Real-World Efficacy and Safety of Disitamab Vedotin (RC48-ADC) in the Treatment of HER2-Overexpressing Advanced Gastric/Gastroesophageal Junction Cancer
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
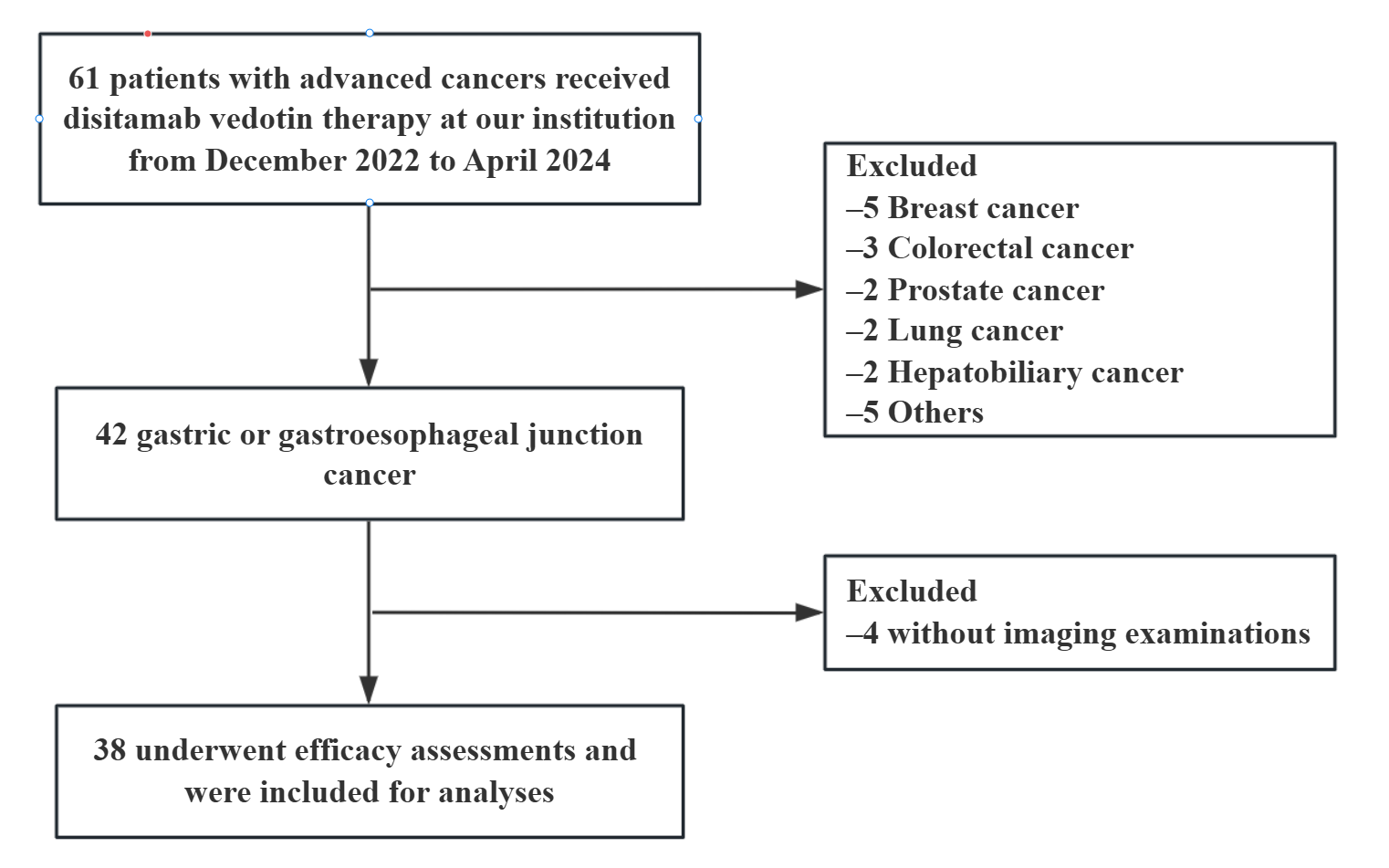
2.1. Patients
2.2. Treatment
2.3. Efficacy and Safety Evaluation
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
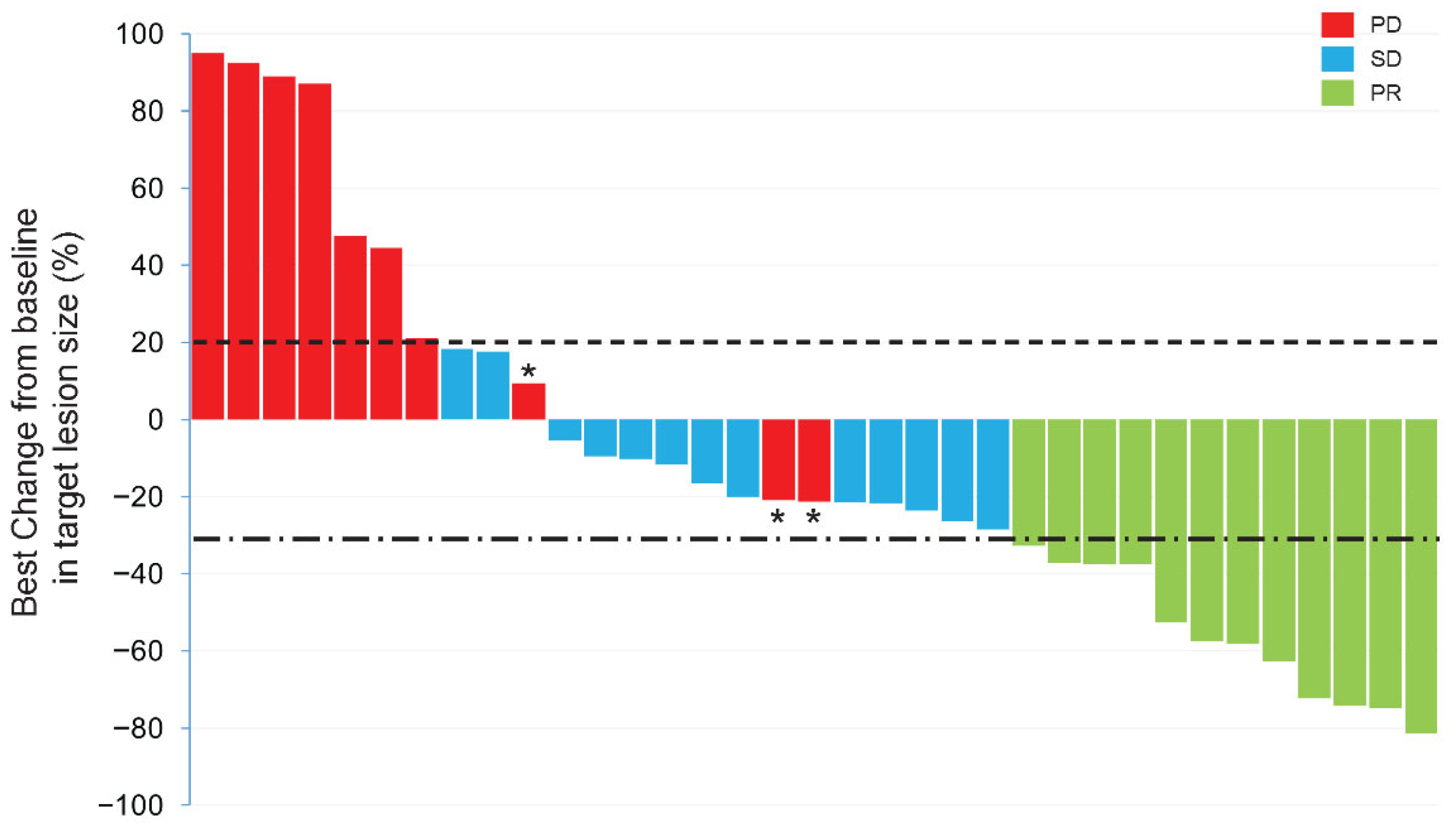
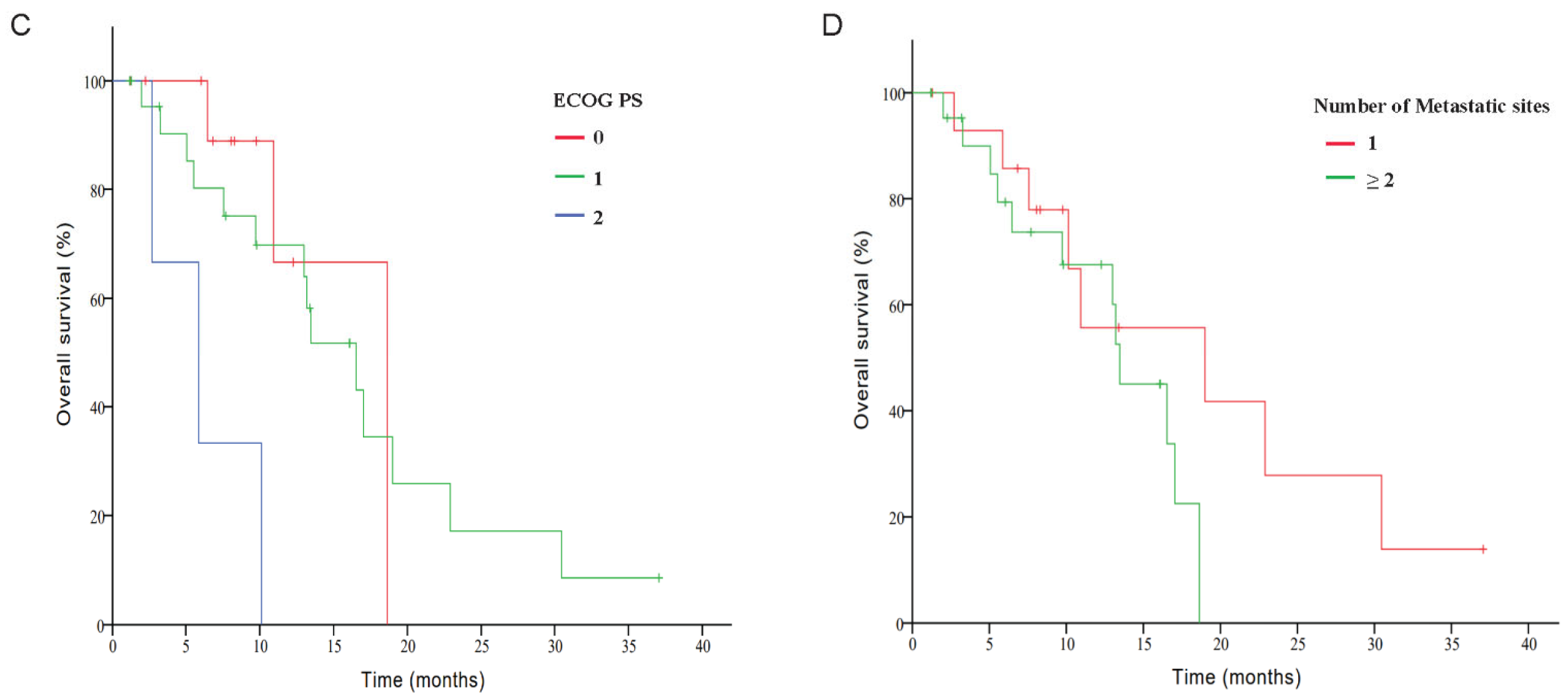
3.2. Efficacy
3.3. Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Li, Y.; Yan, H.; Xie, J.; Wang, J.; Zhao, B. Global burden, risk factors, clinicopathological characteristics, molecular biomarkers and outcomes of microsatellite instability-high gastric cancer. Aging 2024, 16, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Z.; Zhang, X.; Liu, Q.; Cui, X.; Li, L.; Liu, B.; Wei, J. Real-world clinical outcomes of the combination of anti-PD-1 antibody, trastuzumab, and chemotherapy for HER2-positive gastric/gastroesophageal junction cancer. Cancer Med. 2023, 12, 9517–9526. [Google Scholar] [CrossRef]
- Takehana, T.; Kunitomo, K.; Kono, K.; Kitahara, F.; Iizuka, H.; Matsumoto, Y.; Fujino, M.A.; Ooi, A. Status of c-erbB-2 in gastric adenocarcinoma: A comparative study of immunohistochemistry, fluorescence in situ hybridization and enzyme-linked immuno-sorbent assay. Int. J. Cancer 2002, 98, 833–837. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.J.; Feng-Yi, F.; Xu, J.M.; Lee, K.W.; Jiao, S.C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.S.; Fan, X.S.; Wu, H.M.; Liu, J.P.; Sun, W.Y.; Li, S.S.; Hou, Y.Y.; Nie, X.; Li, J.; et al. HER2 status in gastric adenocarcinoma of Chinese: A multicenter study of 40 842 patients. Zhonghua Bing Li Xue Za Zhi 2018, 47, 822–826. [Google Scholar] [CrossRef]
- Kim, W.-H.; Gomez-Izquierdo, L.; Vilardell, F.; Chu, K.-M.; Soucy, G.; dos Santos, L.V.; Monges, G.; Viale, G.; Brito, M.J.; Osborne, S.; et al. HER2 Status in Gastric and Gastroesophageal Junction Cancer: Results of the Large, Multinational HER-EAGLE Study. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 239–245. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Kang, Y.-K.; Park, H.; Uronis, H.E.; Lee, K.-W.; Ng, M.C.H.; Enzinger, P.C.; Park, S.H.; Gold, P.J.; Lacy, J.; et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): A single-arm, phase 1b–2 trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qiu, M.-Z.; Wang, J.-F.; Zhang, Y.-Q.; Shen, A.; Yuan, X.-L.; Zhang, T.; Wei, X.-L.; Zhao, H.-Y.; Wang, D.-S.; et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell Rep. Med. 2022, 3, 100814. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Mayordomo, J.I.; Hanna, D.L.; Ajani, J.A.; Murphy, M.A.B.; Murthy, R.K.; Piha-Paul, S.A.; Bauer, T.M.; Bendell, J.C.; et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J. Clin. Oncol. 2018, 36, 2500. [Google Scholar] [CrossRef]
- Xu, J.; Ying, J.; Liu, R.; Wu, J.; Ye, F.; Xu, N.; Zhang, Y.; Zhao, R.; Xiang, X.; Wang, J.; et al. KN026 (anti-HER2 bispecific antibody) in patients with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancer. Eur. J. Cancer 2022, 178, 1–12. [Google Scholar] [CrossRef]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef]
- Mark, C.; Lee, J.S.; Cui, X.; Yuan, Y. Antibody-Drug Conjugates in Breast Cancer: Current Status and Future Directions. Int. J. Mol. Sci. 2023, 24, 13726. [Google Scholar] [CrossRef]
- Giugliano, F.; Corti, C.; Tarantino, P.; Michelini, F.; Curigliano, G. Bystander effect of antibody-drug conjugates: Fact or fiction. Curr. Oncol. Rep. 2022, 24, 809–817. [Google Scholar] [CrossRef]
- Martín, M.; Pandiella, A.; Vargas-Castrillón, E.; Díaz-Rodríguez, E.; Iglesias-Hernangómez, T.; Martínez Cano, C.; Fernández-Cuesta, I.; Winkow, E.; Perelló, M.F. Trastuzumab deruxtecan in breast cancer. Crit. Rev. Oncol./Hematol 2024, 198, 104355. [Google Scholar] [CrossRef]
- Tsao, L.C.; Wang, J.S.; Ma, X.; Sodhi, S.; Ragusa, J.V.; Liu, B.; McBane, J.; Wang, T.; Wei, J.; Liu, C.X.; et al. Effective extracellular payload release and immunomodulatory interactions govern the therapeutic effect of trastuzumab deruxtecan (T-DXd). Nat. Commun. 2025, 16, 3167. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Mimura, K.; Matsumoto, T.; Thar Min, A.K.; Ito, M.; Nakano, H.; Neupane, P.; Kanke, Y.; Okayama, H.; Saito, M.; et al. The effects of T-DXd on the expression of HLA class I and chemokines CXCL9/10/11 in HER2-overexpressing gastric cancer cells. Sci. Rep. 2021, 11, 16891. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, L.; Li, X.; Zhou, Y.; Han, X.; Zhang, W.; Wang, W.; Guo, W.; Liu, W.; Xu, Q.; et al. A HER2-targeting antibody-MMAE conjugate RC48 sensitizes immunotherapy in HER2-positive colon cancer by triggering the cGAS-STING pathway. Cell Death Dis. 2023, 14, 550. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Gong, J.; Zhang, X.; Peng, Z.; Sheng, X.; Mao, C.; Fan, Q.; Bai, Y.; Ba, Y.; et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody–MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 2021, 24, 913–925. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, T.; Wei, J.; Wang, A.; He, Y.; Yang, L.; Zhang, X.; Fan, N.; Luo, S.; Li, Z.; et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: A single-arm phase II study. Cancer Commun. 2021, 41, 1173–1182. [Google Scholar] [CrossRef]
- Cui, J.; He, Y.; Zhu, F.; Gong, W.; Zuo, R.; Wang, Y.; Luo, Y.; Chen, L.; Wang, C.; Huo, G.; et al. Inetetamab, a novel anti-HER2 monoclonal antibody, exhibits potent synergistic anticancer effects with cisplatin by inducing pyroptosis in lung adenocarcinoma. Int. J. Biol. Sci. 2023, 19, 4061–4081. [Google Scholar] [CrossRef]
- Fu, R.; Qi, R.; Xiong, H.; Lei, X.; Jiang, Y.; He, J.; Chen, F.; Zhang, L.; Qiu, D.; Chen, Y.; et al. Combination therapy with oncolytic virus and T cells or mRNA vaccine amplifies antitumor effects. Signal Transduct. Target. Ther. 2024, 9, 118. [Google Scholar] [CrossRef]
- Ito, Y.; Yamada, D.; Kobayashi, S.; Sasaki, K.; Iwagami, Y.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Takahashi, H.; Shimizu, J.; et al. The combination of gemcitabine plus an anti-FGFR inhibitor can have a synergistic antitumor effect on FGF-activating cholangiocarcinoma. Cancer Lett. 2024, 595, 216997. [Google Scholar] [CrossRef]
- Van Cutsem, E.; di Bartolomeo, M.; Smyth, E.; Chau, I.; Park, H.; Siena, S.; Lonardi, S.; Wainberg, Z.A.; Ajani, J.; Chao, J.; et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): Primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023, 24, 744–756. [Google Scholar] [CrossRef]
- Verhoeven, R.H.A.; Kuijper, S.C.; Lordick, F.; Slingerland, M.; Qin, A.; van Laarhoven, H.W.M. 1574P Trastuzumab deruxtecan versus ramucirumab and paclitaxel as second-line therapy for patients with her2-positive gastric or gastro-esophageal junction adenocarcinoma: A propensity score matched comparison. Ann. Oncol. 2023, 34, S878. [Google Scholar] [CrossRef]
- Scheck, M.K.; Hofheinz, R.D.; Lorenzen, S. HER2-Positive Gastric Cancer and Antibody Treatment: State of the Art and Future Developments. Cancers 2024, 16, 1336. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Citterio, C.; Orlandi, E.; Vecchia, S.; Anselmi, E.; Toscani, I.; Rotolo, M.; Salati, M.; Ghidini, M. Fighting HER2 in Gastric Cancer: Current Approaches and Future Landscapes. Int. J. Mol. Sci. 2025, 26, 7285. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti–ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti–PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, e58–e68. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, R.; Xie, K.; Zhang, J.; Tao, F.; Pi, C.; Feng, Y.; Gu, H.; Fang, J. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res. Treat. 2021, 191, 51–61. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, T.; Zhu, J.; Zhang, Z.; Yang, L.; Zhang, Y.; Hu, C.; Chen, J.; Wang, J.; Tian, X.; et al. Spatiotemporal Quantification of HER2-targeting Antibody-Drug Conjugate Bystander Activity and Enhancement of Solid Tumor Penetration. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 984–997. [Google Scholar] [CrossRef]
- Sheng, X.; Zhou, L.; He, Z.; Guo, H.; Yan, X.; Li, S.; Xu, H.; Li, J.; Chi, Z.; Si, L.; et al. Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2022, 40, 4518. [Google Scholar] [CrossRef]
- Nie, C.; Xu, W.; Guo, Y.; Gao, X.; Lv, H.; Chen, B.; Wang, J.; Liu, Y.; Zhao, J.; Wang, S.; et al. Immune checkpoint inhibitors enhanced the antitumor efficacy of disitamab vedotin for patients with HER2-positive or HER2-low advanced or metastatic gastric cancer: A multicenter real-world study. BMC Cancer 2023, 23, 1239. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Wang, A.; Wei, J.; Peng, Z.; Wang, X.; Zhou, J.; Qi, C.; Liu, D.; Li, J.; et al. Disitamab vedotin (RC48) plus toripalimab for HER2-expressing advanced gastric or gastroesophageal junction and other solid tumours: A multicentre, open label, dose escalation and expansion phase 1 trial. Eclinicalmedicine 2024, 68, 102415. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Liu, Y.; Li, K.; Cong, L.; Cao, F.; Liu, A.; Liu, H.; Li, L.; Qu, L.; et al. Efficacy of disitamab vedotin (RC48) plus tislelizumab and S-1 as first-line therapy for HER2-overexpressing advanced stomach or gastroesophageal junction adenocarcinoma: A multicenter, single-arm, phase II trial (RCTS). J. Clin. Oncol. 2024, 42, 4009. [Google Scholar] [CrossRef]
- Hu, H.; Wang, K.; Jia, R.; Zeng, Z.-X.; Zhu, M.; Deng, Y.-L.; Xiong, Z.-J.; Tang, J.-N.; Xie, H.; Wang, Y.; et al. Current Status in Rechallenge of Immunotherapy. Int. J. Biol. Sci. 2023, 19, 2428–2442. [Google Scholar] [CrossRef]

| Characteristics | No. of Patients (N = 38) | % |
|---|---|---|
| Age (years) | ||
| Median | 66 (range 34–84 years) | |
| <65 | 18 | 47.4 |
| ≥65 | 20 | 52.6 |
| Sex | ||
| Male | 27 | 71.1 |
| Female | 11 | 28.9 |
| ECOG PS | ||
| 0 | 11 | 28.9 |
| 1 | 24 | 63.2 |
| 2 | 3 | 7.9 |
| Family history of cancer | ||
| Yes | 6 | 15.8 |
| No | 32 | 84.2 |
| Primary tumor site | ||
| Gastric | 32 | 84.2 |
| Gastroesophageal junction | 6 | 15.8 |
| HER2 status | ||
| IHC 2+ and FISH-negative | 11 | 28.9 |
| IHC 2+ and FISH-positive | 3 | 7.9 |
| IHC 3+ | 24 | 63.2 |
| Prior anti-HER2 therapy | ||
| Yes | 26 | 68.4 |
| No | 12 | 31.6 |
| Prior surgery | ||
| Yes | 24 | 63.2 |
| No | 14 | 36.8 |
| Prior chemotherapy | ||
| Yes | 38 | 100 |
| No | 0 | 0 |
| Prior radiotherapy | ||
| Yes | 12 | 31.6 |
| No | 26 | 68.4 |
| Prior immunotherapy | ||
| Yes | 35 | 92.1 |
| No | 3 | 7.9 |
| Prior antiangiogenic therapy | ||
| Yes | 6 | 15.8 |
| No | 32 | 84.2 |
| Number of treatment lines | ||
| 2 | 9 | 23.7 |
| ≥3 | 29 | 76.3 |
| Concurrent therapy | ||
| Chemotherapy | 4 | 10.5 |
| Radiotherapy | 5 | 13.2 |
| Immunotherapy | 13 | 34.2 |
| Targeted therapy | 6 | 15.8 |
| None | 16 | 42.1 |
| Number of metastatic sites | ||
| 1 | 15 | 39.5 |
| ≥2 | 23 | 60.5 |
| Metastatic sites | ||
| Liver | 15 | 39.5 |
| Lung | 7 | 18.4 |
| Bone | 6 | 15.8 |
| Lymph nodes | 27 | 71.1 |
| Adrenal gland | 2 | 5.3 |
| Others | 11 | 28.9 |
| Parameters | Best Tumor Response | ORR | p | DCR | p | |||
|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | |||||
| Total | 0 | 12 | 13 | 13 | 31.6 | 65.8 | ||
| Primary tumor site | 0.920 | 0.054 | ||||||
| GC | 0 | 10 | 9 | 13 | 31.3 | 59.4 | ||
| GEJC | 0 | 2 | 4 | 0 | 33.3 | 100 | ||
| Number of metastatic sites | 0.022 | 0.435 | ||||||
| 1 | 0 | 8 | 3 | 4 | 53.3 | 73.3 | ||
| ≥2 | 0 | 4 | 10 | 9 | 17.4 | 60.9 | ||
| HER2 status | 0.715 | 0.858 | ||||||
| Positive | 0 | 9 | 9 | 9 | 33.3 | 66.7 | ||
| Negative | 0 | 3 | 4 | 4 | 27.3 | 63.6 | ||
| Treatment lines | 0.342 | 0.095 | ||||||
| 2 | 0 | 4 | 4 | 1 | 44.4 | 88.9 | ||
| ≥3 | 0 | 8 | 9 | 12 | 27.6 | 58.6 | ||
| Prior anti-HER2 therapy | 0.553 | 0.938 | ||||||
| Yes | 0 | 9 | 8 | 9 | 34.6 | 65.4 | ||
| No | 0 | 3 | 5 | 4 | 25.0 | 66.7 | ||
| Concurrent ICIs therapy | 0.938 | 0.690 | ||||||
| Yes | 0 | 4 | 4 | 5 | 30.8 | 61.5 | ||
| No | 0 | 8 | 9 | 8 | 32.0 | 68.0 | ||
| TRAEs | Grade (N = 38) | |||
|---|---|---|---|---|
| Any Grades (%) | 1 (%) | 2 (%) | ≥3 (%) | |
| Hematologic | ||||
| Anemia | 34 (89.5) | 22 (57.9) | 7 (18.4) | 5 (13.2) |
| Lymphocytopenia | 31 (81.6) | 11 (28.9) | 13 (34.2) | 7 (18.4) |
| Hypoalbuminemia | 27 (71.1) | 25 (65.8) | 2 (5.3) | 0 |
| Increased LDH | 22 (57.9) | 15 (39.5) | 7 (18.4) | 0 |
| Leukopenia | 20 (52.6) | 4 (10.5) | 15 (39.5) | 1 (2.6) |
| Increased γGT | 19 (50.0) | 6 (15.8) | 7 (18.4) | 6 (15.8) |
| Neutropenia | 16 (42.1) | 3 (7.9) | 9 (23.7) | 4 (10.5) |
| Hypophosphatemia | 16 (42.1) | 10 (26.3) | 5 (13.2) | 1 (2.6) |
| Hypothyroidism | 13 (34.2) | 12 (31.6) | 1 (2.6) | 0 |
| Hyperglycemia | 11 (28.9) | 7 (18.4) | 2 (5.3) | 2 (5.3) |
| Hypokalemia | 10 (26.3) | 3 (7.9) | 5 (13.2) | 2 (5.3) |
| Hyponatremia | 10 (26.3) | 7 (18.4) | 2 (5.3) | 1 (2.6) |
| Thrombocytopenia | 8 (21.1) | 5 (13.2) | 0 | 3 (7.9) |
| Increased ALT | 7 (18.4) | 6 (15.8) | 1 (2.6) | 0 |
| Hyperlipemia | 7 (18.4) | 6 (15.8) | 0 | 1 (2.6) |
| Hypercholesteremia | 7 (18.4) | 7 (18.4) | 0 | 0 |
| Increased AST | 6 (15.8) | 4 (10.5) | 1 (2.6) | 1 (2.6) |
| Hyperbilirubinemia | 5 (13.2) | 2 (5.3) | 2 (5.3) | 1 (2.6) |
| Increased ALP | 2 (5.3) | 2 (5.3) | 0 | 0 |
| Non-hematologic | ||||
| Decreased appetite | 24 (63.2) | 17 (44.7) | 4 (10.5) | 3 (7.9) |
| Fatigue | 11 (28.9) | 8 (21.1) | 3 (7.9) | 0 |
| Vomiting | 10 (26.3) | 7 (18.4) | 2 (5.3) | 1 (2.6) |
| Constipation | 9 (23.7) | 7 (18.4) | 2 (5.3) | 0 |
| Abdominal pain | 9 (23.7) | 4 (10.5) | 3 (7.9) | 2 (5.3) |
| Neuropathy peripheral | 8 (21.1) | 5 (13.2) | 3 (7.9) | 0 |
| Pruritus | 7 (18.4) | 3 (7.9) | 3 (7.9) | 1 (2.6) |
| Nausea | 7 (18.4) | 4 (10.5) | 1 (2.6) | 2 (5.3) |
| Pyrexia | 7 (18.4) | 6 (15.8) | 1 (2.6) | 0 |
| Rash | 7 (18.4) | 5 (13.2) | 1 (2.6) | 1 (2.6) |
| Diarrhea | 6 (15.8) | 5 (13.2) | 0 | 1 (2.6) |
| Muscle pain/joint pain | 4 (10.5) | 1 (2.6) | 2 (5.3) | 1 (2.6) |
| Weight loss | 2 (5.3) | 1 (2.6) | 1 (2.6) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shi, Z.; Wang, Y.; Wang, Y.; Liu, S.; Zhang, L.; Xin, K.; Liu, B.; Liu, Q. Real-World Efficacy and Safety of Disitamab Vedotin (RC48-ADC) in the Treatment of HER2-Overexpressing Advanced Gastric/Gastroesophageal Junction Cancer. Curr. Oncol. 2026, 33, 2. https://doi.org/10.3390/curroncol33010002
Shi Z, Wang Y, Wang Y, Liu S, Zhang L, Xin K, Liu B, Liu Q. Real-World Efficacy and Safety of Disitamab Vedotin (RC48-ADC) in the Treatment of HER2-Overexpressing Advanced Gastric/Gastroesophageal Junction Cancer. Current Oncology. 2026; 33(1):2. https://doi.org/10.3390/curroncol33010002
Chicago/Turabian StyleShi, Zhan, Yan Wang, Yumeng Wang, Shutong Liu, Lianru Zhang, Kai Xin, Baorui Liu, and Qin Liu. 2026. "Real-World Efficacy and Safety of Disitamab Vedotin (RC48-ADC) in the Treatment of HER2-Overexpressing Advanced Gastric/Gastroesophageal Junction Cancer" Current Oncology 33, no. 1: 2. https://doi.org/10.3390/curroncol33010002
APA StyleShi, Z., Wang, Y., Wang, Y., Liu, S., Zhang, L., Xin, K., Liu, B., & Liu, Q. (2026). Real-World Efficacy and Safety of Disitamab Vedotin (RC48-ADC) in the Treatment of HER2-Overexpressing Advanced Gastric/Gastroesophageal Junction Cancer. Current Oncology, 33(1), 2. https://doi.org/10.3390/curroncol33010002





