Abstract
Polyhydroxyalkanoates (PHAs), a family of biodegradable polyesters produced through microbial fermentation of carbon-rich residues, are emerging as attractive alternatives to petroleum-based plastics. Their appeal lies in their exceptional biocompatibility, inherent biodegradability, and tunable physicochemical properties across diverse applications. These materials are environmentally friendly not just at the end of their life, but throughout their entire production–use–disposal cycle. This mini-review presents an update on the expanding biomedical relevance of PHAs, with emphasis on their utility in tissue engineering and drug delivery platforms. In addition, current clinical evaluations and regulatory frameworks are briefly discussed, underscoring the translational potential of PHAs in meeting unmet medical needs. As the healthcare sector advances toward environmentally responsible and patient-focused innovations, PHAs exemplify the convergence of waste valorization and biomedical progress, transforming discarded resources into functional materials for repair, regeneration, and healing.
1. Introduction
1.1. Traditional Plastics: Convenience at the Cost of the Planet
Plastics are among the most widely utilized materials worldwide, valued for their adaptability across sectors ranging from large-scale manufacturing to everyday consumer packaging. The most common plastics—made from polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyurethane (PUR), polyethylene terephthalate (PET), and polybutylene terephthalate (PBT)—are primarily derived from petrochemical sources (Table 1) [1,2].

Table 1.
Applications, useful life, and degradation time of different petrochemical plastics are compared to polyhydroxyalkanoates (PHAs). The degradation time varies depending on the shape and thickness of the plastic object, its application, and the environment in which it is located (e.g., land, sea, temperature, etc.) (Reprinted from reference [1], MDPI 2024).
Their success stems from low production costs compared to alternatives, light weight density, mechanical strength, and ease of processing, which have enabled their integration into nearly every aspect of modern economies. However, this reliance has triggered escalating environmental and socio-economic concerns [3]. Petrochemical plastics contribute significantly to greenhouse gas emissions during production and disposal, while their resistance to degradation means they persist in soil, rivers, and oceans for centuries [4]. Current estimates suggest 400 million tonnes of plastic are produced annually, with half designed for single-use applications. Alarmingly, only about 10% of this plastic is recycled, while 19–23 million tonnes leak into aquatic ecosystems each year, a figure projected to rise by 50% by 2040 if no action is taken [5,6,7,8,9]. The linear “produce–use–discard” model, especially in packaging systems, has intensified ecological damage. Plastics fragment into microplastics and nanoplastics, which are now found in virtually every ecosystem—from polar ice to coral reefs—and even in human food, water, and air [10,11,12]. The degradation process is modulated by abiotic factors such as ultraviolet (UV) radiation and mechanical weathering, as well as by biotic activity involving microorganisms [13,14]. Studies estimate that the average person ingests over 50,000 plastic particles annually, with inhalation raising this number significantly [15,16]. These particulate contaminants can be ingested and subsequently bioaccumulate within tissues, where they may induce toxicological or carcinogenic effects [17], and disrupt food chains. Several bacterial genera (Arthrobacter, Pseudomonas, Bacillus, Streptomyces, Rhodococcus, Micrococcus, Corynebacterium, Nocardia) and fungal groups (Fusarium, Aspergillus, Penicillium) produce extracellular enzymes capable of cleaving polymer chains into lower-molecular-weight compounds [18]. Nevertheless, evolutionary processes have not yet yielded highly specialized enzymatic systems capable of efficiently mineralizing synthetic polymers. Complicating this further, many plastics are engineered as complex composites incorporating solubilizers and chemical additives to enhance mechanical strength and physicochemical performance, thereby rendering natural biodegradation even more challenging [19].
1.2. Bioplastics as Sustainable Alternatives to Petroleum-Based Plastics
In recent times, attention has shifted toward finding substitutes for petroleum-based plastics [20]. A major focus has been on bio-based polymers, or bioplastics, which pose fewer risks to human health and the environment [21,22]. These materials are made entirely or partly from natural sources like rubber or latex and have a long history of use—the first man-made biopolymer, based on cellulose, was created in the 1800s [23]. Bioplastics are typically classified into two groups: (1) the first includes biodegradable types, which can break down naturally into simpler compounds; and (2) the second consists of nonbiodegradable types, which do not decompose on their own and must be processed through industrial composting. Biopolymers themselves fall into three main categories: (1) one group comes directly from biological matter, such as starches, proteins like collagen or gelatin, and fats; (2) another group is produced from renewable raw material intermediates, with polylactic acid (PLA) being a common example; and (3) the final group is generated by microorganisms, with polyhydroxyalkanoates (PHAs) considered as the most notable [24].
PHAs stand out due to their role as naturally occurring polyesters, produced in limited quantities by a wide range of prokaryotic species and, to some extent, by certain eukaryotes. Within these organisms, PHAs accumulate as intracellular granules that function as reservoirs of carbon and energy. A key advantage of these biopolymers is their complete biodegradability: microbial processes can convert them back into simple end products such as carbon dioxide and water [25]. Because of this environmentally benign breakdown, PHAs are often highlighted as promising substitutes for petroleum-derived plastics, offering the durability and versatility of conventional polymers while ensuring ecological compatibility throughout their life span. However, despite their potential, large-scale commercialization remains constrained by the high expense of production, which emphasizes the importance of ongoing scientific innovation and technological progress to make them economically viable [26].
While several recent reviews have focused primarily on PHAs as drug-delivery carriers, the present mini-review takes a broader biomedical perspective. We integrate advances in tissue engineering, emerging clinical evaluations, regulatory considerations, and sustainability-driven production strategies to highlight how PHAs uniquely bridge waste valorization with translational medical innovation.
2. Characteristics and Applications of PHAs
PHAs form a wide group of biomaterials, and their characteristics depend on both the kind and sequence of monomers that make up their polymer chains. While more than 150 hydroxyalkanoate monomers with the ability to generate PHAs have been discovered, only a limited number are currently used at an industrial scale [27]. The classification of PHAs is based on the count of carbon atoms present in their main chain or in the side group (R), as shown in Figure 1.
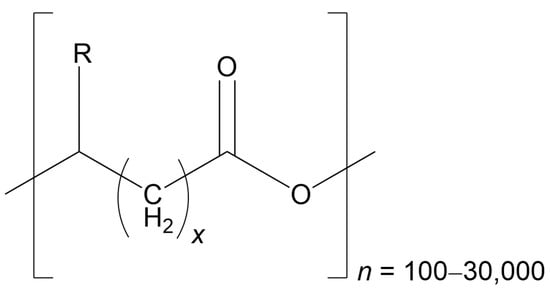
Figure 1.
The general structure of PHAs is represented as follows: R can range from hydrogen to pentadecyl, n represents the number of repeats of the monomer(s) (which can be arranged in tandem or randomly), and x signifies the number of CH2 groups within the main chain of each monomer. For the classification of PHAs, the total number of carbons present in the monomer is used, calculated as (R + x + 2) (Redrawn from reference [1], MDPI 2024).
Biopolyesters are typically classified according to the length of their monomer chains. Short-chain PHAs (scl-PHAs) consist of 4–5 carbon atoms, medium-chain PHAs (mcl-PHAs) fall within 6–14 carbons, and long-chain PHAs (lcl-PHAs) extend beyond 14 carbons. In some research, these boundaries are expanded, with mcl-PHAs defined as having 6–16 carbons, while lcl-PHAs are described as containing more than 16 carbons. The variation in side-chain length significantly influences both the structural behavior of the polymer and its potential fields of application (Table 2) [1].

Table 2.
Properties and applications of different PHAs based on the size of their side chain (R) (Reprinted from reference [1], MDPI 2024).
Another way to classify PHAs is by their molecular composition; homopolyesters are formed from a single monomer type, whereas heteropolyesters incorporate multiple monomers. Heteropolyesters can be further divided into subgroups: copolyesters include two monomers, terpolyesters contain three, and quaterpolyesters are built from four distinct monomers [36]. The incorporation of different monomers produces heteropolymers with properties that are intermediate between those of the individual homopolymers from which they originate.
Another way to classify PHAs is by examining the carbon sources used during their synthesis. They can be produced from traditional raw materials such as sugars, plant oils, or animal-derived fatty acids, which serve as precursors for hydroxy acid monomers [37]. In contrast, altered carbon sources may be utilized to generate polymers containing new functional groups within their side chains, leading to changes in both structure and physical performance [1]. Current studies are investigating chemical modifications—such as carboxylation, hydroxylation, and graft copolymerization—to improve traits like water resistance and compatibility with biological systems, thereby positioning PHAs as highly suitable for biomedical applications [38]. PHAs are particularly valued because of their distinctive benefits, including their ability to degrade naturally, their compatibility with living tissues, and their non-toxic nature [39,40]. These attributes make them versatile materials with applications spanning medicine, agriculture, and renewable energy production [41,42].
3. Waste as a Resource to Produce Bioplastics
The cost of producing polyhydroxybutyrate (PHB) is estimated to fall between 5380 and 18,300 USD per tonne of purified polymer. This high expense is largely dictated by the price of feedstock, which has prompted extensive research into utilizing agricultural byproducts and various microbial strains to enhance economic feasibility [43]. Since the agri-food industry generates vast amounts of waste—often of poor quality or environmentally damaging—these residues can be redirected into PHA production, transforming them into valuable bioplastics.
As an illustration, the cocoa sector generates about 4.2 tonnes annually, with pods making up 75% of the fruit’s weight. This translates into roughly 10 tonnes of waste for every tonne of dry beans produced. Studies have shown that Cupriavidus necator can accumulate PHB at 58.60 ± 4.95% of its cell dry mass when cultivated on alkaline cocoa pod hydrolysates [44]. Likewise, discarded fruits and vegetables have proven to be efficient feedstocks, allowing Cupriavidus necator to achieve PHA yields as high as 79.20% of cell dry mass (w/w) [45].
Cheese whey, the principal byproduct of dairy manufacturing, is composed mainly of water (over 90%), along with lactose (4.5–5.0% w/v), proteins (0.6–0.85% w/v), minor amounts of fat (0.36%), and minerals (0.53%) [46]. Microbial communities capable of generating biohydrogen while simultaneously storing PHAs from whey have shown notable efficiency [47]. Similarly, the wine industry produces around 5 tonnes of waste per hectare each year, which can be repurposed to mitigate environmental damage [48]. Strains such as Cupriavidus necator, Halomonas halophila, and Halomonas organivorans have demonstrated PHB accumulation rates of 70.4 ± 2.4%, 57.0 ± 1.0%, and 55.4 ± 1.4% of cell dry mass, respectively, when cultivated on grape sugar extract [49]. Other genera, including Bacillus, Tepidimonas, Azotobacter, and Pseudomonas, have also exhibited potential in converting wine-derived residues into PHAs [50].
Slaughterhouse byproducts, which are rich in fats, can be metabolized by Cupriavidus necator and Pseudomonas oleovorans to synthesize scl-PHAs [51]. Recombinant strains of Delftia acidovorans have been engineered to utilize substrates such as corn oil, udder, lard, and tallow, achieving yields of 26.72 ± 6.66%, 26.72 ± 6.66%, 39.33 ± 1.04%, and 15.33 ± 6.11% of cell dry mass, respectively [52]. Olive oil production, particularly concentrated in the Mediterranean region, generates approximately 2000 tonnes annually, along with more than 30 tonnes of wastewater from mills [53]. This effluent, which contains carbohydrates, lipids, and volatile fatty acids, provides a cost-effective feedstock for PHA synthesis [54]. Bacillus amyloliquefaciens OM81, capable of accumulating 30.2 ± 0.3% PHAs on glucose, has also been shown to utilize olive mill wastewater, producing yields between 5.6 ± 0.1% and 11.2 ± 0.3%. Additionally, waste frying oils have proven to be effective substrates for PHA production [55].
Beet molasses, a secondary product of sugar beet refining (0.25–0.35 tonnes generated per 7 tonnes of beets), can be directly applied as a substrate in fermentation processes [56]. Cupriavidus necator has been effectively cultivated on beet molasses, leading to the synthesis of P(3HB-co-3HV) [57]. When desugared beet molasses is used, Bacillus megaterium strains have achieved yields of 55–60% bioplastic per dry cell mass [58]. Additionally, species of Parapedobacter have demonstrated the ability to produce PHB from molasses [59].
The wood-processing sector also generates non-edible residues that can serve as low-cost raw materials for producing valuable bioproducts [60]. Among these, lignocellulose stands out as one of the most abundant renewable resources globally, arising as a major byproduct of both agriculture and forestry [61]. Although its utilization requires advanced microbial systems capable of breaking down lignin, microbial consortia have been shown to successfully convert lignocellulosic hydrolysates into PHAs [62].
Another major factor contributing to the higher cost of PHAs compared to petroleum-derived plastics is the extraction process, which represents nearly 30% of the overall production expense [63]. Reported recovery methods are generally classified into chemical and physical techniques, which may be applied independently or in combination [2,64]. The process of isolating PHAs from fermentation cultures typically involves four essential stages: (1) biomass separation; (2) biomass pretreatment; (3) polymer extraction; and (4) purification of the final product (Figure 2). To ensure optimal yield and polymer quality, extraction is commonly carried out during the peak phase of bacterial growth [65]. Among the four stages of PHA isolation, biomass pretreatment and polymer extraction are generally considered the most labor-intensive steps. Pretreatment often requires cell disruption through mechanical (e.g., high-pressure homogenization, bead milling) or chemical means, both of which demand significant energy input and operational time. The greatest loss of biopolymer typically occurs during the extraction stage, particularly when harsh solvents or incomplete cell lysis lead to partial polymer degradation or incomplete recovery.
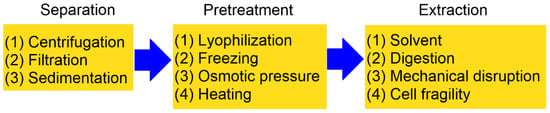
Figure 2.
A schematic diagram illustrating the steps required to obtain PHAs generated by microorganisms (reproduced from reference [1], MDPI, 2024).
Several studies have proposed solutions to mitigate these challenges. Enzymatic digestion and mild surfactant-assisted treatments have been shown to reduce energy consumption while improving polymer integrity. Likewise, non-chlorinated solvent systems, supercritical fluid extraction, and aqueous two-phase extraction have been reported to enhance recovery yields and reduce polymer loss [2]. More recently, genetically engineered strains with weakened cell walls and autolytic systems have been explored as a means to simplify downstream processing and minimize extraction-related losses. These approaches collectively demonstrate promising pathways to improve both the efficiency and sustainability of PHA recovery.
4. Degradation of PHAs
One of the most significant benefits of PHAs compared to other bioplastics is their ability to completely biodegrade, even in marine environments. Research indicated that the biological breakdown of PHAs is 8–20 times faster than non-biological degradation [66]. A wide range of bacteria and fungi can degrade PHAs, and such organisms have been identified in varied ecosystems, including soil, compost, and aquatic environments [67]. Under aerobic conditions, such as in soil or seawater, PHAs decompose into carbon dioxide and water, whereas in anaerobic settings like sediments or landfills, they are converted into carbon dioxide and methane. The suggested degradation pathway is presented in Figure 3.
The process begins with microbial exo-enzymes that break down insoluble PHAs into smaller, water-soluble fragments such as oligomers. These oligomers, along with monomers, are then utilized by microorganisms as sources of carbon and energy, ultimately leading to complete mineralization [68]. This continuous cycle of PHA synthesis and degradation not only helps mitigate plastic pollution but also contributes to a circular economy by enabling materials to be repeatedly produced from renewable biological sources and returned to the environment through natural biodegradation without accumulating as waste [69].
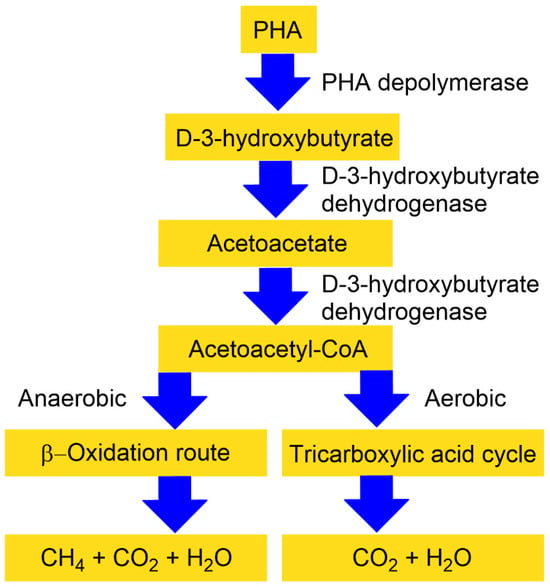
Figure 3.
A simplified representation of extracellular PHA degradation under anaerobic and aerobic conditions. The tricarboxylic acid cycle is also often known as the Krebs cycle, or citric acid cycle (reprinted from reference [69], open access, Elsevier 2023).
5. Medical Application of PHA
PHAs are widely utilized across the medical sector, with applications ranging from preventive healthcare and diagnostic tools to surgical procedures, as evidenced by numerous patents [70]. They are incorporated into single-use medical items such as syringes and blood storage bags, and play a role in surgical materials, including sutures, tissue adhesives, and sealants. In orthopedics, PHAs are fashioned into devices like screws and fixation plates, while in prosthetics, they are employed for intraocular lenses, dental implants, breast implants, and cartilage repair systems. Their versatility extends further to artificial organs designed for either temporary or permanent support, such as synthetic kidneys and hearts. Additional uses include cardiovascular patches, nerve guidance conduits, PHB-coated (PHB: polyhydroxybutyrate) surgical meshes for hernia repair, and a variety of applications in reconstructive and skin-related surgeries.
5.1. Tissue Engineering
Tissue engineering has emerged as a multidisciplinary field that merges biomaterials with medical science to create functional tissue replacements. Its central aim is to regenerate or repair tissues by integrating living cells, bioactive compounds, and supportive biomaterials [71]. For biomaterials to be effective in tissue restoration, they must satisfy two essential criteria: (1) they should possess adequate mechanical strength to sustain organs during healing; and (2) they must provide a surface structure that encourages firm cell attachment and active proliferation. To achieve these requirements, researchers design scaffolds that mimic the architecture, spatial organization, and chemical environment of the natural extracellular matrix, thereby fostering cell growth and differentiation [72]. Tissues are broadly divided into two categories: hard tissue substitutes, such as bone and cartilage, and soft tissue substitutes, including vascular grafts and skin replacements [73]. The natural occurrence of 3-hydroxybutyrate (3HB) in the bloodstream, combined with the excellent biocompatibility of PHB, makes PHB particularly suitable for scaffold construction in tissue and organ repair [74]. Biopolymers like PHAs provide tunable mechanical properties, high compatibility with biological systems, minimal toxicity, and efficient biodegradability within the body. They have been utilized to fabricate scaffolds with improved mechanical resilience—such as screws, pins, sutures, and thin films—that supply nutrients to cells, stimulate tissue growth, and assist in repairing cartilage, skin, nerve pathways, and cardiovascular structures [75]. Furthermore, PHAs can be enhanced by blending with inorganic bioceramics or hydroxyapatite (HAp), producing advanced composite scaffolds and tissue products tailored for therapeutic and medical applications [76].
5.1.1. Bone Tissue
Artificial prostheses, including bone grafts and substitutes, are vital in addressing osteochondral defects, congenital skeletal deformities, and in maintaining separation between articular surfaces and underlying bone layers. Bone tissue engineering, which emphasizes the regeneration of new bone, provides structural reinforcement while simultaneously stimulating cellular activity, thereby aiding in the correction of bone-related disorders. scl-PHAs, particularly PHB and PHBV (PHBV: poly(3-hydroxybutyrate-co-3-hydroxyvalerate), have been successfully applied to fabricate bone-supporting materials with strong mechanical properties [77]. To further enhance their performance, PHAs are frequently combined with inorganic or bioactive components—such as hydrogels, HAp, and other biocompatible polymers—resulting in improved tensile strength, elasticity, stress resistance, and compressive modulus, while also promoting osteoblast adhesion, proliferation, and differentiation in both laboratory and animal studies [78].
Additional bioactive reinforcements, including natural coral (NC) [79], sol–gel bioactive glass (SGBG), tricalcium phosphate (TCP) [80], and wollastonite (W) [81], have been incorporated into PHB and PHBV composites to further optimize bone regeneration. Among these, HAp is particularly significant, as it constitutes 65–70% of the natural bone matrix. In vitro experiments reveal that the mechanical strength, osteoblast activity, inflammatory response, and mineralization of PHB/HAp composites are strongly influenced by the proportion of HAp present [82]. Recent findings demonstrate that combining HAp with PHB produces bioresorbable porous scaffolds capable of enhancing both osteoconductivity and osteoinductivity [83]. In vivo studies further confirm that PHB reinforced with particulate HAp supports healthy bone remodeling without inducing chronic inflammation [84]. Moreover, PHB/HA scaffolds have been shown to maintain the viability and proliferation of human mesenchymal stromal cells (hMSCs) [85]. When PHB scaffolds seeded with hMSCs and coated with collagen-I or collagen-I/chondroitin sulfate were implanted in rats, vascularization occurred within the scaffold, and osteogenic markers such as osteonectin, osteopontin, and collagen I were expressed around PHB fibers—demonstrating effective bone growth and differentiation in vivo [86].
Blends of PHBV with hydroxyapatite have demonstrated significant promise in bone reconstruction at implant sites, where bone thickness increased from approximately 130 mm after one month to nearly 770 mm after six months [87]. When PHBV scaffolds were reinforced with hydroxyapatite nanoparticles, they exhibited notable improvements in compressive stiffness, mechanical strength, and in vitro bioactivity [88]. A novel scaffold design combining poly(lactic-co-glycolic acid) (PLGA) and PHBV—produced by embedding PHBV microspheres into a PLGA matrix through particle leaching—achieved high porosity, strong interconnectivity, enhanced compressive resistance, and promoted robust proliferation of human mesenchymal stem cells (hMSCs) [89]. Table 3 presents other examples of PHAs used for bone tissue engineering.

Table 3.
Some PHAs used for bone tissue engineering applications.
Hydrogels have also been investigated for scaffold construction because of their naturally porous architecture; however, their limited mechanical stability and weak bioactivity reduce their effectiveness. To overcome these shortcomings, researchers have developed tri-layered scaffolds that integrate PHB, hydroxyapatite, and protein-based hydrogels. These composite structures provide superior mechanical strength, enable effective cell encapsulation, and enhance the adaptability of bone cells to the scaffold environment [102] (see Table 4).

Table 4.
Some PHA–hydrogel hybrids used for tissue engineering applications.
Electrospinning techniques have been employed to create hybrid scaffolds incorporating PHB, polycaprolactone (PCL, a biodegradable, semi-crystalline polyester widely used in biomaterials, tissue engineering, and drug-delivery systems), and bioactive glass, effectively merging the rigidity of PHB, the elasticity of PCL, and the biofunctional properties of 58S glass into a unified fibrous matrix (see Table 5) (58S bioactive glass is a sol–gel–derived bioactive glass made of about 60% silica (SiO2), 36% calcium oxide (CaO), and 4% phosphorus pentoxide (P2O5). It’s well known for bonding strongly with bone and stimulating biological activity. These engineered composites exhibited improved osteoblast survival, robust cellular interactions, and heightened alkaline phosphatase activity [77]. Extending beyond PHB and PHBV systems, scaffolds composed of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) have demonstrated notable compatibility with bone marrow cells. In comparison to PHB, PHBHHx scaffolds show superior adhesion to rabbit bone marrow cells, fostering cell proliferation and expressing osteogenic traits such as elevated alkaline phosphatase activity, calcium mineralization, collagen production, and rounded cellular morphology [115]. Furthermore, bone marrow stromal cells readily attach, proliferate, and undergo osteogenic differentiation when cultured on PHBHHx scaffolds in vitro, underscoring their strong biological affinity [116]. Incorporation of collagen into PHBHHx scaffolds has enabled the successful culture of hMSCs, reinforcing their potential utility in bone tissue engineering [117]. Despite hydroxyapatite’s recognized bioactivity and osteoconductivity, its combination with PHBHHx does not yield substantial improvements in mechanical strength or osteoblast performance [118]. A more advanced PHA variant, the terpolymer PHBVHHx, surpasses PHBV and PHBHHx in terms of flexibility, thermal resilience, and mechanical robustness. Its increased surface roughness and hydrophobicity enhance hMSC attachment more effectively than polylactic acid (PLA), PHBHHx, or tricalcium phosphate (TCP), leading to markedly higher cell proliferation rates [119].

Table 5.
Electrospinning-based synthesis of PHAs for tissue engineering applications.
Table 5 summarizes recent developments in the electrospinning-based fabrication of PHA and PHA composite nanofibers for tissue engineering applications. The listed studies highlight the growing diversity of PHA formulations—ranging from pure PHB to hybrid systems incorporating natural polymers, inorganic nanoparticles, and bioactive additives—and demonstrate how electrospinning enables the creation of highly porous, fibrous scaffolds that mimic the extracellular matrix. By comparing materials and publication years, the table illustrates current research trends, emerging composite strategies, and the expanding relevance of electrospun PHAs across various tissue engineering contexts.
To be effectively used in bone tissue engineering, polyhydroxyalkanoate-based composites must meet several essential criteria. First, they require sufficient mechanical strength and stiffness to withstand physiological loading and to provide temporary structural support during bone regeneration. Their biodegradation rate must be well-matched to the pace of new bone formation, ensuring gradual load transfer without premature collapse or prolonged persistence. PHA composites must also exhibit excellent biocompatibility, avoiding chronic inflammation while supporting osteoblast adhesion, proliferation, and differentiation. Incorporation of bioactive components—such as hydroxyapatite, tricalcium phosphate, or bioactive glass—should enhance osteoconductivity and osteoinductivity, promoting vascularization and mineralized tissue formation. Finally, the scaffold architecture must offer high porosity and interconnected pore networks to facilitate nutrient diffusion, cell infiltration, and neovascularization. Together, these criteria define the functional requirements for PHA-based materials to serve as reliable and effective platforms for bone repair and regeneration.
5.1.2. Cartilage Tissue
Cartilage tissue engineering offers innovative approaches for the restoration of damaged articular cartilage. This specialized tissue, composed of chondrocytes, lines the ends of bones within synovial (diarthrodial) joints, where it functions to distribute mechanical stresses, facilitate smooth articulation, and maintain overall joint mobility. Because cartilage possesses the lowest cellular density of any human tissue and exhibits minimal intrinsic regenerative capacity, injury frequently results in impaired joint performance and progression toward osteoarthritis. Traditional surgical interventions—including microfracture, arthroscopic abrasion, and subchondral drilling—have been employed clinically but generally fail to reestablish native cartilage functionality [141,142]. To overcome these limitations, robust three-dimensional porous scaffolds fabricated from polymers such as PHB, PLA [143], polyglycolic acid (PGA) [144], PLGA [145], collagen [146], silk fibroin [147], chitosan [148], and their composites have been designed. These biomaterials provide a conducive framework for cellular proliferation and tissue integration, supporting both biomechanical stability and biochemical signaling [149].
A comprehensive review by Puppi et al. [150] highlighted the contributions of PHAs in cartilage regeneration. Porous scaffolds composed of PHBHHx/PHB blends have served as effective matrices for rabbit articular chondrocytes (RAC), demonstrating enhanced cell growth and proliferation compared to scaffolds of pure PHB. Blends containing 60% PHBHHx by weight exhibited superior mechanical performance relative to either polymer alone. These hybrid constructs not only improved chondrocyte viability and function but also facilitated the anchorage of type II collagen filaments and their penetration into deeper scaffold regions [151]. Complementary findings by Deng et al. [152] revealed that RAC cultured on PHB/PHBHHx blends expressed higher levels of collagen II mRNA compared to cells grown on neat PHB scaffolds. Moreover, extracellular collagen X—a marker associated with endochondral ossification—was progressively reduced as PHBHHx content increased, underscoring the role of blend composition in modulating chondrocyte activity (see Table 6).

Table 6.
Cartilage tissue engineering applications for PHAs.
5.1.3. Cardiac Tissue
Cardiovascular disease continues to be one of the foremost causes of mortality and long-term disability across the globe [161]. To confront this pressing medical burden, cardiac tissue engineering has emerged as a pivotal research focus. Traditional surgical interventions—including defect reconstruction, revascularization, and patch closure—are widely practiced, yet the biomaterials employed must satisfy stringent criteria: they need to be mechanically robust, resistant to infection and degradation, biocompatible and non-toxic, sufficiently flexible, and available in diverse dimensions to support both vascular and cardiac repair procedures [162,163].
Among candidate materials, mcl-PHAs display distinctive attributes that render them highly suitable for cardiac applications. These polymers are elastic, exhibit elevated glass transition temperatures, integrate effectively with myocardial tissue, and possess the capacity to bind bioactive agents such as vascular endothelial growth factor, thereby promoting cellular adhesion, proliferation, and survival [164]. More recently, polymers including poly(4-hydroxybutyrate) (P4HB) and poly(3-hydroxyoctanoate) (P3HO) have been identified as promising options for addressing congenital heart anomalies, constructing vascular grafts, and fabricating heart valves. Additional bioplastics, such as poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) (PHBVHHx) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (PHB4HB), have also been utilized in the development of membranes and cardiac patches, respectively, underscoring their potential in cardiovascular tissue engineering [165] (see Table 7).

Table 7.
Cardiac tissue engineering applications for PHAs.
5.1.4. Heart Valves
Cardiac tissue engineering provides a pathway for generating functional heart valve substitutes by molding biomaterials into valve-like architectures and subsequently seeding them with living cells [179]. Numerous biomaterials have been investigated for this application, including PHA, polylactic acid (PLA) [180], polycaprolactone (PCL) [181], polyglycolic acid (PGA) [182], and decellularized extracellular matrix, each offering a supportive microenvironment conducive to cellular proliferation and differentiation. Notably, mcl-PHAs exhibit superior flexibility compared to PLA and PGA, making them particularly advantageous for constructing leaflets in tri-leaflet valve designs [183]. The copolyester P(3HHx-co-3HO) (poly(3-hydroxyhexanoate-co-3-hydroxyoctanoate)), when paired with autologous cells in the pulmonary position, has yielded promising outcomes, characterized by only mild stenosis and the absence of thrombus formation [184]. Further advancements have been realized through blending PGA with P(3HHx-co-3HO) and reinforcing the composite with PHB, which improved the mechanical and biological properties of the scaffold, ultimately delivering enhanced performance [185].
5.1.5. Vascular Grafts
Vascular grafting has been explored as a therapeutic strategy for managing diverse cardiovascular disorders. Yet, conventional synthetic graft materials frequently encounter limitations, including inadequate endothelialization, mismatched mechanical compliance, and rapid blockage under low-flow conditions, all of which contribute to postoperative complications [186]. In contrast, bioplastics such as PHAs present a compelling alternative, since their monomeric composition can be engineered to deliver optimal mechanical strength, biocompatibility, and predictable degradation behavior. Within this class, PHBHHx stands out for exhibiting enhanced physicochemical characteristics relative to PHB and PHBV (poly(3-hydroxybutyrate-co-3-hydroxyvalerate)) (see Table 8). Its suitability for blood-contact applications is underscored by diminished platelet adhesion, superior cytocompatibility and hemocompatibility, and reduced interactions with erythrocytes alongside lower hemolytic activity [187]. Even so, additional investigations—particularly those involving vascular graft implantation in animal models—remain essential to fully establish its translational potential in vascular tissue engineering and to resolve persistent challenges such as compliance mismatch [188].

Table 8.
Vascular tissue engineering applications for PHAs.
5.1.6. Artificial Blood Vessels
Due to their adjustable mechanical properties—including flexibility, elasticity, tensile strength, and the capacity to stimulate elastin synthesis—PHB and P3HB4HB (poly(3-hydroxybutyrate-co-4-hydroxybutyrate)) have been explored as promising biomaterials for constructing artificial blood vessels [200]. These polymers are regarded as strong contenders for vascular tissue applications. Moreover, advanced block copolymers with meticulously engineered architectures have been developed, notably alternating block polyurethanes synthesized from P3HB4HB-diol and poly(propylene glycol)-poly(ethylene glycol)-poly(propylene glycol), with 1,6-hexamethylene diisocyanate (HDI) serving as the capping agent [201].
5.1.7. Skin Tissue Engineering
The search for advanced biomaterials capable of regenerating injured skin continues to pose a significant challenge in reconstructive medicine [202]. To address wound healing needs, both naturally derived polymers—such as collagen, alginic acid, hyaluronic acid, chitosan, and fucoidan—and synthetic alternatives, including Teflon, polyurethanes, and methyl methacrylate, have been utilized in the fabrication of artificial dressings [203]. Biopolymers are particularly advantageous because they emulate essential physiological roles of intact skin: they provide antimicrobial defense, enable gaseous exchange, sustain a moist healing environment, deliver mechanical reinforcement, exhibit sufficient tensile strength, and possess elasticity that allows them to adapt to irregular wound geometries [204]. Their application not only mitigates inflammation but also stimulates angiogenesis and expedites tissue repair. Within this context, PHAs, especially scl-PHAs, have been investigated for wound management [204]. Notably, PHA and PHB4HB have been employed in the design of wound dressings and surgical sutures, offering adequate mechanical resilience that makes them suitable for repairing muscle-fascial defects and cutaneous injuries [205] (see Table 9).

Table 9.
Wound healing tissue engineering using PHAs.
5.1.8. Nerve Repair
Peripheral nerve damage frequently leads to partial or complete impairment of motor, sensory, and autonomic functions, and restoring these capabilities continues to pose a significant challenge for both clinicians and researchers. Clinical data indicate that only around 3% of patients recover sensory function, while fewer than 25% regain motor function after such injuries [219]. Although surgical repair remains the standard approach, lesions involving tissue gaps greater than 5 mm typically necessitate grafting. In this context, nerve guidance conduits (NGCs) have shown encouraging results, as they help prevent myofibroblast invasion, minimize scar formation, and provide a supportive framework for axonal regrowth [220]. Nevertheless, their clinical use is currently limited to relatively short nerve defects [221]. While several FDA-approved synthetic conduits exist, none are sanctioned for gaps exceeding 3 cm. The most employed natural biomaterials include collagen-based devices (NeuraGen®, Neuroflex®, NeuroMatrix®, RevolNerv®) and chitosan-derived conduits (Reaxon®), as highlighted in the review by Kornfeld et al. [222]. These biomaterial conduits generally facilitate functional recovery, though complications such as adverse effects and incomplete regeneration remain ongoing concerns.
PHAs have been widely explored in the context of nerve regeneration owing to their adaptable mechanical and physicochemical characteristics, which can be fine-tuned through fabrication techniques such as electrospinning, extrusion, and sheet rolling [223]. Among these, PHB has emerged as a particularly promising material in preclinical investigations, attributed to its biocompatibility, biodegradability, elasticity, and inherent piezoelectric behavior [224]. Importantly, PHB has also been shown to stimulate early vascularization following implantation [225]. Although its stability may restrict its utility in short-term applications, PHB remains highly suitable for long-term nerve repair, effectively bridging both small and extensive defects and offering a viable alternative to direct epineural suturing [226]. Evidence from rat sciatic nerve models demonstrates that PHB conduits can successfully restore 10 mm gaps within 30 days, characterized by minimal inflammatory response and robust axonal regrowth comparable to autologous grafts [227]. Complementary studies in rabbits, using the common peroneal nerve model, further validated PHB’s capacity to support regeneration across larger defects [227]. Moreover, the incorporation of trophic factors such as glial growth factor/neuregulin-1 (GGF/NRG1) significantly enhanced repair across 2–4 cm lesions, yielding favorable regenerative outcomes in both short- and long-term evaluations [228].
PHB conduits have been evaluated against fibrin glue conduits and further tested with internal fillers consisting of fibrin matrices seeded with Schwann cells (SCs) or mesenchymal stem cells, achieving successful repair of 10 mm nerve gaps in rat models [229]. In another approach, PHB strips populated with SCs were applied to bridge sciatic nerve defects, facilitating early axonal regeneration and enabling functional recovery within just two weeks [230]. Extended investigations lasting 12 months, in which PHB strips were enriched with either SCs or adipose-derived stem cells, revealed enhanced regenerative outcomes, including greater axonal penetration into distal stumps and reduced muscle atrophy compared to untreated controls [231]. Modifications of PHB through blending with chitosan minimized crystallization and allowed the fabrication of aligned fibers via electrospinning, thereby improving scaffold performance [232]. Additionally, composite structures combining PHB with synthetic polymers such as PCL have produced effective nerve guidance conduits [233].
Although PHB offers several advantages, it is hindered by drawbacks such as brittleness, pronounced crystallization, and limited hydrophilicity, all of which compromise its ductility and flexibility. To overcome these shortcomings, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) was engineered through copolymerization of PHB with hydroxyvalerate, resulting in improved mechanical characteristics [234,235]. Compared to PHB, PHBV exhibits greater flexibility and is easier to process, though it remains constrained by a narrow processing window and a lower strain-at-break relative to petroleum-derived polymers [236]. In vivo studies using rat sciatic nerve models demonstrated that PHBV fibrous conduits successfully bridged 30 mm defects within four months, restoring skeletal muscle innervation and producing fiber diameters similar to those achieved with autografts [237,238]. The addition of SCs further enhanced regenerative outcomes [239,240]. Structurally, PHBV conduits are highly porous (~95%), biocompatible, and mechanically adaptable, capable of bending up to 180° and returning to their original form. Moreover, micropatterned PHBV wafers have facilitated both sensory and motor recovery in rats following 30 mm nerve gap repairs [241]. Beyond conduits, PHBV microspheres integrated with alginate hydrogel have supported the growth, differentiation, and maturation of neuroblastoma cells and fetal cortical neurons, underscoring their potential in neural tissue engineering [242].
Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), a notable PHA derivative, exhibits enhanced flexibility and mechanical resilience owing to the incorporation of hydroxyhexanoate (HHx) units within its polymer backbone. This material demonstrates excellent biocompatibility with neural stem cells and has been engineered into conduits featuring both uniform and non-uniform wall porosity, each successfully bridging 10 mm sciatic nerve gaps in rat models [243]. Although both architectures facilitated nutrient diffusion, conduits with non-uniform porosity offered superior mechanical integrity and more favorable biodegradation profiles. PHBHHx nanofiber scaffolds further revealed a strong affinity for neuronal stem and progenitor cells, effectively supporting their adhesion, viability, differentiation, and synapse formation [244]. Strikingly, when PHBHHx scaffolds seeded with neural stem cell/neural progenitor cell complexes were implanted into rats suffering from traumatic brain injury, they prevented glial scar development and instead promoted neurite outgrowth alongside measurable functional recovery [245]. Table 10 presents some other examples of the PHAs used for nerve regeneration.

Table 10.
Nerve regeneration using PHAs.
5.2. Overview of Drug Delivery Systems and the Emergence of PHAs
In conventional drug administration, compounds disperse systemically rather than concentrating at the intended organ or tissue, which often diminishes therapeutic efficiency. To overcome these shortcomings, drug delivery systems (DDS) were introduced in the United States during the late 1960s. These polymer-based platforms were designed to maintain controlled release of drugs at optimal concentrations, direct therapeutic agents specifically to diseased sites while sparing healthy tissues, and enable externally triggered release mechanisms. They also simplified administration routes, most commonly through dermal or mucosal pathways. Collectively, DDS enhance solubility and bioavailability, mitigate toxicity, minimize adverse effects, and allow precise targeting of particular cells and tissues [253].
By the early 1990s, PHAs had gained recognition as promising materials for drug delivery applications, owing to their inherent biodegradability, biocompatibility, and favorable thermal processing properties. Their versatile chemical structures and modifiable side groups provide opportunities for tailoring functionality, making them suitable for a wide range of delivery devices. Because PHAs combine biocompatibility with biodegradability, they are well tolerated by human cells and tissues [254], which underpins their increasing adoption in drug delivery research and biomedical applications. Of all the PHAs, PHB and PHBV have been the most widely investigated for developing effective drug delivery platforms, including micro- and nanodevices, disks, implants, rods, and films [255].
5.2.1. Antibiotic and Antimicrobial Applications
Microdevices such as microspheres and microcapsules have been developed to enable controlled drug delivery, including the administration of anesthetics, antibiotics, anti-inflammatory drugs, anticancer agents, hormones, steroids, and vaccines [256]. Biopolymeric systems such as P3HB4HB, PHBV, and PHB have been engineered into biodegradable, implantable rods and disks for localized antibiotic release in the treatment of chronic osteomyelitis, thereby reducing risks of post-surgical and implant-associated infections [257,258].
PHB and PHBV have been identified as promising candidates for drug delivery systems owing to their advantageous physicochemical properties, their neutrality toward platelet activity, and their compatibility with other polymers. Matrix-based carriers constructed from PHB and PHBV have been explored for the controlled release of numerous antibiotics and therapeutic compounds, including tetracycline, rifampicin, sulbactam-cefoperazone, gentamicin, sulperazone, rubomycin, and rhodamine B isothiocyanate [259]. Long-term investigations ranging from 15 to 60 days demonstrated that polymer molecular weight plays a critical role, with higher molecular weights correlating to slower drug release rates. Furthermore, the release kinetics and cumulative drug release are strongly influenced by the hydroxyvalerate (HV) content in PHBV copolymers [85]; increasing HV units accelerates drug release, while the amount of drug loaded directly modulates release behavior. Among the different PHA variants examined, P3HB4HB has exhibited the most favorable and controlled release characteristics [260].
5.2.2. Antitumor Applications
Nanostructures derived from PHB and its copolymers have been developed as carriers for a wide range of molecular therapeutics, including anticancer drugs, hormones, and immunomodulators, all of which are capable of penetrating intracellular membranes [257]. PHB microspheres have been explored as carriers for anticancer therapeutics, with rubomycin-loaded formulations demonstrating effective inhibition of cell proliferation in Ehrlich’s carcinoma models [261].
PHBV has been especially prominent in drug delivery research. Docetaxel encapsulated within PHBV-based nanoparticles (NPs) exhibited potent cytotoxicity against MCF-7 breast cancer cells [262]. These nanoparticles, optimized through a Box–Behnken statistical design, displayed enhanced efficacy compared to free docetaxel, while offering improved safety features attributed to their negative surface charge, sustained-release kinetics, and passive tumor targeting via the enhanced permeability and retention (EPR) effect. Furthermore, when PHBV was blended with D-α-tocopheryl polyethylene glycol succinate (TPGS), the resulting docetaxel-loaded nanoparticles provided more controlled and prolonged release, thereby lowering required therapeutic doses and reducing systemic toxicity [263].
Recent progress in drug delivery has introduced targeting approaches, in which doxorubicin (DOX) encapsulated within PHBV nanoparticles is functionalized with cell-targeting peptides. This modification enhances nanoparticle localization in close proximity to cell nuclei, thereby producing stronger cytotoxic effects than either free DOX or non-targeted nanoparticle formulations. Importantly, PHA-based nanoparticles with diameters of approximately 200 nm exhibit intrinsic antiproliferative activity, a property that can be exploited in anticancer treatment strategies [264].
5.2.3. Cardiovascular Applications
In cardiovascular applications, PHB blended with polyhydroxyoctanoate has been incorporated into drug-eluting stent coatings to mitigate arterial restenosis [265]. The intrinsic hydrophobicity and biodegradability of PHAs support the sustained release of hydrophobic cardiovascular drugs, making them suitable for long-term vascular therapies [266].
5.2.4. Immunomodulatory Applications
Multilayered sandwich films composed of PHBHHx copolymers, loaded with thymopentin-phospholipid complexes, achieved controlled drug release lasting up to 42 days and produced enhanced immunomodulatory activity in rat models of immunosuppression [267].
5.2.5. Neurological Applications
Early investigations have highlighted the promise of PHA-based nanoparticles for transporting therapeutic agents in the treatment of neurological disorders. PHBV nanoparticles have been explored for drug delivery in multiple sclerosis [268] and Parkinson’s disease [269], demonstrating their potential for crossing biological barriers and delivering neuroactive compounds.
5.2.6. Manufacturing and Engineering Considerations
A variety of processing strategies are currently employed to fabricate PHA-derived microparticles and capsules for drug delivery applications [270]. Among these, the emulsion/solvent-evaporation method is widely adopted, as it provides enhanced control over drug encapsulation and stability [260]. Despite its utility, this technique suffers from notable drawbacks, particularly its inability to produce particles with a narrow and well-controlled size distribution, which can lead to unpredictable drug-release kinetics and reduced bioavailability [271]. Moreover, the formation of mesopores and micropores during fabrication, together with additional porosity generated through polymer degradation, often accelerates drug release and consequently limits the broader applicability of these delivery systems [36].
To overcome these limitations, innovative strategies have been introduced, including chemical modification, copolymer development, and surface functionalization with targeting ligands, all of which improve encapsulation efficiency, enhance chemical stability, and prolong release profiles [272]. Advances in automated manufacturing have further enabled the production of PHA-based particles with highly uniform sizes and reproducible characteristics, which is essential for ensuring consistent performance in downstream applications such as drug delivery, cell encapsulation, and controlled release studies [273]. Additionally, the conjugation of bioactive molecules or targeting moieties to particle surfaces has been shown to modulate cellular uptake, making these systems particularly valuable in the design of next-generation cancer therapeutics [274].
5.2.7. Material Limitations and Strategies for Improvement
The biodegradability of PHB and PHBV allows them to facilitate controlled drug release via surface erosion. Nonetheless, their relatively high melting points and crystalline nature restrict drug-loading capacity and reduce release efficiency [275]. To overcome these limitations, strategies such as blending PHB with other polymers or creating copolymers have been employed. These modifications yield softer and more resilient materials with reduced melting points, thereby enhancing their practicality and effectiveness in drug delivery applications.
5.2.8. PHB Copolymers
An amorphous copolymer composed of poly(3-hydroxybutyrate)-poly(ethylene glycol)-poly(3-hydroxybutyrate) (PHB-PEG-PHB) has been engineered to function as a delivery platform for hydrophobic therapeutic agents [276]. Relative to pure PHB, this copolymer exhibits enhanced drug-loading efficiency while retaining comparable biodegradability and controlled release behavior. The incorporation of PEG not only prolongs systemic circulation time [277] but has also been applied in the design of copolymer-based formulations for peptide-loaded nanoparticles [278]. Remarkably, in vivo experiments employing PHB-PEG nanoparticles encapsulating the anticancer peptide NuBCP-9 achieved a 90% reduction in tumor size within an Ehrlich syngeneic mouse model.
A biodegradable cationic amphiphilic copolymer, PHB-b-poly(2-(dimethylamino)ethyl methacrylate) (PHB-b-PDMAEMA), has been utilized for the simultaneous delivery of paclitaxel and a Bcl-2 converting gene, successfully overcoming mechanisms of drug resistance [279]. These dual-delivery constructs achieved notable transfection efficiency and significantly increased apoptosis in drug-resistant HepG2 cancer cells. In parallel, PHB-b-PDMAEMA systems were also shown to suppress resistance pathways mediated by both p-glycoprotein and Bcl-2 proteins [280]. An ideal anticancer therapy selectively eliminates malignant cells while sparing healthy ones, and PHB/PEG-Nickel Oxide (NiO) nanocomposites encapsulating norfloxacin have demonstrated this selective cytotoxicity—exerting potent effects against cancer cells while leaving normal cells unaffected [281].
To further minimize adverse impacts on healthy tissues, injectable thermogelling PHB-based copolymers have been engineered. The PEG-PPG-PHB triblock polymer exhibits thermogelling properties, strong biocompatibility, and enables sustained release of chemotherapeutics such as paclitaxel and doxorubicin. Direct intratumoral administration of this PTX-thermogel produced greater tumor regression compared to either free drug or thermogel alone [282].
PHBV copolymers have also been explored for advanced drug delivery. An mPEG-PHBV copolymer synthesized via transesterification generated biocompatible nanoparticles capable of encapsulating and gradually releasing hydrophobic drugs. Furthermore, a PHBV-hyperbranched polyethylenimine (PEI) copolymer has been evaluated as a non-viral vector for siRNA delivery. This platform achieved superior transfection efficiency and reduced cytotoxicity compared to branched-PEI across two cell lines, while demonstrating in vitro luciferase gene silencing on par with Lipofectamine 2000 [283].
5.2.9. PHA Blends
Nanoparticles fabricated from poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(lactic-co-glycolic acid) (PHBV/PLGA) blends and loaded with the recombinant protein teriparatide have been investigated as a therapeutic strategy for osteoporosis [284]. The optimized formulation exhibited a prolonged in vitro release profile, with the encapsulated teriparatide maintaining stability throughout the duration of release. An alternative design involved injectable gelatin-based hydrogels incorporating PHB nanoparticles. In this dual drug-delivery system, naproxen sodium was encapsulated within PHB nanoparticles, while curcumin was integrated into the hydrogel matrix. In vitro evaluations confirmed that this configuration achieved sustained release of both therapeutic agents [285]. Similarly, injectable hydrogels containing PHBV nanoparticles have been developed. In this case, nafarelin-loaded nanoparticles were dispersed within a sodium alginate/Poloxamer 407 solution, and in vitro testing demonstrated continuous release of nafarelin for as long as 60 days [286].
5.2.10. Fiber Membranes
Electrospun PHB-PEO fiber membranes incorporating different concentrations of chlorhexidine (CHX) (1%, 5%, and 10%) demonstrated antibacterial efficacy against E. coli and S. aureus. Interestingly, the 1% CHX formulation achieved both a sustained release profile and a faster initial release compared to higher loadings [287]. In a similar vein, solvent-free melt electrospinning was used to fabricate PLLA-PHB (PLLA: poly(L-lactic acid)) fibers containing dipyridamole. These fibers exhibited irregular surface textures and variable diameters, while the inclusion of PHB reduced PLLA crystallinity. In vitro release experiments revealed that drug release was predominantly controlled by diffusion through the polymer matrix, although ester bond hydrolysis also contributed as the polymer degraded [288].
Moving beyond conventional DDS, PHAs—particularly mcl-PHAs—have been explored for transdermal drug delivery systems (TDDS) (see Table 11). Such systems encompass drug encapsulation, cellular uptake via endocytosis, retention within tissues, controlled release, and activation in vivo [289]. Their performance in these applications is closely tied to physicochemical parameters such as degradation rate, solubility in water, molecular weight, and their capacity to be chemically grafted with other biodegradable polymers like PCL, PLA, and PLGA. A notable illustration is PEG-PCL micelles loaded with gold nanoparticles, which, upon stimulation with near-infrared radiation (NIR), successfully induced apoptosis in cancer cells during in vitro studies [290].

Table 11.
Different PHAs used as drug carriers.
To facilitate comparison among the various PHA-based systems used in drug delivery, Figure 4 summarizes the chemical structures of the principal homopolymers and copolymers discussed in this section. Differences in side-chain length, co-monomer composition, and functional modifications (e.g., PEGylation, PEI grafting, metal-oxide conjugation) directly influence hydrophobicity, crystallinity, degradation rate, and drug-loading capacity. These structural distinctions underpin the diverse performance profiles observed across PHA-based nanoparticle and composite formulations.
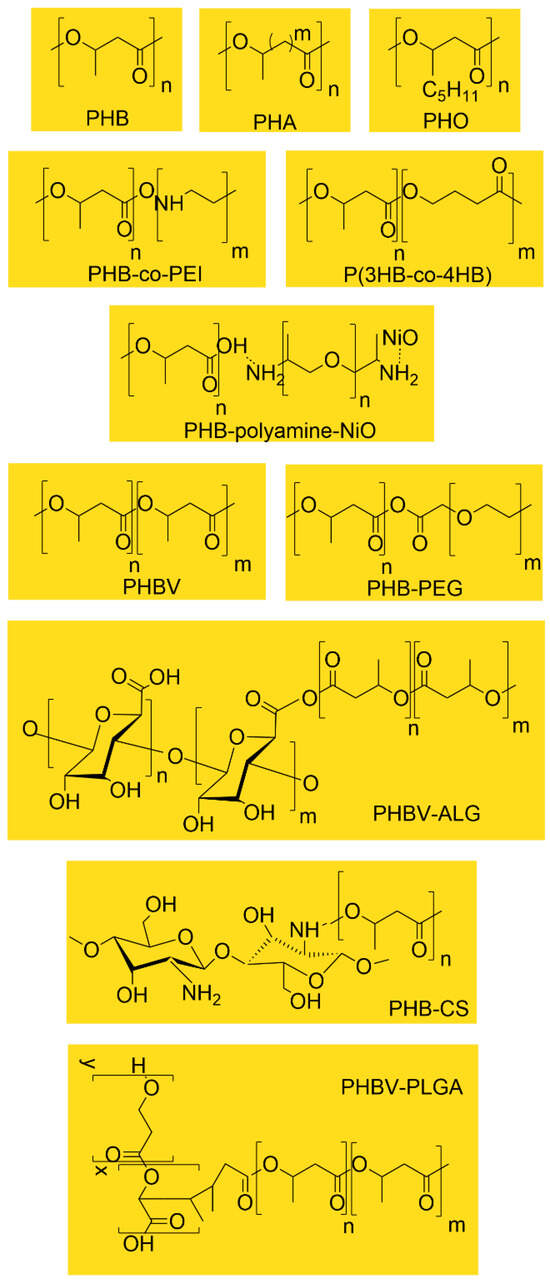
Figure 4.
Representative chemical structures of PHAs and PHA-based copolymers used in drug delivery systems.
6. Clinical Trials and Regulatory Approval of Pha-Based Devices
Regulatory approval has already been secured for P(4HB), a scl-PHAs commercialized under the name TephaFLEX®, in both the United States and Europe [302]. In the U.S., the Food and Drug Administration (FDA) authorized TephaFLEX® for clinical use in surgical sutures as early as 2007 [64]. Another clinically available derivative of P(4HB) is the PHASIX™ plug and patch, specifically developed for the treatment of inguinal hernias [77]. Looking ahead, it is expected that ongoing and future clinical trials will further investigate the therapeutic potential of PHA-derived prototypes [303].
7. Challenges
PHAs have gained significant attention due to their biocompatibility, biodegradability, and versatility, positioning them as promising materials for both large-scale industrial production and advanced medical applications. Their key physicochemical properties—such as glass transition temperature, melting point, tensile strength, elongation capacity, and elastic modulus—are strongly shaped by monomer composition and molecular weight, making careful optimization essential for achieving targeted performance. High molecular weight copolymers, in particular, offer improved mechanical and thermal characteristics, though their use in biomedical settings still requires caution, as certain formulations or impurities may trigger immune responses and therefore demand thorough preclinical evaluation. At the same time, the broader adoption of PHAs in clinical practice continues to be limited by challenges in cost-effective scale-up and consistent production of medical-grade materials. Microbial fermentation and downstream purification remain resource-intensive, with high energy demands and solvent use contributing to elevated costs. Strategies to address these barriers include altering feedstock composition, utilizing low-cost agricultural residues or wastewater organics, refining culture conditions, engineering robust microbial strains, adopting continuous fermentation systems, and developing solvent-free or enzymatic recovery methods. Collaborative efforts across academia, industry, and government, supported by biorefinery integration and public funding, will be essential to make PHA production economically viable. Regulatory approval adds another layer of complexity, as variations in molecular weight, crystallinity, and degradation rates complicate standardization, and current frameworks often require case-by-case evaluations. Long-term biodegradation studies, GMP-compliant manufacturing, and harmonized testing protocols are needed to streamline approval pathways and build clinical trust. Looking ahead, innovations such as smart PHAs responsive to stimuli, hybrid composites with ceramics or nanomaterials, and 3D-printed patient-specific implants are reshaping their role in healthcare. Beyond clinical performance, PHAs also support sustainability by enabling a circular bioeconomy—produced from renewable feedstocks, degrading into non-toxic compounds, and reducing environmental burdens through eco-friendly medical devices. The journey of PHAs from microbial byproducts to multifunctional biomaterials underscores both the technical and regulatory challenges ahead, but continued investment and interdisciplinary collaboration are paving the way for PHAs to evolve into the gold standard of sustainable medical and industrial polymers.
8. Conclusions
PHAs have emerged as one of the most versatile and promising biomaterials in modern medicine. Their rare combination of biocompatibility, biodegradability, mechanical adaptability, and functional tunability positions them as ideal candidates for applications ranging from drug delivery systems and wound dressings to orthopedic implants and tissue engineering scaffolds. PHAs can be fabricated into diverse formats—nanoparticles, microspheres, hydrogels, and films—each offering unique advantages for sustained release, targeted therapy, and regenerative support. Their ability to degrade into non-toxic metabolites, promote cellular activities, and align with therapeutic timelines underscores their clinical relevance, while their integration into sutures, stents, and fixation devices highlights their structural reliability and biological harmony.
The rise in PHAs reflects the power of interdisciplinary collaboration. Advances in biotechnology have enabled scalable microbial production using engineered strains and waste-derived feedstocks. Materials science has refined its mechanical properties, degradation profiles, and bioactivity through blending, copolymerization, and surface modification. Medicine has validated their efficacy in wound healing, tissue regeneration, and implant performance, ensuring that PHA-based devices are both biologically effective and clinically viable. This convergence of disciplines has created a feedback loop of innovation, where biological insights inform material design and material capabilities expand therapeutic possibilities.
At their core, PHAs represent a paradigm shift in how we approach biomaterials—transforming microbial byproducts and waste carbon sources into high-value medical solutions. They embody the principles of circular bioeconomy and regenerative design, offering a model of responsible innovation that bridges clinical performance with ecological stewardship. In a world facing plastic pollution, resource scarcity, and healthcare inequities, PHAs demonstrate that high-performance materials can also be sustainable, safe, and socially responsible.
Looking ahead, PHAs may serve as the foundation for next-generation smart biomaterials—responsive, personalized, and ecologically attuned. Their potential integration with biosensors, targeted therapies, and regenerative platforms places them at the forefront of future medicine. More than just materials, PHAs embody a philosophy of innovation rooted in harmony between biology and technology, resilience in design, and renewal of both human health and planetary well-being.
Realizing their full potential will require continued investment in research, infrastructure, and policy, alongside rethinking production paradigms and regulatory frameworks. The rewards—safer therapies, cleaner environments, and more equitable healthcare—are well worth the effort. Ultimately, PHAs offer a vision of medicine that heals not only the body but also the planet, standing as a powerful example of what is possible when science, sustainability, and humanity converge.
Unlike previous reviews that concentrate mainly on drug-delivery technologies, this review positions PHAs within a wider biomedical and ecological framework. By linking material origin, biodegradability, clinical readiness, and environmental responsibility, we highlight PHAs as a platform that unites sustainable manufacturing with therapeutic function. This integrated perspective underscores their potential not only as drug carriers but as versatile, next-generation biomaterials capable of supporting repair, regeneration, and patient-centered healthcare.
Author Contributions
Conceptualization, F.H.H., R.B. and C.P.; methodology, F.H.H. and R.B.; validation, C.P., L.C. and R.B.; formal analysis, C.P.; investigation, F.H.H., R.B. and P.S.P.; resources, F.H.H., P.S.P. and R.B.; data curation, F.H.H.; writing—original draft preparation, F.H.H., P.S.P. and R.B.; writing—review and editing, C.P. and L.C.; visualization, F.H.H. and P.S.P.; supervision, C.P.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study is a review article and does not report any new data. All data discussed are cited from previously published sources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Getino, L.; Martín, J.L.; Chamizo-Ampudia, A. A Review of Polyhydroxyalkanoates: Characterization, Production, and Application from Waste. Microorganisms 2024, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Salvatori, G.; Alfano, S.; Lorini, L.; Valentino, F.; Villano, M.; Chronopoulou, L.; Palocci, C. Supercritical Carbon Dioxide-Based Approach for the Recovery and Purification of Polyhydroxyalkanoates from Mixed Microbial Cultures: A Green Approach for Bioplastics Production. J. Supercrit. Fluids 2026, 228, 106760. [Google Scholar] [CrossRef]
- Cordier, M.; Uehara, T.; Jorgensen, B.; Baztan, J. Reducing Plastic Production: Economic loss or Environmental Gain? Camb. Prism. Plast. 2024, 2, e2. [Google Scholar] [CrossRef]
- Pottinger, A.S.; Geyer, R.; Biyani, N.; Martinez, C.C.; Nathan, N.; Morse, M.R.; Liu, C.; Hu, S.; De Bruyn, M.; Boettiger, C.; et al. Pathways to Reduce Global Plastic Waste Mismanagement and Greenhouse Gas Emissions by 2050. Science 2024, 386, 1168–1173. [Google Scholar] [CrossRef]
- Lawrence-Brown, A. (Ed.) Turning off the Tap: How the World Can End Plastic Pollution and Create a Circular Economy; United Nations Environment Programme: Nairobi, Kenya, 2023; Available online: https://www.unep.org/resources/turning-off-tap-end-plastic-pollution-create-circular-economy (accessed on 16 May 2023).
- OECD. Global Plastics Outlook: Policy Scenarios to 2060; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Kelly, F.J.; Wright, S.L.; Woodward, G.; Fussell, J.C. Plastic Pollution Under the Influence of Climate Change: Implications for the Abundance, Distribution, and Hazards in Terrestrial and Aquatic Ecosystems. Front. Sci. 2025, 3, 1636665. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution; UNEP: Nairobi, Kenya, 2021; Available online: https://www.unep.org/resources/pollution-solution-global-assessment-marine-litter-and-plastic-pollution (accessed on 21 October 2021).
- Corami, F.; Iannilli, V.; Hallanger, I.G. Editorial: Microplastics and Nanoparticles in Polar Areas: Arctic, Antarctica, and the World’s Glaciers. Front. Mar. Sci. 2025, 12, 1587557. [Google Scholar] [CrossRef]
- Renuka, G.; Devi, U.A.; Srinivas, D.; Vijaya, L.V.; Ugandhar, T. The Impact of Microplastics in Food, Water and Air on Human Health Risks and Future Research. In Microplastics Pollution; Springer: Cham, Switzerland, 2025; pp. 33–66. Available online: https://link.springer.com/chapter/10.1007/978-3-031-99774-7_2 (accessed on 3 January 2025).
- Trevisan, R.; Ranasinghe, P.; Jayasyndara, N.; Di Giulio, R.T. Nanoplastics in Aquatic Environments: Impacts on Aquatic Species and Interactions with Environmental Factors and Pollulants. Toxics 2022, 10, 326. [Google Scholar] [CrossRef]
- Acosta, D.J.; Barth, D.R.; Bondy, J.; Appler, K.E.; De Anda, V.; Ngo, P.H.T.; Alper, H.S.; Baker, B.J.; Marcotte, E.M.; Ellington, A.D. Plastic Degradation by Enzymes from Uncultured Deep Sea Microorganisms. ISME J. 2025, 19, wraf068. [Google Scholar] [CrossRef] [PubMed]
- Saba, Y.; Mujahid, F.; Muhammad, Z.; Nafeesa, N.; Wardha, Z.; Zaki, U.Z.A.; Shehaayaar, F.; Shafaqat, A. Detection and Degradation of Microplastics in the Environment: A Review. Environ. Sci. Adv. 2025, 4, 1142–1165. [Google Scholar] [CrossRef]
- Gao, L.; Su, Y.; Mehmood, T.; Wang, Z.; Peng, L.; Zhang, N. UVA-Induced Weathering of Microplastics in Seawater: Surface Property Transformations and Kinetics. Front. Mar. Sci. 2025, 12, 1519668. [Google Scholar] [CrossRef]
- Pfohl, P.; Santizo, K.; Sipe, J.; Wiesner, M.; Harrison, S.; Svendsen, C.; Wohlleben, W. Environmental Degradation and Fragmentation of Microplastics: Dependence on Polymer Type, Humidity, UV Dose and Temperature. Micropl. Nanopl. 2025, 5, 7. [Google Scholar] [CrossRef]
- Statista Research Department. Microplastics Worldwide—Statistics & Facts. Statista. 2025. Available online: https://www.statista.com/topics/13600/microplastics-worldwide/ (accessed on 17 January 2025).
- Parray, J.A.; Li, W.-J. Mechanism of actions of microbes for degradation of plastics. In Synthesis Lectures on Chemical Engineering and Biochemical Engineering; Springer: Berlin/Heidelberg, Germany, 2025; pp. 35–54. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Maerz, L. Embracing Bioplastics: A Sustainable Alternative to Fossil Fuel-Based Plastics. University of Washington PCC Blog. 2024. Available online: https://pcc.uw.edu/2024/08/27/embracing-bioplastics-a-sustainable-alternative-to-fossil-fuel-based-plastics/ (accessed on 27 August 2024).
- Sharma, S.; Kumar, G.; Bhardwaj, S.K.; Chauhan, A.; Rathi, A.; Kumar, J.; Sahil, K.; Vishavamitera, S. Bioplastics as Next-Generation Eco-Friendly Materials: A Comprehensive Review. Clean Technol. Environ. Policy 2025, 27, 5009–5027. [Google Scholar] [CrossRef]
- Dai, Y.; Xia, Q.; Mao, Z.M.; Mu, J.; Peng, F.; Hao, X. Sustainable Bioplastics Build on D-Xylose Cores: From Backup to the Center Stage. Green Chem. 2025, 27, 4464–4488. [Google Scholar] [CrossRef]
- Samer, M.; Amer, M. Introductory Chapter: Bioplastics as Substitutes to Petroplastics. In Bioplastics within the Circular Bioeconomy; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Paloyan, A.; Tadevosyan, M.; Ghevondyan, D.; Khoyetsyan, L.; Karapetyan, M.; Margaryan, A.; Antranikian, G.; Panosyan, H. Biodegradation of Polyhydroxyalkanoates: Current State and Future Prospects. Front. Microbiol. 2025, 16, 1542468. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and Advances Toward Biotechnological Applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, R.; Wang, Z.; Deng, Y.; Chen, G.-Q. Application of (R)-3-Hydroxyalkanoate Methyl Esters Derived from Microbial Polyhydroxyalkanoates as Novel Biofuels. Biomacromolecules 2009, 10, 707–711. [Google Scholar] [CrossRef]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the Application of Polyhydroxyalkanoates Nanoparticles for Novel Drug Delivery System. BioMed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, Production, Recent Developments and Applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Reddy, V.U.N.; Ramanaiah, S.V.; Reddy, M.V.; Chang, Y.-C. Review of the Developments of Bacterial Medium-Chain-Length Polyhydroxyalkanoates (mcl-PHAs). Bioengineering 2022, 9, 225. [Google Scholar] [CrossRef]
- Basnett, P.; Lukasiewicz, B.; Marcello, E.; Gura, H.K.; Knowles, J.C.; Roy, I. Production of a Novel Medium Chain Length Poly(3-hydroxyalkanoate) Using Unprocessed Biodiesel Waste and Its Evaluation as a Tissue Engineering Scaffold. Microb. Biotechnol. 2017, 10, 1384–1399. [Google Scholar] [CrossRef]
- Aldor, I.S.; Kaesling, J.D. Process Design for Microbial Plastic Factories: Metabolic Engineering of Polyhydroxyalkanoates. Curr. Opin. Biotechnol. 2003, 14, 475–483. [Google Scholar] [CrossRef]
- Song, H.M.; Joo, J.C.; Lim, S.H.; Lim, H.J.; Lee, S.; Park, S.J. Production of Polyhydroxyalkanoates Containing Monomers Conferring Amorphous and Elastomeric Properties from Renewable Resources: Current Status and Future Perspectives. Bioresour. Technol. 2022, 366, 128114. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoates (PHA): Review of Synthesis, Characterization, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- SpecialChem. Polyhydroxyalkanoates (PHAs): Types, Properties, Chemical Structure & Toxicity. In SpecialChem Plastics Guide; SpecialChem: Paris, France, 2025; Available online: https://www.specialchem.com/plastics/guide/polyhydroxyalkanoate-pha (accessed on 14 January 2025).
- Surendran, A.; Lakshmanan, M.; Chee, J.Y.; Sulaiman, A.M.; Van Thuoc, D.; Sudesh, K. Can Polyhydroxyalkanoates Be Produced Efficiently from Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Guzik, M.; Witko, T.; Steinbüchel, A.; Wojnarowska, M.; Sołtysik, M.; Wawak, S. What Has Been Trending in the Research of Polyhydroxyalkanoates? A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 959. [Google Scholar] [CrossRef]
- Mahato, R.P.; Kumar, S.; Singh, P. Production of Polyhydroxyalkanoates from Renewable Resources: A Review on Prospects, Challenges and Applications. Arch. Microbiol. 2023, 205, 172. [Google Scholar] [CrossRef]
- Bano, S.; Aslam, A.A.; Khan, A.; Shabbir, A.; Qayyum, F.; Wahab, N.; Jabar, A.; Ul Islam, I.; Ng, S.L. A Mini-Review on Polyhydroxyalkanoates: Synthesis, Extraction, Characterization, and Applications. Process Biochem. 2024, 146, 250–261. [Google Scholar] [CrossRef]
- Thamarai, P.; Vickram, A.S.; Saravanan, A.; Deivayanai, V.C.; Evangeline, S. Recent Advancements in Biosynthesis, Industrial Production, and Environmental Applications of Polyhydroxyalkanoates (PHAs): A Review. Bioresour. Technol. Rep. 2024, 27, 101957. [Google Scholar] [CrossRef]
- Pandey, A.; Adama, N.; Adjallé, K.; Blais, J.-F. Sustainable Applications of Polyhydroxyalkanoates in Various Fields: A Critical Review. Int. J. Biol. Macromol. 2022, 221, 1184–1201. [Google Scholar] [CrossRef]
- Chalermthai, B.; Ashraf, M.T.; Bastidas-Oyanedel, J.-R.; Olsen, B.D.; Schmidt, J.E.; Taher, H. Techno-Economic Assessment of Whey Protein-Based Plastic Production from a Co-Polymerization Process. Polymers 2020, 12, 847. [Google Scholar] [CrossRef]
- Ramos, L.H.; Cisneros-Yupanqui, M.; Santisteban Soto, D.V.; Lante, A.; Favaro, L.; Casella, S.; Basaglia, M. Exploitation of Cocoa Pod Residues for the Production of Antioxidants, Polyhydroxyalkanoates, and Ethanol. Fermentation 2023, 9, 843. [Google Scholar] [CrossRef]
- Madi, F.; Hachicha, R.; Gamero, J.E.R.; Gupte, A.P.; Gronchi, N.; Haddad, M.; Favaro, L.; Casella, S.; Basaglia, M. Exploitation of Spoilage Dates as Biomass for the Production of Bioethanol and Polyhydroxyalkanoates. Renew. Energy 2024, 220, 119655. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Francini, G.; Lombardi, L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Environmental Life Cycle Assessment of Polyhydroxyalkanoates Production from Cheese Whey. Waste Manag. 2021, 132, 31–43. [Google Scholar] [CrossRef]
- Colombo, B.; Calvo, M.V.; Sciarria, T.P.; Scaglia, B.; Kizito, S.S.; D’Imporzano, G.; Adani, F. Biohydrogen and Polyhydroxyalkanoates (PHA) as Products of a Two-Steps Bioprocess from Deproteinized Dairy Wastes. Waste Manag. 2019, 95, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P. Grape Winery Waste as Feedstock for Bioconversions: Applying the Biorefinery Concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape Winery Waste as a Promising Feedstock for the Production of Polyhydroxyalkanoates and Other Value-Added Products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Andler, R.; González-Arancibia, F.; Vilos, C.; Sepulveda-Verdugo, R.; Castro, R.; Mamani, M.; Valdés, C.; Arto-Paz, F.; Díaz-Barrera, A.; Martínez, I. Production of Poly-3-Hydroxybutyrate (PHB) Nanoparticles Using Grape Residues as the Sole Carbon Source. Int. J. Biol. Macromol. 2024, 261, 129649. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Rodriguez, J.E.G.; Morelli, A.; Ibraheem, O.; Pizzocchero, V.; Casella, S. Bacterial Production of PHAs from Lipid-Rich By-Products. Appl. Food Biotechnol. 2019, 6, 45–52. [Google Scholar] [CrossRef]
- Giovanna Romanelli, M.; Povolo, S.; Favaro, L.; Fontana, F.; Basaglia, M.; Casella, S. Engineering Delftia acidovorans DSM39 to Produce Polyhydroxyalkanoates from Slaughterhouse Waste. Int. J. Biol. Macromol. 2014, 71, 21–27. [Google Scholar] [CrossRef]
- Khdair, A.I.; Abu-Rumman, G.; Khdair, S.I. Pollution Estimation from Olive Mills Wastewater in Jordan. Heliyon 2019, 5, e02386. [Google Scholar] [CrossRef] [PubMed]
- Bacha, S.; Arous, F.; Chouikh, E.; Jaouani, A.; Gtari, M.; Charradi, K.; Attia, H.; Ghorbel, D. Exploring Bacillus amyloliquefaciens Strain OM81 for the Production of Polyhydroxyalkanoate (PHA) Bioplastic Using Olive Mill Wastewater. 3 Biotech 2023, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of Polyhydroxyalkanoates from Waste Frying Oil by Cupriavidus necator. AMB Express 2011, 1, 11. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar Beet Molasses as a Potential C-Substrate for PHA Production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the Polyhydroxyalkanoate Biopolymer by Cupriavidus necator Using Beer Brewery Wastewater Containing Maltose as a Primary Carbon Source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Schmid, M.T.; Song, H.; Raschbauer, M.; Emerstorfer, F.; Omann, M.; Stelzer, F.; Neureiter, M. Utilization of Desugarized Sugar Beet Molasses for the Production of Poly(3-Hydroxybutyrate) by Halophilic Bacillus megaterium Uyuni S29. Process Biochem. 2019, 86, 9–15. [Google Scholar] [CrossRef]
- Tyagi, B.; Takkar, S.; Meena, R.; Thakur, I.S. Production of Polyhydroxybutyrate (PHB) by Parapedobacter sp. ISTM3 Isolated from Mawsmai Cave Utilizing Molasses as Carbon Source. Environ. Technol. Innov. 2021, 24, 101854. [Google Scholar] [CrossRef]
- Li, D.; Ma, X.; Li, J.; Sun, B. Insights Into Enhanced Polyhydroxyalkanoate Production by the Synergistic Use of Waste Wood Hydrolysate and Volatile Fatty Acids by Mixed Microbial Cultures. Bioresour. Technol. 2021, 337, 125488. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Su, Y.; Yan, X.; Wang, F.; Yu, L.; Ma, X. Efficient and Economical Production of Polyhydroxyalkanoate from Sustainable Rubber Wood Hydrolysate and Xylose as Co-Substrate by Mixed Microbial Cultures. Bioresour. Technol. 2022, 355, 127238. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, M.; Cai, C.; Chen, S.; Jin, M. Microbial Polyhydroxyalkanoate Production from Lignin by Pseudomonas putida NX-1. Bioresour. Technol. 2021, 319, 124210. [Google Scholar] [CrossRef]
- Colombo, B.; Pereira, J.; Martins, M.; Torres-Acosta, M.A.; Dias, A.C.R.V.; Lemos, P.C.; Ventura, S.P.M.; Eisele, G.; Alekseeva, A.; Adani, F.; et al. Recovering PHA from Mixed Microbial Biomass: Using Non-Ionic Surfactants as a Pretreatment Step. Sep. Purif. Technol. 2020, 253, 117521. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Eugenia Suárez-Ojeda, M. Recovery of Polyhydroxyalkanoates (PHAs) from Wastewater: A Review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; Pablo López-Gómez, J.; Roy, I. A Sustainable Approach for the Downstream Processing of Bacterial Polyhydroxyalkanoates: State-of-the-Art and Latest Developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B.; Alemtoshi. PHA-Based Bioplastic: A Potential Alternative to Address Microplastic Pollution. Water Air Soil Pollut. 2023, 234, 21. [Google Scholar] [CrossRef]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Najar, I.N.; Sharma, P.; Das, R.; Mondal, K.; Singh, A.K.; Tamang, S.; Hazra, P.; Thakur, N.; Bhanwaria, R.; Gandhi, S.G.; et al. In Search of Poly-3-Hydroxybutyrate (PHB): A Comprehensive Review Unveiling Applications and Progress in Fostering a Sustainable Bio-Circular Economy. Food Bioprod. Process. 2024, 148, 11–30. [Google Scholar] [CrossRef]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) Synthesis and Degradation by Microbes and Applications Towards a Circular Economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Ansari, S.; Sami, N.; Yasin, D.; Ahmad, N.; Fatma, T. Biomedical Applications of Environmental Friendly Poly-Hydroxyalkanoates. Int. J. Biol. Macromol. 2021, 183, 549–563. [Google Scholar] [CrossRef]
- Waghamare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch Based Nanofibrous Scaffolds for Wound Healing Applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional Neuronal Cell Growth and Differentiation on Aligned Polyhydroxyalkanoate Blend Microfibres with Varying Diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef]
- Balogová, A.F.; Hudák, R.; Tóth, T.; Schnitzer, M.; Feranc, J.; Bakoš, D.; Živčák, J. Determination of Geometrical and Viscoelastic Properties of PLA/PHB Samples Made by Additive Manufacturing for Urethral Substitution. J. Biotechnol. 2018, 284, 123–130. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, Synthesis and Medical Application of Bacterial Polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Marques-Calvo, M.S.; Gil, F.J.; Manoero, J.S. Modification of Titanium Surfaces by Adding Antibiotic-Loaded PHB Spheres and PEG for Biomedical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 124. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Khulsov, I.A.; Volova, T.G. A Hybrid PHB-Hydroxyapatite Composite for Biomedical Application: Production, in Vitro and in Vivo Investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kose, G.T.; Korkusuz, F.; Korkusuz, P.; Hasirci, V. In Vivo Tissue Engineering of Bone Using Poly(3-Hydroxybutyric Acid-co-3-Hydroxyvaleric Acid) and Collagen Scaffolds. Tissue Eng. 2004, 10, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, W.; Muller, T. Electrospun Polyhydroxybutyrate/Poly(ε-Caprolactone)/58S Sol-Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef]
- Krobot, Š.; Melčová, V.; Menčík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Mojžišová, E.; Baco, A.; Přikryl, R. Poly(3-hydroxybutyrate) (PHB) and Polycaprolactone (PCL) Based Blends for Tissue Engineering and Bone Medical Applications Processed by FDM 3D Printing. Polymers 2023, 15, 2404. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Y.; Yang, H.; Chen, X.; Chen, Z. Characteristic Comparison of Bioactive Scaffolds Based on Polyhydroxyalkanoate/Bioceramic Hybrids. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 236–243. [Google Scholar] [CrossRef]
- Li, H.; Zhai, W.; Chang, J. In Vitro Biocompatibility Assessment of PHBV/Wollastonite Composites. J. Mater. Sci. Mater. Med. 2008, 19, 67–73. [Google Scholar] [CrossRef]
- Chen, L.J.; Wang, M. Production and Evaluation of Biodegradable Composite Based on PHB-PHV Copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly Porous PHB-Based Bioactive Scaffolds for Bone Tissue Engineering by In Situ Synthesis of Hydroxyapatite. Mater. Sci. Eng. C 2019, 100, 286–296. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.T.; Bonfield, W. In Vitro and In Vivo Evaluation of Polyhydroxybutyrate and of Polyhydroxybutyrate Reinforced with Hydroxyapatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- 2006 Bio Micro and Nanosystems Conference; IEEE Cat. No. 06EX1265C; IEEE: New York, NY, USA, 2006.
- Gursel, I.; Yagmurlu, F.; Korkusuz, F.; Hasirici, V. In Vitro Antibiotic Release from Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Rods. J. Microencapsul. 2008, 19, 153–164. [Google Scholar] [CrossRef]
- Luklinska, Z.B.; Schluckweder, H. In Vivo Response to HA-Polyhydroxybutyrate/Polyhydroxyvalerate Composite. J. Microsc. 2003, 211, 121–129. [Google Scholar] [CrossRef]
- Jack, K.S.; Velayudhan, S.; Luckman, P.; Trau, M.; Grondahl, L.; Cooper-White, J. The Fabrication and Characterization of Biodegradable HA/PHBV Nanoparticle-Polymer Composite Scaffolds. Acta Biomater. 2009, 5, 2657–2667. [Google Scholar] [CrossRef]
- Huang, W.; Shi, X.; Ren, L.; Du, C.; Wang, Y. PHBV Microspheres—PLGA Matrix Composite Scaffold for Bone Tissue Engineering. Biomaterials 2010, 31, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, Y.; Liu, T.; Chen, Z.; Chen, W.; Wu, Z.; Wang, Y.; Li, J.; Li, T.; Jiang, T.; et al. Beta-Tricalcium Phosphate Enhanced Mechanical and Biological Properties of 3D-Printed Polyhydroxyalkanoates Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 209, 1553–1561. [Google Scholar] [CrossRef]
- Injorhor, P.; Trongsatitkul, T.; Wittayakun, J.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Biodegradable Polylactic Acid-Polyhydroxyalkanoate-Based Nanocomposites with Bio-Hydroxyapatite: Preparation and Characterization. Polymers 2023, 15, 1261. [Google Scholar] [CrossRef] [PubMed]
- Marcello, E.; Maqbool, M.; Nigmatullin, R.; Cresswell, M.; Jackson, P.R.; Basnett, P.; Knowles, J.; Boccaccini, A.R.; Roy, I. Antibacterial Composite Materials Based on the Combination of Polyhydroxyalkanoates with Selenium and Strontium Co-Substituted Hydroxyapatite for Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 647007. [Google Scholar] [CrossRef]
- Raziyan, M.S.; Palevicius, A.; Perkowski, D.; Urbaite, S.; Janusas, G. Development and Evaluation of 3D-Printed PLA/PHA/PHB/HA Composite Scaffolds for Enhanced Tissue-Engineering Applications. J. Compos. Sci. 2024, 8, 226. [Google Scholar] [CrossRef]
- Nahanmoghadam, A.; Asemani, M.; Goodarzi, V.; Ebrahimi-Barough, S. In Vivo Investigation of PCL/PHBV/Hydroxyapatite Nanocomposite Scaffold in Regeneration of Critical-Sized Bone Defects. Fibers Polym. 2021, 22, 2507–2516. [Google Scholar] [CrossRef]
- Sarrami, P.; Karbasi, S.; Farahbakhsh, Z.; Bigham, A.; Rafienia, M. Fabrication and Characterization of Novel Polyhydroxybutyrate-Keratin/Nanohydroxyapatite Electrospun Fibers for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2022, 220, 1368–1389. [Google Scholar] [CrossRef] [PubMed]
- Dukle, A.; Sankar, M.R. 3D-Printed Polylactic Acid Scaffolds for Bone Tissue Engineering: Bioactivity Enhancing Strategies Based on Composite Filaments and Coatings. Mater. Today Commun. 2024, 40, 109776. [Google Scholar] [CrossRef]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Duhan, A.A.; et al. Poly(3-hydroxybutyrate)/Hydroxyapatite/Alginate Scaffolds Seeded with Mesenchymal Stem Cells Enhance the Regeneration of Critical/Sized Bone Defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef]
- Karbowniczek, J.E.; Kaniuk, L.; Berniak, K.; Gruszczynski, A.; Stachewicz, U. Enhanced Cells Anchoring to Electrospun Hybrid Scaffolds with PHBV and HA Particles for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 632029. [Google Scholar] [CrossRef]
- Sadat-Shojai, M. Electrospun Polyhydroxybutyrate/Hydroxyapatite Nanoparticles: Microstructure and Bone Cell-Response. J. Mater. Sci. Technol. 2016, 32, 1013–1020. [Google Scholar] [CrossRef]
- Senatov, F.; Anisimova, N.; Kiselevskiy, M.; Kopylov, A.; Tcherdyntsev, V.; Maksimkin, A. Polyhydroxybutyrate/Hydroxyapatite Highly Porous Scaffold for Small Bone Defects Replacement in the Nonload-Bearing Parts. J. Bionic Eng. 2017, 14, 648–658. [Google Scholar] [CrossRef]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Biocomposite Scaffolds for Bone Regeneration: Role of Chitosan and Hydroxyapatite Within Poly-3-Hydroxybutyrate-co-3-Hydroxyvalerate on Mechanical Properties and in Vitro Evaluation. J. Mech. Behav. Biomed. Mater. 2015, 51, 88–98. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. A New Strategy for Fabrication of Bone Scaffolds Using Electrospun Nano-Hap/PHB Fibers and Protein Hydrogels. Chem. Eng. J. 2016, 289, 38–47. [Google Scholar] [CrossRef]
- Gunes, Q.C.; Albayrak, A.Z.; Tasdemir, S.; Sendemir, A. Wet-Electrospun PHBV Nanofiber Reinforced Carboxymethyl Chitosan-Silk Hydrogel Composite Scaffolds for Articular Cartilage Repair. J. Biomater. Appl. 2020, 35, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Cano-Vicent, A.; Tunon-Molina, A.; Aparicio-Collado, J.L.; Salesa, B.; Serra, R.S.; Serrano-Aroca, A. Engineering Alginate Hydrogel Films with Poly(3-Hydroxybutyrate-co-3-Valerate) and Graphene Nanoplatelets: Enhancement of Antiviral Activity, Cell Adhesion and Electroactive Properties. Int. J. Biol. Macromol. 2022, 219, 694–708. [Google Scholar] [CrossRef]
- Baysan, G.; Gunes, O.C.; Akokay, P.; Husemoglu, R.B.; Ertugrulogu, P.; Albayrak, A.Z.; Cecen, B.; Avitcioglu, H. Loofah-Chitosan and Poly(-3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBV) Based Hydrogels Scaffolds for Meniscus Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 221, 1171–1183. [Google Scholar] [CrossRef]
- Pérez-Recalde, M.; Pacheco, E.; Aráoz, B.; Hermida, É.B. Effects of Polyhydroxybutyrate-co-hydroxyvalerate Microparticle Loading on Rheology, Microstructure, and Processability of Hydrogel-Based Inks for Bioprinted and Moulded Scaffolds. Gels 2025, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Getova, V.E.; Pascual, A.; Dijkstra, R.; Gładysz, M.Z.; Ubels, D.; Wlodarczyk-Biegun, M.K.; Burgess, J.K.; Siebring, J.; Harmsen, M.C. Multilayered Tissue Assemblies Through Tuneable Biodegradable Polyhydroxyalkanoate Polymer (Mesh)-Reinforced Organ-Derived Extracellular Matrix Hydrogels. Gels 2025, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Motiee, E.-S.; Shariati, L.; Karbasi, S. Evaluating the Osteogenic Potential of Reduced Graphene Oxide-Reinforced Poly(3-Hydroxybutyrate)-Chitosan Composite Scaffold for Bone Tissue Regeneration Applications. J. Polym. Sci. 2025, 63, 3323–3349. [Google Scholar] [CrossRef]
- Petrovova, E.; Tomco, M.; Holovska, K.; Danko, J.; Kresakova, L.; Vdoviakova, K.; Simaiova, V.; Kolvek, F.; Hornakova, P.; Toth, T.; et al. PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study. Polymers 2021, 13, 1232. [Google Scholar] [CrossRef]
- Ferri, M.; Chiromito, E.M.S.; de Carvalho, A.J.F.; Morselli, D.; Degli Esposti, M.; Fabbri, P. Fine Tuning of the Mechanical Properties of Bio-Based PHB/Nanofibrillated Cellulose Biocomposites to Prevent Implant Failure Due to the Bone/Implant Stress Shielding Effect. Polymers 2023, 15, 1438. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.-C.; et al. Bacterial Cellulose-Modified Polyhydroxyalkanoates Scaffolds Promotes Bone Formation in Critical Size Calvarial Defects in Mice. Materials 2025, 13, 4361. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Che, X.; Yu, L.; Jia, W.; Shen, R.; Chen, J.; Ma, Y.; Chen, G.-Q. Synthesis and Characterization of Polyhydroxyalkanoate Organo/Hydrogels. Biomacromolecules 2019, 20, 3303–3312. [Google Scholar] [CrossRef]
- Sandar, D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Fabrication of Chitin/Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Hydrogel Scaffold. Carbohydr. Polym. 2012, 90, 725–729. [Google Scholar] [CrossRef]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite Hydrogel of Chitosan-Poly(Hydroxybutyrate-co-Valerate) with Chondroitin Sulfate Nanoparticles for Nucleus Pulposus Tissue Engineering. Colloids Surf. B Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, Proliferation and Differentiation of Osteoblasts on Random Biopolyester Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate) Scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Zhu, S. Studies on Bone Marrow Stromal Cells Affinity of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate. Biomaterials 2004, 25, 421–428. [Google Scholar] [CrossRef]
- Lomas, A.J.; Webb, W.R.; Han, J.F.; Chen, G.-Q.; Sun, X.; El Haj, A.J.; Forsyth, N.R. Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate)/Collagen Hybrid Scaffolds for Tissue Engineering Applications. Tissue Eng. Part C Methods 2013, 19, 577–585. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, Q.; Chen, G.Q. Gelatin Blending Improves the Performance of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate) Films for Biomedical Applications. Biomacromolecules 2005, 6, 566–571. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Wei, X.; Zhao, W.; Liu, Y.-S.; Chen, G.-Q. Biocompatibility of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate-co-3-Hydroxyhexanoate) with Bone Marrow Mesenchymal Stem Cells. Acta Biomater. 2009, 5, 1115–1125. [Google Scholar] [CrossRef]
- Uddin, M.M.; Jahan, U.M.; Mohakar, V.; Talukder, A.; Absalan, Y.; Blevins, B.; Yadavalli, N.S.; Reukov, V.; Minko, S.; Sharma, S. Fabrication of Algined Polyhydroxybutyrate Fibrous Scaffolds Via a Touchspinning Apparatus. ACS Omega 2025, 10, 22735–22746. [Google Scholar] [CrossRef]
- Sriram, M.; Priya, S.; Katti, D.S. Polyhydroxybutyrate-Based Osteoinductive Mineralized Electrospun Structures that Mimic Components and Tissue Interfaces of the Osteon for Bone Tissue Engineering. Biofabrication 2024, 16, 025036. [Google Scholar] [CrossRef]
- Raza, Z.A.; Naeem, A.R.; Shafi, R.; Abid, S. Chitosan-Incorporated Poly(Hydroxybutyrate) Porous Electrospun Scaffold for Potential Biomedical Applications. Polym. Bull. 2024, 81, 1691–1705. [Google Scholar] [CrossRef]
- Abdollahi, M.; Karbasi, S.; Beigi-Boroujeni, S.; Benisi, S.Z.; Saeed, M. Polyhydroxybutyrate-Starch/Carbon Nanotube Electrospun Nanocomposite: A Highly Potential Scaffold for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 223, 524–542. [Google Scholar] [CrossRef]
- Mohammadalipour, M.; Behzad, T.; Karbasi, S.; Khorzoghi, M.B.; Mohammadalipour, Z. Osteogenic Potential of PHB-Lignin/Cellulose Nanofiber Electrospun Scaffold as a Novel Bone Regeneration Construct. Int. J. Biol. Macromol. 2023, 250, 126076. [Google Scholar] [CrossRef]
- Ghasemi, S.; Alibabaie, A.; Saberi, R.; Esmaeili, M.; Semnani, D.; Karbasi, S. Evaluation of the Effects of Zein Incorporation on Physical, Mechanical, and Biological Properties of Polyhydroxybutyrate Electrospun Scaffold for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2023, 253, 126843. [Google Scholar] [CrossRef]
- Abdollahi, M.; Karbasi, S.; Beigi-Boroujeni, S.; Benisi, S.Z.; Saeed, M. Evaluation of the Effects of Starch on Polyhydroxybutyrate Electrospun Scaffolds for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2021, 191, 500–513. [Google Scholar] [CrossRef]
- Solarz, D.; Witki, T.; Karcz, R.; Malagurski, I.; Ponjavic, M.; Levc, S.; Nesic, A.; Guzik, M.; Savic, S.; Nikodinovic-Runic, J. Biological and Physiochemical Studies of Electrospun Polylactid/Polyhydroxyoctanoate PLA/P(3HO) Scaffolds for Tissue Engineering Applications. RSC Adv. 2023, 13, 24112–24128. [Google Scholar] [CrossRef]
- Jin, A.; Pérez, G.; Martínez de Ilarduya, A.; del Valle, L.J.; Puiggalí, J. Characterization and Biomedical Applications of Electrospun PHBV Scaffolds Derived from Organic Residues. Int. J. Mol. Sci. 2025, 26, 180. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.; Chen, S.; Wu, S. Designing an Innovative Electrospinning Strategy to Generate PHBV Nanofiber Scaffolds with a Radially Oriented Fibrous Pattern. Nanomaterials 2023, 13, 1150. [Google Scholar] [CrossRef]
- Pryadko, A.S.; Mukhortova, Y.R.; Chernozem, R.V.; Shlapakova, L.E.; Wagner, D.V.; Romanyuk, K.; Gerasimov, E.Y.; Kholkin, A.; Surmenev, R.A.; Surmeneva, M.A. Comprehensive Study on the Reinforcement of Electrospun PHB Scaffolds with Composite Magnetite Fe3O4-rGO Fillers: Structure, Physico-Mechanical Properties, and Piezoelectric Response. ACS Omega 2022, 7, 41392–41411. [Google Scholar] [CrossRef]
- Cortes-Ortiz, E.; Olayo-Valles, R.; Rodriguez-Talavera, R.; Gonzalez-Torres, M.; Vargas-Munoz, S.; Olayo, R.; Godinez-Fernandez, R.; Juarez, O.E.U.; Morales-Corona, J. Plasma Functionalized Scaffolds of Polyhydroxybutyrate Electrospun Fibers for Pancreatic Beta Cell Cultures. Front. Mater. 2021, 8, 600738. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Ola, K.A.A.; Al-Qahtani, S.D. Development of Hydroxyapatite/Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Gelatin Composite Nanofibers to Improve Bone-Forming Cell Proliferation and Mineral Deposition. Polym. Eng. Sci. 2024, 64, 5831–5841. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Valentino, A.; Bonadies, I.; de Almeida, Y.M.B.; Duraccio, D.; Calarco, A.; d’Ayala, G.G. Electrospun Membranes of Polyhydroxybutyrate-Niobium Pentoxide as Potential Scaffolds for Bone Tissue Engineering. Mater. Today Commun. 2025, 49, 114177. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, Y.; Li, Z. Injectable Cell- and Growth Factor-Free Poly(4-Hydroxybutyrate) (P4HB) Microsphere with Open Porous Structures and Great Efficiency of Promoting Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 4432–4440. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Dai, Z.-W.; Chen, S.-Y.; Guo, W.-X.; Wang, Z.-W.; Wei, J.-S. A Novel Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBV)-PEG-Melatonin Composite Scaffold Enhances for Inhibiting Bone Tumor Recurrence and Enhancing Bone Regeneration. Front. Pharmacol. 2023, 14, 1246783. [Google Scholar] [CrossRef]
- Strangis, G.; Labardi, M.; Gallone, G.; Milazzo, M.; Capaccioli, S.; Forli, F.; Cinelli, P.; Berrettini, S.; Seggiani, M.; Danti, S.; et al. 3D Printed Piezoelectric BaTiO3/Polyhydroxybutyrate Nanocomposite Scaffolds for Bone Tissue Engineering. Bioengineering 2024, 11, 193. [Google Scholar] [CrossRef]
- Chaber, P.; Tylko, G.; Włodarczyk, J.; Nitschke, P.; Hercog, A.; Jurczyk, S.; Rech, J.; Kubacki, J.; Adamus, G. Surface Modification of PHBV Fibrous Scaffold via Lithium Borohydride Reduction. Materials 2022, 15, 7494. [Google Scholar] [CrossRef]
- Kaewkong, P.; Kosorn, W.; Sonthithai, P.; Lertwimol, T.; Thavornyutikarn, B.; Chantaweroad, S.; Janvikul, W. Chondrogenic Differentiation of Human Mesenchymal Stem Cells and Macrophage Polarization on 3D-Printed Poly(ε-Caprolactone)/Poly(3-Hydroxubutyrate-co-3-Hydroxyvalerate) Blended Scaffolds with Different Secondary Porous Structures. ACS Appl. Bio Mater. 2022, 5, 2689–2702. [Google Scholar] [CrossRef]
- Sabouri, E.; Rezaie, Z.; Enderami, S.E.; Mirahmadi, M.; Askari, M. Different Osteoconductivity of PLLA/PHB Composite Nanofibers Prepared by One- and Two-Nozzle Electrospinning. Polym. Adv. Technol. 2021, 32, 1783–1792. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Liow, S.S.; Loh, X.J. New Dual Functional PHB-Grafted Lignin Copolymer: Synthesis, Mechanical Properties, and Biocompatibility Studies. ACS Appl. Bio Mater. 2019, 2, 127–132. [Google Scholar] [CrossRef]
- Schulz, R.M.; Bader, A. Cartilage Tissue Engineering and Bioreactor Systems for the Cultivation and Stimulation of Chondrocytes. Eur. Biophys. J. 2007, 36, 539–568. [Google Scholar] [CrossRef]
- LeBaron, R.G.; Athanasiou, K.A. Ex Vivo Synthesis of Articular Cartilage. Biomaterials 2000, 21, 2575–2587. [Google Scholar] [CrossRef]
- Gong, Y.; He, L.; Li, J.; Zhou, Q.; Ma, Z.; Gao, C.; Shen, J. Hydrogel-Filled Polylactide Porous Scaffolds for Cartilage Tissue Engineering. J. Biomed. Mater. Res. Appl. Biomater. 2007, 82, 192–204. [Google Scholar] [CrossRef]
- Moutos, F.T.; Freed, L.F.; Guilak, F. A Biomimetic Three-Dimensional Woven Composite Scaffold for Functional Tissue Engineering of Cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef]
- Hussain, M. Continuing Differentiation of Human Mesenchymal Stem Cells and Induced Chondrogenic and Osteogenic Lineages in Electrospun PLGA Nanofiber Scaffold. Biomaterials 2007, 28, 316–325. [Google Scholar] [CrossRef]
- Galois, L.; Hutasse, S.; Cortial, D.; Rousseau, C.F.; Grossin, L.; Ronziere, M.-C.; Herbage, D.; Freyria, A.-M. Bovine Chondrocyte Behaviour in Three-Dimensional Type I Collagen Gel in Terms of Gel Contraction, Proliferation and Gene Expression. Biomaterials 2006, 27, 79–90. [Google Scholar] [CrossRef]
- Wang, Y.; Blasioli, D.J.; Kim, H.-J.; Kim, H.S.; Kaplan, D.L. Cartilage Tissue Engineering with Silk Scaffolds and Human Articular Chondrocytes. Biomaterials 2006, 27, 4434–4442. [Google Scholar] [CrossRef]
- Xia, W.; Liu, W.; Cui, L.; Liu, Y.; Zhong, W.; Liu, D.; Wu, J.; Chua, K.; Cao, Y. Tissue Engineering of Cartilage with the use of Chitosan-Gelatin Complex Scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 373–380. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.; Li, H.; Chang, J. Macroporous Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Matrices for Cartilage Tissue Engineering. Eur. Polym. J. 2005, 41, 2443–2449. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric Materials for Bone and Cartilage Repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.-Q. Polyhydroxyalkanoate (PHA) Scaffolds with Good Mechanical Properties and Biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, K.; Zhang, X.F.; Hu, P.; Chen, G.Q. Study on the Three-Dimensional Proliferation of Rabbit Articular Cartilage-Derived Chondrocytes on Polyhydroxyalkanoate Scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Huang, Y.; Dai, Y.; Xi, T.; Zhou, Z.; Liu, H. Quercetin Modified Electrospun PHBV Fibrous Scaffold Enhances Cartilage Regeneration. J. Mater. Sci. Mater. Med. 2021, 32, 92. [Google Scholar] [CrossRef]
- Baysan, G.; Yilmaz, P.A.; Albayrak, A.Z.; Havitcioglu, H. Loofah and Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBV) Nano-Fiber-Reinforced Chitosan Hydrogel Composite Scaffolds with Elderberry (Sambucus nigra) and Hawthorn (Crataegus oxyacantha) Extracts as Additive for Osteochondral Tissue Engineering Applications. Polym. Bull. 2024, 81, 10255–10276. [Google Scholar] [CrossRef]
- Xue, K.; Zhang, S.; Ge, J.; Wang, Q.; Qi, L.; Liu, K. Integration of Bioglass Into PHBV Constructed Tissue-Engineered Cartilages to Improve Chondrogenic Properties of Cartilage Progenitor Cells. Front. Bioeng. Biotechnol. 2022, 10, 868719. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, R.; Zhou, Z.; Liu, H.; Dai, Y.; Hu, Y. Ginsenoside Rg1-Modified PHBV Fibrous Scaffold Reduces Interleukin-1 Beta-Induced Dedifferentiation of Articular Chondrocytes. J. Appl. Polym. Sci. 2019, 137, 48378. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Señarís-Rodríguez, J.; Piñeiro-Ramil, M.; Cabarcos-Mouzo, L.; Veiga-Barbazán, M.d.C.; Mejide-Faílde, R.M.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Díaz-Prado, S.M. Encapsulation of Transforming Growth Factor-β3 in Poly(hydroxybutyrate-co-hydroxyvalerate) Nanoparticles for Enhanced Cartilage Tissue Engineering. Int. J. Mol. Sci. 2025, 26, 4997. [Google Scholar] [CrossRef]
- Meenakshi Sundaram, R.S.; Rupert, S.; Srinivasan, P.; Sathyanesan, J.; Govarthanan, K.; Jeyaraman, N.; Ramasubramanian, S.; Jeyaraman, M.; Chung, H.Y.; Gangadaran, P.; et al. Decoding Cytokine Dynamics: Wharton’s Jelly Stromal Cells and Chondro-Differentiates in PHA-Stimulated Co-Culture. Cells 2025, 14, 174. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, Mechanical and Biological Evaluation of Poly(3-Hydroxybutyrate)-Chitosan/MWNTs as a Novel Electrospun Scaffold for Cartilage Tissue Engineering Applications. Polym.-Plast. Technol. Mater. 2020, 59, 429–471. [Google Scholar] [CrossRef]
- Jaicy, J.; Namdev, M.; Choppadandi, M.; Piyush, G.; Kiran, K.; Govinda, K. Smart Piezoelectric Nanohybrid of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) and Barium Titanate for Stimulated Cartilage Regeneration. ACS Appl. Bio Mater. 2019, 2, 4922–4931. [Google Scholar] [CrossRef]
- Morosco, G.J. Conquering Heart Disease: A Call to Action. Prev. Cardiol. 2002, 5, 31–36. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.B.; Roy, I. Biomedical Applications of Polyhydroxyalkanoates, an Overview of Animal Testing and in Vivo Responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef]
- Hirt, M.N.; Hansen, A.; Eschenhagen, T. Cardiac Tissue Engineering: State of the Art. Circ. Res. 2014, 114, 354–367. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Taylor, C.S.; Basnett, P.; Lukasewicz, B.; Paxinou, A.; Lizarraga-Valderrama, L.R.; Haycock, J.W.; Roy, I. Medium Chain Length Polyhydroxyalkanoates as Potential Matrix Materials for Peripheral Nerve Regeneration. Regen. Biomater. 2023, 10, rbad063. [Google Scholar] [CrossRef]
- Shijun, X.; Junsheng, M.; Jianqun, Z.; Ping, B. In Vitro Three-Dimensional Coculturing Poly3-Hydroxybutyrate-co-3-Hydroxyhexanoate with Mouse-Induced Pluripotent Stem Cells for Myocardial Patch Application. J. Biomater. Appl. 2016, 30, 1273–1282. [Google Scholar] [CrossRef]
- Gregory, D.A.; Fricker, A.T.R.; Mitrev, P.; Ray, M.; Asare, E.; Sim, D.; Larpnimitchai, S.; Zhang, Z.; Ma, J.; Tetali, S.S.V.; et al. Additive Manufacturing of Polyhydroxyalkanoate-Based Blends Using Fused Deposition Modelling for the Development of Biomedical Devices. J. Funct. Biomater. 2023, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Sevastianova, V.V.; Antonova, L.V.; Mironov, A.V.; Yuzhalin, A.E.; Silnikov, V.N.; Glushkova, T.V.; Godovikonova, T.S.; Krivkina, E.O.; Bolbasov, E.; Akentyeva, T.N.; et al. Biodegradable Patches for Arterial Reconstruction Modified with RGD Peptides: Results of an Experimental Study. ACS Omega 2020, 5, 21700–21711. [Google Scholar] [CrossRef]
- Antonova, L.V.; Krivkina, E.O.; Sevostianova, V.V.; Mironov, A.V.; Rezvova, M.A.; Shabaev, A.R.; Tkachenko, V.O.; Krutitskiy, S.S.; Khanova, M.Y.; Sergeeva, T.Y.; et al. Tissue-Engineered Carotid Artery Interposition Grafts Demonstrate High Primary Patency and Promote Vascular Tissue Regeneration in the Ovine Model. Polymers 2021, 13, 2637. [Google Scholar] [CrossRef]
- Majid, Q.A.; Pandey, P.; Bellahcene, M.; Grisby, C.L.; Stevens, M.M.; Talman, V.; Stuckey, D.J.; Harding, S.E.; Roy, I.; Foldes, G. Melt Electrowritten Medium Chain Length Polyhydroxyalkanoate Cardiac Patches for Post-MI Cardiac Regeneration. Mater. Today Bio 2025, 43, 102256. [Google Scholar] [CrossRef]
- Fu, Z.; Qiu, H.; Xu, Y.; Tan, C.; Wang, H. Biological Effects, Properties and Tissue Engineering Applications of Polyhydroxyalkanoates: A Review. Int. J. Biol. Macromol. 2024, 293, 139281. [Google Scholar] [CrossRef]
- Bagdadi, A.V.; Safari, M.; Dubey, P.; Basnett, P.; Sofokleous, P.; Humphrey, E.; Locke, I.; Edirisinghe, M.; Terracciano, C.; Boccaccini, A.R.; et al. Poly(3-Hydroxyoctanoate); a Promising New Material for Cardiac Tissue Engineering. J. Tissue Eng. Regen. Med. 2016, 12, e495–e512. [Google Scholar] [CrossRef]
- Constantinides, C.; Basnett, P.; Lukasiewicz, B.; Carnicer, R.; Swider, E.; Majid, Q.A.; Srinivas, M.; Carr, C.A.; Roy, I. In Vivo Tracking and 1H/19F Magnetic Resonance Imaging of Biodegradable Polyhydroxyalkanoate/Polycaprolactone Blend Scaffolds Seeded with Labeled Cardiac Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 25056–25068. [Google Scholar] [CrossRef]
- Dubey, P. Development of Cardiac Patches Using Medium Chain Length Polyhydroxyalkanoates for Cardiac Tissue Engineering. Ph.D. Thesis, University of Westminster, Life Science, London, UK, 2020. [Google Scholar] [CrossRef]
- Castellano, D.; Blandes, M.; Marco, B.; Cerrada, I.; Suiz-Sauri, A.; Pelacho, B.; Arana, M.; Montero, J.A.; Cambra, V.; Prosper, F.; et al. A Comparison of Electrospun Polymers Reveals Poly(3-Hydroxybutyrate) Fiber as a Superior Scaffold for Cardiac Repair. Stem Cells Dev. 2014, 23, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Azuraini, M.J.; Vigneswari, S.; Huong, K.-H.; Khairul, W.M.; Khalil, H.P.S.A.; Ramakrishna, S.; Amirul, A.-A.A. Surface Modification of Sponge-like Porous Poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/Gelatine Blend Scaffolds for Potential Biomedical Applications. Polymers 2022, 14, 1710. [Google Scholar] [CrossRef]
- Vigneswari, S.; Chai, J.M.; Kamarudin, K.H.; Amirul, A.-A.A.; Focarete, M.L.; Ramakrishna, S. Elucidating the Surface Functionality of Biomimetic RGD Peptides Immobilized on Nano-P(3HB-co-4HB) for H9c2 Myoblast Cell Proliferation. Front. Bioeng. Biotechnol. 2020, 8, 567693. [Google Scholar] [CrossRef]
- Sodian, R.; Sperling, J.S.; Martin, D.P.; Egozy, A.; Stock, U.; Mayer, J.E., Jr.; Vacanti, J.P. Technical Report: Fabrication of a Trileaf Heart Valve Scaffold from a Polyhydroxyalkanoate Biopolyester for Use in Tissue Engineering. Tissue Eng. 2004, 6, 183–188. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.-L.; Cui, B.; Qu, X.-Q.; Chen, G.-Q. Study on Decellularized Porcine Aortic Valve/Poly(3-Hydroxybutyrate-co-3-Hydroxyexanoate) Hybrid Heart Valve in Sheep Model. Artif. Organs 2007, 31, 689–697. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Chen, G.-Q. Medical Application of Microbial Biopolyesters Polyhydroxyalkanoates. Artif. Cells Blood Substit. Immobil. Biotechnol. 2009, 37, 1–12. [Google Scholar] [CrossRef]
- Engelmayr, G.; Sutherland, F.W.H.; Mayer, J.E., Jr.; Sacks, M.S. A Novel Bioreactor for the Flexural Stimulation of Tissue Engineered Heart Valve Biomaterials. In Proceedings of the ASME 2002 International Mechanical Engineering Congress and Exposition, New Orleans, LA, USA, 17–22 November 2002; pp. 95–96. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical Properties and Cell Cultural Response of Polycaprolactone Scaffolds Designed and Fabricated Via Fused Deposition Modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Martin, D.P.; Daebritz, S.; Mayer, J.E., Jr.; Vacanti, J.P. Evaluation of Biodegradable, Three-Dimensional Matrices for Tissue Engineering of Heart Valves. ASAIO J. 2000, 46, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Hoerstrup, S.P.; Sodian, R.; Daebritz, S.; Wang, J.; Bacha, E.A.; Martin, D.P.; Moran, A.M.; Guleserian, K.J.; Sperling, J.S.; Kaushal, S.; et al. Functional Living Trileaflet Heart Valves Grown in Vitro. Circulation 2000, 102, III44–III49. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Saebritz, S.; Martin, D.P.; Moran, A.M.; Kim, B.S.; Schoen, F.J.; Vacanti, J.P.; Mayer, J.E., Jr. Early in Vivo Experience with Tissue-Engineered Trileaflet Heart Valves. Circulation 2000, 102, III22–III29. [Google Scholar] [CrossRef]
- Stamm, C.; Khosravi, A.; Grabow, N.; Schmohl, K.; Treckmann, N.; Drechsel, A.; Nan, M.; Schmitz, K.-P.; Haubold, A.; Steinhoff, G. Biomatrix/Polymer Composite Material for Heart Valve Tissue Engineering. Ann. Thorac. Surg. 2004, 78, 2084–2093. [Google Scholar] [CrossRef]
- Cassell, O.C.S.; Hofer, S.O.P.; Morrison, W.A.; Knight, K.R. Vascularisation of Tissue-Engineered Grafts: The Regulation of Angiogenesis in Reconstructive Surgery and in Disease States. Br. J. Plast. Surg. 2002, 55, 603–610. [Google Scholar] [CrossRef]
- Qu, X.-H.; Wu, Q.; Chen, G.-Q. In Vitro Study on Hemocompatibility and Cytocompatibility of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate). J. Biomater. Sci. 2006, 17, 1107–1121. [Google Scholar] [CrossRef]
- Salacinski, H.; Tiwari, A.; Hamilton, G.; Seifalian, A.M. Performance of a Polyurethane Vascular Prosthesis Carrying a Dipyridamole (Persantin) Coating on its Lumenal Surface. J. Biomed. Mater. Res. 2002, 61, 337–338. [Google Scholar] [CrossRef]
- Antonova, L.V.; Mironov, A.V.; Yuzhalin, A.E.; Krivkina, E.O.; Shabaev, A.R.; Rezvova, M.A.; Tkachenko, V.O.; Khanova, M.Y.; Sergeeva, T.Y.; Krutitskiy, S.S.; et al. A Brief Report on an Implantation of Small-Caliber Biodegradable Vascular Grafts in a Carotid Artery of the Sheep. Pharmaceuticals 2020, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostianova, V.V.; Silnikov, V.N.; Krivkina, E.O.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Senokosova, E.A.; Khanova, M.Y.; Glushkova, T.V.; et al. Comparison of the Patency and Regenerative Potential of Biodegradable Vascular Prostheses of Different Polymer Compositions in an Ovine Model. Int. J. Mol. Sci. 2023, 24, 8540. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Miranda, M.; Apsite, I.; Agudo, J.A.R.; Costante, G.; Ionov, L. 4D Biofabrication of Mechanically Stable Tubular Constructs Using Morphing Porous Bilayer for Vascularization Application. Macromol. Biosci. 2022, 23, 2200320. [Google Scholar] [CrossRef]
- Wang, D.; Wang, C.; Bi, Z.; Zhou, B.; Wang, L.; Li, Q.; Turng, L.-S. Expanded Polytetrafluoroethylene/poly(3-Hydroxybutyrate) (ePTFE/PHB) Triboelectric Nanogenerators and their Potential Applications as Self-Powered and Sensing Vascular Grafts. Chem. Eng. J. 2023, 455, 140494. [Google Scholar] [CrossRef]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Mionov, A.V.; Krivkina, E.O.; Shabaev, A.R.; Matveeva, V.G.; Velikanova, E.A.; Sergeeva, E.A.; Burago, A.Y.; et al. Vascular Endothelial Growth Factor Improves Physico.Mechanical Properties and Enhances Endothelialization of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Poly(ε-Caprolactone) Small-Diameter Vascular Grafts In Vivo. Front. Pharmacol. 2016, 7, 230. [Google Scholar] [CrossRef]
- Gladysz, M.Z.; Ubels, D.; Koch, M.; Amirsadeghi, A.; Alleblas, F.; van Vliet, S.; Kamperman, M.; Siebring, J.; Nagelkerke, A.; Wlodarczuk-Biegun, M.K. Melt Electrowriting of Polyhydroxyalkanoates for Enzymatically Degradable Scaffolds. Adv. Healthc. Mater. 2024, 14, 2401504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Li, Z.-H.; Zhang, Y.-D.; Chen, J.; Ki, Y.; Wu, F.-Q.; Wang, W.; Cui, Z.J.; Chen, G.-Q. Ketone Body 3-Hydroxybutyrate Ameliorates Atherosclerosis via Receptor Gpr109a-Mediated Calcium Influx. Adv. Sci. 2021, 8, 2003410. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Sfefaniu, A.; Somoghi, R.; Trusca, R. Medium Chain-Length Polyhydroxyalkanoate Copolymer Modified by Bacterial Cellulose for Medical Devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef]
- Antonova, L.V.; Burago, A.Y.; Mironov, A.V.; Matveeva, V.G.; Velikanova, E.A.; Mukhamadiyarov, R.A.; Gushkova, T.V.; Kudryavtseva, Y.A.; Barbarash, O.L.; Barbarash, L.S. Polyhydroxybutyrate/Valerate/Polycaprolactone Small-Diameter Vascular Graft: Experimental Study of Integration Into Organism. AIP Conf. Proc. 2015, 1683, 020010. [Google Scholar] [CrossRef]
- Gao, J.; Guo, H.; Tian, S.; Qiao, Y.; Han, J.; Li, Y.; Wang, L. Preparation and Mechanical Performance of Small-Diameter PHBHHx Vascular Graft by Electrospinning. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 575–581. [Google Scholar] [CrossRef]
- Adamus, G.; Sikorska, W.; Janeczek, H.; Kwiecien, M.; Sobota, M.; Kowalczuk, M. Novel Block Copolymers of Atactic PHB with Natural PHA for Cardiovascular Engineering: Synthesis and Characterization. Eur. Polym. J. 2012, 48, 621–631. [Google Scholar] [CrossRef]
- Cheng, S.T.; Chen, Z.F.; Chen, G.Q. The Expression of Cross-Linked Elastin by Rabbit Blood Vessel Smooth Muscle Cells Cultured in Polyhydroxyalkanoate Scaffolds. Biomaterials 2008, 29, 4187–4194. [Google Scholar] [CrossRef]
- Li, S.Y.; Dong, C.L.; Wang, S.Y.; Ye, H.M.; Chen, G.Q. Microbial Production of Polyhydroxyalkanoate Block Copolymer by Recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2010, 90, 659–669. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Nikolaeva, E.D.; Vinogradova, O.N.; Volova, T.G. Experimental Wound Dressings of Degradable PHA for Skin Defect Repair. J. Mater. Sci. 2016, 27, 165. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E. Biopolymers as Wound Healing Materials: Challenges and New Strategies. In Biomaterials Applications for Nanomedicine; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Martin, D.P.; Williams, S.F. Medical Applications of Poly-4-Hydroxybutyrate: A Strong Flexible Absorbable Biomaterial. Biochem. Eng. J. 2003, 16, 97–105. [Google Scholar] [CrossRef]
- Brigham, C. Applications of Polyhydroxyalkanoates in the Medical Industry. J. Biomater. Nanobiotechnol. 2012, 1, 27–39. [Google Scholar] [CrossRef]
- Sayaner Taşçı, B.N.; Kozan, S.; Demirel Kars, M.; Çetin, K.; Karslıoğlu, S.; Kars, G. Fabrication and In Vitro Evaluation of LL37-Loaded Electrospun PHB/Collagen Nanofibers for Wound Healing. Polymers 2025, 17, 2486. [Google Scholar] [CrossRef]
- Hamza, K.H.; El-Shanshory, A.A.; Agwa, M.M.; Abo-Alkasem, M.I.; El-Fakharany, E.M.; Abdelsattar, A.S.; El-Bardan, A.A.; Kassem, T.S.; Mo, X.; Soliman, H.M.A. Topically Applied Biopolymer-Based Tri-Layered Hierarchically Structured Nanofibrous Scaffold with a Self-Pumping Effect for Accelerated Full-Thickness Wound Healing in a Rat Model. Pharmaceutics 2023, 15, 1518. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Galván, J.M.; Hernández-Rodríguez, J.E.; Martín-Barrasa, J.L.; Monzón-Mayor, M.; Saavedra-Santana, P.; Romero-Alemán, M.d.M. Macroscopic Evaluation of Poly(3-hydroxybutyrate-co-3-hydroxy valerate), PHBV-Based Nanofiber Scaffolds with Aloe Vera or Honey in Murine Wound Healing. Pharmaceutics 2025, 17, 833. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Fusco, A.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Coltelli, M.-B.; De Clerck, K.; Roy, I.; Macchia, M.; Lazzeri, A.; et al. Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract. Appl. Sci. 2021, 11, 4006. [Google Scholar] [CrossRef]
- Balcucho, J.; Narváez, D.M.; Tarazona, N.A.; Castro-Mayorga, J.L. Microbially Synthesized Polymer-Metal Nanoparticles Composites as Promising Wound Dressings to Overcome Methicillin-Resistance Staphylococcus aureus Infections. Polymers 2023, 15, 920. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Dudaev, A.E.; Volova, T.G. Resorbable Nanomatrices from Microbial Polyhydroxyalkanoates: Design Strategy and Characterization. Nanomaterials 2022, 12, 3843. [Google Scholar] [CrossRef] [PubMed]
- Kalaoglu-Altan, O.I.; Baskan, H.; Meireman, T.; Basnett, P.; Azimi, B.; Fusco, A.; Funel, N.; Donnarumma, G.; Lazzeri, A.; Roy, I.; et al. Silver Nanoparticle-Coated Polyhydroxyalkanoate Based Electrospun Fibers for Wound Dressing Applications. Materials 2021, 14, 4907. [Google Scholar] [CrossRef]
- Dias, Y.J.; Robles, J.R.; Sinha-Ray, S.; Abiade, J.; Pourdeyhimi, B.; Niemczyk-Soczynska, B.; Kolbuk, D.; Sajkiewicz, P.; Yarin, A.L. Solution-Blown Poly(Hydroxybutyrate) and ε-Poly-L-Lysine Submicro- and Microfiber-Based Sustainable Nonwovens with Antimicrobial Activity for Single-Use Application. ACS Biomater. Sci. Eng. 2021, 7, 3980–3992. [Google Scholar] [CrossRef]
- Lampomarda, A.; Degli Esposti, M.; Micalizzi, S.; Fabbri, P.; Galletti, A.M.R.; Morselli, D.; De Maria, C. Valorization of a Levolinic Acid Platform Through Electrospinning of Polyhydroxyalkanoates-Based Fibrous Membranes for In Vitro Modeling of Biological Barriers. ACS Appl. Polym. Mater. 2022, 4, 5872–5881. [Google Scholar] [CrossRef]
- Giubilini, A.; Messori, M.; Bondioli, F.; Minetola, P.; Iuliano, L.; Nystrom, G.; Maniura-Weber, K.; Rottmar, M.; Siqueira, G. 3D-Printed Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate)-Cellulose-Based Scaffolds for Biomedical Applications. Biomacromolecules 2023, 24, 3961–3971. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.-N.; Peng, Z.-X.; Chen, N.-B.; Zhang, P.; Zhang, X.; Chen, G.-Q. Multifunctional Electrospinning Polyhydroxyalkanoates Fibrous Scaffolds with Antibacterial and Angiogenesis Effects for Accelerating Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 364–377. [Google Scholar] [CrossRef]
- Pramanik, N.; De, J.; Basu, R.K.; Rath, T.; Kundu, P.P. Fabrication of Magnetite Nanoparticle Doped Reduced Graphene Oxide Grafted Polyhydroxyalkanoate Nanocomposites for Tissue Engineering Applications. RSC Adv. 2016, 6, 46116–46133. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Al Moustafa, A.-E. Cerium Oxide Nanoparticle Incorporated Electrospun Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Membranes for Diabetic Wound Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 58–70. [Google Scholar] [CrossRef]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef]
- Lundborg, G. A 25-Year Perspective of Peripheral Nerve Surgery: Evolving Neuroscientific Concepts and Clinical Significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Sakiyama-Elbert, S.E. Matrices, scaffolds & Carriers for Cell Delivery in Nerve Regeneration. Exp. Neurol. 2019, 319, 112837. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve Grafting for Peripheral Nerve Injuries with Extended Defect Sizes. Wien. Med. Wochenschr. 2018, 169, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, E.; Morshed, M.; Rasekhian, P.; Karbasi, S.; Kerbalaie, K.; Karamali, F.; Abedi, D.; Razavi, S.; Jafarian-Dehkordi, A.; Nasr-Esfahani, M.H.; et al. Does the Tissue Engineering Architecture of Poly(3-Hydroxybutyrate Scaffold Effects Cell-Material Interactions? J. Biomed. Mater. Res. A 2012, 100, 1907–1918. [Google Scholar] [CrossRef]
- Young, R.C.; Terenghi, G.; Wiberg, M. Poly-3-Hydroxybutyrate (PHB): A Resorbable Conduit for Long-Gap Repair in Peripheral Nerves. Br. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef]
- Mohanna, P.N.; Tereghi, G.; Wiberg, M. Composite PHB-GGF Conduit for Long Nerve Gap Repair: A Long-Term Evaluation. Scand. J. Plast. Reconstr. Surg. Hard Surg. 2005, 39, 129–135. [Google Scholar] [CrossRef]
- Aberg, M.; Ljungberg, C.; Edin, E.; Millqvist, H.; Nordh, E.; Theorin, A.; Tereghi, G.; Wiberg, M. Clinical Evaluation of a Resorbable Wrap-Around Implant as an Alternative to Nerve Repair: A Prospective, Assessor-Blinded, Randomised Clinical Study of Sensory, Motor and Functional Recovery After Peripheral Nerve Repair. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1503–1509. [Google Scholar] [CrossRef]
- Hazari, A.; Wiberg, M.; Johansson-Ruden, G.; Green, C.; Terenghi, G. A Resorbable Nerve Conduit as an Alternative to Nerve Autograft in Nerve Gap Repair. Br. J. Plast. Surg. 1999, 52, 653–657. [Google Scholar] [CrossRef]
- Mohanna, P.N.; Young, R.C.; Wiberg, M.; Terenghi, G. A Composite Poly-Hydroxybutyrate-Glial Growth Factor Conduit for Long Nerve Gap Repairs. J. Anat. 2003, 6, 553–565. [Google Scholar] [CrossRef]
- Kalbermatten, D.F.; Kingham, P.J.; Mahay, D.; Mantovani, C.; Pettersson, J.; Raffoul, W.; Balcin, H.; Pierer, G.; Terenghi, G. Fibrin Matrix for Suspension of Regenerative Cells in an Artificial Nerve Conduit. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Erba, P.; Mahay, D.; Wiberg, M.; Pierer, G.; Terenghi, G. Schwann Cell Strip for Peripheral Nerve Repair. J. Hand Surg. Eur. Vol. 2008, 33, 587–594. [Google Scholar] [CrossRef]
- Schaakxs, D.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Wiberg, M.; Kingham, P.J. Poly-3-Hydroxybutyrate Strips Seeded with Regenerative Cells are Effective Promoters of Peripheral Nerve Repair. J. Tissue Eng. Regen. Med. 2017, 11, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Karbasi, S.; Razavi, S.; Zargar, E.N. Poly(Hydroxybutyrate)/Chitosan Aligned Electrospun Scaffold as a Novel Substrate for Nerve Tissue Engineering. Adv. Biomed. Res. 2018, 7, 277. [Google Scholar] [CrossRef]
- Hinuber, C.; Chwalek, K.; Pan-Montojo, F.J.; Nitschke, M.; Vogel, R.; Brunig, H.; Heinrich, G.; Werner, C. Hierarchically Structured Nerve Guidance Channels Based on Poly-3-Hydroxybutyrate Enhance Oriented Axonal Outgrowth. Acta Biomater. 2014, 10, 2086–2095. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-Alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef]
- Soriano-Cuadrado, B.; Fontecha-Cámara, M.Á.; Mañas-Villar, M.; Delgado-Blanca, I.; Ramírez-Rodríguez, M.D. Mechanical, Thermal and Morphological Study of Bio-Based PLA Composites Reinforced with Lignin-Rich Agri-Food Wastes for Their Valorization in Industry. Polymers 2024, 16, 2462. [Google Scholar] [CrossRef]
- Biazar, E.; Keshel, S.H.; Pauya, M. Efficacy of Nanofibrous Conduits in Repair of Long-Segment Sciatic Nerve Defects. Neural Regen. Res. 2013, 8, 2501–2509. [Google Scholar] [CrossRef]
- Biazar, E.; Keshel, S.H.; Pouya, M.; Rad, H.; Nava, M.O.; Azarbakhsh, M.; Hooshmand, S. Nanofibrous Nerve Conduit for Repair of 30-mm-Long Sciatic Nerve Defects. Neural Regen. Res. 2013, 8, 2266–2274. [Google Scholar] [CrossRef]
- Biazar, E.; Keshel, S.H.; Pouya, M. Behavioral Evaluation of Regenerated Rat Sciatic Nerve by a Nanofibrous PHBV Conduit Filled with Schwann Cells as Artificial Nerve Graft. Cell Commun. Adhes. 2013, 20, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Keshel, S.H. A Nanofibrous PHBV Tube with Schwann Cell as Artificial Nerve Graft Contributing to Rat Sciatic Nerve Regeneration Across a 30-mm Defect Bridge. Cell Commun. Adhes. 2013, 20, 41–49. [Google Scholar] [CrossRef]
- Karimi, M.; Biazar, E.; Keshel, S.H.; Ronaghi, A.; Doostmohamadpour, J.; Janfada, A.; Montazeri, A. Rat Sciatic Nerve Reconstruction Across a 30 mm Defect by an Oriented Porous PHBV Tube with Schwann Cell as Artificial Nerve Graft. ASAIO J. 2014, 60, 224–233. [Google Scholar] [CrossRef]
- Chen, W.; Tong, Y.W. PHBV Microspheres as Neural Tissue Engineering Scaffold Support Neuronal Cell Growth and Axon-Dendrite Polarization. Acta Biomater. 2012, 8, 540–548. [Google Scholar] [CrossRef]
- Bian, Y.Z.; Wang, Y.; Aibaidoula, G.; Chen, G.Q.; Wu, Q. Evaluation of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate) Conduits for Peripheral Nerve Regeneration. Biomaterials 2008, 30, 217–225. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Li, X.-T.; Peng, S.-W.; Xiao, J.-F.; Liu, C.; Fang, G.; Chen, K.C.; Chen, G.-Q. The Behaviour of Neural Stem Cells on Polyhydroxyalkanoate Nanofiber Scaffolds. Biomaterials 2010, 31, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.-Y.; Chen, C.-R.; Sun, Y.-M.; Young, T.-H. The Response of Rat Cerebellar Granule Neurons (rCGNs) to Various Polyhydroxyalkanoate (PHA) Films. Desalination 2009, 245, 639–646. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Ronchi, G.; Nigmatullin, R.; Fregnan, F.; Basnett, P.; Paxinou, A.; Geuna, S.; Roy, I. Preclinical Study of Peripheral Nerve Regeneration Using Nerve Guidance Conduits Based on Polyhydroxyalkanoates. Bioeng. Transl. Med. 2021, 6, e10223. [Google Scholar] [CrossRef] [PubMed]
- Shlapakova, L.E.; Botvin, V.V.; Mukhortovam, Y.R.; Zharkova, I.I.; Alipkina, S.I.; Zeltzer, A.; Dudun, A.A.; Makhina, T.; Bonartseva, G.A.; Voinova, V.V.; et al. Magnetoactive Composite Conduits Based on Poly(3-Hydroxybutyrate) and Magnetite Nanoparticles for Repair of Peripheral Nerve Injury. ACS Appl. Bio Mater. 2024, 7, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, X.; Gonzalez-Perez, F.; Bazan, X.; Diez-Ahedo, R.; Quintana, I.; Rodriguez, F.J.; Basnett, P.; Nigmatullin, R.; Lukasiewicz, B.; Roy, I.; et al. Bioresorbable and Mechanically Optimized Nerve Guidance Conduit Based on a Naturally Derived Medium Chain Length Polyhydroxyalkanoate and Poly(ε-Caprolactone) Blend. ACS Biomater. Sci. Eng. 2021, 7, 672–689. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.S.; Behbehani, M.; Glen, A.; Basnett, P.; Gregory, D.A.; Lukasiewicz, B.B.; Nigmatullin, R.; Claeyssens, F.; Roy, I.; Haycock, J.W. Aligned Polyhydroxyalkanoate Blend Electrospun Fibers as Intraluminal Guidance Scaffolds for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2023, 9, 1472–1485. [Google Scholar] [CrossRef]
- Ozer, H.; Bozkurt, H.; Bozkurt, G.; Demirbilek, M. Regenerative Potential of Chitosan-Coated Poly-3-Hydroxybutyrate Conduits Seeded with Mesenchymal Stem Cells in a Rat Sciatic Nerve Injury Model. Int. J. Neurosci. 2018, 128, 828–834. [Google Scholar] [CrossRef]
- Gao, Y.; Kong, L.; Zhang, L.; Gong, Y.; Chen, G.; Zhao, N.; Zhang, X. Improvement of Mechanical Properties of Poly(DL-Lactide) Films by Blending of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate). Eur. Polym. J. 2006, 42, 764–775. [Google Scholar] [CrossRef]
- Tar, K.A.; Karbasi, S.; Naghashzargar, E.; Salehi, H. Biodegradation and Cellular Evaluation of Aligned and Random Poly(3-Hydroxybutyrate)/Chitosan Electrosun Scaffold for Nerve Tissue Engineering Applications. Mater. Technol. 2020, 35, 92–101. [Google Scholar] [CrossRef]
- Michalak, M.; Marek, A.A.; Zawadiak, J.; Kawalec, M.; Kurcok, P. Synthesis of PHB-Based Carrier for Drug Delivery Systems with pH-Controlled Release. Eur. Polym. J. 2013, 49, 4149–4156. [Google Scholar] [CrossRef]
- Grage, K.; Jahns, A.C.; Parlane, N.; Palanisamy, R.; Rasiah, I.A.; Atwood, J.A.; Rehm, B.H.A. Bacterial Polyhydroxyalkanoate Granules: Biogenesis, Structure, and Potential Use as Nano-/Micro-Beads in Biotechnological and Biomedical Applications. Biomacromolecules 2009, 10, 660–669. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Ciraolo, E.; Stefania, R.; Chen, G.-Q.; Zhang, Y.; Hirsch, E. Sustained Release of PI3K Inhibitor from PHA Nanoparticles and In Vitro Inhibitor of Cancer Cell Lines. Appl. Microbiol. Biotechnol. 2011, 89, 1423–1433. [Google Scholar] [CrossRef]
- Muruevam, A.V.; Shishatskaya, E.I.; Kuzmina, A.M.; Volova, T.G.; Sinskey, A.J. Microparticles Prepared from biodegradable Polyhydroxyalkanoates as Matrix for Encapsulation of Cytostatic Drug. J. Mater. Sci. Mater. Med. 2013, 24, 1905–1915. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-Mediated Poly(3-Hydroxybutyrate-co-3-Hydroxyoctanoate) Nanoparticles for Targeting Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 76, 10–16. [Google Scholar] [CrossRef]
- Rossi, S.; Azghani, A.O.; Omri, A. Antimicrobial Efficacy of a New Antibiotic Loaded Poly(Hydroxybutyric-co-Hydroxyvaleric acid) Controlled Release System. J. Antimicrob. Chemother. 2004, 54, 1013–1018. [Google Scholar] [CrossRef]
- Biondi, M.; Guarnieri, D.; Yu, H.; Belli, V.; Netti, P.A. Sub-100 nm Biodegradable Nanoparticles: In Vitro Release Features and Toxicity Testing in 2D and 3D Cell Cultures. Nanobiotechnology 2013, 24, 045101. [Google Scholar] [CrossRef]
- Turesin, F.; Gursel, I.; Hasirci, V. Biodegradable Polyhydroxyalkanoate Implants for Osteomyelitis Therapy: In Vitro Antibiotic Release. J. Biomater. Sci. Polym. Ed. 2001, 12, 195–207. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Goreva, A.V.; Voinova, O.N.; Inzhevatkin, E.V.; Khlebopros, R.G.; Volova, T.G. Evaluation of Antitumor Activity of Rubomycin Deposited in Absorbable Polymeric Microparticles. Bull. Exp. Biol. Med. 2008, 145, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Mittal, P.; Reddy Adena, S.K.; Upadhyay, M.; Mishra, B. Development of Long-Circulating Docetaxel Loaded Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Nanoparticles: Optimization, Pharmacokinetic, Cytotoxicity and In Vivo Assessment. Int. J. Biol. Macromol. 2017, 103, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Mittal, P.; Reddy Adena, S.K.; Upadhyay, M.; Yadav, S.K.; Mishra, B. Process Optimization and In Vivo Performance of Docetaxel Loaded PHBV-TPGS Therapeutic Vesicles: A Synergistic Approach. Int. J. BIol Macromol. 2018, 108, 729–743. [Google Scholar] [CrossRef]
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of Ellipticine in Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Based Nanoparticles and its In Vitro Application. Mater. Sci. Eng. Mater. Biol. Appl. 2013, 33, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.L.; Locke, I.C.; Roy, I. Aspirin-Loaded P(3HO)(P(3HB) Blend Films: Potential Materials for Biodegradable Drug-Eluting Stents. Bioinspired Biomim. Nanobiomaterials 2013, 2, 141–153. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a Family of Natural Polymers, and Their Applications in Drug Delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Peng, K.; Wu, G.; Wie, G.-G.; Jiang, J.; Zhang, Z.; Sun, X. Implantable Sandwich PHBHHx Film for Burst-Free Controlled Delivery of Thymopentin Peptide. Acta Pharm. Sin. B 2018, 8, 432–439. [Google Scholar] [CrossRef]
- Shirmard, L.R.; Javan, N.B.; Khoshayand, M.R.; Kebriaee-Zadeh, A.; Dinarvand, R.; Dorkoosh, F.D. Nanoparticulate Fingolimod Delivery System Based on Biodegradable Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBV): Design, Optimization, Characterization and In-Vitro Evaluation. Pharm. Dev. Technol. 2017, 22, 860–870. [Google Scholar] [CrossRef]
- Javan, N.B.; Omid, N.J.; Hasab, N.M.; Shirmard, L.R.; Rafiee-Tehrani, M.; Dorkoosh, F. Preparation, Statistical Optimization and In Vitro Evaluation of Pramipexole Prolonged Delivery System Based on Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 44, 82–90. [Google Scholar] [CrossRef]
- Prasad Rao, J.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Orts, W.J.; Nobes, G.A.R.; Kawada, J.; Nguyen, S.; Yu, G.-E.; Ravanelle, F. Poly(Hydroxyalkanoates): Biorefinery Polymers with a Whole Range of Applications. The Work of Robert H. Marchessault. Can. J. Chem. 2008, 86, 628–640. [Google Scholar] [CrossRef]
- Govindasamy, S.; Syafiq, I.M.; Amirul, A.-A.A.; Amin, R.M.; Bhubalan, K. Dataset on Controlled Production of Polyhydroxyalkanoate-Based Microbead Using Double Emulsion Solvent Evaporation Technique. Data Brief 2019, 23, 103675. [Google Scholar] [CrossRef]
- Kiliocay, E.; Demirbilek, M.; Turk, M.; Guven, E.; Hazer, B.; Denkbas, E.B. Preparation and Characterization of Poly(3-Hydroxybutyrate-co-3-Hydroxyhexanoate) (PHBHHX) Based Nanoparticles for Targeted Cancer Therapy. Eur. J. Pharm. Sci. 2011, 44, 310–320. [Google Scholar] [CrossRef]
- Ying, T.H.; Ishii, D.; Mahara, A.; Murakami, S.; Yamaoka, T.; Sudesh, K.; Samian, R.; Fujita, M.; Maeda, M.; Iwata, T. Scaffolds from Electrospun Polyhydroxyalkanoate Copolymers: Fabrication, Characterization, Bioabsorption and Tissue Response. Biomaterials 2008, 29, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Chem, C.; Yu, C.H.; Cheng, Y.C.; Yu, P.H.F.; Cheung, M.K. Preparation and Characterization of Biodegradable Nanoparticles Based on Amphiphilic Poly(3-Hydroxybutyrate)-Poly(Ethylene Glycol)-Poly(3-Hydroxybutyrate) Triblock Copolymer. Eur. Polym. J. 2006, 42, 2211–2220. [Google Scholar] [CrossRef]
- Ueda, H.; Tabata, Y. Polyhydroxyalkanoate Derivatives in Current Clinical Applications and Trials. Adv. Drug Deliv. Rev. 2003, 55, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Gupta, D.; Kumar, M.; Sharma, S.; Gupta, A.K.; Misro, M.M.; Singh, H. Intracellular Delivery of Peptide Cargos Using Polyhydroxybutyrate Based Biodegradable Nanoparticles: Studies on Antitumor Efficacy of BCL-2 Converting Peptide, NuBCP-9. Int. J. Pharm. 2016, 11, 876–889. [Google Scholar] [CrossRef]
- Wang, X.; Liow, S.S.; Wu, Q.; Li, C.; Owh, C.; Li, Z.; Loh, X.J.; Wu, Y.-L. Codelivery for Paclitaxel and Bcl-2 Conversion Gene by PHB-PDMAEMA Amphiphilic Cationic Copolymer for Effective Drug-Resistant Cancer Therapy. Macromol. Biosci. 2017, 17, 1700186. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, Z.; Wu, C.; Wang, X.; Liow, S.S.; Li, Z.; Wu, Y.-L. Overcoming STC2 Mediated Drug Resistance Through Drug and Gene Co-Delivery by PHB-PDMAEMA Cationic Polyester in Liver Cancer Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 210–217. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Salahuddin, N.; Gaber, M.; Mousa, M. Poly(3-Hydroxybutyrate)/Polyethylene Glycol-NiO Nanocomposite for NOR Delivery: Antibacterial Activity and Cytotoxic Effect Against Cancer Cell Lines. Int. J. Biol. Macromol. 2018, 114, 717–727. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Wang, H.; Qiu, Y.-K.; Liow, S.S.; Li, Z.; Loh, X.J. PHB-Based Gels as Delivery Agents of Chemotherapeutic for the Effective Shrinkage of Tumors. Adv. Healthc. Mater. 2016, 5, 2679–2685. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Z.; Chi, W.; Yang, X.; Wang, W.; Zhang, B. Mono-Methoxy-Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate)-Graft-Hyper-Branched Polyethylenimine Copolymers for siRNA Delivery. Biomaterials 2012, 33, 2334–2344. [Google Scholar] [CrossRef]
- Javan, N.B.; Shirmard, L.R.; Omid, N.J.; Javar, H.A.; Tehrani, M.R.; Dorkoosh, F.A. Preparation, Statistical Optimization and In Vitro Characterization of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Poly(Lactic-co-Glycol Acid) Blend Nanoparticles for Prolonged Delivery of Teriparatide. J. Microencapsul. 2016, 33, 460–474. [Google Scholar] [CrossRef]
- Bini, R.A.; Silva, M.F.; Varanda, L.C.; da Silva, M.A.; Dreiss, C.A. Soft Nanocomposites of Gelatin and Poly(3-Hydroxybutyrate) Nanoparticles for Dual Drug Release. Colloids Surf. B Biointerfaces 2017, 157, 191–198. [Google Scholar] [CrossRef]
- Alizadeh, B.; Javan, N.B.; Javar, H.A.; Khoshayand, M.R.; Dorkoosh, F. Prolonged Injectable Formulation of Nafarelin Using In Situ Gel Combination Delivery System. Pharm. Dev. Technol. 2018, 23, 132–144. [Google Scholar] [CrossRef]
- Fernandes, J.G.; Correia, D.M.; Botelho, G.; Padrao, J.; Dourado, F.; Ribeiro, C.; Lanceros-Mendez, S.; Sancadas, V. PHB-PEO Electrospun Fiber Membranes Containing Chlorhexidine for Drug Delivery Applications. Polym. Test. 2014, 34, 64–71. [Google Scholar] [CrossRef]
- Cao, K.; Liu, Y.; Olkhov, A.A.; Siracusa, V.; Iordanskii, A.L. PLLA-PHB Fiber Membranes Obtained by Solvent-Free Electrospinning for Short-Time Drug Delivery. Drug Deliv. Transl. Res. 2018, 8, 291–302. [Google Scholar] [CrossRef]
- Wang, Z.; Itoh, Y.; Hosaka, Y.; Kobayashi, I.; Nakano, Y.; Maeda, I.; Umeda, F.; Yamakawa, J.; Nishimine, M.; Suenobu, T.; et al. Mechanism of Enhancement Effect of Dendrimer on Transfermal Drug Permeation Through Polyhydroxyalkanoate Matrix. J. Biosci. Bioeng. 2003, 96, 537–540. [Google Scholar] [CrossRef]
- Shagan, A.; Croitoru-Sadger, T.; Corem-Salkmon, E.; Mizrahi, B. Near-Infrared Light Induced Phase Transition of Biodegradable Composites for On-Demand Healing and Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 4131–4139. [Google Scholar] [CrossRef]
- Babos, G.; Rydz, J.; Kawalec, M.; Klim, M.; Fodor-Kardos, A.; Trif, L.; Feczkó, T. Poly(3-Hydroxybutyrate)-Based Nanoparticles for Sorafenib and Doxorubicin Anticancer Drug Delivery. Int. J. Mol. Sci. 2020, 21, 7312. [Google Scholar] [CrossRef] [PubMed]
- Faisalina, A.F.; Sonvico, F.; Colombo, P.; Amirul, A.A.; Wahab, H.A.; Majid, M.I.A. Docetaxel-Loaded Poly(3HB-co-4HB) Biodegradable Nanoparticles: Impact of Copolymer Composition. Nanomaterials 2020, 10, 2123. [Google Scholar] [CrossRef]
- Wu, M.; Zhong, C.; Zhang, Q.; Wang, L.; Wang, L.; Liu, Y.; Zhang, X.; Zhao, X. pH-Responsive Delivery Vehicle Based on RGD-Modified Polydopamine-Paclitaxel-Loaded Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Nanoparticles for Targeted Therapy in Hepatocellular Carcinoma. J. Nanobiotechnol. 2021, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, S.Y.; Ku, S.H.; Park, E.J.; Jang, D.-J.; Kim, S.T.; Kim, S.-B. Polyhydroxyalkanoate Decelerates the Release of Paclitaxel from Poly(Lactic-co-Glycolic Acid) Nanoparticles. Pharmaceutics 2022, 14, 1618. [Google Scholar] [CrossRef]
- Salahuddin, N.; Gaber, M.; Mousa, M.; Abdelwahab, M.A. Poly(3-Hydroxybutyrate)/Poly(Amine)-Coated Nickel Oxide Nanoparticles for Norfloxacin Delivery: Antibacterial and Cytotoxicity Efficiency. RSC Adv. 2020, 10, 34046–34058. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Huang, S.; Dai, Z.; Liang, J.; Qiu, Q.; Chen, S.; Guo, W.; Wang, Z.; Wei, J. Poly-3-Hydroxybutyrate-co-3-Hydroxyvalerate (PHBV)-Polyethylene Glycol 20k (PEG20k) as a Promising Delivery System for PT2399 in the Treatment of Disc Degeneration. J. Biol. Eng. 2024, 18, 11. [Google Scholar] [CrossRef]
- Miu, D.-M.; Pavaloiu, R.D.; Sha’at, F.; Vladu, M.-G.; Neagu, G.; Manoiu, V.-S.; Eremia, M.-C. Preparation and Optimization of a Polyhydroxyoctanoate–Hydroxyapatite Composite Available to Scaffolds in Implantable Devices. Molecules 2025, 30, 730. [Google Scholar] [CrossRef]
- Bayrami, S.; Chamani, M.; Jamali Moghadam Siahkali, S.R.; Sayed Alinaghi, S.A.; Shirmard, L.R.; Bayrami, S.; Javar, H.A.; Ghahremani, M.H.; Amini, S.; Tehrani, M.R.; et al. Preparation, Characterization and In Vitro Evaluation of Insulin-PHBV Nanoparticles/Alginate Hydrogel Composite System for Prolonged Delivery of Insulin. J. Pharm. Sci. 2024, 113, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Il Youn, S. Chitosan-reinforced PHB Hydrogel and Aerogel Monoliths Fabricated by Phase Separation with the Solvent-Exchange Method. Carbohydr. Polym. 2022, 284, 119184. [Google Scholar] [CrossRef] [PubMed]
- Javan, N.B.; Montazeri, H.; Shirmard, L.R.; Omid, N.J.; Barbari, G.R.; Amini, M.; Ghahremani, M.H.; Rafiee-Tehrani, M.; Dorkoosh, F.A. Preparation, Characterization and In Vivo Evaluation of a Combination Delivery System Based on Hyaluronic Acid/Jeffamine Hydrogel Loaded with PHBV/PLGA Blend Nanoparticles for Prolonged Delivery of Teriparatide. Eur. J. Pharm. Sci. 2017, 101, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Memari, E.; Maghsoudi, A.; Yazdian, F.; Yousefi, M.; Mohammadi, M. Synthesis of PHB-co-PEI Nanoparticles as Gene Carriers for miR-128-Encoding Plasmid Delivery to U87 Glioblastoma Cells. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124898. [Google Scholar] [CrossRef]
- Gregory, D.A.; Taylor, C.S.; Fricker, A.T.R.; Asare, E.; Tetali, S.S.V.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates and Their Advances for Biomedical Applications. Trends Mol. Med. 2022, 28, 331–342. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Figueroa-Lopez, K.J.; Cabrera-Villamizar, L. Advancements in Reinforcement, Antimicrobial Functionalization, and Biodegradation of Polyhydroxyalkanoates (PHAs) Nanocomposites. In Polyhydroxyalkanoates: Sustainable Production and Biotechnological Applications III; Springer: Singapore, 2025; pp. 15–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.