Abstract
The application of microbe-derived products as natural biocontrol agents to boost systemic disease resistance to virus infections in plants is a prospective strategy to make agriculture more sustainable and environmentally friendly. In the current study, the rhizobacterium Bacillus amyloliquefaciens strain TBorg1 was identified based on 16S rRNA, rpoB, and gyrA gene sequences, and evaluated for its efficiency in conferring protection of tomato from infection by Tobacco mosaic virus (TMV). Under greenhouse circumstances, foliar sprays of TBorg1 culture filtrate (TBorg1-CF) promoted tomato growth, lowered disease severity, and significantly decreased TMV accumulation in systemically infected leaves of treated plants relative to untreated controls. TMV accumulation was reduced by 90% following the dual treatment, applied 24 h before and after TMV infection. Significant increases in levels of total soluble carbohydrates, proteins, and ascorbic acid were also found. In addition, a significant rise in activities of enzymes capable of scavenging reactive oxygen species (PPO and POX), as well as decreased levels of non-enzymatic oxidative stress markers (H2O2 and MDA) were observed, compared to untreated plants. Enhanced systemic resistance to TMV was indicated by significantly increased transcript accumulation of polyphenolic pathway (C4H, HCT, and CHI) and pathogenesis-related (PR-1 and PR-5) genes. Out of the 15 compounds identified in the GC-MS analysis, 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester and phenol, 2,4-bis(1,1-dimethylethyl), as well as L-proline, N-valeryl-, and heptadecyl ester were present in the highest concentrations in the ethyl acetate extract of TBorg1-CF. In addition, significant amounts of n-hexadecanoic acid, pyrrolo [1,2-a] pyrazine-1,4-dione hexahydro-3-(2-methylpropyl)-, nonane, 5-butyl-, and eicosane were also detected. These compounds may act as inducers of systemic resistance to viral infection. Our findings indicate that the newly isolated B. amyloliquefaciens strain TBorg1 could be a potentially useful rhizobacterium for promoting plant growth and a possible source of biocontrol agents for combating plant virus infections.
1. Introduction
Climate change, coupled with global population growth and urbanization, contributes to the rising demand for increasing crop yields and enhanced food quality. Numerous initiatives have already been implemented to increase the rate of agricultural production. On the other hand, plant diseases cause considerable crop losses and stall advances in crop management [1,2]. Plant viral infections pose a significant danger to plant biosecurity, resulting in massive crop losses on a global scale [3,4]. Tobacco mosaic virus (TMV) is one of the most contagious plant diseases, owing to its broad host range (about 66 families with over 900 plant species) and severe infection consequences [5,6,7]. Mechanical transmission of TMV occurs when diseased plants, contaminated agriculture instruments, and/or contaminated seeds come into close physical contact [8]. In addition, TMV virions are exceptionally stable, ensuring persistence for years in infected materials such as fallen leaves or soil, with an intact potential for re-infection [9]. In tomatoes, various morphological abnormalities are caused by TMV infection, including systemic leaf mosaicism and necrosis, and leaf chlorosis [10]. Moreover, if TMV infection becomes more severe, it can cause systemic changes in the blooming organs, delaying fruit ripening, reducing crop output, and ultimately causing crop loss [11].
It is difficult to control TMV infection, depending primarily on adopting resistant plant cultivars and preventing vector spread by the intense application of insecticides, which can negatively affect the environment and public health [12]. The interest in biocontrol agents as environmentally benign alternatives for the toxic chemicals now used in plant pest management strategies is increasing to ensure long-term sustainability in agriculture and the environment [13,14,15]. Natural rhizosphere microbiota called plant growth-promoting rhizobacteria (PGPR) boost plant development and resistance to several diseases. Multiple studies have discovered that PGPR can promote plant growth while increasing resistance to, e.g., viral infection [16,17]. PGPRs promote plant growth by boosting nutrient absorption and producing biomolecules that are important in conferring stress tolerance. Depending on the circumstances, these activities may indirectly suppress the plant’s susceptibility to infections or combat pathogens directly by synthesis of antibiotics or competition for critical nutrients [18,19]. Strains of Bacillus spp., for example, are frequently used as PGPRs, their efficiency attributable to their diverse mechanisms for controlling infections and stimulating plant growth, which are mediated by, e.g., a large number of secondary metabolites [20,21]. Under field conditions, however, the survival and adaptation of PGPR inocula to natural microbiota are strain-specific and significantly influenced by procedures of application, which are frequently associated with ambiguous results in the laboratory [20]. Furthermore, it is unknown whether PGPR inocula impact the native microbial population at the application site, potentially increasing antagonistic resistance [22,23,24]. As a result of these drawbacks, microbial culture filtrates have been developed as an environmentally friendly and dependable alternative to PGPRs [20].
Generally, two basic mechanisms may cause induced resistance in plants. The first is systemic acquired resistance (SAR), which is induced by pathogens or elicitor molecules contacting aerial plant parts (leaves) and is controlled primarily by salicylic acid [7,25]. The second is induced systemic resistance (ISR), which is induced by PGPR (and other beneficial microorganisms) when they interact with plant roots, and is regulated by jasmonic acid and ethylene [25,26]. Both pathways contribute to plant viral resistance. The activation of both SAR and ISR results in numerous, partially overlapping cellular responses, including the activation of defense genes and antioxidant enzymes [25,27,28,29]. For example, the SAR pathway is characterized by the induction of defense genes, including those encoding for the pathogenesis-related proteins, PR-1, PR-2, and PR-5, while ISR is instead marked by elevated levels of PR-3, PR-4, PR-6, and PR-12.
The present study aimed to determine the efficacy of Bacillus amyloliquefaciens strain TBorg1-culture filtrate (TBorg1-CF) to enhance tomato growth and confer protection against TMV infection in tomato plants either directly or by inducing systemic resistance following foliar spraying. We assumed that the application of TBorg1-CF (instead of live bacteria) to tomato leaves (rather than roots) may imply, primarily, the induction of an SAR, rather than an ISR, against TMV. To elucidate the mechanisms of resistance induction, we determined the activities of enzymes involved in ROS scavenging (PPO, SOD, and POX), levels of non-enzymatic oxidative stress indicators (H2O2 and MDA), as well as DPPH (an indicator of free radical scavenging activity), ascorbic acid, and total carbohydrate and protein contents. In addition, the transcript levels of various defense-related genes, including PR-1, PR-2, PR-5, C4H, HCT, and CHI, were estimated in tomato tissues, along with TMV accumulation. Finally, the bioactive ingredients of TBorg1-CF were screened and identified using a gas chromatography-mass spectrometry (GC-MS) approach.
2. Materials and Methods
2.1. Plant Material and Viral Source
A virus-free line of the Carmen cultivar of tomato (Solanum lycopersicum L.), sensitive to TMV infection, was obtained from the Egyptian Agriculture Research Center. A purified isolate of TMV strain KH1 (accession number MG264131) was employed as a source of viral inoculum that has been previously characterized [10].
2.2. Bacterial Isolation
A nutrient agar (NA) medium with peptone (5%), yeast extract (3%), NaCl (5%), and agar (15%) was used for bacterial isolation [30]. Five tomato rhizosphere samples were collected from healthy tomato fields in Alexandria Governorate, Egypt. Each sample (10 g) was a mix of five (2 g) rhizosphere samples derived from different tomato plants and collected at the same site. After that, each sample (10 g) was shaken in 100 mL of 0.9% NaCl solution for 30 min. Then, 100 µL of each serial dilution was aseptically streaked on duplicate NA plates and incubated at 30 °C for 24 h. Single bacterial colonies from a 10−6 dilution were chosen and cultured individually in nutrient broth (agar-free nutrient medium) for 48 h at 30 °C, shaking at 200 rpm. After centrifugation (10 min, 10,000 rpm), the culture filtrate (CF) was collected and filtered with a 0.45 µm pore syringe filter. The purified isolates’ antiviral effects were investigated on Datura stramonium plants, serving as TMV local lesion hosts. The upper right half of the leaves received 100 µL of bacterial CF, whereas the left half received 100 µL of sterilized nutrient broth medium. After 24 h, both leaf halves were inoculated with TMV. The study used three biological replicates. The isolate with the highest antiviral activity was chosen for further testing based on its inhibition percentage relative to local lesion numbers.
2.3. Molecular Bacterial Identification
The Wizard Genomic DNA Purification Kit (Promega, Fitchburg, WI, USA) was used to purify bacterial genomes following the manufacturer’s instructions. The isolate demonstrating highest antiviral efficacy was molecularly identified using the 16S rRNA, rpoB, and gyrA genes (Table 1) and analysis of morphological traits [31]. Sequencing of the purified PCR products was conducted by an Analyzer 3130xl (Applied Biosystems, Foster City, CA, USA), using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA). Following analyses with NCBI-BLAST, the MEGA 11 software was applied, using UPGMA and a bootstrap method with 2000 replications to uncover the chosen isolate’s phylogenetic relationships. Annotated nucleotide sequences were deposited to GenBank.

Table 1.
The nucleotide sequences of the specific primers that were used in this study.
2.4. Design of Greenhouse Experiments and Evaluation of Growth Parameters
Seeds of tomato were grown in plastic pots (30 cm in diameter) under greenhouse conditions. Each pot was supplied with 4 kg of autoclaved sterilized sand and clay (1:1). Day and night temperatures of 28 °C/16 °C were used, with a relative humidity of 70% for the incubation of tomato seedlings. One week following transplantation, on the 28th day after sowing, each tomato seedling’s two uppermost true leaves were mechanically inoculated with semi-purified TMV virions (1 mL), as reported previously [32]. Five treatments were used in the experiments, each treatment with five biological replicates and each biological replicate containing five tomato plants per pot (Figure 1). During all analytical evaluations, three uppermost true leaves per tomato plant were used to create a pool of one biological replicate (15 leaves). For every one biological replicate, three technical replicates were performed. The first treatment was the control (mock treatment). The second treatment was TMV inoculation (TMV treatment). The third treatment was tomato foliar treated with bacterial CF 24 h before TMV inoculation (TB treatment). The fourth treatment was tomato foliar treated with bacterial CF 24 h after TMV inoculation (TA treatment). The fifth treatment was tomato foliar treated with bacterial CF 24 h before and after viral infection (TBA treatment). A handheld pressure sprayer was used for spraying the entire plant with bacterial CF until runoff. All plants were kept in insect-proof greenhouses for over three weeks, and the development of mosaic symptoms was investigated daily.
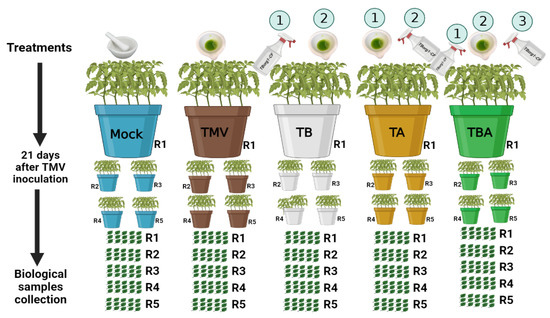
Figure 1.
Greenhouse experiment scheme among different treatments. Mock: tomato plants inoculated with viral inoculation buffer; TMV: tomato plants inoculated with TMV; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF.
2.5. Sample Collection, Disease Assessment, and Determination of Virus Accumulation
Tomato plants from each group were collected at 21 days post-TMV inoculation (dpi), washed many times with running water, weighed, measured, and analyzed for their fresh weight and shoot and root lengths. The plant’s dry weight was calculated after drying at 50 °C. The enzyme activities and gene expression levels were also determined at 21 dpi. Viral accumulation was calculated based on expression levels of the TMV-CP gene (Table 1) in plant samples of different treatments. The disease severity was estimated based on visual observation and a rating scale of 0 to 3. While 0 indicates no symptoms, 1 indicates vein clearing; 2 indicates chlorotic and mild mosaic, and 3 indicates severe mosaic and malformation. The disease severity (DS) index was estimated using the following formula:
2.6. Oxidative Stress Markers
2.6.1. Determination of Hydrogen Peroxide (H2O2)
The determination of H2O2 in the fresh tomato samples was performed using the KI method [33], with a few modifications. A 0.1 g of fresh plant samples were homogenized in 1 mL of 0.1% TCA and centrifuged (10,000 rpm, 15 min, 4 °C) to obtain a clear homogenate. One milliliter of plant homogenate and two milliliters of KI solution (1 M KI in 10 mM potassium phosphate buffer, pH 7.0) were mixed to detect H2O2. After 20 min, the reaction absorbance was assayed at 390 nm. The results were calculated based on the H2O2 extinction coefficient (0.28 M−1 cm−1) and expressed as µmol/g fresh weight.
2.6.2. Determination of Malondialdehyde (MDA)
Thiobarbituric acid (TBA) was used to assay malondialdehyde levels in all treatments, as previously described [34]. One milliliter of 0.1% trichloroacetic acid (TCA) was used to grind 100 mg of tomato leaf samples. The samples were then centrifuged at 10,000 rpm, 30 min at 4 °C, to separate the TCA from the ground-up tomato leaf samples. Each 1 mL of sample supernatant was mixed with 4 mL of TBA solution (0.5% TBA:20% TCA). Then, the samples were put into an oven set at 95 °C for 30 min. The reaction was stopped by immersing the samples in ice. At 600 nm, the color of the product indicated the amount of malondialdehyde present (µmol/g fresh weight).
2.7. Measurement of Antioxidant Enzymatic Activities
2.7.1. Polyphenol Oxidase (PPO)
Quinone techniques were used to determine the PPO activity [35]. In summary, 500 mL of crude plant extract was added to 1 mL of 50 mM quinone and incubated at 25 °C for 10 min. The absorbance of the reaction was determined at 420 nm, where a 0.001 rise in absorbance corresponds to one unit of enzyme activity per minute and is reported as µmol/g fresh weight.
2.7.2. Superoxide Dismutase (SOD)
SOD activity was measured using a modified nitroblue tetrazolium (NBT) photoreduction inhibition technique [36]. The crude plant extract (100 µL in 100 mM of potassium phosphate buffer pH 7.0) was combined with the same amount of 50 µM of NBT, 10 µM of riboflavin, 10 mM EDTA, 50 mM sodium carbonate, and 12 µM L-methionine. To obtain a final reaction volume of 3 mL, a 50 mM phosphate buffer, pH 7.6, was added. As controls, reaction mixtures devoid of plant extract were used. After 15 min of exposure to fluorescent lights to commence the photochemical process, the mixtures were put in the dark, and the absorbance at 560 nm was measured. One unit of enzyme activity was defined as a 50% inhibition of the photochemical reduction [37]. SOD activity was quantified as µmol/g fresh weight.
2.7.3. Peroxidase (POX)
POX activity was determined using the technique of Angelini et al. [38]. In summary, 120 µL of 1 mM hydrogen peroxide and 500 µL of 5 mM guaiacol were added to the crude plant extract (80 µL in K-phosphate buffer, pH 7.0). The final volume of 1.2 mL was adjusted by using 100 mM phosphate buffer pH 7.0. The reaction was incubated at 30 °C for 10 min, and the absorbance at 480 nm was determined. Using an extinction coefficient of 26,600 M−1 cm−1, the POX activity was calculated and expressed as µmol/g fresh weight.
2.8. Total Soluble Protein (TSP) Determination
Amounts of total soluble proteins were determined using the Bradford method and a standard curve of bovine serum albumin [39].
2.9. Detection of Total Soluble Carbohydrates (TSC)
Total soluble carbohydrate contents (TSC) were determined using the anthrone method, as described previously [40]. Plant leaves were first homogenized in 95% ethanol in a solid: liquid ratio of 1:20. Following precipitation (centrifugation at 5000 rpm for 10 min), 100 mL was mixed with 1 mL anthrone solution (200 mg of anthrone in 100 mL of concentrated H2SO4) and incubated for 10 min at 100 °C in a water bath. The reaction absorbance was measured at 625 nm after cooling for 1 h. The contents of total soluble carbohydrates (mg/g dry weight) were calculated using a glucose standard curve.
2.10. Determination of Ascorbic Acid (AsA)
Ascorbic acid contents were determined using Na-molybdate, as previously reported [41]. Samples of fresh leaves were homogenized in 5% sulfosalicylic acid (solid:liquid ratio of 1:5). The homogenate was then centrifuged at 10,000 rpm and 4 °C for 15 min. A 1 mL of clear leaf extract was added to 5 mL of freshly made reaction mixture consisting of 2% of Na-molybdate, 0.075 M of H2SO4, and 1.5 mM of Na2HPO4 (v/v/v). After 40 min of incubation at 60 °C, the absorbance was measured at 660 nm, and the ascorbic acid content (mg/g fresh weight) was determined from an ascorbic acid standard curve.
2.11. Free Radical Scavenging Activity Evaluation
The free radical scavenging capacity was investigated according to Shimada et al. [42], as follows: 2 mL 2,2-Diphenyl-1-picrylhydrazyl (DPPH, 0.05 M in methanol) was added to 100 µL of plant leaves extract (in K-phosphate buffer pH 7.0). The color reduction was measured at 517 nm for 30 min and expressed as scavenging activity (%) using the equation: Scavenging of free radicals (%) = (AI − A30/AI) × 100, where AI is the initial reaction absorbance and A30 is the reaction absorbance after 30 min.
2.12. Analyzing Results of Quantitative RT-PCR (qRT-PCR)
2.12.1. Extraction and Synthesis of cDNA from Plant Total RNA
One hundred milligrams of tomato leaves were used for total RNA extraction by the guanidium isothiocyanate method [43,44]. The purity and concentration of extracted RNA was measured by a SPECTRO Star Nano instrument (BMG Labtech, Ortenberg, Germany) at A260/A280. Moreover, the quality of 28S and 18S rRNA bands separated by electrophoresis on 1.2% agarose gels was used to assess RNA integrity. In order to synthesize cDNA, 1 μg of DNase I-treated RNA was utilized from each sample as a template in a reverse transcription process. The reaction procedure was carried out with oligo (dT) and random hexamer primers, as was reported in a prior study [45]. The final cDNA product was kept at −20 °C until utilized as a template for qRT-PCR.
2.12.2. Expression of Tomato Defense Genes and TMV-CP
The transcript levels of three tomato genes that encode pathogenesis-related proteins (PR-1, PR-2, and PR-5), and three genes encoding proteins involved in polyphenol metabolism (C4H, HCT, and CHI), along with transcript accumulation of the TMV-coat protein gene (TMV-CP) were assessed for all treatments using the qRT-PCR technique. The nucleotide sequences of the primers used are presented in Table 1. Expression values of tomato defense genes and TMV-CP were adjusted by assaying the expression of β-actin as a reference gene. Expression of β-actin was tested during all types of treatments (mock and TMV inoculation with/without CF-treatments), and significant changes in gene expression were not detectable. The qPCR reactions for each biological treatment were performed in a separate batch using a SYBR Green Mix (Thermo Fisher, CA, USA) and run on a Rotor-Gene 6000 real-time thermocycler (QIAGEN, Germantown, MD, USA), as previously described [46]. For each gene studied, relative expression levels were estimated according to the 2−ΔΔCT technique [47].
2.13. GC-MS Analysis of Active Biomolecules in Bacterial Culture Filtrates
After ethyl acetate extraction, gas chromatography-mass spectrometry (GC-MS) was employed to detect the active biomolecules in bacterial culture filtrates (CF), as previously reported [15]. Briefly, the CF was added to ethyl acetate in a ratio of 1:1 and shaken vigorously for 20 min, and then phases were separated using a separating funnel. The ethyl acetate phase was concentrated at a temperature of 50 °C using a rotatory evaporator. By using a GC-MS system (TRACE 1300 Series, Thermo, Waltham, MA, USA) with a split mode mass detector and helium as the carrier gas at a flow rate of 1 mL/min, the concentrated CF extracts were screened for secondary metabolite compounds. The injector was set to 250 °C for two minutes and the oven to 60 °C for two minutes with a scan time of 0.2 s; a mass range of 50–650 amu; and a 20-min ramp to 250 °C. During the 53-min run period, mass spectra at 70 eV were recorded. The CF components were identified by comparison to published data and the GC-MS library.
2.14. Statistical Analyses
Using the GraphPad Prism software, all data were statistically analyzed using a one-way ANOVA. By using the Tukey’s honest significant differences (H.S.D.) method at a probability value (p-Value) ≤ 0.05, significant differences were determined. In the tables and histograms, a column bar is used to show the standard deviation (SD). Compared to mock-inoculated samples, relative gene expression values higher than one depict an increase in transcript accumulation, while values lower than one indicate a decrease in gene expression levels.
3. Results
3.1. Identification of an Isolate of Bacillus amyloliquefaciens from Tomato Rhizosphere
The morphological investigation of the bacterial isolate that was isolated from the rhizosphere of tomato plants revealed gram-positive and endospore-forming characteristics. The appearance of colonies was creamy-white and rough, and the edges of colonies slightly irregular. PCR reaction results revealed that the amplicons of the three bacterial genes tested, 16S rRNA, rpoB, and gyrA, were approximately 1532, 519, and 906 bp in length, respectively. On the basis of NCBI-BLAST alignment and phylogenetic tree analysis, the bacterial isolate was identified as Bacillus amyloliquefaciens and deposited in the GenBank database under the name “Bacillus amyloliquefaciens strain TBorg1” with the accession numbers of ON197102, ON193513, and ON193514 for the 16S rRNA, rpoB, and gyrA genes, respectively. The NCBI-BLAST alignment and phylogenetic tree analysis revealed that the nucleotide sequence of the 16S RNA gene of TBorg1 (Figure 2) displays a 100% similarity to other B. amyloliquefaciens isolates, especially to isolates from India (OL636031 and MZ396973), Taiwan (DQ993675 and EF423605), and Poland (JF412546). On the other hand, the BLAST alignment of the nucleotide sequence of the gyrA gene indicated a high similarity (100% identity) to other B. amyloliquefaciens isolates, especially to an isolate from Taiwan (CP053376). Furthermore, the nucleotide sequence of the rpoB gene exhibited 100% identity with other B. amyloliquefaciens isolates deposited in GenBank, particularly with two isolates from China (CP054415 and CP032146).
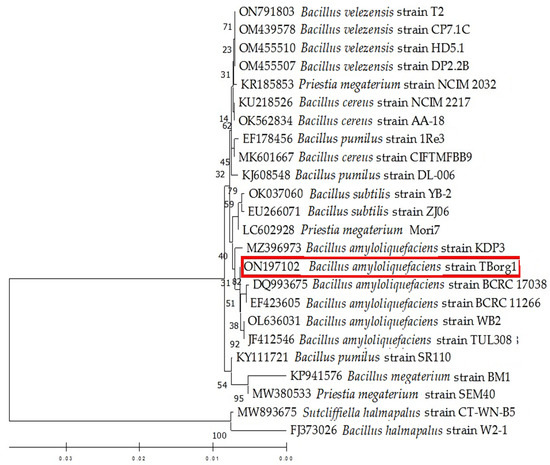
Figure 2.
A phylogenic tree depicting the relationships of the isolated Bacillus amyloliquefaciens strain TBorg1 (indicated by a red rectangle) and other closely related isolates from GenBank based on the 16s rRNA nucleotide sequence. The MEGA 11 software used the UPGMA algorithm, and the bootstrap method with 2000 replicates to create the tree.
3.2. Effect of TBorg1 Culture Filtrate on Tomato Growth and Systemic Accumulation of TMV
The data of tomato growth parameters from greenhouse experiments showed significant reductions in fresh weight, dry weight, shoot length, and root length of tomato plants that were infected with TMV (TMV treatment), recording 6.89 ± 1.53 g, 1.63 ± 0.16 g, 27.33 ± 1.58 cm, and 9.50 ± 1.51 cm, respectively, as compared to mock-treated plants (Table 2). On the other hand, foliar applications of TBorg1 culture filtrate (CF) either 24 h before, 24 h after, or 24 h before and 24 h after TMV inoculation (TB or TA or TBA) resulted in significantly increased tomato fresh weight, dry weight, shoot length, and root length, in comparison to TMV treatment plants (Table 2). The TBA treatment was the most effective in suppressing the adverse effects of the disease by increasing fresh and dry weights to 10.55 ± 1.38 g and 2.15 ± 0.28 g, respectively. Moreover, shoot length, and root length values following TBA treatments were significantly higher than those of the other treatments, recording 38.50 ± 3.04 cm, and 18.00 ± 3.08 cm, respectively, at 21 dpi.

Table 2.
Effects of foliar application of B. amyloliquefaciens strain TBorg1 culture filtrate on tomato plant growth parameters at 21 dpi.
In our greenhouse experiments, we found that the foliar application of B. amyloliquefaciens strain TBorg1-culture filtrate (TBorg1-CF) significantly reduces disease severity and decreases virus accumulation in all treated tomato plants (TB, TA, and TBA), as compared to the TMV treatment. The results showed that TMV-inoculated tomato plants developed characteristic symptoms with severe mosaic appearing by 14 dpi (Figure 3). On the other hand, a delay in symptom development of five and three days were found with TB and TA treatments, respectively (Figure 3). Moreover, dual foliar application of TBorg1-CF (TBA) resulted in a ca. 7 days’ delay in symptom development. No disease symptoms were observed on the mock-inoculated plants. Consistent with symptom appearance, the TMV treatment resulted in disease severity of 93.43 ± 1.98% (Table 3). However, TB and TA treatments significantly reduced disease severity to 32.16 ± 1.41% and 43.28 ± 2.43%, respectively (Table 3). Moreover, the TBA treatment exhibited the lowest disease severity of 17.19 ± 1.67%. Importantly, treatments with TBorg1-CF considerably reduced the accumulation of TMV in tomato leaves. Furthermore, qRT-PCR results revealed that the TMV treatment results in the highest levels of TMV-CP transcripts, reflecting a relative expression of 27.98, indicating the plant’s viral infection (Table 3). In contrast, the relative expression levels of TMV-CP in TBorg1-CF-treated plants were only 4.54, 3.39, and 2.78 for the TA, TB, and TBA treatments, respectively (Table 3). In fact, the small amounts of TMV detected in TBA-treated plants corresponds to a 90% reduction in viral accumulation. These results suggest that TBorg1-CF may indeed confer plant resistance to TMV replication in tomato tissues.

Figure 3.
Effect of foliar application of B. amyloliquefaciens strain TBorg1-CF on developing disease symptoms in tomato leaves at 18 dpi. Mock: tomato plants inoculated with viral inoculation buffer and foliar sprayed with sterile bacteria-free broth medium; TMV: tomato plants inoculated with TMV and foliar sprayed with sterile bacteria-free broth medium; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection.; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF.

Table 3.
Effects of foliar application of B. amyloliquefaciens strain TBorg1-CF on disease severity and accumulation levels of TMV-CP gene in tomato plants at 21 dpi.
3.3. Effect of TBorg1-CF on Oxidative Stress Markers
Levels of two oxidative stress markers, malondialdehyde (MDA) and hydrogen peroxide (H2O2), were evaluated in the five tomato treatment groups (Figure 4). For MDA, the TMV treatment group showed the highest levels (144 ± 2.8 µmol/g f.wt.), while the mock plants exhibited the lowest MDA levels (103 ± 4.9 µM/g f.wt.). Similarly, the TMV treatment and mock treatment plants showed the highest and lowest levels of H2O2 with 9.2 ± 0.3 and 5.7 ± 0.4 µmol/g f.wt., respectively (Figure 4). Compared to the TMV treatment, the foliar applications of TBorg1-CF significantly decreased MDA and H2O2 contents in all treated plants (Figure 4). Among TBorg1-CF treatments, the TBA treatment resulted in the lowest levels of MDA and H2O2 (111 ± 4.1 and 6.2 ± 0.4 µM/g f.wt.), followed by TB (122 ± 1.8 and 6.8 ± 0.4 µM/g f.wt.), and TA (131 ± 2.4 and 7.7 ± 0.9 µM/g f.wt.), respectively.
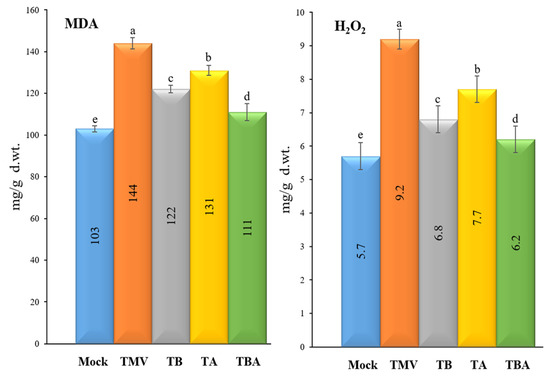
Figure 4.
Effect of foliar application of B. amyloliquefaciens strain TBorg1-CF on two oxidative stress markers of tomato plants at 21 dpi; MDA (left) and H2O2 (right). Mock: tomato plants inoculated with viral inoculation buffer and foliar sprayed with sterile bacteria-free broth medium; TMV: tomato plants inoculated with TMV and foliar sprayed with sterile bacteria-free broth medium; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection.; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF. Each value in a column is the average ± SD of the results from five biological replicates. The average values of the columns with the same letter do not differ significantly.
3.4. Effect of TBorg1-CF on Antioxidant Enzymatic Activities
The assay results of the three antioxidant enzymes (SOD, PPO, and POX) revealed that PPO and POX have significantly different activities upon TMV and TBorg1-CF treatments (Figure 5). The results indicated a remarkable increase in POX activates after treatment of tomato plants with TBorg1-CF (Figure 5). Compared to mock treatment (0.16 ± 0.01 µM/g f.wt.), the TBA treatment exhibited the highest POX levels (0.32 ± 0.02 µM/g f.wt.), followed by TA and TB treatments with levels of 0.25 ± 0.01 µM/g f.wt. and 0.23 ± 0.01 µM/g f.wt., respectively. No significant differences in POX activities were found between TMV treatment (0.16 ± 0.02 µM/g f.wt. min−1) and mock treatment groups. Regarding PPO (Figure 5), our results showed a significant reduction in PPO activities upon TMV infection in all treatment groups except TBA treatment, which showed a slight increase (0.20 ± 0.03 µM/g f.wt.), with no significant changes as compared to mock treatment (0.18 ± 0.02 µM/g f.wt.). The lowest PPO activities were detected in TMV treatment (0.13 ± 0.01 µM/g f.wt.), followed by TA (0.15 ± 0.01 µM/g f.wt.) and TB (0.16 ± 0.04 µM/g f.wt.) treatments. Regarding SOD, no significant changes in SOD activities were found between different treatments (Figure 5).
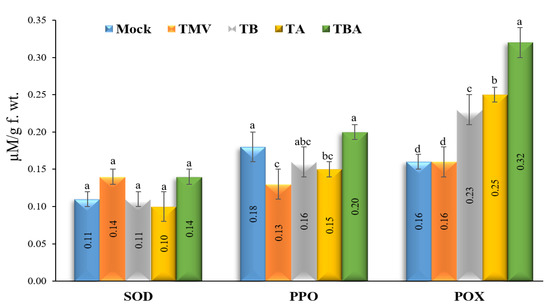
Figure 5.
Effect of foliar application of B. amyloliquefaciens strain TBorg1-CF on three enzymes exhibiting antioxidant activities—SOD (superoxide dismutase), PPO (polyphenol oxidase), and POX (peroxidase)—in tomato plants at 21 dpi. Mock: tomato plants inoculated with viral inoculation buffer and foliar sprayed with sterile bacteria-free broth medium; TMV: tomato plants inoculated with TMV and foliar sprayed with sterile bacteria-free broth medium; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF. Each value in a column is the average ± SD of the results from five biological replicates. The average values of the columns with the same letter do not differ significantly.
3.5. Impact of TBorg1-CF on Total Soluble Carbohydrates and Total Soluble Protein Contents
Regarding the contents of total soluble carbohydrates (TSC), the results revealed that the foliar application of TBorg1-CF significantly elevated TSC contents in the treated tomato plants (Figure 6). The TBA treatment resulted in the highest TSC contents (6.0 ± 0.9 mg/g d.wt.), followed by TA (5.6 ± 0.2 mg/g d.wt.) and TB (5.5 ± 0.3 mg/g d.wt.) treatments, with no significant changes among the three treatments (Figure 6). On the other hand, no significant changes in TSC contents were found between mock (4.1 ± 0.4 mg/g d.wt.) and TMV (3.5 ± 0.1 mg/g d.wt.) treatments. Concerning total soluble protein (TSP) contents, the dual foliar application of TBorg1-CF (TBA treatment) resulted in the highest TSP (2.6 ± 0.2 mg/g d.wt.) among all tomato treatments (Figure 6). Compared to the mock treatment (1.9 ± 0.2 mg/g d.wt.), the TMV, TB, and TA treatment groups exhibited approximately the same TSP contents (2.1 ± 0.1 mg/g d.wt.).
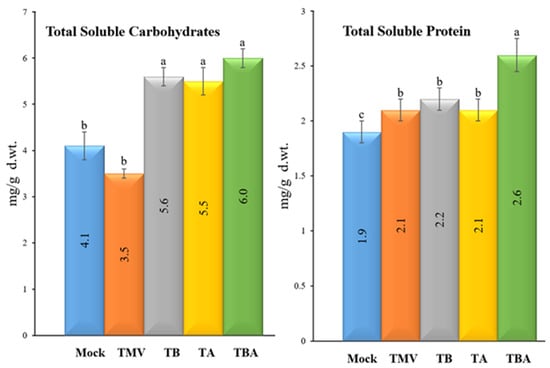
Figure 6.
Effect of foliar application of B. amyloliquefaciens strain TBorg1-CF on total soluble carbohydrates (left) and total soluble protein (right) contents of tomato plants at 21 dpi. Mock: tomato plants inoculated with viral inoculation buffer and foliar sprayed with sterile bacteria-free broth medium; TMV: tomato plants inoculated with TMV and foliar sprayed with sterile bacteria-free broth medium; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection.; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF. Each value in a column is the average ± SD of the results from five biological replicates. The average values of the columns with the same letter do not differ significantly.
3.6. Effect of TBorg1-CF on Free Radical Scavenging Activities and Ascorbic Acid Contents
The free radical scavenging activities were evaluated using the DPPH method in the five tomato experimental conditions (Figure 7). The results indicated a significant activation of free radical scavenging activities after treatments of tomato plants with TBorg1-CF (TB, TA, and TBA treatments) compared to the mock and TMV treatments (Figure 7). The TBA treatment group showed the highest DPPH levels/free radical scavenging activities (35.4 ± 3.2%), followed by TB (33.3 ± 1.2%) and TA (30.5 ± 0.9%) treatments. No significant differences were found between the TMV (27.1 ± 1.3%) and mock (26.5 ± 2.3%) treatments (Figure 7). Remarkably, ascorbic acid contents were significantly reduced upon TMV infection (to 192 ± 9.5 mg/g f.wt.) as compared to the mock treatment (428 ± 18.1 mg/g f.wt.) (Figure 7). In comparison to the TMV treatment, the treatment of tomato plants with TBorg1-CF either before (TB treatment) or after (TA treatment) TMV inoculation considerably increased ascorbic acid contents to 263 ± 16.4 mg/g f.wt. and 284 ± 19.7 mg/g f.wt., respectively, (Figure 7). Interestingly, no significant changes were found in ascorbic acid contents between the TBA treatment (376 ± 21.3 mg/g f.wt.) and the mock treatment (428 ± 18.1 mg/g f.wt.) (Figure 7).
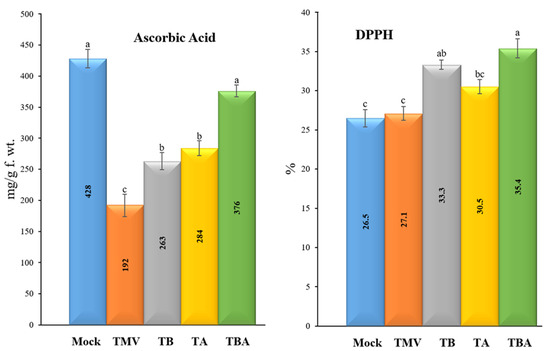
Figure 7.
Effect of foliar application of B. amyloliquefaciens strain TBorg1-CF on the free radical scavenging activities (left) and ascorbic acid contents (right) of tomato plants at 21 dpi. Mock: tomato plants inoculated with viral inoculation buffer and foliar sprayed with sterile bacteria-free broth medium; TMV: tomato plants inoculated with TMV and foliar sprayed with sterile bacteria-free broth medium; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection.; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF. Each value in a column is the average ± SD of the results from five biological replicates. The average values of the columns with the same letter do not differ significantly.
3.7. Effect of TBorg1-CF on the Expression of Tomato Defense Genes
3.7.1. Polyphenol Biosynthetic Pathway Genes
The transcript levels of three genes (C4H, HCT, and CHI) encoding the critical enzymes that regulate polyphenol biosynthesis pathways were studied at 21 dpi after TMV inoculation. (Figure 8). Compared to the mock treatment (control), a significant up-regulation of C4H in the four tomato treatment groups (TMV, TB, TA, and TBA) was observed (Figure 8). The qPCR results revealed that the viral infection, as well as TBorg1-CF applications, induced transcript levels of the C4H gene. The TBA treatment group exhibited the highest expression levels, with a relative expression of 7.73, compared to the control group. The TMV treatment showed a relative expression of 2.82, while TB and TA treatments exhibited relative expressions of 5.50 and 4.20, respectively (Figure 8). Similarly to C4H, HCT was also induced in all tomato treatments compared to the control (Figure 8). A significant up-regulation of HCT was found with relative expression levels of 1.82, 2.38, and 4.01 for TMV, TA, and TB treatments, respectively. The TBA treatment exhibited the highest HCT transcript levels with a relative expression 6.89-fold higher than the control.
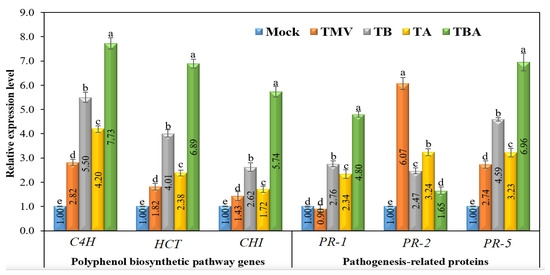
Figure 8.
Effect of foliar application of B. amyloliquefaciens strain TBorg1-CF on the expression of genes encoding polyphenol biosynthetic pathway enzymes (cinnamate 4-hydroxylase, C4H; hydroxycinnamoyl transferase, HCT; chalcone isomerase, CHI) and pathogenesis-related (PR-1, PR-2 and PR-5) proteins of tomato plants at 21 dpi. Mock: tomato plants inoculated with viral inoculation buffer and foliar sprayed with sterile bacteria-free broth medium; TMV: tomato plants inoculated with TMV and foliar sprayed with sterile bacteria-free broth medium; TB: tomato plants sprayed with TBorg1-CF 24 h prior to viral infection; TA: tomato plants sprayed with TBorg1-CF 24 h following viral infection.; TBA: tomato plants were sprayed twice, once 24 h before and once 24 h after viral inoculation, with TBorg1-CF. Each value in a column is the average ± SD of the results from five biological replicates. The average values of the columns with the same letter do not differ significantly.
Regarding CHI, despite the up-regulation of the CHI gene in all treatments compared to the control, it exhibited the lowest transcript levels compared to C4H and HCT (Figure 8). Nevertheless, the dual foliar application of TBorg1-CF resulted in the highest expression levels of CHI with a relative expression of 5.74, followed by TB and TA treatments with relative transcript levels of 2.62 and 1.72, higher than that of the control. Even the TMV treatment group displayed a significant change in CHI expression level of 1.43-fold compared to the mock (control) treatment (Figure 8).
3.7.2. Pathogenesis-Related Protein-Encoding Genes
Compared to the control (mock), a clear difference in transcriptional profiles of three genes (PR-1, PR-2, and PR-5) encoding three pathogenesis-related proteins was observed (Figure 8). Concerning PR-1, its expression was induced only in TBorg1-CF treated plants with relative expressions of 2.76 and 2.34 in TB and TA treatments, respectively (Figure 8). The dual TBorg1-CF application treatment (TBA) showed the best results of PR-1 gene induction with a 4.80-fold higher expression than the mock treatment. The TMV treatment group exhibited a slight decrease in PR-1 relative expression (0.90-fold) compared to the mock treatment (Figure 8). For PR-5 expression, a significant up-regulation (2.74-fold) was observed in TMV-treated plants compared to the mock plants. However, in plants treated with TBorg1-CF either before (TB) or after (TA) infection, expression levels of PR-5 were 4.59- and 3.23-fold higher, respectively, than that of the control (Figure 8). The TBA treatment group showed the highest induction of PR-5 expression, with transcript levels 6.96-fold higher than in the mock treatment group. Thus, treatments with either TMV or TBorg1-CF can effectively trigger PR-5 expression. Regarding PR-2, the TMV treatment group showed the most significant up-regulation of expression with a 6.07-fold higher value than in the mock treatment (Figure 8). However, compared to the TMV treatment, the TBorg1-CF treatment groups (either before or after TMV inoculation or dual CF application) exhibited a significant reduction in transcript levels of PR-2 (Figure 8). The TB and TA treatments resulted in relative expression levels of 2.47 and 3.24, respectively, while the TBA treatment group showed a relative expression level only 1.65-fold higher than that of the control (Figure 8).
3.8. GC-MS Analysis of Bioactive Metabolites of TBorg1-CF
Using a GC-MS equipment, the bioactive components of the ethyl acetate extract of TBorg1-CF were identified. The active ingredients, including their retention time (RT), peak height, chemical formula, molecular weight, and molecular structures are shown in Table 4. Among the 15 bioactive compounds detected in the GC-MS analysis, 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester showed the highest peak area with an RT of 23.369, followed by the compounds phenol, 2,4-bis(1,1-dimethylethyl)- and L-proline, N-valeryl-, heptadecyl ester at RTs of 12.189 and 15.542, respectively (Table 4). The compounds n-hexadecanoic acid, pyrrolo[1,2-a]pyrazine-1,4-dione hexahydro-3-(2-methylpropyl)-, as well as Nonane, 5-butyl-, and eicosane displayed moderate peak areas at RTs of 15.483, 15.437, 13.690, and 15.232, respectively. Moreover, hexadecane, tetradecane, and 1-tridecene were also detected at RTs of 12.795, 12.745, and 14.317, respectively. Other constituents displayed varying retention times and peak areas.

Table 4.
The chemical characteristics of the bioactive components in the ethyl acetate extract of B. amyloliquefaciens strain TBorg1-culture filtrate as analyzed by gas chromatography–mass spectrometry (GC–MS).
4. Discussion
Plant viruses are among the most significant plant disease-causing agents, since over half of all newly developing epidemics have a viral etiology [3,16]. This creates issues regarding food security, and is also accountable for enormous losses in crop productivity. Chemical treatments, such as pesticides and insecticides, must be monitored and controlled due to adverse effects on human health and environmental risks. In agriculture, biological pest management utilizing PGPR (one or more strains) is being evaluated as an improvement over chemical control for fighting plant diseases [48]. Therefore, there is an urgent need for research on novel, environmentally acceptable biocontrol agents that can manage viral plant diseases. The current study evaluated the antiviral activities of a newly identified B. amyloliquefaciens strain, TBorg1, against TMV in tomato plants. The morphological features of the TBorg1 strain were consistent with the description of B. amyloliquefaciens in Bergey’s Manual of Systematic Bacteriology [49]. Furthermore, sequence analyses of representative bacterial genes (16S rRNA, rpoB, and gyrA) of TBorg1 confirmed morphological investigations. However, instead of using the PGPR soil application method for our antiviral biocontrol strategy, we sprayed tomato shoots with the CF of the strain TBorg1, since microbial CFs are regarded as an environmentally friendly and dependable alternative to PGPRs [20]. We have demonstrated that tomato plants treated with TBorg1-CF display a reduced rate of TMV infection, with significantly milder systemic symptoms and disease severity that correlates with the suppression of virus accumulation in tomato leaf tissues. These results demonstrate the efficacy of TBorg1-CF in controlling the disease caused by TMV in tomatoes.
Under greenhouse conditions, the foliar application of TBorg1-CF in TMV-infected tomatoes significantly increased the shoot and root lengths and the fresh weights (TB, TA, and TBA treatments) compared to TMV-infected plants without CF treatments. These findings demonstrate that TBorg1 can significantly improve the growth and development of plant roots and shoots, exhibiting the characteristics of PGPR. Interestingly, the dual application of TBorg1-CF (TBA treatment) resulted in a 7-day delay in TMV-elicited symptoms compared to untreated tomato, which developed severe mosaic symptoms by 14 dpi, as previously described [7,12]. Several authors reported that the foliar application of bacterial culture filtrates results in delayed development of plant viral symptoms [7,15,16,50]. Consistent with symptom appearance, the TA, TB, and TBA treatments significantly reduced TMV disease severity to 43.28, 32.16, and 17.19%, respectively. Furthermore, treatments with TBorg1-CF considerably reduced TMV accumulation by up to 90% in the TBA treatment. Thus, the foliar application of TBorg1 may be capable of mediating induced resistance against TMV in tomato plants. Foliar applications of biological control agents are less widespread than soil treatments, and numerous studies have revealed the effectiveness of B. amyloliquefaciens in increasing the growth of numerous plant species, mediated through a large variety of growth-promoting secondary metabolites and phytohormones [51,52,53]. These findings imply that the CF of B. amyloliquefaciens TBorg1 may contain secondary metabolites that directly suppress TMV and/or play a significant role in inducing SAR. In this context, the foliar application of B. amyloliquefaciens Ba33 resulted in the suppression of TMV and tomato yellow leaf curl virus in tobacco and tomato plants [54,55].
It has been demonstrated that the SAR enhances the production of antioxidant protective enzymes, secondary metabolites, and the expression of plant defense-related genes [16,25]. In the present study, the potential of TBorg1-CF was assessed to induce the activities of antioxidant enzymes and reduce oxidative stress in tomato plants during TMV infection. The considerable increase in H2O2 and MDA in TMV-infected tomato was compatible with the characteristics of viral-infected plants and a high level of reactive oxygen species [56,57]. Interestingly, the foliar applications of TBorg1-CF could be associated with a significant decrease in both oxidative stress markers. The suppression of activities of oxidative stress-generating enzymes was found to maintain the integrity and stability of cell membranes [58]. Moreover, the foliar application of the culture filtrate of B. amyloliquefaciens QSB-6 improved plant stress tolerance by inhibiting MDA buildup in plant tissues [59]. Thus, the considerable decrease in MDA and H2O2 levels demonstrates the efficiency of TBorg1-CF in mitigating oxidative stress in virus-infected plants. Similarly, the increased activities of enzymes with antioxidant functions such as POX and PPO in leaves of virus-infected tomato plants limited tissue oxidation and pathogen penetration by strengthening cell walls [60]. In our study, TMV infection induced a considerable reduction in PPO activity compared to the mock treatment. In contrast, PPO and POX enzyme activities achieved their maximum levels in TBA treatment plants. These alterations indicate that these enzymes might play an important role in ROS detoxification in tomato plants after TBorg1-CF treatment [61,62]. Furthermore, our results imply that the enhancement of PPO and POX enzyme activities by TBorg1-CF could contribute to the arrest of TMV in tomato cells by promoting the establishment of polymerized phenolic barriers around infection sites [63,64]. Activities of such enzymes (limiting tissue oxidation and pathogen penetration) were significantly increased in virus-infected plants after being exposed to culture filtrates of biocontrol agents that cause SAR [57,59]. Moreover, the application of TBorg1-CF significantly increased total soluble carbohydrates, total soluble protein contents, and free-radical quenching activity. Ascorbic acid is a primary non-enzymatic antioxidant essential for plant development and defense. However, the exact mechanism of how ascorbic acid may enhance plants’ resistance to infection is still unclear [65,66]. Compared to TMV infection alone, the TB, TA, and TBA treatments significantly increased the ascorbic acid contents of tomato tissues. An increased accumulation of ascorbic acid in plant tissues alleviates symptoms of viral infection and inhibits RNA virus replication [67,68]. Regarding free radical scavenging activity (DPPH), we have observed that TB and TBA treatments significantly enhance DPPH activity in treated and TMV-infected tomato tissues compared to TMV and mock treatments. It has been reported that increasing the plant’s free radical scavenging potential is part of its defense to microbial infections to mitigate the negative side effects of the surge in oxidative stress [69,70]. The obtained data demonstrate that B. amyloliquefaciens TBorg1 and other beneficial bacteria share comparable strategies for controlling plant viral infections [51,55,57].
Gene expression analysis revealed that the relative expression levels of C4H, HCT, and CHI were significantly increased in all treatments compared to the mock treatment. As secondary metabolites, plants’ polyphenolic compounds are important for plant growth and defence against biotic and abiotic stressors. [16,71]. C4H encodes one of the most important enzymes in the phenylpropanoid biosynthetic pathway that plays a vital role in converting cinnamic acid to p-coumaric acid, followed by the formation of the main intermediate for polyphenolic compounds [72]. HCT is the first enzyme in the chlorogenic acid pathway that catalyzes the conversion of p-coumaroyl CoA to shikimate, which results in chlorogenic acid production [73]. CHI is one of the most important enzymes in the flavonoid pathway. It converts naringenin chalcone into naringenin, a key step in synthesizing additional flavonoid compounds [72]. The up-regulation of such genes in infected tomato tissues reflects their role in defense against viral infection. The highest transcriptional levels were shown in the TBA treatment with relative expression levels of 5.74-, 6.89-, and 7.73-fold, followed by TB treatment, with 2.62-, 4.01-, and 5.50-fold higher than the control for CHI, HCT, and C4H, respectively. The activation of such defense-related tomato genes in TB, TA, and TBA treatments suggests that TBorg1-CF is an effective elicitor of SAR that is linked to biosynthesis and accumulation of polyphenolic compounds in tomato tissues. Moreover, the very high transcript levels of these genes detected by us in the TBA treatment may correspond to the high levels of flavonoid accumulation in plant tissues, suggesting the significance of treatment dosages (dual foliar application) relative to treatment time (single foliar application) in the accumulation of these defense-related compounds [57].
Many reports state that the activation of several PR proteins plays an essential role in activating SAR, and is also effective in reducing pathogen development, multiplication, or dissemination of pathogens [74,75,76]. In line with these observations, the foliar applications of TBorg1-CF, either as TB, TA, or TBA treatments, resulted in a considerable increase in the transcript levels of the PR-1, PR-2, and PR-5 genes. Compared to the mock treatment, the transcript accumulation of PR-1 was significantly induced, with relative expression levels of 2.34, 2.76, and 4.80 in TA, TB, and TBA treatments, respectively. The elevation of PR-1 expression is directly correlated with the activation of salicylic acid during pathogen infection [25,77]. The PR-5 gene encodes thaumatin-like proteins and is involved in the host defense system against biotic and abiotic challenges, and also in regulating physiological processes in several plant species [78]. PR-5 transcriptional profiles in the current study were similar to PR-1, where the expression levels were induced upon TMV challenge during different treatments. TMV infection alone caused a relative expression of PR-5 2.74-fold greater than that of the control. These results agree with a previous report showing that infection of Arabidopsis thaliana with beet severe curly top virus boosted PR-5 expression levels [79]. Moreover, tobacco plants infected with tobacco vein banding mosaic virus also exhibited an up-regulation of the PR-5 gene [80]. In the present study, the foliar application of TBorg1-CF enhanced the expression of PR-5, resulting in maximum expression levels during the TBA treatment (6.96-fold change compared to the control). Therefore, these results suggest that TBorg1-CF could be used as a plant systemic immunity mediator by greatly boosting the expression of SA-inducible genes, which are all considered vital SAR markers [27,76,81]. Regarding the expression of PR-2, our results demonstrated a significant induction of PR-2 in TMV-infected plants, with a relative expression level 6.07-fold greater than in the control. Although PR-2 was up-regulated in TBorg1-CF-treated plants, a significantly lower expression was observed than in TMV infection without TBorg1-CF-treatments. The PR-2 gene encodes β-1,3-glucanase, which may allow viruses to move between plant cells via plasmodesmata. Therefore, TMV might activate this gene to facilitate its mobility and spread within plant cells [10,82], consistent with previous research that found a marked induction of PR-2 during viral infections in potato, Arabidopsis, onion, tobacco, and tomato plants [7]. In addition, the absence of tobacco PR-2 expression decreased sensitivity to viral infection, while the overexpression of PR-2 accelerated the cell-to-cell transmission of potato virus Y [83,84,85]. Foliar treatment of TBorg1-CF may reduce TMV infection by, e.g., inhibiting the cell-to-cell and long-distance movement of the virus by lowering PR-2 expression.
Microbial secondary metabolites are precursors to many biological functions; therefore, monitoring these compounds by various analytical techniques is critical for understanding biological regulation for novel applications [86]. With the aid of a GC-MS instrument, bioactive components in an ethyl acetate extract of TBorg1-CF were identified in the current study. According to the GC-MS analysis results, TBorg1-CF contains 15 distinct components. 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester was present in the highest concentration, followed by the compounds phenol, 2,4-bis(1,1-dimethylethyl)- and L-proline, N-valeryl-, heptadecyl ester. In addition, n-hexadecanoic acid, pyrrolo[1,2-a]pyrazine-1,4-dione hexahydro-3-(2-methylpropyl)-, nonane, 5-butyl-, and eicosane were detected at moderate levels. The results were similar to those previously reported for the culture filtrates of several Bacillus spp. [15,16,57]. Under fungal and bacterial attack, phenol 2,4-bis(1,1-dimethylethyl)- accumulates in plant cells. It is a compound playing a significant role in plant disease resistance, possibly by inhibiting reactive oxygen species (ROS) [87,88]. TMV infection is frequently associated with elevated ROS levels; thus, inhibiting ROS may alleviate the symptoms of viral infection, as shown previously for infections by, e.g., TMV and other plant viruses [70,89]. Eicosane (a long-chain fatty acid) is a biologically active compound derived from different microorganisms and is a potent antimicrobial for controlling different plant pathogens [90,91]. Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- is a heterocyclic compound with various biological activities, including antimicrobial, antiviral, anti-inflammatory, and anticancer properties [92,93,94,95]. According to a recent study, the extract of B. velezensis contains several different pyrrolo[1,2-a]pyrazine-1,4-dione compounds, which inhibit Fusarium oxysporum and increase the systemic resistance to cucumber mosaic virus [15]. It has been found that the mono(2-ethylhexyl) ester of benzenedicarboxylic acid is produced by marine Streptomyces spp. and exerts anticancer action in vitro against human breast adenocarcinoma and hepatocellular liver carcinoma [96]. In summary, the findings of our GC-MS analysis indicated the presence of several physiologically active compounds in the supernatant of B. amyloliquefaciens strain TBorg1 with different antimicrobial properties. Thus, TBorg1 can be suggested as a potentially powerful PGPR for use in agricultural pest management. However, additional research is needed to determine the exact mechanism(s) underpinning the antiviral activity of TBorg1 towards plant viruses such as TMV.
5. Conclusions
The rhizobacterium B. amyloliquefaciens strain TBorg1-CF was found to be an effective growth promoter and tomato defense stimulator against TMV infection. Under greenhouse conditions, the foliar applications of TBorg1-CF significantly improved the growth parameters of tomato (shoot and root length, fresh weight), decreased TMV disease severity, and reduced virus levels by up to 90%. Furthermore, considerable increases in total soluble proteins, total soluble carbohydrates, ascorbic acid, and activities of enzymes capable of scavenging reactive oxygen species (PPO and POX), as well as significantly decreased amounts of non-enzymatic oxidative stress markers (H2O2 and MDA), compared to untreated plants, were also observed. In addition, enhanced systemic resistance to TMV was associated with increased transcript levels of genes encoding polyphenolic pathway enzymes and pathogenesis-related (PR) proteins. The main antimicrobial compounds detected in the TBorg1-CF ethyle acetate extract were 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester, phenol-2,4-bis(1,1-dimethylethyl)-, L-proline-N-valeryl-heptadecyl ester, and pyrrolo[1,2-a]pyrazine-1,4-dione hexahydro-3-(2-methylpropyl)-. Our findings indicate that the newly isolated B. amyloliquefaciens strain TBorg1 is a promising source of plant growth promotion and antiviral compounds for effectively managing plant diseases.
Author Contributions
Conceptualization, A.A.; methodology, A.A. and D.G.A.; software, A.A. and D.G.A.; validation, A.A.A.-A., A.A. and H.M.; formal analysis, A.A.; investigation, D.G.A.; resources, A.A.A.-A. and L.K.; data curation, A.K.; writing—original draft preparation, A.A. and D.G.A.; writing—review and editing, A.A., L.K., A.K. and H.M.; visualization, A.A. and D.G.A.; supervision, H.M. and A.A.A.-A.; project administration L.K. and A.A.A.-A.; funding acquisition, A.A.A.-A., A.K. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by the Science and Technology Development Fund (STDF), Egypt, Grant No 30102. This research was financially supported by the Researchers Supporting Project number (RSP2022R505), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This paper is based upon work supported by the Science, Technology & Innovation Funding Authority (STDF) under grant (30102). This work was supported by grants of the Hungarian National Research, Development and Innovation Office (NKFIH FK131401 and K128868, for A.K. and L.K., respectively). The authors would like to extend their appreciation to the Researchers Supporting Project number (RSP2022R505), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.A.; Macarthur, R.; Boonham, N. The role and challenges of new diagnostic technology in plant biosecurity. Food Secur. 2016, 8, 103–109. [Google Scholar] [CrossRef]
- Roossinck, M.J. Plants, viruses and the environment: Ecology and mutualism. Virology 2015, 479, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Fraile, A.; García-Arenal, F. Tobamoviruses as models for the study of virus evolution. Adv. Virus Res. 2018, 102, 89–117. [Google Scholar]
- Abo-Zaid, G.A.; Matar, S.M.; Abdelkhalek, A. Induction of Plant Resistance against Tobacco mosaic virus Using the Biocontrol Agent Streptomyces cellulosae Isolate Actino 48. Agronomy 2020, 10, 1620. [Google Scholar] [CrossRef]
- McDaniel, L.; Maratos, M.; Farabaugh, J. Infection of plants by Tobacco mosaic virus. Am. Biol. Teach. 1998, 60, 434–439. [Google Scholar] [CrossRef]
- Peng, J.; Song, K.; Zhu, H.; Kong, W.; Liu, F.; Shen, T.; He, Y. Fast detection of Tobacco mosaic virus infected tobacco using laser-induced breakdown spectroscopy. Sci. Rep. 2017, 7, 44551. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to Tobacco mosaic virus. JAPS-J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- Bazzini, A.A.; Hopp, H.E.; Beachy, R.N.; Asurmendi, S. Infection and coaccumulation of Tobacco mosaic virus proteins alter microRNA levels, correlating with symptom and plant development. Proc. Natl. Acad. Sci. USA 2007, 104, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco mosaic virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber mosaic virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef] [PubMed]
- Sorokan, A.; Cherepanova, E.; Burkhanova, G.; Veselova, S.; Rumyantsev, S.; Alekseev, V.; Mardanshin, I.; Sarvarova, E.; Khairullin, R.; Benkovskaya, G.; et al. Endophytic Bacillus spp. as a Prospective Biological Tool for Control of Viral Diseases and Non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front. Microbiol. 2020, 11, 569457. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 12, 634796. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Bernardi, M.; Mattedi, A.; Spera, D.M.; Del Gallo, M. Cell-free supernatants of plant growth-promoting bacteria: A review of their use as biostimulant and microbial biocontrol agents in sustainable agriculture. Sustainability 2020, 12, 9917. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [Green Version]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 529. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Mhamdi, R. Microbial Inoculants and Their Impact on Soil Microbial Communities: A Review. BioMed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Rumyantsev, S.D.; Cherepanova, E.A.; Alekseev, V.Y.; Sarvarova, E.R.; Kasimova, A.R.; Maksimov, I. V By modulating the hormonal balance and ribonuclease activity of tomato plants Bacillus subtilis induces defense response against potato virus X and potato virus Y. Biomolecules 2022, 12, 288. [Google Scholar] [CrossRef]
- Ali, M.I.A.; Ouf, S.A.; Khalil, N.M.; Abd El-Ghany, M.N. Biosynthesis of laccase by Aspergillus flavus NG85 Isolated from Saint Catherine protectorate. Egyptian Journal of Botany 2015, 55, 127–147. [Google Scholar]
- Logan, N.A.; De Vos, P. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Hafez, E.E.; El-Morsi, A.A.; El-Shahaby, O.A.; Abdelkhalek, A.A. Occurrence of iris yellow spot virus from onion crops in Egypt. VirusDisease 2014, 25, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 05, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- CHO, Y.K.; AHN, H.Y.E.K. Purification and characterization of polyphenol oxidase from potato: II. Inhibition and catalytic mechanism. J. Food Biochem. 1999, 23, 593–605. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Kumar, A.; Dutt, S.; Bagler, G.; Ahuja, P.S.; Kumar, S. Engineering a thermo-stable superoxide dismutase functional at sub-zero to >50 °C, which also tolerates autoclaving. Sci. Rep. 2012, 2, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Islam, M.J.; Kim, J.W.; Begum, M.K.; Sohel, M.A.; Lim, Y.-S. Physiological and Biochemical Changes in Sugar Beet Seedlings to Confer Stress Adaptability under Drought Condition. Plants 2020, 9, 1511. [Google Scholar] [CrossRef]
- Oser, B.L.; Hawk, P.B. Hawk’s Physiological Chemistry, 14th ed.; McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Sanan-Mishra, N. Differential expression profiles of tomato miRNAs induced by Tobacco mosaic virus. J. Agric. Sci. Technol. 2019, 21, 475–485. [Google Scholar]
- Abdelkhalek, A.; Ismail, I.A.I.A.; Dessoky, E.S.E.S.; El-Hallous, E.I.E.I.; Hafez, E. A tomato kinesin-like protein is associated with Tobacco mosaic virus infection. Biotechnol. Biotechnol. Equip. 2019, 33, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.E.; Abdelkhalek, A.A.; Abd El-Wahab, A.S.E.-D.; Galal, F.H. Altered gene expression: Induction/suppression in leek elicited by Iris Yellow Spot Virus infection (IYSV) Egyptian isolate. Biotechnol. Biotechnol. Equip. 2013, 27, 4061–4068. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lacava, P.T.; Bogas, A.C.; Cruz, F. de P.N. Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms From the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Firmicutes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 3, ISBN 0387684891. [Google Scholar]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.H.; Ryu, C.-M. Spraying of leaf-colonizing Bacillus amyloliquefaciens protects pepper from Cucumber mosaic virus. Plant Dis. 2016, 100, 2099–2105. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Zhou, G.; Yang, J.; Munir, S.; Ahmed, A.; Liu, Q.; Zhao, Z.; Ji, G. Bacillus amyloliquefaciens WS-10 as a potential plant growth-promoter and biocontrol agent for bacterial wilt disease of flue-cured tobacco. Egypt. J. Biol. Pest Control 2022, 32, 1–14. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—A review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Wang, F.; Liu, Y.; Qian, Y.; Yang, J.; Sun, H. Suppression of Tobacco mosaic virus by Bacillus amyloliquefaciens strain Ba33. J. Phytopathol. 2013, 161, 293–294. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Lou, Y.; Shi, M.; Jiang, Y.; Zhou, J.; Sun, Y.; Xue, Q.; Lai, H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere microecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019, 137, 154–166. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, Q.P.; Che, Y.P.; Zhu, P.X.; Zhang, Q.Q.; Ji, Z.L. Glutathione contributes to resistance responses to TMV through a differential modulation of salicylic acid and reactive oxygen species. Mol. Plant Pathol. 2021, 22, 1668–1687. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Balal, R.M.; Khan, M.M.; Shahid, M.A.; Mattson, N.S.; Abbas, T.; Ashfaq, M.; Garcia-Sanchez, F.; Ghazanfer, U.; Gimeno, V.; Iqbal, Z. Comparative studies on the physiobiochemical, enzymatic, and ionic modifications in salt-tolerant and salt-sensitive citrus rootstocks under NaCl stress. J. Am. Soc. Hortic. Sci. 2012, 137, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Yanan, D.; Ran, C.; Rong, Z.; Jiang, W.; Xuesen, C.; Chengmiao, Y.; Zhiquan, M. Isolation, identification, and antibacterial mechanisms of Bacillus amyloliquefaciens QSB-6 and its effect on plant roots. Front. Microbiol. 2021, 2727, 746799. [Google Scholar]
- Mathioudakis, M.M.; Veiga, R.S.L.; Canto, T.; Medina, V.; Mossialos, D.; Makris, A.M.; Livieratos, I. Pepino mosaic virus triple gene block protein 1 (TGBp1) interacts with and increases tomato catalase 1 activity to enhance virus accumulation. Mol. Plant Pathol. 2013, 14, 589–601. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Paul Bolwell, G. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- Boeckx, T.; Winters, A.L.; Webb, K.J.; Kingston-Smith, A.H. Polyphenol oxidase in leaves: Is there any significance to the chloroplastic localization? J. Exp. Bot. 2015, 66, 3571–3579. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Shimura, H.; Masuta, C.; Sano, S.; Inukai, T. Exogenous ascorbic acid derivatives and dehydroascorbic acid are effective antiviral agents against Turnip mosaic virus in Brassica rapa. J. Gen. Plant Pathol. 2013, 79, 198–204. [Google Scholar] [CrossRef]
- Wang, S.D.; Zhu, F.; Yuan, S.; Yang, H.; Xu, F.; Shang, J.; Xu, M.Y.; Jia, S.D.; Zhang, Z.W.; Wang, J.H.; et al. The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta 2011, 234, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Togawa, S.; Hikawa, T.; Matsuura, H.; Masuta, C.; Inukai, T. Ascorbic acid accumulates as a defense response to Turnip mosaic virus in resistant Brassica rapa cultivars. J. Exp. Bot. 2016, 67, 4391–4402. [Google Scholar] [CrossRef] [Green Version]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Kiraly, L.; Barna, B.; Király, Z. Plant resistance to pathogen infection: Forms and mechanisms of innate and acquired resistance. J. Phytopathol. 2007, 155, 385–396. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.-H. From fighting critters to saving lives: Polyphenols in plant defense and human health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef] [PubMed]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.-F.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef]
- Hoffmann, L.; Maury, S.; Martz, F.; Geoffroy, P.; Legrand, M. Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J. Biol. Chem. 2003, 278, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- ElMorsi, A.; Abdelkhalek, A.; Alshehaby, O.; Hafez, E.E.E.E. Pathogenesis-related genes as tools for discovering the response of onion defence system against iris yellow spot virus infection. Botany 2015, 93, 735–744. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Arishi, A.A.; Behiry, S.I. Trichoderma hamatum Strain Th23 Promotes Tomato Growth and Induces Systemic Resistance against Turnip mosaic virus. J. Fungi 2022, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Klessig, D.F. SOS—Too many signals for systemic acquired resistance? Trends Plant Sci. 2012, 17, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, H.; Wei, X.; Zhang, J.; Wang, H.; Liu, D. Expression analysis and functional characterization of a pathogen-induced thaumatin-like gene in wheat conferring enhanced resistance to Puccinia triticina. J. Plant Interact. 2017, 12, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, Z.; Teng, K.; Lai, J.; Zhang, Y.; Huang, Y.; Li, Y.; Liang, L.; Wang, Y.; Chu, C. Up-regulation of LSB1/GDU3 affects geminivirus infection by activating the salicylic acid pathway. Plant J. 2010, 62, 12–23. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Liu, Y.; Meng, D.; Jin, T.; Zhou, X. HC-Pro viral suppressor from tobacco vein banding mosaic virus interferes with DNA methylation and activates the salicylic acid pathway. Virology 2016, 497, 244–250. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Gurunathan, S.; Rangappa, S.; Huntrike, S.S.; Srivastava, R.K. Elicitation of resistance and associated defense responses in Trichoderma hamatum induced protection against pearl millet downy mildew pathogen. Sci. Rep. 2017, 7, 43991. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins, F.; Meins Jr, F. Movement of plant viruses is delayed in a β-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins Jr, F.; Iglesias, V.A. Local expression of enzymatically active class I β-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef]
- Dobnik, D.; Baebler, Š.; Kogovšek, P.; Pompe-Novak, M.; Štebih, D.; Panter, G.; Janež, N.; Morisset, D.; Žel, J.; Gruden, K. β-1, 3-glucanase class III promotes spread of PVY NTN and improves in planta protein production. Plant Biotechnol. Rep. 2013, 7, 547–555. [Google Scholar] [CrossRef] [Green Version]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by Potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, A.; Bano, A.; Janjua, H.T. Microbial Secondary Metabolites and Defense of Plant Stress. Microb. Serv. Restor. Ecol. 2020, 11, 37–46. [Google Scholar] [CrossRef]
- María Teresa, R.-C.; Rosaura, V.-G.; Elda, C.-M.; Ernesto, G.-P. The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 2014, 87, 32–41. [Google Scholar] [CrossRef]
- Abdullah, A.S.H.; Mirghani, M.E.S.; Jamal, P. Antibacterial activity of Malaysian mango kernel. Afr. J. Biotechnol. 2011, 10, 18739–18748. [Google Scholar] [CrossRef]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Octarya, Z.; Novianty, R.; Suraya, N. Saryono Antimicrobial activity and GC-MS analysis of bioactive constituents of Aspergillus fumigatus 269 isolated from Sungai Pinang hot spring, Riau, Indonesia. Biodiversitas 2021, 22, 1839–1845. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Kumari, N.; Menghani, E.; Mithal, R. GCMS analysis of compounds extracted from actinomycetes AIA6 isolates and study of its antimicrobial efficacy. Indian J. Chem. Technol. 2019, 26, 362–370. [Google Scholar]
- Ser, H.-L.; Palanisamy, U.D.; Yin, W.-F.; Abd Malek, S.N.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Presence of antioxidative agent, Pyrrolo [1,2-a] pyrazine-1,4-dione, hexahydro-in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015, 6, 854. [Google Scholar] [CrossRef] [Green Version]
- Pooja, S.; Aditi, T.; Naine, S.J.; Devi, C.S. Bioactive compounds from marine Streptomyces sp. VITPSA as therapeutics. Front. Biol. 2017, 12, 280–289. [Google Scholar] [CrossRef]
- Krishnan, K.; Mani, A.; Jasmine, S. Cytotoxic Activity of Bioactive Compound 1,2-Benzene Dicarboxylic Acid, Mono 2-Ethylhexyl Ester Extracted from a Marine Derived Streptomyces sp. VITSJK8. Int. J. Mol. Cell. Med. 2014, 3, 246–254. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).