; Fernández, L. Genome Sequence of Lactobacillus gastricus PS3, a Strain Isolated from Human Milk. Genome Announc. 2013, 1, e00489-13. [Google Scholar] [CrossRef] [PubMed]
- Kavita, S.; Pooranachithra, M.; Singh, N.; Prasanth, M.I.; Balamurugan, K.; Goel, G. Lactobacillus gastricus BTM 7 Prevents Intestinal Colonization by Biofilm Forming Cronobacter sakazakii in Caenorhabditis elegans Model Host. Antonie Van Leeuwenhoek 2020, 113, 1587–1600. [Google Scholar] [CrossRef]
- Kamboj, K.; Vasquez, A.; Balada-Llasat, J.-M. Identification and Significance of Weissella Species Infections. Front. Microbiol. 2015, 6, 1204. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Takala, T.M.; Huynh, V.A.; Ahonen, S.L.; Paulin, L.; Björkroth, J.; Sironen, T.; Kant, R.; Saris, P. Comparative Genomics of 40 Weissella paramesenteroides Strains. Front. Microbiol. 2023, 14, 1128028. [Google Scholar] [CrossRef]
- Panthee, S.; Paudel, A.; Blom, J.; Hamamoto, H.; Sekimizu, K. Complete Genome Sequence of Weissella hellenica 0916-4-2 and Its Comparative Genomic Analysis. Front. Microbiol. 2019, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Singh, S.; Singh, V.; Roberts, K.D.; Zaidi, A.; Rodriguez-Palacios, A. The Weissella Genus: Clinically Treatable Bacteria with Antimicrobial/Probiotic Effects on Inflammation and Cancer. Microorganisms 2022, 10, 2427. [Google Scholar] [CrossRef]
- Leong, K.-H.; Chen, Y.-S.; Lin, Y.-H.; Pan, S.-F.; Yu, B.; Wu, H.-C.; Yanagida, F. Weissellicin L, a Novel Bacteriocin from Sian-Sianzih-Isolated Weissella hellenica 4-7. J. Appl. Microbiol. 2013, 115, 70–76. [Google Scholar] [CrossRef]
- Jazi, V.; Foroozandeh, A.D.; Toghyani, M.; Dastar, B.; Rezaie Koochaksaraie, R.; Toghyani, M. Effects of Pediococcus acidilactici, Mannan-Oligosaccharide, Butyric Acid and Their Combination on Growth Performance and Intestinal Health in Young Broiler Chickens Challenged with Salmonella Typhimurium. Poult. Sci. 2018, 97, 2034–2043. [Google Scholar] [CrossRef]
- Seo, H.-J.; Kang, S.-S. Inhibitory Effect of Bacteriocin Produced by Pediococcus acidilactici on the Biofilm Formation of Salmonella Typhimurium. Food Control 2020, 117, 107361. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Dalloul, R.A.; Park, D.W.; Hong, Y.H.; Lin, J.J. Influence of Pediococcus-Based Probiotic on Coccidiosis in Broiler Chickens. Poult. Sci. 2007, 86, 63–66. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yang, N.-S.; Lin, Y.-C.; Ho, S.-T.; Li, K.-Y.; Lin, J.-S.; Liu, J.-R.; Chen, M.-J. A Combination of Lactobacillus mali APS1 and Dieting Improved the Efficacy of Obesity Treatment via Manipulating Gut Microbiome in Mice. Sci. Rep. 2018, 8, 6153. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral Treatment of Chickens with Lactobacilli Influences Elicitation of Immune Responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii Attenuates Inflammatory Responses in Obese Adipose Tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Ogita, T.; Yamamoto, Y.; Mikami, A.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii Strongly Suppresses Th2 Immune Responses in Mice. Front. Immunol. 2020, 11, 379. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Torok, V.A.; Hughes, R.J.; Mikkelsen, L.L.; Perez-Maldonado, R.; Balding, K.; MacAlpine, R.; Percy, N.J.; Ophel-Keller, K. Identification and Characterization of Potential Performance-Related Gut Microbiotas in Broiler Chickens across Various Feeding Trials. Appl. Environ. Microbiol. 2011, 77, 5868–5878. [Google Scholar] [CrossRef]
- Guh, A.Y.; Kutty, P.K. Clostridioides difficile Infection. Ann. Intern. Med. 2018, 169, ITC49–ITC64. [Google Scholar] [CrossRef]
- Bolton, D.; Marcos, P. The Environment, Farm Animals and Foods as Sources of Clostridioides difficile Infection in Humans. Foods 2023, 12, 1094. [Google Scholar] [CrossRef]
- Heise, J.; Witt, P.; Maneck, C.; Wichmann-Schauer, H.; Maurischat, S. Prevalence and Phylogenetic Relationship of Clostridioides difficile Strains in Fresh Poultry Meat Samples Processed in Different Cutting Plants. Int. J. Food Microbiol. 2021, 339, 109032. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct Supplementation with Urolithin A Overcomes Limitations of Dietary Exposure and Gut Microbiome Variability in Healthy Adults to Achieve Consistent Levels across the Population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.J.; Corrigan, A.; Murphy, R.A.; Walsh, F. Effect of Mannan-Rich Fraction Supplementation on Commercial Broiler Intestinum Tenue and Cecum Microbiota. Anim. Microbiome 2022, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, B.J.; Tai, A.Y.; Kotsanas, D.; Francis, M.J.; Roberts, S.A.; Ballard, S.A.; Junckerstorff, R.K.; Korman, T.M. Clinical and Microbiological Characteristics of Eggerthella lenta Bacteremia. J. Clin. Microbiol. 2015, 53, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Ang, Q.Y.; Nayak, R.R.; Bustion, A.E.; Sandy, M.; Zhang, B.; Upadhyay, V.; Pollard, K.S.; Lynch, S.V.; Turnbaugh, P.J. Human Gut Bacterial Metabolism Drives Th17 Activation and Colitis. Cell Host Microbe 2022, 30, 17–30.e9. [Google Scholar] [CrossRef]
- Li, S.; Lin, R.; Chen, J.; Hussain, R.; Zhang, S.; Su, Y.; Chan, Y.; Shi, D. Integrated Gut Microbiota and Metabolomic Analysis Reveals Immunomodulatory Effects of Echinacea Extract and Astragalus Polysaccharides. Front. Vet. Sci. 2022, 9, 971058. [Google Scholar] [CrossRef]
- Patterson, A.M.; Mulder, I.E.; Travis, A.J.; Lan, A.; Cerf-Bensussan, N.; Gaboriau-Routhiau, V.; Garden, K.; Logan, E.; Delday, M.I.; Coutts, A.G.P.; et al. Human Gut Symbiont Roseburia hominis Promotes and Regulates Innate Immunity. Front. Immunol. 2017, 8, 1166. [Google Scholar] [CrossRef]
- Rychlik, I. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Song, L.; Sun, Q.; Zheng, H.; Zhang, Y.; Wang, Y.; Liu, S.; Duan, L. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, 2200164. [Google Scholar] [CrossRef]
- Chiumento, S.; Roblin, C.; Kieffer-Jaquinod, S.; Tachon, S.; Leprètre, C.; Basset, C.; Aditiyarini, D.; Olleik, H.; Nicoletti, C.; Bornet, O.; et al. Ruminococcin C, a Promising Antibiotic Produced by a Human Gut Symbiont. Sci. Adv. 2019, 5, eaaw9969. [Google Scholar] [CrossRef]
- Dabard, J.; Bridonneau, C.; Phillipe, C.; Anglade, P.; Molle, D.; Nardi, M.; Ladiré, M.; Girardin, H.; Marcille, F.; Gomez, A.; et al. Ruminococcin A, a New Lantibiotic Produced by a Ruminococcus gnavus Strain Isolated from Human Feces. Appl. Environ. Microbiol. 2001, 67, 4111–4118. [Google Scholar] [CrossRef]
- Roblin, C.; Chiumento, S.; Jacqueline, C.; Pinloche, E.; Nicoletti, C.; Olleik, H.; Courvoisier-Dezord, E.; Amouric, A.; Basset, C.; Dru, L.; et al. The Multifunctional Sactipeptide Ruminococcin C1 Displays Potent Antibacterial Activity In Vivo as Well as Other Beneficial Properties for Human Health. Int. J. Mol. Sci. 2021, 22, 3253. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Gotoh, Y.; Ogura, Y.; Hayashi, T.; Fukiya, S.; Yokota, A. Comparative Genomic and Physiological Analysis against Clostridium scindens Reveals Eubacterium sp. c-25 as an Atypical Deoxycholic Acid Producer of the Human Gut Microbiota. Microorganisms 2021, 9, 2254. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Reconstitution Restores Bile Acid Mediated Resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Le Roy, T.; Moens de Hase, E.; Van Hul, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Depommier, C.; Druart, C.; Everard, A.; Maiter, D.; et al. Dysosmobacter welbionis Is a Newly Isolated Human Commensal Bacterium Preventing Diet-Induced Obesity and Metabolic Disorders in Mice. Gut 2022, 71, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Moens De Hase, E.; Neyrinck, A.M.; Rodriguez, J.; Cnop, M.; Paquot, N.; Thissen, J.-P.; Xu, Y.; Beloqui, A.; Bindels, L.B.; Delzenne, N.M.; et al. Impact of Metformin and Dysosmobacter welbionis on Diet-Induced Obesity and Diabetes: From Clinical Observation to Preclinical Intervention. Diabetologia 2024, 67, 333–345. [Google Scholar] [CrossRef]
- Moens De Hase, E.; Petitfils, C.; Alhouayek, M.; Depommier, C.; Le Faouder, P.; Delzenne, N.M.; Van Hul, M.; Muccioli, G.G.; Cenac, N.; Cani, P.D. Dysosmobacter Welbionis Effects on Glucose, Lipid, and Energy Metabolism Are Associated with Specific Bioactive Lipids. J. Lipid Res. 2023, 64, 100437. [Google Scholar] [CrossRef]
- Rinkinen, M.L.; Koort, J.M.K.; Ouwehand, A.C.; Westermarck, E.; Björkroth, K.J. Streptococcus alactolyticus Is the Dominating Culturable Lactic Acid Bacterium Species in Canine Jejunum and Feces of Four Fistulated Dogs. FEMS Microbiol. Lett. 2004, 230, 35–39. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Wang, L.; Zhang, K.; Tian, Y.; Wang, X.; Xu, G.; Guo, Z.; Ahmad, S.; Egide, H.; et al. The Antioxidant Activity and Metabolomic Analysis of the Supernatant of Streptococcus alactolyticus Strain FGM. Sci. Rep. 2024, 14, 8413. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, L.; Zhang, K.; Qiu, Z.; Zhang, K.; Yue, C.; Zhang, Y.; Zhao, X.; Li, J. The Safety and Potential Probiotic Properties Analysis of Streptococcus alactolyticus Strain FGM Isolated from the Chicken Cecum. Ann. Microbiol. 2021, 71, 19. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Rui, W.; Li, X.; Wang, L.; Tang, X.; Yang, J. Potential Applications of Blautia wexlerae in the Regulation of Host Metabolism. Probiotics Antimicrob. Proteins 2024, 5, 1866–1874. [Google Scholar] [CrossRef]
- Hosomi, K.; Saito, M.; Park, J.; Murakami, H.; Shibata, N.; Ando, M.; Nagatake, T.; Konishi, K.; Ohno, H.; Tanisawa, K.; et al. Oral Administration of Blautia wexlerae Ameliorates Obesity and Type 2 Diabetes via Metabolic Remodeling of the Gut Microbiota. Nat. Commun. 2022, 13, 4477. [Google Scholar] [CrossRef]
- Magdy Wasfy, R.; Mbaye, B.; Borentain, P.; Tidjani Alou, M.; Murillo Ruiz, M.L.; Caputo, A.; Andrieu, C.; Armstrong, N.; Million, M.; Gerolami, R. Ethanol-Producing Enterocloster bolteae Is Enriched in Chronic Hepatitis B-Associated Gut Dysbiosis: A Case–Control Culturomics Study. Microorganisms 2023, 11, 2437. [Google Scholar] [CrossRef]
- Werner, C. Chapter 4—Metabolic Pathways and Cycles. In The Scientist’s Guide to Cardiac Metabolism; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The Pentose Phosphate Pathway: An Antioxidant Defense and a Crossroad in Tumor Cell Fate. Free Radic. Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Abriouel, H.; Pérez Montoro, B.; Casimiro-Soriguer, C.S.; Pérez Pulido, A.J.; Knapp, C.W.; Caballero Gómez, N.; Castillo-Gutiérrez, S.; Estudillo-Martínez, M.D.; Gálvez, A.; Benomar, N. Insight into Potential Probiotic Markers Predicted in Lactobacillus pentosus MP-10 Genome Sequence. Front. Microbiol. 2017, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Lee, C.; Seo, M.-J.; Roh, S.W.; Lee, S.H. Characterization of a Potential Probiotic Bacterium Lactococcus raffinolactis WiKim0068 Isolated from Fermented Vegetable Using Genomic and in Vitro Analyses. BMC Microbiol. 2020, 20, 136. [Google Scholar] [CrossRef]
- Nishimura, J. Probiotic Characteristics and Carbohydrate Metabolism of Lactobacillus reuteri. Milk Sci. 2020, 69, 71–82. [Google Scholar] [CrossRef]
- Zan, Z.; Mao, Q.; Han, Z.; Sun, J. Changes in the Intestinal Microbiota of Farmed Northern Sheatfish (Silurus Soldatovi) Associated with Natural Bacterial Infection. J. World Aquac. Soc. 2023, 54, 1575–1591. [Google Scholar] [CrossRef]
- Lamas, A.; Regal, P.; Vázquez, B.; Cepeda, A.; Franco, C.M. Short Chain Fatty Acids Commonly Produced by Gut Microbiota Influence Salmonella enterica Motility, Biofilm Formation, and Gene Expression. Antibiotics 2019, 8, 265. [Google Scholar] [CrossRef]
- Alshawabkeh, K.; Tabbaa, M.J. Using Dietary Propionic Acid to Limit Salmonella Gallinarum Colonization in Broiler Chicks. Asian Australas. J. Anim. Sci. 2002, 15, 243–246. [Google Scholar] [CrossRef]
- Al-Tarazi, Y.H.; Alshawabkeh, K. Effect of Dietary Formic and Propionic Acids on Salmonella Pullorum Shedding and Mortality in Layer Chicks after Experimental Infection. J. Vet. Med. Ser. B 2003, 50, 112–117. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, Y.; Liu, Z.; Hu, Z.; Guo, Y.; Deng, M.; Liu, G.; Sun, B. Effects of Malic Acid and Sucrose on the Fermentation Parameters, CNCPS Nitrogen Fractions, and Bacterial Community of Moringa oleifera Leaves Silage. Microorganisms 2021, 9, 2102. [Google Scholar] [CrossRef]
- Wang, H.; McElfresh, G.; Wijesuriya, N.; Podgorny, A.; Hecht, A.D.; Ray, J.C.J. Overlapping Stimulons Arising in Response to Divergent Stresses in Escherichia coli. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kraimi, N.; Lormant, F.; Calandreau, L.; Kempf, F.; Zemb, O.; Lemarchand, J.; Constantin, P.; Parias, C.; Germain, K.; Rabot, S.; et al. Microbiota and Stress: A Loop That Impacts Memory. Psychoneuroendocrinology 2022, 136, 105594. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Muraoka, A.; Bedenbaugh, M.; Childress, B.; Pernot, L.; Wiencek, M.; Peterson, Y.K. The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition. Front. Microbiol. 2022, 13, 807955. [Google Scholar] [CrossRef]
- Jan, T.-R.; Lin, C.-S.; Wang, S.-Y.; Yang, W.-Y. Cytokines and Cecal Microbiome Modulations Conferred by a Dual Vaccine in Salmonella-Infected Layers. Poult. Sci. 2023, 102, 102373. [Google Scholar] [CrossRef]
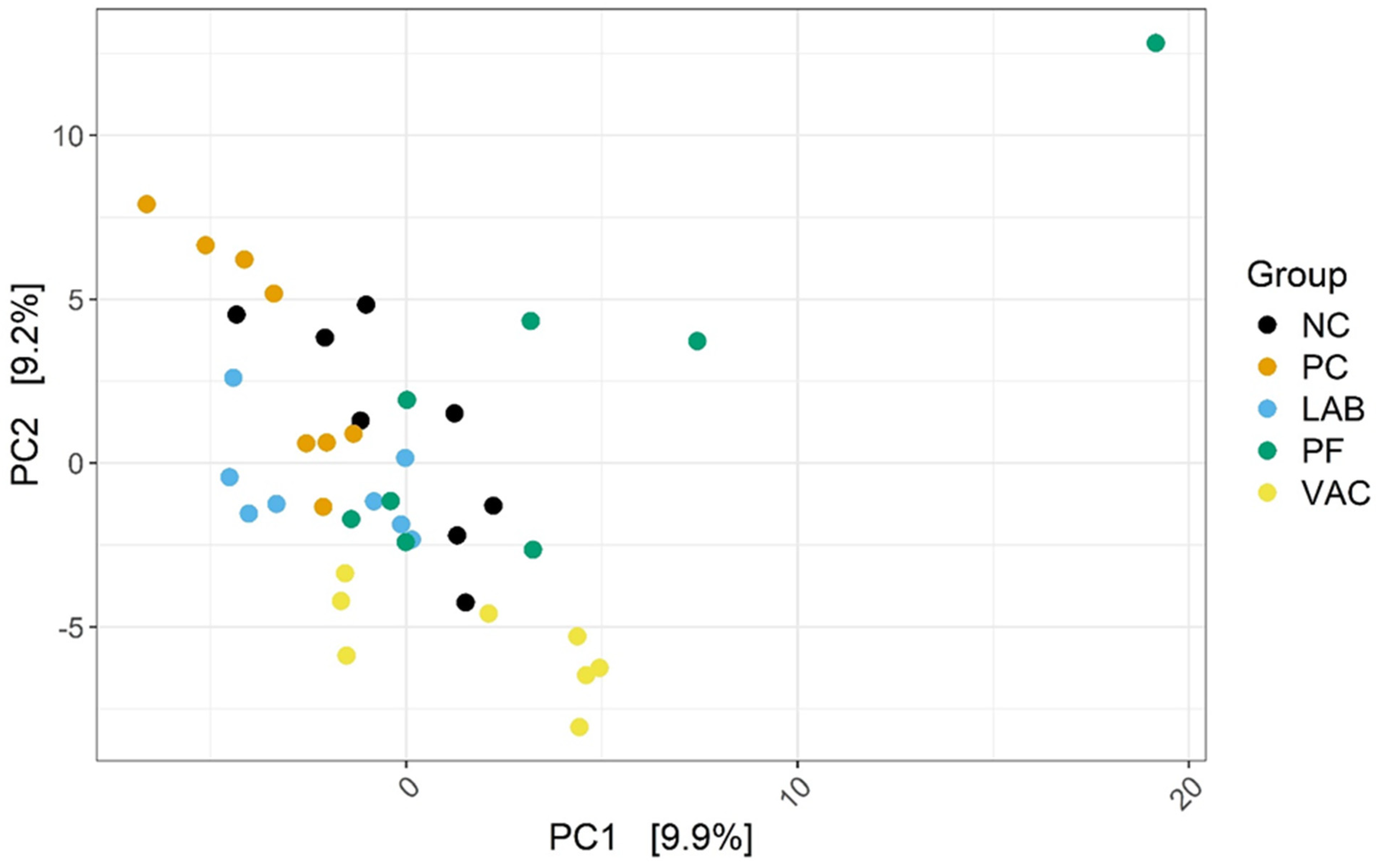
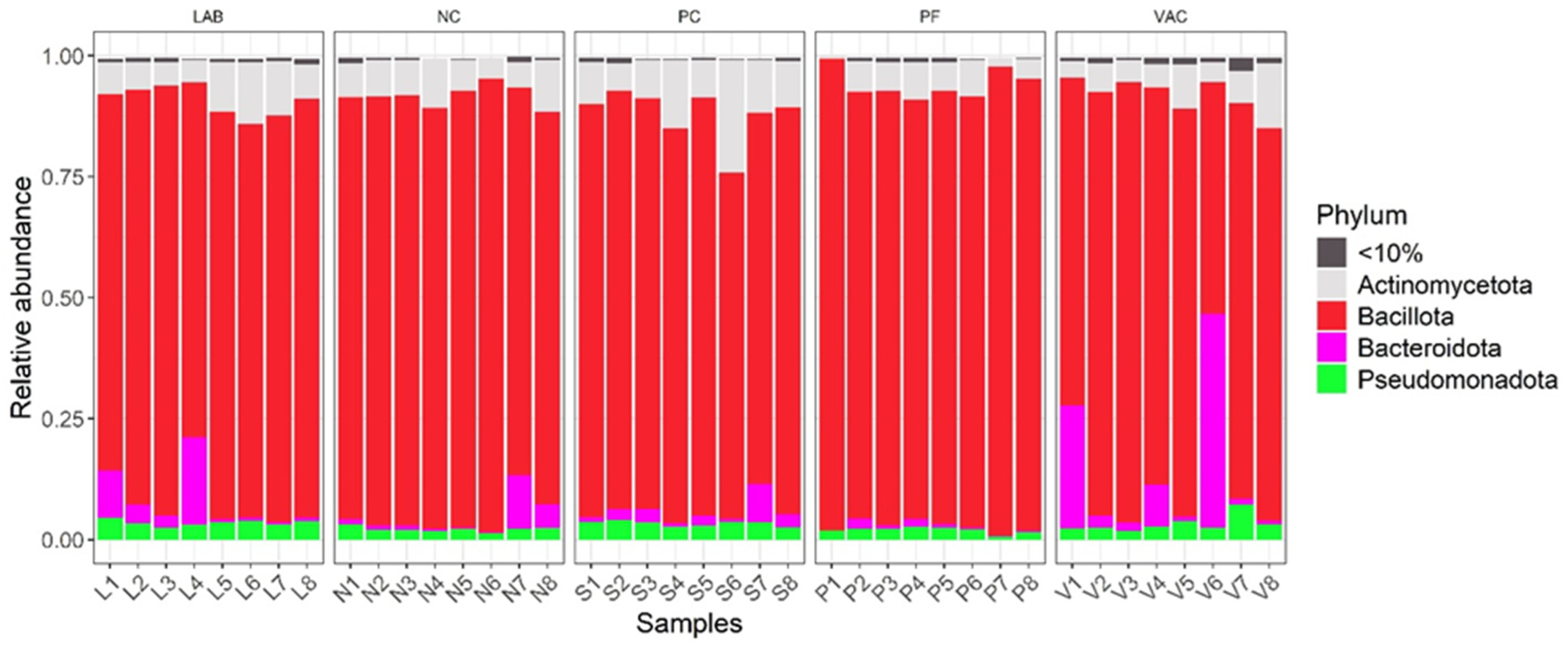
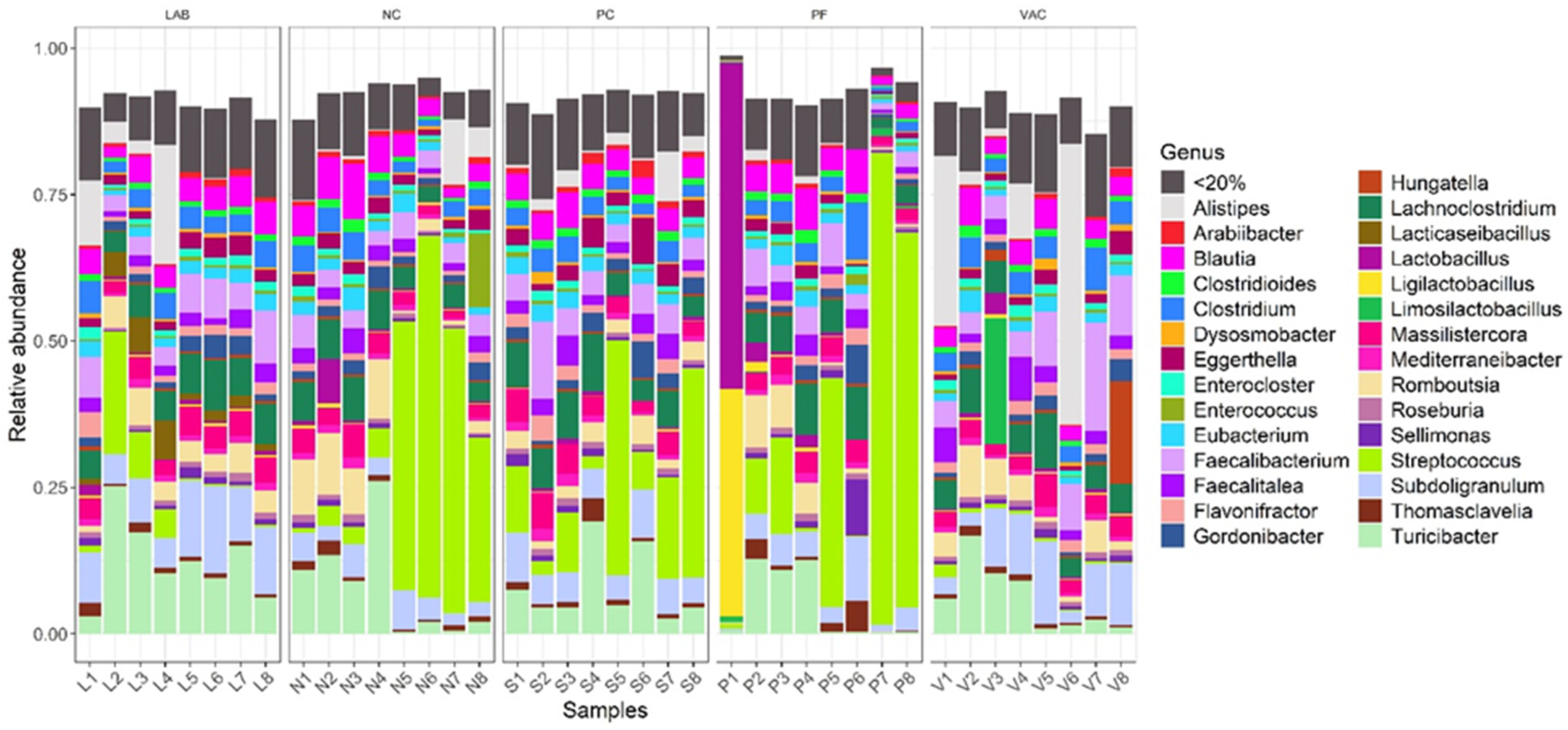
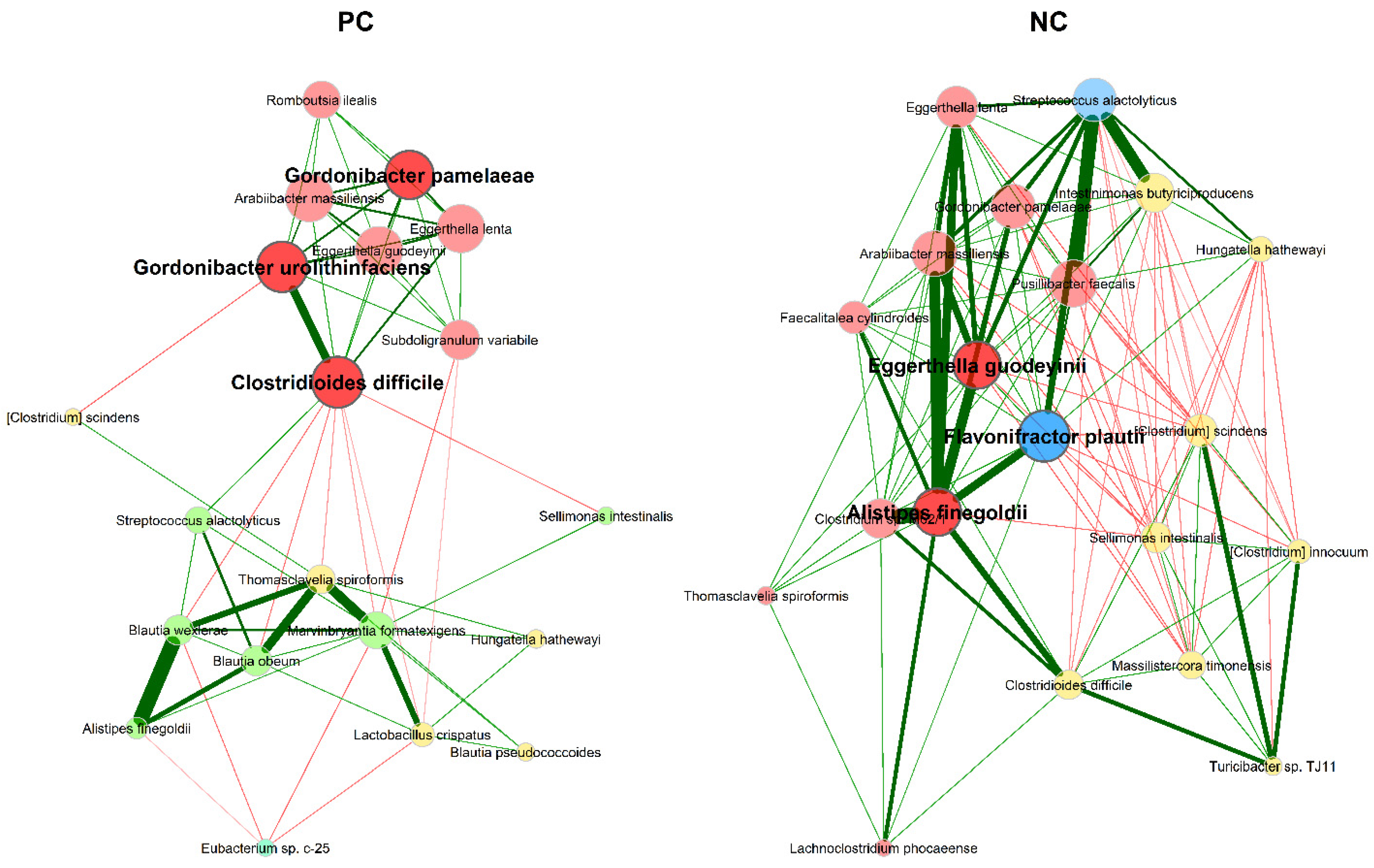
| Species | Treatments | lfc | q-Value |
|---|---|---|---|
| Limosilactobacillus gastricus | LAB | 3.02 | <0.0001 |
| PF | 2.96 | 0.0067 | |
| VAC | 3.60 | <0.0001 | |
| Lactobacillus sp. ESL0681 | LAB | 2.85 | 0.0003 |
| PF | 3.19 | 0.0095 | |
| VAC | 3.51 | <0.0001 | |
| Weissella hellenica | LAB | 2.36 | 0.0011 |
| PF | 2.81 | 0.0011 | |
| VAC | 3.80 | 0.0004 | |
| Pediococcus acidilactici | LAB | 1.93 | <0.0001 |
| PF | 2.24 | 0.0086 | |
| VAC | 2.59 | <0.0001 | |
| Liquorilactobacillus mali | LAB | 1.85 | 0.0006 |
| PF | 2.28 | 0.0013 | |
| VAC | 2.51 | <0.0001 | |
| Massilia forsythiae | LAB | −1.17 | 0.0013 |
| PF | −1.70 | 0.006 | |
| VAC | −1.55 | 0.0022 | |
| Tsuneonella dongtanensis | LAB | −1.46 | 0.0002 |
| PF | −2.19 | 0.0009 | |
| VAC | −1.65 | 0.0024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muringattu Prabhakaran, D.; Kollanoor Johny, A.; Nair, D.V.T.; Manjankattil, S.; Johnson, T.J.; Noll, S.; Reed, K.M. Beneficial Cecal Microbiome Modulation in Turkeys Exposed to Probiotics and Vaccine After Multidrug-Resistant Salmonella Heidelberg Challenge. Microbiol. Res. 2025, 16, 136. https://doi.org/10.3390/microbiolres16070136
Muringattu Prabhakaran D, Kollanoor Johny A, Nair DVT, Manjankattil S, Johnson TJ, Noll S, Reed KM. Beneficial Cecal Microbiome Modulation in Turkeys Exposed to Probiotics and Vaccine After Multidrug-Resistant Salmonella Heidelberg Challenge. Microbiology Research. 2025; 16(7):136. https://doi.org/10.3390/microbiolres16070136
Chicago/Turabian StyleMuringattu Prabhakaran, Dhananjai, Anup Kollanoor Johny, Divek V. T. Nair, Shijinaraj Manjankattil, Timothy J. Johnson, Sally Noll, and Kent M. Reed. 2025. "Beneficial Cecal Microbiome Modulation in Turkeys Exposed to Probiotics and Vaccine After Multidrug-Resistant Salmonella Heidelberg Challenge" Microbiology Research 16, no. 7: 136. https://doi.org/10.3390/microbiolres16070136
APA StyleMuringattu Prabhakaran, D., Kollanoor Johny, A., Nair, D. V. T., Manjankattil, S., Johnson, T. J., Noll, S., & Reed, K. M. (2025). Beneficial Cecal Microbiome Modulation in Turkeys Exposed to Probiotics and Vaccine After Multidrug-Resistant Salmonella Heidelberg Challenge. Microbiology Research, 16(7), 136. https://doi.org/10.3390/microbiolres16070136






