Effect of Short-Term Nitrogen Addition on N and P Stoichiometry of Herbaceous Leaves and Roots in the Understory of Larix principis-rupprechtii Plantation in Northern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design
2.3. Sampling Methods
2.4. Indicator Measurements
2.5. Statistical Analysis
3. Results
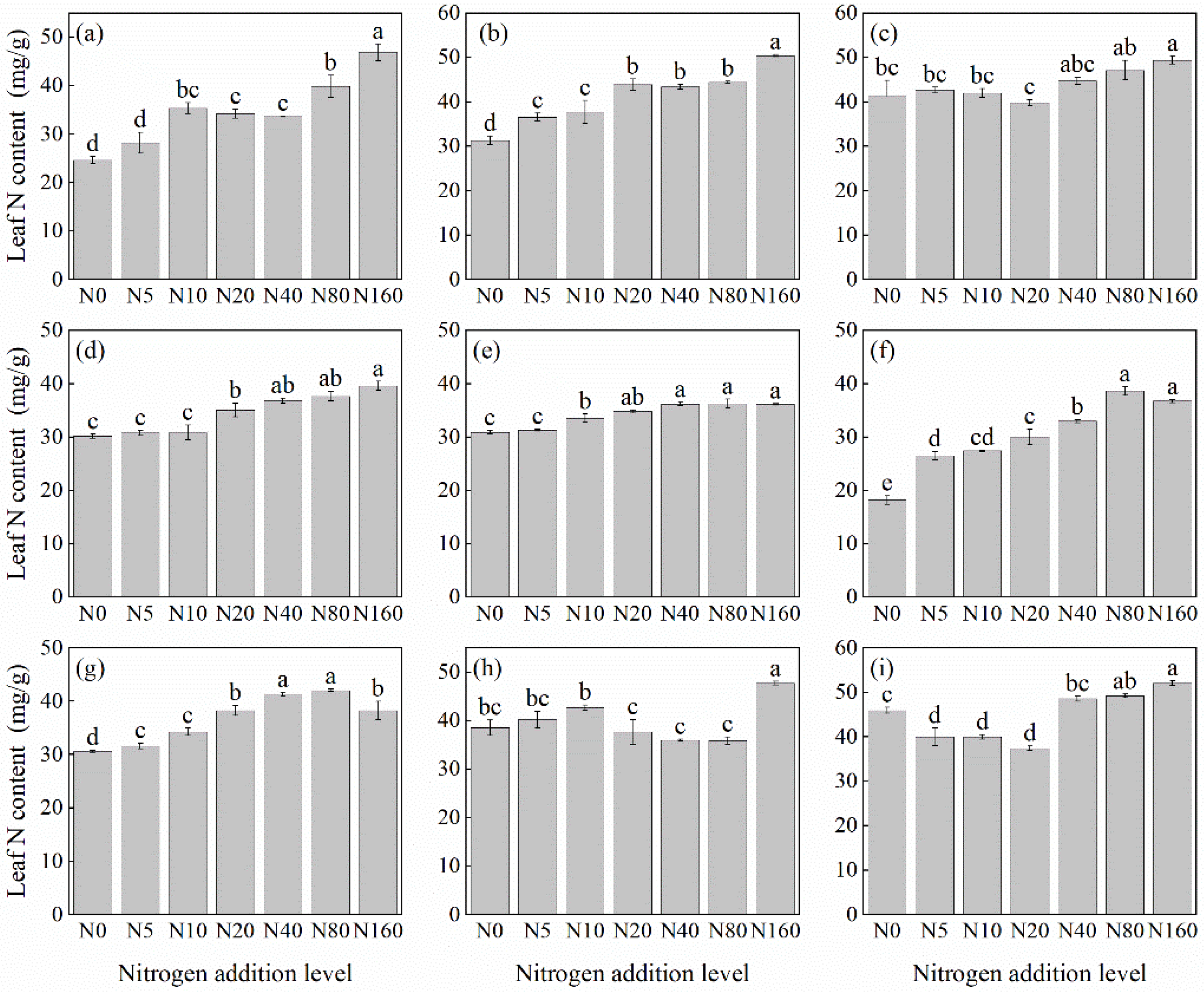
3.1. Plant N Concentration in Response to N Addition
3.1.1. Leaf N Concentration
3.1.2. Root N Concentration
3.2. Plant P Concentration in Response to N Addition
3.2.1. Leaf P Concentration
3.2.2. Root P Concentration
3.3. Plant N/P Response to N Addition
3.3.1. Leaf N/P Ratio
3.3.2. Root N/P Ratio
3.4. Correlation of Plant N and P Concentrations and Their Ratios with Soil Nutrients
3.4.1. Leaf N and P Concentrations and N/P Ratio
3.4.2. Root N and P Concentrations and N/P Ratio
4. Discussion
4.1. Effects of N Addition on the N Concentration of Herbaceous Plants
4.2. Effects of N Addition on the P Concentration of Herbaceous Plants
4.3. Effect of N Addition on N/P Ratio of Herbaceous Plants
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.X.; Jia, Y.L.; Yu, G.R.; Wang, Q.; He, N.P.; Chen, Z.; He, H.L.; Zhu, X.J.; Li, P.; Zhang, F.S.; et al. Changing Patterns of Global Nitrogen Deposition Driven by Socio-Economic Development. Nat. Commun. 2025, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.L.; et al. Stabilization of Atmospheric Nitrogen Deposition in China over the Past Decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhou, G.Y.; Liu, J.X.; Zhang, D.Q.; Xu, Z.H.; Liu, S.Z. Effects of Elevated Carbon Dioxide and Nitrogen Addition on Foliar Stoichiometry of Nitrogen and Phosphorus of Five Tree Species in Subtropical Model Forest Ecosystems. Environ. Pollut. 2012, 168, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dinis, M.D.L.; In, M. Spatial Variability of Atmosphere Dust Fallout Flux in Urban–Industrial Environments. Atmosphere 2020, 11, 1069. [Google Scholar] [CrossRef]
- Zhang, W.J.; She, W.W.; Qin, S.G.; Qiao, Y.G.; Zhang, Y.Q. Effects of nitrogen and water addition on leaf nitrogen and phosphorus stoichiometry of the dominant species in an Artemisia ordosica community. Chin. J. Plant Ecol. 2024, 48, 590–600. [Google Scholar]
- Mo, Q.F.; Zou, B.; Li, Y.W.; Chen, Y.; Zhang, W.X.; Mao, R.; Ding, Y.Z.; Wang, J.; Lu, X.K.; Li, X.B.; et al. Response of Plant Nutrient Stoichiometry to Fertilization Varied with Plant Tissues in a Tropical Forest. Sci. Rep. 2015, 5, 14605. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial Phosphorus Limitation: Mechanisms, Implications, and Nitrogen–Phosphorus Interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Bu, W.S.; Chen, F.S.; Wang, F.C.; Fang, X.M.; Mao, R.; Wang, H.M. The Species-Specific Responses of Nutrient Resorption and Carbohydrate Accumulation in Leaves and Roots to Nitrogen Addition in a Subtropical Mixed Plantation. Can. J. For. Res. 2019, 49, 826–835. [Google Scholar] [CrossRef]
- Su, Y.; Ma, X.F.; Le, J.J.; Li, K.H.; Han, W.X.; Liu, X.J. Decoupling of Nitrogen and Phosphorus in Dominant Grass Species in Response to Long-Term Nitrogen Addition in an Alpine Grassland in Central Asia. Plant Ecol. 2021, 222, 261–274. [Google Scholar] [CrossRef]
- Mao, Q.G.; Lu, X.K.; Mo, H.; Gundersen, P.; Mo, J.M. Effects of Simulated N Deposition on Foliar Nutrient Status, N Metabolism and Photosynthetic Capacity of Three Dominant Understory Plant Species in a Mature Tropical Forest. Sci. Total Environ. 2018, 610–611, 555–562. [Google Scholar] [CrossRef]
- Yan, T.; Qu, T.T.; Song, H.H.; Ciais, P.; Piao, S.L.; Sun, Z.Z.; Zeng, H. Contrasting Effects of N Addition on the N and P Status of Understory Vegetation in Plantations of Sapling and Mature Larix Principis-Rupprechtii. J. Plant Ecol. 2018, 11, 843–852. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Lu, J.; Su, Y.Q.; Yang, Q.S.; Lin, Y.H.; Liu, H.M.; Yang, J.; Huang, H.; Wang, X.H. Differential Effects of N and P Additions on Foliar Stoichiometry between Species and Community Levels in a Subtropical Forest in Eastern China. Ecol. Indic. 2020, 117, 106537. [Google Scholar] [CrossRef]
- Wang, Q.S.Y.; Zheng, C.Y.; Zhang, X.Y.; Zeng, F.X.; Xing, J. Impacts of Nitrogen Addition on Foliar Nitrogen and Phosphorus Stoichiometry in a Subtropical Evergreen Broad-Leaved Forest in Mount Wuyi. Chin. J. Plant Ecol. 2016, 40, 1124–1135. [Google Scholar]
- Lu, M.; Yang, Y.H.; Luo, Y.Q.; Fang, C.M.; Zhou, X.H.; Chen, J.K.; Yang, X.; Li, B. Responses of Ecosystem Nitrogen Cycle to Nitrogen Addition: A Meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.C.; Yang, L.; Li, Q.R.; Li, X.; Xu, G.Q.; Xu, Z.Q.; Jia, Y.L. Short-Term Responses of Soil Nutrients and Enzyme Activities to Nitrogen Addition in a Larix Principis-Rupprechtii Plantation in North China. Front. Ecol. Evol. 2023, 11, 1105150. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; Van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global Patterns of Terrestrial Nitrogen and Phosphorus Limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Yang, L.; Jia, Y.L.; Li, Q.R.; Cui, H.N.; Lu, J.P.; Ma, J.J.; Xu, Z.Q. Ecoenzymatic Stoichiometry in the Rhizosphere and Bulk Soil of a Larix Principis-Rupprechtii Plantation in North China. Forests 2023, 14, 1315. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.T.; Chen, H.Y.H.; Ruan, H.H. Responses of C:N Stoichiometry in Plants, Soil, and Microorganisms to Nitrogen Addition. Plant Soil. 2020, 456, 277–287. [Google Scholar] [CrossRef]
- Bennett, L.T.; Adams, M.A. Response of a Perennial Grassland to Nitrogen and Phosphorus Additions in Sub-Tropical, Semi-Arid Australia. J. Arid. Environ. 2001, 48, 289–308. [Google Scholar] [CrossRef]
- Zeng, W.J.; Zhang, J.Y.; Wang, W. Strong Root Respiration Response to Nitrogen and Phosphorus Addition in Nitrogen-Limited Temperate Forests. Sci. Total Environ. 2018, 642, 646–655. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, C.; Ren, F.; Jing, X.; Ma, W.H.; He, J.S.; Jiang, L. Asymmetric Response of Aboveground and Belowground Temporal Stability to Nitrogen and Phosphorus Addition in a Tibetan Alpine Grassland. Glob. Change Biol. 2023, 29, 7072–7084. [Google Scholar] [CrossRef] [PubMed]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Temperate Forest Ecosystems. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Xia, J.Y.; Wan, S.Q. Global Response Patterns of Terrestrial Plant Species to Nitrogen Addition. New Phytol. 2008, 179, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.T.; Reed, S.C.; Yu, Q.; Han, X.G. Nutrient Resorption Helps Drive Intra-Specific Coupling of Foliar Nitrogen and Phosphorus under Nutrient-Enriched Conditions. Plant Soil 2016, 398, 111–120. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wang, J.; Wu, H.H.; Yang, T.; An, Y.X.; Zhang, Y.L.; Bian, J.L.; Li, Y.; Ren, H.Y.; Lkhagva, A.; et al. Nitrogen Addition Alters Aboveground C:N:P Stoichiometry of Plants but Not for Belowground in an Inner Mongolia Grassland. J. Plant Ecol. 2024, 17, rtad041. [Google Scholar] [CrossRef]
- You, C.M.; Wu, F.Z.; Yang, W.Q.; Xu, Z.F.; Tan, B.; Yue, K.; Ni, X.Y. Nutrient-Limited Conditions Determine the Responses of Foliar Nitrogen and Phosphorus Stoichiometry to Nitrogen Addition: A Global Meta-Analysis. Environ. Pollut. 2018, 241, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.F.; McNeil, B.E.; Lovett, G.M.; Canham, C.D.; Driscoll, C.T.; Rustad, L.E.; Denny, E.; Hallett, R.A.; Arthur, M.A.; Boggs, J.L.; et al. Do Nutrient Limitation Patterns Shift from Nitrogen Toward Phosphorus with Increasing Nitrogen Deposition Across the Northeastern United States? Ecosystems 2012, 15, 940–957. [Google Scholar] [CrossRef]
- Dong, J.L.; Xu, H.; Xie, Y.X.; Chen, J.; Li, Y.P.; Lei, J. Effects of nitrogen addition on root morphology and nutrient content of roots and leaves of leguminous seedlings with different nitrogen requirements. Chin. J. Ecol. 2023, 43, 1255–1262. [Google Scholar]
- Rocci, K.S.; Barker, K.S.; Seabloom, E.W.; Borer, E.T.; Hobbie, S.E.; Bakker, J.D.; MacDougall, A.S.; McCulley, R.L.; Moore, J.L.; Raynaud, X.; et al. Impacts of Nutrient Addition on Soil Carbon and Nitrogen Stoichiometry and Stability in Globally-Distributed Grasslands. Biogeochemistry 2022, 159, 353–370. [Google Scholar] [CrossRef]
- Kou, L.; Wang, H.M.; Gao, W.L.; Chen, W.W.; Yang, H.; Li, S.G. Nitrogen Addition Regulates Tradeoff between Root Capture and Foliar Resorption of Nitrogen and Phosphorus in a Subtropical Pine Plantation. Trees 2017, 31, 77–91. [Google Scholar] [CrossRef]
- He, X.J.; Hou, E.Q.; Liu, Y.; Wen, D.Z. Altitudinal Patterns and Controls of Plant and Soil Nutrient Concentrations and Stoichiometry in Subtropical China. Sci. Rep. 2016, 6, 24261. [Google Scholar] [CrossRef] [PubMed]
- Tessier, J.T.; Raynal, D.J. Use of Nitrogen to Phosphorus Ratios in Plant Tissue as an Indicator of Nutrient Limitation and Nitrogen Saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, B.; Yue, Z.W.; Zeng, F.J.; Li, X.Y.; Li, L. Effects of Short-Term Nitrogen and Phosphorus Addition on Leaf Stoichiometry of a Dominant Alpine Grass. PeerJ 2021, 9, e12611. [Google Scholar] [CrossRef]
- He, J.S.; Fang, J.Y.; Wang, Z.H.; Guo, D.L.; Flynn, D.F.B.; Geng, Z. Stoichiometry and Large-Scale Patterns of Leaf Carbon and Nitrogen in the Grassland Biomes of China. Oecologia 2006, 149, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf Nitrogen and Phosphorus Stoichiometry across 753 Terrestrial Plant Species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.B.; Wang, H.Y.; Lü, X.T.; Wang, Z.W. Effects of nitrogen and phosphorus addition on C : N : P stoichiometry in roots and leaves of four dominant plant species in a meadow steppe of Hulunbuir. Chin. J. Ecol. 2017, 36, 80–88. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhang, T.; Li, Z.; Liu, X.; Liu, Z.; Lv, C.; Lu, J.; Ma, J.; Xu, Z.; Jia, Y. Effect of Short-Term Nitrogen Addition on N and P Stoichiometry of Herbaceous Leaves and Roots in the Understory of Larix principis-rupprechtii Plantation in Northern China. Forests 2025, 16, 320. https://doi.org/10.3390/f16020320
Wu S, Zhang T, Li Z, Liu X, Liu Z, Lv C, Lu J, Ma J, Xu Z, Jia Y. Effect of Short-Term Nitrogen Addition on N and P Stoichiometry of Herbaceous Leaves and Roots in the Understory of Larix principis-rupprechtii Plantation in Northern China. Forests. 2025; 16(2):320. https://doi.org/10.3390/f16020320
Chicago/Turabian StyleWu, Shujing, Tingting Zhang, Zuhong Li, Xiao Liu, Zehua Liu, Chunxi Lv, Jinping Lu, Jiaojiao Ma, Zhongqi Xu, and Yanlong Jia. 2025. "Effect of Short-Term Nitrogen Addition on N and P Stoichiometry of Herbaceous Leaves and Roots in the Understory of Larix principis-rupprechtii Plantation in Northern China" Forests 16, no. 2: 320. https://doi.org/10.3390/f16020320
APA StyleWu, S., Zhang, T., Li, Z., Liu, X., Liu, Z., Lv, C., Lu, J., Ma, J., Xu, Z., & Jia, Y. (2025). Effect of Short-Term Nitrogen Addition on N and P Stoichiometry of Herbaceous Leaves and Roots in the Understory of Larix principis-rupprechtii Plantation in Northern China. Forests, 16(2), 320. https://doi.org/10.3390/f16020320



