Tracking Mycoviruses in Public RNAseq Datasets of Malassezia: Three Original Totiviruses Revealed
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
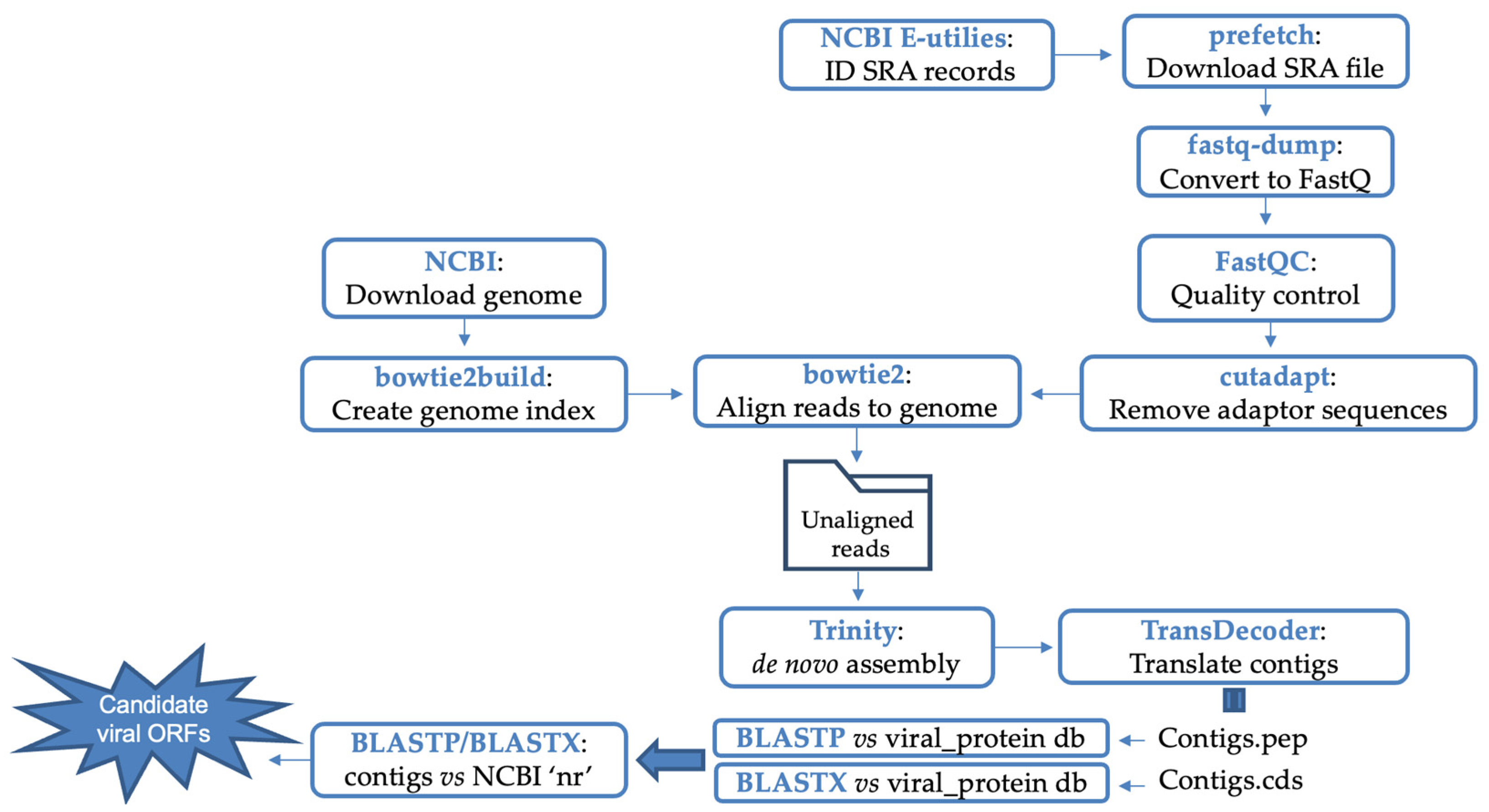
2.2. Pipeline for Identification of Viral-like Sequences
2.3. Phylogenetic Tree Construction
3. Results
3.1. Mycoviruses in Malassezia spp. Transcriptomes
3.2. Assignment of Identified Sequences to Their Taxonomic Group
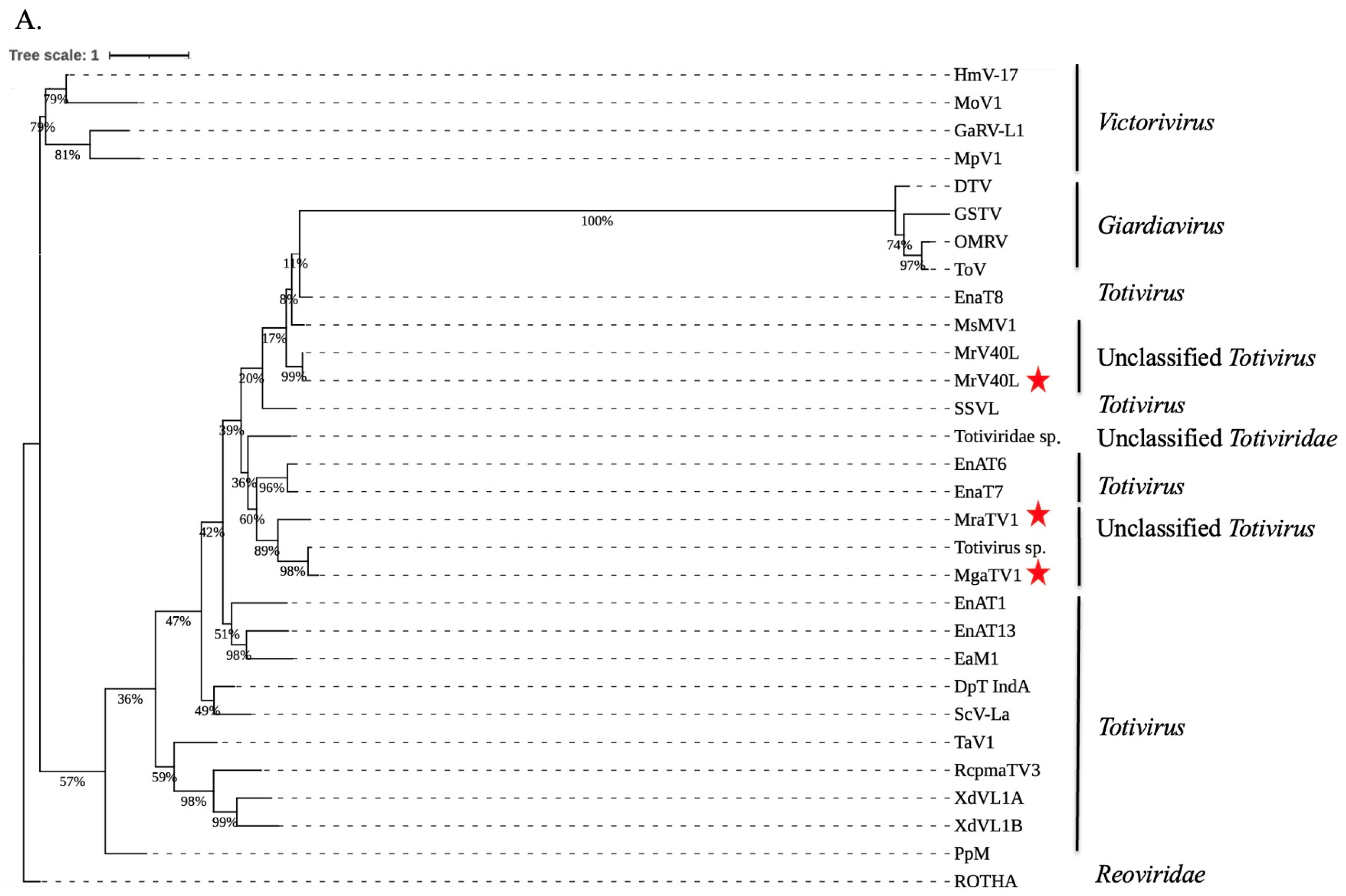
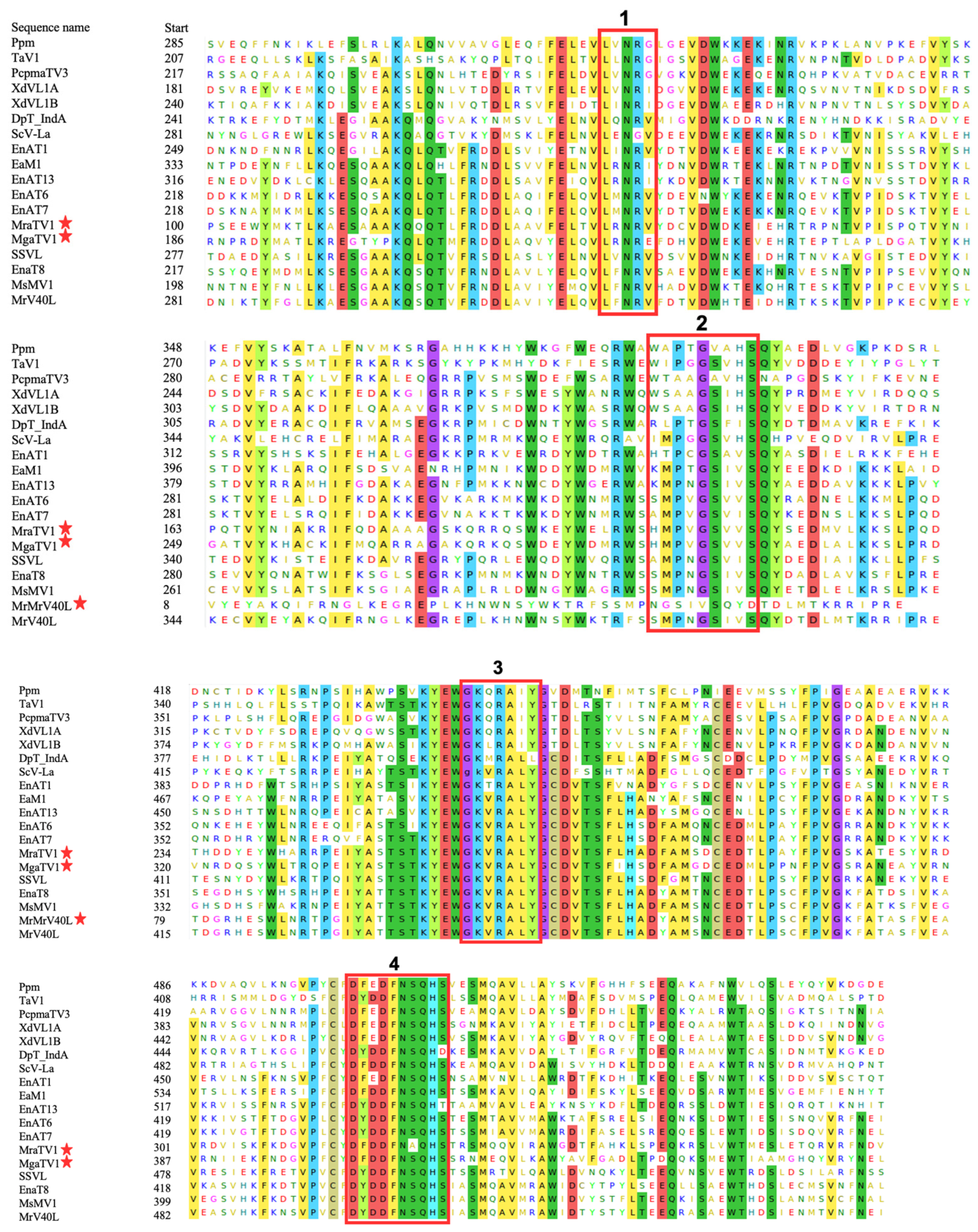
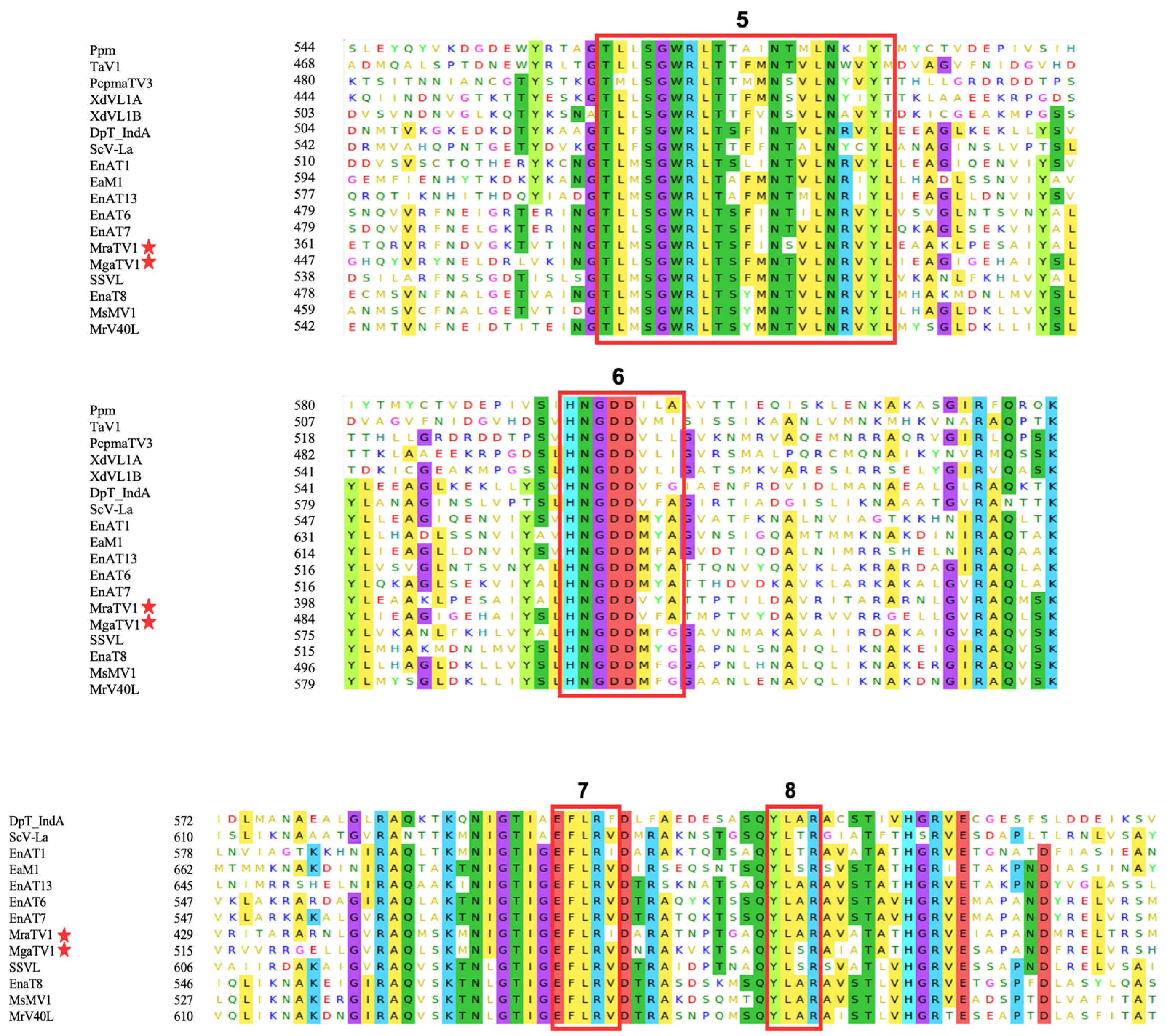
3.3. Phylogenetic Analysis and Characterisation of Conserved RdRp and CP Regions of Totiviruses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Ongena, M.; Qiu, D.; Guo, L. Fungal viruses: Promising fundamental research and biological control agents of fungi. SM Virol. 2017, 2, 1011. [Google Scholar]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef] [PubMed]
- Jacquat, A.G.; Theumer, M.G.; Cañizares, M.C.; Debat, H.J.; Iglesias, J.; García Pedrajas, M.D.; Dambolena, J.S. A Survey of Mycoviral Infection in Fusarium spp. Isolated from Maize and Sorghum in Argentina Identifies the First Mycovirus from Fusarium verticillioides. Viruses 2020, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Cho, Y.-J.; Kim, D.; Yang, C.-S.; Lee, S.M.; Dawson, T.L.; Nakamizo, S.; Kabashima KLee, Y.W.; Jung, W.H. A Novel Virus Alters Gene Expression and Vacuolar Morphology in Malassezia Cells and Induces a TLR3-Mediated Inflammatory Immune Response. mBio 2020, 11, e01521-20. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef]
- Picarelli, M.A.S.C.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme Diversity of Mycoviruses Present in Isolates of Rhizoctonia solani AG2-2 LP From Zoysia japonica From Brazil. Front. Cell. Infect. Microbiol. 2019, 9, 244. [Google Scholar] [CrossRef]
- Nuss, D.L. Biological control of chestnut blight: An example of virus-mediated attenuation of fungal pathogenesis. Microbiol. Rev. 1992, 56, 561–576. [Google Scholar] [CrossRef]
- Ghabrial, S.A.; Castón, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479, 356–368. [Google Scholar] [CrossRef]
- Sutela, S.; Piri, T.; Vainio, E.J. Discovery and Community Dynamics of Novel ssRNA Mycoviruses in the Conifer Pathogen Heterobasidion parviporum. Front. Microbiol. 2021, 12, 770787. [Google Scholar] [CrossRef]
- Özkan, S.; Coutts, R.H.A. Aspergillus fumigatus mycovirus causes mild hypervirulent effect on pathogenicity when tested on Galleria mellonella. Fungal Genet. Biol. 2015, 76, 20–26. [Google Scholar] [CrossRef]
- Crabtree, A.M.; Kizer, E.A.; Hunter, S.S.; Van Leuven, J.T.; New, D.D.; Fagnan, M.W.; Rowley, P.A. A Rapid Method for Sequencing Double-Stranded RNAs Purified from Yeasts and the Identification of a Potent K1 Killer Toxin Isolated from Saccharomyces cerevisiae. Viruses 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-M.; Banerjee, N.; Koltin, Y.; Bruenn, J.A. The Ustilago maydis virally encoded KP1 killer toxin. Mol. Microbiol. 1996, 20, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Thapa, V.; Turner, G.G.; Hafenstein, S.; Overton, B.E.; Vanderwolf, K.J.; Roossinck, M.J. Using a novel partitivirus in Pseudogymnoascus destructans to understand the epidemiology of white-nose syndrome. PLoS Pathog. 2016, 12, e1006076. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, H.; Chu, H.; Cho, W.K. Identification of viruses from fungal transcriptomes. BioRxiv 2020. [Google Scholar] [CrossRef]
- Gilbert, K.B.; Holcomb, E.E.; Allscheid, R.L.; Carrington, J.C. Hiding in plain sight: New virus genomes discovered via a systematic analysis of fungal public transcriptomes. PLoS ONE 2019, 14, e0219207. [Google Scholar] [CrossRef]
- Applen Clancey, S.; Ruchti, F.; LeibundGut-Landmann, S.; Heitman, J.; Ianiri, G. A Novel Mycovirus Evokes Transcriptional Rewiring in the Fungus Malassezia and Stimulates Beta Interferon Production in Macrophages. Mbio 2020, 11, e01534-20. [Google Scholar] [CrossRef]
- Babraham Bioinformatics-FastQC. A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 27 November 2022).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods Nat. Publ. Group 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Home TransDecoder/TransDecoder Wiki. GitHub. Available online: https://github.com/TransDecoder/TransDecoder (accessed on 27 November 2022).
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Adams, M.; Carstens, E.; Lefkowitz, E. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9. [Google Scholar]
- Bruenn, J.A. A closely related group of RNA-dependent RNA polymerases from double-stranded RNA viruses. Nucleic Acids Res. 1993, 21, 5667–5669. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, H.; Chu, H.; Cho, W.K. Unveiling Mycoviromes Using Fungal Transcriptomes. Int. J. Mol. Sci. 2022, 23, 10926. [Google Scholar] [CrossRef]
- Ali, M. Ninth Report of the International Committee on Taxonomy of Viruses. Available online: https://www.academia.edu/8097730/Ninth_Report_of_the_International_Committee_on_Taxonomy_of_Viruses (accessed on 27 November 2022).
- Hewson, I.; Johnson, M.R.; Tibbetts, I.R. An Unconventional Flavivirus and Other RNA Viruses in the Sea Cucumber (Holothuroidea; Echinodermata) Virome. Viruses 2020, 12, 1057. [Google Scholar] [CrossRef]
- Suzaki, K.; Ikeda, K.; Sasaki, A.; Kanematsu, S.; Matsumoto, N.; Yoshida, K. Horizontal transmission and host-virulence attenuation of totivirus in violet root rot fungus Helicobasidium mompa. J. Gen. Plant Pathol. 2005, 71, 161–168. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulanouar, F.; Ranque, S.; Levasseur, A. Tracking Mycoviruses in Public RNAseq Datasets of Malassezia: Three Original Totiviruses Revealed. Viruses 2023, 15, 1368. https://doi.org/10.3390/v15061368
Boulanouar F, Ranque S, Levasseur A. Tracking Mycoviruses in Public RNAseq Datasets of Malassezia: Three Original Totiviruses Revealed. Viruses. 2023; 15(6):1368. https://doi.org/10.3390/v15061368
Chicago/Turabian StyleBoulanouar, Fatima, Stéphane Ranque, and Anthony Levasseur. 2023. "Tracking Mycoviruses in Public RNAseq Datasets of Malassezia: Three Original Totiviruses Revealed" Viruses 15, no. 6: 1368. https://doi.org/10.3390/v15061368
APA StyleBoulanouar, F., Ranque, S., & Levasseur, A. (2023). Tracking Mycoviruses in Public RNAseq Datasets of Malassezia: Three Original Totiviruses Revealed. Viruses, 15(6), 1368. https://doi.org/10.3390/v15061368






