Orally Dispersible Swallowed Topical Corticosteroids in Eosinophilic Esophagitis: A Paradigm Shift in the Management of Esophageal Inflammation
Abstract
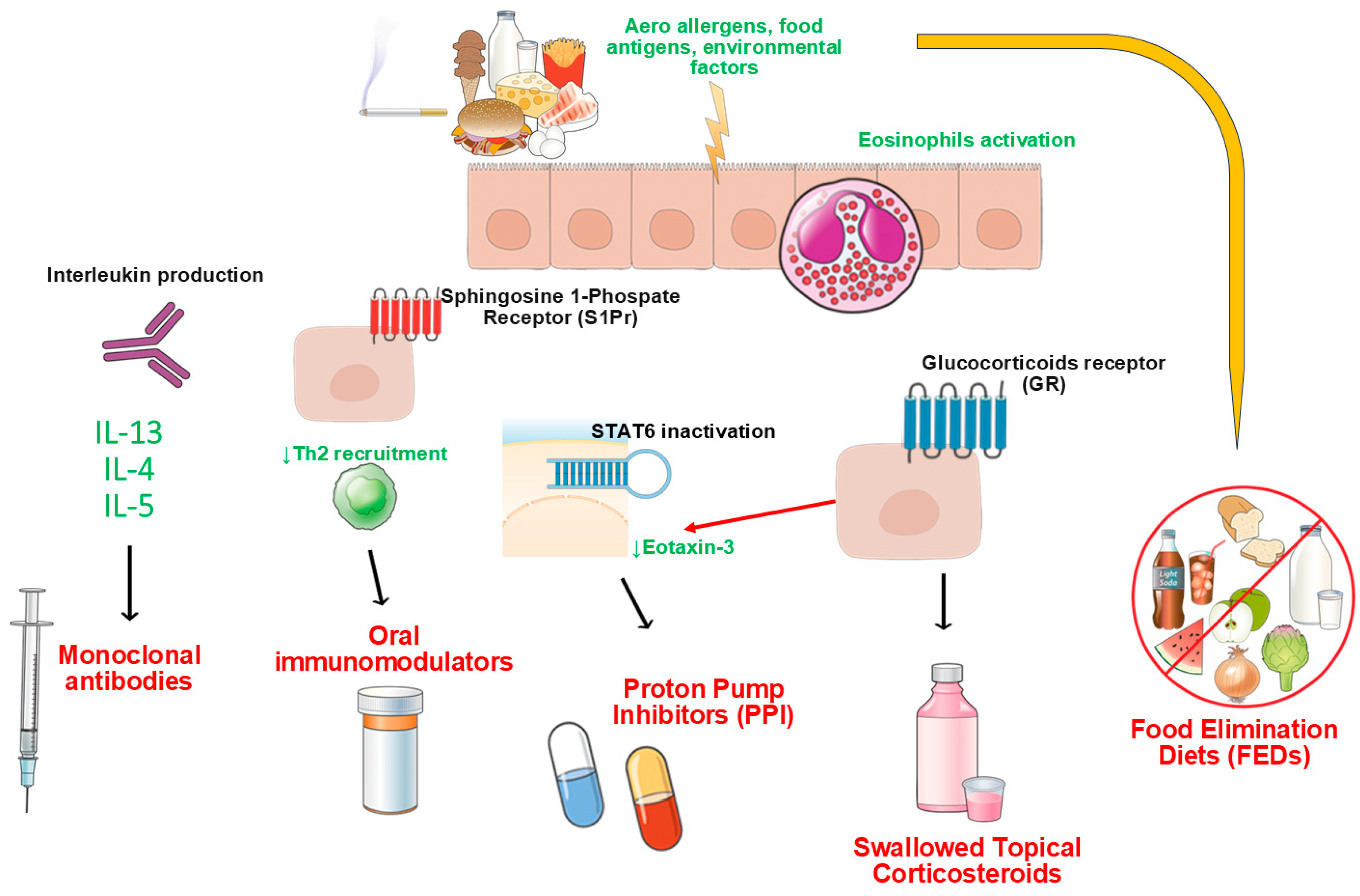
1. Introduction
2. Off-Label (ol)-STCs Formulations
3. Budesonide (BOT) and Fluticasone (FOT) Orally Dispersible Tablets
| Study (First Author, Year) | Study Design | Intervention | Duration | Population (Age) | Primary Outcome | Key Results |
|---|---|---|---|---|---|---|
| Lucendo et al. (2019) [55] | Phase 3, RCT, DB, PC (EOS-1) | BOT 1 mg BID vs. Placebo | 6–12 weeks | 88 adults (18–75 years) | Complete CHR: <16 eos/mm2 HPF (<5 eos HPF) Symptom severity ≤2 points on NRS scale | Complete remission: 58% BOT vs. 0% Placebo; Histologic remission: 93% BOT vs. 0% Placebo (p < 0.0001) |
| Miehlke et al. (2016) [56] | Phase 2, RCT, DB, PC | BOT 2 mg QD/2 mg BID/OVB 2 mg QD vs. Placebo | 2 weeks | 77 adults (18–75 years) | Histological remission: <16 eos/mm2 HPF (<5 eos HPF) Change in mean PEC (eos/mm2 HPF) | Histological response (<65 eos/mm2 HPF/<20 eos HPF) in 100% and 94.7% for both BOT dosages. 0% in placebo Histological remission was 84.2% and 89.5% compared to 73.7% in OVB Higher tolerance and satisfaction for BOT compared to OVB |
| Miehlke et al. (2021) [57] | Phase 3, open-label induction for RCT, DB, PC (EOS-2) | BOT 1 mg BID | 6 weeks | 181 patients (18–75 years) | Complete CHR: <16 eos/mm2 HPF (<5 eos HPF) Symptom severity ≤2 points on NRS scale | -CHR 69.6% Histological remission: 90.1% (deep remission 0 eos HPF 84.5%) Clinical remission 75.1%. Significant endoscopic improvement (p < 0.0001) |
| Straumann et al. (2020) [61] | Phase 3 maintenance RCT, DB, PC (EOS-2) | BOT 0.5/1.0 mg BID vs. Placebo | 48 weeks | 204 patients (18–75 years) | Maintenance of remission: n° of pts not in clinical relapse (≥4 points on NRS scale) n° of pts not in histologic relapse (≥48 eos/mm2 HPF/≥15 eos HPF) | Maintained remission: 73.5% (0.5 mg BID), 75% (1.0 mg BID), 4.4% (Placebo) |
| Biedermann et al. (2025) [62] | OLE of a randomized, DB, PC, 48-week maintenance trial (EOS-2) | BOT 0.5 or 1.0 mg BID or 2.0 mg BID for OLRI | 96 weeks | 186 patients (extension of previous RCT) (18–75 years) | Maintenance of remission: n° of pts not in clinical relapse (≥4 points on NRS scale) n° of pts not in histologic relapse (≥48 eos/mm2 HPF/≥15 eos HPF) | Clinical remission: 81.9% Histological remission: 80.1% (deep remission 0 eos HPF 78.8%) CHR 78.1% Histological relapses 15.1% EREFS stable through week 48 to 96 High patient satisfaction |
4. Budesonide Oral Suspension (BOS)
| Study (First Author, Year) | Study Design | Intervention | Duration | Population (Age) | Primary Outcome | Key Results |
|---|---|---|---|---|---|---|
| Dellon et al., 2017 [73] | Phase 2, RCT, DB, PC | BOS 2 mg BID vs. placebo | 12 weeks | 93 patients (11–40 years) | Histological response (≤6 eos/hpf); DSQ score improvement | Histological response: 39% BOS vs. 3% placebo (p < 0.0001); DSQ improvement: −14.3 BOS vs. −7.5 placebo (p = 0.0096). |
| Hirano et al., 2022 [74] | Phase 3, RCT, DB, PC (ORBIT1) | BOS 2 mg BID vs. Placebo | 12 weeks | 318 patients (11–55 years) | Histologic response (≤6 eos/hpf); DSQ symptom response (≥30%) | Histological response: 53.5% BOS vs. 1% placebo (p < 0.001); Symptom response: 52.6% BOS vs. 39.1% Placebo (p = 0.024). |
| Gupta et al., 2015 [71] | Phase 2, RCT, DB, PC | Low, medium, high dose BOS vs. Placebo | 12 weeks | 71 patients (2–18 years) | Histological and symptom compound response | Responder in medium-dose: 52.6%; Responder in high-dose: 47.1%; Responder in placebo: 5.6% (p < 0.01). No significant difference in percentages of responders between the low-dose BOS (11.8%) and placebo groups (p = 0.5282). |
| Collins et al., 2019 [75] | Phase 2, RCT, DB, PC | BOS 2 mg BID vs. Placebo | 12 weeks | 87 patients (11–40 years) | EoEHSS (grade and stage) improvement | EoEHSS total scores improved for 6 of the 8 and 5 of the 8 histopathologic features for grade and stage, respectively, versus placebo. Change in EoEHSS total scores correlated moderately but significantly with change in endoscopic severity (p < 0.0001). The change in EoE HSS stage total score correlated weakly with the change in DSQ. |
| Dellon et al., 2019 [77] | Open-label extension study of a multicenter, randomized, DB, PC trial. | BOS 2 mg QD, then optional 1.5–2 mg BID | 24 weeks | 82 patients (11–40 years) | Histological response (≤6 eos/hpf) and change in mean peak eosinophil counts after 24 weeks | 42% of patients maintained histologic response; 4% of non-responders gained response. |
| Dellon et al., 2022 [78] | Phase 3, RCT, DB (ORBIT2) | BOS 2 mg BID vs. Placebo | 36 weeks | 48 patients (11–55 years) | Relapse rate (≥15 eos/hpf and ≥4 dysphagia days) by 36 week | More BOS–Placebo than BOS–BOS patients relapsed over 36 weeks (43.5% vs. 24.0%; p = 0.131). |
5. Pharmacological Characteristics of Conventional and Novel Steroid Formulations in EoE
5.1. Old Formulations of Budesonide and Fluticasone (SAF/FNDS via MDI and OVB)
5.2. Orally Dispersible Tablets (ODT) and Oral Suspension Formulations (OS)
5.2.1. Drug Release Mechanisms
5.2.2. Role of Excipients in Enhancing Contact Time
| Delivery System | Esophageal Mucosa Contact Time (min) | Mucosal Surface Contact Area (Relative Scale) | Notes |
|---|---|---|---|
| MDI Swallowed [33] | ~3 | Low | Aerosol swallowed; minimal mucosal contact |
| Slurry Viscous [54,56] | 10–15 | Moderate | Liquid flows down the esophagus; moderate contact |
| Orodispersible Tablets (ODT) [33] | 20–30 | High | Slowly dissolves in the mouth; adheres well to the mucosa |
| Budesonide Oral Suspension (BOS) [75] | 15–20 | High | Viscous liquid formulation; prolonged contact |
5.2.3. Impact of pH and Saliva on Drug Dissolution and Distribution
5.3. ESOCAP System
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Muir, A.; Falk, G.W. Eosinophilic esophagitis: A review. J. Am. Med. Assoc. 2021, 326, 1310–1318. [Google Scholar] [CrossRef]
- Barchi, A.; Vespa, E.; Passaretti, S.; Dell’Anna, G.; Fasulo, E.; Yacoub, M.-R.; Albarello, L.; Sinagra, E.; Massimino, L.; Ungaro, F.; et al. The Dual Lens of Endoscopy and Histology in the Diagnosis and Management of Eosinophilic Gastrointestinal Disorders-A Comprehensive Review. Diagnostics 2024, 14, 858. [Google Scholar] [CrossRef]
- Furuta, G.T.; Katzka, D.A. Eosinophilic Esophagitis. N. Engl. J. Med. 2015, 373, 1640–1648. [Google Scholar] [CrossRef]
- de Bortoli, N.; Visaggi, P.; Penagini, R.; Annibale, B.; Baiano Svizzero, F.; Barbara, G.; Bartolo, O.; Battaglia, E.; Di Sabatino, A.; De Angelis, P.; et al. The 1st EoETALY Consensus on the Diagnosis and Management of Eosinophilic Esophagitis—Definition, Clinical Presentation and Diagnosis. Dig. Liver Dis. 2024, 56, 951–963. [Google Scholar] [CrossRef]
- Dellon, E.S.; Hirano, I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.W.; Lee, K.; Shin, J.I.; Cho, S.H.; Turner, S.; Shin, J.U.; Yeniova, A.Ö.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Global Incidence and Prevalence of Eosinophilic Esophagitis, 1976–2022: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 3270–3284.e77. [Google Scholar] [CrossRef] [PubMed]
- Mona, R.; Hruz, P. Epidemiology of eosinophilic esophagitis: Really a novel and evolving disease? Inflamm. Intest. Dis. 2025, 10, 34–40. [Google Scholar] [CrossRef]
- Straumann, A.; Spichtin, H.-P.; Grize, L.; Bucher, K.A.; Beglinger, C.; Simon, H.-U. Natural history of primary eosinophilic esophagitis: A follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003, 125, 1660–1669. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef]
- Mukkada, V.; Falk, G.W.; Eichinger, C.S.; King, D.; Todorova, L.; Shaheen, N.J. Health-Related Quality of Life and Costs Associated with Eosinophilic Esophagitis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 495–503.e8. [Google Scholar] [CrossRef]
- Dellon, E.S.; Muir, A.B.; Katzka, D.A.; Shah, S.C.; Sauer, B.G.; Aceves, S.S.; Furuta, G.T.; Gonsalves, N.; Hirano, I. ACG Clinical Guideline: Diagnosis and Management of Eosinophilic Esophagitis. Am. J. Gastroenterol. 2025, 120, 31–59. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Gutiérrez-Ramírez, L.; Tejera-Muñoz, A.; Molina-Infante, J.; Arias, Á.; EUREOS Guidelines Committee. Proton Pump Inhibitors for Inducing and Maintaining Remission in Eosinophilic Esophagitis: An Updated Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Barchi, A.; Massimino, L.; Mandarino, F.V.; Yacoub, M.-R.; Albarello, L.; Savarino, E.V.; Ungaro, F.; Danese, S.; Passaretti, S.; Bredenoord, A.J.; et al. Clinical, histological and safety outcomes with long-term maintenance therapies for eosinophilic esophagitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2024, 23, 1890–1904.e7. [Google Scholar] [CrossRef]
- Alexander, R.; Kassmeyer, B.; Lennon, R.; Alexander, J.; Snyder, D.; Ravi, K. Eoe recurrence on PPI maintenance therapy: You do not know if you do not look! Dig. Dis. Sci. 2024, 69, 4048–4052. [Google Scholar] [CrossRef]
- Hartnett, D.A.; Muftah, M.; Leung, R.; Hiramoto, B.; Flanagan, R.; Cai, J.X.; Lo, W.-K.; Chan, W.W. Distribution of esophageal eosinophilia as a predictor of proton pump inhibitor response in eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2025, in press. [Google Scholar] [CrossRef]
- Muftah, M.; Goldin, A.H.; Barshop, K.; Hsu Blatman, K.; Hamilton, M.J.; Lo, W.-K.; Hornick, J.L.; Chan, W.W. Twice-Daily Proton Pump Inhibitor Induces Higher Remission Rate in Eosinophilic Esophagitis Than Once-Daily Regimen Regardless of Total Daily Dose. Am. J. Gastroenterol. 2024, 119, 991–995. [Google Scholar] [CrossRef]
- Kliewer, K.L.; Gonsalves, N.; Dellon, E.S.; Katzka, D.A.; Abonia, J.P.; Aceves, S.S.; Arva, N.C.; Besse, J.A.; Bonis, P.A.; Caldwell, J.M.; et al. One-food versus six-food elimination diet therapy for the treatment of eosinophilic oesophagitis: A multicentre, randomised, open-label trial. Lancet Gastroenterol. Hepatol. 2023, 8, 408–421. [Google Scholar] [CrossRef]
- Mayerhofer, C.; Kavallar, A.M.; Aldrian, D.; Lindner, A.K.; Müller, T.; Vogel, G.F. Efficacy of Elimination Diets in Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2197–2210.e3. [Google Scholar] [CrossRef] [PubMed]
- Bredenoord, A.J.; Dellon, E.S.; Hirano, I.; Lucendo, A.J.; Schlag, C.; Sun, X.; Glotfelty, L.; Mannent, L.; Maloney, J.; Laws, E.; et al. Dupilumab demonstrated efficacy and was well tolerated regardless of prior use of swallowed topical corticosteroids in adolescent and adult patients with eosinophilic oesophagitis: A subgroup analysis of the phase 3 LIBERTY EoE TREET study. Gut 2024, 73, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Collins, M.H.; Rothenberg, M.E.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.; Straumann, A.; Safroneeva, E.; Rodriguez, C.; et al. Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients with Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2021, 19, 473–483.e17. [Google Scholar] [CrossRef]
- Bredenoord, A.; Houbiers, J.G.; Vanuytsel, T.; Chvatchko, Y.; Hoff, D.A.; Conchillo, J.M.; Dellon, E.S.; Tran, I.; Guyon-Gellin, N.; Holz, J.-B.; et al. 962 caly-002, an anti-il-15 antibody, results in histological and clinical improvement in patients with eosinophilic esophagitis in a phase 1a/b study. Gastroenterology 2024, 166, S–228–S–229. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Dellon, E.S.; Collins, M.H.; Bredenoord, A.J.; Hirano, I.; Peterson, K.A.; Brooks, L.; Caldwell, J.M.; Fjällbrant, H.; Grindebacke, H.; et al. Eosinophil Depletion with Benralizumab for Eosinophilic Esophagitis. N. Engl. J. Med. 2024, 390, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Collins, M.H.; Bredenoord, A.J.; Philpott, H.; Biedermann, L.; Dulcine, M.; Nguyen-Cleary, T.; Su, C.; Yu, J.; Tan, H.; et al. Etrasimod as a treatment for eosinophilic oesophagitis (VOYAGE): A double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2025, 10, 622–633. [Google Scholar] [CrossRef]
- Dell’Anna, G.; Fanizza, J.; Mandarino, F.V.; Barchi, A.; Fasulo, E.; Vespa, E.; Fanti, L.; Azzolini, F.; Battaglia, S.; Puccetti, F.; et al. The endoscopic management of anastomotic strictures after esophagogastric surgery: A comprehensive review of emerging approaches beyond endoscopic dilation. J. Pers. Med. 2025, 15, 111. [Google Scholar] [CrossRef]
- Pellegatta, G.; Giugliano, F.P.; Mastrorocco, E.; Baiardini, I.; Hassan, C.; Repici, A. Switch From Off-Label Swallowed Topical Corticosteroids to Budesonide Orodispersible Tablets in Eosinophilic Esophagitis Patients. Clin. Gastroenterol. Hepatol. 2025, 23, 1058–1060.e3. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.P.; Gordon, M.; Sinopoulou, V.; Dellon, E.S.; Gupta, S.K.; Reed, C.C.; Gutiérrez-Junquera, C.; Venkatesh, R.D.; Erwin, E.A.; Egiz, A.; et al. Medical treatment of eosinophilic esophagitis. Cochrane Database Syst. Rev. 2023, 7, CD004065. [Google Scholar] [CrossRef]
- Gold, B.D.; Goodwin, B.; Davis, K.; Sweeney, C.; Ziemiecki, R.; Jiang, J.; Fan, T.; Boules, M.; Chen, S.-T.; Katzka, D.A. Satisfaction with and Adherence to Off-Label Corticosteroids in Adolescents and Adults with Eosinophilic Esophagitis: Results of a Web-Based Survey in the United States. J. Clin. Gastroenterol. 2025, 59, 138–146. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Noel, R.J.; Blanchard, C.; Kirby, C.; Jameson, S.C.; Buckmeier, B.K.; Akers, R.; Cohen, M.B.; Collins, M.H.; Assa’ad, A.H.; et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006, 131, 1381–1391. [Google Scholar] [CrossRef]
- Peterson, K.A.; Thomas, K.L.; Hilden, K.; Emerson, L.L.; Wills, J.C.; Fang, J.C. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig. Dis. Sci. 2010, 55, 1313–1319. [Google Scholar] [CrossRef]
- Moawad, F.J.; Veerappan, G.R.; Dias, J.A.; Baker, T.P.; Maydonovitch, C.L.; Wong, R.K.H. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am. J. Gastroenterol. 2013, 108, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.A.; Jung, K.W.; Arora, A.S.; Enders, F.; Katzka, D.A.; Kephardt, G.M.; Kita, H.; Kryzer, L.A.; Romero, Y.; Smyrk, T.C.; et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2012, 10, 742–749.e1. [Google Scholar] [CrossRef]
- Andreae, D.A.; Hanna, M.G.; Magid, M.S.; Malerba, S.; Andreae, M.H.; Bagiella, E.; Chehade, M. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children with Eosinophilic Esophagitis. Am. J. Gastroenterol. 2016, 111, 1187–1197. [Google Scholar] [CrossRef]
- Laserna-Mendieta, E.J.; Navarro, P.; Casabona-Francés, S.; Savarino, E.V.; Amorena, E.; Pérez-Martínez, I.; Guagnozzi, D.; Blas-Jhon, L.; Betoré, E.; Guardiola-Arévalo, A.; et al. Swallowed topical corticosteroids for eosinophilic esophagitis: Utilization and real-world efficacy from the EoE CONNECT registry. United Eur. Gastroenterol. J. 2024, 12, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Affrime, M.B.; Kosoglou, T.; Thonoor, C.M.; Flannery, B.E.; Herron, J.M. Mometasone furoate has minimal effects on the hypothalamic-pituitary-adrenal axis when delivered at high doses. Chest 2000, 118, 1538–1546. [Google Scholar] [CrossRef][Green Version]
- Bergquist, H.; Larsson, H.; Johansson, L.; Bove, M. Dysphagia and quality of life may improve with mometasone treatment in patients with eosinophilic esophagitis: A pilot study. Otolaryngol. Head Neck Surg. 2011, 145, 551–556. [Google Scholar] [CrossRef]
- Syverson, E.P.; Hait, E.; McDonald, D.R.; Rubinstein, E.; Goldsmith, J.D.; Ngo, P.D.; Mitchell, P.D.; Lee, J.J. Oral viscous mometasone is an effective treatment for eosinophilic esophagitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1107–1109. [Google Scholar] [CrossRef]
- Tytor, J.; Larsson, H.; Bove, M.; Johansson, L.; Bergquist, H. Topically applied mometasone furoate improves dysphagia in adult eosinophilic esophagitis—Results from a double-blind, randomized, placebo-controlled trial. Scand. J. Gastroenterol. 2021, 56, 629–634. [Google Scholar] [CrossRef]
- Krause, J.; Rosenbaum, C.; Grimm, M.; Rump, A.; Keßler, R.; Hosten, N.; Weitschies, W. The EsoCap-system—An innovative platform to drug targeting in the esophagus. J. Control. Release 2020, 327, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.J.; Nantes-Castillejo, Ó.; Straumann, A.; Biedermann, L.; Bredenoord, A.J.; Guagnozzi, D.; Blas-Jhon, L.; Wiechowska-Kozlowska, A.; Weidlich, S.; von Arnim, U.; et al. Clinical trial: Safety and efficacy of a novel oesophageal delivery system for topical corticosteroids versus placebo in the treatment of eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2025, 61, 444–455. [Google Scholar] [CrossRef]
- Dohil, R.; Newbury, R.; Fox, L.; Bastian, J.; Aceves, S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010, 139, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Conus, S.; Degen, L.; Felder, S.; Kummer, M.; Engel, H.; Bussmann, C.; Beglinger, C.; Schoepfer, A.; Simon, H.-U. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010, 139, 1526–1537, 1537.e1. [Google Scholar] [CrossRef]
- Straumann, A.; Conus, S.; Degen, L.; Frei, C.; Bussmann, C.; Beglinger, C.; Schoepfer, A.; Simon, H.-U. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2011, 9, 400–409.e1. [Google Scholar] [CrossRef]
- Oliva, S.; Volpe, D.; Russo, G.; Veraldi, S.; Papoff, P.; Giordano, C.; Ruggiero, C.; Trovato, C.M.; Terrin, G.; Rossetti, D. Maintenance therapy with the lowest effective dose of oral viscous budesonide in children with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2022, 20, 2905–2907.e2. [Google Scholar] [CrossRef]
- Greuter, T.; Safroneeva, E.; Bussmann, C.; Biedermann, L.; Vavricka, S.R.; Katzka, D.A.; Schoepfer, A.M.; Straumann, A. Maintenance Treatment Of Eosinophilic Esophagitis with Swallowed Topical Steroids Alters Disease Course Over A 5-Year Follow-up Period In Adult Patients. Clin. Gastroenterol. Hepatol. 2019, 17, 419–428.e6. [Google Scholar] [CrossRef]
- Greuter, T.; Godat, A.; Ringel, A.; Almonte, H.S.; Schupack, D.; Mendoza, G.; McCright-Gill, T.; Dellon, E.S.; Hirano, I.; Alexander, J.; et al. Effectiveness and Safety of High- vs. Low-Dose Swallowed Topical Steroids for Maintenance Treatment of Eosinophilic Esophagitis: A Multicenter Observational Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2514–2523.e2. [Google Scholar] [CrossRef] [PubMed]
- McCallen, J.D.; Dave, M.; LaFata, S.S.; Cameron, B.A.; Xue, A.Z.; Kiran, A.; Ocampo, A.A.; Lee, C.J.; Borinsky, S.A.; Redd, W.D.; et al. Topical Steroids Are Effective and Safe in Patients with Eosinophilic Esophagitis Over a Median of 6.5 Years of Chronic Use. J. Clin. Gastroenterol. 2025, 59, 737–743. [Google Scholar] [CrossRef]
- Reed, C.C.; LaFata, S.S.; Gee, T.S.; Thel, H.L.; Cameron, B.A.; Xue, A.Z.; Kiran, A.; Ocampo, A.A.; McCallen, J.; Lee, C.J.; et al. Worsening Disease Severity as Measured by I-SEE Associates with Decreased Treatment Response to Topical Steroids in Eosinophilic Esophagitis Patients. Clin. Gastroenterol. Hepatol. 2025, 23, 1737–1745.e3. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Woosley, J.T.; Arrington, A.; McGee, S.J.; Covington, J.; Moist, S.E.; Gebhart, J.H.; Tylicki, A.E.; Shoyoye, S.O.; Martin, C.F.; et al. Efficacy of budesonide vs. fluticasone for initial treatment of eosinophilic esophagitis in a randomized controlled trial. Gastroenterology 2019, 157, 65–73.e5. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, T.; Kang, Y.; Zhong, Y.; Chen, S.; Zhang, Y.; Chai, X. Oral viscous budesonide solution for enhanced localized treatment of eosinophilic esophagitis through improved mucoadhesion and permeation. J. Pharm. Sci. 2024, 113, 3384–3392. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Sheikh, A.; Speck, O.; Woodward, K.; Whitlow, A.B.; Hores, J.M.; Ivanovic, M.; Chau, A.; Woosley, J.T.; Madanick, R.D.; et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012, 143, 321–324.e1. [Google Scholar] [CrossRef]
- Rubinstein, E.; Hait, E.E.; Mitchell, P.D.; Lee, J.J. Every-other-day Dosing of Oral Viscous Budesonide Is not Effective in the Management of Eosinophlic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 395–397. [Google Scholar] [CrossRef]
- Greuter, T.; Bussmann, C.; Safroneeva, E.; Schoepfer, A.M.; Biedermann, L.; Vavricka, S.R.; Straumann, A. Long-Term Treatment of Eosinophilic Esophagitis with Swallowed Topical Corticosteroids: Development and Evaluation of a Therapeutic Concept. Am. J. Gastroenterol. 2017, 112, 1527–1535. [Google Scholar] [CrossRef]
- Barchi, A.; Mandarino, F.V.; Yacoub, M.-R.; Albarello, L.; Massimino, L.; Savarino, E.V.; Ungaro, F.; Passaretti, S.; Masclee, G.M.C.; Danese, S.; et al. From Pathogenesis to Treatment: Targeting Type-2 Inflammation in Eosinophilic Esophagitis. Biomolecules 2024, 14, 1080. [Google Scholar] [CrossRef]
- Miehlke, S.; Lucendo, A.J.; Straumann, A.; Jan Bredenoord, A.; Attwood, S. Orodispersible budesonide tablets for the treatment of eosinophilic esophagitis: A review of the latest evidence. Therap. Adv. Gastroenterol. 2020, 13, 1756284820927282. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Miehlke, S.; Schlag, C.; Vieth, M.; von Arnim, U.; Molina-Infante, J.; Hartmann, D.; Bredenoord, A.J.; Ciriza de Los Rios, C.; Schubert, S.; et al. Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo-Controlled Trial. Gastroenterology 2019, 157, 74–86.e15. [Google Scholar] [CrossRef] [PubMed]
- Miehlke, S.; Hruz, P.; Vieth, M.; Bussmann, C.; von Arnim, U.; Bajbouj, M.; Schlag, C.; Madisch, A.; Fibbe, C.; Wittenburg, H.; et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 2016, 65, 390–399. [Google Scholar] [CrossRef]
- Miehlke, S.; Schlag, C.; Lucendo, A.J.; Biedermann, L.; Vaquero, C.S.; Schmoecker, C.; Hayat, J.; Hruz, P.; Ciriza de Los Rios, C.; Bredenoord, A.J.; et al. Budesonide orodispersible tablets for induction of remission in patients with active eosinophilic oesophagitis: A 6-week open-label trial of the EOS-2 Programme. United Eur. Gastroenterol. J. 2022, 10, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Lucendo, A.; Schlag, C.; Straumann, A.; Vieth, M.; Mueller, R.; Greinwald, R.; Miehlke, S. Mo1130—Budesonide Orodispersible Tablets Can Effectively Induce Complete Remission of Endoscopic and Histologic Mucosal Abnormalities and Can Induce Deep Disease Remission in Active Eosinophilic Esophagitis: Results from a Post-Hoc Analysis of the Randomized, Double-Blind, Placebo-Controlled Eos-1 Trial. Gastroenterology 2019, 156, S-715–S-716. [Google Scholar]
- Rawla, P.; Sunkara, T.; Thandra, K.C.; Gaduputi, V. Efficacy and Safety of Budesonide in the Treatment of Eosinophilic Esophagitis: Updated Systematic Review and Meta-Analysis of Randomized and Non-Randomized Studies. Drugs R D 2018, 18, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Barberio, B.; Del Corso, G.; de Bortoli, N.; Black, C.J.; Ford, A.C.; Savarino, E. Comparison of drugs for active eosinophilic oesophagitis: Systematic review and network meta-analysis. Gut 2023, 72, 2019–2030. [Google Scholar] [CrossRef]
- Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C.S.; Ciriza de Los Rios, C.; Schmoecker, C.; Madisch, A.; et al. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients with Eosinophilic Esophagitis. Gastroenterology 2020, 159, 1672–1685.e5. [Google Scholar] [CrossRef]
- Biedermann, L.; Schlag, C.; Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Santander, C.; Ciriza de Los Rios, C.; Schmöcker, C.; Madisch, A.; et al. Efficacy and Safety of Budesonide Orodispersible Tablets for Eosinophilic Esophagitis up to 3 Years: An Open-Label Extension Study. Clin. Gastroenterol. Hepatol. 2024, in press. [Google Scholar] [CrossRef]
- Eluri, S.; Runge, T.M.; Hansen, J.; Kochar, B.; Reed, C.C.; Robey, B.S.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Diminishing Effectiveness of Long-Term Maintenance Topical Steroid Therapy in PPI Non-Responsive Eosinophilic Esophagitis. Clin. Transl. Gastroenterol. 2017, 8, e97. [Google Scholar] [CrossRef]
- Nennstiel, S.; Schlag, C. Treatment of eosinophlic esophagitis with swallowed topical corticosteroids. World. J. Gastroenterol. 2020, 26, 5395–5407. [Google Scholar] [CrossRef]
- Visaggi, P.; Ghisa, M.; Vespa, E.; Barchi, A.; Mari, A.; Pasta, A.; Marabotto, E.; de Bortoli, N.; Savarino, E.V. Optimal Assessment, Treatment, and Monitoring of Adults with Eosinophilic Esophagitis: Strategies to Improve Outcomes. Immunotargets. Ther. 2024, 13, 367–383. [Google Scholar] [CrossRef]
- Hirano, I.; Safroneeva, E.; Roumet, M.C.; Comer, G.M.; Eagle, G.; Schoepfer, A.; Falk, G.W. Randomised clinical trial: The safety and tolerability of fluticasone propionate orally disintegrating tablets versus placebo for eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2020, 51, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Lucendo, A.J.; Schlag, C.; Schoepfer, A.M.; Falk, G.W.; Eagle, G.; Nezamis, J.; Comer, G.M.; Knoop, K.; Hirano, I. Fluticasone Propionate Orally Disintegrating Tablet (APT-1011) for Eosinophilic Esophagitis: Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 2485–2494.e15. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Pujols, L.; Alobid, I.; Pérez-Gonzalez, M.; Fuentes, M.; de Borja Callejas, F.; Valero, A.; Picado, C.; Roca-Ferrer, J. Fluticasone furoate inhibits cytokine secretion from nasal epithelial cells and reduces eosinophil survival in an in vitro model of eosinophilic inflammation. Int. Arch. Allergy Immunol. 2014, 163, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Nistel, M.; Furuta, G.T.; Pan, Z.; Hsu, S. Impact of dose reduction of topical steroids to manage adrenal insufficiency in pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 786–792. [Google Scholar] [CrossRef]
- Gupta, S.K.; Hill, M.; Vitanza, J.M.; Farber, R.H.; Desai, N.K.; Williams, J.; Song, I.H. Pharmacokinetics of budesonide oral suspension in children and adolescents with eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 186–191. [Google Scholar] [CrossRef]
- Gupta, S.K.; Vitanza, J.M.; Collins, M.H. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2015, 13, 66–76.e3. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Ahmed, N.S.; Yuan, Y.; Qasim, A.; O’Gorman, D.B.; Feagan, B.G.; Jairath, V.; Bredenoord, A.J.; Dellon, E.S.; Ma, C. Meta-Analysis: Evaluating Placebo Rates Across Outcomes in Eosinophilic Oesophagitis Randomised Controlled Trials. Aliment. Pharmacol. Ther. 2025, 61, 32–43. [Google Scholar] [CrossRef]
- Dellon, E.S.; Katzka, D.A.; Collins, M.H.; Hamdani, M.; Gupta, S.K.; Hirano, I. MP-101-06 Investigators Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology 2017, 152, 776–786.e5. [Google Scholar] [CrossRef]
- Hirano, I.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Morey, R.; Desai, N.K.; Lan, L.; Williams, J.; Dellon, E.S.; et al. Budesonide Oral Suspension Improves Outcomes in Patients with Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 525–534.e10, Correction in Clin. Gastroenterol. Hepatol. 2022, 20, 2418. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Dellon, E.S.; Katzka, D.A.; Hirano, I.; Williams, J.; Lan, L. Budesonide Oral Suspension Significantly Improves Eosinophilic Esophagitis Histology Scoring System Results: Analyses From a 12-Week, Phase 2, Randomized, Placebo-controlled Trial. Am. J. Surg. Pathol. 2019, 43, 1501–1509. [Google Scholar] [CrossRef]
- Collins, M.H.; Martin, L.J.; Alexander, E.S.; Boyd, J.T.; Sheridan, R.; He, H.; Pentiuk, S.; Putnam, P.E.; Abonia, J.P.; Mukkada, V.A.; et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Dellon, E.S.; Katzka, D.A.; Collins, M.H.; Gupta, S.K.; Lan, L.; Williams, J.; Hirano, I. Safety and efficacy of budesonide oral suspension maintenance therapy in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2019, 17, 666–673.e8. [Google Scholar] [CrossRef]
- Dellon, E.S.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Morey, R.; Goodwin, B.; Eisner, J.D.; Lan, L.; Desai, N.K.; et al. Long-Term Treatment of Eosinophilic Esophagitis with Budesonide Oral Suspension. Clin. Gastroenterol. Hepatol. 2022, 20, 1488–1498.e11. [Google Scholar] [CrossRef]
- Dellon, E.S.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Zhang, W.; Goodwin, B.; Terreri, B.; Boules, M.; Desai, N.K.; et al. Effect of randomized treatment withdrawal of budesonide oral suspension on clinically relevant efficacy outcomes in patients with eosinophilic esophagitis: A post hoc analysis. Therap. Adv. Gastroenterol. 2024, 17, 17562848241307602. [Google Scholar] [CrossRef]
- Barchi, A.; Massimino, L.; Mandarino, F.V.; Vespa, E.; Sinagra, E.; Almolla, O.; Passaretti, S.; Fasulo, E.; Parigi, T.L.; Cagliani, S.; et al. Microbiota profiling in esophageal diseases: Novel insights into molecular staining and clinical outcomes. Comput. Struct. Biotechnol. J. 2024, 23, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, J.E.; Fox, V.L.; Twarog, F.J.; Nurko, S.; Antonioli, D.; Gleich, G.; Badizadegan, K.; Furuta, G.T. Eosinophilic esophagitis in children: Immunopathological analysis and response to fluticasone propionate. Gastroenterology 2002, 122, 1216–1225. [Google Scholar] [CrossRef]
- Wales, D.; Makker, H.; Kane, J.; Mc Dowell, P.; O’Driscoll, B.R. Systemic Bioavailability and Potency of High-Dose Inhaled Corticosteroids. Chest 1999, 115, 1278–1284. [Google Scholar] [CrossRef]
- Daley-Yates, P.T. Inhaled corticosteroids: Potency, dose equivalence and therapeutic index. Brit. J. Clin. Pharma. 2015, 80, 372–380. [Google Scholar] [CrossRef]
- Sharpe, M.; Jarvis, B. Inhaled Mometasone Furoate: A Review of its Use in Adults and Adolescents with Persistent Asthma. Drugs 2001, 61, 1325–1350. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, E.; Thalén, A.; Brattsand, R.; Gustafsson, J.A.; Johansson, U.; Roempke, K.; Saartok, T. Correlation between chemical structure, receptor binding, and biological activity of some novel, highly active, 16 alpha, 17 alpha-acetal-substituted glucocorticoids. Mol. Pharmacol. 1984, 25, 70–78. [Google Scholar] [CrossRef]
- Edsbäcker, S.; Bengtsson, B.; Larsson, P.; Lundin, P.; Nilsson, Å.; Ulmius, J.; Wollmer, P. A pharmacoscintigraphic evaluation of oral budesonide given as controlled-release (Entocort) capsules. Aliment. Pharmacol. Ther. 2003, 17, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.; Otley, A. Budesonide in the treatment of inflammatory bowel disease. Expert Rev. Clin. Immunol. 2011, 7, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, Å.; Edsbäcker, S.; Löfberg, R.; Nilsson, Å.; Nyman-Pantelidis, M.; Olsson, O.; Suhr, O.; Willén, R. Pharmacokinetics of budesonide enema in patients with distal ulcerative colitis or proctitis. Aliment. Pharmacol. Ther 1993, 7, 401–407. [Google Scholar] [CrossRef]
- Thorsson, L.; Borgå, O.; Edsbäcker, S. Systemic availability of budesonide after nasal administration of three different formulations: Pressurized aerosol, aqueous pump spray, and powder. Brit. J. Clin. Pharma. 1999, 47, 619–624. [Google Scholar] [CrossRef]
- Donnelly, R.; Seale, J.P. Clinical Pharmacokinetics of Inhaled Budesonide. Clin. Pharmacokinet. 2001, 40, 427–440. [Google Scholar]
- Simeoli, R.; Lava, S.A.G.; Di Deo, A.; Roversi, M.; Cairoli, S.; Tambucci, R.; Rea, F.; Malamisura, M.; Angelino, G.; Biondi, I.; et al. Pharmacokinetic Evaluation of Oral Viscous Budesonide in Paediatric Patients with Eosinophilic Oesophagitis in Repaired Oesophageal Atresia. Pharmaceutics 2024, 16, 872. [Google Scholar] [CrossRef] [PubMed]
- Soulele, K.; Macheras, P.; Karalis, V. On the pharmacokinetics of two inhaled budesonide/formoterol combinations in asthma patients using modeling approaches. Pulm. Pharmacol. Ther. 2018, 48, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Edsbäcker, S.; Andersson, T. Pharmacokinetics of Budesonide (Entocort™ EC) Capsules for Crohn’s Disease. Clin. Pharmacokinet. 2004, 43, 803–821. [Google Scholar]
- Back, H.; Lee, J.B.; Kim, A.; Park, S.-J.; Kim, J.; Chae, J.; Sheen, S.S.; Kagan, L.; Park, H.-S.; Ye, Y.-M.; et al. Exposure-Response and Clinical Outcome Modeling of Inhaled Budesonide/Formoterol Combination in Asthma Patients. Pharmaceutics 2020, 12, 336. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, C.; Yu, H.; Shen, K.; Assam, P.N.; Gillen, M.; Liu, Y.; Dorinsky, P. Pharmacokinetics and Tolerability of Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate and Glycopyrronium/Formoterol Fumarate Dihydrate Metered Dose Inhalers in Healthy Chinese Adults: A Randomized, Double-blind, Parallel-group Study. Clin. Ther. 2019, 41, 897–909.e1. [Google Scholar] [CrossRef] [PubMed]
- Tambucci, R.; Roversi, M.; Rea, F.; Malamisura, M.; Angelino, G.; Biondi, I.; Simeoli, R.; Goffredo, B.M.; Francalanci, P.; Simonetti, A.; et al. Oral Viscous Budesonide in Children with Eosinophilic Esophagitis After Repaired Esophageal Atresia: A Clinical Trial. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 249–255. [Google Scholar] [CrossRef]
- Song, I.H.; Finkelman, R.D.; Lan, L. A Pharmacokinetic Bridging Study to Compare Systemic Exposure to Budesonide between Budesonide Oral Suspension and ENTOCORT EC in Healthy Individuals. Drugs R D 2020, 20, 359–367. [Google Scholar] [CrossRef]
- O’Donnell, S.; O’Morain, C.A. Therapeutic benefits of budesonide in gastroenterology. Ther. Adv. Chronic Dis. 2010, 1, 177–186. [Google Scholar] [CrossRef]
- Hefner, J.N.; Howard, R.S.; Massey, R.; Valencia, M.; Stocker, D.J.; Philla, K.Q.; Goldman, M.D.; Nylund, C.M.; Min, S.B. A Randomized Controlled Comparison of Esophageal Clearance Times of Oral Budesonide Preparations. Dig. Dis. Sci 2016, 61, 1582–1590. [Google Scholar] [CrossRef]
- Dellon, E.S.; Veerappan, R.; Selitsky, S.R.; Parker, J.S.; Higgins, L.L.; Beitia, R.; Genta, R.M.; Lash, R.H. A Gene Expression Panel is Accurate for Diagnosis and Monitoring Treatment of Eosinophilic Esophagitis in Adults. Clin. Transl. Gastroenterol. 2017, 8, e74. [Google Scholar] [CrossRef]
- Wu, S.; Fang, X.; Zhao, J.; Liu, G.; Liao, P. Nutrient regulation targeting macrophage-controlled intestinal mucosal healing: A promising strategy against intestinal mucositis induced by deoxynivalenol. Toxicon 2025, 264, 108434. [Google Scholar] [CrossRef] [PubMed]

| Active Principle | Formulation Type | Dosage and Posology | CHR | Efficacy in Maintenance Phase | Tolerability and Safety | Limitations | |
|---|---|---|---|---|---|---|---|
| OlSTCs | Fluticasone | -Aerosolized swallowed with MDI -Nasal drop suspension -Home-made oral viscous solution | ≤0.25 mg/day to ≥1.6 mg/day | Up to ~60–65% (dose-dependent, particularly for ≥0.8 mg/day | Decreased remission with dose reduction: 46% for fluticasone [PMID: 38284792] | Generally well tolerated; oral candidiasis reported in ~10–15% | -Non-intuitive administration methods -Home-made preparation -Non-standardized dosages -Nebulized suspensions with less mucosal adherence compared to viscous slurry compounds |
| Budesonide | -OVB (budesonide nebulizer suspension + sucralose or cellulose) -Aerosolized swallowed with MDI | -OVB 1–2 mg/day (0.25 mg BID in maintenance) | -~72–80% for OVB (2 mg/day); no added benefit above 4 mg/day | -OVB maintained remission (>65% overall and over discontinuation registered a significant increase in PEC in placebo, p = 0.024) -No difference in High Vs. Lower dosage but early relapses at low dose | |||
| Mometasone | -Aerosolized mometasone with MDI | up to 1500 μg/day | -Median eos HPF change from baseline (−50, p < 0.001) -significant improvement in dysphagia score, not QoL | -No data available | -No major adverse events reported | -10× lower bioavailability compared to fluticasone and >300× lower than budesonide -No data on histologic and endoscopic outcomes | |
| Novel STCs | Fluticasone | -FOT (APT-1011)(tablets to be merged with saliva and swallowed) | -3 mg BID -3 mg HS -1.5 mg BID -1.5 mg HS | ->65% up to 100% of histologic remission -Significant improvement in dysphagia scores for all dosages -Good response also for EREFS in fibrostenotic patients | -Histological response maintained (up to 84%) at 52 weeks with 1.5 mg BID. Lower (30%) for 1.5 mg QD | -Safe profile overall with candidiasis as the most frequent event (12–16%, usually mild) | -Enhanced esophageal targeting -Favorable tolerability -High patient adherence due to the ease of administration -No need for compounding -Higher patient satisfaction with OVB |
| Budesonide | -BOT (tablets to be merged with saliva and swallowed) -BOS (syrup-like consistency with two viscosity-modifying agents) | -BOT: 1–2 mg/day (0.5–1 mg BID for maintenance) -BOS: 2 mg BID (2 mg QD for maintenance) | -From 70% to 100% CHR with BOT (2 mg/day); OR 18.9 for remission (p < 0.001) in EoE -BOS 45–50% of histological response in pediatric (clinical improvement non-significant) -BOS in adults showed significant CHR (p < 0.0001 and p = 0.0096) | -BOT reported CHR up to 75% at 52 weeks and >80% at 96 weeks -Relapse rates were lower in the BOS group (24%) compared to placebo (43.5%) | |||
| Mometasone | -Mometasone (ESO-101) | -Histological remission in 48% compared to 0% in placebo -Endoscopic improvement with ESO-101 compared to placebo but not for clinical symptoms | -No trials available | No adverse event reported |
| Findings | Clinical Implications | |
|---|---|---|
| Absorption mechanisms | Two parallel absorption pathways: zero-order esophageal absorption and first-order gastrointestinal absorption | Explains variability in drug exposure and highlights the role of esophageal mucosal residence time in determining efficacy and safety. |
| Systemic exposure | Considerably higher than with inhaled budesonide (≈5×) and capsules for Crohn’s disease (≈3–10×). AUC and Cmax are also greater than those of the oral suspension for EoE. | Risk of unwanted systemic effects (e.g., cortisol suppression, growth impairment). |
| Variability | High inter-individual and inter-occasion variability, not explained by age, weight, or dose. | Suggests influence of posture, motility, and mucosal status. Dose adjustments may not solve variability. |
| Mucosal residence | Zero-order absorption lasted ≈ 8 h, suggesting prolonged esophageal contact. | Once-daily dosing may be sufficient to maintain therapeutic effect. |
| Safety | Short-term and even long-term treatment is well tolerated by both for pediatric and adult populations | A longer follow-up is needed to confirm safety in chronic use. |
| Dose rationale | Exposure is not linearly related to dose; higher doses are not justified. Lower doses (0.25–0.5 mg/day) and supine administration are recommended. | Supports re-evaluation of standard dosing strategies to minimize systemic absorption. |
| Function | Excipients | Possible Contributions to the Formulation |
|---|---|---|
| Effervescence/disintegration/pH buffer | Sodium acid citrate, Disodium (or sodium di-) hydrogen citrate, Sodium hydrogen carbonate | These agents react in the presence of saliva to produce an effervescent effect, helping the tablet to disintegrate/dissolve rapidly in the mouth. The citrate/bicarbonate system creates mild fizz and promotes saliva production, which enhances the dispersal of the active ingredient. |
| Plasticizer/surfactant/wetting | Docusate sodium | Helps with wetting, perhaps aiding uniform distribution of saliva and aiding disintegration. |
| Polymer binder/film forming | Povidone (K25) | Acts as a binder to help tablet cohesion; it may also affect the dissolution profile. |
| Filler/diluent/bulking agent | Mannitol (E421) | Used to give mass/volume to the tablet; often gives a pleasant mouthfeel; relatively water-soluble, so it contributes to dissolution. |
| Lubricant | Magnesium stearate | To reduce friction during tablet manufacture, avoid sticking in the tablet press; this ensures the tablets can be produced with uniformity. |
| Polyethylene glycol (PEG)/Macrogol | Macrogol 6000 | Acts as a PEG/disperser; helps in dissolution/disintegration; can enhance the wetting properties of the tablet. |
| Function | BOS—Suspension | BOT—Orally Dispersible Tablets |
|---|---|---|
| Vehicle/Base | Purified water | Mannitol (filler/diluent) |
| Sweeteners and Flavors | Acesulfame K, Magnasweet®, dextrose, maltodextrin, cherry flavor | Sucralose |
| Viscosity/Mucoadhesion | Avicel® RC-591 (microcrystalline cellulose + Na-CMC), glycerine | Povidone K25, Macrogol 6000, Docusate sodium |
| Disintegration | no needed | CO2 gas disintegrant |
| Stabilizer (antioxidants/ chelators) | Ascorbic acid, sodium ascorbate, disodium EDTA | Not reported |
| Lubricants (manufacturing) | Not required (liquid formulation) | Magnesium stearate |
| Buffering/pH control | Citric acid + sodium citrate | Sodium dihydrogen citrate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchi, A.; Girelli, M.; Ventimiglia, A.; Mandarino, F.V.; Danese, S.; Passaretti, S.; Yacoub, M.-R.; Nannipieri, S.; Ciliberto, A.F.; Albarello, L.; et al. Orally Dispersible Swallowed Topical Corticosteroids in Eosinophilic Esophagitis: A Paradigm Shift in the Management of Esophageal Inflammation. Pharmaceutics 2025, 17, 1325. https://doi.org/10.3390/pharmaceutics17101325
Barchi A, Girelli M, Ventimiglia A, Mandarino FV, Danese S, Passaretti S, Yacoub M-R, Nannipieri S, Ciliberto AF, Albarello L, et al. Orally Dispersible Swallowed Topical Corticosteroids in Eosinophilic Esophagitis: A Paradigm Shift in the Management of Esophageal Inflammation. Pharmaceutics. 2025; 17(10):1325. https://doi.org/10.3390/pharmaceutics17101325
Chicago/Turabian StyleBarchi, Alberto, Marina Girelli, Antonio Ventimiglia, Francesco Vito Mandarino, Silvio Danese, Sandro Passaretti, Mona-Rita Yacoub, Serena Nannipieri, Ambra Federica Ciliberto, Luca Albarello, and et al. 2025. "Orally Dispersible Swallowed Topical Corticosteroids in Eosinophilic Esophagitis: A Paradigm Shift in the Management of Esophageal Inflammation" Pharmaceutics 17, no. 10: 1325. https://doi.org/10.3390/pharmaceutics17101325
APA StyleBarchi, A., Girelli, M., Ventimiglia, A., Mandarino, F. V., Danese, S., Passaretti, S., Yacoub, M.-R., Nannipieri, S., Ciliberto, A. F., Albarello, L., Bartolucci, A., Vespa, E., & Dell’Anna, G. (2025). Orally Dispersible Swallowed Topical Corticosteroids in Eosinophilic Esophagitis: A Paradigm Shift in the Management of Esophageal Inflammation. Pharmaceutics, 17(10), 1325. https://doi.org/10.3390/pharmaceutics17101325







