The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Inhibitor Characterization
2.2. Conversion Coating Characterization
3. Results and Discussion
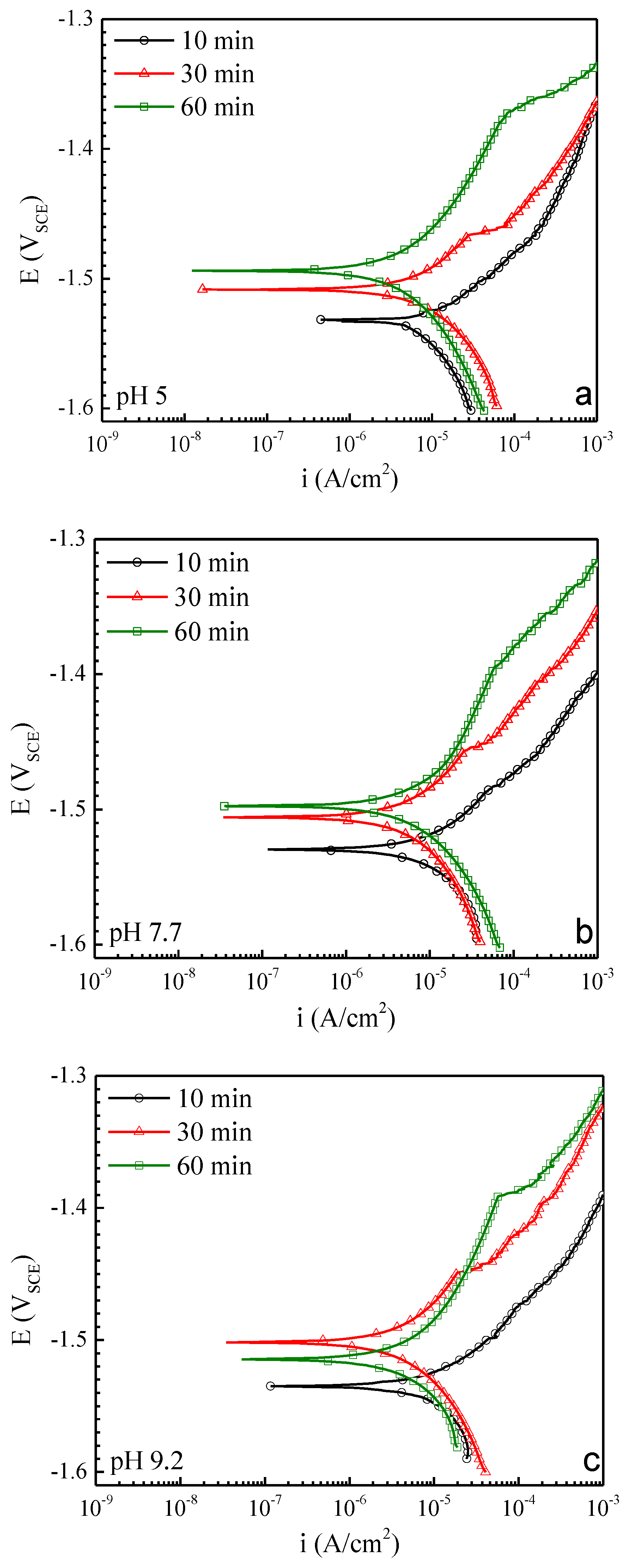
3.1. Characterization of AZ31 Response in Chloride-Only Solutions
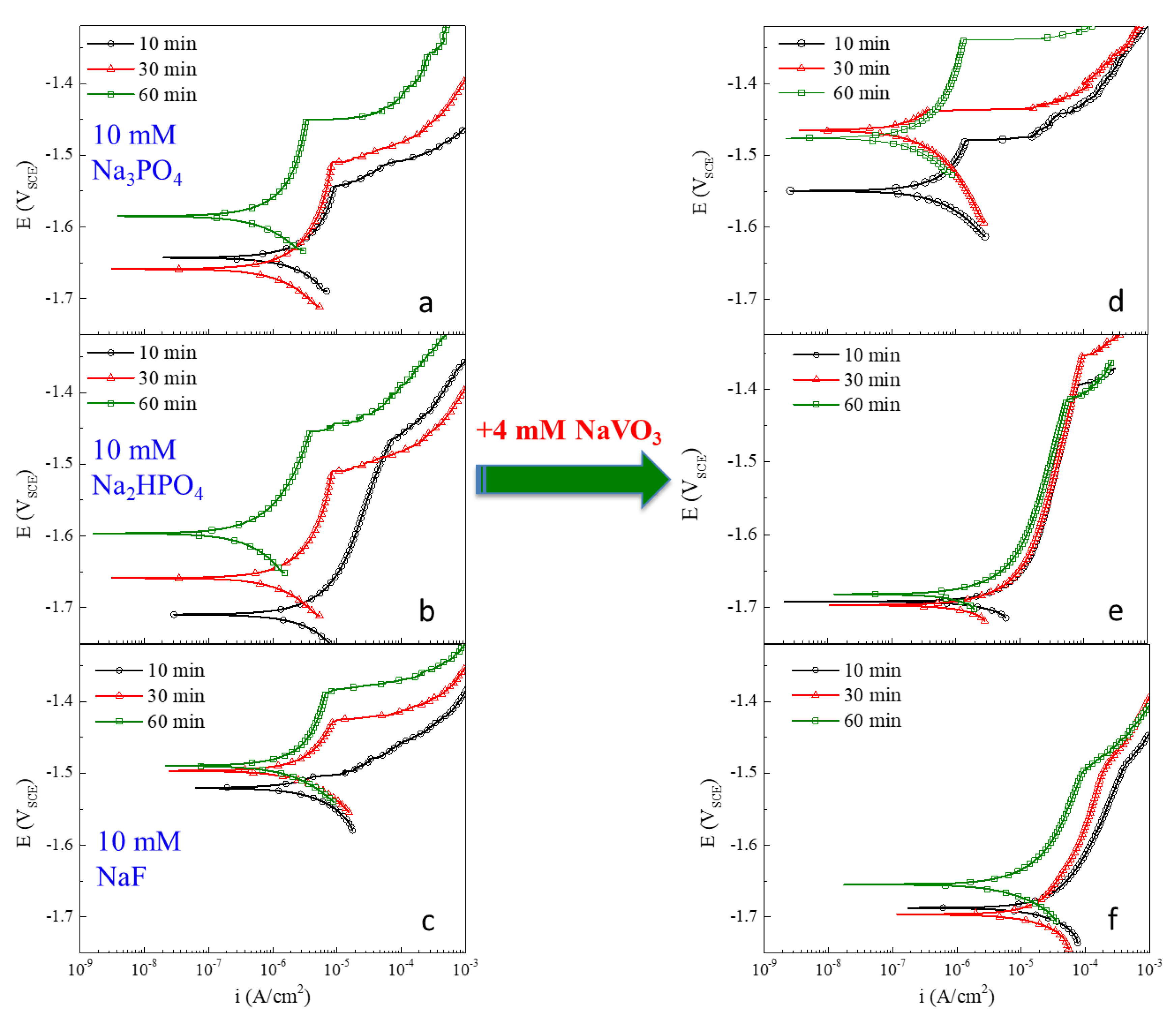
3.2. Inhibitor Characterization of AZ31 Polarization Response in Chloride-Only Solutions in Single and Mixed Inhibitors
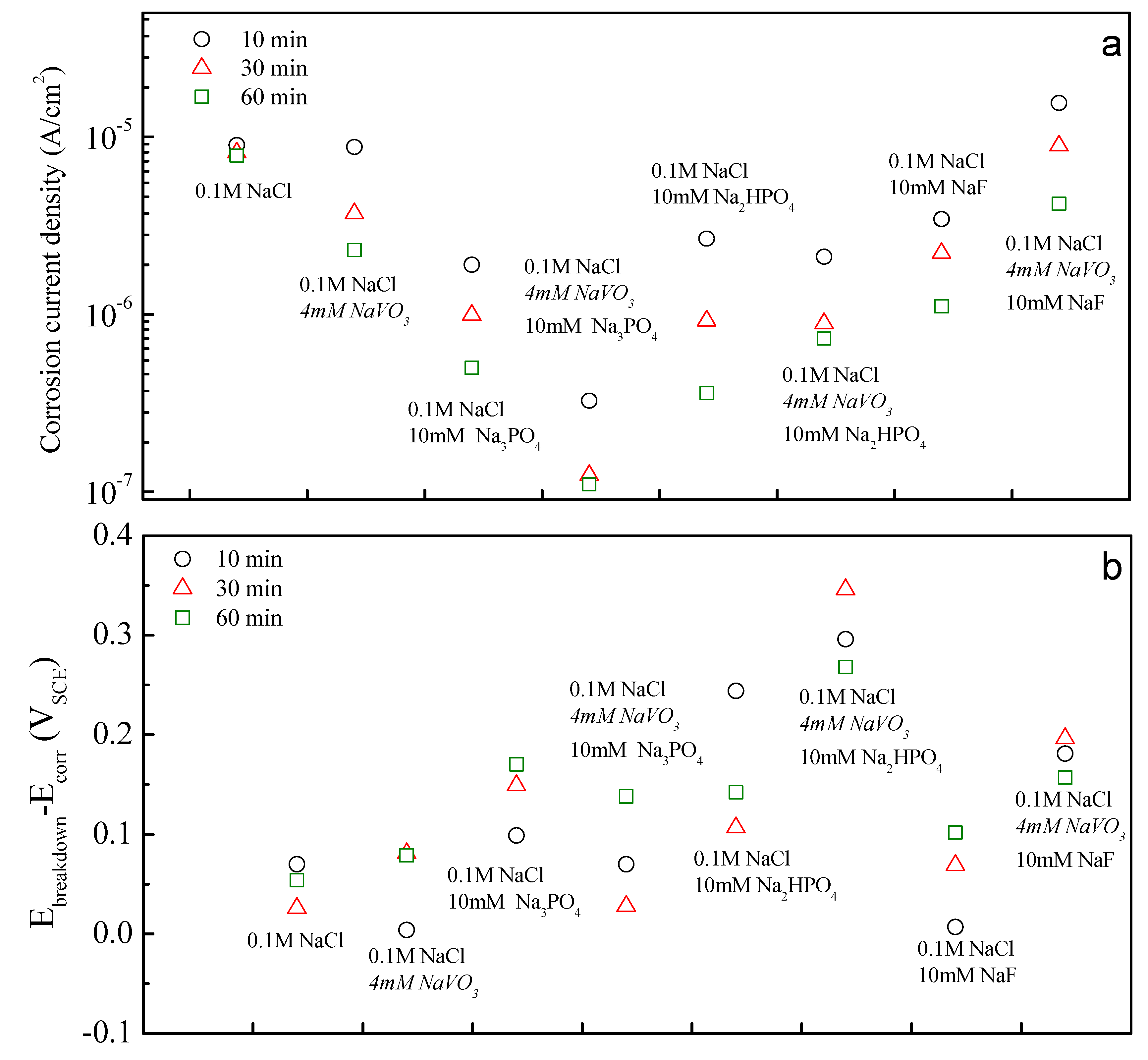
3.3. Assessing the Strength of Inhibitor Pair Interactions
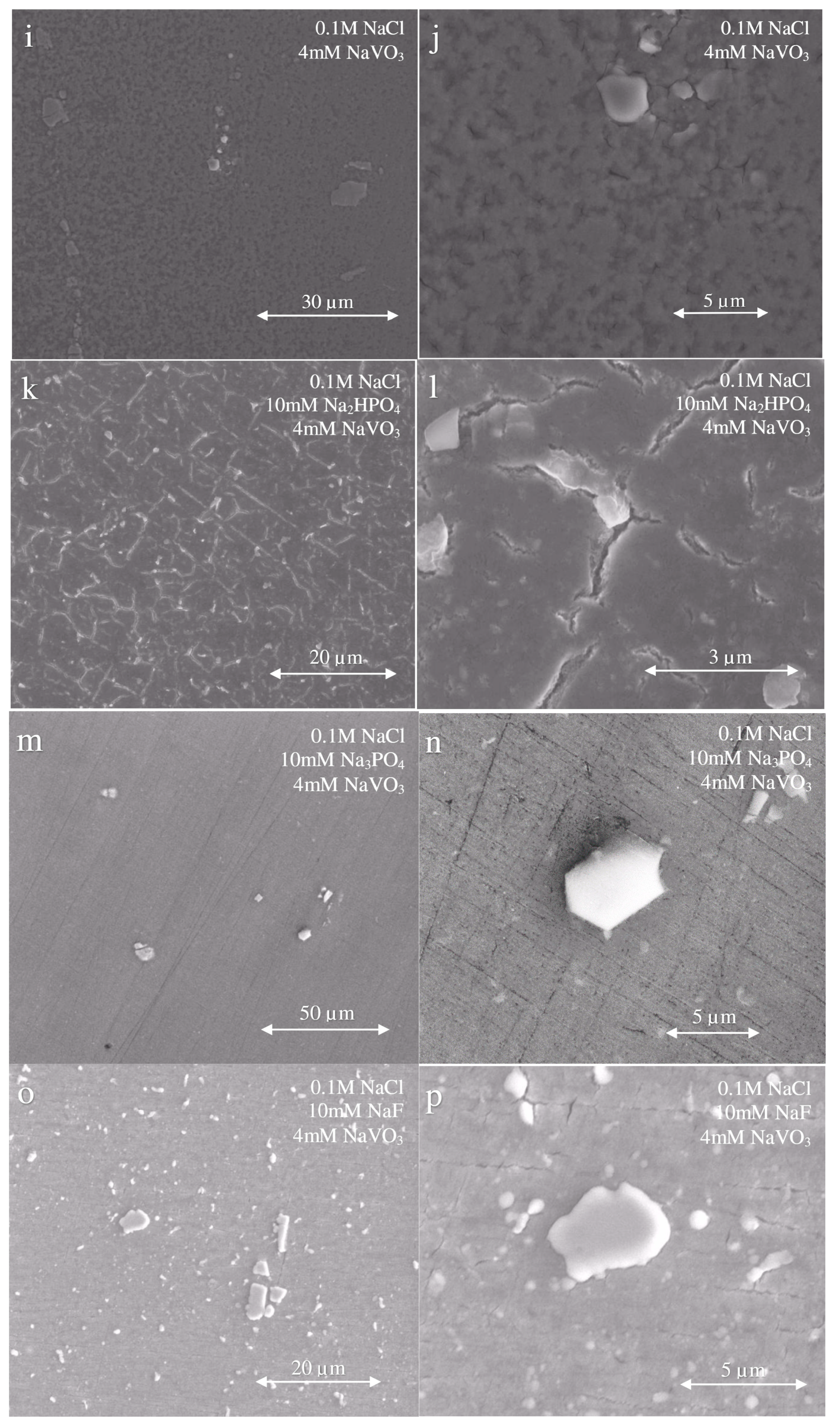
3.4. Post-Exposure Surface Morphology
3.5. Conceptual Conversion Coatings for AZ31
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Feng, Z. Corrosion inhibition study of AZ31 Mg alloy by Vanadate, Selenite and Phosphate. Doctor’s Thesis, The Ohio State University, Columbus, OH, USA, 2019. [Google Scholar]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Review of corrosion-resistant conversion coatings for magnesium and its alloys. Corrosion 2011, 67, 035005-1–035005-16. [Google Scholar] [CrossRef]
- Esmaily, M.; Mortazavi, N.; Shahabi-Navid, M.; Svensson, J.E.; Johansson, L.G.; Halvarsson, M. On the capability of in-situ exposure in an environmental scanning electron microscope for investigating the atmospheric corrosion of magnesium. Ultramicroscopy 2015, 153, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Birbilis, N.; Sha, G.; Wang, Y.; Daniels, J.E.; Xiao, Y.; Ferry, M. A high-specific-strength and corrosion-resistant magnesium alloy. Nat. Mater. 2015, 14, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Lamaka, S.V.; Knörnschild, G.; Snihirova, D.V.; Taryba, M.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C.; Wang, F.; Lou, H.; Cao, C. Study on the environmentally friendly anodizing of AZ91D magnesium alloy. Surf. Coat. Technol. 2002, 161, 36–43. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Yi, G. Electrochemical measurement, modeling, and prediction of corrosion inhibition efficiency of ternary mixtures of homologous surfactants in salt solution. Corros. Sci. 2015, 98, 417–429. [Google Scholar] [CrossRef]
- Zhu, Y.; Free, M.L.; Yi, G. The effects of surfactant concentration, adsorption, aggregation, and solution conditions on steel corrosion inhibition and associated modeling in aqueous media. Corros. Sci. 2016, 102, 233–250. [Google Scholar] [CrossRef]
- Zhang, W.; Hurley, B.; Buchheit, R.G. Characterization of chromate conversion coating formation and breakdown using electrode arrays. J. Electrochem. Soc. 2002, 149, B357–B365. [Google Scholar] [CrossRef]
- Lamaka, S.V.; Vaghefinazari, B.; Mei, D.; Petrauskas, R.P.; Höche, D.; Zheludkevich, M.L. Comprehensive screening of Mg corrosion inhibitors. Corros. Sci. 2017, 128, 224–240. [Google Scholar] [CrossRef]
- Park, R.M.; Bena, J.F.; Stayner, L.T.; Smith, R.J.; Gibb, H.J.; Lees, P.S.J. Hexavalent chromium and lung cancer in the chromate industry: A quantitative risk assessment. Risk Anal. 2004, 24, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- JANG, S.-K.; ICHINO, R.; KIM, S.-J.; OKIDO, M. Surface characteristics of chemical conversion coating for Mg-Al alloy. Trans. Nonferrous Met. Soc. China 2009, 19, 892–897. [Google Scholar]
- Yong, Z.; Zhu, J.; Qiu, C.; Liu, Y. Molybdate/phosphate composite conversion coating on magnesium alloy surface for corrosion protection. Appl. Surf. Sci. 2008, 255, 1672–1680. [Google Scholar] [CrossRef]
- Dabalà, M.; Brunelli, K.; Napolitani, E.; Magrini, M. Cerium-based chemical conversion coating on AZ63 magnesium alloy. Surf. Coat. Technol. 2003, 172, 227–232. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Zhang, B.; Yang, K. Preparation and characterization of fluoride conversion coating on biodegradable AZ31B magnesium alloy. J. Optoelectron. Adv. Mater. 2014, 16, 562–567. [Google Scholar]
- Niu, L.; Chang, S.-H.; Tong, X.; Li, G.; Shi, Z. Analysis of characteristics of vanadate conversion coating on the surface of magnesium alloy. J. Alloys Compd. 2014, 617, 214–218. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Doench, I.; Möhwald, H. Assessment of a one-step intelligent self-healing vanadia protective coatings for magnesium alloys in corrosive media. Electrochim. Acta 2011, 56, 2493–2502. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Doench, I.; Möhwald, H. Smart self-healing anti-corrosion vanadia coating for magnesium alloys. Prog. Org. Coatings 2011, 72, 387–393. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Li, J.; Buchheit, R. Corrosion Inhibition Study of Aqueous Vanadate on Mg Alloy AZ31. J. Electrochem. Soc. 2018, 165, C94–C102. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Buchheit, R. Corrosion Inhibition of Mg Alloy AZ31 By Selenite (SeO32-). Meet. Abstr. 2017, MA2017-02, 782. [Google Scholar] [CrossRef]
- Feng, Z.; Hurley, B.; Zhu, M.; Yang, Z.; Hwang, J.; Buchheit, R. Corrosion Inhibition of AZ31 Mg Alloy by Aqueous Selenite (SeO 3 2− ). J. Electrochem. Soc. 2019, 166, C520–C529. [Google Scholar] [CrossRef]
- Ralston, K.D.; Chrisanti, S.; Young, T.L.; Buchheit, R.G. Corrosion Inhibition of Aluminum Alloy 2024-T3 by Aqueous Vanadium Species. J. Electrochem. Soc. 2008, 155, C350. [Google Scholar] [CrossRef]
- Guan, H.; Buchheit, R.G. Corrosion protection of aluminum alloy 2024-T3 by vanadate conversion coatings. Corrosion 2004, 60, 284–296. [Google Scholar] [CrossRef]
- Li, J.; Hurley, B.; Buchheit, R. Inhibition Performance Study of Vanadate on AA2024-T3 at High Temperature by SEM, FIB, Raman and XPS. J. Electrochem. Soc. 2015, 162, C219–C227. [Google Scholar] [CrossRef]
- Salman, S.A.; Kuroda, K.; Okido, M. Formation of Vanadate Conversion Coating on AZ31 Magnesium Alloy. In Magnesium Technology 2013; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 181–187. ISBN 9781118605523. [Google Scholar]
- Atsdr Toxicological Profile for Vanadium. In ATSDR’s Toxicological Profiles; CRC Press: Boca Raton, FL, USA, 2002.
- Abbasi, F.; Sarabi, A.A. Electrochemical Properties of Phosphate Coating on AZ31 Magnesium Alloy: Effect of Phosphating Process Parameters. Corrosion 2012, 68, 835–843. [Google Scholar] [CrossRef]
- Hiromoto, S. Corrosion of Calcium Phosphate Coated AZ31 Magnesium Alloy under a Salt Spray Test. Mater. Trans. 2012, 53, 700–706. [Google Scholar] [CrossRef]
- Jayaraj, J.; Amruth Raj, S.; Srinivasan, A.; Ananthakumar, S.; Pillai, U.T.S.; Gopala, N.; Dhaipule, K.; Mudali, U.K. Composite magnesium phosphate coatings for improved corrosion resistance of magnesium AZ31 alloy. Eval. Program Plann. 2016, 113, 104–115. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Grace, R. Inhibition of magnesium localised corrosion in chloride containing electrolyte. Electrochim. Acta 2010, 55, 7824–7833. [Google Scholar] [CrossRef]
- Greene, J.; Vonk, D. Conversion Coatings Including Alkaline Earth Metal Fluoride Complexes. US6749694B2 6 November 2003. [Google Scholar]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Esmaily, M.; Mortazavi, N.; Svensson, J.E.; Halvarsson, M.; Blucher, D.B.; Jarfors, A.E.W.; Wessen, M.; Johansson, L.G. Atmospheric Corrosion of Mg Alloy AZ91D Fabricated by a Semi-Solid Casting Technique: The Influence of Microstructure. J. Electrochem. Soc. 2015, 162, C311–C321. [Google Scholar] [CrossRef]
- Hurley, B.L.; Qiu, S.; Buchheit, R.G. Raman Spectroscopy Characterization of Aqueous Vanadate Species Interaction with Aluminum Alloy 2024-T3 Surfaces. J. Electrochem. Soc. 2011, 158, C125–C131. [Google Scholar] [CrossRef][Green Version]
- BLISS, C.I. THE TOXICITY OF POISONS APPLIED JOINTLY. Ann. Appl. Biol. 1939, 26, 585–615. [Google Scholar] [CrossRef]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Synergistic corrosion inhibition effect of metal cations and mixtures of organic compounds: A Review. J. Environ. Chem. Eng. 2017, 5, 246–273. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Zhang, S.; Li, W.; Yu, S.; Tan, J. Synergistic effect of tartaric acid with 2,6-diaminopyridine on the corrosion inhibition of mild steel in 0.5 M HCl. Sci. Rep. 2016, 6, 33305. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Dai, Y.; Luo, F.; Zhang, H.X. High efficiency corrosion inhibitor 8-hydroxyquinoline and its synergistic effect with sodium dodecylbenzenesulphonate on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 1603–1609. [Google Scholar] [CrossRef]
- Thomas, S.; Medhekar, N.V.; Frankel, G.S.; Birbilis, N. Corrosion mechanism and hydrogen evolution on Mg. Curr. Opin. Solid State Mater. Sci. 2015, 19, 85–94. [Google Scholar] [CrossRef]
- Rodríguez-Diaz, R.A.; Uruchurtu-chavarín, J.; Cotero-Villegas, A.M.; Valdez, S.; Juárez-Islas, J.A. Corrosion behavior of AlMgSi alloy in aqueous saline solution. Int. J. Electrochem. Sci. 2015, 10, 1792–1808. [Google Scholar]
- Ishizaki, T.; Kamiyama, N.; Watanabe, K.; Serizawa, A. Corrosion resistance of Mg(OH)2/Mg-Al layered double hydroxide composite film formed directly on combustion-resistant magnesium alloy AMCa602 by steam coating. Corros. Sci. 2015, 92, 76–84. [Google Scholar] [CrossRef]
- Rosalbino, F.; Scavino, G.; Mortarino, G.; Angelini, E.; Lunazzi, G. EIS study on the corrosion performance of a Cr(III)-based conversion coating on zinc galvanized steel for the automotive industry. J. Solid State Electrochem. 2011, 15, 703–709. [Google Scholar] [CrossRef]

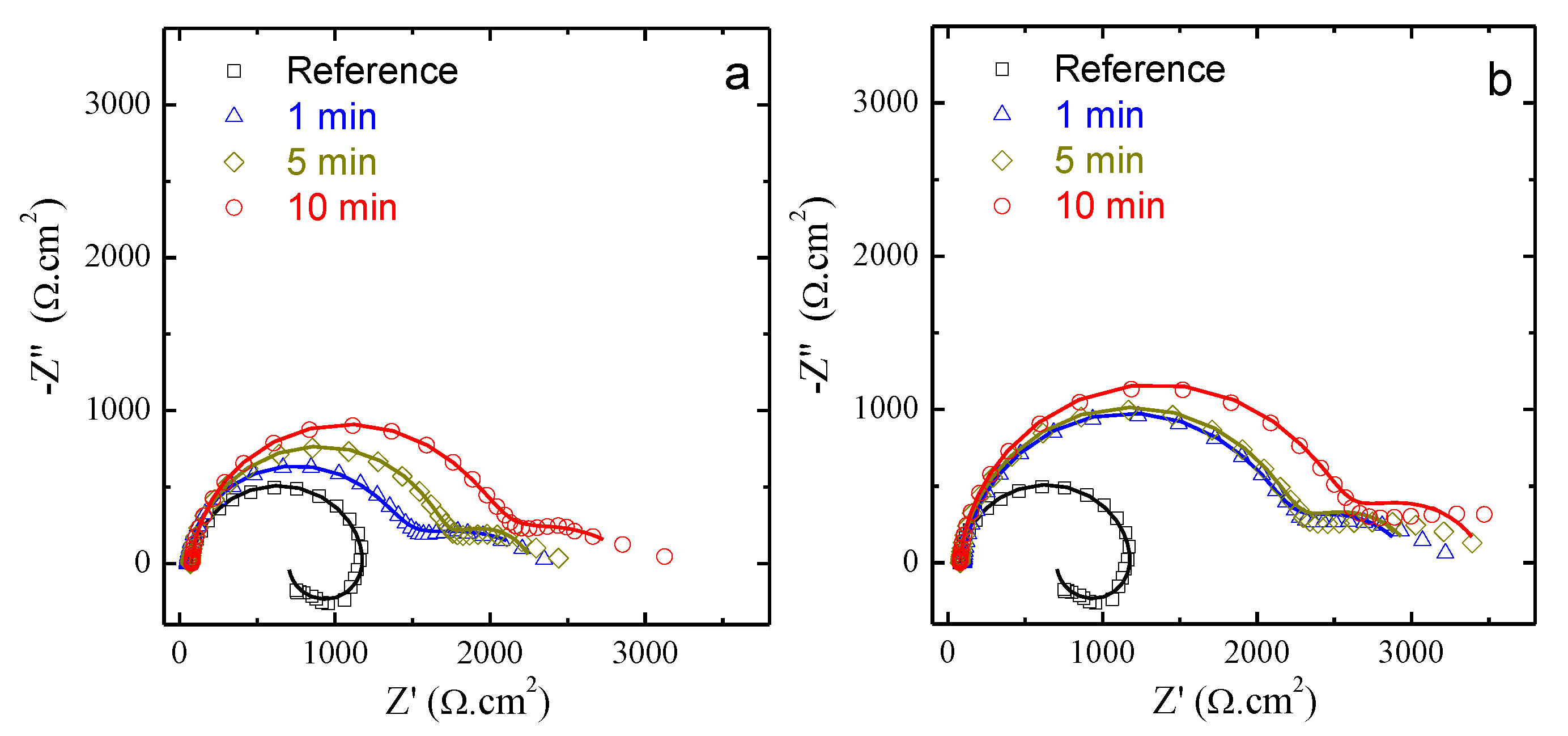
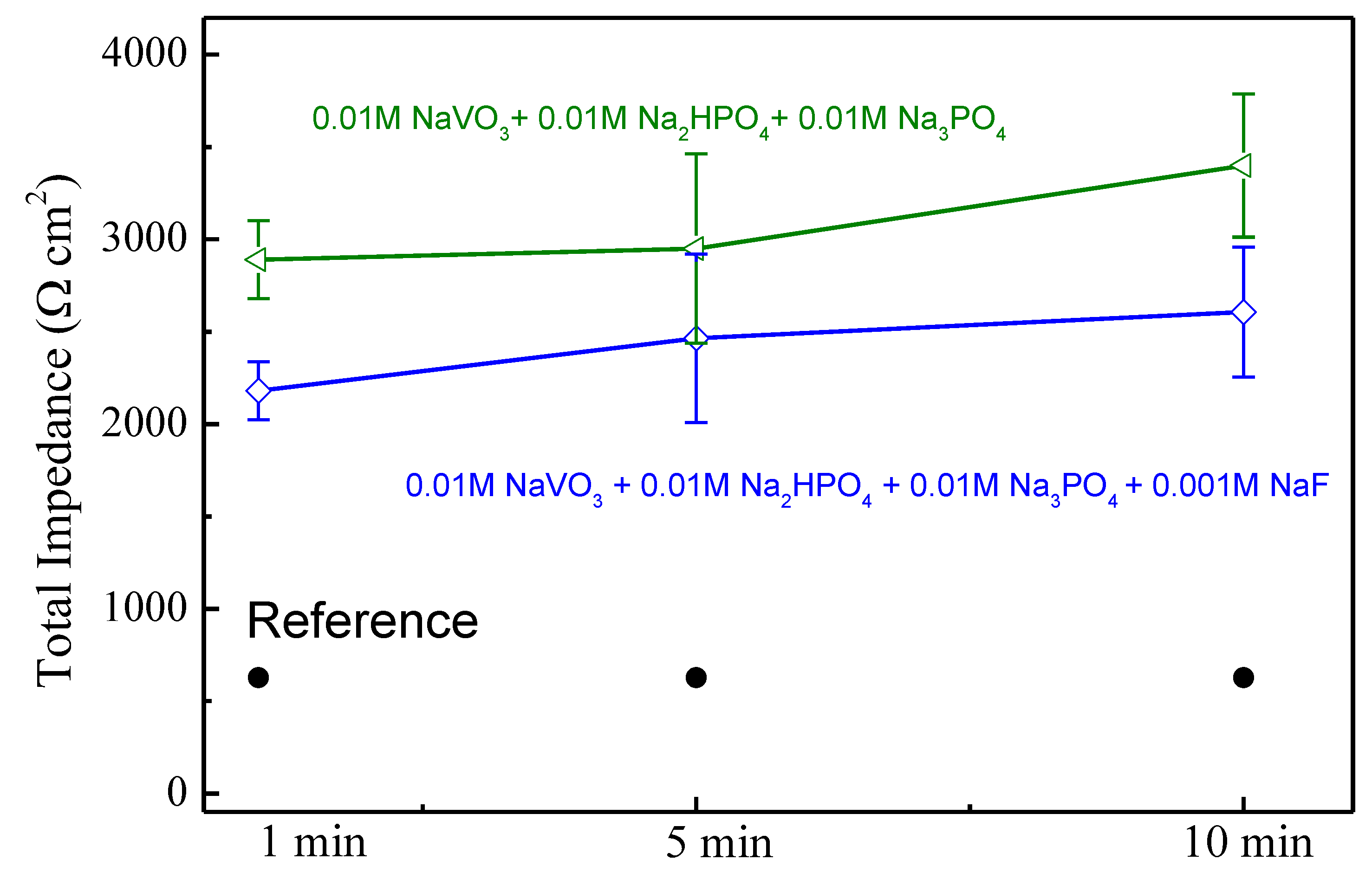
| No. | Inhibitor Content |
|---|---|
| 1 | 0.1 M NaCl + 10 mM Na3PO4 |
| 2 | 0.1 M NaCl + 10 mM Na2HPO4 |
| 3 | 0.1 M NaCl + 10 mM NaF |
| 4 | 0.1 M NaCl + 4 mM NaVO3 + 10 mM Na3PO4 |
| 5 | 0.1 M NaCl + 4 mM NaVO3 + 10 mM Na2HPO4 |
| 6 | 0.1 M NaCl + 4 mM NaVO3 + 10 mM NaF |
| Inhibitors | icorr Mixing Effect 1 | icorr Bliss Test | ||||
|---|---|---|---|---|---|---|
| Time | 10 min | 30 min | 60 min | 10 min | 30 min | 60 min |
| NaVO3 + Na3PO4 | + | + | + | 5.69 | 3.63 | 1.33 |
| NaVO3 + Na2HPO4 | + | + | − | 1.24 | 0.47 | 0.14 |
| NaVO3 + NaF | − | − | − | 0.22 | 0.11 | 0.08 |
| Inhibitors | Ebreakdown-Ecorr Mixing Effect 1 | Ebreakdown-Ecorr Bliss Test | ||||
|---|---|---|---|---|---|---|
| Time | 10 min | 30 min | 60 min | 10 min | 30 min | 60 min |
| NaVO3 + Na3PO4 | − | − | − | 0.68 | 0.78 | 0.89 |
| NaVO3 + Na2HPO4 | + | + | + | 1.63 | 5.41 | 4.31 |
| NaVO3 + NaF | + | + | + | 1.58 | 1.82 | 1.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Li, J.; Yang, Z.; Buchheit, R. The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions. Materials 2020, 13, 1325. https://doi.org/10.3390/ma13061325
Feng Z, Li J, Yang Z, Buchheit R. The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions. Materials. 2020; 13(6):1325. https://doi.org/10.3390/ma13061325
Chicago/Turabian StyleFeng, Zhiyuan, Jichao Li, Zi Yang, and Rudolph Buchheit. 2020. "The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions" Materials 13, no. 6: 1325. https://doi.org/10.3390/ma13061325
APA StyleFeng, Z., Li, J., Yang, Z., & Buchheit, R. (2020). The Effect of Vanadate, Phosphate, Fluoride Compounds on the Aqueous Corrosion of Magnesium Alloy AZ31 in Dilute Chloride Solutions. Materials, 13(6), 1325. https://doi.org/10.3390/ma13061325






