Abstract
As a zero-carbon fuel, ammonia holds significant potential for achieving the “dual carbon” strategic goals. However, its extremely low laminar burning velocity (LBV) limits its direct application in combustion systems. This work systematically reviews the research progress on the LBV of ammonia flames, focusing on three key aspects: measurement methods, effects of combustion conditions, and reaction kinetic models. In terms of measurement methods, the principles, applicability, and limitations of the spherical outwardly propagating flame method, Bunsen-burner method, counter-flow flame method, and heat flux method are discussed in detail. It is pointed out that the heat flux method and counter-flow flame method are more suitable for the accurate measurement of ammonia flame LBV due to their low stretch rate and high stability. Regarding the effects of combustion conditions, the LBV characteristics of pure ammonia flames under ambient temperature and pressure are summarized. The influence patterns of three factors on LBV are analyzed systematically: blending high-reactivity fuels (e.g., hydrogen and methane), oxygen-enriched conditions, and variations in temperature and pressure. This analysis reveals effective approaches to improve ammonia combustion performance. Furthermore, the promoting effect of high-reactivity fuel blending on liquid ammonia combustion was also summarized. For reaction kinetic models, various chemical reaction mechanisms applicable to pure ammonia and ammonia-blended fuels (ammonia/hydrogen, ammonia/methane, etc.) are sorted out. The performance and discrepancies of each model in predicting LBV are evaluated. It is noted that current models still have significant uncertainties under specific conditions, such as high pressure and moderate blending ratios. This review aims to provide theoretical references and data support for the fundamental research and engineering application of ammonia combustion, promoting the development and application of ammonia as a clean fuel.
1. Introduction
The combustion of conventional fossil fuels releases substantial amounts of carbon dioxide, exerting adverse impacts on the global climate [1,2,3,4,5,6]. Consequently, researchers in both industry and academia worldwide have been intensively seeking economically viable and environmentally benign alternatives. In this context, several member states of the European Union and Japan have pledged to achieve carbon neutrality by 2050, while China has committed to peak its carbon emissions before 2030 and attain carbon neutrality by 2060 [7]. To fulfill the “dual carbon” strategy, the adoption of zero-carbon ammonia as a combustible fuel has emerged as a crucial measure [8]. Under ideal conditions, the complete combustion of ammonia yields only nitrogen and water, with no carbon emissions compared to traditional hydrocarbon fuels. Moreover, ammonia demonstrates additional advantages in terms of economic feasibility and safety when utilized as a substitute energy carrier.
1.1. Characteristics and Advantages of Ammonia Fuel
Natural gas is the primary gaseous fuel at the current stage, and the zero-carbon candidates proposed to replace it are hydrogen and ammonia. The physicochemical properties of the three fuels are compared in Table 1 [9]. Hydrogen is extremely difficult to liquefy at room temperature and has a low volumetric energy density (12.1 MJ/m3). Additionally, hydrogen suffers from issues such as high flammability and explosiveness, as well as great challenges in large-scale storage and transportation. As an efficient carrier of hydrogen (H) element, ammonia is referred to as “the other hydrogen” and exhibits the following prominent advantages:

Table 1.
Thermal property parameters of gas fuel.
- (1)
- It is a carbon-free fuel, with no greenhouse gas emissions during its application;
- (2)
- Its energy density is 22.5 MJ/kg, which is comparable to that of conventional fossil fuels. For instance, the calorific value of low-rank coal is approximately 20 MJ/kg;
- (3)
- At room temperature its condensation pressure is only 0.99 MPa, and its boiling point is −33.4 °C under atmospheric pressure. Moreover, the volumetric energy density of liquid ammonia exceeds that of liquid hydrogen by 45% and is virtually identical to that of gasoline, making it a prospective transportation fuel;
- (4)
- Ammonia has a pungent odor. Humans can detect this odor at volume fractions as low as 5 × 10−6, which provides an inherent leak-warning capability;
- (5)
- The large-scale industrial production of ammonia is relatively mature and well-suited to China’s national conditions. China processes abundant renewable energy resources, which can be used to synthesize green ammonia through a fully decarbonized industrial process.
1.2. Challenges in Ammonia Combustion
Research on the application of ammonia fuel in gas turbines, power plant boilers, industrial boilers, and other fields is led by Japan, which is at an advanced level [10,11]. However, in practical applications, ammonia combustion exhibits certain drawbacks, with two key challenges being the most prominent. First, ammonia has an extremely low laminar burning velocity (LBV). When ammonia burns in air, the maximum LBV occurs at an equivalence ratio of approximately 1.1. Its peak value is only 7 cm/s. Under the same conditions, this is merely one-fifth of methane’s LBV and less than one-fortieth of hydrogen’s. This extremely low LBV makes it difficult to maintain stable combustion of ammonia in internal combustion engines and gas turbines, resulting in reduced combustion efficiency, impaired power output, and increased pollutant emissions. Second, ammonia-fueled systems are predisposed to high nitrogen-oxide (NOx) formation. Due to the presence of nitrogen atoms in ammonia, large amounts of fuel-bound NOx are generated during its combustion. The formation mechanism of this fuel-bound NOx differs from that of thermal NOx. Thermal NOx is produced by the combustion of hydrocarbon fuels. This difference means that traditional NOx control methods may be ineffective.
Although ammonia combustion faces the aforementioned challenges, satisfactory performance can still be attained. This is possible if appropriate technical measures are adopted. Particularly for addressing the challenge of ammonia’s low LBV, a variety of methods have been developed. These methods include co-combustion with high-reactivity fuels, oxygen-enriched combustion, and adjustment of combustion conditions (e.g., temperature and pressure). Therefore, it is necessary to conduct a comprehensive review of the research progress on the LBV of ammonia combustion.
1.3. Research Value of Laminar Burning Velocity of Ammonia Flames
The LBV is an inherent parameter of ammonia fuel that quantifies the amount of ammonia consumed per unit area of the flame front per unit time. It is controlled by the equivalence ratio, temperature, and pressure. As a crucial indicator for ammonia and ammonia-blended fuels, LBV conveys fundamental information on fuel reactivity, diffusivity, and exothermicity. Additionally, LBV is closely associated with combustion oscillations, flashback, blow-off characteristics, and the development of kinetic mechanisms, and it also serves as the basis for studying turbulent flame velocity. In practical engineering applications, to enhance the combustion heat intensity of combustion equipment and reduce the size of such equipment, efforts should be made to maximize LBV as much as possible. Consequently, LBV research on ammonia combustion should focus on three aspects: the measurement methods of LBV, the specific characteristics of LBV, and the reaction kinetic models related to ammonia.
Owing to the extremely low LBV of ammonia, its accurate measurement poses multiple challenges—specifically, stringent requirements for minimizing heat losses from the experimental apparatus and precisely controlling flame stretch. From the perspective of methodological evolution, common approaches include the spherical outwardly propagating flame method, the Bunsen-burner method, the counter-flow and stagnation-flame method, and the heat flux method. Ammonia combustion research has developed in a diversified way. For example, operating parameters (e.g., blended components, initial temperature, and pressure) have changed. These changes exert complex impacts on LBV measurement. Consequently, the applicability and accuracy boundaries of existing measurement methods have been continuously re-examined. A systematic review of their technical evolution, core principles, and application limitations is therefore urgently needed to provide guidance for future experimental investigations and engineering applications.
The specific characteristics of ammonia’s LBV exhibit diversity. Pure ammonia flames possess extremely weak flame propagation capability, thus, summarizing relevant experimental data can provide a fundamental reference for comparative studies under other operating conditions. Blending ammonia with high-reactivity fuels has emerged as the most straightforward method to enhance its LBV, with common high-reactivity fuels including hydrogen and methane, whereas oxygen-enriched combustion also yields a pronounced enhancement of ammonia’s LBV. In terms of thermodynamic parameter regulation, temperature and pressure exert distinctly different influences on ammonia’s LBV. However, there is still a gap in summarizing two aspects: the effects of various factors on ammonia flame LBV, and the action mechanisms of these factors. Consequently, two tasks are essential for improving the theoretical system of ammonia combustion and guiding its engineering applications. First, systematically collate neat-ammonia LBV data obtained at room temperature and pressure. Second, conduct an in-depth analysis of how four factors (fuel blending, oxygen enrichment, temperature, pressure) regulate both the magnitude of LBV enhancement and its underlying mechanisms.
Corresponding to experimental measurements, calculating the ammonia-related LBV through chemical reaction kinetics has become a common research method. For this purpose, a large number of kinetic models have been developed. It is necessary to summarize the development of these kinetic models, categorizing them by fuel type—such as pure ammonia, ammonia/hydrogen, ammonia/methane, and ammonia/hydrogen/methane blends—to provide a reference for researchers in selecting and applying the most appropriate ones.
1.4. Objectives and Structure of This Work
This work aims to provide a comprehensive review of the research progress on the LBV of ammonia fuel. It offers references for researchers in this field from three aspects:
(1) Selecting appropriate LBV measurement methods for ammonia flames based on experimental conditions;
(2) Understanding the laws and mechanisms of how combustion conditions affect the LBV of ammonia, which is conducive to improving the theory of ammonia combustion and promoting the engineering application of ammonia combustion;
(3) Systematically organizing the various ammonia combustion models to facilitate researchers in searching for and downloading the models.
The work consists of three main parts: Section 2 surveys advances in LBV measurement methods for ammonia combustion; Section 3 presents the specific characteristics of LBV related to ammonia combustion, covering most of the conditions that currently exert an influence on LBV; and Section 4 elaborates on the development of reaction kinetic models associated with ammonia fuel. Finally, concluding remarks and recommendations for future research are provided.
2. Measurement Methods of Laminar Burning Velocity for Ammonia Flames
The laminar burning velocity (LBV) is defined as the velocity of a premixed laminar flame relative to the unburned incoming gas in an infinitely long one-dimensional space. In their extensive review, Konnov et al. [12] proposed that the ideal flame conditions for LBV measurement should simultaneously satisfy three criteria:
- (1)
- Steady state: Neither the flame nor the flow field varies with time;
- (2)
- Adiabaticity: Heat released by the flame is solely used to heat unburned substances—no losses by radiation, conduction or convection to the surroundings;
- (3)
- One-dimensional free diffusion: The flow field is unobstructed, with infinitely long spaces both upstream and downstream of the flame.
However, under laboratory conditions, it is impossible to create flames that strictly meet the above criteria for ammonia combustion. Consequently, current mainstream LBV measurement methods for ammonia all incorporate specific assumptions and approximations, which can be systematically analyzed based on different measurement principles.
2.1. Spherical Outwardly Propagating Flame Method
The spherical outwardly propagating flame method was proposed by Nair and Gupta in 1974 [13]. For LBV measurement, a combustible mixture is used. This mixture has a prescribed equivalence ratio, temperature, and pressure, and it is filled into a sealed, optically accessible vessel. After central ignition, the ensuing outwardly propagating spherical flame is recorded by high-speed schlieren cinematography. By analyzing the variation in the flame propagation radius with time, the LBV can be derived [14], and the experimental system is shown in Figure 1.
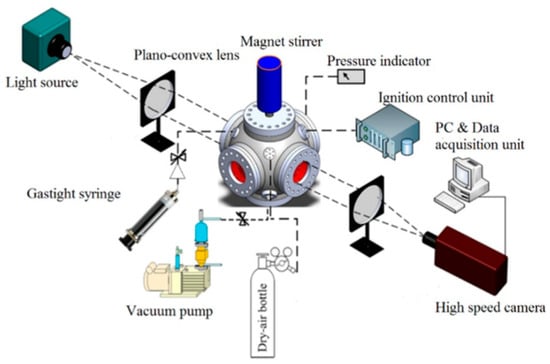
Figure 1.
Schematic of the experimental system for the spherical outwardly propagating flame method [15].
The spherical outwardly propagating flame method offers quasi-adiabatic conditions and unobstructed flame propagation, which facilitate the measurement of flame speeds under high-pressure environments. However, it also has notable drawbacks: the flame is subject to stretch effects, necessitating extrapolation correction for stretch; the ignition energy and initial flame propagation time are difficult to determine accurately; and flame instability can interfere with the identification of the flame edge. In the LBV measurement of pure ammonia and ammonia-blended fuels, the impact of such instability becomes even more prominent. Due to the low LBV of ammonia, the flame is severely affected by buoyancy-induced instability [16], with the visual manifestation shown in Figure 2. Therefore, for ammonia flames, the spherical outwardly propagating flame method is not recommended for LBV measurement. This recommendation can be revised only if specific measures are employed, such as blending with high-reactivity fuels, oxygen-enriched combustion, and elevated temperature, to mitigate instability and enhance flame propagation.
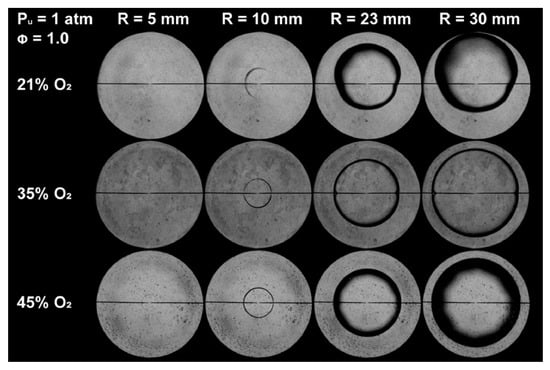
Figure 2.
Instability observed in ammonia flame during LBV measurement using the spherical outwardly propagating flame method [16].
2.2. Bunsen-Burner Method
The Bunsen-burner method, originally developed by Bunsen in 1855, is the simplest and most mature method for measuring the LBV of stable premixed flames. A large number of early studies adopted this method, and its measurement system is illustrated in Figure 3 [17]. The combustion front of a Bunsen flame can be simplified as a three-dimensional conical surface. The LBV can be calculated using either the cone angle method or the area method, as shown in Figure 4 and Equations (1) and (2). In these equations, SL denotes the LBV, ug is the flow velocity of the unburned mixture, Q represents the volumetric flow rate of the premixed gas, and Af stands for the surface area of the flame. However, the method has a notable downside that counterbalances its advantages of experimental simplicity and ease of operation. It introduces systematic errors, which stem from excessive simplifications inherent in the Bunsen-burner method. Real flames rarely assume an ideal cone shape; unavoidable stretch effects exist across different regions of the flame. Meanwhile, heat dissipation from the flame is also difficult to quantify accurately, and flame stabilization becomes problematic at elevated pressures [18].
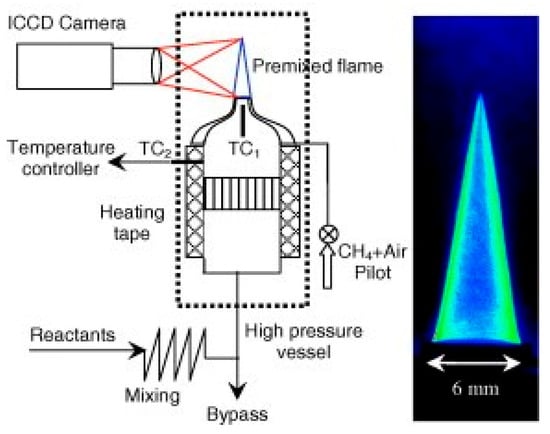
Figure 3.
Schematic of the experimental system for the Bunsen-burner method [17].
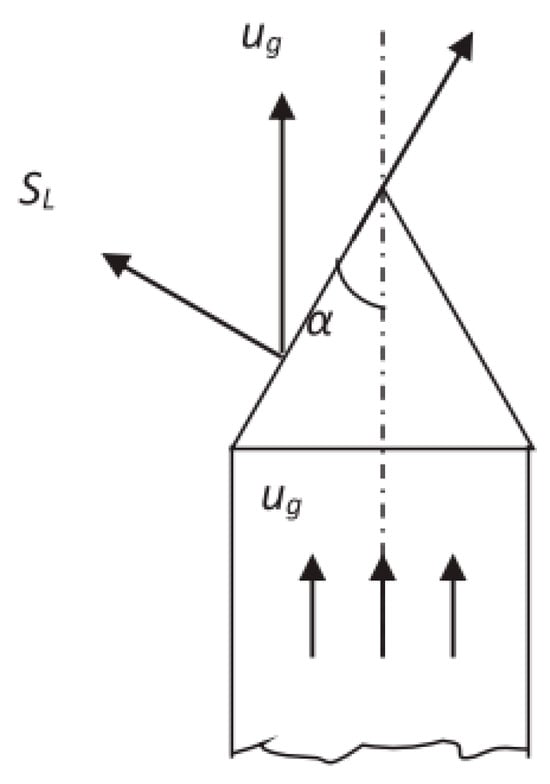
Figure 4.
Schematic illustration of the principle of the cone-angle method for LBV measurement.
To minimize these errors, improvements can be made from three aspects: First, the conical flame area method should be adopted instead of the cone angle method. Due to the excessively high local stretch rates at the top and bottom of the flame, the LBV values calculated at different positions of the flame front vary significantly [12]. The area method averages the effect of flame stretch over the entire flame, thereby avoiding large errors caused by this issue. Second, the flame edge identification method should be optimized to improve the accuracy of flame area calculation, which can further reduce errors induced by stretch. Precise identification of the flame edge ensures that the measured flame surface area more closely reflects the actual combustion front, thereby enhancing the reliability of LBV calculations. Third, according to reports by Natarajan et al. [17,19], flames with a larger aspect ratio (flame height/nozzle width > 1) can form a longer flame shoulder, which allows the stretch effect to be neglected. The extended flame shoulder region exhibits more uniform flow and stretch characteristics, making it more suitable for approximating the ideal one-dimensional flame condition required for accurate LBV measurement. Meanwhile, reconfiguring the traditional Bunsen burner into a Mache–Hebra nozzle burner—by means of structural optimization—can also improve flame stability and mitigate flame stretch effects. As shown in Figure 3, the Mache–Hebra nozzle burner has two key structural features: a relatively wide inner diameter at the inlet section, and a tapered shape near the nozzle exit. This structure ensures a more uniform spatial gas velocity distribution at the outlet.
Despite the above improvements, a critical issue persists when measuring the LBV of pure ammonia fuel: the extremely low LBV of pure ammonia flames—combined with heat dissipation effects—makes it nearly impossible for the Bunsen flame to stabilize at the burner outlet under atmospheric temperature and pressure. In most cases, flame blow-off is highly likely to occur. Therefore, similar to the spherical outwardly propagating flame method, the Bunsen burner method is recommended for use in studying ammonia flames under modified conditions—such as ammonia blended with high-reactivity fuels, oxygen-enriched combustion, elevated temperature, or increased pressure. Meanwhile, to enhance the accuracy of flame edge identification, it is preferable to utilize radicals such as OH* and CH* for this purpose [17,20], rather than relying on visible light. This approach avoids the inaccuracies associated with visible light detection, which can be easily affected by flame luminosity variations and background interference, especially for low-intensity pure ammonia flames.
2.3. Counter-Flow Flame and Stagnation Flame Method
In 1957, Simmons and Wolfhard first proposed the counter-flow flame configuration [21], and since 1986, Law and co-workers have further advanced its development [13,22]. Currently, the counter-flow flame method for measuring LBV and stretch rate has become quite mature, with a large number of relevant literature reports available [23,24]. For premixed combustion, the premixed fuel/air mixture is supplied through two nozzles, creating two premixed flat flames separated by a stagnation plane (as shown in Figure 5). At the flame surface, the flow velocity of the premixed gas is equal in magnitude but opposite in direction to the LBV. A stagnation flame can be regarded as a special type of counter-flow flame. The key difference lies in that one of the opposing nozzles is replaced with a bluff body, which serves to stabilize the flame. This modification optimizes the flow field distribution near the flame, which enables more stable combustion. This stability is an advantage that is particularly valuable for measuring the LBV of low-reactivity fuels like ammonia.
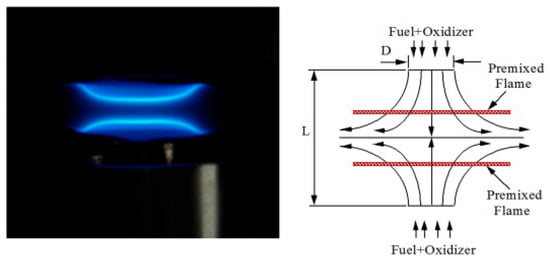
Figure 5.
Schematic diagram of the counter-flow flame and its experimental system [23].
The counter-flow flame offers the following advantages: it exhibits high stability and can maintain a steady flame for an extended period. Meanwhile, in the dual-flame configuration, due to symmetry, there is no heat conduction loss downstream of the stable flame, and radiative heat loss is negligible [23]. Thus, the only external influence is the hydrodynamic stretch caused by the gas flow field. However, the flame’s stretch rate can be adjusted by modifying the gas flow rate or nozzle distance. Considering the characteristics of ammonia flames—such as low LBV, high susceptibility to flame extinction due to gas flow disturbances, and sensitivity to flow stretch rate—the counter-flow flame method is well-suited for measuring the LBV of ammonia flames.
2.4. Heat Flux Method
Around 2000, De Goey and co-workers developed the heat flux method [25,26], as illustrated in Figure 6. The heat flux method can also generate a planar flame. However, it differs from the counter-flow flame and stagnation flame methods. Unlike the planar flames formed by those two methods, the flame from the heat flux method is free from stretch effects. Although stretch complicates data post-processing, it helps stabilize the flame during experiments [18]. Consequently, a stretch-free planar flame is inherently unstable. It readily blows off or flashes back unless special precautions are taken. In the heat flux method, the design of the heat flux burner is the most critical component. This burner ensures that all heat released by the flame is used to heat the unburned mixture, creating an adiabatic flame. This design guarantees that the incoming flow velocity of the fuel is equivalent to the unstretched adiabatic laminar burning velocity.
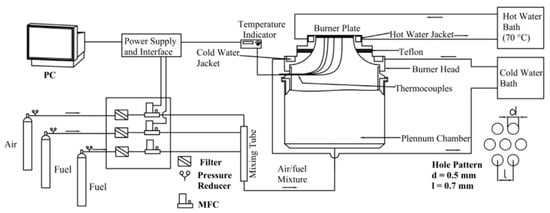
Figure 6.
Schematic of the experimental system for LBV measurement using the heat flux method [12].
The most prominent advantage of the heat flux method lies in its ability to directly obtain an adiabatic, unstretched flame without the need for extrapolation or other analytical techniques. Its disadvantages, however, include difficulties in measuring the LBV of fuels with extremely high LBV and in LBV measurements under high-pressure operating conditions. Given that ammonia has an extremely low LBV, it is highly suitable for LBV measurement using the heat flux method. The research team led by Zhihua Wang from Zhejiang University systematically measured the LBV of pure ammonia and ammonia-blended fuels using the heat flux method—findings that verified the reliability of this method [27,28,29,30].
In summary, four methods for measuring ammonia flame LBV are summarized in Table 2. This summary includes the advantages and disadvantages of each method, as well as the applicability of each method under the conditions of high-reactivity fuel blending and high pressure.

Table 2.
Comparison and summary of LBV measurement methods for ammonia flames.
3. Characteristics of Laminar Burning Velocity for Ammonia Flames
3.1. LBV of Pure Ammonia Flames Under Ambient Temperature and Pressure
Extensive research has been conducted on the LBV of pure ammonia premixed flames. A chronological overview of the relevant research progress is presented in Table 3. Owing to their early date, studies by Ronney [31], Pfahl et al. [32], and Jabbour et al. [33] neglected the influence of flame stretch on the final results. However, flame stretch causes the local flame temperature to deviate from the adiabatic flame temperature (the direction of deviation depends on the Lewis number of the mixture). This temperature variation significantly alters the reaction rate within the flame, leading to a discrepancy between the measured LBV and the actual LBV. In Table 3, most studies since 2007 have incorporated stretch corrections into their experimental designs or data post-processing. Nevertheless, ammonia has an extremely low LBV. When measuring its LBV using the spherical outwardly propagating flame method, the flame often exhibits a typical “tulip” shape. This shape leads to substantial fluctuations in the measured LBV values.

Table 3.
Summary of LBV research progress for pure ammonia/air flames.
The experimental results in Table 3 are summarized in Figure 7. It can be observed that the LBV of pure ammonia flames follows a consistent trend: as the equivalence ratio increases, the LBV first rises and then falls, reaching its maximum value under slightly fuel-rich conditions. Based on the data presented in Figure 7, the average values of LBV for pure ammonia flames as a function of equivalence ratio were calculated and fitted. The resulting correlation is: SL = −132Φ3 + 349Φ2 − 288 + 78. This fitted equation can be used to provide a preliminary prediction of LBV prior to conducting experiments. Notably, under atmospheric temperature and pressure, the LBV of pure ammonia flames at the stoichiometric is only between 6 and 7 cm/s—extremely low compared to other common fuels. For instance, under the same conditions, the LBV of methane flames is approximately 36 cm/s [42]. This significant discrepancy clearly demonstrates that ammonia has extremely low reactivity, making it highly unfavorable for standalone use as a combustion fuel. On the fuel-rich side, the LBVs reported by Ronney et al. [31] and Mei et al. [16] are higher than those measured by other researchers. This discrepancy can be attributed to the absence of flame stretch correction in Ronney et al.’s study [31], as well as the more pronounced influence of buoyancy and hydrodynamic instabilities in Mei et al.’s experiments [16]. These findings demonstrate that even when the same measurement technique is employed, differences in experimental implementation details, data extraction, and processing procedures can lead to variations in LBV results.
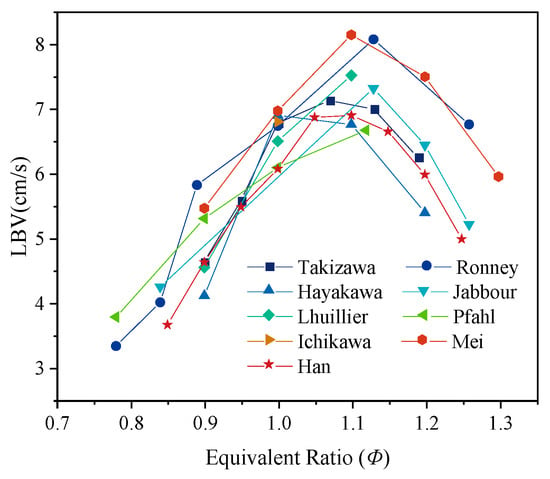
Figure 7.
Variation in LBV of ammonia flames with equivalence ratio under atmospheric temperature and pressure.
3.2. Effect of Blending High-Reactivity Fuels on the LBV of Ammonia Flames
Given the extremely low LBV of ammonia, co-firing it with other high-reactivity fuels to boost the blended fuel’s LBV is a widely adopted approach to ensure ammonia’s stable combustion. Among these high-reactivity fuels, hydrogen and methane have been the most extensively studied [43,44,45,46].
In terms of hydrogen blending, Table 4 summarizes LBV research progress for ammonia-hydrogen blended fuels. Lee et al. [47] showed that hydrogen addition increases ammonia LBV at both lean-fuel and fuel-rich conditions. Li et al. [48] and Lhuillier et al. [49] also observed a similar trend in LBV. Ichikawa et al. [37] found in their study that when the volume fraction of blended hydrogen reaches 40%, the LBV of the gas mixture reaches 35 cm/s, which is roughly equivalent to the LBV of natural gas burning in air. Goldmann et al. [50] also carried out similar calculation work, but greatly expanded the initial combustion conditions and obtained an LBV database under these conditions, providing a reference for finding blended components with LBV similar to methane under specific conditions. From the opposite perspective, Kumar et al. [18] found that blending ammonia into hydrogen flames leads to a decrease in the LBV of the blended fuel.

Table 4.
Summary of LBV research progress for ammonia/hydrogen/air flames.
In a follow-up study, Han et al. [27] measured the LBV of ammonia/hydrogen/air flames under different hydrogen blending ratios and equivalence ratios using the heat flux method, and compared the results with those from the aforementioned relevant studies, as shown in Figure 8. The LBV values under different operating conditions were generally consistent with previous studies, exhibiting an exponential increase with increasing hydrogen blending ratio.
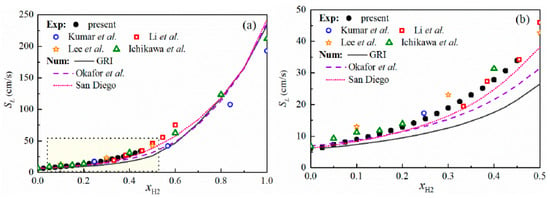
Figure 8.
(a) Variation in LBV of ammonia/hydrogen/air flames with hydrogen blending ratio at stoichiometric equivalence ratio; (b) Locally enlarged view for hydrogen blending ratio less than 0.5 [27].
In Table 4, Wang et al. [29], Shrestha et al. [41], Ichikawa et al. [37], and Goldmann et al. [50] all examined the LBV of ammonia/hydrogen/air flames under high pressure. Their studies showed that while increasing hydrogen content increases the LBV, increasing pressure decreases it. In terms of numerical calculations, a number of models for ammonia/hydrogen flames have been developed, but their predictive accuracies differ markedly [46,51,52,53], requiring further optimization to enhance this accuracy.
Compared to the gaseous ammonia combustion mode, the high-pressure direct liquid ammonia injection mode has greater potential in reducing unburned ammonia and nitrogen oxide emissions. Liquid ammonia is typically injected into the combustion chamber as a turbulent spray. After injection, it undergoes evaporation and mixing processes. These processes form a combustible mixture, which then undergoes exothermic combustion. This process is a typical spray combustion process. Wu et al. [54] found that spray flames can be divided into four stages: (1) the ignition process, where auto-ignition nuclei appear at the jet front; (2) the flame propagation process, where auto-ignition nuclei spread toward the center of the spray; (3) the fully developed combustion process, where the flame fills the core region; and (4) the post-combustion process, where the flame area rapidly decreases.
Although spray can enhance the mixing of ammonia with air and intensify the combustion reaction rate, it is still necessary to blend it with other highly reactive fuels to strengthen combustion. Huang et al. [55] proposed a method of directly injecting high-pressure ammonia into a premixed lean hydrogen-air mixture, using spark-ignited hydrogen flame to ignite and enhance liquid ammonia spray combustion. They also investigated the combustion and emission characteristics of high-pressure direct-injection liquid ammonia under different pressures and hydrogen concentrations. The results showed that when the excess air ratio was 3.0 and the pressure was 2.5 MPa, high-pressure direct-injection ammonia achieved near-complete combustion with nitrous oxide emissions below 10 ppm. Ishihara et al. [56,57] investigated the co-combustion characteristics of ammonia and coal in utility boilers. The blended fuel reduced carbon emissions. However, two main challenges emerged. First, NO emissions became an issue when the ammonia blending ratio was 10%. Second, ammonia slip occurred when the ratio exceeded 40%. Furthermore, the substantial demand for liquid ammonia has heightened the need for research on integrated intelligent ammonia supply technology for boilers and the compatibility of ammonia-coal co-firing in large-scale coal-fired boilers.
In terms of methane blending, Table 5 summarizes LBV research progress for ammonia-methane blended fuels. Compared with ammonia blending with hydrogen, fewer studies address methane addition. This is because even at extremely high methane blending ratios, the LBV of ammonia/methane/air flames remains lower than that of pure methane flames. Experiments by Han et al. [27] and Shu et al. [58] both indicate that the LBV of ammonia/methane/air flames shows an almost linear increase with the proportion of methane, valid for all equivalence ratios, as shown in Figure 9. Based on the above conclusions, blending methane makes the combustion and utilization of ammonia feasible. However, the proportion of ammonia in the blended fuel still needs to be considered cautiously. For example, Henshaw et al. [59] found that when the volume fraction of ammonia in the mixture is only 4%, the LBV of the mixture decreases by 10–20%. In their study, the maximum proportion of ammonia cannot exceed 5%. Okafor et al. [60] discovered that under 0.5 MPa, blending methane can increase the maximum ammonia blending ratio to 30%.

Table 5.
Summary of LBV research progress for ammonia/methane/air flames.
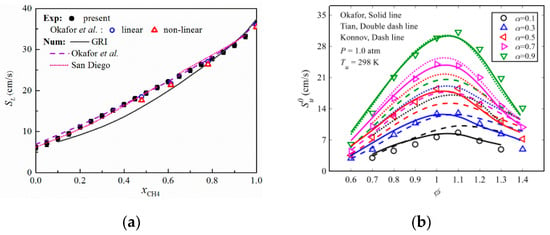
Figure 9.
LBV of ammonia/methane/air flames versus methane blending ratio: (a) Experimental and simulation results by Han et al. [27]; (b) Experimental and simulation results by Shu et al. [58] (where α represents methane blending ratio).
3.3. Effect of Oxygen-Enriched Combustion on the LBV of Ammonia Flames
Recently, the use of oxygen-enriched air or even pure oxygen to assist ammonia combustion has also received considerable research attention. In the industrial sector, the Japan Science and Technology Agency (JST) and Osaka University conducted ammonia oxygen-enriched combustion experiments in a 10 kW industrial furnace. They found that when the oxygen concentration in the oxidizer reaches 30%, ammonia can achieve stable combustion, and the combustion temperature is higher than that of methane/air non-premixed combustion [10]. Grannell et al. [62] reported that when the oxygen fraction in the oxidizer reaches 33%, an ammonia spark-ignition engine delivered the same power as a gasoline/air engine. The above industrial cases imply that oxygen enrichment has a pronounced effect on the LBV of ammonia flames.
In 2015, Takeishi et al. [63] measured the LBV of oxygen-enriched ammonia flames. The results showed that when the oxygen concentration reached 35%, the LBV of the ammonia flame could reach 36.1 cm/s at an equivalence ratio of 1.1, which was already close to the LBV of methane flames; however, stretch corrections were not applied in their study, leaving the accuracy uncertain. In 2019, Mei et al. [16] used the spherical outwardly propagating flame method in a constant-volume bomb to measure the LBV of oxygen-enriched ammonia flames under different oxygen enrichment levels and equivalence ratios, with flame stretch correction considered. The experimental and simulation results are shown in Figure 10. They extended the oxygen enrichment concentration to 45% and found that the LBV of the ammonia flame increased with the rise of oxygen concentration, which was consistent with the conclusion of Takeishi et al. [63]. The difference, however, was that a 40% oxygen concentration was required to match methane’s LBV. Meanwhile, the measurement results of Shrestha et al. [41] and Xia et al. [64] were also basically consistent with those of Mei et al. [16]. Xia et al. [64] found that at 40% oxygen concentration, the LBV of the oxygen-enriched ammonia flame was 33.7 cm/s—approximately 4.9 times that of the ammonia/air flame.
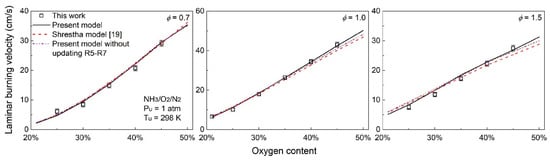
Figure 10.
LBV of ammonia/oxygen/nitrogen flames at different oxygen contents, including experimental and simulation results [16,41].
In current research on oxygen-enriched ammonia combustion, the oxygen concentration range is typically below 40%, with insufficient research on higher concentrations or even pure oxygen combustion. Liu et al. [65] studied ammonia/pure oxygen flames and found that the LBV of ammonia was maximum under stoichiometric conditions, reaching 1.05 m/s. Wang et al. [66] conducted a similar study but drew different conclusions: they argued that the LBV of ammonia was maximum at an equivalence ratio of 0.9, reaching 1.25 m/s, with the maximum value shifting toward the lean-burn side. Therefore, more LBV test data for oxygen-enriched ammonia flames are needed to verify the accuracy of existing reports and assess the predictive accuracy of ammonia oxidation mechanisms under oxygen-enriched conditions.
3.4. Effect of Temperature on the LBV of Ammonia Flames
According to the gas molecular collision theory, increasing the temperature of unburned gas enhances the kinetic energy of gas molecules and strengthens heat transfer. This significantly accelerates the chemical reaction rate, thereby increasing the flame propagation speed. This expectation has been confirmed experimentally and numerically for ammonia flames in numerous studies.
For pure ammonia flames, Han et al. [67] measured the LBV at different initial temperatures (Figure 11), and the LBV increases monotonically with temperature. Kohansal et al. [68] reported that compared with the LBV at room temperature, the LBV of the flame at 373 K and 473 K increased by 66% and 151%, respectively.
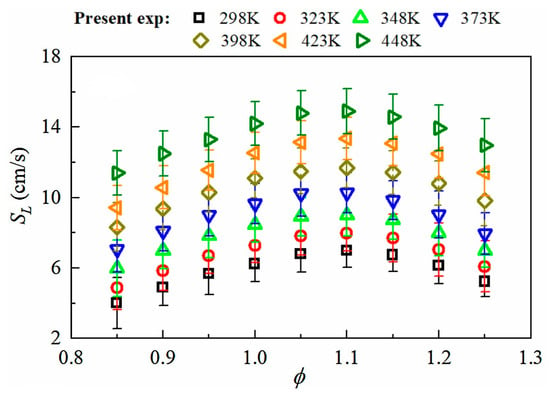
Figure 11.
LBV of ammonia flames versus equivalence ratio at various initial temperatures [67].
For ammonia/hydrogen/air flames, Lhuillier et al. [49] measured the LBV in the temperature range of 298 K to 473 K using the spherical outwardly propagating flame method, as shown in Figure 12. Under all investigated equivalence ratio conditions, the LBV increased significantly with increasing temperature.
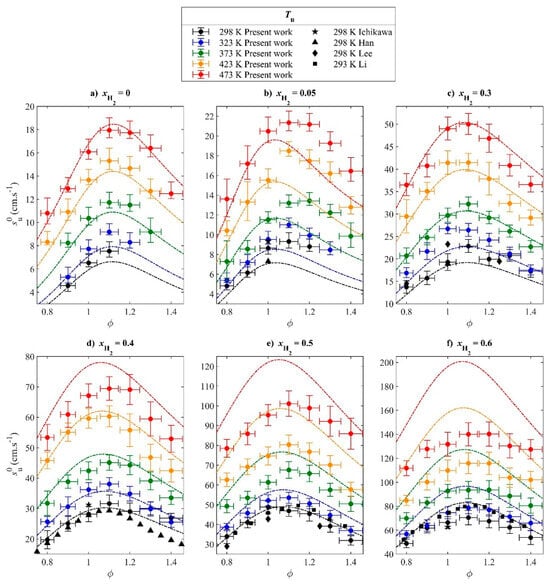
Figure 12.
LBV of ammonia/air and ammonia/hydrogen/air flames at various initial temperatures [49].
For ammonia/methane/air flames, Kohansal et al. [68] extended measurements to 1000 K and observed the same monotonic LBV increase. A more interesting finding, however, is that ammonia enhances the preheating effect: as the ammonia concentration in the binary fuel increases, the sensitivity of LBV to initial temperature becomes progressively stronger.
For oxygen-enriched ammonia flames, Karan et al. [69] investigated the propagation of ammonia/oxygen flames under elevated temperature and pressure. They found that the LBV exhibited a good linear relationship with both temperature and pressure, increasing synchronously as temperature and pressure rose. Sensitivity analysis further confirmed that temperature is the dominant factor affecting the LBV, whereas the LBV exhibits a weak dependence on pressure.
3.5. Effect of Pressure on the LBV of Ammonia Flames
Theoretically, the effect of pressure on LBV can be characterized by the overall reaction order n, as shown in Equation (3). Analysis of experimental results indicates that for low LBV (less than 50 cm/s) and when the overall reaction order is generally less than 2, the LBV decreases with increasing pressure [70].
Mitu et al. [71] derived an LBV correlation (Equation (4)) by analyzing the thermal theory of flame propagation, which describes the effect of pressure on LBV. In the correlation, λ denotes thermal conductivity, ρ denotes density, Cp denotes the specific heat capacity of unburned gas, C0 denotes the initial fuel concentration, Tf denotes the flame front temperature, and n and Ea denote the reaction order and activation energy, respectively. With constant fuel concentration and flame front temperature, increasing pressure leads to higher gas density. However, thermal conductivity and specific heat capacity are insensitive to pressure. This combination of effects ultimately results in a decrease in LBV [72].
Building on theoretical analyses, numerous experimental studies have been conducted. Hayakawa et al. [36] used the spherical outwardly propagating flame method to investigate the LBV of ammonia flames at 0.1, 0.3, and 0.5 MPa. The results (Figure 13) indicate that the maximum LBV of unstretched ammonia flames is less than 7 cm/s—much lower than that of hydrocarbons—and decreases with increasing pressure, consistent with the behavior of hydrocarbons. Meanwhile, the authors also found that in ammonia flames under variable pressure conditions, the accuracy of LBV simulated by five existing ammonia combustion models (Miller et al., Lindstedt et al., Konnov et al. without all carbon reactions, Tian et al., and GRI-Mech 3.0) [73,74,75,76,77] is insufficient. Therefore, it is necessary to refine the high-pressure combustion model for ammonia.
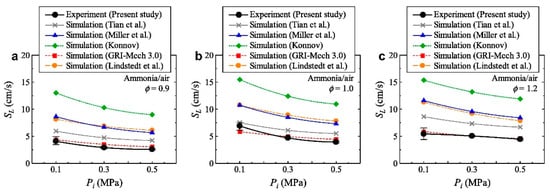
Figure 13.
Variation in unstretched LBV of ammonia flame with initial mixture pressure [36].
For ammonia/methane flames, Okafor et al. [60] studied the LBV of ammonia/methane/air flames at 1, 3, and 5 atm. The equivalence ratio was fixed at 1, with the ammonia concentration set to 0.05 (heat fraction basis). The LBV values of the blended fuel at 1, 3, and 5 atm were 28.91, 20.10, and 16.51 cm/s, respectively; This phenomenon is associated with the increase in overall reaction rate induced by elevated pressure. Analysis of the simulation results shows that under elevated pressure conditions, the concentration of OH radicals becomes critical for accurate prediction of LBV. Mei et al. [16] also observed the same trend and calculated the pressure dependence coefficient. The results indicate that the pressure dependence of ammonia/methane/air flames is weaker than that of hydrocarbon and biomass fuel flames.
Ichikawa et al. [37] studied the LBV of ammonia/hydrogen/air flames at pressures ranging from 1 to 5 atm and hydrogen blending ratios of 0–100%; the LBV also decreased as pressure rose. Wang et al. [29] systematically investigated the high-pressure LBV of ammonia blended with different fuels (including syngas, CO, and hydrogen), with combustion pressures up to 5 atm. For kinetic simulation, four mechanisms were adopted: Han [28], Shrestha [78], UC-San Diego [79], and Stagni [80]. The experimental and simulation results are summarized in Figure 14. For all three fuels, the LBV declined with pressure; however, the decline was more pronounced for ammonia/hydrogen blends than for ammonia/CO blends. On the other hand, in the comparison between experimental and simulation results, good consistency was observed under both high ammonia proportion conditions (60–100%) and low ammonia proportion conditions (0–20%). In contrast, a large discrepancy existed under medium ammonia proportions (20–60%), which is attributable to uncertainties in ammonia chemistry.
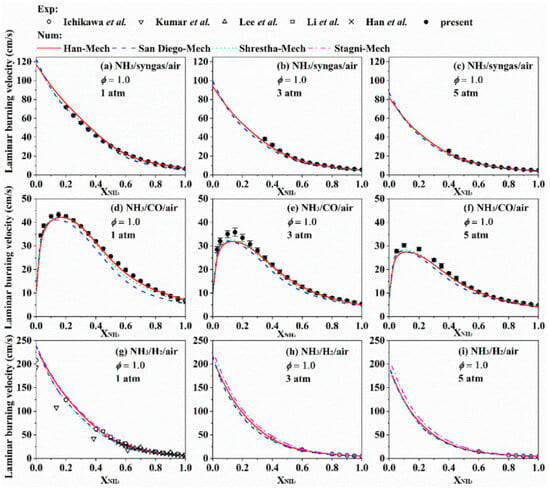
Figure 14.
LBV of stoichiometric ammonia/syngas/air, ammonia/CO/air, and ammonia/hydrogen/air flames as a function of XNH3 at 1, 3 and 5 atm [29].
As indicated by the aforementioned literature review, research on the effect of pressure on the LBV of ammonia flames has focused on high-pressure conditions, while research on low-pressure conditions (below atmospheric pressure) remains scarce. Nevertheless, low-pressure ammonia combustion holds research value in high-altitude regions and the aerospace field. In terms of high-pressure conditions, there is a consistent conclusion that the LBV of ammonia-related flames decreases with increasing pressure. However, the following issues require further investigation: measurable discrepancies between experimentally measured LBV and numerically simulated LBV persist, and some ammonia oxidation mechanisms still exhibit significant uncertainty under high-pressure conditions. More high-pressure LBV data for ammonia-blended fuels are therefore urgently needed to validate ammonia chemistry and reduce its uncertainty.
In summary, the effects of combustion conditions on the LBV of ammonia flames are summarized in Figure 15, which is used to analyze the relationship between combustion parameters and LBV.
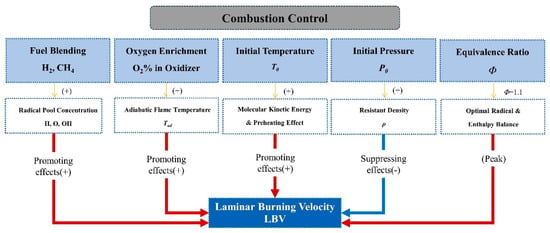
Figure 15.
Flow chart illustrating the effects of key combustion parameters on the LBV of ammonia-based flames. Arrows indicate the direction of influence, with ‘+’ and ‘–’ symbols denoting promoting and suppressing effects on LBV, respectively.
4. Development of Reaction Kinetic Models for Ammonia Combustion
In addition to experimental measurement, chemical kinetic modeling is also indispensable for determining the LBV of ammonia flames. Beyond LBV, chemical kinetic calculations can also yield data such as NO emissions and ignition delay times—information crucial for advancing ammonia combustion-related technologies. Conversely, the LBV obtained through experimental measurements also serves as a basis for verifying and optimizing the accuracy of chemical kinetic models.
In the chemical reaction kinetic calculations of ammonia combustion, the chemical reaction kinetic model is critical. A complete chemical reaction kinetic model should include three types of data files: chemical reaction mechanisms, thermodynamic parameters, and transport parameters. Among these, the core lies in the chemical reaction mechanism—a collection of information describing the elementary reactions involving species and their corresponding reaction rate constants. In general, chemical kinetic models related to ammonia combustion have received extensive attention. Early chemical kinetic models for ammonia mainly focused on NOx formation and ammonia’s role as a reducing agent in selective non-catalytic reduction (SNCR) reactions [81,82], with relatively little attention given to LBV. Over time, an increasing number of chemical kinetic mechanisms have been developed for the ammonia combustion process. Table 6, Table 7 and Table 8 summarize the chemical kinetic models applicable to the combustion calculations of pure ammonia, ammonia/hydrogen/air, and ammonia/methane/air, respectively.

Table 6.
The reaction kinetic models applicable to pure ammonia combustion calculations.

Table 7.
The reaction kinetic models applicable to ammonia/hydrogen/air combustion calculations.

Table 8.
The reaction kinetic models applicable to ammonia/methane/air combustion calculations.
Once a chemical kinetic model is obtained, it is imported into kinetic simulation software. By configuring simulation settings (e.g., different reactor physical models) and input parameters (such as boundary conditions), kinetic simulations of ammonia combustion can be performed to calculate the LBV of ammonia flames. Currently, several simulation tools are available, including the commercial software Chemkin-Pro and open-source codes such as OpenSMOKE++, Cantera, and FlameMaster. Chemkin-Pro, in particular, has undergone years of development and updates. It features a user-friendly interface, comprehensive post-processing functions, and excellent operability, which makes it widely used for simulating zero-dimensional or one-dimensional reactors [107]. Wang [70] predicted and analyzed the variation in LBV with ammonia content for ammonia/methane/air and ammonia/hydrogen/air flames at 0.1 and 0.5 MPa using different mechanisms, as shown in Figure 16. This work is relatively comprehensive, involving eight chemical kinetic models and covering conditions discussed in Section 3 (e.g., blending of high-reactivity fuels and pressure effects). As observed in Figure 16, the simulated LBV variation trends of ammonia flames are consistent with the experimental results presented in Section 3—this confirms the effectiveness of chemical kinetic calculations.
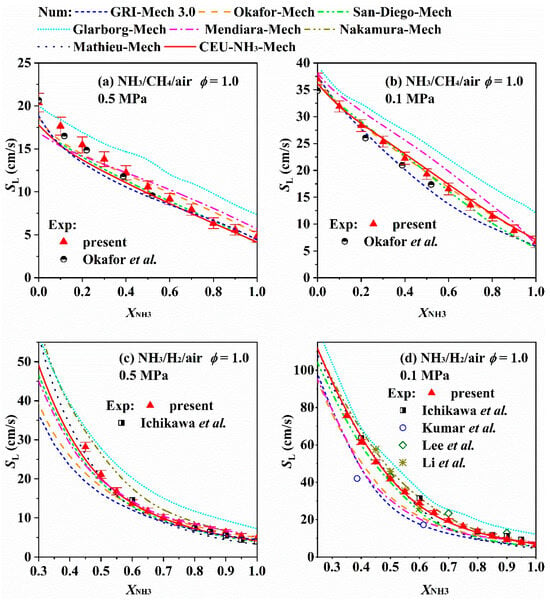
Figure 16.
Variation in LBV with XNH3 for ammonia/methane/air and ammonia/hydrogen/air flames at 0.1 and 0.5 MPa [70].
Beyond directly obtaining LBV through reaction kinetic calculations, sensitivity analysis can identify the elementary reactions and corresponding species that most strongly influence LBV. This enables targeted exploration of the mechanisms by which combustion conditions affect LBV, such as those mentioned in Section 3: blending with high-reactivity fuels, oxygen enrichment, temperature, and pressure. For instance, to investigate the effect of equivalence ratio on the LBV of ammonia/hydrogen/air flames, Wang [70] identified the eleven most sensitive reactions, as shown in Figure 17. The Stagni model identifies H2 + M = H + H + M and H2O2 (+M) = OH + OH (+M) as the elementary reactions with the most pronounced impact on LBV, whereas the Han model considers H + O2 = O + OH as the most influential. These discrepancies underscore the need for further comparative studies to reach a consensus on the key sensitive reactions.
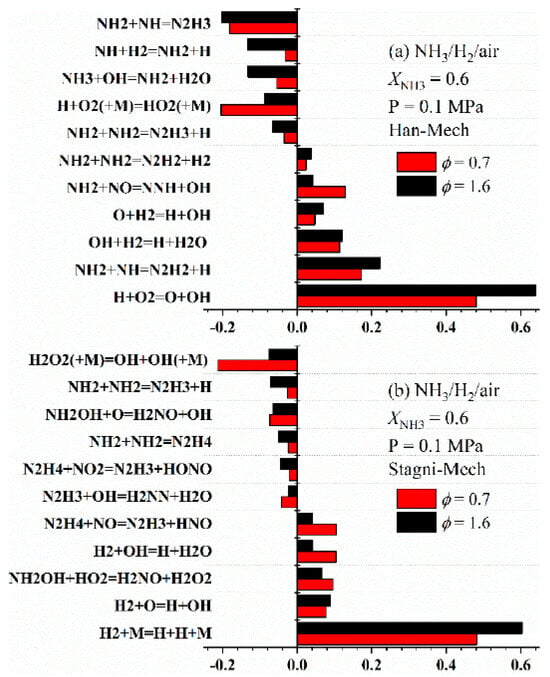
Figure 17.
Sensitivity analysis of LBV for ammonia/hydrogen/air flames at XNH3 = 0.6, equivalence ratios of 0.7 and 1.6 [70].
In addition to sensitivity analysis, chemical kinetic pathway analysis and pathway contribution analysis also help clarify how combustion conditions affect the LBV of ammonia flames. As shown in Figure 18, Mei et al. [16] elucidated the mechanism by which oxygen-enriched combustion enhances the LBV of ammonia flames under both lean-burn and fuel-rich conditions. The main reaction pathways are identical for ammonia flames and enriched oxygen ammonia flames, but their relative contributions change with oxygen content—particularly the contribution of hydrogen atoms to the reaction pathways. Specifically, the abstraction reaction of ammonia by hydrogen atoms under oxygen-enriched conditions is more than three times more active than that under air conditions, whereas the contribution of that by hydroxyl radicals exhibits the opposite trend.
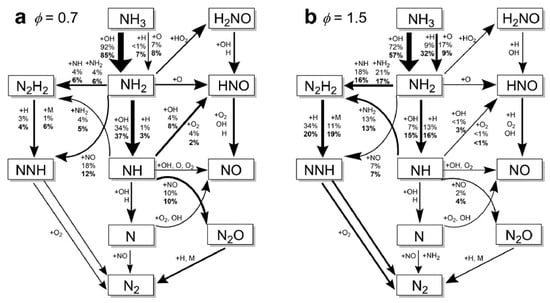
Figure 18.
Chemical reaction pathway and contribution analysis of ammonia/oxygen/nitrogen flames under normal air and oxygen-enriched conditions [16].
As chemical kinetic models are developed, another critical issue lies in their comprehensive validation. Taking pressure as an example, model calculations indicate that the rate constants of unimolecular decomposition reactions and chemically activated reactions exhibit strong pressure dependence. Although mechanism files typically use pressure-dependent rate expressions to describe how reaction rate constants vary with pressure, discrepancies remain widespread. These expressions include Troe, PLOG, and Chebyshev correlations. The discrepancies exist in two aspects: on one hand, between predictions of different models; on the other hand, between model predictions and experimental values.
5. Conclusions and Recommendations
As a highly promising zero-carbon fuel, ammonia’s LBV is a key parameter for evaluating its combustion characteristics and reaction activity. This review systematically summarizes the research status of LBV for ammonia flames and presents the following main conclusions:
- (1)
- The extremely low LBV and flame instability of ammonia impose strict requirements on LBV measurement techniques. The spherical outwardly propagating flame method is significantly affected by buoyancy effects and stretch correction. This influence leads to relatively large measurement errors, especially for pure ammonia flames. The Bunsen-burner method is also unsuitable for measuring the LBV of low-velocity pure ammonia flames due to issues of flame stability and heat dissipation. In contrast, the counter-flow flame method and heat flux method are currently the more reliable choices for measuring the LBV of pure ammonia and ammonia-blended fuels. Each of these two methods leverages distinct advantages: the counter-flow flame method has high stability and low stretch rate, while the heat flux method has quasi-adiabatic characteristics.
- (2)
- The LBV of pure ammonia flames is extremely low (approximately 6–7 cm/s under atmospheric temperature and pressure), which severely limits its direct application in combustion systems. While liquid ammonia spray combustion plays a role in promoting ammonia combustion. Blending high-reactivity fuels (e.g., hydrogen, methane) is an effective way to increase LBV, among which hydrogen exhibits a particularly significant enhancement effect—with LBV showing an exponential growth trend as the hydrogen blending ratio increases. Furthermore, hydrogen blending also facilitates the ignition of liquid ammonia and promotes its spray combustion. Oxygen-enriched combustion can greatly increase the LBV of ammonia flames—when the oxygen concentration exceeds 40%, the LBV can reach the level of conventional hydrocarbon fuels. Increasing the initial temperature significantly promotes LBV, whereas higher pressure generally leads to a decrease in LBV.
- (3)
- Although numerous chemical reaction kinetic models have been developed for systems such as pure ammonia, ammonia/hydrogen, and ammonia/methane, and most of them can well predict LBV under atmospheric pressure and typical blending ratios, there are discrepancies in prediction results between different models under complex conditions (e.g., high pressure, moderate blending ratios), and deviations also exist between model predictions and experimental data. This reveals that significant uncertainties remain in current ammonia combustion kinetic mechanisms, particularly regarding key nitrogen-containing reaction pathways and their pressure-dependent behaviors.
Based on the limitations of current research and the development needs of ammonia fuel applications, the following recommendations for future work are proposed:
- (1)
- Current experimental data on ammonia flame LBV are mostly concentrated under atmospheric pressure, moderate-to-low temperatures, and moderate-to-low blending ratios. Urgent efforts are needed to systematically perform accurate LBV measurements of ammonia and its blends with diverse fuels (e.g., syngas, alcohols) under extreme operating conditions—such as high pressure (>5 atm), low pressure (<1 atm), high temperature (>1000 K), and high-to-extreme oxygen concentrations (including pure oxygen). Such data will provide critical support for practical application scenarios including internal combustion engines, gas turbines, aerospace, and high-altitude areas.
- (2)
- Further development is needed for high-precision, low-uncertainty LBV measurement methods highly suitable for ammonia flames—especially under high-pressure and extremely high-oxygen-concentration conditions. Efforts should be directed toward integrating advanced optical measurement techniques and radical imaging technology to improve the accuracy of flame structure identification and LBV extraction.
- (3)
- Current models still exhibit errors in LBV prediction under high-pressure, moderate blending ratio, and high-temperature conditions. In the future, by leveraging sensitivity and pathway analysis, focus should be placed on verifying the rate constants of key elementary reactions (e.g., H + O2 = O + OH, etc.), and developing unified simplified mechanisms highly applicable across a wide range of conditions.
- (4)
- Future research should place greater emphasis on applying optimized kinetic models to CFD simulations of key actual combustion devices (e.g., gas turbines, internal combustion engines). This application will enable cross-scale accurate prediction and optimization. The optimization range covers from fundamental combustion parameters to system-level combustion performance, thereby accelerating the practical application of ammonia fuel.
Author Contributions
Conceptualization, X.Y.; validation, Z.X. and R.H.; data curation, Z.X.; writing—original draft preparation, X.Y.; visualization, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coal-Major Project (2025ZD1701100) and Fundamental Research Funds for the Central Universities (2022ZFJH04).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest nor any personal circumstances or interests that may be perceived as inappropriately influencing the representation or interpretation of the reported research results. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wang, Y.; Zhou, Y. Numerical optimization of the influence of multiple deep air-staged combustion on the NOx emission in an opposed firing utility boiler using lean coal. Fuel 2020, 269, 116996. [Google Scholar] [CrossRef]
- Chen, Z.; You, C.; Wang, H.; Liu, Q. Experimental study on the synergetic removal of fine particles by wet flue gas desulfurization tower with a flow pattern control device. Powder Technol. 2019, 343, 122–128. [Google Scholar] [CrossRef]
- Akihama, K.; Takatori, Y.; Inagaki, K.; Sasaki, S.; Dean, A.M. Mechanism of the Smokeless Rich Diesel Combustion by Reducing Temperature; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Ishida, M.; Yamamoto, S.; Ueki, H.; Sakaguchi, D. Remarkable improvement of NOx–PM trade-off in a diesel engine by means of bioethanol and EGR. Energy 2010, 35, 4572–4581. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Shang, Q.; Feng, D.; Zhang, X.; Cheng, Z.; Wang, S.; Zhao, Y.; Sun, S. Synergistic effects of biomass/plastics and multi-step regulation of H2O in the co-production of H2-CNTs by gasification of biomass and plastic wastes. Appl. Catal. B Environ. Energy 2025, 379, 125695. [Google Scholar] [CrossRef]
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The role of new energy in carbon neutral. Petrol. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.; Okafor, E. Science and technology of ammonia combustion. P. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Murai, R.; Omori, R.; Kano, R.; Tada, Y.; Higashino, H.; Nakatsuka, N.; Hayashi, J.; Akamatsu, F.; Iino, K.; Yamamoto, Y. The radiative characteristics of NH3/N2/O2 non-premixed flame on a 10 kW test furnace. Energy Procedia 2017, 120, 325–332. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3–CH4–air combustion gas-turbine power generations. P. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.I.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Nair, M.R.S.; Gupta, M.C. Burning velocity measurement by bomb method. Combust. Flame 1974, 22, 219–221. [Google Scholar] [CrossRef]
- San Diego Mechanism. Available online: http://combustion.ucsd.edu (accessed on 9 October 2025).
- Elbaz, A.M.; Giri, B.R.; Issayev, G.; Shrestha, K.P.; Mauss, F.; Farooq, A.; Roberts, W.L. Experimental and Kinetic Modeling Study of Laminar Flame Speed of Dimethoxymethane and Ammonia Blends. Energy Fuel 2020, 34, 14726–14740. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and kinetic modeling investigation on the laminar flame propagation of ammonia under oxygen enrichment and elevated pressure conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Natarajan, J.; Kochar, Y.; Lieuwen, T.; Seitzman, J. Pressure and preheat dependence of laminar flame speeds of H2/CO/CO2/O2/He mixtures. P. Combust. Inst. 2009, 32, 1261–1268. [Google Scholar] [CrossRef]
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
- Natarajan, J.; Lieuwen, T.; Seitzman, J. Laminar flame speeds of H2/CO mixtures: Effect of CO2 dilution, preheat temperature, and pressure. Combust. Flame 2007, 151, 104–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Yu, B.; Li, X.; Zhang, L.; Zhao, Y.; Sun, S. Experimental and kinetic modeling study on laminar flame speeds and emission characteristics of oxy-ammonia premixed flames. Int. J. Hydrogen Energy 2024, 63, 857–870. [Google Scholar] [CrossRef]
- Simmons, R.F.; Wolfhard, H.G. Some limiting oxygen concentrations for diffusion flames in air diluted with nitrogen. Combust. Flame 1957, 1, 155–161. [Google Scholar] [CrossRef]
- Law, C.K.; Zhu, D.L.; Yu, G. Propagation and extinction of stretched premixed flames. Symp. Combust. 1988, 21, 1419–1426. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Hansen, N.; Ju, Y.; Kohse-Höinghaus, K.; Law, C.K.; Qi, F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Prog. Energy Combust. Sci. 2014, 43, 36–67. [Google Scholar] [CrossRef]
- Niemann, U.; Seshadri, K.; Williams, F.A. Accuracies of laminar counterflow flame experiments. Combust. Flame 2015, 162, 1540–1549. [Google Scholar] [CrossRef]
- Bosschaart, K.J.; de Goey, L.P.H. The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method. Combust. Flame 2004, 136, 261–269. [Google Scholar] [CrossRef]
- de Goey, L.P.H.; van Maaren, A.; Quax, R.M. Stabilization of Adiabatic Premixed Laminar Flames on a Flat Flame Burner. Combust. Sci. Technol. 1993, 92, 201–207. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/syngas/air premixed flames. Combust. Flame 2020, 213, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed flames at elevated pressures. Combust. Flame 2020, 221, 270–287. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; He, Y.; Zhu, R.; Zhu, Y.; Zhou, Z.; Cen, K. Experimental and kinetic study on the laminar burning velocities of NH3 mixing with CH3OH and C2H5OH in premixed flames. Combust. Flame 2021, 229, 111392. [Google Scholar] [CrossRef]
- Ronney, P.D. Effect of Chemistry and Transport Properties on Near-Limit Flames at Microgravity. Combust. Sci. Technol. 1988, 59, 123–141. [Google Scholar] [CrossRef]
- Pfahl, U.J.; Ross, M.C.; Shepherd, J.E.; Pasamehmetoglu, K.O.; Unal, C. Flammability limits, ignition energy, and flame speeds in H2–CH4–NH3–N2O–O2–N2 mixtures. Combust. Flame 2000, 123, 140–158. [Google Scholar] [CrossRef]
- Jabbour, T.; Clodic, D.F. Burning velocity and refrigerant flammability classification. ASHRAE Trans. 2004, 110, 522–533. Available online: https://www.researchgate.net/publication/279588720_Burning_velocity_and_refrigerant_flammability_classification (accessed on 9 October 2025).
- Zakaznov, V.F.; Kursheva, L.A.; Fedina, Z.I. Determination of normal flame velocity and critical diameter of flame extinction in ammonia-air mixture. Combust. Explos. Shock. Waves 1978, 14, 710–713. [Google Scholar] [CrossRef]
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning velocity measurements of nitrogen-containing compounds. J. Hazard. Mater. 2008, 155, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Kunkuma Amila Somarathne, K.D.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/hydrogen/air premixed flames at elevated pressures. Int. J. Hydrogen Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Davis, S.G.; Pagliaro, J.L.; Debold, T.F.; van Wingerden, M.; van Wingerden, K. Flammability and explosion characteristics of mildly flammable refrigerants. J. Loss Prev. Process Ind. 2017, 49, 662–674. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Li, B.; Gao, W. Explosion behaviors of ammonia–air mixtures. Combust. Sci. Technol. 2018, 190, 1804–1816. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An experimental and modeling study of ammonia with enriched oxygen content and ammonia/hydrogen laminar flame speed at elevated pressure and temperature. P. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Mitu, M.; Movileanu, C.; Giurcan, V. The Laminar Burning Velocities of Stoichiometric Methane-Air Mixture from Closed Vessels Measurements. Energies 2022, 15, 5058. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Morris, S.; Runyon, J.; Pugh, D.G.; Marsh, R.; Beasley, P.; Hughes, T. Ammonia, Methane and Hydrogen for Gas Turbines. Energy Procedia 2015, 75, 118–123. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P.J. Study on Reduced Chemical Mechanisms of Ammonia/Methane Combustion under Gas Turbine Conditions. Energy Fuel 2016, 30, 8701–8710. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling Combustion of Ammonia/Hydrogen Fuel Blends under Gas Turbine Conditions. Energy Fuel 2017, 31, 8631–8642. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Nagai, Y. Study on using hydrogen and ammonia as fuels: Combustion characteristics and NOx formation. Int. J. Energy Res. 2014, 38, 1214–1223. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Goldmann, A.; Dinkelacker, F. Approximation of laminar flame characteristics on premixed ammonia/hydrogen/nitrogen/air mixtures at elevated temperatures and pressures. Fuel 2018, 224, 366–378. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; Wang, C.; Yuan, H. Numerical study on laminar burning velocity and ignition delay time of ammonia flame with hydrogen addition. Energy 2017, 126, 796–809. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Deng, L.; He, Z.; Osaka, Y.; Kobayashi, N. Effect of hydrogen addition on combustion and heat release characteristics of ammonia flame. Energy 2019, 175, 604–617. [Google Scholar] [CrossRef]
- da Rocha, R.C.; Costa, M.; Bai, X.-S. Chemical kinetic modelling of ammonia/hydrogen/air ignition, premixed flame propagation and NO emission. Fuel 2019, 246, 24–33. [Google Scholar] [CrossRef]
- Wu, H.; Qian, Y.; Zhang, T.; Zhu, J.; Lu, X. Ignition and flame development of high-pressure liquid ammonia spray combustion with simultaneous high-speed OH* and NH2* chemiluminescence imaging. Combust. Flame 2025, 272, 113899. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Li, S.; Yi, P.; Chen, R.; Wang, X.; Wu, Z.; Liu, S.; Wang, N.; Li, T. High-pressure ammonia spray combustion in lean premixed hydrogen mixture. Energy 2025, 336, 138520. [Google Scholar] [CrossRef]
- Ishihara, S.; Zhang, J.; Ito, T. Numerical calculation with detailed chemistry on ammonia co-firing in a coal-fired boiler: Effect of ammonia co-firing ratio on NO emissions. Fuel 2020, 274, 117742. [Google Scholar] [CrossRef]
- Zhang, J.; Ito, T.; Ishii, H.; Ishihara, S.; Fujimori, T. Numerical investigation on ammonia co-firing in a pulverized coal combustion facility: Effect of ammonia co-firing ratio. Fuel 2020, 267, 117166. [Google Scholar] [CrossRef]
- Shu, T.; Xue, Y.; Zhou, Z.; Ren, Z. An experimental study of laminar ammonia/methane/air premixed flames using expanding spherical flames. Fuel 2021, 290, 120003. [Google Scholar] [CrossRef]
- Henshaw, P.F.; D’Andrea, T.; Mann, K.R.C.; Ting, D.S.K. Premixed Ammonia-Methane-Air Combustion. Combust. Sci. Technol. 2005, 177, 2151–2170. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Grannell, S.; Stack, C.; Gille, D. A Comparison of Combustion Promoters for Ammonia and Two Ways to Run Engines on Ammonia as the Only Fuel. Available online: https://www.ammoniaenergy.org/wp-content/uploads/2021/01/shawngrannellammoniaconfpres2010.pdf (accessed on 9 October 2025).
- Takeishi, H.; Hayashi, J.; Kono, S.; Arita, W.; Iino, K.; Akamatsu, F. Characteristics of ammonia/N2/O2 laminar flame in oxygen-enriched air condition. Trans. JSME 2015, 81, 14-00423. [Google Scholar] [CrossRef]
- Xia, Y.; Hashimoto, G.; Hadi, K.; Hashimoto, N.; Hayakawa, A.; Kobayashi, H.; Fujita, O. Turbulent burning velocity of ammonia/oxygen/nitrogen premixed flame in O2-enriched air condition. Fuel 2020, 268, 117383. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Huang, J.; Shen, Y.; Zhang, Y.; Liu, Z. The characteristics of flame propagation in ammonia/oxygen mixtures. J. Hazard. Mater. 2019, 363, 187–196. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, Z.; Wang, S.; Zhang, T.; Yang, J. Measurement of oxy-ammonia laminar burning velocity at normal and elevated temperatures. Fuel 2020, 279, 118425. [Google Scholar] [CrossRef]
- Xinlu, H. Research on Fundamental Laminar Combustion Characteristics and Reaction Kinetic Mechanism of Innovative Carbon-Free Ammonia Fuel; Zhe Jiang University: Hangzhou, China, 2021. [Google Scholar]
- Kohansal, M.; Kiani, M.; Masoumi, S.; Nourinejad, S.; Ashjaee, M.; Houshfar, E. Experimental and Numerical Investigation of NH3/CH4 Mixture Combustion Properties under Elevated Initial Pressure and Temperature. Energy Fuel 2023, 37, 10681–10696. [Google Scholar] [CrossRef]
- Karan, A.; Dayma, G.; Chauveau, C.; Halter, F. Experimental study and numerical validation of oxy-ammonia combustion at elevated temperatures and pressures. Combust. Flame 2022, 236, 111819. [Google Scholar] [CrossRef]
- Wang, S. Experimental and Kinetic Study of Gaseous Fuel Combustion Characteristics Under Elevated Pressures Based on Heat Flux Method; Zhejiang University: Hangzhou, China, 2020. [Google Scholar]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Law, C.K. Combustion Physics; Cambridge University: New York, NY, USA, 2006. [Google Scholar]
- Miller, J.A.; Smooke, M.D.; Green, R.M.; Kee, R.J. Kinetic Modeling of the Oxidation of Ammonia in Flames. Combust. Sci. Technol. 2007, 34, 149–176. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 1994, 99, 253–276. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M. GRI-Mech 3.0. Available online: http://www.me.berkeley.edu/gri_mech (accessed on 9 October 2025).
- Shrestha, K.P.; Seidel, L.; Zeuch, T.; Mauss, F. Detailed Kinetic Mechanism for the Oxidation of Ammonia Including the Formation and Reduction of Nitrogen Oxides. Energy Fuel 2018, 32, 10202–10217. [Google Scholar] [CrossRef]
- Burke, U.; Somers, K.P.; O’Toole, P.; Zinner, C.M.; Marquet, N.; Bourque, G.; Petersen, E.L.; Metcalfe, W.K.; Serinyel, Z.; Curran, H.J. An ignition delay and kinetic modeling study of methane, dimethyl ether, and their mixtures at high pressures. Combust. Flame 2015, 162, 315–330. [Google Scholar] [CrossRef]
- Stagni, A.; Cavallotti, C.; Arunthanayothin, S.; Song, Y.; Herbinet, O.; Battin-Leclerc, F.; Faravelli, T. An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia. React. Chem. Eng. 2020, 5, 696–711. [Google Scholar] [CrossRef]
- Cremer, M.A.; Montgomery, C.J.; Wang, D.H.; Heap, M.P.; Chen, J.-Y. Development and implementation of reduced chemistry for computional fluid dynamics modeling of selective non-catalytic reduction. P. Combust. Inst. 2000, 28, 2427–2434. [Google Scholar] [CrossRef]
- Haynes, B.S. Reactions of ammonia and nitric oxide in the burnt gases of fuel-rich hydrocarbon-air flames. Combust. Flame 1977, 28, 81–91. [Google Scholar] [CrossRef]
- Konnov, A.A.; Ruyck, J.D. A Possible New Route for No Formation Via N2H3. Combust. Sci. Technol. 2001, 168, 1–46. [Google Scholar] [CrossRef]
- Dagaut, P.; Glarborg, P.; Alzueta, M. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The role of NNH in NO formation and control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Bertolino, A.; Fürst, M.; Stagni, A.; Frassoldati, A.; Pelucchi, M.; Cavallotti, C.; Faravelli, T.; Parente, A. An evolutionary, data-driven approach for mechanism optimization: Theory and application to ammonia combustion. Combust. Flame 2021, 229, 111366. [Google Scholar] [CrossRef]
- Singh, A.S.; Mohapatra, S.; Boyapati, R.; Elbaz, A.M.; Dash, S.K.; Roberts, W.L.; Reddy, V.M. Chemical Kinetic Modeling of the Autoignition Properties of Ammonia at Low–Intermediate Temperature and High Pressure using a Newly Proposed Reaction Mechanism. Energy Fuel 2021, 35, 13506–13522. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical kinetic modeling of ammonia oxidation with improved reaction mechanism for ammonia/air and ammonia/hydrogen/air combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Chan, S.H.; Shahsavari, M. Tailoring reduced mechanisms for predicting flame propagation and ignition characteristics in ammonia and ammonia/hydrogen mixtures. Energy 2022, 260, 125090. [Google Scholar] [CrossRef]
- Zhu, Y.; Curran, H.J.; Girhe, S.; Murakami, Y.; Pitsch, H.; Senecal, K.; Yang, L.; Zhou, C.-W. The combustion chemistry of ammonia and ammonia/hydrogen mixtures: A comprehensive chemical kinetic modeling study. Combust. Flame 2024, 260, 113239. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. A Detailed Kinetic Study of Ammonia Oxidation. Combust. Sci. Technol. 1995, 108, 231–254. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Contino, F.; Vandooren, J.; Jeanmart, H. Modeling of ammonia combustion at low pressure. Combust. Flame 2012, 159, 2799–2805. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Nakamura, H.; Hasegawa, S.; Tezuka, T. Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile. Combust. Flame 2017, 185, 16–27. [Google Scholar] [CrossRef]
- Zhang, X.; Moosakutty, S.P.; Rajan, R.P.; Younes, M.; Sarathy, S.M. Combustion chemistry of ammonia/hydrogen mixtures: Jet-stirred reactor measurements and comprehensive kinetic modeling. Combust. Flame 2021, 234, 111653. [Google Scholar] [CrossRef]
- Gotama, G.J.; Hayakawa, A.; Okafor, E.C.; Kanoshima, R.; Hayashi, M.; Kudo, T.; Kobayashi, H. Measurement of the laminar burning velocity and kinetics study of the importance of the hydrogen recovery mechanism of ammonia/hydrogen/air premixed flames. Combust. Flame 2022, 236, 111753. [Google Scholar] [CrossRef]
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia chemistry in oxy-fuel combustion of methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Li, R.; Konnov, A.A.; He, G.; Qin, F.; Zhang, D. Chemical mechanism development and reduction for combustion of NH3/H2/CH4 mixtures. Fuel 2019, 257, 116059. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Chen, C.; Elbaz, A.M.; Sun, Z.; Roberts, W.L. Applying heat flux method to laminar burning velocity measurements of NH3/CH4/air at elevated pressures and kinetic modeling study. Combust. Flame 2022, 236, 111788. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, S.; Sui, R.; Lu, Q. Impact of ammonia addition on soot and NO/N2O formation in methane/air co-flow diffusion flames. Combust. Flame 2023, 247, 112483. [Google Scholar] [CrossRef]
- He, X.; Li, M.; Shu, B.; Fernandes, R.; Moshammer, K. Exploring the Effect of Different Reactivity Promoters on the Oxidation of Ammonia in a Jet-Stirred Reactor. J. Phys. Chem. A 2023, 127, 1923–1940. [Google Scholar] [CrossRef]
- Shi, X.X. Research on Combustion Enhancement Method of Ammonia by Cofiring Dimethyl Ether with Insights into Pollutant Formation Mechanism; Shanghai Jiao Tong University: Shanghai, China, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).