Abstract
In the context of mounting concerns over carbon emissions and the need to accelerate the energy transition, green hydrogen has emerged as a strategic solution for decarbonizing hard-to-abate sectors. This paper introduces a methodological innovation by proposing the Green Hydrogen Efficiency Index (GHEI), a unified and quantitative framework that integrates multiple stages of the hydrogen value chain into a single comparative metric. The index encompasses six core criteria: electricity source, water treatment, electrolysis efficiency, compression, end-use conversion, and associated greenhouse gas emissions. Each are normalized and weighted to reflect different performance priorities. Two weighting profiles are adopted: a first profile, which assigns equal importance to all criteria, referred to as the balanced profile, and a second profile, derived using the analytic hierarchy process (AHP) based on structured expert judgment, named the AHP profile. The methodology was developed through a systematic literature review and was applied to four representative case studies sourced from the academic literature, covering diverse configurations and geographies. The results demonstrate the GHEI’s capacity to distinguish the energy performance of different green hydrogen routes and support strategic decisions related to technology selection, site planning, and logistics optimization. The results highlight the potential of the index to contribute to more sustainable hydrogen value chains and advance decarbonization goals by identifying pathways that minimize energy losses and maximize system efficiency.
1. Introduction
The global scientific consensus underscores the critical need to limit global warming to 1.5 °C to mitigate the most severe impacts of climate change. The Intergovernmental Panel on Climate Change (IPCC) emphasizes that achieving this target necessitates rapid and deep reductions in greenhouse gas (GHG) emissions across all sectors by mid-century. Despite advancements in renewable energy, global energy-related CO2 emissions reached a record high of 37.4 billion tonnes in 2023, marking a 1.1% increase from the previous year. This upward trend highlights the urgency for comprehensive decarbonization strategies, particularly in hard-to-abate sectors such as heavy industry and long-distance transport.
Green hydrogen has emerged as a promising solution to address these challenges due to its potential to decarbonize sectors where direct electrification is not feasible. However, the production and utilization of green hydrogen involve complex processes that can vary significantly in terms of energy efficiency and environmental impact. Therefore, establishing a standardized metric to evaluate the energy performance of green hydrogen systems is crucial. The Green Hydrogen Efficiency Index (GHEI) aims to fill this gap by providing a comprehensive framework to assess and compare the efficiency of different green hydrogen production pathways, thereby informing policy decisions and guiding investments toward more sustainable energy solutions.
The energy transition refers to the systemic shift from fossil-based energy sources to renewables, aiming to reduce greenhouse gas emissions and environmental impact. The generation of energy from renewable sources has been driven by advances in technologies such as solar and wind. Using these two sources as examples, improvements in the efficiency of photovoltaic modules, as well as the development of wind turbines with greater capacity and efficiency, have helped lower the cost of energy generation. This has made these sources more competitive and, consequently, reduced the global environmental impact of the energy sector. Solar photovoltaic technology has seen remarkable progress, with efficiency improvements of 5% over the past decade. These advancements, along with a 67% cost reduction, have made solar energy more accessible and viable. Wind energy has also experienced significant technological progress, with turbine efficiency increasing by 10% and costs decreasing by 64%. These developments have made wind power a competitive alternative to fossil fuels in many scenarios [1,2].
In this context, energy storage plays a key role, as it enables increased use of renewable energy by mitigating the intermittency of sources like solar and wind. Storage adds stability and reliability to energy supply. According to [3], energy storage enhances the economic penetration of renewable energy, reduces total investments in nuclear and gas-fired power plants, and improves the utilization of installed capacity in the energy matrix. However, deployment costs of energy storage systems remain a challenge to large-scale implementation and economic feasibility. Furthermore, the choice of storage technology must be preceded by an in-depth analysis of application requirements, such as the type of renewable energy source, location, and infrastructure [3,4,5].
Beyond the expansion of renewable energy generation and storage, another emerging factor in the energy transition is the pursuit of complementary solutions that enhance the feasibility and applicability of clean energy sources across different sectors. In this context, the development of sustainable energy carriers such as green hydrogen has gained prominence due to its ability to store and transport energy, as well as replace fossil fuels in sectors that are difficult to electrify. The growing adoption of green hydrogen highlights the need for integrated strategies that combine technological innovation, public policy, and infrastructure to maximize its potential in decarbonizing the global economy.
Hydrogen is a versatile element that can be used across various sectors [6]. It has a high energy content per unit of mass, making it an efficient energy carrier [7,8]. Hydrogen can be produced using various methods, both sustainable and non-sustainable. To differentiate its origin, color codes have been adopted. Green hydrogen is a clean energy carrier produced through the electrolysis of water using electricity generated from renewable sources. This process results in no direct greenhouse gas emissions, positioning green hydrogen as a clean and sustainable solution for the energy transition [9,10,11].
Due to its characteristics as an energy carrier, green hydrogen is considered a crucial element in the energy transition. It enables the use of renewable energy in sectors responsible for significant portions of emissions, such as transportation and heavy industry [9,12,13]. Hydrogen also functions as a buffer for intermittent renewable energy generation, allowing excess energy to be stored and later converted into heat or electricity [14,15]. Additionally, green hydrogen facilitates the integration of renewable generation, helping address the growing mismatch between generation and load throughout the day, improving grid stability and optimizing the use of renewable resources [16,17].
However, there are challenges to the adoption of green hydrogen. The primary barrier is cost, as hydrogen production remains more expensive than fossil fuels, particularly in the case of green hydrogen [8,18]. Storage and safety challenges are also significant, as hydrogen’s low density makes storage complex and costly, requiring advanced technologies to ensure safety and efficiency, such as metal hydrides and the use of advanced materials [6,19]. In terms of production, methods like electrolysis, although cleaner, are less efficient compared to conventional hydrogen production methods [6,18]. Given these challenges, the deployment of infrastructure for hydrogen production, storage, and distribution is both a technical and economic challenge, limiting its large-scale adoption [8,19].
Despite significant advancements in hydrogen research, much of the existing literature tends to focus on isolated technical components, such as electrolyzer efficiency, water electrolysis technology, or storage infrastructure, without adopting a comprehensive perspective on the full green hydrogen value chain. Consequently, few tools currently exist to compare hydrogen production routes based on energy efficiency across all stages, from electricity sourcing to end-use conversion. This gap highlights the need for an integrated and quantifiable approach that can assess performance holistically. Addressing this need, the present study proposes a novel framework, the GHEI, capable of evaluating different green hydrogen production configurations using unified energy efficiency criteria across the full value chain.
Therefore, replacing fossil fuels with green hydrogen requires a comprehensive analysis of the entire value chain. This includes not only hydrogen generation but also its compression, storage, transportation, and end use. It is essential to adopt metrics that can evaluate the energy performance of each stage of this chain. An efficiency index can serve as a strategic tool to compare different technologies, identifying ideal production locations, and optimizing logistics routes. This, in turn, ensures maximum energy conversion throughout the process.
The aim of this article is to provide a holistic view of the green hydrogen value chain and to present a methodology for analyzing the energy efficiency of different production topologies. The study will cover the process stages, including energy supply for electrolysis, water treatment, hydrogen generation and compression, as well as its final conversion into electricity via fuel cells. This work proposes an efficiency index that enables performance comparison across the various stages of the production chain using a single metric, as well as evaluation of different configurations and logistical routes for the production and use of green hydrogen.
The remainder of this paper is structured as follows: Section 2 provides a brief overview of the current landscape and challenges of green hydrogen production. Section 3 introduces the methodological framework for the proposed index, including the definition of evaluation criteria, scoring logic, and weighting profiles. Section 4 applies the GHEI to four representative case studies from the literature and discusses the comparative results using the two proposed profiles. Finally, Section 5 outlines the main conclusions of the study, including insights on planning and policy, and suggests directions for future research.
2. Literature Review
Analogous to electricity, hydrogen can be used as an energy carrier rather than a primary energy source. Its production can originate from various energy sources, although currently the largest share is predominantly derived from fossil fuels.
In the current context, hydrogen is classified into different “colors” to indicate its origin and production process. Each color represents a distinct production pathway, ranging from the most polluting, based on fossil fuels, to the cleanest, relying exclusively on renewable sources. This differentiation is crucial for assessing the sustainability and economic feasibility of each type of hydrogen, as well as for determining their potential applications across different sectors.
Although there is no broad consensus on the exact meaning of each color, Table 1 presents a summary of the main color variations and their corresponding production methods.

Table 1.
Hydrogen color classification and production technology [20,21].
According to Arcos and Santos [21], the challenges for widespread use of low-CO2 hydrogen, or sustainable hydrogen, include the need to develop more efficient technologies, reduce renewable electricity costs, and establish an adequate infrastructure for storage and distribution.
2.1. Green Hydrogen Chain Overview
Green hydrogen, produced from renewable sources, is the cleanest form of hydrogen. It is obtained through the electrolysis of water, a process that separates hydrogen from oxygen using electrical energy. However, unlike electricity, which, as an energy carrier, is represented solely through electric charges (electrons and protons), green hydrogen can serve as an energy carrier in multiple forms. The energy it stores can be converted into heat, electricity, and other forms of energy, depending on the application [22]. In this alternative energy chain, one possible configuration of which is illustrated in Figure 1, hydrogen plays multiple roles. One of the most important is enabling the use of renewable energy in sectors that are currently powered almost exclusively by fossil fuels.
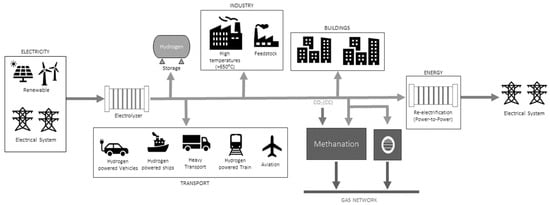
Figure 1.
Green hydrogen chain illustration [22].
In industry, green hydrogen can replace hydrogen derived from fossil fuels; however, it is not yet widely adopted due to its high cost [23]. In the long term, other fossil-based feedstocks may also be replaced by green hydrogen, significantly reducing CO2 emissions.
In the commercial and residential gas sectors, injecting hydrogen into the gas grid can reduce the consumption of liquefied petroleum gas (LPG) and natural gas. This integration has the potential to create new business models and expand the green hydrogen market [24,25,26].
In the transport sector, fuel cell vehicles powered by green hydrogen can shift the sector toward a low-carbon economy. For commercial transportation, hydrogen is particularly suitable for long-distance travel and high usage rates, overcoming some of the limitations of battery-powered alternatives.
In the energy sector, hydrogen production and storage can help make the electrical system more flexible by absorbing excess renewable energy. However, there are still technical and economic challenges to overcome, such as increasing the efficiency of electrolysis, reducing the cost of electrolyzers, and developing more feasible storage systems. Achieving the effective deployment of green hydrogen will require balancing cost and availability.
While Figure 1 provides a schematic view of the green hydrogen value chain, it is also important to understand how the existing academic literature addresses each stage of this chain. Table 2 presents a synthesis of selected studies, categorized by their primary focus within the hydrogen chain. It highlights which phases are typically assessed, indicates the methodological approaches used, and identifies the main strengths and limitations of each study.

Table 2.
Comparative review of the literature on the hydrogen chain stage.
This analysis underscores the novelty of the GHEI, which integrates multiple chain stages using a unified metric for energy efficiency assessment.
2.2. Green Hydrogen Chain Energy Efficiency Review
The energy balance in the production and use of green hydrogen is a critical factor in assessing its viability as a sustainable energy source. Green hydrogen, produced using energy from renewable sources, has the potential to significantly reduce GHG emissions and dependence on fossil fuels. However, achieving a positive energy balance along this pathway remains a challenge, particularly when considering production methods, the efficiency of key process stages, and the integration of various energy systems.
To investigate the energy efficiency of the green hydrogen value chain and establish a robust foundation for the proposed index, a comprehensive literature review was carried out. The review followed a systematic approach and was conducted across several strategic and internationally recognized scientific databases, including Google Scholar, Web of Science, Scopus, ScienceDirect, IEEE Xplore, and SpringerLink. These platforms were selected for their extensive coverage of high-impact journals and peer-reviewed conference proceedings in the fields of energy systems, sustainability, and industrial engineering.
The search strategy employed a combination of targeted keywords and Boolean operators to capture the breadth and depth of the green hydrogen research landscape. Key search terms included “green hydrogen production”, “green hydrogen plant”, “green hydrogen chain”, “electrolysis efficiency”, “hydrogen compression”, “renewable hydrogen systems”, and “lifecycle emissions of hydrogen”. These terms were used individually and in various combinations to identify both broad surveys and specialized studies that address different stages of the hydrogen value chain.
To ensure the inclusion of the most relevant and up-to-date findings, the review focused on publications from the past 10–15 years, emphasizing those with quantitative analysis of energy efficiency, system-level evaluations, or multi-criteria assessments of hydrogen technologies. Studies were selected based on their methodological rigor, transparency in assumptions, and their relevance to one or more of the criteria proposed in the GHEI framework, such as electricity sourcing, water treatment, electrolysis technologies, compression strategies, end-use applications, and greenhouse gas emissions.
The literature review ultimately led to the identification of a diverse body of work, encompassing technical, economic, and environmental analyses. These studies not only informed the conceptual structure of the GHEI, but also guided the definition of scoring thresholds and the development of realistic evaluation profiles. In doing so, the review process provided a comprehensive understanding of the current state of the art in green hydrogen production and highlighted key areas where energy efficiency and sustainability can be enhanced across the full value chain.
The study presented in Pashchenko [27] investigates the energy efficiency of using green hydrogen as a fuel for energy generation. It analyzes hydrogen’s efficiency by comparing its lower heating value (LHV) and higher heating value (HHV). The LHV refers to the amount of energy released when a fuel is burned, excluding the energy contained in the water vapor produced during combustion. It measures the usable energy available from the fuel, as it does not consider the latent heat of vaporization of water. In contrast, the HHV includes this energy, accounting for the recovery of latent heat during the condensation of combustion-generated steam. Thus, HHV represents the total energy released by the complete combustion of the fuel and is especially relevant when technologies are capable of recovering this additional heat. The study examines three main stages involved in using green hydrogen as a fuel for power generation, along with their respective efficiencies: hydrogen production, hydrogen compression, and hydrogen utilization. However, the study focuses on the thermodynamic analysis of hydrogen and does not evaluate other important stages of the green hydrogen chain, such as energy supply and water treatment, using the same metric.
The study by Petrakopoulou and García-Tenorio [28] proposes an analysis of electricity generation from hydrogen using the concept of total efficiency, which encompasses the life cycle stages of the fuel: production, processing, and transport. Two case studies were conducted at thermal power plants in Spain and Germany, exploring nine different scenarios based on various hydrogen production routes (such as methane reforming, electrolysis, and pyrolysis) and logistical options (such as pipelines, trucks, and ships). The study found that the production stage consumes the most energy, with the conversion of natural gas into hydrogen being a critical factor. Local green hydrogen production using surplus renewable energy was found to be the most efficient option. Hydrogen derived from fossil fuels, especially when transported over long distances, resulted in the lowest efficiencies. Similar to the study presented by Mukherjee et al. [26], this work emphasizes the thermodynamic analysis of hydrogen production for electricity generation, without including stages such as energy generation evaluation or water treatment.
The articles by Pashchenko [27] and Petrakopoulou and García-Tenorio [28] showed the greatest alignment with the efficiency analysis of the green hydrogen chain, providing a foundation for proposing a green hydrogen chain efficiency index. However, both studies focus on thermal efficiency, a component that depends heavily on the configuration of the hydrogen generation system, which complicates comparisons between different systems.
Other studies evaluate hydrogen production efficiency more generally, often involving new technologies. For instance, the work presented by Zhang et al. [29] analyzes efficiency from a chemical reaction perspective. The research aims to increase the economic viability of green hydrogen by integrating chemical production with hydrogen generation, thereby addressing current limitations in hydrogen’s competitiveness against fossil fuels. The study applies a comprehensive net energy life cycle assessment (LCA) to evaluate the energy flows and efficiency of the proposed coupled photoelectrochemical device.
In Bao et al. [30], an equilibrium optimization model is proposed to assess the spatial potential, capacity, and benefits of hydrogen consumption. The study demonstrates that using curtailed renewable energy for hydrogen production improves both energy efficiency and economic benefits, thereby enhancing the overall energy balance in green hydrogen systems. The article explores a renewable-based system for hydrogen production, storage, and use, focusing on addressing wind and solar energy curtailment in northwest China. In this region, curtailment occurs when generated renewable energy is not fully utilized or integrated into the grid, due to factors such as grid infrastructure limitations, geographic disparities between renewable resource availability and demand, and low local energy consumption. The study highlights the potential of hydrogen production technology in advancing green energy transformation and improving overall energy utilization.
A significant portion of hydrogen efficiency studies focus primarily on economic aspects [31,32,33]. However, no studies were identified that specifically conduct comparative analyses of electrical energy consumption across the various stages of green hydrogen production chains.
In summary, it is evident that a comprehensive analysis of hydrogen efficiency is multifaceted, incorporating production methods, energy consumption for compression, fuel utilization efficiencies, and overall energy conversion rates to assess hydrogen’s viability as a sustainable energy source.
3. Methodology
3.1. Research Design and Methodological Steps
This study followed a structured methodology designed to support the development, justification, and application of the GHEI. The research process was divided into four main phases, as shown in Figure 2.
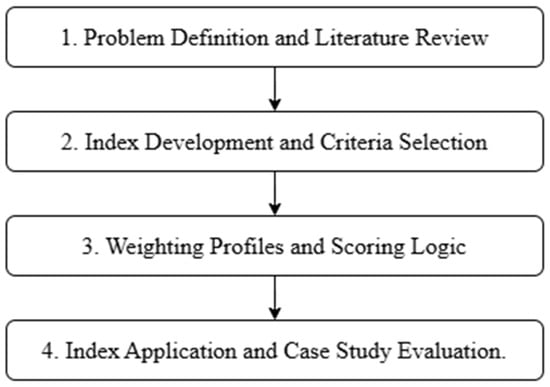
Figure 2.
Research methodology flowchart for the development and application of the Green Hydrogen Efficiency Index (GHEI).
- Problem definition and literature review: The study began with a comprehensive literature review focused on energy efficiency in green hydrogen production, identifying critical stages of the hydrogen value chain and reviewing existing evaluation methods. This phase established the conceptual and technical basis for the GHEI criteria.
- Index development and criteria selection: Six performance criteria were defined based on energy intensity and relevance across the hydrogen chain: electricity source, water treatment, electrolysis technology, compression, end use, and associated GHG emissions. Scoring thresholds were established for each criterion based on data ranges and benchmarks extracted from the literature.
- Weighting profiles and scoring logic: Two weighting profiles were defined: a balanced profile, where all six criteria are equally weighted, providing a neutral perspective, and an AHP profile, developed using the analytic hierarchy process, which assigns weights based on structured expert comparisons of importance across criteria. Normalization and weighted average logic were used to integrate the scores.
- Index application and Case Study Evaluation: The GHEI was applied to four international case studies selected from the literature. Each project was assessed against the six criteria, and GHEI scores were calculated using both profiles. The results were analyzed comparatively to evaluate the utility of the index in distinguishing project performance.
This research design ensures methodological rigor, transparency, and replicability in evaluating energy efficiency across complete green hydrogen production chains.
3.2. GHEI Structure and Calculation
This section presents the proposed methodology for implementing the GHEI, designed to assess the energy efficiency of the green hydrogen production chain. The index encompasses a broad scope of the value chain, covering the following stages: renewable energy generation, water treatment for electrolysis, electrolysis itself, hydrogen compression, and final conversion into electricity through a fuel cell. The goal is to provide a holistic view of the energy performance of the entire chain.
The methodology was based on a comprehensive literature review and on case studies of implemented green hydrogen production and usage systems, which, although still limited in number, have been growing significantly. Various efficiency metrics found in the literature were analyzed, but the focus was placed on electrical energy consumption, a common input across all stages of the chain.
The central idea of the index is to deepen the analysis of green hydrogen production across different production chain structures. That is, depending on the specific configuration of the chain, two identical hydrogen molecules may require vastly different amounts of energy to be produced and made available. How can this variation be measured and incorporated into decision-making regarding hydrogen application?
Developing a methodology to assess this type of energy chain poses significant challenges, especially when compared to the electricity value chain, which is characterized by a relatively linear flow from generation to end use. In contrast, the green hydrogen value chain is more complex: electricity is used to power electrolyzers that produce hydrogen gas, which can subsequently be utilized directly in various applications or reconverted into electricity through additional processes.
The green hydrogen value chain can follow multiple distinct pathways, depending on the intended end use of the hydrogen and the intermediate steps involved. As illustrated in Figure 3, three potential routes can be identified, each beginning with electricity generation that powers the electrolysis process. In Route 1, the hydrogen produced is immediately made available as a feedstock. In Route 2, the hydrogen is also used as a feedstock but undergoes compression before use, introducing an additional stage. Route 3 differs more significantly, as the hydrogen is utilized to generate electricity via a hydrogen fuel cell, completing a circular flow of energy.
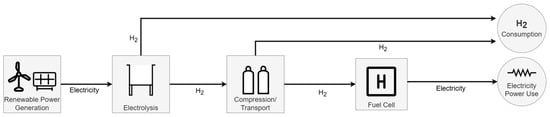
Figure 3.
Overview of the green hydrogen production chain.
Given this diversity of pathways, the methodology was developed to enable a comparative analysis of the energy consumption involved in the production and use of green hydrogen across different routes. It considers all steps, from generation to final application, allowing for a comprehensive evaluation of the energy efficiency of the green hydrogen chain.
The core metric employed is the specific energy consumption (kWh/kg H2), which quantifies the amount of electrical energy required to process 1 kg of hydrogen at each stage of the chain. This approach allows for standardized comparisons between different routes and helps identify the stages with the greatest impact on the overall system efficiency.
3.2.1. Analysis of Energy Efficiency in the Stages of the Green Hydrogen Chain
In order to present the bases on which the GHEI was developed, this section presents an analysis of each stage of the chain considered in the proposed methodology: power supply, water treatment, electrolysis, compression, fuel cells, and greenhouse gas emissions. The selection of these stages for the formulation of the GHEI was based on a comprehensive review of the various topologies and configurations of green hydrogen production chains found in the academic literature and observed in commercial-scale projects. This approach ensures that the index reflects real-world systems and captures the most energy-relevant and operationally significant stages of the value chain.
- Power supply
Electricity is the central input in the electrolysis process and the main cost determinant in the final price of green hydrogen [34]. For hydrogen to be classified as “green”, the electricity used must be renewable and traceable, which can be ensured through certification systems such as International Renewable Energy Certificates (I-RECs) [35].
From a technical standpoint, the electricity supply has a direct impact on the efficiency of the green hydrogen production chain. In this context, for the purpose of analyzing electricity supply for electrolysis, three main scenarios were considered: Scenario 1 involves surplus energy from curtailment; Scenario 2 involves on-site power generation; and Scenario 3 involves electricity from the power grid.
- Scenario 1—Curtailment
The rapid growth of intermittent renewable sources such as solar and wind has posed technical challenges to power systems worldwide, especially related to the phenomenon of curtailment, the forced reduction of available renewable generation, even when natural resources (sun or wind) are sufficient. This occurs due to limitations in the electrical infrastructure or oversupply during low-demand periods. Although unavoidable in contexts with high renewable penetration, curtailment represents a loss of clean energy and a significant economic challenge for grid operators and renewable power generation investors. In systems with limited flexibility, such as grids with a high share of inflexible thermal plants or without storage, curtailment can occur frequently, resulting in inefficiencies and wasted energy [36].
In Brazil, this issue is particularly evident in the Northeast region, where high installed wind capacity contrasts with transmission bottlenecks and the limited flexibility of the National Interconnected System. Studies have shown that high wind penetration in the region can be significantly constrained by operational and transmission limitations, with curtailments already documented during periods of low demand [37]. This scenario highlights the need for infrastructure modernization, expansion of the transmission network, and integration of storage technologies to fully and efficiently harness Brazil’s renewable potential. In this scenario, electrolysis uses surplus renewable energy that could not be injected into the grid, generally due to transmission constraints or system operation limitations.
- Scenario 2—Local Energy Generation
In this arrangement, renewable electricity is generated on-site, at the same location as the electrolysis plant. This scenario minimizes transmission losses and provides greater control over the renewable source. Despite its energy efficiency advantages, this model may face economic limitations, as energy consumption is constrained by the demand of local hydrogen production. This can result in underutilization of the electrical generation potential [38].
- Scenario 3—Energy from the Power Network
In this case, electricity is sourced from the power grid. Although this is the most flexible and often the most economically viable option, it is associated with transmission losses and presents challenges in tracking the true origin of the energy, given the complexity of power systems. I-REC certification is a strategy used to ensure the renewable origin of the energy. However, the energy supply efficiency in this scenario may be lower than in Scenario 1, due to the inherent losses in long-distance infrastructure [39].
The analysis of these three energy supply scenarios reveals that the efficiency of the electrolysis stage in the green hydrogen value chain is closely linked to how electricity is generated and, more importantly, how it is delivered. While the curtailment scenario offers the highest energy utilization potential and the lowest marginal cost, its intermittency limits production predictability. On the other hand, local generation provides better control and efficiency, although it brings economic and operational challenges. Electricity from the grid, while more flexible, imposes limitations related to the traceability of renewable sources and transmission losses.
- 2.
- Water treatment
The United Nations’ Sustainable Development Goals (SDGs) emphasize the critical role of water in sustaining life and promoting development. Specifically, SDG 6 aims to ensure the availability and sustainable management of water and sanitation for all. This goal highlights water not only as a vital resource for life but also as a key enabler of social and economic development [40].
Globally, the energy sector is one of the largest industrial consumers of water, often surpassing agriculture in terms of water use per unit of energy produced [41]. Water plays an essential role in various stages of the energy chain, from fossil fuel extraction and refining to cooling in thermal and nuclear power plants, as well as in hydroelectric generation. This high level of dependence makes the sector particularly vulnerable to water shortage events, as demonstrated by the temporary shutdowns of nuclear power plants in Europe during heatwaves and droughts in 2022 [42].
With the intensification of extreme weather events, such as prolonged droughts and heatwaves driven by climate change, these risks are expected to become more severe [43]. In response, the energy sector has begun adopting water management best practices and transitioning to renewable sources that are less water intensive, such as photovoltaic solar and wind energy, which require significantly less water compared to conventional thermal power plants [44].
To assess the efficiency of water treatment for green hydrogen production, a comparative analysis was conducted based on a literature review of the electricity consumption associated with the water treatment processes required to supply different electrolysis technologies.
The quality of purified water is classified into Types I, II, and III, based on chemical purity and electrical conductivity. This standard, defined by the American Society for Testing and Materials (ASTM), is widely used in laboratories and pharmaceutical industries and is increasingly being applied to hydrogen production via electrolysis.
Water purity requirements vary by electrolysis technology. Proton exchange membrane (PEM) and anion exchange membrane (AEM) technologies require ultrapure water (Type I), with electrical conductivity below 1 μS/cm. This imposes the need for sophisticated purification systems, such as reverse osmosis combined with electrodeionization (EDI) and UV treatment [45,46]. In contrast, alkaline electrolysis permits the use of purified water (Type II or III), with greater tolerance to contaminants, and requires simpler processes like filtration, demineralization, and the use of concentrated potassium hydroxide (KOH) [47]. The solid oxide electrolyzer cell (SOEC), which operates with high-temperature steam (600–850 °C), requires lower chemical water purity but demands additional thermal energy to generate and maintain steam [48].
Based on the observed data, the energy consumption for water treatment in electrolysis can be grouped into three categories: 1. PEM and AEM Technologies: Due to their high-water purity requirements, they involve reverse osmosis, electrodeionization, and ultraviolet (UV) treatment, with a typical energy consumption of 0.036 to 0.054 kWh to produce 1 kg of H2 by processing 9 L of water [49]. 2. Alkaline Electrolysis: Using simpler processes such as filtration and basic ion exchange, it requires approximately 0.013 to 0.023 kWh/kg H2 [47]. 3. SOEC Technology: While the water treatment is relatively simple, the vaporization process demands considerable thermal energy, with an estimated consumption of 0.08 to 0.12 kWh/kg H2 [50].
A comparative summary of treatment methods, energy consumption, and water quality requirements for each electrolysis technology is presented in Table 3.

Table 3.
Comparison of water treatment, consumption, and requirements of electrolysis technologies.
- 3.
- Electrolysis
The efficiency of an electrolyzer is a key parameter in determining electrical energy consumption and, consequently, the production cost of hydrogen. The choice of electrolyzer technology directly influences its efficiency. As in the previous analyses, the metric used to evaluate the efficiency of each technology is electrical energy consumption per kilogram of hydrogen produced (kWh/kg H2). Although other factors, such as the equipment’s nominal power and balance of plant consumption, can also affect overall efficiency, this metric allows for direct comparisons across different technologies and stages of the hydrogen value chain.
Alkaline electrolysis is one of the most mature and widely adopted technologies for large-scale industrial hydrogen production. It employs an aqueous solution of potassium hydroxide (KOH) as the electrolyte and nickel or stainless-steel electrodes. The specific energy consumption reported in most references ranges from 47 to 50 kWh per kg of hydrogen, with a typical efficiency of 65% to 70%. However, alkaline electrolyzers exhibit lower current density and slower response times, especially when compared to PEM technology. Nonetheless, alkaline electrolysis is recognized for its operational robustness, simple design, and lower initial capital cost [51].
PEM electrolysis is a more modern alternative with superior operational characteristics, including higher current density and faster response to load variations. PEM technology operates in an acidic environment and requires noble materials such as platinum and iridium, which contribute to higher acquisition costs. The average energy consumption for PEM electrolysis ranges from 43 to 50 kWh per kg of hydrogen, a level comparable to that of alkaline electrolysis. Due to its faster dynamic response, PEM offers advantages for integration with intermittent energy sources such as solar and wind [51].
AEM electrolysis is a relatively new approach developed to combine the advantages of both alkaline and PEM electrolysis. It operates in an alkaline environment with a structure similar to that of PEM. Although it is less technologically mature than alkaline and PEM systems, AEM shows competitive potential, with estimated energy consumption in the range of 46 to 54 kWh per kg of hydrogen. The main appeal of AEM electrolysis lies in the possibility of using non-noble materials, which may reduce costs and enable large-scale deployment in the long term. However, challenges related to membrane durability and electrochemical stability still limit its widespread adoption [52].
SOECs differ by operating at high temperatures (600–850 °C) and using steam rather than liquid water. This characteristic allows thermal energy sources to supply part of the energy required for the electrochemical reaction. As a result, SOECs exhibit higher thermodynamic efficiency and lower direct electrical consumption, typically in the range of 35 to 40 kWh per kg of hydrogen, considering the electrical component. Although SOEC technology is still in the early stages of maturity, it holds promise for integration with industrial processes that generate waste heat [48].
Figure 4 presents a comparative chart showing the reported energy consumption for each electrolysis technology analyzed. The more efficient technology is emphasized in green.
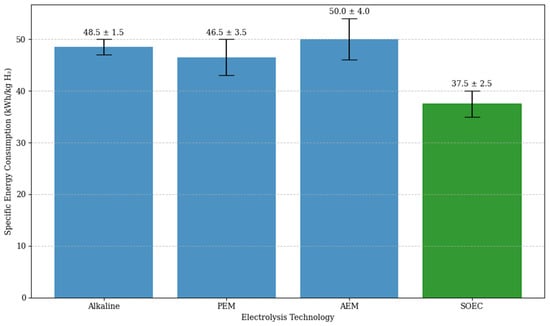
Figure 4.
Comparison of electricity consumption by electrolysis technology [48,51,52].
The chart highlights that the choice of electrolysis technology has a relevant impact on the energy efficiency of the green hydrogen value chain. SOEC is the most efficient, followed by PEM and alkaline electrolysis, while AEM, although promising, shows the highest average energy consumption and the greatest uncertainty.
- 4.
- Compression
Compression and storage are often overlooked stages in hydrogen supply chain studies, yet they are essential components in assessing the overall efficiency of the green hydrogen value chain. Hydrogen compression is the process of increasing the volumetric density of the gas to facilitate its storage and transportation. In the stages of transport, storage, and usage at refueling stations, compression is fundamental to optimizing space and system efficiency. Compression at refueling stations, particularly in transportation applications, is critical to ensure the appropriate pressure levels for such activities.
Hydrogen can be compressed across various pressure ranges, from 20 to 1000 bar, depending on the application. The following sections summarize energy consumption characteristics for the most commonly observed compression ranges in green hydrogen production chains:
- Compression to 20–30 bar
Most commercial PEM and alkaline electrolyzers operate with hydrogen output pressures between 20 and 30 bar. This pressure is generated directly in the electrolysis process, thus requiring no additional energy consumption for compression at this stage [53]. However, for most practical applications, such as storage, transport, or vehicle refueling, this pressure level is insufficient, making a subsequent compression stage necessary to reach higher pressures.
- Compression to 100 bar
Compression of hydrogen up to 100 bar is considered an intermediate stage, commonly used in supply chains that rely on pressurized tanks for stationary storage or local distribution networks. Energy consumption to reach this range is relatively low, varying between 0.6 and 1.8 kWh/kg H2, depending on the technology. At this pressure level, electrochemical compression, as discussed in Bouwman [54], emerges as an energy-efficient alternative, reducing maintenance costs and mechanical friction losses. When performed as part of a larger compression process up to 600 bar, total consumption for the 20–100 bar stage can be significantly lower, potentially below 1 kWh/kg H2 [27].
- Compression to 350 bar
Compression to 350 bar is widely used for hydrogen transport via trailers and in refueling stations for heavy-duty fuel cell vehicles, such as buses. This pressure level enables the storage of a reasonable quantity of hydrogen in relatively compact tanks while meeting industrial safety requirements. The specific energy consumption for this pressure level depends on compressor efficiency and operational conditions (speed, compression volume, etc.). According to the DOE Hydrogen and Fuel Cells Program [55], the estimated specific consumption for this pression ranges from 1.7 to 2.5 kWh/kg H2. Variations are due to differences in heat dissipation and mechanical inefficiencies, especially in faster compression processes.
- Compression to 700 bar
This is the most common pressure level for refueling light-duty fuel cell vehicles. Energy demand increases proportionally with the pressure level. Compression to 700 bar may consume between 4.0 and 6.4 kWh/kg H2 in real-world applications, particularly when pre-cooling of the gas is required to prevent tank overheating during rapid refueling operations [55,56].
- Compression to 875–1000 bar
Compression up to 875–1000 bar is considered an ultra-high-pressure range, mainly used in innovative storage and transport solutions, such as pressurized cryogenic trailers that eliminate the need for compressors at the final delivery points. Electrochemical compression is a promising alternative for this pressure range, with energy consumption around 3.0 kWh/kg H2. This technology uses membranes and electrolytes to pump hydrogen without moving parts, reducing friction and heat losses. It is much more efficient than traditional mechanical compression, especially at high pressures. However, it remains a low-maturity technology for large-scale applications [57].
A comparative summary of pressure levels and corresponding specific energy consumption for compressing 1 kg of hydrogen is presented in Table 4.

Table 4.
Comparative summary of hydrogen compression levels and specific consumption.
Table 4 shows that the specific energy consumption for hydrogen compression is directly related to the desired final pressure level, varying significantly depending on the application. The natural outlet pressure of electrolyzers (30 bar) does not require additional compression energy. Intermediate storage at 100 bar presents a moderate consumption, ranging from 0.6 to 1.8 kWh/kg H2, making it suitable for logistical applications and system buffering. Energy demand increases substantially for transportation applications: For heavy-duty vehicles (such as buses) with compression to 350 bar, specific consumption ranges from 1.7 to 2.5 kWh/kg H2. For light-duty vehicles using storage at 700 bar, the energy requirement rises significantly, reaching up to 6.4 kWh/kg H2.
- 5.
- Fuel Cells
Although not a mandatory stage in the green hydrogen value chain, fuel cells are an important component due to their role in converting the chemical energy of hydrogen into electrical energy, either for stationary applications or mobility. This conversion occurs through an electrochemical reaction with oxygen, without combustion, and the only by-product is water, with no atmospheric pollutant emissions [58].
Conceptually, the efficiency of a fuel cell is defined as the ratio between the useful electrical energy generated and the chemical energy available in the hydrogen. This efficiency can be analyzed from different perspectives: thermodynamic efficiency, based on the Gibbs free energy-to-enthalpy limit of the process; actual electrical efficiency, which considers losses due to internal resistance, reaction kinetics, and mass transport; and system efficiency, which incorporates auxiliary subsystems such as cooling, control, and gas compression, collectively known as the balance of plant (BoP) [59].
Among the existing types of hydrogen fuel cells, the following technologies are considered for comparison: PEMFCs, alkaline fuel cells (AFCs), phosphoric acid fuel cells (PAFCs), and solid oxide fuel cells (SOFCs).
- PEM Fuel Cells
Proton exchange membrane fuel cells (PEMFCs) use a polymer membrane as the electrolyte and operate at relatively low temperatures (60–80 °C). They are relatively lightweight, compact, offer fast response, and have a high-power density, making them ideal for automotive and portable applications. However, they require high-purity hydrogen and use platinum catalysts, which increases costs and sensitivity to contaminants. Their efficiency typically ranges from 40% to 60% [60].
- Alkaline Fuel Cells (AFCs)
AFCs use an aqueous solution of potassium hydroxide as the electrolyte. Known for their high efficiency and low material cost, they were historically used by NASA in space missions. Operating between 60 °C and 90 °C, their main limitation is their sensitivity to carbon dioxide, which necessitates highly purified fuel and air. They have an efficiency range similar to PEMFCs, typically between 45% and 60% [61].
- Phosphoric Acid Fuel Cells (PAFCs)
PAFCs use liquid phosphoric acid as the electrolyte and operate at temperatures between 150 and 200 °C. They are more robust against impurities and are primarily used for stationary applications, such as power generation for buildings. Although they have lower power density than PEMFCs, they offer better tolerance to contaminants and stable operation. Reported efficiencies are generally lower than the previous technologies, ranging from 35% to 45%, but can be significantly higher with the use of residual heat in combined heat and power (CHP) applications [62].
- Solid Oxide Fuel Cells (SOFCs)
SOFCs operate at high temperatures, typically 600–1000 °C, and use solid ceramic electrolytes. They are capable of using various fuels (hydrogen, natural gas, methane) and offer high thermal and electrical efficiency. Suitable for stationary and industrial applications, they require long start-up times and corrosion-resistant materials due to high temperatures. The characteristic efficiency range is 50% to 65%, and like PAFCs, they can achieve even higher efficiencies when used in cogeneration systems [63,64].
Table 5 shows a comparison of efficiency for each fuel cell technology. This table details the efficiency of fuel cells in different temperature ranges and membrane types.

Table 5.
Comparison of the characteristics of the main hydrogen fuel cell technologies.
- 6.
- Greenhouse Gas Emissions
While green hydrogen is characterized by zero direct GHG emissions during production via renewable-powered electrolysis, a comprehensive environmental assessment must consider indirect emissions associated with the broader life cycle. Green hydrogen projects can exhibit significant variation in CO2-equivalent emissions when system boundaries extend beyond the electrolysis step [65,66].
Indirect GHG emissions primarily arise from upstream and downstream processes. Key sources include the manufacturing of electrolyzers (particularly PEM and SOEC technologies that rely on rare metals such as platinum and iridium), energy use in water treatment (especially desalination), compression, storage, and transportation. These stages often involve electricity use, material processing, or logistics that emit CO2, especially when powered by non-renewable energy sources [67,68].
The electricity source is a major determinant of life-cycle GHG performance. When electrolyzers draw from uncertified renewable electricity, such as through power purchase agreements (PPAs) or I-RECs, they can achieve emissions below 1 kg CO2eq/kg H2, aligning with regulatory definitions of green hydrogen [66,69].
System boundary definitions are critical to GHG quantification. A cradle-to-gate assessment includes electricity and water use up to hydrogen production, while cradle-to-grave or well-to-wheel boundaries account for compression, transport, and end-use emission. For example, transporting hydrogen as liquefied gas or ammonia can substantially increase life-cycle GHG due to additional processing and fuel requirements.
Geographic context also affects emissions. Electrolyzer deployment in regions with low renewable capacity or significant water scarcity may lead to higher emissions. Conversely, co-locating hydrogen facilities with abundant renewable energy and water resources minimizes life-cycle impacts.
Including the Energy Score for GHG Emissions (ESGHG) as a GHEI criterion ensures projects are assessed holistically, recognizing that zero direct emissions do not always equate to net-zero climate impact. This approach aligns with recent research and policy trends emphasizing life-cycle carbon intensity as a determinant of green hydrogen classification.
3.2.2. Green Hydrogen Efficiency Index (GHEI)
The growing demand for sustainable energy solutions positions green hydrogen as a strategic vector in the global energy transition. However, the rush to implement green hydrogen production chains and systems can sometimes shift the focus away from the efficient use of natural resources. On the other hand, an integrated assessment of key attributes, such as energy efficiency, environmental impact, and operational viability, requires robust and adaptable metrics.
In this context, the present work proposes a methodology for implementing the GHEI as a flexible tool to compare diverse projects or configurations within the green hydrogen value chain.
The index is proposed as a composite metric for evaluating the overall performance of green hydrogen production chains. Its primary objective is to support decision-making by providing a holistic view of energy efficiency and environmental impact across various process configurations. The GHEI enables comparisons among projects and technologies by aggregating critical performance attributes into a single, interpretable index.
Following the proposed methodology, the index incorporates six key criteria representing the primary stages and impacts of green hydrogen production and use. The criteria are presented in Table 6.

Table 6.
GHEI criteria definitions and descriptions.
Based on the literature review and project information, these criteria were quantitatively evaluated. Finally, they were normalized to values ranging from 0, corresponding to minimum performance, to 1, corresponding to maximum performance, as presented in Table 7.

Table 7.
GHEI evaluation criteria.
Therefore, the GHEI is calculated as a weighted average of the six criteria as follows (Equation (1)):
where is the efficiency score for the criterion (ESSE, ESWT, ESET, ESCO, ESEU, and ESGHG), and is the weight of the criterion i (subjected to ). After defining the performance scores for each of the six criteria, the weights to be applied to each of the GHEI Efficiency Scores should be defined.
- Weighting Profiles
To ensure flexibility while maintaining methodological rigor, two distinct weighting profiles are proposed: the AHP profile and the balanced profile. Each reflects a different approach to prioritizing performance dimensions in the hydrogen value chain.
Incorporating weights into the GHEI methodology allows for tailored assessments that align with decision making objectives. Weighting provides the ability to emphasize or de-emphasize individual criteria based on contextual priorities, such as technical performance, environmental impact, or overall system balance. This flexibility supports the development of distinct evaluation profiles, each underpinned by a clear logical or methodological foundation. For instance, the AHP profile reflects expert-driven prioritization based on structured comparisons, while the balanced profile offers a neutral reference where all criteria are treated equally. By enabling these variations, the GHEI becomes a more versatile tool capable of adapting to different stakeholder perspectives and project evaluation goals.
- AHP Profile
To improve the robustness of the GHEI, an alternative weighting scheme was developed using the analytic hierarchy process (AHP). AHP is a well-established method in decision science, introduced by Saaty [70], that enables quantitative prioritization of criteria based on pairwise comparisons. This method has been extensively used in energy planning and sustainability evaluations to structure subjective judgment with mathematical consistency [71].
AHP uses and ordinal scale to capture how much more important one criterion is over another. Table 8 shows the meaning of each value on the Saaty scale.

Table 8.
Values and interpretation of each value on the Saaty scale.
For this study, AHP was used to assign weights to the six criteria of the GHEI, based on judgement of relative importance. The following steps explain how the AHP weights were calculated.
- Step 1—Constructing the Pairwise Comparison Matrix
The six criteria have been defined as the key dimensions of green hydrogen project performance, as defined in the GHEI: ESSE (source of electricity), ESWT (water treatment), ESET (electrolysis technology), ESCO (compression), ESEU (hydrogen end use), and ESGHG (CO2 equivalent emissions).
The criteria were compared pairwise and assigned levels of importance based on the Saaty scale. The authors performed the importance judgment, considering analyses observed in the academic and technical literature.
The pairwise comparisons yielded the following assessments based on Saaty’s scale: ESET was judged moderately more important (3) compared to ESCO, ESEU, ESGHG, and ESSE; ESEU was judged moderately more important (3) compared to ESCO, ESGHG, and ESWT; ESGHG was judged moderately more important (3) compared to ESCO and ESEU; ESCO was considered equally important (1) to ESWT; ESGHG was considered equally important (1) to ESSE; ESSE was judged strongly more important (5) compared to ESWT; ESET was judged strongly more important (5) compared to ESWT; and ESSE was judged moderately more important (3) compared to ESCO and ESEU. A summary of the comparison is provided as follows: ESET was considered of moderate importance compared to ESCO, ESEU, ESGHG, and ESSE; ESEU was considered of moderate importance compared to ESCO, ESGHG, and ESWT; ESGHG was considered of moderate importance compared to ESCO and ESEU; ESCO was considered as important as ESWT; ESGHG was considered as important as ESSE; ESSE was considered to have strong importance compared to ESWT; ESET was considered to have strong importance compared to ESWT; and ESSE was considered of moderate importance compared to ESCO and ESEU.
The judgements were used to populate a 6 × 6 reciprocal matrix. Each element in the matrix reflects the relative importance of criterion compared to criterion . For instance, ESET was judged to be strongly more important than ESWT, leading to an entry of 5 in the matrix, while the reciprocal value (1/5) was placed in the opposite cell.
- Step 2—Normalizing the Matrix
The matrix was normalized by summing each column and dividing each element by the column total. This ensures all values are scaled proportionally.
- Step 3—Calculating Weights
The AHP weight for each criterion was computed as the mean of the normalized row values, yielding the priority vector presented in Table 9.

Table 9.
AHP profile weights.
The results emphasize ESET as the most critical factor, followed by ESSE, ESEU, and ESGHG, reflecting their dominant contributions to energy losses and environmental performance in the green hydrogen chain. ESWT is assigned the lowest weight, consistent with its relatively minor influence on overall system performance in most configurations.
- Step 4—Consistency Check
To verify the logical coherence of the expert judgements in the pairwise comparison matrix, a consistency check was performed. This involves several calculations: the weighted sum vector, the consistency vector, the consistency index (CI) and the consistency ratio (CR).
Weighted Sum Vector: The weighted sum vector is obtained by multiplying the original pairwise comparison matrix by the AHP vector (Equation (2))
Each element represents the combined relative importance of criterion , aggregated across all comparisons, weighted by the AHP-derived weights of the other criteria.
Consistency Vector : It is calculated by dividing each element of the weighted sum vector by the corresponding AHP weight (Equation (3)).
Each gives an individual consistency measure for criterion i. Conceptually, it reflects how closely the judgments for that criterion agree with the overall pattern of weights. The values obtained for the weighted sum and are presented in Table 10.

Table 10.
Weighted sum and for each criterion.
Ideally, all values would be equal, suggesting perfect consistency. However, slight variations are acceptable if the overall consistency is within bounds.
Calculate (Principal Eigenvalue): The principal eigenvalue is calculated as the average of the values, according to Equation (4), yielding a result of 6.5162.
Consistency Index (: is calculated using Equation (5) and gives the value = 0.1032.
Consistency Ratio (): Using the random inconsistency () index, defined by Saaty, for = 6, which is 1.24, and Equation (6), a = 0.0833 is obtained.
Based on the value obtained for the 6.5162, consistency index () = 0.1032 and consistency ratio () = 0.0833 were derived. Since CR < 0.10, the matrix satisfies Saaty’s threshold for acceptable consistency, indicating that the expert comparisons used to derive the AHP profile weights are both logically consistent and mathematically sound, supporting the validity of the resulting GHEI weighting scheme.
- 2.
- Balanced Profile
The balanced profile offers a neutral and transparent weighting scheme by assigning equal importance to all six criteria. It serves as a benchmark configuration to evaluate projects without bias toward any specific performance dimension. Table 11 shows the meaning of each value on the Saaty scale.

Table 11.
Balanced weights for GHEI criteria.
In Figure 5, a graphical comparison between the two weighting profiles is presented.
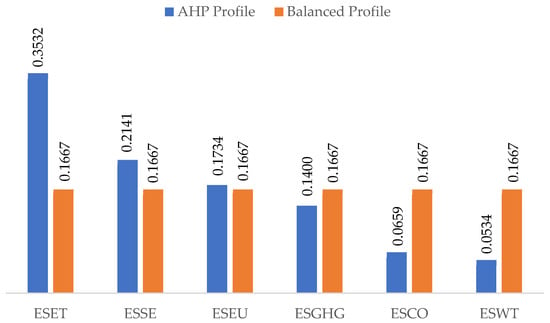
Figure 5.
Comparison between the AHP and balanced profile weights.
Together, these two profiles (AHP and balanced) offer a versatile analytical foundation for assessing green hydrogen projects under both expert-prioritized and stakeholder-neutral conditions. The following sections apply both profiles to real-world case studies to demonstrate the utility of the GHEI across diverse contexts.
4. Results
To test the applicability and usefulness of the Green Hydrogen Efficiency Index (GHEI), the proposed methodology was applied to a set of four representative case studies drawn from the academic literature. The selection of these projects was based on three main criteria: (i) technological diversity, including different electrolysis technologies and end-use applications; (ii) geographical variation, capturing projects in both developed and developing regions with different renewable resource profiles; and (iii) data availability, ensuring that the minimum required technical parameters could be inferred or sourced to support GHEI scoring. This approach ensured that the selected cases offer a realistic and comparative cross-section of the current landscape of green hydrogen initiatives. The projects examined include hydrogen applications in port logistics, passenger transport, hydropower integration, and large-scale solar-based ammonia production.
4.1. Project Descriptions and Assigned Efficiency Scores
The projects evaluated include applications in port logistics, maritime transport, hydropower-based hydrogen production, and ammonia export. The GHEI criteria scores were attributed based on project specifications available in the literature, supported by assumptions aligned with the performance categories presented in Table 6.
- Valparaíso (Chile): A port-based pilot utilizing local renewable electricity for hydrogen production via alkaline electrolysis, targeting hydrogen fuel supply for port operations [72]. ESSE and ESGHG scores reflect moderate renewable integration and relatively clean energy sourcing.
- Ferry (Europe): A maritime transport project converting a passenger ferry to hydrogen propulsion using grid electricity and PEM fuel cells [73]. The reliance on grid electricity limits the ESSE score, although water treatment is assumed to be efficient.
- Indonesia (hydropower): A decentralized hydrogen system that leverages surplus hydropower on remote islands. The project scores highest in ESSE and ESGHG due to the clean energy source, although other stages are standard in efficiency [74].
- Australia (export-oriented): A large-scale initiative producing hydrogen using solar-powered electrolysis, targeting ammonia export to Asian markets [75]. It scores well in ESEU and ESGHG, reflecting optimized downstream energy use and a relatively clean upstream source.
A summary of the key characteristics of the case study projects analyzed in this study is presented in Table 12.

Table 12.
Summary of case study projects.
Based on the analysis of the characteristics of each project, the efficiency scores assigned to each criterion for all case studies are presented in Table 13.

Table 13.
Efficiency scores for each project.
4.2. GHEI Scores and Profile Comparison
The efficiency scores were combined with the respective weightings from each profile to calculate GHEI values for the four projects. The result is shown in Figure 6.
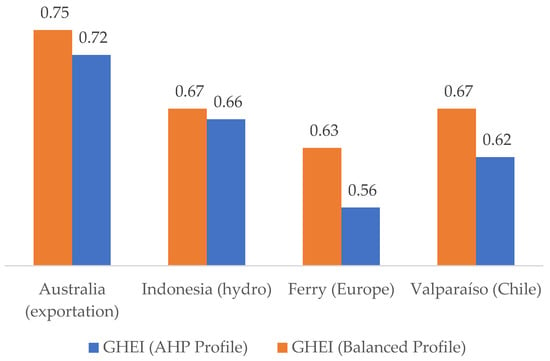
Figure 6.
Comparison between the GHEI obtained for the projects considering the two profiles.
The AHP profile places higher emphasis on ESET, ESSE, ESEU, and ESGHG, favoring technically and environmentally optimized systems. The balanced profile maintains equal weight across all criteria, providing a baseline for comparison.
4.3. Detailed Analysis and Interpretation
The results demonstrate a clear distinction between project configurations when evaluated under different profiles. Notably, the Australia project achieves the highest GHEI across both profiles. Its superior performance stems from strong downstream efficiency (ESEU = 1.00), supported by a solar energy source that contributes to moderate ESSE and ESGHG scores. The AHP profile, which prioritizes technical and environmental efficiency, slightly moderates its advantage due to average performance in electrolysis and compression, yet it remains top-ranked. This case exemplifies the synergy between renewable input, efficient conversion, and strategic end-use.
Indonesia also performs strongly, ranking second overall. Its reliance on hydropower ensures top ESSE and ESGHG scores, which are heavily weighted in the AHP profile. Despite average performance in other stages, the project maintains robust scores due to the environmental cleanliness of its primary energy source. This case highlights how location-specific natural resources can drive low-emission hydrogen production with minimal technological complexity.
Valparaíso shows mid-range performance, with higher water treatment efficiency and a reasonably clear energy input. Its lower scores in end-use and compression moderately reduces its GHEI, particularly under the AHP profile. The project reflects a typical industrial application where partial decarbonization and operational constraints balance out to yield consistent but not leading performance.
Ferry consistently ranks lowest in both profiles. The primary reason lies in its dependence on grid electricity (ESSE = 0.50), which underperforms in both carbon intensity and traceability. Even though the project shows favorable water treatment efficiency, its lack of differentiation in the other criteria highlights the sensitivity of the GHEI to upstream energy source and downstream application.
4.4. Profile Sensitivity and Policy Relevance
Comparing the AHP and balanced profiles illustrates how different stakeholder priorities can influence project evaluation. The AHP profile, grounded in structured pairwise comparisons, aligns closely with technical and climate-related metrics. It favors projects that optimize emissions and conversion efficiency, providing a useful tool for private investors or industrial operators focused no return and compliance.
In contrast, the balanced profile offers a fair and transparent baseline, treating each criterion equally. This configuration is ideal for early-stage project screening, public funding assessment, or comparisons across diverse regions where no single performance category dominates. It is particularly useful in ensuring that trade-offs across the hydrogen value chain are not masked by single-dimensional optimization.
Ultimately, both profiles rank Australia and Indonesia at the top, confirming that projects with clear renewable sourcing and high-impact end uses deliver robust GHEI scores. Valparaíso and Ferry remain viable but illustrate the importance of context-specific optimization. The dual-profile analysis reinforces the decision-making capacity of the GHEI and highlights its adaptability across varying technical, environmental, and industrial priorities.
5. Conclusions
This study introduced the Green Hydrogen Efficiency Index (GHEI), a novel methodological tool designed to evaluate the energy efficiency of green hydrogen production chains through a single, integrated, and quantitative metric. The GHEI’s core innovation lies in its holistic assessment of hydrogen systems across six critical dimensions, electricity sourcing, water treatment, electrolysis efficiency, compression, end-use application, and greenhouse gas emissions. These dimensions are evaluated using scoring thresholds. The index supports two weighting profiles, including a balanced profile with equal weight for each criterion and an AHP profile based on structured expert judgment, enabling adaptable analysis across technical and policy contexts. Unlike prior studies focused on isolated stages, the GHEI enables full-chain comparisons using a single, replicable metric adaptable to various stakeholder perspectives.
By applying the index to four diverse case studies, spanning different geographies, energy inputs, and end uses, the GHEI demonstrated its utility in identifying performance strengths and weaknesses across multiple configurations. For instance, the GHEI awarded the Australia solar-powered ammonia export project a top scorer (0.771 under balanced; 0.739 under AHP), while the hydropower-based Indonesian system followed closely (0.750 and 0.752, respectively), illustrating the influence of clean energy sourcing and efficient downstream use on overall system performance. Projects with clean and traceable electricity sources, efficient end-use applications, and minimized emissions consistently achieved higher scores, especially under the AHP weighting logic. The results affirm the capacity of the index to inform technology selection, site planning, and strategy design in the context of green hydrogen implementation.
It is important to emphasize that the GHEI is not proposed as a definitive or all-encompassing tool for evaluating every aspect of green hydrogen systems. Rather, it serves as a first-step framework, designed to highlight the importance of integrated energy efficiency assessment and to demonstrate the feasibility of using a standardized metric to compare heterogeneous hydrogen value chains. Its role is to complement, not replace, broader economic, environmental, or life-cycle analysis tools. One of the current limitations of the GHEI lies in the availability and reliability of performance data for the equipment used across different configurations. In many cases, particularly in pilot or early commercial projects, real-world efficiency data for electrolysis systems, compression technologies, or end-use applications are limited, which constrains the granularity and precision of scoring. Additionally, the estimation of upstream and downstream GHG emissions remains highly dependent on modeled assumptions, which may not always reflect local realities.
Despite these challenges, the GHEI provides a flexible and replicable structure that can evolve with sectoral developments. Future enhancements may include the incorporation of expert-derived input into the AHP profile through targeted surveys or stakeholder engagement. This would strengthen the weight assignments and ensure that the index reflects the priorities and experiences of industry practitioners and policy advisors.
Importantly, the GHEI also holds promise as a decision-support tool for policymakers, offering a transparent means to evaluate and compare hydrogen production scenarios according to energy performance and emissions reduction potential. In this way, it may inform policy formulation, investment prioritization, funding prioritization, and regulatory strategies aimed at scaling up low-carbon hydrogen systems effectively.
In summary, the GHEI offers a replicable and adaptable methodology to support more informed, transparent, and technically grounded decision-making for green hydrogen deployment. As the hydrogen economy matures, tools like the GHEI will be increasingly important in guiding sustainable development aligned with global decarbonization goals.
Author Contributions
Conceptualization, L.T.B., P.H.R.P.G., and P.A.C.R.; writing—original draft preparation, L.T.B.; writing—review and editing, L.T.B., S.D.V., P.A.C.R., D.C.P.B., and J.F.C.C.; visualization, P.A.C.R., D.C.P.B., S.D.V., J.F.C.C., and D.C.P.B.; supervision, L.T.B., J.F.C.C., P.H.R.P.G., and P.A.C.R.; project administration, L.T.B.; funding acquisition, L.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Research and Development Program regulated by ANEEL the Brazilian National Electric Energy Agency and the EDP Company under the project code PD-07267-0022/2021.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are grateful to the Research and Development Program regulated by ANEEL, the EDP Company, Federal University of Pernambuco, and the Advanced Institute of Technology and Innovation (IATI), Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pawar, S. Harnessing the Power of Renewable Energy: A Study of Sustainable Sources and Technologies. J. Res. Appl. Sci. Biotechnol. 2024, 3, 163–169. [Google Scholar] [CrossRef]
- Wheatley, M.C. Advancements in Renewable Energy Technologies: A Decade in Review. Science 2024, 1, 100013. [Google Scholar] [CrossRef]
- Sisternes, F.; Jenkins, J.; Botterud, A. The Value of Energy Storage in Decarbonizing the Electricity Sector. Appl. Energy 2016, 175, 368–379. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M. Energy Storage Systems towards 2050. Energy 2020, 219, 119634. [Google Scholar] [CrossRef]
- Gallo, A.B.; Simões-Moreira, J.R.; Costa, H.K.M.; Santos, M.M.; dos Santos, E.M. Energy Storage in the Energy Transition Context: A Technology Review. Renew. Sustain. Energy Rev. 2016, 65, 800–822. [Google Scholar] [CrossRef]
- Rampai, M.; Mtshali, C.; Seroka, N.; Khotseng, L. Hydrogen Production, Storage, and Transportation: Recent Advances. RSC Adv. 2024, 14, 6699–6718. [Google Scholar] [CrossRef]
- Etezadi, R.; Wang, R.; Tsotsis, T. Hydrogen, a Versatile Chemical for the Future: Applications and Production Methods. AIChE J. 2024, 71, e18645. [Google Scholar] [CrossRef]
- Beschkov, V.; Ganev, E. Perspectives on the Development of Technologies for Hydrogen as a Carrier of Sustainable Energy. Energies 2023, 16, 6108. [Google Scholar] [CrossRef]
- Gómez, J.; Castro, R. Green Hydrogen Energy Systems: A Review on Their Contribution to a Renewable Energy System. Energies 2024, 17, 3110. [Google Scholar] [CrossRef]
- Sharma, M.; Tyagi, V.; Kouser, R.; Kumari, K.; Chopra, K.; Kothari, R. Green Hydrogen and Climatic Change: Current Status and Future Outlook. In Green Hydrogen Economy for Environmental Sustainability. Volume 2: Applications, Challenges, and Policies; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; Volume 1474, pp. 31–54. [Google Scholar]
- Islam, A.; Islam, T.; Mahmud, H.; Raihan, O.; Islam, M.S.; Marwani, H.M.; Rahman, M.M.; Asiri, A.M.; Hasan, M.M.; Hasan, M.N.; et al. Accelerating the Green Hydrogen Revolution: A Comprehensive Analysis of Technological Advancements and Policy Interventions. Int. J. Hydrogen Energy 2024, 67, 458–486. [Google Scholar] [CrossRef]
- Jumah, A.B. A Comprehensive Review of Production, Applications, and the Path to a Sustainable Energy Future with Hydrogen. RSC Adv. 2024, 14, 26400–26423. [Google Scholar] [CrossRef] [PubMed]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of Renewable-Energy-Based Green Hydrogen into the Energy Future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in Energy Transition: A Review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S. Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Jaradat, M.; Almashaileh, S.; Bendea, C.; Juaidi, A.; Bendea, G.; Bungau, T. Green Hydrogen in Focus: A Review of Production Technologies, Policy Impact, and Market Developments. Energies 2024, 17, 3992. [Google Scholar] [CrossRef]
- Angelico, R.; Giametta, F.; Bianchi, B.; Catalano, P. Green Hydrogen for Energy Transition: A Critical Perspective. Energies 2025, 18, 19961073. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Abe, J.; Popoola, A.; Ajenifuja, E.; Popoola, O. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- IRENA. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Barbosa, L.T.; Vasconcelos, S.D.; Rosas, P.A.C.; Castro, J.F.C.; Barbosa, D.C.P. Assessment of Green Hydrogen as Energy Supply Alternative for Isolated Power Systems and Microgrids. Energies 2024, 17, 4774. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Green Hydrogen Cost Reduction Scaling up Electrolysers to Meet the 1.5 °C Climate Goal H2O2; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Quintino, F.M.; Nascimento, N.; Fernandes, E.C. Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment. Hydrogen 2021, 2, 301–318. [Google Scholar] [CrossRef]
- Mejia, A.H.; Brouwer, J.; Mac Kinnon, M. Hydrogen Leaks at the Same Rate as Natural Gas in Typical Low-Pressure Gas Infrastructure. Int. J. Hydrogen Energy 2020, 45, 8810–8826. [Google Scholar] [CrossRef]
- Mukherjee, U.; Elsholkami, M.; Walker, S.; Fowler, M.; Elkamel, A.; Hajimiragha, A. Optimal Sizing of an Electrolytic Hydrogen Production System Using an Existing Natural Gas Infrastructure. Int. J. Hydrogen Energy 2015, 40, 9760–9772. [Google Scholar] [CrossRef]
- Pashchenko, D. Green Hydrogen as a Power Plant Fuel: What Is Energy Efficiency from Production to Utilization? Renew. Energy 2024, 223, 120033. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; García-Tenorio, E. Evaluating Hydrogen-Based Electricity Generation Using the Concept of Total Efficiency. Energy Convers. Manag. 2023, 293, 117438. [Google Scholar] [CrossRef]
- Zhang, X.; Schwarze, M.; Schomäcker, R.; van de Krol, R.; Abdi, F.F. Life Cycle Net Energy Assessment of Sustainable H2 Production and Hydrogenation of Chemicals in a Coupled Photoelectrochemical Device. Nat. Commun. 2023, 14, 991. [Google Scholar] [CrossRef]
- Bao, C.; Liu, D.; Wang, Y.; Mao, J.; Tan, Y.; Zhang, Q. Modeling, Optimization and Evaluation of Production-Storage-Utilization Hydrogen System Based on Renewable Energy. In Proceedings of the 2023 3rd International Conference on Intelligent Power and Systems (ICIPS), Shenzhen, China, 20–22 October 2023; pp. 832–838. [Google Scholar]
- Lăzăroiu, G.; Mihăescu, L.; Negreanu, G.; Grigoriu, R.M.; Simion, I. An Analysis of the Economic Production and Use of Green Hydrogen. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Galati, Romania, 2023; Volume 1290, p. 012015. [Google Scholar]
- Salam, M.A.; Ahmed, K.; Marufuzzaman; Muhammad Sayem, A.S. Chapter 5—Techno-Economic Prospects of Green Hydrogen Production. In Hydrogen Energy Conversion and Management; Khan, M.M.K., Azad, A.K., Oo, A.M.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 133–155. ISBN 978 0 443 15329 7. [Google Scholar]
- Majewski, P.; Salehi, F.; Xing, K. Green Hydrogen. AIMS Energy 2023, 11, 878–895. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Ferraro, M.; Gallo, P.; Ippolito, M.G.; Massaro, F.; Sanseverino, E.R.; Ruffino, S.; Santamaria, M.; Sciumè, G. A Test Bench for the Production of Green Hydrogen and Its Traceability and Certification Using Blockchain Technology. In Proceedings of the 2023 IEEE International Conference on Environment and Electrical Engineering and 2023 IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Madrid, Spain, 6–9 June 2023; pp. 1–6. [Google Scholar]
- Henriot, A. Economic Curtailment of Renewable Energy Sources. In Proceedings of the Sustainable Energy Policy and Strategies for Europe, 14th IAEE European Conference, Rome, Italy, 28–31 October 2014. [Google Scholar]
- Miranda, R.; Soria, R.; Schaeffer, R.; Szklo, A.; Saporta, L. Contributions to the Analysis of “Integrating Large Scale Wind Power into the Electricity Grid in the Northeast of Brazil” [Energy 100 (2016) 401–415]. Energy 2017, 118, 1198–1209. [Google Scholar] [CrossRef]
- Stockl, F.; Schill, W.-P.; Zerrahn, A. Optimal supply chains and power sector benefits of green hydrogen. arXiv 2021, arXiv:2005.03464. [Google Scholar] [CrossRef]
- May, T.W.; Yeap, Y.M.; Ukil, A. Comparative Evaluation of Power Loss in HVAC and HVDC Transmission Systems. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 637–641. [Google Scholar]
- Irena, B. Water for Hydrogen Production: International Renewable Energy Agency; Bluerisk: Abu Dhabi, United Arab Emirates, 2023. [Google Scholar]
- Inhaber, H. Water Use in Renewable and Conventional Electricity Production. Energy Sources 2004, 26, 309–322. [Google Scholar] [CrossRef]
- Khamis, I.; El-Emam, R. Towards Efficient Water Management in Nuclear Power Plants. Desalination Water Treat. 2020, 176, 395–398. [Google Scholar] [CrossRef]
- Mapes, A.S.; Larsen, M. Projected Future European Power Sector Water Usage across Power Scenarios and Corresponding Trends in Water Availability. J. Environ. Manage. 2023, 343, 118208. [Google Scholar] [CrossRef]
- Jayabal, R. Next-Generation Solutions for Water Sustainability in Nuclear Power Plants: Innovations and Challenges. Nucl. Eng. Des. 2025, 432, 113757. [Google Scholar] [CrossRef]
- Mulk, W.U.; Aziz, A.; Ismael, M.; Ghoto, A.A.; Ali, S.A.; Younas, M.; Gallucci, F. Electrochemical Hydrogen Production through Anion Exchange Membrane Water Electrolysis (AEMWE): Recent Progress and Associated Challenges in Hydrogen Production. Int. J. Hydrogen Energy 2024, 94, 1174–1211. [Google Scholar] [CrossRef]
- Padgett, E.; Badgett, A.; Brauch, J.; Kreider, M.; Volk, E.; Alia, S.; Shviro, M.; Pivovar, B.S. Comparing and Contrasting the Advantages and Challenges of Catalysts for Low Temperature AEM, AEL, and PEM Electrolysis. In Electrochemical Society Meeting Abstracts; IOP Publishing Ltd.: Bristol, UK, 2024. [Google Scholar] [CrossRef]
- Gallandat, N.; Romanowicz, K.; Züttel, A. An Analytical Model for the Electrolyser Performance Derived from Materials Parameters. J. Power Energy Eng. 2017, 5, 34–49. [Google Scholar] [CrossRef]
- Kim, S.-D.; Choi, Y.; Park, H. Advances in Solid Oxide Electrolysis Cell (SOEC) Technology for Sustainable, Efficient, and Durable Hydrogen Production. In Electrochemical Society Meeting Abstracts; IOP Publishing Ltd.: Bristol, UK, 2024. [Google Scholar] [CrossRef]
- Shaya, N.; Glöser-Chahoud, S. A Review of Life Cycle Assessment (LCA) Studies for Hydrogen Production Technologies through Water Electrolysis: Recent Advances. Energies 2024, 17, 3968. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, X.; Zhang, C.; Jun, K.; Kim, S.K.; Park, H.-G.; Gao, Y.; Zhu, Y.; et al. A Comparative Study on Hybrid Power-to-Liquids/Power-to-Gas Processes Coupled with Different Water Electrolysis Technologies. Energy Convers. Manag. 2022, 263, 115671. [Google Scholar] [CrossRef]
- Bühler, L.; Möst, D. Projecting Technological Advancement of Electrolyzers and the Impact on the Competitiveness of Hydrogen. Int. J. Hydrogen Energy 2025, 98, 1174–1184. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Belghiti, A.; Laasri, S.; Kaya, S.; Hlil, E.; Rachidi, S.; Hajjaji, A. A Comprehensive Review of Recent Advances in Alkaline Water Electrolysis for Hydrogen Production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Wirkert, F.; Roth, J.; Rost, U.; Brodmann, M. Hydraulic Cell Compression for Performance Preserving Upscaling of PEM Electrolyzers. Int. J. Smart Grid Clean Energy 2017, 6, 171–176. [Google Scholar] [CrossRef][Green Version]
- Bouwman, P. Advances in Electrochemical Hydrogen Compression and Purification. ECS Trans. 2016, 75, 503–510. [Google Scholar] [CrossRef]
- DOE. Hydrogen and Fuel Cells Program; EERE Publication and Product Library: Washington, DC, USA, 2012. [Google Scholar]
- Susetyo, M.A.; Reza, A.; Juangsa, F.; Abdurrahman, G. Thermodynamic Analysis of Hydrogen Supply Chain for Fuel Cell Electric Vehicle Application. In Proceedings of the 2024 International Conference on Technology and Policy in Energy and Electric Power (ICTPEP), Bali, Indonesia, 3–5 September 2024; pp. 421–425. [Google Scholar] [CrossRef]
- Brenkman, J. High Pressure Hydrogen Dehydration Using Ionic Liquids; Delft University of Technology: Delft, The Netherlands, 2019. [Google Scholar]
- Ghasemi, M.; Nassef, A.M.; Al-Dhaifallah, M.; Rezk, H. Performance Improvement of Microbial Fuel Cell through Artificial Intelligence. Int. J. Energy Res. 2021, 45, 342–354. [Google Scholar] [CrossRef]
- Giorgi, L.; Leccese, F. Fuel Cells: Technologies and Applications. Open Fuel Cells J. 2013, 6, 1–20. [Google Scholar] [CrossRef]
- Jiménez, H.; Las, A. Contribuciones a Las Pilas de Combustible de Electrolito Polimérico (PEFC) de Potencia Media y Refrigeradas Por Aire: Fundamentación Teórica Del Proceso de Fabricación Del Stack y Estudio Experimental Del Balance de Planta; Universidad de Huelva: Huelva, Spain, 2018; Volume 1. [Google Scholar]
- Zeng, W.; Guan, B.; Zhuang, Z.; Chen, J.; Zhu, L.; Ma, Z.; Hu, X.; Zhu, C.; Zhao, S.; Shu, K.; et al. Comprehensive Review on the Advances and Comparisons of Proton Exchange Membrane Fuel Cells (PEMFCs) and Anion Exchange Membrane Fuel Cells (AFCs): From Fundamental Principles to Key Component Technologies. Int. J. Hydrogen Energy 2025, 102, 222–246. [Google Scholar] [CrossRef]
- Mollaamin, F.; Pham, T.T.T.; Dang, D.; Monajjemi, M.; Dang, C. Modelling and Controlling of Ion Transport Rate Efficiency in Proton Exchange Membrane (PEMFC), Alkaline (AFC), Direct Methanol (DMFC), Phosphoric Acid (PAFC), Direct Forming Acid (DFAFC) and Direct Carbon (DCFC) Fuel Cells. Biointerface Res. Appl. Chem. 2019, 9, 4050–4059. [Google Scholar] [CrossRef]
- Melinescu, A.; Velciu, G.; Marinescu, V.; Preda, M. ELEMENTE CONSTITUTIVE PENTRU CELULELE SOFC-IT MAIN CONSTITUENT ELEMENTS FOR CELLS SOFC-IT. Rev. Română Mater./Rom. J. Mater. 2011, 41, 262–268. [Google Scholar]
- Nascimento, A.C.; Mohallem, N.D.S. Materials Used in the Manufacture of the Main Components of Solid Oxide Fuel Cells. Cerâmica 2009, 55, 46–52. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life Cycle Assessment of Hydrogen Production Methods: A Review; Institut für Energie-und Klimaforschung, Systemforschung und Technologische: Jülich, Germany, 2012. [Google Scholar]
- Global Hydrogen Review 2021—Analysis. Available online: https://www.iea.org/reports/global-hydrogen-review-2021 (accessed on 14 May 2025).
- Ajanovic, A.; Haas, R. Prospects and Impediments for Hydrogen and Fuel Cell Vehicles in the Transport Sector. Hydrog. Fuel Cells 2021, 46, 10049–10058. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydro-gen and Fuel Cells in the Global Energy System. Energy Environ. Science 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Council, H. Hydrogen Insights—A Perspective on Hydrogen Investment. In Market Development and Cost Competitiveness; Hydrogen Council: Brussels, Belgium, 2021. [Google Scholar]
- Saaty, T.L. How to Make a Decision: The Analytic Hierarchy Process. Eur. J. Oper. Res. 1990, 48, 9–26. [Google Scholar] [CrossRef]
- Pohekar, S.D.; Ramachandran, M. Application of Multi-Criteria Decision Making to Sustainable Energy Planning—A Review. Renew. Sustain. Energy Rev. 2004, 8, 365–381. [Google Scholar] [CrossRef]
- Masip Macía, Y.; Rodríguez Machuca, P.; Rodríguez Soto, A.A.; Carmona Campos, R. Green Hydrogen Value Chain in the Sustainability for Port Operations: Case Study in the Region of Valparaiso, Chile. Sustainability 2021, 13, 13681. [Google Scholar] [CrossRef]
- Perna, A.; Jannelli, E.; Di Micco, S.; Romano, F.; Minutillo, M. Designing, Sizing and Economic Feasibility of a Green Hydrogen Supply Chain for Maritime Transportation. Energy Convers. Manag. 2023, 278, 116702. [Google Scholar] [CrossRef]
- Siburian, C.P.; Simanullang, F.N.T.P. Techno-Economic Analysis of Green Hydrogen Production from Excess Power Hydropower Plant. In Proceedings of the 2024 International Seminar on Intelligent Technology and Its Applications (ISITIA), Mataram, Indonesia, 10–12 July 2024; pp. 92–97. [Google Scholar]
- Benalcazar, P.; Komorowska, A. Techno-Economic Analysis and Uncertainty Assessment of Green Hydrogen Production in Future Exporting Countries. Renew. Sustain. Energy Rev. 2024, 199, 114512. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).