Abstract
Macroalgae (seaweeds) produce unique bioactive metabolites that have enabled their survival for millions of years, offering significant potential for human benefits. In the Israeli Mediterranean Sea, no comprehensive systematic surveys of seaweeds have been published since the 1990s, and their chemical composition remains largely unexplored. This study presents an extensive survey of intertidal seaweed communities along the shallow Israeli coastline, documenting their spatial, temporal, and biochemical diversity. Of the 320 specimens collected, 55 seaweed species were identified: 29 red (Rhodophyta), 14 brown (Phaeophyceae), and 12 green (Chlorophyta). A significant shift in species abundance was documented, with a single dominant annual bloom occurring during spring, unlike previously reported biannual blooms. Chemical analysis of the dominant species revealed significant seasonal variations in compound levels, with higher protein content in winter and increased antioxidant capacity during spring. Phenolic and natural sunscreen compounds (mycosporine-like amino acids, MAAs) showed no general seasonal trend. These findings highlight the optimal environmental conditions for seaweed growth and underscore their potential for aquaculture and biotechnology. We hypothesize that the ecologically unique conditions of the Israeli Mediterranean Sea may foster resilient seaweed species enriched with distinctive chemical properties, suitable for nutritional, health, pharmaceutical, and nutraceutical applications, particularly as climate-adaptive bioresources.
1. Introduction
Alongside their significant ecosystem services and nutritious ingredients, marine macroalgae (seaweeds), are among nature’s key sustainable resources, with rapidly growing human interest in their use as food, nutraceutical, cosmeceuticals, nutricosmetic, and pharmaceutical applications [1]. Seaweeds form the basis of many coastal marine ecosystems worldwide, providing shelter, nursery sites, and food for diverse marine species [2]. They are among the planet’s most productive primary producers, generating life-supporting oxygen and fixing carbon, attributes that help reduce greenhouse gases and counter global warming [3,4]. One of the most important ecosystem services of seaweeds is their unique ability to assimilate and purify dissolved nutrients and pollutants from the aquatic environment, thus helping in maintaining the ecological balance [5].
With the global population increasing, farmland decreasing, and climate change intensifying, edible seaweeds present a promising sustainable food source from the sea, potentially ensuring food security for future generations. For example, certain red seaweeds (Rhodophyta) can contain nearly 50% protein of their dry weight (DW) [6]. Generally, seaweed proteins are rich in essential amino acids, meeting FAO requirements, with species such as Palmaria sp., Porphyra sp., and Ulva spp. offering comparable essential amino acid levels to eggs, soybeans, and other leguminous plants [7]. Seaweeds are also a natural source of carotenoids and omega-3 fatty acids, and are the most abundant source of polysaccharides such as alginate, agar, fucoidan, ulvan, agarose, and carrageenan, used for many biomedical, cosmetic, and food applications. Some of these polysaccharides are used as dietary fibers important for a healthy digestion due to their prebiotic properties [8,9]. Furthermore, seaweeds are rich sources of vitamins A, B, C, and E, as well as essential minerals such as I, Fe, Zn, and Mg, often exhibiting concentrations 10–100 times higher than those found in land vegetables [10].
To overcome the fluctuating conditions present in the intertidal zone, seaweeds have evolved an arsenal of unique natural chemical defenses and other diverse adaptations. These adaptations include secondary metabolites that possess strong bioactive attributes such as antioxidants, phenolic compounds, and natural ultraviolet radiation (UVR) sunscreen substances such as mycosporine-like amino acids (MAAs), which we have evaluated in the current study [8,11].
Environmental oxidative stress in seaweeds can lead to the generation of reactive oxygen species (ROS), which may damage essential cellular components, including proteins, lipids, nucleic acids, and the photosynthetic apparatus. In response, seaweeds increase their antioxidant defenses, including ROS-scavenging enzymes (e.g., superoxide dismutase, catalase) and nonenzymatic secondary metabolites such as phenolic compounds, α-tocopherol (vitamin E), ascorbic acid (vitamin C), carotenoids, phycobiliproteins, and MAAs [12,13]. In the human body, ROS are associated with various clinical conditions such as aging, arthritis, strokes, atherosclerosis, diabetes, cancers, and neurodegenerative disorders. Seaweeds, with their relatively high antioxidant levels, represent a promising natural antioxidant resource, documented to mitigate oxidative damage, boost the immune system, and reduce disease risk [13].
Phenolic compounds, secondary metabolites found in terrestrial plants, algae, and many seaweed species, play vital roles in growth and survival. They are synthesized as a defense response to environmental stressors like UV radiation, metal contamination, and attacks by pathogens and insects. These compounds offer diverse beneficial biological functions, including wound healing, antioxidant, anti-inflammatory, antitumoral, antiviral, and antimicrobial properties [14].
One of the important algal photoprotective molecules are the MAAs, found largely in red seaweeds [15]. MAAs are thought to have evolved as a defense mechanism against chronic exposure to UVR in sunlight-rich, shallow-water habitats. These natural photoprotectors safeguard living cells by absorbing harmful UVR (310–362 nm range) and converting its energy into heat, thereby reducing the production of ROS [16,17]. Additionally, MAAs possess diverse bioactive properties, including potent antioxidant, anti-aging, and anti-inflammatory effects. In human skin, UVR exposure can cause photo-aging, lipid peroxidation, loss of skin resilience, wrinkles, and skin cancer. MAAs present a promising environmentally friendly natural alternative for the production of UV filters [18].
That being said, the biochemical composition of seaweeds is not stable and can vary greatly depending on environmental factors, including geographic location, seasonality, depth, irradiance, salinity, and nutrient availability, as well as on developmental stages, and biotic stressors. Currently, a significant knowledge gap remains regarding how such factors may influence the biochemical response of seaweeds, leading to the formation of specific compounds [19,20,21]. Acquiring this knowledge is especially essential in light of the rising public demands for natural, high-quality, health-promoting products that can offer an alternative to synthetic chemical materials. Currently, seaweed production is primarily focused on raw commoditized biomass, with less than 1% utilized for high-value, health-promoting products [22,23]. Moreover, out of the thousands of known seaweed species, only a relatively small number are currently being exploited. This leaves a vast underexplored biodiversity of seaweeds, offering the potential for additional utilizations [24].
The Levantine Basin, in which the Israeli Mediterranean coast is located, is environmentally unique, characterized by oligotrophic conditions and relatively high levels of temperature, salinity, and irradiance, which may encourage the development of chemically distinct seaweed species [25,26,27,28,29]. Despite the potential of seaweeds as a globally and regionally valuable natural resource, comprehensive studies examining the distribution, biodiversity, and chemodiversity of natural seaweed populations along the Israeli Mediterranean coast have remained relatively limited and infrequent, with the last systematic field survey conducted several decades ago [30,31].
In this study we had two main goals: to explore the biodiversity and seasonal variation of intertidal seaweed communities along the Israeli Mediterranean Sea coast; and to investigate the seasonal variability of the chemical constituents, including protein, polyphenol, MAA, and antioxidant levels, for the local seaweeds collected over time.
2. Results
2.1. Seaweed Survey
The distribution of red, brown, and green seaweeds across the survey collection sites and throughout the sampling period is presented in Figure 1 and Figure 2.
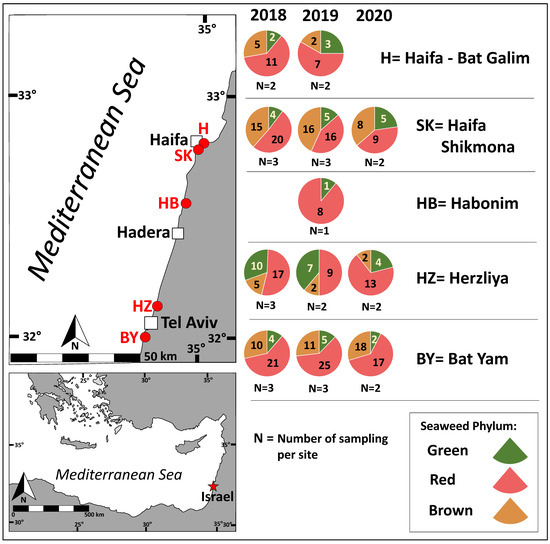
Figure 1.
Seaweed phyla abundance according to sampling site. Chlorophyta (green), Heterokontophyta class Phaeophyceae (brown), and Rhodophyta (red). Sampling sites are labeled in red on the map. The number of species sampled per phylum is indicated (each species was counted only once), along with the sampling effort per site (N).
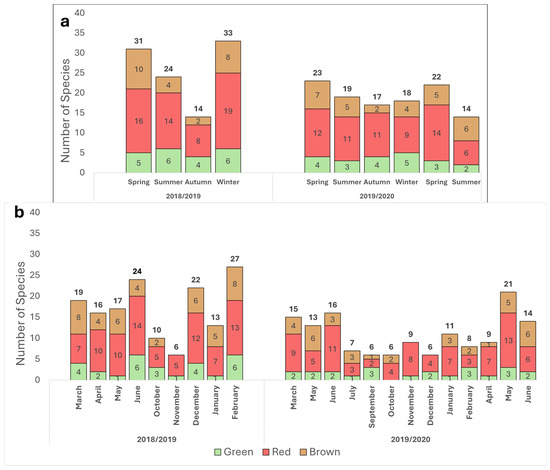
Figure 2.
Seasonal (a) and monthly (b) distribution of seaweeds by phyla, from March 2018 through June 2020. Chlorophyta (green), Heterokontophyta class Phaeophyceae (brown) and Rhodophyta (red). The number of species per phylum is indicated inside each column, and total number of species recorded is noted at the top. Each species was counted only once per the defined period.
Red seaweeds were dominant throughout the survey for the different seasons and geographical locations (Figure 1 and Figure 2). Brown seaweeds were observed in larger numbers during winter and spring. The general number of seaweeds increased gradually with the progress of the seasons, throughout the winter period, reaching its maximal peak during spring (Figure 2). A characteristic spatial and seasonal subdivision of the intertidal euphotic zone was also observed for the different species. The most abundant species were Jania rubens, Corallina elongata, Laurencia papillosa, Hypnea musciformis, and Gelidium pusillum for the red seaweeds; Padina pavonica, Dictyota dichotoma, and Sargassum vulgare for the brown seaweeds; and all Ulva species (Ulva rigida, Ulva compressa, Ulva fasciata) and Codium adhaerens for the green seaweeds. The species J. rubens, L. papillosa, and P. pavonica were observed year-round, in all seasons (Supplementary Table S1).
A total of 55 seaweed species were identified in situ during the current survey– comprising 29 red seaweeds (Rhodophyta), 14 brown seaweeds (Heterokontophyta, class Phaeophyceae), and 12 green seaweeds (Chlorophyta), all originating primarily from the intertidal zone (Table 1).

Table 1.
Seaweed species sampled in the survey classified according to phylum. Chlorophyta (green seaweeds), Heterokontophyta, class Phaeophyceae (brown seaweeds), and Rhodophyta (red seaweeds). In total 55 species.
Supplementary Table S1 presents the seaweed species seasonal checklist. Supplementary Figure S1 presents images of all the surveyed seaweeds. As characterized for the eastern Mediterranean Levant region, the highest number of species observed during the current study belonged to the Phylum Rhodophyta, red seaweeds (53%), followed by Heterokontophyta (Phaeophyceae), brown seaweeds (25%), and Chlorophyta, green seaweeds (22%).
2.2. Biochemical Composition
The most dominant species from each phylum were selected for biochemical analysis, as presented in Figure 3.

Figure 3.
The eight seaweed species that were chemically evaluated: (a) Hypnea musciformis, (b) Ulva rigida, (c) Sargassum vulgare, (d) Jania rubens, (e) Dictyota dichotoma, (f) Padina pavonica, (g) Laurencia papillosa, and (h) Codium adhaerens.
2.2.1. Protein Content
Except for Hypnea musciformis and Codium adhaerens which showed similar protein percentages throughout the year (Permutation Anova, F3,22 = 1.36, p = 0.32, One-way Anova, F3,17 = 0.9, p = 0.49, respectively), all other seaweeds presented significantly higher protein levels in winter compared to the other seasons (Supplementary Table S2, Table 2). Different species showed significantly different protein levels. Overall, Dictyota dichotoma had the highest protein levels followed by H. musciformis (Permutation Anova, F7,178 = 36.6, p < 0.0001, Table 2).

Table 2.
Evaluated compounds, including total protein (% of DW), antioxidant activity (mg TE g−1 of DW), and polyphenol levels (mg PE g−1 of DW) for the eight seaweed species sampled throughout the survey, along with the indications of their seasonal variation trends. ‘NA’ indicates the absence of the species in a given season or insufficient sample material for chemical analysis. A significant increase in compound content compared to other seasons is highlighted in bold.
2.2.2. Antioxidant Activity
For most seaweeds evaluated, a general seasonal trend was observed, showing significantly higher antioxidant capacity during spring (One-way Anova, Permutation Anova, p < 0.01, Supplementary Table S2, Table 2). The elevated antioxidant levels in spring were observed for all brown seaweeds and additionally for Codium adhaerens and Jania rubens. Maximal differences reached 286% for Padina pavonica (brown seaweeds), 170% for C. adhaerens (green seaweeds), and 132% for J. rubens (red seaweeds). Laurencia papillosa had the highest antioxidant levels during the summer season. Significantly lower antioxidant capacity was observed for L. papillosa and U. rigida during autumn. Hypnea musciformis showed similar antioxidant capacity throughout the year (Permutation Anova, F3,29 = 1.8, p = 0.19, Table 2). Overall, significant differences were found among the species antioxidant levels. Brown seaweeds displayed the highest antioxidant capacity, followed by the red, and green seaweed species (Permutation ANOVA, F7,235 = 109.6, p < 0.0001, Table 2).
2.2.3. Phenolic Compounds
Phenolic compound content was evaluated for the brown and green seaweed species. Overall, no general seasonal trend was observed for the different species in their phenolic levels. Padina pavonica showed similar phenolic content across seasons with relatively higher content during autumn (One-way Anova, F3,32 = 2.49, p = 0.08, Table 2). Significant differences in the phenolic content were observed for the other seaweed species during different seasons (One-way Anova, Permutation Anova, p < 0.01, Supplementary Table S2, Table 2). Sargassum vulgare showed the highest polyphenol levels during spring (reaching a seasonal average of 37 mg PE g−1 DW). Dictyota dichotoma showed significantly higher polyphenol levels during autumn. In contrast, Ulva rigida had significantly low phenolic content in autumn, while Codium adhaerens had the lowest during winter. Generally, the brown seaweeds presented significantly higher phenolic content compared to the green seaweed species, with the highest values displayed by S. vulgare (Permutation Anova, F4,166 = 113.4, p < 0.0001, Table 2).
2.2.4. Mycosporine-like Amino Acids (MAAs)
MAAs were evaluated for the red seaweeds. Jania rubens showed significant seasonal variation in total MAA content, reaching a peak during winter (One-way ANOVA, F3,32 = 53.8, p < 0.0001, Table 3), while both Laurencia papillosa and Hypnea musciformis presented similar total MAA levels across seasons (One-way ANOVA, Permutation Anova, F3,32 = 2.03, 0.11, p = 0.13, 0.95, respectively, Table 3). Generally, all three red seaweeds contained the MAA palythine. J. rubens also contained shinorine, and small amounts of asterina-330 (0.01–0.09 mg g−1 DW). L. papillosa also contained asterina-330 as a main MAA. H. musciformis contained in addition to palythine, large quantities of palythinol (as the main MAA), shinorine, and, during winter, also exhibited the MAA porphyra-334 (Table 3, Figure 4). A seasonal effect was also evident in the MAA composition of J. rubens; for example, palythine fluctuated between seasons ranging between 43 and 65%, increasing during spring. Asterina-330 altered between 7 and 20%, peaking during winter, and shinorine levels changed from 27% in spring to 47% in autumn. In contrast, L. papillosa and H. musciformis showed only small seasonal variation in their MAA composition (Table 3, Figure 4). Overall, H. musciformis displayed significantly higher MAA content compared to J. rubens and L. papillosa (permutation ANOVA, F2,105 = 153.3, p < 0.0001, Table 3).

Table 3.
Mycosporine-like amino acids (MAAs) average content (mg g−1 DW) in Jania rubens, Laurencia papillosa, and Hypnea musciformis sampled across different seasons during the survey. The total MAA content for each species in each season is highlighted in bold. ‘NA’ indicates the absence of a given MAA.
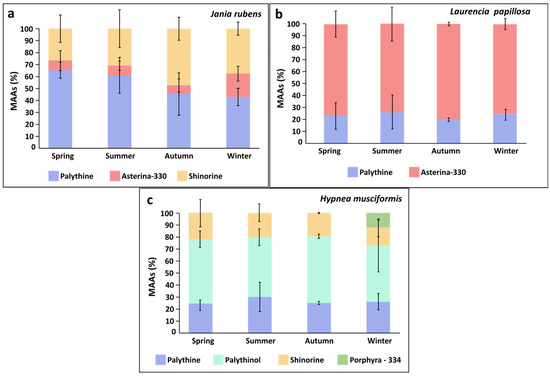
Figure 4.
Seasonal average proportions (%) of Mycosporine-like Amino Acids (MAAs) throughout the sampling period for (a) Jania rubens, (b) Laurencia papillosa, and (c) Hypnea musciformis.
3. Discussion
3.1. The Seaweed Survey: Ecological and Functional Insights
Overall, a total of 55 seaweed species were identified in this study out of about 300 marine macroalgae previously described for this geographical area [31]. Along the Mediterranean Sea, as one travels eastward, nutrients level decrease and seawater temperature and evaporation increase. As a result, the eastern Mediterranean Sea, particularly the Levantine Basin, represents an oligotrophic marine environment, characterized by relatively higher salinity and temperature compared to the western basin. Key nutrients, such as nitrogen and phosphorus, are generally limiting algal growth [27,29]. Seaweed biodiversity is therefore lower in the eastern Mediterranean basin than in the western basin, although it still exhibits a relatively impressive level of diversity [26,29]. Additionally, the Levantine Basin has long been exposed to increasing anthropogenic pressures, including the introduction of invasive species, pollution, and urban development [32,33].
The seaweeds inhabiting the intertidal are constantly influenced by tidal fluctuations that shape their communities. During the calm seas and low tides common in this area, seaweeds may be exposed to heavy desiccation, and high irradiance, temperature, and salinity. Conversely, in high sea conditions, turbulence and wave forces can supply the seaweeds with minerals, nutrients, and dissolved CO2, benefiting their growth. Biotic factors such as competition, epiphytism and herbivory, also play a fundamental part in the location and boundaries of different seaweeds [34,35]. Along the Israeli coast in particular, the dynamic environmental conditions encourage the growth of species that have developed unique physiological, biological, and chemical adaptations [36,37].
Leaf-like species such Ulva spp. and Porphyra sp. (now classified as Neopyropia) thrive in the upper intertidal due to their high tolerance to desiccation and their ability to maintain photosynthesis during exposure to dry conditions. Ulva has a worldwide distribution, is typically opportunistic with high temperature resilience, and hence can grow rapidly and spread both in summer and winter [35,38]. Codium spp. were also observed in the current survey as a dominant species occupying the subtidal zone, concealed beneath shaded rocky environments. Acanthophora najadiformis and Hypnea musciformis are seasonal red species and were usually observed during the colder seasons, dominating the opportunistic summer species. They were documented to be less resilient, and sensitive to high temperature, desiccation, and salinity [36]. The most dominant brown seaweed species were Padina pavonica, followed by Dictyota dichotoma and Sargassum vulgare. These species typically inhabit the subtidal zone. P. pavonica exhibited a relatively broad distribution and was observed year-round. This widespread presence is likely attributed to its ability to perform photosynthesis both when submerged and when exposed during low tides [34,36]. Jania rubens is a well-known prevalent red opportunistic species, and is probably the most common red seaweed in the eastern Mediterranean, occupying the intertidal and subtidal zones. Acting also as an epiphyte upon other seaweeds, it was observed year-round in the current survey and is usually closely accompanied by another dominant species - Corallina elongata. Both are calcareous red algae, with a robust skeletal bio-structure that enable them to endure strong wave conditions [39]. The red seaweed Laurencia papillosa is also one of the most abundant seaweeds of the Israeli upper tidal zone, occupying the edge of the abrasion platform, and was observed year-round in the current study. It has a unique physical ability to adapt to both light and shade, enabling it to thrive under changing irradiance levels, and was described to possess a unique mucus layer filled with a beneficial symbiotic microbiome that enhances its endurance [40].
In contrast to previous works that have described a seaweed seasonal biodiversity trend of two annual high growth seasons, in spring and autumn [35], the current survey suggests a different pattern, with only one growth maximum occurring during springtime. This growth peak diminishes through summer and autumn and then swiftly resumes towards the end of winter. This shift in the local seaweed bloom may be attributed to the impact of regional environmental changes and global warming. It is widely understood that the spring season offers optimal balanced growth conditions for seaweeds to prosper. The abiotic factors of temperature and irradiance are favorable, coupled with a high level of nutrients following the winter mixing and the run-off from terrestrial sources. Through this mechanism, nutrients are elevated from the sea bottom layer to the upper water column and sea surface, nourishing the algal flora [41,42]. Interestingly, this seasonal cycle was recently similarly described for the phytoplankton communities in the upper 50 m in the eastern Israeli Mediterranean [27,43]. Finally, the high seaweed growth documented during the winter of 2018 likely occurred due to intense winter storms in this particular year, which generated a plume of coastal freshwater rich in nutrients and submarine groundwater discharge. This phenomenon increased the general autotrophic activity and may have significantly impacted the local macroalgae community, thereby promoting their growth [44].
3.2. Protein Content in the Surveyed Seaweeds
Seasonality has been recorded to have a significant effect on seaweed protein content. Generally, seaweed protein is highest during the period of winter–early spring and lowest during summer–early autumn. Such fluctuations are believed to be particularly associated with a rich nutrient supply and nitrogen availability [45]. For example, the protein content of Palmaria palmata collected from the French Atlantic coast, exhibited the highest values during the winter and spring months. A similar pattern has also been reported for other green and brown seaweed species [6,46]. In the current work, most of the species presented significantly higher protein levels during the winter. The seaweeds exploit the dissolved nutrients and nitrogen in their environment to generate proteins and build their tissues [47,48]. Additionally, during autumn and winter, certain seaweeds have been reported to store nitrogen (N) and phosphorus (P) as reserve resources to contend with seasonal light and nutrient limitation periods. This is suggested to be an ecological strategy to enhance their competitive ability [49]. However, not all species demonstrate this trend, as observed in the case of Codium adhaerens and Hypnea musciformis in the current survey, as well as in other species described in previous studies [50].
Generally, green and red seaweeds are reported to have high protein concentrations, comprising approximately 10–30% and 35–47% of their dry weight, respectively, while brown seaweeds typically have lower protein content, averaging 5–15% on a DW basis [6,51]. In the present survey, Dictyota dichotoma revealed a relatively high protein concentration, consistent with values close to 14% DW previously reported for this species [52]. Padina pavonica and Sargassum vulgare also had values within the range of those in earlier studies (9–13.6, 7.7–16.3% DW, respectively) [53,54]. Among the red seaweeds, Jania rubens displayed the lowest values (4–6.5% DW) and H. musciformis presented the highest protein content (12.7–14% DW), both within the range of previously reported protein values for these species [55,56]. Laurencia papillosa protein levels were lower than the 34% DW value described by Kumar and Murugesan (2018) [57]. Among the green seaweeds, Ulva rigida mean protein content was higher than the values of 6.6 and 4.6% DW noted in earlier works [58,59]. C. adhaerens displayed similar or higher protein levels compared to other Codium spp. protein contents of 7 and 6.1% DW reported by McDermid and Stuercke (2003) [60] and Manivannan et al. (2008) [61], respectively. Variations in seaweed protein content in comparison to those in the literature can also be attributed to specimen handling, different drying techniques and evaluation methodologies [7]. In the current study, a nitrogen-to-protein conversion factor of 5 was applied, specifically calculated for seaweed protein nutritional evaluation. This contrasts with the factor of 6.25 used in many previous studies, which has been suggested to be significantly overestimated [62,63].
Despite the encouraging potential of seaweed proteins, there are still aspects that hinder their possible contribution and utilization, including digestibility and the presence of toxic compounds. Future studies will need to focus on novel and viable extraction and pre-treatments methods that could help to overcome these issues, improving seaweed proteins’ safety and bioavailability [64,65,66,67].
3.3. Antioxidant Activity of Natural Seaweeds
In the current study seasonality had a significant effect on seaweed antioxidant activity. For most species evaluated, particularly the brown seaweeds, the antioxidant capacity was highest during the spring. A similar trend was observed in work conducted on seaweeds collected from a coastal marine ecosystem located in Alexandria, Egypt, in the eastern Mediterranean [68]. Celis-Plá et al. (2016) [69], similarly found that Cystoseira tamariscifolia accumulated a high concentration of antioxidant and phenolic compounds during spring, while in contrast, during summer, photoinhibition and low nutrient levels led to an opposite trend. The spring season in the Israeli Mediterranean Sea may provide ideal conditions for the synthesis of high levels of antioxidant substances in seaweeds. As noted, the nutrient-rich seawater resulting from the preceding winter mixing, followed by increasing irradiance, serve as building blocks and energy supply for the synthesis of the necessary antioxidant metabolites [27,70]. Elevated nutrient levels have already been documented to play a key role in enhancing seaweeds’ antioxidant defenses against abiotic stressors [71,72]. Lesser et al. (2006) [73] demonstrated that nitrogen-supplied algae were more resilient to high ultraviolet radiation (UVR) compared to nitrogen-limited algae, aiding the algal cell to repair radiation damage and increase the turnover of critical proteins, photosynthetic pigment complexes, and necessary antioxidant defense compounds.
In the current survey, Codium adhaerens exhibited antioxidant levels similar to those reported by Pedro et al. (2022) [74] for Codium sp., 2.22–2.75 mg TE g−1 DW. The antioxidant level of Ulva rigida was slightly lower than values described elsewhere (2.36–3.55 mg TE g−1 DW) [70]. In general, the antioxidant capacity of the studied red and brown seaweeds either aligned with or surpassed that of other seaweed species from the same phyla in previous studies, which presented antioxidant values ranging from 1.2 to 15 mg TE g−1 DW [75]. In the current work, the brown species exhibited significantly high antioxidant levels compared to the red and green species. Sargassum vulgare in particular exhibited a relatively high antioxidant capacity of 56.98 ± 12.25 mg TE g−1 DW. Brown seaweeds have been widely acknowledged for their rich content of antioxidant compounds, including sulfated polysaccharides, phenolic compounds, flavonoids, fucans, fucoxanthin, β-carotene, and more. This indicates their high potential for medical and nutritional antioxidant applications [53,76,77,78]. Antioxidant activity in general may be associated with compounds that provide photoprotection and the neutralization of ROS such as carotenoids and phycobiliproteins pigments, phenolic compounds, MAAs, and others [79].
3.4. Phenolic Compounds in the Surveyed Seaweeds
Among the seaweed phyla, the brown seaweeds have been identified as an outstanding and the highest source of phenolic compounds, from simple phenolic acids to more complex polymers such as tannins (mainly phlorotannins). In the present work, brown seaweeds demonstrated the highest relative phenolic content, with Sargassum vulgare showing the highest levels. The phenol levels observed were in line with previously reported values for both brown and green seaweeds [79]. Throughout this work, it became evident that the phenolic content of seaweeds may vary significantly due to environmental factors, making chemical variability often complex and difficult to predict [80]. Recent studies have highlighted the significant impact of seasonality on the nutritional and chemical profiles of brown seaweeds [19,81]. It is generally assumed that a higher phenolic content, along with increased nutritional content, including polyunsaturated fatty acids (PUFA), vitamins, and minerals, and an enhanced general bioactivity of brown algae, may occur during the hot and dry summer seasons, associated with higher sea temperatures and irradiance [82,83,84]. For example, Čagalj et al. (2022) [19] found that the highest phenolic content in Cystoseira compressa was observed during June. However, other seaweed studies have not indicated a uniform seasonal effect on the concentration of phenolic substances, including in genera such as Padina, Colpomenia, Saccharina, and Dictyota [81,85,86]. Phenolic compounds were shown to present a photoprotective role and to increase under climate change scenarios such as elevated CO2 and temperature [87].
From the current survey, it appears that the different seaweeds do not exhibit a common seasonality trend in their phenolic content. Each species presented a different response, accumulating phenols in different seasons. For example, S. vulgare exhibited the highest phenol levels during spring and Dictyota dichotoma reached its highest levels in autumn, while Padina pavonica maintained relatively constant phenolic content throughout the year. It is possible that each species employs a different ecological strategy or activates varying levels or forms of phenolic compounds based on its biological needs and life history. Given the diversity of polyphenols, such as phenolic acids, flavonoids, stilbenes, tannins, and lignans, more precise monitoring and chemical evaluation would appear to be necessary.
3.5. Mycosporine-like Amino Acids (MAAs) in Israeli Seaweeds: An Untapped Potential
The production of MAAs, nitrogenous photoprotective compounds, largely depends on the availability of nutrients, particularly inorganic nitrogen, and solar irradiance, especially blue wavelengths of photosynthetically active radiation (PAR) and UVR [71,88,89]. The composition and formation of MAA molecules may change according to seaweed habitat or culture conditions, even within the same algal species [70,88,90,91]. Red seaweeds in intertidal zones generally exhibit higher MAA content compared to species in subtidal or deep shaded habitats [90,92,93]. Similarly, seaweeds in low-latitude regions may present higher MAA content compared to those in high-latitude regions [94], positioning the Israeli Mediterranean in the Levantine Basin as a promising region for high MAA occurrence. In the current evaluation, a significant seasonality effect was observed for Jania rubens, which presented a significantly higher total MAA content during winter. The other studied red seaweeds maintained constant total MAA levels throughout the year. However, Hypnea musciformis presented the MAA Porphyra-334 exclusively during winter. Although higher MAA levels are typically expected in more sunny seasons such as summer and spring, it seems that the Israeli winter too can provide the necessary solar energy for MAA synthesis, accompanied by nutrient-enriched seawater. Previous studies have reported a significant seasonal trend in seaweed MAAs, with levels usually increasing in spring but sometimes in winter, attributed to higher daily irradiance and nitrogen-enriched waters [95,96].
The MAA composition and content evaluated in the current work, characterizing each red seaweed species, generally align with those observed in previous studies [70,97]. The MAA palythine was consistently observed in all three red seaweed species throughout the year, with particularly elevated levels in J. rubens during spring and summer. This observation may be associated with the significant antioxidant capacity observed in J. rubens, particularly during the hot sunlit seasons. Previous studies have demonstrated that palythine exhibits the highest antioxidant capacity among common red seaweed MAAs and also serves as a multifunctional photoprotective molecule [98,99]. This suggests that the relatively challenging environmental conditions experienced by seaweeds prompt them to utilize palythine as a protective antioxidant agent, indicating a physiological response to stress [70].
An annual investigation of MAAs could provide valuable insights into the environmental factors influencing their synthesis, both at the species level and at the level of the individual MAA molecule. Sun-rich regions like the Israeli coastline offer a strong potential for the natural production of photoprotective molecules. Such knowledge could help to determine optimal harvesting periods for a high-MAA seaweed biomass in nutrient-enriched coastal areas and serve as a guide for controlled aquaculture cultivation [18,70].
3.6. Practical and Future Perspectives
An important next step will be to conduct extensive routine surveys of local marine flora, expanding the analysis to include additional phytochemical compounds and identifying additional seaweed species as potential candidates for aquaculture. Such efforts will support both ecological assessments and the growing industrial demand for sustainable marine resources.
In this study, in line with previous findings, we suggest that red seaweeds, for example, can be particularly well-suited for cosmetic applications due to their natural sunscreen and antioxidant compounds (e.g., MAAs). Brown seaweeds show strong potential for both cosmetic and pharmaceutical use, owing to their high phenolic content and antioxidant capacity. Green and red seaweeds, with their relatively high protein levels, appear promising for functional foods and nutraceutical applications. Ultimately, each seaweed species offers distinct bioactive properties that can be harnessed for specific health-promoting and nutritional uses.
4. Materials and Methods
4.1. Seaweed Survey and Sample Collections
A seaweed field survey was conducted at five different sites along the Israeli Mediterranean Sea shoreline, between March 2018 and June 2020 (Figure 1 and Figure 2). During this period, one or two surveys were conducted each month, totaling 30 sampling days, and 320 individual seaweeds were surveyed. Sampling was carried out mainly from the intertidal and shallow subtidal zones of the abrasion platforms. We categorized four seasons, each comprising a three-month period, as follows: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February) (Figure 1 and Figure 2).
Taxonomic Descriptions
Species were described taxonomically mainly following visual and microscopic observations. Literature descriptions and herbarium specimens were used as taxonomical keys for comparison and identification (https://www.seaweedherbarium.com, accessed on 1 May 2025). Prior to identification, species were pretreated as detailed below. Specimens that could not be positively identified to the species level were labeled as sp. and maintained for further genetic analysis (Table 1, Supplementary Figure S1).
4.2. Chemical Analysis Sample Preparation
Eight dominant, representative species were evaluated for seasonal changes in their chemical constitutes: two greens, Ulva rigida and Codium adhaerens, three reds, Jania rubens, Laurencia papillosa and Hypnea musciformis, and three brown seaweeds, Dictyota dichotoma, Padina pavonica and Sargassum vulgare (Figure 3). Freshly collected seaweed material was carefully washed with tap water to discard salt, debris, and epiphytes, and finally centrifuged with a kitchen salad spinner to remove excess water. Samples were then freeze-dried using a lyophilizer (Christ, Alpha 1–2 LD plus, Osterode am Harz, Germany), grounded to a fine homogenized powder and stored at −20 °C prior to further chemical analyses. Triplicates from each sample were taken for the different chemical analyses. All seaweed samples were also photographed and documented (Figure 3, Supplementary Figure S1).
4.3. Protein Content Evaluation
Protein content (% of DW) was evaluated using Kjeldahl nitrogen analysis [100]. Dried samples were evaluated for nitrogen and converted to total protein content using a corrected nitrogen-to-protein factor of five, as proposed by Angell et al. (2016) [62].
4.4. Determination of Antioxidant Activity
The seaweed antioxidant activity was evaluated using the ABTS method [101]. Seaweed samples were first extracted by adding 1 mL of DDW to 20–50 mg of dry seaweed powder. The samples were vortexed, placed in an ultrasonic bath for 30 min, and then remained overnight in darkness at 25 °C. On the following day, extracts were centrifuged, and supernatants were used for analyses. ABTS reagent was prepared in sodium phosphate buffer (0.1 M, pH 6.5), using ABTS (2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid, 7 mM) and potassium persulfate (K2S2O8, 2.45 mM). The reagent was incubated in darkness at room temperature for 12–16 h, allowing complete formation of the radical. The assay reaction was performed by adding 950 µL of diluted ABTS reagent with 50 µL seaweed sample extract. The samples were agitated, and absorbance was read via an Agilent Cary 60 UV-Vis Spectrophotometer (Santa Clara, CA, USA) at 727 nm after 8 min of incubation. The blank was phosphate buffer. Antioxidant activity was calculated using the following formula:
4.5. Determination of Phenolic Content
Quantification of phenolic compounds was performed according to the Folin–Ciocalteu method [102] with some modifications. Seaweed samples were first extracted by adding 1 mL of phosphate buffer (0.1 M, pH = 6.5) to 20 mg of dry seaweed powder. The samples were vortexed and remained overnight in darkness at 4 °C. The reaction was performed by adding 100 µL of each seaweed extract to 700 µL distilled water, 50 µL of the Folin–Ciocalteu reagent, and, finally, 150 µL 20% anhydrous sodium carbonate (Na2CO3). The solution was vortexed and incubated at 4 °C in darkness for 2 h. Absorbance was measured at 760 nm using a UV-visible spectrophotometer (UV-2700i Shimadzu, Duisburg, Germany). The blank included all reagents, and the crude extract was replaced by distilled water. Phenolic content was evaluated by constructing a standard curve using different phloroglucinol concentrations. Results were expressed as mg of phloroglucinol equivalent (PE) per g of seaweed dry weight (mg PE g−1 DW).
4.6. Analysis of Mycosporine-like Amino Acids (MAAs)
MAAs were assayed according to Korbee–Peinado et al. (2004) [103]. An amount of 50 mg of dried seaweed was incubated in 20% methanol (1 mL) in a water bath at 45 °C for 2 h. Then, 700 μL of the supernatant was taken and evaporated under vacuum at 45 °C (SpeedVac SPD210 Vacuum Concentrator, Thermo Scientific, Waltham, MA, USA). Dried extracts were redissolved in 700 μL of 100% methanol and vortexed for 30 s. After passing through a 0.2-μm membrane filter, samples were analyzed with an Agilent UHPLC system (1260 Agilent InfinityLab Series, Santa Clara, CA, USA) by using a Luna C8 column (Phenomenex) with an isocratic flow of 0.5 mL min and mobile phase of 1.5% methanol and 0.15% acetic acid in ultrapure water. Isolated MAAs through HPCCC [104] were used as standards. Results were expressed as (mg g−1 DW).
4.7. Statistical Analysis
Statistical analyses were performed using the R statistic program, version 4.3.1, Vienna, Austria. One-way ANOVA (α = 0.05) was used to compare parameters between experiments. Tukey’s HSD test was used for post hoc pairwise comparisons. Data were tested for normality (Shapiro–Wilk test) and homogeneity of variance (Levene’s test). When ANOVA assumptions were not met, a permutation ANOVA test of 5000 repetitions was used, and a Games–Howell test was applied for post hoc comparison. Data in tables and figures are expressed as mean ± SD.
The complete statistical data are summarized in Supplementary Table S2. In the results chapter, when the results of multiple tests are mentioned together, they are referred to Supplementary Table S2.
5. Conclusions
Belonging to ancient and distinct evolutionary dynasties, seaweeds have evolved unique molecules that enable them to thrive in challenging fluctuating environments. They contribute to nearly 3000 different natural products, encompassing around 20% of the entire chemistry of the marine realm [105]. From a biotechnological perspective, seaweeds may be defined as ‘biological machines’, able to produce valuable nutritional and bioactive materials that can be harnessed for human benefit [1,51,106]. This study focused on natural seaweed communities along the Israeli Mediterranean Sea, specifically within the Levantine Basin, the easternmost and one of the most ecologically distinctive regions of the Mediterranean Sea [25,31]. As discussed throughout this work, environmental variability may stimulate the synthesis of protective substances and secondary metabolites in seaweeds. The concentrations of active and beneficial compounds measured in the surveyed species were generally within or above the known range for their genera. Notably, certain species, particularly brown seaweeds, exhibited especially high levels of antioxidants and phenolic compounds.
We suggest that this region may function as a unique ‘incubator habitat’ serving as a hotspot for adapted seaweed species. It may offer insight into potential climate change scenarios and help to predict or ‘foster’ resilient seaweed species capable of enduring future global warming, potentially becoming dominant in other regions under such conditions [42,107]. Those species are likely to be highly tolerant and will possess the necessary physiological, structural, and chemical adaptations, including enrichment in distinctive and beneficial bioactive compounds [108,109,110]. In the current survey we identified the dominant species and highlighted some of their chemical advantages. Our findings indicate how the different seasons, characterized by various environmental factors, may affect the seaweeds’ chemistry. Such knowledge can be translated into aquaculture cultivation protocols to define optimal growth conditions for different seaweed species, enabling the enhancement of their beneficial metabolites for use in nutrition and health applications, including superfoods, dietary supplements, cosmetics, nutraceuticals, and pharmaceuticals [48,70]. Beyond the seaweeds’ practical advantages, their primary benefit lies in their environmental attributes. Cultivating seaweed helps to conserve and balance the natural marine ecosystems. To address environmental degradation, humanity must undergo a paradigm shift and rely on science-based solutions and sustainable technologies [111,112]. Consequently, seaweeds can be defined as a sustainable resource that, if utilized correctly will assist humanity in tackling ongoing global challenges such as the climate crisis, food security, pollution, resource depletion, and the crossing of planetary boundaries.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/md23080320/s1, Figure S1: Images of the seaweed species sampled throughout the survey; Table S1: Seaweeds seasonal checklist; Table S2: Statistical summary.
Author Contributions
Author contributions: Conceptualization, D.Y.A., A.A., S.B.-V., F.L.F. and Á.I.; methodology, D.Y.A., S.B.-V., A.A. and Á.I.; validation, D.Y.A. and J.V.; formal analysis, D.Y.A.; investigation, D.Y.A.; resources, A.A., E.S., F.L.F. and Á.I.; visualization, D.Y.A., A.A., G.P. and Á.I.; supervision, A.A., E.S. and Á.I.; project administration, D.Y.A., A.A., E.S. and Á.I.; funding acquisition, A.A., E.S., F.L.F. and Á.I.; writing—original draft preparation, D.Y.A.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Ministry of Science and Innovation of Spain [ALGA-HUB project; TED2021-131555B-C22], and The Prince Albert II de Monaco Foundation, grant number [2664].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are available in the main text or the Supplementary Materials.
Acknowledgments
We would like to thank the Prince Albert II de Monaco Foundation for their financial support. DYA would also like to express its gratitude for the financial support provided by the Zvi Keinan z”l scholarship of the Institute for Maritime Policy & Strategy (MPS), the Admiral Yohay Ben-Nun Foundation for Marine and Freshwater Sciences, and the Yehoshua Salti Foundation. FLF would like to thank the Ministry of Science and Innovation (project: Algae for more sustainable and healthy functional foods, ALGA-HUB, TED2021-131555B) for their financial aid. We are also grateful to Karen Madmoni and the Interuniversity Institute for Marine Sciences in Eilat (IUI) for providing accommodation and logistical support. Special gratitude to Alla Zalmanzon for her help with the protein analyses. DYA and AA would like to dedicate this work to Itzchak (Itzik) Brickner of blessed memory, with warm appreciation for his guidance, friendship, and inspiration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DW | Dry Weight |
| MAAs | Mycosporine-like Amino Acids |
| FAO | Food and Agriculture Organization |
| UV | Ultraviolet |
| UVR | Ultraviolet Radiation |
| ROS | Reactive Oxygen Species |
| TE | Trolox Equivalent |
| PE | Phloroglucinol Equivalent |
References
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent Advances in Industrial Applications of Seaweeds. Crit. Rev. Food Sci. Nutr. 2023, 63, 4979–5008. [Google Scholar] [CrossRef]
- Pereira, L.; Neto, J.M. Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4665-8167-8. [Google Scholar]
- Krause-Jensen, D.; Duarte, C.M. Substantial Role of Macroalgae in Marine Carbon Sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Israel, Á.; Shpigel, M. Photosynthetic CO2 Uptake by Ulva (Chlorophyta) as a Potential Contribution to Global Warming Containment. J. Appl. Phycol. 2023, 35, 1987–1994. [Google Scholar] [CrossRef]
- Cotas, J.; Gomes, L.; Pacheco, D.; Pereira, L. Ecosystem Services Provided by Seaweeds. Hydrobiology 2023, 2, 75–96. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Černá, M. Chapter 24—Seaweed Proteins and Amino Acids as Nutraceuticals. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 297–312. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive Components from Seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 345–378. ISBN 978-0-12-408062-1. [Google Scholar]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging Prospects of Macro- and Microalgae as Prebiotic. Microb. Cell Fact. 2021, 20, 112. [Google Scholar] [CrossRef]
- Circuncisão, A.; Catarino, M.; Cardoso, S.; Silva, A. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Hu, Z.-M.; Fraser, C. Seaweed Phylogeography: Adaptation and Evolution of Seaweeds Under Environmental Change; Springer: Berlin, Germany, 2016; ISBN 978-94-017-7534-2. [Google Scholar]
- Cruces, E.; Rautenberger, R.; Rojas-Lillo, Y.; Cubillos, V.M.; Arancibia-Miranda, N.; Ramírez-Kushel, E.; Gómez, I. Physiological Acclimation of Lessonia Spicata to Diurnal Changing PAR and UV Radiation: Differential Regulation among down-Regulation of Photochemistry, ROS Scavenging Activity and Phlorotannins as Major Photoprotective Mechanisms. Photosynth. Res. 2017, 131, 145–157. [Google Scholar] [CrossRef]
- Cornish, M.L.; Garbary, D.J. Antioxidants from Macroalgae: Potential Applications in Human Health and Nutrition. ALGAE 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main Bioactive Phenolic Compounds in Marine Algae and Their Mechanisms of Action Supporting Potential Health Benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Richa; Sinha, R.; Singh, S.P.; Häder, D. Photoprotective Compounds from Marine Organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, X.; Zhou, X.-F.; Yang, X.; Liu, Y. Chemical and Biological Aspects of Marine Cosmeceuticals. In Marine Cosmeceuticals; CRC Press: Boca Raton, FL, USA, 2011; pp. 11–38. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-Like Amino Acids from Red Macroalgae: UV-Photoprotectors with Potential Cosmeceutical Applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira Compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- Israel, A.; Martinez-Goss, M.; Friedlander, M. Effect of Salinity and pH on Growth and Agar Yield of Gracilaria tenuistipitata Var. Liui in Laboratory and Outdoor Cultivation. J. Appl. Phycol. 1999, 11, 543–549. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal Chemodiversity and Bioactivity: Sources of Natural Variability and Implications for Commercial Application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Ioannou, E.; Roussis, V. Natural Products from Seaweeds. In Plant-Derived Natural Products: Synthesis, Function, and Application; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 51–81. ISBN 978-0-387-85498-4. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Chopin, T.; Tacon, A.G.J. Importance of Seaweeds and Extractive Species in Global Aquaculture Production. Rev. Fish. Sci. Aquac. 2021, 29, 139–148. [Google Scholar] [CrossRef]
- Krom, M.D.; Groom, S.; Zohary, T. The Eastern Mediterranean. In Biogeochemistry of Marine Systems; Blackwell: Hoboken, NJ, USA, 2003; ISBN 978-0-367-81242-3. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Reich, T.; Ben-Ezra, T.; Belkin, N.; Tsemel, A.; Aharonovich, D.; Roth-Rosenberg, D.; Givati, S.; Bialik, M.; Herut, B.; Berman-Frank, I.; et al. A Year in the Life of the Eastern Mediterranean: Monthly Dynamics of Phytoplankton and Bacterioplankton in an Ultra-Oligotrophic Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2022, 182, 103720. [Google Scholar] [CrossRef]
- Finné, M.; Holmgren, K.; Sundqvist, H.S.; Weiberg, E.; Lindblom, M. Climate in the Eastern Mediterranean, and Adjacent Regions, during the Past 6000 Years—A Review. J. Archaeol. Sci. 2011, 38, 3153–3173. [Google Scholar] [CrossRef]
- Israel, A.; Golberg, A.; Neori, A. The Seaweed Resources of Israel in the Eastern Mediterranean Sea. Bot. Mar. 2020, 63, 85–95. [Google Scholar] [CrossRef]
- Lundberg, B. Composition of the Seaweed Vegetation along the Mediterranean Coast of Israel. In Nature Conservation in Israel, Research and Surveys; Nature Reserves Authority: Jerusalem, Israel, 1996; Supplement 3. [Google Scholar]
- Einav, R.; Israel, A. Checklist of Seaweeds from the Israeli Mediterranean: Taxonomical and Ecological Approaches. Isr. J. Plant Sci. 2008, 56, 127–191. [Google Scholar] [CrossRef]
- Spanier, E.; Zviely, D. Key Environmental Impacts along the Mediterranean Coast of Israel in the Last 100 Years. J. Mar. Sci. Eng. 2023, 11, 2. [Google Scholar] [CrossRef]
- Hoffman, R. Alien Benthic Algae and Seagrasses in the Mediterranean Sea and Their Connection to Global Warming. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 159–181. ISBN 978-94-007-6704-1. [Google Scholar]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; John Wiley & Sons: Hoboken, NJ, USA, 1990; ISBN 978-0-471-62434-9. [Google Scholar]
- Einav, R.; Israel, A. Seaweeds on the Abrasion Platforms of the Intertidal Zone of Eastern Mediterranean Shores. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Cellular Origin, Life in Extreme Habitats and Astrobiology; Springer: Dordrecht, The Netherlands, 2007; pp. 193–207. ISBN 978-1-4020-6112-7. [Google Scholar]
- Einav, R.; Breckle, S.; Beer, S. Ecophysiological Adaptation Strategies of Some Intertidal Marine Macroalgae of the Israeli Mediterranean Coast. Oceanogr. Lit. Rev. 1996, 5, 500. [Google Scholar] [CrossRef]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. Chapter Eight—The Stressful Life of Red and Brown Seaweeds on the Temperate Intertidal Zone: Effect of Abiotic and Biotic Parameters on the Physiology of Macroalgae and Content Variability of Particular Metabolites. In Advances in Botanical Research; Bourgougnon, N., Ed.; Seaweeds Around the World: State of Art and Perspectives; Academic Press: Cambridge, MA, USA, 2020; Volume 95, pp. 247–287. [Google Scholar]
- Lipkin, Y.; Beer, S.; Eshel, A. The Ability of Porphyra linearis (Rhodophyta) to Tolerate Prolonged Periods of Desiccation. Bot. Mar. 1993, 36, 517–524. [Google Scholar] [CrossRef]
- Bianco-Stein, N.; Polishchuk, I.; Lang, A.; Atiya, G.; Villanova, J.; Zaslansky, P.; Katsman, A.; Pokroy, B. Structural and Chemical Variations in Mg-Calcite Skeletal Segments of Coralline Red Algae Lead to Improved Crack Resistance. Acta Biomater. 2021, 130, 362–373. [Google Scholar] [CrossRef]
- Bar-Gil, A. Aspects of the Rhodophyte Laurencia papillosa (Macroalgae) and Microalgae Porphyridium cruentum: Photosynthesis, Symbiosis, Physiology and Biochemical Parameters. Ph.D. Thesis, University of Haifa (Israel), Haifa, Israel, 2018. [Google Scholar]
- Behrenfeld, M.J. Abandoning Sverdrup’s Critical Depth Hypothesis on Phytoplankton Blooms. Ecology 2010, 91, 977–989. [Google Scholar] [CrossRef]
- Ben Ezra, T.; Krom, M.D.; Tsemel, A.; Berman-Frank, I.; Herut, B.; Lehahn, Y.; Rahav, E.; Reich, T.; Thingstad, T.F.; Sher, D. Seasonal Nutrient Dynamics in the P Depleted Eastern Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, 176, 103607. [Google Scholar] [CrossRef]
- Ben-Ezra, T.; Reich, T.; Tsemel, A.; Berman-Frank, I.; Lehahn, Y.; Sher, D.; Suari, Y.; Krom, M.D. Nutrient Dynamics across the Israeli Coastal Shelf: An Unusual Oligotrophic Coastal System. Cont. Shelf Res. 2023, 266, 105103. [Google Scholar] [CrossRef]
- Rahav, E.; Raveh, O.; Yanuka-Golub, K.; Belkin, N.; Astrahan, P.; Maayani, M.; Tsumi, N.; Kiro, Y.; Herut, B.; Silverman, J.; et al. Nitrate-Enrichment Structures Phytoplankton Communities in the Shallow Eastern Mediterranean Coastal Waters. Front. Mar. Sci. 2020, 7, 611497. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from Macroalgae1. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional Value of Proteins from Edible Seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, D.Y.; Israel, A.; Abelson, A. A Novel Two-Stage Seaweed Integrated Multi-Trophic Aquaculture. Rev. Aquac. 2019, 11, 246–262. [Google Scholar] [CrossRef]
- Ashkenazi, D.Y.; Segal, Y.; Ben-Valid, S.; Paz, G.; Tsubery, M.N.; Salomon, E.; Abelson, A.; Israel, Á. Enrichment of Nutritional Compounds in Seaweeds via Abiotic Stressors in Integrated Aquaculture. Innov. Food Sci. Emerg. Technol. 2022, 80, 103067. [Google Scholar] [CrossRef]
- Martínez, B.; Rico, J.M. Changes in Nutrient Content of Palmaria palmata in Response to Variable Light and Upwelling in Northern Spain1. J. Phycol. 2008, 44, 50–59. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Imran, M.; Iqbal, A.; Badshah, S.L.; Sher, A.A.; Ullah, H.; Ayaz, M.; Mosa, O.F.; Mostafa, N.M.; Daglia, M. Chemical and Nutritional Profiling of the Seaweed Dictyota Dichotoma and Evaluation of Its Antioxidant, Antimicrobial and Hypoglycemic Potentials. Mar. Drugs 2023, 21, 273. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal Variation in the Chemical Composition of Two Tropical Seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Manivannan, K.; Thirumaran, G.; Devi, G.K.; Anantharaman, P.; Balasubramanian, T. Proximate Composition of Different Group of Seaweeds from Vedalai Coastal Waters (Gulf of Mannar): Southeast Coast of India. Middle-East J. Sci. Res. 2009, 4, 72–77. [Google Scholar]
- Balamurugan, M.; Selvam, G.G.; Thinakaran, T.; Sivakumar, K. Biochemical Study and GC-MS Analysis of Hypnea musciformis (Wulf.) Lamouroux. Am.-Eurasian J. Sci. Res. 2013, 8, 117–123. [Google Scholar]
- Elangovan, M.; Anantharaman, P. Nutritional Composition and Phytochemistry Profile of Seaweeds Collected from Rameshwaram Coast. Int. J. Sci. Technol. Res. 2019, 8, 3137–3140. [Google Scholar]
- Kumar, B.S.; Murugesan, S. Proximate and Amino Acid Compositions of Marine Red Alga Laurencia papillosa (C. agardh) Greville from South East Coast of India. J. Glob. Trends Pharm. Sci. 2018, 9, 4987–4995. [Google Scholar]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein Content, Amino Acid Composition and Nitrogen-to-Protein Conversion Factors of Ulva rigida and Ulva capensis from Natural Populations and Ulva lactuca from an Aquaculture System, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Satpati, G.; Pal, R. Biochemical Composition and Lipid Characterization of Marine Green Alga Ulva rigida- a Nutritional Approach. J. Algal Biomass Util. 2011, 2, 10–13. [Google Scholar]
- McDermid, K.J.; Stuercke, B. Nutritional Composition of Edible Hawaiian Seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Manivannan, K.; Thirumaran, G.; Devi, G.; Hemalatha, A. Biochemical Composition of Seaweeds from Mandapam Coastal Regions along Southest Coast of India. Am.-Eurasian J. Bot. 2008, 1, 32–37. [Google Scholar]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The Protein Content of Seaweeds: A Universal Nitrogen-to-Protein Conversion Factor of Five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino Acid Composition, Protein Content, and Nitrogen-to-Protein Conversion Factors of 21 Seaweed Species from Norwegian Waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Urbano, M.G.; Goñi, I. Bioavailability of Nutrients in Rats Fed on Edible Seaweeds, Nori (Porphyra tenera) and Wakame (Undaria pinnatifida), as a Source of Dietary Fibre. Food Chem. 2002, 76, 281–286. [Google Scholar] [CrossRef]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen Content, Dietary Fiber, and Digestibility in Algal Food Products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Samarathunga, J.; Wijesekara, I.; Jayasinghe, M. Seaweed Proteins as a Novel Protein Alternative: Types, Extractions, and Functional Food Applications. Food Rev. Int. 2023, 39, 4236–4261. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Bases, E.; El Shafay, S.M.; El-shenody, R. Influence of Seasonal Variations on Extract Yield and Antioxidant Activities of Some Seaweed Species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 915–923. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Bouzon, Z.L.; Hall-Spencer, J.M.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal Biochemical and Photophysiological Responses in the Intertidal Macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, D.Y.; Figueroa, F.L.; Korbee, N.; García-Sánchez, M.; Vega, J.; Ben-Valid, S.; Paz, G.; Salomon, E.; Israel, Á.; Abelson, A. Enhancing Bioproducts in Seaweeds via Sustainable Aquaculture: Antioxidant and Sun-Protection Compounds. Mar. Drugs 2022, 20, 767. [Google Scholar] [CrossRef] [PubMed]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Effect of Light Quality on the Accumulation of Photosynthetic Pigments, Proteins and Mycosporine-like Amino Acids in the Red Alga Porphyra leucosticta (Bangiales, Rhodophyta). J. Photochem. Photobiol. B Biol. 2005, 80, 71–78. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Bedoux, G.; Boulho, R.; Taupin, L.; Freile-Pelegrín, Y.; Bourgougnon, N.; Robledo, D. Stress Tolerance and Photoadaptation to Solar Radiation in Rhodymenia pseudopalmata (Rhodophyta) through Mycosporine-like Amino Acids, Phenolic Compounds, and Pigments in an Integrated Multi-Trophic Aquaculture System. Algal Res. 2019, 41, 101542. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative Stress in Marine Eniviroments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Pedro, J.; Cardoso, C.; Afonso, F.; Bandarra, N.M. Season Affects Three Insufficiently Studied Seaweed Species (Bifurcaria bifurcata, Codium Sp., Ericaria selaginoides): Bioactivity Alterations. Appl. Phycol. 2022, 3, 98–108. [Google Scholar] [CrossRef]
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean Macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) Bioprospection: A Study of Fatty Acid Profiles and Bioactivity. Int. J. Food Sci. Technol. 2019, 54, 880–890. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential Antioxidant Capacity of Sulfated Polysaccharides from the Edible Marine Brown Seaweed Fucus Vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, Antitumor and Antioxidant Activities in Vitro of Polysaccharides from the Brown Seaweed Sargassum Pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef]
- Airanthi, M.K.W.-A.; Hosokawa, M.; Miyashita, K. Comparative Antioxidant Activity of Edible Japanese Brown Seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef]
- Schneider, G.; Lopez Figueroa, F.; Vega, J.; Chaves, P.; Álvarez-Gómez, F.; Korbee, N.; Bonomi Barufi, J. Photoprotection Properties of Marine Photosynthetic Organisms Grown in High Ultraviolet Exposure Areas: Cosmeceutical Applications. Algal Res. 2020, 49, 101956. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Marinho, G.S.; Sørensen, A.-D.M.; Safafar, H.; Pedersen, A.H.; Holdt, S.L. Antioxidant Content and Activity of the Seaweed Saccharina latissima: A Seasonal Perspective. J. Appl. Phycol. 2019, 31, 1343–1354. [Google Scholar] [CrossRef]
- Mancuso, F.P.; Messina, C.M.; Santulli, A.; Laudicella, V.A.; Giommi, C.; Sarà, G.; Airoldi, L. Influence of Ambient Temperature on the Photosynthetic Activity and Phenolic Content of the Intertidal Cystoseira compressa along the Italian Coastline. J. Appl. Phycol. 2019, 31, 3069–3076. [Google Scholar] [CrossRef]
- Praiboon, J.; Palakas, S.; Noiraksa, T.; Miyashita, K. Seasonal Variation in Nutritional Composition and Anti-Proliferative Activity of Brown Seaweed, Sargassum oligocystum. J. Appl. Phycol. 2018, 30, 101–111. [Google Scholar] [CrossRef]
- Britton, D.; Schmid, M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L.; Mundy, C.N. Seasonal and Site-Specific Variation in the Nutritional Quality of Temperate Seaweed Assemblages: Implications for Grazing Invertebrates and the Commercial Exploitation of Seaweeds. J. Appl. Phycol. 2021, 33, 603–616. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of Nutritional Value in a Brown Seaweed Sargassum wightii and Their Seasonal Variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A. Seasonal Variation of Fucoxanthin Content in Four Species of Brown Seaweeds from Qeshm Island, Persian Gulf and Evaluation of Their Antibacterial and Antioxidant Activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Martínez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Ecophysiological Responses to Elevated CO2 and Temperature in Cystoseira tamariscifolia (Phaeophyceae). Clim. Change 2017, 142, 67–81. [Google Scholar] [CrossRef]
- Karsten, U.; Franklin, L.A.; Lüning, K.; Wiencke, C. Natural Ultraviolet Radiation and Photosynthetically Active Radiation Induce Formation of Mycosporine-like Amino Acids in the Marine Macroalga Chondrus crispus (Rhodophyta). Planta 1998, 205, 257–262. [Google Scholar] [CrossRef]
- Barufi, J.B.; Korbee, N.; Oliveira, M.C.; Figueroa, F.L. Effects of N Supply on the Accumulation of Photosynthetic Pigments and Photoprotectors in Gracilaria tenuistipitata (Rhodophyta) Cultured under UV Radiation. J. Appl. Phycol. 2011, 23, 457–466. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of Sunscreen Compounds in Antarctic Macroalgae by Different Radiation Conditions. Mar. Biol. 2002, 141, 619–627. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Kamiya, M.; West, J.; Ganzera, M. Chemotaxonomic Study of Bostrychia Spp. (Ceramiales, Rhodophyta) Based on Their Mycosporine-Like Amino Acid Content. Molecules 2020, 25, 3273. [Google Scholar] [CrossRef]
- Karsten, U.; Wiencke, C. Factors Controlling the Formation of UV-Absorbing Mycosporine-like Amino Acids in the Marine Red Alga Palmaria palmata from Spitsbergen (Norway). J. Plant Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]
- Diehl, N.; Michalik, D.; Zuccarello, G.C.; Karsten, U. Stress Metabolite Pattern in the Eulittoral Red Alga Pyropia Plicata (Bangiales) in New Zealand—Mycosporine-like Amino Acids and Heterosides. J. Exp. Mar. Biol. Ecol. 2019, 510, 23–30. [Google Scholar] [CrossRef]
- Véliz, K.; Chandía, N.; Karsten, U.; Lara, C.; Thiel, M. Geographic Variation in Biochemical and Physiological Traits of the Red Seaweeds Chondracanthus Chamissoi and Gelidium Lingulatum from the South East Pacific Coast. J. Appl. Phycol. 2019, 31, 665–682. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and Spatial Variability of Mycosporine-like Amino Acids and Pigments in Three Edible Red Seaweeds from Western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Yamamoto, R.; Mune Mune, M.A.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation in Mycosporine-like Amino Acids from Red Alga Dulse (Devaleraea Inkyuleei, Formerly Palmaria palmata in Japan). Phycology 2023, 3, 127–137. [Google Scholar] [CrossRef]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.O.; Nunes, J.M.C.; Schmitz, C.; Ramlov, F.; Maraschin, M.; Korbee, N.; et al. The Influence of Environmental Features in the Content of Mycosporine-like Amino Acids in Red Marine Algae along the Brazilian Coast. J. Phycol. 2018, 54, 380–390. [Google Scholar] [CrossRef] [PubMed]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant Activity of Mycosporine-like Amino Acids Isolated from Three Red Macroalgae and One Marine Lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular Photoprotection of Human Keratinocytes in Vitro by the Naturally Occurring Mycosporine-like Amino Acid Palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds Inot Percentages of Protein; US Department of Agriculture: Washington, DC, USA, 1931; pp. 1–21. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Trytophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Peinado, N.K.; Díaz, R.T.A.; Figueroa, F.L.; Helbling, E.W. Ammonium and Uv Radiation Stimulate the Accumulation of Mycosporine-Like Amino Acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina1. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef]
- Vega, J.; Bárcenas-Pérez, D.; Fuentes-Ríos, D.; López-Romero, J.M.; Hrouzek, P.; Figueroa, F.L.; Cheel, J. Isolation of Mycosporine-like Amino Acids from Red Macroalgae and a Marine Lichen by High-Performance Countercurrent Chromatography: A Strategy to Obtain Biological UV-Filters. Mar. Drugs 2023, 21, 357. [Google Scholar] [CrossRef]
- Amsler, C.D. (Ed.) Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74180-0. [Google Scholar]
- Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T.; Pérez-Lloréns, J.L. Saved by Seaweeds: Phyconomic Contributions in Times of Crises. J. Appl. Phycol. 2020, 33, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Einav, R.; Seckbach, J. Seaweeds and Their Role in Globally Changing Environments; Springer Science & Business Media: Berlin, Germany, 2010; ISBN 978-90-481-8569-6. [Google Scholar]
- Neiva, J.; Assis, J.; Coelho, N.C.; Fernandes, F.; Pearson, G.A.; Serrão, E.A. Genes Left Behind: Climate Change Threatens Cryptic Genetic Diversity in the Canopy-Forming Seaweed Bifurcaria bifurcata. PLoS ONE 2015, 10, e0131530. [Google Scholar] [CrossRef]
- Bothwell, J. Seaweeds of the World: A Guide to Every Order; Princeton University Press: Oxford, UK, 2023; ISBN 978-0-691-22854-9. [Google Scholar]
- Wesselmann, M.; Hendriks, I.E.; Johnson, M.; Jordà, G.; Mineur, F.; Marbà, N. Increasing Spread Rates of Tropical Non-Native Macrophytes in the Mediterranean Sea. Glob. Change Biol. 2024, 30, e17249. [Google Scholar] [CrossRef]
- Nakhate, P.; van der Meer, Y. A Systematic Review on Seaweed Functionality: A Sustainable Bio-Based Material. Sustainability 2021, 13, 6174. [Google Scholar] [CrossRef]
- Mishra, H.; Kumar, K.S.; Pratibha, K.; Periyasamy, C.; Rao, P.V.S. Seaweeds Aid in Carbon Sequestration to Combat Global Warming: A Glimpse. In Algae Mediated Bioremediation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2024; pp. 505–520. ISBN 978-3-527-84336-7. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).