The Synergistic Role of Sargassum horneri Fucoidan and Lactobacillus plantarum: Microbiome and Gut Barrier Restoration in Zebrafish Colitis
Abstract
1. Introduction
2. Results
2.1. S. horneri Fucoidan Increases the Growth of L. plantarum
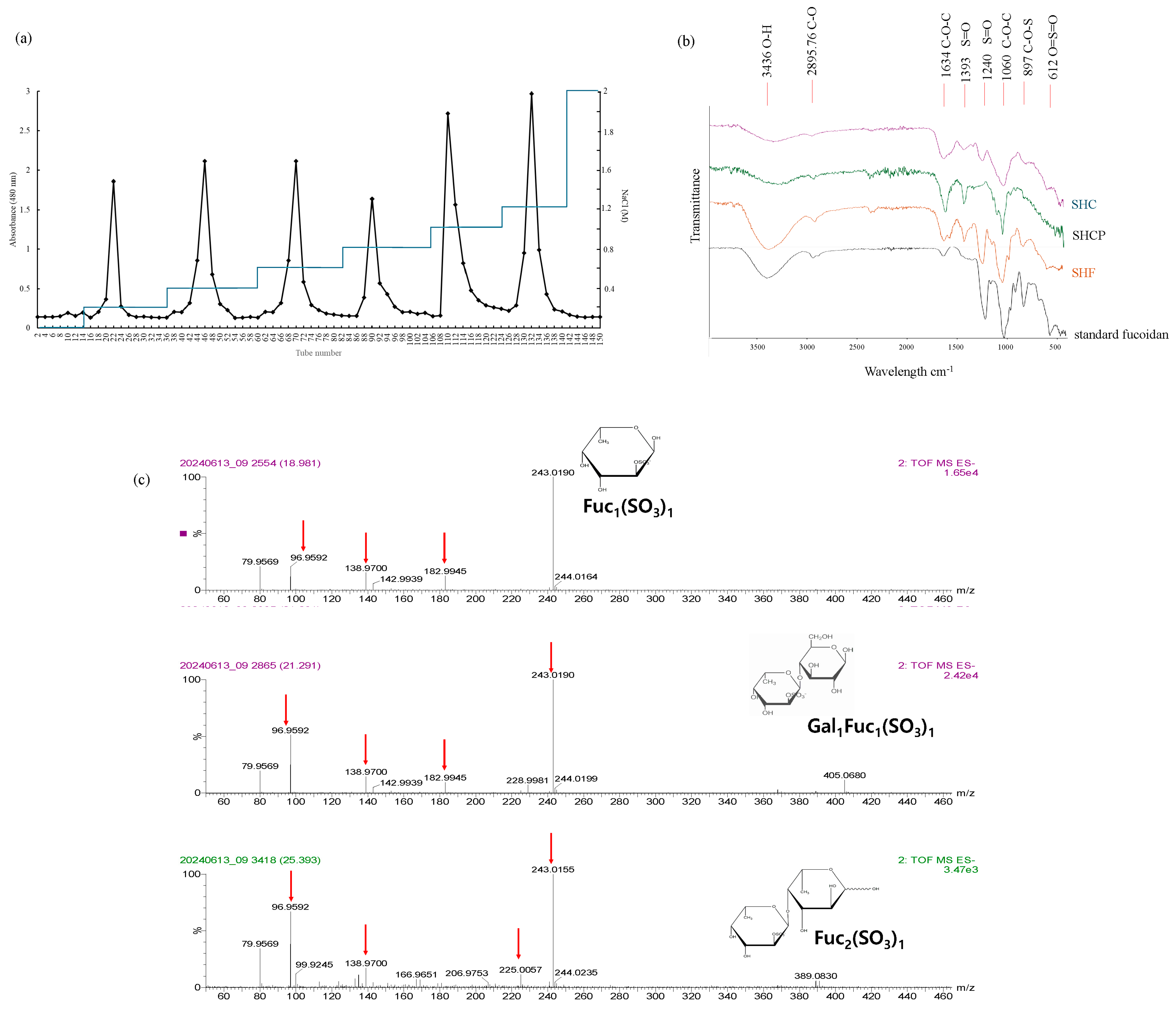
2.2. Purified S. horneri Fucoidan Fraction by Anion-Exchange Chromatography
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4. UPLC-QTOF-MS Analysis
2.5. Linkage Pattern Analysis
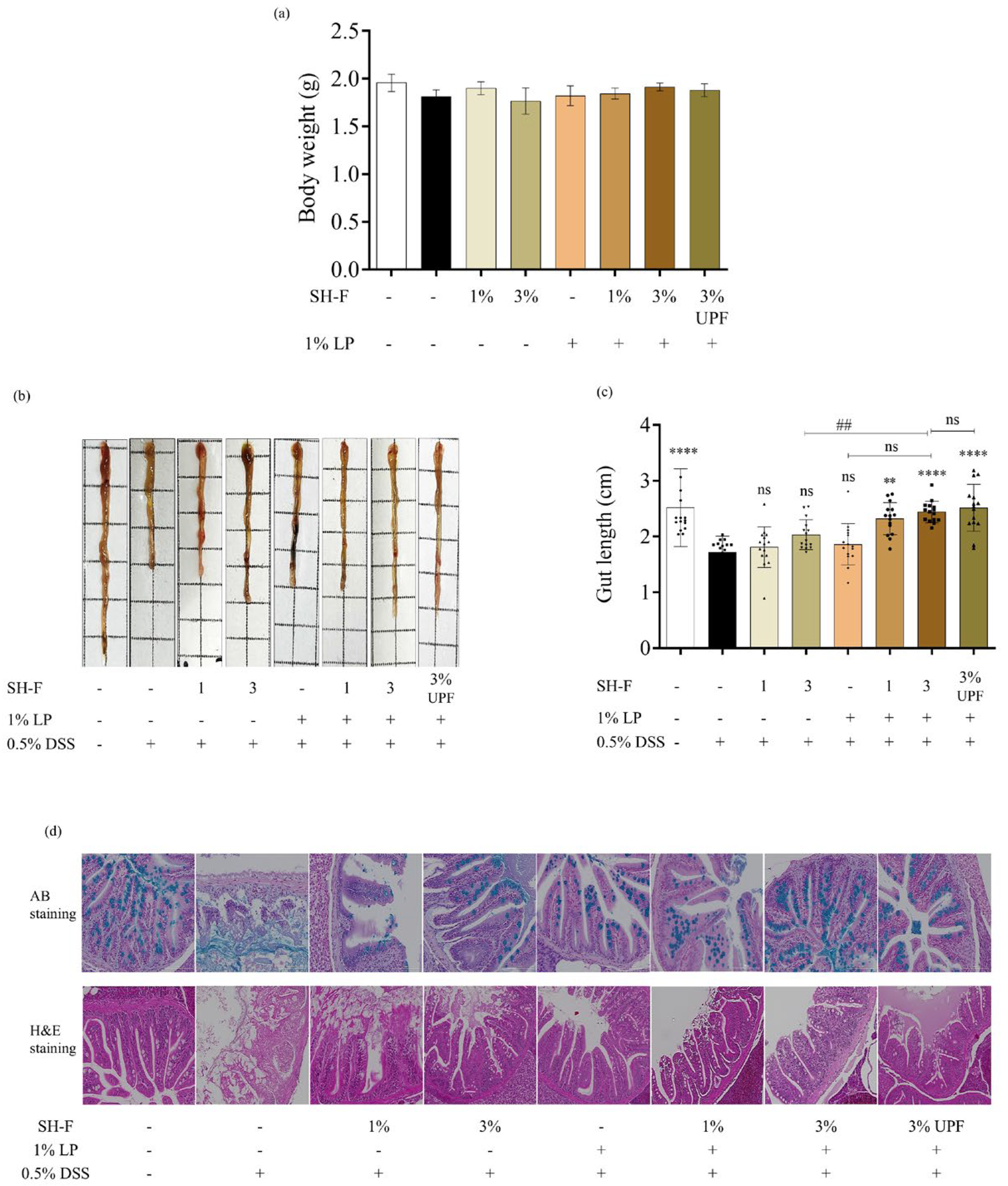
2.6. Synbiotic Treatment of L. plantarum and SH-F on Body Weight and Mortality of Zebrafish
2.7. Synbiotic Treatment of SH-F and L. plantarum Reduced Morphological Changes and Injuries in the Intestine
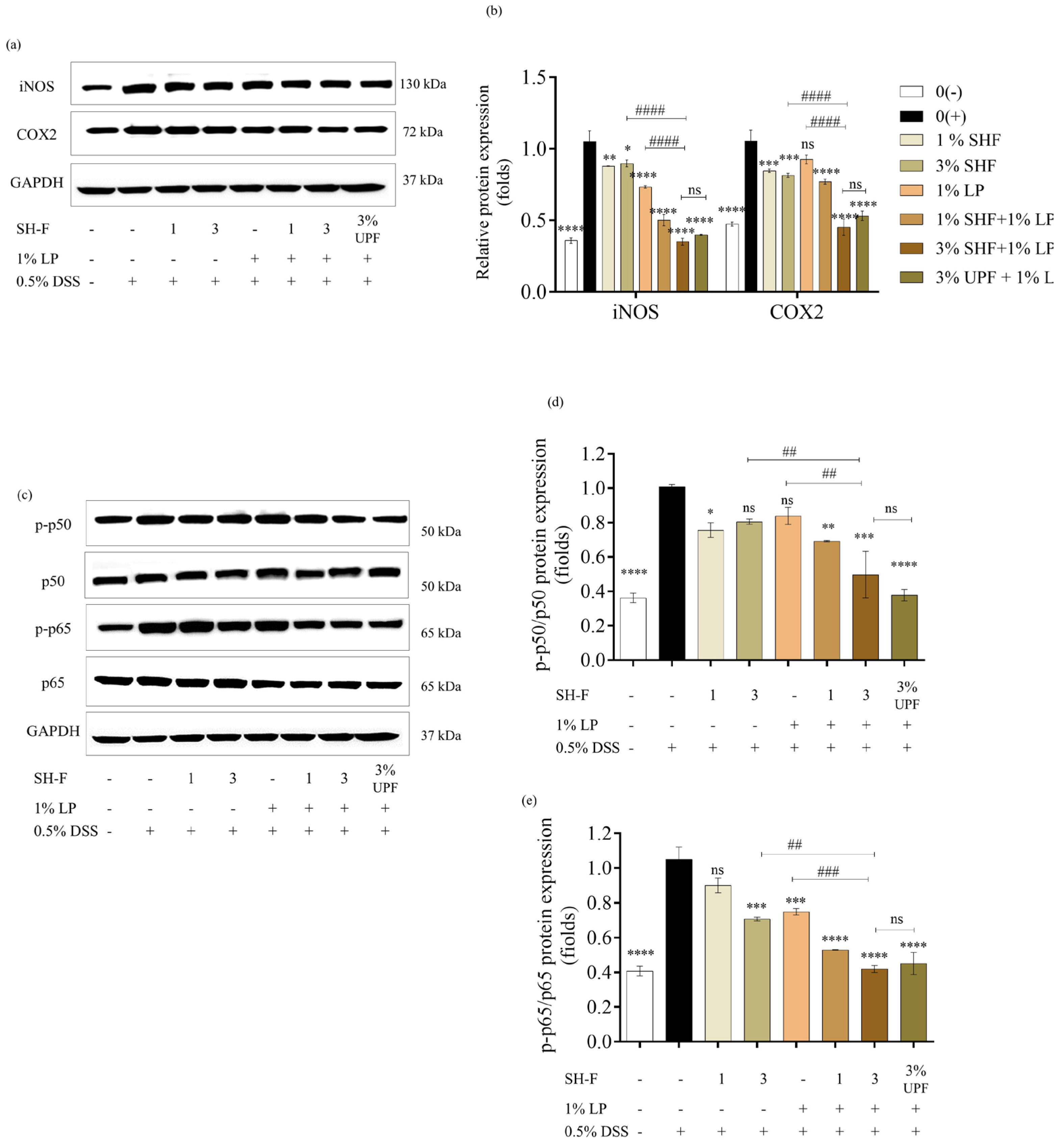
2.8. SH-F and L. plantarum Inhibited the Expression of iNOS, COX2, and NF-kB Pathway Proteins
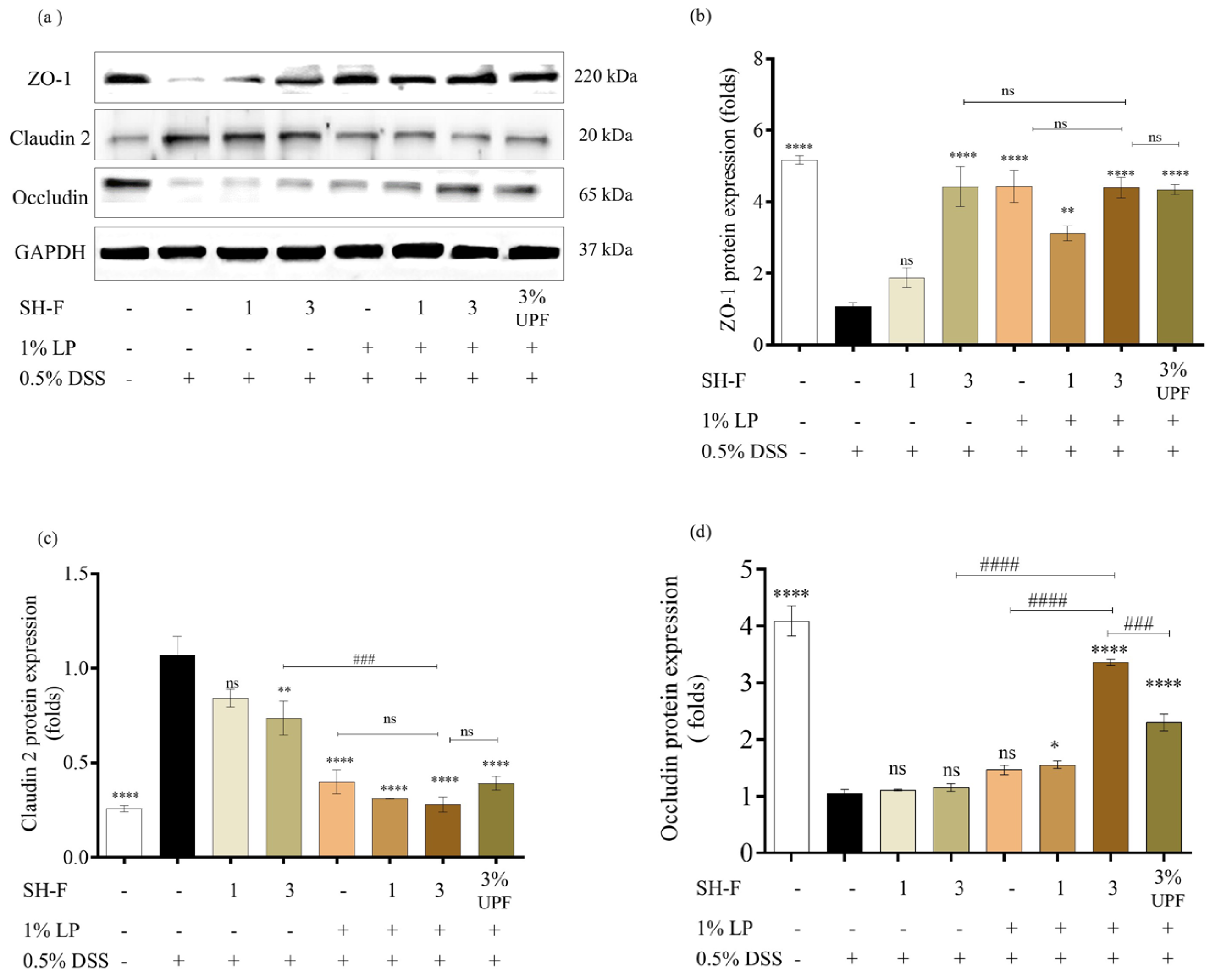
2.9. Synbiotic Treatment of SH-F and L. plantarum Restored Intestinal Barrier Function
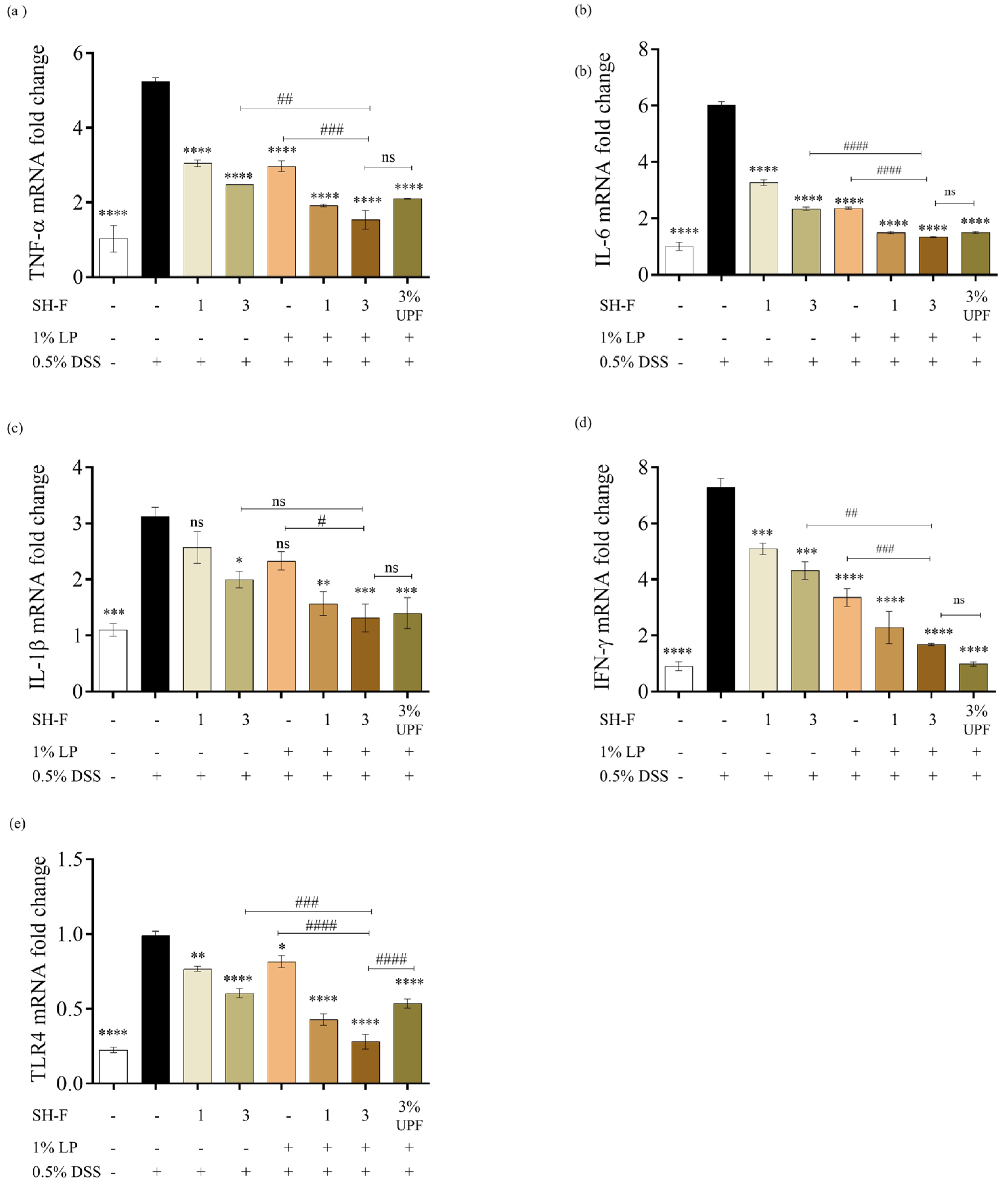
2.10. SH-F and L. plantarum Synbiotic Treatment Inhibited the Gene Expression of Pro-Inflammatory Cytokines in DSS-Induced Zebrafish UC Model
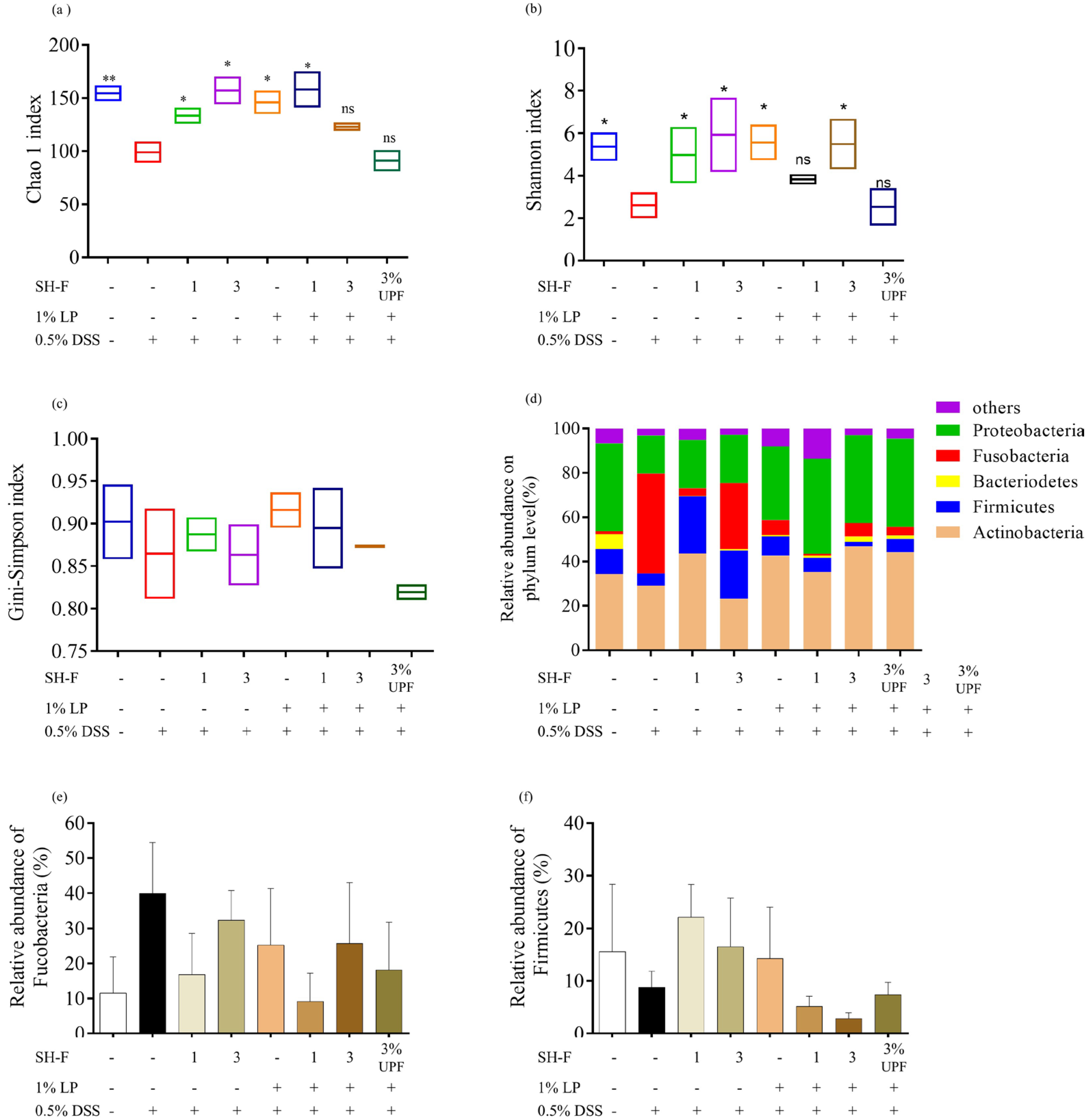
2.11. SH-F and L. plantarum Combined Treatment Regulated Microbial Composition and Prevented Microflora Dysbiosis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of S. horneri Fucoidan
4.3. S. horneri Fucoidan on L. plantarum Growth
4.4. Chemical Composition and Monosaccharide Composition Analysis of S. horneri Fucoidan
4.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis and Molecular Weight Determination
4.6. Methylation Analysis
4.7. UPLC-Q-TOF MS/MS Analysis
4.8. Maintenance of Adult Zebrafish
4.9. Experimental Diet Preparation
4.10. Zebrafish Experiment Design
4.11. Measurement of Body Weight
4.12. Histological Analysis
4.13. qPCR Analysis
4.14. Western Blot Analysis
4.15. 16S rRNA Sequencing and Data Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A. Use of Synbiotics for Ulcerative Colitis Treatment. Curr. Clin. Pharmacol. 2020, 15, 174–182. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta-analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Shalaby, M.; Abdelaziz, R.R.; Ghoneim, H.A.; Suddek, G.M. Imatinib mitigates experimentally-induced ulcerative colitis: Possible contribution of NF-kB/JAK2/STAT3/COX2 signaling pathway. Life Sci. 2023, 321, 121596. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, Z.; Zhao, Z.; Chen, Y.; Zhang, L. Native and Engineered Probiotics: Promising Agents against Related Systemic and Intestinal Diseases. Int. J. Mol. Sci. 2022, 23, 594. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef]
- Pan, Y.; Ning, Y.; Hu, J.; Wang, Z.; Chen, X.; Zhao, X. The Preventive Effect of Lactobacillus plantarum ZS62 on DSS-Induced IBD by Regulating Oxidative Stress and the Immune Response. Oxidative Med. Cell. Longev. 2021, 2021, 9416794. [Google Scholar] [CrossRef] [PubMed]
- Prantera, C.; Scribano, M.L.; Falasco, G.; Andreoli, A.; Luzi, C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: A randomised controlled trial with Lactobacillus GG. Gut 2002, 51, 405. [Google Scholar] [CrossRef] [PubMed]
- Hasannejad-Bibalan, M.; Mojtahedi, A.; Eshaghi, M.; Rohani, M.; Pourshafie, M.R.; Talebi, M. The effect of selected Lactobacillus strains on dextran sulfate sodium-induced mouse colitis model. Acta Microbiol. Immunol. Hung. 2020, 67, 138–142. [Google Scholar] [CrossRef]
- Wang, T.; van Dijk, L.; Rijnaarts, I.; Hermes Gerben, D.A.; de Roos Nicole, M.; Witteman Ben, J.M.; de Wit Nicole, J.W.; Govers, C.; Smidt, H.; Zoetendal Erwin, G. Methanogen Levels Are Significantly Associated with Fecal Microbiota Composition and Alpha Diversity in Healthy Adults and Irritable Bowel Syndrome Patients. Microbiol. Spectr. 2022, 10, e01653-22. [Google Scholar] [CrossRef]
- Vu, V.; Muthuramalingam, K.; Singh, V.; Hyun, C.; Kim, Y.M.; Unno, T.; Cho, M. Effects of β-glucan, probiotics, and synbiotics on obesity-associated colitis and hepatic manifestations in C57BL/6J mice. Eur. J. Nutr. 2022, 61, 793–807. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef]
- Hwang, E.K.; Lee, S.J.; Ha, D.S.; Park, C.S. Sargassum golden tides in the Shinan-gun and Jeju Island, Korea. Korean J. Fish. Aquat. Sci. 2016, 49, 689–693. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Kim, Y.-S.; Nagahawatta, D.P.; Jin, H.; Yang, H.-W.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Jeon, Y.-J. Sargassum horneri as a Prebiotic Dietary Supplement for Immunity Development in Streptococcus parauberis Infected Zebrafish Model. Front. Mar. Sci. 2022, 9, 901676. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Nagahawatta, D.P.; Yang, F.; Kim, Y.-S.; Kim, D.; Jeon, Y.-J. Synbiotic regulation of chronic intestinal inflammation by Sargassum horneri fucoidan and Lactobacillus plantarum. J. Funct. Foods 2025, 133, 106988. [Google Scholar] [CrossRef]
- Zheng, W.; Jia, J.; Zhang, C.; Zhang, P.; Song, S.; Ai, C. Undaria pinnatifida fucoidan ameliorates dietary fiber deficiency-induced inflammation and lipid abnormality by modulating mucosal microbiota and protecting intestinal barrier integrity. Int. J. Biol. Macromol. 2023, 247, 125724. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Sharma, J.; Babu, S.; Rizwanulla; Singla, A. Role of probiotics in health and disease: A review. J. Pak. Med. Assoc. 2013, 63, 253–257. [Google Scholar]
- Zaylaa, M.; Al Kassaa, I.; Alard, J.; Peucelle, V.; Boutillier, D.; Desramaut, J.; Dabboussi, F.; Pot, B.; Grangette, C. Probiotics in IBD: Combining in vitro and in vivo models for selecting strains with both anti-inflammatory potential as well as a capacity to restore the gut epithelial barrier. J. Funct. Foods 2018, 47, 304–315. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.-S.; Lee, D.-S.; Jeon, Y.-J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-T.; Lin, J.-H.; Chang, T.-X.; Lin, Y.-L.; Lee, S.-J.; Zheng, Y.-Y.; Hsueh, Y.-H. Incorporation of high molecular weight gamma-polyglutamic acid in maltodextrin-microencapsulated Bifidobacterium bifidum enhances resistance to simulated gastrointestinal fluids. Process Biochem. 2023, 133, 285–291. [Google Scholar] [CrossRef]
- Yoo, H.J.; You, D.-J.; Lee, K.-W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Fedorak, R.N. Understanding Why Probiotic Therapies Can be Effective in Treating IBD. J. Clin. Gastroenterol. 2008, 42, S111–S115. [Google Scholar] [CrossRef]
- Ma, L.; Yinhua, N.; Zhe, W.; Wenqing, T.; Liyang, N.; Fen, Z.; Aqian, Z.; Luting, H.; Yufeng, Z.; Liujie, Z.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial Tight Junctions in Intestinal Inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Song, Y.; Gao, Y.; Zhao, F.; Luo, Y.; Qian, F.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020, 11, 5205–5222. [Google Scholar] [CrossRef]
- Shi, J.; Xie, Q.; Yue, Y.; Chen, Q.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Gut microbiota modulation and anti-inflammatory properties of mixed lactobacilli in dextran sodium sulfate-induced colitis in mice. Food Funct. 2021, 12, 5130–5143. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Zheng, L.; Rong, N.; Yang, Y.; Gong, P.; Yang, Y.; Siwu, X.; Zhang, C.; Zhu, L.; et al. Bifidobacterium and Lactobacillus improve inflammatory bowel disease in zebrafish of different ages by regulating the intestinal mucosal barrier and microbiota. Life Sci. 2023, 324, 121699. [Google Scholar] [CrossRef]
- Yi, R.; Yang, B.; Zhu, H.; Sun, Y.; Wu, H.; Wang, Z.; Lu, Y.; He, Y.-W.; Tian, J. Quorum-Sensing Signal DSF Inhibits the Proliferation of Intestinal Pathogenic Bacteria and Alleviates Inflammatory Response to Suppress DSS-Induced Colitis in Zebrafish. Nutrients 2024, 16, 1562. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.J.; Radford-Smith, G. Intestinal Barrier Dysfunction in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-Q. Regulation of TLR4 Expression Is a Tale About Tail. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2582–2584. [Google Scholar] [CrossRef]
- Huang, X.; Hu, J.; Zhang, H.; Li, J.; Zhu, X.; Liu, Y.; Liang, Y.; Mei, Y. Clostridium butyricum and Chitooligosaccharides in Synbiotic Combination Ameliorate Symptoms in a DSS-Induced Ulcerative Colitis Mouse Model by Modulating Gut Microbiota and Enhancing Intestinal Barrier Function. Microbiol. Spectr. 2023, 11, e04370-22. [Google Scholar] [CrossRef]
- Nishikawa, J.; Takahiko, K.; Shinji, S.; Yoshimi, B.; and Sugiyama, T. Diversity of mucosa-associated microbiota in active and inactive ulcerative colitis. Scand. J. Gastroenterol. 2009, 44, 180–186. [Google Scholar] [CrossRef]
- Wu, Y.; Jha, R.; Li, A.; Liu, H.; Zhang, Z.; Zhang, C.; Zhai, Q.; Zhang, J. Probiotics (Lactobacillus plantarum HNU082) Supplementation Relieves Ulcerative Colitis by Affecting Intestinal Barrier Functions, Immunity-Related Gene Expression, Gut Microbiota, and Metabolic Pathways in Mice. Microbiol. Spectr. 2022, 10, e01651-22. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef] [PubMed]
- Vilela, E.G.; Ferrari, M.D.L.D.A.; Torres, H.O.D.G.; Pinto, A.G.; Aguirre, A.C.C.; Martins, F.P.; Goulart, E.M.A.; Da Cunha, A.S. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Pereira Guedes, T.; Alves Silva, J.; Neves, S.; Falcão, D.; Costa, P.; Lago, P.; Pedroto, I.; Salgado, M. Positioning Aeromonas Infection in Inflammatory Bowel Disease: A Retrospective Analysis. GE-Port. J. Gastroenterol. 2023, 30, 20–28. [Google Scholar] [CrossRef]
- Lee, W.; Ahn, G.; Oh, J.Y.; Kim, S.M.; Kang, N.; Kim, E.A.; Kim, K.-N.; Jeong, J.B.; Jeon, Y.-J. A prebiotic effect of Ecklonia cava on the growth and mortality of olive flounder infected with pathogenic bacteria. Fish Shellfish Immunol. 2016, 51, 313–320. [Google Scholar] [CrossRef]
- Je, J.-G.; Kim, C.-Y.; Sim, J.; Roh, Y.; Kurera, M.J.M.S.; Liyanage, N.M.; Jung, S.; Jeon, Y.-J.; Ryu, B. Anti-inflammatory activity and structural analysis of fucoidan extracted from Sargassum fusiforme via ESI-TOF MS spectrometry. Food Biosci. 2024, 60, 104414. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Liyanage, N.M.; Lee, H.-G.; Nagahawatta, D.P.; Jayawardhana, H.H.A.C.K.; Ryu, B.; Jeon, Y.-J. Characterization and therapeutic effect of Sargassum coreanum fucoidan that inhibits lipopolysaccharide-induced inflammation in RAW 264.7 macrophages by blocking NF-κB signaling. Int. J. Biol. Macromol. 2022, 223, 500–510. [Google Scholar] [CrossRef]
- Xiao, H.; Feng, J.; Peng, J.; Wu, P.; Chang, Y.; Li, X.; Wu, J.; Huang, H.; Deng, H.; Qiu, M.; et al. Fuc-S—A New Ultrasonic Degraded Sulfated α-l-Fucooligosaccharide—Alleviates DSS-Inflicted Colitis through Reshaping Gut Microbiota and Modulating Host–Microbe Tryptophan Metabolism. Mar. Drugs 2023, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Kesbiç, O.S.; Acar, Ü.; Yilmaz, S.; Aydin, Ö.D. Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish. Physiol. Biochem. 2020, 46, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sireswar, S.; Dey, G.; Biswas, S. Influence of fruit-based beverages on efficacy of Lacticaseibacillus rhamnosus GG (Lactobacillus rhamnosus GG) against DSS-induced intestinal inflammation. Food Res. Int. 2021, 149, 110661. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Lee, H.-G.; Heo, M.-S.; Jeon, Y.-J. Eckmaxol Isolated from Ecklonia maxima Attenuates Particulate-Matter-Induced Inflammation in MH-S Lung Macrophage. Mar. Drugs 2022, 20, 766. [Google Scholar] [CrossRef]
- Nagahawatta, D.P.; Liyanage, N.M.; Jayawardhana, H.H.A.C.K.; Lee, H.-G.; Jayawardena, T.U.; Jeon, Y.-J. Anti-Fine Dust Effect of Fucoidan Extracted from Ecklonia maxima Leaves in Macrophages via Inhibiting Inflammatory Signaling Pathways. Mar. Drugs 2022, 20, 413. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Yang, J.H.; Park, J.W.; Kim, H.S.; Lee, S.; Yerke, A.M.; Jaiswal, Y.S.; Williams, L.L.; Hwang, S.; Moon, K.H. Effects of Antibiotic Residues on Fish Gut Microbiome Dysbiosis and Mucosal Barrier-Related Pathogen Susceptibility in Zebrafish Experimental Model. Antibiotics 2024, 13, 82. [Google Scholar] [CrossRef]
- Yang, F.; Nagahawatta, D.P.; Yang, H.W.; Ryu, B.; Lee, H.G.; Je, J.G.; Heo, S.M.; Jeon, Y.J. In vitro and in vivo immuno-enhancing effect of fucoidan isolated from non-edible brown seaweed Sargassum thunbergii. Int. J. Biol. Macromol. 2023, 253, 127212. [Google Scholar] [CrossRef] [PubMed]
| Retention Time (min) | Partially Methylated Alditol Acetates (PMAA) | Methylated Sugar | Mass Fragment (m/z) | Molar Ratio | Type of Linkage | |
|---|---|---|---|---|---|---|
| 1 | 3.95 | 1,5,6-Tri-O-acetyl-1-deuterio-2,3,4-tri-O-methyl-D-galactitol | 6-linked-D-galactopyranosyl residue | 41, 43, 85, 103, 131, 160, 176, 198, 220, 258, 272, 298 | 0.11 | →6)-Gal(1→ |
| 2 | 4.11 | 1,4,5-Tri-O-acetyl-1-deuterio-6-deoxy-2,3-di-O-methyl-L-galactitol | 4-linked-6-deoxy-L-galactopyranosyl (Fuc) residue | 41, 43, 85, 103, 120, 145, 176, 198, 220, 258, 268, 300 | 0.16 | →4)-Fucp(1→ |
| 3 | 4.25 | 1,3,5-Tri-O-acetyl-1-deuterio-6-deoxy-2,4-di-O-methyl-L-galactitol | 3-linked-6-deoxy-L-galactopyranosyl(Fuc) residue | 41, 43, 85, 103, 119, 148, 190, 213, 220, 258, 272, 300 | 0.44 | →3)-Fucp(1→ |
| 4 | 5.33 | 1,4,5,6-Tetra-O-acetyl-1-deuterio-2,3-di-O-methyl-D-galactitol | 4,6-linked-D-galactopyranosyl residue | 41, 43, 72, 114, 118, 161, 185, 199, 241, 243, 288, 326, 368 | 0.13 | →4,6)-Gal(1→ |
| 5 | 9.25 | 1,3,4,5-Tetra-O-acetyl-1-deuterio-6-deoxy-2-O-methyl-L-galactitol | 3,4-linked-6-deoxy-L-galactopyranosyl (Fuc) residue | 41, 43, 73, 97, 129, 157, 185, 213, 227, 258, 281, 299 | 0.16 | →3,4)-Fucp(1→ |
| Sum of molar ratio | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyanage, N.M.; Nagahawatta, D.P.; Jayawardhana, H.H.A.C.K.; Je, J.-G.; Yiqiao, L.; Yang, F.; Kim, Y.-S.; Ko, K.Y.; Jeon, Y.-J. The Synergistic Role of Sargassum horneri Fucoidan and Lactobacillus plantarum: Microbiome and Gut Barrier Restoration in Zebrafish Colitis. Mar. Drugs 2025, 23, 372. https://doi.org/10.3390/md23100372
Liyanage NM, Nagahawatta DP, Jayawardhana HHACK, Je J-G, Yiqiao L, Yang F, Kim Y-S, Ko KY, Jeon Y-J. The Synergistic Role of Sargassum horneri Fucoidan and Lactobacillus plantarum: Microbiome and Gut Barrier Restoration in Zebrafish Colitis. Marine Drugs. 2025; 23(10):372. https://doi.org/10.3390/md23100372
Chicago/Turabian StyleLiyanage, N. M., D. P. Nagahawatta, H. H. A. C. K. Jayawardhana, Jun-Geon Je, Li Yiqiao, Fengqi Yang, Young-Sang Kim, Kyung Yuk Ko, and You-Jin Jeon. 2025. "The Synergistic Role of Sargassum horneri Fucoidan and Lactobacillus plantarum: Microbiome and Gut Barrier Restoration in Zebrafish Colitis" Marine Drugs 23, no. 10: 372. https://doi.org/10.3390/md23100372
APA StyleLiyanage, N. M., Nagahawatta, D. P., Jayawardhana, H. H. A. C. K., Je, J.-G., Yiqiao, L., Yang, F., Kim, Y.-S., Ko, K. Y., & Jeon, Y.-J. (2025). The Synergistic Role of Sargassum horneri Fucoidan and Lactobacillus plantarum: Microbiome and Gut Barrier Restoration in Zebrafish Colitis. Marine Drugs, 23(10), 372. https://doi.org/10.3390/md23100372








