Optimisation of Pressurised Liquid Extraction and Subsequent Hydrolysate Fermentation by Lactiplantibacillus plantarum for Integrated Bioprocessing of Ulva sp.
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Ulva sp. Biomass
2.2. PLE Optimisation of Ulva sp. Biomass
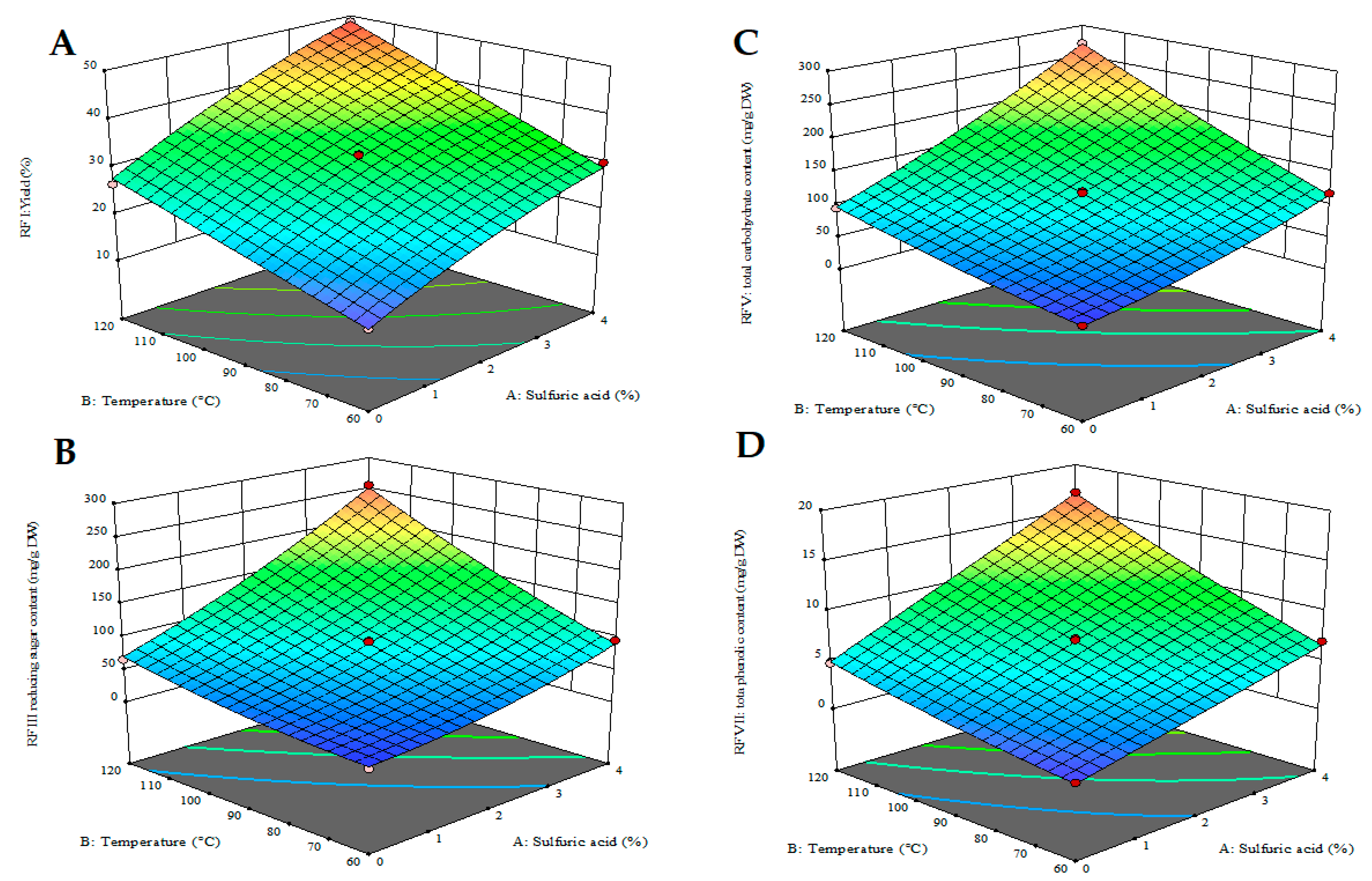
2.2.1. Box–Behnken Design and Response Surface Plots of PLE
2.2.2. Simultaneous Response Optimisation and Model Validation
2.3. Fermentation of Ulva sp. Hydrolysate Obtained by PLE
2.3.1. Growth Kinetics and pH Changes
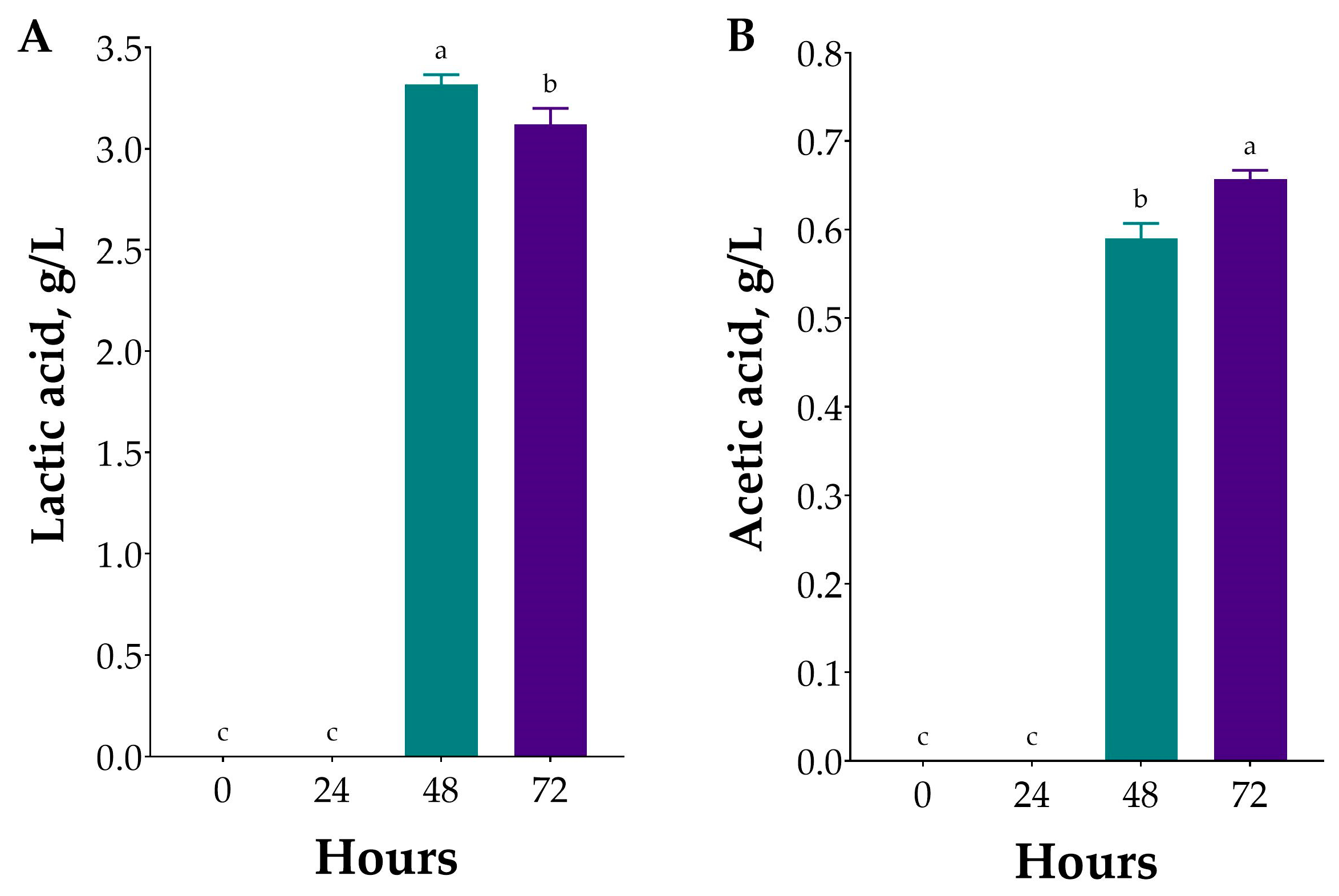
2.3.2. Consumption of Monosaccharides and Production of Organic Acids During Fermentation of Ulva sp. Hydrolysate
2.3.3. Changes in the In Vitro Antioxidant Capacity During Fermentation of Ulva sp. Hydrolysate
3. Materials and Methods
3.1. Ulva sp. Biomass and Reagents
3.2. Proximate Composition of Ulva sp. Biomass
3.3. PLE of Ulva sp. Biomass by Experimental Design
3.4. Determination of Reducing Sugar Content
3.5. Determination of Total Carbohydrate Content
3.6. Determination of Total Phenolic Content
3.7. Fermentation of Ulva sp.Hydrolysate
3.7.1. Bacterial Strain and Media Preparation
3.7.2. Fermentation of PLE Extract with Lactic Acid Bacteria
3.7.3. Microbial Growth and pH Analysis
3.8. Monosaccharide and Organic Acid Analysis
3.9. In Vitro Antioxidant Activity Assessment
3.9.1. Determination of the ABTS•+ Scavenging Capacity
3.9.2. Determination of the Cupric Reducing Antioxidant Capacity
3.9.3. Determination of the DPPH• Scavenging Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Francezon, N.; Tremblay, A.; Mouget, J.-L.; Pasetto, P.; Beaulieu, L. Algae as a Source of Natural Flavors in Innovative Foods. J. Agric. Food Chem. 2021, 69, 11753–11772. [Google Scholar] [CrossRef]
- Segaran, T.C.; Azra, M.N.; Mohd Noor, M.I.; Danish-Daniel, M.; Burlakovs, J.; Lananan, F.; Xu, J.; Kari, Z.A.; Wei, L.S. Knowledge Mapping Analysis of the Global Seaweed Research Using CiteSpace. Heliyon 2024, 10, e28418. [Google Scholar] [CrossRef]
- Hofmann, L.C.; Strauss, S.; Shpigel, M.; Guttman, L.; Stengel, D.B.; Rebours, C.; Gjorgovska, N.; Turan, G.; Balina, K.; Zammit, G.; et al. The Green Seaweed Ulva: Tomorrow’s “Wheat of the Sea” in Foods, Feeds, Nutrition, and Biomaterials. Crit. Rev. Food Sci. Nutr. 2025, 65, 3728–3763. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Pari, R.F.; Uju, U.; Hardiningtyas, S.D.; Ramadhan, W.; Wakabayashi, R.; Goto, M.; Kamiya, N. Ulva Seaweed-Derived Ulvan: A Promising Marine Polysaccharide as a Sustainable Resource for Biomaterial Design. Mar. Drugs 2025, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Dave, N.; Varadavenkatesan, T.; Singh, R.S.; Giri, B.S.; Selvaraj, R.; Vinayagam, R. Evaluation of Seasonal Variation and the Optimization of Reducing Sugar Extraction from Ulva prolifera Biomass Using Thermochemical Method. Environ. Sci. Pollut. Res. 2021, 28, 58857–58871. [Google Scholar] [CrossRef]
- Qarri, A.; Israel, A. Seasonal Biomass Production, Fermentable Saccharification and Potential Ethanol Yields in the Marine Macroalga Ulva sp. (Chlorophyta). Renew. Energy 2020, 145, 2101–2107. [Google Scholar] [CrossRef]
- Bayu, A.; Warsito, M.F.; Putra, M.Y.; Karnjanakom, S.; Guan, G. Macroalgae-Derived Rare Sugars: Applications and Catalytic Synthesis. Carbon Resour. Convers. 2021, 4, 150–163. [Google Scholar] [CrossRef]
- Tong, K.T.X.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Lim, S.; Lee, K.T. Advancement of Biorefinery-Derived Platform Chemicals from Macroalgae: A Perspective for Bioethanol and Lactic Acid. Biomass Conv. Bioref. 2024, 14, 1443–1479. [Google Scholar] [CrossRef] [PubMed]
- Teh, Y.Y.; Lee, K.T.; Chen, W.-H.; Lin, S.-C.; Sheen, H.-K.; Tan, I.S. Dilute Sulfuric Acid Hydrolysis of Red Macroalgae Eucheuma denticulatum with Microwave-Assisted Heating for Biochar Production and Sugar Recovery. Bioresour. Technol. 2017, 246, 20–27. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Dave, N.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. A Critical Review on Production of Bioethanol from Macroalgal Biomass. Algal Res. 2019, 42, 101606. [Google Scholar] [CrossRef]
- Salvador, R.; Eriksen, M.L.; Kjaersgaard, N.C.; Hedegaard, M.; Knudby, T.; Lund, V.; Larsen, S.B. From Ocean to Meadow: A Circular Bioeconomy by Transforming Seaweed, Seagrass, Grass, and Straw Waste into High-Value Products. Waste Manag. 2025, 200, 114753. [Google Scholar] [CrossRef] [PubMed]
- Rajak, R.C.; Jacob, S.; Kim, B.S. A Holistic Zero Waste Biorefinery Approach for Macroalgal Biomass Utilization: A Review. Sci. Total Environ. 2020, 716, 137067. [Google Scholar] [CrossRef] [PubMed]
- Bikker, P.; van Krimpen, M.M.; van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the Green Seaweed Ulva lactuca to Produce Animal Feed, Chemicals and Biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal Fermentation—The Seed for a New Fermentation Industry of Foods and Related Products. Jpn. Agric. Res. Q. 2013, 47, 53–63. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chang, J.H.; Lee, S.B. Chemical Composition, Saccharification Yield, and the Potential of the Green Seaweed Ulva pertusa. Biotechnol. Bioprocess Eng. 2014, 19, 1022–1033. [Google Scholar] [CrossRef]
- Bokhtiar, S.M.; Sarker, D.; Akter, A.; Salam, M.A.; Ahmed, K.U.; Anwar, M.M.; Hossain, M.F.; Ahmed, M.; Bhuiyan, M.S.; Kanta, R.A.; et al. Nutritional Profiling, Phytochemical Screening, Cytotoxicity, and Antioxidant Content Analysis for Different Crude Extracts of Ulva lactuca from Coast of Bangladesh. Future Foods 2024, 10, 100513. [Google Scholar] [CrossRef]
- Balar, N.; Sharnagat, P.; Kumari, P.; Mantri, V.A. Variation in the Proximate Composition of Edible Marine Macroalga Ulva rigida Collected from Different Coastal Zones of India. J. Food Sci. Technol. 2019, 56, 4749–4755. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Flores Ramos, L.; Oscanoa Huaynate, A.I.; Ruíz Soto, A.; Ramírez, M.E. Biochemical and Nutritional Characterization of Edible Seaweeds from the Peruvian Coast. Plants 2023, 12, 1795. [Google Scholar] [CrossRef]
- Keramane, B.; Sánchez-Camargo, A.d.P.; Montero, L.; Laincer, F.; Bedjou, F.; Ibañez, E. Pressurized Liquid Extraction of Bioactive Extracts with Antioxidant and Antibacterial Activity from Green, Red and Brown Algerian Algae. Algal Res. 2023, 76, 103293. [Google Scholar] [CrossRef]
- Rudke, A.R.; Zanella, E.; Stambuk, B.U.; de Andrade, C.J.; Ferreira, S.R.S. Deconstruction of Kappaphycus alvarezii Biomass by Pressurized Solvents to Increase the Carrageenan Purity. Food Hydrocoll. 2024, 155, 110204. [Google Scholar] [CrossRef]
- Zonfrillo, B.; Bellumori, M.; Digiglio, I.; Innocenti, M.; Orlandini, S.; Furlanetto, S.; Khatib, M.; Papini, A.; Mainente, F.; Zoccatelli, G.; et al. Multivariate Optimization of Ulvan Extraction Applying Response Surface Methodology (RSM): The Case of Ulva lactuca L. from Orbetello Lagoon. Carbohydr. Polym. 2025, 354, 123340. [Google Scholar] [CrossRef]
- Park, Y.-S.; Roy, V.C.; Park, J.-S.; Zhang, W.; Chun, B.-S. Optimization of Subcritical Water Extraction Parameters of Phlorotannins from Brown Alga (Ecklonia stolonifera): Bipotentialities and Possible Applications. J. Supercrit. Fluids 2025, 218, 106502. [Google Scholar] [CrossRef]
- Boisvert, C.; Beaulieu, L.; Bonnet, C.; Pelletier, É. Assessment of the Antioxidant and Antibacterial Activities of Three Species of Edible Seaweeds. J. Food Biochem. 2015, 39, 377–387. [Google Scholar] [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of Pressurised Liquid Extraction and Solid–Liquid Extraction Methods on the Phenolic Content and Antioxidant Activities of Irish Macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869. [Google Scholar] [CrossRef]
- Hebbale, D.; Ramachandra, T.V. Optimal Sugar Release from Macroalgal Feedstock with Dilute Acid Pretreatment and Enzymatic Hydrolysis. Biomass Convers. Biorefin. 2023, 13, 8287–8300. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Reddy, C.R.K.; Jha, B. Enzymatic Hydrolysis and Production of Bioethanol from Common Macrophytic Green Alga Ulva fasciata Delile. Bioresour. Technol. 2013, 150, 106–112. [Google Scholar] [CrossRef]
- Choi, W.Y.; Han, J.G.; Lee, C.G.; Song, C.H.; Kim, J.S.; Seo, Y.C.; Lee, S.E.; Jung, K.H.; Kang, D.H.; Heo, S.J.; et al. Bioethanol Production from Ulva pertusa Kjellman by High-Temperature Liquefaction. Chem. Biochem. Eng. Q. 2012, 26, 15–21. [Google Scholar]
- Brandão, M.; Marques, D.J.; Sousa, S.; Mateus, M.; Pinheiro, H.M.; da Fonseca, M.M.R.; Pires, C.; Nunes, M.L.; Marques, A.; Cesário, M.T. Lactic Acid Bacteria and Yeast Fermentation to Improve the Nutritional Value of Ulva rigida. Mar. Drugs 2025, 23, 106. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Helmes, R.J.K.; López-Contreras, A.M.; Benoit, M.; Abreu, H.; Maguire, J.; Moejes, F.; van den Burg, S.W.K. Environmental Impacts of Experimental Production of Lactic Acid for Bioplastics from Ulva spp. Sustainability 2018, 10, 2462. [Google Scholar] [CrossRef]
- Nagarajan, D.; Nandini, A.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Lactic Acid Production from Renewable Feedstocks Using Poly(vinyl alcohol)-Immobilized Lactobacillus plantarum 23. Ind. Eng. Chem. Res. 2020, 59, 17156–17164. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Dharani, G. Evaluation of Seaweed for the Production of Lactic Acid by Fermentation Using Lactobacillus plantarum. Bioresour. Technol. Rep. 2022, 17, 100890. [Google Scholar] [CrossRef]
- Nagarajan, D.; Oktarina, N.; Chen, P.-T.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Fermentative Lactic Acid Production from Seaweed Hydrolysate Using Lactobacillus sp. and Weissella sp. Bioresour. Technol. 2022, 344, 126166. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Li, D.-Y.; Cheng, Y.-S. Application of Ensilage as a Green Approach for Simultaneous Preservation and Pretreatment of Macroalgae Ulva lactuca for Fermentable Sugar Production. Clean Technol. Environ. Policy 2018, 20, 2057–2065. [Google Scholar] [CrossRef]
- Takei, M.; Kuda, T.; Eda, M.; Shikano, A.; Takahashi, H.; Kimura, B. Antioxidant and Fermentation Properties of Aqueous Solutions of Dried Algal Products from the Boso Peninsula, Japan. Food Biosci. 2017, 19, 85–91. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Duman, H.; Karav, S. Nutritional and Functional Aspects of Fermented Algae. Int. J. Food Sci. Technol. 2024, 59, 5270–5284. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chen, C.-Y.; Ariyadasa, T.U.; Lee, D.-J.; Chang, J.-S. Macroalgal Biomass as a Potential Resource for Lactic Acid Fermentation. Chemosphere 2022, 309, 136694. [Google Scholar] [CrossRef]
- Steinbruch, E.; Drabik, D.; Epstein, M.; Ghosh, S.; Prabhu, M.S.; Gozin, M.; Kribus, A.; Golberg, A. Hydrothermal Processing of a Green Seaweed Ulva sp. for the Production of Monosaccharides, Polyhydroxyalkanoates, and Hydrochar. Bioresour. Technol. 2020, 318, 124263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Linzon, Y.; Vitkin, E.; Yakhini, Z.; Chudnovsky, A.; Golberg, A. Thermochemical Hydrolysis of Macroalgae Ulva for Biorefinery: Taguchi Robust Design Method. Sci. Rep. 2016, 6, 27761. [Google Scholar] [CrossRef] [PubMed]
- Polikovsky, M.; Gillis, A.; Steinbruch, E.; Robin, A.; Epstein, M.; Kribus, A.; Golberg, A. Biorefinery for the Co-Production of Protein, Hydrochar and Additional Co-Products from a Green Seaweed Ulva sp. with Subcritical Water Hydrolysis. Energy Convers. Manag. 2020, 225, 113380. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, M.; Zheng, Y.; Miao, K.; Qu, X. The Carbohydrate Metabolism of Lactiplantibacillus Plantarum. Int. J. Mol. Sci. 2021, 22, 13452. [Google Scholar] [CrossRef]
- Mao, B.; Yin, R.; Li, X.; Cui, S.; Zhang, H.; Zhao, J.; Chen, W. Comparative Genomic Analysis of Lactiplantibacillus plantarum Isolated from Different Niches. Genes 2021, 12, 241. [Google Scholar] [CrossRef]
- Uchida, M.; Murata, M. Isolation of a Lactic Acid Bacterium and Yeast Consortium from a Fermented Material of Ulva spp. (Chlorophyta). J. Appl. Microbiol. 2004, 97, 1297–1310. [Google Scholar] [CrossRef]
- Hwang, H.J.; Lee, S.Y.; Kim, S.M.; Lee, S.B. Fermentation of Seaweed Sugars by Lactobacillus Species and the Potential of Seaweed as a Biomass Feedstock. Biotechnol. Bioprocess Eng. 2011, 16, 1231–1239. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alva, A.; MacIntosh, A.J.; Baigts-Allende, D.K.; García-Torres, R.; Ramírez-Rodrigues, M.M. Fermentation of Algae to Enhance Their Bioactive Activity: A Review. Algal Res. 2022, 64, 102684. [Google Scholar] [CrossRef]
- Putra, N.R.; Fajriah, S.; Qomariyah, L.; Dewi, A.S.; Rizkiyah, D.N.; Irianto, I.; Rusmin, D.; Melati, M.; Trisnawati, N.W.; Darwati, I.; et al. Exploring the Potential of Ulva lactuca: Emerging Extraction Methods, Bioactive Compounds, and Health Applications—A Perspective Review. S. Afr. J. Chem. Eng. 2024, 47, 233–245. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaitė, J.; Paškauskas, R.; Bašinskienė, L.; Venskutonis, P.R. Recovery of Lipophilic Products from Wild Cyanobacteria (Aphanizomenon flos-aquae) Isolated from the Curonian Lagoon by Means of Supercritical Carbon Dioxide Extraction. Algal Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Teixeira, R.S.S.; da Silva, A.S.; Ferreira-Leitão, V.S.; da Silva Bon, E.P. Amino Acids Interference on the Quantification of Reducing Sugars by the 3,5-Dinitrosalicylic Acid Assay Mislead Carbohydrase Activity Measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A New Method for Rapid Determination of Carbohydrate and Total Carbon Concentrations Using UV Spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Nagybákay, N.E.; Sarapinaitė, L.; Syrpas, M.; Venskutonis, P.R.; Kitrytė-Syrpa, V. Optimization of Pressurized Ethanol Extraction for Efficient Recovery of Hyperoside and Other Valuable Polar Antioxidant-Rich Extracts from Betula pendula Roth Leaves. Ind. Crops Prod. 2023, 205, 117565. [Google Scholar] [CrossRef]
- Aboobacker, S.; Kitrytė-Syrpa, V.; Šipailienė, A.; Rutkaitė, R.; Syrpas, M. Fermentation-Induced Nutritional and in Vitro Antioxidant Capacity Changes in Arthrospira platensis (Spirulina). Food Biosci. 2025, 68, 106747. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
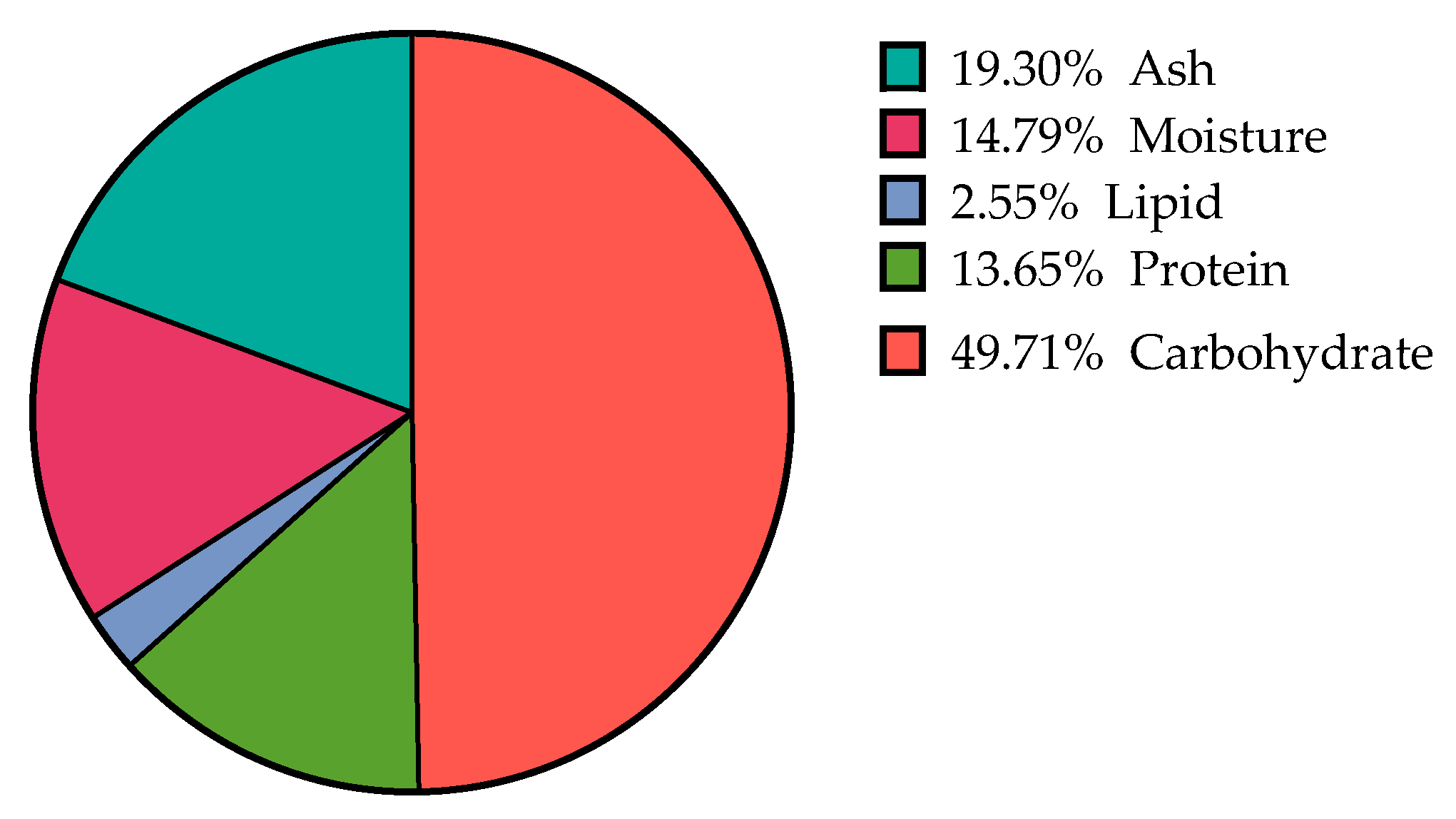


| Run | Independent PLE Variables | Response Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C * | RFI | RFII | RFIII | RFIV | RFV | RFVI | RFVII | |
| H2SO4, % (v/v) | T, °C | τ, min/cycle | Yield (g/100 g DW) | TRS (mg/g E) | TRS (mg/g DW) | TCC (mg/g E) | TCC (mg/g DW) | TPC (mg GAE/g E) | TPC (mg GAE/g DW) | |
| 1 | 2 | 90 | 10 | 30.9 ± 0.3 | 278.5 ± 3.5 | 86.1 ± 0.8 | 375.8 ± 1.3 | 116.2 ±0.5 | 21.9 ± 0.2 | 6.8 ± 0.4 |
| 2 | 2 | 90 | 10 | 32.3 ± 0.2 | 301.6 ± 3.4 | 97.4 ± 1.0 | 382.8 ± 1.4 | 123.6 ±0.6 | 22.5 ± 0.2 | 7.3 ± 0.1 |
| 3 | 4 | 120 | 10 | 48.7 ± 0.4 | 524.6 ± 2.5 | 255.3 ± 2.6 | 556.0 ± 5.7 | 270.6 ± 1.8 | 34.9 ± 0.4 | 17.0 ± 0.6 |
| 4 | 0 | 120 | 10 | 26.4 ± 0.3 | 249.5 ± 7.5 | 65.9 ± 0.8 | 358.5 ± 2.3 | 94.80 ± 0.3 | 17.8 ± 0.3 | 4.7 ± 0.0 |
| 5 | 2 | 120 | 15 | 43.7 ± 0.1 | 393.6 ± 2.6 | 172.0 ± 1.9 | 460.9 ± 4.8 | 201.4 ±1.2 | 28.0 ± 0.2 | 12.2 ± 0.45 |
| 6 | 4 | 90 | 5 | 34.1 ± 0.1 | 376.1 ± 2.9 | 128.4 ± 1.4 | 470.9 ± 4.9 | 160.7 ± 0.9 | 27.7 ± 0.1 | 9.4 ± 0.1 |
| 7 | 0 | 90 | 5 | 17.8 ± 0.4 | 189.3 ± 1.5 | 33.8 ± 0.2 | 293.5 ± 5.1 | 52.40 ± 0.2 | 9.6 ± 0.2 | 1.7 ± 0.0 |
| 8 | 2 | 90 | 10 | 30.8 ± 0.2 | 306.4 ± 3.5 | 94.5 ± 1.0 | 391.5 ± 1.4 | 120.8 ± 0.6 | 21.6 ± 0.1 | 6.7 ± 0.0 |
| 9 | 0 | 60 | 10 | 13.4 ± 0.2 | 172.5 ± 6.0 | 23.2 ± 0.1 | 281.3 ± 3.2 | 37.80 ± 0.1 | 6.6 ± 0.4 | 0.9 ± 0.0 |
| 10 | 2 | 60 | 5 | 17.6 ± 0.1 | 187.6 ± 6.9 | 33.1 ± 0.2 | 294.1 ± 3.6 | 51.80 ± 0.2 | 12.8 ± 0.2 | 2.2 ± 0.0 |
| 11 | 4 | 90 | 15 | 39.9 ± 0.2 | 454.6 ± 7.7 | 181.6 ± 2.3 | 520.8 ± 9.2 | 208.0 ± 1.3 | 30.5 ± 0.2 | 12.2 ± 0.4 |
| 12 | 4 | 60 | 10 | 30.0 ± 0.1 | 316.7 ± 6.0 | 95.1 ± 1.0 | 392.0 ± 3.4 | 117.7 ± 0.6 | 23.1 ± 0.2 | 6.9 ± 0.0 |
| 13 | 2 | 120 | 5 | 34.3 ± 0.3 | 353.7 ± 5.4 | 121.2 ± 1.2 | 427.1 ± 5.4 | 146.4 ± 0.9 | 24.8 ± 0.0 | 8.5 ± 0.1 |
| 14 | 2 | 60 | 15 | 23.6 ± 0.2 | 217.3 ± 6.0 | 51.4 ± 0.5 | 320.2 ± 5.2 | 75.70 ± 0.3 | 14.9 ± 0.2 | 3.5 ± 0.1 |
| 15 | 0 | 90 | 15 | 21.8 ± 0.4 | 216.7 ± 6.9 | 47.2 ± 0.4 | 315.9 ± 4.7 | 68.80 ± 0.2 | 12.0 ± 0.6 | 2.6 ± 0.1 |
| 16 | 2 | 90 | 10 | 32.1 ± 0.2 | 289.5 ± 3.4 | 92.9 ± 0.9 | 379.0 ± 1.4 | 121.7 ± 0.5 | 22.1 ± 0.2 | 7.1 ± 0.1 |
| 17 | 2 | 90 | 10 | 30.9 ± 0.2 | 293.1 ± 3.3 | 90.7 ± 0.94 | 381.9 ± 1.5 | 118.0 ± 0.6 | 21.9 ± 0.1 | 6.8 ± 0.2 |
| Response Factors | Predicted Mean | 95% PI Low | Experimental Value | 95% PI High |
|---|---|---|---|---|
| RFI: Yield (g/100 g DW) | 46.4 | 43.1 | 46.9 ± 0.1 | 49.7 |
| RFII: TRS (mg/g E) | 491.1 | 460.1 | 520.2 ± 1.9 | 522.1 |
| RFIII: TRS (mg/g DW) | 229.1 | 210.5 | 244.0 ± 0.9 | 247.7 |
| RFIV: TCC (mg/g E) | 531.7 | 503.4 | 555.1 ± 0.8 | 559.9 |
| RFV: TCC (mg/g DW) | 249.8 | 237.6 | 260.3 ± 0.4 | 262.0 |
| RFVI: TPC (mg GAE/g E) | 32.3 | 31.0 | 32.9 ± 0.1 | 33.6 |
| RFVII: TPC (mg GAE/g DW) | 15.2 | 14.1 | 15.4 ± 0.2 | 16.3 |
| Fermentation Time | TEACCUPRAC, mg TE/g DW | TEACDPPH, mg TE/g DW | TEACABTS, mg TE/g DW |
|---|---|---|---|
| 0 h | 49.3 ± 0.2 a,b | 36.9 ± 0.3 a | 17.11 ± 0.2 a |
| 24 h | 46.0 ± 1.2 b | 31.7 ± 3.3 b | 17.01 ± 0.3 a |
| 48 h | 52.5 ± 2.4 a | 38.5 ± 0.6 a | 18.0 ± 0.6 a |
| 72 h | 54.2 ± 2.7 a | 38.3 ± 1.7 a | 17.6 ± 1.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dave, A.D.; Bilgin, H.; Kitrytė-Syrpa, V.; Syrpas, M. Optimisation of Pressurised Liquid Extraction and Subsequent Hydrolysate Fermentation by Lactiplantibacillus plantarum for Integrated Bioprocessing of Ulva sp. Mar. Drugs 2025, 23, 371. https://doi.org/10.3390/md23100371
Dave AD, Bilgin H, Kitrytė-Syrpa V, Syrpas M. Optimisation of Pressurised Liquid Extraction and Subsequent Hydrolysate Fermentation by Lactiplantibacillus plantarum for Integrated Bioprocessing of Ulva sp. Marine Drugs. 2025; 23(10):371. https://doi.org/10.3390/md23100371
Chicago/Turabian StyleDave, Aniruddh Dayanand, Hakki Bilgin, Vaida Kitrytė-Syrpa, and Michail Syrpas. 2025. "Optimisation of Pressurised Liquid Extraction and Subsequent Hydrolysate Fermentation by Lactiplantibacillus plantarum for Integrated Bioprocessing of Ulva sp." Marine Drugs 23, no. 10: 371. https://doi.org/10.3390/md23100371
APA StyleDave, A. D., Bilgin, H., Kitrytė-Syrpa, V., & Syrpas, M. (2025). Optimisation of Pressurised Liquid Extraction and Subsequent Hydrolysate Fermentation by Lactiplantibacillus plantarum for Integrated Bioprocessing of Ulva sp. Marine Drugs, 23(10), 371. https://doi.org/10.3390/md23100371







