Screening and Identification of Drought-Sensitive and Drought-Tolerant Poplar Germplasm Based on Short-Term Physiological and Biochemical Differences
Abstract
1. Introduction
2. Results
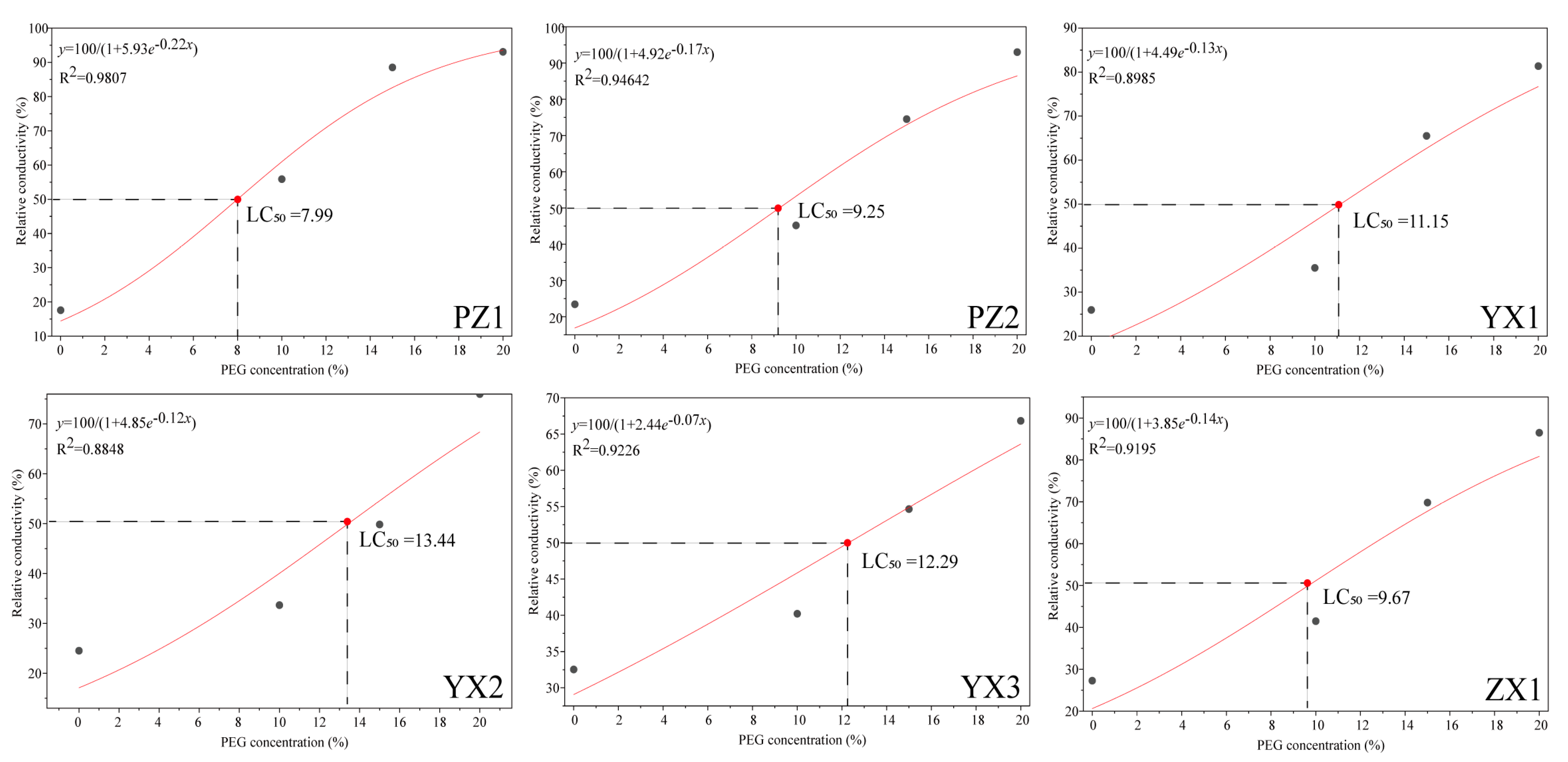
2.1. Changes in REC in Different Poplar Varieties Under PEG-Induced Drought Stress
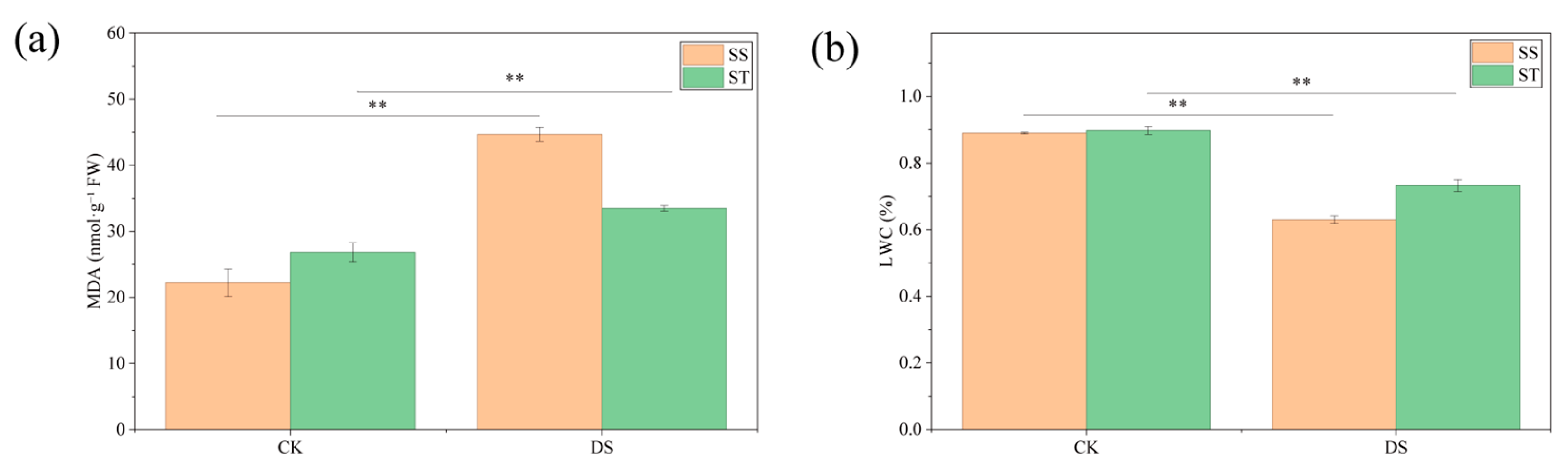
2.2. Changes in MDA and LWC of Poplar Under PEG-Induced Drought Stress
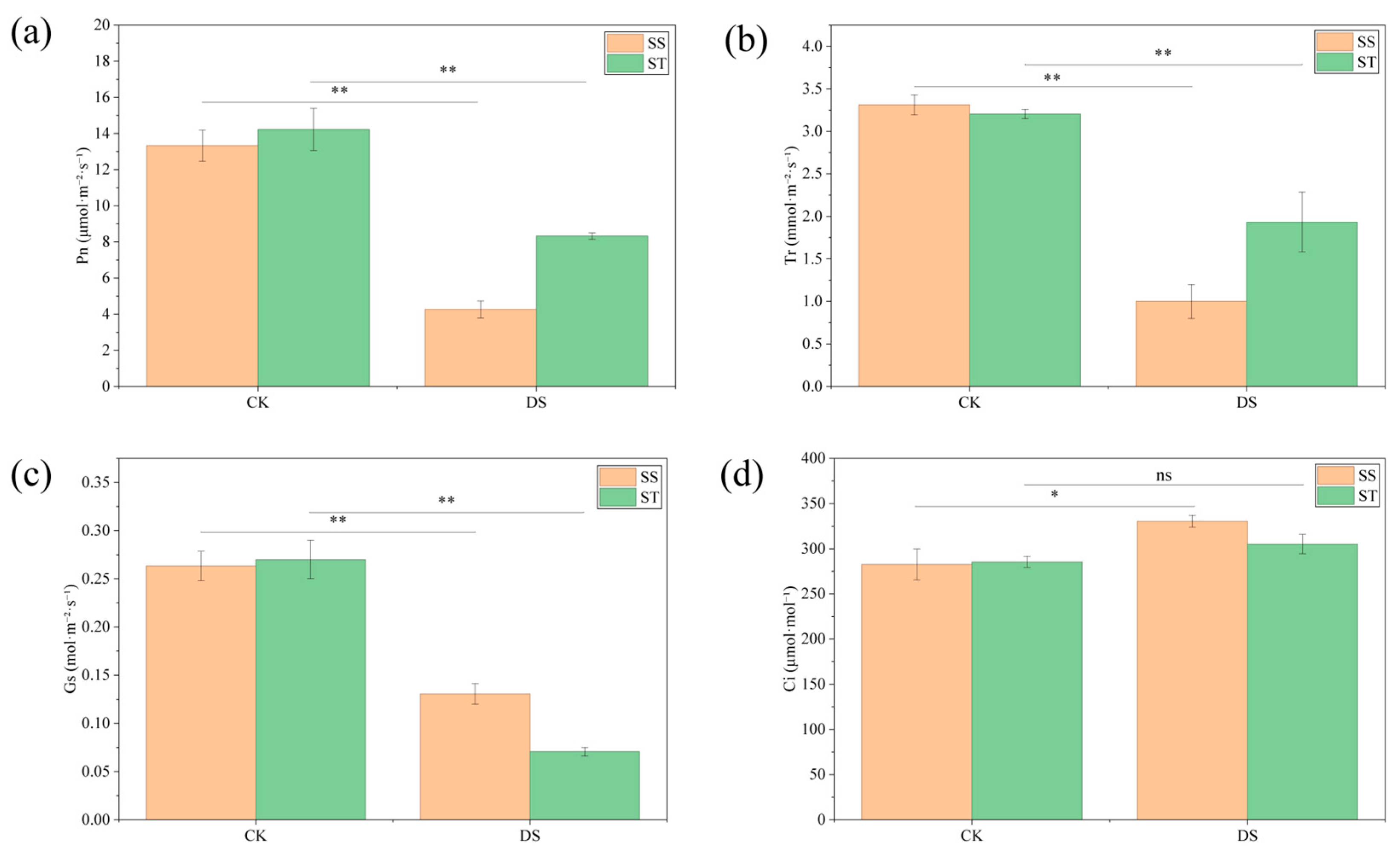
2.3. Changes in Photosynthetic Parameters of Poplar Under PEG-Induced Drought Stress
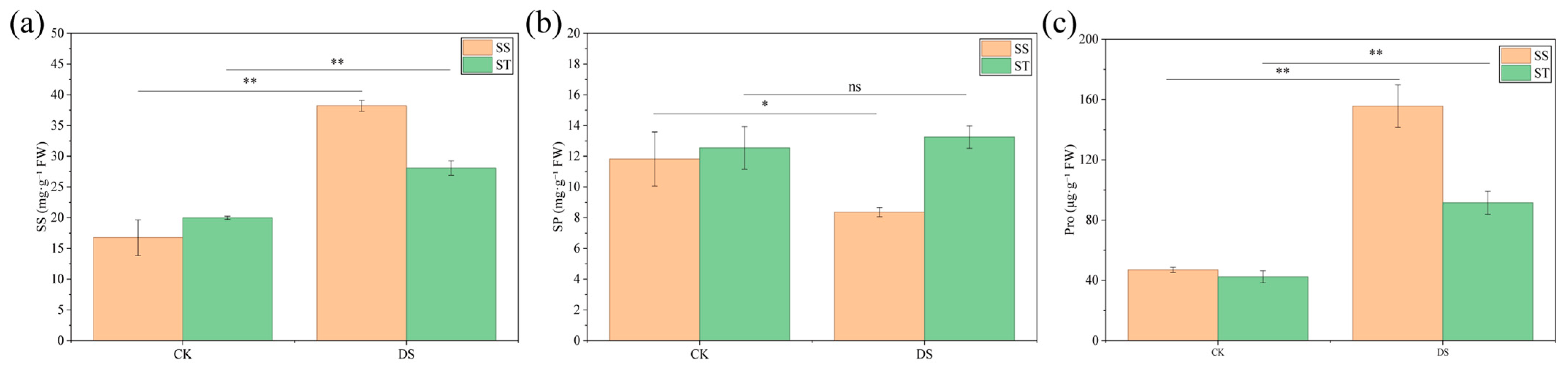
2.4. Changes in Osmolyte Content of Poplar Under PEG-Induced Drought Stress
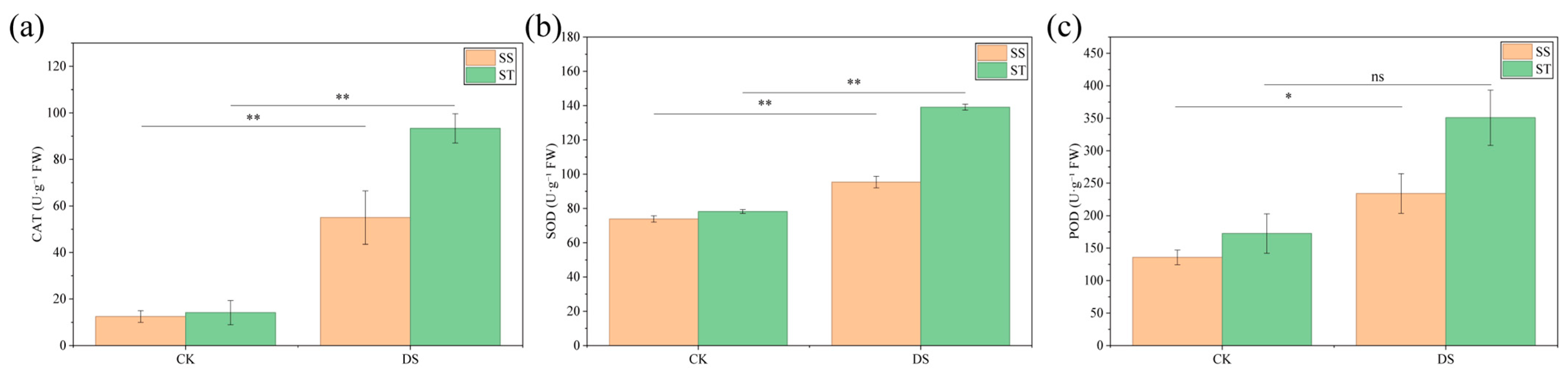
2.5. Changes in Antioxidant Enzyme Activities of Poplar Under PEG-Induced Drought Stress
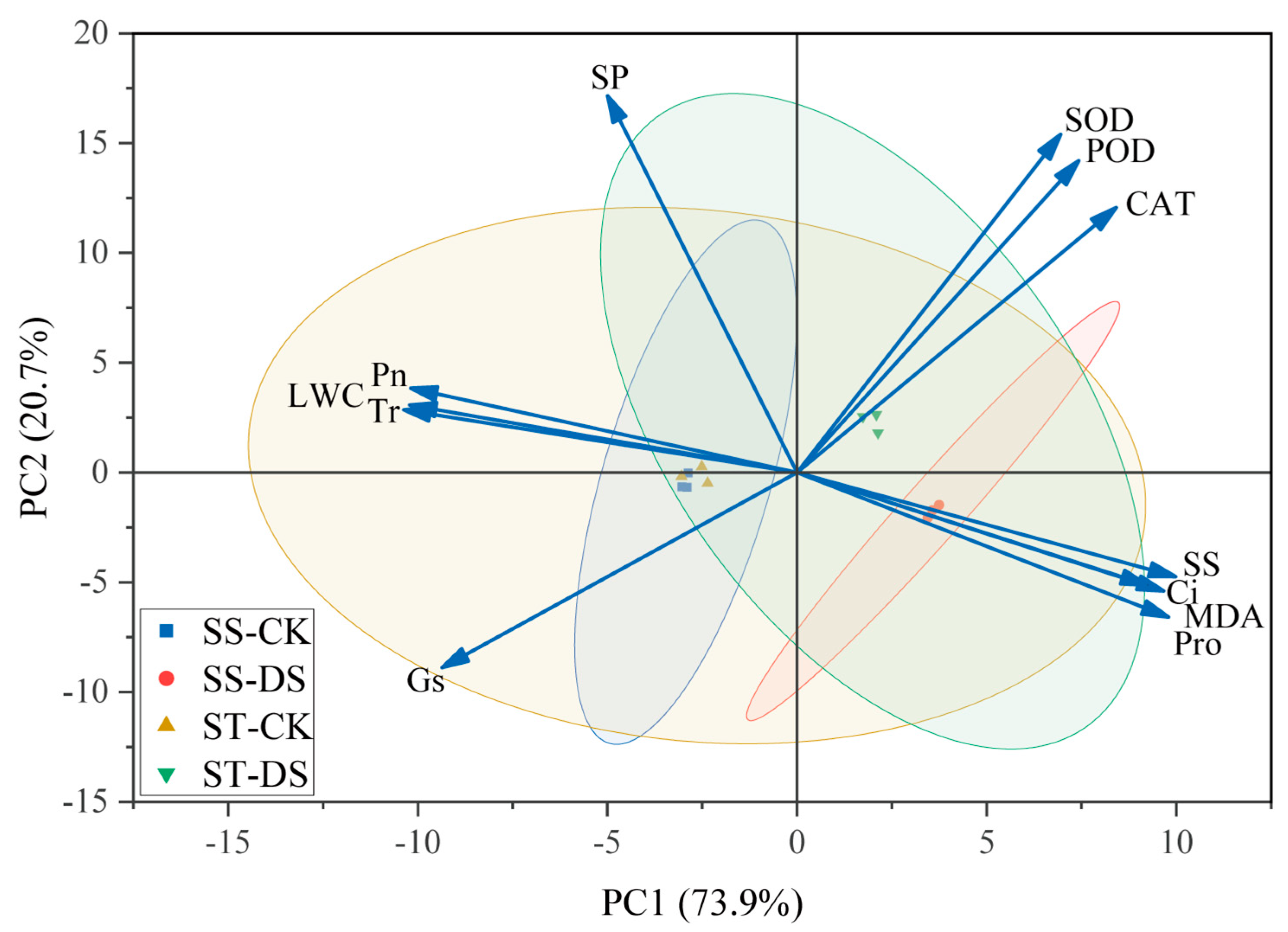
2.6. Principal Component Analysis of Indicators
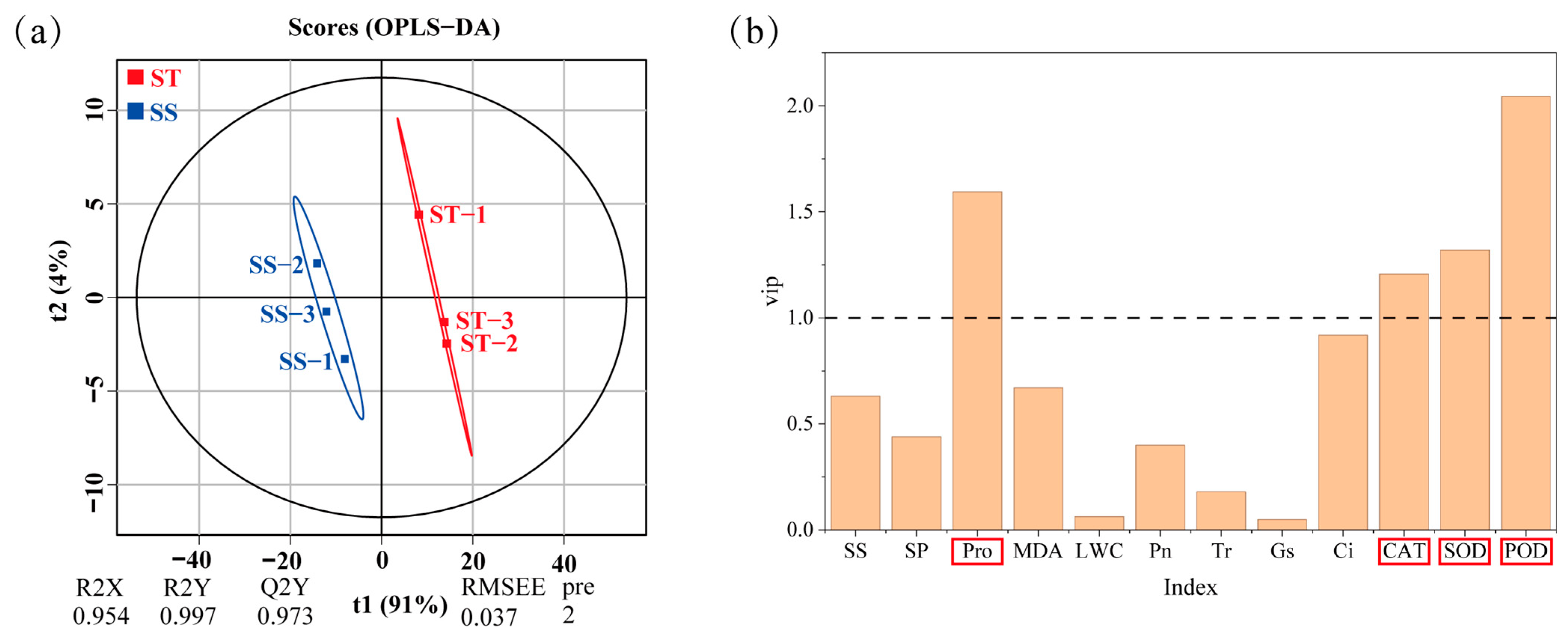
2.7. OPLS-DA Profiling of Indicators
3. Discussion
3.1. Differential Responses of Relative Water Content and Photosynthesis Between SS and ST Poplar Cultivars Under Drought Stress
3.2. Differentia Responses of Osmolyte Accumulation Between SS and ST Poplar Cultivars Under Drought Stress
3.3. Differential Antioxidant Enzyme Activities Between SS and ST Poplar Cultivars Under Drought Stress
3.4. Screening of Key Physiological Indicators in Poplar Responding to Drought Stress
4. Materials and Methods
4.1. Experimental Materials and Pre-Treatment
4.2. Experimental Design and Sampling
4.3. Experimental Indicators and Measurement Methods
4.3.1. REC and LC50 Determination
4.3.2. Physiochemical Factors
4.3.3. Key Physiological Indicators Screening
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Lu, G.; Long, W.; Zou, X.; Li, F.; Nishio, T. Recent progress in drought and salt tolerance studies in Brassica crops. Breed. Sci. 2014, 64, 60–73. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhou, J.; Quan, M.; Xiao, L.; Lu, W.; Qin, S.; Fang, Y.; Wang, D.; Li, P.; Du, Q.; et al. Transcriptome and association mapping revealed functional genes respond to drought stress in Populus. Front. Plant Sci. 2022, 13, 829888. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, X.; Li, H.; Guo, L.; Lv, T.; Wu, P. Exotic shrub species (Caragana korshinskii) is more resistant to extreme natural drought than native species (Artemisia gmelinii) in a semiarid revegetated ecosystem. Agric. For. Meteorol. 2018, 263, 207–216. [Google Scholar] [CrossRef]
- Kar, R.K. Plant responses to water stress: Role of reactive oxygen species. Plant Signal. Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Wang, X.; Ge, J.; He, M.; Li, Q.; Cai, J.; Zhou, Q.; Zhong, Y.; Wollenweber, B.; Jiang, D. Enhancing crop resilience: Understanding the role of drought priming in wheat stress response. Field Crops Res. 2023, 302, 109083. [Google Scholar] [CrossRef]
- Emami Bistgani, Z.; Barker, A.V.; Hashemi, M. Physiology of medicinal and aromatic plants under drought stress. Crop J. 2024, 12, 330–339. [Google Scholar] [CrossRef]
- Aalam, F.; Rezaei Nejad, A.; Mousavi-Fard, S.; Raji, M.; Nikoloudakis, N.; Goumenaki, E.; Fanourakis, D. Water Deficit Severity during the Preceding Year Determines Plant Tolerance to Subsequent Year Drought Stress Challenges: A Case Study in Damask Rose. Horticulturae 2024, 10, 462. [Google Scholar] [CrossRef]
- Sajad, A.; Rakeeb, A.M.; Md Azizul, H.; Danishuddin; Mohammed, A.A.; Mohammad, A.; Ashraf, K.; Henda, M.; Mohammad, S.; Anshika, T.; et al. Exploring physiological and molecular dynamics of drought stress responses in plants: Challenges and future directions. Front. Plant Sci. 2025, 16, 1565635. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.L.; Lim, H.; Denison, M.I.J.; Oh, C. Transcriptomic and Physiological Analysis Reveals Genes Associated with Drought Stress Responses in Populus alba × Populus glandulosa. Plants 2023, 12, 3238. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tahir, S.; Hassan, S.S.; Lu, M.; Wang, X.; Quyen, L.T.Q.; Zhang, W.; Chen, S. The Role of Phytohormones in Mediating Drought Stress Responses in Populus Species. Int. J. Mol. Sci. 2025, 26, 3884. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhao, Q.Y.; Ma, C.L.; Zhang, Z.H.; Cao, H.L.; Kong, Y.M.; Yue, C.; Hao, X.Y.; Chen, L.; Ma, J.Q.; et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef]
- Yang, W.-H.; Yong, S.-H.; Park, D.-J.; Ahn, S.-J.; Kim, D.-H.; Park, K.-B.; Jin, E.-J.; Choi, M.-S. Efficient Cold Tolerance Evaluation of Four Species of Liliaceae Plants through Cell Death Measurement and Lethal Temperature Prediction. Horticulturae 2023, 9, 751. [Google Scholar] [CrossRef]
- Shreyas, R.; Narasimhan, S. PEG-induced Drought Stress in Plants: A Review. Res. J. Pharm. Technol. 2021, 14, 6173–6178. [Google Scholar] [CrossRef]
- Tang, D.F.; Wei, F.; Qin, S.X.; Khan, A.; Kashif, M.H.; Zhou, R.Y. Polyethylene glycol induced drought stress strongly influences seed germination, root morphology and cytoplasm of different kenaf genotypes. Ind. Crops Prod. 2019, 137, 180–186. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Liu, Y.; Dai, C.; Zhang, Y.; Zhou, F.; Zhu, Y. Salicylic Acid Modulates the Osmotic System and Photosynthesis Rate to Enhance the Drought Tolerance of Toona ciliata. Plants 2023, 12, 4187. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Stockle, C. Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Singapore, 2017; ISBN 978-981-10-8247-8. [Google Scholar]
- Ma, P.; Bai, T.-H.; Ma, F.-W. Effects of progressive drought on photosynthesis and partitioning of absorbed light in apple trees. J. Integr. Agric. 2015, 14, 681–690. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C(3) plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ali, V.; Ali, Y.; Mehdi, Y. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Biophys. Struct. Biol. 2010, 6, 4–16. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; Van Montagu, M.; Inzé, D.; Van Camp, W. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Galindo-Prieto, B.; Eriksson, L.; Trygg, J. Variable influence on projection (VIP) for OPLS models and its applicability in multivariate time series analysis. Chemom. Intell. Lab. Syst. 2015, 146, 297–304. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Z. Effects of Drought Stress and Postdrought Rewatering on Winter Wheat: A Meta-Analysis. Agronomy 2024, 14, 298. [Google Scholar] [CrossRef]
- Secchi, F.; Pagliarani, C.; Zwieniecki, M. The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ. 2017, 40, 858–871. [Google Scholar] [CrossRef]
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Gennaro, M.; Nervo, G.; Secchi, F.; Carra, A. Responses to drought stress in poplar: What do we know and what can we learn? Life 2023, 13, 533. [Google Scholar] [CrossRef]
- Masud, M.; Matin, M.; Mst, F.; Ahamed, M.; Chowdhury, M.R.; Paul, S.; Karmakar, S.; Kang, S.G.; Hossain, M. Screening for drought tolerance and diversity analysis of Bangladeshi rice germplasms using morphophysiology and molecular markers. Biologia 2021, 77, 21–37. [Google Scholar] [CrossRef]
- Li, P.; Lin, P.P.; Zhao, Z.L.; Li, Z.H.; Liu, Y.M.; Huang, C.H.; Huang, G.Q.; Xu, L.N.; Deng, Z.H.; Zhang, Y.; et al. Gene Co-Expression Analysis Reveals Transcriptome Divergence between Wild and Cultivated Sugarcane under Drought Stress. Int. J. Mol. Sci. 2022, 23, 569. [Google Scholar] [CrossRef]
- Li, W.L.; Liu, Z.W.; Feng, H.; Yang, J.L.; Li, C.H. Characterization of the Gene Expression Profile Response to Drought Stress in Populus ussuriensis Using PacBio SMRT and Illumina Sequencing. Int. J. Mol. Sci. 2022, 23, 3840. [Google Scholar] [CrossRef]
- Dexter, S.T.; Tottingham, W.E.; Graber, L.F. Preliminary Results in Measuring the Hardiness of Plants. Plant Physiol. 1930, 5, 215–223. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Wang, Y.; Jiang, A.; Meng, F.; Wang, B.; Chen, M. Integrated physio-biochemical and transcriptomic analyses reveal the mechanism underlying ABA-mediated alleviation of salt stress in Limonium bicolor seedlings. Environ. Exp. Bot. 2024, 220, 105707. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na(+) uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Tang, L.; Zuo, Z.; Fan, W.; Yang, H.; Zhou, Q. Screening and Identification of Drought-Sensitive and Drought-Tolerant Poplar Germplasm Based on Short-Term Physiological and Biochemical Differences. Forests 2025, 16, 1750. https://doi.org/10.3390/f16111750
Fan L, Tang L, Zuo Z, Fan W, Yang H, Zhou Q. Screening and Identification of Drought-Sensitive and Drought-Tolerant Poplar Germplasm Based on Short-Term Physiological and Biochemical Differences. Forests. 2025; 16(11):1750. https://doi.org/10.3390/f16111750
Chicago/Turabian StyleFan, Lili, Luozhong Tang, Zheng Zuo, Wei Fan, Haiqing Yang, and Qi Zhou. 2025. "Screening and Identification of Drought-Sensitive and Drought-Tolerant Poplar Germplasm Based on Short-Term Physiological and Biochemical Differences" Forests 16, no. 11: 1750. https://doi.org/10.3390/f16111750
APA StyleFan, L., Tang, L., Zuo, Z., Fan, W., Yang, H., & Zhou, Q. (2025). Screening and Identification of Drought-Sensitive and Drought-Tolerant Poplar Germplasm Based on Short-Term Physiological and Biochemical Differences. Forests, 16(11), 1750. https://doi.org/10.3390/f16111750




