Abstract
Microalgae are considered promising sustainable feedstocks for the production of food, food additives, feeds, chemicals and various high-value products. Marine microalgae Phaeodactylum tricornutum, Isochrysis galbana and Nitzschia laevis are rich in fucoxanthin, which is effective for weight loss and metabolic diseases. The selection of microalgae species with outstanding nutritional profiles is fundamental for novel foods development, and the nutritional value of P. tricornutum, I. galbana and N. laevis are not yet fully understood. Hence, this study investigates and analyzes the nutritional components of the microalgae by chromatography and mass spectrometry, to explore their nutritional and industrial application potential. The results indicate that the three microalgae possess high nutritional value. Among them, P. tricornutum shows significantly higher levels of proteins (43.29%) and amino acids, while I. galbana has the highest content of carbohydrates (25.40%) and lipids (10.95%). Notwithstanding that P. tricornutum and I. galbana have higher fucoxanthin contents, N. laevis achieves the highest fucoxanthin productivity (6.21 mg/L/day) and polyunsaturated fatty acids (PUFAs) productivity (26.13 mg/L/day) because of the competitive cell density (2.89 g/L) and the advantageous specific growth rate (0.42/day). Thus, compared with P. tricornutum and I. galbana, N. laevis is a more promising candidate for co-production of fucoxanthin and PUFAs.
1. Introduction
Microalgae fix CO2 through the Calvin cycle to convert it into valuable organic compounds through various intracellular metabolic pathways, such as pigments, proteins, polysaccharides and lipids [1,2,3,4]. Many of these compounds, including carotenoids, polyunsaturated fatty acids (PUFAs), bioactive poly/monosaccharides, proteins and peptides containing essential amino acids (EAAs), present high nutritional value and health-beneficial functions, such as anti-oxidation, anti-inflammatory, anti-cancer, anti-obesity and anti-diabetes activities [4]. Accordingly, microalgae are promising to take the place of traditional food sources as new food and functional food products, and due to their well-rounded chemical composition, they can be used to enhance the nutritional value of foods. For example, adding Spirulina in bread improved its nutritional quality, increasing protein content and certain essential amino acids (threonine, methionine, isoleucine and leucine) by 39.04% compared to untreated bread [5]. Incorporating 5.41% Spirulina significantly enhanced the nutritional value of donuts, with protein content rising from 7.37% to 12.19% and lipid content increasing from 5.82% to 12.11% [6]. Moreover, marine microalgae, in particular, offer unique bioactive compounds due to their ability to thrive in diverse marine environments [7]. Hence, it is essential to acquire adequate knowledge about the biochemical composition of marine microalgal species in order to exploit their potential in different fields.
Among the typically bioactive components in microalgae, proteins and polysaccharides constitute a significant proportion of the dry cell weight (DCW). However, the types and amounts of nutrients vary greatly among different microalgae species. For instance, the protein content of Chlorella sp. is around 40–50% of the DCW, whereas Spirulina sp. has a protein content as high as 70% [8]. The chrysolaminarin content of Odontella aurita is notably high [9,10], constituting important nutritional components of its cellular sugars, while some microalgae have higher levels of fatty acids instead [11,12,13,14]. The nutritional value of microalgae depends not only on the nutrients content in cells but also on the proportions and composition of each nutrient component. For example, the level of EAAs within proteins determines their nutritional value, and the content of essential PUFAs, such as arachidonic acid (ARA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in fatty acids reflects the nutritional value of algal oil. Among the fatty acids, PUFAs have more than one double bond in their carbon chain structure, and they are considered as high-value compounds in health food industry. There are two classes of PUFAs, n-6 and n-3, which are synthesized from linoleic acid and linolenic acid, respectively. In addition, alpha-linolenic acid, EPA and DHA are the key n-3 fatty acids. DHA and EPA are nutritionally significant PUFAs produced in significant amounts by some marine microalgae, and they have been paid particular interest due to their bioactivities for human fitness [6,15]. It was reported that regular consumption of EPA and DHA supplements could reduce inflammation and prevent cardiovascular disease [16,17,18,19]. The production of microalgal PUFAs has become more cost-effective compared to the production of biofuel, and in recent years, a large number of producers have shifted their focus towards PUFAs production.
Apart from these common nutrients, species like Phaeodactylum tricornutum, Isochrysis galbana, Nitzschia laevis and O. aurita are rich in fucoxanthin [20,21,22], which cannot be synthesized artificially. It is one of the primary carotenoids in marine brown seaweeds, diatoms and golden algae [23,24]. Recent studies have focused on synthesizing fucoxanthin using P. tricornutum and I. galbana, given its potential for beneficial activities, including anti-oxidation and anti-obesity effects [24,25,26,27]. Furthermore, the selection of industrial microalgae species with outstanding nutritional profiles is fundamental for the successful development of novel foods. Hence, a detailed analysis and evaluation of the nutritional value of the microalgae is essential for the selection of the suitable microalgae for specific food technology applications, consequently facilitating the food industry. Therefore, this study selects three marine microalgae species (P. tricornutum, I. galbana and N. laevis) rich in fucoxanthin and PUFAs to investigate and analyze their growth characteristics and main nutritional components, thereby exploring their potential in the industrial production of high-value products.
2. Results and Discussion
2.1. Growth Characteristics and Carbon Partitioning of P. tricornutum, I. galbana and N. laevis
The industrialization potential of microalgae mainly depended on the productivities of high-value products [28,29,30], which were closely related with their growth characteristics and the biosynthesis of target products [31,32]. Among the three microalgae, N. laevis UTEX 2047 was able to grow under mixotrophic cultivation, heterotrophic cultivation and autotrophic cultivation, but the biomass concentration in autotrophic cultivation was rare low. During the cultivation process, the addition of light usually incurred significantly higher costs. Thus, considering the production cost, heterotrophic cultivation was chosen for N. laevis UTEX 2047 in this study. In terms of cell growth, N. laevis UTEX 2047 exhibited a notably higher specific growth rate compared to P. tricornutum UTEX 646 and I. galbana UTEX 2307, as shown in Figure 1, indicating faster proliferation to achieve higher final cell densities. Studies revealed that both P. tricornutum and I. galbana required light for growth and struggled to achieve high biomass concentrations [33,34,35], limiting their development and utilization to some extent. Thus, mixotrophic cultivation was chosen for P. tricornutum and I. galbana due to the ability to achieve higher cell densities with the addition of organic substrates compared with autotrophic cultivation. Previous research utilizing flat-panel photobioreactors achieved maximum biomass concentrations of only 4.10 g/L and 3.99 g/L for P. tricornutum and I. galbana, respectively [36,37]. In contrast, N. laevis could be cultured under both light and dark conditions, with high glucose utilization rates facilitating the attainment of high biomass concentrations [38,39]. Early studies by Wen et al. demonstrated that the high cell density of 40 g/L could be achieved by using perfusion cultivation methods for N. laevis [40].
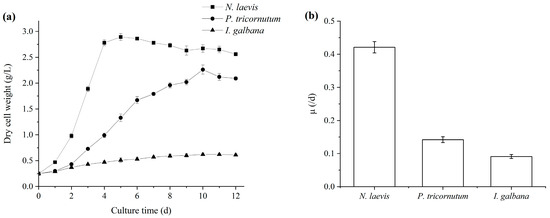
Figure 1.
Growth curves (a) and maximum specific growth rates (b) of N. laevis, P. tricornutum and I. galbana under mixotrophic cultivation. Values are mean ± SD of at least three independent experiments.
Biochemical composition of microalgae cells that included proteins, carbohydrates, lipids and pigments could provide an indication of their nutritional value. The intracellular composition of total proteins, carbohydrates, lipids and pigments in three microalgae are summarized in Table 1. It was observed that P. tricornutum UTEX 646 exhibited the highest protein content at 43.29% of the DCW, which was significantly higher than that of the other two species. I. galbana showed the highest total carbohydrate content at 25.40%, followed by N. laevis at 21.97% and P. tricornutum at 14.85%. All three microalgae were rich in lipids, with the highest lipid content found in I. galbana, followed by P. tricornutum and N. laevis. As for pigment content, P. tricornutum showed significantly higher levels compared to I. galbana and N. laevis, exceeding their pigment contents by two-fold and three-fold, respectively. The nutritional value of the proteins, polysaccharides and lipids mainly depended on amino acid composition, monosaccharide composition and the presence of PUFAs.

Table 1.
The carbon partitioning of P. tricornutum, I. galbana and N. laevis a.
2.2. Amino Acid Composition of P. tricornutum, I. galbana and N. laevis
The total protein content of microalgae varied due to different species and up to 70% of the DCW [8,41,42,43]. The amino acid profile, including the content, proportion and availability of amino acids, was an important parameter of the protein quality and nutritional value [44]. The amino acid profiles of P. tricornutum, I. galbana and N. laevis were compiled in Table 2, and the Food and Agriculture Organization of the United Nations (FAO/WHO) proposed a reference standard with the recommended content of the EAAs in a protein or a mixture of proteins.

Table 2.
Amino acid composition of P. tricornutum, I. galbana and N. laevis a.
As shown in Table 2, the amino acid profiles of P. tricornutum, I. galbana and N. laevis were deficient for tryptophan. It was evident that P. tricornutum exhibited higher concentrations of most amino acids compared to the others, consistent with its highest protein content as shown in Table 1. Among non-essential amino acids (NEAA), alanine, serine, aspartic acid, glutamic acid and arginine were presented in notable amounts, exceeding 1% of the DCW in all three microalgae. EAAs, except for methionine, which is below 0.5% of the DCW, were generally presented in higher concentrations, particularly leucine, which exceeds 2% of the DCW in both P. tricornutum and I. galbana. Additionally, histidine, which was unable to be synthesized by infants, was sometimes considered essential. All three microalgae contained varying amounts of histidine, with similar and relatively high levels observed in P. tricornutum and I. galbana. However, the lysine in three marine microalgae were lower than those reported in Chlorella vulgaris, Arthrospira maxima and Arthrospira platensis, which might suggest freshwater microalgae are more suitable as feed because lysine was typically the first limiting amino acid in aquaculture [44]. The lack or low content of tryptophan might be due to the high-salinity environment being unfavorable for its biosynthesis. The total content of EAAs was notably higher in P. tricornutum (9.00%) and I. galbana (8.17%) compared to N. laevis. Therefore, considering the protein nutrition, P. tricornutum UTEX 646 and I. galbana UTEX 2307 demonstrated advantages over N. laevis UTEX 2047 among the three microalgae.
According to the ideal protein model proposed by FAO/WHO standard, the ratio of EAAs to total amino acids (EAA/TAA) of good quality was about 40%, and the ratio of essential to non-essential amino acids (EAA/NEAA) was more than 60% [45]. As presented in Table 2, P. tricornutum and I. galbana showed better qualities compared with N. laevis. The EAA indices reflected the average supply of EAAs in food proteins. The higher EAA index (EAAI) value of food protein is to 100, the higher the nutritional value. Hence, the protein of P. tricornutum presented more valuable than that of the other two microalgae. According to Table 2, N. laevis UTEX 2047 may not be the best choice to obtain protein for food and feed application.
2.3. Monosaccharide Profiles of P. tricornutum, I. galbana and N. laevis
Compared to other sources, polysaccharides derived from microalgae were reported as stable, safe, versatile, biocompatible and biodegradable [46]. They presented complex biochemical structures according to each microalgal species. In addition, they exhibited beneficial biological characteristics that included anti-oxidant, anti-inflammatory, anti-tumor and anti-microbial activities [47]. Microalgal polysaccharides were predominantly composed of pentose and hexose monosaccharide subunits with many glycosidic bonds [46].
The monosaccharide profiles of three microalgae after polysaccharide hydrolysis are shown in Table 3. It was observed that glucose was the predominant monosaccharide among the 14 identified monosaccharides and uronic acids in all three microalgae. In addition to glucose, mannose content was also notably high in P. tricornutum and N. laevis, exceeding 40 mg/g. Although I. galbana exhibited relatively high proportions of glucose and mannose in its own cellular polysaccharides, these amounts were several times lower compared to those exhibited by P. tricornutum and N. laevis. This discrepancy could be attributed to the lack of a cell wall in I. galbana, resulting in lower polysaccharide content compared to P. tricornutum and N. laevis. However, I. galbana contained comparable or higher levels of galacturonic acid, ribose, galactose, xylose, arabinose and fucose compared to the other two species, and especially notable high levels of arabinose (7.01 mg/g) and fucose (3.39 mg/g) which were significantly higher than those in P. tricornutum and N. laevis. Galacturonic acid and galactose content were more than twice as high in I. galbana compared to N. laevis.

Table 3.
Monosaccharide profiles of P. tricornutum, I. galbana and N. laevis a.
N. laevis exhibited the highest levels of glucose (529.10 mg/g), mannose (44.26 mg/g), ribose (4.25 mg/g), glucosamine (2.25 mg/g) and galacturonic acid (2.85 mg/g), which were easily to be digested and absorbed deep. Studies indicated that microalgal cell walls consisted of heteropolysaccharides or multiple types of polysaccharides, with glucose, mannose, xylose and galactose typically being the most abundant monosaccharides [47,48,49]. For instance, in C. vulgaris, glucogalactan was prevalent in the cell wall polysaccharides, making glucose and galactose the most abundant monosaccharides [50]. The ratio of galacturonic acid to glucuronic acid in cells depended significantly on the microalgae species. While P. tricornutum and N. laevis had higher total monosaccharide content compared to I. galbana, I. galbana exhibited advantages in specific monosaccharides such as arabinose and fucose.
2.4. Fatty Acids Profiles of P. tricornutum, I. galbana and N. laevis
The characteristic of microalgae to store lipids was extremely valuable to meet the growing demand for the production of food, feed and biofuel. According to the analysis, lipids were the most frequently extracted compounds from microalgae and had the greatest potential for commercialization [11,14,15,51]. The diverse lipid composition of microalgae opens up broad applications, such as biofuel production, animal husbandry/aquaculture feeds, food products and food additives [52].
In this study, all three microalgae were rich in lipids, with P. tricornutum and N. laevis containing high levels of EPA, as shown in Table 4, while I. galbana was abundant in DHA and stearidonic acid (SDA). Linoleic acid and gamma-linolenic acid were essential fatty acids in humans and precursors for the synthesis of ARA, EPA and DHA, the physiological functions of which had been well-known in the medical and health industries, and many PUFAs had already been put into production and utilization. Among the three microalgae, I. galbana contained the highest level of C18 unsaturated fatty acid, with C18:1 at 3.17%, C18:3 at 1.05% and C18:4 at 1.20% (DCW), respectively. In addition, the C14:0 content of 1.71% and C16:0 content of 1.82% of I. galbana were higher than the other two microalgae. Differently, P. tricornutum and N. laevis presented high contents of C16:0, C16:1 and C20:5, and the EPA contents even reached over 2% of the DCW, making them the highest natural sources of EPA, and valuable for EPA industrial applications. Overall, the PUFAs within the cells of these three microalgae possessed significant nutritional value.

Table 4.
Fatty acids profiles of P. tricornutum, I. galbana and N. laevis a.
2.5. Fucoxanthin and PUFAs Productivities of P. tricornutum, I. galbana and N. laevis
Fucoxanthin-rich microalgae, including P. tricornutum, I. galbana, Odontella aurita and Mallomonas sp., have been reported to have high fucoxanthin content (1.65% to 2.66%), but their fucoxanthin productivities were low, with the maximum productivity ranging from 1.75 to 7.96 mg/L/day [20,28,29,30,53,54,55]. Industrial-scale fermentation of microalgae for producing high-value products necessitated considerations beyond the intracellular content of the target product, including biomass concentration and the fermentation cost. Especially, despite higher fucoxanthin content in I. galbana cells, its slow growth and low biomass concentration resulted in lower fucoxanthin productivity. Although N. laevis had the lowest fucoxanthin content among the three microalgae, its rapid growth and ability to achieve high biomass concentrations contributed to the highest fucoxanthin productivity. As shown in Figure 2, the fucoxanthin content of N. laevis (0.89%, DCW) was lower than that of P. tricornutum (2.43%, DCW) and I. galbana (0.98%, DCW), but its fucoxanthin productivity (6.21 mg/L/day) was significantly higher compared to P. tricornutum and I. galbana. Besides the fucoxanthin, the PUFAs produced by microalgae were also worth to be co-produced for the high value utilization.
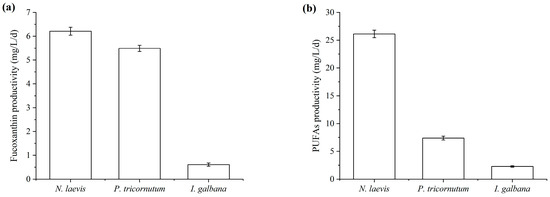
Figure 2.
Fucoxanthin productivities (a) and PUFAs productivities (b) of N. laevis, P. tricornutum and I. galbana. Values are mean ± SD of at least three independent experiments.
Due to the similar physicochemical properties of some microalgal metabolites (such as fatty acids and carotenoids), including molecular weight, polarity, solubility and hydrophobicity, multiple compounds could be extracted from microalgal cells simultaneously. Therefore, many efforts had been devoted to co-production of high-value metabolites by microalgae. For example, Synechococcus nidulans and Spirulina sp. were used to co-produce phycobiliprotein and carbonic anhydrase [56]; Dunaliella salina was selected as a candidate producer of carotenoid and protein [57]. In view of the great commercial application prospect of fucoxanthin and PUFAs, several marine microalgae were explored for the co-production potential [58]. It was reported that N. laevis and P. tricornutum were promising producers for EPA [36,38,40,54], and I. galbana was abundant in DHA and SDA. However, the production process was still faced with many problems such as high production costs and low productivities. As shown in Table 4 and Figure 2, although there were no large differences between the PUFAs contents of P. tricornutum, I. galbana and N. laevis, the PUFAs productivity of N. laevis (26.13 mg/L/day) was much higher than that of P. tricornutum and I. galbana. Given the advantage in fucoxanthin and PUFAs productivities, N. laevis was expected to be more attractive for industrial applications compared to the other two microalgae. Nevertheless, each microalgae species has its own benefits, such as the differential production of EPA (P. tricornutum), DHA (I. galbana) and both (N. laevis) omega-3 PUFA species, which command high interest in the food and feed sector.
3. Materials and Methods
3.1. Strains and Mediums
The Nitzschia laevis UTEX 2047, Phaeodactylum tricornutum UTEX 646 and Isochrysis galbana UTEX 2307 were purchased from the Culture Collection of Algae at the University of Texas-Austin, USA. N. laevis was cultured in the modified LDM medium; P. tricornutum and I. galbana were cultured in the modified f/2 medium.
Modified LDM medium (per L) comprised 892 mL artificial seawater, tryptone 1 g, NaNO3 1 g, Na2SiO3·9H2O 120 mg, K2HPO4 7.5 mg, KH2PO4 17.5 mg, MgSO4·7H2O 7.5 mg, Na2EDTA 4.5 mg, CaCl2·2H2O 2.5 mg, FeCl3·6H2O 0.582 mg, ZnCl2 0.03 mg, CoCl2·6H2O 0.012 mg, Na2MoO4·2H2O 0.024 mg, MnCl2·4H2O 0.246 mg, biotin 0.025 mg and VB12 0.015 mg. The medium was adjusted to pH 8.5.
Modified f/2 medium (per L) comprised NaNO3 300 mg, NaH2PO4·H2O 20 mg, Na2EDTA·2H2O 4.36 mg, FeCl3·6H2O 3.15 mg, MnCl2·4H2O 0.18 mg, CoCl2·6H2O 0.01 mg, ZnSO4·7H2O 22 μg, CuSO4·5H2O 9.8 μg, Na2MoO4·2H2O 6.3 μg, thiamin·HCl 100 μg, biotin 0.5 μg and cyanocobalamin 0.5 μg. The culture medium of P. tricornutum contain sea salt 20 g/L, Na2SiO3·9H2O 30 mg/L, and the medium was adjusted to pH 8.5. The culture medium of I. galbana contain sea salt (Sigma Co., Kawasaki, Japan) 20 g/L, and the medium was adjusted to pH 8.2.
Artificial sea water (per L) comprised NaCl 18 g, MgSO4·7H2O 2.44 g, KCl 0.6 g, NaNO3 1 g, CaCl2·2H2O 0.3 g, KH2PO4 0.05 g, Tris buffer (Sigma Co.) 1 g, NH4Cl 0.0267 g, VB12 0.15 μg, PI metal solution 10 mL and chelated iron solution 3 mL; pH was 8.08.
PI metal solution (per L) comprised H3BO3 3.426 g, CoCl2·6H2O 1.215 mg, MnCl2·4H2O 0.432 mg, ZnCl2 31.5 mg, H2SO4 1 mL and (NH4)6Mo7O24·4H2O 31.19 mg; chelated iron solution (per L) comprised Na2EDTA 10 g and FeCl3·6H2O 0.81 g.
3.2. Culture Conditions
Before inoculation, the microalgal seeds were observed under a microscope to check for bacterial contamination. The diatom N. laevis was cultured in 500 mL Erlenmeyer flasks containing 200 mL of fresh LDM medium, under dark condition with orbital shaking at 150 rpm at 22 °C. P. tricornutum was cultured in a photobioreactor containing 200 mL fresh f/2 medium. Light intensity was set at 50 μmol photons/m2/s and temperature was maintained at 22 °C. I. galbana was cultured in 500 mL Erlenmeyer flasks containing 200 mL of fresh f/2 medium and maintained in a stationary incubator under a 12/12 light/dark cycle, with the flasks manually shaken three times daily. All the culture mediums were supplemented with 5 g/L glucose.
3.3. Determination of Microalgal Biomass
Cell suspension (5 mL) was centrifuged at 5000 rpm for 3 min. Then, the pellet was washed twice with distilled water and filtered through a pre-weighed filter paper (Whatman GF/C). Cells on the filter paper were dried to a constant weight at 80 °C in a vacuum oven. The specific growth rates of N. laevis, P. tricornutum and I. galbana were calculated using the following equation:
where was the biomass concentration of the culture after t days (g/L) and was the initial biomass concentration (g/L).
3.4. Analysis of Protein and Amino Acids
For the determination of intracellular protein, 10 mg lyophilized cells were added to 200 μL of 1 M NaOH, and heated in water bath at 80 °C for 10 min. Then, the samples were added with 1.8 mL distilled water and mixed well, centrifuged at 12,000× g for 30 min, and then, the supernatants were transferred to new centrifuge tubes. The extraction procedure was repeated twice, and all supernatants were collected to measure protein concentration by Protein assay kit (Bio-Rad, Hercules, CA, USA).
For the analysis of amino acids, 20 mg lyophilized cells were added to 6 M HCl and digested at 110 °C for 21 h. Then, 4 M NaOH solution was added to 50 μL digestion solution. Next, 50 μL protein precipitant was added to 50 μL mixed solution of the standards and samples, and then, 8 μL supernatant was mixed with 42 μL labeling buffer. The separated sample was mixed with 20 μL 6-Aminoquinolyl-N-hydroxysccinimidyl carbamate (AQC) as derivatization reagent, and incubated at 55 °C for 15 min. After derivatization, the samples were cooled in a refrigerator. To analyze the compositions and contents of amino acids, high-performance liquid chromatography (Ultimate 3000, Thermo Scientific, Waltham, MA, USA)-tandem mass spectrometry (API 3200 Q-TRAP, Applied Biosystems Inc., Foster City, CA, USA) (HPLC-MS/MS) was used [59].
The nutritional quality of protein from the microalgae was evaluated on the basis of EAAI [60]. The EAAI correlated the content of each EAA of a protein and the content of the same amino acid in the FAO/WHO standard (2013). The EAAI of microalgae examined here was calculated using the following equation:
where U was the content of EAA (g/100 g), V was the content of the same amino acid in the FAO/WHO standard (g/100 g) and n was the number of analyzed EAAs.
3.5. Analysis of Carbohydrates and Monosac Charides
For the determination of total carbohydrates, 10 mg lyophilized cells were incubated with glacial acetic acid (0.1 mL) at 80 °C for 20 min. Then, 2 mL acetone was added followed by centrifugation at 5000 rpm for 10 min. Then, it was digested using 4 M trifluoroacetic acid by incubating in boiling water for 4 h. The suspension was cooled to room temperature and the supernatant was used to determine total sugar content by phenol sulphuric acid method [61]. To quantify the starch content, glucose was used to establish the standard curve.
For the analysis of monosaccharides, 5 mg lyophilized cells were added with 1 mL 2 M trifluoroacetic acid solution, then hydrolyzed in an oven at 110 °C for 6 h. After cooling to room temperature, the volume of sample solution was adjusted to 10 mL. Methanol solution was added to 2 mL sample solution and blown dry with nitrogen gas at 70 °C water bath. This step was repeated twice to remove trifluoroacetic acid. To obtain the polysaccharide hydrolysate, 1 mL 0.3 M NaOH solution was added to dissolve the residue. Next, 400 μL polysaccharide hydrolysate solution was added with 400 μL PMP methanol solution and incubated at 70 °C water bath for 2 h. When the solution was cooled to room temperature, 400 μL 0.3 M HCl (pH 6–7), 200 μL water, and 200 μL chloroform were added to the solution for extraction. High performance liquid chromatography (HPLC) was used to analyze the compositions and contents of monosaccharides [62,63].
3.6. Analysis of Lipids and Fatty Acids
For the determination of lipid content, 20 mg lyophilized cells were used with a mixture of chloroform, methanol and water (4:2:1.5, by volume). The chloroform layer was washed with 5% (w/v) sodium chloride and then evaporated under nitrogen gas; after that, it was dried at 60 °C in a vacuum oven to a constant weight and the samples were weighed. This extraction process was repeated multiple times until the weight before and after extraction remained constant to ensure complete extraction. The lipid content was determined based on the results from these multiple measurements. Chloroform was added to test tubes to adjust the final concentration at 10 μg chloroform/mg biomass [64].
For the analysis of fatty acids, 10 μL of total lipids was added to 2 mL 1% methanolic sulfuric acid solution (v/v, with 0.05% BHT) and 0.2 mL heptadecanoic acid methyl ester (HAME) solution (0.1 mg/mL n-hexane, w/v). The mixed solution was heated at 85 °C for 2.5 h with shaking every 30 min. The chloroform layer was taken to be methylated to fatty acid methyl esters (FAMEs) by incubating with 1% sulphuric acid in methanol [65]. After cooling to room temperature, the mixed solution was added to 1 mL 0.75% NaCl aqueous solution, and then extracted with 2 mL hexane. The FAMEs were analyzed by a gas chromatography-mass spectrometry (GC-MS, GC-MS-QP 2010 SE, Shimadzu, Japan), and quantified by a Stabliwas-DA capillary column (30 m × 0.25 mm × 0.25 μm, Shimadzu, Japan). A standard mixture of 37 FAMEs (C4:0-C22:6, Supleco Inc.) dissolved in n-hexane (30 mg/mL) was used to identify the methylated fatty acid species. HAME dissolved with n-hexane was used as the internal standard to quantify the contents of fatty acids. The injector temperature was 250 °C and injection volume was 1 μL.
3.7. Analysis of Pigments and Fucoxanthin
For the determination of pigment content, 20 mg lyophilized cells were dissolved in 99.9% methanol and incubated at 45 °C in the dark condition for 24 h. The mixture was centrifuged, and the absorbance of the supernatant was measured separately at 470, 652.4 and 665.2 nm. Pigment concentrations were calculated according to the previous study [66].
Fucoxanthin was extracted and the content was determined by HPLC according to our previous study [64]. The lyophilized cell samples were grounded and subsequently extracted with pure ethanol until the pellet was almost colorless. The ethanol layer was filtered through a 0.22-µm Millipore membrane before subjecting to HPLC.
3.8. Statistical Analysis
To ensure the accuracy of experimental analysis, the data of all the measurements were obtained from three repetitions and expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with subsequent post hoc multiple-comparison LSD tests were used for significance analysis with SPSS 19.0 software.
4. Conclusions
This study analyzed and compared the cell growth and product content of P. tricornutum, I. galbana and N. laevis to assess the marine microalgae in the industrial applications. It was indicated that all the three microalgae possessed high nutritional value and promised to be natural sources of EAAs, fucoxanthin and PUFAs. P. tricornutum showed significantly higher levels of proteins and amino acids than the other two microalgae, while I. galbana had the highest total content of carbohydrates and lipids. P. tricornutum and I. galbana had higher fucoxanthin content compared to N. laevis, while among the three microalgae, N. laevis achieved the highest fucoxanthin and PUFAs productivities because of the competitive high cell density and the advantageous specific growth rate. Thus, among P. tricornutum, I. galbana and N. laevis, N. laevis was a more promising candidate for co-production of fucoxanthin and PUFAs at the industrial scale.
Author Contributions
Conceptualization, methodology and writing—original draft, X.L. and S.Y.; data curation and investigation, W.Z. and M.N.; methodology, Y.H.; conceptualization and writing—review and editing, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the Financial Program of BJAST (Nos. 24CB011-01 and 23CB107), and National Natural Science Foundation of China (32302059), and Guangdong Natural Science Foundation (2024A1515011807).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Sun, H.; Wu, T.; Chen, S.H.Y.; Ren, Y.; Yang, S.; Huang, J.; Mou, H.; Chen, F. Powerful tools for productivity improvements in microalgal production. Renew. Sustain. Energy Rev. 2021, 152, 111609. [Google Scholar] [CrossRef]
- Morales, M.; Sánchez, L.; Revah, S. The impact of environmental factors on carbon dioxide fixation by microalgae. FEMS Microbiol. Lett. 2018, 365, fnx262. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xiao, M.; Liang, Q.; Yang, S.; Liu, J.; Mou, H.; Sun, H. Upcycling food waste into biorefinery production by microalgae. Chem. Eng. J. 2024, 484, 149532. [Google Scholar] [CrossRef]
- Figueira, F.d.S.; Crizel, T.d.M.; Silva, C.R.; Salas-Mellado, M.d.l.M. Pão sem gluten enriquecido com a microalga Spirulina platensis. Braz. J. Food Technol. 2011, 14, 308–316. [Google Scholar] [CrossRef]
- Rabelo, S.F.; Lemes, A.C.; Takeuchi, K.P.; Frata, M.T.; Carvalho, J.C.M.d.; Danesi, E.D.G. Development of cassava doughnuts enriched with Spirulina platensis biomass. Braz. J. Food Technol. 2013, 16, 42–51. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wang, Y.; Yang, S.; Wang, J.; Wu, T.; Lu, X.; Chu, Y.; Chen, F. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresour. Technol. 2021, 347, 126401. [Google Scholar] [CrossRef]
- Abreu, A.P.; Martins, R.; Nunes, J. Emerging applications of Chlorella sp. and Spirulina (Arthrospira) sp. Bioengineering 2023, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yao, R.; He, X.S.; Liao, Z.H.; Liu, Y.T.; Gao, B.Y.; Zhang, C.W.; Niu, J. Beneficial contribution of the microalga Odontella aurita to the growth, immune response, antioxidant capacity, and hepatic health of juvenile golden pompano (Trachinotus ovatus). Aquaculture 2022, 555, 738206. [Google Scholar] [CrossRef]
- Xia, S.; Gao, B.; Li, A.; Xiong, J.; Ao, Z.; Zhang, C. Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar. Drugs 2014, 12, 4883–4897. [Google Scholar] [CrossRef]
- Behrens, P.W.; Kyle, D.J. Microalgae as a source of fatty acids. J. Food Lipids 1996, 3, 259–272. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Kumar, B.R.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- Huerlimann, R.; de Nys, R.; Heimann, K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae1. J. Phycol. 1994, 30, 972–979. [Google Scholar] [CrossRef]
- Mason, R.P.; Sherratt, S.C.R.; Eckel, R.H. Omega-3-fatty acids: Do they prevent cardiovascular disease? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101681. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Bernasconi, A.A.; Lavie, C.J. Bringing the potential benefits of omega-3 to a higher level. Mayo Clin. Proc. 2024, 99, 520–523. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage carbon metabolism of Isochrysis zhangjiangensis under different light intensities and its application for co-production of fucoxanthin and stearidonic acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Zarekarizi, A.; Hoffmann, L.; Burritt, D. Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. J. Appl. Phycol. 2019, 31, 281–299. [Google Scholar] [CrossRef]
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from algae to human, an extraordinary bioresource: Insights and advances in up and downstream processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Li, Y.L.; Yang, S.F.; Chen, D.D.; Tu, Y.D.; Liu, J.; Sun, Z. Synthetic biology in microalgae towards fucoxanthin production for pharmacy and nutraceuticals. Biochem. Pharmacol. 2024, 220, 115958. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. BBA Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Pocha, C.K.R.; Chia, W.Y.; Chew, K.W.; Munawaroh, H.S.H.; Show, P.L. Current advances in recovery and biorefinery of fucoxanthin from Phaeodactylum tricornutum. Algal Res. 2022, 65, 102735. [Google Scholar] [CrossRef]
- Seth, K.; Kumar, A.; Rastogi, R.P.; Meena, M.; Vinayak, V. Bioprospecting of fucoxanthin from diatoms—Challenges and perspectives. Algal Res. 2021, 60, 102475. [Google Scholar] [CrossRef]
- Pang, Y.; Duan, L.; Song, B.; Cui, Y.; Liu, X.; Wang, T. A Review of fucoxanthin biomanufacturing from Phaeodactylum tricornutum. Bioprocess Biosyst. Eng. 2024, 1–22. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Celi, C.; Fino, D.; Savorani, F. Phaeodactylum tricornutum as a source of value-added products: A review on recent developments in cultivation and extraction technologies. Bioresour. Technol. Rep. 2022, 19, 101122. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, W.; Wang, J.; He, Y.; Yang, S.; Sun, H. A comprehensive review on the heterotrophic production of bioactive compounds by microalgae. World J. Microb. Biot. 2024, 40, 210. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef]
- Gao, B.; Chen, A.; Zhang, W.; Li, A.; Zhang, C. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean U. China 2017, 16, 916–924. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, H.; Zhou, Z.; Ashokkumar, M.; Liu, J. Screening of Isochrysis strains and utilization of a two-stage outdoor cultivation strategy for algal biomass and lipid production. Appl. Biochem. Biotechnol. 2018, 185, 1100–1117. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, Y.; Chen, F. Fatty acid and lipid class composition of the eicosapentaenoic acid-producing microalga, Nitzschia laevis. Food Chem. 2007, 104, 1580–1585. [Google Scholar] [CrossRef]
- Lu, X.; Sun, H.; Zhao, W.; Cheng, K.W.; Chen, F.; Liu, B. A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar. Drugs 2018, 16, 219. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Chen, F. Perfusion culture of the diatom Nitzschia laevis for ultra-high yield of eicosapentaenoic acid. Process Biochem. 2002, 38, 523–529. [Google Scholar] [CrossRef]
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2023, 29, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as sources of high-quality protein for human food and protein supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Wild, K.J.; Steingaß, H.; Rodehutscord, M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1306–1319. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Wang, J.; Cheng, K.; Liu, J.; He, Y.; Zhang, Y.; Mou, H.; Sun, H. Microalgal protein for sustainable and nutritious foods: A joint analysis of environmental impacts, health benefits and consumer’s acceptance. Trends Food Sci. Technol. 2024, 143, 104278. [Google Scholar] [CrossRef]
- Yin, M.; Wang, X. Impact of temperature fluctuations on fatty acid composition and nutritional quality of tilapia fillet (Oreochromis niloticus). J. Food Meas. Charact. 2024, 18, 1679–1689. [Google Scholar] [CrossRef]
- Moreira, J.B.; Vaz, B.D.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.D.; Costa, J.A.V.; Morais, M.G.D. Microalgae polysaccharides: An alternative source for food production and sustainable agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Bratchkova, A.; Kroumov, A.D. Microalgae as producers of biologically active compounds with antibacterial, antiviral, antifungal, antialgal, antiprotozoal, antiparasitic and anticancer activity. Acta Microbiol. Bulg. 2020, 36, 79–89. [Google Scholar]
- Synytsya, A.; Sushytskyi, L.; Saloň, I.; Babayeva, T.; Čopíková, J. Intracellular and extracellular carbohydrates in microalgae. In Handbook of Food and Feed from Microalgae, 1st ed.; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 87–102. [Google Scholar]
- Trincone, A. Polysaccharides produced by microalgae. In Polysaccharides of Microbial Origin: Biomedical Applications, 1st ed.; Oliveira, J.M., Radhouani, H., Reis, R.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 341–362. [Google Scholar]
- Sui, Z.; Gizaw, Y.; BeMiller, J.N. Extraction of polysaccharides from a species of Chlorella. Carbohydr. Polym. 2012, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae-A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sust. Energ. Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Bio/Technol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- Ores, J.D.C.; Amarante, M.C.A.; Kalil, S.J. Co-production of carbonic anhydrase and phycobiliproteins by Spirulina sp. and Synechococcus nidulans. Bioresour. Technol. 2016, 219, 219–227. [Google Scholar] [CrossRef]
- Sui, Y.; Muys, M.; Van de Waal, D.B.; D’Adamo, S.; Vermeir, P.; Fernandes, T.V.; Vlaeminck, S.E. Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour. Technol. 2019, 287, 121398. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; You, Y.; Liu, X.; Ho, S.H.; Xie, Y.; Chen, J. Highly efficient co-production of fucoxanthin and eicosapentaenoic acid by heterotrophic cultivation of a newly isolated microalga Nitzschia sp. FZU62. Algal Res. 2023, 71, 103046. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Reproductive sterility increases the capacity to exploit the green seaweed Ulva rigida for commercial applications. Algal Res. 2017, 24, 64–71. [Google Scholar] [CrossRef]
- Oser, B.L. Method for integrating essential amino acid content in the nutritional evaluation of protein. J. Am. Diet. Assoc. 1951, 27, 396–402. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Fan, Y.; Lu, X.; Zhao, W.; Chen, F. Systematic metabolic tools reveal underlying mechanism of product biosynthesis in Chromochloris zofingiensis. Bioresour. Technol. 2021, 337, 125406. [Google Scholar] [CrossRef]
- Búriová, E.; Medová, M.; Macášek, F.; Brúder, P. Separation and detection of oxidation products of fluorodeoxyglucose and glucose by high-performance liquid chromatography–electrospray ionisation mass spectrometry. J. Chromatogr. A 2004, 1034, 133–137. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.W.; Zhu, S.; Yin, H.P.; Wang, M.; Tang, J.A. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Lu, X.; Liu, B.; He, Y.J.; Guo, B.B.; Sun, H.; Chen, F. Novel insights into mixotrophic cultivation of Nitzschia laevis for co-production of fucoxanthin and eicosapentaenoic acid. Bioresour. Technol. 2019, 294, 122145. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Wang, X.F.; Zhang, Y.; Guo, Z.; Jiang, Y.; Chen, F. Enzymatic ethanolysis subjected to Schizochytrium biomass: Sequential processing for DHA enrichment and biodiesel production. Energy Convers. Manag. 2019, 184, 159–171. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels 2017, 10, 60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).