Abstract
Tribulus terrestris is a rich source of bioactive molecules and thrives in Mediterranean and desert climate regions worldwide. In this study, Tribulus terrestris methanolic HPLC fractions were evaluated for bioactive compounds and PBP2a transpeptidase inhibitors against methicillin-resistant Staphylococcus epidermidis (MRSE). Among the collected HPLC fractions, F02 of the methanol extract demonstrated potential activity against MRSE01 (15 ± 0.13 mm), MRSE02 (13 ± 0.21 mm), and MRSE03 (16 ± 0.14 mm) isolates. GC-MS analysis of the F02 fraction identified seventeen compounds. Among seventeen compounds, eight have favorable pharmacokinetics and medicinal chemistry; however, on the basis of in silico high water solubility, high GI absorption, blood–brain barrier non-permeability, lack of toxicity, and potential drug-likeness, 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl), were processed for molecular docking. 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane formed three hydrogen bonds with Ser-452, Thr-584, and Asn-454 residues of the PBP2a transpeptidase. Similarly, phthalimide, N-(1-hydroxy-2-propyl)-formed four hydrogen bonds with Ser-396, Asn-454, Lys-399, and Ser-452 residues of PBP2a transpeptidase. These two compounds are proposed as novel putative PBP2a transpeptidase inhibitors. Further characterization of compounds extracted from Tribulus terrestris may aid in identifying novel PBP2a inhibitory agents for managing MRSE infections.
1. Introduction
Plants are a significant source of pharmacologically active compounds that benefit human and animal health. Approximately 50% of the population worldwide relies on traditional medicine [1]. The therapeutic potential of medicinal plants is largely ascribed to their secondary metabolites, including flavonoids, alkaloids, phenolics, and other bioactive compounds [2,3]. These metabolites, often produced in response to microbial infections, have demonstrated in vitro medicinal activity against a wide range of pathogens [4].
Plants used as traditional medicine have been evident for decades. Among these, Tribulus terrestris (TT) has been used traditionally in Asia to treat gastrointestinal and cardiovascular problems and urinary tract infections [5]. TT, a widespread plant from the Zygophyllaceae family, is found in tropical regions, including the Mediterranean, Asia, the United States, and Mexico. In Saudi Arabia, TT grows abundantly, particularly in the Southern Hejaz and Eastern Najd regions [6,7]. In Chinese medicine, TT fruits and roots have been used for thousands of years to treat eye infections, respiratory tract infections, and mastitis [8]. Moreover, TT leaves, flowers, and seeds have been utilized in folk medicine for their analgesic, antidiabetic, anti-inflammatory, anticancer, antispasmodic, antibacterial, and other therapeutic purposes [6,7,8,9]. The medicinal potential of TT is due to diverse bioactive phytochemicals, not limited to flavonoids, saponins, terpenoids, tannins, and proteins [10].
The emergence of multidrug-resistant (MDR) bacterial pathogens has necessitated the exploration of alternative antibacterial agents. The limitations of current antibiotics, including issues of efficacy, safety, and cost, keep medicinal plants as an important source of antibacterial compounds. Staphylococcus epidermidis is a common skin-associated opportunistic pathogen that frequently causes nosocomial bacteremia and exhibits resistance to multiple antibiotics [11].
It has been reported that S. epidermidis is an important gene reservoir that disseminates through horizontal transfer to other bacteria and facilitates virulence and antibiotic resistance [12]. Methicillin-resistant Staphylococcus epidermidis (MRSE) possesses resistance to beta-lactam antibiotics due to penicillin-binding protein 2a (PBP2a), a product of the mecA gene. PBP2a, with transpeptidase and transglycosylase activities, is critical for cell wall peptidoglycan synthesis in resistant strains [13,14,15,16]. MRSE infections are further complicated by biofilm formation, which enhances bacterial survival and resistance to therapeutic molecules. Despite advancements in antimicrobial therapies, vancomycin remains the primary treatment against MRSE infections [16,17].
Recently, computational approaches have been significantly employed to design and develop medicinal molecules from natural products. Prediction of water solubility, Lipinski’s rule of five, absorption, distribution, metabolism, excretion, and toxicity (ADMET) are useful tools with which to explore the biological and synthetic chemistry, drug-likeness, and toxicity of lead molecules [18,19,20]. Similarly, molecular docking analysis is important in the evaluation of the lead compounds’ conformation with the target active sites. These tools provided insights into medicinal molecules and allowed for the exploration of ligand–receptor interactions [21,22,23]. As far as literature mining is concerned, there is a lack of data on the anti-MRSE and anti-PBP2a potential of the HPLC fractions of TT fruit extract.
This study focuses on the methanolic extract of TT fruits to identify bioactive compounds with anti-MRSE activity. Selected compounds were further analyzed for their potential as PBP2a inhibitors, aiming to uncover druggable molecules for combating MRSE infections.
2. Materials and Methods
2.1. Tribulus terrestris Fruits Collection
Tribulus terrestris fruits were collected and authenticated by the botany expert at Abdul Wali Khan University, Mardan, Pakistan. The TT fruit was deposited with a voucher specimen (24-05/TT). The fruits were properly washed, air-dried, and minced into small pieces [24].
2.2. Preparation of TT Extracts
TT extracts were prepared as described [25], with some modifications. Briefly, 100 g of powdered TT fruit was soaked in 500 mL methanol and incubated at room temperature for 30 days. The extract was passed through Whatman paper and concentrated via rotary evaporation at 44 °C to remove the solvent.
2.3. Identification of Methicillin-Resistant S. epidermidis (MRSE)
Previously collected Staphylococcus epidermidis isolates (n = 15) from the Department of Microbiology, AWKUM, Mardan, Pakistan, were processed for MRSE detection. Gram staining and biochemical tests, including catalase, coagulase, DNase, and cefoxitin disc assays, were performed. For the molecular detection of S. epidermidis, DNA was extracted using Chelax 100 resins (Biorad, Hercules, CA, USA). Briefly, Chelax 100 resins were added to sample suspension. The mixture was heated at 95 °C for 10 min, and the resins were separated via centrifugation. The supernatant was used for PCR. Species-specific primers targeting the rdr gene (forward: AAGAGCGTGGAGAAAAGTATCAAG; reverse: TCGATACCATCAAAAAGTTGG, 130 bp) were used for S. epidermidis detection. mecA gene primers (forward: AAAATCGATGGTAAAGGTTGGC; reverse: AGTTCTGCAGTACCGGATTTGC, 533 bp) were used for the detection of methicillin resistance previously described [26].
2.4. Antimicrobial Susceptibility Testing of MRSE
The Kirby–Bauer disc diffusion method was employed to evaluate the antibiotic susceptibility of S. epidermidis isolates [27]. Bacterial isolates were inoculated onto Muller–Hinton agar plates; and antibiotic discs (Oxoid, Hampshire, UK), including penicillin, augmentin, methicillin, vancomycin, ciprofloxacin, linezolid, erythromycin, gentamicin, tetracycline, azithromycin, and clarithromycin, were used. Methicillin resistance was phenotypically confirmed using a cefoxitin disc. The zone diameter of inhibition was measured in mm and interpreted as per CLSI guidelines [28].
2.5. Well Diffusion Assay for Anti-MRSE Activity of Methanolic Fruit Extract
The methanolic extracts were checked against methicillin-resistant S. epidermidis [29]. Briefly, an overnight MRSE growth was inoculated on Muller–Hinton agar. The TT dried extract was dissolved in dimethyl sulfoxide (DMSO). A 20 µL plant extract was added into each well and incubated overnight at 37 °C. DMSO was incorporated as a solvent control, while augmentin disc was applied as standard antibiotic control. Inhibitory zones were documented in mm.
2.6. TT Fractionation Using Gradient HPLC
Methanolic TT extracts were subjected to gradient HPLC fractionation as described earlier [30]. Solidified TT methanolic extracts were dissolved in a distilled water–methanol mixture (ratio: 1:80) and were fractionated using a C-18 column (PerkinElmer, Shelton, CT, USA). The HPLC system was configured with an isocratic LC pump, solvent manager, degassing, and tubing kit. The system default pressure and fractionation pressure were set at 2000 psi and 1820/1950 psi, respectively. The monochromator wavelength was configured at 250 nm. Multiple HPLC fractions were collected and stored for downstream processing.
2.7. Well Diffusion Assay for Anti-MRSE Activity of HPLC Fractions
The anti-MRSE activity of HPLC fractions was assessed using the well diffusion method [29]. Briefly, bacterial cultures were refreshed in nutrient broth and incubated at 37 °C for 2 h. Bacterial suspensions, standardized with 0.5 McFarland, were inoculated onto Muller–Hinton agar. Wells were loaded with HPLC fractions, while DMSO was incorporated as a solvent control, and augmentin was incorporated as a standard antibiotic control. All the plates were incubated overnight at 37 °C, and zone of inhibition was measured in mm. The experiment was run in triplicate.
2.8. Identification of Bioactive Compounds
GC-MS (Agilent Technologies, Santa Clara, CA, USA) was used to identify bioactive compounds. Samples were injected via a GC automatic liquid sampler into a column (30 m × 250 µm × 0.25 µm). The ESI source, helium as the mobile phase (1 mL/min), and pressure (8.8085 psi) were set accordingly. The oven’s temperature was adjusted from 70 °C to 270 °C. Data acquisition and mass spectral library searches were conducted using MassHunter software (NIST11.L). The three best hits from each library were documented.
2.9. Prediction of Lead Hits
The structural parameters of bioactive molecules were retrieved (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 20 October 2024)). Drug-likeness, medicinal chemistry, and ADMET properties were assessed using online tools such as SWISS-ADME (http://www.swissadme.ch/ (accessed on 1 November 2024)) [19] and ProTox 3.0 (https://tox.charite.de/protox3/ (accessed on 5 November 2024)) [23].
2.10. Preparation of PBP2a for Docking Assessment
The PBP2a protein sequence of MRSE was obtained from the UniProt database (Accession ID: A0A5E9MEW2_STAEP). The three-dimensional structure of PBP2a protein was obtained from the Protein Data Bank (PDB ID: AF-A0A5E9MEW2-F1-v4). The protein structure was prepared by removing water molecules, adding Gasteiger charges, and minimizing energy. Docking analysis were conducted using MOE software (2016.08) to evaluate the interactions of ligands with the active site residues of the PBP2a enzyme.
2.11. Statistical Analysis
Experimental results were reported as the standard error of the mean.
3. Results
3.1. Confirmation of MRSE
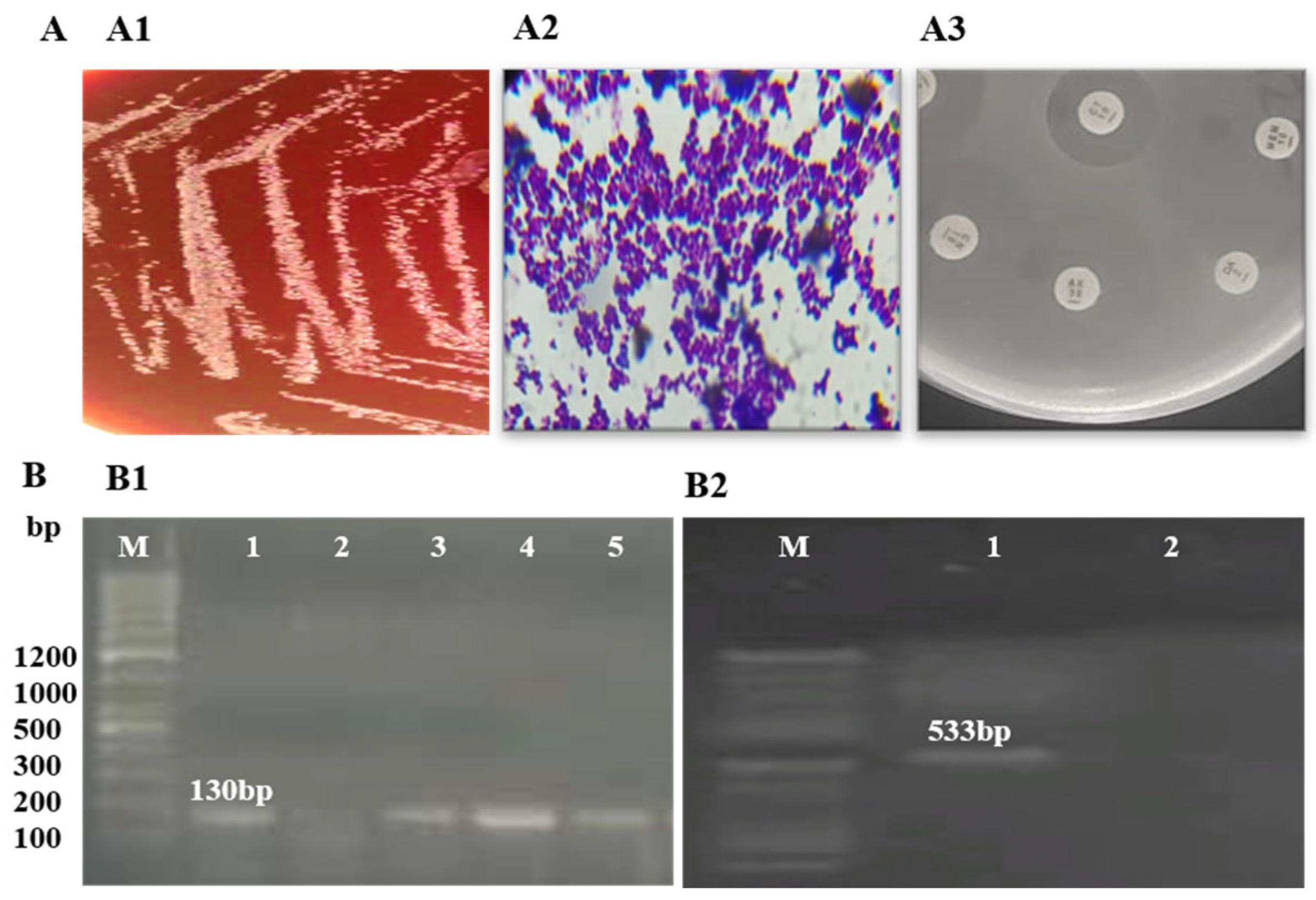
Identification of S. epidermidis isolates was performed using Gram staining and biochemical tests. Amplification of the rdr gene, resulting in a 130 bp product on agarose gel electrophoresis, confirmed the identification of S. epidermidis. Detection of the mecA gene, with a 533 bp amplification product, confirmed methicillin resistance in all three isolates. Further analysis of these MRSE isolates revealed resistance to methicillin, penicillin, augmentin, and erythromycin (Figure 1, Table 1).
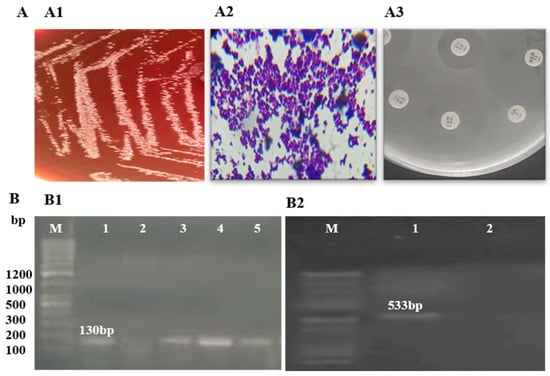
Figure 1.
Phenotypic and molecular identification of MRSE: (A) phenotypic identification of MRSE; (A1) growth on blood agar; (A2) Gram staining showing Gram-positive cocci in clusters; (A3) antibiotic susceptibility on MHA agar; (B) molecular identification of MRSE; (B1) amplification of rdr (130 bp) for the detection of S. epidermidis (M: ladder; 1, 3, 4, 5: rdr-positive; 2: rdr-negative); (B2) amplification of mecA (533 bp) (M: ladder; 1: mecA-positive; 2: mecA-negative).

Table 1.
Detection and antibiotic susceptibility of MRSE and anti-MRSE activity of Tribuls terrestris HPLC methanolic fractions.
3.2. HPLC Fractions of TT Methanol Extract Against MRSE
The methanol extract of TT, fractionated via HPLC, was tested for anti-MRSE activity. Among the 15 fractions collected, three fractions demonstrated a zone of inhibition against all MRSE isolates. The F02 fraction exhibited the highest inhibitory activity, with inhibition zones of 15 ± 0.13 mm, 13 ± 0.21 mm, and 16 ± 0.14 mm against MRSE01, MRSE02, and MRSE03, respectively (Table 1). It was noted that the TT fractions exhibited potential activity against MRSE as compared to augmentin (standard antibiotic).
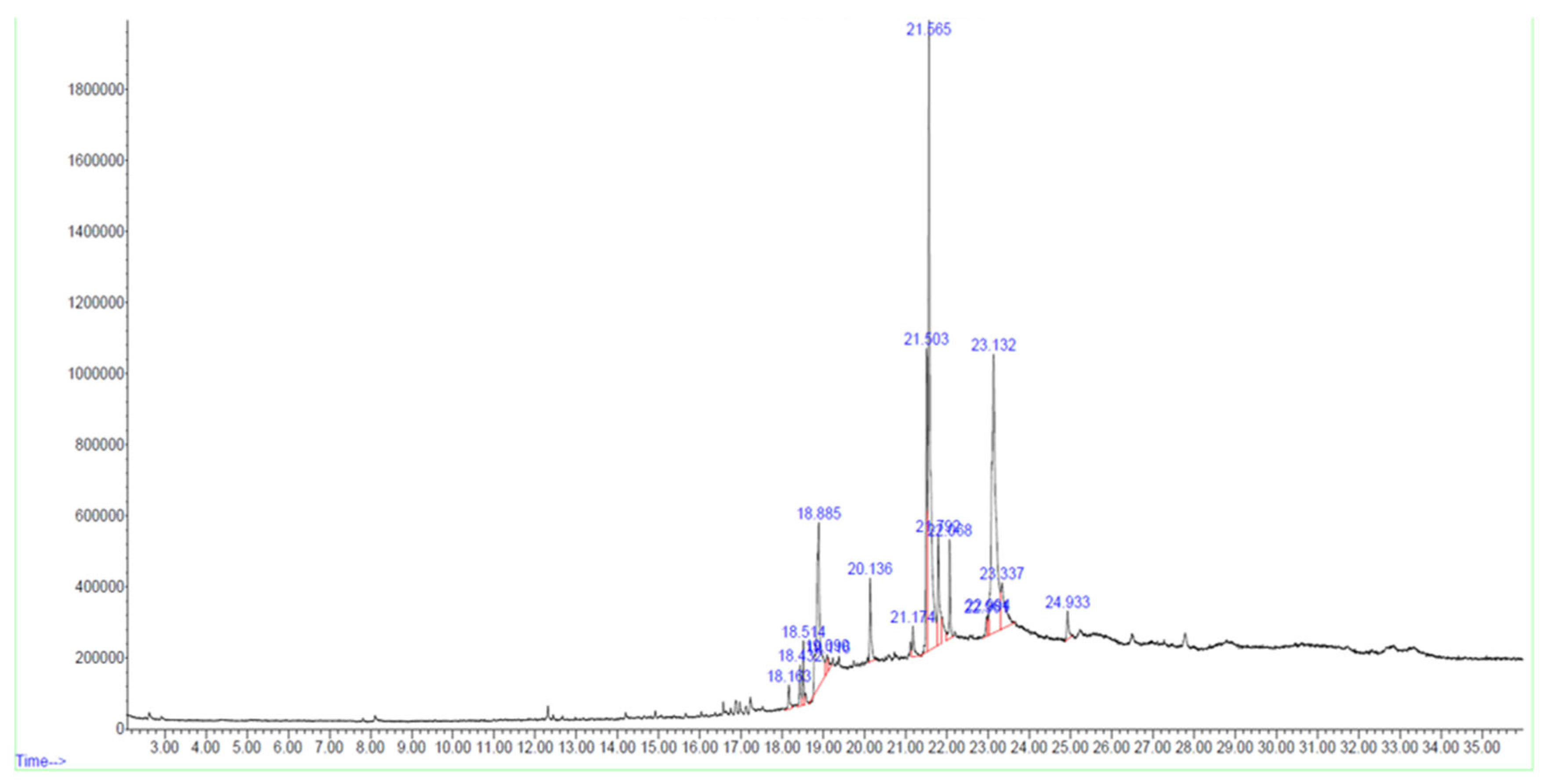
3.3. Identification of Bioactive Compounds by GC-MS Analysis
In the F02 fraction, 17 compounds were identified based on spectral library searches. The compounds, along with their molecular formulae, retention times, CAS numbers, m/z ratios, and molecular structures, are detailed in Figure 2, Table 2 and Supplementary Figure S1.
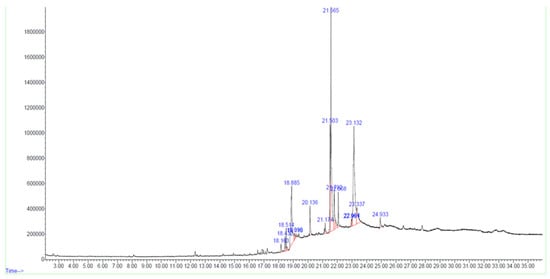
Figure 2.
GC-MS chromatogram of bioactive HPLC fraction of Tribulus terrestris fruit methanolic extract.

Table 2.
Identification of bioactive compounds from Tribulus terrestris fruit methanolic extracts.
3.4. Druggable Characteristics of Bioactive Compounds
The identified 17 compounds were analyzed for druggability using ADMET profiling. Eight compounds, including 13-Hexyloxacyclotridec-10-en-2-one, Bicyclo[5.3.1]undecan-11-one, 13-Oxabicyclo[10.1.0]tridecane, 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane, N-[[2-p-Tolylsulfonyl]ethyl]phthalimide, Phthalimide, N-(1-hydroxy-2-propyl)-, Oxacyclododecan-2-one, Pyridine-3-carboxamide and oxime, N-(2-trifluoromethylphenyl), exhibited favorable pharmacokinetics, drug-likeness, and medicinal potential (Table 3).

Table 3.
Druggable compounds of Tribulus terrestris fruit methanolic extracts.
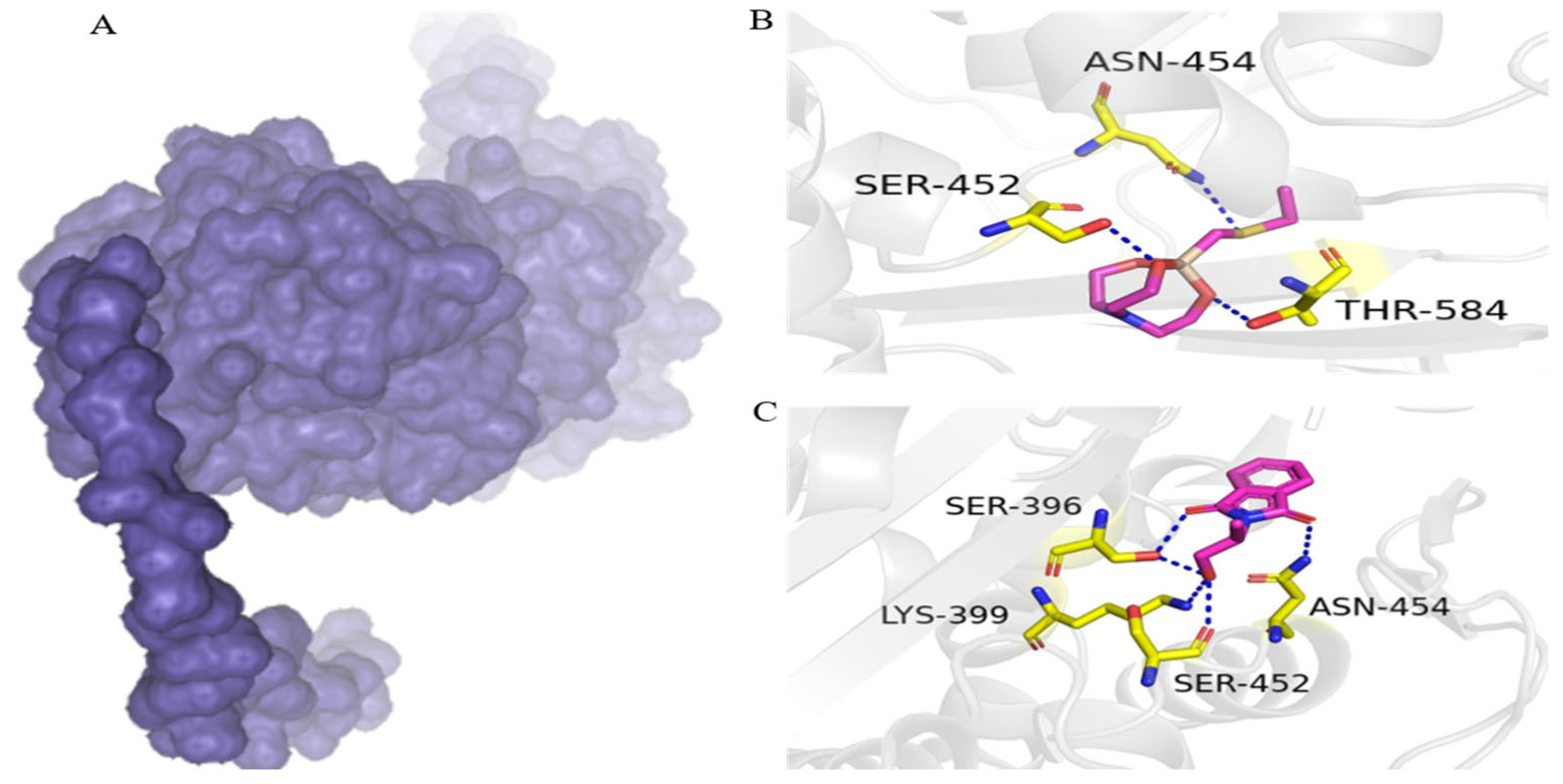
3.5. Putative PBP2a Inhibitors
On the basis of high water solubility, high GI absorption, blood–brain barrier non-permeability, lack of toxicity, and drug-likeness, two compounds, including 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and Phthalimide, N-(1-hydroxy-2-propyl)-, were assessed for molecular interaction with PBP2a transpeptidase of MRSE. 1-Ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane interacts with Ser-452, Thr-584, and Asn-454 residues of PBP2a transpeptidase via hydrogen bonds. Similarly, phthalimide, N-(1-hydroxy-2-propyl)-formed four hydrogen bonds with PBP2a transpeptidase amino acid residues of Ser-396, Asn-454, Lys-399, and Ser-452 (Figure 3).
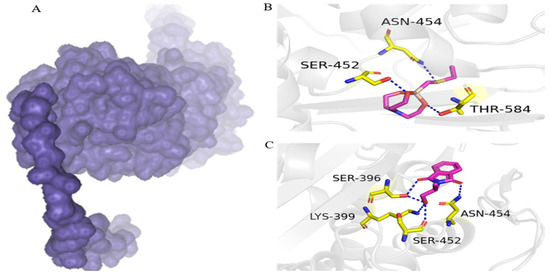
Figure 3.
Binding conformation and chemical interaction network of 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl)- within the binding pocket of PBP2a enzyme: (A) PBP2a surface; (B) interaction map of 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane with active site residues of PBP2a; (C) phthalimide, N-(1-hydroxy-2-propyl)- with active site residues of PB.
4. Discussion
The emergence of drug resistance in Gram-positive bacteria, particularly methicillin resistance, has posed significant challenges to the therapeutic efficacy of beta-lactam antibiotics. Staphylococcus epidermidis is a well-documented nosocomial pathogen capable of thriving in hospital and community environments. Methicillin-resistant Staphylococcus epidermidis (MRSE) expresses PBP2a, which confers resistance to beta-lactam [14]. Given the increasing resistance to conventional antibiotics, the exploration of alternative therapeutic sources, such as medicinal plants, is imperative.
Medicinal plants, including Tribulus terrestris (TT), have long been recognized as reservoirs of bioactive compounds with diverse pharmacological activities. TT contains secondary metabolites, such as flavonoids, alkaloids, and steroidal saponins, which contribute to its broad-spectrum antimicrobial, anticancer, and anti-inflammatory properties [8]. Prior studies have demonstrated TT’s efficacy against other bacterial species. For instance, a recent study from Saudi Arabia reported TT’s cytotoxic role against cancer cell lines, as well as its antibacterial and antibiofilm activities against Streptococcus and Lactobacillus species [31].
No reports to date have evaluated its anti-MRSE potential, which underscores the novelty of the present study. One study identified bioactive molecules in TT leaf ethanolic extracts [32], while another reviewed the phytochemical and pharmacological potential of TT [33]. In this study, methanolic HPLC fractions of TT exhibiting anti-MRSE activity were processed for GC-MS analysis. Seventeen compounds were identified, and most are reported here for the first time in TT fruit.
ADMET analysis revealed that eight compounds showed drug-likeness characteristics and medicinal potential. Among these, 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl)- showed excellent water solubility, high GI absorption, and non-permeability across the blood–brain barrier, fulfilling the five rules of drug-likeness. The compound 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane was previously identified in A. chinense bulb hexane extracts with activity against Staphylococcus aureus and Pseudomonas aeruginosa [34]; however, it is reported here for the first time in TT fruit methanolic extract. Phthalimide, N-(1-hydroxy-2-propyl) has previously shown anti-inflammatory activity in murine models of chronic inflammation [35].
Docking studies are essential for evaluating the medicinal potential of lead compounds for medicinal potential [21]. These methods allow for the exploration of ligand–receptor affinities and interactions [22]. In this study, 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl)- exhibited multiple interactions with the key residues of the PBP2a transpeptidase enzyme of MRSE. The PBP2a transpeptidase expression in MRSE exhibits resistance to beta-lactam antibiotics [14]. It was found that 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl)- strongly interacted with the PBP2a enzyme; therefore, further studies are recommended to validate their efficacy and safety against MRSE.
In the current study, anti-MRSE methanolic TT fractions were evaluated for bioactive compounds, and processed for ADMET and docking analysis; however, in vitro and in vivo validation will be required to explore the potential and efficacy of the identified compounds. Further characterization of the 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl) will help to elucidate these compounds as new anti-infective agents against MRSE.
5. Conclusions
Seventeen compounds were identified in the TT methanolic extract of the F02 fraction, of which eight compounds exhibited favorable druggable characteristics. Among the eight compounds, 1-ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane and phthalimide, N-(1-hydroxy-2-propyl) were highly water-soluble and made hydrogen bonds with multiple residues of the PBP2a transpeptidase. The findings of this study may open new avenues for natural product research, and they emphasize the need for further preclinical and clinical investigations to validate their therapeutic potential against MRSE and other resistant pathogens.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cimb47010052/s1.
Funding
This research was funded by Taif University, Taif, Saudi Arabia (TU-DSPP-2024-05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data access statements are included in the publication.
Acknowledgments
The author extends their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-05). Thanks are given to Hazir Rehman, Abdul Wali Khan University Mardan, for his technical support.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Doughari, J.H.; El-mahmood, A.M.; Tyoyina, I. Antimicrobial activity of leaf extracts of Senna obstusifolia. Afr. J. Pharm. Pharmacol. 2008, 2, 344–346. [Google Scholar]
- Kivanç, M.; Kunduhoğlu, B. Antimıcrobial activity of fresh plant juice on the growth of bacteria and yeast. J. Qafqaz Univ. 1997, 1, 27–35. [Google Scholar]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu India. BMC Complement. Altern. Med. 2006, 6, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Dey, P.; Lamb, C. Phytoalexins: Enzymology and molecular biology. Adv. Enzymol. 1983, 55, 1–69. [Google Scholar]
- Gauthaman, K.; Adaikan, P.G.; Prasad, R.N. Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002, 71, 1385–1396. [Google Scholar] [CrossRef]
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–51. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Al-Elaiwi, A.M.; Athar, M.T.; Tariq, M.; Al Eid, A.; Al-Asmary, S.M. A review of hepatoprotective plants used in Saudi traditional medicine. Evid.-Based Complement. Altern. Med. 2014, 2014, 890842. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Alkaloids and other constituents from Tribulus terrestris. Phytochemistry 1999, 50, 1411–1415. [Google Scholar] [CrossRef]
- Shahid, M.; Riaz, M.; Talpur, M.M.; Pirzada, T. Phytopharmacology of Tribulus terrestris. J. Biol. Regul. Homeost. Agents 2016, 30, 785–788. [Google Scholar]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 60. [Google Scholar] [CrossRef]
- Xu, Z.; Cave, R.; Chen, L.; Yangkyi, T.; Liu, Y.; Li, K.; Meng, G.; Niu, K.; Zhang, W.; Tang, N.; et al. Antibiotic resistance and molecular characteristics of methicillin-resistant Staphylococcus epidermidis re-covered from hospital personnel in China. J. Glob. Antimicrob. Resist. 2020, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: Staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. Bioessays 2013, 35, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32, S114–S132. [Google Scholar] [PubMed]
- Gaisford, W.C.; Reynolds, P.E. Methicillin resistance in Staphylococcus epidermidis Relationship between the additional penicillin-binding protein and an attachment transpeptidase. Eur. J. Biochem. 1989, 185, 211–218. [Google Scholar] [CrossRef]
- Mariana, G.P.; Hermínia de, L.; Alexander, T. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. USA 2001, 98, 10886–10891. [Google Scholar]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial bio-films. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Liu, L.G.; Zhu, Y.L.; Hu, L.F.; Cheng, J.; Ye, Y.; Li, J.B. Comparative study of the mutant prevention concentrations of vancomycin alone and in combination with levofloxacin, rifampicin and fosfomycin against methicillin-resistant Staphylococcus epidermidis. J. Antibiot. 2013, 66, 709–712. [Google Scholar] [CrossRef]
- Baldi, A. Computational Approaches for Drug Design and Discovery: An Overview. Syst. Rev. Pharm. 2010, 1, 99. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Bouamrane, S.; Khaldan, A.; Hajji, H.; El-mernissi, R.; Maghat, H.; Ajana, M.A.; Sbai, A.; Bouachrine, M.; Lakhlifi, T. 3D-QSAR, molecular docking, molecular dynamic simulation, and ADMET study of bioactive compounds against candida albicans. Mor. J. Chem. 2022, 10, 523–541. [Google Scholar]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Lahyaoui, M.; El-Idrissi, H.; Saffaj, T.; Ihssane, B.; Saffaj, N.; Mamouni, R.; Rodi, Y.K. QSAR modeling, molecular docking and molecular dynamic simulation of phosphorus-substituted quinoline derivatives as topoisomerase I inhibitors. Arab. J. Chem. 2023, 16, 104783. [Google Scholar] [CrossRef]
- Priyanka, B.; Emanuel, K.; Mathias, D.; Robert, P. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, 513–520. [Google Scholar]
- Wendakoon, C.; Calderon, P.; Gagnon, D. Evaluation of selected medicinal plants extracted in different ethanol concentrations for antibacterial activity against human pathogens. J. Med. Act. Plants 2012, 1, 60–68. [Google Scholar]
- Alipour, M.; Khanmohammadi, O. Antibacterial activity of plant extracts against oral and skin pathogens. Afr. J. Microbiol. Res. 2011, 5, 2909–2911. [Google Scholar]
- Ahmad, S.; Rahman, H.; Mumtaz, S.; Qasim, M.; Rahman, Z.U.; Alsuwat, M.A.; Halawani, I.F.; Alzahrani, F.M.; Ali, S. mecA and fdh: Markers of pathogenicity and commensalism in Staphylococcus epidermidis of pediatric origin from Pakistan. Diagn. Microbiol. Infect. Dis. 2024, 108, 116109. [Google Scholar] [CrossRef]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M.M. Single disc antibiotic sensitivity testing of Staphylococci. Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Anti-Microbial Susceptibility Testing, 30th ed.; M100; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Azarm, A.; Ayoobi, F.; Zare-Bidaki, M.; Taheri, M.; Zarandi, E.R. Antibacterial and antibiofilm activities of Tribulus terrestris methanolic extract against Streptococcus mutans, Streptococcus sobrinus, and Lactobacillus acidophilus: An in vitro study. Dent. Res. J. 2024, 21, 57. [Google Scholar] [CrossRef]
- Khan, I.; Rahman, H.; Abd El-Salam, N.M.; Tawab, A.; Hussain, A.; Khan, T.A.; Khan, U.A.; Qasim, M.; Adnan, M.; Azizullah, A. Punica granatum peel extracts: HPLC fractionation and LC MS analysis to quest compounds having activity against multidrug resistant bacteria. BMC Complement. Altern. Med. 2017, 17, 247. [Google Scholar] [CrossRef]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity. Separations 2022, 9, 383. [Google Scholar] [CrossRef]
- Amani, A.T.; Tahany, A.T.; Zahraa, A.E.; Al, N.; ZIinah, A.K. Phytochemical Investigation and GC-MS analysis of Tribulus terrestris L. cultivated in Iraq. OBAT J. Ris. Ilmu Farm. Dan. Kesehat. 2024, 2, 22–29. [Google Scholar] [CrossRef]
- Stefanescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.-E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Rhetso, T.; Shubharani, R.; Roopa, M.S.; Sivaram, V. Chemical constituents, antioxidant, and antimicrobial activity of Allium chinense G. Don. Futur. J. Pharm. Sci. 2020, 6, 102. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Godin, A.M.; Melo, I.S.F.; Coura, G.M.E.; Matsui, T.C.; Dutra, M.B.; Brito, A.M.S.; Canhestro, W.G.; Alves, R.J.; Araujo, D.P.; et al. The phthalimide analogues N-3-hydroxypropylphthalimide and N-carboxymethyl-3-nitrophthalimide exhibit activity in experimental models of inflammatory and neuropathic pain. Pharmacol. Rep. 2019, 71, 1177–1183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).