Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation
Abstract
1. Introduction
2. Characteristics of VZV Latency
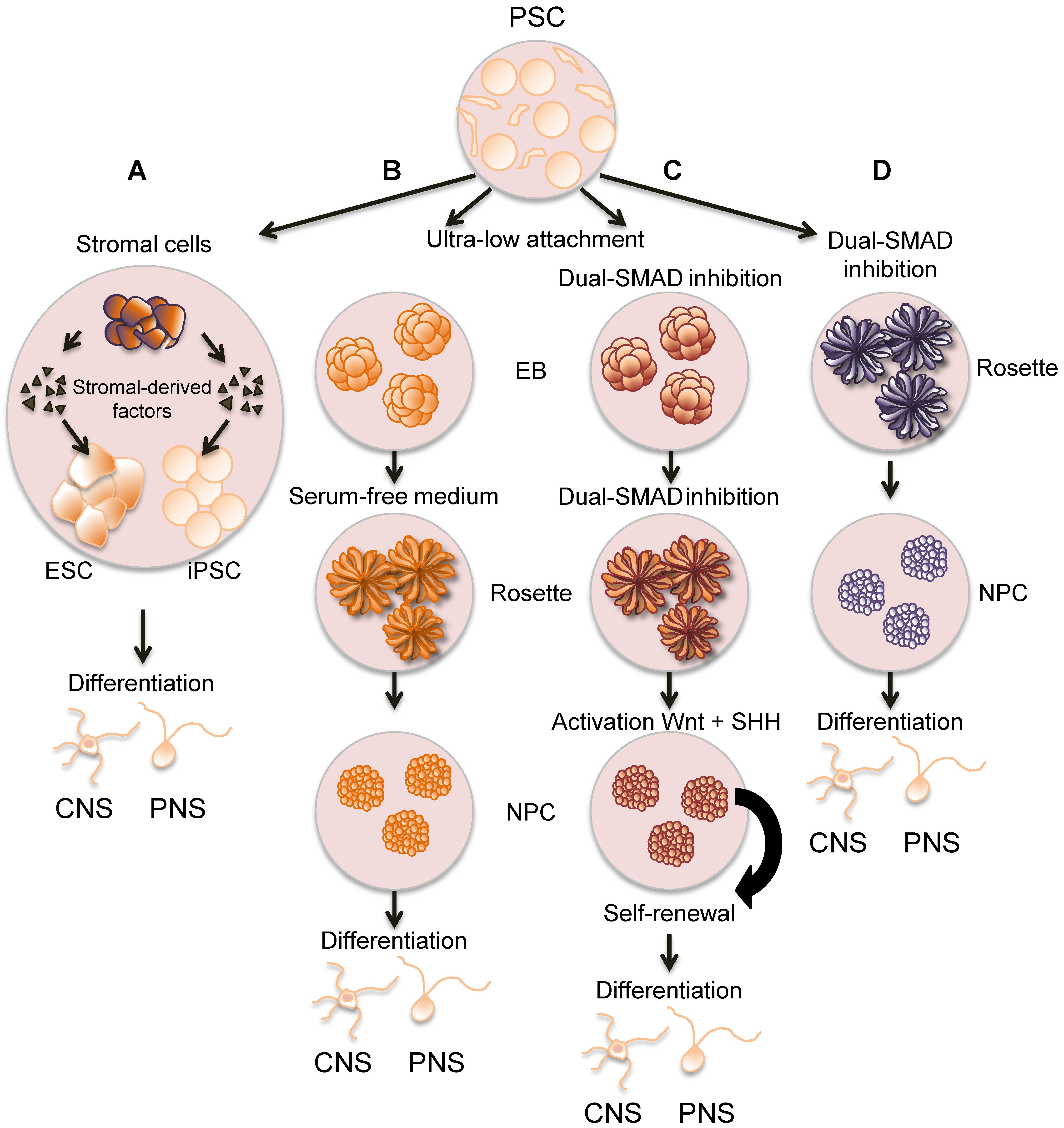
3. Generation and Characterization of Human Neurons to Study VZV
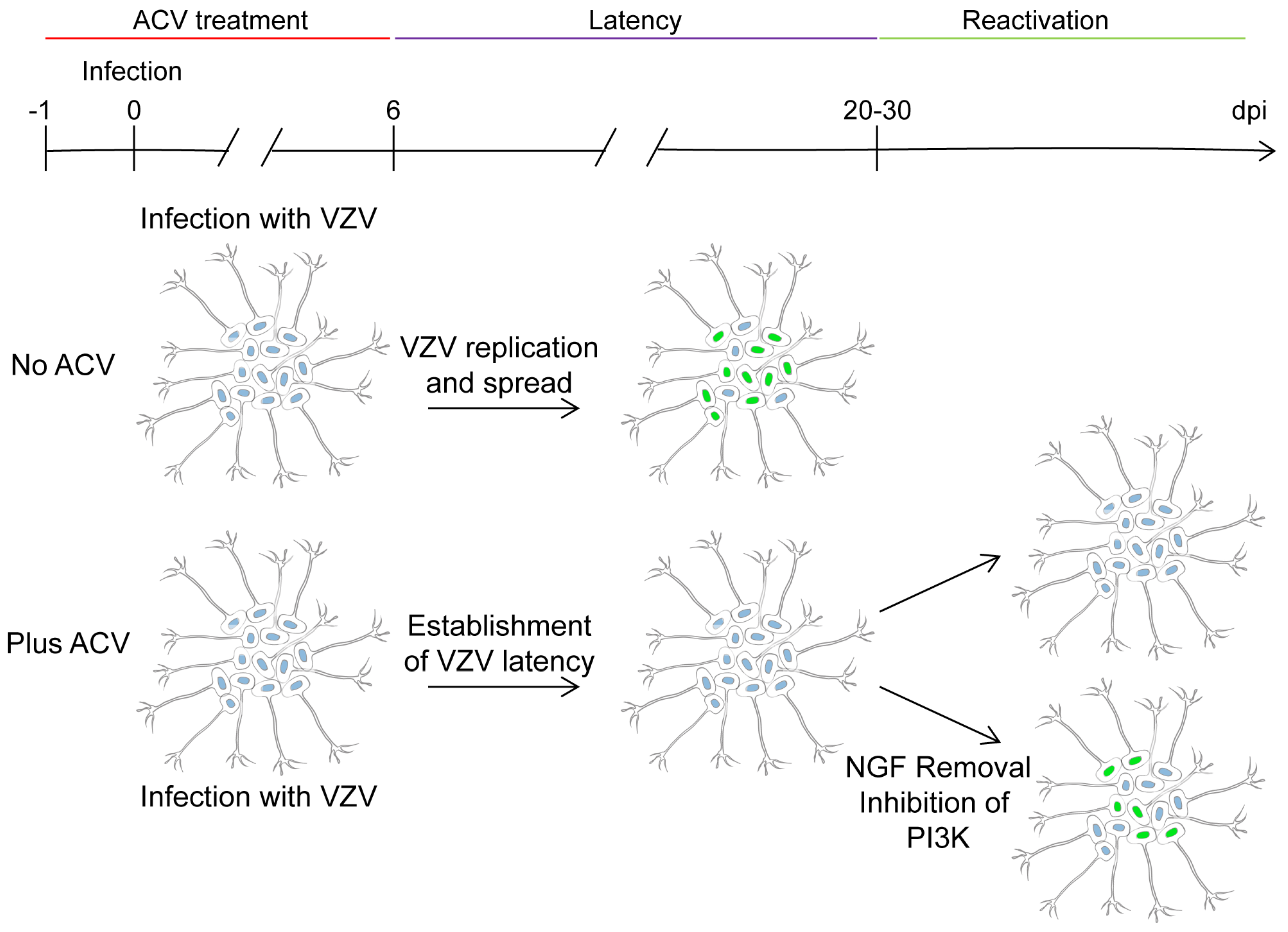
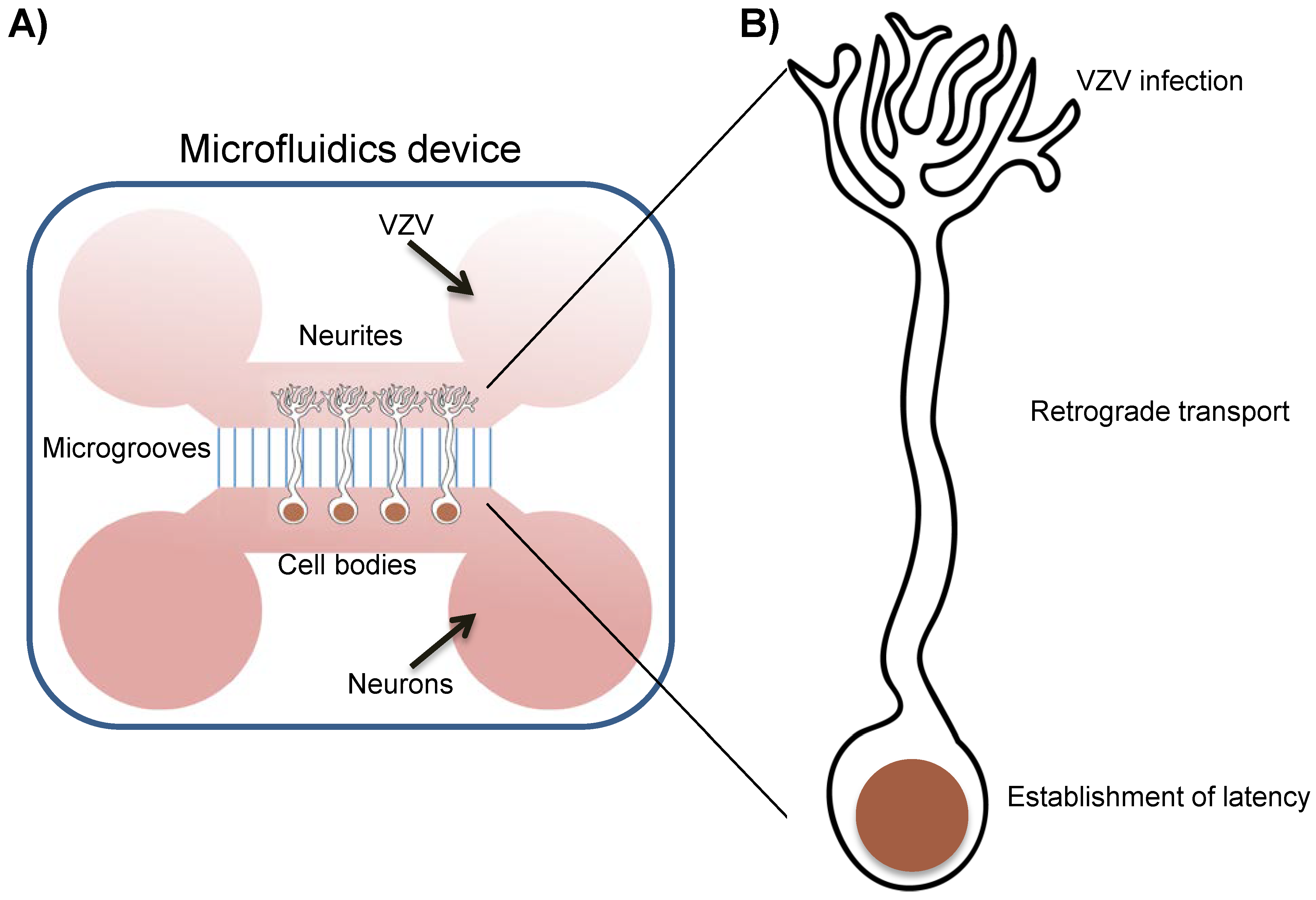
4. In vitro Latency/Reactivation Models
5. The Relevance of NGF in VZV Latency and Reactivation
6. Concluding Remarks and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Gershon, A.A.; Chen, J.; Davis, L.; Krinsky, C.; Cowles, R.; Reichard, R.; Gershon, M. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans. Am. Clin. Climatol. Assoc. 2012, 123, 17–33, discussion 33–35. [Google Scholar] [PubMed]
- Nagel, M.A.; Rempel, A.; Huntington, J.; Kim, F.; Choe, A.; Gilden, D. Frequency and abundance of alphaherpesvirus DNA in human thoracic sympathetic ganglia. J. Virol. 2014, 88, 8189–8192. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Chen, J.; Gershon, M.D. Use of saliva to identify varicella zoster virus infection of the gut. Clin. Infect. Dis. 2015, 61, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Gershon, A.A.; Li, Z.; Cowles, R.A.; Gershon, M.D. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J. Neurovirol. 2011, 17, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Azarkh, Y.; Bos, N.; Gilden, D.; Cohrs, R.J. Human trigeminal ganglionic explants as a model to study alphaherpesvirus reactivation. J. Neurovirol. 2012, 18, 456–461. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Badani, H.; Bos, N.; Scianna, C.; Hoskins, I.; Baird, N.L.; Gilden, D. Alphaherpesvirus DNA replication in dissociated human trigeminal ganglia. J. Neurovirol. 2016, 22, 688–694. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Badani, H.; Baird, N.L.; White, T.M.; Sanford, B.; Gilden, D. Induction of varicella zoster virus DNA replication in dissociated human trigeminal ganglia. J. Neurovirol. 2017, 23, 152–157. [Google Scholar] [CrossRef]
- Ouwendijk, W.J.; Choe, A.; Nagel, M.A.; Gilden, D.; Osterhaus, A.D.; Cohrs, R.J.; Verjans, G.M. Restricted varicella-zoster virus transcription in human trigeminal ganglia obtained soon after death. J. Virol. 2012, 86, 10203–10206. [Google Scholar] [CrossRef]
- Depledge, D.P.; Ouwendijk, W.J.D.; Sadaoka, T.; Braspenning, S.E.; Mori, Y.; Cohrs, R.J.; Verjans, G.; Breuer, J. A spliced latency-associated VZV transcript maps antisense to the viral transactivator gene 61. Nat. Commun. 2018, 9, 1167. [Google Scholar] [CrossRef]
- Depledge, D.P.; Sadaoka, T.; Ouwendijk, W.J.D. Molecular aspects of varicella-zoster virus latency. Viruses 2018, 10, 349. [Google Scholar] [CrossRef]
- Gilden, D.H.; Dueland, A.N.; Devlin, M.E.; Mahalingam, R.; Cohrs, R. Varicella-zoster virus reactivation without rash. J. Infect. Dis. 1992, 166 (Suppl. 1), S30–S34. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Gershon, A.A. Clinical features of varicella-zoster virus infection. Viruses 2018, 10. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Mehta, S.K.; Schmid, D.S.; Gilden, D.H.; Pierson, D.L. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J. Med. Virol. 2008, 80, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Cohrs, R.J.; Forghani, B.; Zerbe, G.; Gilden, D.H.; Pierson, D.L. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J. Med. Virol. 2004, 72, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Gershon, M.D. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin. Microbiol. Rev. 2013, 26, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Sharp, M.; Maecker, H.T.; Maino, V.C.; Arvin, A.M. Frequencies of memory t cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 2000, 181, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Koenig, H.C.; Garland, J.M.; Weissman, D.; Mounzer, K. Vaccinating hiv patients: Focus on human papillomavirus and herpes zoster vaccines. AIDS Rev. 2013, 15, 77–86. [Google Scholar] [PubMed]
- Levin, M.J.; Smith, J.G.; Kaufhold, R.M.; Barber, D.; Hayward, A.R.; Chan, C.Y.; Chan, I.S.; Li, D.J.; Wang, W.; Keller, P.M.; et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose vzv vaccine. J. Infect. Dis. 2003, 188, 1336–1344. [Google Scholar] [CrossRef]
- Saylor, D.; Thakur, K.; Venkatesan, A. Acute encephalitis in the immunocompromised individual. Curr. Opin. Infect. Dis. 2015, 28, 330–336. [Google Scholar] [CrossRef]
- Weinberg, A.; Levin, M.J. Vzv t cell-mediated immunity. Curr. Top. Microbiol. Immunol. 2010, 342, 341–357. [Google Scholar] [PubMed]
- Zhang, Y.; Cosyns, M.; Levin, M.J.; Hayward, A.R. Cytokine production in varicella zoster virus-stimulated limiting dilution lymphocyte cultures. Clin. Exp. Immunol. 1994, 98, 128–133. [Google Scholar] [CrossRef]
- Kristie, T.M.; Liang, Y.; Vogel, J.L. Control of alpha-herpesvirus ie gene expression by hcf-1 coupled chromatin modification activities. Biochim. Biophys. Acta 2010, 1799, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.M.; Cliffe, A. Chromatin control of herpes simplex virus lytic and latent infection. Nat. Rev. Microbiol. 2008, 6, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kristie, T.M.; Vogel, J.L.; Sears, A.E. Nuclear localization of the c1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc. Natl. Acad. Sci. USA 1999, 96, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, W.; Lorentzen, E.U.; Eing, B.R.; Muller, M.; King, N.J.; Klupp, B.; Mettenleiter, T.C.; Kuhn, J.E. Entry of herpes simplex virus type 1 (HSV-1) into the distal axons of trigeminal neurons favors the onset of nonproductive, silent infection. PLoS Pathog. 2012, 8, e1002679. [Google Scholar] [CrossRef]
- Gary, L.; Gilden, D.H.; Cohrs, R.J. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J. Virol. 2006, 80, 4921–4926. [Google Scholar] [CrossRef]
- Clarke, P.; Beer, T.; Cohrs, R.; Gilden, D.H. Configuration of latent varicella-zoster virus DNA. J. Virol. 1995, 69, 8151–8154. [Google Scholar] [PubMed]
- Nagel, M.A.; Choe, A.; Traktinskiy, I.; Cordery-Cotter, R.; Gilden, D.; Cohrs, R.J. Varicella-zoster virus transcriptome in latently infected human ganglia. J. Virol. 2011, 85, 2276–2287. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Grinfeld, E.; Bell, J.E. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 2000, 74, 11893–11898. [Google Scholar] [CrossRef] [PubMed]
- Cohrs, R.J.; Gilden, D.H.; Kinchington, P.R.; Grinfeld, E.; Kennedy, P.G. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 2003, 77, 6660–6665. [Google Scholar] [CrossRef] [PubMed]
- Lungu, O.; Panagiotidis, C.A.; Annunziato, P.W.; Gershon, A.A.; Silverstein, S.J. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. USA 1998, 95, 7080–7085. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Wellish, M.; Cohrs, R.; Debrus, S.; Piette, J.; Rentier, B.; Gilden, D.H. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 2122–2124. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, M.P.; Proenca, J.T.; Efstathiou, S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, L.; Sobel, R.A.; Lai, M.; Triglia, R.; Steain, M.; Abendroth, A.; Arvin, A. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group a determinants in sensory neurons. J. Virol. 2012, 86, 578–583. [Google Scholar] [CrossRef]
- Ouwendijk, W.J.; Flowerdew, S.E.; Wick, D.; Horn, A.K.; Sinicina, I.; Strupp, M.; Osterhaus, A.D.; Verjans, G.M.; Hufner, K. Immunohistochemical detection of intra-neuronal vzv proteins in snap-frozen human ganglia is confounded by antibodies directed against blood group a1-associated antigens. J. Neurovirol. 2012, 18, 172–180. [Google Scholar] [CrossRef]
- Gershon, A.A.; Chen, J.; Gershon, M.D. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J. Infect. Dis. 2008, 197 (Suppl. 2), S61–S65. [Google Scholar] [CrossRef]
- Ferenczy, M.W.; Ranayhossaini, D.J.; Deluca, N.A. Activities of icp0 involved in the reversal of silencing of quiescent herpes simplex virus 1. J. Virol. 2011, 85, 4993–5002. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Kemp, C.D.; Isler, J.A.; Davido, D.J.; Schaffer, P.A. Icp0, icp4, or vp16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 2001, 75, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Schaffer, P.A. Icp0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 2001, 75, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Astor, T.L.; Liptak, L.M.; Cho, C.; Coen, D.M.; Schaffer, P.A. The herpes simplex virus type 1 regulatory protein icp0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 1993, 67, 7501–7512. [Google Scholar]
- Preston, C.M. Reactivation of expression from quiescent herpes simplex virus type 1 genomes in the absence of immediate-early protein icp0. J. Virol. 2007, 81, 11781–11789. [Google Scholar] [CrossRef]
- Miller, C.S.; Danaher, R.J.; Jacob, R.J. Icp0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells. J. Virol. 2006, 80, 3360–3368. [Google Scholar] [CrossRef]
- Thompson, R.L.; Sawtell, N.M. Evidence that the herpes simplex virus type 1 icp0 protein does not initiate reactivation from latency in vivo. J. Virol. 2006, 80, 10919–10930. [Google Scholar] [CrossRef]
- Christensen, J.; Steain, M.; Slobedman, B.; Abendroth, A. Differentiated neuroblastoma cells provide a highly efficient model for studies of productive varicella-zoster virus infection of neuronal cells. J. Virol. 2011, 85, 8436–8442. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Montague, P.; Scott, F.; Grinfeld, E.; Ashrafi, G.H.; Breuer, J.; Rowan, E.G. Varicella-zoster viruses associated with post-herpetic neuralgia induce sodium current density increases in the nd7-23 nav-1.8 neuroblastoma cell line. PLoS ONE 2013, 8, e51570. [Google Scholar] [CrossRef]
- Baird, N.L.; Yu, X.; Cohrs, R.J.; Gilden, D. Varicella zoster virus (VZV)-human neuron interaction. Viruses 2013, 5, 2106–2115. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, S.C. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell 2016, 19, 573–586. [Google Scholar] [CrossRef]
- Kawasaki, H.; Mizuseki, K.; Nishikawa, S.; Kaneko, S.; Kuwana, Y.; Nakanishi, S.; Nishikawa, S.I.; Sasai, Y. Induction of midbrain dopaminergic neurons from es cells by stromal cell-derived inducing activity. Neuron 2000, 28, 31–40. [Google Scholar] [CrossRef]
- Pomp, O.; Brokhman, I.; Ben-Dor, I.; Reubinoff, B.; Goldstein, R.S. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells 2005, 23, 923–930. [Google Scholar] [CrossRef]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef]
- Zhang, S.C.; Wernig, M.; Duncan, I.D.; Brustle, O.; Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human es and ips cells by dual inhibition of smad signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Reinhardt, P.; Glatza, M.; Hemmer, K.; Tsytsyura, Y.; Thiel, C.S.; Hoing, S.; Moritz, S.; Parga, J.A.; Wagner, L.; Bruder, J.M.; et al. Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS ONE 2013, 8, e59252. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lonnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell rna sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Li, C.L.; Li, K.C.; Wu, D.; Chen, Y.; Luo, H.; Zhao, J.R.; Wang, S.S.; Sun, M.M.; Lu, Y.J.; Zhong, Y.Q.; et al. Somatosensory neuron types identified by high-coverage single-cell rna-sequencing and functional heterogeneity. Cell Res. 2016, 26, 967. [Google Scholar] [CrossRef]
- Zerboni, L.; Arvin, A. Neuronal subtype and satellite cell tropism are determinants of varicella-zoster virus virulence in human dorsal root ganglia xenografts in vivo. PLoS Pathog. 2015, 11, e1004989. [Google Scholar] [CrossRef]
- Yu, X.; Seitz, S.; Pointon, T.; Bowlin, J.L.; Cohrs, R.J.; Jonjic, S.; Haas, J.; Wellish, M.; Gilden, D. Varicella zoster virus infection of highly pure terminally differentiated human neurons. J. Neurovirol. 2013, 19, 75–81. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Nair, S.; Velmurugan, K.; Liang, Q.; Mahalingam, R.; Cohrs, R.J.; Nagel, M.A.; Gilden, D. Varicella-zoster virus infection of differentiated human neural stem cells. J. Virol. 2011, 85, 6678–6686. [Google Scholar] [CrossRef]
- Baird, N.L.; Bowlin, J.L.; Hotz, T.J.; Cohrs, R.J.; Gilden, D. Interferon gamma prolongs survival of varicella-zoster virus-infected human neurons in vitro. J. Virol. 2015, 89, 7425–7427. [Google Scholar] [CrossRef]
- Baird, N.L.; Bowlin, J.L.; Yu, X.; Jonjic, S.; Haas, J.; Cohrs, R.J.; Gilden, D. Varicella zoster virus DNA does not accumulate in infected human neurons. Virology 2014, 458–459, 1–3. [Google Scholar] [CrossRef]
- Goodwin, T.J.; McCarthy, M.; Osterrieder, N.; Cohrs, R.J.; Kaufer, B.B. Three-dimensional normal human neural progenitor tissue-like assemblies: A model of persistent varicella-zoster virus infection. PLoS Pathog. 2013, 9, e1003512. [Google Scholar] [CrossRef]
- Baird, N.L.; Bowlin, J.L.; Cohrs, R.J.; Gilden, D.; Jones, K.L. Comparison of varicella-zoster virus rna sequences in human neurons and fibroblasts. J. Virol. 2014, 88, 5877–5880. [Google Scholar] [CrossRef]
- Sloutskin, A.; Kinchington, P.R.; Goldstein, R.S. Productive vs non-productive infection by cell-free varicella zoster virus of human neurons derived from embryonic stem cells is dependent upon infectious viral dose. Virology 2013, 443, 285–293. [Google Scholar] [CrossRef]
- Gowrishankar, K.; Slobedman, B.; Cunningham, A.L.; Miranda-Saksena, M.; Boadle, R.A.; Abendroth, A. Productive varicella-zoster virus infection of cultured intact human ganglia. J. Virol. 2007, 81, 6752–6756. [Google Scholar] [CrossRef]
- Markus, A.; Grigoryan, S.; Sloutskin, A.; Yee, M.B.; Zhu, H.; Yang, I.H.; Thakor, N.V.; Sarid, R.; Kinchington, P.R.; Goldstein, R.S. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: Direct demonstration of axonal infection, transport of vzv, and productive neuronal infection. J. Virol. 2011, 85, 6220–6233. [Google Scholar] [CrossRef]
- Lee, K.S.; Zhou, W.; Scott-McKean, J.J.; Emmerling, K.L.; Cai, G.Y.; Krah, D.L.; Costa, A.C.; Freed, C.R.; Levin, M.J. Human sensory neurons derived from induced pluripotent stem cells support varicella-zoster virus infection. PLoS ONE 2012, 7, e53010. [Google Scholar] [CrossRef]
- Harkness, J.M.; Kader, M.; DeLuca, N.A. Transcription of the herpes simplex virus 1 genome during productive and quiescent infection of neuronal and nonneuronal cells. J. Virol. 2014, 88, 6847–6861. [Google Scholar] [CrossRef]
- Wilcox, C.L.; Johnson, E.M., Jr. Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J. Virol. 1988, 62, 393–399. [Google Scholar]
- Wilcox, C.L.; Johnson, E.M., Jr. Nerve growth factor deprivation results in the reactivation of latent herpes simplex virus in vitro. J. Virol. 1987, 61, 2311–2315. [Google Scholar]
- Cliffe, A.R.; Wilson, A.C. Restarting lytic gene transcription at the onset of herpes simplex virus reactivation. J. Virol. 2017, 91, e01419-16. [Google Scholar] [CrossRef]
- Camarena, V.; Kobayashi, M.; Kim, J.Y.; Roehm, P.; Perez, R.; Gardner, J.; Wilson, A.C.; Mohr, I.; Chao, M.V. Nature and duration of growth factor signaling through receptor tyrosine kinases regulates hsv-1 latency in neurons. Cell Host Microbe 2010, 8, 320–330. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mandarino, A.; Chao, M.V.; Mohr, I.; Wilson, A.C. Transient reversal of episome silencing precedes vp16-dependent transcription during reactivation of latent hsv-1 in neurons. PLoS Pathog. 2012, 8, e1002540. [Google Scholar] [CrossRef]
- Markus, A.; Lebenthal-Loinger, I.; Yang, I.H.; Kinchington, P.R.; Goldstein, R.S. An in vitro model of latency and reactivation of varicella zoster virus in human stem cell-derived neurons. PLoS Pathog. 2015, 11, e1004885. [Google Scholar] [CrossRef]
- Grose, C.; Brunel, P.A. Varicella-zoster virus: Isolation and propagation in human melanoma cells at 36 and 32 degrees c. Infect. Immun. 1978, 19, 199–203. [Google Scholar]
- Sadaoka, T.; Depledge, D.P.; Rajbhandari, L.; Venkatesan, A.; Breuer, J.; Cohen, J.I. In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc. Natl. Acad. Sci. USA 2016, 113, E2403–E2412. [Google Scholar] [CrossRef]
- Kurapati, S.; Sadaoka, T.; Rajbhandari, L.; Jagdish, B.; Shukla, P.; Ali, M.A.; Kim, Y.J.; Lee, G.; Cohen, J.I.; Venkatesan, A. Role of the jnk pathway in varicella-zoster virus lytic infection and reactivation. J. Virol. 2017, 91, e00640-17. [Google Scholar] [CrossRef]
- Cliffe, A.R.; Arbuckle, J.H.; Vogel, J.L.; Geden, M.J.; Rothbart, S.B.; Cusack, C.L.; Strahl, B.D.; Kristie, T.M.; Deshmukh, M. Neuronal stress pathway mediating a histone methyl/phospho switch is required for herpes simplex virus reactivation. Cell Host Microbe 2015, 18, 649–658. [Google Scholar] [CrossRef]
- Koyuncu, O.O.; MacGibeny, M.A.; Hogue, I.B.; Enquist, L.W. Compartmented neuronal cultures reveal two distinct mechanisms for alpha herpesvirus escape from genome silencing. PLoS Pathog. 2017, 13, e1006608. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. Ngf and prongf: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27. [Google Scholar] [CrossRef]
- Barbacid, M. Neurotrophic factors and their receptors. Curr. Opin. Cell Biol. 1995, 7, 148–155. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Yanez, A.A.; Harrell, T.; Sriranganathan, H.J.; Ives, A.M.; Bertke, A.S. Neurotrophic factors ngf, gdnf and ntn selectively modulate hsv1 and hsv2 lytic infection and reactivation in primary adult sensory and autonomic neurons. Pathogens 2017, 6. [Google Scholar] [CrossRef]
- St Leger, A.J.; Hendricks, R.L. Cd8+ t cells patrol hsv-1-infected trigeminal ganglia and prevent viral reactivation. J. Neurovirol. 2011, 17, 528–534. [Google Scholar] [CrossRef]
- De Regge, N.; Van Opdenbosch, N.; Nauwynck, H.J.; Efstathiou, S.; Favoreel, H.W. Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Pourchet, A.; Modrek, A.S.; Placantonakis, D.G.; Mohr, I.; Wilson, A.C. Modeling HSV-1 latency in human embryonic stem cell-derived neurons. Pathogens 2017, 6, 24. [Google Scholar] [CrossRef]
- Como, C.N.; Pearce, C.M.; Cohrs, R.J.; Baird, N.L. Interleukin-6 and type 1 interferons inhibit varicella zoster virus replication in human neurons. Virology 2018, 522, 13–18. [Google Scholar] [CrossRef]
- Decman, V.; Kinchington, P.R.; Harvey, S.A.; Hendricks, R.L. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 2005, 79, 10339–10347. [Google Scholar] [CrossRef]
- Linderman, J.A.; Kobayashi, M.; Rayannavar, V.; Fak, J.J.; Darnell, R.B.; Chao, M.V.; Wilson, A.C.; Mohr, I. Immune escape via a transient gene expression program enables productive replication of a latent pathogen. Cell Rep. 2017, 18, 1312–1323. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baird, N.L.; Zhu, S.; Pearce, C.M.; Viejo-Borbolla, A. Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation. Viruses 2019, 11, 103. https://doi.org/10.3390/v11020103
Baird NL, Zhu S, Pearce CM, Viejo-Borbolla A. Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation. Viruses. 2019; 11(2):103. https://doi.org/10.3390/v11020103
Chicago/Turabian StyleBaird, Nicholas L., Shuyong Zhu, Catherine M. Pearce, and Abel Viejo-Borbolla. 2019. "Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation" Viruses 11, no. 2: 103. https://doi.org/10.3390/v11020103
APA StyleBaird, N. L., Zhu, S., Pearce, C. M., & Viejo-Borbolla, A. (2019). Current In Vitro Models to Study Varicella Zoster Virus Latency and Reactivation. Viruses, 11(2), 103. https://doi.org/10.3390/v11020103




