Harnessing Nature for Breast Cancer Management: Effects of Fisetin-Loaded Nigellasomes Embedded in Microneedles Improve Tumor Suppression and Reduce Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fixed Oil Extraction and Separation from Seeds of Nigella sativa
2.3. GC/MS Analysis of the Isolated Nigella sativa Oil
2.4. Fisetin High-Performance Liquid Chromatography Analysis
2.5. Animals
2.6. Methods
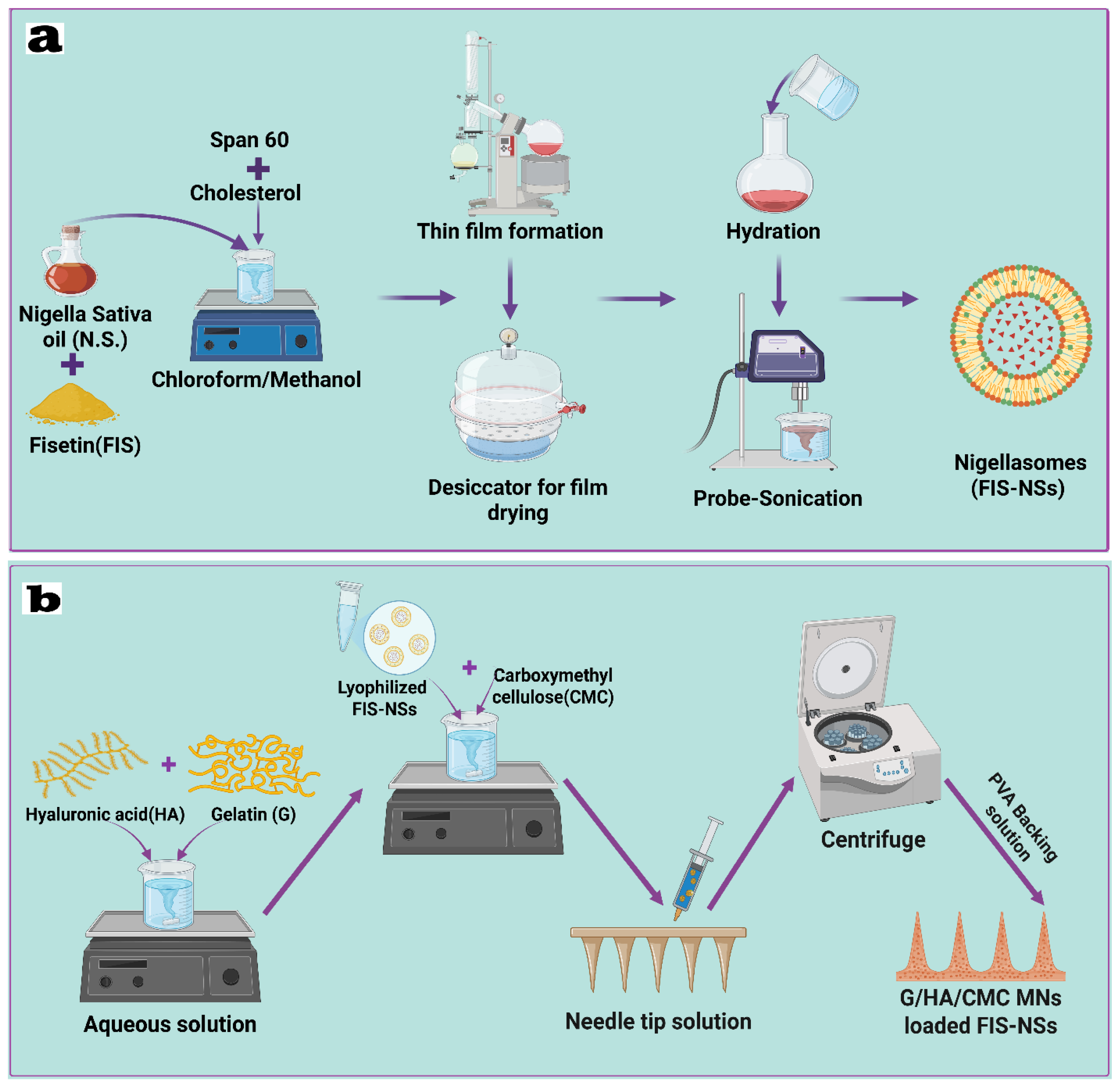
2.6.1. Design of Experiments Fisetin–Nigellasomes (FIS-NSs)
2.6.2. Preparation and Optimization of Fisetin–Nigellasomes (FIS-NSs)
2.6.3. Physicochemical Characterization of Fisetin–Nigellasomes (FIS-NSs)
Particle Size, Polydispersity Index, and Zeta Potential
Entrapment Efficiency
2.6.4. Evaluation of the Optimized Fisetin–Nigellasomes (FIS-NSs)
Transmission Electron Microscopy (TEM)
Fourier Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
Stability Study of the Optimized Fisetin–Nigellasomes (FIS-NSs)
2.7. Fabrication of Carbohydrate-Based Hybrid Dissolving Microneedles (FIS-NSs-Loaded G/HA/CMC MNs)
2.8. Physical Characteristics of the Carbohydrate-Based Hybrid Dissolving Microneedles (G/HA/CMC MNs)
2.8.1. Mechanical Strength and Penetration Capability Test
2.8.2. Determination of Drug Content
2.8.3. Dissolution Time Assessment
2.8.4. Water Loss During Drying (LOD) Test
2.8.5. Characterization of Optimized Carbohydrate-Based Hybrid Dissolving Microneedles
Scanning Electron Microscopy (SEM)
Fourier Transform Infrared (FTIR) Spectroscopy
Differential Scanning Calorimetry (DSC)
In Vitro Drug Release Study
Ex Vivo Permeation Study and Kinetic Analysis
2.9. In Vitro Cell Culture Studies
2.9.1. Cell Line
2.9.2. Cellular Cytotoxicity
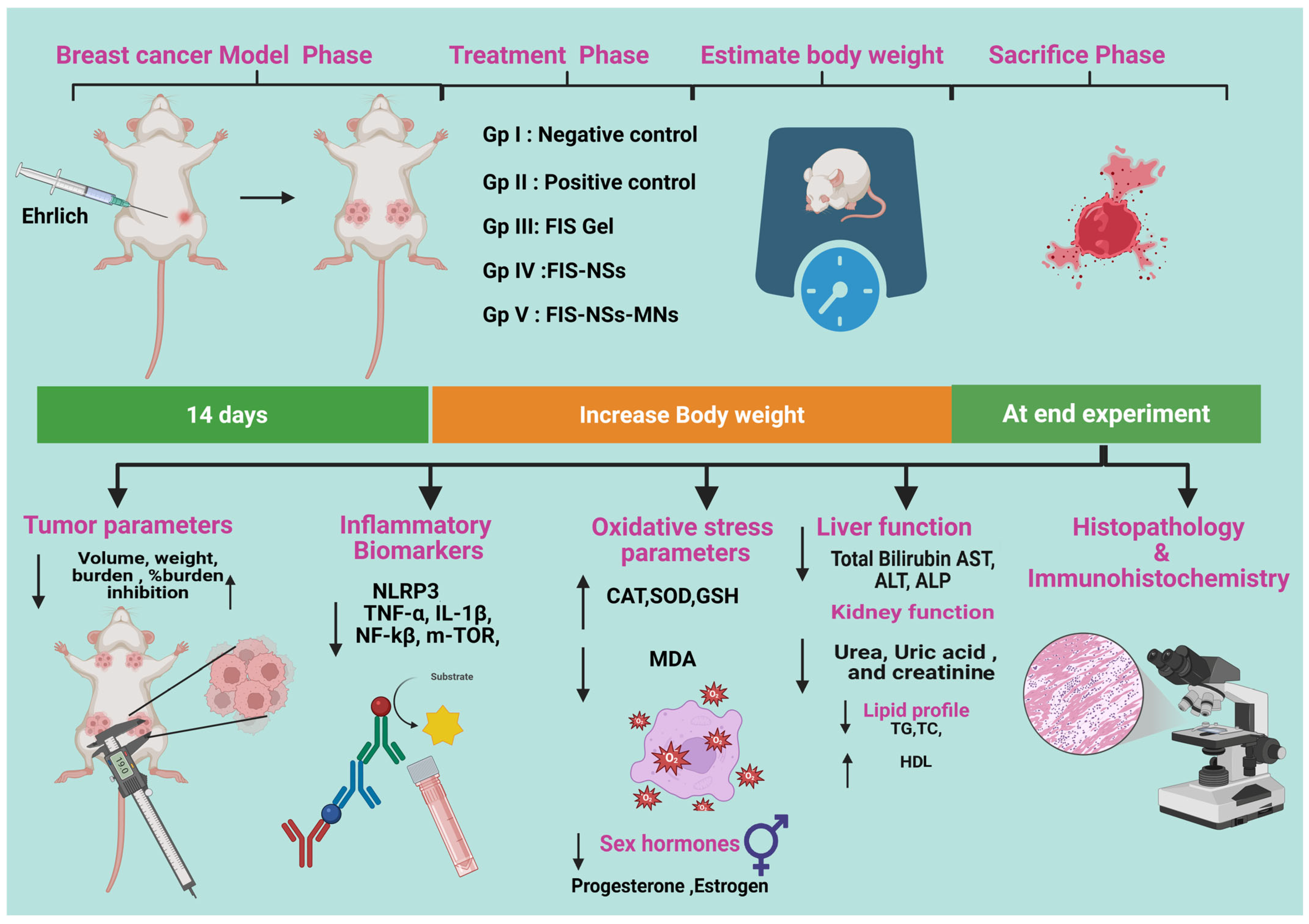
2.10. In Vivo Study
2.10.1. Induction of Breast Cancer in Mice
2.10.2. Estimation of Skin Morphology and Body Weight
2.10.3. Estimation of Tumor Parameters
2.10.4. Assessment of Inflammatory Biomarkers
2.10.5. Assay for Oxidative Stress Parameters
Assessment of Oxidant Biomarkers
Enzymatic Antioxidant Parameters
Non-Enzymatic Antioxidant Parameters
2.10.6. Liver Function Tests (LFTs), Kidney Function Tests and Sex Hormone Assessment
2.10.7. Lipid Profile
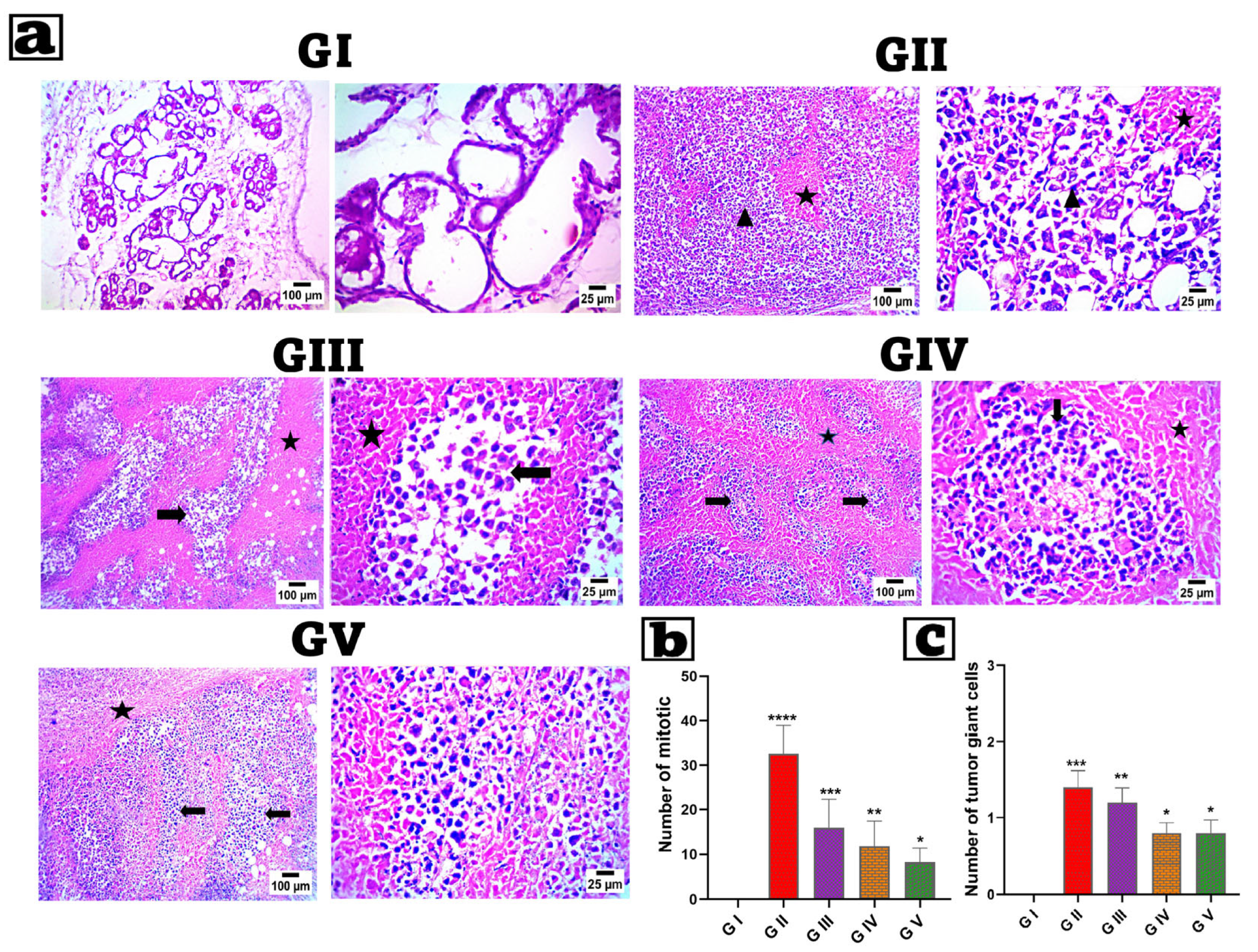
2.11. Histopathological Analysis
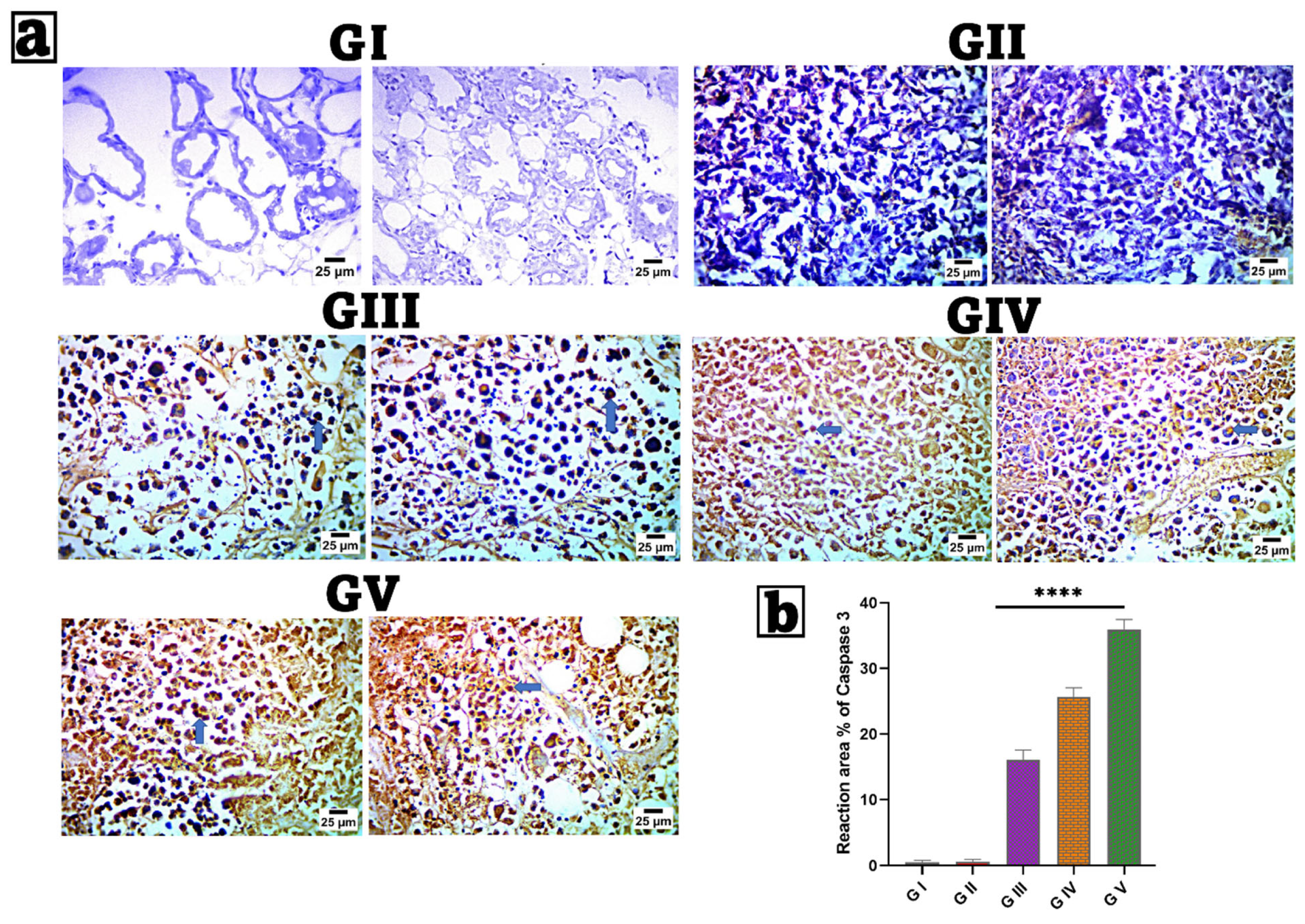
2.12. Immunohistochemical (IHC) Examination of Caspase-3
2.13. Statistical Analysis
3. Results and Discussion
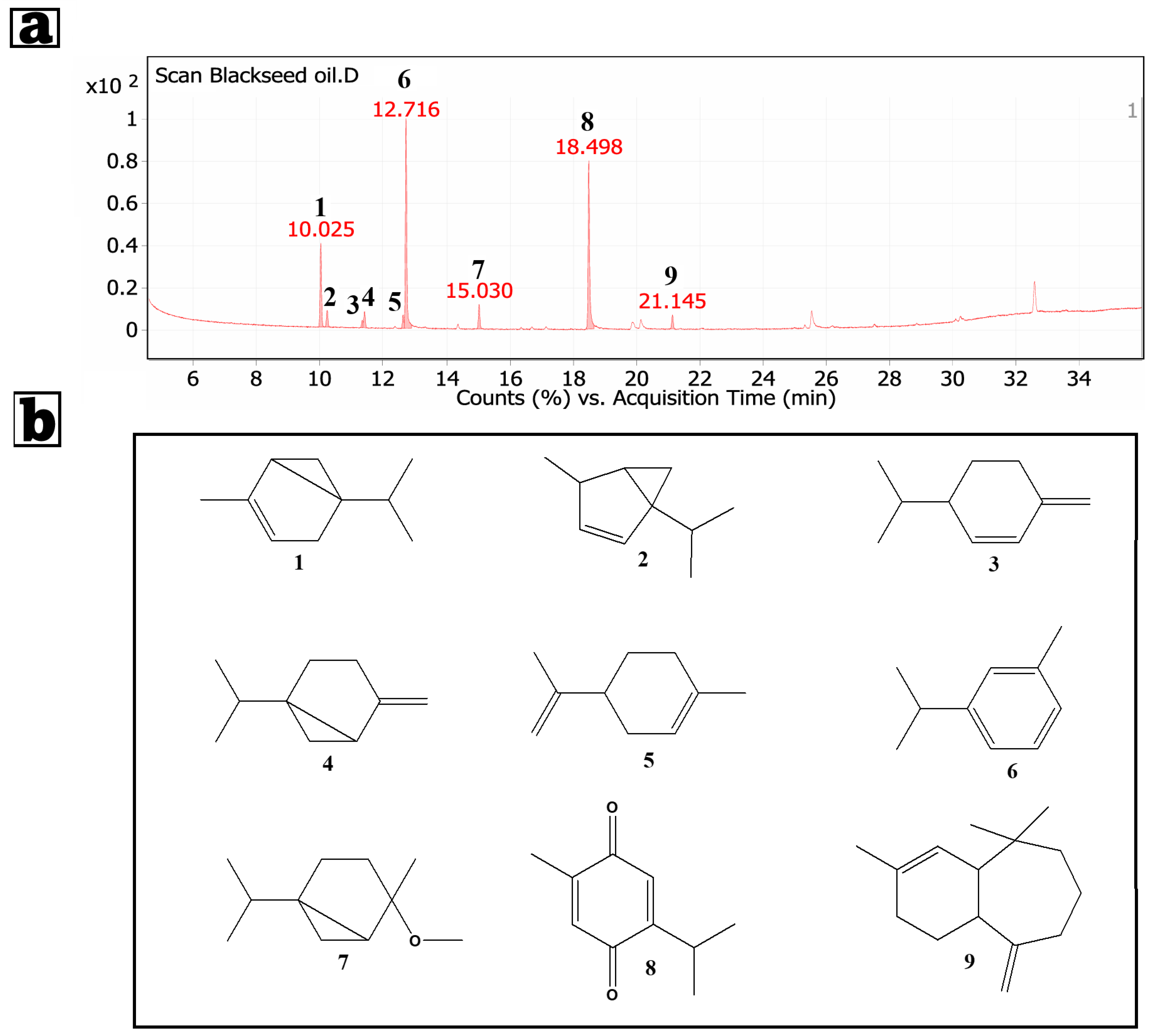
3.1. GC/MS Analysis of the Petroleum Ether Extract of Nigella sativa Seeds
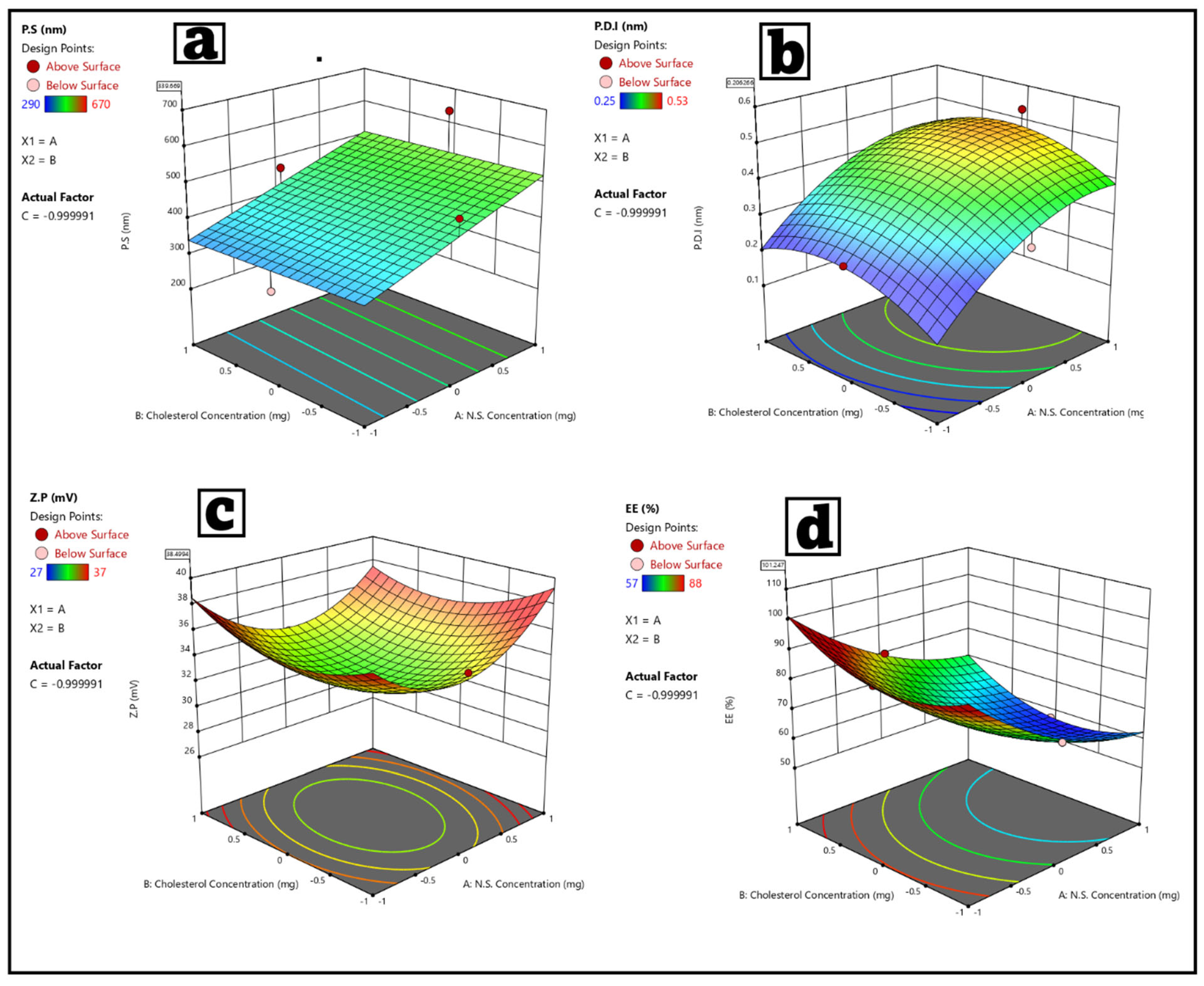
3.2. Statistical Analysis of the Factorial Experimental Design Fisetin–Nigellasomes (FIS-NSs)
3.3. Evaluation of FIS-NSs Formulations
3.3.1. Particle Size, Polydispersity Index, and Zeta Potential
Particle Size (P.S)
Polydispersity Index (P.D.I)
Zeta Potential (Z.P)
3.3.2. Entrapment Efficiency (E.E%)
3.3.3. Optimization of FIS-NSs Formulations
3.4. Evaluation of the Optimized Fisetin–Nigellasomes (FIS-NSs)
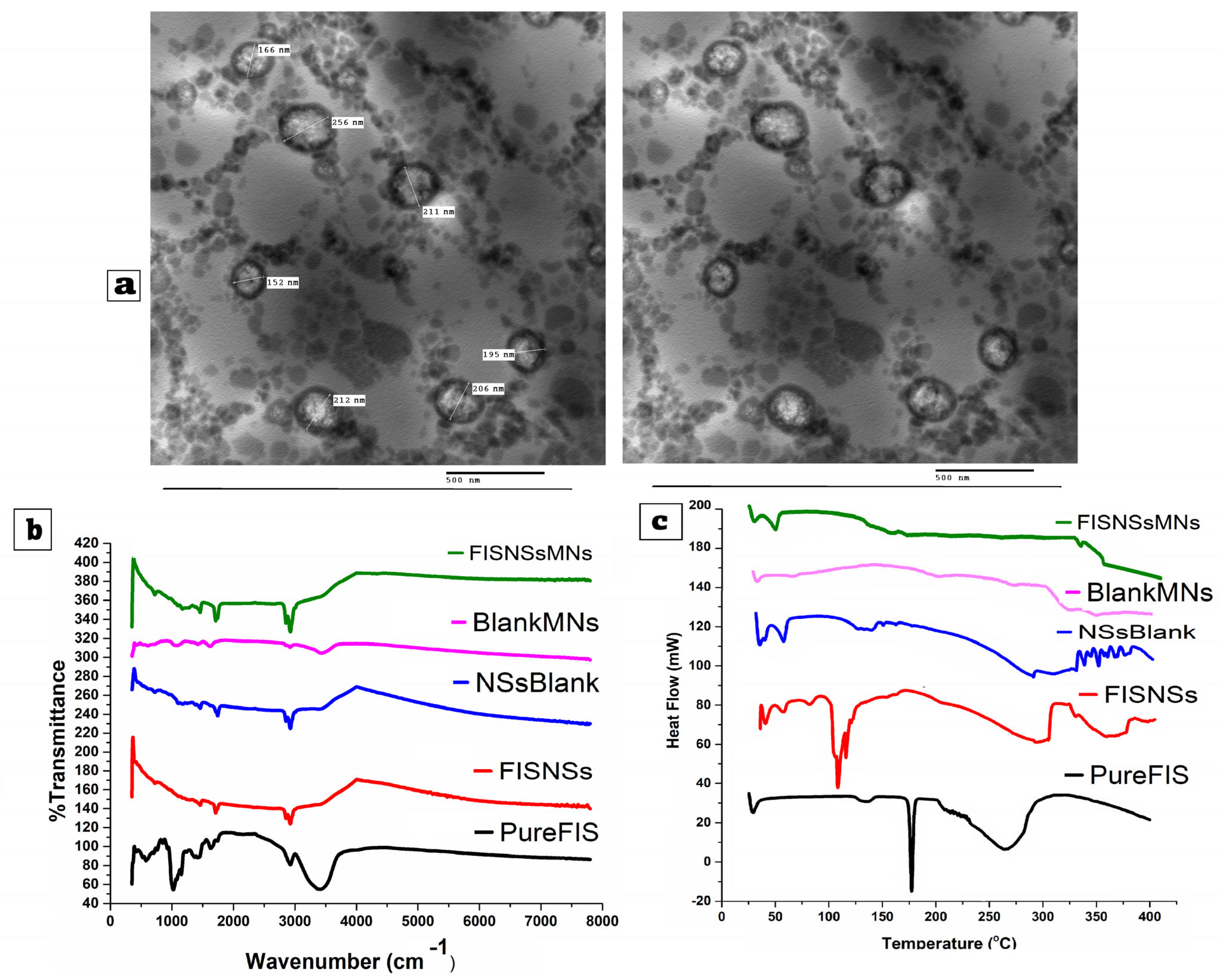
3.4.1. Transmission Electron Microscopy (TEM)
3.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.3. Differential Scanning Calorimetry (DSC)
3.5. Stability Study of the Optimized Fisetin–Nigellasomes (FIS-NSs)
3.6. Physical Characteristics of the Carbohydrate-Based Hybrid Dissolving Microneedles (G/HA/CMC MNs)
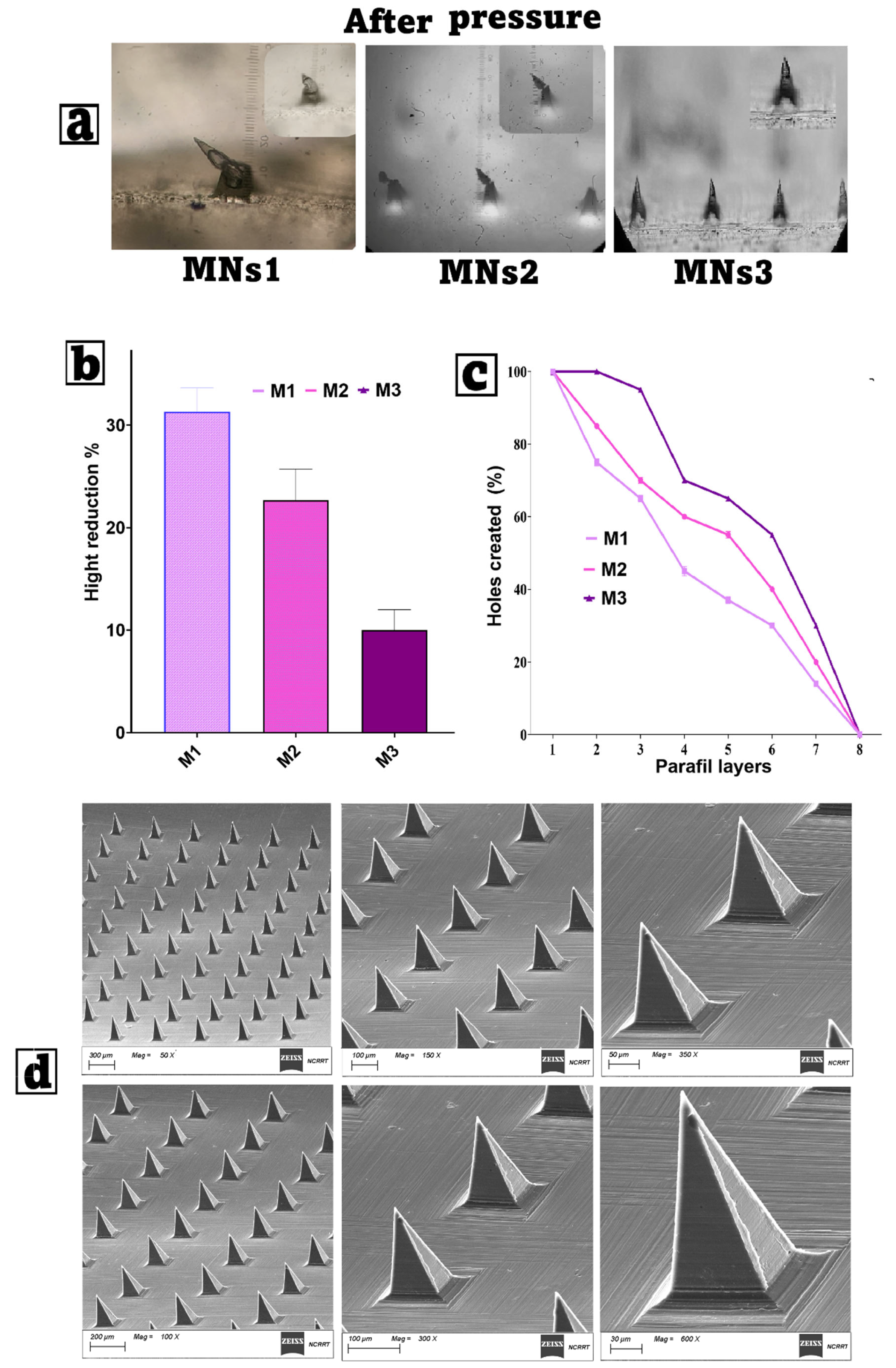
3.6.1. Mechanical Strength and Penetration Capability Test
3.6.2. Determination of Drug Content
3.6.3. Dissolution Time Assessment
3.6.4. Water Loss During Drying (LOD) Test
3.7. Characterization of Optimized Carbohydrate-Based Hybrid Dissolving Microneedles
3.7.1. Scanning Electron Microscopy (SEM)
3.7.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.7.3. Differential Scanning Calorimetry (DSC)
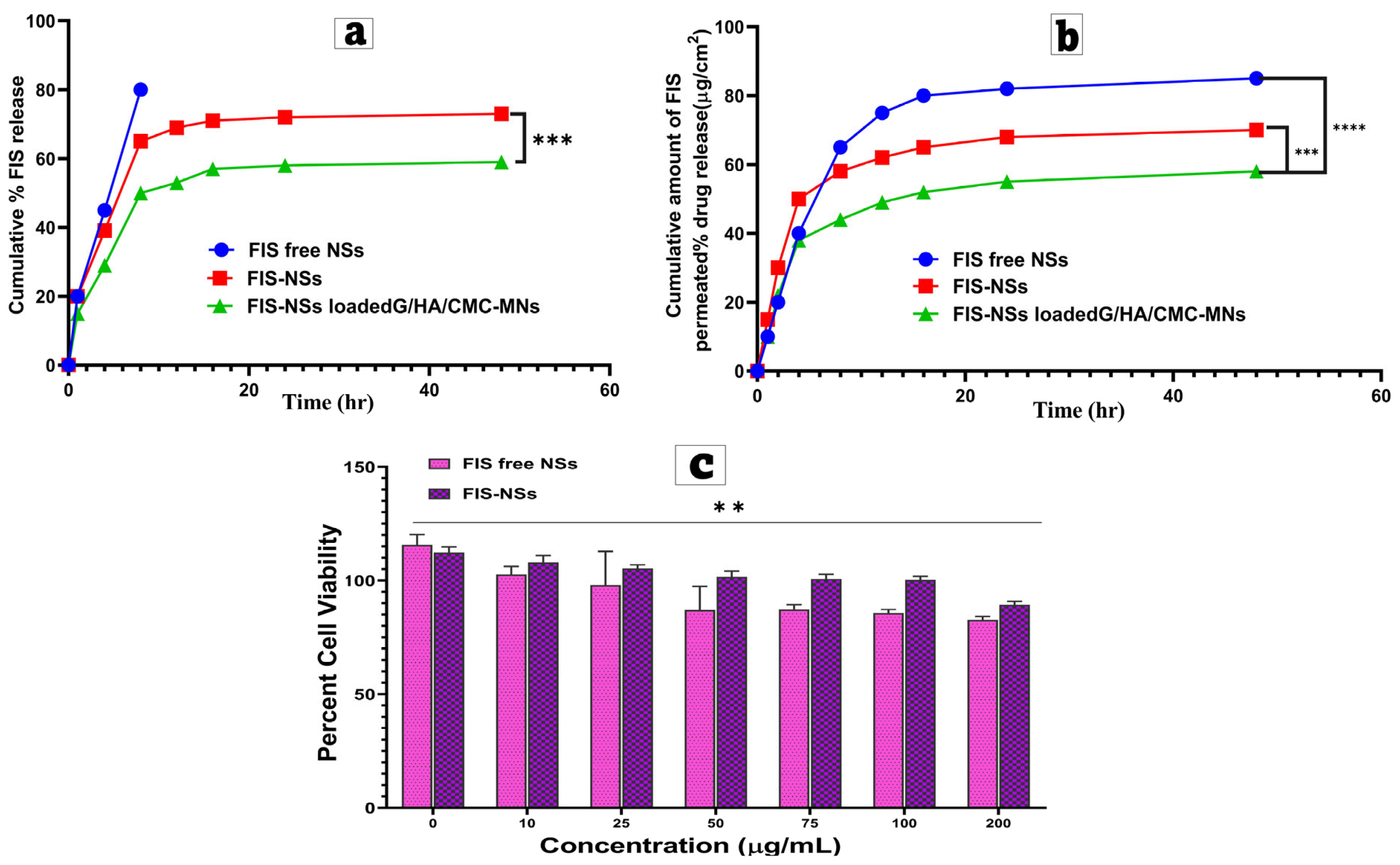
3.8. In Vitro Drug Release Study
3.9. Ex Vivo Permeation Study and Kinetic Analysis
3.10. In Vitro Cell Culture Studies
3.11. In Vivo Study
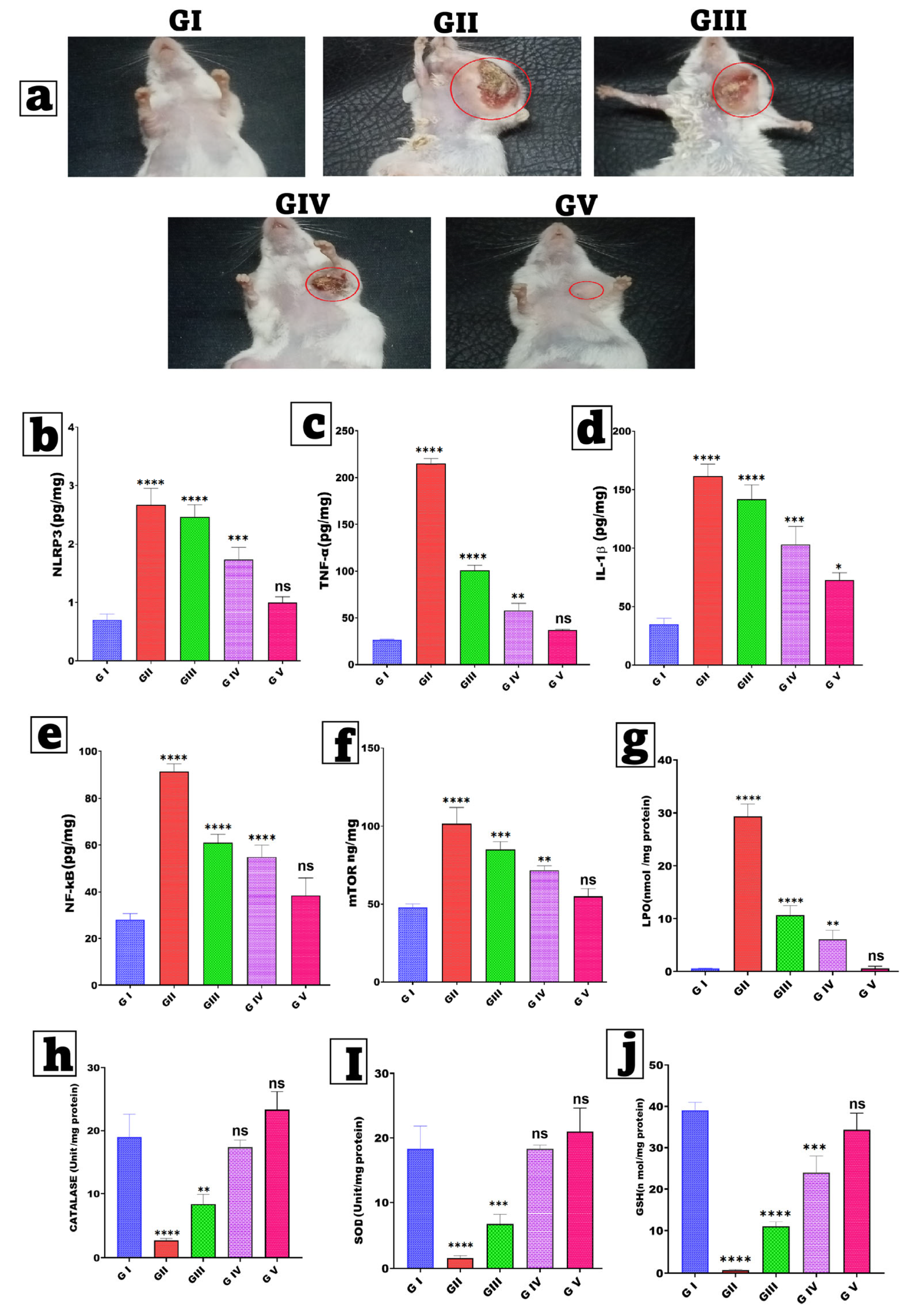
3.11.1. Estimation of Skin Morphology and Body Weight
3.11.2. Assessment of Inflammatory Biomarkers
3.11.3. Estimation of Tumor Parameters
3.11.4. Assay for Oxidative Stress Parameters
3.11.5. Liver Function Tests (LFTs), Kidney Function Tests, Sex Hormones, and Lipid Profile
3.12. Histopathological Analysis
3.13. Immunohistochemical (IHC) Examination of Caspase-3
4. Future Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FIS | Fisetin |
| NSs | Nigella sativa Nanovesicles |
| FIS-NSs | Fisetin-loaded Nigella sativa Nanovesicles |
| MNs | Microneedles |
| FIS-NSs-MNs | Fisetin-loaded Nigella sativa Nanovesicle Microneedles |
| GC | Gas Chromatography Analysis |
| HA | Hyaluronic Acid |
| CMC | Carboxymethyl Cellulose |
| SDC | Sodium Deoxycholate |
| TEM | Transmission Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| DSC | Differential Scanning Calorimetry |
| PDI | Polydispersity Index |
| IC50 | Half Maximal Inhibitory Concentration |
| MMT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide Assay |
| MDA | Malondialdehyde |
| CAT | Catalase |
| SOD | Superoxide Dismutase |
| GSH | Reduced Glutathione |
| TNF-α | Tumor Necrosis Factor-Alpha |
| IL-1β | Interleukin-1 Beta |
| NF-κB | Nuclear Factor Kappa B |
| mTOR | Mechanistic Target of Rapamycin |
| BNP | Benoxinate Hydrochloride |
References
- Jeganathan, A.; Arunachalam, K.; Byju, A.; Rani George, A.; Sajeev, S.; Thangasamy, K.; Natesan, G. Chitosan Nanoparticle-Mediated Delivery of Alstonia venenata R.Br. Root Methanolic Extract: A Promising Strategy for Breast Cancer Therapy in DMBA-Induced Breast Cancer in Sprague Dawley Rats. Antioxidants 2024, 13, 1513. [Google Scholar] [CrossRef]
- Avtandilyan, N.; Javrushyan, H.; Ginovyan, M.; Karapetyan, A.; Trchounian, A. Anti-Cancer Effect of in Vivo Inhibition of Nitric Oxide Synthase in a Rat Model of Breast Cancer. Mol. Cell. Biochem. 2023, 478, 261–275. [Google Scholar] [CrossRef]
- Meiyanto, E.; Hermawan, A.; Anindyajati, A. Natural Products for Cancer-Targeted Therapy: Citrus Flavonoids as Potent Chemopreventive Agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Karnam, K.C.; Ellutla, M.; Bodduluru, L.N.; Kasala, E.R.; Uppulapu, S.K.; Kalyankumarraju, M.; Lahkar, M. Preventive Effect of Berberine against DMBA-Induced Breast Cancer in Female Sprague Dawley Rats. Biomed. Pharmacother. 2017, 92, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.K.S.; How, C.W.; Low, L.E.; Ong, B.H.; Loh, J.S.; Lim, S.Y.; Ong, Y.S.; Foo, J.B. Magnetic-Guided Targeted Delivery of Zerumbone/SPION Co-Loaded in Nanostructured Lipid Carrier into Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2023, 87, 104830. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Seabra, H.A.; Oliveira, M.S.; Carregal, V.M.; Vilela, J.M.C.; Andrade, M.S.; Townsend, D.M.; Colletti, P.M.; Leite, E.A.; et al. α- Tocopherol Succinate Loaded Nano-Structed Lipid Carriers Improves Antitumor Activity of Doxorubicin in Breast Cancer Models in Vivo. Biomed. Pharmacother. 2018, 103, 1348–1354. [Google Scholar] [CrossRef]
- Karim, S.; Alghanmi, A.N.; Jamal, M.; Alkreathy, H.; Jamal, A.; Alkhatabi, H.A.; Bazuhair, M.; Ahmad, A. A Comparative in Vitro Study on the Effect of SGLT2 Inhibitors on Chemosensitivity to Doxorubicin in MCF-7 Breast Cancer Cells. Oncol. Res. 2024, 32, 817. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; Ibrahim, Y.F.; Ewedah, T.M.; Mousa, I.S.; Allam, K.M.; Hamdan, A.M.E. Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer. Pharmaceutics 2025, 17, 611. [Google Scholar] [CrossRef]
- Al-Zuhairy, S.A.K.S.; Elhabal, S.F.; Mohamed Elrefai, M.F.; Hababeh, S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; Ewedah, T.M.; Mousa, I.S.; Hamdan, A.M.E. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a PH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals 2025, 18, 381. [Google Scholar] [CrossRef]
- Hamza, A.A.; Khasawneh, M.A.; Elwy, H.M.; Hassanin, S.O.; Elhabal, S.F.; Fawzi, N.M. Salvadora persica Attenuates DMBA-Induced Mammary Cancer through Downregulation Oxidative Stress, Estrogen Receptor Expression and Proliferation and Augmenting Apoptosis. Biomed. Pharmacother. 2022, 147, 112666. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Al-Zuhairy, S.A.; El-Nabarawi, M.A.; Mohamed Elrefai, M.F.; Shoela, M.S.; Hababeh, S.; Nelson, J.; Khalek, M.A.; Fady, M.; Elzohairy, N.A.; et al. Enhancing Photothermal Therapy for Antibiofilm Wound Healing: Insights from Graphene Oxide-Cranberry Nanosheet Loaded Hydrogel in Vitro, in Silico, and in Vivo Evaluation. Int. J. Nanomed. 2024, 19, 12999–13027. [Google Scholar] [CrossRef]
- Tamizmaran, V.; Mannur, V.S.; Koli, R.; Biradar, P. Novel Intranasal Fisetin-Loaded Mucoadhesive Microemulsion for Schizophrenia Management: A Nanotherapeutic Approach to Enhance Brain Bioavailability and Improved Efficacy. Mol. Pharm. 2025, 22, 4293–4313. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Lin, C.C.; Hsieh, Y.S.; Wu, Y.T. Nanoformulation Development to Improve the Biopharmaceutical Properties of Fisetin Using Design of Experiment Approach. Molecules 2021, 26, 3031. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and Optimization of Fisetin Loaded Glycerol Based Soft Nanovesicles by Box-Behnken Design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef]
- Kansom, T.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Opanasopit, P.; Suriyaamporn, P. Development and Evaluation of Mangosteen Extract-Loaded Dissolving Microneedle for Acne Treatment. J. Drug Deliv. Sci. Technol. 2025, 112, 107273. [Google Scholar] [CrossRef]
- Telrandhe, U.B.; Hasnain, A.N.; Kothawade, S.N.; Telange, D.R. Recent Advancement of Fisetin-Based Nanoformulations in the Management of Psoriasis. Discov. Nano 2025, 20, 105. [Google Scholar] [CrossRef]
- Yadav, N.; Mohanty, S.K.; Chandramohan, S.; Archana, V.K.; Suchiang, K.; Rajagopalan, R. Exploration of Mechanism of Anticancer Potential of Fisetin Nanoformulations: Nanotized Fisetin/Fisetin Loaded Polymannose Nanoparticles in the Treatment of Triple Negative Breast Carcinoma. 3 Biotech 2025, 15, 267. [Google Scholar] [CrossRef]
- Akash, S.; Rahman, M.M.; Lima, C.M.G.; Bin Emran, T.; Sultana, S.; Naz, S.; Aziz, T.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Design and Development of New Inhibitors against Breast Cancer, Monkeypox and Marburg Virus by Modification of Natural Fisetin via in Silico and SAR Studies. Acta Biochim. Pol. 2023, 70, 599–608. [Google Scholar] [CrossRef]
- Lin, M.T.; Lin, C.L.; Lin, T.Y.; Cheng, C.W.; Yang, S.F.; Lin, C.L.; Wu, C.C.; Hsieh, Y.H.; Tsai, J.P. Synergistic Effect of Fisetin Combined with Sorafenib in Human Cervical Cancer HeLa Cells through Activation of Death Receptor-5 Mediated Caspase-8/Caspase-3 and the Mitochondria-Dependent Apoptotic Pathway. Tumor Biol. 2016, 37, 6987–6996. [Google Scholar] [CrossRef]
- Sherif, A.H.; Elkasef, M.; Mahfouz, M.E.; Kasem, E.A. Impacts of Dietary Zinc Oxide Nanoparticles on the Growth and Immunity of Nile Tilapia Could Be Ameliorated Using Nigella sativa Oil. J. Trace Elem. Med. Biol. 2023, 79, 127265. [Google Scholar] [CrossRef]
- Dalli, M.; Daoudi, N.E.; Azizi, S.; Roubi, M.; Alami Merrouni, I.; Souna, F.; Choukri, M.; Kim, B.; Gseyra, N. Harnessing the Hepatoprotective and Nephroprotective Potential of Nigella sativa Fractions via per Os Administration in CCl4-Intoxicated Wistar Rats: A Mixed Approach. Pharmaceuticals 2025, 18, 1147. [Google Scholar] [CrossRef]
- Osanloo, A.-M.; Hamze, F.; Haghgoo, R.; Mashhadi-Abbas, F.; Papi, F. Evaluating the Cytotoxicity and Efficacy of Nanoemulsion Containing Propolis plus Nigella sativa Nanoparticles for Pulpotomy of Primary Teeth: Randomised Split-Mouth Clinical Trial Pilot Study. Eur. Arch. Paediatr. Dent. 2025, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Luff, M.; Evans, L.; Hiyari, S.; Kwan, K.; Cameron, B.; Miller, A.; St. John, M.; Alhiyari, Y. Nigella sativa Oil Mitigates Xerostomia and Preserves Salivary Function in Radiotherapy-Treated Mice. Laryngoscope Investig. Otolaryngol. 2023, 8, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Bakr, M.A. Will Nigella sativa Oil Protect Parotid Glands of Rats against Cranium Gamma Irradiation? Histological and Immunohistochemical Evaluation. BMC Complement. Med. Ther. 2024, 24, 111. [Google Scholar] [CrossRef] [PubMed]
- Mouket, S.; Demir, E.; Yucel, A.; Taysi, S. Nigella sativa Oil Reduces Oxidative/Nitrosative Stress in the Salivary Gland of Rats Exposed to Total Cranial Irradiation. Drug Chem. Toxicol. 2023, 46, 1051–1056. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured Lipid Carriers, Solid Lipid Nanoparticles, and Polymeric Nanoparticles: Which Kind of Drug Delivery System Is Better for Glioblastoma Chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef]
- Heikal, L.A.; Ashour, A.A.; Aboushanab, A.R.; El-Kamel, A.H.; Zaki, I.I.; El-Moslemany, R.M. Microneedles Integrated with Atorvastatin-Loaded Pumpkisomes for Breast Cancer Therapy: A Localized Delivery Approach. J. Control. Release 2024, 376, 354–368. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Hicks, M. Ufasomes Are Stable Particles Surrounded by Unsaturated Fatty Acid Membranes. Nature 1973, 243, 232–234. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Farahat, M.S.; Teaima, M.H.; Elzohairy, N.A.; El-Nabarawi, M. Innovate Sodium Alginate Microneedle Patches Integrated with Soft Lidocaine Invasomes: Advanced Strategies for Oral Ulcerative Mucositis Treatment via TNF-α/NF-ΚB Pathways. Drug Deliv. Transl. Res. 2025, 15, 1–26. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Al-Zuhairy, S.A.-K.S.; Elrefai, M.F.M.; El-Nabarawi, M.A.; Hababeh, S.; Attalla, K.Z.; Shoela, M.S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; et al. Chitosan-Based Intelligent Microneedles for Delivery of Amphotericin B Loaded Oleosomes: Antifungal Ocular Patch Targeting for Effective Against Fungal Keratitis Using Rabbit Model via TLR4/NLRP3 Pathway. Int. J. Nanomed. 2025, 20, 5949–5981. [Google Scholar] [CrossRef]
- Mahdy, N.K.; El-Mosallamy, A.E.M.K.; Mohamed, E.A.; Teaima, M.H.; El-Said Azzazy, H.M.; El-Nabarawi, M.; Elhabal, S.F. Carvedilol-Loaded Propolis Nanoparticles Embedded in Gel-Casted Film and 3D Electrospun Nanofiber Film—An in Vivo Study to Enhance the Bioavailability via the Intranasal Route. J. Drug Deliv. Sci. Technol. 2025, 112, 107254. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; AbdelGhany Morsy, S.A.; Ewedah, T.M.; Mousa, I.S.; Fouad, M.A.; Hamdan, A.M.E. Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease. Pharmaceutics 2025, 17, 653. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Ashour, H.A.; Elrefai, M.F.M.; Teaima, M.H.; Elzohairy, N.A.; El-Nabarawi, M. Innovative Transdermal Delivery of Microneedle Patch for Dual Drugs Febuxostat and Lornoxicam: In Vitro and in Vivo Efficacy for Treating Gouty Arthritis. J. Drug Deliv. Sci. Technol. 2025, 110, 107053. [Google Scholar] [CrossRef]
- Elhabal, S.F.; El-Nabarawi, M.; Elrefai, M.F.M.; Teaima, M.H.; Shoela, M.S.; Khamis, G.M.; Faheem, A.M.; Kholeif, N.A.; Sanad, M.T. Nano-Spanlastics-Loaded Dissolving Microneedle Patches for Ketotifen Fumarate: Advanced Strategies for Allergic Conjunctivitis Treatment and Molecular Insights. Drug Deliv. Transl. Res. 2025, 15, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yue, J.; Guo, S.; Rahmatulla, A.; Li, S.; Liu, Y.; Chen, Y. Dissolving Microneedles: A Transdermal Drug Delivery System for the Treatment of Rheumatoid Arthritis. Int. J. Pharm. 2025, 671, 125206. [Google Scholar] [CrossRef]
- Nagra, U.; Barkat, K.; Ashraf, M.U.; Shabbir, M. Feasibility of Enhancing Skin Permeability of Acyclovir through Sterile Topical Lyophilized Wafer on Self-Dissolving Microneedle-Treated Skin. Dose-Response 2022, 20, 15593258221097594. [Google Scholar] [CrossRef]
- Sartawi, Z.; Blackshields, C.; Faisal, W. Dissolving Microneedles: Applications and Growing Therapeutic Potential. J. Control. Release 2022, 348, 186–205. [Google Scholar] [CrossRef]
- Demir, E.; Taysi, S.; Ulusal, H.; Kaplan, D.S.; Cinar, K.; Tarakcioglu, M. Nigella sativa Oil and Thymoquinone Reduce Oxidative Stress in the Brain Tissue of Rats Exposed to Total Head Irradiation. Int. J. Radiat. Biol. 2020, 96, 228–235. [Google Scholar] [CrossRef]
- Akyuz, M.; Taysi, S.; Baysal, E.; Demir, E.; Alkis, H.; Akan, M.; Binici, H.; Karatas, Z.A. Radioprotective Effect of Thymoquinone on Salivary Gland of Rats Exposed to Total Cranial Irradiation. Head Neck 2017, 39, 2027–2035. [Google Scholar] [CrossRef]
- Ali, M.L.; Jaber, M.A.; Hasan, Z.; Proma, N.M.; Hoque, N.; Tipu, M.T.R.; Ahamed, K.U.; Akter, B.; Khanam, B.H.; Hossain, M.K. Phytochemical and Bioactivity Profiling of Unconventional Food Plant, Boehmeria Caudata Leaves: FT-IR, GC-MS, Experimental, and In Silico Investigation. Food Sci. Nutr. 2025, 13, e70953. [Google Scholar] [CrossRef]
- Chung, K.X.; Wei, P.L.Y.; Akowuah, G.A. Different Extraction Methods and Solvents for Thymoquinone from Nigella sativa L. Seeds. Curr. Trends Biotechnol. Pharm. 2023, 17, 1–7. [Google Scholar] [CrossRef]
- Taleuzzaman, M. Liquid-Liquid Extraction Method Developed for Thymoquinone from Seed Powder of Nigella sativa, Characterized It by UVspectrophotometer. J. Med. Org. Chem. 2018, 1, 2. [Google Scholar]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of Transethosomes Formulation for Dermal Fisetin Delivery: Box–Behnken Design, Optimization, in Vitro Skin Penetration, Vesicles–Skin Interaction and Dermatokinetic Studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; El-Nabarawi, M.; Teaima, M.H.; Abdel-Haleem, K.M.; Ragab, G.M.; Abo-Zalam, H.B.; Elhabal, S.F. Intranasal Delivery of Levocetirizine via Transethosomal Thermosensitive in Situ Gel: A Promising Strategy for Enhanced Nasal Absorption and Antihistamine Action in Allergic Rhinitis. J. Drug Deliv. Sci. Technol. 2025, 114, 107452. [Google Scholar] [CrossRef]
- ELhabal, S.F.; El-Nabarawi, M.A.; Hassanin, S.O.; Hassan, F.E.; Abbas, S.S.; Gebril, S.M.; Albash, R. Transdermal Fluocinolone Acetonide Loaded Decorated Hyalurosomes Cellulose Acetate/Polycaprolactone Nanofibers Mitigated Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats. J. Pharm. Investig. 2024, 55, 113–132. [Google Scholar] [CrossRef]
- Azhari, H.; Younus, M.; Hook, S.M.; Boyd, B.J.; Rizwan, S.B. Cubosomes Enhance Drug Permeability across the Blood–Brain Barrier in Zebrafish. Int. J. Pharm. 2021, 600, 120411. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.M.; Abdelmonem, R.A.A.B.; El-Nabarawi, M.A.; Elhabal, S.F. Sucrose Acetate Isobutyrate (SAIB) and Glyceryl Monooleate (GMO) Hybrid Nanoparticles for Bioavailability Enhancement of Rivaroxaban: An Optimization Study. Pharm. Dev. Technol. 2023, 28, 928–938. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Abdelmonem, R.A.A.B.; El Nashar, R.M.; Mohamed Elrefai, M.F.; Hamdan, A.M.; Safwat, N.A.; Shoela, M.S.; Hassan, F.E.S.; Rizk, A.; Kabil, S.L.; et al. Enhanced Antibacterial Activity of Clindamycin Using Molecularly Imprinted Polymer Nanoparticles Loaded with Polyurethane Nanofibrous Scaffolds for the Treatment of Acne vulgaris. Pharmaceutics 2024, 16, 947. [Google Scholar] [CrossRef]
- Zarif Attalla, K.; Hassan, D.H.; Teaima, M.H.; Yousry, C.; El-Nabarawi, M.A.; Said, M.A.; Elhabal, S.F. Enhanced Intranasal Delivery of Atorvastatin via Superparamagnetic Iron-Oxide-Loaded Nanocarriers: Cytotoxicity and Inflammation Evaluation and In Vivo, In Silico, and Network Pharmacology Study for Targeting Glioblastoma Management. Pharmaceuticals 2025, 18, 421. [Google Scholar] [CrossRef]
- Awadeen, R.H.; Boughdady, M.F.; Zaghloul, R.A.; Elsaed, W.M.; Abu Hashim, I.I.; Meshali, M.M. Plausible Role of Oral Fisetin-Loaded Chitosan Oligosaccharide Nanoparticles in Amelioration of Benign Prostatic Hypertrophy: In Vitro and in Vivo Assessments. J. Drug Deliv. Sci. Technol. 2024, 93, 105423. [Google Scholar] [CrossRef]
- Ewedah, T.M.; Abdalla, A.M.; Hagag, R.S.; Elhabal, S.F.; Teaima, M.H.M.; El-Nabarawi, M.A.; Schlatter, G.; Shoueir, K.R. Enhancing Cellular Affinity for Skin Disorders: Electrospun Polyurethane/Collagen Nanofiber Mats Coated with Phytoceramides. Int. J. Pharm. 2024, 663, 124541. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of Nanosuspensions in Drug Delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Barkat, K.; Ashraf, M.U.; Nagra, U. Development of a Novel Self-Dissolving Microneedle-Assisted Percutaneous Delivery System of Diacerein through Solid Dispersion Gel: Solubility Enhancement, Proof of Anti-Inflammatory Activity and Safety. Curr. Drug Deliv. 2022, 20, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; Sutrisna, L.F.P.; Harahap, Y.; Putri, K.S.S.; Ulayya, F.; Hartrianti, P.; Anjani, Q.K.; Donnelly, R.F. Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers. Pharmaceutics 2023, 15, 289. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Udomsom, S.; Rachtanapun, P.; Piboon, P.; Jantrawut, P. Determination of Vat-Photopolymerization Parameters for Microneedles Fabrication and Characterization of HPMC/PVP K90 Dissolving Microneedles Utilizing 3D-Printed Mold. Sci. Rep. 2024, 14, 16174. [Google Scholar] [CrossRef]
- Matadh, A.V.; Jakka, D.; Pragathi, S.G.; Poornima, K.; Shivakumar, H.N.; Murthy, R.N.; Rangappa, S.; Shivanna, M.; Murthy, S.N. Polymer Coated Polymeric Microneedles for Intravitreal Delivery of Dexamethasone. Exp. Eye Res. 2023, 231, 109467. [Google Scholar] [CrossRef]
- Faizi, H.S.; Nasiri, M.I.; Wu, Y.; Mishra, D.; Donnelly, R.F.; Minhas, M.U.; Vora, L.K.; Singh Thakur, R.R. Deferasirox Nanosuspension Loaded Dissolving Microneedles for Ocular Drug Delivery. Int. J. Pharm. 2024, 664, 124614. [Google Scholar] [CrossRef]
- Rad, Z.F.; Nordon, R.E.; Anthony, C.J.; Bilston, L.; Prewett, P.D.; Arns, J.Y.; Arns, C.H.; Zhang, L.; Davies, G.J. High-Fidelity Replication of Thermoplastic Microneedles with Open Microfluidic Channels. Microsyst. Nanoeng. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, G.; Chen, Y.; Lin, N.; Ma, J. Poly(Lactide Acid)-Based Microneedles Enhanced by Tunicate Cellulose Nanocrystals for Potential Diabetic Periodontitis Treatment. Carbohydr. Polym. 2025, 361, 123629. [Google Scholar] [CrossRef]
- Abu Ershaid, J.M.; Zhang, H.; Tayyem, M.; Sabri, A.H.; Donnelly, R.F.; Vora, L.K. Sodium Alginate Microneedles Loaded with Vancomycin for Skin Infections. J. Funct. Biomater. 2024, 15, 316. [Google Scholar] [CrossRef]
- Asaf, M.B.; Khairiyah; Kurniawan, I.; Achmad, N.A.; Tuna, R.W.; Himawan, A.; Rahman, L.; Aliyah; Agustina, R.; Dominguez-Robles, J.; et al. Amphotericin B Nanocrystals Integrated with Bilayer Dissolving Microneedles: A New Strategy for Transmucosal Delivery of Amphotericin B to Improve the Effectiveness of Oral Candidiasis Therapy. J. Pharm. Investig. 2025, 55, 1–19. [Google Scholar] [CrossRef]
- Saady, M.; Shoman, N.A.; Teaima, M.H.M.; Abdelmonem, R.A.A.B.; El-Nabarawi, M.A.; Elhabal, S.F. Fabrication of Gastro-Floating Sustained-Release Etoricoxib and Famotidine Tablets: Design, Optimization, in-Vitro, and in-Vivo Evaluation. Pharm. Dev. Technol. 2024, 29, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Kral, Ö.; Ilbasmis-Tamer, S.; Han, S.; Tirnaksiz, F. Development of Dermal Lidocaine Nanosuspension Formulation by the Wet Milling Method Using Experimental Design: In Vitro/In Vivo Evaluation. ACS Omega 2024, 9, 50992–51008. [Google Scholar] [CrossRef]
- Todo, H. Transdermal Permeation of Drugs in Various Animal Species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef]
- Qaiser, R.; Pervaiz, F.; Noreen, S.; Hanan, H.; Shoukat, H.; Mahmood, H.; Ashraf, M.A. Optimizing Lornoxicam-Loaded Poly(Lactic-Co-Glycolic Acid) and (Polyethylene Glycol) Nanoparticles for Transdermal Delivery: Ex Vivo/in Vivo Inflammation Evaluation. Nanomedicine 2024, 19, 1471–1485. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, T.; Tan, D.; Liu, J.; Gao, Y.; Wang, H.; Gao, F.; Yang, Z. Preparation, Characterization, and Ex Vivo Evaluation of Isoxanthohumol Nanosuspension. Int. J. Pharm. 2024, 667, 124909. [Google Scholar] [CrossRef]
- Raghav, S.S.; Kumar, B.; Sethiya, N.K.; Pahwa, S. Development and Optimization of Kaempferol Loaded Ethosomes Using Box–Behnken Statistical Design: In Vitro and Ex-Vivo Assessments. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35394. [Google Scholar] [CrossRef]
- Mohanty, D.L.; Divya, N.; Zafar, A.; Warsi, M.H.; Parida, G.R.; Padhi, P.; Khalid, M.; Yasir, M.; Mujtaba, M.A. Development of Etoricoxib-Loaded Mesoporous Silica Nanoparticles Laden Gel as Vehicle for Transdermal Delivery: Optimization, Ex Vivo Permeation, Histopathology, and in Vivo Anti-Inflammatory Study. Drug Dev. Ind. Pharm. 2025, 51, 506–521. [Google Scholar] [CrossRef]
- Raab, C.; Brugger, S.; Lechner, J.-S.; Barbalho, G.N.; Gratieri, T.; Agarwal, P.; Rupenthal, I.D.; Keck, C.M. Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations. Pharmaceutics 2024, 16, 1302. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Macedo, C.; Estevinho, B.; Sut, S.; Dall’Acqua, S.; Delerue-Matos, C.; Costa, P.C.; Rodrigues, F. Design of Advanced Buccal Films with Kiwiberry Extract to Prevent Oral Mucositis: From in Vitro Buccal Models to Ex Vivo Studies. J. Drug Deliv. Sci. Technol. 2024, 96, 105725. [Google Scholar] [CrossRef]
- Eliaa, S.G.; Al-Karmalawy, A.A.; Saleh, R.M.; Elshal, M.F. Empagliflozin and Doxorubicin Synergistically Inhibit the Survival of Triple-Negative Breast Cancer Cells via Interfering with the MTOR Pathway and Inhibition of Calmodulin: In Vitro and Molecular Docking Studies. ACS Pharmacol. Transl. Sci. 2020, 3, 1330–1338. [Google Scholar] [CrossRef]
- Wang, W.-J.; Liu, J.-S.; Kha, N.H.A.; Shen, W.-J.; Chen, C.-J.; Chen, S.-C.; Liao, P.-C.; Huang, C.-Y.; Kuo, W.-W. MiR-6126 Modulates GRP78 to Suppress the Warburg Effect and Mitochondrial Dynamics in Triple-Negative Breast Cancer. Int. J. Med. Sci. 2025, 22, 3598–3616. [Google Scholar] [CrossRef]
- Bidabad, S.; Ahmadpour Yazdi, H.; Zolghadr, L.; Valivand, N.; Gheibi, N. Evaluation of Liposome Encapsulated Propolis Nanoparticles on Cell Proliferation and Apoptosis in A375 Melanoma Cancer Cell Line. Food Sci. Nutr. 2025, 13, e70303. [Google Scholar] [CrossRef]
- Quiroz, F.G.; Posada, O.M.; Gallego-Perez, D.; Higuita-Castro, N.; Sarassa, C.; Hansford, D.J.; Agudelo-Florez, P.; López, L.E. Housekeeping Gene Stability Influences the Quantification of Osteogenic Markers during Stem Cell Differentiation to the Osteogenic Lineage. Cytotechnology 2010, 62, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Ünalmış Aykar, D.; Ülger, H.; Doganyiğit, Z.; Şeker Karatoprak, G.; Pandır, D.; Uçar, S.; Kaymak, E.; Okan Oflamaz, A.; Yılmaz, S. Antitumor Effects of Dadas Cress (Lepidium sativum var. sativum) on Ehrlich Ascites Tumor Cells: An in Vitro and in Vivo Study. J. Mol. Histol. 2025, 56, 307. [Google Scholar] [CrossRef]
- Radulski, D.R.; Stipp, M.C.; Galindo, C.M.; Acco, A. Features and Applications of Ehrlich Tumor Model in Cancer Studies: A Literature Review. Transl. Breast Cancer Res. 2023, 4, 22. [Google Scholar] [CrossRef]
- Alsunbul, M.; El-Masry, T.A.; El-Bouseary, M.M.; El Zahaby, E.I.; El-Sheekh, M.M.; Gaballa, M.M.S.; Wahsh, E.; Badawy, H.K.; Alamoudi, J.A.; ALQahtani, R.; et al. Evaluating the Anticancer Efficacy of Chara Vulgaris Ethanolic Extract and Selenium Nanoformulation in Ehrlich Carcinoma Mice: Role of Autophagy and Apoptosis. BMC Biotechnol. 2025, 25, 53. [Google Scholar] [CrossRef]
- Teaima, M.H.; Badawi, N.M.; Attia, D.A.; El-Nabarawi, M.A.; Elmazar, M.M.; Mousa, S.A. Efficacy of Pomegranate Extract Loaded Solid Lipid Nanoparticles Transdermal Emulgel against Ehrlich Ascites Carcinoma. Nanomedicine 2022, 39, 102466. [Google Scholar] [CrossRef]
- Aboushanab, A.R.; El-Moslemany, R.M.; El-Kamel, A.H.; Mehanna, R.A.; Bakr, B.A.; Ashour, A.A. Targeted Fisetin-Encapsulated β-Cyclodextrin Nanosponges for Breast Cancer. Pharmaceutics 2023, 15, 1480. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabanah, O.A.; Osman, A.M.; Al-Harbi, M.M.; Al-Gharably, N.M.; Al-Bekairi, A.M. Enhancement of Doxorubicin-Induced Cytotoxicity by Hyperthermia in Ehrlich Ascites Cells. Chemotherapy 1994, 40, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lo, C.L.; Lin, Y.F.; Hsiue, G.H. Rapamycin Encapsulated in Dual-Responsive Micelles for Cancer Therapy. Biomaterials 2013, 34, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Fotsing Fongang, Y.S.; Ossomba, E.R.; Tatsinda, V.; Silihe, K.K.; Mbou, W.D.; Fogang, B.; Essomba, R.; Chouna, J.R.; Njamen, D.; et al. Oligandrin from Croton oligandrus (Euphorbiaceae) Exhibits Anti-Breast Cancer Activity through Immune-Boosting Mechanisms: In Vitro and in Vivo Study. Heliyon 2024, 10, e35000. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Song, C.; Ma, J.; Cui, J.; Zhan, J.; Zhao, J.; Zhang, Y.; Zhu, Z.; Du, X. Quercetin Prevents Hyperuricemia Associated with Gouty Arthritis by Inactivating the NLRP3/NF-ΚB Signaling Pathway. Chem. Biol. Drug Des. 2025, 105, e70103. [Google Scholar] [CrossRef]
- Valenzuela, A. The Biological Significance of Malondialdehyde Determination in the Assessment of Tissue Oxidative Stress. Life Sci. 1991, 48, 301–309. [Google Scholar] [CrossRef]
- Reilly, C.A.; Aust, S.D. Measurement of Lipid Peroxidation. Curr. Protoc. Toxicol. 1999, 2, 2–4. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Elwy, H.M.; Hassanin, S.O.; El-Rashedy, A.A.E.; Hamza, A.A.; Khasawneh, M.A. Biosynthesis and Characterization of Gold and Copper Nanoparticles from Salvadora persica Fruit Extracts and Their Biological Properties. Int. J. Nanomed. 2022, 17, 6095–6112. [Google Scholar] [CrossRef]
- Ukeda, H.; Maeda, S.; Ishii, T.; Sawamura, M. Spectrophotometric Assay for Superoxide Dismutase Based on Tetrazolium Salt 3’-{1-[(Phenylamino)-Carbonyl]-3,4-Tetrazolium}-Bis(4-Methoxy-6- Nitro)Benzenesulfonic Acid Hydrate Reduction by Xanthine-Xanthine Oxidase. Anal. Biochem. 1997, 251, 206–209. [Google Scholar] [CrossRef]
- Ewing, J.F.; Janero, D.R. Microplate Superoxide Dismutase Assay Employing a Nonenzymatic Superoxide Generator. Anal. Biochem. 1995, 232, 243–248. [Google Scholar] [CrossRef]
- Stoian, I.A.-M.; Vlad, A.; Gilca, M.; Dragos, D. Modulation of Glutathione-S-Transferase by Phytochemicals: To Activate or Inhibit-That Is the Question. Int. J. Mol. Sci. 2025, 26, 7202. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of Glutathione, Glutathione Reductase and Glutathione S-Transferase Activities in Rat Lung and Liver. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Rice, E.W.; Epstein, M.B.; Witter, R.F.; Platt, H.A. Triglycerides (“Neutral Fat”) in Serum. Stand. Methods Clin. Chem. 1970, 6, 215–222. [Google Scholar] [CrossRef]
- Burstein, M.; Scholnick, H.R.; Morfin, R. Rapid Method for the Isolation of Lipoproteins from Human Serum by Precipitation with Polyanions. J. Lipid Res. 1970, 11, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Burstein, M.; Fine, A.; Atger, V.; Wirbel, E.; Girard-Globa, A. Rapid Method for the Isolation of Two Purified Subfractions of High Density Lipoproteins by Differential Dextran Sulfate-Magnesium Chloride Precipitation. Biochimie 1989, 71, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Abouelezz, H.M.; El-Kashef, D.H.; Abdelaziz, R.R.; Nader, M.A. Tiron Enhances the Anti-Cancer Activity of Doxorubicin in DMBA-Induced Breast Cancer: Role of Notch Signaling/Apoptosis/Autophagy/Oxidative Stress. Food Chem. Toxicol. 2024, 193, 114968. [Google Scholar] [CrossRef]
- Huynh, T.M.H.; Huang, P.X.; Wang, K.L.; Tran, N.T.; Iao, H.M.; Pan, W.C.; Chang, Y.H.; Lien, H.W.; Lee, A.Y.L.; Chou, T.C.; et al. Reprogramming Immunodeficiency in Lung Metastases via PD-L1 SiRNA Delivery and Antigen Capture of Nanosponge-Mediated Dendritic Cell Modulation. ACS Nano 2025, 19, 25134–25153. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Assiri, A.M.A.; Alzohairy, A.M.; Oraby, H.F. Health-Promoting Value and Food Applications of Black Cumin Essential Oil: An Overview. J. Food Sci. Technol. 2015, 52, 6136–6142. [Google Scholar] [CrossRef]
- Degu, B.; Tesfaye, B.; Abebe, W.; Assefa, K. Diversity of Ethiopian Black Cumin (Nigella sativa L.) Based on Compositions of Essential Oil. Biochem. Res. Int. 2025, 2025, 2065593. [Google Scholar] [CrossRef]
- Tabassum, H.; Ahmad, A.; Ahmad, I.Z. Nigella sativa L. and Its Bioactive Constituents as Hepatoprotectant: A Review. Curr. Pharm. Biotechnol. 2018, 19, 43–67. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Tekbaş, A.; Bremer-Streck, S.; Wissenbach, D.K.; Peters, F.T.; von Lilienfeld-Toal, M.; Soonawalla, Z.; Rauchfuß, F.; Settmacher, U.; Dahmen, U. Gas Chromatography-Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies. Int. J. Mol. Sci. 2023, 24, 16431. [Google Scholar] [CrossRef]
- Abo-Neima, S.E.; El-Sheekh, M.M.; Al-Zaban, M.I.; EL-Sayed, A.I.M. Antibacterial and Anti-Corona Virus (229E) Activity of Nigella sativa Oil Combined with Photodynamic Therapy Based on Methylene Blue in Wound Infection: In Vitro and in Vivo Study. BMC Microbiol. 2023, 23, 274. [Google Scholar] [CrossRef]
- Hossain, M.S.; Sharfaraz, A.; Dutta, A.; Ahsan, A.; Masud, M.A.; Ahmed, I.A.; Goh, B.H.; Urbi, Z.; Sarker, M.M.R.; Ming, L.C. A Review of Ethnobotany, Phytochemistry, Antimicrobial Pharmacology and Toxicology of Nigella sativa L. Biomed. Pharmacother. 2021, 143, 112182. [Google Scholar] [CrossRef]
- Xing, M.; Wang, X.; Zhao, L.; Zhou, Z.; Liu, H.; Wang, B.; Cheng, A.; Zhang, S.; Gao, Y. Novel dissolving microneedles preparation for synergistic melasma therapy: Combined effects of tranexamic acid and licorice extract. Int. J. Pharm. 2021, 600, 120406. [Google Scholar] [CrossRef]
- El-Nawawy, T.M.; Adel, Y.A.; Teaima, M.H.M.; Nassar, N.N.; Nemr, A.A.; Al-Samadi, I.; El-Nabarawi, M.A.; Elhabal, S.F. Intranasal Bilosomes in Thermosensitive Hydrogel: Advancing Desvenlafaxine Succinate Delivery for Depression Management. Pharm. Dev. Technol. 2024, 29, 663–674. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Cellulose Nanocrystals-silver Nanoparticles-Reduced Graphene Oxide Based Hybrid PVA Nanocomposites and Its Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 191, 445–456. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, G.A.; Girgis, G.N.S.; Hamed, M.F.; Soliman, O.A.E.A.; El Gawad, A.E.G.H.A. Formulation and Pathohistological Study of Mizolastine–Solid Lipid Nanoparticles–Loaded Ocular Hydrogels. Int. J. Nanomed. 2021, 16, 7775–7799. [Google Scholar] [CrossRef] [PubMed]
- Schaffazick, S.R.; Guterres, S.S.; De Lucca Freitas, L.; Pohlmann, A.R. Physicochemical Characterization and Stability of the Polymeric Nanoparticle Systems for Drug Administration. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, J.S.; Lee, H.G. Nanoencapsulation of Synergistic Antioxidant Fruit and Vegetable Concentrates and Their Stability during in Vitro Digestion. J. Sci. Food Agric. 2020, 100, 1056–1063. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.M.; Bondy, B.J.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Effect of Osmotic Pressure on the Stability of Whole Inactivated Influenza Vaccine for Coating on Microneedles. PLoS ONE 2015, 10, e0134431. [Google Scholar] [CrossRef]
- Garidel, P.; Pevestorf, B.; Bahrenburg, S. Stability of Buffer-Free Freeze-Dried Formulations: A Feasibility Study of a Monoclonal Antibody at High Protein Concentrations. Eur. J. Pharm. Biopharm. 2015, 97, 125–139. [Google Scholar] [CrossRef]
- Hamed, R.; Alhadidi, H.F.I. Minoxidil Nanosuspension-Loaded Dissolved Microneedles for Hair Regrowth. AAPS PharmSciTech 2024, 25, 75. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Yadav, K.S. Applications of Microneedles in Delivering Drugs for Various Ocular Diseases. Life Sci. 2019, 237, 116907. [Google Scholar] [CrossRef] [PubMed]
- Anjani, Q.K.; Pandya, A.K.; Demartis, S.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Li, H.; Gavini, E.; Patravale, V.B.; Donnelly, R.F. Liposome-Loaded Polymeric Microneedles for Enhanced Skin Deposition of Rifampicin. Int. J. Pharm. 2023, 646, 123446. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Yoo, D.G.; Bondy, B.J.; Quan, F.S.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Stability of influenza vaccine coated onto microneedles. Biomaterials 2012, 33, 3756–3769. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Ex Vivo Transdermal Delivery of Nicotinamide Mononucleotide Using Polyvinyl Alcohol Microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef]
- Aroche, A.F.; Nissan, H.E.; Daniele, M.A. Hydrogel-Forming Microneedles and Applications in Interstitial Fluid Diagnostic Devices. Adv. Healthc. Mater. 2025, 14, e2401782. [Google Scholar] [CrossRef]
- Duarah, S.; Sharma, M.; Chen, S.; Proft, T.K.; Loh, J.; Wen, J. Design, Optimization and Evaluation of Dexamethasone-Loaded Microneedles for Inflammatory Disorders. Int. J. Pharm. 2023, 635, 122690. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Liu, S.; He, Z.; Yu, S.; Ruan, Z.; Jin, N. Fabrication and Characterization of Dissolving Microneedles for Transdermal Drug Delivery of Allopurinol. Drug Dev. Ind. Pharm. 2021, 47, 1578–1586. [Google Scholar] [CrossRef]
- Zong, Q.; Wang, G.; Zhao, Z.; Li, W.; Hou, X.; Yao, M.; Tang, D.; Sheng, C.; Liu, Z.; Zheng, Y.; et al. Fabrication and Characterization of Dissolving Microneedles for Transdermal Delivery of Hypocrellin A. J. Drug Deliv. Sci. Technol. 2024, 95, 105594. [Google Scholar] [CrossRef]
- Alrbyawi, H.; Annaji, M.; Fasina, O.; Palakurthi, S.; Boddu, S.H.S.; Hassan, N.; Tiwari, A.K.; Suryawanshi, A.; Babu, R.J. Rapidly Dissolving Trans-Scleral Microneedles for Intraocular Delivery of Cyclosporine A. AAPS PharmSciTech 2024, 25, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, S.; Zhao, X.; Lian, J.; Gao, Y. Preparation, Characterization, and in Vivo Evaluation of Levonorgestrel-Loaded Thermostable Microneedles. Drug Deliv. Transl. Res. 2022, 12, 944–956. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride. Pharmaceutics 2021, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Ananda, P.W.R.; Elim, D.; Zaman, H.S.; Muslimin, W.; Tunggeng, M.G.R.; Permana, A.D. Combination of Transdermal Patches and Solid Microneedles for Improved Transdermal Delivery of Primaquine. Int. J. Pharm. 2021, 609, 121204. [Google Scholar] [CrossRef]
- Suriyaamporn, P.; Pornpitchanarong, C.; Pamornpathomkul, B.; Patrojanasophon, P.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Ganciclovir Nanosuspension-Loaded Detachable Microneedles Patch for Enhanced Drug Delivery to Posterior Eye Segment. J. Drug Deliv. Sci. Technol. 2023, 88, 104975. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohammadi, M.; Shafei, S.; Banafshe, H.R.; Malekshahi, Z.V.; Ebrahimi-Barough, S.; Ai, J. Impact of Atorvastatin Loaded Exosome as an Anti-Glioblastoma Carrier to Induce Apoptosis of U87 Cancer Cells in 3D Culture Model. Biochem. Biophys. Rep. 2020, 23, 100792. [Google Scholar] [CrossRef]
- Saraswati, S.; Agrawal, S.S.; Alhaider, A.A. Ursolic Acid Inhibits Tumor Angiogenesis and Induces Apoptosis through Mitochondrial-Dependent Pathway in Ehrlich Ascites Carcinoma Tumor. Chem. Biol. Interact. 2013, 206, 153–165. [Google Scholar] [CrossRef]
- El-Lakkany, N.M.; Elkattan, H.H.; Elsisi, A.E. The Ponatinib/Gossypol Novel Combination Provides Enhanced Anticancer Activity against Murine Solid Ehrlich Carcinoma via Triggering Apoptosis and Inhibiting Proliferation/Angiogenesis. Toxicol. Appl. Pharmacol. 2021, 432, 115767. [Google Scholar] [CrossRef]
- Li, H.; Yu, B.; Lin, J.; Wang, Q.; Zhang, L.; Li, Y.; Liu, X.; Liu, Y.; Li, C.; Zhao, G. Piperine Inhibits Fungal Growth and Protects against Pyroptosis in Aspergillus Fumigatus Keratitis by Regulating the MTOR/HIF-1α Pathway. Int. Immunopharmacol. 2025, 150, 114286. [Google Scholar] [CrossRef]
- Baig, W.A.; Alwosaibai, K.; Al-Jubran, K.M.; Chaudhry, T.M.; Al-Dowish, N.; Alsaffar, F.; Alam, M.A. Synergistic Anti-Cancer Effects of Nigella sativa Seed Oil and Conventional Cytotoxic Agent against Human Breast Cancer. Drug Metab. Pers. Ther. 2022, 37, 315–321. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Liu, Y.; Zheng, T.; Chen, N.; Du, P.; Ye, H. Exploration of Key Genes Associated with Oxidative Stress in Polycystic Ovary Syndrome and Experimental Validation. Front. Med. 2025, 12, 1493771. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mohammed-Geba, K.; Tawfic, A.A.; El-Magd, M.A. Camel Milk Exosomes Modulate Cyclophosphamide-Induced Oxidative Stress and Immuno-Toxicity in Rats. Food Funct. 2019, 10, 7523–7532. [Google Scholar] [CrossRef]
- Alkis, H.; Demir, E.; Taysi, M.R.; Sagir, S.; Taysi, S. Effects of Nigella sativa Oil and Thymoquinone on Radiation-Induced Oxidative Stress in Kidney Tissue of Rats. Biomed. Pharmacother. 2021, 139, 111540. [Google Scholar] [CrossRef]
- Cikman, O.; Ozkan, A.; Aras, A.B.; Soylemez, O.; Alkis, H.; Taysi, S.; Karaayvaz, M. Radioprotective Effects of Nigella sativa Oil against Oxidative Stress in Liver Tissue of Rats Exposed to Total Head Irradiation. J. Investig. Surg. 2014, 27, 262–266. [Google Scholar] [CrossRef]
- Tang, M.; Guo, C.; Sun, M.; Zhou, H.; Peng, X.; Dai, J.; Ding, Q.; Wang, Y.; Yang, C. Effective Delivery of Osteopontin Small Interference RNA Using Exosomes Suppresses Liver Fibrosis via TGF-Β1 Signaling. Front. Pharmacol. 2022, 13, 882243. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, Y.; Shen, Y.; Ao, H.; Guo, Y.; Han, M.; Wang, X. Preparation of High Drug-Loading Celastrol Nanosuspensions and Their Anti-Breast Cancer Activities in Vitro and in Vivo. Sci. Rep. 2020, 10, 8851. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Arribada, R.G.; Silva, J.O.; Silva-Cunha, A.; Townsend, D.M.; Ferreira, L.A.M.; Barros, A.L.B. In Vitro and In Vivo Effect of PH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics 2022, 14, 2394. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, W.; Tan, X.; Liang, H.; Li, J.; Yun, H.; He, C.; Chen, J.; Ma, X.; Xie, Y.; et al. Ganoderma Lucidum Spore Oil Induces Apoptosis of Breast Cancer Cells in Vitro and in Vivo by Activating Caspase-3 and Caspase-9. J. Ethnopharmacol. 2020, 247, 112256. [Google Scholar] [CrossRef]
- Bhat, A.A.; Thapa, R.; Afzal, O.; Agrawal, N.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Altamimi, A.S.A.; Prasher, P.; Singh, S.K.; et al. The Pyroptotic Role of Caspase-3/GSDME Signalling Pathway among Various Cancer: A Review. Int. J. Biol. Macromol. 2023, 242, 124832. [Google Scholar] [CrossRef]
- Dou, H.; Yu, P.Y.; Liu, Y.Q.; Zhu, Y.; Li, F.C.; Wang, Y.Y.; Chen, X.Y.; Xiao, M. Recent Advances in Caspase-3, Breast Cancer, and Traditional Chinese Medicine: A Review. J. Chemother. 2024, 36, 370–388. [Google Scholar] [CrossRef]
- Shehatta, N.H.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Baicalin; a promising chemopreventive agent, enhances the antitumor effect of 5-FU against breast cancer and inhibits tumor growth and angiogenesis in Ehrlich solid tumor. Biomed. Pharmacother. 2022, 146, 112599. [Google Scholar] [CrossRef]
| Factors (Independent Variables) | Design Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| X1: N.S. Conc (mg) | 12 | 30 | 45 |
| X2: Cholesterol Conc (mg) | 15 | 30 | 45 |
| X3: Span 60 Conc (mg) | 50 | 100 | 150 |
| Responses (Dependent variables) | Goal | ||
| Y1: P.S (nm) | Minimize | ||
| Y2: P.D.I | Minimize | ||
| Y3: Z.P (mV) | Maximize | ||
| Y4: E.E (%) | Maximize | ||
| Formulations | Gelatin (% w/v) | Hyaluronic Acid (% w/v) | CMC (% w/v) | PVA (% w/v) |
|---|---|---|---|---|
| MNs1 | 3 | 0.25 | 0.25 | 3 |
| MNs2 | 5 | 0.5 | 0.5 | 6 |
| MNs3 | 8 | 1 | 1 | 9 |
| Peak | Rt | Identified Compound | Mol. Formula | Area | Area Sum % |
|---|---|---|---|---|---|
| 1 | 10.025 | α-Thujene | C10H16 | 2,937,557.5 | 14.12 |
| 2 | 10.225 | Bicyclo [3.1.0] hex-2-ene, 4-methyl-1-(1-methylethyl)- | C10H16 | 656,059.91 | 3.15 |
| 3 | 11.33 | β-Phellandrene | C10H16 | 254,740.8 | 1.22 |
| 4 | 11.406 | Bicyclo [3.1.0] hexane, 4-methylene-1-(1-methylethyl)- | C10H16 | 620,005.12 | 2.98 |
| 5 | 12.621 | D-Limonene | C10H16 | 442,968.13 | 2.13 |
| 6 | 12.716 | Benzene, 1-methyl-3-(1-methylethyl)- | C10H14 | 7,727,949.5 | 37.14 |
| 7 | 15.03 | cis-4-methoxy thujane | C11H20O | 894,216.03 | 4.3 |
| 8 | 18.498 | Thymoquinone | C10H12O2 | 6734417 | 32.36 |
| 9 | 21.145 | 1H-Benzocycloheptene, 2,4a,5,6,7,8,9,9a-octahydro-3,5,5-trimethyl-9-methylene-, (4aS-cis)- | C15H24 | 542110.99 | 2.61 |
| Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Run | A: N.S. Conc (mg) | B: Cholesterol Conc (mg) | C: Span 60 Conc (mg) | P.S (nm) | P.D.I | Z.P (mV) | EE (%) |
| 1 | 12 | 30 | 45 | 550 ± 0.93 | 0.48 ± 0.04 | 30 ± 0.69 | 66 ± 0.96 |
| 2 | 30 | 45 | 45 | 615 ± 0.75 | 0.46 ± 0.08 | 27 ± 0.83 | 74 ± 0.46 |
| 3 | 45 | 45 | 100 | 460 ± 0.99 | 0.35 ± 0.01 | 35 ± 0.85 | 71 ± 0.61 |
| 4 | 30 | 15 | 45 | 515 ± 0.86 | 0.49 ± 0.10 | 28 ± 0.54 | 65 ± 0.75 |
| 5 | 30 | 45 | 50 | 470 ± 0.51 | 0.38 ± 0.06 | 34 ± 0.92 | 80 ± 0.66 |
| 6 | 30 | 30 | 100 | 430 ± 1.09 | 0.51 ± 0.09 | 28 ± 0.85 | 59 ± 0.57 |
| 7 | 45 | 15 | 100 | 560 ± 0.98 | 0.39 ± 0.04 | 36 ± 08 | 65 ± 0.82 |
| 8 | 12 | 45 | 100 | 385 ± 0.46 | 0.37 ± 0.06 | 33 ± 0.07 | 85 ± 0.76 |
| 9 | 45 | 30 | 45 | 670 ± 0.76 | 0.36 ± 0.01 | 29 ± 0.86 | 67 ± 0.84 |
| 10 | 12 | 15 | 100 | 420 ± 0.91 | 0.39 ± 0.05 | 31 ± 0.46 | 78 ± 0.67 |
| 11 | 12 | 30 | 50 | 190 ± 0.74 | 0.25 ± 0.07 | 37 ± 0.57 | 88 ± 0.77 |
| 12 | 45 | 30 | 50 | 635 ± 0.96 | 0.53 ± 0.05 | 35 ± 0.61 | 57 ± 0.75 |
| 13 | 30 | 30 | 100 | 430 ± 0.67 | 0.53 ± 0.06 | 28 ± 0.75 | 59 ± 0.79 |
| 14 | 30 | 15 | 50 | 485 ± 0.73 | 0.33 ± 0.02 | 35 ± 0.98 | 70 ± 0.88 |
| 15 | 30 | 30 | 100 | 430 ± 0.52 | 0.52 ± 0.04 | 28 ± 0.43 | 59 ± 0.24 |
| Responses | P.S | P.D.I | Z.P | E.E% |
| Minimum | 190 ± 0.74 | 0.25 ± 0.07 | 27 ± 0.83 | 57 ± 0.57 |
| Maximum | 670 ± 0.76 | 0.53 ± 0.07 | 37 ± 0.57 | 88 ± 0.77 |
| p-value | 0.0078 | 0.0084 | 0.0074 | <0.0001 |
| F-value | 10.5091 | 17.74 | 18.8811 | 135.65 |
| R2 | 0.5862 | 0.8975 | 0.9208 | 0.9957 |
| Adjusted R2 | 0.4734 | 0.7132 | 0.7782 | 0.9879 |
| Predicted R2 | 0.1865 | 0.6136 | 0.2673 | 0.9310 |
| Adequate precision | 7.5072 | 7.2860 | 6.5546 | 34.54 |
| Significant factors | A,C | A,B | A,C | A,B,C |
| Storage Time | Refrigerated Temperature (4 ± 1 °C) | Ambient Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PS (nm) | PDI (nm) | ZP (mV) | EE (%) | Drug Retention (%) | PS (nm) | P.D.I | ZP (mV) | EE (%) | Drug Retention (%) | |
| Zero time | 190 ± 0.23 | 0.25 ± 0.07 | 37 ± 0.57 | 88.00 ± 0.77 | 100 ± 0.00 | 190 ± 0.23 | 0.25 ± 0.07 | 37 ± 0.57 | 88.00 ± 0.77 | 100 ± 0.00 |
| 1 month | 191 ± 0.87 | 0.25 ± 0.19 | 37 ± 0.54 | 87.00 ± 0.98 | 99.87 ± 0.10 | 194 ± 0.34 | 0.35 ± 0.79 | 37 ± 0.87 | 87.00 ± 0.98 | 99.01 ± 0.34 |
| 2 months | 193 ± 0.76 | 0.24 ± 0.09 | 36 ± 0.93 | 86.98 ± 0.16 | 98.09 ± 0.76 | 199 ± 0.23 | 0.54 ± 0.99 | 38 ± 0.09 | 86.98 ± 0.16 | 97.12 ± 0.32 |
| 3 months | 195 ± 0.32 | 0.23 ± 0.05 | 35 ± 0.39 | 86.00 ± 0.54 | 97.89 ± 0.34 | 210 ± 0.87 | 0.68 ± 0.45 | 39 ± 0.23 | 86.00 ± 0.54 | 95.45 ± 0.15 |
| 4 months | 197 ± 0.77 | 0.25 ± 0.09 | 33 ± 0.89 | 85.00 ± 0.90 | 96.87 ± 0.29 | 230 ± 0.77 | 0.76 ± 0.01 | 40 ± 0.13 | 85.00 ± 0.90 | 90.01 ± 0.27 |
| 5 months | 199 ± 0.53 | 0.27 ± 0.05 | 34 ± 0.34 | 84.00 ± 0.15 | 96.12 ± 0.83 | 249 ± 0.53 | 0.81 ± 0.07 | 42 ± 0.14 | 84.00 ± 0.15 | 89.32 ± 0.33 |
| 6 months | 200 ± 0.43 | 0.29 ± 0.15 | 34 ± 0.11 | 83.01 ± 0.49 | 95.34 ± 0.54 | 250 ± 0.43 | 0.88 ± 0.01 | 43 ± 0.01 | 83.01 ± 0.49 | 86.32 ± 0.12 |
| Parameter | FIS-Free NSs | FIS-NSs | FIS-NSs Loaded G/HA/CMC MNs |
|---|---|---|---|
| Zero order R2 | 0.892 | 0.875 | 0.902 |
| First order R2 | 0.945 | 0.936 | 0.911 |
| Higuchi R2 | 0.973 | 0.979 | 0.968 |
| Korsmeyer–Peppas R2 | 0.981 | 0.987 | 0.984 |
| n (diffusion exponent) | 0.45 | 0.58 | 0.83 |
| Weibull R2 | 0.993 | 0.996 | 0.997 |
| β (Weibull exponent) | 0.62 | 0.71 | 0.88 |
| Best-fit Model | Weibull | Weibull | Weibull |
| Release Mechanism | Fickian diffusion | Fickian diffusion | Anomalous (diffusion + erosion) |
| Groups | Initial Body Weight (g) | Body Weight After Treatment |
|---|---|---|
| Group I | 20 ± 0.34 | 40 ± 0.14 |
| Group II | 19 ± 0.07 | 19.9 ± 0.15 |
| Group III | 20 ± 0.12 | 26 ± 0.03 |
| Group IV | 19 ± 0.14 | 31± 0.05 |
| Group V | 20 ± 0.09 | 35 ± 0.19 |
| Parameters | GI | GII | GIII | GIV | GV |
|---|---|---|---|---|---|
| Tumor Weight (g) | - | 2.5 ± 0.37 | 1.96 ± 0.31 * | 1.49 ± 0.34 * | 0.78 ± 0.17 *** |
| Tumor Volume (cm3) | - | 2.17 ± 0.64 | 1.67 ± 0.54 * | 1.16 ± 0.51 * | 0.72 ± 0.38 *** |
| Tumor Burden (g) | - | 25 ± 0.39 | 19.61 ± 0.75 * | 14.89 ± 0.36 * | 7.82 ± 0.18 *** |
| Tumor burden inhibition (%) | - | 26.14 ± 0.42 | 42.35 ± 0.54 * | 56.66 ± 0.54 * | 77 ± 0.8 *** |
| Parameter | GI | GII | GIII | GIV | GV |
|---|---|---|---|---|---|
| Total Bilirubin (mg/dL) | 3.23 ± 0.15 | 45.10 ± 0.43 **** | 21.37 ± 0.89 ** | 10.56 ± 0.98 *** | 5.92 ± 0.99 **** |
| SGOT (μ/L) | 33 ± 1.32 | 129 ± 0.35 **** | 97.43 ± 0.24 ** | 75.34 ± 0.12 *** | 49 ± 0.98 **** |
| SGPT (μ/L) | 37.23 ± 0.64 | 110.88 ± 0.87 **** | 91 ± 0.75 * | 70.94 ± 0.35 *** | 49.19 ± 0.34 **** |
| ALP (μ/L) | 131 ± 1.23 | 350 ± 1.32 **** | 280 ± 0.14 ** | 179 ± 0.34 *** | 151 ± 0.56 **** |
| Total Cholesterol (mg/dL) | 155 ± 0.91 | 236 ± 0.56 **** | 205 ± 0.99 * | 189 ± 0.98 ** | 162 ± 0.90 *** |
| Triglycerides (mg/dL) | 101 ± 0.47 | 199 ± 0.57 **** | 171 ± 0.57 ** | 131 ± 0.49 *** | 120 ± 0.78 *** |
| HDL Cholesterol (mg/dL) | 71 ± 0.89 | 29 ± 0.57 **** | 41 ± 0.89 * | 51 ± 0.43 ** | 69 ± 0.08 *** |
| Urea (mg/dL) | 31 ± 0.79 | 97 ± 0.48 **** | 67 ± 0.93 * | 59 ± 0.56 ** | 46 ± 0.38 *** |
| Uric acid (mg/dL) | 3.34 ± 0.11 | 45 ± 0.29 **** | 24 ± 0.37 ** | 16 ± 0.34 *** | 5.42 ± 0.88 **** |
| Creatinine (mg/dL) | 0.59 ± 0.01 | 30 ± 0.49 **** | 14.51 ± 0.14 ** | 6.12 ± 0.34 *** | 2.34 ± 0.56 **** |
| Progesterone.H (ng/mL) | 47.23 ± 0.65 | 88.45 ± 0.23 **** | 77.51 ± 0.47 * | 68.59 ± 0.82 ** | 54.51 ± 0.98 *** |
| Estrogen.H (Pg/mL) | 72.3 ± 0.98 | 101 ± 0.45 **** | 91.89 ± 0.19 * | 82.59 ± 0.93 ** | 79.91 ± 0.93 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhabal, S.F.; Mohammed Ali, E.; Hababeh, S.; Hassan, F.E.; Morsy, S.A.A.; Ahmed Elbahy, D.; Ali, S.K.; Allam, K.M.; Mousa, I.; Fouad, M.A.; et al. Harnessing Nature for Breast Cancer Management: Effects of Fisetin-Loaded Nigellasomes Embedded in Microneedles Improve Tumor Suppression and Reduce Oxidative Stress. Pharmaceutics 2025, 17, 1392. https://doi.org/10.3390/pharmaceutics17111392
Elhabal SF, Mohammed Ali E, Hababeh S, Hassan FE, Morsy SAA, Ahmed Elbahy D, Ali SK, Allam KM, Mousa I, Fouad MA, et al. Harnessing Nature for Breast Cancer Management: Effects of Fisetin-Loaded Nigellasomes Embedded in Microneedles Improve Tumor Suppression and Reduce Oxidative Stress. Pharmaceutics. 2025; 17(11):1392. https://doi.org/10.3390/pharmaceutics17111392
Chicago/Turabian StyleElhabal, Sammar Fathy, Eman Mohammed Ali, Sandra Hababeh, Fatma E. Hassan, Suzan Awad AbdelGhany Morsy, Dalia Ahmed Elbahy, Sahar K. Ali, Khaled M. Allam, Ibrahim Mousa, Marwa A. Fouad, and et al. 2025. "Harnessing Nature for Breast Cancer Management: Effects of Fisetin-Loaded Nigellasomes Embedded in Microneedles Improve Tumor Suppression and Reduce Oxidative Stress" Pharmaceutics 17, no. 11: 1392. https://doi.org/10.3390/pharmaceutics17111392
APA StyleElhabal, S. F., Mohammed Ali, E., Hababeh, S., Hassan, F. E., Morsy, S. A. A., Ahmed Elbahy, D., Ali, S. K., Allam, K. M., Mousa, I., Fouad, M. A., & Mohsen Elsaid Hamdan, A. (2025). Harnessing Nature for Breast Cancer Management: Effects of Fisetin-Loaded Nigellasomes Embedded in Microneedles Improve Tumor Suppression and Reduce Oxidative Stress. Pharmaceutics, 17(11), 1392. https://doi.org/10.3390/pharmaceutics17111392










