1. Introduction
Deformed Wing Virus (DWV) is arguably—in concert with its vector the ectoparasitic mite
Varroa destructor—the most important pathogen of the European honey bee (
Apis mellifera). DWV has a near-global distribution (excluding Australia, where it is either absent or present at lower levels without
Varroa transmission [
1]) and, in the absence of
Varroa, persists at low levels and is rarely pathogenic [
2]. In contrast, when transmitted by mites, DWV titres become highly elevated, infested pupae may develop characteristic symptoms and significant levels of overwintering colony losses occur [
3,
4,
5,
6], attributable to the reduction of honey bee longevity [
7]. Evidence on the selective evolution of DWV through vector transmission bottlenecking [
8,
9], selection of
Varroa propagating DWV variants [
10], and synergistic action of the mite and the virus on the host [
11] have been reported. However, there remain conflicting studies on the ability of the mite to support DWV replication with some indicating biological [
10] and others favouring non-propagative transmission routes [
12]. Although there is a clear correlation between the mite-borne transmission and symptomatic outcome of the DWV infection the underlying mechanisms of the cooperative action of the two pathogens needs further clarification. A better understanding of DWV pathogenesis is needed to further develop intervention strategies to prevent and control disease.
DWV is a picorna-like virus from the
Iflaviridae family [
13,
14]. The single-stranded positive-sense RNA genome encodes a polyprotein flanked by 5′- and 3′-untranslated regions (UTR). Based upon our understanding of related viruses, the polyprotein is processed by viral and/or cellular enzymes into the structural and non-structural proteins required to complete the virus life cycle. The structural proteins form the virus capsid [
14], whereas the non-structural proteins modify the cellular milieu and replicate the genome. Like other RNA viruses, DWV is genetically diverse, with a related complex of viruses divided into two or three groups sharing ~84–97% genetic identity. DWV A [
14] and Kakugo virus [
13] exhibit 97% identity in their RNA sequences and form the type A subgroup. Another master variant of DWV was initially isolated from
Varroa and named Varroa Destructor Virus type 1 (VDV-1) [
15]. As a consequence of its high sequence similarity (84/95% identity at the RNA and protein levels respectively to DWV A) [
16] and its ability to infect the same host (honey bee), it is often referred to as DWV type B [
17,
18]. A third master variant of the virus designated as DWV C has also been reported [
18]. A range of differences in host preference, tissue tropism, morbidity, and pathogenicity have been suggested for the two master variants [
9,
10,
19,
20,
21,
22]. For example, the predominance of DWV A in a landscape-scale study on Hawaii following the introduction of
Varroa to naïve colonies with a diverse virus population was interpreted as an indication that this variant was more virulent [
17,
22]. Conversely, in side-by-side studies in laboratory experiments, DWV A had a less pronounced effect on adult honey bee survival compared to DWV B or a mixture of both variants [
20]. Further studies using field sourced inoculates of DWV A and B showed that they were equally virulent and generated similar levels of morbidity in emerged adult bees [
23]. In addition to these so-called master variants, a range of recombinants between DWV A and B have been reported [
21,
24,
25,
26]. For example, VDV-1
DVD (GenBank HM067437) and VDV-1
VVD (VDV-1-DWV-No-9, GenBank HM067438), both bearing the DWV A capsid proteins coding region and DWV B non-structural coding region [
26]. In some studies, these accumulated to a higher level in infected honey bees than the parental strains and it has been suggested that evolution of the DWV quasispecies is driven by
Varroa transmission toward the emergence of variants with enhanced virulence [
21,
26]. All of these reports are based on virus field isolates, and it remains unclear whether the DWV master variants and recombinants fundamentally differ in their phenotypes or if the differences reported reflect local strain variation or the experimental system used [
9,
17]. Therefore, further studies are required to associate the virulence with a particular genotype. A direct way to address this, and one that allows the propagation of near-clonal viral stocks for analysis, is to generate viruses using a reverse genetic (RG) system.
In virology, RG involves the manipulation of the genotype, the recovery of the virus, and the investigation of the phenotype. Over almost four decades, it has become the
de facto standard approach to address questions about virus replication, virulence and pathogenesis [
27,
28]. To facilitate these studies, a range of genome modifications (e.g. reporter genes) have been used to allow the sensitive quantification and localization of the virus [
29]. Molecular cloning of individual genetic variants of DWV is required to establish a direct connection between infection, and the observed symptoms. RG systems for type A DWV have been reported [
12,
30]. In this study, we exploit an extended RG toolbox for both DWV A and B master variants and a type B/A recombinant to investigate their comparative transmission, tropism and pathogenesis in honey bees. Using these resources, we also provide direct evidence of DWV replication in
Varroa destructor. Finally, by introducing a reporter encoding sequence to the virus genome we visualise the
in vivo tissue distribution of DWV in infected honey bee brood. The results of this study provide new insights into understanding the nature of DWV infection and introduce new molecular tools for honey bee research.
2. Materials and Methods
2.1. RG System for Three DWV Variants
RG constructs used in this study were based on the cDNA clone of a recombinant DWV variant VDV-1-DWV-No-9 (GenBank HM067438.1). VDV-1-DWV-No-9 sequence was rescued using samples from a
Varroa-infested honey bee colony from Warwick-HRI apiary (2014), and a cloned full-length cDNA was incorporated into a plasmid vector containing all required elements for the transcription of the viral RNA. VVD RG clone is identical to the source VDV-1-DWV-No-9, with the exception of two nucleotide substitutions (positions 5277 and 5280 in the VVD clone cDNA, which corresponds to positions 5124 and 5127 in the GenBank HM067438.1 sequence lacking the very 5‘-end of the virus genome), resulting in creation of an
HpaI restriction site. In order to obtain VDD and VVV constructs bases 2727–4888 (capsid proteins encoding region) and 4885-9783 (non-structural proteins encoding region) were replaced with corresponding DWV A and B fragments respectively (
Figure 1,
supplementary text S1). Inserts encoding DWV A capsid proteins and DWV B non-structural proteins were based on published data [
14,
15] and obtained by custom gene synthesis (IDT, Leuven, Belgium). The replacement sequences were amplified with High Fidelity Phusion DNA polymerase (Thermo Fisher Scientific, Winsford, UK) and incorporated into the initial construct using NEBuilder Hifi assembly reaction (New England Biolabs, Hitchin, UK). The nomenclature used for the corresponding virus clones in this study is as follows: “VDD” —type A DWV (protein coding part), “VVV”—type B, “VVD”—B/A recombinant (see
Figure 1 for details). New restriction sites were introduced into each plasmid either by standard site-directed mutagenesis or by including the modification into the synthetic sequence:
HpaI (5275-5280 nt in VVD variant),
Kpn2I and
AvrII (2751-2756 and 4884-4889 nt in VDD variant), and
AvrII,
PflFI and
BglII (4884-4889, 6087-6095, and 9783-9788 nt, respectively, in VVV variant).
EGFP-encoding RG constructs (DWV
E) were obtained by NEBuilder Hifi assembly (New England Biolabs, Hitchin, UK) reaction applied to previously made RG constructs. EGFP sequence flanked by 32 additional nucleotides (ATGGATAACCCT from 5’ and GCAAAACCAGAG from 3’ end) resulting in duplication of the protease cleavage site (amino acid composition of the site is AKPEMDNP [
14]) was inserted between nucleotides 1785–1786 of DWV cDNA. All plasmids were subjected to Sanger sequencing and the results were aligned with data from
in silico cloning simulation. Full cDNA sequences of DWV clones used in this study are available in
Text S1 (
Supplementary Materials) and online (GenBank accession numbers: DWV-VDD - MT415949, DWV-VVD - MT415950, DWV-VVD_truncated - MT415951, DWV-VVV - MT415952, DWV-VDD-eGFP - MT415948, DWV-VVD-eGFP - MT415953).
2.2. Viral RNA Synthesis
DWV RNA was synthesized using linearized plasmid templates. Full length and truncated templates were linearised with Pme I (cutting at the end of the sequence encoding the poly-A tail) or Nru I (nt 9231 located within the sequence encoding the viral polymerase) respectively. Linearized DNA was purified by phenol-chloroform extraction and ethanol precipitation and T7 transcription was performed with T7 RiboMAXTM Express Large Scale RNA Production System (Promega, Southampton, UK) according to the manufacturer’s protocol. In order to account for the stabilization effect of the poly-A tail truncated transcripts were subjected to an additional polyadenylation step with poly(A) Tailing Kit (Thermo Fisher Scientific, Winsford, UK). RNA transcripts were purified with GeneJet RNA Purification Kit (Thermo Fisher Scientific, Winsford, UK) using clean up protocol and eluted in RNAse free H2O. All transcripts were analysed for integrity by gel electrophoresis and stored at −80 °C.
2.3. Virus Stocks
DWV stocks were prepared from honey bee pupae injected with in vitro transcribed RNA. Homogenized tissue was diluted with sterile PBS in 1:1 (w:v) ratio and centrifuged at 13 000× g, 4 °C for 10 min. The supernatant was sterilized by passing through 0.22 μM PES filter (Merck Millipore, Watford, UK) and treated with RNase A to destroy all non-encapsidated RNA. RNA was extracted from 100 μL of the virus stock using RNeasy kit (Qiagen, Manchester, UK) and analysed by RT-qPCR.
2.4. Honey Bees
All honey bee (Apis mellifera) brood in this study was obtained from the research apiary of the University of St Andrews. Hives used for sampling were regularly treated for Varroa with an appropriate miticide and routinely screened for DWV. DWV level in bees obtained from these hives was found to range within 102–106 genome equivalents (GE) per 1 μg of total RNA. No phenotypic evidence for other honey bee viruses being present was found within the colonies throughout the course of the studies. Pupae and larvae of the required age were collected from the comb and transferred to the incubator set at 34.5 °C and 90% relative humidity. Pupae were kept on folded sheets of filter paper, and larvae were transferred into 96-well plates with round bottom wells containing feeding diet (6% (w/v) glucose, 6% (w/v) fructose, 1% (w/v) yeast extract, and 50% (w/v) royal jelly in H2O).
2.5. Varroa Feeding
Varroa mites were collected from the brood frames of infested colonies kept at the University of Aberdeen. Mites were placed in Petri dishes in groups of 10 with a single honey bee pupa and kept overnight in the incubator (34.5 °C, 90% humidity). On the next day, feeding packets containing artificial feeding diet and supplemented with DWV stock or equal amount of PBS were prepared. The feeding packets were prepared by wrapping a 175 μL drop of the diet containing RNase A treated DWV inoculate (prepared as described in
Section 2.3). The diet is 75:25 holidic:locust haemolymph (the detailed description of the feeding diet to be published separately [
31]). To prepare the diet the locust (fourth instar
Locusta migratoria) haemolymph was frozen-thawed, heat-treated, and centrifuged for 30 s at 7500×
g.
Mites were placed in groups of 5–10 mites per feeding packet with four replicates for each treatment and kept in the incubator for four days. At the end of incubation live mites were collected, snap-frozen in liquid nitrogen and stored at −80 °C until further analysis. Total RNA was extracted from pooled mite samples using the standard TriReagent protocol (Thermo Fisher Scientific, Winsford, UK).
2.6. RNA Injections and Virus Inoculations
RNA and virus injections into pupae were performed with insulin syringes (BD Micro Fine Plus, 1 mL, 30 G (Becton Dickinson, Oxford, UK)). 5 μL injections were introduced by inserting the syringe needle between the second and third abdominal segments of a pupa. All pupae were maintained on a warm heating plate during the injections.
0.5 and 5 μg (equivalent to 8.7 × 1010 and 8.7 × 1011 of DWV RNA copies) of in vitro transcribed RNA was injected individually into white-eyed honey bee pupae. Truncated VVD transcript injections were used as a negative control in RNA transcript injections. RNA-injected pupae were analysed at 72 h post-injection.
Injections of pupae with virus stocks were performed using the same technique as for RNA transcripts. Serial dilutions of DWV stocks in sterile PBS were prepared immediately before the injections. Mock control groups were injected with PBS only, while non-injected controls were left intact throughout the duration of the experiment.
For oral infection, larvae were placed into 96 well plates with a diet supplemented with DWV. Fresh diet without virus was added after 24 h or when all virus-supplemented diet was consumed.
2.7. RNA Extraction, Reverse Transcription and PCR
Total RNA was extracted individually from all bee samples, Varroa mites were analysed in pools of 5–10 according to the treatment group. Samples were homogenized using a Precellys Evolution instrument (Bertin Instruments, Montigny-le-Bretonneux, France). Total RNA was extracted with GeneJet RNA Purification Kit (Thermo Fisher Scientific, Winsford, UK) using a protocol adapted for vacuum manifold application. cDNA was prepared with qScript cDNA Synthesis Kit (Quanta Biosciences, VWR International Ltd, Lutterworth, UK) from 1 μg of total RNA following the manufacturer’s protocol with both oligo dT and random hexamer primers included in the reaction mixture in the reaction volume of 20 μL.
All sequences of primers used in this study are shown in
Table S1. Detection of DWV and honey bee
actin, used as an internal RNA quality control, was carried out by end-point PCR with
Taq DNA polymerase (New England Biolabs, Hitchin, UK) and 2 μL of cDNA. DWV_RTPCR primers were designed to detect all three DWV variants under study. To amplify the cDNA regions containing restriction site tags in VDD and VVV virus variants Kpn2I_F/R and PflFI_F/R primer sets were used respectively. PCR cycling conditions were 30 cycles of 95 °C (15 s), 55 °C (15 s), 68 °C (2 min) with an initial 95 °C step (30 s), and a final extension at 68 °C (5 min). PCR samples were analysed on a 1% agarose gel stained with ethidium bromide. When required, DWV PCR products were subjected to restriction digest prior to loading on the gel.
The quantification of DWV genome copies was performed by SYBR-Green Real-Time Quantitative PCR (qPCR). Reactions were carried out in a C1000 Thermal Cycler (Bio-Rad Laboratories, Deeside, UK) using Luna Universal qPCR master mix (New England Biolabs, Hitchin, UK), 0.25 μM forward and reverse DWV_qPCR primers, and 2 μL of cDNA with the following thermal profile: 1 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C with a final post-amplification melting curve analysis step. Accumulation of DWVE RNA was assayed by a set of primers amplifying a junction region between EGFP and viral sequence (primers EGFP_qPCR_F and VDD_VP2qPCR_RP or VVV_VP2qPCR_RP depending on the virus variant used). DWV and DWVE titers were calculated by relating the resulting Ct value to the standard curve generated by performing qPCR from a serial dilution of the cDNA obtained from 1 μg of VVD or DWVE RNA transcript respectively.
2.8. Negative Strand Assay
Strand-specific detection of DWV RNA was performed as described earlier [
10]. Briefly, 1 µg of total RNA was used in reverse transcription reaction carried out with Superscript III reverse transcriptase (Invitrogen, Thermo Fisher Scientific, Winsford, UK) and the adapter extended primer DWV(-RNA)_RT designed to anneal to the negative strand RNA of DWV. The PCR step was carried out by
Taq DNA polymerase (New England Biolabs, Hitchin, UK) using a forward primer identical to the adapter sequence (primer 388) and DWV(-RNA)_RT R primer. PCR was run for 35 cycles in the same conditions as described above.
2.9. Microscopy
All imaging to detect EGFP signal in samples infected with DWVE was done using Leica TCS SP8 confocal microscope with 10× HC PL FLUOTAR objective (Leica Biosystems, Newcastle, UK).
2.10. Cryosection of Larvae Samples
Live larvae were washed with increasing concentrations of aqueous ethanol solution and fixed in 4% paraformaldehyde in PBS for 2 h at 4 °C. Fixed larvae were allowed to sink in 30% sucrose in PBS, mounted in NEG-50 Frozen Section Medium (Thermo Fisher Scientific, Winsford, UK) and subjected to microsectioning on CM1860 Cryostat (Leica Biosystems, Newcastle, UK). Sections of 50–80 µm thickness were placed on Superfrost Plus microscope slides (Thermo Fisher Scientific, Winsford, UK), mounted in ProLong Gold antifade medium, and analysed by microscopy.
4. Discussion
The inexorable rise in global honey bee colony numbers masks increasing levels of colony losses, which are regularly reported to exceed 30% per annum and predominantly occur during the winter in temperate regions [
39,
40,
41]. For almost a decade the major cause of these losses, the ectoparasitic mite
Varroa destructor and the smorgasbord of viruses it transmits, has been well known [
7]. Of these, the most important virus associated with overwintering colony loss is DWV. Although DWV is a single stranded, positive sense RNA virus—a group that includes poliovirus which has been dissected at the molecular level for almost four decades—molecular methods to study its biology have developed slowly. One of the major reasons for this is the absence of a usable
in vitro cell culture system enabling virus propagation [
42]. The recent development of an RG system [
30] allowing the recovery of infectious virus from a cDNA for the type A variant of DWV provided the first tractable approach to a better understanding of the biology and pathogenesis of DWV.
We report here the extension of the genetic tools to study the biology of DWV including the type B variant (also designated VDV-1) and a recombinant that has previously been reported to predominate in
Varroa-infested colonies [
9,
26]. We additionally demonstrate how the RG approach can be exploited to study host-pathogen and host-vector-pathogen interactions, and—with the development of reporter gene-expressing variants of the virus—to study tissue distribution in developing honey bee larvae, pupae, and eclosed adults.
Using standard molecular cloning techniques combined with
in vitro gene synthesis, we constructed infectious cDNAs for type A (protein coding sequence), type B and a recombinant variant of DWV, recovered molecularly tagged virus after RNA inoculation and investigated the kinetics of virus replication in honey bee pupae. Replication was rapid, amplifying to ~10
8 GE/μg of RNA within 24 h, and plateauing at ~10
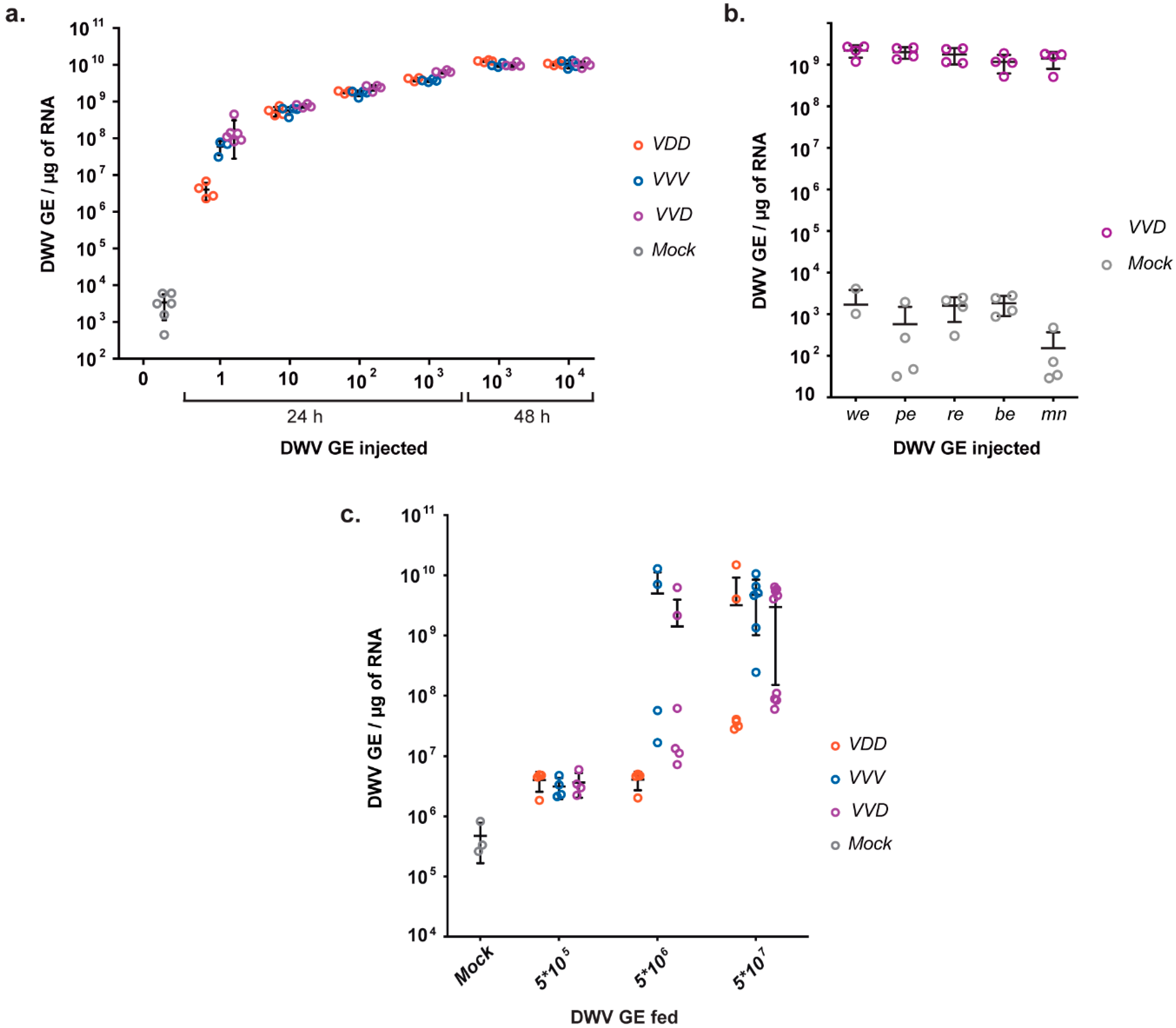
10 GE per μg of total cellular RNA within 48 h. There were no major differences between the replication rates of the three DWV variants under study, and all stages of pupal development tested appeared equally susceptible to virus infection (
Figure 2). This implies that, although pupae are only naturally exposed to mite-borne virus upon capping, even very low amounts of virus inocula introduced by the mite have the capacity to replicate to very high levels before eclosion. The rapid kinetics of DWV replication also means that all progeny mites produced by a single pupa are likely to almost exclusively carry the virus population representative of that introduced by the foundress mite, as these (and not the endogenous virus population) are what are amplified following direct injection.
Horizontal virus transmission within the colony also occurs during larval feeding by nurse bees. We show that larvae exhibit higher resistance to infection with clonal DWV inoculates
per os when compared with the susceptibility of pupae to injected virus. Greater than 10
6-10
7 GE of virus was needed to reliably infect more than 50% of larvae with any DWV variants tested (
Figure 2c). This result indicates that high levels of DWV present in the colony due to amplification by
Varroa parasitized individuals can boost the development of a symptomatic infection state both through pupal infestation by mites and feeding of larvae by diseased nurse bees. A better understanding of this will require the levels of virus transmitted orally, or present in bee bread fed to developing larvae, to be determined. Interestingly, inoculation with VDD clone containing DWV type A structural proteins sequence required higher virus concentrations to achieve efficient infection in larvae. This result may indicate that the 5’ part of the protein encoding sequence of DWV B genome enables higher infectivity either due to enhanced binding and penetration into the target host cells or via other interactions caused by clone-specific secondary or tertiary structures of viral RNA.
As honey bee pupae appear very sensitive to infection by direct injection (recapitulating transmission by
Varroa), any virus replication in the mite would likely guarantee the transmitted dose would significantly exceed the ID
50. The viral load in mites has been reported to be high [
43] though this may reflect the level of virus in the preceding host pupa rather than replication
per se. Previous analysis of virus replication in
Varroa has produced conflicting results. Mass spectroscopy studies failed to detect viral non-structural proteins implying that DWV may not replicate in the mite [
44]. Conversely, although detection of viral negative strand replication intermediates [
15,
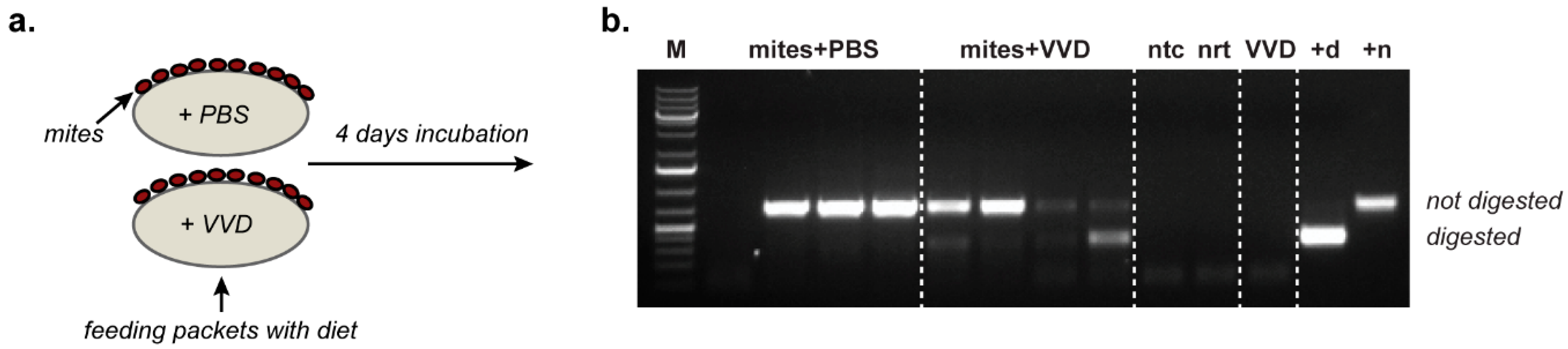
45] in mites may indicate replication, they may also reflect carry-over from the previous pupal feed. To address this, we maintained mites
in vitro on a diet containing no honey bee-derived components. The only DWV virus (and viral negative strand RNA) present would therefore be derived from the last pupa the mites had fed on, together with any subsequent replication in the mite. We supplemented the diet with the RG-derived VVD variant of DWV carrying a unique genetic tag allowing its unambiguous identification. The presence of genetically tagged negative strand RNA of DWV in the pooled mites samples fed on this diet demonstrates that VVD does replicate in
Varroa and that newly acquired virus can replicate in the presence of a pre-existing virus population in the mite (
Figure 3). The latter point is significant as it suggests that the virus population in the mite reflects its historical diet from successive infested pupae, potentially influenced by any differential virus replication in the mite.
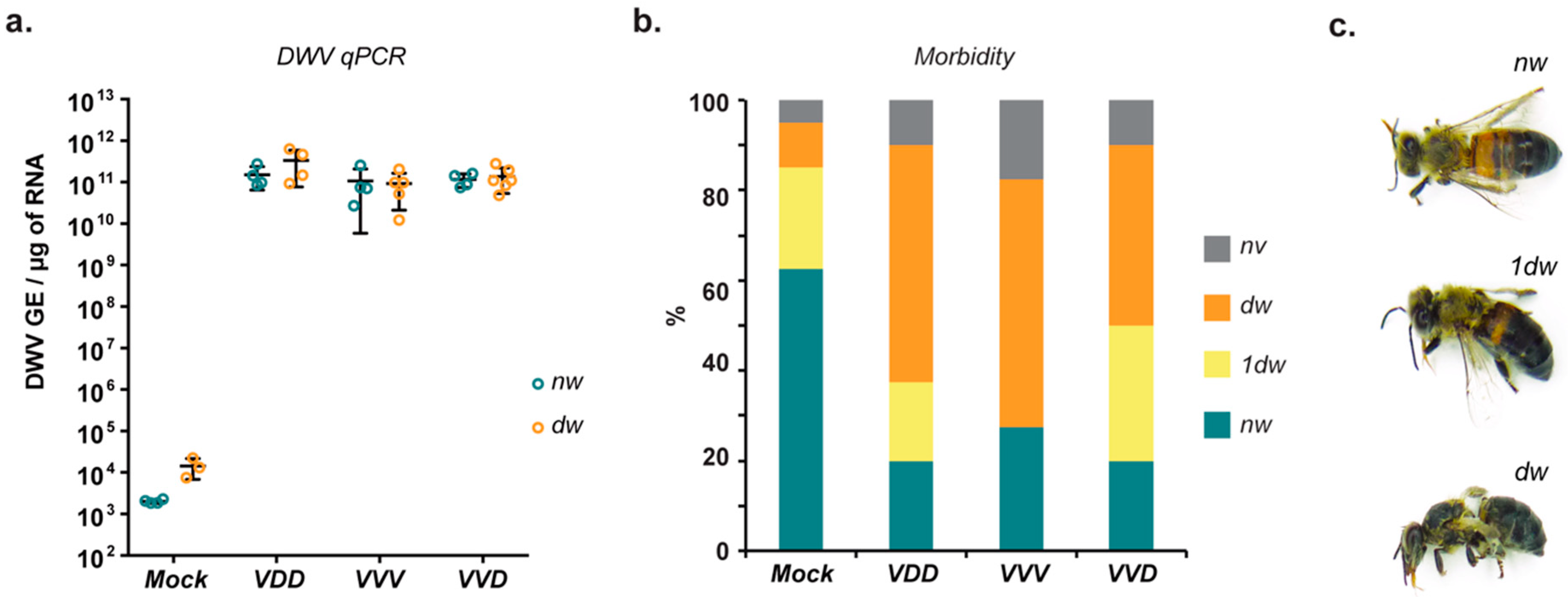
Although mite transmission of DWV is associated with overt symptoms such as wing deformities these are not the inevitable consequence of high viral loads [
23,
30]. Approximately 25% of inoculated pupae had normal wings on eclosion, similar to another recent report [
23], despite having viral loads in excess of 10
10 GE/μg RNA. While apparently developmentally normal, evidence indicates that workers with high viral loads are impaired in foraging ability, cognitive functions, and die prematurely [
7,
10,
46,
47]. In contrast to the pupal injections where a significant proportion eclosed and appeared developmentally normal despite high levels of virus replication, no virus-fed larvae which eclosed exhibited normal wings, although >50% reached the adult stage (
Figure S4). These observations suggest that the earlier stages of honey bee development may be more susceptible to DWV-mediated damage. Additionally, this signifies that the occurrence of high DWV levels in bees with normal wings in the field may be due to
Varroa-mediated transmission, while the outcome of oral infection in larvae depends on the amount of virus ingested and results in either benign asymptomatic infection or in high morbidity due to high levels of DWV replication and consequential developmental damage. Apart from horizontal transmission addressed in this study, DWV can be transmitted vertically from an infected queen [
48]. Further studies are required to elucidate the exact impact of this route on the phenotypic outcome of the infection.
It remains unclear what determines whether virus exposure results in overt disease or asymptomatic infection [
43] although results presented here suggest that the timing of infection (larvae
vs. pupae) is probably critical. Analysis of whether infection of all stages of pupal development are as likely to result in wing deformities may be informative in this regard, though
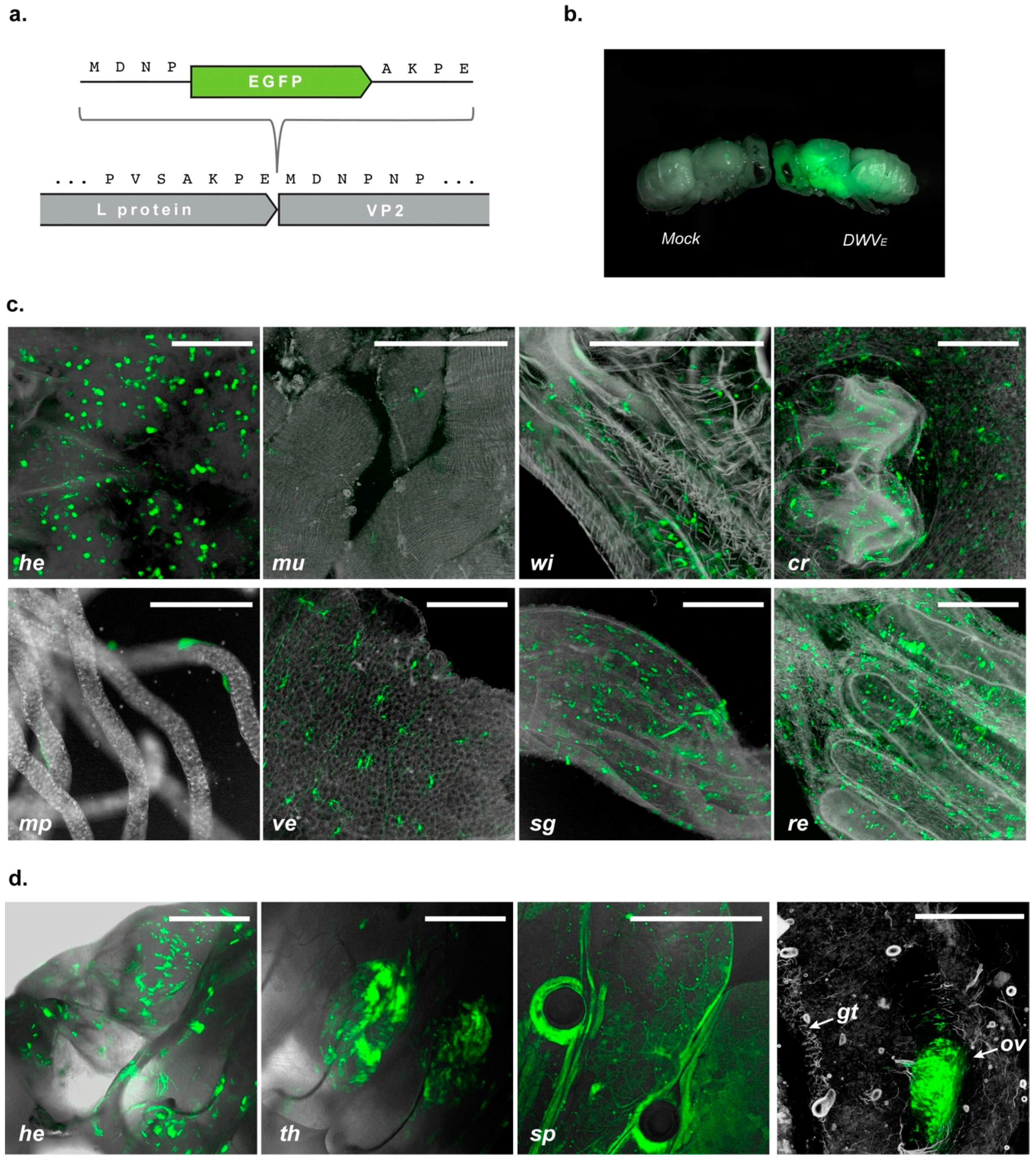
Varroa-mediated virus transmission will initially occur when the foundress mite feeds on the just-capped pre-pupa. One of the factors which can potentially influence the development of overt disease are the sites of virus replication in the developing honey bee. This may result in direct cytopathicity and tissue damage or indirectly by dysregulation leading to damage at remote locations. To address the tissue tropism of DWV, we constructed a modified virus genome co-translationally expressing the green fluorescent protein. Inoculation of pupae, or ingestion by larvae, resulted in distinct fluorescence in a range of organs and tissues. In fed larvae, the EGFP signal accumulated in thoracic segments where the developing wing buds are located [
49], and infection of these presumably accounts for the characteristic symptoms of DWV infection. It was notable that larvae fed with large amounts of DWV invariably developed with malformed wings. The developing ovary of worker larvae also appears to be a site of virus replication. Worker ovaries—other than in laying workers—never produce eggs, so further studies will be required to determine whether the ovary of developing queens also shows evidence of virus replication. This, together with the known presence of DWV in drone semen [
50], would presumably explain the vertical transmission of virus.
Our observations indicate that DWV selectively targets organs most favourable for its oral and vertical transmission. Sites of DWV replication colocalize with exocrine glands, which are responsible for the secretion of larval diet components, and digestive tract tissues, from which it can presumably be transmitted in faeces [
51] and, following regurgitation, orally. These results support previous studies using anti-capsid antibodies or riboprobes targeting the virus genome [
30,
35] where DWV-specific signals were found in honey bee brains, exocrine glands, midgut, fat bodies, and reproductive organs [
30,
35,
52,
53]. Since the emergence of
Varroa as a vector the oral route no longer plays the decisive role in DWV spread, but the observed tropism of DWV infection indicates the initial evolutionary trait used by the virus. Robust virus replication in the primary tissues that enable subsequent transmission often results in spillover to secondary sites that are permissive for virus infection, but that offer no further route to a new host, e.g., the neurotropism of the faecal-orally transmitted poliovirus [
54]. It is therefore unsurprising that DWV infection was additionally found in a range of sites in the developing larva or pupa, including the spiracles and nascent wing tissues. The availability of EGFP-markers for sites of virus replication will enable further
in vivo time course studies of virus dissemination and facilitate host-vector transmission studies. More generally, the ability to introduce a ‘payload’ to the virus genome may also be exploited by using genetically modified DWV as a virus-based gene delivery system [
37]. Our results clearly demonstrate that DWV is abundant in many tissues in honey bees and a EGFP, or similar reporter, would enable tissue-specific RNAi responses to be quantified and optimized. Likewise, reporter-expressing derivatives of DWV have potential in tropism and transmission studies in other species in which the virus is known or suspected of replicating, including
Varroa or
Bombus [
32] or, more speculatively, as gene delivery vectors for mite control.
In many single stranded positive sense RNA viruses, the development of reverse genetic systems was facilitated by a good understanding of virus replication in vitro. With no system for propagating DWV in vitro these studies have had to be conducted in vivo. We show that the availability of a reverse genetic system allows the kinetics of virus replication in larvae and pupae to be examined. Comparative studies demonstrate that the two predominant variants of DWV essentially replicate equivalently, and that the symptomatic outcome of DWV infection is not linked to a particular genotype of the virus nor to the route of transmission. Unique genetic tags introduced to the genome enabled discrimination of input from the endogenous virus population and provide unequivocal evidence for virus replication in Varroa following detection of de novo synthesised negative-sense viral RNA. A viable GFP-tagged DWV genome supplies the basis for a better understanding of virus tropism and pathogenesis in infected brood and will provide a useful tool for elucidating the mechanisms behind symptomatic DWV infection in developing honey bees.