Abstract
Macadamia integrifolia is a vital cash crop. The shells of its nuts serve multiple purposes in both agricultural practices and waste management initiatives. In this research, transcriptome analysis was carried out on three macadamia nut varieties with significantly different shell thicknesses, namely ‘A38’, ‘Guire No.1’ (‘GR1’), and HAES842 (‘842’), at the same stage of maturity. The results revealed remarkable differences in their gene expression profiles. A total of 4311 novel genes were identified, among which 1631 were functionally annotated. Analyses using Gene Ontology (GO), Clusters of Orthologous Groups (COGs), and the Kyoto Encyclopedia of Genes and Genomes (KEGG) indicated that the main categories of differentially expressed genes (DEGs) were associated with plant–pathogen interactions. Additionally, 10 members of the heat shock protein 90 (MiHSP90) family were identified and classified into subgroups A, B, and C by comparing them with the HSP90 gene family members of Arabidopsis and rice. Among these, the MiHSP90.1, MiHSP90.2, and MiHSP90.9 proteins were differentially highly expressed in the three macadamia nut varieties. These findings provide fundamental insights into the regulatory mechanisms underlying shell formation in macadamia nuts.
1. Introduction
The macadamia (Macadamia integrifolia) is an evergreen fruit tree that belongs to the Proteaceae family. It is native to Australia []. The shell, the primary byproduct of the macadamia nut, constitutes approximately 50% to 60% of the total fruit weight. Although it was long considered waste, it can be utilized as a carbon support material in fuel cell applications in the present day []. Macadamia shells have a distinctive three-layer architecture. The exocarp is a dense outer layer, 200–300 μm thick, featuring surface pores measuring 5–10 μm in diameter; the mesocarp is a 1.5–2.0 mm fibrous middle layer with radial vascular bundles; and the endocarp is a 0.5–1.0 mm inner layer with helical cellulose reinforcement. Studies have indicated that macadamia shells are primarily composed of cellulose (34.65%), lignin (39.75%), and hemicellulos and contain trace amounts of minerals such as calcium, potassium, and iron. When moisture levels are controlled, shell thickness follows a normal distribution, with 63 percent of the values falling between 24 and 26 mm. The shell thickness of the selected varieties varies significantly. With the rise in resource recycling and green chemistry, notable progress has been achieved in the research on macadamia nut shells in recent years. Specifically, scanning electron microscopy (SEM) and specific surface area analysis (BET) have shown that the shells possess a rich hierarchical pore structure, suggesting the potential to utilize them as an adsorption material, such as a filler in the fused deposition modeling (FDM) of poly lactic acid (PLA) composite filaments []. Moreover, Fourier-transform infrared spectroscopy (FTIR) analysis has revealed that the shell surface contains numerous active functional groups, such as hydroxyl and carboxyl groups, which endow it with potential in chemical modification. There are seven products and processes in which recycled macadamia shells can be reused: biochar [,], renewable energy generation, carbon filters, life-saving medical treatments, particle boards, industrial nanopowders [], and homeware. The thick shells of macadamia nuts can be utilized to produce biochar, while the thin shells are amenable to processing. Nevertheless, the key genes that govern shell formation in macadamia nuts have not been reported to date.
Shell formation occurs to defend against insects and fungi. For instance, the ZjMYB44-ZjPOD51 module boosts the defense response of jujube against phytoplasma by upregulating lignin biosynthesis []. Research on the HSP90 protein family in plants is mainly based on the molecular chaperone theory. This theory posits that HSP90 acts as a key regulator of protein homeostasis. It does so by assisting in the folding, assembly, transport, and degradation of client proteins []. As a highly conserved molecular chaperone, HSP90 plays a crucial role in maintaining the structural integrity and functional activity of various signaling proteins, kinases, and transcription factors in eukaryotic cells []. The molecular chaperone function of HSP90 is mediated by its capacity to bind ATP and engage with a complex network of co-chaperones, thereby enabling the precise regulation of protein folding and maturation processes [,]. HSP90 has been demonstrated to partake in the regulation of hormone signaling pathways [], heat stress responses [], and immune defense mechanisms in plants, further underscoring its multifaceted role as a molecular regulator [,]. Classical theoretical investigations have offered valuable insights into the mechanisms underpinning HSP90 function. Both domestically and internationally, notable advancements have been achieved in research regarding the structure and function of the HSP90 protein family in plants. For example, research has indicated that the HSP90 family is encoded by multiple genes in different plant species. Specifically, Arabidopsis thaliana harbors seven HSP90 genes [,], whereas Oryza sativa comprises nine [,]. This establishes a foundation for further research.
Accordingly, we propose the following scientific hypothesis: the formation mechanism of the macadamia nutshell is closely associated with the structural characteristics of the HSP90 protein. To validate this hypothesis, we employed integrated analytical approaches, including transcriptomic profiling, cluster analysis, and bioinformatics, to conduct a detailed comparative examination of transcriptomic variations across three distinct varieties exhibiting significant differences in shell thickness. This investigation provides a valuable reference for further research on shell development in macadamia nuts.
2. Materials and Methods
2.1. Macadamia Variety and Study Area
This experiment was carried out at the Guangxi South Subtropical Agricultural Sciences Research Institute experimental station (106°79′85″ E, 22°34′13″ N). Three macadamia nut varieties, ‘A38’, ‘GR1’, and ‘842’, those that exhibit remarkable disparities in shell thickness were selected as the research materials. Pericarp thickness (mm), shell thickness (mm), fresh fruit weight (g), and nut weight (g) were measured using calipers and an electronic balance. The full-blossom stage of all three macadamia nut varieties took place from 24–27 March 2024. Fruit samples were concurrently collected on 10 September 2024.
2.2. RNA-Seq and Bioinformatics Analysis
The RNA-seq analysis of three macadamia nut varieties, ‘A38’, ‘GR1’, and ‘842’ as well as bioinformatics analysis of MiHSP90 family members were according to our previously methods []. A basic analysis of the mapped data included gene expression quantification, alternative splicing analysis, novel gene prediction, and gene structure optimization. DEGs with a fold change ≥ 1.5 and a p-value < 0.05 were selected to analyze GO and KEGG enrichment. The filtered sequences were aligned to the reference genome of ‘Guire No.1’ which have been deposited in the Genome Warehouse in China National Center for Bioinformation, under accession number PRJCA008938 that is publicly accessible at https://ngdc.cncb.ac.cn/gwh (accessed on 11 May 2024). The transcriptome sequence data were assembled using StringTie software v3.0.0 https://ccb.jhu.edu/software/stringtie/ (accessed on 1 June 2025).
2.3. RT-qPCR Verification
Real-time polymerase chain reaction (PCR) was carried out using SYBR qPCR Master Mix (Vazyme, Nanjing, China) as previously methods []. MiActin (GenBank accession: HQ260674.1) served as the reference gene (Table 1). The synthesized cDNA was serially diluted by 10−1, 10−2, 10−3, 10−4, 10−5, and 10−6 fold using EASY Dilution from SYBR® Premix Ex TaqTM (Takara Biomedical Technology (Beijing) Co., Ltd. Beijing, China), which served as the template. A no-template control (ddH2O) was included in the reaction. Three biological replicates, each comprising three technical replicates, were analyzed per sample.

Table 1.
RT-qPCR primers.
3. Results
3.1. Macadamia Nuts Fruit Development Analysis
The initial flowering period of macadamia trees in Guangxi Province, China, usually ranges from February 28 to March 18 each year. Taking the variety ‘GR1’ as an example, both the weight and size of the shell and kernel stabilize approximately 60 d after pollination. The entire fruit continues to grow until a stable state is reached at 165 d (Figure 1). Sixty days after pollination, the shell weight, transverse diameter, and longitudinal diameter were 7.83 g, 20.43 mm, and 20.83 mm, respectively. At 165 d after pollination, the shell weight, transverse diameter, and longitudinal diameter were 8.75 g, 23.44 mm, and 24.3 mm, respectively. Similarly, the ‘A38’ and ‘842’ varieties also follow similar developmental patterns.
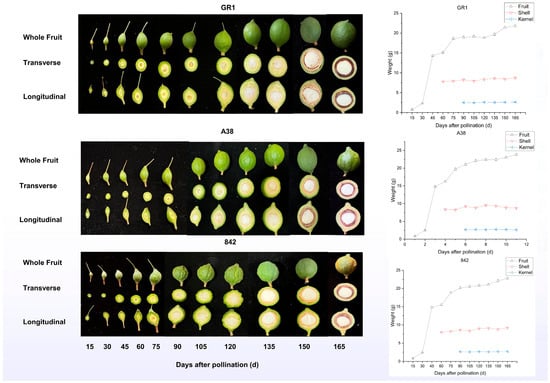
Figure 1.
The weight, transverse, and longitudinal image of 3 macadamia varieties after pollination.
For the comparative transcriptome analysis, fruits at consistent developmental and maturation stages were selected on 10 September 2023. As shown in Figure 2, the pericarp thicknesses of the ‘A38’, ‘GR1’, and ‘842’ varieties were 4.41 ± 0.15, 3.42 ± 0.11, and 3.16 ± 0.12 mm, respectively; the shell thicknesses of the three varieties were 2.87 ± 0.14 mm, 2.14 ± 0.09 mm, and 2.44 ± 0.08 mm, respectively; the fresh fruit weight of the three varieties was 26.1 ± 3.52, 19.41 ± 2.47, and 15.64 ± 2.14 g, respectively; and the nut weight of the three varieties was 11.06 ± 1.24, 9.6 ± 0.84, and 7.28 ± 0.74 g, respectively.
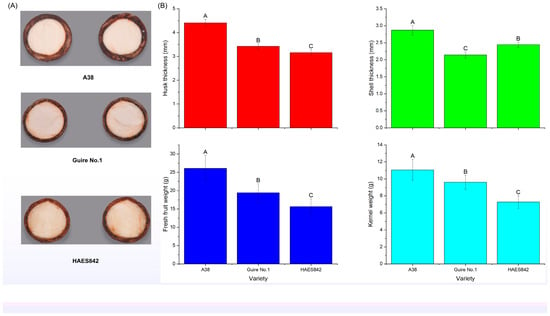
Figure 2.
The pericarp thickness, shell thickness, fresh fruit weight, and nut weight of the three macadamia varieties. (A) The transverse images of shell and nut; (B) The bar graph of the pericarp thickness, shell thickness, fresh fruit weight, and nut weight. The capital letters (A, B and C) on the column chart indicate that the differences are at an extremely significant level p < 0.01.
3.2. DEGs of the Three Macadamia Varieties
Transcriptome analysis was conducted on nine samples derived from the ‘A38’, ‘GR1’, and ‘842’ macadamia varieties, characterized by differential shell thicknesses, with three biological replicates per variety. Sequencing generated 69.74 Gb of high-quality clean data, yielding an average of 7.10 Gb per sample. The Q30 base call quality score exceeded 94.20% across all samples. The alignment of clean reads against the designated reference genome demonstrated alignment efficiencies ranging from 86.74% to 93.00%. Pearson’s correlation coefficient analysis confirmed exceptionally high reproducibility among the triplicate samples within each variety (Figure 3A). Principal component analysis (PCA) further indicated the distinct clustering of biological replicates corresponding to each variety within separate quadrants (Figure 3B and Supplementary Materials).
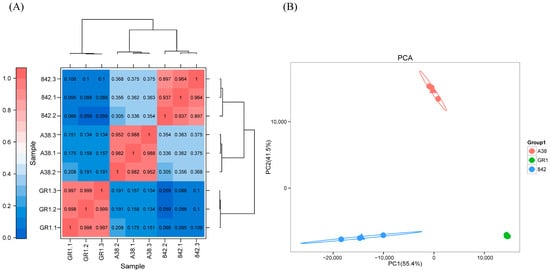
Figure 3.
Pearson’s correlation coefficient (A) and principal component analysis (PCA) (B) of all the genes in the three macadamia varieties.
A total of 4311 novel genes were identified, among which 1631 were functionally annotated. As depicted in Figure 4, the number of differentially expressed genes (DEGs) identified in the transcriptome analysis of the three varieties reached a significant level. The total number of DEGs between ‘A38’ and ‘GR1’ was 7275, with 3738 upregulated genes and 3537 downregulated genes. Between ‘A38’ and ‘842’, there were 4095 DEGs, comprising 2044 upregulated and 2051 downregulated genes. The comparison between ‘GR1’ and ‘842’ revealed 7404 DEGs, including 3569 upregulated and 3835 downregulated genes (Figure 4A). The DEGs between ‘A38’ and ‘842’ were primarily associated with general function prediction only and signal transduction mechanisms (Figure 4B); the DEGs between ‘A38’ and ‘GR1’ were mainly involved in signal transduction mechanisms and carbohydrate transport and metabolism (Figure 4C); and the DEGs between ‘GR1’ and ‘842’ were predominantly related to general function prediction only, signal transduction mechanisms, and carbohydrate transport and metabolism (Figure 4D).
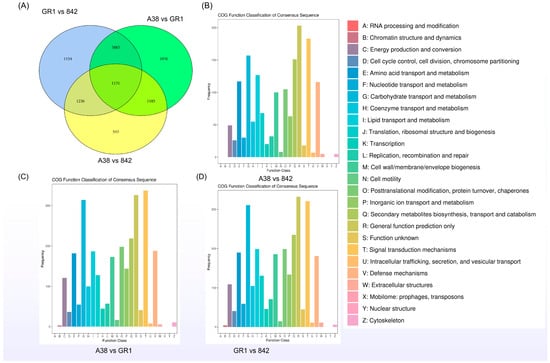
Figure 4.
Venn diagram (A) and COG analysis of differentially expressed genes (DEGs) in three macadamia varieties with varying shell thicknesses (B–D).
As depicted in Figure 5, the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis indicated that the differentially expressed genes (DEGs) were mainly enriched in the plant–pathogen interaction, plant hormone signal transduction, and starch and sucrose metabolism pathways. The numbers of DEGs in these three pathways between the pairs ‘A38’ and ‘GR1’, ‘A38’ and ‘842’, and ‘GR1’ and ‘842’ were 281, 192, and 182, respectively (Figure 5). Overall, 956 DEGs were identified in the plant–pathogen interaction pathway. The expression analysis showed that the expression levels of these 956 genes were significantly different among the three macadamia varieties (Figure 6).
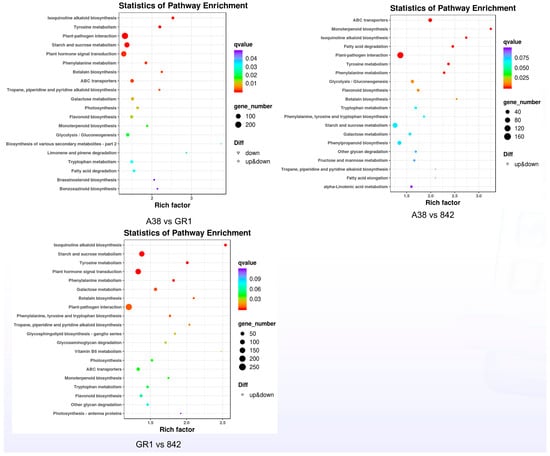
Figure 5.
KEGG analysis of DEGs in the three macadamia varieties.
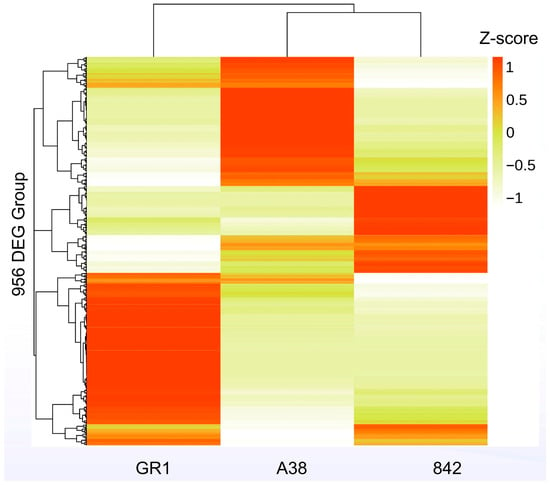
Figure 6.
Heatmap of the expression level of DEGs in the three macadamia varieties.
3.3. Characterization of 10 MiHSP90 Protein Members
Among these differentially expressed genes (DEGs), we identified 10 MiHSP90 family proteins (see Supplementary Materials) using the PF00183 protein domain via HMMER 3.4, http://hmmer.org/ (10 March 2025). Noticeable differences were observed in the expression levels, especially for MiHSP90.1, MiHSP90.2, and MiHSP90.9 (Figure 7A). Additionally, we carried out a phylogenetic analysis of the 10 MiHSP90s, comparing them with their orthologs from Arabidopsis thaliana and Oryza sativa. As shown in Figure 7B, these proteins were classified into three distinct subgroups, namely A, B, and C. Subgroup A includes MiHSP1, 3, 5, 7, and 9; subgroup B consists of MiHSP4, 6, and 10; and subgroup C is composed of MiHSP2 and 8.
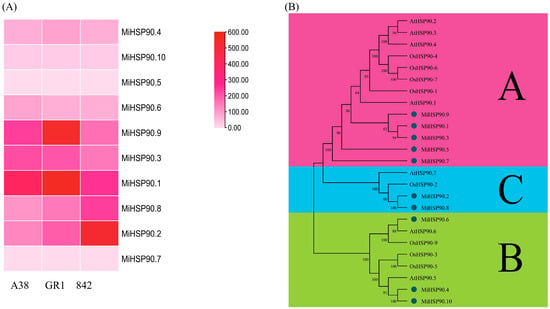
Figure 7.
Expression (A) and phylogenetic analysis (B) of 10 MiHSP90s in the three macadamia varieties. The capital letters (A, B and C) mean the different subgroup of plant HSP90 protein family.
According to the ‘GR1’ genome, seven MiHSP90 genes are located on five chromosomes: MiHSP90.1 on chromosome 14, MiHSP90.5 on chromosome 6, MiHSP90.6 on chromosome 5, MiHSP90.8 on chromosome 7, and MiHSP90.9 and MiHSP90.10 on chromosome 9. MiHSP90.2, MiHSP90.3, MiHSP90.4, and MiHSP90.7 could not be assigned to any of the chromosomes (Figure 8). The promoter and motif analyses revealed that all 10 MiHSP90 proteins contain motifs such as the G-box, ABRE, and HD-Zip. The motif analysis showed that they had 10 motifs; of these, 4 of the main motifs were PRK05218, HATPase_Hsp90-like, HSP90, and the PRK14083 superfamily (Figure 9).
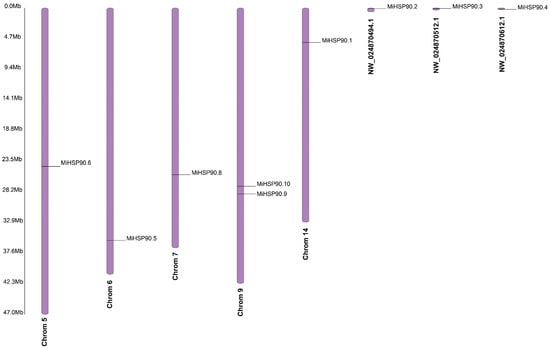
Figure 8.
The chromosomal location analysis of 10 MiHSP90s in the macadamia variety ‘GR1’.
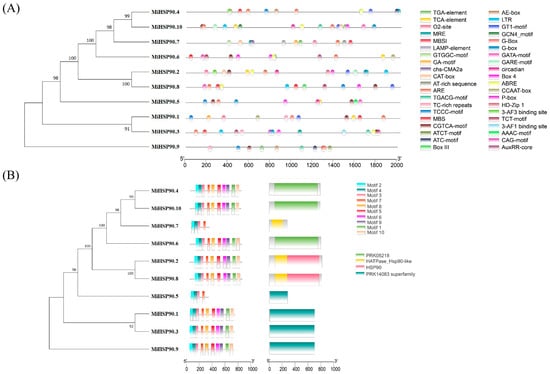
Figure 9.
The promoter region (A) and protein motif analysis (B) of 10 MiHSP90s in macadamia nuts.
As depicted in Figure 10, the outcomes of the fluorescence quantitative analysis were in accordance with those of the transcriptome analysis. There were notable differences in the expression levels, especially for MiHSP90.1, MiHSP90.2, MiHSP90.3, MiHSP90.6, MiHSP90.7, and MiHSP90.10. Nevertheless, the gene expression of MiHSP90.4, MiHSP90.5, MiHSP90.8, and MiHSP90.9 did not exhibit significant differences.
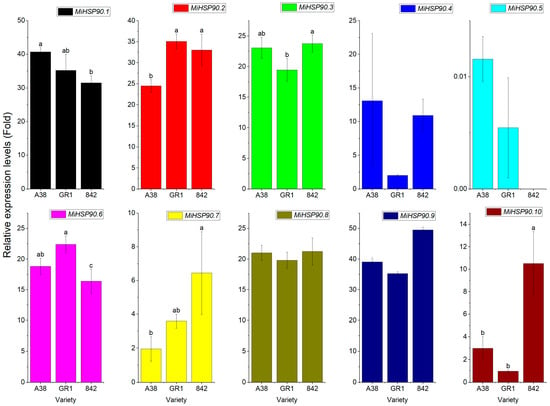
Figure 10.
RT-qPCR verification of the gene expression of 10 MiHSP90s in the nutshells of the three macadamia nut varieties. The lowercase letters (a, b and c) on the column chart indicate that the differences are at significant level p < 0.05.
4. Discussion
Macadamia nuts have become one of the most valuable tree nut crops worldwide, with their annual production surpassing 300,000 metric tons. Their hard shell, which accounts for 50%–60% of the total fruit weight, poses both a challenge in terms of disposal, but also an opportunity, as it is an untapped resource [,,]. Our research indicates that macadamia nut shells undergo gradual growth from 60 days post pollination until the harvest period at 180 days, with significant differences among different varieties (Figure 1 and Figure 2). This lays a solid foundation for the further efficient utilization of these fruit shells and the selection and breeding of new varieties []. Previous studies have shown that the application of boron and brassinosteroids (BRs) can increase the fruit set of macadamia trees []. In our transcriptome analysis, we found that the main categories of differentially expressed genes (DEGs) were related to plant–pathogen interactions (Figure 5 and Figure 6). Based on this, we found 10 MiHSP90 protein family members (Figure 7, Figure 8 and Figure 9).
The HSP90 protein family is a highly conserved group of molecular chaperones that play crucial roles in various cellular processes, including protein folding, signal transduction, and stress response []. We speculate that MIHSP90 is closely related to the formation of macadamia nut shells. Firstly, the HSP90 protein is related to plant growth and development. For instance, wheat HSP90.2 enhances CO2 assimilation rates and grain weight [,]. HSP90 modulates developmental processes and abiotic stress responses in ginger []. Knockout of the endoplasmic reticulum-localized molecular chaperone HSP90.7 leads to impaired development in Arabidopsis seedlings []. Secondly, HSP90 is involved in plant–pathogen interactions. In cassava, HSP90 interacts with autophagy-related genes to enhance disease resistance []. Arabidopsis requires HSP90 interaction with RAR1 and SGT1 for disease resistance mediation []. In tobacco, Hsp90 interacts with the Tm-22 protein, conferring resistance to the Tobacco mosaic virus []. Thirdly, HSP90 plays a role within the signaling pathways of plant hormones. Specifically, in Arabidopsis, jasmonate signaling involves SGT1b–HSP70–HSP90 chaperone complexes []. HSP90 modulates brassinosteroid signaling in Arabidopsis through its interaction with the BES1/BZR1 transcription factor []. This study identified the proteins MiHSP90.1, MiHSP90.2, and MiHSP90.9 as being putatively involved in shell formation. HbHSP90.1 is a gene that plays a role in stress response in rubber trees []. In Arabidopsis, ROF1 modulates thermotolerance by interacting with HSP90.1, which is essential for survival at high temperatures []. HSP90.2 plays a function both in plant growth [,] and disease resistance []. The cassava MeHSP90.9–MeSGT1–MeRAR1 chaperone complex engages with MeATGs and subsequently initiates autophagy signaling, thereby enhancing the resistance of cassava to bacterial blight []. MeHSP90.9 positively regulates the activity of MeCatalase1, and MeHSP90.9-silenced cassava leaves accumulate more H2O2 under drought stress []. MeHSP90.9-interacting proteins in both cassava and the bacterial pathogen revealed that MeHSP90.9 plays a dual role in cassava–Xam interaction []. Also, salicylic acid (SA) upregulates HSP90.9 gene expression and enhances resistance to Sri Lankan Cassava Mosaic Virus []. At present, the research on the regulatory genes of plant fruit shells is still at an early stage. It has now been confirmed that it is a quantitative trait [,,]. This study found that the MiHSP90 protein family is related to the thickness of the macadamia nut shells, but it is not the main regulatory gene yet. Further experiments are needed for verification. These findings are in accordance with our results, but further experiments need to be performed in order for the functions of the MiHSP90 gene family in macadamia nuts to be proven.
5. Conclusions
Over the years, research on macadamia nut shells has gradually evolved from a focus on waste disposal to a multidisciplinary field of study. GO, COG, and KEGG analyses revealed that the main categories of differentially expressed genes (DEGs) are related to plant–pathogen interactions. Additionally, 10 heat shock protein 90 (MiHSP90s) transcription factors were identified and classified into A, B, and C subgroups when compared with those in the Arabidopsis and rice HSP90 gene family members. Among these, the MiHSP90.1, MiHSP90.2, and MiHSP90.9 proteins exhibit high differential expression in these macadamia nuts. Given that the shell thickness is a quantitative trait, various subsequent techniques, such as map cloning, mutation analysis, and genetic transformation, are necessary to determine the main regulatory functions of these genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16121775/s1.
Author Contributions
Conceptualization, H.C., W.W. and L.W.; methodology, X.H.; software, X.H.; formal analysis, X.H., X.T., C.Z., X.Y., Y.W. and Q.T.; investigation, X.H., X.T., C.Z., X.Y., Y.W. and Q.T.; resources, W.W.; writing—original draft preparation, X.H.; writing—review and editing, H.C., W.W. and L.W.; funding acquisition, H.C. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangxi Natural Science Foundation of China (2025GXNSFAA069142); the Science and Technology Development Fund of Guangxi Academy of Agricultural Sciences (Guangxi Agricultural Science and Technology 2021YT156); and the National Key Research and Development Program of China (2022YFF1300705).
Data Availability Statement
The raw transcriptome data have been uploaded to the National Genomics Data Center. The BioProject accession number is PRJCA047895.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gong, L.; Zhang, H.; Ma, J.; Li, Z.; Li, T.; Wu, C.; Li, Y.; Tao, L. Unravel the molecular basis underlying inflorescence color variation in Macadamia based on widely targeted metabolomics. Front. Plant Sci. 2025, 16, 1533187. [Google Scholar] [CrossRef]
- Mojapelo, N.A.; Seroka, N.S.; Khotseng, L. Macadamia Nut Bio-Waste: An Agricultural Waste with Potential to Be Used as Carbon Support Material in Fuel Cell Applications. Coatings 2023, 13, 1545. [Google Scholar] [CrossRef]
- Song, X.; He, W.; Qin, H.; Yang, S.; Wen, S. Fused Deposition Modeling of Poly (lactic acid)/Macadamia Composites-Thermal, Mechanical Properties and Scaffolds. Materials 2020, 13, 258. [Google Scholar] [CrossRef]
- Na, P.T.L.; Tuyen, N.D.K.; Dang, B.T. Sorption of four antibiotics onto pristine biochar derived from macadamia nutshell. Bioresour. Technol. 2024, 394, 130281. [Google Scholar] [CrossRef]
- Wang, H.J.; Al-Kurdhani, J.M.H.; Ma, J.J.; Wang, Y.D. Adsorption of Zn2+ ion by macadamia nut shell biochar modified with carboxymethyl chitosan and potassium ferrate. J. Environ. Chem. Eng. 2023, 11, 110150. [Google Scholar] [CrossRef]
- Moyo, M.; Modise, S.J.; Pakade, V.E. Palladium nanoparticles dispersed on functionalized macadamia nutshell biomass for formic acid-mediated removal of chromium(VI) from aqueous solution. Sci. Total Environ. 2020, 743, 140614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Wei, X.; Li, Y.; Liu, Z.; Liu, M.; Huang, W.; Wang, H.; Zhao, J. The ZjMYB44-ZjPOD51 module enhances jujube defense response against phytoplasma by upregulating lignin biosynthesis. Hortic. Res. 2025, 12, uhaf083. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Jiang, Y.; Wang, Z.; Li, X.; Li, H.; Tang, S.; Zhang, J.; Xia, M.; Zhang, M.; Deng, X.; et al. Genome-Wide Identification and Expression Analysis of the HSP90 Gene Family in Relation to Developmental and Abiotic Stress in Ginger (Zingiber officinale Roscoe). Plants 2025, 14, 1660. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.Y.; Feng, Q.; Man, X.Y.; Qi, D.Q.; Qing, Y.S.; Yang, Z.W.; Elbagory, M.; Kasem, E.S.; Yasir, M.; Rong, J.Y. Genome-wide identification and characterization of HSP90 family gene in cotton and their potential role in salt stress tolerance. Front. Plant Sci. 2025, 16, 1574604. [Google Scholar] [CrossRef]
- Guo, Y.T.; Yan, Y.; Zhang, G.L.; Gou, J.Y. Dataset of wheat HSP90.2 chaperome. Data Brief. 2024, 55, 110583. [Google Scholar] [CrossRef]
- Chiosis, G.; Digwal, C.S.; Trepel, J.B.; Neckers, L. Structural and functional complexity of HSP90 in cellular homeostasis and disease. Nat. Rev. Mol. Cell Biol. 2023, 24, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Millet, Y.A.; Cheng, Z.; Bush, J.; Ausubel, F.M. Jasmonate signalling in Arabidopsis involves SGT1b-HSP70-HSP90 chaperone complexes. Nat. Plants 2015, 1, 15049. [Google Scholar] [CrossRef] [PubMed]
- Zabka, M.; Lesniak, W.; Prus, W.; Kuznicki, J.; Filipek, A. Sgt1 has co-chaperone properties and is up-regulated by heat shock. Biochem. Biophys. Res. Commun. 2008, 370, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, W.; Jaskani, M.J.; Maqbool, R.; Chattha, W.S.; Ali, Z.; Naqvi, S.A.; Haider, M.S.; Khan, I.A.; Vincent, C.I. Heat shock protein and aquaporin expression enhance water conserving behavior of citrus under water deficits and high temperature conditions. Environ. Exp. Bot. 2021, 181, 104270. [Google Scholar] [CrossRef]
- Samakovli, D.; Roka, L.; Dimopoulou, A.; Plitsi, P.K.; Zukauskaite, A.; Georgopoulou, P.; Novák, O.; Milioni, D.; Hatzopoulos, P. HSP90 affects root growth in Arabidopsis by regulating the polar distribution of PIN1. New Phytol. 2021, 231, 1814–1831. [Google Scholar] [CrossRef]
- Bao, F.; Huang, X.; Zhu, C.; Zhang, X.; Li, X.; Yang, S. Arabidopsis HSP90 protein modulates RPP4-mediated temperature-dependent cell death and defense responses. New Phytol. 2014, 202, 1320–1334. [Google Scholar] [CrossRef]
- Hubert, D.A.; He, Y.; McNulty, B.C.; Tornero, P.; Dangl, J.L. Specific Arabidopsis HSP90.2 alleles recapitulate RAR1 cochaperone function in plant NB-LRR disease resistance protein regulation. Proc. Natl. Acad. Sci. USA 2009, 106, 9556–9563. [Google Scholar] [CrossRef]
- Wang, H.; Charagh, S.; Dong, N.; Lu, F.; Wang, Y.; Cao, R.; Ma, L.; Wang, S.; Jiao, G.; Xie, L.; et al. Genome-Wide Analysis of Heat Shock Protein Family and Identification of Their Functions in Rice Quality and Yield. Int. J. Mol. Sci. 2024, 25, 11931. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.H.; Ye, T.Z.; Chen, R.J.; Gao, X.L.; Xu, Z.J. Molecular characterization, expression pattern and function analysis of the OsHSP90 family in rice. Biotechnol. Biotechnol. Equip. 2016, 30, 669–676. [Google Scholar] [CrossRef]
- Tan, Q.; Huan, X.; Pan, Z.; Yang, X.; Wei, Y.; Zhou, C.; Wang, W.; Wang, L. Comparative Transcriptome Analysis Reveals Key Functions of MiMYB Gene Family in Macadamia Nut Pericarp Formation. Int. J. Mol. Sci. 2024, 25, 6840. [Google Scholar] [CrossRef]
- Kim, J.H.; Chan, K.L.; Hart-Cooper, W.M.; Ford, D.; Orcutt, K.; Palumbo, J.D.; Tam, C.C.; Orts, W.J. Valorizing Tree-Nutshell Particles as Delivery Vehicles for a Natural Herbicide. Methods Protoc. 2024, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Islam, M.M.; Epaarachchi, J.; Shibata, S. Exploring the Prospects of Macadamia Nutshells for Bio-Synthetic Polymer Composites: A Review. Polymers 2023, 15, 4007. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.S.; Balieiro, L.C.S.; Teixeira, E.J.O.; Rodrigues, L.M.; Rosa, D.S.; Mulinari, D.R. Revalorization of Macadamia nutshell residue as a filler in eco-friendly castor polyol-based polyurethane foam. J. Mater. Cycles Waste Manag. 2023, 25, 2295–2311. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Zhao, Z.X.; Zhou, J.J.; Yang, F.; Zhang, J.Z. Boron Supplementation and Phytohormone Application: Effects on Development, Fruit Set, and Yield in Macadamia Cultivar ‘A4’ (Macadamia integrifolia, M. tetraphylla). Plants 2025, 14, 2461. [Google Scholar] [CrossRef]
- Kadota, Y.; Shirasu, K. The HSP90 complex of plants. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 689–697. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, M.L.; Guo, Y.T.; Ding, C.H.; Niu, K.X.; Li, X.M.; Sun, C.; Dong, Z.; Cui, D.; Rasheed, A.; et al. HSP90.2 promotes CO2 assimilation rate, grain weight and yield in wheat. Plant Biotechnol. J. 2023, 21, 1229–1239. [Google Scholar] [CrossRef]
- Noureddine, J.; Mu, B.; Hamidzada, H.; Mok, W.L.; Bonea, D.; Nambara, E.; Zhao, R. Knockout of endoplasmic reticulum-localized molecular chaperone HSP90.7 impairs seedling development and cellular auxin homeostasis in Arabidopsis. Plant J. 2024, 119, 218–236. [Google Scholar] [CrossRef]
- Wei, Y.X.; Zeng, H.Q.; Liu, W.; Cheng, X.; Zhu, B.B.; Guo, J.R.; Shi, H.T. Autophagy-related genes serve as heat shock protein 90 co-chaperones in disease resistance against cassava bacterial blight. Plant J. 2021, 107, 925–937. [Google Scholar] [CrossRef]
- Takahashi, A.; Casais, C.; Ichimura, K.; Shirasu, K. HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 11777–11782. [Google Scholar] [CrossRef]
- Qian, L.C.; Zhao, J.P.; Du, Y.M.; Zhao, X.J.; Han, M.; Liu, Y.L. Hsp90 Interacts With Tm-22 and Is Essential for Tm-22-Mediated Resistance to Tobacco mosaic virus. Front. Plant Sci. 2018, 9, 411. [Google Scholar] [CrossRef]
- Shigeta, T.; Zaizen, Y.; Sugimoto, Y.; Nakamura, Y.; Matsuo, T.; Okamoto, S. Heat shock protein 90 acts in brassinosteroid signaling through interaction with BES1/BZR1 transcription factor. J. Plant Physiol. 2015, 178, 69–73. [Google Scholar] [CrossRef]
- Liu, M.Y.; Wang, L.F.; Ke, Y.H.; Xian, X.M.; Wang, J.L.; Wang, M.; Zhang, Y. Identification of HbHSP90 gene family and characterization HbHSP90.1 as a candidate gene for stress response in rubber tree. Gene 2022, 827, 146475. [Google Scholar] [CrossRef] [PubMed]
- Meiri, D.; Breiman, A. Arabidopsis ROF1 (FKBP62) modulates thermotolerance by interacting with HSP90.1 and affecting the accumulation of HsfA2-regulated sHSPs. Plant J. 2009, 59, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.X.; Liu, W.; Hu, W.; Yan, Y.; Shi, H.T. The chaperone MeHSP90 recruits MeWRKY20 and MeCatalase1 to regulate drought stress resistance in cassava. New Phytol. 2020, 226, 476–491. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, B.; Zhang, Y.; Ma, G.; Wu, J.; Tang, L.; Shi, H. CPK1-HSP90 phosphorylation and effector XopC2-HSP90 interaction underpin the antagonism during cassava defense-pathogen infection. New Phytol. 2024, 242, 2734–2745. [Google Scholar] [CrossRef]
- Pattanavongsawat, C.; Malichan, S.; Vannatim, N.; Chaowongdee, S.; Hemniam, N.; Paemanee, A.; Siriwan, W. Enhancing Plant Resistance to Sri Lankan Cassava Mosaic Virus Using Salicylic Acid. Metabolites 2025, 15, 261. [Google Scholar] [CrossRef]
- Purohit, A. Unravelling Genetic Determinants of Shell Thickness in Groundnut: Insights from a Genome-Wide Association Study. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Arab, M.M.; Marrano, A.; Abdollahi-Arpanahi, R.; Leslie, C.A.; Askari, H.; Neale, D.B.; Vahdati, K. Genome-wide patterns of population structure and association mapping of nut-related traits in Persian walnut populations from Iran using the Axiom J. regia 700K SNP array. Sci. Rep. 2019, 9, 6376. [Google Scholar] [CrossRef]
- Valentini, N.; Pavese, V.; Martina, M.; Acquadro, A.; Torello Marinoni, D.; Botta, R.; Portis, E. Genomic investigation of traits associated with nut and kernel in a full-sib population of European hazelnut. Sci. Hortic. 2025, 339, 113871. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).