Generation of Well-Defined Micro/Nanoparticles via Advanced Manufacturing Techniques for Therapeutic Delivery
Abstract
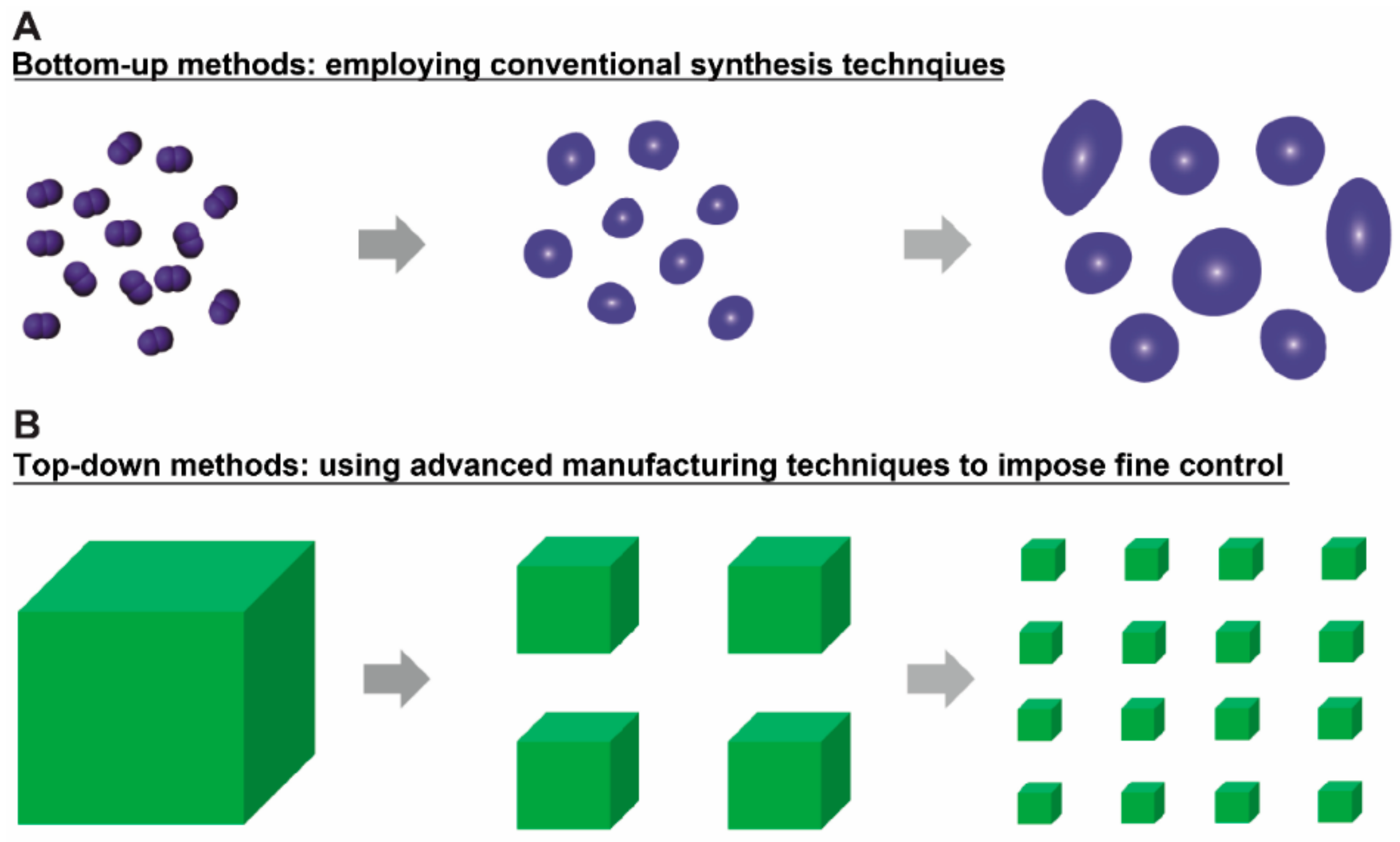
1. Introduction
2. Key Characteristics of Nanomaterials for Therapeutic and Imaging Applications
2.1. Size
2.2. Surface Property
2.3. Component Materials
2.4. Shape
2.5. Nanomaterial-Induced Cellular Toxicity
3. Advanced Manufacturing Techniques in Designing Micro/Nano Particles for Therapeutic Delivery
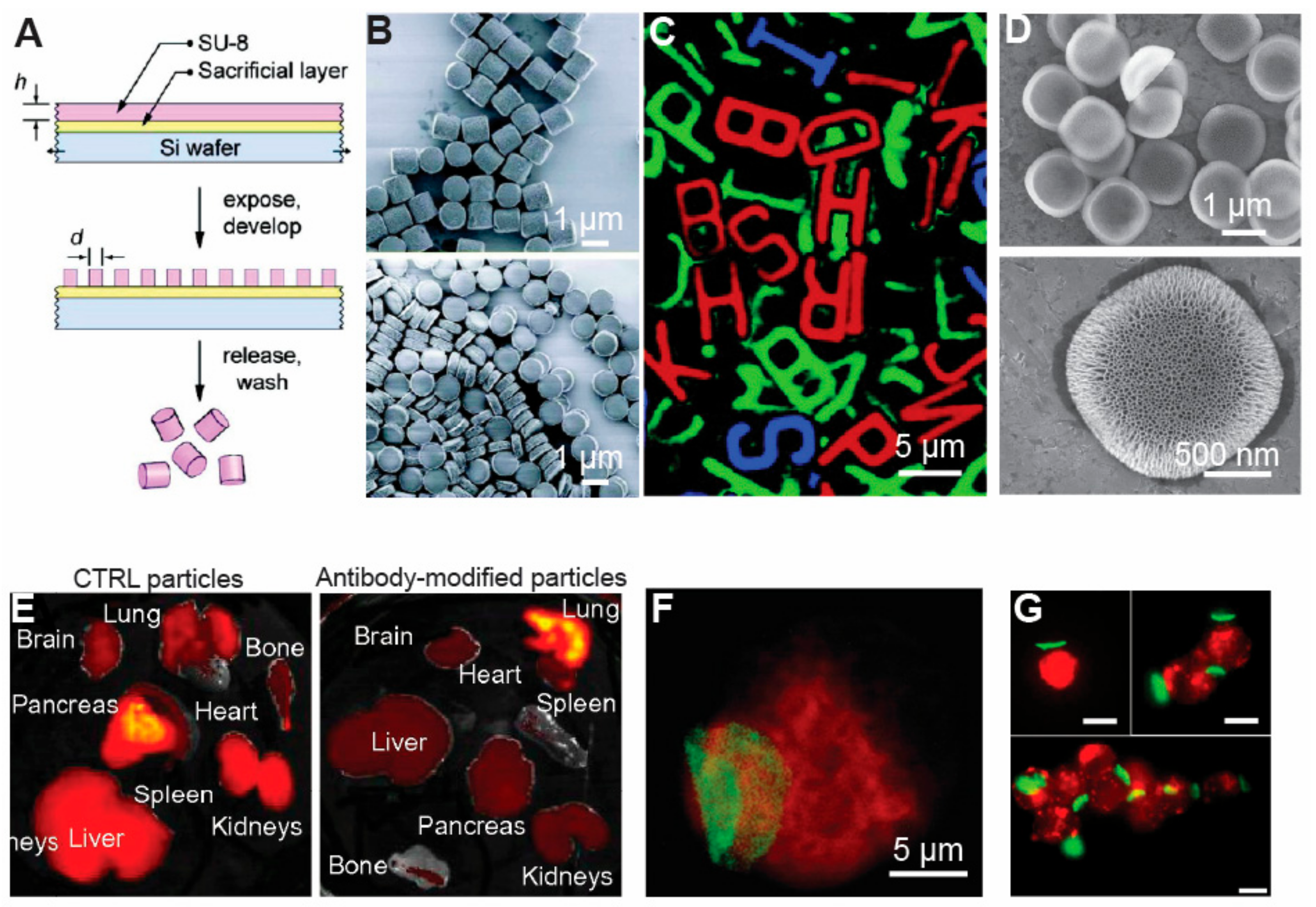
3.1. Photolithography
3.2. Soft Lithography
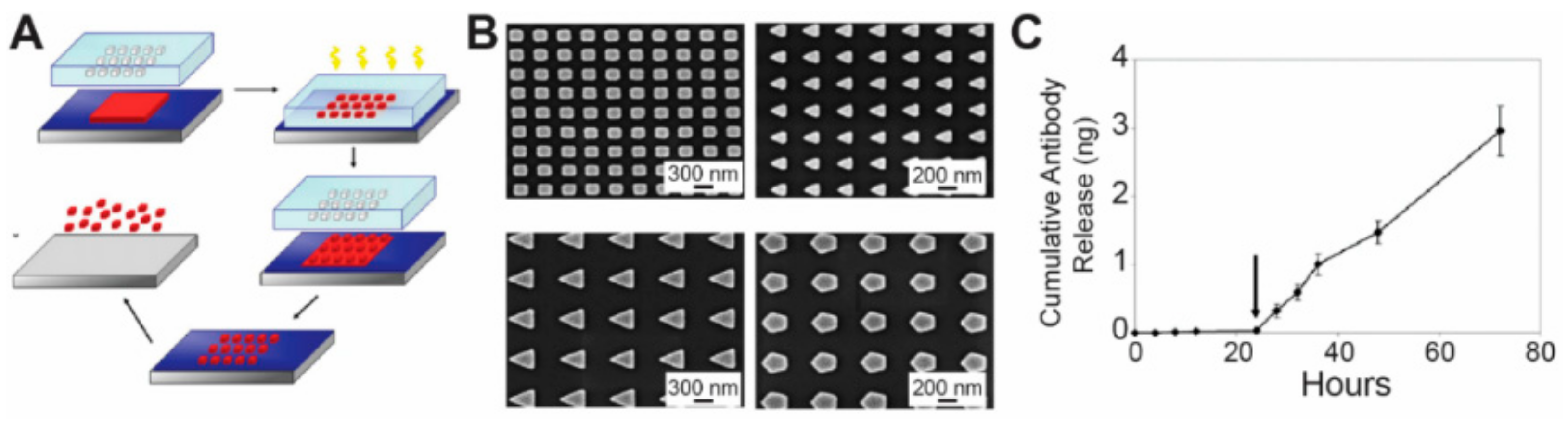
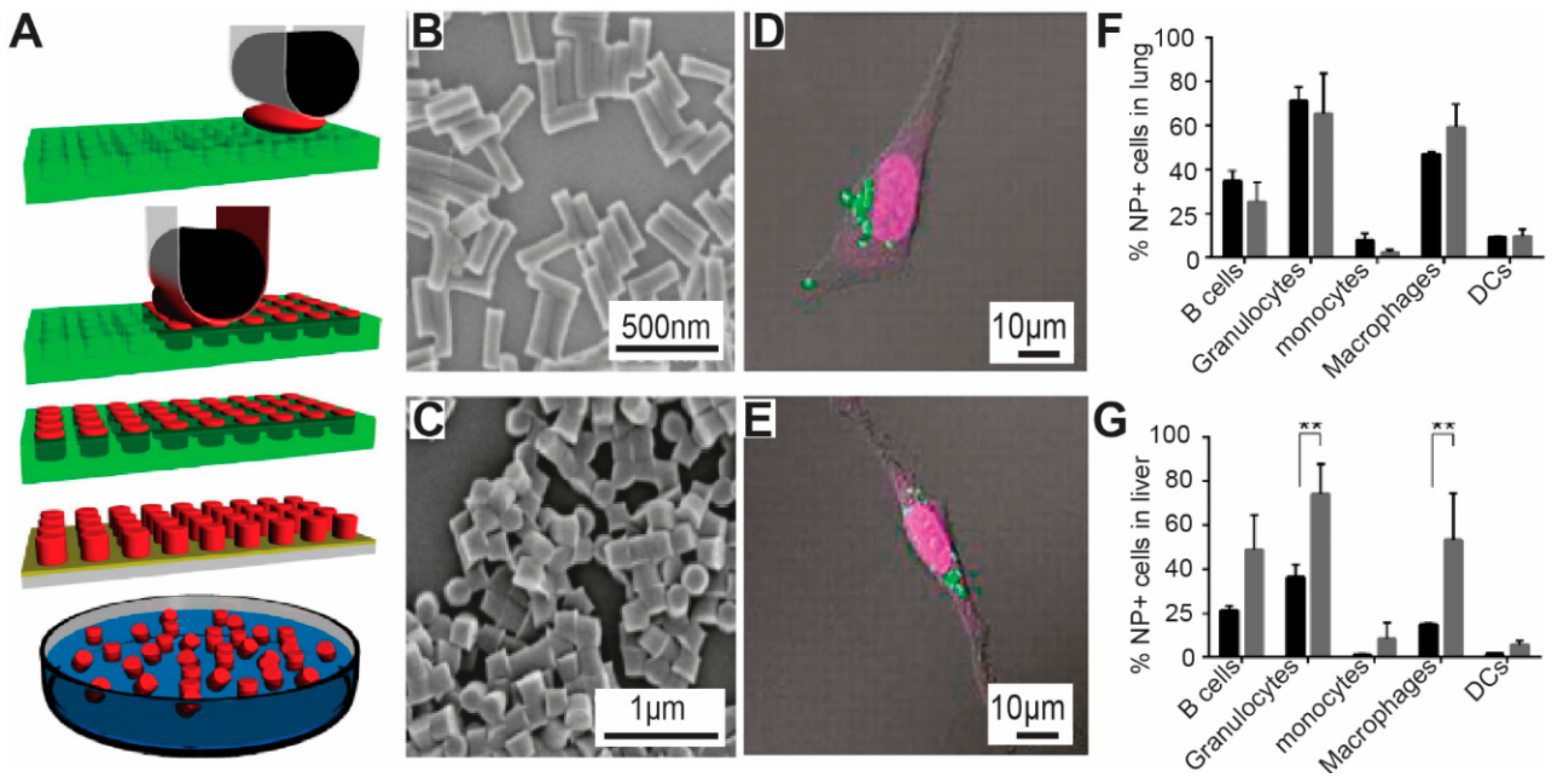
3.3. Nano-Imprint Lithography
3.4. Mechanical Stretching
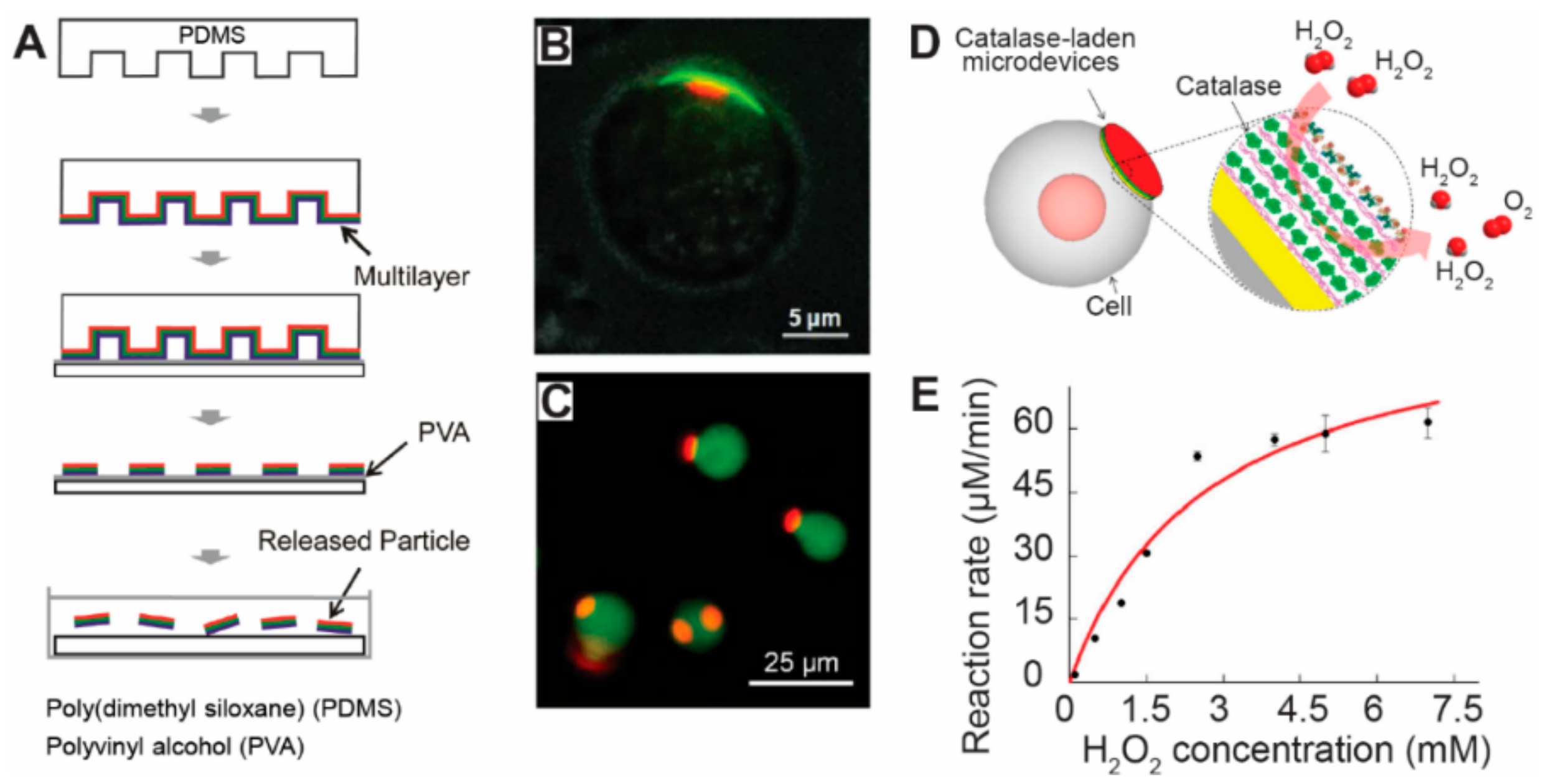
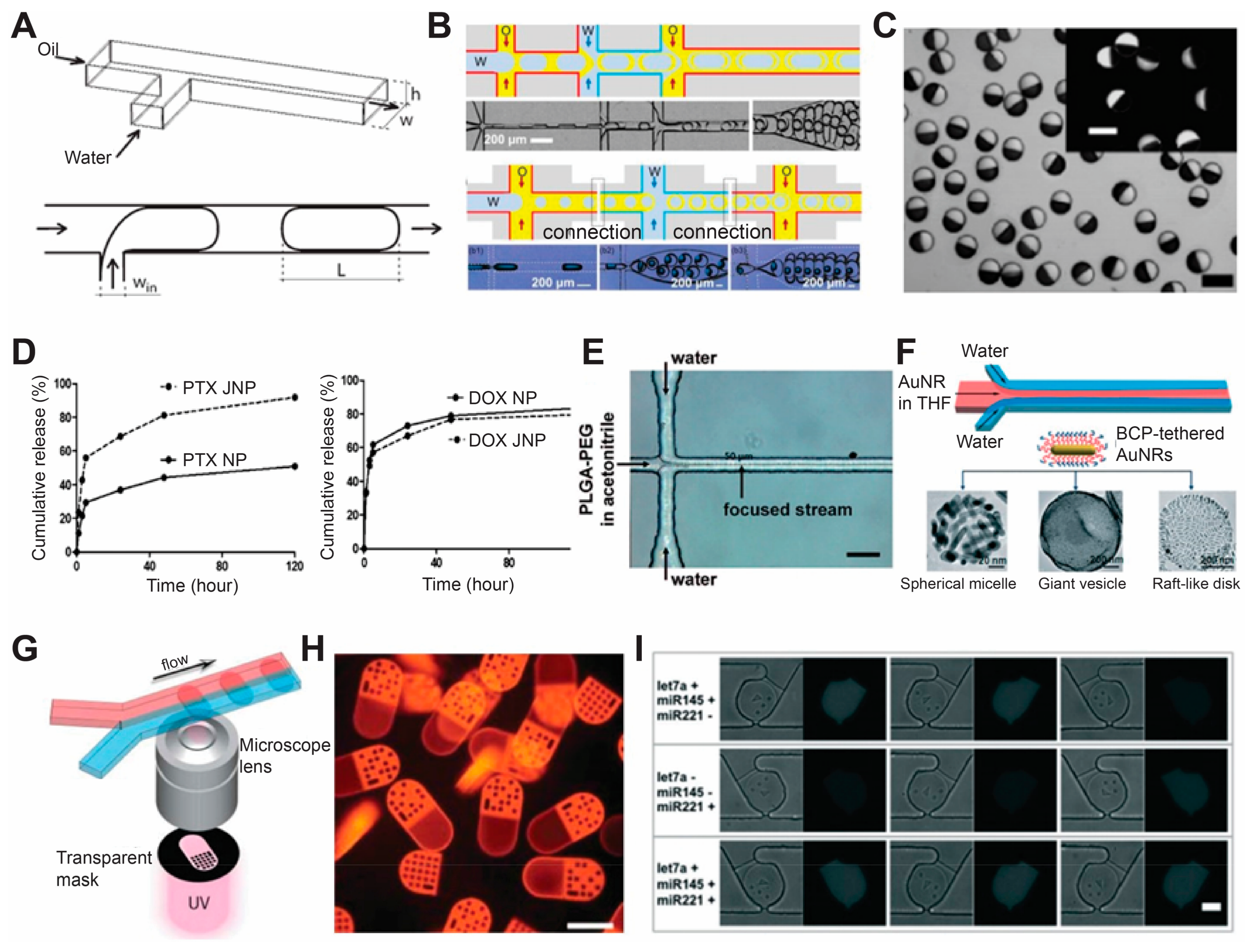
3.5. Microfluidic Fabrication
4. Conclusions
Conflicts of Interest
References
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Han, J.; Guck, J.; Espinosa, H. Micro and nanotechnology for biological and biomedical applications. Med. Biol. Eng. Comput. 2010, 48, 941–943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leucuta, S.E. Nanotechnology for delivery of drugs and biomedical applications. Curr. Clin. Pharmacol. 2010, 5, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Pieper, S.; Langer, K. Doxorubicin-loaded PLGA nanoparticles—A systematic evaluation of preparation techniques and parameters. Mater. Today Proc. 2017, 4, S188–S192. [Google Scholar] [CrossRef]
- Li, Z.B.; Huang, H.; Tang, S.Y.; Li, Y.; Yu, X.F.; Wang, H.Y.; Li, P.H.; Sun, Z.B.; Zhang, H.; Liu, C.L.; et al. Small gold nanorods laden macrophages for enhanced tumor coverage in photothermal therapy. Biomaterials 2016, 74, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Ali, H.R.; Rankin, C.R.; El-Sayed, M.A. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials 2016, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Liebman, H.A.; Bauer, K.A.; Caughey, T.; Campigotto, F.; Rosovsky, R.; Mantha, S.; Kessler, C.M.; Eneman, J.; Raghavan, V.; et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: A randomized-controlled phase II trial (the Microtec study). Br. J. Haematol. 2013, 160, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Merrett, K.; Gerasimov, M.; Griffith, M. Ocular applications of bioresorbable polymers-from basic research to clinical trials. Bioresorbable Polym. Biomed. Appl. Fundam. Transl. Med. 2017, 120, 497–523. [Google Scholar]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, G.; Farokhzad, O.C.; Mahmoudi, M. Biological Identity of Nanoparticles In Vivo: Clinical Implications of the Protein Corona. Trends Biotechnol. 2017, 35, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Maisel, K.; Ensign, L.; Reddy, M.; Cone, R.; Hanes, J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J. Control. Release 2015, 197, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.D.; Regulacio, M.D.; Ye, E.; Han, M.Y. Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 2013, 42, 6006–6018. [Google Scholar] [CrossRef] [PubMed]
- Mathaes, R.; Winter, G.; Engert, J.; Besheer, A. Application of different analytical methods for the characterization of non-spherical micro- and nanoparticles. Int. J. Pharm. 2013, 453, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Mathaes, R.; Winter, G.; Besheer, A.; Engert, J. Non-spherical micro- and nanoparticles: Fabrication, characterization and drug delivery applications. Expert Opin. Drug Deliv. 2015, 12, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Beletskii, A.; Galloway, A.; Rele, S.; Stone, M.; Malinoski, F. Engineered PRINT® nanoparticles for controlled delivery of antigens and immunostimulants. Hum. Vaccines Immunother. 2014, 10, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top-down and bottom-up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [PubMed]
- Hansen, H.N.; Carneiro, K.; Haitjema, H.; De Chiffre, L. Dimensional micro and nano metrology. CIRP Ann. Manuf. Technol. 2006, 55, 721–743. [Google Scholar] [CrossRef]
- Zammuto, R.F.; Oconnor, E.J. Gaining Advanced Manufacturing Technologies Benefits—The Roles of Organization Design and Culture. Acad. Manag. Rev. 1992, 17, 701–728. [Google Scholar]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.M.; Aizenberg, J.; Analoui, M.; Andrews, A.M.; Bisker, G.; Boyden, E.S.; Kamm, R.D.; Karp, J.M.; Mooney, D.J.; Oklu, R.; et al. Emerging Trends in Micro- and Nanoscale Technologies in Medicine: From Basic Discoveries to Translation. ACS Nano 2017, 11, 5195–5214. [Google Scholar] [CrossRef] [PubMed]
- Barkalina, N.; Charalambous, C.; Jones, C.; Coward, K. Nanotechnology in reproductive medicine: Emerging applications of nanomaterials. Nanomedicine 2014, 10, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.H.; Ho, D. Cancer Nanomedicine: From Drug Delivery to Imaging. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials (Basel) 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Maestas, D.R., Jr.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Leary, J.F. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int. J. Nanomed. 2014, 9, 711–726. [Google Scholar]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997, 14, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stoter, M.; et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.L.; Ho, Y.C.; Chen, Y.M.; Peng, S.F.; Ke, C.J.; Chen, K.J.; Mi, F.L.; Sung, H.W. The characteristics, cellular uptake and intracellular trafficking of nanoparticles made of hydrophobically-modified chitosan. J. Control. Release 2010, 146, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.L.; Chen, C.Y.; Zhao, Y.L. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Achouri, S.; Yoon, Y.Z.; Herre, J.; Bryant, C.E.; Cicuta, P. Phagocytosis Dynamics Depends on Target Shape. Biophys. J. 2013, 105, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Pratten, M.K.; Lloyd, J.B. Pinocytosis and Phagocytosis—The Effect of Size of a Particulate Substrate on Its Mode of Capture by Rat Peritoneal-Macrophages Cultured Invitro. Biochim. Biophys. Acta 1986, 881, 307–313. [Google Scholar] [CrossRef]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri, S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J. Control. Release 2015, 220, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and Immunological Response of Gold and Silver Nanoparticles of Different Sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.; Chow, A.H. Particle size analysis in pharmaceutics: Principles, methods and applications. Pharm. Res. 2007, 24, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Pradal, J.; Maudens, P.; Gabay, C.; Seemayer, C.A.; Jordan, O.; Allemann, E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int. J. Pharm. 2016, 498, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Restani, R.B.; Silva, A.S.; Pires, R.F.; Cabral, R.; Correia, I.J.; Casimiro, T.; Bonifacio, V.D.B.; Aguiar-Ricardo, A. Nano-in-Micro POxylated Polyurea Dendrimers and Chitosan Dry Powder Formulations for Pulmonary Delivery. Part. Part. Syst. Charact. 2016, 33, 851–858. [Google Scholar] [CrossRef]
- Malcolmson, R.J.; Embleton, J.K. Dry powder formulations for pulmonary delivery. Pharm. Sci. Technol. Today 1998, 1, 394–398. [Google Scholar] [CrossRef]
- Devrim, B.; Bozkir, A.; Canefe, K. Preparation and evaluation of poly(lactic-co-glycolic acid) microparticles as a carrier for pulmonary delivery of recombinant human interleukin-2: II. In vitro studies on aerodynamic properties of dry powder inhaler formulations. Drug Dev. Ind. Pharm. 2011, 37, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Dasari, R.K.; Berson, R.E. The effect of particle size on hydrolysis reaction rates and rheological properties in cellulosic slurries. Appl. Biochem. Biotechnol. 2007, 137, 289–299. [Google Scholar] [PubMed]
- Zhu, Z.J.; Carboni, R.; Quercio, M.J.; Yan, B.; Miranda, O.R.; Anderton, D.L.; Arcaro, K.F.; Rotello, V.M.; Vachet, R.W. Surface Properties Dictate Uptake, Distribution, Excretion, and Toxicity of Nanoparticles in Fish. Small 2010, 6, 2261–2265. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle-Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guan, J. Fabrication of multilayered microparticles by integrating layer-by-layer assembly and microcontact printing. Small 2011, 7, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Lestini, B.J.; Sagnella, S.M.; Xu, Z.; Shive, M.S.; Richter, N.J.; Jayaseharan, J.; Case, A.J.; Kottke-Marchant, K.; Anderson, J.M.; Marchant, R.E. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J. Control. Release 2002, 78, 235–247. [Google Scholar] [CrossRef]
- Zhang, P.P.; Qiao, Y.; Wang, C.M.; Ma, L.Y.; Su, M. Enhanced radiation therapy with internalized polyelectrolyte modified nanoparticles. Nanoscale 2014, 6, 10095–10099. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Fine, R.L.; Demento, S.; Bockenstedt, L.K.; Fahmy, T.M. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials 2011, 32, 3094–3105. [Google Scholar] [CrossRef] [PubMed]
- Bosker, W.T.E.; Iakovlev, P.A.; Norde, W.; Stuart, M.A.C. BSA adsorption on bimodal PEO brushes. J. Colloid Interface Sci. 2005, 286, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Blattler, T.M.; Pasche, S.; Textor, M.; Griesser, H.J. High salt stability and protein resistance of poly(L-lysine)-g-poly(ethylene glycol) copolymers covalently immobilized via aldehyde plasma polymer interlayers on inorganic and polymeric substrates. Langmuir 2006, 22, 5760–5769. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.; Mcintosh, T.J.; Lasic, D.D. Repulsive Interactions and Mechanical Stability of Polymer-Grafted Lipid-Membranes. Biochim. Biophys. Acta 1992, 1108, 40–48. [Google Scholar] [CrossRef]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2017, 7, 8798–8799. [Google Scholar] [CrossRef]
- Xu, Q.G.; Ensign, L.M.; Boylan, N.J.; Schon, A.; Gong, X.Q.; Yang, J.C.; Lamb, N.W.; Cai, S.T.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Alhalafi, A.M. Applications of polymers in intraocular drug delivery systems. Oman J. Ophthalmol. 2017, 10, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Yang, H.M.; Han, S.Y.; Zhao, D.Y.; Wang, G.Y. Adjuvant effect of polysaccharide from fruits of Physalis alkekengi L. in DNA vaccine against systemic candidiasis. Carbohydr. Polym. 2014, 109, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax (TM), a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S. Could recent advances in DNA-loaded nanoparticles lead to effective inhaled gene therapies? Nanomedicine 2016, 11, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, E.; Li, T.I.N.G.; Senesi, A.J.; Schmucker, A.L.; Pals, B.C.; de la Cruz, M.O.; Mirkin, C.A. DNA-mediated nanoparticle crystallization into Wulff polyhedra. Nature 2014, 505, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-gamma in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.K.; Suresh, P.K. Recent Advances in Ocular Drug Delivery, with Special Emphasis on Lipid Based Nanocarriers. Recent Pat. Nanotechnol. 2015, 9, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Y.; Du, J.; Li, Y.; Zhou, Y.; Fu, Q.; Zhang, J.; Wang, X.; Zhan, L. Different-Sized Gold Nanoparticle Activator/Antigen Increases Dendritic Cells Accumulation in Liver-Draining Lymph Nodes and CD8+ T Cell Responses. ACS Nano 2016, 10, 2678–2692. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Andorko, J.I.; Jewell, C.M. Impact of dose, route, and composition on the immunogenicity of immune polyelectrolyte multilayers delivered on gold templates. Biotechnol. Bioeng. 2017, 114, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Chiu, Y.C.; Tostanoski, L.H.; Jewell, C.M. Polyelectrolyte Multilayers Assembled Entirely from Immune Signals on Gold Nanoparticle Templates Promote Antigen-Specific T Cell Response. ACS Nano 2015, 9, 6465–6477. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gao, J.H.; Ai, H.; Chen, X.Y. Applications and Potential Toxicity of Magnetic Iron Oxide Nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Han, K.; Tang, Y.G.; Wang, B.D.; Lin, T.; Cheng, W.B. Recent advances in carbon nanodots: Synthesis, properties and biomedical applications. Nanoscale 2015, 7, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Niidome, T. Polyelectrolyte-coated gold nanorods and their biomedical applications. Nanoscale 2015, 7, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.L.; Desai, T.A. Micromachined devices: The impact of controlled geometry from cell-targeting to bioavailability. J. Control. Release 2005, 109, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Gallud, A.; Bondarenko, O.; Feliu, N.; Kupferschmidt, N.; Atluri, R.; Garcia-Bennett, A.; Fadeel, B. Macrophage activation status determines the internalization of mesoporous silica particles of different sizes: Exploring the role of different pattern recognition receptors. Biomaterials 2017, 121, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Mitragotri, S. Polymer particles that switch shape in response to a stimulus. Proc. Natl. Acad. Sci. USA 2010, 107, 11205–11210. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl. Acad. Sci. USA 2007, 104, 11901–11904. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-Based Drug Delivery Devices Using Phagocytosis-Resistant Backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.L.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Lee, S.; Bhushan, B.; Ferrari, M. A theoretical model for the margination of particles within blood vessels. Ann. Biomed. Eng. 2005, 33, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Prabhakarpandian, B.; Rea-Ramsey, A.; Pant, K.; Sundaram, S.; Mitragotri, S. Flow and adhesion of drug carriers in blood vessels depend on their shape: A study using model synthetic microvascular networks. J. Control. Release 2010, 146, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Adriani, G.; de Tullio, M.D.; Ferrari, M.; Hussain, F.; Pascazio, G.; Liu, X.W.; Decuzzi, P. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials 2012, 33, 5504–5513. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G. Nanotoxicology: In Vitro-in Vivo Dosimetry. Environ. Health Perspect. 2012, 120, A13. [Google Scholar] [CrossRef] [PubMed]
- Seleverstov, O.; Zabirnyk, O.; Zscharnack, M.; Bulavina, L.; Nowicki, M.; Heinrich, J.M.; Yezhelyev, M.; Emmrich, F.; O’Regan, R.; Bader, A. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 2006, 6, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.; Feng, C.; Wen, L.P. Nano neodymium oxide induces massive vacuolization and autophagic cell death in non-small cell lung cancer NCI-H460 cells. Biochem. Biophys. Res. Commun. 2005, 337, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Thubagere, A.; Reinhard, B.M. Nanoparticle-Induced Apoptosis Propagates through Hydrogen-Peroxide-Mediated Bystander Killing: Insights from a Human Intestinal Epithelium In Vitro Model. ACS Nano 2010, 4, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Davoren, M.; Lyng, F.M.; Byrne, H.J. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol. Appl. Pharmacol. 2010, 246, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Liu, H.L.; Sun, Y.; Wang, H.L.; Guo, F.; Rao, S.A.; Deng, J.J.; Zhang, Y.L.; Miao, Y.F.; Guo, C.Y.; et al. PAMAM Nanoparticles Promote Acute Lung Injury by Inducing Autophagic Cell Death through the Akt-TSC2-mTOR Signaling Pathway (vol 1, pg 37, 2009). J. Mol. Cell Biol. 2010, 2, 103. [Google Scholar] [CrossRef]
- Bottini, M.; Bruckner, S.; Nika, K.; Bottini, N.; Bellucci, S.; Magrini, A.; Bergamaschi, A.; Mustelin, T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006, 160, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Stilwell, J.; Zhang, T.; Elboudwarej, O.; Jiang, H.; Selegue, J.P.; Cooke, P.A.; Gray, J.W.; Chen, F.F. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005, 5, 2448–2464. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.B.; Smith, C.G.; Rennie, A.R. Fabricating colloidal particles with photolithography and their interactions at an air-water interface. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62 Pt B, 951–960. [Google Scholar] [CrossRef]
- Revzin, A.; Russell, R.J.; Yadavalli, V.K.; Koh, W.G.; Deister, C.; Hile, D.D.; Mellott, M.B.; Pishko, M.V. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir 2001, 17, 5440–5447. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Park, C.; Whitesides, G.M. Generation of submicrometer structures by photolithography using arrays of spherical microlenses. J. Colloid Interface Sci. 2003, 265, 304–309. [Google Scholar] [CrossRef]
- Badaire, S.; Cottin-Bizonne, C.; Woody, J.W.; Yang, A.; Stroock, A.D. Shape selectivity in the assembly of lithographically designed colloidal particles. J. Am. Chem. Soc. 2007, 129, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Conley, W.; Garza, C. Trends in photolithography materials. Photochemistry 2010, 38, 369–387. [Google Scholar]
- Hernandez, C.J.; Mason, T.G. Colloidal alphabet soup: Monodisperse dispersions of shape-designed LithoParticles. J. Phys. Chem. C 2007, 111, 4477–4480. [Google Scholar] [CrossRef]
- Tasciotti, E.; Liu, X.; Bhavane, R.; Plant, K.; Leonard, A.D.; Price, B.K.; Cheng, M.M.; Decuzzi, P.; Tour, J.M.; Robertson, F.; et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat. Nanotechnol. 2008, 3, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Mann, A.; Ferrari, M.; Tanaka, T. Mesoporous silicon particles for sustained gene silencing. Methods Mol. Biol. 2013, 1049, 481–493. [Google Scholar] [PubMed]
- Tanaka, T.; Mangala, L.S.; Vivas-Mejia, P.E.; Nieves-Alicea, R.; Mann, A.P.; Mora, E.; Han, H.D.; Shahzad, M.M.; Liu, X.; Bhavane, R.; et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010, 70, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Godin, B.; Oborn, C.J.; Alexander, J.F.; Liu, X.; Fidler, I.J.; Ferrari, M. Porous silicon nanocarriers for dual targeting tumor associated endothelial cells and macrophages in stroma of orthotopic human pancreatic cancers. Cancer Lett. 2013, 334, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Godin, B.; Bhavane, R.; Nieves-Alicea, R.; Gu, J.; Liu, X.; Chiappini, C.; Fakhoury, J.R.; Amra, S.; Ewing, A.; et al. In vivo evaluation of safety of nanoporous silicon carriers following single and multiple dose intravenous administrations in mice. Int. J. Pharm. 2010, 402, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Swiston, A.J.; Cheng, C.; Um, S.H.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008, 8, 4446–4453. [Google Scholar] [CrossRef] [PubMed]
- Polak, R.; Lim, R.M.; Beppu, M.M.; Pitombo, R.N.; Cohen, R.E.; Rubner, M.F. Liposome-Loaded Cell Backpacks. Adv. Healthc. Mater. 2015, 4, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Gilbert, J.B.; Kumar, S.; Gupta, V.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: A generalized strategy to deliver drugs to treat inflammation. J. Control. Release 2015, 199, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Bookstaver, M.L.; Jewell, C.M. Engineering Cell Surfaces with Polyelectrolyte Materials for Translational Applications. Polymers 2017, 9, 40. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Bhise, N.S.; Evangelista, M.B.; Rouwkema, J.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Vrana, N.E.; Khademhosseini, A. Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends Biotechnol. 2016, 34, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Hortensius, R.A.; Harley, B.A. Naturally derived biomaterials for addressing inflammation in tissue regeneration. Exp. Biol. Med. (Maywood) 2016, 241, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Csucs, G.; Michel, R.; Lussi, J.W.; Textor, M.; Danuser, G. Microcontact printing of novel co-polymers in combination with proteins for cell-biological applications. Biomaterials 2003, 24, 1713–1720. [Google Scholar] [CrossRef]
- Sathish, S.; Ricoult, S.G.; Toda-Peters, K.; Shen, A.Q. Microcontact printing with aminosilanes: Creating biomolecule micro- and nanoarrays for multiplexed microfluidic bioassays. Analyst 2017, 142, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Shuster, M.J.; Vaish, A.; Cao, H.H.; Guttentag, A.I.; McManigle, J.E.; Gibb, A.L.; Martinez-Rivera, M.; Nezarati, R.M.; Hinds, J.M.; Liao, W.S.; et al. Patterning small-molecule biocapture surfaces: Microcontact insertion printing vs. photolithography. Chem. Commun. 2011, 47, 10641–10643. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Bhushan, R. Microcontact printing in bioanalysis: Where are we and where shall we be? Bioanalysis 2016, 8, 2093–2095. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; He, H.; Lee, L.J.; Hansford, D.J. Fabrication of particulate reservoir-containing, capsulelike, and self-folding polymer microstructures for drug delivery. Small 2007, 3, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Ferrell, N.; James Lee, L.; Hansford, D.J. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials 2006, 27, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Shin, C.S.; McDermott, M.; Mishra, H.; Park, H.; Kwon, I.C.; Park, K. The hydrogel template method for fabrication of homogeneous nano/microparticles. J. Control. Release 2010, 141, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Acharya, G.; Shin, C.S.; Vedantham, K.; McDermott, M.; Rish, T.; Hansen, K.; Fu, Y.; Park, K. A study of drug release from homogeneous PLGA microstructures. J. Control. Release 2010, 146, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sturek, M.; Park, K. Microparticles produced by the hydrogel template method for sustained drug delivery. Int. J. Pharm. 2014, 461, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Xia, J.; Wang, Z.; Kirkland, B.; Guan, J. Top-down fabrication of polyelectrolyte-thermoplastic hybrid microparticles for unidirectional drug delivery to single cells. Adv. Healthc. Mater. 2013, 2, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.F.; Wang, Z.B.; Huang, D.T.; Yan, Y.W.; Li, Y.; Guan, J.J. Asymmetric Biodegradable Microdevices for Cell-Borne Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 6293–6299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xia, J.; Wang, Z.; Guan, J. Gold nanoparticle-packed microdisks for multiplex Raman labelling of cells. Nanoscale 2014, 6, 8762–8768. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.F.; Wang, Z.B.; Yan, Y.W.; Cheng, Z.J.; Sun, L.; Li, Y.; Ren, Y.; Guan, J.J. Catalase-Laden Microdevices for Cell-Mediated Enzyme Delivery. Langmuir 2016, 32, 13386–13393. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, S.; Li, Z. Nanoimprint lithography for nanodevice fabrication. Nano Converg. 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Glangchai, L.C.; Caldorera-Moore, M.; Shi, L.; Roy, K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J. Control. Release 2008, 125, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Pease, R.F. Nanoimprint lithography 20 years on. Nanotechnology 2015, 26, 182501. [Google Scholar] [CrossRef] [PubMed]
- Traub, M.C.; Longsine, W.; Truskett, V.N. Advances in Nanoimprint Lithography. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Truskett, V.N.; Watts, M.P. Trends in imprint lithography for biological applications. Trends Biotechnol. 2006, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.G.; Perry, J.L.; Kim, D.; Luft, J.C.; Liu, R.; DeSimone, J.M. Targeted PRINT Hydrogels: The Role of Nanoparticle Size and Ligand Density on Cell Association, Biodistribution, and Tumor Accumulation. Nano Lett. 2015, 15, 6371–6378. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.P.; Brighton, H.E.; Fromen, C.A.; Shen, T.W.; Luft, J.C.; Luft, Y.E.; Keeler, A.W.; Robbins, G.R.; Ting, J.P.; Zamboni, W.C.; et al. Tumor Presence Induces Global Immune Changes and Enhances Nanoparticle Clearance. ACS Nano 2016, 10, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Fromen, C.A.; Rahhal, T.B.; Robbins, G.R.; Kai, M.P.; Shen, T.W.; Luft, J.C.; DeSimone, J.M. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomedicine 2016, 12, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Padmore, T.; Stark, C.; Turkevich, L.A.; Champion, J.A. Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages. Biochim. Biophys. Acta 2017, 1861, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Sunshine, J.C.; Perica, K.; Kosmides, A.K.; Aje, K.; Schneck, J.P.; Green, J.J. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small 2015, 11, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, B. Controlled formation of double-emulsion drops in sudden expansion channels. J. Colloid Interface Sci. 2014, 415, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up (vol 6, pg 437, 2006). Lab Chip 2006, 6, 693. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.H.; Li, W.; Seo, M.; Xu, S.Q.; Kumacheva, E. Janus and ternary particles generated by microfluidic synthesis: Design, synthesis, and self-assembly. J. Am. Chem. Soc. 2006, 128, 9408–9412. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; She, Z.G.; Wang, S.; Sharma, G.; Smith, J.W. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir 2012, 28, 4459–4463. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wei, Z.J.; Wang, L.; Tomova, Z.; Babu, T.; Wang, C.Y.; Han, X.J.; Fourkas, J.T.; Nie, Z.H. Hydrodynamically Driven Self-Assembly of Giant Vesicles of Metal Nanoparticles for Remote-Controlled Release. Angew. Chem. Int. Ed. 2013, 52, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.L.; Lin, Y.; Yang, C.X.; Manocchi, A.K.; Yuet, K.P.; Doyle, P.S.; Yi, H. Microfluidic Fabrication of Hydrogel Microparticles Containing Functionalized Viral Nanotemplates. Langmuir 2010, 26, 13436–13441. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, D.C.; Chapin, S.C.; Doyle, P.S. Multiplexed Protein Quantification with Barcoded Hydrogel Microparticles. Anal. Chem. 2011, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar] [CrossRef]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry Can Make Strict and Fuzzy Controls for Bio-Systems: DNA Nanoarchitectonics and Cell-Macromolecular Nanoarchitectonics. Bull. Chem. Soc. Jpn. 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Ariga, K.; Kawakami, K.; Ebara, M.; Kotsuchibashi, Y.; Ji, Q.M.; Hill, J.P. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J. Chem. 2014, 38, 5149–5163. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Xia, J.; Luo, S. Generation of Well-Defined Micro/Nanoparticles via Advanced Manufacturing Techniques for Therapeutic Delivery. Materials 2018, 11, 623. https://doi.org/10.3390/ma11040623
Zhang P, Xia J, Luo S. Generation of Well-Defined Micro/Nanoparticles via Advanced Manufacturing Techniques for Therapeutic Delivery. Materials. 2018; 11(4):623. https://doi.org/10.3390/ma11040623
Chicago/Turabian StyleZhang, Peipei, Junfei Xia, and Sida Luo. 2018. "Generation of Well-Defined Micro/Nanoparticles via Advanced Manufacturing Techniques for Therapeutic Delivery" Materials 11, no. 4: 623. https://doi.org/10.3390/ma11040623
APA StyleZhang, P., Xia, J., & Luo, S. (2018). Generation of Well-Defined Micro/Nanoparticles via Advanced Manufacturing Techniques for Therapeutic Delivery. Materials, 11(4), 623. https://doi.org/10.3390/ma11040623




