Ammonia/Ethanol Mixture for Adsorption Refrigeration
Abstract
1. Introduction
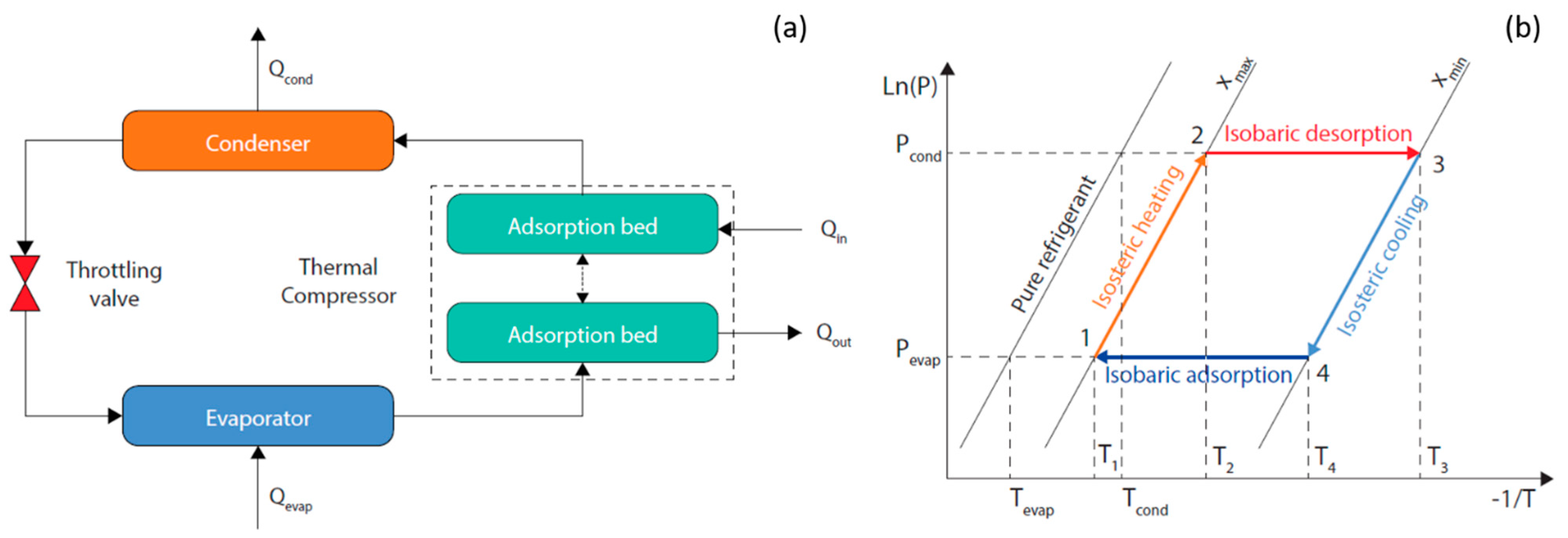
- Isosteric heating (1−2): As a result of low-grade heating the pressure in the adsorption bed increases from Pevap to Pcond while the adsorption bed temperature increases from T1 to T2.
- Isobaric desorption (2−3): The adsorption bed continues to receive heat and its temperature keeps raising from T2 to T3, which results in the desorption of the refrigerant vapor to the condenser under a constant vapor pressure. The working fluid concentration shifts from xmax to xmin.
- Isosteric cooling (3−4): As a result of cooling, the pressure in the adsorption bed decreases from Pcond to Pevap while the adsorption bed temperature decreases from T3 to T4.
- Isobaric adsorption (4−1): The adsorption bed continues to be cooled and its temperature keeps lowering from T4 to T1, which results in the adsorption of the refrigerant vapor from the evaporator under a constant vapor pressure. The working fluid concentration shifts back from xmin to xmax.
2. Thermodynamic Model
2.1. Case Study A: Complete cycle modelling with PRSV + IAST
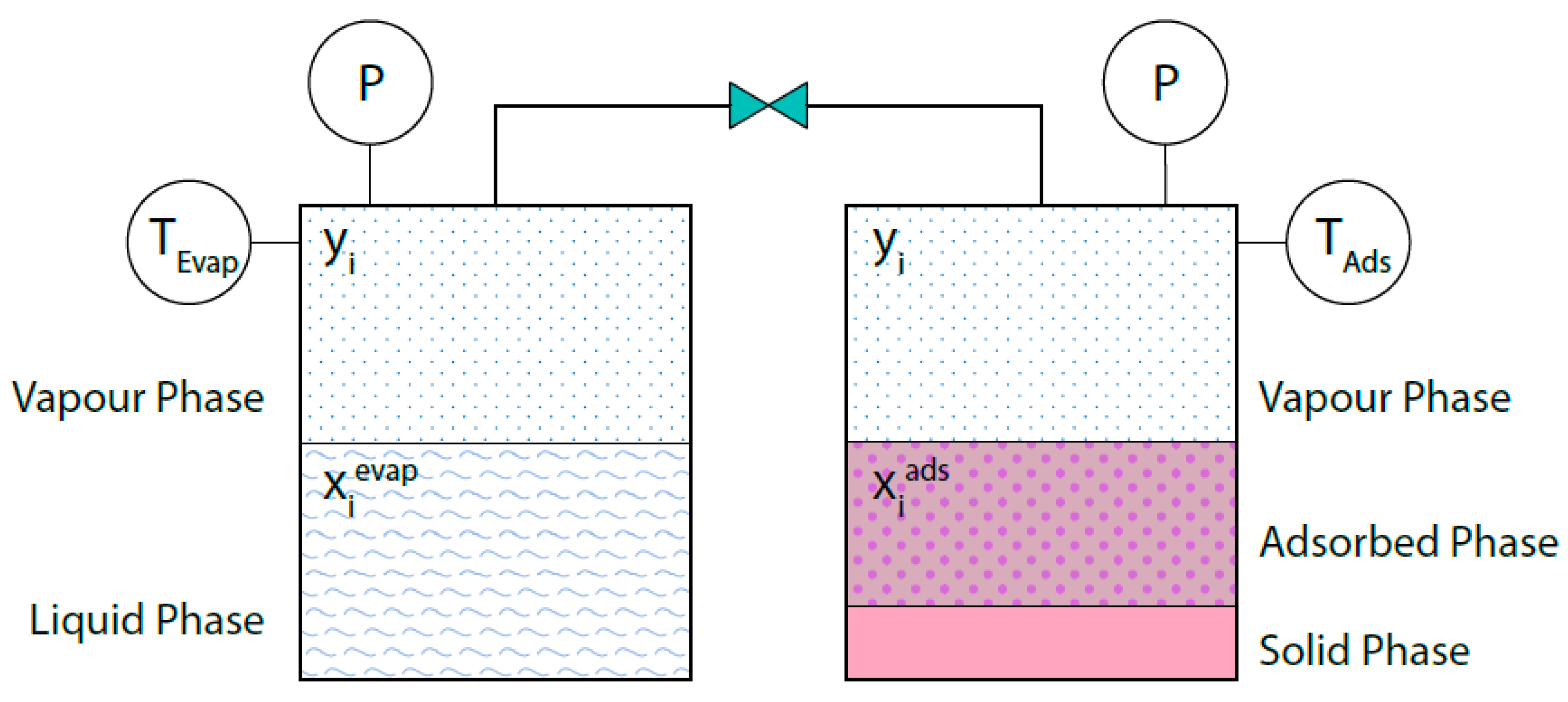
2.2. Case Study B: Adsorption bed/Evaporator Connection Modelling with PRSV + MPTA
3. Results and Discussion
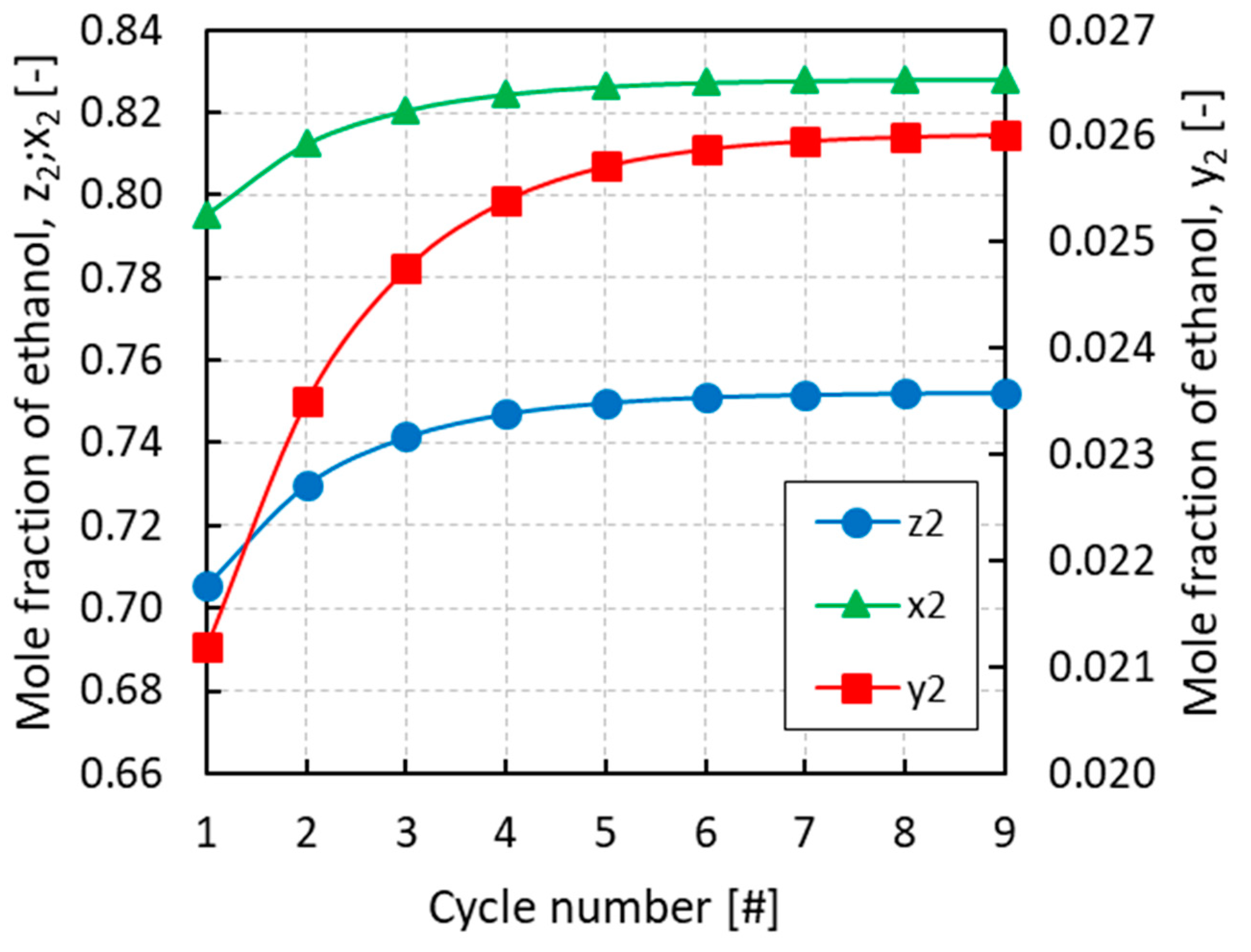
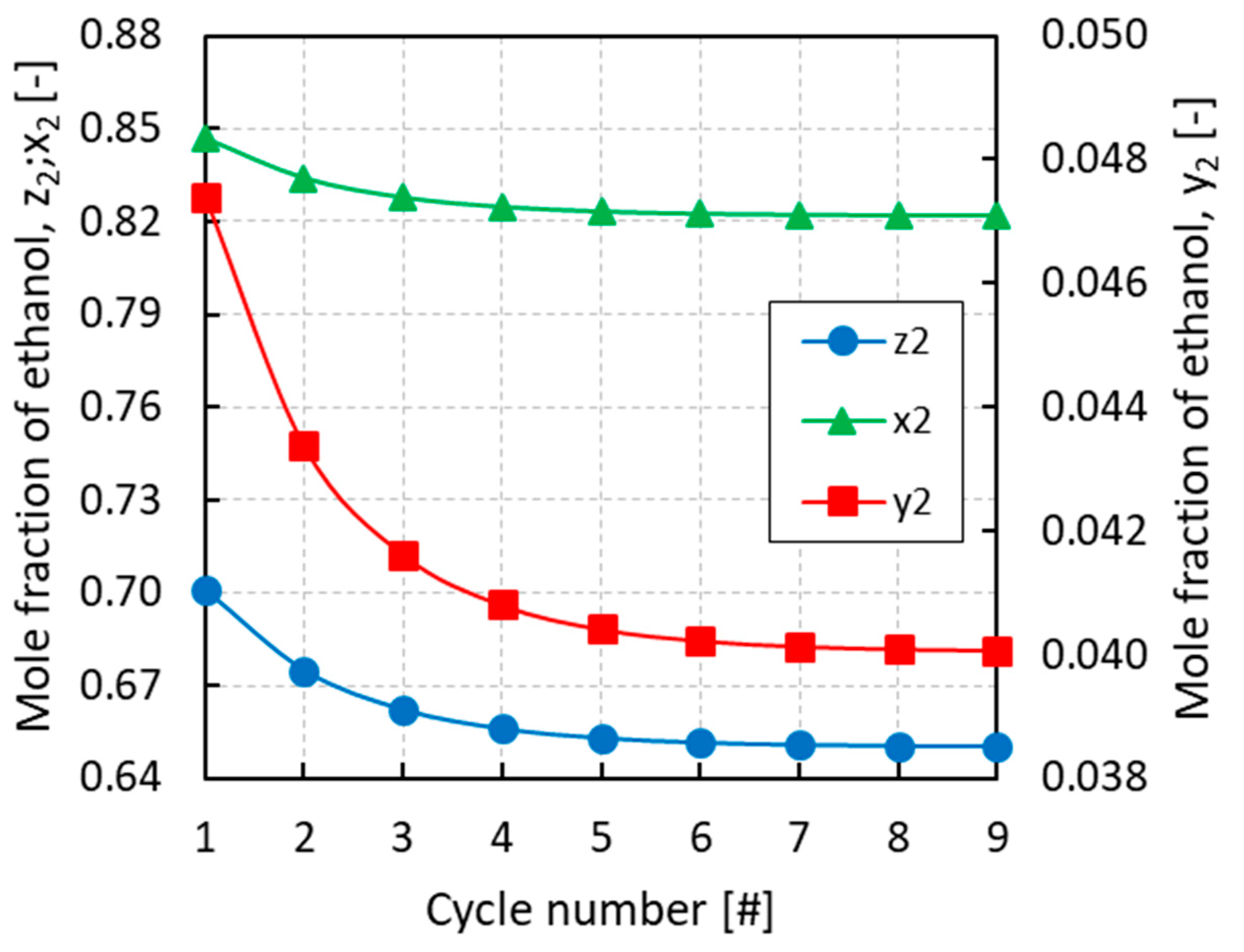
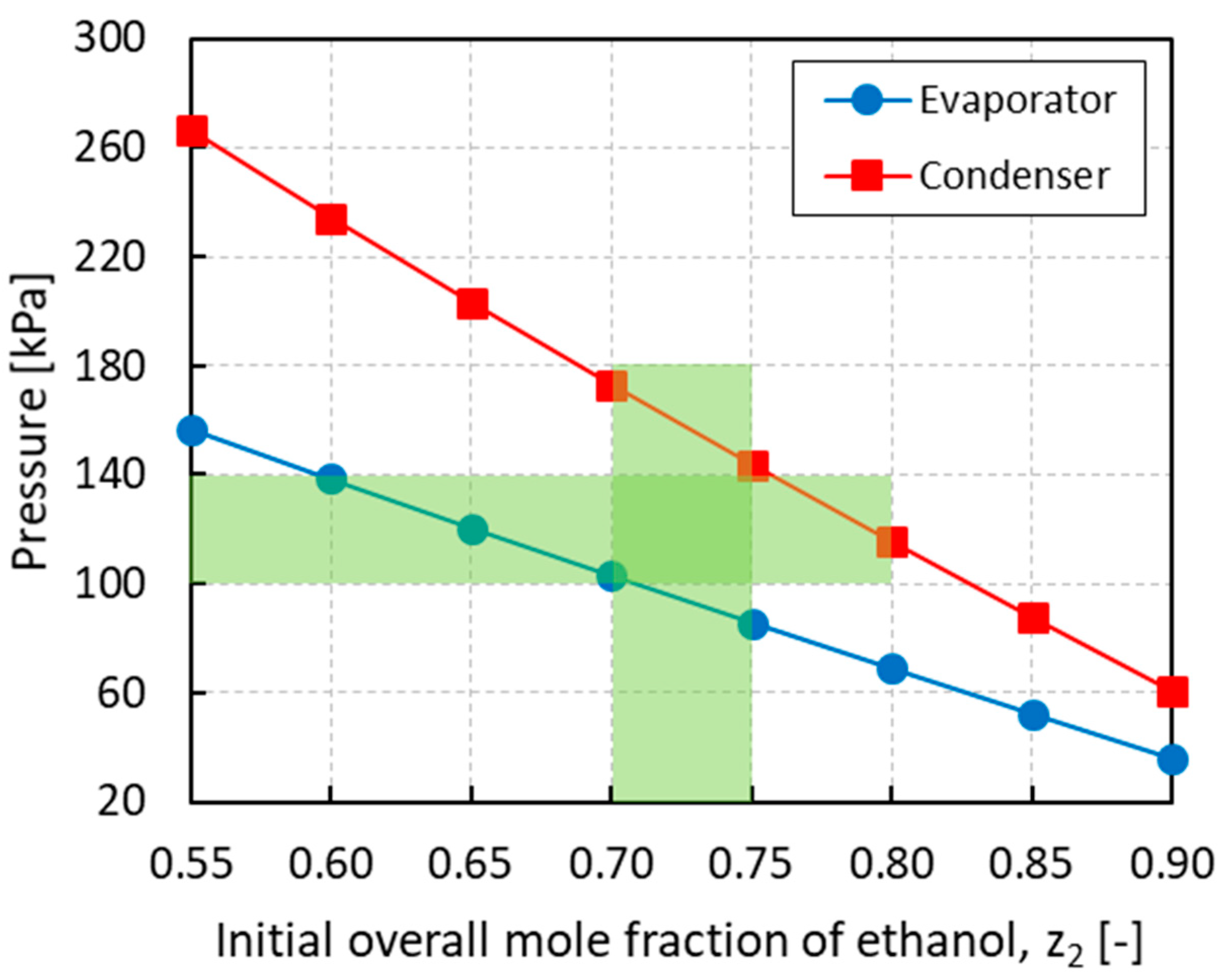
3.1. Case Study A: PRSV + IAST
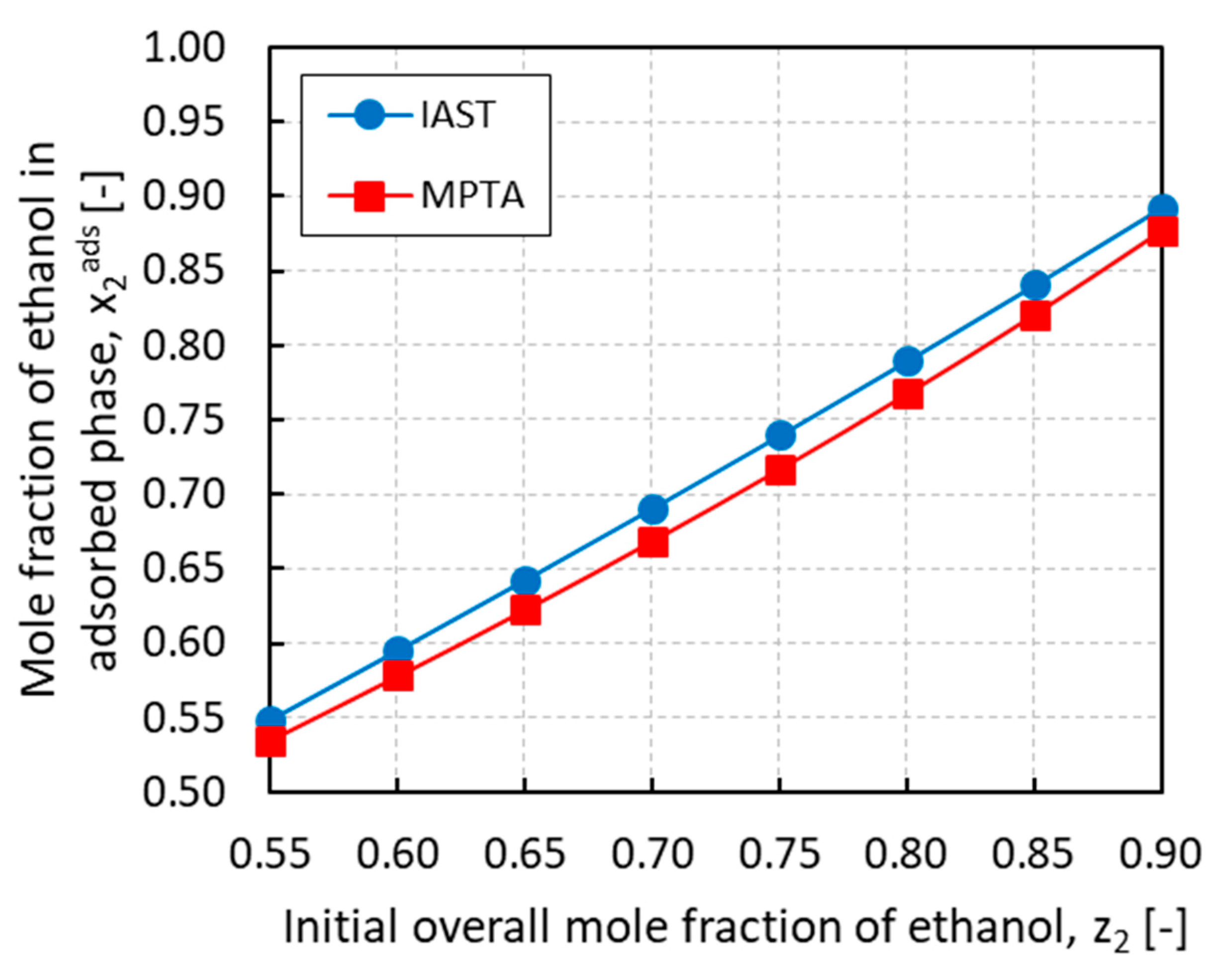
3.2. Case Study B: PRSV + MPTA
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | Polynomial coefficient A of PRSV equation of state [-] |
| a | Second virial coefficient mixing parameter of PRSV equation of state [m6 mol−2] |
| ai | Second virial coefficient of PRVS equation of state [m6 mol−2] |
| B | Polynomial coefficient B of PRSV equation of state [-] |
| b | Covolume mixing parameter of PRSV equation of state [m3 mol−1] |
| bi | Covolume of PRSV equation of state [m3 mol−1] |
| b0,j | Pre-exponential adsorption equilibrium constant of site j in the dual-site Langmuir model [kPa−1] |
| COP | Coefficient of performance of refrigeration cycle [-] |
| cp0,cr | Critical ideal gas molar heat capacity [kJ mol−1 K−1] |
| cp,ads | Molar heat capacity of the adsorbent [kJ mol−1 K−1] |
| cp,ref | Molar heat capacity of the refrigerant [kJ mol−1 K−1] |
| Fads | Total number of moles in the adsorption bed [mol] |
| Fcond | Total number of moles in the condenser [mol] |
| Fevap | Total number of moles in the evaporator [mol] |
| Gads | Number of moles of the vapor phase in the adsorption bed [mol] |
| Gcond | Number of moles of the vapor phase in the condenser [mol] |
| Gevap | Number of moles of the vapor phase in the evaporator [mol] |
| ΔHdes | Enthalpy of desorption [kJ mol−1] |
| ΔHevap | Enthalpy of vaporization of refrigerant in the evaporator [kJ mol−1] |
| ΔHj | Enthalpy of adsorption of site j in the dual-site Langmuir model [kJ mol−1] |
| kiads | Equilibrium constant of component i in the adsorption bed [-] |
| kicond | Equilibrium constant of component i in the condenser [-] |
| kievap | Equilibrium constant of component i in the evaporator [-] |
| Lcond | Number of moles of the liquid phase in the condenser [mol] |
| Levap | Number of moles of the liquid phase in the evaporator [mol] |
| Lrec | Recirculated moles of liquid phase from the condenser to the evaporator [mol] |
| mads | Mass of adsorbent [kg] |
| N | Number of moles of the adsorbed phase [mol] |
| P | Equilibrium pressure [kPa] |
| Pi0 | Surface pressure of component i [kPa] |
| Pcond | Condenser pressure [kPa] |
| Pcr | Critical pressure [kPa] |
| Pevap | Evaporator pressure [kPa] |
| Psat | Saturation pressure [kPa] |
| Pz | Local adsorption pressure [kPa] |
| Qdes | Heat of desorption for adsorbent regeneration [kJ mol−1] |
| Qevap | Heat removed from the evaporator [kJ mol−1] |
| QH | Total heat supplied to the system [kJ mol−1] |
| Qref | Heat to bring the adsorbed phase from Tint,h to Treg [kJ mol−1] |
| Qsol | Heat to bring the adsorbent from Tint,h to Treg [kJ mol−1] |
| qi | Amount adsorbed of component i [mol kg−1] |
| qs,j | Saturation adsorption capacity of site j in the dual-site Langmuir model [mol kg−1] |
| R | Ideal gas constant [L kPa mol−1 K−1] |
| Tads | Adsorption temperature [K] |
| Tcond | Condenser temperature [K] |
| Tcr | Critical temperature [K] |
| Tdes | Desorption temperature [K] |
| Tevap | Evaporator temperature [K] |
| Tint,h | Intermediate temperature of isosteric heating [K] |
| Treg | Maximum temperature of adsorbent regeneration [K] |
| Vads | Volume of the adsorption bed [L] |
| Vcond | Volume of the condenser [L] |
| Vevap | Volume of the evaporator [L] |
| xmax | Mole fraction in the adsorbed phase at the end of adsorption [-] |
| xmin | Mole fraction in the adsorbed phase at the end of desorption [-] |
| xiads | Mole fraction of component i in the adsorbed phase [-] |
| xicond | Mole fraction of component i in the liquid phase of the condenser [-] |
| xievap | Mole fraction of component i in the liquid phase of the evaporator [-] |
| yi | Equilibrium mole fraction of component i in the vapor phase [-] |
| yicond | Mole fraction of component i in the vapor phase of the condenser [-] |
| yievap | Mole fraction of component i in the vapor phase of the evaporator [-] |
| Z | Compressibility factor [-] |
| Zads | Compressibility factor of the vapor phase in the adsorption bed [-] |
| Zcond | Compressibility factor of the vapor phase in the condenser [-] |
| Zevap | Compressibility factor of the vapor phase in the evaporator [-] |
| z | Pore volume [cm3 g−1] |
| z0 | Pore volume at saturation [cm3 g−1] |
| ziads | Overall mole fraction of component i in the adsorption bed [-] |
| zicond | Overall mole fraction of component i in the condenser [-] |
| zievap | Overall mole fraction of component i in the evaporator [-] |
| β | Dubinin potential parameter [-] |
| Γi | Surface excess of component i [mol m−2] |
| εb | Adsorption bed porosity [-] |
| εi | Potential field of component i [kJ mol−1] |
| εi0 | Characteristic adsorption energy of component i [kJ mol−1] |
| εp | Adsorbent porosity [-] |
| λvap | Latent heat of vaporization [kJ mol−1] |
| ρ | Density of the vapor phase [mol m−3] |
| ρb | Adsorption bed density [mol m−3] |
| ρcr | Critical density [mol m−3] |
| ρz | Local density of the adsorbed phase [mol m−3] |
| φi | Fugacity coefficient of component i [-] |
| φiL,evap | Fugacity coefficient of component i in the liquid phase of the evaporator [-] |
| φiL,cond | Fugacity coefficient of component i in the liquid phase of the condenser [-] |
| φiV,ads | Fugacity coefficient of component i in the vapor phase of the adsorption bed [-] |
| φiV,evap | Fugacity coefficient of component i in the vapor phase of the evaporator [-] |
| φiV,cond | Fugacity coefficient of component i in the vapor phase of the condenser [-] |
| ψeq | Reduced grand potential at equilibrium [mol kg−1] |
| ψi | Reduced grand potential of component i [mol kg−1] |
| ω | Acentric factor [-] |
References
- Critoph, R.E. Solid sorption cycles: A short history. Int. J. Refrig. 2012, 35, 490–493. [Google Scholar] [CrossRef]
- Critoph, R.E.; Zhong, Y. Review of trends in solid sorption refrigeration and heat pumping technology. J. Process Mech. Eng. 2005, 219, 285–300. [Google Scholar] [CrossRef]
- Wang, R.Z.; Oliveira, R.G. Adsorption refrigeration: An efficient way to make good use of waste heat and solar energy. Prog. Energy Combust. Sci. 2006, 32, 424–458. [Google Scholar] [CrossRef]
- Freni, A.; Maggio, G.; Vasta, S.; Santori, G.; Polonara, F.; Restuccia, G. Optimization of a solar-powered adsorptive ice-maker by a mathematical method. Sol. Energy 2008, 82, 965–976. [Google Scholar] [CrossRef]
- Santori, G.; Sapienza, A.; Freni, A. A dynamic multi-level model for adsorptive solar cooling. Renew. Energy 2012, 43, 301–312. [Google Scholar] [CrossRef]
- Santori, G.; Santamaria, S.; Sapienza, A.; Brandani, S.; Freni, A. A stand-alone solar adsorption refrigerator for humanitarian aid. Sol. Energy 2014, 100, 172–178. [Google Scholar] [CrossRef]
- Tzabar, N. Mixed-refrigerant Joule-Thomson (MR JT) mini-cryocoolers. Adv. Cryog. Eng. 2014, 1573, 148–154. [Google Scholar]
- Tzabar, N. Binary mixed-refrigerant for steady cooling temperatures between 80 K and 150 K with Joule-Thomson cryocoolers. Cryogenics 2014, 64, 70–76. [Google Scholar] [CrossRef]
- Santori, G.; Di Santis, C. Optimal fluids for adsorptive cooling and heating. Sustain. Mater. Technol. 2017, 12, 52–61. [Google Scholar] [CrossRef]
- Freni, A.; Maggio, G.; Sapienza, A.; Frazzica, A.; Restuccia, G.; Vasta, S. Comparative analysis of promising adsorbent/adsorbate pairs for adsorptive heat pumping, air conditioning and refrigeration. Appl. Therm. Eng. 2016, 104, 85–95. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, D. Adsorption heat pump using an innovative coupling refrigeration cycle. Adsorption 2004, 10, 47–55. [Google Scholar] [CrossRef]
- Tzabar, N.; Holland, H.J.; Vermeer, C.H.; ter Brake, H.J.M. Modeling the adsorption of mixed gases based on pure gas adsorption properties. IOP Conf. Ser. Mater. Sci. Eng. 2015, 101, 012169. [Google Scholar] [CrossRef]
- Tzabar, N.; Grossman, G. Analysis of an activated-carbon sorption compressor operating with gas mixtures. Cryogenics 2012, 52, 491–499. [Google Scholar] [CrossRef]
- Stryjek, R.; Vera, J.H. PRSV: An improved Peng-Robinson equation of state for pure compounds and mixtures. Can. J. Chem. Eng. 1986, 64, 323–333. [Google Scholar] [CrossRef]
- Stryjek, R.; Vera, J.H. PRSV: An improved Peng-Robinson equation of state with new mixing rules for strongly nonideal mixtures. Can. J. Chem. Eng. 1986, 64, 334–340. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Santori, G.; Luberti, M.; Ahn, H. Ideal adsorbed solution theory solved with direct search minimisation. Comput. Chem. Eng. 2014, 71, 235–240. [Google Scholar] [CrossRef]
- Shapiro, A.A.; Stenby, E.H. Potential theory of multicomponent adsorption. J. Colloid Interface Sci. 1998, 201, 146–157. [Google Scholar] [CrossRef]
- Santori, G.; Luberti, M.; Brandani, S. Common tangent plane in mixed-gas adsorption. Fluid Phase Equilibria 2015, 392, 49–55. [Google Scholar] [CrossRef]
- Santori, G.; Luberti, M. Thermodynamics of thermally-driven adsorption compression. Sustain. Mater. Technol. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Sandler, S.I. Chemical, Biochemical, and Engineering Thermodynamics, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rachford, H.H., Jr.; Rice, J.D. Procedure for use of electronic digital computers in calculating flash vaporization hydrocarbon equilibrium. J. Pet. Technol. 1952, 195, 327–328. [Google Scholar] [CrossRef]
- MATLAB R2018a. MathWork. Available online: https://www.mathworks.com/products/matlab.html (accessed on 29 January 2020).
- Mikyska, J.; Firoozabadi, A. A new thermodynamic function for phase-splitting at constant temperature, moles, and volume. AIChE J. 2011, 57, 1897–1904. [Google Scholar] [CrossRef]
- Critoph, R.E. Performance limitations of adsorption cycles for solar cooling. Sol. Energy 1988, 41, 21–31. [Google Scholar] [CrossRef]
- Siperstein, F.R.; Myers, A.L. Mixed-gas adsorption. AIChE J. 2001, 47, 1141–1159. [Google Scholar] [CrossRef]
- REFPROP v10.0. Available online: https://www.nist.gov/srd/refprop (accessed on 29 January 2020).
- Figueira, F.L.; Derjani-Bayeh, S.; Olivera-Fuentes, C. Prediction of the thermodynamic properties of {ammonia + water} using cubic equations of state with the SOF cohesion function. Fluid Phase Equilibria 2011, 303, 96–102. [Google Scholar] [CrossRef]
- Tillner-Roth, R.; Friend, D.G. A Helmholtz free energy formulation of the thermodynamic properties of the mixture {water + ammonia}. J. Phys. Chem. Ref. Data 1998, 27, 63–96. [Google Scholar] [CrossRef]
- Brancato, V.; Frazzica, A.; Sapienza, A.; Gordeeva, L.; Freni, A. Ethanol adsorption onto carbonaceous and composite adsorbents for adsorptive cooling system. Energy 2015, 84, 177–185. [Google Scholar] [CrossRef]
- Tamainot-Telto, Z.; Metcalf, S.J.; Critoph, R.E.; Zhong, T.; Thorpe, R. Carbon-ammonia pairs for adsorption refrigeration applications: Ice making, air conditioning and heat pumping. Int. J. Refrig. 2009, 32, 1212–1229. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schicktanz, M.; Hügenell, P.P.C.; Sievers, H.; Henning, H.-M. Evaluation of methanol adsorption on activated carbons for thermally driven chillers part I: Thermophysical characterisation. Int. J. Refrig. 2012, 35, 543–553. [Google Scholar] [CrossRef]
- Sudibandriyo, M.; Pan, Z.; Fitzgerald, J.E.; Robinson, R.L., Jr.; Gasem, K.A.M. Adsorption of methane, nitrogen, carbon dioxide, and their binary mixtures on dry activated carbon at 318.2 K and pressures up to 13.6 MPa. Langmuir 2003, 19, 5323–5331. [Google Scholar] [CrossRef]
- Monsalvo, M.A.; Shapiro, A.A. Study of high-pressure adsorption from supercritical fluids by the potential theory. Fluid Phase Equilibria 2009, 283, 56–64. [Google Scholar] [CrossRef]
- Qian, J.-W.; Privat, R.; Jaubert, J.-N.; Duchet-Suchaux, P. Enthalpy and heat capacity changes on mixing: Fundamental aspects and prediction by means of the PPR78 cubic equation of state. Energy Fuels 2013, 27, 7150–7178. [Google Scholar] [CrossRef]
- Mejbri, K.; Bellagi, A. Modelling of the thermodynamic properties of the water–ammonia mixture by three different approaches. Int. J. Refrig. 2006, 29, 211–218. [Google Scholar] [CrossRef]
- Dechema, Detherm. Available online: https://i-systems.dechema.de/detherm/mixture.php (accessed on 29 January 2020).



| Fluid | λvap (@283 K) [kJ mol−1] | Psat (@283 K) [kPa] | Tcr [K] | Pcr [kPa] | ρcr [mol m−3] | ω [-] | cp0,cr [kJ mol−1 K−1] |
|---|---|---|---|---|---|---|---|
| Water | 44.6 | 1.2 | 647.3 | 22,048 | 17,857 | 0.344 | 0.037 |
| Ammonia | 21.4 | 611.2 | 405.7 | 11,300 | 13,889 | 0.253 | 0.038 |
| Methanol | 37.6 | 7.4 | 512.6 | 8140 | 8547 | 0.566 | 0.061 |
| Ethanol | 43.9 | 3.1 | 513.9 | 6120 | 5952 | 0.643 | 0.098 |
| Isopropanol | 46.8 | 2.2 | 508.3 | 4790 | 4525 | 0.670 | 0.133 |
| Variable | Evaporator | Condenser | Adsorption Bed |
|---|---|---|---|
| Temperature, T [K] | 283.15 | 298.15 | 298.15 (ads); 353.15 (des) |
| Volume, V [L] | 10 | 10 | 0.213 |
| Total number of moles, F [mol] | 7 | 7 | Calculated |
| Overall mole fraction, z2 [-] | 0.55 | 0.55 | Calculated |
| 0.60 | 0.60 | ||
| 0.65 | 0.65 | ||
| 0.70 | 0.70 | ||
| 0.75 | 0.75 | ||
| 0.80 | 0.80 | ||
| 0.85 | 0.85 | ||
| 0.90 | 0.90 |
| Form | Origin | Particle Size [mm] | Surface Area [m2 g−1] | mads [kg] | εb [-] | εp [-] | ρb [kg m−3] | cp,ads [kJ kg−1 K−1] |
|---|---|---|---|---|---|---|---|---|
| Grains | Coconut shell | 0.5−2 | 2613 | 0.1 | 0.35 | 0.84 | 420 | 0.95 |
| Component | qs1 [mol kg−1] | b01 [kPa−1] | ΔH1 [kJ mol−1] | qs2 [mol kg−1] | b02 [kPa−1] | ΔH2 [kJ mol−1] |
|---|---|---|---|---|---|---|
| Ammonia (1) | 53.27 | 8.97 × 10−8 | 23.45 | 5.10 | 5.78 × 10−12 | 55.04 |
| Ethanol (2) | 11.96 | 1.58 × 10−10 | 58.53 | 3.76 | 1.21 × 10−8 | 41.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luberti, M.; Di Santis, C.; Santori, G. Ammonia/Ethanol Mixture for Adsorption Refrigeration. Energies 2020, 13, 983. https://doi.org/10.3390/en13040983
Luberti M, Di Santis C, Santori G. Ammonia/Ethanol Mixture for Adsorption Refrigeration. Energies. 2020; 13(4):983. https://doi.org/10.3390/en13040983
Chicago/Turabian StyleLuberti, Mauro, Chiara Di Santis, and Giulio Santori. 2020. "Ammonia/Ethanol Mixture for Adsorption Refrigeration" Energies 13, no. 4: 983. https://doi.org/10.3390/en13040983
APA StyleLuberti, M., Di Santis, C., & Santori, G. (2020). Ammonia/Ethanol Mixture for Adsorption Refrigeration. Energies, 13(4), 983. https://doi.org/10.3390/en13040983





