Abstract
Exercise is related to many individual health outcomes but impact evaluations of exercise programmes are seldom conducted. The purpose of the study is to evaluate the feasibility of an exercise prescription intervention in primary health-care settings (CAMINEM Programme) located in two socially disadvantaged neighbourhoods. The CAMINEM was a pragmatic-driven intervention with opportunistic recruitment. It followed the 5As framework for health promotion and also the exercise training principles. Feasibility was evaluated using the RE-AIM framework (Reach, Effectiveness, Adoption, Implementation, and Maintenance). Patients with non-communicable chronic diseases participated in a 12-month home-based moderate-intensity exercise program, counselled by exercise physiologists. Participants were grouped according to their physical activity behaviour at baseline and 6-month adherence. CAMINEM reached 1.49% (n = 229) of the eligible population (N = 15374) and included a final sample of 178. Health outcomes for adhered participants followed positive patterns. Non-adhered participants visited their practitioner more compared to adhered participants. Thirty-three practitioners (40%) referred patients. Nurses referred four times more than physicians (81% and 19% respectively). The delivery of exercise prescriptions proved to be easy to complete and record by participants as well as easy to monitor and adjust by the exercise physiologists. One out of four participants adhered during the 12-month intervention. This intervention has been feasible in primary care in Catalonia, Spain, to safely prescribe home-based exercise for several conditions.
1. Introduction
Physical inactivity was identified as the fourth leading risk factor for global mortality [1] and trend data showed limited improvement between 2001 and 2016 [2] and between 2007 and 2017 [3]. Consequently, member states of the WHO agreed to achieve a 10% relative reduction in the prevalence of physical inactivity by 2050, as one of the global targets for the prevention and treatment of non-communicable diseases [4]. They also suggested that some differences in physical activity levels within and between countries can be explained by inequities in opportunities to be physically active, as was identified by Bauman et al. [5]. Even low levels of physical activity may reduce morbidity, all-cause mortality, and length life expectancy [6]. Accordingly, physical activity promotion is already issued in clinical guidelines, even excluding physical activity-specific guidelines [7]. Health-enhancing physical activity (HEPA) promotion includes primary health-care (PHC) settings because they reach a substantial number of people who overall, are more inactive and may benefit more [8,9]. In Spain, roughly 46% of the population are advised to walk as HEPA promotion [10].
Practitioners need systematic work to achieve habit change such as sedentary behaviour [11]. Written physical activity prescriptions have been feasible for practitioners and patients in health-care settings [8,12,13]. However, Spanish interventions of HEPA are seldom detailed and reported [10]. Catalan general practitioners rarely see HEPA promotion as a priority in 5-min consultations [14]. Moreover, 55% of the physicians who already promote HEPA consider there to be not enough time. Recently, Martínez–Gómez et al. [10] suggested that Spanish physicians may tailor counselling and provide written directions of HEPA, but there is a challenge to structuring this counselling (e.g., frequency and duration of walking) given time constraints and lack of staff training. Additionally, pre-exercise medical clearance is generally unnecessary [15], especially when the intensity of the activity is planned to be light to moderate [16].
Feasibility of the approaches is not universal; policies and legal frameworks differ between countries, and even between regions within the same country (e.g., Spain). The Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework [17] was first designed to estimate the public health impact of an intervention and it has been accepted to target the feasibility of HEPA interventions in community settings, such as PHC centres [18,19].
Recently, the European Commission, through the Public Health Agency, made a call for countries to transfer the Swedish Physical Activity on Prescription Model to other European regions, and the EUPAP Project (www.eupap.org, accessed on 1 February 2021) is actually ongoing and aiming to implement the Swedish Physical Activity on Prescription Model into nine other countries: Catalonia (Spain), Denmark, Flanders (Belgium), Germany, Italy, Lithuania, Malta, Portugal, and Romania [20]. The health sector is the gate for citizens to receive HEPA prescriptions, mostly primary healthcare settings, and the first option is that the physical activity is performed outside the healthcare setting. The core components of the Swedish model to be adapted by the EUPAP Project include: individualised patient-centered counselling, written prescription, evidence-based physical activity recommendations, follow-up, and collaboration with activity organisers [21].
The overall aim of this pragmatic study was to evaluate the feasibility (i.e., external validity) of the CAMINEM (Let’s Walk) Programme as an exercise-on-prescription, interdisciplinary approach for 12 months in two socially disadvantaged neighbourhoods. The specific aims were to (i) determine if CAMINEM reached the citizens with chronic health conditions that may benefit from exercising (Reach), (ii) assess clinical and quality-of-life outcomes and the use of health services (Effects), (iii) determine the adoption of CAMINEM by the practitioners (Adoption), (iv) evaluate participants’ retention and compliance (Implementation), and (v) describe if the practitioners prescribe exercise post-intervention (Maintenance).
2. Materials and Methods
The study was a pragmatic-driven intervention with opportunistic recruitment by general practitioners (GPs) or community nurses during 18 months–that is, regular patients aged 18 and above who visited their GP or community nurse may be invited. Participants received an exercise written prescription and were followed-up by an exercise physiologist for twelve months before being discharged. The rationale and design of the CAMINEM programme, including detailed procedures (referral, first face-to-face contact, follow-up, discharge) is published elsewhere [22,23]. Supplementary material Figures S1–S4 and Table S5 illustrates them.
There were no fixed inclusion criteria for the patients but practitioners focused on diseases in which exercise is known to be beneficial (i.e., overweight/obesity, hypertension, diabetes mellitus, dyslipidaemia, musculoskeletal pain, respiratory diseases, and minor mental health problems). Exclusion criteria were overt cardiovascular disease, uncontrolled hypertension, uncontrolled insulin-dependent diabetes, or other conditions that prevented participation in a walking programme determined by the practitioner or the exercise physiologist.
The settings of intervention were selected following a convenience sampling; that is, two primary health-care settings in the city of Lleida that offered health services to the two highest socially disadvantaged neighbourhoods, out of a total of seven primary health-care settings. The reason for this is that Catalan health policies encourage health promotion in such neighbourhoods, and it has eventually been found positive by WHO [24,25]. All GPs and community nurses working at least 3 months in any of these two settings were invited to participate by referring patients to the CAMINEM Programme.
The CAMINEM Programme provides a set of tools for practitioners to individualise exercise prescriptions: validated urban routes to monitor HEPA [26], a short questionnaire to asses physical activity level (ClassAF) [27], and a prescription form/logbook to monitor adherence (i.e., exercising following the prescriptions). This pragmatic study included the participation of a non-staffed exercise physiologist who counselled the participants previously referred by their GP or nurse. Exercise counselling relied on the 5As framework (Ask, Advise, Agree, Assist, Arrange) for health promotion [28] and the exercise training principles. Exercise periodization followed the health-oriented guidelines from the American College of Sports Medicine [29] and the Catalan exercise prescription handbook [27]. The conditioning period goal (up to two months) was to ensure that participants followed the WHO recommendations on physical activity for health for adults and older adults with a minimum of 150 minutes weekly of moderate-intensity aerobic physical activity [1]. The improvement period (two to six months) focused on increasing the total amount of exercise volume (first frequency, then duration), and on keeping adherence. The maintenance period (six to 12 months) aimed to maintain or increase exercise volume.
The exercise was unsupervised, individually based, moderate-intensity, continuous, and aerobic; that is, walking. Individualization of prescriptions was set during the first meeting with the exercise physiologist who underwent a motivational interview with each participant. Together they set goals, chose the specific urban route, set the frequency and duration of each walk, and scheduled the next follow-up meeting. The exercise physiologist delivered the written exercise prescription and then explained how to self-control the intensity of exercising using the Talk Test [30], as it has recently been suggested [31]. Follow-up meetings were for monitoring the compliance (adherence), giving advice to overcome possible barriers, and discussing the exercise prescription to finally deliver a new update on the written exercise prescription. Participants completed the full intervention after 12 months, were discharged and encouraged to keep an active lifestyle, and monitor their HEPA. Specific criteria for decision making are shown in Supplementary Material S5.
For the feasibility study we evaluated the five dimensions of the RE-AIM framework [17](see Table 1).

Table 1.
Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) Dimensions and their evaluative questions. Based on Glasgow [32] and Estabrooks & Gyurcsik [33].
Reach referred the participation rate among eligible patients, taken from the health provider medical records. Reasons for exclusion and dropout were collected in a Microsoft® Access database created ad hoc.
Effects outcomes included clinical health, health-related quality of life (HRQOL), and health services use. Clinical health outcomes (BMI, waist circumference, blood pressure, resting heart rate, and biochemical compounds from blood samples [glucose, triglycerides, glycated haemoglobin, HDL, LDL, and total cholesterol]) were collected before (M1 pre-test), during (M2 6 months), and after (M3 post-test) the intervention by nurses during their regular routine unless no data had been recorded within 6 months prior to the referral. To measure HRQOL we used the Short Form 12 Health Survey version 2 (SF-12v2) in Catalan [34], and a simple question comparing self-perceived well-being in two separate moments: ‘What do you think about your overall health, is it better, worse, or the same as the day you started the CAMINEM?’. Health services use (i.e., the number of monthly visits with practitioners) data were obtained from the health provider database, and was measured as a ratio of number of the visits to the physician or nurse per month. We focused on the effects and not efficacy/effectiveness because efficacy trials can be obtained under optimal conditions (e.g., RCT design) (Flay, 1986, as cited in Estabrooks & Gyurcsik, 2003, p. 43) and effectiveness is ”the extent to which the intended effect or benefits that could be achieved under optimal conditions are achieved in practice” [35]. Since none of the previous had been done before, we relied on the health effects.
Adoption referred as the participation rate among the total number of potential participant agents (i.e., staffed GPs and nurses).
Implementation referred to participants’ adherence, which included (i) retention days, (ii) attendance at follow-up meetings, and (iii) exercise compliance. For participants to be compliant they had to complete more than 50% of the prescribed exercise sessions. The 6-month assessment was considered a critical breakpoint since most dropouts occur within that time when starting a physical activity program [36]. Participants included longer than six months were considered as retained but not necessarily adhered to the intervention. They may attend follow-up meetings but may not exercise as intended (e.g., less frequently). Safety was measured as the number of adverse events reported by either participants or practitioners while exercising.
Maintenance data at the individual level were to be assessed 3-months post-intervention by regards of clinical, HRQOL and physical activity level. Assesments at 6- and 12-months post-intervention were set as the number of prescriptions delivered by the practitioners. Unfortunately, these assessments were not done due to a high amount of missing data that were supposed to be collected by regular practitioners.
Variables were analysed between 4 cohort groups distributed according to their basal physical activity level (AC/IN, previously active/inactive) and their adherence for a minimum of 6 months (AD/NA, adhered/non adhered). Dropout participants were evaluated as intention-to-treat. Categorical variables are presented as percentages. Pearson’s chi-squared (χ2) tests were used to determine differences between groups. Continuous variables are presented as the mean value and standard deviation (SD). Statistical differences were assessed by analyses of variance (ANOVA) by time in relation to the four groups using the Schefée test, a contrast coefficient test when comparing two subgroups out of all four, and the t-test when comparing two groups only (i.e., effects for the adhered groups only). Normally distributed variables were tested according the Shapiro–Wilk test for samples lower than 30, and the Kolmogorov–Smirnov test if higher than 30. Analyses pre- and post-intervention were done if the sample was higher than five. Continuous variables that statistically differed between groups at baseline (i.e., confounding factors) were used as covariates. ANOVA was used for variables following normal distribution. Non-parametric tests were applied for variables that were not normally distributed: the Mann–Whitney U test between groups, the Wilcoxon signed-rank test (Wilcoxon’s Z) for two measurements within groups, and the Kendall’s coefficient of concordance (Kendall’s W) for three measurements within groups. Mean differences between moments were shown as total difference and the percentage of difference. PASW statistics (release 18.0.0) was used for data treatment and for all analyses. Statistical significance was set at p < 0.05 with 95% of confidence intervals.
The Clinical Investigation Ethics Committee of the IDIAP, Jordi Gol, Barcelona, approved this study, and it followed the ethical principles of the Declaration of Helsinki. Participants gave their verbal consent for referral and signed an informed consent before inclusion. Clinical data collection was part of the ordinary healthcare practice and written permission to use these data was obtained from the healthcare provider. The exercise physiologists were members of the national exercise professional association in accordance with Catalan regulations.
3. Results
3.1. Reach
Figure 1 shows the flowchart of the 178 participants included in the study (mean age 58.1 ± 12.2, women n = 115), the number of retained participants (i.e., not dropout even without being compliant with the exercise prescriptions), participants who adhered to the program (i.e., were compliant with the prescriptions), and the reasons for dropout. Note that 19 participants were still adhering to the intervention by the time this feasibility study ended; that is, they did not complete the minimum of 6-month adherence but did not drop out either.
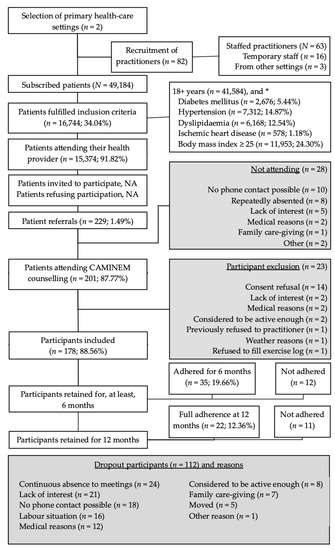
Figure 1.
Flowchart of the participants included in the CAMINEM intervention. * Patients may have more than one condition. The CAMINEM Programme addressed patients from two primary health-care settings in socially disadvantaged neighbourhoods. All practitioners (physicians and community nurses) were invited to participate. Patients to be included had to be visited by their practitioner during a regular visit, accepted their oral invitation, being referred to the exercise physiologist and finally sign the informed consent. Retention refers to not drop out while adherence shows fulfillment of the exercise prescription.
The most common reasons for referral were dyslipidaemia and diabetes mellitus in men, and musculoskeletal diseases and mental health conditions in women. Patients with high blood pressure were referred almost exclusively by nurses: 48% of patients suffering from it were referred by nurses, compared to 3% by GPs. Practitioners referred from one healthcare setting (PHC_A) mostly referred patients with one (45.5%) or two (31.2%) conditions, while practitioners from the other healthcare setting (PHC_B) referred patients with more than one condition (82.2%). The youngest age group (18 to 44 years) was mostly referred due to overweight (84%) and was diagnosed with one or two conditions only (88%). Patients from 45 to 64 years were referred due to respiratory diseases, mental ill-health, and other reasons in a greater proportion than other age groups. Almost one-third (31.1%) of older adults (65 years and above) were diagnosed with a cardiovascular condition, while the percentage in other age groups was below 10%. Participants reporting lower levels of PA were generally referred with more than one health condition, and the sufficiently active were referred with a lower number of conditions (see Table 2).

Table 2.
Participants grouping and reasons for prescription.
3.2. Effects
Complete collection of clinical parameters was obtained from 43 participants (24%), and 29 (16%) including HRQOL. Table 3 shows descriptive statistics for baseline clinical parameters, HRQOL and healthcare attendance among groups. Participant characteristics did not differ significantly when comparing two subgroups in relation to their basal PA behaviour. Although overweight participants were the majority in most groups, the AD-AC group (adhered and previously active) statistically showed a lower proportion (χ2 = 16.3, p = 0.001) of overweight participants than other groups. The most common profile for participants in all groups was a woman aged 45–64 being referred by her nurse.

Table 3.
Homogeneity for continuous variables at baseline among groups.
Due to a low sample for four measurements, basal (M1) and post-intervention intention-to-treat follow-up (MOT3) were chosen for pre-post analyses and grouping adhered participants and non-adhered participants. Thus, the adhered group (AD-Group) comprised of AD-IN and AD-AC and the non-adhered group (NA-Group) comprised of NA-AC and NA-IN. Homogeneity between these two groups was calculated by contrast coefficient tests not assuming equal variances for age (t = 1.552, p = 0.126) and assuming them for BMI (t = 0.731, p = 0.466). As a result, adhered and non-adhered groups were homogeneous at baseline for all clinical parameters, as well as for categorical variables except those diagnosed with dyslipidaemia and overweight (see Table 4).

Table 4.
Homogeneity at baseline (M1) between groups by adherence.
There were no statistical differences between the two groups on baseline data, neither for continuous variables nor for qualitative data (gender, practitioner referring, PHC centre) except those diagnosed with dyslipidaemia or overweight.
Comparative analyses within and between groups were done using ANOVA for variables following normal distribution, which resulted to be: resting heart rate, total blood cholesterol, and LDL-cholesterol. Non-parametric tests were applied for variables that were not normally distributed: body mass index, waist circumference, systolic and diastolic blood pressure, triglycerides, HDL-cholesterol, glucose, and glycated haemoglobin.
As shown in Table 5, most clinical parameters improved over time for both groups: participants who adhered and who did not adhere. Improvements in total blood cholesterol (p = 0.008, 85% CI [4.3, 26.9]) and BMI (p = 0.013) were found over time regardless of grouping. Adhered participants saw a significant decrease in their triglycerides level (28.77%, p = 0.009) when comparing means range. The NA-Group showed significant improvements in total blood cholesterol (11.71%, p = 0.001, 95% CI [9.7, 36.9]). The range mean for BMI also improved significantly for the NA-Group (1.91%, p = 0.002); although it did not for the AD-Group, most likely due to a larger sample. Differences in range mean for systolic blood pressure were also significant for the NA-Group (3.50%, p = 0.019) while it did not improve for the AD-Group.

Table 5.
Intention-to-treat multivariate analyses of variance
After the intervention all participants answered feeling equal or better than the inclusion day. None of the respondents reported feeling worse compared to their first day and 100% of previously inactive participants reported feeling better than their first day. No participants (n = 0) reported any health problem due to the exercise prescriptions.
Participant health-care attendance was similar before, during, and after the CAMINEM intervention. Globally, participants averaged 1.5 visits per month in the year before their inclusion. Participants who finally adhered averaged 1.3 visits to their health practitioner during the time the CAMINEM was delivered, less than participants who did not adhere (1.5), although these differences were not statistically significant (p > 0.05). See Figure 2.
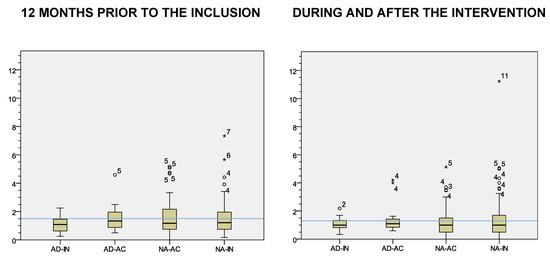
Figure 2.
Health-care provider visits per month by intervention groups. Note. Blue lines = mean. AD-IN = adhered and previously inactive, AD-AC = adhered and previously active, NA-AC = non adhered and previously active, NA-IN = non adhered and previously inactive * stands for “Outliers”.
3.3. Adoption
A total of 82 health practitioners were invited to refer patients and none of them formally refused to participate, although 49 (60%) did not refer any patients throughout the 18-month intervention. Three patients registered at other primary health-care settings prompted their GP to be referred, and were finally included. Nurses referred 186 (81%) patients and GPs referred 43 (19%). Ten practitioners referred more than ten patients while another ten referred only one. No supply physicians referred patients, whereas nurses referred patients regardless of their employment situation: eight out of twelve substitute nurses referred (66.67%) and 19 out of 34 regular nurses (55.88%) did.
3.4. Implementation
Thirty-five participants (19.66%) adhered for more than 180 days. Twelve other participants were retained for more than six months without being compliant. Almost half of non-adhered participants (42.3%) followed the exercise prescription before dropout. Table 6 shows the distribution of adherence of the four groups. Adhered participants were of similar age compared to the non-adhered, although the AD-AC group was the oldest, with a mean value over 64 years and 95% CI [61.38, 67.52]. Ninety-eight participants (56.60%) dropped out within the first three months. The main reasons for dropping out were not attending three consecutive meetings or not answering three phone calls.

Table 6.
Distribution of CAMINEM adherence by intervention groups.
4. Discussion
This study was, to our knowledge, the first pragmatic study assessing the feasibility of an exercise prescription intervention in primary health-care settings based on matching exercise training principles and public health promotion. The feasibility of pragmatic interventions intends to answer the recommended evaluative questions suggested by Estabrooks & Gyurcsik across the RE-AIM dimensions [37]. The “efficacy/effectiveness” dimension was substituted by the “effects” due to lack of a previously efficacy trial (i.e., under optimal conditions).
The overall aim of this pragmatic study was to evaluate the feasibility (i.e., external validity) of the CAMINEM (Let’s Walk) Programme as an exercise-on-prescription, interdisciplinary approach for 12 months in two socially disadvantaged neighbourhoods. The specific aims were to (i) determine if the CAMINEM reached the citizens with chronic health conditions that may benefit from exercising (Reach), (ii) assess clinical and quality-of-life outcomes and the use of health services (Effects), (iii) determine the adoption of CAMINEM by the practitioners (Adoption), (iv) evaluate participants’ retention and compliance (Implementation), and (v) describe if the practitioners prescribe exercise post-intervention (Maintenance). As stated previously, maintenance could not be finally evaluated due to a low sample for data collection.
4.1. Did the CAMINEM Programme Reach the Target Population?
The results flagged up 16,744 patients who fulfilled the inclusion criteria, of which 15,374 (91.82%) had visited their health practitioner at least once during the intervention. The proportion is slightly higher than the average of 87% of Spanish citizens who visited their practitioners in 2005 [38], and similar to 94% of citizens who lived in the same region where the study was implemented, based on self-reported data from 2018-19 [39]. Selected nurses volunteered to record the number of patients who were invited, and who did not wish to take part in the study. However, they did not report any data. They were reminded several times but eventually the research group decided not to insist due to practitioners’ time constraints. If we could analyse the number of withdrawals at this stage, then we may drive the intervention to: more stress on behaviour counselling (if high number of patients refusing) or more emphasis on recruitment (if low number of invitations). The lack of a concrete number of target participants refusing to take part in the physical activity referral intervention was also reported in the EXERT study [40]. In contrast to the EXERT, all referrals (n = 229) reached the exercise physiologist, which is the 1.49% of the eligible population (N = 15374). Of referrals, 28 (12.23%) patients did not attend the first counselling meeting and the final number of included participants was 178, 1.16% of the eligible population.
A similar proportion is reported in other studies. Of all participants referred by a health professional, 27% never made contact with the exercise referral scheme in a British study [41]. More recently, data of seven years of the Welsh referral programme showed a reach of 3.3% of the population [42], which is an average of roughly 0.7% each 18 months, the length of the CAMINEM study. In Sweden, the FaR® scheme reached 1.5% of the total population, and 1.3% of those who attended their primary health-care setting [43]. Less than 1% was reported in a revision in the UK [44]. More intensive interventions reached a higher proportion of the target population. One intervention in Switzerland aimed at increasing physical activity in inactive regular patients invited all patients attending five volunteer GP for an RCT [45]. The practitioners were reimbursed with 25 CHF (€ 18) for each questionnaire that was filled out in their office. The Newcastle exercise project reached 17% for their RCT, but the researcher was in the practice to initiate recruitment daily [46].
In sum, 1.16% of the eligible population reached the programme which is a similar proportion to other physical activity prescription intervention studies.
4.2. What were the Effects of the CAMINEM PROGRAMME?
Clinical health and quality-of-life outcomes for adhered participants followed positive patterns, although few were statistically significant. From a pragmatic point of view, note that non-adhered participants visited their practitioner more compared to adhered participants. Also, none of the participants reported any injury or complication of their disease due to the exercise participation, which let us confirm that the intervention was safe even without supervision by exercise professionals. This programme followed global and regional recommendations on health-enhancing physical activity [1,15,24,27] and its results could be useful for future interventions. A systematic review of exercise referral schemes did not find consistent evidence in favour of the interventions in outcomes based on health-related quality of life, blood pressure, serum lipid levels, indices of obesity, or glycemic control, among others [47].
Despite the aforementioned, every increase in physical activity, even small, entails health gains at both an individual level and public health level regardless of whether they reach the recommendations of physical activity levels [6,48].
4.3. Was the CAMINEM Programme Adopted by the Health Practitioners?
None of the practitioners formally refused to participate and nurses referred four times more than physicians (81% and 19% respectively). One GP and one community nurse form the primary health-care unit patients can visit. Nurses were more engaged in the intervention. However, nurses were in charge of collecting the clinical data at baseline, during and after the intervention but they did not collect as it was agreed due to time constraints. Regular referral by the 18-month timeframe of the implementation suggests that the CAMINEM procedures facilitated the Catalan practitioners to cope with the barriers for HEPA promotion [14].
4.4. Could the CAMINEM Programme Be Implemented the Way it was Designed?
Our challenge was to monitor exercise prescription adherence and collect reported data from the participants to adapt new exercise prescriptions following individualised progressions to achieve greater benefits. Adhered and non-adhered participants were completing their prescriptions, so dropouts were due to other reasons rather than challenges on understanding or following the prescriptions. Adherence to the walking intervention showed no relation to age or diagnose. Probably the type of exercise prescribed, walking, was not encouraging enough for some individuals and future interventions may include other types of physical activity (e.g., cycling, swimming, callisthenics), individually or as group-based activities (e.g., dancing, team sports, instruction-led group exercise), to improve aerobic and other physical capacities (e.g., strength, flexibility).
The CAMINEM Programme tools [22,26] were implemented and interdisciplinary work was achieved in the real setting, as previous experiences pointed out as a main challenge to cope with, such as lack of time, training and protocols [10,11,14,49]. Follow-up of the exercise prescriptions was possible using the CAMINEM Programme tools.
The CAMINEM procedures for implementation have been the framework to adapt in Catalonia the Swedish Physical Activity on Prescription model (FaR, in Swedish) under a 3rd Health Programme grant by the European Commission [50].
4.5. Study Limitations
The CAMINEM practice-driven, rather than research-driven, procedures resulted in poor data collection. In the eighteen-month intervention period, the recruitment of patients was the responsibility of health practitioners and was undertaken during their regular consultations. Adoption of the programme relied on practitioners who were motivated to participate during their regular practice, even though they did not have formal recommendations from the healthcare provider. This resulted in a relatively low number of referrals and a high proportion of missing data related to clinical health outcomes, which limited the impact of the clinical results. Perhaps tighter control on data collection and referral procedures co-ordinated by the steering group may have shown more positive outcomes. Organisational instructions by decision-makers may have encouraged motivated practitioners and served as a guarantee for some practitioners who did not refer patients or only referred a few.
Both primary health-care settings are located in socially deprived neighbourhoods and some practitioners reported that health promotion interventions are rarely adopted by some ethnic minorities. The overall intervention was strongly associated with the personal interest of all agents involved, which had to cope with the political and financial situation which led to structural changes in the public administration (both Catalan and Spanish) in general and health-care administration in particular, such as, staff layoffs or budget reduction, among other organisational issues.
4.6. Practical Implications
- This study assessed tools and procedures to encourage practitioners during their regular consultations to prescribe exercise to cope with the most prevalent chronic diseases.
- Regular practitioners had severe time constraints to strictly collect relevant health data to monitor the effects of the exercise prescriptions.
- The procedures to prescribe exercise were easy to understand and complete by the participants, easy to deliver, to monitor and to adapt by the exercise physiologists.
- The intervention framework may provide practice-based evidence to adapt exercise-on-prescriptions approaches in real settings to achieve public health goals.
5. Conclusions
The overall goal of this study was to evaluate the applicability of an exercise-on-prescription intervention in primary health-care settings: the CAMINEM Programme. CAMINEM used an interdisciplinary approach comprised of primary health-care practitioners (general practitioners and nurses) and exercise physiologists. The intervention was carried out with the underlying aim of improving regular patients’ health by facilitating the adoption and maintenance of exercise levels with safe, home-based exercises (walking). Two separate study objects provided the focus for this study. First, regular patients, with chronic health conditions that may benefit from exercise, that attended their primary health-care centre were assessed for intervention effects. On the other hand, both patients and health practitioners (i.e., general practitioners and nurses) were to be evaluated for intervention feasibility.
The CAMINEM Programme has been found to be feasible in real practice. Exercise prescriptions were for moderate-intensity aerobic activity in the form of walking outdoors following specific urban routes. It reached the target population, it was adopted by health practitioners, and it was implemented following the procedures. Health outcomes were positive although few clinical effects showed statistically significant differences. Health-related quality-of-life of adhered participants improved and they were less likely to visit their GP or nurse.
Future research-oriented initiatives may target selected health practitioners to collect all data or include an external researcher with this responsibility and use objective measures for physical activity levels, sedentary behaviour, and health-related fitness evaluation. All of the beforementioned requires specific funding and may not be feasible in real practice.
On the other hand, future practice-oriented interventions may design specific and safe procedures to prescribe, monitor, and follow-up home-based exercise to improve other fitness capacities (e.g., muscle strength, balance, flexibility) or to improve aerobic capacity by walking or other activities according to the participants’ preferences or accessibility. Selection of feasible fitness tests to apply in real practice may also be of interest.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/6/3192/s1, Figure S1: CAMINEM Referral procedure, Figure S2: CAMINEM First contact procedure, Figure S3: CAMINEM Follow-up procedure, Figure S4: CAMINEM Discharge procedure, Table S5: Criteria to determine exercise prescription progression.
Author Contributions
Conceptualization, S.M.-A., A.P.-A., X.P.-T. and G.G.-O.; Data curation, S.M.-A.; Formal analysis, S.M.-A., A.P.-A. and J.R.-G.; Funding acquisition, A.P.-A. and X.P.-T.; Investigation, S.M.-A. and J.R.-G.; Methodology, S.M.-A., X.P.-T. and G.G.-O.; Project administration, S.M.-A.; Resources, A.P.-A. and G.G.-O.; Supervision, A.P.-A. and X.P.-T.; Visualization, S.M.-A. and A.P.-A.; Writing—original draft, S.M.-A.; Writing review & editing, S.M.-A., J.R.-G. and G.G.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Secretaria General de l’Esport and the Departament d’Innovació, Universitats i Empresa, Generalitat de Catalunya with a competitive grant for junior researchers, grant number 006780 Ordre 001.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of IDIAP Jordi Gol. The Ethics Committee of IDIAP Jordi Gol institutional review board deemed this study to be a part of regular procedures in health care and waived specific statement albeit informed consent was required.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from Institut Català de la Salut and are available on request from the corresponding authors with the permission of Institut Català de la Salut.
Acknowledgments
The authors warmly thank the contributions of Carles Vallbona (†) from the Baylor College of Medicine, Houston TX, and the HEPA-Europe Networks colleagues Minna Aittasalo, Matti Leijon and Lena Kallings who made their insight into public health considerations. We also acknowledge David Carreras, Francesc Corbi and Divina Farreny for their feedback of the study design. Finally, we thank the health practitioners of the steering group Inés Cruz, Mari-Àngels Revés and Dolors Tomás.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Gakidou, E.; Lim, S.S.; Abate, D.; Abate, K.H.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Dise. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Bauman, A.E.; Reis, R.S.; Sallis, J.F.; Wells, J.C.; Loos, R.J.F.; Martin, B.W. Correlates of physical activity: Why are some people physically active and others not? Lancet 2012, 380, 258–271. [Google Scholar] [CrossRef]
- Wen, C.P.; Wai, J.P.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.; Lee, M.C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Weiler, R.; Feldschreiber, P.; Stamatakis, E. Medicolegal neglect? The case for physical activity promotion and Exercise Medicine. Br. J. Sport Med. 2012, 46, 228–232. [Google Scholar] [CrossRef]
- Aittasalo, M. Physical activity counselling in primary health care. Scand. J. Med. Sci. Sport 2008, 18, 261–262. [Google Scholar] [CrossRef]
- Glanz, K.; Rimer, B.K.; Viswanath, K. Health Behavior and Health Education. Theory, Research, and Practice, 4th ed.; Jossey-Bass: San Francisco, CA, USA, 2008. [Google Scholar]
- Martínez-Gómez, D.; León-Muñoz, L.M.; Guallar-Castillón, P.; López-García, E.; Aguilera, M.T.; Banegas, J.R.; Rodríguez-Artalejo, F. Reach and equity of primary care-based counseling to promote walking among the adult population of Spain. J. Sci. Med. Sport 2013, 16, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Laitakari, J.; Miilunpalo, S. How can physical activity be changed—Basic concepts and general principles in the promotion of health-related physical activity. Patient Educ. Couns. 1998, 33, S47–S59. [Google Scholar] [CrossRef]
- Leijon, M.E.; Bendtsen, P.; Ståhle, A.; Ekberg, K. Activating People. In Physical Activity in the General Population and Referral Schemes among Primary Health Care Patients in a Swedish County; Linköping University: Linköping, Sweden, 2009. [Google Scholar]
- Sørensen, J.B.; Skovgaard, T.; Puggaard, L. Exercise on prescription in general practice: A systematic review. Scand. J. Prim. Health Care 2006, 24, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Puig-Ribera, A.; McKenna, J.; Riddoch, C. Attitudes and practices of physicians and nurses regarding physical activity promotion in the Catalan primary health-care system. Eur. J. Public Health 2005, 15, 569–575. [Google Scholar] [CrossRef]
- WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-001512-8.
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sport. Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, B.; Shoup, J.A.; Glasgow, R.E. The RE-AIM framework: A systematic review of use over time. Am. J. Public Health 2013, 103, e38–e46. [Google Scholar] [CrossRef]
- Paez, D.C.; Reis, R.S.; Parra, D.C.; Hoehner, C.M.; Sarmiento, O.L.; Barros, M.; Brownson, R.C. Bridging the gap between research and practice: An assessment of external validity of community-based physical activity programs in Bogotá, Colombia, and Recife, Brazil. Transl. Behav. Med. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mas-Alòs, S.; Aleknavičius, K.; Avelar Rosa, B.; Banzer, W.; Borg Buontempo, M.; Carraro, N.; Clausen, M.M.; Demeyer, D.; Domnariu, C.; Farías-Torbidoni, E.I.; et al. EUPAP Feasibility Study. Final Report; Mas-Alòs, S., Ed.; Institut Nacional d’Educació Física de Catalunya, Departament de Presidència, Generalitat de Catalunya: Lleida, Catalonia, 2020; ISBN 978-84-18199-33-2. [Google Scholar]
- Kallings, L.V. Physical Activity on Prescription. In Studies on Physical Activity Level, Adherence and Cardiovascular Risk Factors; Karolinska Institutet: Stockholm, Sweden, 2008. [Google Scholar]
- Mas Alòs, S.; Galindo Ortego, G.; Planas Anzano, A.; Peirau Terés, X.; Real, J. Health promotion and exercise prescription in primary health-care settings—Rationale and design of the Let’s Walk Program (CAMINEM). Apunts. Educ. Fís. Deporte 2017, 130, 40–57. [Google Scholar] [CrossRef][Green Version]
- Mas Alòs, S. Exercise on Prescription and Exercise Planning in Primary Health-Care Settings an Approach By the ‘Let’S Walk Programme’ (Programa Caminem); University of Lleida: Lleida, Catalonia, Spain, 2012. [Google Scholar]
- Departament de Salut, Generalitat de Catalunya. Pla de Salut de Catalunya a L’horitzó 2010: Els 5 Eixos Estratègics que Articulen les Polítiques de Salut. 2a Part; Departament de Salut, Generalitat de Catalunya, Ed.; Departament de Salut: Barcelona, Spain, 2009; Volume II. [Google Scholar]
- World Health Organization Thirty-Year Retrospective of Catalan Health Planning; WHO Regional Office for Europe: Copenhagen, Denmark, 2020.
- Planas, A.; Peirau, X.; Pujol, J.; Farreny, D. Validation of Urban Itineraries for the Prescription of Physical Exercise. Apunts. Educ. Fís. Deporte 2010, 100, 12–20. [Google Scholar]
- Departament de Salut, Generalitat de Catalunya. Guia de Prescripció de L’exercici Físic per a la Salut (PEFS); Vallbona Calbó, C., Roure Cuspinera, E., Violan Fors, M., Peirau Terés, X., González Peris, M., Eds.; Direcció General de Salut Pública, Secretaria General de l’Esport: Barcelona, Spain, 2007. [Google Scholar]
- Aittasalo, M.; Miilunpalo, S.; Kukkonen-Harjula, K.; Pasanen, M. A randomized intervention of physical activity promotion and patient self-monitoring in primary health care. Prev. Med. 2006, 42, 40–46. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Thompson, W.R., Gordon, N.F., Pescatello, L.S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; ISBN1 9780781745062. ISBN2 0781745063. [Google Scholar]
- Foster, C.; Porcari, J.P.; Anderson, J.; Paulson, M.; Smaczny, D.; Webber, H.; Doberstein, S.T.; Udermann, B. The talk test as a marker of exercise training intensity. J. Cardiopulm. Rehabil. Prev. 2008, 28, 24. [Google Scholar] [CrossRef]
- Freene, N.; Waddington, G.; Chesworth, W.; Davey, R.; Cochrane, T. Validating two self-report physical activity measures in middle-aged adults completing a group exercise or home-based physical activity program. J. Sci. Med. Sport 2014, 17, 611–616. [Google Scholar] [CrossRef]
- Glasgow, R.E. Evaluation of theory-based interventions. The RE-AIM Model. In Health Behavior and Health Education: Theory, Research, and Practice; Glanz, K., Rimer, B.K., Lewis, F.M., Eds.; Jossey-Bass: San Francisco, CA, USA, 2002; pp. 530–544. ISBN 0-7879-5715-1. [Google Scholar]
- Estabrooks, P.A.; Gyurcsik, N.C. Evaluating the impact of behavioral interventions that target physical activity: Issues of generalizability and public health. Psychol. Sport Exerc. 2003, 4, 41–55. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; Lopez-Garcia, E.; Alonso, J. Interpretation of SF-36 and SF-12 questionnaires in Spain: Physi.cal and mental components. Med Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Aittasalo, M.; Miilunpalo, S.; Kannas, L.; Laitakari, J.; Bull, F.; van Poppel, M.; Bauman, A. Promoting Physical Activity of Working Aged Adults with Selected Personal Approaches in Primary Health Care. In Feasibility, Effectiveness and an Example of Nationwide Dissemination; University of Jyväskylä: Jyväskylä, Finland, 2008. [Google Scholar]
- Robison, J.I.; Rogers, M.A. Adherence to exercise programmes. Recommendations. Sport Med. 1994, 17, 39–52. [Google Scholar] [CrossRef]
- Estabrooks, P.A.; Glasgow, R.E. Translating Effective Clinic-Based Physical Activity Interventions into Practice. Am. J. Prev. Med. 2006, 31, 45–56. [Google Scholar] [CrossRef]
- Ministerio de Sanidad y Consumo Encuesta Nacional de Salud de España. Available online: http://www.ine.es (accessed on 1 February 2021).
- Departament de Salut. Generalitat de Catalunya Enquesta de Salut de Catalunya (ESCA). Available online: https://salutweb.gencat.cat/ca/el_departament/estadistiques_sanitaries/enquestes/esca/resultats_enquesta_salut_catalunya/ (accessed on 1 February 2021).
- Isaacs, A.J.; Critchley, J.A.; See Tai, S.; Buckingham, K.; Westley, D.; Harridge, S.D.R.; Smith, C.; Gottlieb, J.M. Exercise Evaluation Randomised Trial (EXERT): A randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only. Health Technol. Assess 2007, 11, 1–165. [Google Scholar] [CrossRef] [PubMed]
- Dugdill, L.; Graham, R.C.; McNair, F. Exercise referral: The public health panacea for physical activity promotion? A critical perspective of exercise referral schemes; their development and evaluation. Ergonomics 2005, 48, 1390–1410. [Google Scholar] [CrossRef]
- Morgan, K.; Rahman, M.; Moore, G. Patterning in Patient Referral to and Uptake of a National Exercise Referral Scheme (NERS) in Wales from 2008 to 2017: A Data Linkage Study. Int. J. Environ. Res. Public Health 2020, 17, 3942. [Google Scholar] [CrossRef] [PubMed]
- Leijon, M.E.; Bendtsen, P.; Nilsen, P.; Ekberg, K.; Ståhle, A. Physical activity referrals in Swedish primary health care—Prescriber and patient characteristics, reasons for prescriptions, and prescribed activities. BMC Health Serv Res 2008, 8, 201. [Google Scholar] [CrossRef]
- Fox, K.; Biddle, S.; Edmunds, L.; Bowler, I.; Killoran, A. Physical activity promotion through primary health care in England. Br. J. Gen. Pract. 1997, 47, 367–369. [Google Scholar]
- Jimmy, G.; Martin, B.W. Implementation and effectiveness of a primary care based physical activity counselling scheme. Patient Educ. Couns. 2005, 56, 323–331. [Google Scholar] [CrossRef]
- Harland, J.; White, M.; Drinkwater, C.; Chinn, D.; Farr, L.; Howel, D. The Newcastle exercise project: A randomised controlled trial of methods to promote physical activity in primary care. Br. Med. J. 1999, 319, 828–832. [Google Scholar] [CrossRef][Green Version]
- Pavey, T.G.; Taylor, A.H.; Fox, K.R.; Hillsdon, M.; Anokye, N.; Campbell, J.L.; Foster, C.; Green, C.; Moxham, T.; Mutrie, N.; et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: Systematic review and meta-analysis. BMJ 2011, 343, 6462. [Google Scholar] [CrossRef] [PubMed]
- Kallings, L.V.; Leijon, M.E.; Kowalski, J.; Hellénius, M.-L.; Ståhle, A. Self-reported adherence: A method for evaluating prescribed physical activity in primary health care patients. J. Phys. Act. Health 2009, 6, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Petrella, R.J.; Lattanzio, C.N.; Overend, T.J. Physical Activity Counseling and Prescription Among Canadian Primary Care Physicians. Arch. Intern. Med. 2007, 167, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- EUPAP Consortium EUPAP—A European Physical Activity on Prescription Model. Available online: www.eupap.org (accessed on 1 February 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).