Factors Influencing NO2 Adsorption/Reduction on Microporous Activated Carbon: Porosity vs. Surface Chemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Chemically Activated Carbon
2.2. Modification of the Surface Chemistry
2.2.1. Wet Oxidation
2.2.2. NaOH Neutralization
2.2.3. Thermal Treatment
2.3. Morphological and Textural Properties Characterization
2.4. Surface Chemistry Characterization
2.4.1. Temperature Programmed Desorption-Mass Spectroscopy (TPD-MS)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Boehm Titration
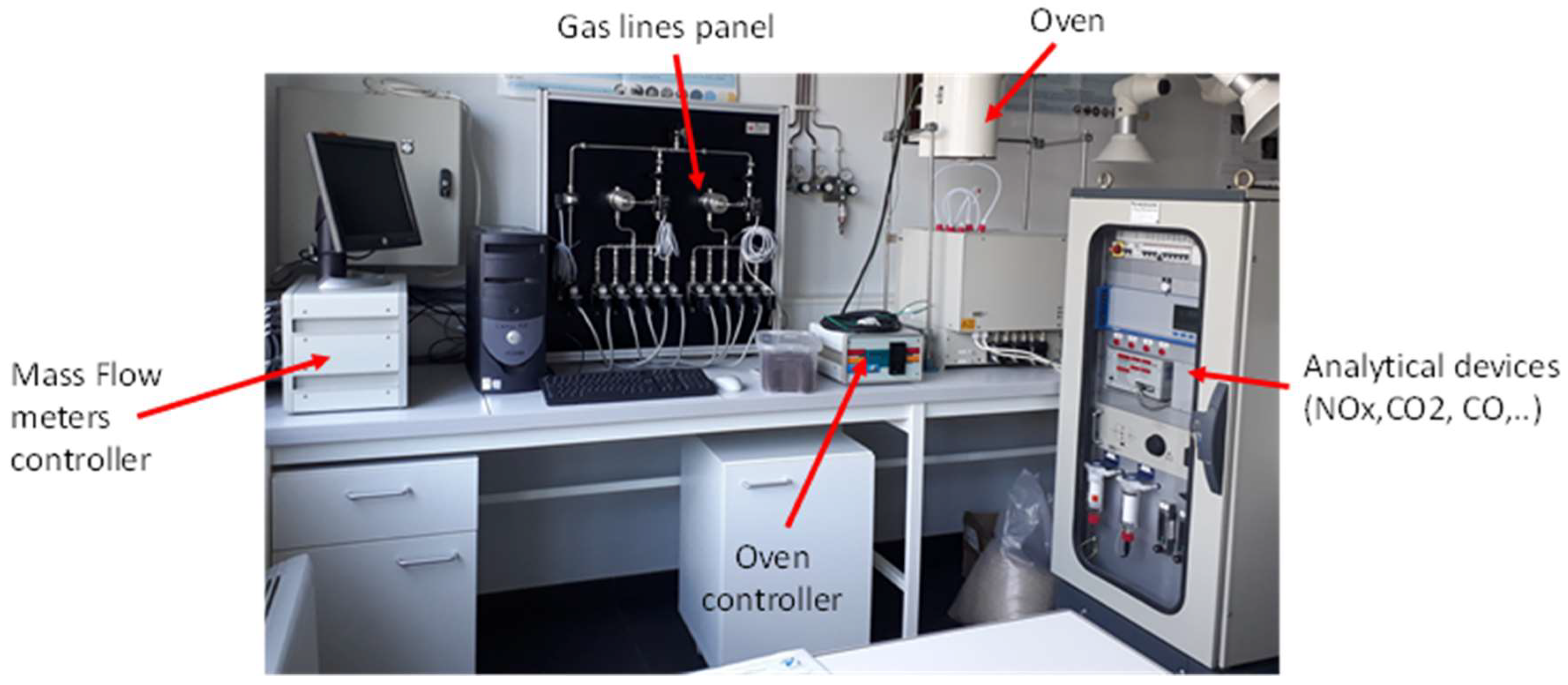
2.5. NO2 Adsorption Tests
3. Results and Discussions
3.1. Activated Carbons Characterization
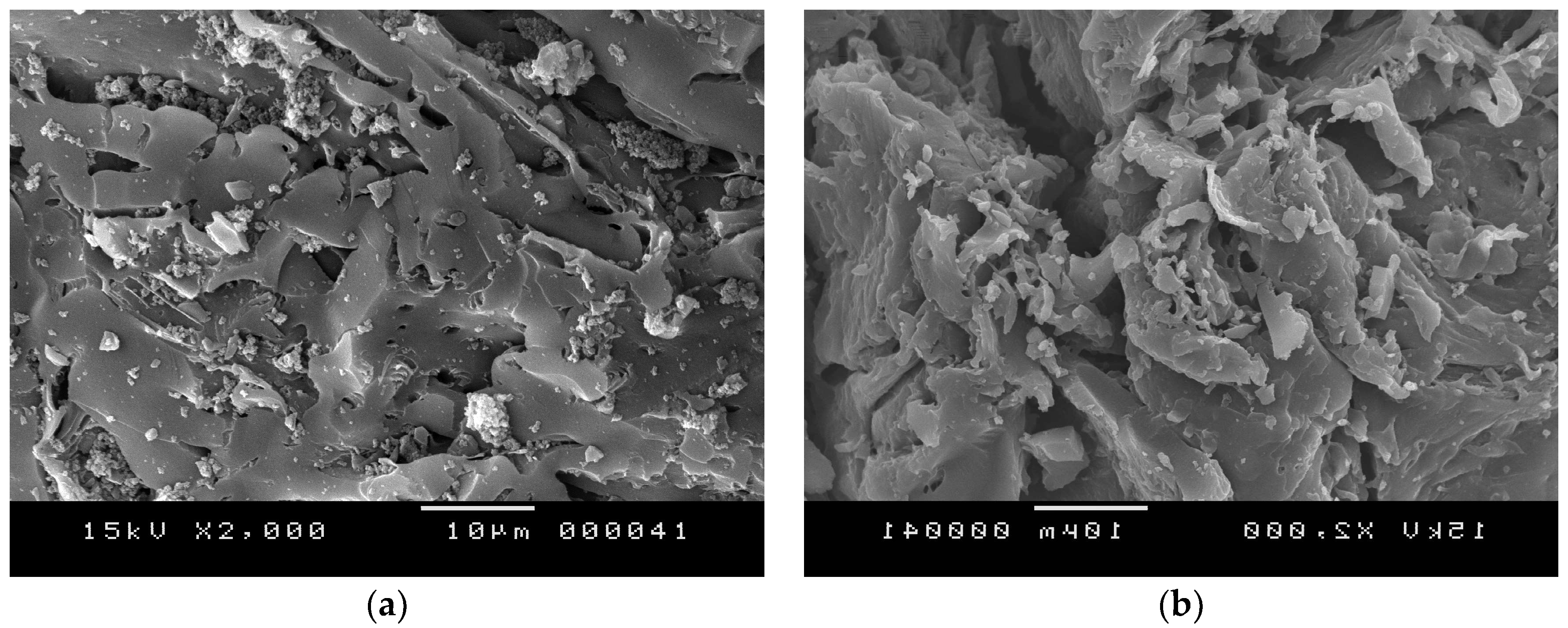
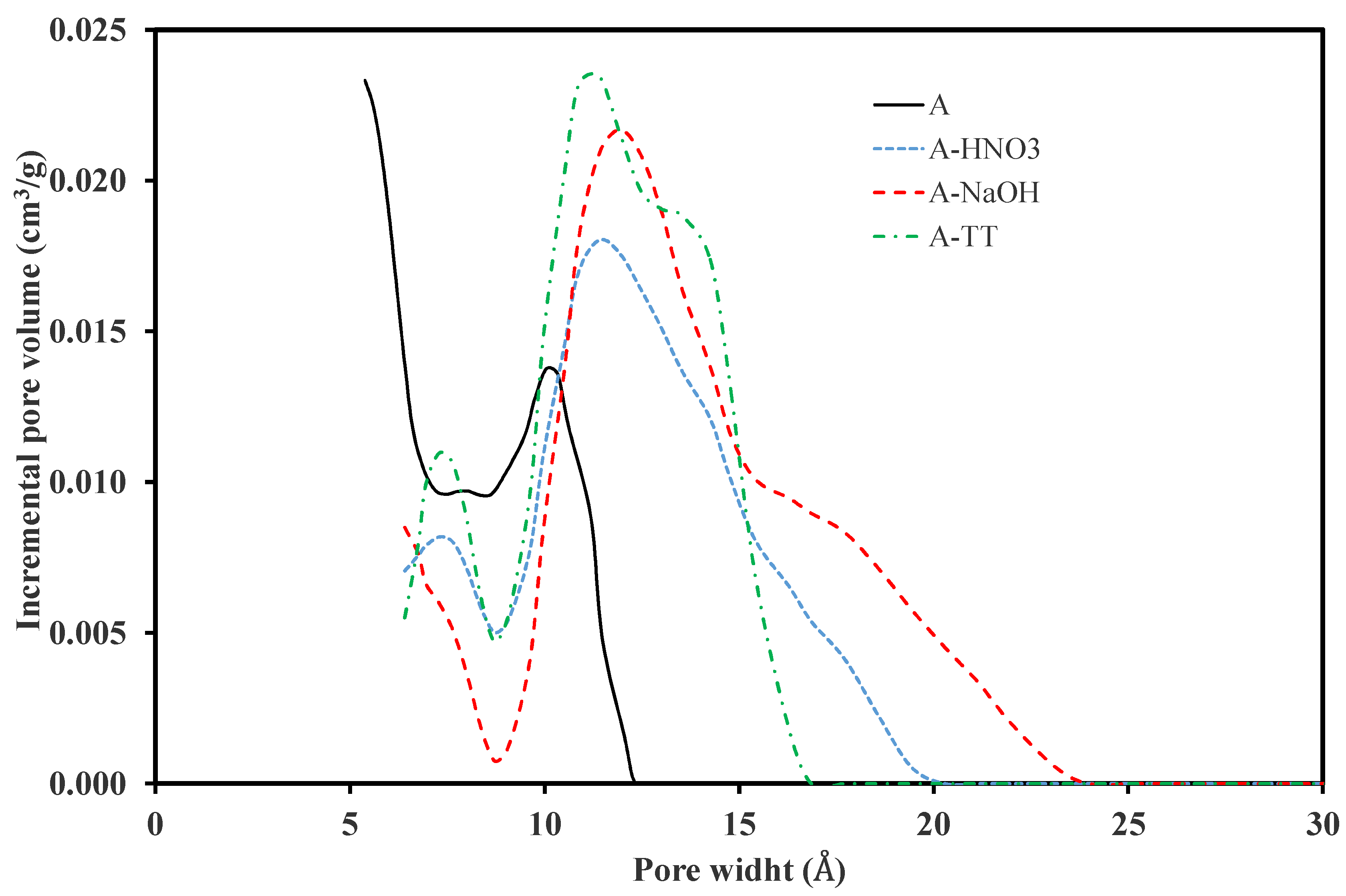
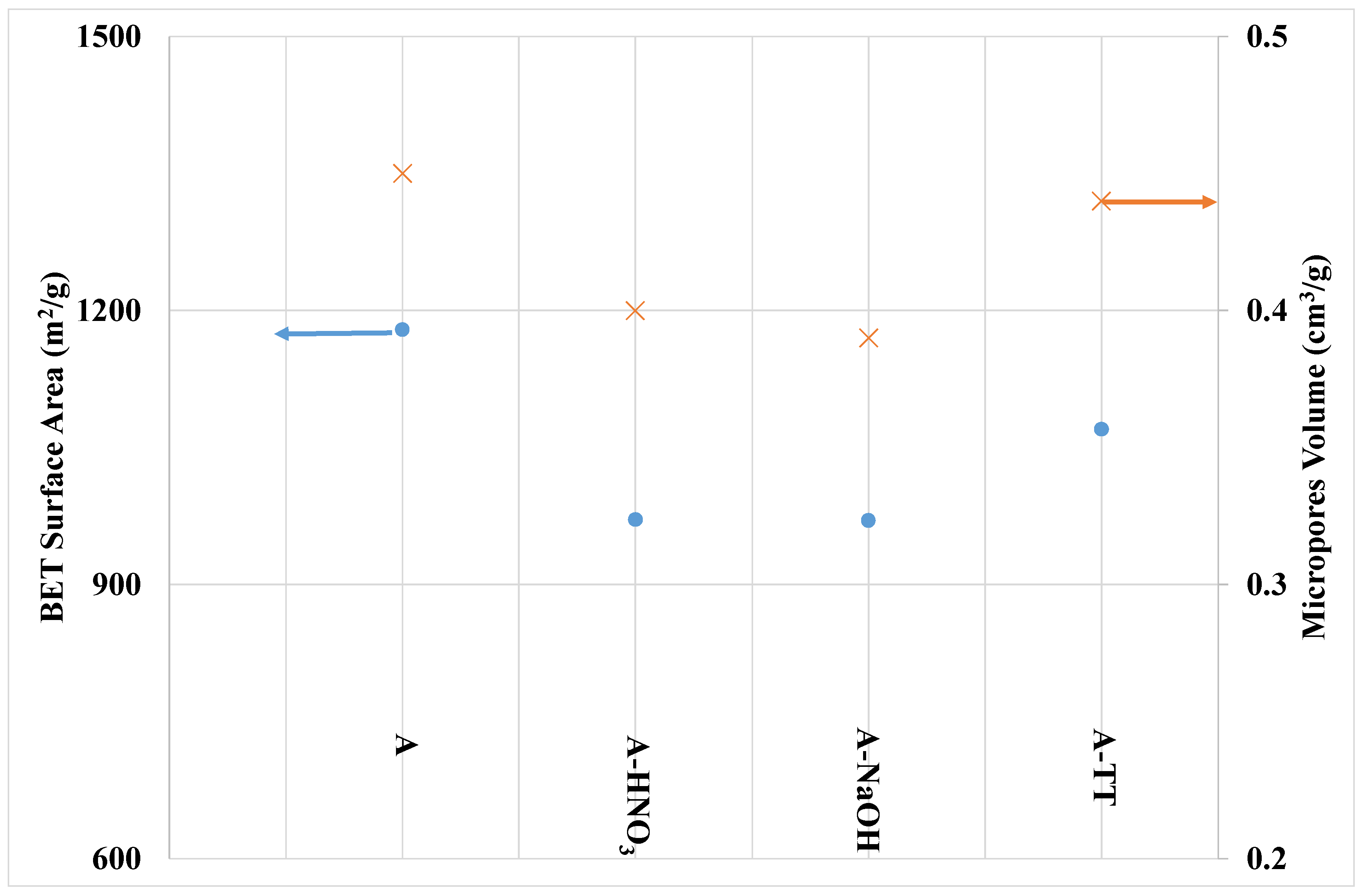
3.1.1. Morphological and Textural Properties
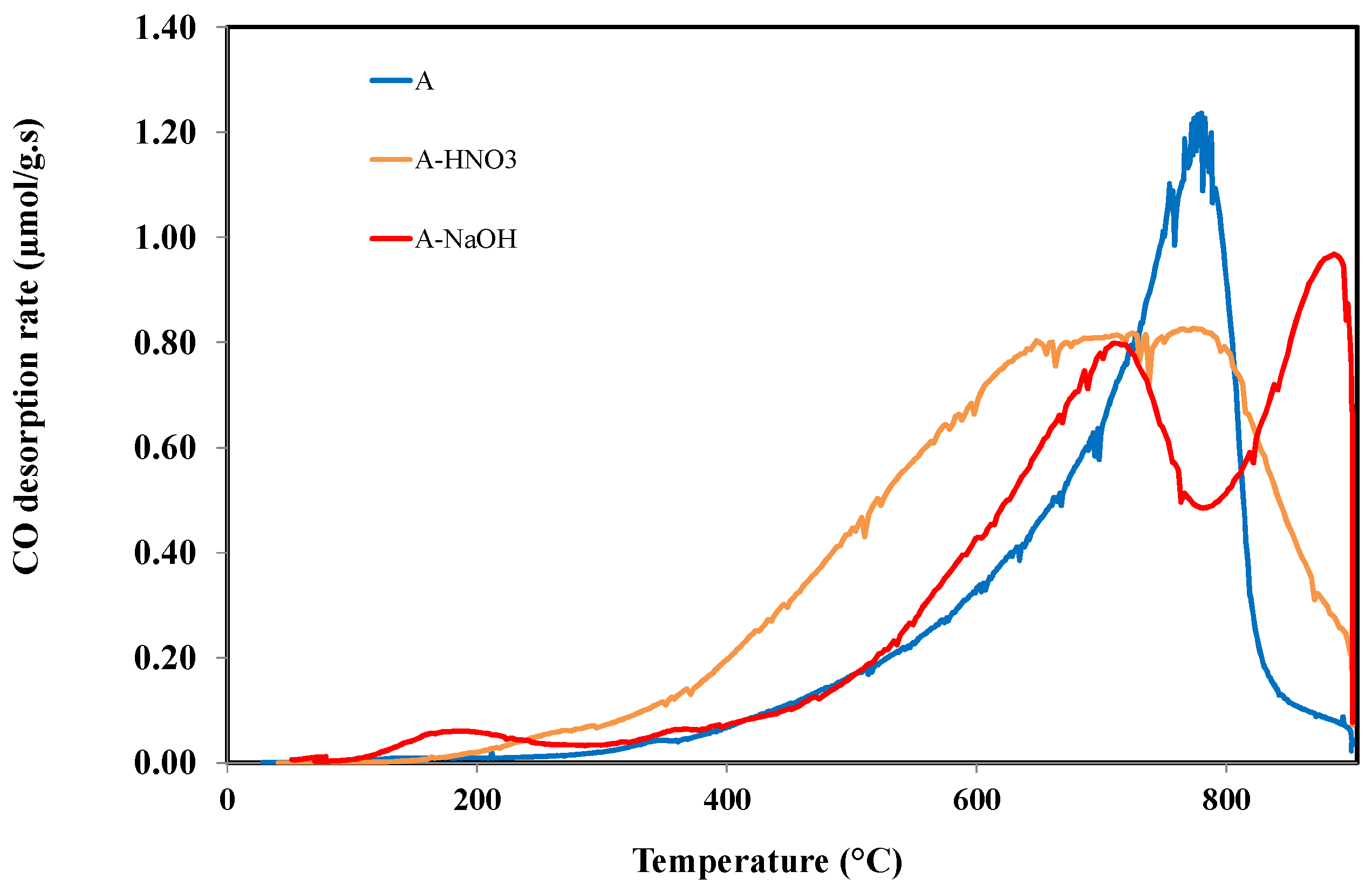
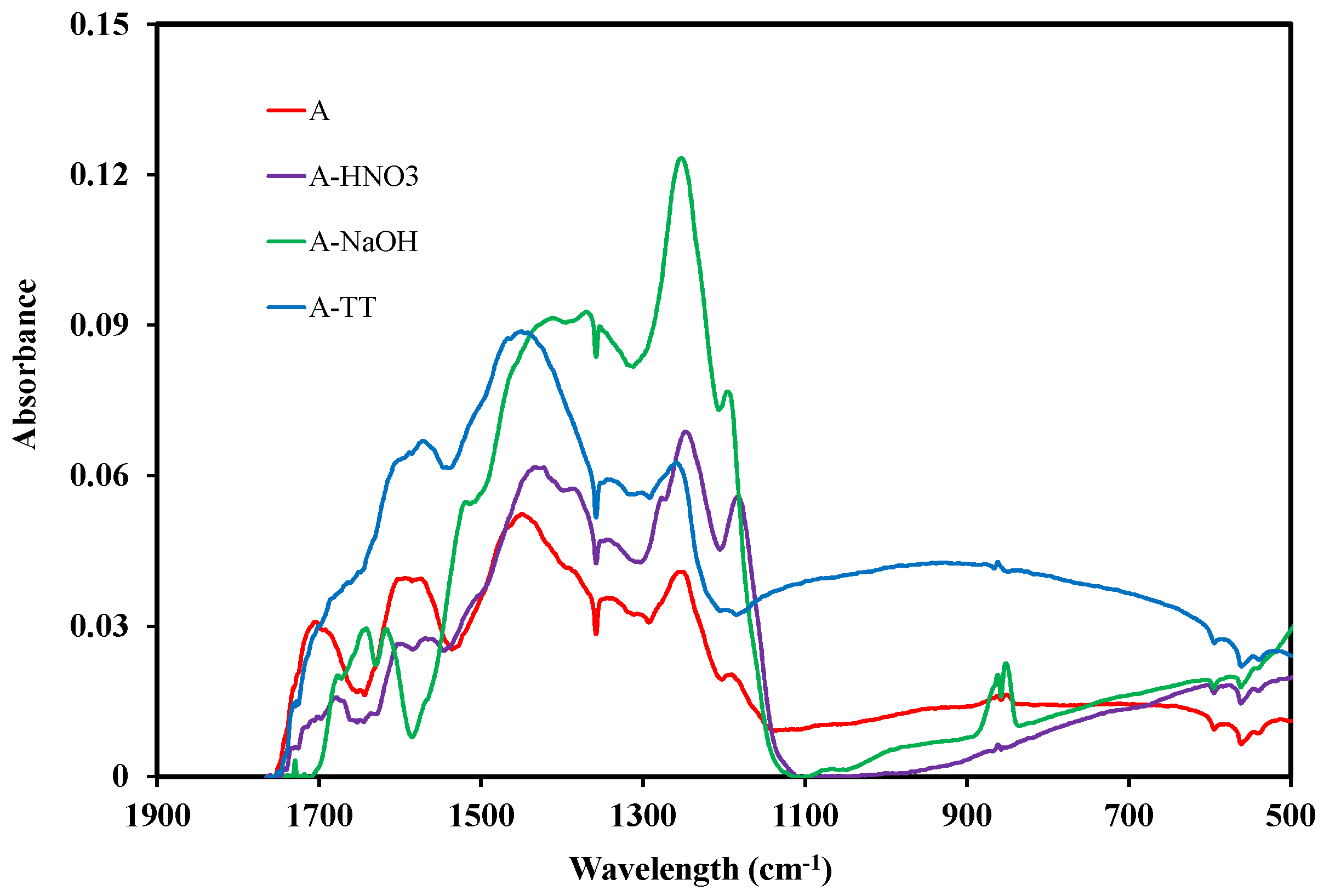
3.1.2. Surface Chemistry
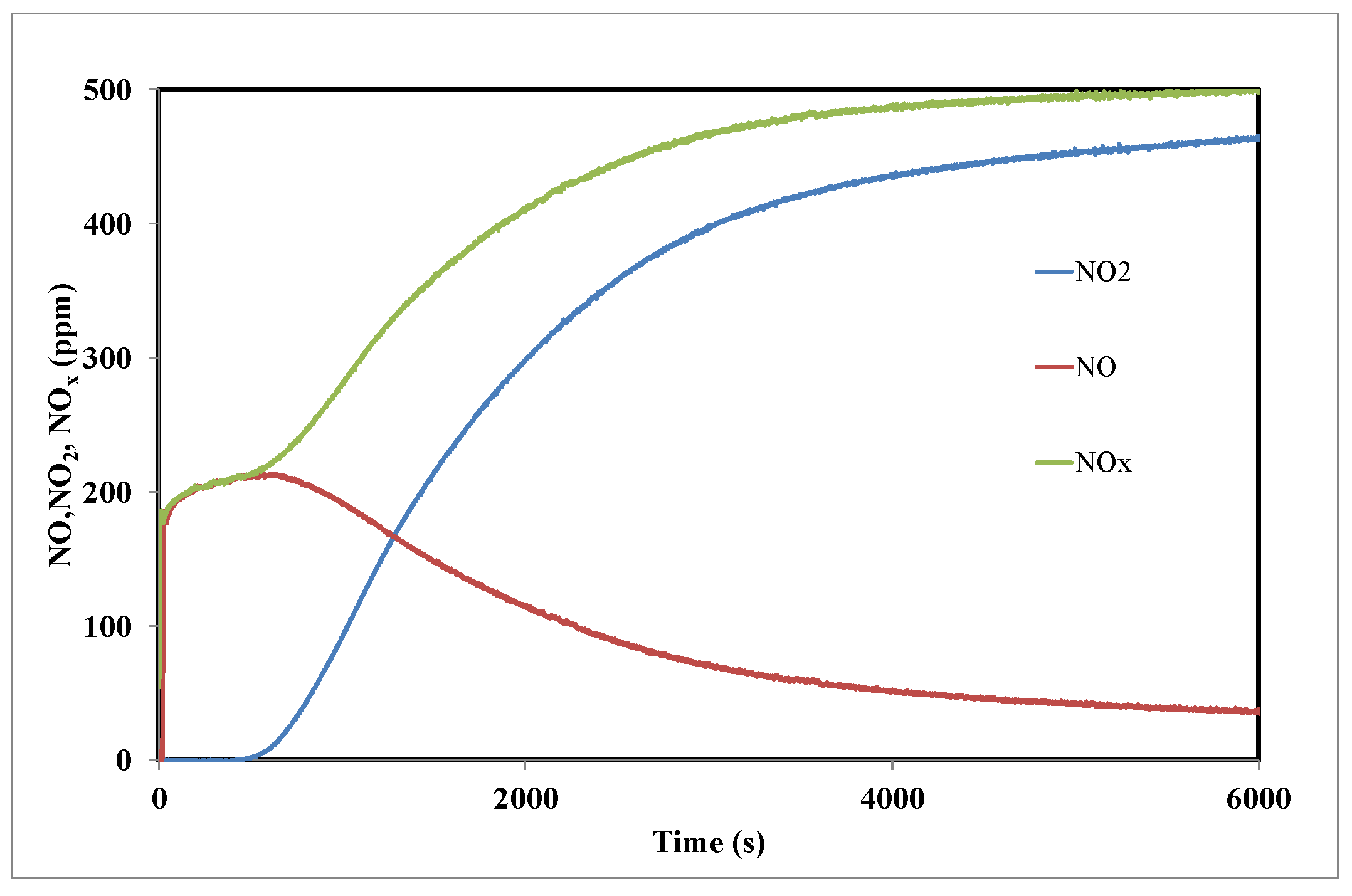
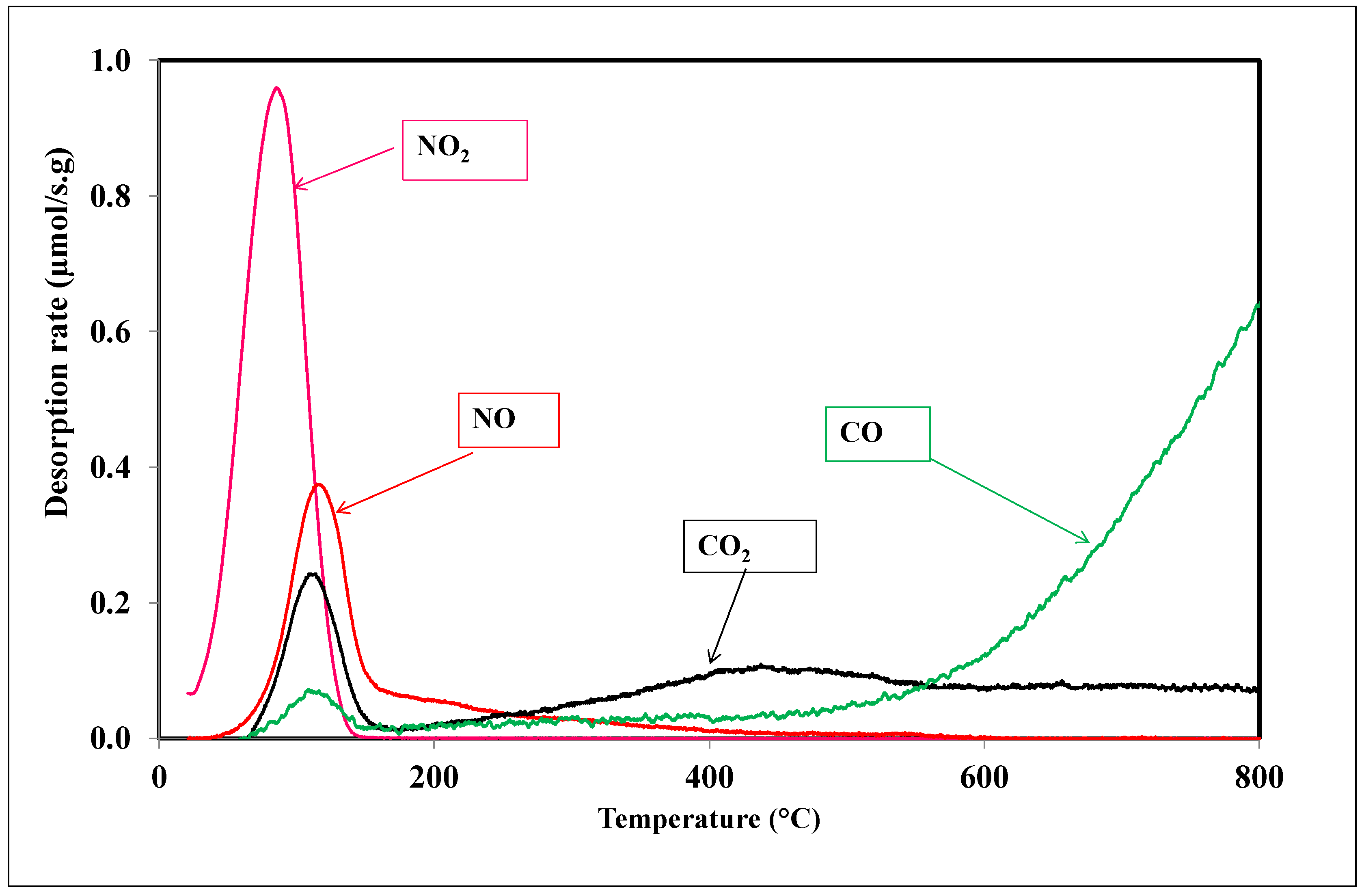
3.2. NO2 Adsorption on the Activated Carbon A
3.3. Effect of Surface Modification on the Adsorption Performances of Activated Carbons
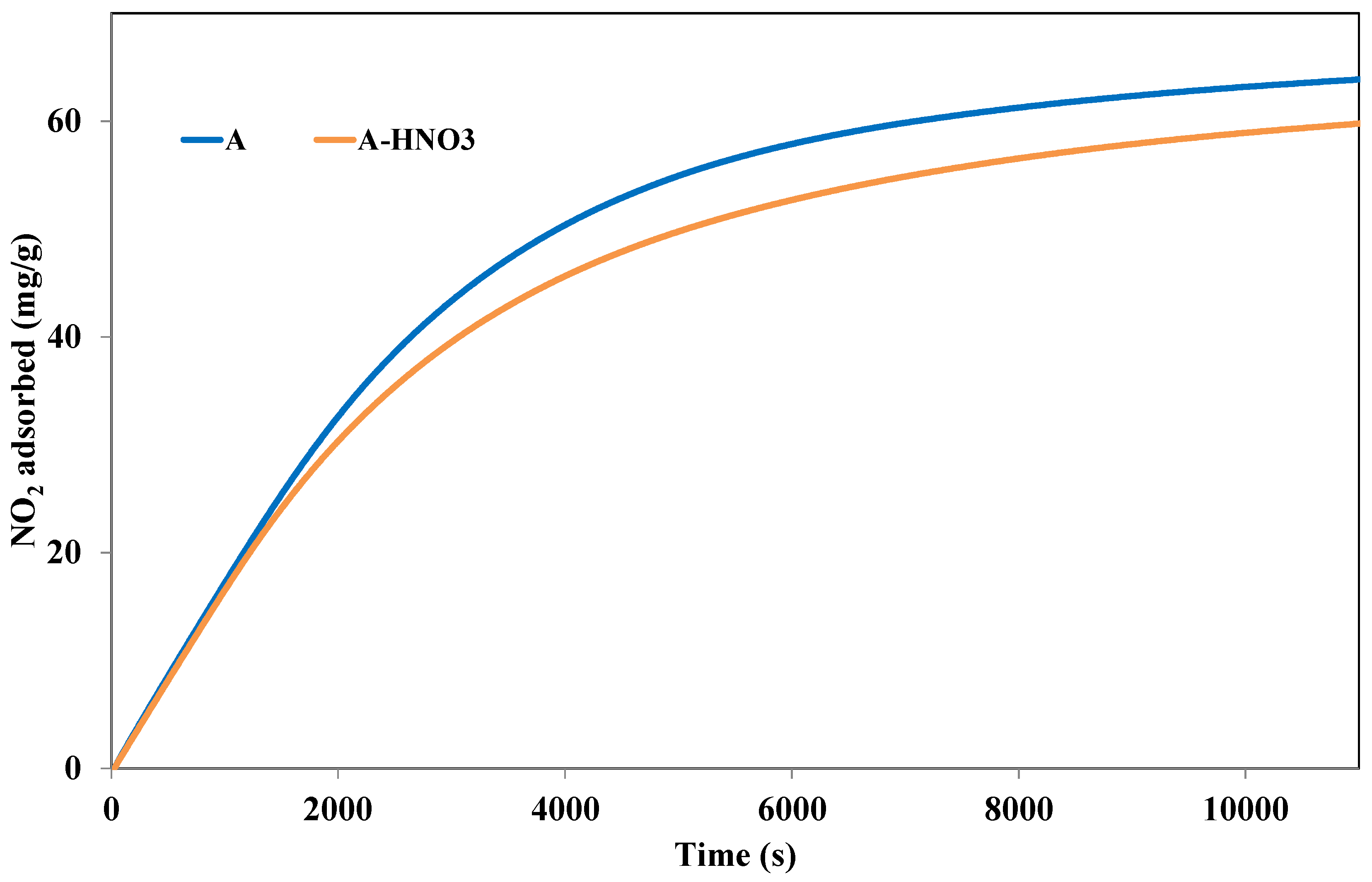
3.3.1. Effect of Acidic Group Incorporation
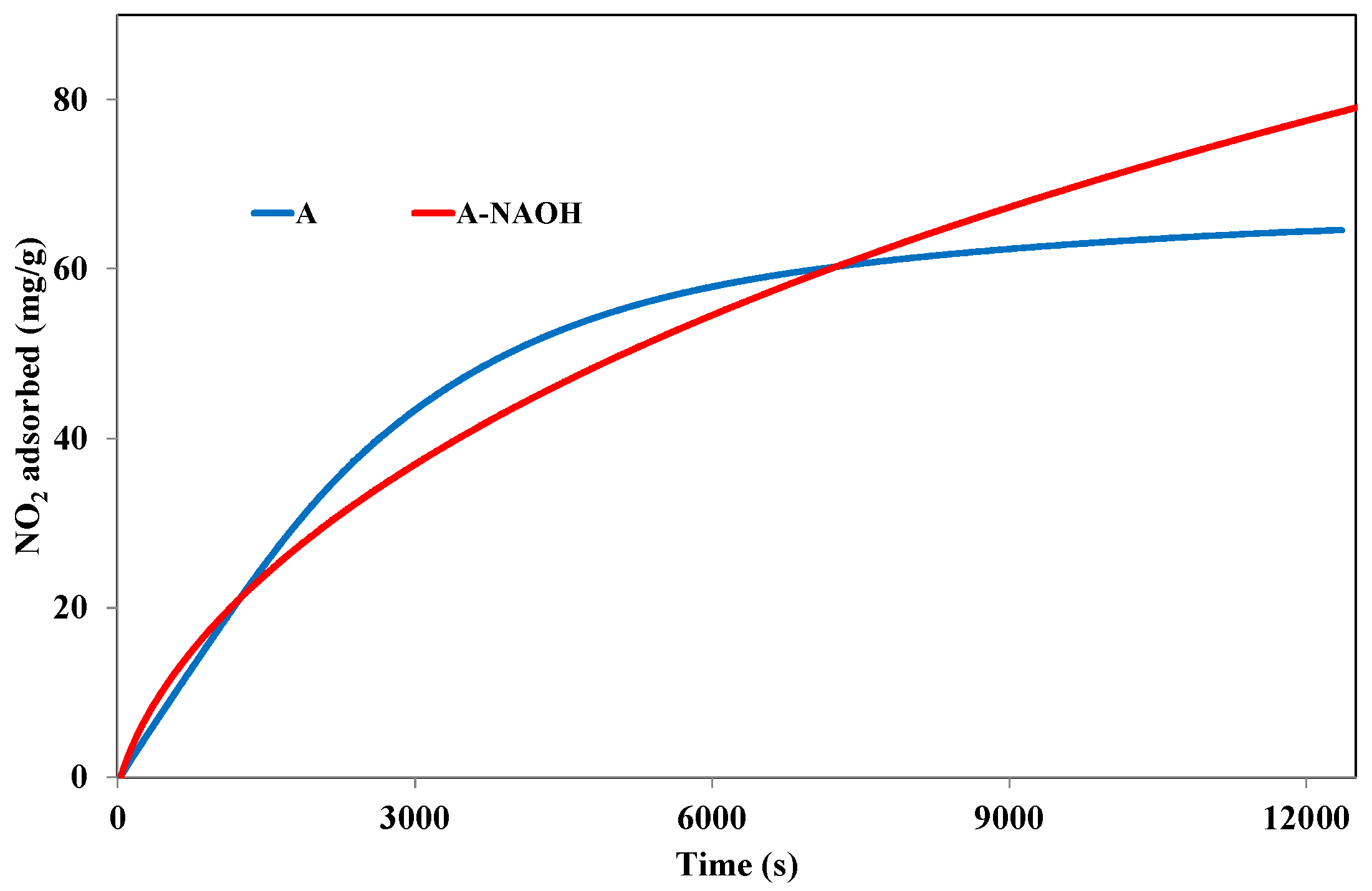
3.3.2. Effect of NaOH Treatment
3.3.3. Effect of Acidic Surface Groups Elimination through Thermal Treatment
3.4. Role of the Surface Oxygen Groups and the Textural Properties on the Interaction of NO2 with the Activated Carbon Surface
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Levasseur, B.; Ebrahim Amani, M.; Burress, J.; Bandosz, T. Interactions of NO2 at ambient temperature with cerium–zirconium mixed oxides supported on SBA-15. J. Hazard. Mater. 2011, 197, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Labaki, M.; Issa, M.; Smeekens, S.; Heylen, S.; Kirschhock, C.E.A.; Villani, K.; Jeguirim, M.; Martens, J.A. Modeling of NOx Adsorption-Desorption-Reduction Cycles on a Ruthenium Loaded Na-Y Zeolite. Appl. Catal. B Environ. 2010, 97, 13–20. [Google Scholar] [CrossRef]
- Sager, U.; Schmidt, W.; Schmidt, F.; Suhartiningsih, S. Catalytic reduction of nitrogen oxides via nanoscopic oxide catalysts within activated carbons at room temperature. Adsorption 2013, 19, 1027–1033. [Google Scholar] [CrossRef]
- Pietrzak, R. Sawdust pellets from coniferous species as adsorbents for NO2 removal. Bioresour. Technol. 2010, 101, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, R. Active Carbons Obtained from Bituminous Coal for NO2 Removal under Dry and Wet Conditions at Room Temperature. Energy Fuels 2009, 23, 3617–3624. [Google Scholar] [CrossRef]
- Sumathi, S.; Bhatia, S.; Lee, K.T.; Mohamed, A.R. Selection of best impregnated palm shell activated carbon (PSAC) for simultaneous removal of SO2 and NOx. J. Hazard. Mater. 2010, 176, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Skibiszewska, P.; Pietrzak, R. NO2 removal on adsorbents prepared from coffee industry waste materials. Adsorption 2013, 19, 521–528. [Google Scholar] [CrossRef]
- Belala, Z.; Belhachemi, M.; Jeguirim, M. Activated Carbon Prepared from Date Pits for the Retention of NO2 at Low Temperature. Int. J. Chem. React. Eng. 2014, 12, 717. [Google Scholar] [CrossRef]
- Belhachemi, M.; Jeguirim, M.; Limousy, L.; Addoun, F. Comparison of NO2 removal using date pits activated carbon and modified commercialized activated carbon via different preparation methods: Effect of porosity and surface chemistry. Chem. Eng. J. 2014, 253, 121–129. [Google Scholar] [CrossRef]
- Pietrzak, R.; Bandosz, T.J. Activated carbons modified with sewage sludge derived phase and their application in the process of NO2 removal. Carbon 2007, 45, 2537–2546. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R. Carbonaceous adsorbents prepared by physical activation of pine sawdust and their application for removal of NO2 in dry and wet conditions. Bioresour. Technol. 2010, 101, 5802–5807. [Google Scholar] [CrossRef] [PubMed]
- Bazan, A.; Nowicki, P.; Pietrzak, R. Removal of NO2 by carbonaceous adsorbents obtained from residue after supercritical extraction of marigold. Adsorption 2016, 22, 465–471. [Google Scholar] [CrossRef][Green Version]
- Nowicki, P.; Wachowska, H.; Pietrzak, R. Active carbons prepared by chemical activation of plum stones and their application in removal of NO2. J. Hazard. Mater. 2010, 181, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-W.; Choi, D.-K.; Park, J.-W. Performance of fixed-bed KOH impregnated activated carbon adsorber for NO and NO2 removal in the presence of oxygen. Carbon 2002, 40, 1409–1417. [Google Scholar] [CrossRef]
- Akhter, M.S.; Chughtai, A.R.; Smith, D.M. Reaction of hexane soot with nitrogen dioxide/nitrogen oxide (N2O4). J. Phys. Chem. 1984, 88, 5334–5342. [Google Scholar] [CrossRef]
- Shirahama, N.; Moon, S.H.; Choi, K.-H.; Enjoji, T.; Kawano, S.; Korai, Y.; Tanoura, M.; Mochida, I. Mechanistic study on adsorption and reduction of NO2 over activated carbon fibers. Carbon 2002, 40, 2605–2611. [Google Scholar] [CrossRef]
- Kante, K.; Deliyanni, E.; Bandosz, T.J. Interactions of NO2 with activated carbons modified with cerium, lanthanum and sodium chlorides. J. Hazard. Mater. 2009, 165, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Kazmierczak, J.; Sawicka, K.; Pietrzak, R. Nitrogen-enriched activated carbons prepared by the activation of coniferous tree sawdust and their application in the removal of Nitrogen dioxide. Int. J. Environ. Sci. Technol. 2015, 12, 2233–2244. [Google Scholar] [CrossRef][Green Version]
- Sager, U.; Däuber, E.; Bathen, D.; Asbach, C.; Schmidt, F.; Tseng, J.-C.; Pommerin, A.; Weidenthaler, C.; Schmidt, W. Influence of the degree of infiltration of modified activated carbons with CuO/ZnO on the separation of NO2 at ambient temperatures. Adsorpt. Sci. Technol. 2016, 34, 307–319. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Park, C.J.; Kim, K.Y.; Son, Y.-S.; Kang, C.-M.; Wolfson, J.M.; Jung, I.-H.; Lee, S.-J.; Koutrakis, P. Development of an activated carbon filter to remove NO2 and HONO in indoor air. J. Hazard. Mater. 2015, 289, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ghouma, I.; Jeguirim, M.; Sager, U.; Limousy, L.; Bennici, S.; Däuber, E.; Asbach, C.; Ligotski, R.; Schmidt, F.; Ouederni, A. The Potential of Activated Carbon Made of Agro-Industrial Residues in NOx Immissions Abatement. Energies 2017, 10, 1508. [Google Scholar] [CrossRef]
- Jeguirim, M.; Tschamber, V.; Brilhac, J.F.; Ehrburger, P. Interaction mechanism of NO2 with carbon black: Effect of surface oxygen complexes. J. Anal. Appl. Pyrolysis 2004, 72, 171–181. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Zhang, Z.; Guo, Y.; Wang, Y.; Lu, G. The role of surface properties of activated carbon in the catalytic reduction of NO by carbon. Appl. Surf. Sci. 2008, 255, 2591–2595. [Google Scholar] [CrossRef]
- Bashkova, S.; Bandosz, T.J. The effects of urea modification and heat treatment on the process of NO2 removal by wood-based activated carbon. J. Colloid Interface Sci. 2009, 333, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Limousy, L.; Ghouma, I.; Ouederni, A.; Jeguirim, M. Amoxicillin removal from aqueous solution using activated carbon prepared by chemical activation of olive stone. Environ. Sci. Pollut. Res. 2017, 24, 9993–10004. [Google Scholar] [CrossRef] [PubMed]
- Ghouma, I.; Jeguirim, M.; Dorge, S.; Limousy, L.; Matei Ghimbeu, C.; Ouederni, A. Activated carbon prepared by physical activation of olive stones for the removal of NO2 at ambient temperature. Comptes Rendus Chim. 2015, 18, 63–74. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Khelifi, A.; Almazán-Almazán, M.C.; Pérez-Mendoza, M.; Domingo-García, M.; López-Domingo, F.J.; Temdrara, L.; López-Garzón, F.J.; Addoun, A. Influence of nitric acid concentration on the characteristics of active carbons obtained from a mineral coal. Fuel Process. Technol. 2010, 91, 1338–1344. [Google Scholar] [CrossRef]
- Shim, J.-W.; Park, S.-J.; Ryu, S.-K. Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers. Carbon 2001, 39, 1635–1642. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catalysis Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Carabineiro, S.A.C.; Chen, X.; Delgado, J.J.; Figueiredo, J.L.; Pereira, M.F.R.; Órfão, J.J.M. Selective Oxidation of Glycerol Catalyzed by Rh/Activated Carbon: Importance of Support Surface Chemistry. Catal. Lett. 2011, 141, 420–431. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon 2003, 41, 713–722. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X. Peanut Shell Activated Carbon: Characterization, Surface Modification and Adsorption of Pb2+ from Aqueous Solution. Chin. J. Chem. Eng. 2008, 16, 401–406. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, B.; Yuan, S.; Wu, X.; Chen, J.; Wang, L. Adsorptive removal of chloramphenicol from wastewater by NaOH modified bamboo charcoal. Bioresour. Technol. 2010, 101, 7661–7664. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, Z.; Liu, Y. Surface chemical functional groups modification of porous carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Radovic, L.; Moreno-Castilla, C.; Rivera-Utrilla, J. Carbon Materials as Adsorbents in Aqueous Solutions. Chem. Phys. Carbon 2000, 27, 227–405. [Google Scholar]




| Carbons | CO2 (µmol/g) | CO (µmol/g) | H2O (µmol/g) | H2 (µmol/g) |
|---|---|---|---|---|
| A | 719 | 3430 | 3460 | 1800 |
| A-HNO | 1560 | 3770 | 2160 | 1310 |
| A-NaOH | 217 | 3190 | 3300 | 4410 |
| A-TT | 0.25 | 3.04 | 1.98 | 2.05 |
| Sample | Carboxyl | Lactones | Phenols | Total Acid Sites | Total Basic Sites |
|---|---|---|---|---|---|
| A | 1.45 | 0.05 | 0.7 | 2.20 | 0 |
| A-HNO3 | 2.00 | 0.50 | 1.10 | 3.60 | 0.15 |
| Sample | A | A-HNO3 |
|---|---|---|
| NO2 adsorption capacities (mg/g) | 64.5 | 57.2 |
| NO2 physisorbed (mg/g) | 51.6 | 45.3 |
| NO2 Chemisorbed (mg/g) | 12.9 | 11.9 |
| Sample | A | A-NaOH |
|---|---|---|
| NO2 adsorption capacities (mg/g) | 64.5 | 79.1 |
| NO2 physisorbed (mg/g) | 51.6 | 42.9 |
| NO2 chemisorbed (mg/g) | 12.9 | 36.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghouma, I.; Jeguirim, M.; Limousy, L.; Bader, N.; Ouederni, A.; Bennici, S. Factors Influencing NO2 Adsorption/Reduction on Microporous Activated Carbon: Porosity vs. Surface Chemistry. Materials 2018, 11, 622. https://doi.org/10.3390/ma11040622
Ghouma I, Jeguirim M, Limousy L, Bader N, Ouederni A, Bennici S. Factors Influencing NO2 Adsorption/Reduction on Microporous Activated Carbon: Porosity vs. Surface Chemistry. Materials. 2018; 11(4):622. https://doi.org/10.3390/ma11040622
Chicago/Turabian StyleGhouma, Imen, Mejdi Jeguirim, Lionel Limousy, Najoua Bader, Abdelmottaleb Ouederni, and Simona Bennici. 2018. "Factors Influencing NO2 Adsorption/Reduction on Microporous Activated Carbon: Porosity vs. Surface Chemistry" Materials 11, no. 4: 622. https://doi.org/10.3390/ma11040622
APA StyleGhouma, I., Jeguirim, M., Limousy, L., Bader, N., Ouederni, A., & Bennici, S. (2018). Factors Influencing NO2 Adsorption/Reduction on Microporous Activated Carbon: Porosity vs. Surface Chemistry. Materials, 11(4), 622. https://doi.org/10.3390/ma11040622








