Abstract
We investigated the effects of polysaccharides from the brown seaweed Sargassum graminifolium (Turn.) (SGP) on calcium oxalate crystallization, and determined its antioxidant activities. To examine the effects of SGP on calcium oxalate crystallization, we monitored nucleation and aggregation of calcium oxalate monohydrate crystals, using trisodium citrate as a positive control. We assessed antioxidant activities of SGP by determining its reducing power, its ability to scavenge superoxide radicals, and its activity in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. The nucleation inhibition ratio of trisodium citrate and SGP was 58.5 and 69.2%, respectively, and crystal aggregation was inhibited by 71.4 and 76.8%, respectively. Increasing concentrations of SGP resulted in increased scavenging of superoxide anions and DPPH radicals (IC50 = 1.9 and 0.6 mg/mL, respectively). These results suggest that SGP could be a candidate for treating urinary stones because of its ability to inhibit calcium oxalate crystallization and its antioxidant properties.
Abbreviations
| SGP | polysaccharide from Sargassum graminifolium |
| IR | infrared spectrum |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl free radical |
| Ox | oxalate |
| CaOx | calcium oxalate |
| OD | optical density |
| SN | maximum increase of optical density with time |
| SA | rate of aggregation derived from the maximum decrease in optical density |
| tmax | maximum time |
| COM | Calcium oxalate monohydrate |
| COD | Calcium oxalate dehydrate |
| COT | Calcium oxalate trihydrate |
1. Introduction
In recent years, there has been much interest in isolating novel bioactive compounds with beneficial effects on human health from marine resources. Marine algae are valuable sources of structurally diverse bioactive compounds. Sulfated polysaccharides are widespread in marine algae, especially brown seaweeds. Sulfated polysaccharides show various biological activities, including anticoagulant, antioxidant, antiviral, anticancer and immunomodulating activities [1,2,3,4,5]. Zhang et al. [6] reported that sulfated polysaccharides play important roles as free-radical scavengers and antioxidants, which can prevent oxidative damage in living organisms. The low molecular weight polysaccharides heparin and fucoidan provided protection against oxalate (Ox)-induced oxidative renal injury [3,7]. Ox and calcium oxalate monohydrate (COM) induce the generation of free radicals, which are major mediators of the pathologic consequences of the formation of kidney stones [8]. Hence, sulfated polysaccharides may be a potential candidate for treating urinary stones because of their protective role in Ox-mediated peroxidative injury.
Urinary stones affect a large proportion of the population. Approximately 85% of urinary stones are calcium stones, which consist of Ox and phosphate, either alone or in combination [9,10]. Crystallization is a physical and chemical process in which there is a change of state from solution to solid. This involves several physicochemical events, i.e., nucleation, growth and aggregation, but the mechanisms controlling these events are not fully understood [11,12].
It is widely known that urinary stones frequently reoccur, and despite progress in medical therapies, there is no satisfactory drug for treatment of this condition [13,14,15]. Recent research has shown that exposure of renal cells to high concentrations of Ox and/or calcium oxalate (CaOx) crystals leads to the production of reactive oxygen species (ROS) in tissue culture and animal model studies [16,17,18]. ROS play critical roles as signaling molecules; however, an overproduction of ROS and/or a reduction in cellular antioxidant capacities due to downregulation of antioxidant enzymes results in oxidative stress [19]. Some studies in vitro and in vivo showed that treatments with antioxidants and free-radical scavengers can reduce Ox/CaOx crystal-induced injuries [18,20]. Some well-known antioxidants, such as vitamin E, have shown promising effects in populations of recurrent stone-formers [21]. These results suggest that there is great potential for the therapeutic application of antioxidants and free-radical scavengers to reduce the occurrence and reoccurrence of urinary stones and to provide superior renal protection [22]. Since the conventional treatment methods vary in their effectiveness, it is worth searching for alternative treatments, e.g., different diets or medicinal plants [8,23] for the treatment of urinary stones.
Brown seaweeds have been used in traditional Chinese medicine for more than 1000 years [24]. Sargassum graminifolium, a brown seaweed extensively distributed along the coasts of the South China Sea and the East China Sea, is commonly consumed as seafood, and as medical resource for its antiallergic effects [25]. To fully utilize this rich resource, it is meaningful to evaluate the polysaccharide bioactivity of S. graminifolium. The aim of the present study was to investigate the antioxidant properties of a polysaccharide extract from S. graminifolium, and to determine its ability to inhibit crystallization. These properties are useful attributes for compounds that could be used to treat urinary stones.
2. Results and Discussion
2.1. Properties of SGP
The results show the total sugar content is 75.68%, protein content is 0.12% and sulfate is 10.13%. Figure 1 shows that SGP in 3471, 2900, 1648 and 1253 cm−1 are absorption peaks, they are the stretching vibration of sugar ring O–H, C–H, C=O and S=O, so this result indicates the SGP contains high sulfate content and it is consistent with chemical analysis result.
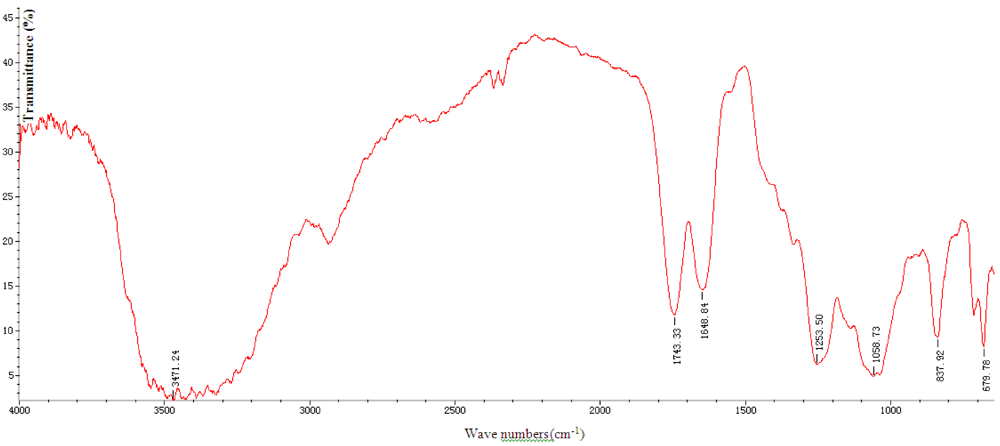
Figure 1.
IR spectra of Sargassum graminifolium (Turn.) (SGP).
2.2. Effect on Calcium Oxalate Crystallization
The inhibitory effect of SGP and trisodium citrate on CaOx crystallization is shown in Table 1. At concentrations of 4 mmol/L calcium and 0.5 mmol/L Ox, addition of 0.25 mmol/L trisodium citrate or 0.01 g/100 mL SGP resulted in a nucleation percentage inhibition ratio of 58.5 and 69.2%, respectively, and crystal aggregation was inhibited by 71.4 and 76.8%, respectively. Compared with control conditions, both SGP and trisodium citrate significantly inhibited CaOx crystallization. As shown in Table 1, addition of both SGP and trisodium citrate resulted in increases in tmax and decreased slopes of CaOx crystal growth (SN and SA) (P < 0.05).

Table 1.
Effects of SGP on calcium oxalate crystallization.
| 4 mmol/L calcium/ 0.5 mmol/L oxalate | 4 mmol/L calcium/ 0.5 mmol/L oxalate + 0.25 mmol/L trisodium citrate | 4 mmol/L calcium/ 0.5 mmol/L oxalate + 0.01 g/100 mL SGP | P | |
|---|---|---|---|---|
| tmax (min) | 8.67 ± 0.94 | 13.44 ± 0.57 | 14.02 ± 0.82 | P < 0.05 a |
| P < 0.05 b | ||||
| SN (×10−3/min) | 5.30 ± 1.23 | 2.20 ± 0.36 | 1.63 ± 0.28 | P < 0.05 a |
| P < 0.05 b | ||||
| SA (×10−3/min) | 1.87 ± 0.62 | 0.53 ± 0.19 | 0.43 ± 0.05 | P < 0.05 a |
| P < 0.05 b |
a 4 mmol/L calcium/0.5 mmol/L oxalate compared with 4 mmol/L calcium/0.5 mmol/L oxalate + 0.25 mmol/L trisodium citrate; b 4 mmol/L calcium/0.5 mmol/L oxalate compared with 4 mmol/L calcium/0.5 mmol/L oxalate + 0.01 g/100 mL SGP.
2.3. Effect on Crystal Morphology
The amount of crystal formation, as estimated from the turbidity of the solution, is shown in Figure 2. Incubating solutions of Ca2+ and Ox resulted in the formation of CaOx crystals (Figure 2A) that consisted largely of hexagonal COM. Both SGP (0.5 mg/mL) and trisodium citrate (1 mM) resulted in the shape changes of CaOx crystals, as shown in Figure 2B,C; a more rounded polygonal crystals shape. This shape may prevent the formation of kidney stones, because crystals with this shape are more easily excreted in the urine compared with the COM.
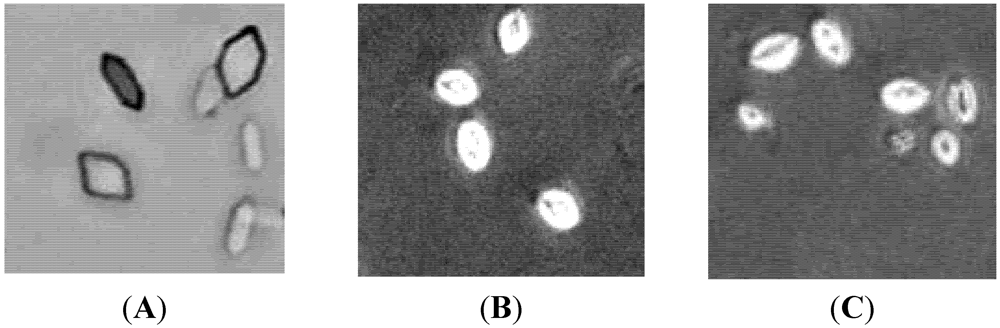
Figure 2.
The CaOx crystals, observed under inverted microscope (100×), formed in the metastable solution of CaOx in the absence (A) and the presence of (B) trisodium citrate (1 mM) (C) SGP (0.5 mg/mL).
Microphotography studies verified that SGP resulted in the formation of round CaOx crystals. CaOx develops in three different hydrated forms: COM, dehydrate (COD), and trihydrate (COT). COM is the most thermodynamically stable phase, followed by tetragonal COD and then triclinic COT. COM and COD are the major forms found in most urinary calculi [26,27]. SGP inhibited the growth of COM crystals, prevented the aggregation of COM crystals, and induced the formation of spherical COD crystals. These spherical COD crystals are the thermodynamically less stable phase and have weaker affinity for cell membranes than COM crystals [28].
2.4. Antioxidant Effects of SGP
The antioxidant properties of SGP are shown in Figure 3, Figure 4, Figure 5. Superoxide anion radicals are formed in cellular oxidation reactions, and these radicals can result in the production of hydrogen peroxide and hydroxyl radicals through dismutation and other chemical reactions. Superoxide anions have a longer lifetime and can move over greater distances than other oxygen radicals; hence, they are more damaging [29]. Therefore, the ability to scavenge superoxide anions is an important biological property for a therapeutic compound. The superoxide anion scavenging activity of different concentrations of SGP is shown in Figure 3. The scavenging effects of SGP increased with increasing concentrations; the IC50 was 1.9 mg/mL.
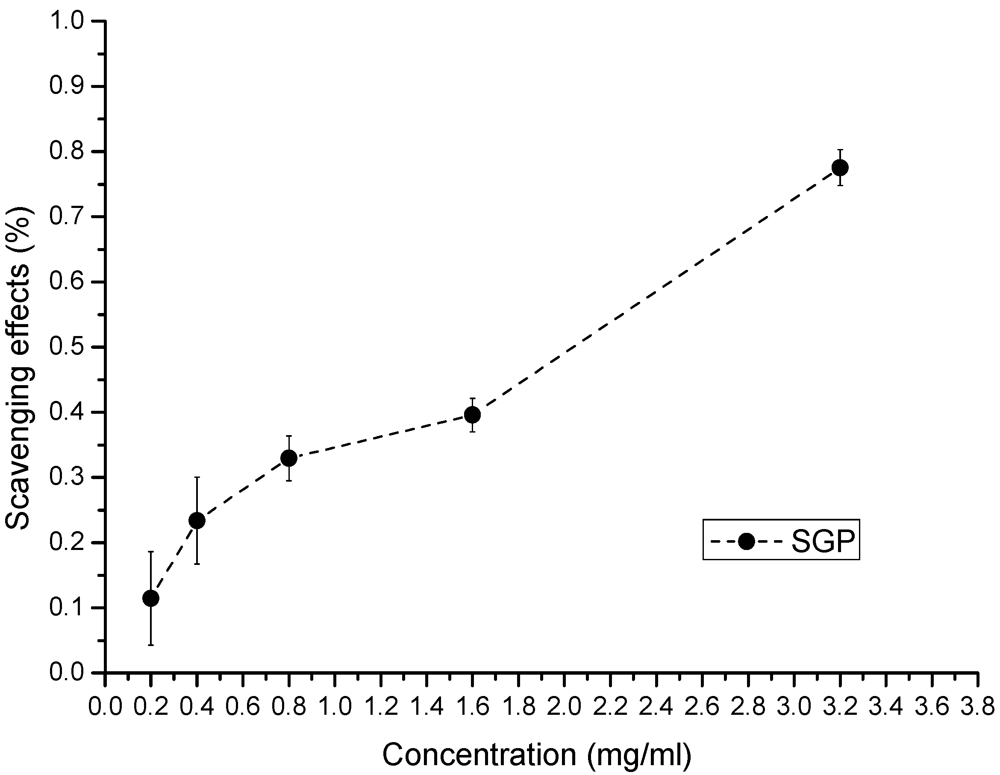
Figure 3.
Scavenging of superoxide anion by various concentrations of SGP.
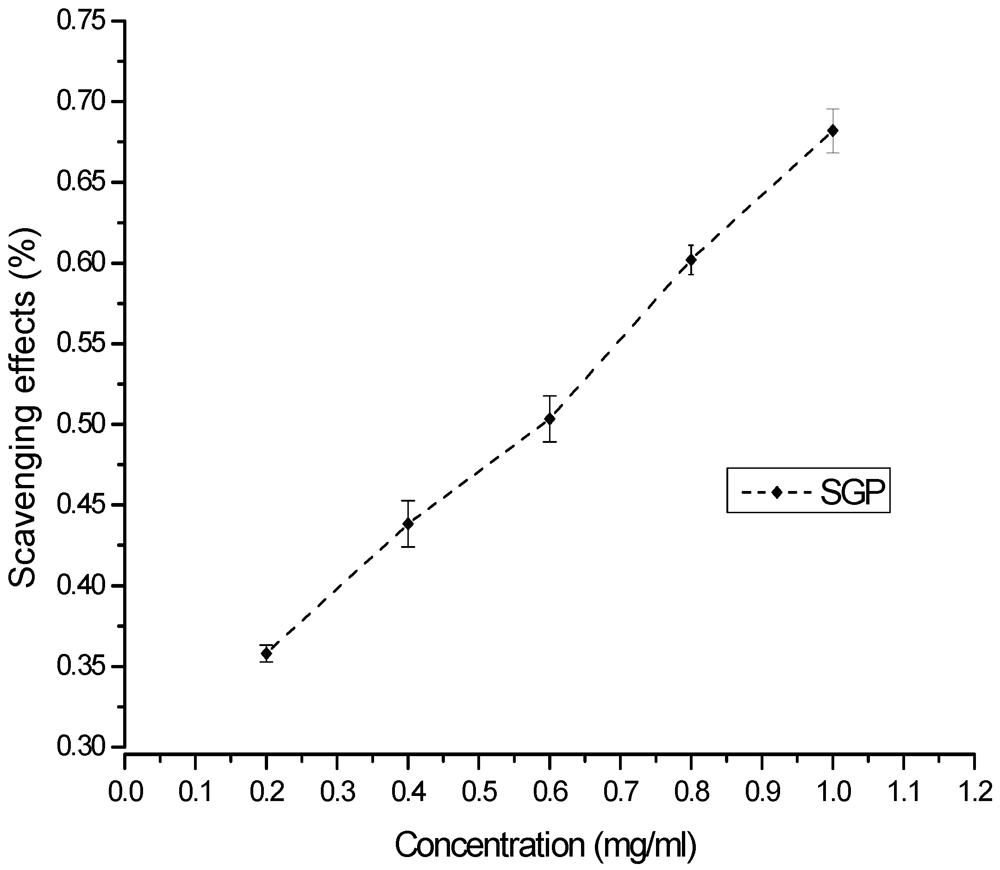
Figure 4.
Scavenging of DPPH radical by various concentrations of SGP.
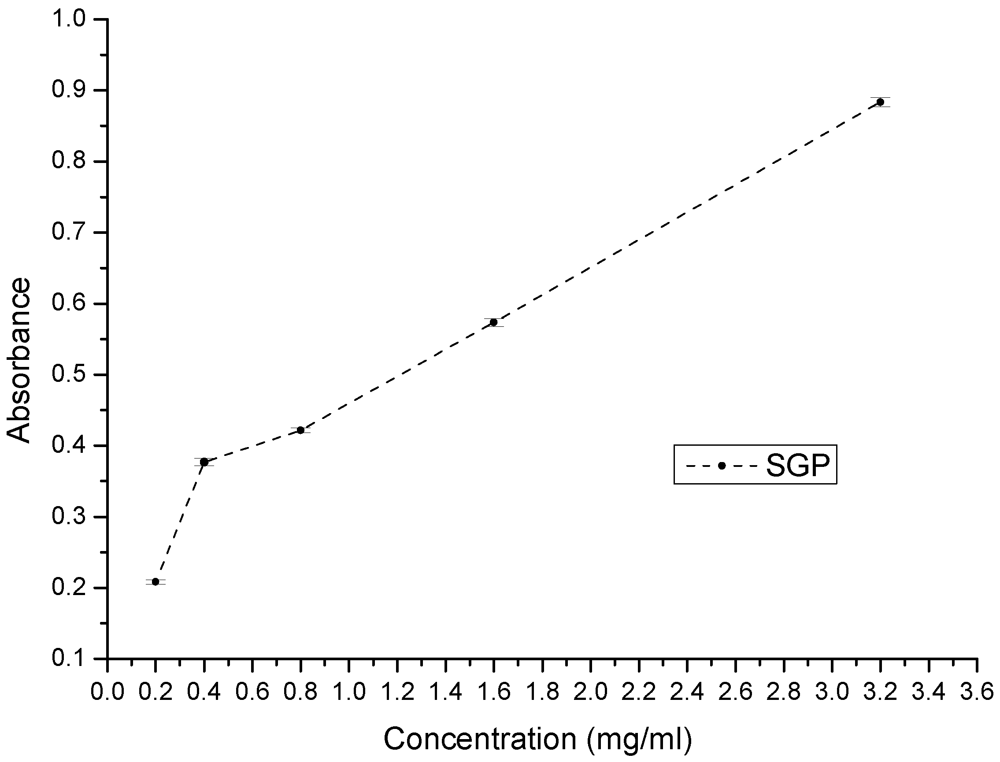
Figure 5.
Reducing power of various concentrations of SGP.
The DPPH free radical is a stable free radical that is widely used as a tool for estimating the free-radical scavenging activities of antioxidants [30]. Free radicals are highly reactive species of atoms or molecules that are unstable because of single or unbalanced electrons. DPPH is a compound with a proton free radical that has a characteristic absorption. The absorption of a DPPH solution decreases significantly in the presence of proton radical scavengers. The scavenging of the DPPH free radical by antioxidants is due to their hydrogen-donating ability [31,32]. The DPPH radical scavenging ability of SGP is shown in Figure 4. The ability to scavenge the DPPH radical increased with increasing concentrations of SGP in a concentration-dependent manner. The IC50 was 0.6 mg/mL.
Reducing power assays are used to evaluate the capacity of natural antioxidants to donate an electron. Natural antioxidants are believed to break free-radical chain reactions by donating an electron or hydrogen atom to free radicals. Therefore, the reducing power of a compound is a significant indicator of its potential antioxidant activity [33,34]. The reducing power of SGP is shown in Figure 5. At concentrations of 0.2, 0.4, 0.8, 1.6 and 3.2 mg/mL, the reducing power of SGP was 0.21, 0.37, 0.42, 0.57 and 0.89, respectively. These results showed that SGP is able to donate electrons, which may be involved in its antioxidant activity.
3. Experimental Section
3.1. Chemicals and Reagents
Dry S. graminifolium (Turn.) was obtained from Zhuhai, Guangdong Province, China. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents were of analytical grade.
3.2. Extraction of Polysaccharides from S. graminifolium and Its Properties
The polysaccharide extract from S. graminifolium (SGP) was prepared as described by Zhuang et al. [35]. Briefly, crude polysaccharides were extracted from the powdered seaweed by chloroform extraction, boiling water extraction, and ethanol precipitation. The degreased seaweed powder was incubated in a water bath at 90 °C for 3 h, and the residue was re-extracted twice and then concentrated to one-third of the original volume at 80 °C, adding 95% ethanol to the water extract until the ethanol concentration reached 80%. After standing overnight, the mixture was centrifuged at 2775 g for 15 min. The precipitate containing crude polysaccharides was washed with 95% ethanol, then with ethyl ether, and finally with acetone. Proteins were removed by adding trichloroacetic acid and centrifuging the mixture at 2775 g for 15 min, then added some sodium hydroxide in the centrifuged solution, to neutralize the remained TCA, and took dialysis operation for it. The resulting products were concentrated and freeze-dried, then kept it in the refrigerator.
To analyze the constituents of SGP 3 methods were used: the phenol sulfuric acid method for the determination of total sugar content, Folin-Phenol Determination method for protein content, and the barium sulfate turbidimetric method for sulfate content. Furthermore, functional groups of SGP were determined by infrared spectrum (IR).
3.3. Calcium Oxalate Crystallization Assay
The effect of SGR on CaOx crystallization was measured spectrophotometrically over 30 min at 620 nm. This assay quantifies crystal nucleation and aggregation in metastable solutions of Ca2+ and Ox. There are three parameters that characterize the crystallization process [36]: first, the maximum increase of optical density (OD) over time, termed SN, mainly reflects the maximum rate of formation of new particles and thus, represents crystal nucleation. Second, the rate of aggregation, SA, is derived from the maximum decrease in optical density. Third, the maximum time, tmax, is the time at which crystals can neither nucleate nor grow. All three parameters are measurable in the crystallization process of CaOx.
We used linear regression analyses for slope measurements. The percentage inhibition was calculated from the nucleation and aggregation rates, as follows: [1 − (SNm/SNc)] × 100 for the rate of nucleation and [(1 − SAm/SAc)] × 100, for the rate of aggregation (where Sm is the slope in the presence of the test material and Sc is the slope of the control experiment).
CaOx monohydrate crystallization was achieved using a mixture of calcium chloride (8 mmol/L) and sodium oxalate (1 mmol/L), containing 200 mmol/L sodium chloride and 10 mmol/L sodium acetate, adjusted to pH 5.7. The concentrations of compounds in this mixture are close to physiological urinary concentrations. The CaCl2 solution (1.0 mL) was stirred constantly at 37 °C both in the absence and presence of different concentrations of the test material or trisodium citrate as the positive control. After obtaining a stable base line, crystallization was induced by the addition of Na2C2O4 solution (1.0 mL) to reach final concentrations of 4 mmol/L calcium and 0.5 mmol/L Ox. Modifiers of CaOx crystallization were compared in assays containing 4 mmol/L calcium and 0.5 mmol/L Ox. The change in turbidity over time was measured. All experiments were run in triplicate.
3.4. Image Analysis of Crystal Morphology
In this study, we used imaging techniques to observe the size and morphology of the crystals and to verify the effect of incubation with the test material on CaOx crystal formation. We used stock solutions of CaCl2 and Na2C2O4 with compositions similar to those in the kinetic study. Aliquots (0.5 mL) of CaCl2 solutions containing SGP (0.5 mg/mL) or trisodium citrate (1 mmol/L) were added to wells in a 24-well plate. To each of the wells, Na2C2O4 solution (0.5 mL) was added to obtain final concentrations of 4.25 mmol Ca2+ and 0.75 mmol Ox [18]. Each concentration of the test material was prepared in triplicate. The plates were then incubated in a shaking water bath at 90 oscillations/min at 37 °C for 45 min. Each well was then observed under an inverted microscope (Olympus Corporation, Japan). Crystal morphology was examined in five randomly selected fields at 100× magnification. Images were captured from different fields. The most representative images are shown in Figure 2.
3.5. Antioxidant Assays
3.5.1. DPPH Assay
The DPPH scavenging activity of the samples was measured according to the method described by Ye et al. [37]. Briefly, a 0.1 mmol solution of DPPH was prepared in ethanol. Then, a 2.0 mL aliquot of the DPPH solution (0.1 mM) was incubated with different concentrations of test samples (each 2.0 mL). The reaction mixture was shaken well and incubated for 30 min in the dark, and the absorbance of the resulting solution was measured at 517 nm against a blank. Measurements were performed at least in triplicate. The percentage of DPPH that was scavenged by the tested extracts was calculated using the following formula:
Scavenging effect = [1 − (Asample − Ablank)/Acontrol)] × 100%
Here, ethanol (2.0 mL) plus sample solution (2.0 mL) was used as a blank and 2 mL DPPH-ethanol solution plus ethanol (2.0 mL) was used as a negative control.
3.5.2. Superoxide Radical Scavenging Assay
The ability of SGP to scavenge superoxide anions was measured using a chemiluminescence method with a BPCL ultra-weak luminescence analyzer (Institute of Biophysics, Beijing, China). The chemiluminescent reaction was conducted in a Na2CO3–NaHCO3 (pH 10.20, 0.5 M) buffer solution. Scavenging activity of the samples was evaluated according to their quenching effects on the chemiluminescence signal of the luminal-pyrogallol system [38]. The ability to scavenge superoxide anion was calculated as follows:
Scavenging effect = (CLblank − CLsample)/CLblank × 100%
Here, CLblank and CLsample represent chemiluminescence peak areas of the blank group and test group, respectively. The luminous intensity was recorded at 2-s intervals and the total luminous integrated intensity was determined for 150 s.
3.5.3. Reducing Powers of SGP
Reducing powers of SGP were evaluated by the method described by Sun et al. [38]. Briefly, an aliquot (2.0 mL) of each sample (at different concentrations) was mixed with 2.0 mL phosphate buffer (0.2 mol/L, pH 6.6) and 2.0 mL potassium ferricyanide (1% w/v). The reaction mixture was incubated at 50 °C for 20 min, then 2.5 mL trichloroacetic acid (10% w/v) was added, and then the mixture was centrifuged at 22.2 g for 10 min. The supernatant was mixed with 2.5 mL distilled water and 0.5 mL ferric chloride solution (0.1% w/v), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated greater reducing power.
3.6. Statistical Analysis
All values shown are means ± SD. P-values less than 0.05 were regarded as significant. All statistical comparisons between groups were made using one-way analysis of variance with Dunnett’s post hoc test or by Student’s t-test. Statistical analyses were carried out using Origin 8 software.
4. Conclusions
The ability of urine to inhibit CaOx crystallization is an important mechanism against formation of urinary stones. Various physicochemical techniques, including turbidimetry methods, conductometric and nephelometric titrations, UV-vis and IR spectroscopy techniques, and potential measurements have been used to evaluate crystal formation [8,39]. For the current study, we used a turbidimetry method to induce and monitor crystallization because it is rapid and reproducible, and allows measurements of nucleation, growth, and aggregation of CaOx crystals [40,41]. We found that SGP inhibited CaOx crystal nucleation and aggregation at similar rates as trisodium citrate, a well-known inhibitor of CaOx crystallization that is widely used to prevent urinary stones.
SGP has many negatively charged –OSO3–, –COO–, and –OH groups, and these anions strongly coordinate with Ca2+ ions [42]. The numerous negatively charged groups of SGP were able to chelate Ca2+ ions, resulting in a rapid increase in the concentration of Ca2+ ions on the surface of the SGP molecules. This resulted in a higher energy interface on the surface of SGP molecules. The adsorption of Ca2+ ions would result in a simultaneous decrease in free Ca2+ ions and an increase in the energy state of Ca2+ ions. Both the high energy interface and high energy state Ca2+ ions would then promote the formation of thermodynamically metastable COD [8].
Our findings indicate that natural substances such as SGP could contribute to the prevention of urinary stones. Sulfated polysaccharides from marine algae possess numerous pharmacological properties, but there is little research on sulfated polysaccharides from S. graminifolium. Further studies on natural substances should be carried out to assess their effects on CaOx crystallization in vivo.
Acknowledgments
The authors thank Wenhui Wu (Institute of Marine Sciences, Shanghai Ocean University) for assistance with the microscopic analysis. This study was supported by the National 863 Plans Projects of China (2011AA09070109).
- Samples Availability: Available from the authors.
References
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar]
- Karnjanapratum, S.; You, S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2010, 48, 311–318. [Google Scholar]
- Rajeswari, A.; Varalakshmi, P. Low molecular weight heparin protection against oxalate-induced oxidative renal insult. Clin. Chim. Acta 2006, 370, 108–114. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar]
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2009, 45, 22–26. [Google Scholar]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar]
- Coothan, K.V.; Anthony, J.; Sreenivasan, P.; Preetha, P.V.; Rajaguru, S. Renal peroxidative changes mediated by oxalate: The protective role of fucoidan. Life Sci. 2006, 79, 1789–1795. [Google Scholar]
- Das, I.; Gupta, S.K.; Ansari, S.A.; Pandey, V.N.; Rastogi, R.P. In vitro inhibition and dissolution of calcium oxalate by edible plant Trianthema monogyna and pulse Macrotyloma uniflorum extracts. J. Cryst. Growth 2005, 273, 546–554. [Google Scholar]
- Basavaraj, D.R.; Biyani, C.S.; Browning, A.J.; Cartledge, J.J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones. EAU-EBU Update Ser. 2007, 5, 126–136. [Google Scholar] [CrossRef]
- Bihl, G.; Meyers, A. Recurrent renal stone disease—advances in pathogenesis and clinical management. Lancet 2001, 358, 651–656. [Google Scholar]
- Atmani, F.; Khan, S.R. Effects of an extract from Herniaria hirsuta on calcium oxalate crystallization in vitro. BJU Int. 2000, 85, 621–625. [Google Scholar]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar]
- Borghi, L.; Meschi, T.; Guerra, A.; Bergamaschi, E.; Mutti, A.; Novarini, A. Effects of urinary macromolecules on the nucleation of calcium oxalate in idiopathic stone formers and healthy controls. Clin. Chim. Acta 1995, 239, 1–11. [Google Scholar]
- Edyvane, K.A.; Hibberd, C.M.; Harnett, R.M.; Marshall, V.R.; Ryall, R.L. Macromolecules inhibit calcium oxalate crystal growth and aggregation in whole human urine. Clin. Chim. Acta 1987, 167, 329–338. [Google Scholar]
- Liu, J.; Wang, T.; Chen, J.; Wang, S.; Ye, Z. Decreased inhibitory activity of prothrombin to calcium oxalate crystallization by specific chemical modification of its gamma-carboxyglutamic acid residues. Urology 2006, 67, 201–203. [Google Scholar]
- Hackett, R.L.; Shevock, P.N.; Khan, S.R. Madin-Darby ca nine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol. Res. 1994, 22, 197–203. [Google Scholar]
- Koul, H.; Kenington, L.; Honeyman, T.; Jonassen, J.; Menon, M.; Scheid, C.R. Activation of the c-myc gene mediates the mitogenic effects of Ox in LLC-PK1 cells, a line of renal epithelial cells. Kidney Int. 1996, 50, 1525–1530. [Google Scholar]
- Bashir, S.; Gilani, A.H. Antiurolithic effect of Bergenia ligulata rhizome: An explanation of the underlying mechanisms. J. Ethnopharmacol. 2009, 122, 106–116. [Google Scholar]
- Saeed, R.K. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol. Res. 2005, 33, 349–357. [Google Scholar]
- Vanachayangkul, P.; Byer, K.; Khan, S.; Butterweck, V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine 2010, 17, 653–658. [Google Scholar]
- Srinivasan, S.; Pragasam, V.; Jenita, X.; Kalaiselvi, P.; Muthu, V.; Varalakshmi, P. Oxidative stress in urogenital tuberculosis patients: a predisposing factor for renal stone formation—amelioration by vitamin E supplementation. Clin. Chim. Acta 2004, 350, 57–63. [Google Scholar]
- Rodrigo, R.; Rivera, G. Renal damage mediated by oxidative stress: A hypothesis of protective effects of red wine. Free Radic. Biol. Med. 2002, 33, 409–422. [Google Scholar]
- Siener, R.; Hesse, A. The effect of different diets on urine composition and the risk of calcium oxalate crystallisation in healthy subjects. Eur. Urol. 2002, 42, 289–296. [Google Scholar]
- Wang, J.; Zhang, Q.B.; Zhang, Z.S.; Zhang, H.; Niu, X.Z. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar]
- Samee, H.; Li, Z.X.; Lin, H.; Khalid, J.; Wang, B.P. In vivo study of antiallergenicity of ethanol extracts from Sargassum tenerrimum, Sargassum cervicorne and Sargassum graminifolium Turn. Eur. Food Res. Technol. 2009, 229, 435–441. [Google Scholar]
- Ouyang, J.M.; Zheng, H.; Deng, S.P. Simultaneous formation of calcium oxalate (mono-, di-, and trihydrate) induced by potassium tartrate in gelatinous system. J. Cryst. Growth 2006, 293, 118–123. [Google Scholar]
- Yu, J.; Tang, H.; Cheng, B. Influence of PSSS additive and temperature on morphology and phase structures of calcium oxalate. J. Colloid Interface Sci. 2005, 288, 407–411. [Google Scholar]
- Liang, M.; Bai, Y.; Huang, L.; Zheng, W.; Liu, J. Inhibition of the crystal growth and aggregation of calcium oxalate by elemental selenium nanoparticles. Colloids Surf. B Biointerfaces 2009, 74, 366–369. [Google Scholar]
- Liochev, S.I.; Fridovich, I. The effects of superoxide dismutase on H2O2 formation. Free Radic. Biol. Med. 2007, 42, 1465–1469. [Google Scholar]
- Hu, F.L.; Lu, R.L.; Huang, B.; Ming, L. Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia 2004, 75, 14–23. [Google Scholar]
- Bortolomeazzi, R.; Sebastianutto, N.; Toniolo, R.; Pizzariello, A. Comparative evaluation of the antioxidant capacity of smoke flavouring phenols by crocin bleaching inhibition, DPPH radical scavenging and oxidation potential. Food Chem. 2007, 100, 1481–1489. [Google Scholar]
- Fagali, N.; Catal, A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys. Chem. 2008, 137, 56–62. [Google Scholar]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009, 114, 717–726. [Google Scholar]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar]
- Zhuang, C.; Itoh, H.; Mizuno, T.; Ito, H. Antitumor active fucoidan from the brown seaweed, umitoranoo (Sargassum thunbergii). Biosci. Biotechnol. Biochem. 1995, 59, 563–567. [Google Scholar]
- Kulaksizoglu, S.; Sofikerim, M.; Cevik, C. In vitro effect of lemon and orange juices on calcium oxalate crystallization. Int. Urol. Nephrol. 2008, 40, 589–594. [Google Scholar]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar]
- Sun, T.; Yao, Q.; Zhou, D.X.; Mao, F. Antioxidant activity of N-carboxymethyl chitosan oligosaccharides. Bioorg. Med. Chem. Lett. 2008, 18, 5774–5776. [Google Scholar]
- Mushtaq, S.; Siddiqui, A.A.; Naqvi, Z.A.; Rattani, A.; Talati, J.; Palmberg, C.; Shafqat, J. Identification of myeloperoxidase, [alpha]-defensin and calgranulin in calcium oxalate renal stones. Clin. Chim. Acta 2007, 384, 41–47. [Google Scholar]
- Hess, B.; Meinhardt, U.; Zipperle, L.; Giovanoli, R.; Jaeger, P. Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol. Res. 1995, 23, 231–238. [Google Scholar]
- Gohel, M.D.; Wong, S.P. Chinese herbal medicines and their efficacy in treating renal stones. Urol. Res. 2006, 34, 365–372. [Google Scholar]
- Kavanagh, J.P.; Jones, L.; Rao, P.N. Calcium oxalate crystallization kinetics studied by oxalate-induced turbidity in fresh human urine and artificial urine. Clin. Sci. (Lond.) 2000, 98, 151–158. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).