Divergent Effects of Forest Canopy Opening on Soil Fauna and Microbes: A Global Meta-Analysis
Abstract
1. Introduction
2. Methods and Materials
2.1. Data
2.2. Statistical Analysis
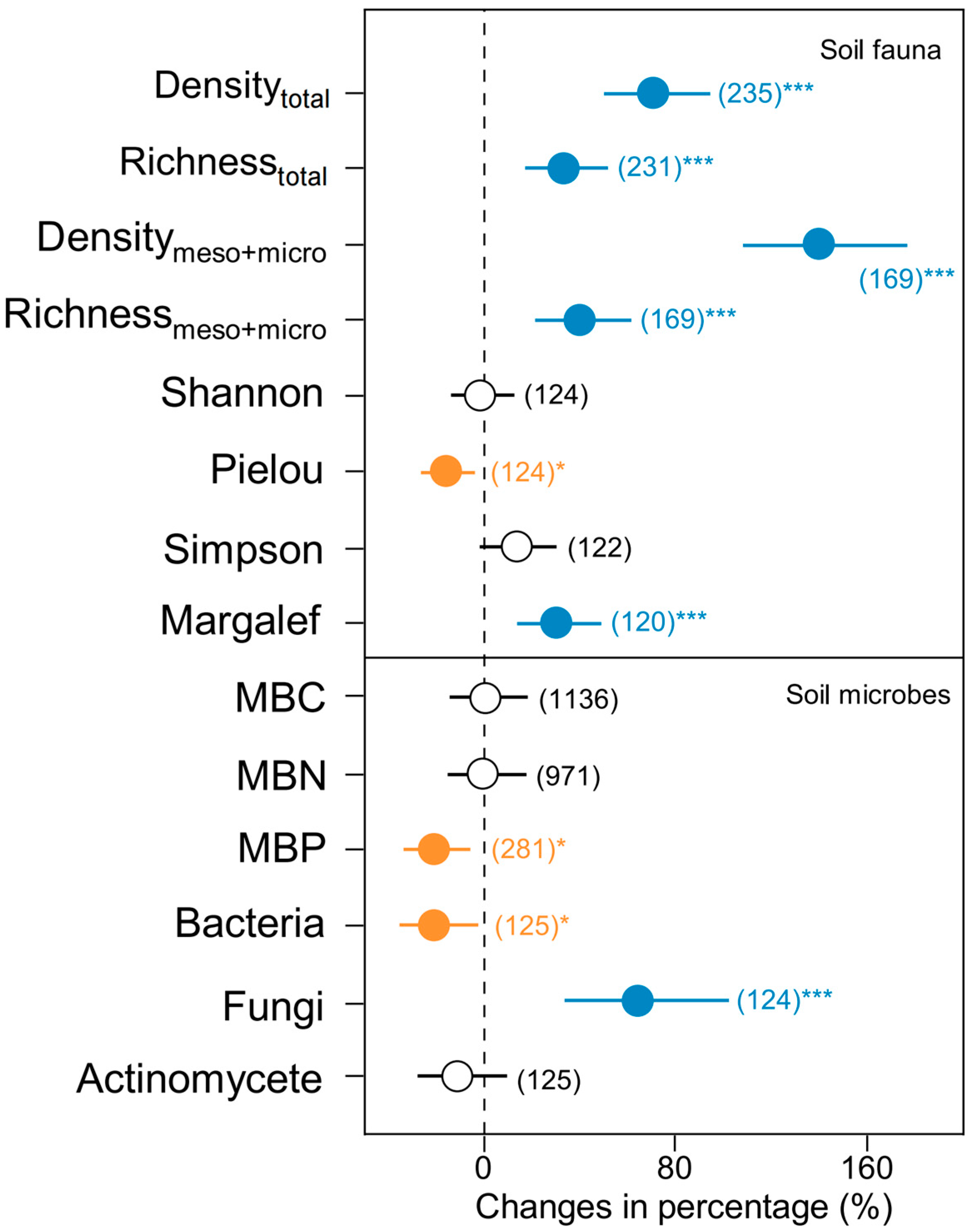
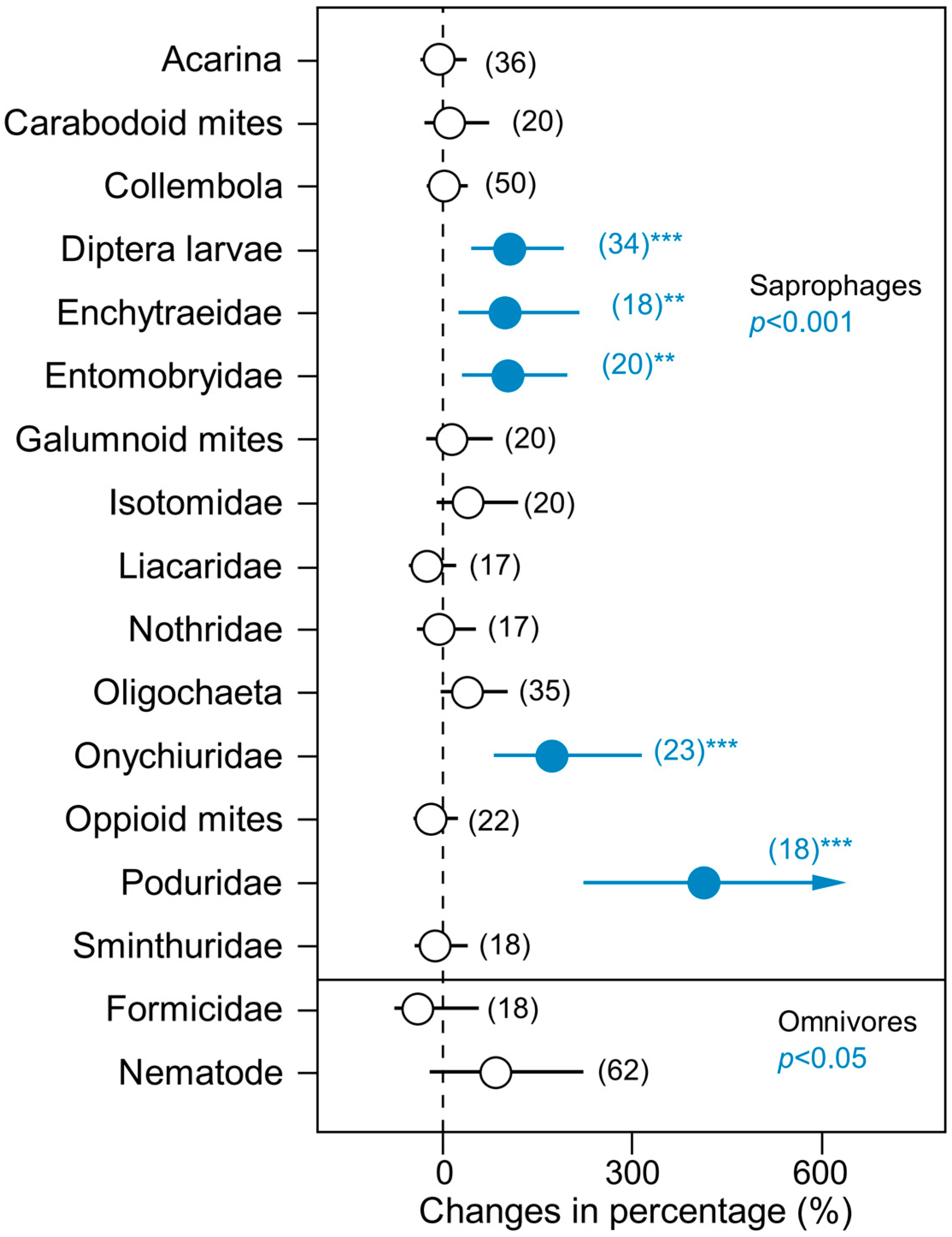
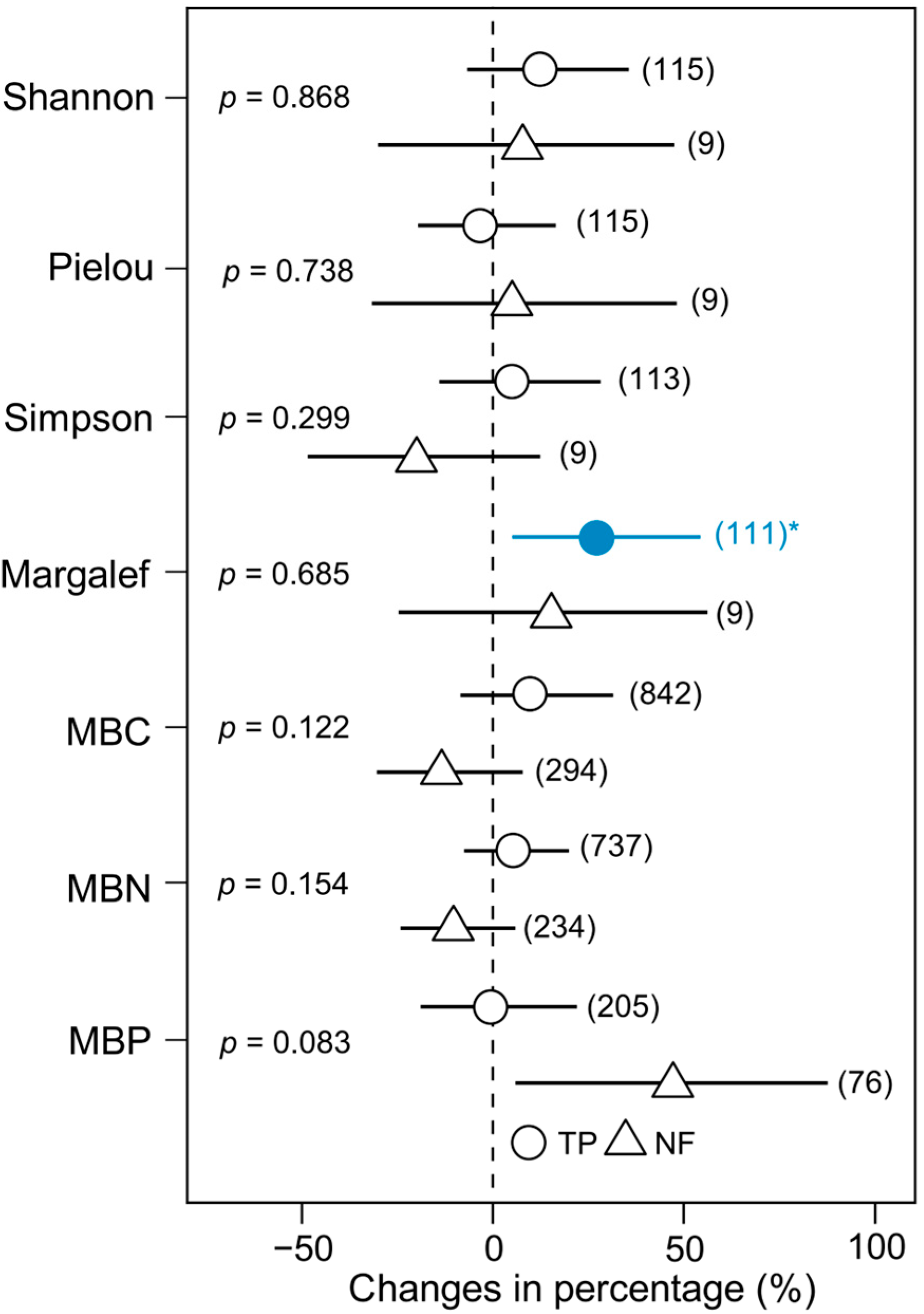
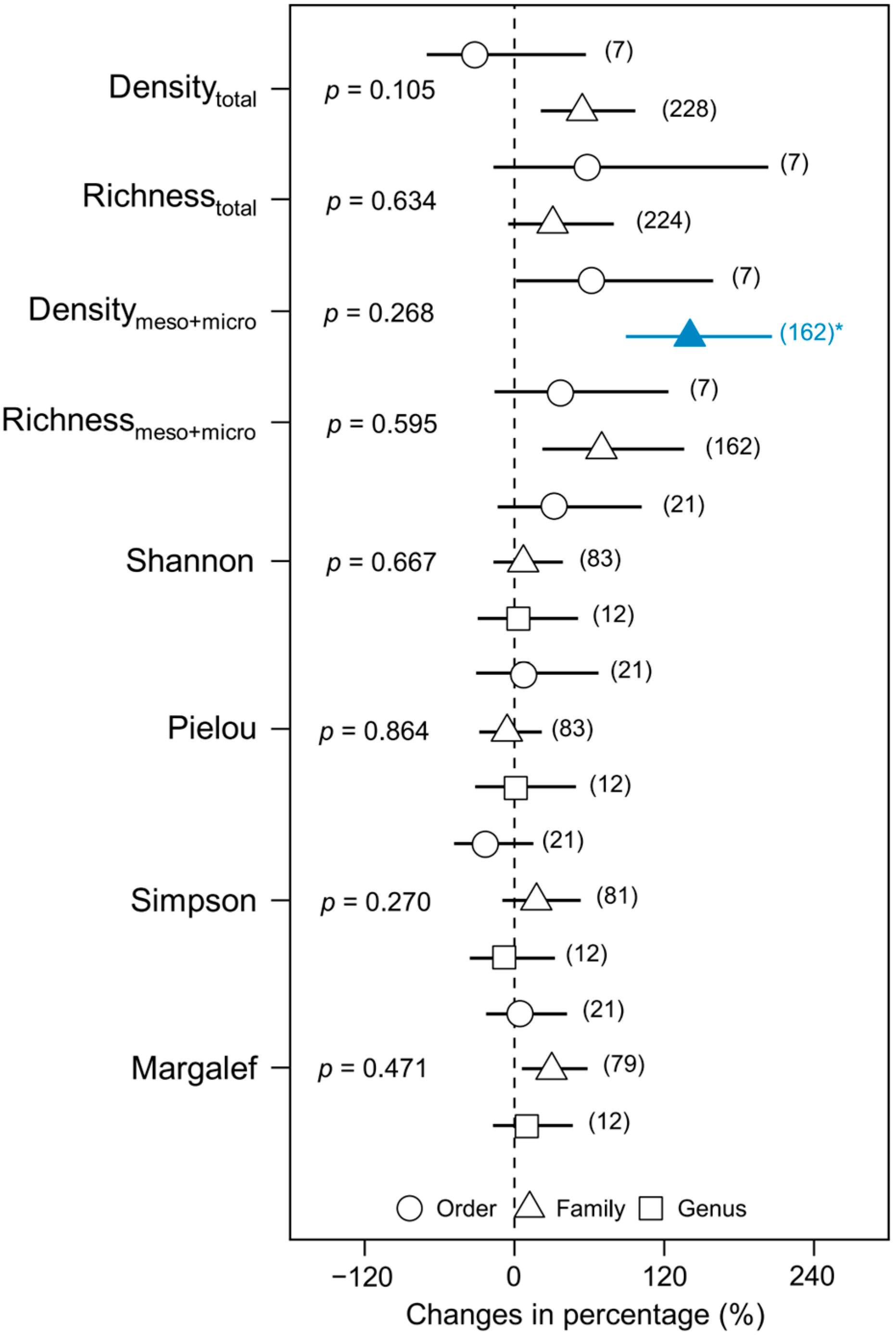
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.; Fang, J.; Houghton, R.; Kauppi, P.; Kurz, W.; Phillips, O.; Shvidenko, A.; Lewis, S.; Canadell, J.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Smith, P.; Cotrufo, M.; Rumpel, C.; Paustian, K.; Kuikman, P.; Elliott, J.; McDowell, R.; Griffiths, R.I.; Asakawa, S.; Bustamante, M.; et al. Biogeochemical cycles and biodiversity as key drivers of ecosystem services provided by soils. Soil 2015, 1, 665–685. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Seidl, R. Disturbances catalyze the adaptation of forest ecosystems to changing climate conditions. Glob. Change Biol. 2017, 23, 269–282. [Google Scholar] [CrossRef]
- Brice, M.; Vissault, S.; Vieira, W.; Gravel, D.; Legendre, P.; Fortin, M. Moderate disturbances accelerate forest transition dynamics under climate change in the temperate-boreal ecotone of eastern North America. Glob. Change Biol. 2020, 26, 4418–4435. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Valbuena, R.; Pinagé, E.; Mohan, M.; de Almeida, D.; Broadbent, E.N.; Jaafar, W.S.W.M.; Papa, D.d.A.; Cardil, A.; Klauberg, C. ForestGapR: An R Package for forest gap analysis from canopy height models. Methods Ecol. Evol. 2019, 10, 1347–1356. [Google Scholar] [CrossRef]
- Tong, R.; Ji, B.; Wang, G.; Lou, C.; Ma, C.; Zhu, N.; Yuan, W.; Wu, T. Canopy gap impacts on soil organic carbon and nutrient dynamic: A meta-analysis. Ann. For. Sci. 2024, 81, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Hedenec, P.; Yue, K.; Ni, X.; Wei, X.; Chen, Z.; Yang, J.; Wu, F. Global forest gaps reduce litterfall but increase litter carbon and phosphorus release. Commun. Earth Environ. 2024, 5, 288. [Google Scholar] [CrossRef]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Su, S.; Zheng, S.; Xu, D.; Wu, Z.; Hong, W.; Wang, J. Effects of Forest Gaps on Soil Properties in Castanopsis kawakamii nature forest. PLoS ONE 2015, 10, e0141203. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Liu, Z.; Cui, L.; Chen, P.; Cai, T.-G.; Li, G.; Ding, L.; Qiao, M.; Zhu, Y.; et al. Biological interactions mediate soil functions by altering rare microbial communities. Environ. Sci. Technol. 2024, 58, 5866–5877. [Google Scholar] [CrossRef]
- Coleman, D.; Geisen, S.; Wall, D. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. In Soil Microbiology, Ecology and Biochemistry, 5th ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 131–159. [Google Scholar]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.; Zellweger, F.; Aalto, J.; Ashcroft, M.; Christiansen, D.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef]
- Kneeshaw, D.; Harvey, B.; Reyes, G.; Caron, M.; Barlow, S. Spruce budworm, windthrow and partial cutting: Do different partial disturbances produce different forest structures? For. Ecol. Manag. 2011, 262, 482–490. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Zhang, D.; Zhang, J. The effects of gap size and litter species on colonization of soil fauna during litter decomposition in Pinus massoniana plantations. Appl. Soil Ecol. 2020, 155, 103611. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Yang, W.Q.; Wu, F.Z.; Xu, Z.F.; Tan, B.; Zhang, L.; He, X.H.; Guo, L. Canopy gaps accelerate soil organic carbon retention by soil microbial biomass in the organic horizon in a subalpine fir forest. Appl. Soil Ecol. 2018, 125, 169–176. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.; Hu, B.; Bao, W.; Tian, G. Does thinning-induced gap size result in altered soil microbial community in pine plantation in eastern Tibetan Plateau? Ecol. Evol. 2017, 7, 2986–2993. [Google Scholar] [CrossRef]
- Bolat, I. The effect of thinning on microbial biomass C, N and basal respiration in black pine forest soils in Mudurnu, Turkey. Eur. J. For. Res. 2014, 133, 131–139. [Google Scholar] [CrossRef]
- Scharenbroch, B.; Bockheim, J. Impacts of forest gaps on soil properties and processes in old growth northern hardwood-hemlock forests. Plant Soil 2007, 294, 219–233. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil macro-and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Siira-Pietikäinen, A.; Haimi, J. Changes in soil fauna 10 years after forest harvestings: Comparison between clear felling and green-tree retention methods. For. Ecol. Manag. 2009, 258, 332–338. [Google Scholar] [CrossRef]
- Schliemann, S.; Bockheim, J. Influence of gap size on carbon and nitrogen biogeochemical cycling in Northern hardwood forests of the Upper Peninsula, Michigan. Plant Soil 2014, 377, 323–335. [Google Scholar] [CrossRef]
- Xu, J.; Xue, L.; Su, Z. Impacts of forest gaps on soil properties after a severe ice storm in a Cunninghamia lanceolata stand. Pedosphere 2016, 26, 408–416. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, C.; Bai, Y.; Wang, H.; Jiang, C.; Huang, K.; Guo, L.; Zeng, S.; Wang, S. Effects of Forest Gap on Soil Microbial Communities in an Evergreen Broad-Leaved Secondary Forest. Forests 2022, 13, 2015. [Google Scholar] [CrossRef]
- Guo, X.; Endler, A.; Poll, C.; Marhan, S.; Ruess, L. Independent effects of warming and altered precipitation pattern on nematode community structure in an arable field. Agric. Ecosyst. Environ. 2021, 316, 107467. [Google Scholar] [CrossRef]
- Peng, Y.; Peñuelas, J.; Vesterdal, L.; Yue, K.; Peguero, G.; Fornara, D.; Heděnec, P.; Steffens, C.; Wu, F. Responses of soil fauna communities to the individual and combined effects of multiple global change factors. Ecol. Lett. 2022, 25, 1961–1973. [Google Scholar] [CrossRef]
- Yu, X.; Yang, L.; Fei, S.; Ma, Z.; Hao, R.; Zhao, Z. Effect of soil layer and plant–soil interaction on soil microbial diversity and function after canopy gap disturbance. Forests 2018, 9, 680. [Google Scholar] [CrossRef]
- Yue, K.; De Frenne, P.; Fornara, D.; Van Meerbeek, K.; Li, W.; Peng, X.; Ni, X.; Peng, Y.; Wu, F.; Yang, Y.; et al. Global patterns and drivers of rainfall partitioning by trees and shrubs. Glob. Change Biol. 2021, 27, 3350–3357. [Google Scholar] [CrossRef]
- Niu, F.; Pan, C.; Ma, L.; Cui, Y. Efficacies of vegetation litter and roots in strengthening rainfall infiltration for different stand ages on the Loess Plateau. Catena 2024, 247, 108502. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Lu, Y.; Chen, L.; Zhang, Z.; Zhang, H.; Wang, X.; Shu, L.; Yang, Q.; Song, Q.; et al. When microclimates meet soil microbes: Temperature controls soil microbial diversity along an elevational gradient in subtropical forests. Soil Biol. Biochem. 2022, 166, 108566. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hedges, L.; Gurevitch, J.; Curtis, P. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Lin, G.; McCormack, M.; Ma, C.; Guo, D. Similar below—Ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 2017, 213, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Yue, K.; De Frenne, P.; Van Meerbeek, K.; Ferreira, V.; Fornara, D.; Wu, Q.; Ni, X.; Peng, Y.; Wang, D.; Hedenec, P.; et al. Litter quality and stream physicochemical properties drive global invertebrate effects on instream litter decomposition. Biol. Rev. 2022, 97, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Vangansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Cernecká, L.; Mihál, I.; Gajdos, P.; Jarcuska, B. The effect of canopy openness of European beech (Fagus sylvatica) forests on ground-dwelling spider communities. Insect Conserv. Divers. 2020, 13, 250–261. [Google Scholar] [CrossRef]
- He, Z.; Wang, L.; Jiang, L.; Wang, Z.; Liu, J.; Xu, D.; Hong, W. Effect of Microenvironment on Species Distribution Patterns in the Regeneration Layer of Forest Gaps and Non-Gaps in a Subtropical Natural Forest, China. Forests 2019, 10, 90. [Google Scholar] [CrossRef]
- Tan, B.; Yin, R.; Zhang, J.; Xu, Z.; Liu, Y.; He, S.; Zhang, L.; Li, H.; Wang, L.; Liu, S.; et al. Temperature and moisture modulate the contribution of soil fauna to litter decomposition via different pathways. Ecosystems 2021, 24, 1142–1156. [Google Scholar] [CrossRef]
- Aupic-Samain, A.; Baldy, V.; Delcourt, N.; Krogh, P.; Gauquelin, T.; Fernandez, C.; Santonja, M. Water availability rather than temperature control soil fauna community structure and prey-predator interactions. Funct. Ecol. 2021, 35, 1550–1559. [Google Scholar] [CrossRef]
- Peng, Y.; Holmstrup, M.; Schmidt, I.; De Schrijver, A.; Schelfhout, S.; Heděnec, P.; Zheng, H.; Bachega, L.; Yue, K.; Vesterdal, L. Litter quality, mycorrhizal association, and soil properties regulate effects of tree species on the soil fauna community. Geoderma 2022, 407, 115570. [Google Scholar] [CrossRef]
- Dias, N.; Hassall, M.; Waite, T. The influence of microclimate on foraging and sheltering behaviours of terrestrial isopods: Implications for soil carbon dynamics under climate change. Pedobiologia 2012, 55, 137–144. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Wang, Z.; Xing, C.; Chen, B.; Wang, X.; Wei, C.; Liu, J.; He, Z.; Xu, D. Canopy gaps control litter decomposition and nutrient release in subtropical forests. Forests 2023, 14, 673. [Google Scholar] [CrossRef]
- Mueller, K.; Blumenthal, D.; Carrillo, Y.; Cesarz, S.; Ciobanu, M.; Hines, J.; Pabst, S.; Pendall, E.; Tomasel, C.; Wall, D.; et al. Elevated CO2 and warming shift the functional composition of soil nematode communities in a semiarid grassland. Soil Biol. Biochem. 2016, 103, 46–51. [Google Scholar] [CrossRef]
- Bach, L.; Grytnes, J.; Halvorsen, R.; Ohlson, M. Tree influence on soil microbial community structure. Soil Biol. Biochem. 2010, 42, 1934–1943. [Google Scholar] [CrossRef]
- Olatunji, O.; Gong, S.; Tariq, A.; Pan, K.; Sun, X.; Chen, W.; Zhang, L.; Dakhil, M.; Huang, D.; Tan, X. The effect of phosphorus addition, soil moisture, and plant type on soil nematode abundance and community composition. J. Soils Sediments 2019, 19, 1139–1150. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, W.; Zhang, J.; Xu, Z.; Zhang, L.; Liu, Y.; Li, H.; You, C.; Tan, B. Forest Gap Size Alters the Functional Diversity of Soil Nematode Communities in Alpine Forest Ecosystems. Forests 2019, 10, 806. [Google Scholar] [CrossRef]
- Ren, S.; Ali, A.; Liu, H.; Yuan, Z.; Yang, Q.; Shen, G.; Zhou, S.; Wang, X. Response of community diversity and productivity to canopy gap disturbance in subtropical forests. For. Ecol. Manag. 2021, 502, 119740. [Google Scholar] [CrossRef]
- Goncharov, A.; Leonov, V.; Rozanova, O.; Semenina, E.; Tsurikov, S.; Uvarov, A.; Zuev, A.; Tiunov, A. A meta-analysis suggests climate change shifts structure of regional communities of soil invertebrates. Soil Biol. Biochem. 2023, 181, 109014. [Google Scholar] [CrossRef]
- Johnston, A.; Sibly, R. Multiple environmental controls explain global patterns in soil animal communities. Oecologia 2020, 192, 1047–1056. [Google Scholar] [CrossRef]
- Fang, X.; Wang, G.; Xu, Z.; Zong, Y.; Zhang, X.; Li, J.; Wang, H.; Chen, F. Litter addition and understory removal influenced soil organic carbon quality and mineral nitrogen supply in a subtropical plantation forest. Plant Soil 2021, 460, 527–540. [Google Scholar] [CrossRef]
- Meehan, M.; Barreto, C.; Turnbull, M.; Bradley, R.; Bellenger, J.; Darnajoux, R.; Lindo, Z. Response of soil fauna to simulated global change factors depends on ambient climate conditions. Pedobiologia 2020, 83, 150672. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, W.; Chen, L.; Ouyang, S.; Xiao, W.; Li, S.; Forrester, D.; Lei, P.; Zeng, Y.; Deng, X.; et al. Soil phosphorus bioavailability and recycling increased with stand age in Chinese fir plantations. Ecosystems 2020, 23, 973–988. [Google Scholar] [CrossRef]
- Joshi, S.; Tfaily, M.; Young, R.; McNear Jr, D. Root exudates induced coupled carbon and phosphorus cycling in a soil with low phosphorus availability. Plant Soil 2024, 498, 371–390. [Google Scholar] [CrossRef]
- Ganault, P.; Nahmani, J.; Hättenschwiler, S.; Gillespie, L.; David, J.; Henneron, L.; Iorio, E.; Mazzia, C.; Muys, B.; Pasquet, A.; et al. Relative importance of tree species richness, tree functional type, and microenvironment for soil macrofauna communities in European forests. Oecologia 2021, 196, 455–468. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, C.; Wu, Q.; Hedenec, P.; Zhao, Z.; Yue, K.; Ni, X.; Wu, F.; Peng, Y. Effects of transforming multiple ecosystem types to tree plantations on soil microbial biomass carbon, nitrogen, phosphorus and their ratios in China. Appl. Soil Ecol. 2024, 193, 105145. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Han, W.; Fang, J. Global patterns of soil microbial nitrogen and phosphorus stoichiometry in forest ecosystems. Glob. Ecol. Biogeogr. 2014, 23, 979–987. [Google Scholar] [CrossRef]
- Balandier, P.; Collet, C.; Miller, J.; Reynolds, P.; Zedaker, S. Designing forest vegetation management strategies based on the mechanisms and dynamics of crop tree competition by neighbouring vegetation. Forestry 2006, 79, 3–27. [Google Scholar] [CrossRef]
| Predict Variables | Latitude (°) | Elevation (m) | Stand Age (yr) | MAT (°C) | MAP (mm) | Gap Size (m2) | Soil Temp (°C) | Soil pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | n | Estimate | n | Estimate | n | Estimate | n | Estimate | n | Estimate | n | Estimate | n | Estimate | n | |
| Densitytotal | 0.01 | 235 | 0.001 | 235 | 0.005 | 46 | 0.017 | 235 | 0.001 | 235 | 0.01 ** | 208 | ||||
| Richnesstotal | 0.01 | 231 | 0.001 | 231 | 0.01 | 42 | 0.017 | 231 | 0.001 | 231 | 0.001 *** | 204 | ||||
| Densitymeso+micro | 0.027 * | 169 | 0.002 * | 169 | 0.019 * | 169 | 0.042 * | 169 | 0.001 * | 169 | 0.001 ** | 154 | 0.18 | 154 | ||
| Richnessmeso+micro | 0.016 | 169 | 0.001 | 169 | 0.011 | 169 | 0.025 | 169 | 0.001 | 169 | 0.001 | 154 | 0.086 *** | 154 | ||
| Shannon | 0.004 | 124 | 0.001 | 121 | 0.001 | 69 | 0.007 | 124 | 0.001 | 124 | −0.001 | 116 | 0.021 | 9 | 0.024 | 55 |
| Pielou | −0.001 | 124 | 0.001 | 121 | 0.001 | 69 | −0.001 | 124 | 0.001 | 124 | −0.001 | 116 | 0.011 | 9 | −0.001 | 55 |
| Simpson | 0.001 | 122 | −0.001 | 119 | −0.002 | 69 | 0.002 | 122 | −0.001 | 122 | 0.001 | 114 | 0.005 | 7 | −0.001 | 55 |
| Margalef | 0.008 * | 120 | 0.001 | 117 | 0.002 | 69 | 0.012 | 120 | 0.001 * | 120 | 0.001 | 112 | 0.005 | 5 | 0.056 | 51 |
| MBC (mg/kg) | −0.001 | 1136 | 0.001 | 1114 | 0.002 | 672 | −0.003 | 1136 | −0.001 | 1133 | −0.001 *** | 754 | 0.001 | 150 | −0.002 | 552 |
| MBN (mg/kg) | 0.001 | 971 | 0.001 | 967 | −0.001 | 573 | −0.005 | 971 | −0.001 | 971 | −0.001 * | 711 | −0.002 | 147 | 0.001 | 554 |
| MBP (mg/kg) | 0.004 | 281 | 0.001 | 281 | −0.002 *** | 127 | 0.009 | 281 | 0.001 | 281 | 0.001 | 196 | 0.02 | 98 | ||
| Bacteria (CFU/g) | −0.019 | 125 | −0.001 | 125 | −0.004 | 92 | −0.054 | 125 | −0.001 | 125 | −0.002 | 17 | −0.014 | 17 | ||
| Fungi (CFU/g) | 0.031 | 124 | 0.001 | 124 | −0.003 | 92 | −0.005 | 124 | 0.001 | 124 | 0.066 | 17 | −0.135 | 17 | ||
| Actinomycete (CFU/g) | −0.001 | 125 | −0.001 | 125 | −0.003 | 92 | −0.003 | 125 | −0.001 | 125 | −0.026 | 17 | −0.036 | 17 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wu, F.; Li, C.; Wu, Q.; Yue, K.; Heděnec, P.; Chen, D.; An, N.; Peng, Y. Divergent Effects of Forest Canopy Opening on Soil Fauna and Microbes: A Global Meta-Analysis. Forests 2025, 16, 1749. https://doi.org/10.3390/f16111749
Wu S, Wu F, Li C, Wu Q, Yue K, Heděnec P, Chen D, An N, Peng Y. Divergent Effects of Forest Canopy Opening on Soil Fauna and Microbes: A Global Meta-Analysis. Forests. 2025; 16(11):1749. https://doi.org/10.3390/f16111749
Chicago/Turabian StyleWu, Shuai, Fuzhong Wu, Cuihuan Li, Qiqian Wu, Kai Yue, Petr Heděnec, Dixin Chen, Nannan An, and Yan Peng. 2025. "Divergent Effects of Forest Canopy Opening on Soil Fauna and Microbes: A Global Meta-Analysis" Forests 16, no. 11: 1749. https://doi.org/10.3390/f16111749
APA StyleWu, S., Wu, F., Li, C., Wu, Q., Yue, K., Heděnec, P., Chen, D., An, N., & Peng, Y. (2025). Divergent Effects of Forest Canopy Opening on Soil Fauna and Microbes: A Global Meta-Analysis. Forests, 16(11), 1749. https://doi.org/10.3390/f16111749








