Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food
Abstract
1. Introduction
1.1. Biochemical Studies
1.2. Cell Studies
1.3. In Silico
1.4. Animal Studies
1.5. Clinical Trials
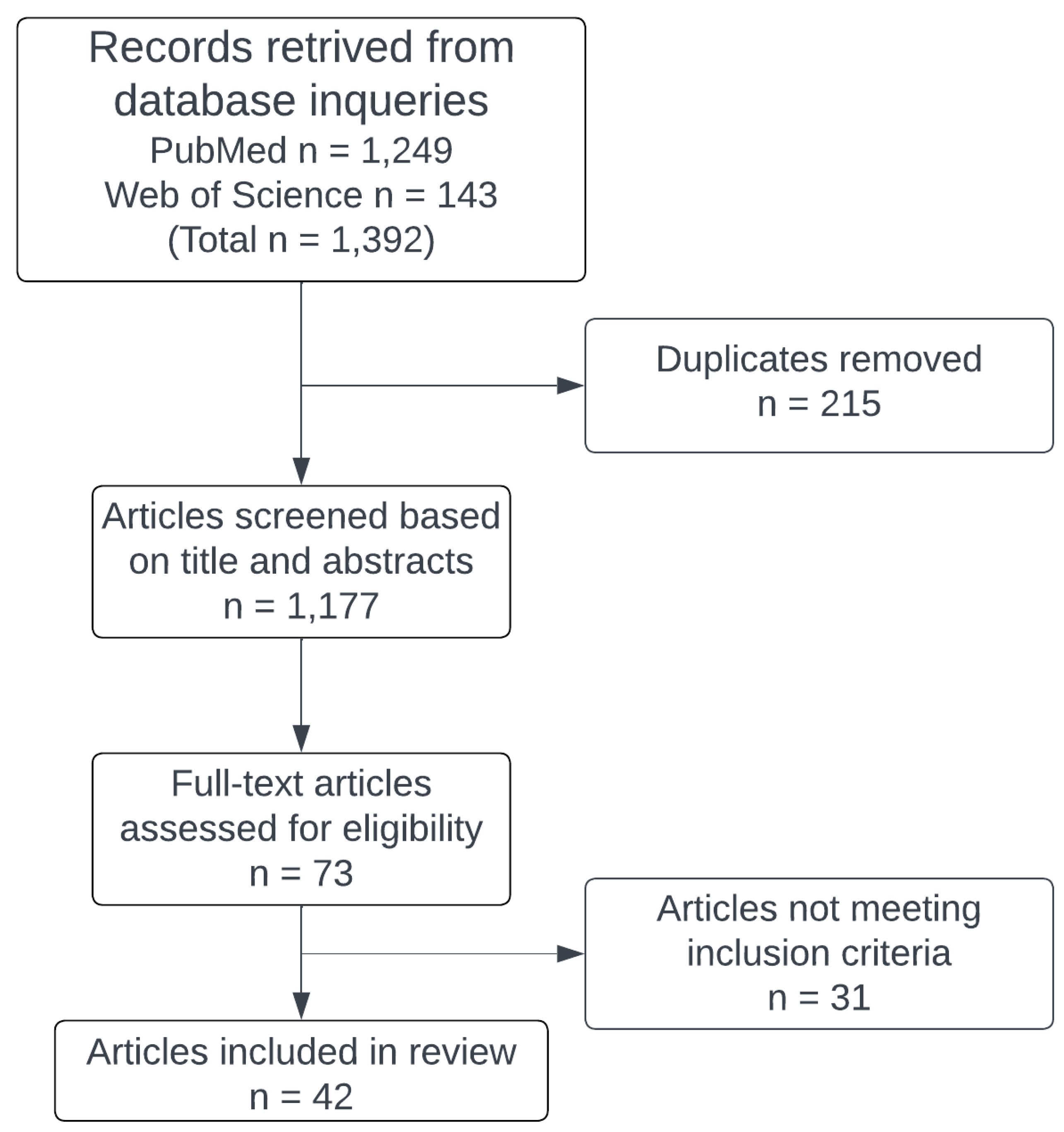
2. Materials and Methods
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 31 October 2023).
- Albasri, A.; Hattle, M.; Koshiaris, C.; Dunnigan, A.; Paxton, B.; Fox, S.E.; Smith, M.; Archer, L.; Levis, B.; Payne, R.A.; et al. Association between antihypertensive treatment and adverse events: Systematic review and meta-analysis. BMJ 2021, 372, n189. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, E.A.; Bhagavathula, A.S.; Abebe, T.B.; Tefera, Y.G.; Abegaz, T.M. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin. Hypertens. 2019, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yano, Y.; Cho, S.M.J.; Heo, J.E.; Kim, D.-W.; Park, S.; Lloyd-Jones, D.M.; Kim, H.C. Adherence to Antihypertensive Medication and Incident Cardiovascular Events in Young Adults with Hypertension. Hypertension 2021, 77, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Isath, A.; Hahn, J.; Wang, Z.; Narasimhan, B.; Kaplin, S.L.; Jneid, H.; Virani, S.S.; Tang, W.H.W. Fish Consumption and Cardiovascular Health: A Systematic Review. Am. J. Med. 2021, 134, 713–720. [Google Scholar] [CrossRef]
- Golden, C.D.; Allison, E.H.; Cheung, W.W.L.; Dey, M.M.; Halpern, B.S.; McCauley, D.J.; Smith, M.; Vaitla, B.; Zeller, D.; Myers, S.S. Nutrition: Fall in fish catch threatens human health. Nature 2016, 534, 317–320. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef]
- Rosenberg, G. A New Critical Estimate of Named Species-Level Diversity of the Recent Mollusca. Am. Malacol. Bull. 2014, 32, 308–322. [Google Scholar] [CrossRef]
- Haszprunar, G.; Wanninger, A. Molluscs. Curr. Biol. 2012, 22, R510–R514. [Google Scholar] [CrossRef] [PubMed]
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, C.T.; Miyake, T.; Rast, J.P. Echinoderms. Curr. Biol. 2005, 15, R944–R946. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, M.K.; Matas Serrato, L.A.; Blanchoud, S. Artificial seawater based long-term culture of colonial ascidians. Dev. Biol. 2021, 480, 91–104. [Google Scholar] [CrossRef]
- Hardoim, C.C.; Costa, R.; Araújo, F.V.; Hajdu, E.; Peixoto, R.; Lins, U.; Rosado, A.S.; van Elsas, J.D. Diversity of bacteria in the marine sponge Aplysina fulva in Brazilian coastal waters. Appl. Environ. Microbiol. 2009, 75, 3331–3343. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-associated microorganisms: Evolution, ecology, and biotechnological potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.W.F.S.; Cunha, N.B.d.; Carneiro, J.A.; Costa, R.A.d.; Alencar, S.A.d.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Ye, H.; Tao, X.; Zhang, W.; Chen, Y.; Yu, Q.; Xie, J. Food-derived bioactive peptides: Production, biological activities, opportunities and challenges. J. Future Foods 2022, 2, 294–306. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2021, 8, 815640. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: A systematic review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Ramakrishnan, S.R.; Jeong, C.R.; Park, J.W.; Cho, S.S.; Kim, S.J. A review on the processing of functional proteins or peptides derived from fish by-products and their industrial applications. Heliyon 2023, 9, e14188. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Patten, G.S.; Abeywardena, M.Y.; Bennett, L.E. Inhibition of Angiotensin Converting Enzyme, Angiotensin II Receptor Blocking, and Blood Pressure Lowering Bioactivity across Plant Families. Crit. Rev. Food Sci. Nutr. 2016, 56, 181–214. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Yanuar, A.; Mulia, K.; Mun’im, A. Review of Angiotensin-converting Enzyme Inhibitory Assay: Rapid Method in Drug Discovery of Herbal Plants. Pharmacogn. Rev. 2017, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ben Henda, Y.; Labidi, A.; Arnaudin, I.; Bridiau, N.; Delatouche, R.; Maugard, T.; Piot, J.M.; Sannier, F.; Thiéry, V.; Bordenave-Juchereau, S. Measuring angiotensin-I converting enzyme inhibitory activity by micro plate assays: Comparison using marine cryptides and tentative threshold determinations with captopril and losartan. J. Agric. Food Chem. 2013, 61, 10685–10690. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; de Castro, R.; Coscueta, E.R.; Pintado, M. Hydrolysate from Mussel Mytilus galloprovincialis Meat: Enzymatic Hydrolysis, Optimization and Bioactive Properties. Molecules 2021, 26, 5228. [Google Scholar] [CrossRef]
- Neves, A.; Harnedy, P.; FitzGerald, R.; Neves, A.C.; Harnedy, P.A.; FitzGerald, R.J. Angiotensin Converting Enzyme and Dipeptidyl Peptidase-IV Inhibitory, and Antioxidant Activities of a Blue Mussel (Mytilus edulis) Meat Protein Extract and Its Hydrolysates. J. Aquat. Food Prod. Technol. 2016, 25, 1221–1233. [Google Scholar] [CrossRef]
- CunhaNeves, A.; Harnedy-Rothwell, P.; FitzGerald, R.; CunhaNeves, A.; Harnedy-Rothwell, P.A.; FitzGerald, R.J. In vitro angiotensin-converting enzyme and dipeptidyl peptidase-IV inhibitory, and antioxidant activity of blue mussel (Mytilus edulis) byssus collagen hydrolysates. Eur. Food Res. Technol. 2022, 248, 1721–1732. [Google Scholar] [CrossRef]
- Sasaki, C.; Tamura, S.; Tohse, R.; Fujita, S.; Kikuchi, M.; Asada, C.; Nakamura, Y. Isolation and identification of an angiotensin I-converting enzyme inhibitory peptide from pearl oyster (Pinctada fucata) shell protein hydrolysate. Process Biochem. 2019, 77, 137–142. [Google Scholar] [CrossRef]
- Li, J.; Su, J.; Chen, M.; Chen, J.; Ding, W.; Li, Y.; Yin, H. Two novel potent ACEI peptides isolated from Pinctada fucata meat hydrolysates using in silico analysis: Identification, screening and inhibitory mechanisms. RSC Adv. 2021, 11, 12172–12182. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef]
- Paul, A.; Eghianruwa, Q.; Oparinde, O.; Adesina, A.; Osoniyi, O. Enzymatic protein hydrolysates, and ultrafiltered peptide fractions from two molluscs: Tympanotonus fuscatus var. radula (L.) and Pachymelania aurita (M.), with angiotensin-I-converting enzyme inhibitory and DPPH radical scavenging activities. Int. J. Appl. Basic Med. Res. 2021, 11, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Lu, D.; Han, J.; Lu, X.; Tian, Z.; Wang, Z. Angiotensin Converting Enzyme Inhibitory, Antioxidant Activities, and Antihyperlipidaemic Activities of Protein Hydrolysates From Scallop Mantle (Chlamys farreri). Int. J. Food Prop. 2015, 18, 33–42. [Google Scholar] [CrossRef]
- Chun, B.-S.; Lee, S.-C.; Ho, T.-C.; Micomyiza, J.-B.; Park, J.-S.; Nkurunziza, D.; Lee, H.-J. Subcritical Water Hydrolysis of Comb Pen Shell (Atrina pectinata) Edible Parts to Produce High-Value Amino Acid Products. Mar. Drugs 2022, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Roy, V.C.; Ho, T.C.; Park, J.-S.; Jeong, Y.-R.; Lee, S.-C.; Kim, S.-Y.; Chun, B.-S. Amino Acid Profiles and Biopotentiality of Hydrolysates Obtained from Comb Penshell (Atrina pectinata) Viscera Using Subcritical Water Hydrolysis. Mar. Drugs 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jang, J.; Ye, B.; Kim, M.; Choi, I.; Park, W.; Heo, S.; Jung, W. Purification and molecular docking study of angiotensin I-converting enzyme (ACE) inhibitory peptides from hydrolysates of marine sponge Stylotella aurantium. Process Biochem. 2017, 54, 180–187. [Google Scholar] [CrossRef]
- Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870–28885. [Google Scholar] [CrossRef] [PubMed]
- Dewi, A.; Patantis, G.; Fawzya, Y.; Irianto, H.; Sa’diah, S. Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Protein Hydrolysates from Indonesian Sea Cucumbers. Int. J. Pept. Res. Ther. 2020, 26, 2485–2493. [Google Scholar] [CrossRef]
- Quaisie, J.; Ma, H.; Guo, Y.; Tuly, J.A.; Igbokwe, C.J.; Ekumah, J.-N.; Akpabli-Tsigbe, N.D.K.; Yanhua, D.; Liu, D. Highly stable, antihypertensive, and antioxidative peptide production from Apostichopus japonicus by integrated enzymatic membrane reactor and nanofilter-purification mechanism. Food Funct. 2022, 13, 2306–2322. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Zhao, Y.; Zhu, X.; Yu, R.; Dong, S.; Wu, H.; Li, J.; Liu, Z.; Zhao, Y.; et al. Novel Natural Angiotensin Converting Enzyme (ACE)-Inhibitory Peptides Derived from Sea Cucumber-Modified Hydrolysates by Adding Exogenous Proline and a Study of Their Structure-Activity Relationship. Mar. Drugs 2018, 16, 271. [Google Scholar] [CrossRef]
- So, P.B.; Rubio, P.; Lirio, S.; Macabeo, A.P.; Huang, H.Y.; Corpuz, M.J.; Villaflores, O.B. In vitro angiotensin I converting enzyme inhibition by a peptide isolated from Chiropsalmus quadrigatus Haeckel (box jellyfish) venom hydrolysate. Toxicon 2016, 119, 77–83. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Shi, Y.; Qiao, R.; Tang, W.; Sun, Z. Production of the angiotensin I converting enzyme inhibitory peptides and isolation of four novel peptides from jellyfish (Rhopilema esculentum) protein hydrolysate. J. Sci. Food Agric. 2016, 96, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, L.; Li, B. Production of the Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptide from Hydrolysates of Jellyfish (Rhopilema esculentum) Collagen. Food Bioprocess. Technol. 2012, 5, 1622–1629. [Google Scholar] [CrossRef]
- Ko, S.; Kang, M.; Lee, J.; Byun, H.; Kim, S.; Lee, S.; Jeon, B.; Park, P.; Jung, W.; Jeon, Y. Effect of angiotensin I-converting enzyme (ACE) inhibitory peptide purified from enzymatic hydrolysates of Styela plicata. Eur. Food Res. Technol. 2011, 233, 915–922. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, J.-K.; Byun, H.-G.; Lee, S.-C.; Jeon, Y.-J. Purification and characterization of angiotensin I-converting enzyme inhibitory peptide from enzymatic hydrolysates of Styela clava flesh tissue. Process Biochem. 2012, 47, 34–40. [Google Scholar] [CrossRef]
- Rivas-Vela, C.I.; Amaya-Llano, S.L.; Castaño-Tostado, E.; Castillo-Herrera, G.A. Protein Hydrolysis by Subcritical Water: A New Perspective on Obtaining Bioactive Peptides. Molecules 2021, 26, 6655. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Ulug, S.K.; Jahandideh, F.; Wu, J. Novel technologies for the production of bioactive peptides. Trends Food Sci. Technol. 2021, 108, 27–39. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhang, Y.; Liu, G. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar. Drugs 2012, 10, 417–426. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Suo, S.K.; Zhao, Y.Q.; Wang, Y.M.; Pan, X.Y.; Chi, C.F.; Wang, B. Seventeen novel angiotensin converting enzyme (ACE) inhibitory peptides from the protein hydrolysate of Mytilus edulis: Isolation, identification, molecular docking study, and protective function on HUVECs. Food Funct. 2022, 13, 7831–7846. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, C.; Liu, X.; Qiao, B.; Song, S.; Fu, Y. ACE inhibitory activities of two peptides derived from Volutharpa ampullacea perryi hydrolysate and their protective effects on H2O2 induced HUVECs injury. Int. Food Res. 2022, 157, 111402. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Ezquerra-Brauer, J.M.; Aubourg, S.P. Characterization of the Jumbo Squid (Dosidicus gigas) Skin By-Product by Shotgun Proteomics and Protein-Based Bioinformatics. Mar. Drugs 2019, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, C.; Song, Y.; Zhu, J.; Zhang, X. Discovery of Novel Angiotensin-Converting Enzyme Inhibitory Peptides from Todarodes pacificus and Their Inhibitory Mechanism: In Silico and In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 4159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Mendez, R.L.; Kwon, J.Y. In Silico Prospecting for Novel Bioactive Peptides from Seafoods: A Case Study on Pacific Oyster (Crassostrea gigas). Molecules 2023, 28, 651. [Google Scholar] [CrossRef] [PubMed]
- Kamble, A.; Srinivasan, S.; Singh, H. In-Silico Bioprospecting: Finding Better Enzymes. Mol. Biotechnol. 2019, 61, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.E.; Turner, R.J. A pharmacological study of the toxin in a Cnidarian, Chironex fleckeri Southcott. Br. J. Pharmacol. 1969, 35, 510–520. [Google Scholar] [CrossRef]
- Freeman, S.E.; Turner, R.J. Cardiovascular effects of toxins isolated from the cnidarian Chironex fleckeri Southcott. Br. J. Pharmacol. 1971, 41, 154–166. [Google Scholar] [CrossRef]
- Freeman, S.E.; Turner, R.J. Cardiovascular effects of cnidarian toxins: A comparison of toxins extracted from Chiropsalmus quadrigatus and Chironex fleckeri. Toxicon 1972, 10, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Park, P.-J.; Byun, H.-G.; Jung, W.-K.; Kim, S.-K. Angiotensin I converting enzyme (ACE) inhibitory peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Bioresour. Technol. 2005, 96, 1624–1629. [Google Scholar] [CrossRef]
- Feng, J.; Dai, Z.; Zhang, Y.; Meng, L.; Ye, J.; Ma, X.; Feng, J.; Dai, Z.; Zhang, Y.; Meng, L.; et al. Alteration of Gene Expression Profile in Kidney of Spontaneously Hypertensive Rats Treated with Protein Hydrolysate of Blue Mussel (Mytilus edulis) by DNA Microarray Analysis. PLoS ONE 2015, 10, e0142016. [Google Scholar] [CrossRef] [PubMed]
- Yamanushi, M.; Shimura, M.; Nagai, H.; Hamada-Sato, N.; Yamanushi, M.; Shimura, M.; Nagai, H.; Hamada-Sato, N. Antihypertensive effects of abalone viscera fermented with Lactiplantibacillus pentosus SN001 via angiotensin-converting enzyme inhibition. Food Chem. 2022, 13, 100239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, X.; Wei, Y.; Liu, Q.; Jiang, Y.; Zhao, L.; Ulaah, S. Isolation, purification and the anti-hypertensive effect of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from Ruditapes philippinarum fermented with Bacillus natto. Food Funct. 2018, 9, 5230–5237. [Google Scholar] [CrossRef]
- Song, Y.H.; Yu, J.; Song, J.L.; Wang, S.L.; Cao, T.F.; Liu, Z.M.; Gao, X.; Wei, Y.X. The antihypertensive effect and mechanisms of bioactive peptides from Ruditapes philippinarum fermented with Bacillus natto in spontaneously hypertensive rats. J. Funct. Foods 2021, 79, 104411. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Zhao, L.; Yu, J.; Wang, S.; Cao, T.; Gao, X.; Wei, Y. Identification and Antihypertension Study of Novel Angiotensin I-Converting Enzyme Inhibitory Peptides from the Skirt of Chlamys farreri Fermented with Bacillus natto. J. Agric. Food Chem. 2021, 69, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nishizono, S.; Kugino, K.; Tamari, M.; Kurumiya, M.; Abe, N.; Ikeda, I. Effects of Dietary Oyster Extract on Lipid Metabolism, Blood Pressure, and Blood Glucose in SD Rats, Hypertensive Rats, and Diabetic Rats. Biosci. Biotechnol. Biochem. 2006, 70, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, K.; Shiozaki, M.; Masuda, J.; Yamauchi, A.; Ohwada, S.; Nakano, T.; Yamaguchi, T.; Saito, T.; Muramoto, K.; Sato, M. Identification of oyster-derived hypotensive peptide acting as angiotensin-I-converting enzyme inhibitor. Fish. Sci. 2010, 76, 865–872. [Google Scholar] [CrossRef]
- Xie, C.; Kim, J.; Ha, J.; Choung, S.; Choi, Y.; Xie, C.-L.; Kim, J.-S.; Ha, J.-M.; Choung, S.-Y.; Choi, Y.-J. Angiotensin I-Converting Enzyme Inhibitor Derived from Cross-Linked Oyster Protein. Biomed. Res. Int. 2014, 2014, 379234. [Google Scholar] [CrossRef]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada fucata martensii) Meat Protein Hydrolysate. Mar. Drugs 2019, 17, 463. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, M. A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate. Peptides 2009, 30, 1028–1033. [Google Scholar] [CrossRef]
- Sadegh Vishkaei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Hypertensive Effect of Protein Hydrolysate from Actinopyga lecanora (Sea Cucumber) in Rats. Mar. Drugs 2016, 14, 176. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Zhang, C.; Liu, C. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from Rhopilema esculentum. Food Chem. 2012, 134, 2134–2140. [Google Scholar] [CrossRef]
- Ko, S.C.; Kim, D.G.; Han, C.H.; Lee, Y.J.; Lee, J.K.; Byun, H.G.; Lee, S.C.; Park, S.J.; Lee, D.H.; Jeon, Y.J. Nitric oxide-mediated vasorelaxation effects of anti-angiotensin I-converting enzyme (ACE) peptide from Styela clava flesh tissue and its anti-hypertensive effect in spontaneously hypertensive rats. Food Chem. 2012, 134, 1141–1145. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.C.; Kim, H.S.; Yang, H.W.; Ahn, G.; Lee, S.C.; Lee, T.G.; Lee, J.S.; Jeon, Y.J. Structural Evidence for Antihypertensive Effect of an Antioxidant Peptide Purified from the Edible Marine Animal Styela clava. J. Med. Food 2020, 23, 132–138. [Google Scholar] [CrossRef]
- Ko, S.; Jung, W.; Lee, S.; Lee, D.; Jeon, Y.; Ko, S.-C.; Jung, W.-K.; Lee, S.-H.; Lee, D.H.; Jeon, Y.-J. Antihypertensive effect of an enzymatic hydrolysate from Styela clava flesh tissue in type 2 diabetic patients with hypertension. Nutr. Res. Pract. 2017, 11, 396–401. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
| Common Name | Scientific Name | Tissue | Method | Control | IC50-Values or % ACE Inhibition | Peptide Sequence | Reference |
|---|---|---|---|---|---|---|---|
| Mollusca | |||||||
| Mediterranean mussel | Mytilus galloprovincialis | Meat | EH with subtilisin and corolase | - | 3.7 ± 0.22 and 1.0 ± 0.56 mg/mL | - | [27] |
| Blue mussel | Mytilus edulis | Meat from co-products * | EH with Alcalase®, Alcalase® + flavourzyme, corolase PP or Promod 144 MG | Unhydrolyzed protein | 1.13–3.34 mg/mL | - | [28] |
| Blue mussel | Mytilus edulis | Byssus | EH with Alcalase®, Alcalase® + flavourzyme, corolase PP, Promod 144 MG or An-PEP | Unhydrolyzed protein | 0.77–1.37 mg/mL | - | [29] |
| Akoya Pearl oyster | Pinctada fucata | Shell | EH with orientase 22 BF | - | 5.82 ± 0.56 μg/mL | GVGSPY | [30] |
| Akoya pearl oyster | Pinctada fucata | Meat | EH with Alcalase® | - | 18.34 and 116.26 μM | FRVW and LPYY | [31] |
| Chinese Venus | Cyclina sinensis | Meat | EH with trypsin | - | 0.789 mM | WPMGF | [32] |
| West African mud creeper and Nigerian periwinkles | Tympanotonus fuscatus var. radula and Pachymelania aurita | Meat and hemolymph | Simulated GI digestion model with pepsin, trypsin, and chymotrypsin | Captopril | 54.93 ± 2.83, 291.7 ± 8.6, 65.2 ± 6.4, and 301.9 ± 59.1 μg/mL | - | [33] |
| Scallop | Chlamys Farreri | Mantle | EH with neutral protease and trypsin | - | 10.28 mg/mL | - | [34] |
| Comb Pen Shell | Atrina pectinate | Edible parts | SCWH | Captopril 1% | 85.85 ± 0.67, 84.55 ± 0.18, and 82.15 ± 0.85% | - | [35] |
| Comb Pen Shell | Atrina pectinate | Viscera | SCWH | Captopril 0.1% | 96.77 ± 0.14–92.16 ± 0.04% | - | [36] |
| Porifera | |||||||
| Sponge | Stylotella aurantium | Whole body | EH with pepsin | - | 273.2 and 306.4 μM | YR and IR | [37] |
| Echinodermata | |||||||
| Stonefish | Actinopyga lecanora | Gutted whole body | EH with Alcalase®, bromelain, trypsin, papain, pepsin or flavourzyme | Captopril | 1.50, 1.73, 2.04, 2.18, 2.31, and 2.54 mg/mL | - | [38] |
| Indonesian sea cucumbers | Holothuria atra, Holothuria leucospilota, and Bohadschia marmorata | Gutted whole body | EH with Alcalase® or bromelain | - | 0.32–0.58 mg/mL and 0.64–0.79 mg/mL | - | [39] |
| Sea cucumber | Argyrosomus japonicus | Whole body | EH with Alcalase® | - | 58.87–80.38% | - | [40] |
| Sea cucumber | Acaudina molpadioidea | Body wall | EH with trypsin, and papain | Captopril | 8.18 and 13.16 μM | PNVA and PNLG | [41] |
| Cnidaria | |||||||
| Box Jellyfish | Chiropsalmus quadrigatus | Venom | EH with pepsin and papain | - | 2.03 μM | ACPGPNPGRP | [42] |
| Flame Jellyfish | Rhopilema esculentum | Whole body | Compound proteinase AQ hydrolysis | EH with other enzymes | 8.4, 23.42, 21.15, and 19.11 μmol/L | VGPY, FTYVPG, FTYVPGA, and FQAVWAG | [43] |
| Flame Jellyfish | Rhopilema esculentum | Collagen | EH with Alcalase® | - | 43 μg/mL | - | [44] |
| Chordata | |||||||
| Solitary tunicate | Styela plicata | Tissue | EH with Protamex | EH with other enzymes | 24.7 μM | MLLCS | [45] |
| Club tunicate | Styela clava | Flesh tissue | EH with Protamex | EH with other enzymes | 37.1 μM | AHIII | [46] |
| Common Name | Scientific Name | Tissue | Method | Control or Databased Used | IC50-Values or % ACE Inhibition | Peptide Sequence | Reference |
|---|---|---|---|---|---|---|---|
| Mollusca | |||||||
| Blue mussel | Mytilus edulis | Proteins | Cell model HUVECs | Captopril and norepinephrine | 0.77 ± 0.020, 0.19 ± 0.010, and 0.32 ± 0.017 mg/mL | IK, YEGDP, and SWISS | [53] |
| Deep sea snail | Volutharpa ampullacea perryi | Edible parts | Cell model HUVECs | Bradykinin enhancer B, octapeptide angiotensin II, and lisinopril | 76.34 ± 0.79 and approximately 40% | IVTNWDDMEK and VGPAGPRG | [54] |
| Jumbo squid | Dosidicus giga | Skin | In silico with pepsin and trypsin | PeptideRanker | - | - | [55] |
| Japanese flying squid | Todarodes pacificus | Myosin heavy chain | In silico and in vitro with papain, ficin, and in combination | BIOPEP-UWM and AHTpin | pIC50 = 4.58 and 4.41 | IIY and NPPK | [56] |
| Pacific oyster | Crassostrea gigas | Large proteins | In silico with pepsin, trypsin, and chemo-trypsin | AHTpDB | - | - | [57] |
| Common Name | Scientific Name | Tissue | Method | Control | IC50-Values or ACE Inhibition % | Peptide Sequence | Animal Model | Dosage | Duration | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Mollusca | ||||||||||
| Blue mussel | Mytilus edulis | Muscle | Fermentation 6 months | Captopril and saline solution | 19.34 μg/mL | EVMAGNLYPG | SHR | 10 mg/kg bw, oral injection * | 9 h | [62] |
| Blue mussel | Mytilus edulis | Muscle | EH with Alcalase® | Water | VW, LGW, and MVWT | SHR | 10 or 20 mg/kg/day hydrolysate, daily oral injection | 28 days | [63] | |
| Abalone | Haliotidae rubra | Viscera | Fermentation with Lactiplantibacillus pentosus SN001 | Standard diet | 80% | - | SHRs | 5% hydrolysate in the diet, ad libitum | 9 weeks | [64] |
| Japanese littleneck clam | Ruditapes phillippinarum | Meat | Fermentation with Bacillus natto | Undisclosed model group | 8.16 μM | VISDEDGVTH | SD rats | 8 mg/ kg bw and 32 mg/kg bw peptide, oral gavage * | 6 days | [65] |
| Japanese littleneck clam | Ruditapes phillippinarum | Meat | Fermentation with Bacillus natto | 10 mg/ kg bw saline and captopril | - | - | SHRs | 100 mg/ kg bw peptide, daily oral gavage | 8 weeks | [66] |
| Scallop | Chlamys farreri | Skirt | Fermentation with Bacillus natto | 1 mL/kg solution and 10 mg/kg captopril | 0.12 ± 0.01 mg/mL | AGFAGDDAPR, CDVDIR, IIAPPER, IWHHTFYNGLR and GIQTAVR | SHRs | 25, 50, or 100 mg/kg fraction in the diet, ad libitum | 24 h and 8 weeks | [67] |
| Oyster | - | Meat | EH with aloase and pancitase | Control diet without hydrolysate | - | - | SHRs | 5% oyster extracts in the diet, ad libitum | 4 weeks | [68] |
| Oyster | Crassostrea gigas | Whole body and striate muscle | EH with trypsin | Control diet without hydrolysate | 143 and 28 nmol/mL | DLTDY and DY | SHRs | 50, 100, and 1000 mg/kg day, single oral injection and in the diet, ad libitum | 6 h, 24 h, and 9 weeks | [69] |
| Oyster | - | Cross-linked protein | EH | Untreated SHRs, Sardine hydrolysate and captopril | 16.7, 29.0, 51.5, 68.2, and 93.9 μM | TAY, VK, KY, FYN, and YA | SHRs | Hydrolysate, single oral gavage | 24 h | [70] |
| Pearl oyster | Pinctada fucata martensii | Meat | EH with alkaline protease | 10 mg/kg captopril and saline solution | 458 ± 3.24 and 109 ± 1.45 μM | HLHT and GWA | SD rats | 10 mg/kg bw hydrolysate, single intravenous administration | 45 min | [71] |
| Echinodermata | ||||||||||
| Sea cucumber | Acaudina molpadioidea | Body wall protein | EH with bromelain and Alcalase® | 3 μM/kg captopril and saline solution | 15.9 and 4.5 μM | MEGAQEAQGD | SHRs | 3 μM/kg, one-shot oral injection | 6 h | [72] |
| Sea cucumber | Actinopyga lecanora | Muscle | EH with bromelain | 50 mg/kg captopril, water, and saline solution | - | - | SD rats | 200, 400, and 800 mg/kg bw, single oral gavage | 3 h | [73] |
| Cnidaria | ||||||||||
| Flame jellyfish | Rhopilema esculentum | Flesh | Two-step EH with pepsin and papain | 50 mg/kg captopril and distilled water | 1.28 mg/mL | - | SHRs | 200, 400, and 800 mg/kg hydrolysate, single oral gavage, and daily oral gavage | 8 h and 5-weeks | [74] |
| Flame jellyfish | Rhopilema esculentum | Collagen | EH with Alcalase® | Captopril and control diet | 43 μg/mL | - | Wistar strain rats | 25 and 100 mg/kg bw, daily oral gavage | 4 weeks | [50] |
| Chordata | ||||||||||
| Club tunicate | Styela clava | Flesh | EH with Protamex | 30 mg/kg bw amlodipine and saline solution | - | AHIII | SHRs and SD rats | 100 mg/kg bw peptide, single oral gavage | 24 h | [75] |
| Club tunicate | Styela clava | Flesh | Synthesized | Captopril and saline solution | 16.4 ± 0.45 μM | LWHTH | SHRs | 40 mg/kg bw peptide, single oral injection | 9 h | [76] |
| Club tunicate | Styela clava | Flesh | Randomized placebo-controlled double-blind study | Not disclosed | - | - | Human | 500 mg/day, capsule | 4 weeks | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walquist, M.J.; Eilertsen, K.-E.; Elvevoll, E.O.; Jensen, I.-J. Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food. Mar. Drugs 2024, 22, 140. https://doi.org/10.3390/md22040140
Walquist MJ, Eilertsen K-E, Elvevoll EO, Jensen I-J. Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food. Marine Drugs. 2024; 22(4):140. https://doi.org/10.3390/md22040140
Chicago/Turabian StyleWalquist, Mari Johannessen, Karl-Erik Eilertsen, Edel Oddny Elvevoll, and Ida-Johanne Jensen. 2024. "Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food" Marine Drugs 22, no. 4: 140. https://doi.org/10.3390/md22040140
APA StyleWalquist, M. J., Eilertsen, K.-E., Elvevoll, E. O., & Jensen, I.-J. (2024). Marine-Derived Peptides with Anti-Hypertensive Properties: Prospects for Pharmaceuticals, Supplements, and Functional Food. Marine Drugs, 22(4), 140. https://doi.org/10.3390/md22040140







