Abstract
(1) Background: Sleep apnea may be a risk factor for deep neck infection (DNI). The objective of this study was to investigate the effects of sleep apnea on DNI. (2) Methods: In this first nationwide retrospective cohort study on the sleep apnea–DNI correlation, we obtained data from the Longitudinal Health Insurance Database 2005, a subset of the Taiwan National Health Insurance Research Database. Patients who were newly diagnosed with sleep apnea between 1997 and 2012 were identified, and patients without sleep apnea were matched at a 1:4 ratio in age, sex, socioeconomic status, and urbanization level. The primary outcome of this study was DNI occurrence. The treatment modalities for sleep apnea and the comorbidities that occurred during the study period were also analyzed. (3) Results: Our sleep apnea and comparison (non-sleep apnea) cohorts comprised 6114 and 24,456 patients, respectively. We compared the cumulative incidence of DNI between these cohorts and found a greater incidence of DNI in the sleep apnea cohort (p < 0.001). A strong sleep apnea–DNI association was found following analysis via the adjusted Cox proportional-hazards model (full model hazard ratio, 1.71; 95% confidence interval, 1.28–2.28; p < 0.001). In the subgroup analysis, sleep apnea increased DNI risk in men, in those aged < 50 years, and in those without diabetes mellitus, end-stage renal disease, liver cirrhosis, autoimmune disease, obesity, tonsillectomy, or adenotonsillectomy. (4) Conclusions: Our results confirmed sleep apnea to be an independent risk factor for DNI. Physicians should be aware of the potential occurrence of DNI in patients with sleep apnea.
1. Introduction
Deep neck infection (DNI) can be detrimental to one’s health and fatal in some cases. The most common causes of DNI include dental infections, upper respiratory tract infections, esophageal foreign body damage, and other factors causing bacterial infection and invasion of the membrane space of the deep neck muscles resulting in inflammation, swelling, and neck abscess. DNIs are typically characterized by a progressive sore throat, dysphagia, trismus, fever, and chills. In severe cases, thick fluid and swollen tissues may compress the airway, thereby suffocating the patient and even causing death. DNIs may also invade adjacent organs and structures, such as the brain, spine, and mediastinum, or spread throughout the body to cause sepsis. According to the literature, once DNI has spread to the mediastinum in a patient with diabetes or an immune-related disease, the mortality rate may be as high as 50% [1,2,3,4,5]. Compared with non-immunocompromised patients, immunocompromised patients, including those with decompensated liver cirrhosis (LC), systemic lupus erythematous, and diabetes mellitus (DM), have been reported to be more likely to develop DNI without the typical symptoms, which may lead to more severe complications and a higher likelihood of mortality [1,3,4,5].
Scholars have discovered strong associations between sleep apnea and cardiovascular disease, such as arrhythmia and stroke, as well as a higher likelihood of stroke- and heart-failure-induced mortality [6,7,8,9,10,11,12]. As a chronic low-grade immunoinflammatory disease [13,14], sleep apnea can increase a patient’s risk of oral infection and periodontal disease [15,16,17]. Given the strong correlation between oral infection and DNI, we believe that the development rate of DNI may be higher in patients with sleep apnea than in those without sleep apnea. A few case reports on this issue have been published, but the exact relationship between OSA and DNI remains unknown. Thus, the objective of the present study is to investigate the effect of sleep apnea on DNI.
2. Methods
2.1. Data Origin
The National Health Insurance (NHI) Research Database (NHIRD) was established by the Taiwan government and provides electronically generated medical claims data for all NHI enrollees, including drug prescriptions, inpatient and outpatient disease diagnoses, procedures and examinations, surgical information, payment information, income levels, and residential location [1]. In 2017, the NHIRD included information of 99.6% of Taiwan’s population [18]. In the NHIRD, diagnostic coding is based on codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [2]. All of the insured individuals’ information was deidentified prior to the release of the data to the researchers to ensure no violation of the patients’ rights and welfare [2,18].
The Longitudinal Health Insurance Database 2005 (LHID2005), a sub-dataset of the NHIRD, contains the data of 1 million Taiwanese NHI-insured individuals selected randomly from the NHIRD in 2005. The National Health Research Institutes reported no significant differences in sex, age distribution, or healthcare cost between the data of LHID2005 and those of all NHI-insured individuals in Taiwan [18]. Several validation studies based on this database have been performed [19,20,21,22], and many scholars conducting population-based studies have employed the LHID2005 in their work [23].
2.2. Study Cohort
We extracted from the LHID2005 a cohort comprising patients aged ≥18 years who received a new sleep apnea diagnosis (ICD-9-CM: 780.51, 780.53, 780.57, 327.20, 327.21, 327.23, 327.26, 327.27, and 327.29; Figure 1) between 1 January 2000 and 31 December 2012 [24]. Because the polysomnography report was not available in the database, we could not distinguish between obstructive, central, and mixed sleep apnea. Therefore, this study considered the diagnosis of all types of sleep apnea [25]. The date of the initial sleep apnea diagnosis was defined as the enrollment date [24]. Individuals who received a sleep apnea diagnosis after 2012 were not included in the analysis to ensure that patients were followed up for at least 1 year. Patients who developed DNI within 1 month of their sleep apnea diagnosis were also excluded from the analysis. A patient was included in the sleep apnea cohort only if their ICD-9-CM codes were recorded in three or more outpatient claims or in an inpatient setting. The diagnosis of sleep apnea was confirmed using strict criteria.
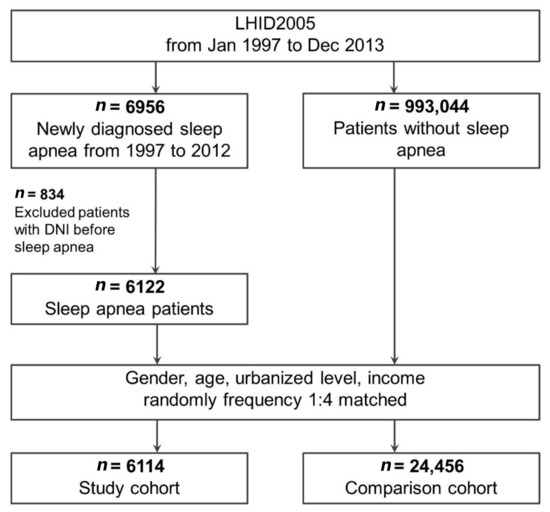
Figure 1.
Flow of study patient identification and enrollment. Abbreviations: DNI, deep neck infection; LHID2005, Longitudinal Health Insurance Database 2005.
2.3. Comparison Cohort
Another group of patients without sleep apnea were randomly selected from the LHID2005 and categorized into the comparison cohort.
2.4. Cohort Matching
A 1:4 ratio was established between the sleep apnea and comparison cohorts. Patients were matched in sex, age, and urbanization and income levels. Patients in the comparison cohort were assigned an index date that matched those in the sleep apnea cohort.
2.5. DNI Incidence (Primary Outcome)
Our primary outcome was DNI incidence, which was defined as hospitalization with one of the following diagnoses: Peritonsillar abscess, parapharyngeal abscess, retropharyngeal abscess, cellulitis and abscess of oral soft tissues (Ludwig’s angina), and cellulitis and abscess of the neck (ICD-9-CM 475, 478.22, 478.24, 528.3, and 682.1) [3]. Patients in both cohorts were followed-up until DNI occurrence, mortality, or the end of 2013.
2.6. Treatment Modalities for Sleep Apnea
Treatments for sleep apnea, including continuous positive airway pressure (CPAP; based on CPAP titration codes in the claims data) and surgical procedures (i.e., uvulopalatopharyngoplasty, submucosal turbinoplasty, septoplasty, septomeatoplasty, and partial glossectomy), were also analyzed.
2.7. Comorbidities
We identified the following comorbidities from the claims data by using ICD-9-CM codes: End-stage renal disease (ESRD; ICD-9-CM 585.6, 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93, and V45.1), diabetes mellitus (DM; ICD-9-CM 250, 357.2, 362.0x, and 366.41), liver cirrhosis (LC; ICD-9-CM 571.2 and 571.5–571.6), autoimmune disease (ICD-9-CM 710.0, 710.1, 710.3, 710.4, 710.2, 714.30–714.33, 446.0, 446.2, 446.4, 446.5, 446.7, 443.1, 136.1, 694.4, 555, 556.0–556.6, and 556.8–556.9), and obesity (ICD-9-CM 278); tonsillectomy or adenotonsillectomy were also identified [26,27,28,29]. We included one of these comorbidities only if its corresponding code was present in at least three outpatient diagnoses or one inpatient diagnosis. Comorbidities that occurred during the study period were included, and the earliest day the diagnosis code appeared in the claims data was regarded as the diagnosis date.
2.8. Statistical Analysis
Unpaired Student’s t- and Pearson’s chi-squared tests were used for between-group comparisons of continuous and categorical variables, respectively. We used the Kaplan–Meier method to determine the cohorts’ cumulative DNI incidence and identified differences by using the (two-tailed) log-rank test. When comparing the cohorts, we estimated hazard ratios (HRs) for DNI through multivariable Cox proportional-hazards regression. The stability of the hazard ratio (HR) was tested through sensitivity and subgroup analyses to assess whether comorbid diseases exert a significant coactive effect with sleep apnea on DNI development. SAS (version 9.4) from the SAS Institute (Cary, NC, USA) was employed for all analyses, and statistical significance was defined using a p of < 0.05.
3. Results
This study enrolled 6114 and 24,456 patients to the sleep apnea and comparison cohorts, respectively. After matching for sex, age, and urbanization and income levels, the sleep apnea group showed higher rates of DM, ESRD, autoimmune disease, obesity, and tonsillectomy/adenotonsillectomy than the comparison group (Table 1). The DNI incidence rate in the sleep apnea group was 2.05 per 100,000 person-years (mean duration of follow-up: 5.7 ± 3.3 years), while that in the comparison group was 1.18 per 100,000 person-years (mean duration of follow-up: 5.8 ± 3.3 years). The DNI incidence rate ratio (95% confidence interval (CI)) was 1.74 (1.32–2.29). Thus, DNI is significantly more common in the sleep apnea cohort than in the comparison cohort (p < 0.001).

Table 1.
Baseline characteristics of our cohorts.
Cumulative DNI incidence was investigated using Kaplan–Meier analysis. The results in Figure 2 reveal a significantly higher cumulative DNI incidence rate in the sleep apnea group than in the comparison group (p < 0.001). According to the Cox proportional-hazards model (Table 2), the DNI risk of patients with sleep apnea was 1.71 times higher than that of patients without sleep apnea (adjusted HR [95% CI]: 1.71 [1.28–2.28], p < 0.001). The main Cox model was adjusted for age, sex, and urbanization and income levels, and the full Cox model was adjusted for age, sex, urbanization and income levels, DM, ESRD, LC, autoimmune disease, obesity, and tonsillectomy/adenotonsillectomy. Sensitivity analysis reflected a stable effect between DNI and sleep apnea. Subgroup analyses revealed that patients with sleep apnea and of male sex, younger than 50 years, and without DM, ESRD, LC, autoimmune disease, obesity, or tonsillectomy/adenotonsillectomy are at great risk of developing DNI (adjusted HR [95% CI] for the male subgroup: 1.85 [1.33–2.57]; for age <50 years: 2.06 [1.42–2.99]; for the non-DM group: 1.77 [1.29–2.44]; for the non-ESRD group: 1.69 [1.27–2.25]; for the non-LC group: 1.73 [1.31–2.30]; for the non-autoimmune disease group: 1.87 [1.41–2.47]; for the non-obesity group: 1.78 [1.34–2.36]; and for the non-tonsillectomy/adenotonsillectomy group: 1.74 [1.31–2.31], all p ≤ 0.001). Apnea patients of female sex with age greater than 50 years and DM, ESRD, LC, or obesity showed increased DNI risk, although the difference noted was non-significant. In the autoimmune disease and tonsillectomy/adenotonsillectomy subgroups, sleep apnea may even decrease DNI risk, although the difference observed was also non-significant.
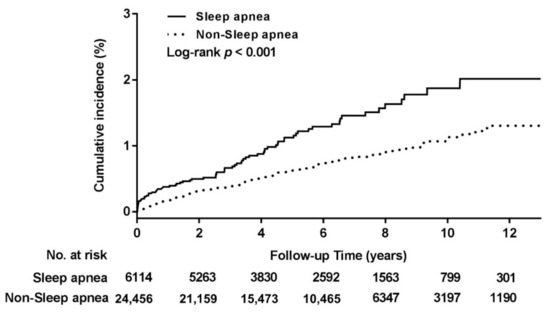
Figure 2.
Cumulative incidence of deep neck infection in the study cohorts.

Table 2.
Multivariable Cox proportional hazard model of sleep apnea and DNI risk.
Finally, patients with sleep apnea with and without treatment showed significantly higher DNI incidence than non-sleep apnea patients (Table 3). The sensitivity test adjusted for DM, ESRD, LC, autoimmune disease, tonsillectomy/adenotonsillectomy, and obesity also showed a significant association between DNI and sleep apnea patients with and without treatment.

Table 3.
Multivariable Cox proportional hazard model for DNI risk in sleep apnea patients with and without treatment.
4. Discussion
This work is the first nationwide study employing a real-world database to investigate the influence of sleep apnea on DNI. We discovered that sleep apnea represents a definite DNI risk factor; specifically, patients with sleep apnea have a 1.71-fold higher risk of developing DNI than patients without sleep apnea. According to our subgroup analysis, sleep apnea increases DNI risk in males, those aged younger than 50 years, and those without DM, ESRD, LC, autoimmune disease, obesity, or tonsillectomy/adenotonsillectomy. Sleep apnea in females, those older than 50 years, and those with comorbidities of DM, ESRD, LC, and obesity was also associated with higher DNI risk, although the results were generally without significance. Interestingly, a non-significant protective trend against DNI was discovered in the autoimmune disease and tonsillectomy/adenotonsillectomy subgroups; we attribute this finding to the limited number of patients on these subgroups. Autoimmune disease affected only 2.6% and 4.8% of the comparison and study cohorts, respectively, and the limited availability of samples may have led to estimation bias with a wider CI. The results of this study showed that sleep apnea, with or without treatment, results in a higher risk of DNI. This finding is likely due to the greater severity of sleep apnea in patients receiving treatment compared with that in patients not receiving such treatment. Unfortunately, because the severity of sleep apnea could not be determined from the database used in this study, further research on this matter remains necessary. DNI was strongly associated with odontogenic infections in one study [30]. Marioni et al. showed that DNI of dental origin most often affects the submandibular space (73/85 patients, 85.9%). Sleep apnea has been strongly associated with oral infections, such as periodontal disease [15,31]. Periodontitis is associated with mild sleep apnea in male patients and severe sleep apnea in female patients [31]. These findings support our detection of a high DNI incidence rate in patients with sleep apnea.
Researchers are increasingly recognizing sleep apnea as an immunocompromising disease that can lead to more severe infections, including pneumonia and upper airway infection [16,17,32]. Mok et al. discovered that patients with untreated sleep apnea who then develop influenza infection have longer hospital stays than those with treated sleep apnea [33]. Patients with sleep apnea have been found to have higher CD8+ T lymphocyte levels, CD4+/CD8+ ratios, and humoral immune-related indices, such as interleukins 4, 6, and 10 and interferon-γ. Scholars have shown that patients with sleep apnea have poor immune functions and systemic inflammatory status [13,14]. We believe that changes in the oral environment caused by sleep apnea, in combination with a decrease in immunity, could explain the increased incidence of DNI noted in this study.
Our study encourages doctors to consider DNI when patients with sleep apnea report a sore throat, trismus, or swelling of the neck. Proper treatment of sleep apnea may improve the patient’s oral environment and general immunity, thereby reducing the risk of DNI and its associated infections. However, the present findings cannot establish the exact mechanism between sleep apnea and DNI. Additional research should be conducted to confirm whether sleep apnea treatment can actually reduce DNI risk.
The present study includes several advantages, such as a large sample size representing a national population of 6114 patients with sleep apnea and an extensive follow-up period, but also has certain limitations that may affect the generalizability of the findings. First, neither physical examination results nor clinical symptoms were available for analysis. Second, we had no access to medical records, imaging data, or laboratory findings when attempting to understand the relation between DNI and sleep apnea severity. Finally, because no bacterial culture reports were available, we were unable to investigate the drug sensitivity and bacterial spectrum of patients with DNI. The influences of these factors on sleep apnea and DNI must be investigated in future work.
5. Conclusions
In this nationwide study, we used data from a real-world database to investigate for the first time whether sleep apnea is associated with higher DNI risk. Our findings confirmed that sleep apnea independently increases DNI risk. Moreover, patients with treated or untreated sleep apnea are at higher risk of developing DNI than those without sleep apnea. Physicians should be aware of the potential occurrence of DNI in patients with sleep apnea accompanied by neck swelling, dysphagia, breathing difficulties, and fever.
Author Contributions
Conceptualization, C.-M.H., H.-Y.L. and M.-S.T.; Data curation, M.-C.D., G.-H.C. and Y.-H.Y.; Formal analysis, M.-C.D. and C.-Y.L.; Funding acquisition, G.-H.C. and Y.-H.Y.; Investigation, Y.-C.L. and M.-S.T.; Methodology, Y.-C.L., L.-A.L. and P.-R.Y.; Project administration, C.-M.H. and M.-S.T.; Software, Y.-T.T.; Supervision, S.Y.-C.L., M.-S.T. and H.-Y.L.; Writing—original draft, M.-C.D., Y.-T.T. and L.-A.L.; Writing—review & editing, S.Y.-C.L., H.-Y.L. and M.-S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by from the Ministry of Science and Technology, Taiwan, grant number MOST 109-2314-B-182A-054-, and by Chang Gung Memorial Hospital, Taoyuan, Taiwan, grant numbers CMRPG6K0301 and CMRPG6J0331.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital (IRB number 201801126B1).
Informed Consent Statement
This study was granted exemption from the need for obtaining informed consent from participants because the data were de-identified.
Data Availability Statement
The datasets analyzed in the current study are available in the Taiwan National Health Insurance Research Database repository.
Acknowledgments
We thank the Health Information and Epidemiology Laboratory (CLRPG6G0041) at Chiayi Chang Gung Memorial Hospital for comments and assistance in data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CI | confidence interval |
| DM | diabetes mellitus |
| DNI | deep neck infection |
| ESRD | end-stage renal disease |
| HR | hazard ratio |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| LC | liver cirrhosis |
| LHID2005 | Longitudinal Health Insurance Database 2005 |
| NHI | National Health Insurance |
| NHIRD | National Health Insurance Research Database |
| NTD | New Taiwan Dollar |
References
- Chang, G.H.; Ding, M.C.; Chen, Y.C.; Yang, Y.H.; Liu, C.Y.; Chang, P.J.; Lee, C.P.; Lin, M.H.; Hsu, C.M.; Wu, C.Y. Real-world evidence for increased deep neck infection risk in patients with rheumatoid arthritis. Laryngoscope 2019, 130, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Su, Y.C.; Lin, K.M.; Liu, C.Y.; Yang, Y.H.; Chang, P.J.; Lin, M.H.; Lee, C.P.; Hsu, C.M.; Tsai, Y.T. Deep neck infection in Systemic Lupus erythematosus patients: Real-World evidence. Sci. Rep. 2020, 10, 4133. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Chang, G.H.; Chen, W.M.; Liu, C.Y.; Lin, M.H.; Chang, P.J.; Huang, T.Y.; Tsai, Y.T.; Wu, C.Y.; Hsu, C.M.; et al. The Association between Decompensated Liver Cirrhosis and Deep Neck Infection: Real-World Evidence. Int. J. Environ. Res. Public Health 2019, 16, 3863. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Liu, T.C.; Chen, P.R.; Tseng, F.Y.; Yeh, T.H.; Chen, Y.S. Deep neck infection: Analysis of 185 cases. Head Neck J. Sci. Spec. Head Neck 2004, 26, 854–860. [Google Scholar] [CrossRef]
- Sakarya, E.U.; Kulduk, E.; Gundogan, O.; Soy, F.K.; Dundar, R.; Kilavuz, A.E.; Ozbay, C.; Eren, E.; Imre, A. Clinical features of deep neck infection: Analysis of 77 patients. Kulak Burun Bogaz Ihtis. Derg. 2015, 25, 102–108. [Google Scholar] [CrossRef]
- Sowerby, L.J.; Rotenberg, B.; Brine, M.; George, C.F.; Parnes, L.S. Sleep apnea, daytime somnolence, and idiopathic dizziness—A novel association. Laryngoscope 2010, 120, 1274–1278. [Google Scholar] [CrossRef]
- Sorajja, D.; Gami, A.S.; Somers, V.K.; Behrenbeck, T.R.; Garcia-Touchard, A.; Lopez-Jimenez, F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. CHEST J. 2008, 133, 927–933. [Google Scholar] [CrossRef]
- Mehra, R.; Stone, K.L.; Varosy, P.D.; Hoffman, A.R.; Marcus, G.M.; Blackwell, T.; Ibrahim, O.A.; Salem, R.; Redline, S. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: Outcomes of sleep disorders in older men (MrOS sleep) study. Arch. Intern. Med. 2009, 169, 1147–1155. [Google Scholar] [CrossRef]
- Wang, H.; Parker, J.D.; Newton, G.E.; Floras, J.S.; Mak, S.; Chiu, K.L.; Ruttanaumpawan, P.; Tomlinson, G.; Bradley, T.D. Influence of obstructive sleep apnea on mortality in patients with heart failure. J. Am. Coll. Cardiol. 2007, 49, 1625–1631. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Valham, F.; Mooe, T.; Rabben, T.; Stenlund, H.; Wiklund, U.; Franklin, K.A. Increased risk of stroke in patients with coronary artery disease and sleep apnea: A 10-year follow-up. Circulation 2008, 118, 955–960. [Google Scholar] [CrossRef]
- Calvin, A.D.; Somers, V.K. Obstructive sleep apnea and risk of stroke: Time for a trial. Nat. Rev. Cardiol. 2009, 6, 90–91. [Google Scholar] [CrossRef]
- Arnaud, C.; Dematteis, M.; Pepin, J.L.; Baguet, J.P.; Lévy, P. Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin. Immunopathol. 2009, 31, 113–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C. Immune status of children with obstructive sleep apnea/hypopnea syndrome. Pak. J. Med. Sci. 2017, 33, 195–199. [Google Scholar] [CrossRef]
- Latorre, C.; Escobar, F.; Velosa, J.; Rubiano, D.; Hidalgo-Martinez, P.; Otero, L. Association between obstructive sleep apnea and comorbidities with periodontal disease in adults. J. Indian Soc. Periodontol. 2018, 22, 215–220. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; González, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef]
- NHRI. Sampling Method and Representativeness of Longitudinal Health Insurance Database (LHID). Available online: http://nhird.nhri.org.tw/en/Data_Subsets.html (accessed on 1 October 2017).
- Cheng, C.L.; Chien, H.C.; Lee, C.H.; Lin, S.J.; Yang, Y.H. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int. J. Cardiol. 2015, 201, 96–101. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chen, C.H.; Li, C.Y.; Lai, M.L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J. Formos. Med. Assoc. 2015, 114, 254–259. [Google Scholar] [CrossRef]
- Wu, C.S.; Lai, M.S.; Gau, S.S.; Wang, S.C.; Tsai, H.J. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS ONE 2014, 9, e112257. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, P.C.; Hsu, C.W.; Chang, S.L.; Lee, C.C. Validity of the age-adjusted charlson comorbidity index on clinical outcomes for patients with nasopharyngeal cancer post radiation treatment: A 5-year nationwide cohort study. PLoS ONE 2015, 10, e0117323. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Yang, Y.H.; Liu, C.Y.; Lin, M.H.; Chang, G.H.; Tsai, Y.T.; Li, H.Y.; Tsai, Y.H.; Hsu, C.M. Unilateral Vocal Fold Paralysis and Risk of Pneumonia: A Nationwide Population-Based Cohort Study. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. 2018, 158, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Li, H.Y.; Huang, C.G.; Wang, R.Y.; Chuang, L.P.; Chen, N.H.; Liu, C.H.; Yang, Y.H.; Liu, C.Y.; Hsu, C.M. Risk of alzheimer’s disease in obstructive sleep apnea patients with or without treatment: Real-world evidence. Laryngoscope 2020, 130, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lee, L.A.; Tsai, Y.T.; Yang, Y.H.; Liu, C.Y.; Lin, M.H.; Hsu, C.M.; Chen, C.K.; Li, H.Y. Sleep apnea and risk of vertigo: A nationwide population-based cohort study. Laryngoscope 2018, 128, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Lin, C.L.; Peng, C.L.; Chen, Y.F.; Lu, C.C.; Sung, F.C.; Kao, C.H. Rheumatoid arthritis and risk of acute myocardial infarction—A nationwide retrospective cohort study. Int. J. Cardiol. 2013, 168, 4750–4754. [Google Scholar] [CrossRef]
- Camacho, M.; Li, D.; Kawai, M.; Zaghi, S.; Teixeira, J.; Senchak, A.J.; Brietzke, S.E.; Frasier, S.; Certal, V. Tonsillectomy for adult obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2016, 126, 2176–2186. [Google Scholar] [CrossRef]
- Todd, C.A.; Bareiss, A.K.; McCoul, E.D.; Rodriguez, K.H. Adenotonsillectomy for obstructive sleep apnea and quality of life: Systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2017, 157, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Suclla-Velásquez, J.A.; Smedts, C. Obesity: A Risk Factor for Infection after Surgery. In Weight Management; IntechOpen: London, UK, 2020. [Google Scholar]
- Marioni, G.; Rinaldi, R.; Staffieri, C.; Marchese-Ragona, R.; Saia, G.; Stramare, R.; Bertolin, A.; Dal Borgo, R.; Ragno, F.; Staffieri, A. Deep neck infection with dental origin: Analysis of 85 consecutive cases (2000–2006). Acta Oto-Laryngol. 2008, 128, 201–206. [Google Scholar] [CrossRef]
- Tremblay, C.; Beaudry, P.; Bissonnette, C.; Gauthier, C.A.; Girard, S.; Milot, M.P.; Durand, R.; Huynh, N. Periodontitis and Obstructive Sleep Apnea: A Literature Review. J. Dent. Sleep Med. 2018, 4, 103–110. [Google Scholar]
- Chiner, E.; Llombart, M.; Valls, J.; Pastor, E.; Sancho-Chust, J.N.; Andreu, A.L.; Sánchez-de-la-Torre, M.; Barbé, F. Association between Obstructive Sleep Apnea and Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0152749. [Google Scholar] [CrossRef]
- Mok, E.M.; Greenough, G.; Pollack, C.C. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2020, 16, 2003–2007. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).