Abstract
Extensive research has focused on enhancing the energy storage density of polypropylene (PP) to meet the demands of high-power and compact electronic devices and electrical systems. However, there is a lack of studies addressing the delicate balance between energy storage density and dielectric loss. Dielectric loss can lead to excessive heat generation, posing a threat to the operation of energy storage capacitors. In this study, PP grafted with glycidyl methacrylate (GMA) was used as a compatibilizer and incorporated into a PP/nano ZrO2 blend to form a ternary system of PP/nano ZrO2/PP grafted GMA. A comparative study was conducted to analyze the effects of GMA grafting and individual doping of nano ZrO2 on the dielectric performance of PP. The results demonstrate that the ternary system not only ensures a high breakdown voltage (382.29 MV/m) but also possesses a high dielectric constant (2.67), thereby achieving an energy storage density of 1.7275 J/cm3 while maintaining low dielectric loss. Furthermore, grafting GMA introduces a significant number of deep traps, a phenomenon substantiated by the results of thermal stimulated depolarization current tests and molecular simulation calculations. However, the ternary system partially avoids the introduction of excessive deep traps associated with GMA grafting. This ternary system exhibits excellent energy storage performance, ease of fabrication, and stability, thereby enriching the research on polymer-based high-energy density dielectric materials.
1. Introduction
Polypropylene (PP) is widely used in energy storage capacitors due to its excellent mechanical properties, thermal stability, and dielectric performance [1,2,3]. In order to meet the demands of power systems and modern electronic devices, it is necessary to enhance the energy storage density of PP, enabling energy storage capacitors to achieve greater capacity while further miniaturizing [4]. For linear dielectric materials like PP, the energy density is directly proportional to the relative permittivity (εr) and the square of the breakdown strength (BD) [5,6,7]. Despite having a high BD, PP is limited by its low εr, which restricts its energy density [8,9,10]. To improve the εr and BD of PP, most researchers currently employ the strategy of nanoparticle doping [11,12]. On one hand, a significant amount of research has already demonstrated that doping nanoparticles can effectively enhance the breakdown strength. Zhou et al. [13] doped PP with different amounts of nano MgO, TiO2, ZnO, and Al2O3 and found that all four types of nanoparticles improved the electrical performance of PP, with TiO2 nanoparticles showing a 43% increase in DC breakdown strength compared to pure PP. On the other hand, enhancing the permittivity of PP through nanoparticle doping is also beneficial for improving the energy storage performance of the composite material. D. J. Sharmila et al. [14] prepared a composite material of PP and nano ZnO and found the addition of a low content of ZnO significantly increased the permittivity of the composite material. Although nanoparticle doping holds great prospects for enhancing the energy density of PP, the effectiveness of this approach can be hindered by nanoparticle agglomeration. Severe agglomeration can lead to non-uniform properties of the composite material and fluctuations in performance across different batches, posing significant challenges for the industrial production of composite materials.
Therefore, inhibiting the agglomeration of nanoparticles and improving their dispersion is of paramount importance in enhancing the energy storage performance of composite materials [15,16]. An effective approach involves utilizing PP composite material grafted with polar monomers as a compatibilizer, which is then blended with PP and supplemented with nanoparticles to form a ternary system. This ternary system effectively suppresses the agglomeration of nanoparticles. Presently, polar monomers that have been proven to be suitable for grafting onto PP include maleic anhydride (MAH) [17], glycidyl methacrylate (GMA) [18], and so on. Liu et al. [19] established a ternary system comprising PP/MAH grafted PP/nano ZrO2, resulting in improved crystallization performance and aggregate structure of the composite material. Another graftable monomer, GMA, has attracted researchers’ attention owing to its high reactivity towards carboxyl, hydroxyl, or amino groups, and its ability to enhance the crystallization capability of the PP matrix after grafting [20,21]. Zhang et al. [22] demonstrated that utilizing GMA-grafted PP (PP-g-GMA) as a compatibilizer in a ternary system with polyamide 1010 and PP improves the homogeneity of the doped blend. Tao and Mai [23] incorporated PP grafted with MAH and GMA, respectively, as compatibilizers into PP/recycled polyethylene terephthalate (PP/rPET) and compared the effects of these two addition methods on the melt performance of PP/rPET. The Differential Scanning Calorimetry results demonstrated that both approaches are conducive to enhancing the melting peak temperature. In a comparative study by Xia et al. [24], the non-isothermal melt behavior of PP grafted with acrylic acid (AA), GMA, and MAH was investigated. The crystallization rate of PP-g-GMA, as measured by the Mo method, was only surpassed by that of PP-g-AA. Furthermore, the absolute value of the crystallization activation energy for PP-g-GMA was determined to be 302.5 kJ (higher than the pure PP value of 200.9 kJ).
Although numerous scholars have conducted research on the development of polymeric composites using compatibilizers, most efforts have been focused on enhancing mechanical and thermal properties. In this study, we proposed a ternary system comprising PP/PP-g-GMA/nano ZrO2. ZrO2 possesses a dielectric constant of 25, thus its inclusion not only increases the dielectric constant of the composite medium, but also mitigates severe field distortions that may arise due to significant disparities in the dielectric constants of different materials [12]. Hence, we opted for ZrO2. The objective of this research is to investigate the impact of different modification approaches within the ternary system on the performance of the composite medium. The study on a system with diverse concentration gradients of both PP-g-GMA and ZrO2 would be excessively vast. It has been reported in [25] and [26] that the best enhancement in mechanical properties of modified PP was achieved with 1 wt.% of nano SiO2 inclusion. Additionally, in [19], it was demonstrated that the best improvement in breakdown strength was observed when ZrO2 inclusion concentration was 0.5 wt%. At low concentrations of nano ZrO2 inclusion, negligible particle aggregation occurs, facilitating the analysis of the individual effect of ZrO2 inclusion on the performance of the composite medium. Furthermore, in the preliminary experiments, we performed breakdown tests on PP-xZrO2 (x = 0, 0.5, 1, 3, 5). The experimental results, as shown in Figure S1 in Supplementary Materials, indicate that a doping concentration of 0.5 wt% yields the most significant enhancement in breakdown voltage. Moreover, we have taken note of previous studies that investigated the role of modified components in enhancing performance, which similarly only prepared samples with a single doping concentration [27,28,29]. Based on these considerations, we chose 0.5 wt% as the doping concentration of ZrO2 in our experimental design, without considering other doping concentrations. In this work, we employed FTIR to confirm the grafting of GMA, while the thermal stability of the ternary system was analyzed through XRD and DSC. Furthermore, we systematically compared the dielectric properties, trap characteristics, and energy storage density of PP, PP-g-GMA, PP with nano ZrO2 inclusion (PP-0.5Zr), and the ternary system. Moreover, molecular simulations were employed to investigate the electronic state and electrostatic potential of PP and PP-g-GMA. This study provides a solution and guidance for the co-modification of PP, thereby accumulating valuable experience for subsequent research on the modification of PP.
2. Experimental Section
2.1. Sample Preparation
2.1.1. Preparation of PP-g-GMA Dielectric Composite
The raw materials used were pure PP particles and GMA monomer. First, a solution of xylene was added to a three-necked flask, followed by 25 g of pure PP particles. In order to avoid the change of material properties caused by different chemical reagents used, the xylene solution mentioned here and in the following sample preparation process is the same batch of reagents produced. The mixture was heated in an oil bath to 140 °C. The PP was stirred in the xylene solution until it was completely dissolved and the solution was transparent without any visible solid particles. The temperature was then adjusted to 120 °C. When the temperature stabilized at 120 °C, 5 g of GMA monomer was slowly added with continuous stirring. After the mixture was stirred evenly, 20 mL of a xylene solution was slowly added dropwise under a nitrogen atmosphere and stirred for 2 h. After the reaction was complete, the solution was transferred to a beaker, and an appropriate amount of acetone was added to dissolve the xylene and other impurities. The solution was then filtered until the residue became a white to pale yellow powder without any clumping. Finally, the powder was dried in a vacuum oven for 48 h to obtain PP-g-GMA.
2.1.2. Preparation of PP-0.5Zr Dielectric Composite
Experimental results from references [25,26] indicate that low doping concentrations are more favorable for enhancing the performance of composite materials. Therefore, before sample preparation, we measured the breakdown voltages of low-concentration PP-xZrO2 (x = 0, 0.5, 1, 3, 5). As shown in Figure S1 in Supplementary Materials, the improvement in PP-0.5Zr was the most significant. Hence, we chose 0.5 wt% as the concentration of ZrO2.
First, the temperature of the torque rheometer was set to 185 °C, and the rotation speed was set to 15 r/min. Once the temperature stabilized, a certain amount of pure PP particles was added. After the torque value dropped to a stable low value, the rotation speed was increased to 60 r/min, and 0.5 wt% of nano ZrO2 powder and 0.3 wt% of antioxidant powder were slowly added. After sufficient mixing for 20 min, the PP-0.5Zr was obtained.
2.1.3. Preparation of PP-gGMA-0.5Zr Dielectric Composite
The PP-gGMA-0.5 ternary dielectric composite was obtained in two steps. First, a certain amount of PP-g-GMA powder was added to the torque rheometer at 185 °C, and the rotation speed of the torque rheometer was set to 40 r/min. After the torque value dropped to a stable low value, nano ZrO2 powder and 0.3 wt% of antioxidant powder were slowly and uniformly added. The rotation speed was increased to 60 r/min, and the mixture was melted and mixed for 10 min. Then, the rotation speed was reduced to 15 r/min, and an equal amount of PP particles was added to the mixture. Finally, after the torque stabilized at a low value, the rotation speed was increased to 60 r/min, and the mixture was melted and mixed for 20 min to obtain the PP-gGMA-0.5Zr. Finally, the dielectric composite film with a thickness of 110 μm was obtained by hot pressing.
2.2. Microstructure Observation
The chemical groups present on the surfaces of PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr, indicating the successful grafting of GMA monomers in the ternary composite, were analyzed using a Fourier Transform Infrared Spectrometer (FTIR, ThermoScientific Nicolet iN10). The scanning mode employed was attenuated total reflectance, with a scanning range of 400–4000 cm−1.
2.3. Crystallization Measurement
The crystalline structures of PP, PP-g-GMA, PP-0.5Zr, and PP-g-GMA-0.5Zr were analyzed using an X-ray Diffractometer (XRD, D8Advance, Bruker, Karlsruhe, Germany). Cu target was used as the X-ray source, with a scanning range of 2θ from 10° to 30°, a scanning step size of 0.0102°, and a dwell time of 0.1 s per step.
The thermal properties of the materials were investigated using Differential Scanning Calorimetry (DSC; DSC822e, METTLER TOLEDO, Zurich, Switzerland). In order to nullify the influence of prior thermal conditions, all specimens were subjected to a thermal treatment wherein they were heated to a temperature of 200 °C and maintained at this level for a duration of 5 min. Subsequently, the temperature was gradually reduced to ambient conditions at a cooling rate of 10 °C/min, thereby facilitating the process of recrystallization. Following this, the specimens were subjected to a heating process at a rate of 10 °C/min until reaching a temperature of 200 °C, and the relevant data pertaining to the melting phenomenon were meticulously recorded.
2.4. Frequency Domain Spectroscopy Test
Dielectric constant and dielectric loss were tested by Novocontrol technologies concept 80 broadband dielectric spectrometer. Prior to testing, gold layers with diameters of 22 mm and 25 mm were sprayed on the upper and lower surfaces of the samples, respectively, followed by drying for 12 h. The AC voltage for the tests was set at 1 V, the electrode diameter was 30 mm, and the frequency range was 10−1 to 105 Hz. The tests were performed at a temperature of 30 °C.
2.5. Thermal Stimulated Depolarization Current Test
Thermal Stimulated Depolarization Current (TSDC) tests were carried out using a Novocontrol concept 40 testing system, with the sample thickness being 110 μm. In the experiment, the system raised the sample temperature to 150 °C and applied a polarization voltage of 250 V for 10 min. The sample was then rapidly cooled to −50 °C and maintained for 5 min before being heated back up to 150 °C at a rate of 3 °C/min. During the heating process, the polarization current was recorded at every 0.25 °C step. Finally, the depolarization current was processed using a MATLAB 2022 program based on the method described in reference [30] to obtain the trap energy levels and trap densities of the material. The TSDC reflects the accumulation of space charges under the influence of an electric field. The measured current peaks correspond to the temperature and intensity related to the depth and density of charge traps. The referenced MATLAB program allows the calculation of trap levels and density distribution based on the measured depolarization current. In this program, a new function is defined to weight the contribution of each trap energy level to the external current, as shown in Equation (1), where G2 is an approximate calculation of the weighted contribution of electrons to the current temperature T for trap energy level E:
where k is the Boltzmann constant, e is the elementary charge, β is the heating rate, E is the trap energy, and v is the escape frequency, typically in the range of 1012–1014 s−1 [30]. In this work, the value of v is taken as 1012 s−1.
The program calculates trap levels and trap density based on Equation (2), requiring only input of current density and temperature during program execution.
where d is the sample thickness, and f0 is the initial occupancy of trap levels and is a constant, assuming all traps are initially filled and f0 = 1. The G2 is plotted as an energy function for different T values, yielding an asymmetric bell-shaped curve with a maximum at Em, which is a function of A.
2.6. DC Breakdown Experiments
DC breakdown experiments were conducted using a teslamanhv TD2202P20-1000 (Dalian, China) DC power supply, with copper electrodes of 25 mm diameter. The voltage was increased at a rate of 500 V/s. To prevent surface flashover and air breakdown, the electrodes and samples were immersed in silicone oil during the measurements. Each sample underwent 30 breakdown tests, and the data were statistically analyzed using a two-parameter Weibull distribution.
2.7. DFT Calculation
Density Functional Theory (DFT) is based on first-principles calculations, and the wave functions of computed molecules are derived by solving Schrodinger’s equation [31]. We performed the first-principles calculations using the Materials Studio 8.0 (MS), the calculation of the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) was performed using the Orbitals feature within the Properties module of the Dmol3 software. The TS method for DFT-D correction was used in this study. The SCF tolerance was set as 1 × 10−5 eV/atom. Precision of the grid resolution was set as fine, and grid interval was set as 0.15 Å. In this study, the isosurface of electron density was set to 0.02 e/bohr3. All calculations optimize the structure before calculating the properties.
3. Results and Discussion
3.1. Quantum Chemical Computation
Compared to the original material, PP-g-GMA has lower LUMO and HOMO levels and is able to generate a strong electrostatic attraction to trap free charge carriers. The band structures of PP and PP-g-GMA are illustrated in Figure 1a. The LUMO energy level of PP measures 7.565 eV, displaying a higher status compared to that of PP-g-GMA, quantified at 4.000 eV. Given that the LUMO energy level governs the electron affinity of a material, this implies that in PP-g-GMA, characterized by a lower LUMO energy level, there is an increased propensity for the capture of free electrons. The measurement of electron trap defects can be established as the disparity in energy levels between the LUMO states of the unmodified polymer and the polymer that has undergone grafting. The substantial modification in the electron energy states gives rise to profound energy traps. Utilizing alterations in the electron band structure subsequent to polypropylene grafting, the electron trap depth is approximated to be 3.565 eV. Correspondingly, employing the substitution of the HOMO level in lieu of the LUMO level, the hole trap depth Ehole is computed to be 0.054 eV.
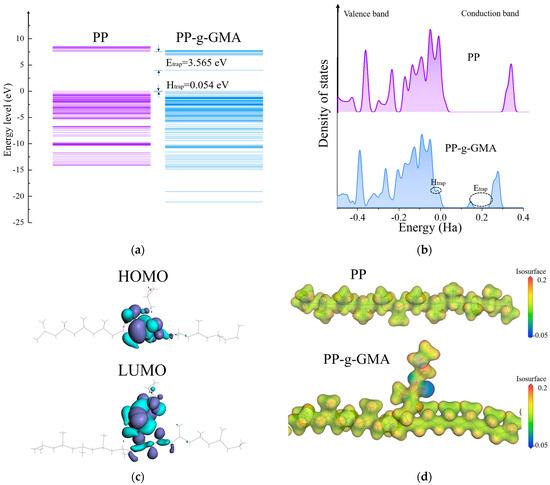
Figure 1.
(a) Energy band structure. (b) DOS for PP and PP-g-GMA. (c) LUMO and HOMO molecular orbitals in PP-g-GMA. (d) Electrostatic potential distribution. Blue and red colors represent positive and negative potentials, respectively.
The density of states (DOS) plots in Figure 1b can also serve as an indicator for assessing the impact of grafting on trap performance. According to the DOS representation, PP-g-GMA exhibits a prominent presence of two distinct energy states in the immediate vicinity of the conduction and valence bands, which can be explicitly associated with electron and hole traps, respectively. Of particular interest is the remarkably low energy level at 4.000 eV in PP-g-GMA, which corresponds to a generated electron trap capable of influencing charge injection and transport processes.
Figure 1c depicts an illustrative representation of the frontier molecular orbitals of PP-g-GMA. The findings indicate that the electronic delocalization primarily concentrates on the grafted GMA moiety. Consequently, it can be affirmed that the grafting of GMA units facilitates the introduction of deep-level traps at the molecular scale, which is conducive to the capture or excitation of free electrons. This, in turn, enables the formation of a fixed electronic coupling between the molecule’s side chain surface, thereby impeding electron transfer efficiency. Given that the hole mobility in insulating materials is significantly lower than that of electron carriers, the introduction of electron traps serves as an effective approach to restrain leakage current, thereby restricting the process of electron carrier migration.
The distribution of electrostatic potential is depicted in Figure 1d. Surface analysis of the grafted GMA molecules reveals that, in comparison to PP, the electrostatic potential region of grafted GMA is predominantly negative and positive. This further validates the attractive characteristic of grafted GMA towards positive and negative charge carriers.
3.2. Microstructure Characterization
Figure 2 shows the infrared spectra of PP and modified PP. The differences in the curves of the four composite media are mainly concentrated in the range of 500~1800 cm−1, so the horizontal coordinate range is limited to 500~2500 cm−1. In comparison to pure PP, the infrared spectra of PP-g-GMA and PP-gGMA-0.5Zr exhibit the emergence of a carbonyl group stretching vibration band around 1740 cm−1. This provides evidence of a bonding reaction between GMA and the polypropylene backbone, indicating the successful grafting of GMA. The absorption peaks observed in the range of 1570–1680 cm−1 correspond to the stretching vibrations of C=C bonds [32], representing both symmetric and antisymmetric stretching. In this range, the absorption peaks of PP-g-GMA and PP-gGMA-0.5Zr are more pronounced, indicating an increase in the content of C=C bonds within the ternary mixture, further confirming the successful grafting of GMA. Furthermore, the changes observed in the spectra of PP-g-GMA and PP-gGMA-0.5Zr in the range of 500–780 cm−1 are primarily attributed to ZrO2, as the peaks associated with Zr-O bonds are located around 650 cm−1 [19].
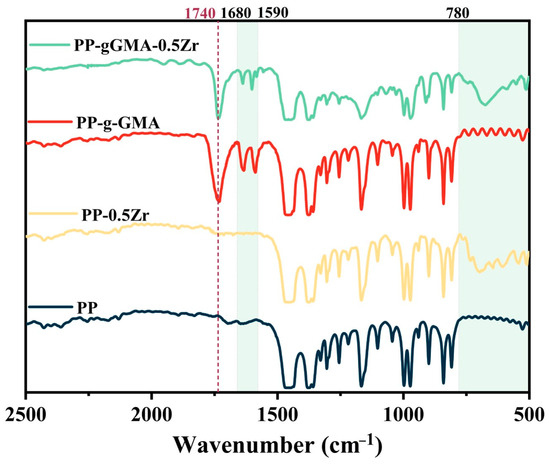
Figure 2.
FTIR patterns of PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
3.3. Crystallization Properties
PP is a semicrystalline polymer, characterized by different crystalline forms including α, β, and γ phases. Among these, the α phase is the most common and is associated with PP’s excellent thermodynamic stability. The formation of α crystals in PP may also be accompanied by the presence of trace amounts of β crystals. XRD analysis can be used to study the crystal symmetry of PP, while DSC allows for investigating the material’s melting behavior. The XRD diffraction pattern of PP, as depicted in Figure 3, exhibits characteristic peaks located at 2θ = 14.1°, 16.9°, 18.5°, 21.2°, and 21.8°, which correspond to the monoclinic α phase’s (110), (040), (130), (111), and (041) crystal planes, respectively. The peak at 16.2° represents the (300) crystal plane of β phase in PP [7]. The incorporation of 0.5 weight percent of nano ZrO2 into PP exerts a discernible inhibitory effect on the formation of the β phase, while concurrently promoting the formation of the α phase. The grafting of GMA effectively suppresses the characteristic peaks associated with the β phase, resulting in a pronounced inhibition of its formation. However, it is noteworthy that the characteristic peaks of the α phase also exhibit a slight weakening. Within the ternary composite medium, it becomes evident that the characteristic peaks of the α phase are significantly amplified, whereas the characteristic peaks of the β phase nearly vanish.
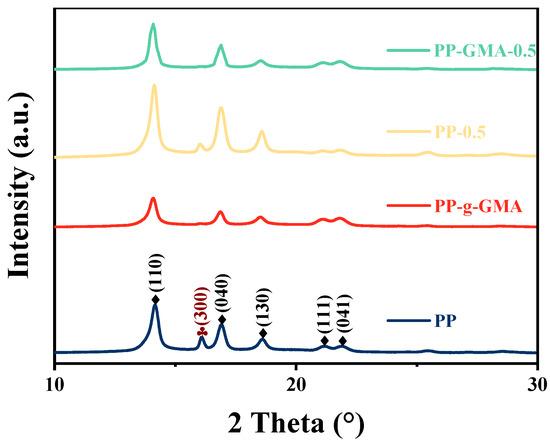
Figure 3.
XRD patterns of sample of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
The melting behavior of PP, PP-0.5Zr, PP-g-GMA, and PP-gGMA-0.5Zr was investigated using DSC. The DSC thermograms during heating from 120 °C to 180 °C are shown in Figure 4. From Figure 4, it can be observed that the addition of ZrO2 slightly increases the melting temperature. This can be attributed to the reinforcement of the α phase, as the melting temperature of α phase is 165 °C, while that of β phase is 154 °C. On the other hand, the grafting of GMA onto PP leads to a slight decrease in its melting temperature, as observed from Figure 3, primarily attributed to the weakening of the α phase. Notably, the ternary system comprising PP, PP-g-GMA, and 0.5 wt% ZrO2 exhibits a higher melt temperature compared to other conditions. XRD analysis reveals the strengthening of the α phase and the reduction of the β phase, indicating the superior thermal stability of this ternary system.
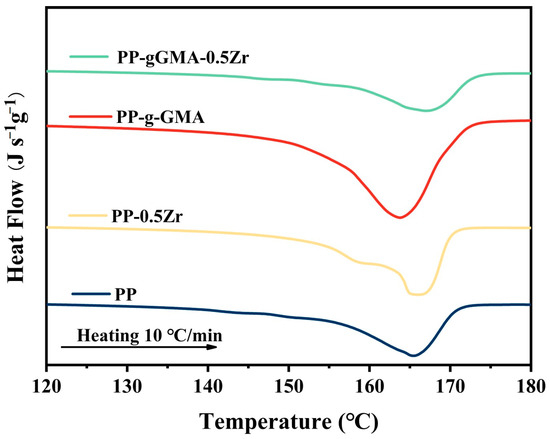
Figure 4.
DSC melting curves of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
3.4. Dielectric Properties
Figure 5 illustrates the dielectric constant of PP and three types of composite media. At lower frequencies, the FDS results effectively reflect the overall information of the materials. In this case, all three modification methods enhance the dielectric constant of PP. Among them, PP-gGMA-0.5Zr exhibits the most significant improvement, reaching approximately 2.67. The dielectric constants of all four composite media decrease to varying degrees as the frequency increases, with the modified PP showing a more pronounced decrease in the high-frequency region compared to pure PP. Pure PP belongs to non-polar polymers, hence its dielectric constant remains relatively stable with increasing frequency. In contrast, the modified PP maintains a stable dielectric constant at lower frequencies, but as the frequency increases, some internal polarization processes cannot establish completely, resulting in a slight decrease in the dielectric constant in the high-frequency region. This phenomenon can be attributed to the interface polarization between ZrO2 nanoparticles and the PP matrix, as well as the orientation polarization of the polar groups of GMA monomers within the matrix. Both of these polarization processes cannot fully establish themselves at higher frequencies. Consequently, as the frequency of the external electric field increases, the polarization strength decreases, leading to a decrease in the dielectric constant.
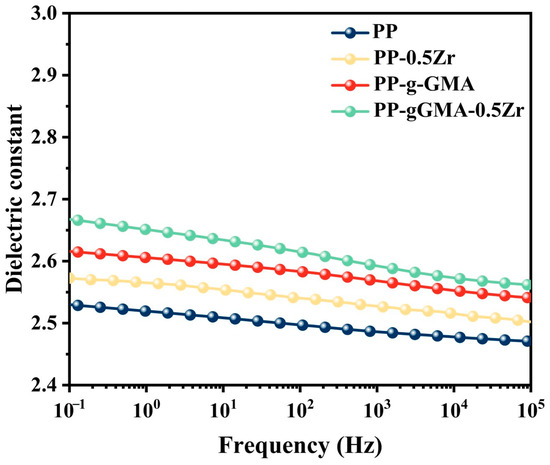
Figure 5.
Dielectric constant of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
Figure 6 presents the dielectric loss curves of PP and three types of composite media. The orientation polarization occurs within the range of 1 to 108 Hz, and the specific range varies for different polymers due to variations in their dipole properties [33]. In the case of PP-g-GMA, an increase in dielectric loss is observed in the range of 101 to 103 Hz, which is likely attributed to the dipolar orientation polarization induced by the polar groups of GMA. PP-0.5Zr exhibits a prominent loss peak in the range of 102 to 104 Hz, possibly due to the enhanced orientation polarization of the composite medium resulting from the interaction forces between ZrO2 and PP molecules upon nanoparticle doping, leading to an increase in the dielectric loss. In the case of PP-gGMA-0.5Zr, a significant increase in dielectric loss is observed within the range of 101 to 103 Hz, which can be attributed to the combined effect of GMA’s polar groups and ZrO2 [12]. Additionally, the increased loss in the low-frequency range (<1 Hz) is likely caused by interface polarization between different media. As the frequency of the external electric field increases, the establishment of both interface polarization and orientation polarization becomes incomplete, resulting in a decrease in dielectric loss within this frequency range to similar levels. Overall, all three modification methods lead to an increase in the dielectric constant while maintaining a minimal dielectric loss (generally < 0.007).
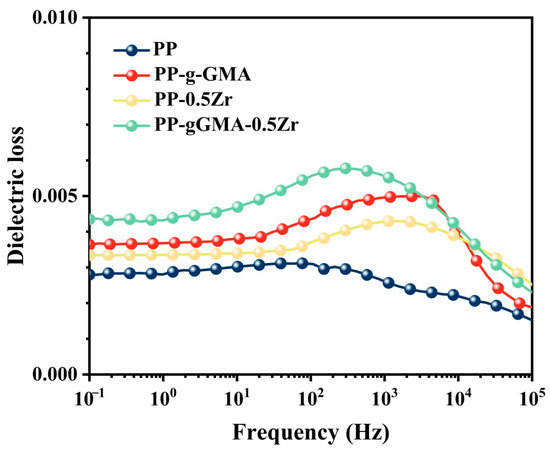
Figure 6.
Dielectric loss of sample of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
3.5. TSDC Analysis
In Figure 7, the TSDC curves of four PP composite materials are shown. It is observed that all three modification methods result in an increase in the depolarization current. The current density of PP-g-GMA exhibits exponential growth after 350 K as depicted in the figure. In the TSDC experiment, there is a strong correlation between the current density and traps [30]. The high-temperature current peak is believed to originate from charge detrapping [34]. PP is a semi-crystalline polymer, and its charge traps arise from the amorphous regions and the crystalline–amorphous boundaries. Physical defects such as voids and pores introduce shallow traps, while chemical defects such as broken chemical bonds and polar groups give rise to deep traps [35,36,37,38]. The introduction of polar groups through GMA grafting leads to an increase in deep traps. The changes in deep traps induced by GMA grafting can also be observed in the results of molecular simulations, as shown in Figure 1a,b. Through molecular simulations, we investigated the trap changes upon grafting GMA onto a PP molecular chain. The calculations indicate that the introduction of GMA onto PP results in the introduction of higher energy level traps near 3.565 eV. These deep traps cause a sharp increase in current density at high temperatures. After doping with ZrO2, the depolarization current increases in the mid–low temperature range (<100 °C), which may be attributed to the introduction of more shallow traps. The peak positions of the depolarization current in the TSDC spectra are observed to be around 260 K and 315 K, which may be associated with the dipole reorientation polarization-induced glass transition, while the peaks in the high temperature region can be attributed to electronic traps.
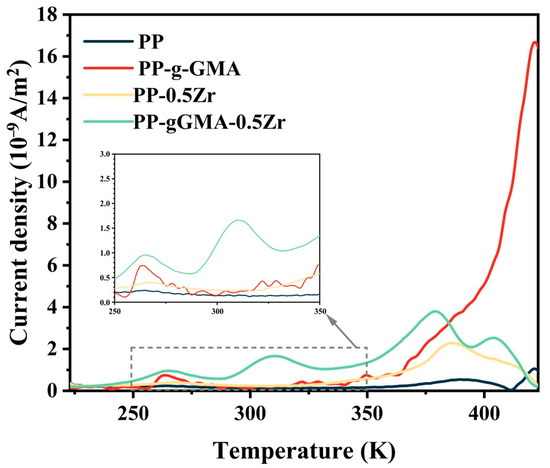
Figure 7.
TSDC curves of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
In accordance with the computational procedure outlined in reference [30], the trap distribution curves for the four polypropylene (PP) composite materials were computed, as depicted in Figure 8. In comparison to unmodified PP, all three modes of modification result in a significant increase in trap density. Notably, PP-gGMA-0.5Zr exhibits a more distinct peak within the energy range of 0.80–0.95 eV. The trap density of PP-g-GMA surpasses the other three samples at energy levels greater than 1.1 eV, while PP-gGMA-0.5Zr demonstrate the most pronounced enhancement of trap density within the sub-1.1 eV range. The abovementioned molecular simulations also corroborate the introduction of deep traps through the grafting of GMA onto PP.
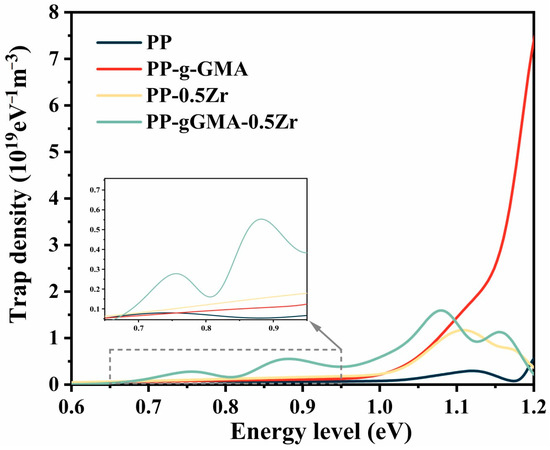
Figure 8.
The trap distribution curves of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
The variations in trap density and trap energy levels can impact the transport of charge carriers within the material. On one hand, deep traps capture charge carriers, impeding the process of carrier transport and consequently diminishing carrier mobility. On the other hand, deep traps exacerbate the accumulation of space charges near the electrodes, potentially leading to severe electric field distortions. A high density of deep traps intensifies electric field distortions, thereby facilitating breakdown. Both groups of samples doped with ZrO2 exhibited an increasing trend in shallow trap density. Carriers captured by shallow traps are more prone to release, hence an increase in shallow trap density promotes carrier migration, thereby mitigating the extent of electric field distortions. However, the presence of a substantial number of shallow traps results in significant leakage current and reduces breakdown voltage. From the test results, it can be observed that grafting GMA introduces a considerable number of deep traps, whereas ZrO2 doping introduces shallow traps. The ternary composite not only amplifies trap density, but also, to some extent, mitigates the excessive occurrence of deep traps resulting from GMA grafting.
3.6. DC Breakdown Strength
To assess the electrical strength of the four samples, the two-parameter Weibull distribution probability function is used to calculate the breakdown strength of the material in a direct current electric field [19]:
where E represents the experimentally measured breakdown voltage, and P(E) denotes the probability of breakdown for samples subjected to applied voltages not exceeding E. Ub serves as a scale parameter, characterizing the characteristic breakdown strength, or electrical strength of the material. On the other hand, β functions as a shape parameter, reflecting the rate of change in breakdown probability as the applied voltage increases, as well as the dispersion of experimental data.
P(E) = 1 − exp[−(E/Ub)β]
Figure 9 presents the Weibull distribution of the composite medium, while Table 1 displays the corresponding shape and scale parameters of the Weibull distribution. The addition of a lower concentration of ZrO2 leads to a slight increase in the electrical strength of the PP composite medium. However, the grafting of GMA onto PP results in a significant decrease in electrical strength. Changes in trap energy and trap density can lead to different charge transport behaviors, thereby affecting direct current breakdown strength [12]. A significant increase in deep traps was observed in PP-g-GMA. Carrier mobility was severely restricted, leading to carrier accumulation and local electric field distortion, making breakdown more likely to occur [29,39,40]. Grafting GMA alone introduced a large number of deep traps, suppressing carrier mobility, leading to a decrease in the breakdown voltage of PP-g-GMA. Molecular simulation results indicated the introduction of new electronic traps at around 3.656 eV after grafting GMA onto PP. Figure 8 also demonstrates an order-of-magnitude increase in deep traps for PP-g-GMA in the region above 1.1 eV. It is these high-energy deep traps that led to the characteristic decrease in breakdown voltage after grafting GMA alone. The doping of ZrO2 induces an increase in the density of shallow traps, which, to a certain extent, inhibits the degree of electric field distortion during the breakdown process, thereby delaying breakdown. As discussed earlier, the ternary modification approach not only increases the density of shallow traps but also avoids the introduction of excessive deep traps through GMA grafting. This contributes to a certain degree of improvement in the breakdown voltage.
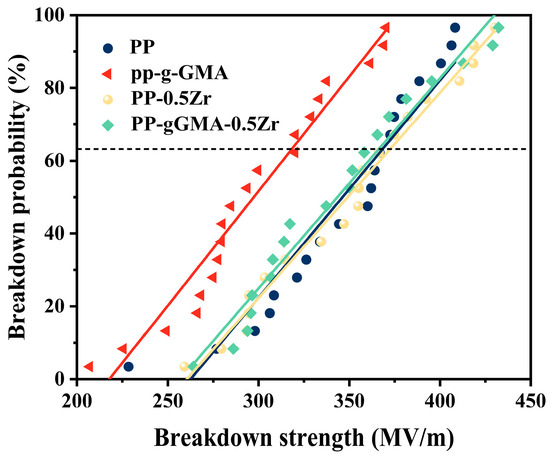
Figure 9.
Weibull distributions of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.

Table 1.
Weibull parameters.
3.7. Energy Storage Density
We anticipate that by constructing a ternary system consisting of PP-gGMA-0.5Zr, the composite medium can exhibit both high breakdown strength and an improved dielectric constant. The energy storage density of a linear dielectric can be calculated using the following Equation (4) [41,42]:
where ε0 represents the vacuum dielectric constant (approximately 8.854 × 10−12 F/m), εr is the relative dielectric constant, and Ub is the electrical strength of PP, which refers to the characteristic breakdown strength of PP.
U = 1/2ε0εrUb2
The energy storage densities of the four samples are illustrated in Figure 10. Pure PP exhibits a high electrical strength of 368.66 MV/m, with a relative permittivity of 2.53, resulting in an energy storage density of approximately 1.5222 J/cm3. Incorporating 0.5 wt% of ZrO2 leads to a slight increase in energy storage density (1.6302 J/cm3). The grafting of GMA onto the composite medium induces a slight increase in the dielectric constant. However, the introduction of excessive deep traps causes a significant decrease in the characteristic breakdown voltage. Hence, grafting GMA alone proves less ideal for enhancing the energy storage density of PP, only 1.2655 J/cm3. Nevertheless, the ternary system achieves a balanced improvement in overall performance. The energy storage density of PP-gGMA-0.5Zr reaches 1.7275 J/cm3, the highest among all samples, presenting an improvement of approximately 13.49% compared to pure PP.
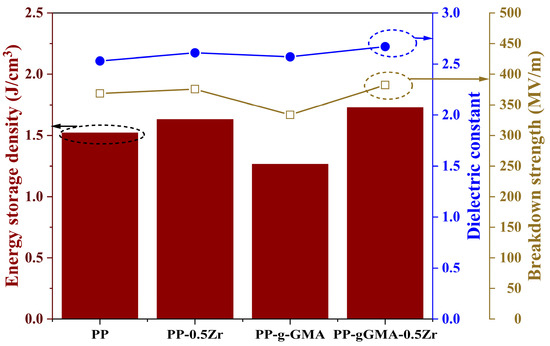
Figure 10.
Energy storage density of neat PP, PP-g-GMA, PP-0.5Zr, and PP-gGMA-0.5Zr.
4. Conclusions
A ternary composite system comprising PP, PP-g-GMA, and 0.5 wt% ZrO2 has been fabricated to enhance energy storage density and thermal stability. Additionally, the mechanism for improving thermal stability has been investigated through molecular simulation calculations, complemented by measurements using infrared spectroscopy, X-ray diffraction, and DSC. Based on the findings, the following conclusions can be drawn:
- PP-g-GMA demonstrates a lower LUMO energy level compared to PP, rendering it more prone to electron capture. The grafted GMA moieties, on a molecular scale, introduce deep traps that form fixed electronic coupling with the side surfaces of the molecules, consequently suppressing electron transfer efficiency. This is further supported by the TSDC results of PP-g-GMA, which indeed validate the introduction of deep traps in the composite medium due to the grafted GMA.
- When PP is blended with PP-g-GMA and 0.5 wt% ZrO2, the α-crystalline phase is enhanced while the β-crystalline phase is nearly eliminated compared to pristine PP. Furthermore, the melting temperature is higher than in other instances, indicating its exceptional thermal stability.
- When compared to other conditions, PP-gGMA-0.5Zr not only demonstrates a higher breakdown strength but also possesses a dielectric constant of 2.67. Its energy storage density reaches 1.7275 J/cm3, the highest among all samples. This signifies an improvement of approximately 13.49% compared to the energy storage density of pure PP at 1.5222 J/cm3.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en16227621/s1, Figure S1: Weibull distributions of PP-xZr.
Author Contributions
Conceptualization, L.C. and C.Z.; methodology, Z.X.; software, L.C., C.Z., and W.L.; validation, S.G., H.L. and L.C.; formal analysis, C.Z., Z.X. and S.G.; investigation, C.Z. and W.L.; resources, S.G., H.L. and W.L.; data curation, S.G., X.C. and H.L.; writing—original draft preparation, C.Z. and Z.X.; writing—review and editing, L.C. and W.L.; visualization, S.G., H.L., X.C. and L.C.; supervision, L.C. and W.L.; project administration, W.L.; funding acquisition, C.Z., W.L. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by State grid smart grid research institute Co., Ltd., grant number 5600007471.
Data Availability Statement
All of the data are available within the manuscript. Additional data will be provided upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. Authors C.Z., Z.X., X.C. and S.G. were employed by the company State grid Smart Grid Research Institute Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmed Dabbak, S.Z.; Illias, H.A.; Ang, B.C.; Abdul Latiff, N.A.; Makmud, M.Z.H. Electrical Properties of Polyethylene/Polypropylene Compounds for High-Voltage Insulation. Energies 2018, 11, 1448. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, Y.; Hu, S.; Yuan, H.; Yuan, J.; Yang, C.; Hu, J.; Li, Q.; He, J. Comprehensive Properties of Grafted Polypropylene Insulation Materials for AC/DC Distribution Power Cables. Energies 2023, 16, 4701. [Google Scholar] [CrossRef]
- Michelazzi, M.; Fabiani, D. Electrical Conduction in Thin-Film Polypropylene Capacitors. Energies 2023, 16, 6631. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L. Polymer nanocomposites for electrical energy storage. J. Polym. Sci. B Polym. Phys. 2011, 49, 1421–1429. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q. Advanced polymer dielectrics for high temperature capacitive energy storage. J. Appl. Phys. 2020, 127, 240902. [Google Scholar] [CrossRef]
- Ma, C.; Min, D.M.; Li, S.T.; Zheng, X.; Li, X.Y.; Min, C.; Zhan, H.X. Trap distribution and direct current breakdown characteristics in polypropylene/Al2O3 nanodielectrics. Acta Phys. Sin. 2017, 66, 281–289. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Liu, H.B.; Ma, Y.W.; Cheng, L.; Liu, W.F.; Chen, D.Y.; Li, S.T. Electrical Properties and Energy Storage Characteristic of Barium Zirconate Titanate/Polypropylene Nanocomposites. Insul. Mater. 2022, 55, 61–68. [Google Scholar] [CrossRef]
- Min, D.; Yan, C.; Mi, R.; Ma, C.; Huang, Y.; Li, S.; Wu, Q.; Xing, Z. Carrier Transport and Molecular Displacement Modulated dc Electrical Breakdown of Polypropylene Nanocomposites. Polymers 2018, 10, 1207. [Google Scholar] [CrossRef]
- Han, L.J.; Wang, H.R.; Tang, Q.; Lang, X.R.; Wang, X.M.; Zong, Y.X.; Zong, C.Z. Preparation of graphene/polypropylene composites with high dielectric constant and low dielectric loss via constructing a segregated graphene network. RSC Adv. 2021, 11, 38264–38272. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chang, C.-W. Dielectric Constant Enhancement with Low Dielectric Loss Growth in Graphene Oxide/Mica/Polypropylene Composites. J. Compos. Sci. 2021, 5, 52. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.Z.; Liu, F.H.; Han, K.; Gadinski, M.R.; Xiong, C.X.; Wang, Q. Solution-processed ferroelectric terpolymer nanocomposites with high breakdown strength and energy density utilizing boron nitride nanosheets. Energy Environ. Sci. 2015, 8, 922–931. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, W.F.; Liu, C.M.; Liu, X.W.; Li, S.T. Enhanced energy storage properties of polypropylene/maleic anhydride-grafted polypropylene/nano-ZrO2 ternary system. J. Appl. Polym. Sci. 2019, 136, 48211. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Dang, B.; He, J.L. Effect of different nanoparticles on tuning electrical properties of polypropylene nanocomposites. IEEE Trans. Dielect. Electr. Insul. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Sharmila, D.J.; Brijitta, J.; Sampathkumar, R. Enhanced Dielectric Properties of Polypropylene based Composite using Zinc Oxide Nanorods Filler. J. Surf. Sci. Technol. 2017, 33, 115–120. [Google Scholar] [CrossRef]
- Khedher, N.B.; Ghalambaz, M.; Alghawli, A.S.; Hajjar, A.; Sheremet, M.; Mehryan, S.A.M. Study of tree-shaped optimized fins in a heat sink filled by solid-solid nanocomposite phase change material. Int. Commun. Heat Mass Transf. 2022, 136, 106195. [Google Scholar] [CrossRef]
- Khedher, N.B.; Bantan, R.A.; Kolsi, L.; Omri, M. Performance investigation of a vertically configured LHTES via the combination of nano-enhanced PCM and fins: Experimental and numerical approaches. Int. Commun. Heat Mass Transf. 2022, 137, 106246. [Google Scholar] [CrossRef]
- Luo, W.; Liu, X.; Fu, Y. Melt grafting of maleic anhydride onto polypropylene with assistance of α-methylstyrene. Polym. Eng. Sci. 2012, 52, 814–819. [Google Scholar] [CrossRef]
- Burton, E.L.; Woodhead, M.; Coates, P.; Gough, T. Reactive grafting of glycidyl methacrylate onto polypropylene. J. Appl. Polym. Sci. 2010, 117, 2707–2714. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Liu, X.; Liu, C.; Chi, X.; Li, S. Correlation between morphology and electrical breakdown strength of the polypropylene/maleic anhydride grafted polypropylene/nano-ZrO2 ternary system. J. Appl. Polym. Sci. 2018, 135, 46842. [Google Scholar] [CrossRef]
- Song, X.J.; Hu, J.; Wang, C.C. Synthesis of highly surface functionalized monodispersed poly(St/DVB/GMA) nanospheres with soap-free emulsion polymerization followed by facile “click chemistry” with functionalized alkylthiols. Colloid Surf. A 2011, 380, 250–256. [Google Scholar] [CrossRef]
- Zheng, X.; He, L.; Yu, G.; Li, Y. Effect of Tea Polyphenols on the Melt Grafting of Glycidyl Methacrylate onto Polypropylene. Polymers 2022, 14, 5253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, Z.; Na, T.; Yin, J. Morphology, mechanical properties and interfacial behaviour of PA1010/PP/PP-g-GMA ternary blends. Polymers 1997, 38, 5905–5912. [Google Scholar] [CrossRef]
- Tao, Y.J.; Mai, K.C. Non-isothermal crystallization and melting behavior of compatibilized poly-propylene/recycled poly(ethylene terephthalate) blends. Eur. Polym. J. 2007, 43, 3538–3549. [Google Scholar] [CrossRef]
- Xia, X.F.; Zhang, J.H.; Fan, J.M.; Jiang, Q.L.; Xu, S.A. Effect of functionalization on non-isothermal crystallization behavior of polypropylene. Int. J. Polym. Anal. Charact. 2016, 21, 697–707. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, J.; Yang, K.; Guo, J.; Wang, W.; Pan, R.; Wu, G. Simultaneous enhancements of mechanical properties and hydrophilic properties of polypropylene via nano-silicon dioxide modified by polydopamine. J. Appl. Polym. Sci. 2017, 134, 45004. [Google Scholar] [CrossRef]
- Jacob, S.; Suma, K.K.; Mendez, J.M.; George, K.E. Reinforcing effect of nanosilica on polypropylene–nylon fibre composite. Mater. Sci. Eng. B 2010, 168, 245–249. [Google Scholar] [CrossRef]
- Chi, X.H.; Cheng, L.; Liu, W.F.; Zhang, X.H.; Li, S.T. Dynamic mechanism of breakdown in polypropylene-based nano-dielectric. AIP Adv. 2019, 9, 015135. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Z.; Ma, L.; Gao, J.; Guo, N. Enhanced breakdown strength and electrical tree resistance properties of MMT/SiO2/LDPE multielement composites. J. Appl. Polym. Sci. 2019, 136, 47364. [Google Scholar] [CrossRef]
- Lv, Y.; Ge, Y.; Wang, L.; Sun, Z.; Zhou, Y.; Huang, M.; Qi, B. Effects of nanoparticle materials on prebreakdown and breakdown properties of transformer oil. Appl. Sci. 2018, 8, 601. [Google Scholar] [CrossRef]
- Tian, F.Q.; Bu, W.B.; Shi, L.S.; Yang, C.; Wang, Y.; Lei, Q.Q. Theory of modified thermally stimulated current and direct determination of trap level distribution. J. Electrostat. 2011, 69, 7–10. [Google Scholar] [CrossRef]
- Yuan, C.; Zhou, Y.; Zhu, Y.; Hu, S.; Liang, J.; Luo, Z.; Li, Q. Improved high-temperature electrical properties of polymeric material by grafting modification. ACS Sustain. Chem. Eng. 2022, 10, 8685–8693. [Google Scholar] [CrossRef]
- Wang, S.; Shao, L.; Song, Z.; Zhao, J.; Feng, Y. Preparation of epoxy functionalized PP with unique structure and its post-ring open reaction. J. Appl. Polym. Sci. 2012, 124, 4827–4837. [Google Scholar] [CrossRef]
- Zhu, L. Exploring strategies for high dielectric constant and low loss polymer dielectrics. J. Phys. Chem. Lett. 2014, 5, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yuan, H.; Zhang, Q.; Li, J.; Wang, M.; Huang, S.; He, J. Deep trap origins, characteristics, and related mechanisms in chemically grafted polypropylene with enhanced direct current volume resistivity. J. Phys. Chem. C 2022, 126, 16280–16288. [Google Scholar] [CrossRef]
- Yuan, H.; Hu, S.X.; Zhou, Y.; Yuan, C.; Song, W.B.; Shao, Q.; Shi, H.W.; Li, J.; Hu, J.; Li, Q.; et al. Enhanced Electrical Properties of Styrene-grafted Polypropylene Insulation for Bulk Power Transmission HVDC Cable. CSEE J. Power Energy Syst. 2021, 12, 11–18. [Google Scholar] [CrossRef]
- Zhai, J.T.; Li, W.K.; Zha, J.W.; Cheng, Q.; Bian, X.M.; Dang, Z.M. Space Charge Suppression of Polyethylene Induced by Blending with Ethylene-butyl Acrylate Copolymer. CSEE J. Power Energy Syst. 2020, 6, 152–159. [Google Scholar] [CrossRef]
- Tian, F.Q.; Lei, Q.Q.; Wang, X.; Wang, Y. Effect of Deep Trapping States on Space Charge Suppression in Polyethylene/ZnO Nanocomposite. Appl. Phys. Lett. 2011, 99, 142903. [Google Scholar] [CrossRef]
- Peng, S.; Dang, B.; Zhou, Y.; Hu, J.; He, J. Functionalized TiO2 nanoparticles tune the aggregation structure and trapping property of polyethylene nanocomposites. J. Phys. Chem. C 2016, 120, 24754–24761. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, J.; Chi, M.; Zhang, J.; Tan, P. Experimental study on trap characteristics of nano-montmorillonite composite pressboards. Energies 2018, 11, 1732. [Google Scholar] [CrossRef]
- Han, T.; Du, B.; Ma, T.; Wang, F.; Gao, Y.; Lei, Z.; Li, C. Electrical tree in HTV silicone rubber with temperature gradient under repetitive pulse voltage. IEEE Access 2019, 7, 41250–41260. [Google Scholar] [CrossRef]
- Xie, H.; Luo, H.; Pei, Z.; Chen, S.; Zhang, D. Improved discharge energy density and efficiency of polypropylene-based dielectric nanocomposites utilizing BaTiO3@TiO2 nanoparticles. Mater. Today Energy 2022, 30, 2468–6069. [Google Scholar] [CrossRef]
- Ai, D.; Li, H.; Zhou, Y.; Ren, L.; Han, Z.; Yao, B.; Zhou, W.; Zhao, L.; Xu, J.; Wang, Q. Tuning Nanofillers in In Situ Prepared Polyimide Nanocomposites for High-Temperature Capacitive Energy Storage. Adv. Energy Mater. 2020, 10, 1903881. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).