Antibacterial Polysaccharides in Dental Implantology
Abstract
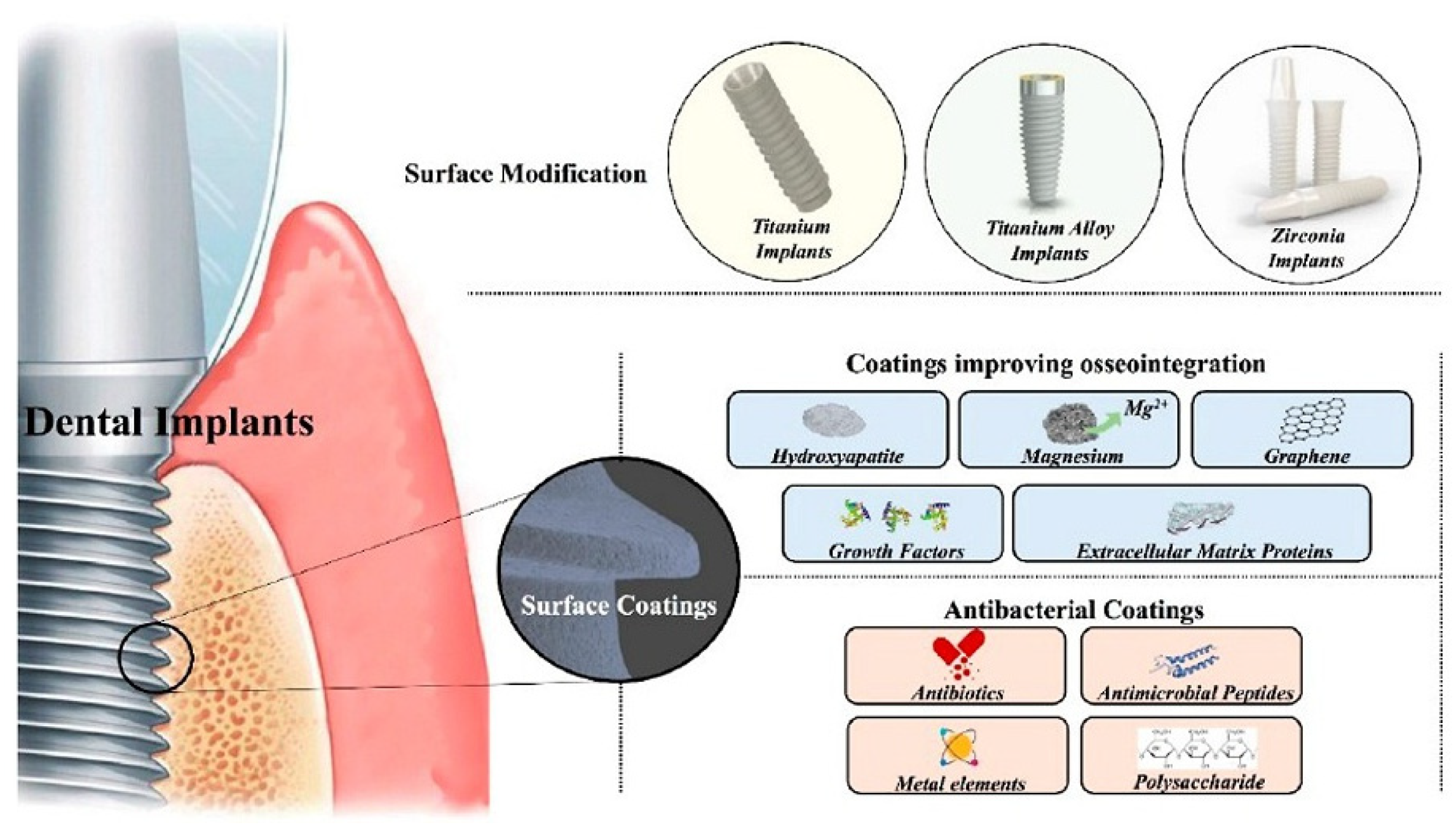
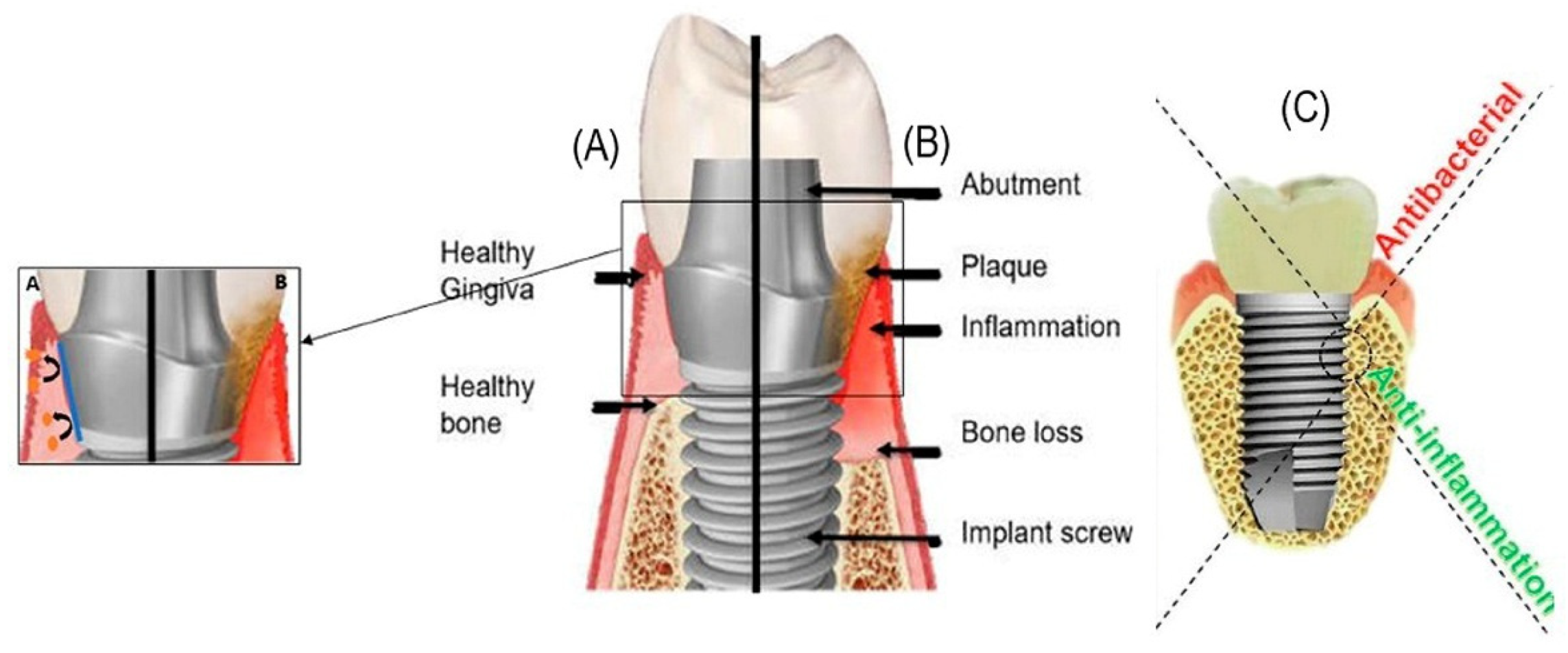
1. Introduction
2. Methods
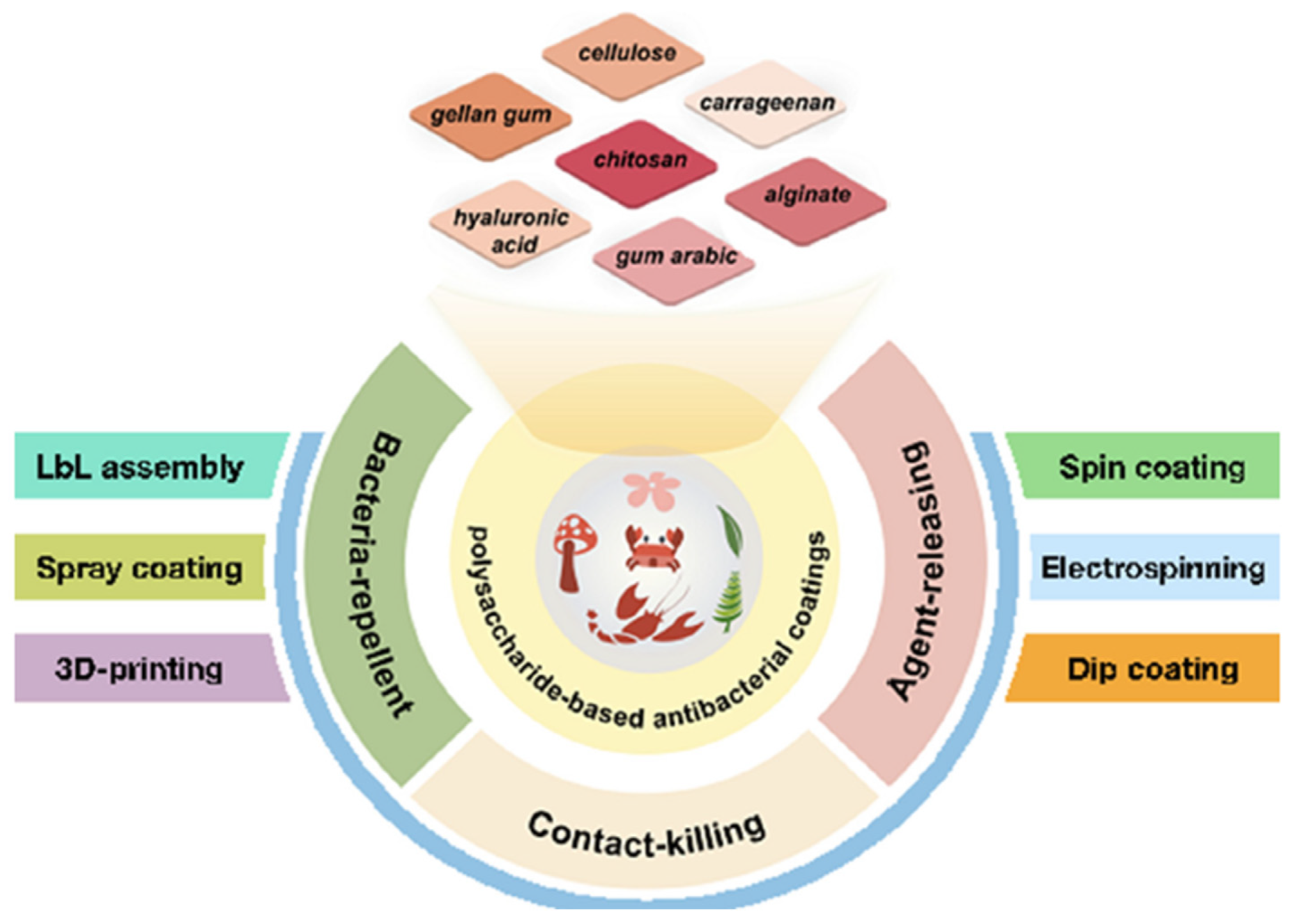
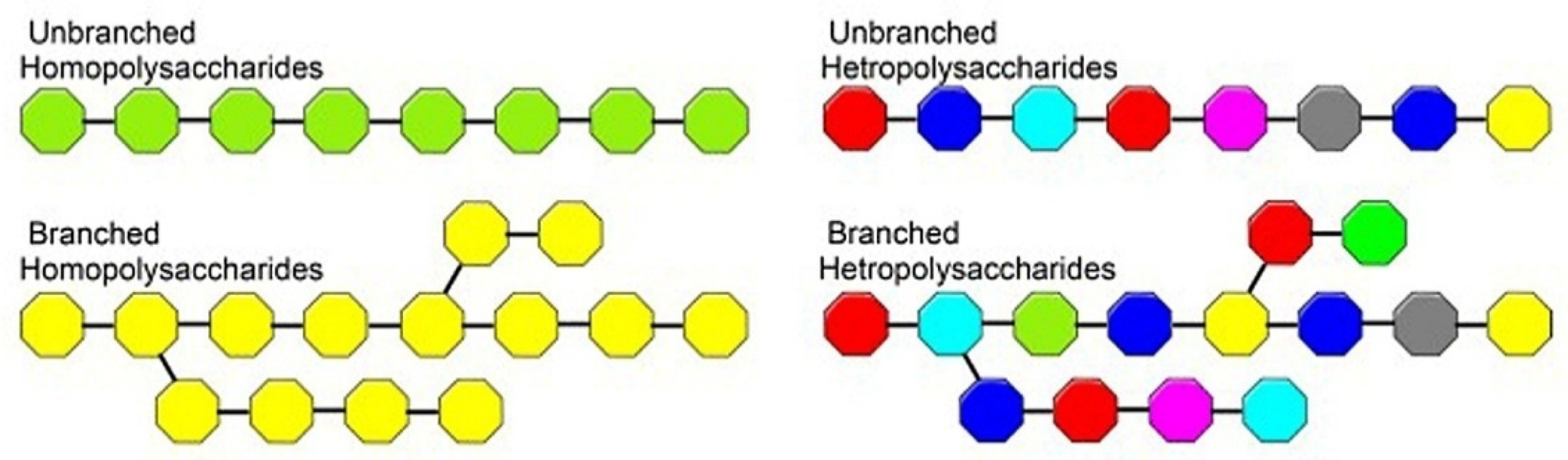
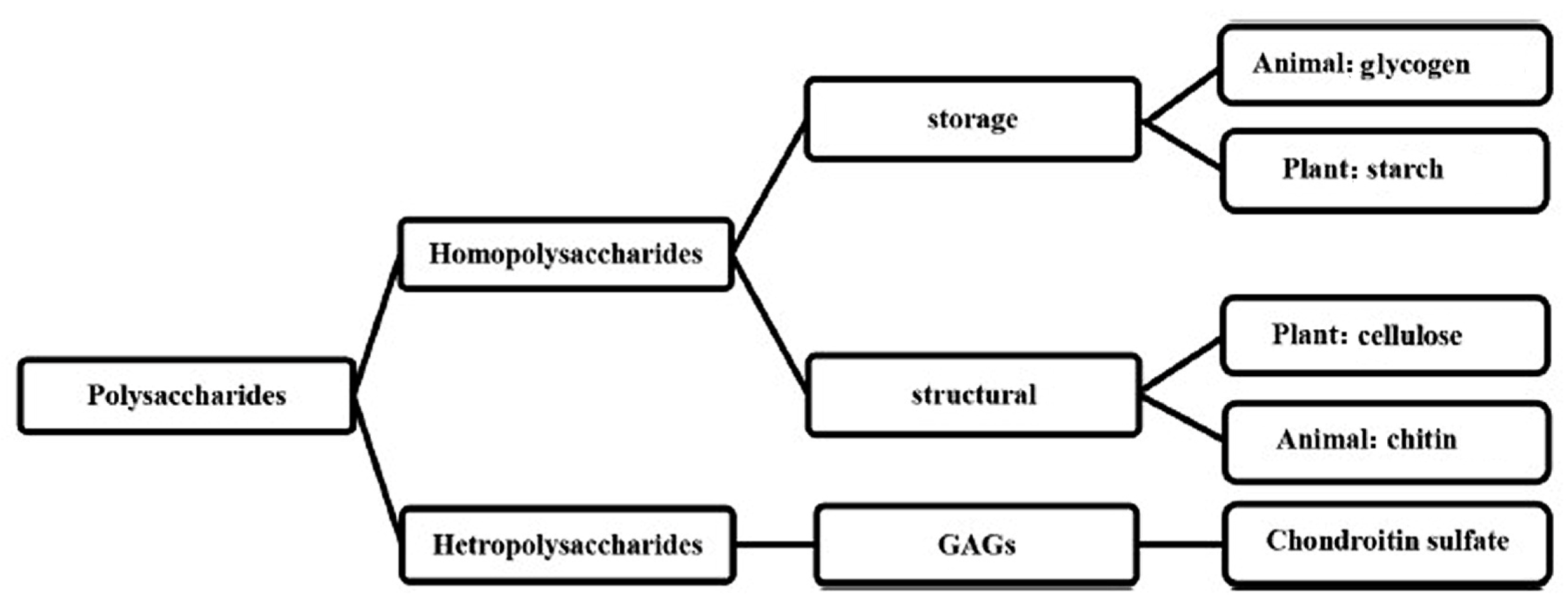
3. Polysaccharides
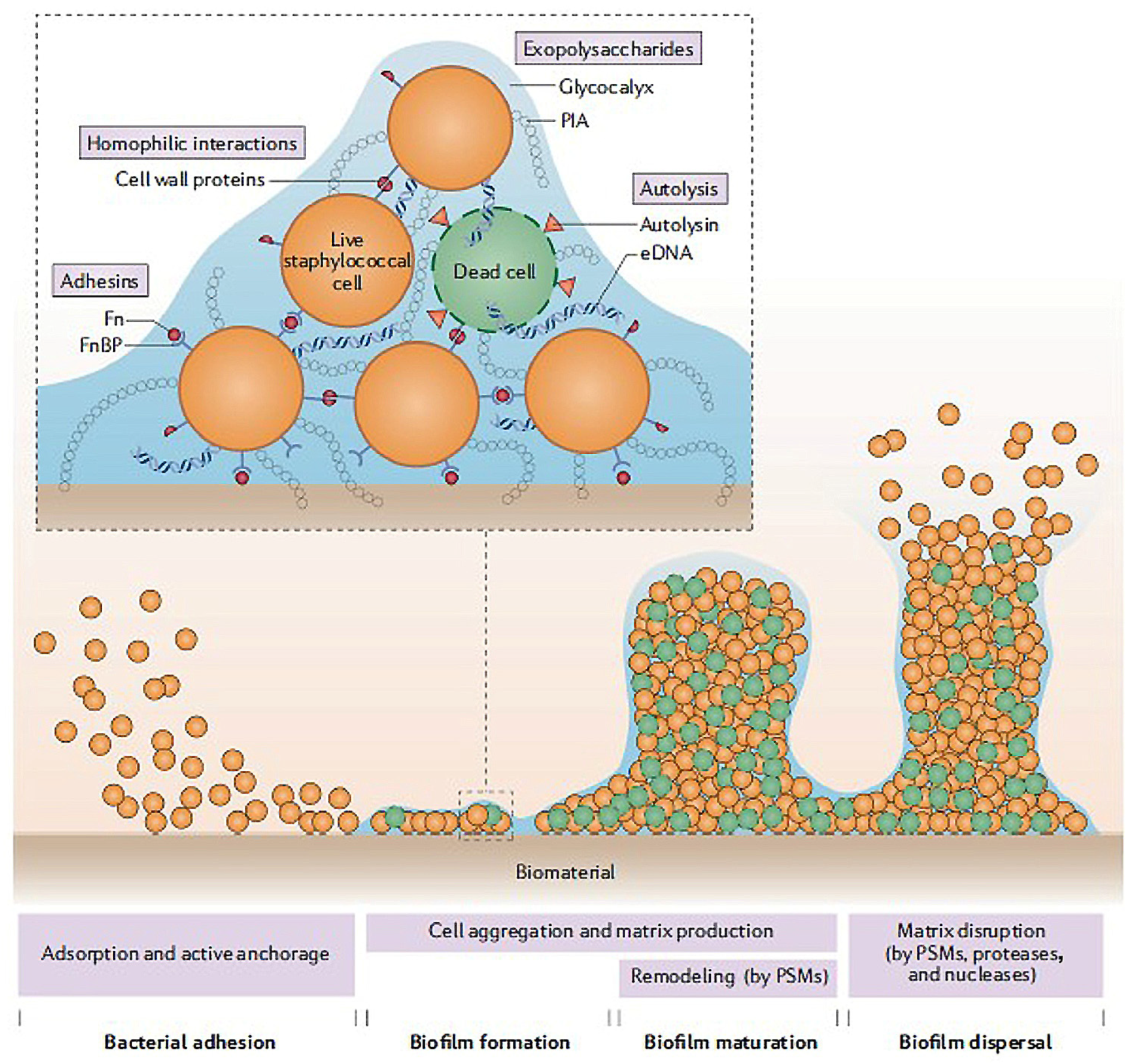
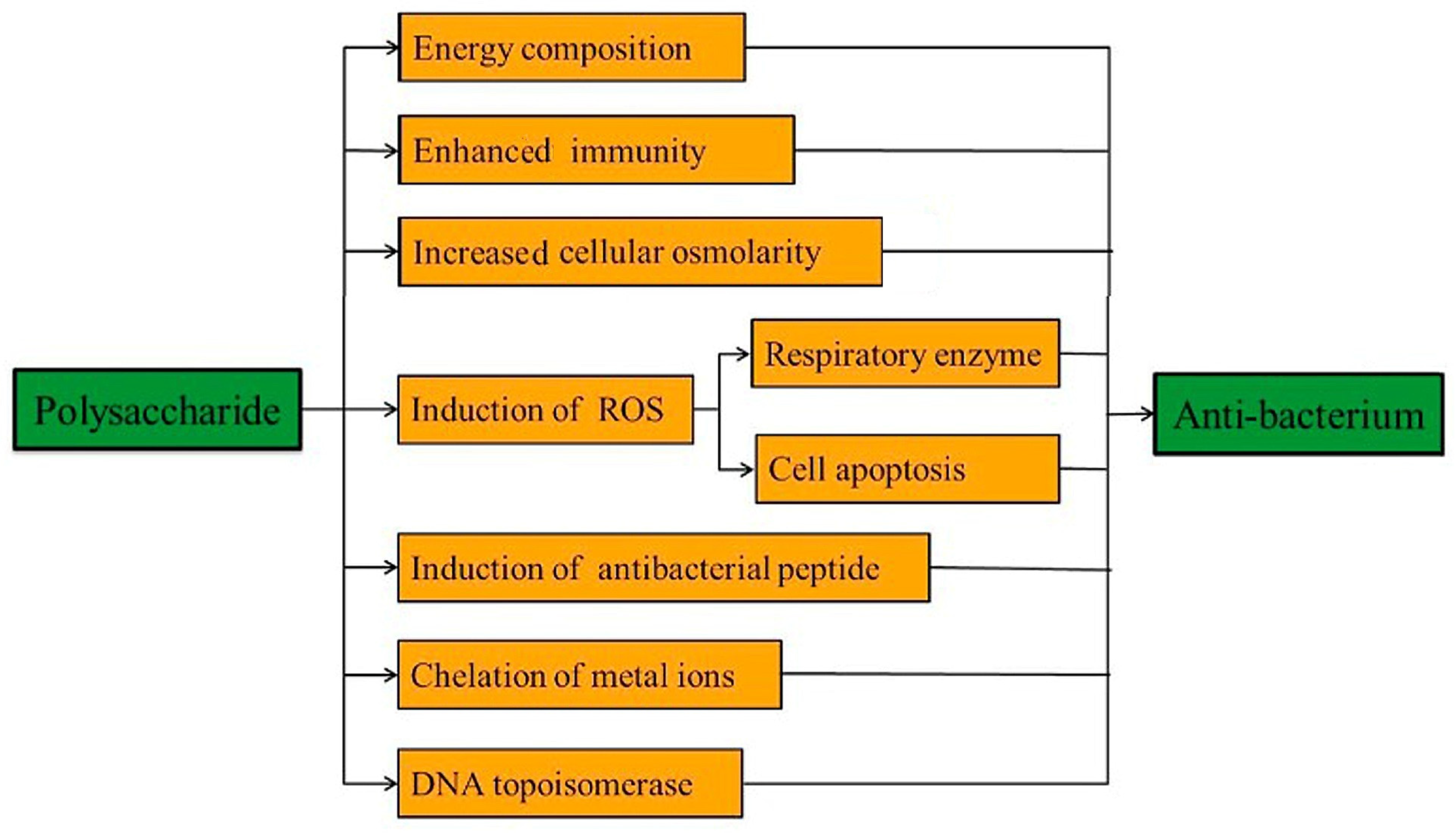
3.1. Antibacterial Polysaccharides
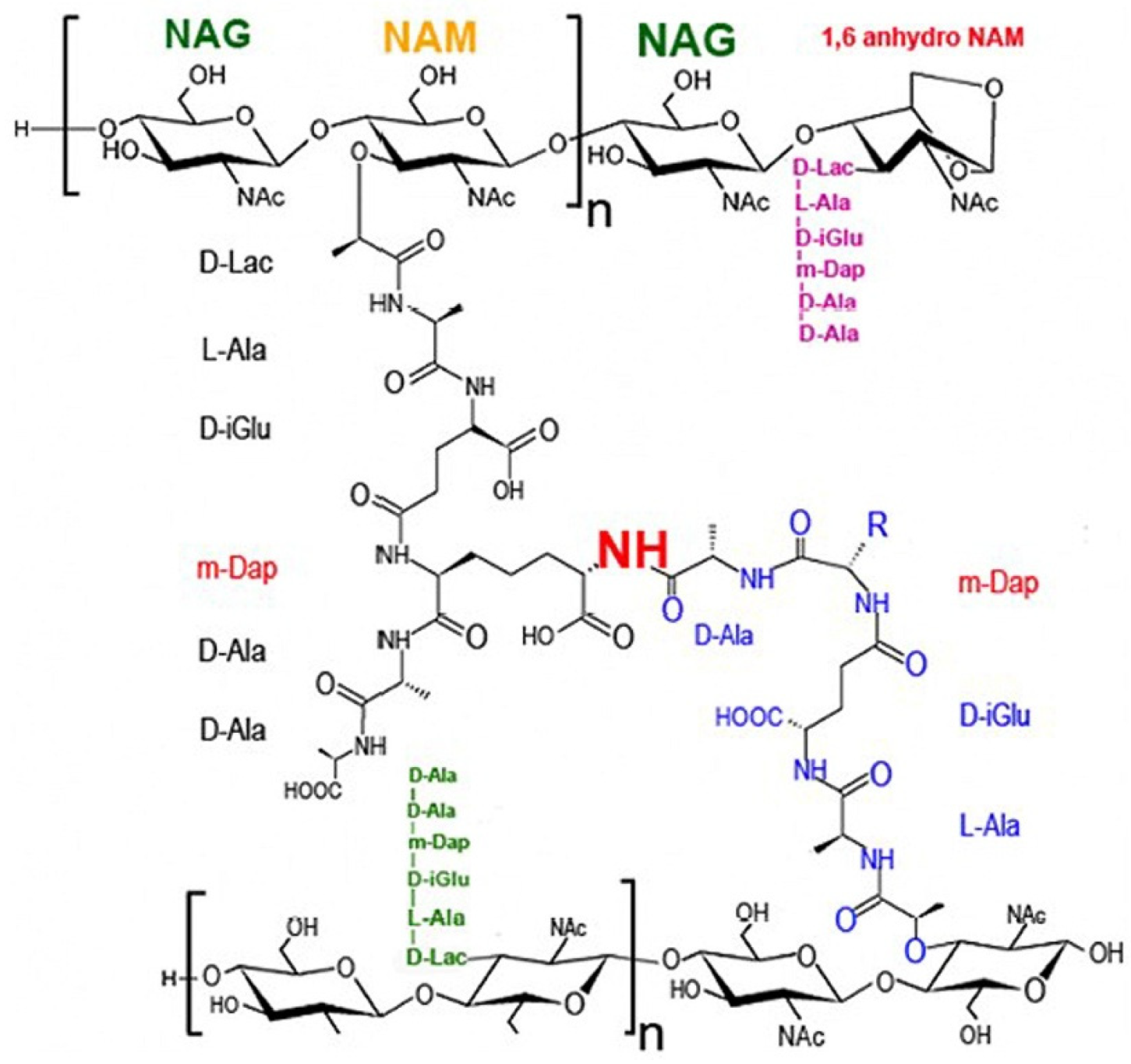
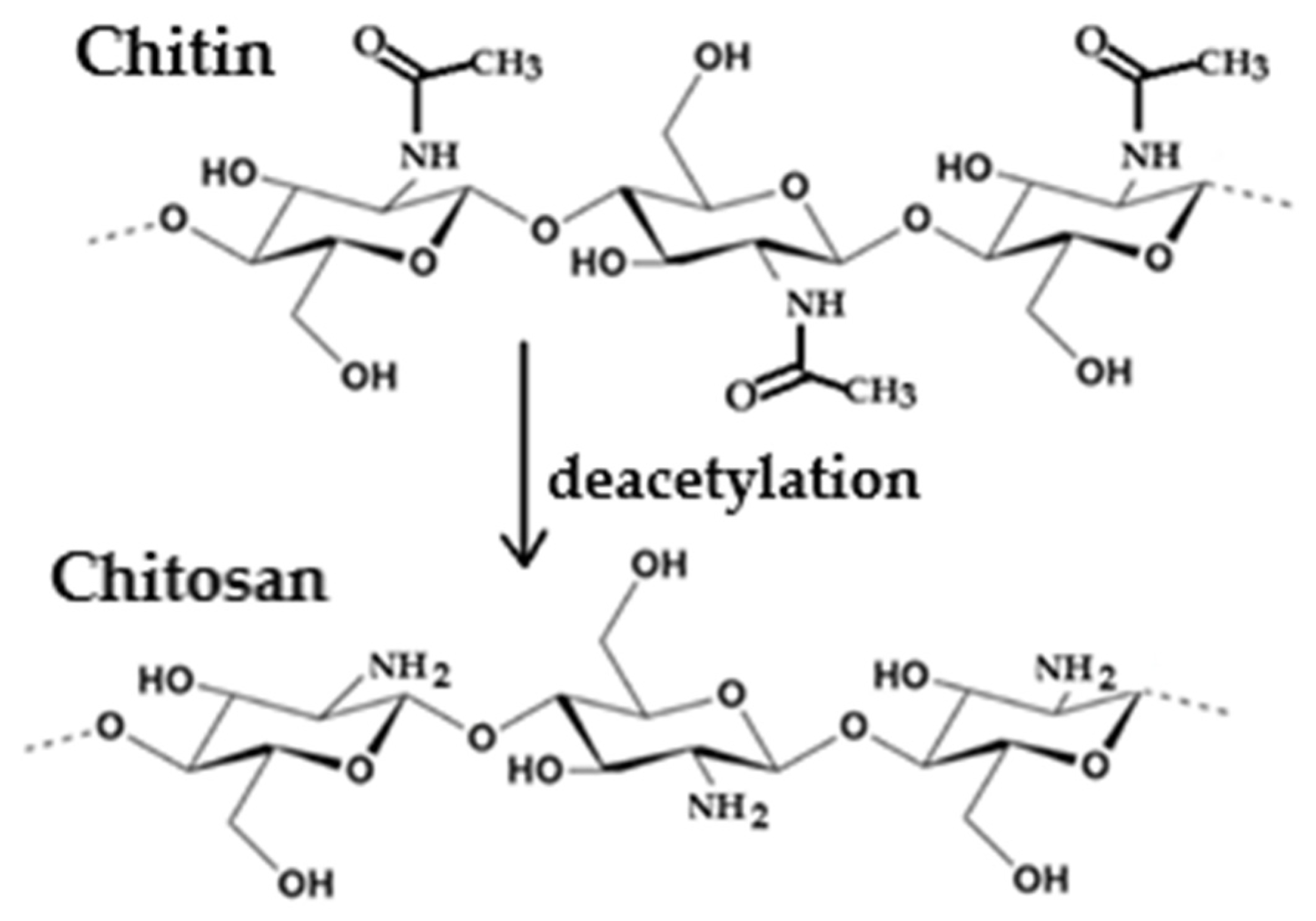
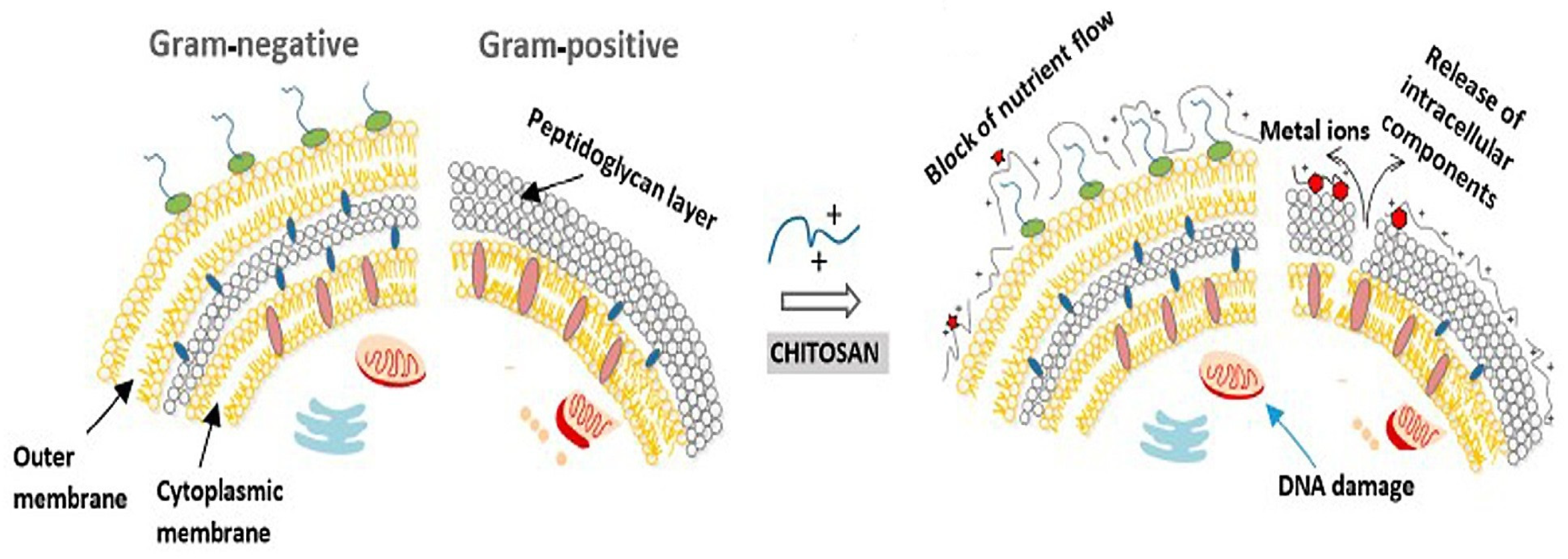
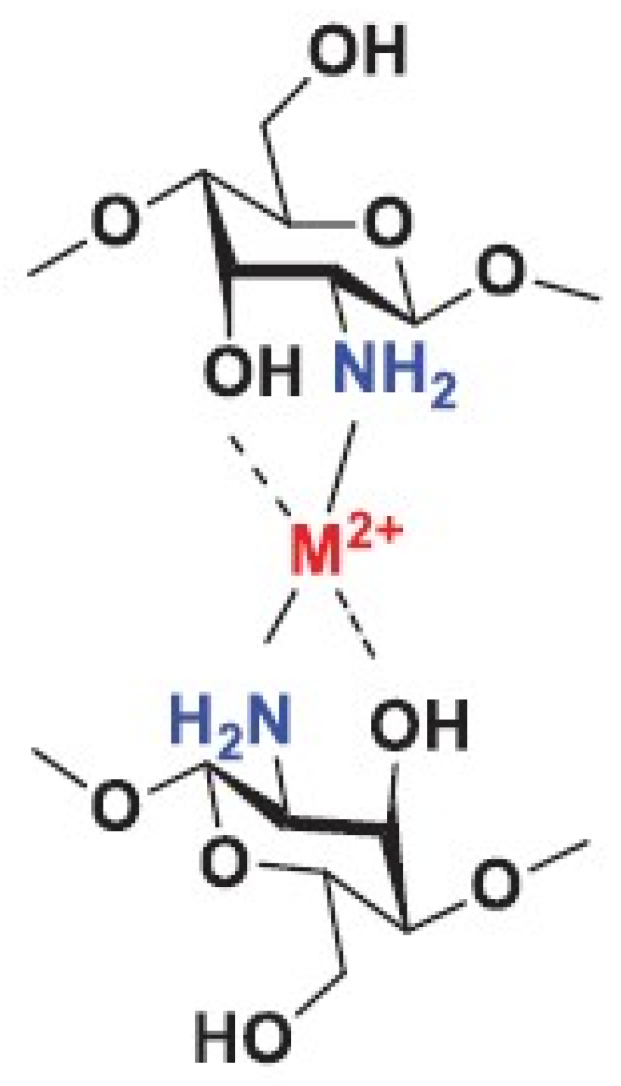
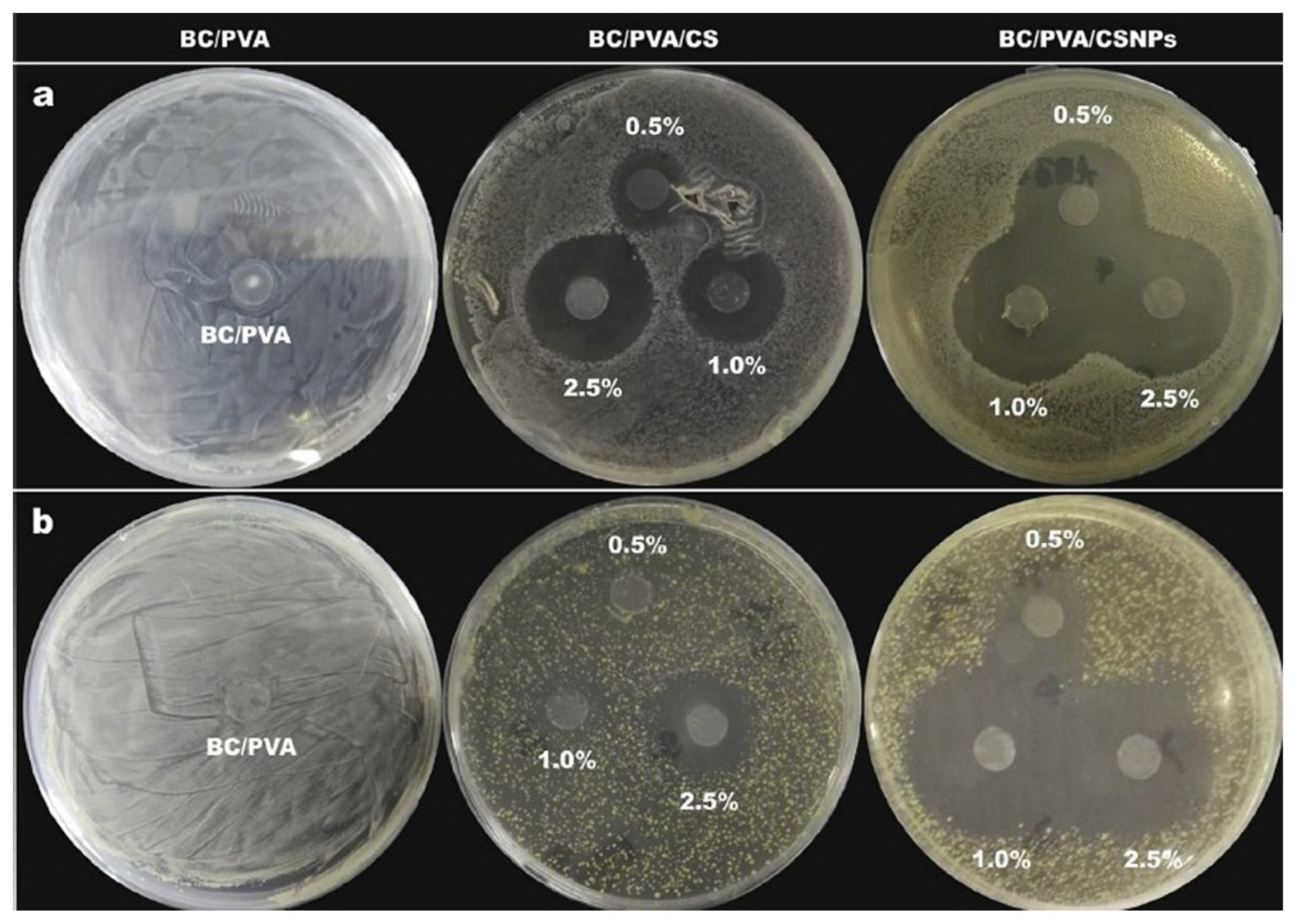
3.1.1. Chitosan
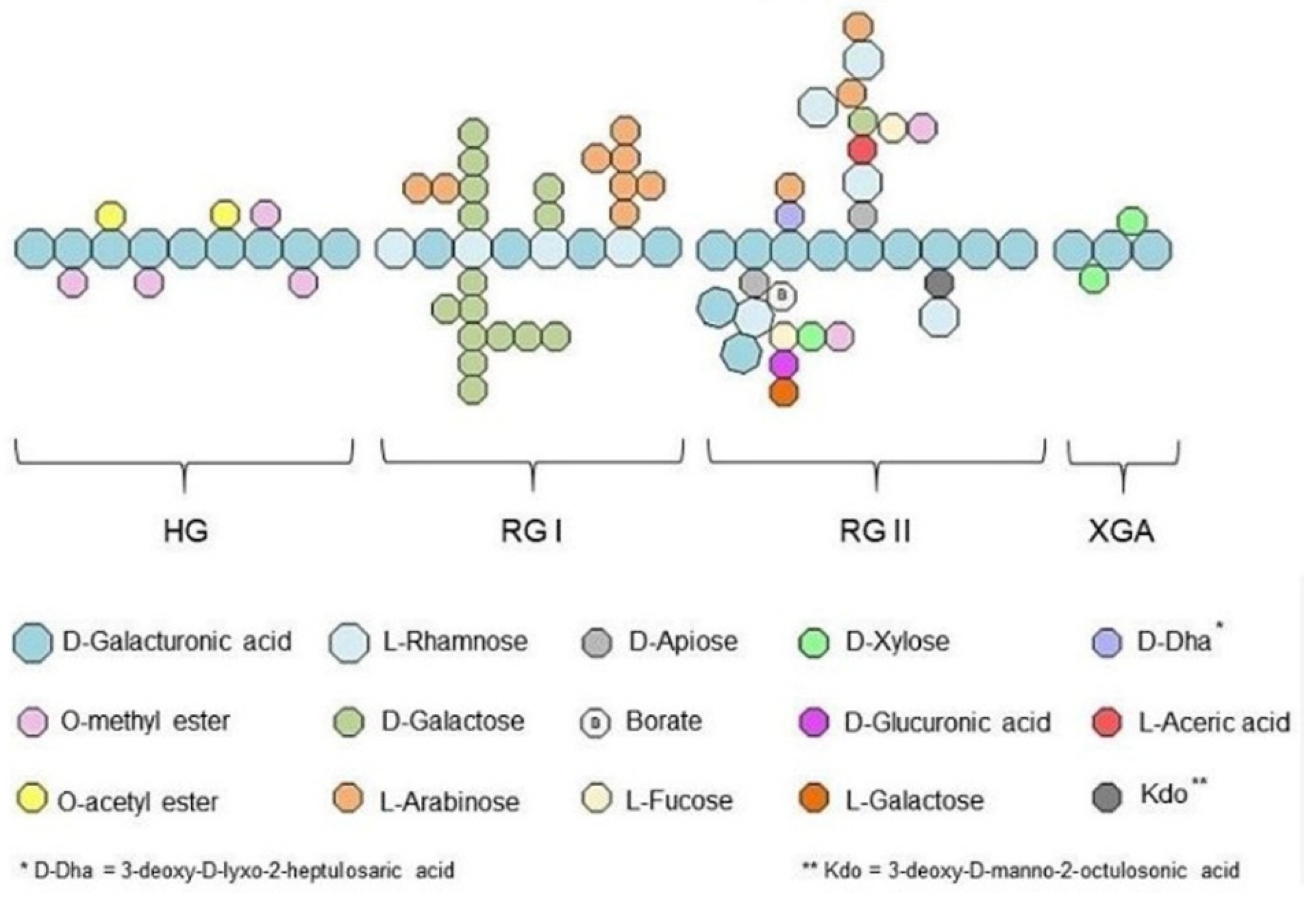
3.1.2. Pectin
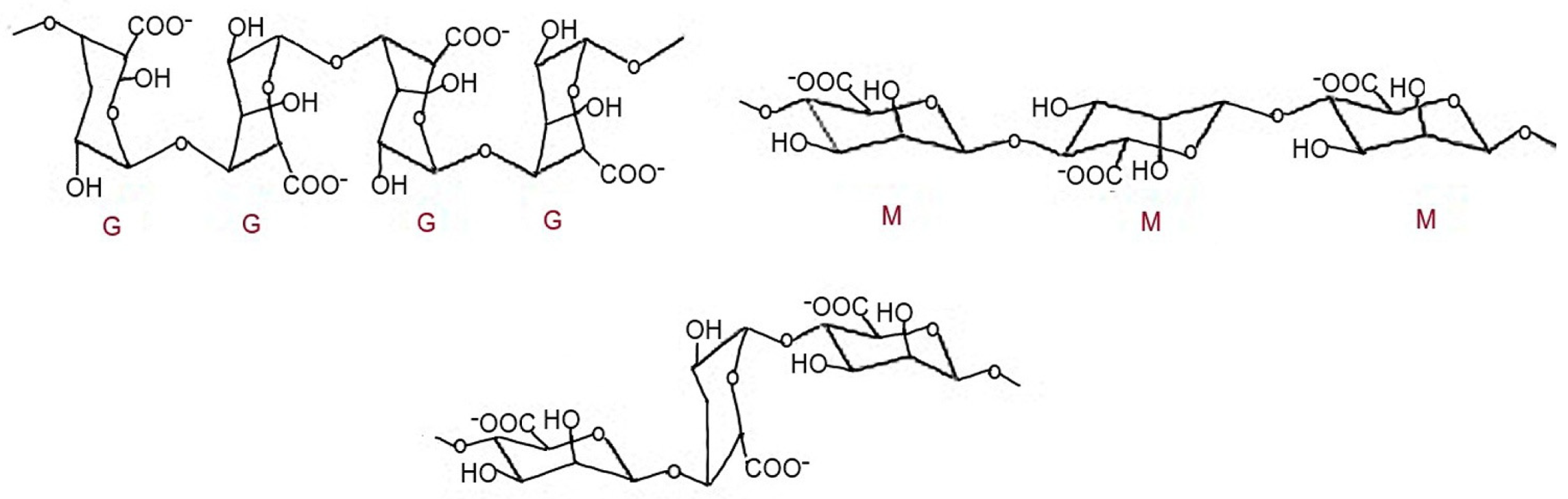
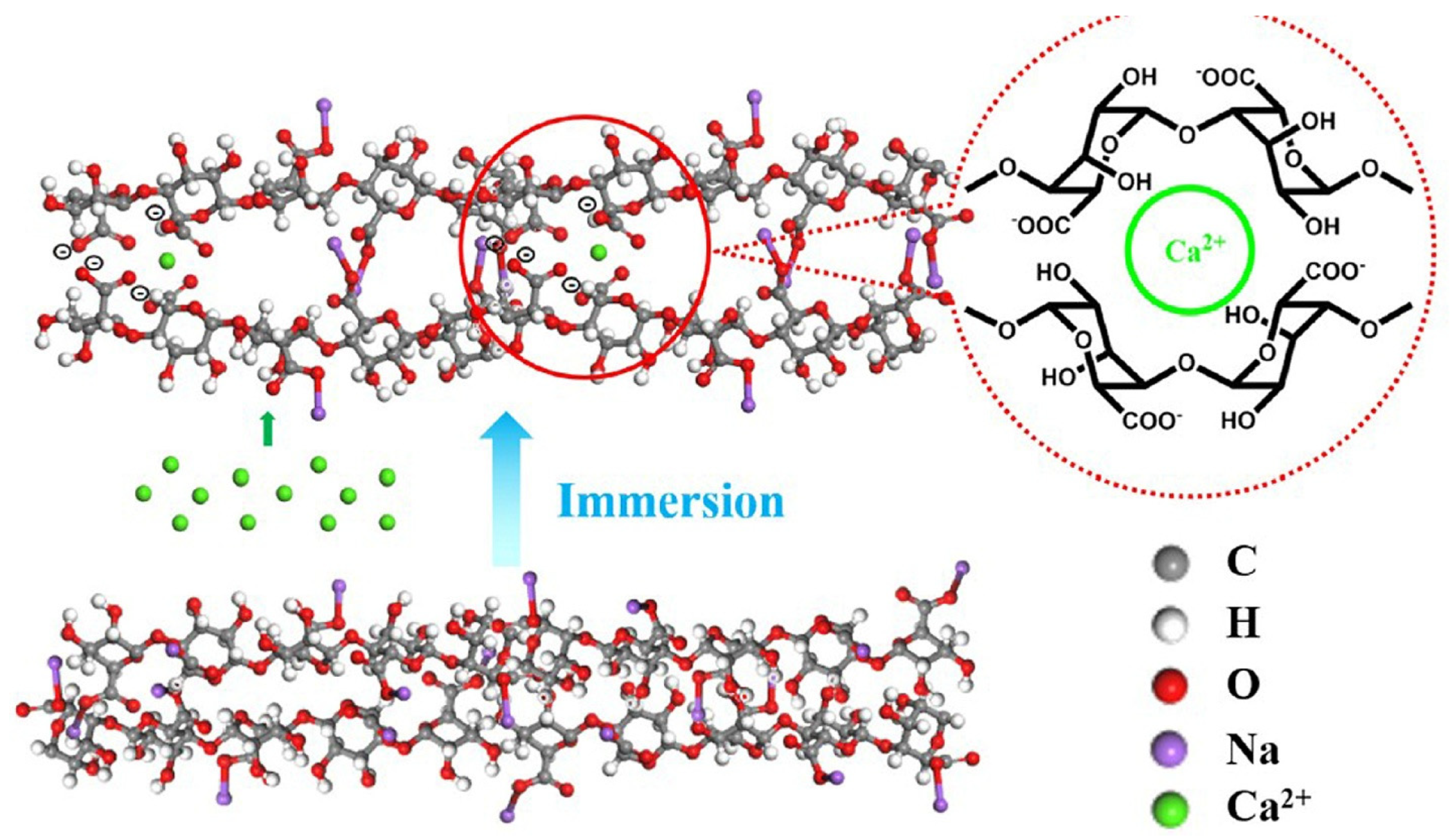
3.1.3. Alginate
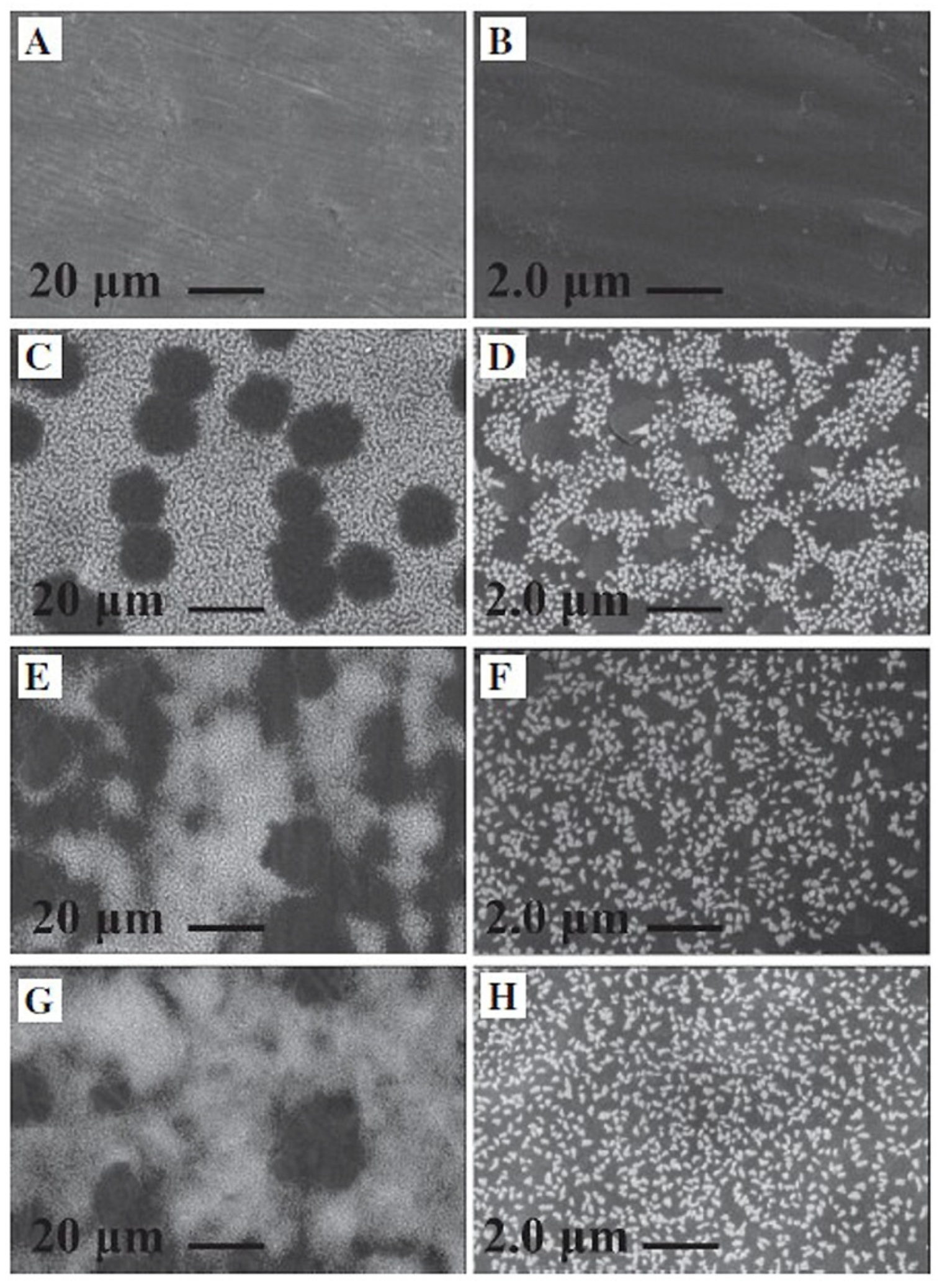
3.1.4. Chitosan Nanoparticles
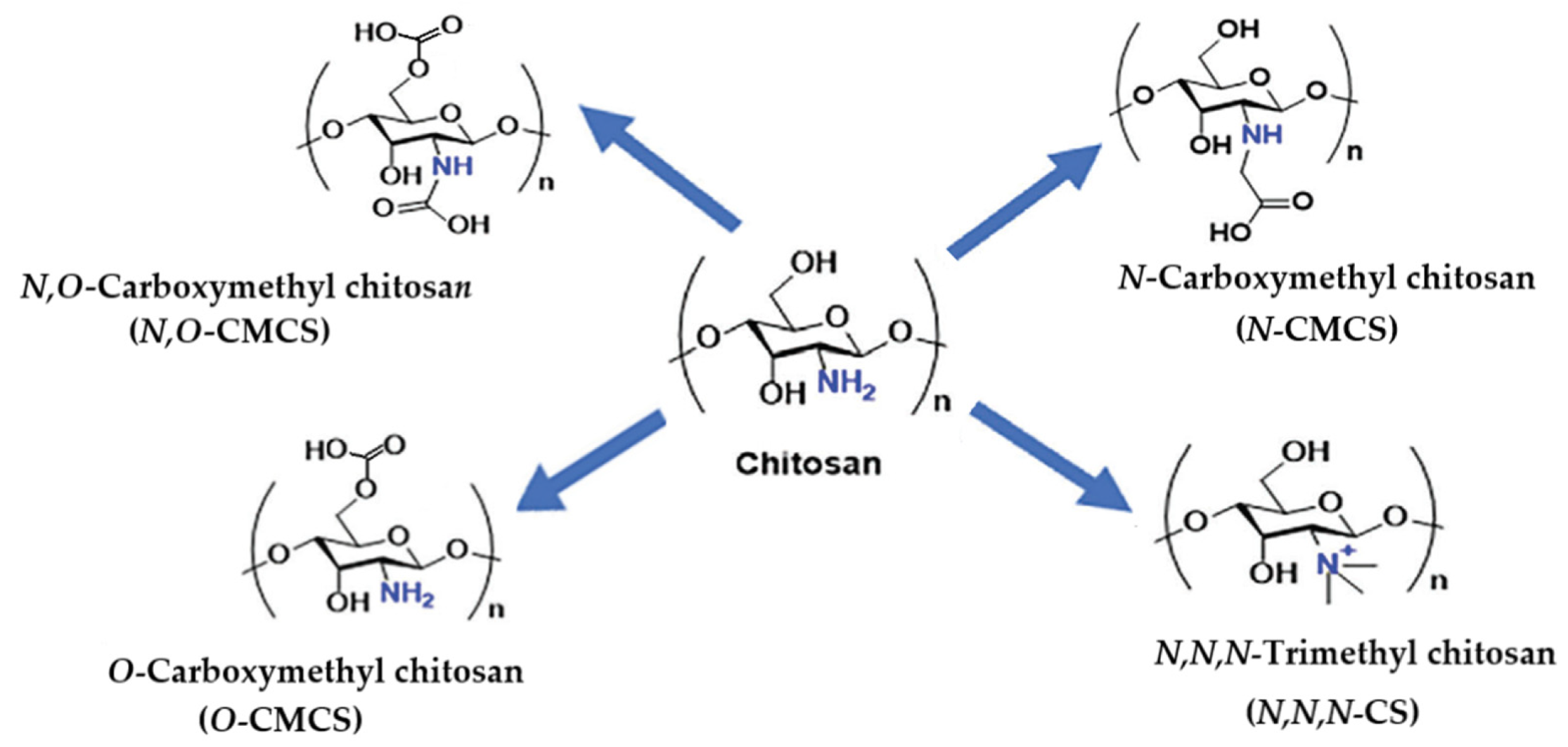
3.1.5. Carboxymethyl Chitosan
3.1.6. Xanthan Oligosaccharide
4. Perspectives for the Application of Antibacterial Polysaccharides in Dental Implantology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, J.D.; Romanos, G.E. The use of bioactive proteins and peptides to promote osseointegration and success of dental implants: A narrative review. Front. Oral Maxillofac. Med. 2024, 7, 1–23. [Google Scholar]
- Kravanja, K.A.; Finšgar, M. Analytical techniques for the characterization of bioactive coating for orthopedic implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef] [PubMed]
- Łosiewicz, B.; Osak, P.; Novinska, D.; Maszybrocka, J. Developments in dental implant surface modification. Coatings 2025, 15, 109. [Google Scholar] [CrossRef]
- Mutreja, I.; Lan, C.; Li, Q.; Aparicio, C. Chemoselective coating of GL13K antimicrobial peptides for dental implants. Pharmaceutics 2023, 15, 2418. [Google Scholar] [CrossRef]
- Spirito, F.D.; Giordano, F.; Palo, M.P.; D´Ambroso, F.; Scognamiglio, B.; Sangiovanni, G.; Caggiano, M.; Gasparro, R. Microbiota of peri-implant healthy tissues, peri-implant muosities, and peri-implantitis: A comprehensive review. Microorganisms 2024, 12, 1137. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Aroso, C.; Salazar, F.; López-Jarana, P.; Rios-Santos, J.V.; Herreo-Climent, M. Review of the mechanical behavior of different implant-abutment connections. Int. J. Environ. Res. Public Health 2020, 17, 8685. [Google Scholar] [CrossRef] [PubMed]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and antibiofilm coating of dental implants-past and new perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef]
- Accioni, F.; Vanquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest trends in surface modification for dental implantology: Innovative developments and analytical applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Giron, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef]
- Jurczak, P.; Witkowska, J.; Rodziewicz-Motowidło, S.; Lach, S. Proteins, peptides and peptidomimetrics as active agents in implant surface functionalization. Adv. Colloid Interface Sci. 2020, 276, 102083. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.; Yeo, I.S.L. Three interfaces of the dental implant system and their clinical effects on hard and soft tissues. Mater. Horiz. 2022, 9, 1387–1411. [Google Scholar] [CrossRef]
- Tuikampee, S.; Chaijareenont, P.; Rungsiyakull, P.; Yavirach, A. Titanium surface modification techniques to enhance osteoblasts and bone formation for dental implants: A narrative review on current advances. Metals 2024, 14, 515. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Ting, M.; Suzuki, J.B. Peri-implantitis. Dent. J. 2024, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Dieckow, S.; Szafrański, S.P.; Grischke, J.; Qu, T.; Doll-Nikutta, K.; Steglich, M.; Yang, I.; Häussler, S. Structure and composition of early biofilms formed on dental implants are complex, diverse, subject-specific and dynamic. NPJ Biofilms Microbiomes 2024, 10, 155. [Google Scholar] [CrossRef]
- Haq, I.U.; Krukiewicz, K. Antimicrobial approaches for medical implants coating to prevent implants associated infections: Insights to develop durable antimicrobial implants. Appl. Surf. Sci. Adv. 2023, 18, 100532. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; Bagnato, V.S.; Reis, A.C. Bacterial adhesion strength on titanium surfaces quantified by atom force microscopy: A systematic review. Antibiotics 2023, 12, 994. [Google Scholar] [CrossRef]
- Viljoen, A.; Mignolet, J.; Viela, F.; Guinet, M.M.; Dufrene, Y.F. How microbes use force to control adhesion. J. Bacteriol. 2020, 202, e00125-20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Shree, P.; Sigh, C.K.; Sodhi, K.K.; Suraya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. J. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. J. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-positive bacterial wall. Microbiol. Spectr. 2019, 7, GPP3-0044-2018. [Google Scholar] [CrossRef]
- Tetteh, J.N.A.; Matthäus, F.; Hernandez-Vargas, E.A. A survey of within-host and between-hosts modelling for antibiotic resistance. BioSistems 2020, 196, 104182. [Google Scholar] [CrossRef]
- Insua, A.; Galindo-Moreno, P.; Miron, R.J.; Wang, H.-L.; Monje, A. Emerging factors affecting peri-implant bone metabolism. Periodontol. 2000 2023, 94, 27–78. [Google Scholar] [CrossRef] [PubMed]
- Körtvelyessy, G.; Tarjanyi, T.; Barath, Z.L.; Minarovits, J. Bioactive coating for dental implants: A review of alternative strategies to prevent peri-implantitis induced by anaerobic bacteria. Anaerobe 2021, 70, 102404. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Tejada, G.M.; Gomes, P.A.C.; Martins, M.C.; Costa, F. Antimicrobial peptides in the battle against orthopedic implant-related infections: A review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef]
- Wang, X.; Liddell, R.S.; Wen, H.B.; Daviedes, J.E.; Ajami, E. The role of implant coronal surface properties on early adhesion of streptococcus oralis-an in vitro comparative study. Biomed. Mater. Res. A 2025, 113, e37866. [Google Scholar] [CrossRef]
- Ghimire, A.; Song, J. Anti-periprosthetic infection strategies: From implant surface topographical engineering to smart drug-releasing coating. J. ACS Appl. Mater. Interfaces 2021, 13, 20921–20937. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Chan, H.M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. J. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 228080002110403. [Google Scholar] [CrossRef]
- Lee, S.; Inzerillo, S.; Lee, G.Y.; Bosire, E.M.; Mahato, S.K.; Song, J. Glycan-mediated molecular interactions in bacterial pathogenesis. Trends Mictobiol. 2022, 30, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.X.; Wu, Y.M.; Bi, Y.F.; Chen, K.; Zhang, W.W.; Liu, A.Q.; Zhang, W.J.; Liu, R.H. Antimicrobial properties and application of polysaccharides and their derivates. Chin. J. Polym. Sci. 2021, 39, 133–146. [Google Scholar] [CrossRef]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef]
- Yu, Y.M.; Lu, Y.P.; Zhang, T.; Zheng, Y.F.; Liu, Y.S.; Xia, D.D. Biomaterial science and surface engineering strategies for dental peri-implantities management. Mil. Med. Res. 2024, 11, 29. [Google Scholar] [PubMed]
- Teulé-Trull, M.; Altuna, P.; Arregui, M.; Rodriguez-Ciiiurana, X.; Aparicio, C. Antibacterial coating for dental implants: A systematic review. Dent. Mater. 2025, 41, 229–247. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Qui, W.; Fang, F. Overview of antibacterial strategies of dental implant materials for the prevention of peri-implantitis. J. Bioconjugate Chem. 2021, 32, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B.; Groza, A. Polymeric coatings and antimicrobial peptides as efficient systems for treating implantable medical devices associated-infections. J. Polym. 2022, 14, 1611. [Google Scholar] [CrossRef]
- Villegas, M.; Bayat, F.; Kramer, T.; Schwarz, E.; Wilson, D.; Hosseinidoust, Z.; Didar, T.F. Emerging strategies to prevent bacterial infections on titanium-based implants. Nano-Micro Small 2024, 20, 2404351. [Google Scholar] [CrossRef]
- Alkarri, S.; Saad, H.B.; Soliman, M. On antimicrobial polymers: Development, mechanism of action, international testing procedures, and applications. Polymers 2024, 16, 771. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Yu, Q.; Chen, H. Dual-function antibacterial surfaces to resist and kill bacteria: Painting a picture with two brushes simultaneously. J. Mater. Sci. Technol. 2021, 70, 24–38. [Google Scholar] [CrossRef]
- Zheng, T.X.; Li, W.; Gu, Y.Y.; Zhao, D.; Qi, M.C. Classification and research progress of implant surface antimicrobial techniques. J. Dent. Sci. 2022, 17, 1–7. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Wang, L.; Lyu, C.; Chen, H.; Lyu, Y.; Nie, B. Preparation and performance of superhydrophobic surfaces with low surface energy modified attapulgite. J. Mol. Struct. 2024, 1, 136586. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RCS Adv. 2021, 11, 15467. [Google Scholar] [CrossRef]
- Puzas, V.M.V.; Carter, L.N.; Schröder, C.; Colavita, P.E.; Hoey, D.A.; Webber, M.A.; Addison, O.; Shepherd, D.E.T.; Attallah, M.M.; Grover, L.M.; et al. Surface free energy dominates the biological interactions of postprocessed additively manufactured Ti-6Al-4V. ACS J. Biomater. Sci. Eng. 2022, 8, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.W.; Dillon, J.T.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Different methods to modify the hydrophilicity of titanium implants with biomimetic surface topography to induce variable response in bone marrow stromal cells. J. Biomim. 2024, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, S.; Taylor, M.; Castillo, A.; Zevallos, L.C.; Oh, J.K.; et al. Influence of surface roughness, nanostructure, and wetting on bacterial adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Frano, R.; Rosa, A.; Lupi, E.; Capogreco, M. The influence of dental implant roughness on biofilm formation: A comprehensive strategy. Dent. Hypotheses 2023, 14, 90–92. [Google Scholar] [CrossRef]
- Botticelli, G.; Falisi, G.; Rastelli, S.; Iacomino, E.; Bruni, A.; Gerardi, D.; Fabio, G.D.; Severino, M.; Bernardi, S. A morphological evaluation of the antibiofilma ctivity on an implant surface using a new electric device: An in vitro study. Dent. J. 2025, 13, 140. [Google Scholar] [CrossRef]
- Sun, X.D.; Liu, T.T.; Wang, Q.Q.; Zhang, J.; Cao, M.S. Surface modification and functionalities for titanium dental implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Nagay, B.E.; Martins, R.; Bertolini, M.; Shibli, J.A.; Aparicio, C.; Feres, M.; Barão, V.A.R. Engineered surface strategies to manage dental-related infections. Periodontol. 2000 2025, 1–56. [Google Scholar] [CrossRef]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface design for antibacterial materials: From fundamentals to advanced strategies. J. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant denstistry: Which are the factors affecting the clinical outcome? Periodontol. 2000 2023, 93, 26–55. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Safael, M.; Mohammadi, H.; Beddu, S.; Mozaffari, H.R.; Rezaei, R.; Sharifi, R.; Moradpoor, H.; Fallahnia, N.; Ebadi, M.; Jamil, M.S.; et al. Surface topography steer soft tissue response and antibacterial function at the transmucosal region of titanium implant. Inter. J. Nanomed. 2024, 19, 4835–4856. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Seth, J.; Aeran, M.; Sani, A. From surface to success: How implant surface shape osseointegration. Int. J. Periodontol. Implantol. 2024, 9, 58–63. [Google Scholar] [CrossRef]
- Hsiao, V.K.S.; Shih, M.H.; Wu, H.C.; Wu, T.-I. Comparative study of surface modification techniques for enhancing biocompatibility of Ti-6Al-4V alloy in dental implants. J. Appl. Sci. 2024, 14, 10904. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.; Li, J.J. Advanced in implant surface modifications to improve osseointegration. J. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Su, q.; Xue, Y.; Wang, C.; Zhou, Q.; Zhao, Y.; Su, J.; Zhu, B. Strategies and applications of antibacterial surface-modified biomaterials. Bioact. Mater. 2025, 53, 114–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental implant nano-engineering: Advances, limitations and future directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Ghodrati, H.; Goodarzi, A.; Golrokhian, M.; Fattahi, F.; Anzabi, R.M.; Mohammadikhah, M.; Sadeghi, S.; Mirhadi, S. A narrative review of recent developments in osseointegration and anti-corrosion of titanium dental implant with nano surface. Bone Rep. 2025, 25, 101846. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The mechanisms and the applications of antibacterial polymers in surface modification on medical devices. Front. Bioeng. Biotechnol. 2020, 8, 910. [Google Scholar] [CrossRef]
- Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of dental implant surface modifications on osseointegration and biofilm attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. J. Acta Biomater. 2021, 127, 80–101. [Google Scholar] [CrossRef]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials. What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Duan, S.; Wu, R.; Xiong, Y.H.; Ren, H.M.; Lei, C.; Zhao, Y.Q.; Zhang, X.Y.; Xu, F.J. Multifunctional antimicrobial materials: From rational design to biomedical applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, L.; Sun, X.; Alastair, A.J.; O’Brien-Simpson, N.M.; Li, W. The overview of antimicrobial peptide-coated implants against oral bacterial infections. Aggregate 2023, 4, e309. [Google Scholar] [CrossRef]
- Authokoralalage, S.S.A.; Datson, Z.; Darwish, N.; Zhu, Y.; Chung, K.H.K.; Chew, K.; Rowan, A.E. Dual-functional antimicrobial and low-fouling cellulose coating. ACS Appl. Mater. 2025, 17, 16027–16039. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Yaghmur, A. Recent advances in antibacterial coatings to combat orthopedic implant-associated infections. Molecules 2024, 29, 1172. [Google Scholar] [CrossRef] [PubMed]
- Kungwani, N.A.; Panda, J.; Mishra, A.K.; CHavda, N.; Shukla, S.; Vikke, K.; Sharma, G.; Mohanta, Y.K.; Sharifi-Rad, M. Combating bacterial biofilms and related drug resistance: Role of phyto-derived adjuvant and nanomaterials. Microb. Pathog. 2024, 195, 106874. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Cai, Q.; Li, X.; Yin, Z.; Li, B.; Li, L.; Meng, W. Advanced and prospects in antibacterial-osteogenic multifunctional dental implant surface. Front. Bioeng. Biotechnol. 2022, 10, 921338. [Google Scholar] [CrossRef]
- Ruan, H.; Aulova, A.; Ghai, V.; Pandit, S.; Lovmar, M.; Mijakovic, I.; Kádár, R. Polysaccharide-based antibacterial coating technologies. J. Acta Biomater. 2023, 168, 42–77. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef] [PubMed]
- Chaisuwan, W.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Wangtueai, S.; Sommano, S.R.; You, S.G.; et al. The antiviral activity of bacterial, fungal and algal polysaccharides as bioactive ingredients: Potential uses for enhancing immune systems and preventing viruses. J. Front. Nutr. 2021, 8, 772033. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Zhang, H.; Wang, J.; Fu, Q.; Qiao, H.; Wang, Q. Insight into antibacterial mechanism of polysaccharides: A review. LWT 2021, 150, 111929. [Google Scholar] [CrossRef]
- Šćepanović, M.B.; Maršić-Lučić, J.; Beloša, R.; Tomšić, S. Preliminary findings on antibacterial activity of selected marine invertebrates. Appl. Sci. 2025, 15, 3101. [Google Scholar] [CrossRef]
- Rofael, M.; Abdelmalek, F.; Steinbüchel, A. Naturally-sourced antibacterial polymeric nanomaterials special reference to modified polymer variants. Int. J. Mol. Sci. 2022, 23, 4101. [Google Scholar] [CrossRef]
- Dmochowska, A.S.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamula, E.; Dobrzynski, P. Biodegradable polymers and polymer composites with antibacterial properties. Int. J. Mol. Sci. 2023, 24, 7473. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trend in Food Sci. Technnol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- Zhai, S.; Tian, Y.; Shi, X.; Liu, Y.; You, J.; Yang, Z.; Wu, Y.; Chu, S. Overview of strategies to improve the antibacterial properties of dental implants. Front. Bioeng. Biotechnol. 2023, 11, 1267128. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides-Natural occurring immune modulators. Polymer 2023, 15, 2373. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Zhu, T.; Liu, Y.; Chu, J.; Chen, N. Structural characterization of polysaccharides of marine origin: A review. Int. J. Biolog. Macromol. 2025, 317, 144979. [Google Scholar] [CrossRef]
- Mohamed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; classification, chemical properties, and future perspective applications in field of pharmacology and biological medicine (a review of current applications and upcoming potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.N.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. J. Colloid Interface Sci. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The antioxidant activity of polysaccharides derived from marine organisms: A review. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Noor, A. Biological activities of plant polysaccharides, mechanism of action and biomedical applications. Res. J. Biotech. 2021, 16, 255–272. [Google Scholar]
- Wang, Z.; Zhu, J.; Li, W.; Li, R.; Wang, X.; Qiao, H.; Sun, Q.; Zhang, H. Antibacterial mechanism of the polysaccharide produced by Chaetomium globosum CGMCC 6882 against staphylococcus aureus. Int. J. Biol. Macromol. 2020, 159, 231–235. [Google Scholar] [CrossRef]
- Jabeen, N.; Atif, M. Polysaccharides based biopolymers for biomedical applications: A review. Polym. Adv. Technol. 2024, 35, e6203. [Google Scholar] [CrossRef]
- Jun, J.-Y.; Jung, M.-J.; Jeong, I.-H.; Yamazaki, K.; Kawai, Y.; Kim, B.-M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Liang, L.; Su, Q.; Ma, Y.; Zhao, S.; Zhang, H.; Gao, X. Research progress on the polysaccharide extraction and antibacterial activity. J. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Lai, F.; Min, T.; Wu, H.; Zhan, Q. The Influence and Mechanisms of Natural Plant Polysaccharides on Intestinal Microbiota-Mediated Metabolic Disorders. Foods 2024, 13, 3882. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Khan, F.; Kim, D.-K.; Cho, K.-J.; Tabassum, N.; Choudhury, A.; Hassan, M.d.I.; Jung, W.-K.; Kim, H.-W.; Kim, Y.-M. Marine polysaccharides for antibiofilm application: A focus on biomedical fields. Int. J. Biol. Macromol. 2024, 283, 137786. [Google Scholar] [CrossRef]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-derived polysaccharide hydrogels as delivery platforms for natural bioactive compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, W.; Liu, H.; Li, R.; Chang, L.; Kann, S.; Hao, M.; Wang, D. The applications of polysaccharides in dentistry. Front. Bioeng. Biotechnol. 2022, 10, 970041. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.G.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. J. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, H.; Wang, X.; Wu, S.; Wang, Y.; Wang, C.; Zhang, Y.; Zhao, H. Unraveling the web of defense: The crusial role of polysaccharides in immunity. Front. Immunol. 2024, 15, 1406213. [Google Scholar] [CrossRef]
- Carrasqueira, J.; Berandino, S.; Alfonso, C. Marine-derived polysaccharides and their potential health benefits in nutraceutical applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef]
- Mansour, F.B.; Guermazi, W.; Chamkha, M.; Bellassoued, K.; Salah, H.B.; Harrath, A.H.; Aldahmash, W.; Rahman, M.A.; Ayadi, H. Bioactive potential of the sulfated exopolysaccharides from the brown microalga Halamphora sp.: Antioxidant, antimicrobial and antiapoptotic profiles. Anal. Sci. Adv. 2024, 5, e202400030. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Wang, J.; Chen, Z.; Xie, J.; Cui, Z.; Xu, D.; Zhang, X.; Yao, W. Advanced in polysaccharide-based antibacterial materials. Int. J. Biol. Macromol. 2025, 308, 142598. [Google Scholar]
- Alsalhi, A. Applications of selected polysaccharides and proteins in dentistry: A review. Int. J. Biol. Macromol. 2024, 269, 129215. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kirichuk, A.A.; Jr Rubanik, V.V.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan and ist derivates: Preparation and antibacterial properties. Materials 2023, 16, 6076. [Google Scholar] [CrossRef] [PubMed]
- Alfinaikh, R.S.; Alamry, K.A.; Hussein, M.A. Sustainable and biocompatible hybrid materials based sulfated polysaccharides for biomedical applications: A review. RSC Adv. 2025, 15, 4708–4767. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yan, Z.; Wu, Z.; Li, J.; Zhong, W.; Ding, L.; Zhong, T.; Jiang, T. Recent advances in the preparation, antibacterial mechanisms, and applications of chitosan. J. Funct. Biomater. 2024, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Scarcelli, E.; Catalano, A.; Iacopetta, D.; Ceramella, J.; Sinicropi, M.S.; Aiello, F. Chitin, chitosan and ist derivatives: Antibacterial and/or mitigatiors of water. Macromol 2025, 5, 15. [Google Scholar] [CrossRef]
- Dhlamini, K.S.; Selepe, C.T.; Ramalapa, B.; Tshweu, L.; Ray, S.S. Reimagining chitosan-based antimicrobial biomaterials to mitigate antibiotic resistance and alleviate antibiotic overuse: A review. Macromol. Mater. Eng. 2024, 309, 2400018. [Google Scholar] [CrossRef]
- Confederal, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and antimicrobial activity of chitosan and its derivates: A concise review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Chen, G.; Gao, H.; Peng, Q. Chitosan-based multifunctional biomaterials as active agent or delivery systems for antibiotic therapy. Bioengineering 2024, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Nrgrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tondevic, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antibacterial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Mirbagheri, V.S.; Alishari, A.; Ahmadian, G.; Petroudi, S.H.H.; Ojagh, S.M.; Romanazzi, G. Toward understanding the antibacterial mechanism of chitosan: Experimental approach and in silico analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadied-Yazdi, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestani, M. Factors influencing the antibacterial mechanism of chitosan action and its derivates: A review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Santos, R.T.; Lima, M.; Gomes, L.C.; Mergulhao, F.J. Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, S.; Samasundaram, A.; Ramasamy, P. A comprehensive review on nanochitosan and its diverse applications in various industries. Int. J. Biol. Macromol. 2025, 305 Pt 1, 141150. [Google Scholar] [CrossRef]
- Anwar, A.; Imran, M.; Warsi, M.F.; Alsafari, I.A.; Parra-Saldivar, R.; Gutiérrez-Soto, G.; Iqbal, H.M.N. Chitosan and its derivates-based nanostructures in conjunction with their versatile applications in biomedicine for alleviating contiguous diseases. Front. Mater. 2025, 12, 1588627. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packing. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivates in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Mahmood, A.; Maher, N.; Amin, F.; Alqutaibi, A.Y.; Kumar, N.; Zafar, M.S. Chitosan-based materials for dental implantology: A comprehensive review. Int. J. Biolog. Macromol. 2024, 268 Pt 2, 131823. [Google Scholar] [CrossRef]
- Olivera, W.F.d.; Albuquerque, P.B.S.; Rodrigues, N.E.R.; Silva, P.M.d.S.; Kennedy, J.F.; Correira, M.T.d.S.; Coelho, L.C.B.B. Pharmaceutical application of chitosan on medical implants. A viable alternative for construction of new materials. Carbohyd. Polym. Technol. Appl. 2024, 7, 100407. [Google Scholar]
- Tardelli, J.D.C.; Schiavon, M.A.; Reis, A.C.D. Chitosan coating on titanium-based implant from development to characterization and behavior: A systematic review. Carbohydr. Polym. 2024, 344, 122496. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.S.; Sousa, R.C.S. Structural applications of pectin in food, biomedical and pharmaceutical industry; a review. Coating 2021, 11, 922. [Google Scholar] [CrossRef]
- Abdelgawand, R.M.; Teixeira, N.D.; Comis, K.G.; Alghamidi, A.; Mahran, A.; Elbackly, R.; Do, T.; Gendy, R.E. Pectin as a biomaterial in regenerative endodontics-assessing biocompatibility and antibacterial efficacy against common endodontic pathogens: An in vitro study. Bioengineering 2024, 11, 653. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; IIharco, L.M.; Pagliaro, M. Pectin: A long-neglected broad-spectrum antibacterial. ChemMedChem 2020, 15, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Picone, P.; Albanese, L.; Meneguzzo, F.; IIharco, L.M.; Nuzzo, D.; Pagliaro, M. Antibacterial activity of lemon integropectin against Escherichia coli. ChemistrySelect 2024, 9, e202404844. [Google Scholar] [CrossRef]
- Alsharbaty, M.H.M.; Naji, G.; Ghani, B.A.; Schagerl, M.; Khalil, M.A.; Ali, S.S. Cytotoxicity and antibacterial susceptibility assessment of a new developed pectin-chitosan polyelectrolyte composite for dental implants. Sci. Rep. 2024, 14, 16968. [Google Scholar] [CrossRef]
- Mania, S.; Banach-Kopec, A.; Staszczyk, K.; Kulesza, J.; Augustin, E.; Tylingo, R. An influence of molecular weight, deacetylation degree of chitosan xerogels on their antimicrobial activity and cytotoxicity. Comparision of chitosan materials obtained using lactic acid and CO2 saturation. Carbohy. Res. 2023, 534, 108973. [Google Scholar] [CrossRef]
- Kruczkowska, W.; Kłosinski, K.K.; Grabowska, K.H.; Gałeziewska, J.; Gromek, P.; Kciuk, M.; Kołat, Z.K.; Kołat, D.; Wach, R.A. Medical applications and cellular mechanisms of action of carboxymethyl chitosan hydrogels. Molecules 2024, 29, 4360. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Y.; Chen, X.; Li, H.; Lu, Y.; Yu, H.; Zheng, D. O-carboxymethyl chitosan in biomedicine: A review. Int. J. Biol. Macromol. 2024, 274, 133465. [Google Scholar] [CrossRef]
- Loukelis, K.; Tsampallas, V.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Synthesis of N, N,O and O-carboxymethyl chitosan derivates of controllable substitution degrees and their utilization as electrospun scaffolds for bone tissue engineering. Carbohydr. Polym. 2025, 348 Pt A, 122775. [Google Scholar] [CrossRef]
- Garcia, L.; Braccini, S.E.; Gronchio, V.D.; Gioia, D.D.; Peniche, H.; Peniche, C.; Puppi, D. Ionically-crosslinked carboxymethyl chitosan scaffolds by additive manufacturing for antimicrobial wound dressing applications. Carbohydr. Polym. 2024, 346, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Dohendou, M.; Dekamin, M.G. Synthesis of carboxymethyl chitosan and its derivates using KI and/or ultrasonication. Chem. Proc. 2022, 12, 90. [Google Scholar] [CrossRef]
- Andreica, B.I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
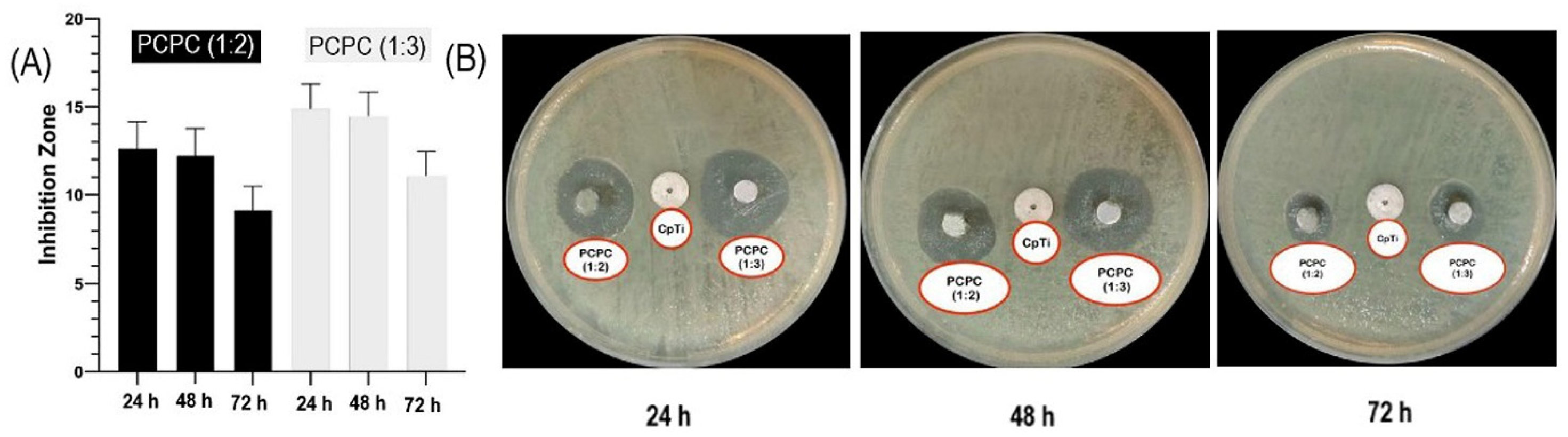
- Rivera-Cortés, M.A.; Niño-Martínez, N.; Ruiz, F.; Félix-Sicairos, B.K.; Martínez-Castañón, G.A. Evaluation of deformation and antibacterial properties of dental alginates mixed with silver nanoparticles. Materials 2025, 18, 2069. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Wang, X.; Li, R.; Qiao, H.; Ma, P.; Sun, Q.; Zhang, H. Antibacterial actvity of xanthan-oligosaccharide against Staphylococcus aureus via targeting biofilm and cell membrane. Inter. J. Biol. Macromol. 2020, 153, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Tafti, F.; Suyogan, S.; Pinge, S.; Thorat, R.; Sharma, V. Hazards associated with nanotechnology in clinical dentistry. Cureus 2023, 15, e46978. [Google Scholar] [CrossRef]
- Ibrahim, A.; Moodley, D.; Uche, C.; Maboza, E.; Olivier, A.; Petrik, L. Antimicrobial and cytotoxic activity of electrosprayed chitosan nanoparticles against endodontic pathogens and Balb/c 3T3 fibroblast cells. Sci. Rep. 2021, 11, 24487. [Google Scholar] [CrossRef] [PubMed]
- Alhomrany, R.; Zhang, C.; Chou, L. Cytotoxic effect of chitosan nanoparticles on normal human dental pulp cells. Nanosci. Nanotechnol. 2019, 3, 16. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Ghotaslou, R.; Kordi, S.; Khoramdel, A.; Aeenfar, A.; Kahjough, S.T.; Akbarzadeh, A. Antibacterial and antifungal effects of chitosan nanoparticles on tissue conditioners of complete dentures. Int. J. Biol. Macromol. 2018, 118 Pt A, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 29, 1312–1338. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Krenek, T.; Kovarik, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valverde, N.; Aragoneses, J.; Lopez-Valverde, A.; Rodrigues, C.; Sousa, B.M.d.; Aragoneses, J.M. Role of chitosan in titanium coatings, trend and new generations of coatings. Front. Bioeng. Biotechnol. 2022, 10, 907589. [Google Scholar] [CrossRef]
- Li, J.; Fan, H.; Li, H.; Hua, L.; Du, J.; He, Y.; Jin, Y. Recent advancements in the surface modification of additively manufactured metallic bone implants. Addit. Manuf. Front. 2025, 4, 200195. [Google Scholar] [CrossRef]
- Tang, W.; Fischer, N.G.; Kong, X.; Sang, T.; Ye, Z. Hybrid coating on dental and orthopedic titanium implants: Current advances and challenges. BMeMAT 2024, 2, e12105. [Google Scholar] [CrossRef]
- Qosim, N.; Dai, Y.; Williams, G.R.; Edirisinghe, M. Structure, properties, forming, and applications of alginate fibers: A review. J. Int. Mater. Rev. 2024, 69, 309–333. [Google Scholar] [CrossRef]
- Khajouei, A.R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaued, P. Structure, properties and applications of alginate. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Wathoni, N.; Cecep, S.; Purnama, M.F.G.P.; Mutmainnah, A.; Nurbaniyah, N.S.; Syafra, D.W.; Elamin, K.M. Alginate and chitosan-based hydrogel enhance antibacterial agent activity on topical application. Infect. Drug Resist. 2024, 17, 791–805. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, Y.; Yan, R.; Lin, M.; Sun, S.; Ma, H. Chitosan-sodium alginate-based coating for self-strengthening anticorrosion and antibacterial protection of titanium substrate in artificial saliva. Inter. J. Biolog. Macromol. 2021, 184, 109–117. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Sardroud, H.A.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Inter. J. Biolog. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- Vakili, N.; Asefnejad, A. Titanium coating: Introducing an antibacterial and bioactive chitosan-alginate film on titanium by spin coating. Biomed. Eng./Biomed. Tech. 2020, 65, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary ammonium chitosans: The importance of the positive fixed charge of the drug delivery systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Ma, Y.; Wu, Y.; Lan, F.; Weir, M.D.; Li, M.; Ren, B.; Oates, T.W.; Xu, H.H.K.; et al. Two-staged time-dependent materials for the prevention of implant-related infections. Acta Biomater. 2020, 101, 128–140. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Huang, Y.-C.; Ding, S.-J. Primary stability of implant placement and loading related to dental implant materials and designs: A literature review. J. Dent. Sci. 2023, 18, 1467–1476. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Z.; Li, Z.; Gu, L. A two-phase and long-lasting multi-antibacterial coating enables titanium biomaterials to prevent implants-related infections. Mater. Today Bio 2022, 15, 100330. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridids, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Berezina, O.V.; Rykov, S.V.; Schwarz, W.H.; Liebl, W. Xanthan: Enzymatic degradation and novel perspectives of applications. Appl. Microbiol. Biotechnol. 2024, 108, 227. [Google Scholar] [CrossRef]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in xanthatan gum-based systems for the delivery of theraupetic agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.M.; Rocha, D.N.; Souza, R.F.B.; Santos, J.L.; Ferreira, J.R.M.; Moraes, A.M. Cell-friendly chitosan-xanthan gum membranes incorporating hydroxyapatite designed for periodontal tissue regeneration. Pharmaceutics 2023, 15, 705. [Google Scholar] [CrossRef]
- Hu, X.; Wang, K.; He, P.; Qiao, H.; Zhang, H.; Wang, Z. Characterization and antioxidant activity of a low molecular weight xanthan gum. Biomolecules 2019, 9, 730. [Google Scholar] [CrossRef]
- Bauer, T.M.; Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 2020, 126, 280–293. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Borbolis, F.; Ploumi, C.; Palikaras, K. Calcium-mediated regulation of mitophagy: Implications in neurodegenerative diseases. npj Metab. Health Dis. 2025, 3, 4. [Google Scholar] [CrossRef] [PubMed]
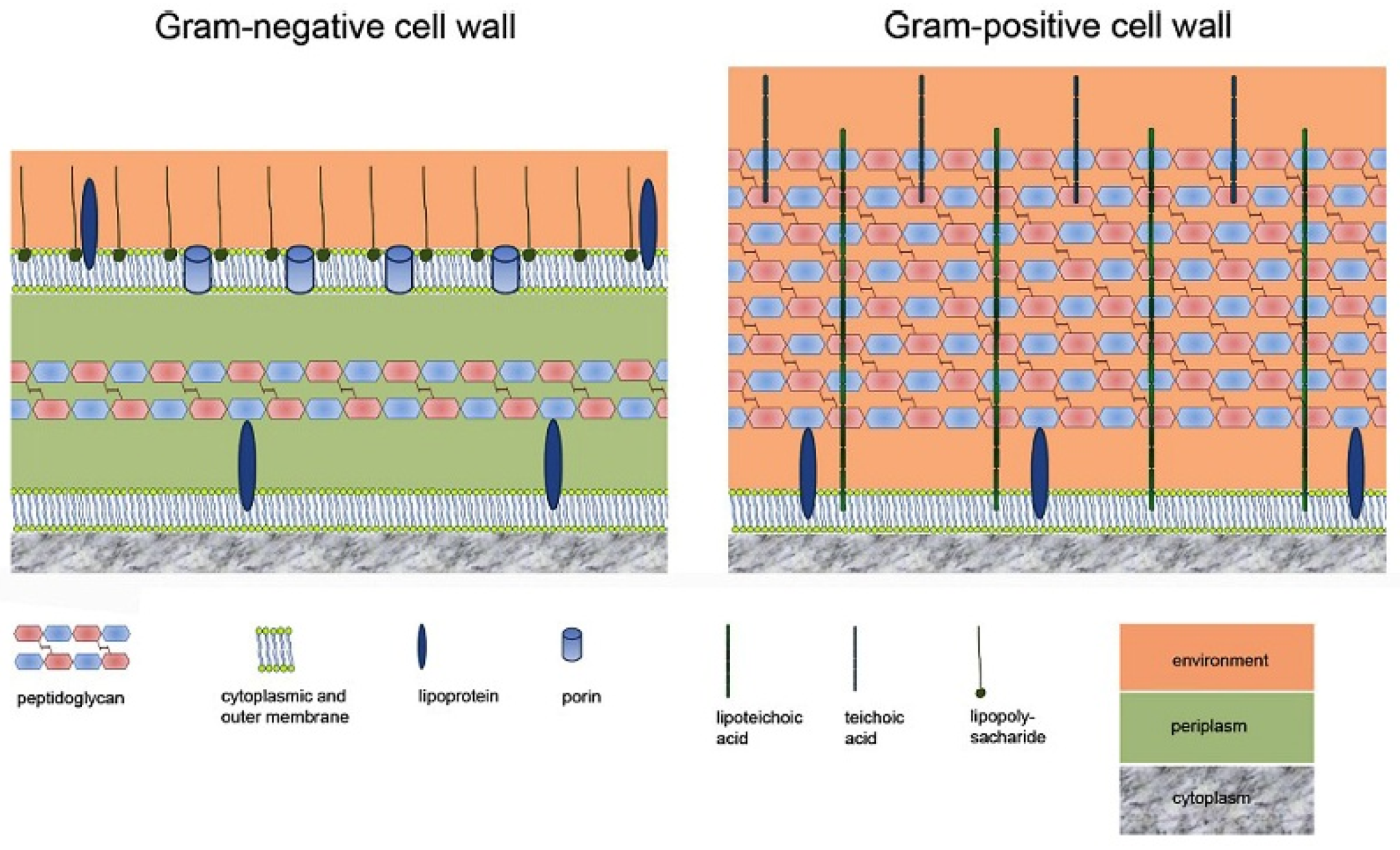
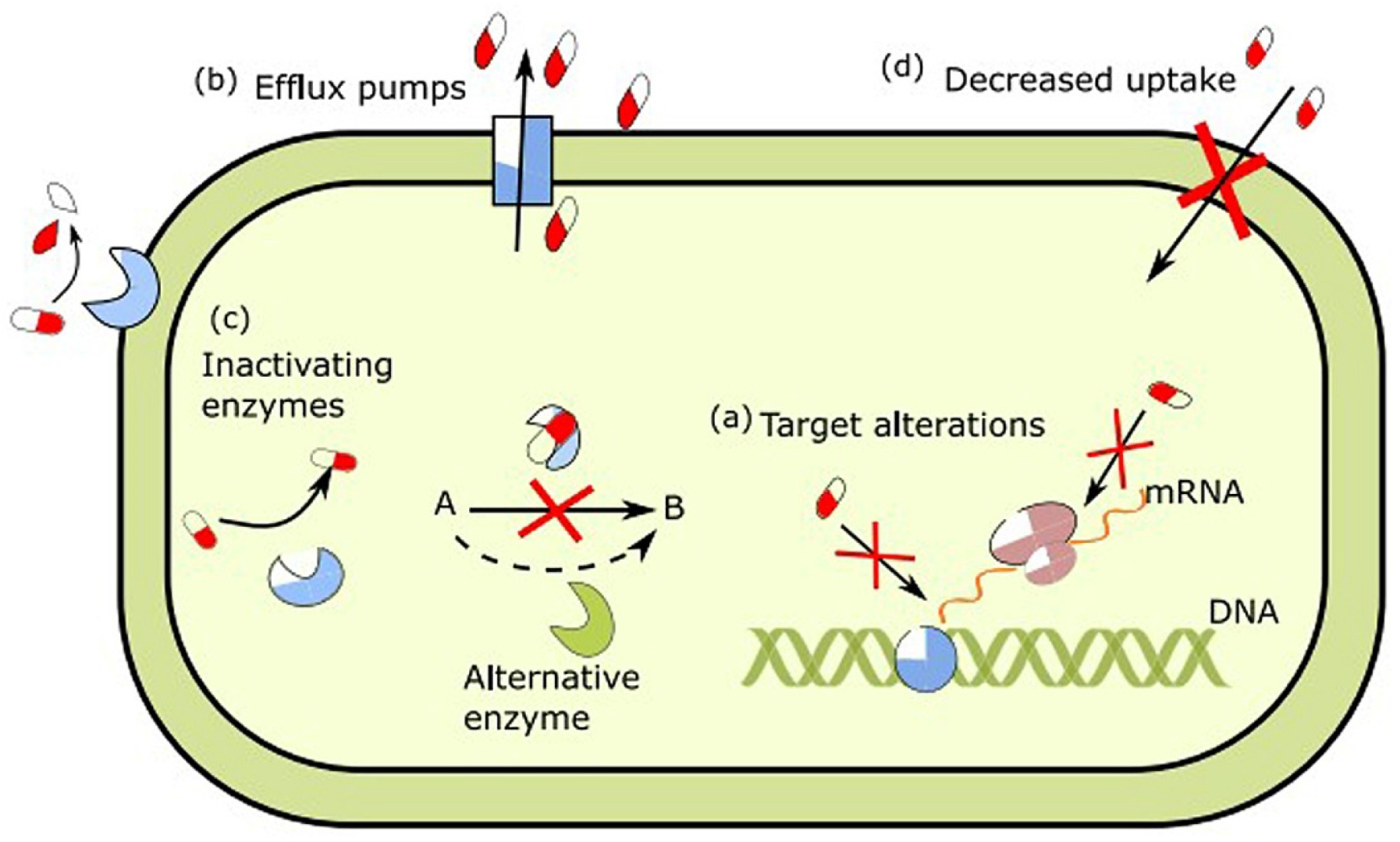
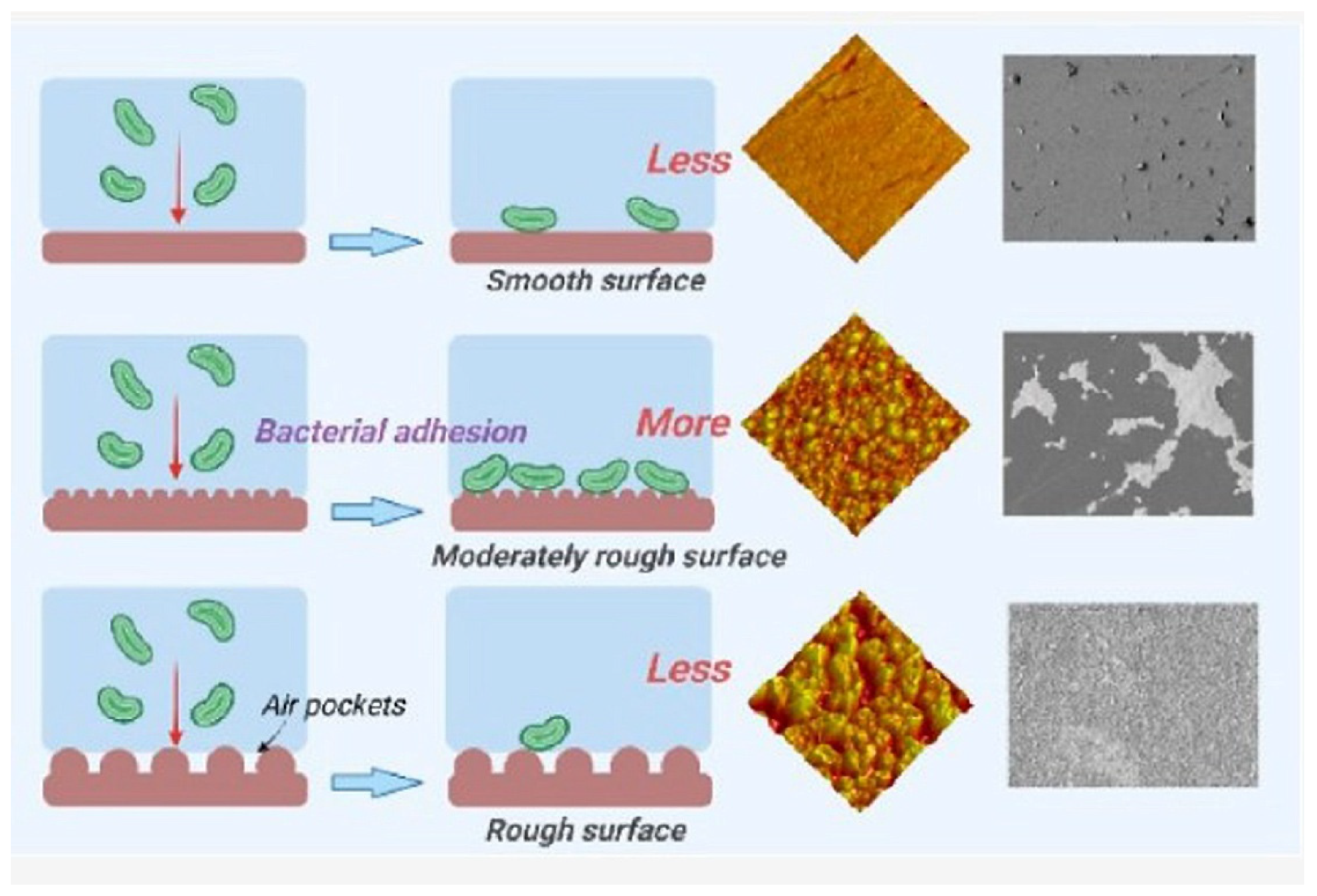
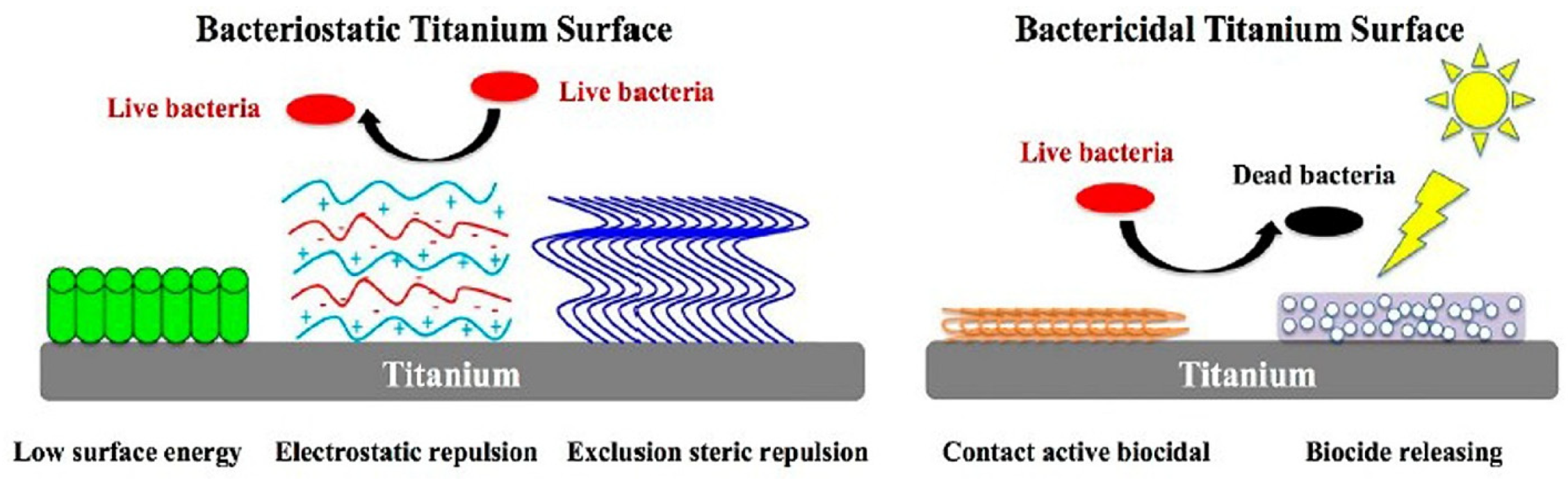


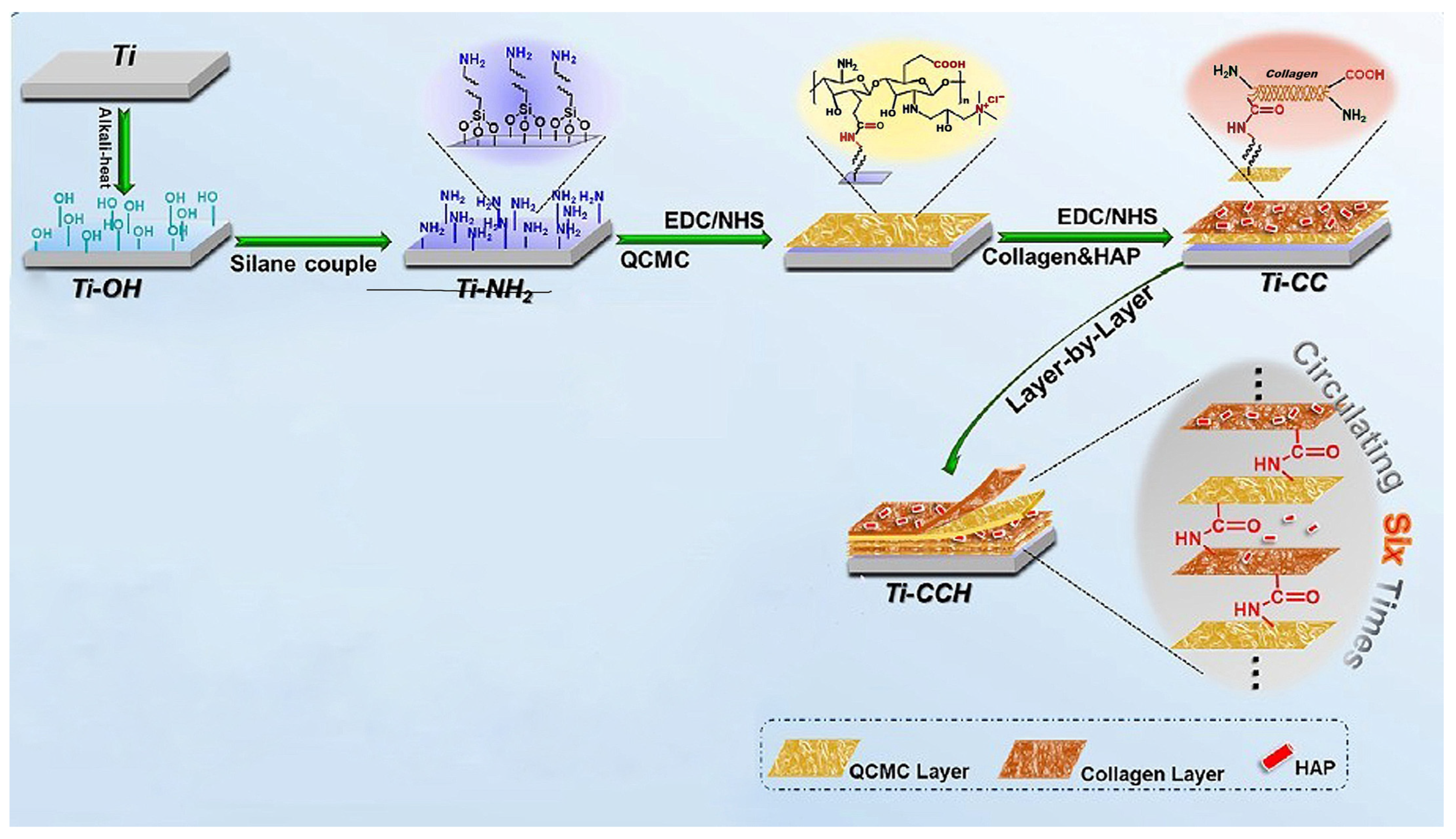
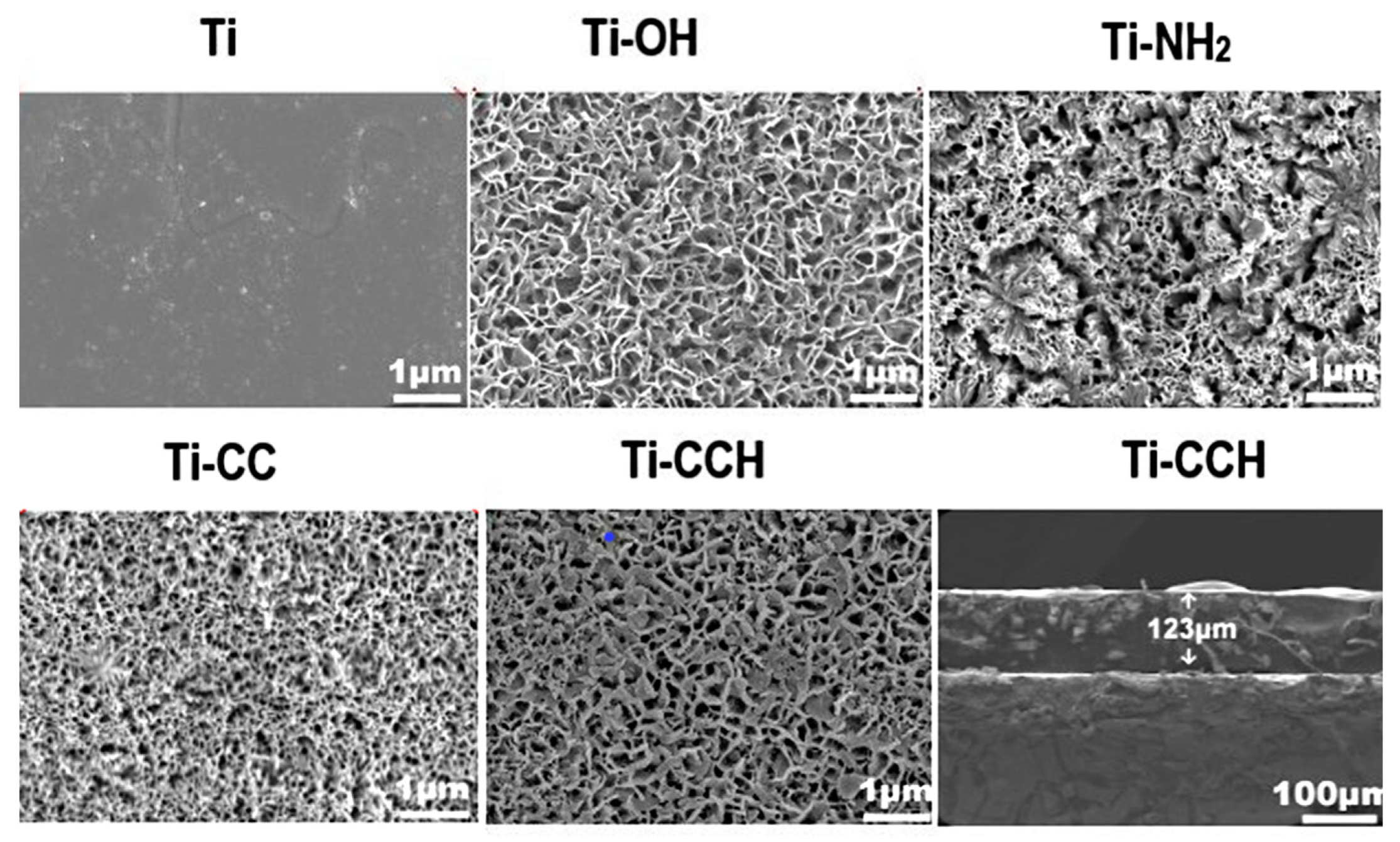
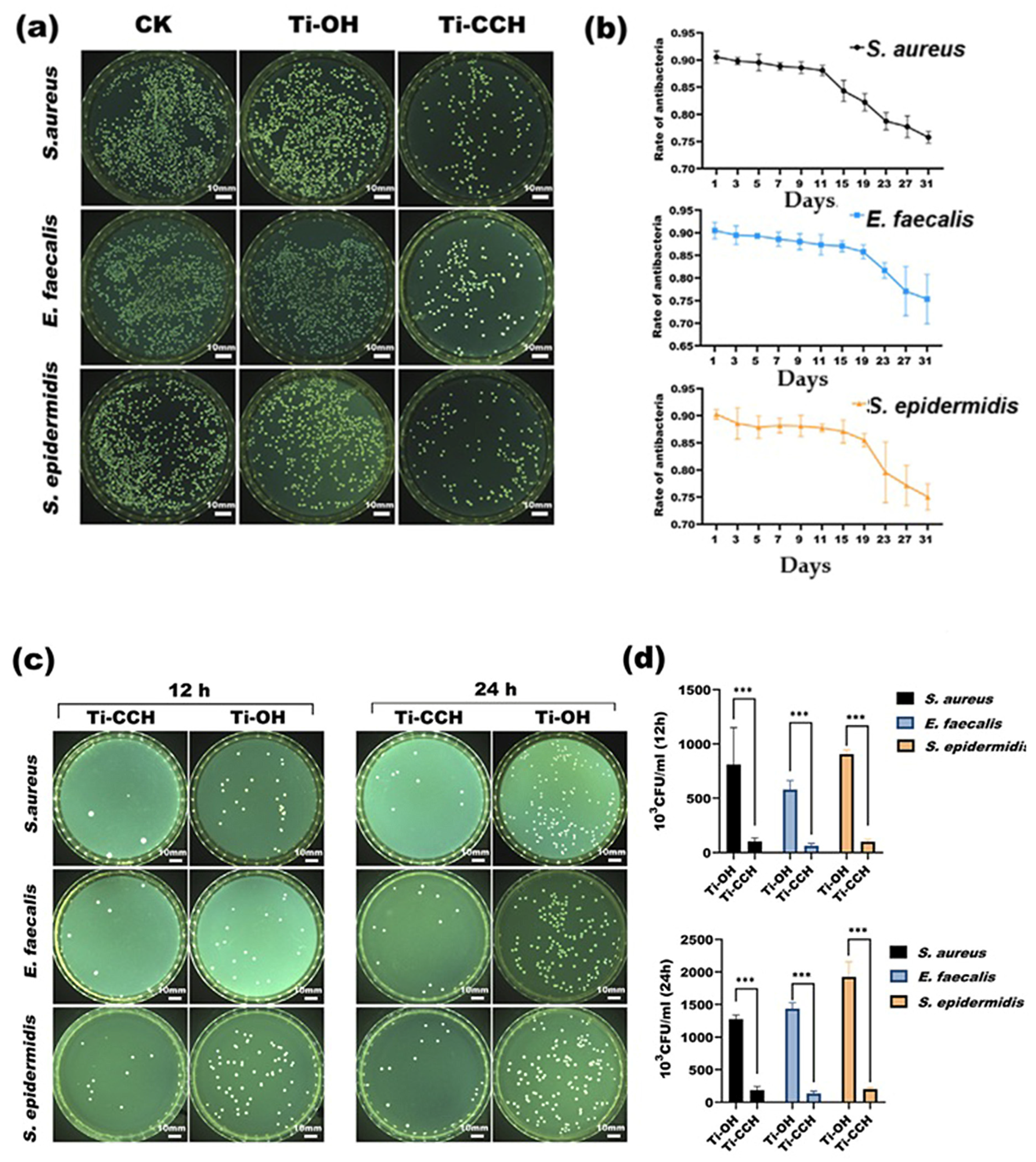

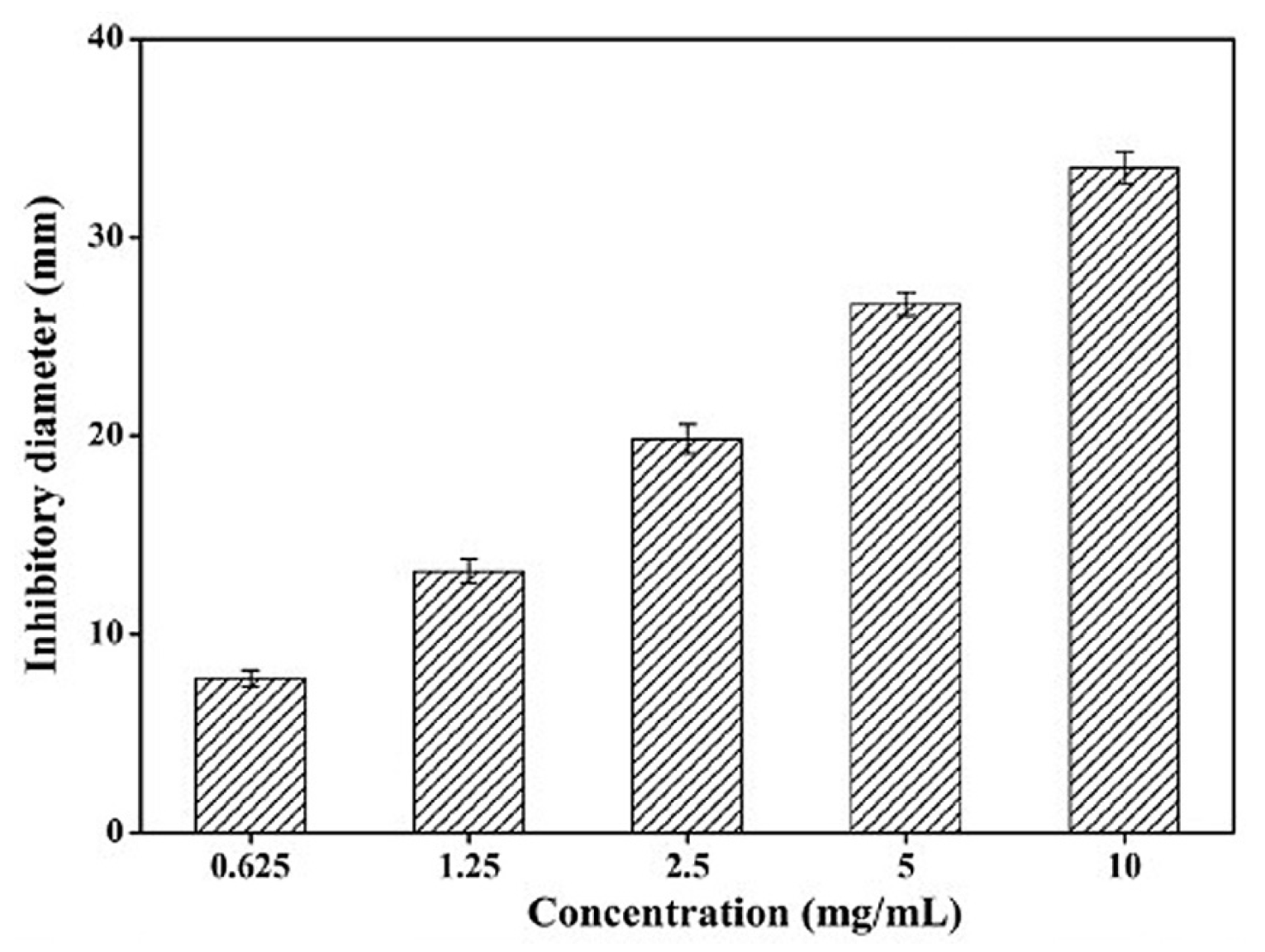
| Material | Properties | Modification | Antibacterial Activity | Mechanism | Ref. |
|---|---|---|---|---|---|
| Chitosan | high antibacterial activity, biocompatibility, biodegradability, nontoxicity, hemostatic effect, rigidity, and brittleness | no | S. aureus E. coli S. gordonii S. epidermis Agg. actinomycetemcomitans P. gingivalis | Electrostatic interaction between chitosan and bacteria. | [100,104,114,117,130] |
| Chitosan | biocompatibility with a positive effect on fibroblast proliferation, hemostatic properties, anti-inflammatory effect, antibacterial activity, anticancer, antitumor | carboxymethyl chitosan (O-CMS) (N-CMS) (N,O-CMS) | S. aureus E. coli P. aeruginosa | Electrostatic interaction between carboxymethyl chitosan. | [131,132,133,134,135] |
| Chitosan | biocompatibility, soluble in physiologic pH, antibacterial | quaternary ammonium trimethyl chitosan (QCMC) | E, Coli P. aeruginosa S. Aureus E. faecalis | Electrostatic interaction between positively charged ammonium groups with negatively charged bacteria. | [131,136,137] |
| Pectin (low-methoxyl commercial citrus pectin) | gelling, thickening, biocompatibility, biodegradability, anticancer, antibacterial, corrosion inhibitor, used to load and control drug release | no | Planktonic bacteria at low pH E. coli S. pyogenes S. aureus E. faecalis | The mechanisms of antibacterial activity are still not understood. The complexity of the structure, differences in extraction methods, and different fragmentation techniques make it hard to determine the active groups. | [126,127] |
| Lemon IntegroPectin | high content of polyphenols, flavonols such as kaempferol, phenolic acids (p-coumaric acid and gallic acid), and monoterpenoid saffron | no | E. coli P. aeruginosa | Synergistic mechanisms involving the intrinsic antibacterial activity of the pectic polymer and antibacterial activity of citrus flavonoids and terpenes at the surface of IntegroPectin. | [128] |
| Alginate (brown seaweeds) | film-forming ability, gelling, nontoxicity, pH responsiveness, hydrophilicity, biocompatibility, biodegradability, wound healing, carriers for drug delivery | mix with AgNPs | S. aureus E. coli S. Epidermis | Ag+ can bind the thiol group of bacteria proteins and interfere with DNA replication. | [138] |
| Xanthan oligosaccharide | lowering of the transcriptional levels of genes (fnbA, fnbB, and dfB) related to biofilm formation | biodegradation of commercial xanthan | S. aureus | Increase in cell membrane permeability. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallmann, L.; Gerngroß, M.D. Antibacterial Polysaccharides in Dental Implantology. Mar. Drugs 2025, 23, 321. https://doi.org/10.3390/md23080321
Hallmann L, Gerngroß MD. Antibacterial Polysaccharides in Dental Implantology. Marine Drugs. 2025; 23(8):321. https://doi.org/10.3390/md23080321
Chicago/Turabian StyleHallmann, Lubica, and Mark Daniel Gerngroß. 2025. "Antibacterial Polysaccharides in Dental Implantology" Marine Drugs 23, no. 8: 321. https://doi.org/10.3390/md23080321
APA StyleHallmann, L., & Gerngroß, M. D. (2025). Antibacterial Polysaccharides in Dental Implantology. Marine Drugs, 23(8), 321. https://doi.org/10.3390/md23080321





