Abstract
Tropical almond tree (Terminalia catappa L.), belonging to the Combretaceae family, is an unfurling tree with different edible parts. This review discussed the nutritional content, ethnopharmacological applications, main bioactive components, biological effects and economic potential of T. catappa. T. catappa shows essential applications in medicine, cosmetics and pharmaceutics. The nutritional values of T. catappa are associated with its contents of carbohydrates, minerals, proteins, lipids, vitamins and amino acids. It is used in many ethnopharmacological applications, including a heart stimulator, anti-diarrhoeal, bactericidal, anti-parasitic and anti-stress. T. catappa is used to treat angina pectoris, asthma attacks and bronchitis. The main reported biological activities for T. catappa were antioxidant, antidiabetic, anti-atherosclerosis, antitumor, antimicrobial, anthelmintic, antimalaria, hepatoprotective, insecticidal, anti-inflammatory and antihyperlipidemic activities. The main bioactive components reported in T. catappa encompassed phenolic compounds, alkaloids, diterpenes, fatty acids, galloyl glucose and derivatives, steroids and coumarins. T. catappa shows great economic opportunities which need to be expanded and diversified, taking into account its sustainability.
1. Introduction
Africa has a rich and varied flora that has always been an inexhaustible source of culinary discoveries. Among this diversity, the tropical almond tree (Terminalia catappa), emblematic of arid lands, stands out. Belonging to the Combretaceae family, T. catappa is a fruit tree. It can reach a height of around twenty meters. T. catappa is a great and unfurling tree that normally emerges in drained and well-aerated sandy soils [1]. It propagates from the seeds and grows faster with minimum maintenance; however, the breeding and improvement lack documentation [2]. It has tiered branches with large and smooth leaves forming a bunch. The leaves are deciduous, simple, slightly oval, with a short and thick stalk. They turn from yellow to red before dropping. T. catappa loses almost all its leaves twice a year, in January–February and July–August. The fruits have an ellipsoidal form, a thin epicarp and fibrous mesocarp [3]. They turn from green to yellow or red or purple when they ripen. The smooth outer skin of the fruits covers a layer of cork-like fibers surrounding the flesh. T. catappa has a kernel containing a very pleasant almond. T. catappa is usually used for ornaments, shading and the production of biofuels [4]. It is also a good source for the production of briquettes, with excellent physical and mechanical properties [5].
In African countries and Asia, T. catappa is used for medicinal purposes [6,7]. In recent decades, an increasing interest has been shown to T. catappa due to its high content of phytochemicals and nutrients [8]. T. catappa displayed beneficial health properties, including antifungal, antioxidant, anti-inflammatory, antibacterial and nephroprotective [6,7,9,10,11]. Many phytochemicals, including phenolic acids, flavonoids, fatty acids and tannins, have been reported in various parts of T. catappa [12,13]. The kernel contains good amounts of lipids, proteins and minerals (magnesium, potassium, calcium and zinc) [14,15].
This review attempts to comprehensively analyze the phytochemical and nutrition diversities, ethnopharmacological basis, biological activities and economic potential of T. catappa L. This comprehensive review compiled all the relevant data in the period between 2000 and 2025, which dealt with the different parts of T. catappa L. and their phytochemical constituents, nutritional values and biological activities.
2. Data Collection Methodology
An online thorough literature search was conducted by combining the relevant keywords, including tropical almond tree, Terminalia catappa, traditional uses, ethnopharmacology, nutrient contents, bioactive compounds, biological activity and economic potentials. The search was carried out by using “AND”/“OR”. The search was extended from 2000 to 2025 by using PubMed, ScienceDirect, Web of Science, Google Scholar and Reaxys. The final search was performed on 23 December 2025. References were arranged using the Mendeley software (Mendeley Desktop1.19.8).
3. Traditional Uses and Ethnopharmacology
T. catappa (Figure 1) is a characteristic tree of the savannahs of West and Central Africa which has become naturalized in many tropical regions including Benin, Ghana, Nigeria, Brazil, Côte d’Ivoire, India and Thailand. T. catappa is widespread in open deciduous forests and scrubby grassy savannahs. It is often found near rivers on poorly drained clay soils. T. catappa can be propagated by its seed and by cuttings. Although its thick, corky bark makes it fairly fire-resistant, T. catappa is threatened locally by overexploitation. T. catappa nuts contain an edible kernel with a delicate flavor. They are eaten at the foot of the tree, after breaking the shell between two stones. They can also be sold dried in urban markets. These kernels can also produce drinks and roasted kernels coated in caramel [16]. Moreover, fresh or dried, the kernel can be eaten directly [17]. These kernels can also be used to produce drinks and roasted kernels coated in caramel [16].
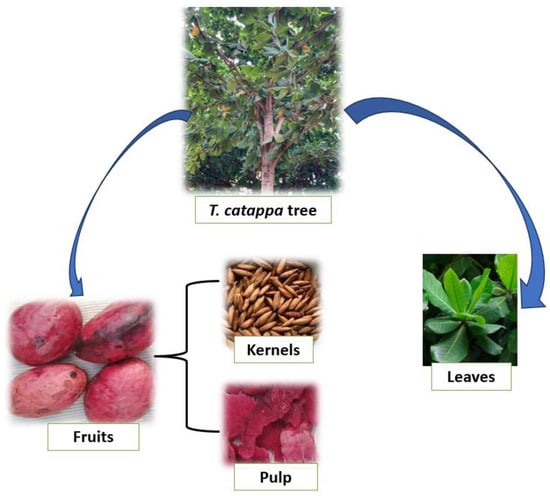
Figure 1.
T. catappa and by-products.
Recently, the WHO emphasized the importance of traditional and complementary medicine for the health of the population, as well as the increasing demand for these medical practices due to the elevated number of chronic diseases [18]. Different parts of T. catappa are traditionally used for the treatment of various illnesses [19,20]. T. catappa is widely used in traditional medicine across Africa, Asia and Latin America. Its ethnopharmacological importance is linked to the extensive use of its leaves, bark, fruits and seeds to treat various ailments. The leaf juice or ointments are applied for the relief of inflammatory and skin diseases—for leprosy, scabies, dermatitis, cutaneous infections, wounds and ulcers [21,22,23]. The bark and leaves are used for the treatment of gastrointestinal disorders such as dysentery, diarrhea, colitis, dyspepsia and intestinal parasites [22,24,25]. In folk medicine, the leaves or barks of Terminalia species are used for the treatment of skin diseases, hepatitis and other liver problems [23,24,26]. Furthermore, the bark and leaves of T. catappa are traditionally used as a heart stimulator, anti-diarrhoeal, bactericidal, anti-parasitic and anti-stress. T. catappa is used to treat angina pectoris, asthma attacks and bronchitis. The fruits or leaves are used for the treatment of metabolic and cardiovascular conditions, including diabetes, hypertension, hypercholesterolemia and cardiovascular disorders [9,12,22,27]. Moreover, in Ayurveda, T. catappa is used to cure cough, asthma, fever, rheumatism, headache, general tonic and “restoring the power of senses” [21,22,24,28].
Abiodun et al. [10] stated that T. catappa is frequently used in tropical regions as having anti-inflammatory, anti-diarrhoeal and antioxidant properties. In West African regions, many parts of the plant are commonly used in traditional medicine to treat a wide range of illnesses, including malaria, hepatitis, venereal diseases and conjunctivitis. The roots are used to treat depression, coughs, syphilis, urinary infections, female sterility, tuberculosis, snake bites and skin diseases, fever and high blood pressure. In India, T. catappa leaves are used for the treatment of many illnesses including scabies, leprosy wounds and other skin diseases. They are also traditionally used for the treatment of diarrhea and fever in many Asian regions [29]. Silva et al. [30] mentioned T. catappa as a medicinal plant from the Caribbean to treat gastritis.
4. Nutrient Contents of T. catappa
The nutritional content is one of the determining criteria to be taken into account to assess the fruit quality and its application in industry. The nutritional content of fruit is dependent on its intrinsic characteristics and the environmental conditions in which the tree grows. The nutritional content of T. catappa differed greatly from each plant organ (Table 1; Figure 2). The highest moisture content was found in the pulp (16.54%), followed by the leaves (10.6%), kernel (6.23–6.92%) and flower (5%), respectively [14,31,32,33,34]. However, the highest protein content was detected in the kernel, being 21.98–25.95%, followed by the leaves (8.73%), kernel (8.28%) and pulp (2.54%), respectively (Table 1). The carbohydrate content also varied in each plant organ, with the flower displaying the highest value (30.85%), followed by the pulp (11.27%) and kernel (6.88–7.01%), respectively (Table 1).
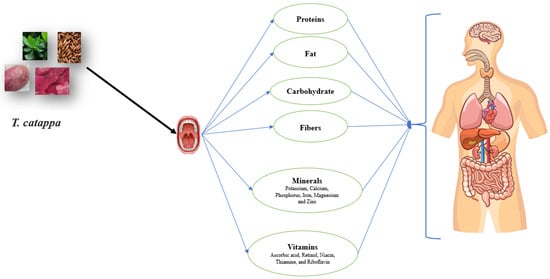
Figure 2.
Nutritional properties T. catappa by-products.
A study addressed the nutritional content of T. catappa seeds from Malaysia [33]. The seeds contained moisture of 6.23%, ash of 3.78%, lipid of 54.68%, protein of 17.66%, total dietary fiber of 9.97%, carbohydrate of 7.68, reducing sugar of 1.36% and starch of 1.22%. The seeds contained considerable amounts of amino acids, including glutamic acid (5.69 ± 1.61 g/100 g), arginine (3.95 g/100 g), aspartic acid (2.15 g/100 g), isoleucine (1.79 g/100 g), glycine acid (1.69 g/100 g), cysteine (1.40 g/100 g), leucine (1.15 g/100 g), phenylalanine (1.14 g/100 g), serine (1.12 g/100 g), threonine (1.04 g/100 g), valine (1.03 g/100 g), alanine (1.02 g/100 g) and proline (1.02 g/100 g) [33].
Dos Santos et al. [32] reported the proximate composition of T. catappa almond pulp to be moisture (16.54%), ashes (4.11%), proteins (2.54%), lipids (14.95%), carbohydrate (11.27%), total fibers (31.68%) and total energy value (268.07 Kcal/100 g). Similarly, Jahurul et al. [14] reported the nutritional contents of T. catappa kernel as moisture (6.87–6.92%), ash (4.77–4.54%), protein (22.44–21.98%), total fat (54.47–49.65%), carbohydrate (6.88–7.01%) and total fiber (5.13–5.36%). The kernel of T. catappa almond from Sri Lanka contained moisture (2.44–2.88%), protein (25.22–25.95%), fat (60.3666.25%), ash (4.03–4.30%) and total carbohydrate (1.62–6.95%) [35]. The mineral composition of the kernel of T. catappa was evaluated and it was revealed that the major minerals are potassium (6861.41–7575 mg/kg), the most abundant element, followed by calcium (2294–2687.52 mg/kg) and magnesium (2273.92–2529.37 mg/kg) [35].
Dos Santos et al. [32] reported the proximate composition of T. catappa almond pulp to be moisture (16.54%), ashes (4.11%), proteins (2.54%), lipids (14.95%), carbohydrate (11.27%), total fibers (31.68%) and total energy value (268.07 Kcal/100 g). Similarly, Jahurul et al. [14] reported the nutritional contents of T. catappa kernel as moisture (6.87–6.92%), ash (4.77–4.54%), protein (22.44–21.98%), total fat (54.47–49.65%), carbohydrate (6.88–7.01%) and total fiber (5.13–5.36%). The kernel of T. catappa almond from Sri Lanka contained moisture (2.44–2.88%), protein (25.22–25.95%), fat (60.3666.25%), ash (4.03–4.30%) and total carbohydrate (1.62–6.95%) [35]. The mineral composition of the kernel of T. catappa was evaluated and it was revealed that the major minerals are potassium (6861.41–7575 mg/kg), the most abundant element, followed by calcium (2294–2687.52 mg/kg) and magnesium (2273.92–2529.37 mg/kg) [35].
One study addressed the vitamin and mineral contents of the endocarp of T. catappa fruit from Nigeria [34]. The flour of the fruit endocarp of T. catappa contained elevated content of moisture (5.00%), ash (11.03%), crude fiber (36.33%), crude fat (8.53%), crude protein (8.28%) and carbohydrate (30.85%). Potassium and calcium were found at higher levels compared to magnesium, phosphorus and sodium, while ascorbic acid was found as the most abundant vitamin, followed by retinol, niacin, thiamine and riboflavin [34]. A study was carried out on the nutritional composition of the leaves of T. catappa from Ghana [31]. They reported dry matter of 89.4%, organic matter of 92.4%, ash of 7.63%, crude protein of 8.73% and crude fiber of 18.3%. The leaves of T. catappa contained nitrate (1710 mg/kg), nitrite (5912.5 mg/kg), sulfate (1612.5 mg/kg), phosphorous (469.77 mg/kg), copper (41.5 mg/kg), magnesium (825 mg/kg), nickel (729.5 mg/kg), iron (5 mg/kg) and ammonium (9.05 mg/kg) [25]. In short, the nutritional values of T. catappa vary greatly depending on the plant organ considered, as well as the origin of the raw material. For instance, the studied data revealed that the kernel, pulp and leaves of T. catappa are high-energy and nutrient-rich foods suitable for oil extraction and formulation of functional foods.

Table 1.
Nutrient composition of leaves, pulp, kernel, and flower of T. catappa.
Table 1.
Nutrient composition of leaves, pulp, kernel, and flower of T. catappa.
| Plant Part | Nutrient Content | References |
|---|---|---|
| Leaves | ||
| Moisture | 10.6% | [31] |
| Crude protein | 8.73% | [31] |
| Crude fibers | 18.3% | [31] |
| Ash | 7.63% | [31] |
| Phosphorous (P) | 469.77 mg/kg FW | [25] |
| Calcium (Ca) | 49,656 mg/kg DW | [36] |
| Magnesium (Mg) | 825 mg/kg FW | [25] |
| Total phenolic | 612.26 g T.A/kg DM | [37] |
| Total tannins | 586.19 g T.A/kg DM | [37] |
| Pulp | ||
| Moisture | 16.54% | [3] |
| Ash | 4.11% | [3] |
| Protein | 2.54% | [3] |
| Lipids | 14.95% | [3] |
| Carbohydrate | 11.27% | [3] |
| Starch | 19.57% | [3] |
| Total fibers (%) | 31.68% | [3] |
| Total phenolic | 361.18 g T.A/kg DM | [37] |
| Total tannins | 298.93 g T.A/kg DM | [37] |
| Kernel | ||
| Moisture | 6.23–6.92% | [14,33] |
| Protein | 21.98–25.95% | [14,33,35] |
| Starch | 1.22% | [33] |
| Total fiber | 5.13–5.36% | [14] |
| Carbohydrate | 6.88–7.01% | [14] |
| Oil content | 49.45–60.45% | [14,38] |
| Saturated fatty acids | 36.16 to 41.33% | [14,35,38] |
| Unsaturated fatty acid | 59.17% to 63.2% | [14,35,38] |
| Ash | 4.54 to 4.77% | [14] |
| Potassium (K) | 6861.41–7575 mg/kg | [35] |
| Calcium (Ca) | 2294–2687.52 mg/kg | [35] |
| Magnesium (Mg) | 2273.92–2529.37 mg/kg | [35] |
| Zinc (Zn) | 32.72–42.57 mg/kg | [35] |
| Copper (Cu) | 22.09–22.31 mg/kg | [35] |
| Manganese (Mn) | 13.91–20.95 mg/kg | [35] |
| Barium (Ba) | 10.28–10.97 mg/kg | [35] |
| Strontium (Sr) | 4.30–7.26 mg/kg | [35] |
| Boron (B) | 2.95–3.72 mg/kg | [35] |
| Cobalt (Co) | 0.34–1.08 mg/kg | [35] |
| Silver | 0.02–0.04 mg/kg | [35] |
| Total phenolic | 39.10–55.97 mg GAE/g | [14] |
| Total tannins | 282.84 g T.A/kg DM | [37] |
| Aspartic acid | 2.15 g/100 g | [33] |
| Glutamic acid | 5.69 g/100 g | [33] |
| Serine | 1.12 g/100 g | [33] |
| Glycine acid | 1.69 g/100 g | [33] |
| Histidine | 0.52 g/100 g | [33] |
| Arginine | 3.95 g/100 g | [33] |
| Threonine | 1.04 g/100 g | [33] |
| Alanine | 1.02 g/100 g | [33] |
| Proline | 1.02 g/100 g | [33] |
| Tyrosine | 0.76 g/100 g | [33] |
| Valine | 1.03 g/100 g | [33] |
| Methionine | 0.26 g/100 g | [33] |
| Cysteine | 1.40 g/100 g | [33] |
| Isoleucine | 1.79 g/100 g | [33] |
| Leucine | 1.15 g/100 g | [33] |
| Phenylalanine | 1.14 g/100 g | [33] |
| Lysine | 0.42 g/100 g | |
| Flower | ||
| Moisture | 5% | [34] |
| Crude protein | 8.28% | [34] |
| Crude fibers | 36.33% | [34] |
| Oil content | 8.53% | [34] |
| Carbohydrate | 30.85% | [34] |
| Ascorbic acid | 14.58 mg/100 g FW | [34] |
| Ash | 11% | [34] |
| Phosphorous (P) | 41.87 mg/100 g | [34] |
| Potassium (K) | 326.32 mg/100 g | [34] |
| Calcium (Ca) | 220.19 mg/100 g | [34] |
| Magnesium (Mg) | 43.66 mg/100 g | [34] |
T.A: Tannic acid, GAE: Gallic acid equivalent, DW: Dry weight, FW: Fresh weight.
5. Phytochemical Composition
5.1. Phenolics
T. catappa is a significant source of bioactive components (Figure 3) [14,39]. However, the bioactive content of T. catappa trees is subject to several considerations. The phenolic content of the kernel oil of Kota Kinabalu and Keningau cultivars was reported to be 55.97 mg GAE/g, while a total phenolic content of 39.10 mg GAE/g was found for the Keningau cultivar [14]. Hence, the higher phenolic content in the oil coincided with low rainfall in the Kota Kinabalu cultivar. In contrast, high rainfall regions may cause high oil content in T. catappa kernels [39]. In T. catappa leaves, a significant amount of alkaloids, tannins, steroids, cardiac glycosides, flavonoids, saponins and coumarins have been identified [40,41]. Although terpenoids were absent in Mwangi et al. [41], they were determined as a sesquiterpene (C15H18O3) in a previous study [42]. Uchida et al. [43] recommended the use of the water–ethanol mixtures as an effective solvent to increase the extraction yields from T catappa fruit pulp. It showed high concentrations of phenolic compounds were 2.98 mg/mL, flavonoids were 1.644 mg/g, anthocyanins were 0.505 mg/g and flavonols were 0.123 mg/g. The quantitative analysis of T. catappa leaves has significant phenolic contents in the ethanolic extract. The results of the ethanolic extract showed that the highest phenol and flavonoid content (21.56 mg GAE/g and 1.31 mg QE/g dry matter) was compared to ethanolic–water and aqueous extracts. [44] Total phenols (GAE) and flavonoids (QE) contents from the ethanol extract of leaves were found to be 354.02 and 51.67 mg/g extract. [45] Chromatograms of the hot aqueous extract (HAE) and room temperature of T. catappa leaves showed similar chemical compounds; however, higher levels of compounds were in HAE. Chemical compounds are identified as gallic acid, ellagic acid and punicalagin. [46] Moderate phenolic contents were found in both fermented and unfermented T. catappa leaves from Ondo State, Nigeria. [47] In both ethanolic extracts, saponin and p-coumaric acid are the most abundant compounds at 1009.24 mg/g–436.95 mg/g and 1666.03–552.02 mg/g, respectively. Other compounds include quercetin (53.50–217.81 mg/g) and naringenin (61.08–56.14 mg/g). Caffeic acid (2.05 mg/g) and tannic acid (5.24 mg/g) were the least concentrated in fermented and unfermented extracts, respectively. Studies showed higher phenolic contents in the unfermented T. catappa leaves; however, additional bioactive compounds were observed in the fermented type, including salicylic acid (14.59 mg/g) and apigenin (72.27 mg/g). In the unfermented and fermented T. catappa seeds, the total phenolic content, flavonoid and tannin were quantified to be 3.214–3.003 mg (GAE)/g, 0.756–0.696 mg (RE)/g, and 0.103–0.087 mg (TAE)/g have been reported, respectively [48]. T. catappa leaves are tannin-rich components, especially ellagitannins (hydrolyzable tannins), which are linked to the antitumor [26,49]. Moreover, phytochemicals present in T. catappa, such as tannins, flavonoids, gallotannins, cyanidins and ellagitannins, showed anti-HIV activity [50]. The chemical analysis of T. catappa leaves (São Luís, Brazil) detected the presence of hydrolyzable tannins (punicalin and punicaligin), gallic acid and C-flavonoid glycosides [26]. Moreover, Chen et al. [49] reported that T. catappa leaves water extract contains 21% tannin. Tannins and flavonoids, constituents of the aqueous leaf extract of T. catappa, were 0.32 ± 0.00 and 1.68%, respectively [51]. The percentage of tannins (0.11%) is found in the gum of T. catappa (Almendron, in Venezuela) [52]. Raw T. catappa nut demonstrated tannins with 27.13 μg/g, which was better than roasted nuts [8]. In T. catappa fruit (Nigeria), the pericarp was found rich with phenol (149.33 GAE/100 g), while the seed was saponin (84.15 mg/100 g) [53]. Study on T. catappa bark (Karnataka, India) highlights its potential as a source of bioactive compounds [54]. Alcoholic extract (TCE) and water extract (TCW) were prepared. The TPC of both extracts was found to be 287 and 175 mg/g, respectively. Thus, the TFC was calculated as 10.2 and 61.7 mg QE/kg for TCE and TCW, respectively. T. catappa can well compete with P. amygdalus in terms of bioactive compounds. P. amygdalus (sweet almond) was found to be higher in phytochemicals than T. catappa (tropical almond). In sweet almonds, tannin, phenols, flavonoids and saponins were 748.49, 1781.50, 456.38 and 158.70 μg/g, respectively. In tropical almonds they were 388.95, 410.83, 73.28 and 86.32 μg/g, respectively [55]. The differences are attributed to several factors, such as drying, climatic conditions and other processing methods.
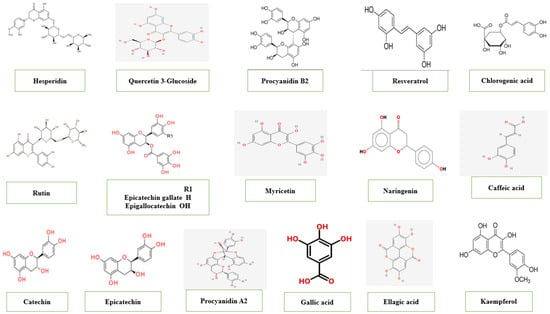
Figure 3.
Major phenolic compounds of T. catappa.
5.2. Alkaloids
T. catappa can well compete with P. amygdalus in terms of phytochemicals such as alkaloids. In both tropical almonds and sweet almonds, no significant differences were found (p > 0.05). Alkaloids were found to be 210.65 μg/g and 240.11 μg/g in tropical almond and sweet almond, respectively [55]. In the aqueous leaf extract of T. catappa, the alkaloid constituent was 0.37% [51]. Nuts of both the yellow and red varieties of T. catappa were investigated. Results indicated that alkaloids are present in the red variety, such as anthraquinones, while terpenoids and steroids are in the yellow variety [56]. T. catappa nuts presented an abundance of alkaloids such as ribalinidine, quinine and sparteine, with anti-malaria and antioxidant potential [8]. Raw T. catappa nut demonstrated quinine, ribalinidine and spartein with 7.18, 45.57 and 7.33 μg/g, which was better than roasted nuts. In T. catappa fruit (Nigeria), alkaloids were present in the pericarp (34.42 mg/100 g), but not found in the seed. [16] Moreover, glycoside and phlobatannin were found to be absent in the seed. T. catappa was reported to contain alkaloids with pharmacological activities such as antimicrobial activity [41,57]. It is able to interchelate with the DNA of both Gram-negative and Gram-positive bacteria [58]. Alkaloid in unfermented and fermented T. catappa seeds was determined to be 24.51% and 20.61%, respectively [48].
5.3. Fatty Acids
One more important part of T. catappa is its kernel. T. catappa kernel has a relatively high oil yield. The kernel oil of T. catappa is commonly referred to as T. catappa kernel oil (TCKO). TCKO yield ranges from 49 to 65% (Table 2) [59,60]. Compared to other commercial oil sources, TCKO yield is higher than cottonseed and soybean [2]. In addition to seeds containing high levels of oil content (600 g/kg), it has the ideal ratio of fatty acids as recommended by dietary guidelines [61]. Despite this importance, it is still considered underutilized. Therefore, it can be used as one potential alternative to some conventional oils in countries where it is available. Several factors play a role in oil yields, including (1) territorial origin, (2) soil nature, (3) harvest time, (4) extraction processing, (5) the age of the tree and fruit maturity or (6) precipitation rate [39,62]. Although Mbah et al. [63] indicated that the fat of the sample did not change concerning the process and location, Jahurul et al. [14] confirmed that the total oil content of T. catappa kernel cultivated from Kota Kinabalu was higher than that of Keningau. Table 2. represents the fatty acid composition of different T. catappa kernel oil. The oil extraction yield in T. catappa (Sabah, Malaysia) ranges from 49.45 to 54.47%. Moreover, the result showed that free fatty acid values are quantified to be 1.17 and 2.42% [14]. Soxhlet extraction of T. catappa kernel oil yielded higher than the maceration methods. The result showed that the major SFAs and MUFAs in T. catappa kernel oil are palmitic acid (C16:0) and oleic acid (C18:1). A study by Iha et al. [59] quantified total saturated fatty acid and total unsaturated fatty acid in T. catappa kernel to be 34.2% and 64.5%, respectively. In the Brazilian T. catappa, castanhola (60%) was found as the predominant fatty acids [64]. Moreover, within unsaturated fatty acids in the T. catappa fruit seed oils, oleic and linoleic acids were predominant [65]. The oleic and linoleic acids together account for 53.47% of T. catappa seed oil [66]. Cuticular waxes in T. catappa leaves include mainly fatty acids [67]. In Malaysian-grown tropical almond nuts, the seed oil content was determined to be 54.68% [33]. Fatty acid composition of Nigerian-grown tropical almonds was 43.71% [55]. GC-MS analysis of n-hexane extract of T. catappa nuts from Nigeria revealed the presence of several notable bioactive compounds. The most abundant component identified was cis-vaccenic acid (24.493%). Overall, fat represented 56.71% of the nut composition [68]. Results of T. catappa seeds (Malaysia) revealed that palmitic acid and oleic acid were the major saturated and unsaturated fatty acids, respectively [33]. The proximate analysis found that the crude fat content of plant-based milk using local almond nuts was higher than that of cow’s milk [69].

Table 2.
Fatty acid composition of different sources T. catappa kernel oil.
Table 2.
Fatty acid composition of different sources T. catappa kernel oil.
| Fatty Acids | Origins | ||||||
|---|---|---|---|---|---|---|---|
| Nigeria | Brazil | Malaysia | Nigeria | Malaysia | Brazil | Ivory Coast | |
| Lauric acid | 0.94 | - | - | - | - | - | |
| Myristic acid | 0.54 | 0.10 | 0.08 | - | 0.09 | - | 0.17 |
| Palmitic acid | 36.01 | 28.30 | 31.32 | 34.82 | 28.98 | 35.0 | 36.20 |
| Palmitoleic acid | - | 0.90 | 0.31 | 0.34 | 0.41 | ||
| Stearic acid | 6.4 | 4.90 | 5.17 | 6.79 | 7.23 | 5.0 | 4.02 |
| Oleic acid | 33.25 | 30.00 | 28.62 | 30.13 | 39.28 | 32.0 | 27.97–32.40 |
| Linoleic acid | 22.26 | 32.80 | 32.25 | 23.44 | 23.01 | 28.0 | 24.65–31.67 |
| Linolenic acid | 0.59 | 1.70 | 0.09 | - | 0.07 | 0.55 | |
| Saturated fatty acids (%) | 43.89 | 34.20 | 37.57 | 43.92 | - | - | 40.22–42.98 |
| Mono-unsaturated fatty acid (%) | 33.25 | 30.00 | 29.03 | - | - | - | 27.97–32.81 |
| Polyunsaturated fatty acid (%) | 22.85 | 34.50 | 32.34 | - | - | - | 25.20–32.17 |
| Unsaturated fatty acids (%) | 56.10 | 74.50 | 61.37 | 56.08 | - | - | - |
| References | [38] | [59] | [14] | [66] | [33] | [64] | [65] |
5.4. Volatile Constituents and Essential Oil Compositions
Essential oils are vital ingredients of the tropical almond tree. They are abundantly present in the leaves, barks, seeds and nuts of the T. catappa. The investigation of T. catappa leaves essential oil via hydrodistillation and GC/MS analysis revealed the presence of volatile components. Ogunmoye et al. [70] detected 40, of which the most abundant were identified as hexahydrofarnesyl acetone (12.34%), 1,3,8-p-menthatriene (9.38%) and 1,2-dimethyl-cyclooctene (7.30%). Another study with the same approach found that (Z)-phytol (41.2%), palmitic acid (11.0%) and (E)-nerolidol (4.7%) were the main identified components [71]. In fruit oil extraction, α-farnesene (21.3%), octadecane (11.7%) and palmitic acid (9.5%) were the major constituents [72]. Hydrocarbons (21.61%) were identified as an abundant class of compounds in T. catappa air-dried leaves oil [70]. Twelve volatile constituents in almond methanol leaf extract were detected; the main identified component was 9, 17-Octadecadienal-Z [73]. DL-α-tocopherol (28.67%), phytol (23.30%), squalene (14.83%) and β-sitosterol (13.92%) were identified in the ethanolic leaf extract of T. catappa through GC-MS analysis [74]. By employing GC-MS, palmitic acid (33.2%), linoleoyl chloride (29.1%) and pentadecanolide (16.2%) were revealed as the main components in T. catappa nuts [75]. A total of 21 volatile compounds in T. catappa seed oil were identified. The n-Hexadecanoic acid (17.96%), oleic acid (22.42%) and 2,9-octadecadienoic acid (22.82%) were detected [76]. Twenty phytochemicals could be identified in both raw and roasted tropical almond nuts from Nigeria. Upon roasting, results showed that quinine, ribalinidine, sapogenin, flavan-3-ol and tannin were reduced, whereas catechin seemed enhanced [8]. Ripe fruits of T. catappa in Brazil were screened for volatile constituents using GC-MS profiling. Results showed that the majority were phenols (50%), furan derivatives and cyclic ketones, with a minor amount of alcohols and esters [77].
5.5. Triterpenoid
Triterpenoids are organic compounds of 30 carbon atoms, which are formed by the polymerization of six isoprene units, and are widely found in nature in different chemical structures [78]. T. catappa L. contains triterpenoids in various structures including ursolic acid and asiatic acid. These compounds have demonstrated hepatoprotective properties through the protection of liver mitochondria and the scavenging action on free radicals [79]. Generally, terpenoid compounds are commonly reported in the leaves, seed and fruit pulp of T. catappa at a concentration of up to 0.22–0.31 mg/g in the fruits [80]. Common terpenoid compounds reported in this plant include oleanolic acid, 2-alpha, 3-beta, 23-trihydroxyurs-12-en-28-oic acid (DHUA), taraxerol, Ssqualene, sesquiterpenes and loliolide have been isolated in T. catappa [42,81,82].
5.6. Lignan Glucoside
Lignan glucosides are lignan compounds that are glycosylated (i.e., bound to sugar molecules, typically glucose). They are a class of polyphenolic compounds found in plants, particularly in seeds, fruits and bark steam, and are known for their antioxidant, anti-inflammatory and estrogenic properties [83]. Although T. catappa is known to contain various bioactive compounds, only a few studies reported the presence of specific lignans in this plant. Sowmya and Raveesha [84] reported the (8R, 8R) Secoisorlariciresinol 9-glucoside as a specific lignan glucoside in the leaves of T. catappa. Moreover, lignan glucosides are identified in other members of the Terminalia genus such as T. chebula var., which showed the presence of termitomenins F and G and T. citrina containing tomentella and terminalosides [85,86].
5.7. Coumarins
Coumarins are a group of aromatic compounds with divers’ structure, found in various plants. These compounds are known for their potential therapeutic properties, including anti-inflammatory and anticoagulant effects [87]. Limited studies have reported coumarins in T. catappa. Those few studies include Mwangi et al. [41], who reported coumarins in T. catappa with demonstrated antimicrobial and antioxidant properties.
5.8. Steroids
Steroids are organic compounds with a characteristic structure of four carbon rings called the steroid nucleus and play vital roles in biological processes in both plants and animals [88]. Several studies reported the presence of steroids in T. catappa at a concentration up to 8.07–6.24 3 mg/g reported in the fruits [41,80,89]. Such steroidal compounds include stigmastane-3, 6-diol identified in the stem barks and demonstrating antibacterial, antifungal, cytotoxic and antioxidant activities [82], and estrogenic steroids including estrone, estriol, equilin and equilin Sulfate (Figure 4) [82,90].
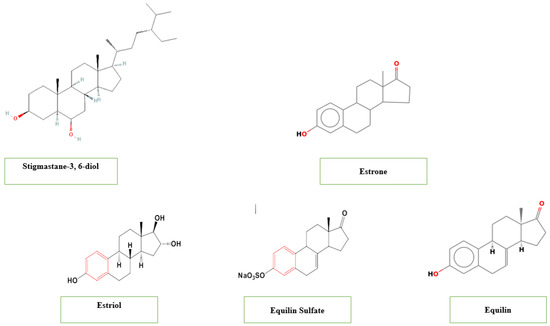
Figure 4.
Steroids contained in T. catappa.
5.9. Polysaccharides (Galloyl Glucose and Derivatives)
Galloyl glucose and derivatives are a type of hydrolyzable tannin formed by the esterification of gallic acid with glucose, reported to be present in some spices of the Terminalia genus, including T. myriocarpa, T. bellirica, T. chebula and T. arjuna. [91] The galloyl glucose and derivatives reported in T. catappa include 2,6-digalloylglucose, 1,3,6-trigalloyl glucose, gallicacid3-O-6 galloyl glucoside and 1-O-galloylfructose. [84] These compounds were reported to exhibit antimicrobial activity with the potential to inhibit multidrug-resistant bacteria such as Staphylococcus aureus, and fungi such as Trichophyton rubrum and Candida spp., which are commonly detected in fermented foods. [84,92]
6. Bioactivities
6.1. Antioxidant Activity
The seeds, leaves, fruits, stems and bark of T. catappa are used for medicinal purposes, offering biological properties and high levels of polyphenolic compounds [93,94] (Table 3). Among the ethanolic extract from T. Catappa ripped fruit and leaves, the latter exhibited the highest levels of DPPH radical scavenging activity with an IC50 value of 43.34 µg/mL and reducing power potential, 2512.89 mM Fe (II)/g, respectively [95]. The significantly higher antioxidant properties in almond leaves, attributed to their rich content of flavonoids and tannins, suggest their more significant potential for managing oxidative stress and neurodegenerative conditions [94]. The antioxidant activity of T. catappa fruit flour (TCF) was measured as 13.06 μmol TEAC/g and 2.07 μmol TEAC/g using the ABTS and the FRAP method, respectively [6]. TCF, a source of polyphenolic compounds such as hesperidin, demonstrated activity against DPPH radicals [96]. Overall, antioxidant activity is associated with phenolic compounds such as rutin, quercetin and kaempferol [97,98,99]. Various studies on the antioxidant activity of oils have shown a strong linear correlation between phenolic content and antioxidant activity. Therefore, the high TPC values of T. catappa kernel oil (TCKO) from the Kota Kinabalu cultivar indicate its strong antioxidant activity [14]. Despite exhibiting comparable antioxidant properties, T. catappa remains an untapped and underutilized tree [2]. The GC-MS profiles of raw and roasted nuts revealed a promising abundance of naringin and flavan-3-ol, both known for their antioxidant potential [8]. Research suggests that polyphenols are the most abundant dietary antioxidants, contributing up to 90% of the total antioxidant capacity in most fruits and vegetables [100]. Numerous pharmacological studies have confirmed the antidiabetic and antioxidant activities of T. catappa, supporting its traditional uses [21,100]. T. catappa L. kernel oil from the purple oil variety exhibited notable antioxidant activity (38.6 ± 2.2 μg TE/g), as determined by the TEAC assay [3]. The ethanolic leaf extract of T. catappa exhibits anti-tumor activity with high antioxidant levels, likely attributed to its phenolic and flavonoid components [45]. Chakkalakal et al. [101] stated that tannin components from Indian almonds possess multiple antioxidant effects, capable of preventing lipid peroxidation (LPO) and superoxide formation. The methanol leaf extract of T. catappa was used to synthesize ZnO-NPs [73]. Both the extract and ZnO-NPs exhibited antioxidant scavenging activity, with the T. catappa extract showing better DPPH radical scavenging properties than the ZnO-NPs.
6.2. Antidiabetic Effect
Diabetes mellitus is a major global cause of mortality linked to hyperglycemia-induced blood disorders and thromboembolic risks [102]. T. catappa has a traditional use in both food and medicine [9]. All parts of Terminalia catappa are used in traditional medicine [103]. The seed kernel of T. catappa is nutrient-rich and can serve as a raw material for developing foods for diabetic patients [35]. Flavonoids and tannins in T. catappa seeds may enhance their anti-diabetic effects by (1) acting as antioxidants to reduce cellular damage [104], (2) improving insulin action, and (3) modulating glucose metabolism pathways [105]. Iheagwam et al. [106] investigated the effect of the aqueous extract of T. catappa leaf on hematological and coagulation disturbances in type 2 diabetic rats. The findings suggest it may reverse diabetes-related blood anomalies via anticoagulant and anti-anemic effects. T. catappa leaf aqueous extract suggests activating the Nrf-2 gene, an antioxidant-related gene [107]. As a result, hyperglycemia-induced oxidative stress and inflammation were reduced. Chinaka et al. [108] suggest that cis-9-hexadecanoic acid in T. catappa may enhance insulin sensitivity and prevent the destruction of insulin-secreting pancreatic beta cells. The extract powder of Vietnamese T. catappa leaves effectively inhibits α-glucosidase activity, making it a potential natural anti-diabetic agent [100]. GC-MS and FT-IR analysis of the n-hexane fraction identified compounds like eugenol, urs-12-en-24-oic acid, and squalene as potential antidiabetic agents, suggesting that T. catappa leaf extract contains α-glucosidase inhibitors and is a valuable source for natural anti-diabetic agents [109]. Despite its anti-diabetic and anti-inflammatory benefits, the aqueous extract of Terminalia catappa leaves may negatively affect male reproductive functions, altering sperm indices and hormones [110]. Because of its established therapeutic potential, it should be used with caution.
6.3. Anti-Inflammatory Effect
Rheumatoid arthritis, a chronic inflammatory autoimmune disorder, is characterized by ongoing synovial inflammation, bone and cartilage degradation, and joint destruction [111]. One of the factors contributing to rheumatoid arthritis is the protein. In vivo protein denaturation may be the cause of auto-antigen production in several rheumatic disorders. Electrostatic, hydrogen, hydrophobic and disulfide bonding changes are most likely part of the denaturation mechanism. Kumar et al. [112] in their study investigated the anti-inflammatory activity of T. catappa leaves aqueous extract. Data showed that the aqueous extract from T. catappa leaves can regulate the synthesis of auto-antigens caused by the in vivo denaturation of proteins in rheumatic disorders. Results thus support the importance of T. catappa leaves in the prevention and treatment of inflammatory disorders such as arthritis [112]. Likewise, protease inhibitory activity is another mechanism that the aqueous extract of T. catappa leaves exhibits anti-inflammatory properties. Synovial fibroblasts release proteases as the pannus spreads into adjacent bone and cartilage. These enzymes actively break down the collagen and proteoglycan matrix, contributing to the degradation of bone and cartilage [113]. Kumar et al. [112] demonstrated that the aqueous extract of T. catappa leaves exhibits significant anti-proteinase activity. Data showed that the aqueous extract of T. catappa leaves inhibited trypsin activity with IC50 values of 384.02 μg/mL. Human trypsin is activated in some types of rheumatoid arthritis, according to previous research [113].
6.4. Hepatoprotective Effect
The hepatoprotective properties of the T. catappa from Gwadar were examined and the results showed that the fruit part had hepatoproective effect [94]. In another study [94] investigating the hepatoprotective effect of T. catappa, the ethanol extract from the leaves helps mice whose livers have been acutely damaged by carbon tetrachloride (CCl4). Mice that received oral doses of 10 or 30 mg/kg before CCl4 injection showed a protective effect. The restoration of the altered activities of serum aspartate aminotransferase and sALT, which are markers of liver injury, showed a protective impact. Additionally, the study examined how the liver tissues of mice exposed to CCl4-induced hepatotoxicity changed in terms of the amount of IL-6 that appeared. The study’s conclusions suggest that the leaf extract’s protective effect against CCl4-induced acute liver damage in mice may be related to its ability to inhibit IL-6 overexpression in liver tissue [94].
6.5. Antimicrobial Activity
T. catappa has been shown to possess a range of pharmacological properties, including antibacterial properties [94]. Kumar et al. [112] investigated the antibacterial potential of T. catappa leaves aqueous extract using the agar well diffusion method against Gram-negative and Gram-positive bacteria. Generally, the extract exhibited a wide range of antimicrobial activity against every bacterium examined in this study, with notable differences noted between Gram-positive bacteria (Staphylococcus and Bacillus species), which are known to be more sensitive than Gram-negative bacteria (E. coli, Klebsiella species and Pseudomonas species). The greatest inhibitory impact was for Staphylococcus species in the Gram-positive group, and for E. coli in the Gram-negative group, with the zone of inhibition 12.0 mm and 9.5, respectively. However, among the bacteria that were studied, Pseudomonas spp. was shown to be the most resistant (5.5 mm) [112]. Other studies also showed that leaves and fruit extract have antibacterial properties against Salmonella, Shigella, Enterococci and Corynebacteria [94].
6.6. Anti-Atherosclerosis and Anti-Hyperlipidemia
Atherosclerosis is a condition where lipids build up in the arteries. Angina pectoris, myocardial infarction and stroke are the main side effects of atherosclerosis [114], whereas hyperlipidemia represents the early development of atherosclerosis and its cardiovascular complications [115]. The fruit of T. catappa demonstrated anti-atherosclerotic, anti-hyperlipidemic and hypolipidemic properties in the in vivo atherosclerosis model on Wistar rats [80]. According to the phytochemical screening, the ethanol and aqueous extracts of T. catappa fruit showed the presence of alkaloids, flavonoids, tannins, saponins, cardiac glycosides, phenols, terpenoids, anthraquinones and steroids. Tannins found in T. catappa act as anti-inflammatory agents, lowering the risk of atherosclerosis [80,116]. The findings of Tabansi et al. [80] demonstrated that in comparison to the healthy control group, which included treatment with T. catappa fruit extracts, the activity of alanine transaminase (ALT), aspartate transaminase (AST) and creatine kinase (CK) was considerably elevated under atherogenic settings. Data indicated that atherosclerosis had a regression impact after six weeks of T. catappa treatment. As well, T. catappa aqueous extract significantly decreased the activity of lactate dehydrogenase (LDH) when compared to atherogenic control. Likewise, the same study demonstrated that T. catappa fruit considerably reduced low-density lipoprotein (LDL) (200 and 300 mg/kg), indicating its effectiveness in blocking atherogenic pathways.
The antihyperlipidemic activity of the T. catappa fruit could be attributed to the significant amount of saponins in the extracts [80]. Saponins have antihyperlipidemic effects through their ability to interact with cholesterol in the intestinal lumen and reduce its absorption. The conversion of cholesterol to sterols by fecal secretion is accelerated by decreased bile acids in their extrahepatic circulation [117]. The in vivo treatment of T. catappa extract resulted in better histopathology, lipid profile, enzyme biomarkers, cardioprotective and atherogenic index [80].
6.7. Antitumor
The anti-tumorogenic activity of T. catappa is due to the free radical quenching property of its phytoconstituents [2]. T. catappa exhibited an antitumor effect by modulating lipid peroxidation and augmenting antioxidant defense systems in Ehrlich ascites carcinoma-bearing mice [45]. In experimental oncology, Ehrlich’s tumor is typically employed to assess the antitumoral activity of various natural compounds or for insight into the therapeutic potential of various synthesized chemotherapeutic drugs [118]. According to the result, the phenolic and flavonoid components in this extract may be responsible for antitumor activity [45].
6.8. Anthelmintic Activity
Paramphistomes are the cause of paramphistomosis, a serious helminthic disease that affects ruminants’ reticulums and rumens. Due to severe acute gastroenteritis, ulcerative ruminitis, dehydration, poor digestion, decreased nutrition conversion and decreased milk and meat production, the disease results in significant economic losses and a high host mortality rate, especially in young animals [119].
Minsakorn et al. [119] investigated the anthelmintic effect and bioassay-guided fractionation of T. catappa plant extracts against G. Crumenifer parasite. The results showed that the adult G. crumenifer could be suppressed by T. catappa leaf water extract (12–90% mortality); however, after 24 h of incubation, T. catappa leaf n-butanal extract demonstrated 100% mortality at 1000 μg/mL. As well, the anthelmintic activity of the T. catappa plant has been reported recently in a published study [94].
6.9. Antimalarial Activity
Malaria is one of the most deadly parasitic infectious diseases, which is caused by Plasmodium protozoa, and in many tropical places, notably West African nations, it is one of the main causes of mortality [120]. In 2023, there were an anticipated 263 million cases and 597,000 malaria deaths globally, according to the World Health Organization’s most recent World Malaria Report [121].
Meanwhile, specific recent studies directly assessing the antimalarial activity of T. catappa were not identified. The plant is well-known for having a high concentration of flavonoids and phenolic chemicals, [2] which have been linked to antimalarial qualities in other plant species. For instance, Terminalia mantaly H. Perrier belongs to the same family of T. catappa, which is Combretaceae, [2120], and has been reported to have antimalarial properties against in vitro β-hematin synthesis. Therefore, it is suggested that T. catappa could have antimalarial activities, and further research is needed to explore this potential.
In short, the bioactivity of T. catappa is largely attributed to its high concentration of bioactive compounds, which explains its potent antioxidant, antimalarial, antimicrobial, antidiabetic, hepatoprotective, anti-atherosclerosis and anti-inflammatory properties. The phytochemical and bioactivity properties richness make T. catappa a promising source for functional foods, nutraceuticals, pharmaceuticals and cosmetic studies.

Table 3.
Biological properties of different parts of T. catappa.
Table 3.
Biological properties of different parts of T. catappa.
| Biological Activity | Plant Organs | Main Findings | Reference(s) |
|---|---|---|---|
| Antioxidant activity | Seeds | -The low peroxide value of the oil confirmed the presence of antioxidants in the seed oil. -T. catappa kernel flour showed significant antioxidant properties. | [105,122] |
| Nuts | Adding almond nut flour to kunu beverage improved its antioxidant properties. | [123] | |
| Antidiabetic activity | Leaf | High antidiabetic activities were related to higher phenolic constituents of almond leaf. | [124] |
| Seed | -Modulation of lipid profile. -LDL reduction. | [125] | |
| Fruit | Methanolic and aqueous extracts exhibited significant antihyperglycemic activities. | [126] | |
| Anti-atherosclerotic, Anti-hyperlipidemic | Fruit | -Tannins found in T. catappa act as anti-inflammatory agents, lowering the risk of atherosclerosis. -Reduced low-density lipoprotein (LDL) (200 and 300 mg/kg). | [80] |
| Antitumor activity | Leaves | T. catappa exhibited antitumor effect by modulating lipid peroxidation and augmenting antioxidant defense systems in Ehrlich ascites carcinoma bearing mice. | [2,45] |
| Anthelmintic activity | Leaves | -G. crumenifer parasite could be suppressed by T. catappa leaf water extract (12–90% mortality). | [94] |
| Anti-inflammatory activity | Leaves | -T. catappa leaves can regulate the synthesis of auto-antigens caused by the in vivo denaturation of proteins in rheumatic disorders. -Showed protease inhibitory activity. | [112] |
| Leaves and fruit | -Antibacterial properties against Staphylococcus, Bacillus spp. E. coli, Klebsiella spps, Pseudomonas salmonella, shigella, enterococci and corynebacteria. | [94,112] | |
| Hepatoprotective effect | Leaves and fruit | T. Catappa, the ethanol extract from the leaves helps mice whose livers have been acutely damaged by carbon tetrachloride (CCl4). | [94] |
| Antimicrobial activity | Leaves | -Gram-positive bacteria (Staphylococcus and Bacillus species) are more sensitive than Gram-negative bacteria (E. coli, Klebsiella species and Pseudomonas species). | [94,112] |
7. Economic Potentials
The global market for products derived from T. catappa is growing significantly, with important economic implications at various levels. The global market for products derived from T. catappa was valued at 450 million USD in 2023, with growth projected to 750 million USD by 2028 [82]. As estimated global market value, the market segments break down as follows: (i) Pharmaceuticals: 45% (202.5 million USD), (ii) Cosmetics: 30% (135 million USD), (iii) Food products: 15% (67.5 million USD) and (iv) Environmental applications: 10% (45 million USD). Trade in T. catappa and its derivatives show positive trends, with global exports of up to 280 million USD (2023). The main exporting countries are India (35%), Indonesia (25%) and the Philippines (15%), with countries such as the United States (30%), the European Union (25%) and Japan (20%). T. catappa harvesting generates significant income for local communities, with an average income estimated at between 1200 and 2500 USD/year per grower. Around 50,000 direct jobs have been created in producing countries, and the activity contributes between 0.5 and 1.2% to the agricultural GDP of the main producing regions. In Indonesia, for example, processing cooperatives saw their revenues increase by 35% between 2020 and 2023 thanks to the valorization of by-products [9].
8. Challenges and Prospects
8.1. Current Challenges
Standardization and quality control are major obstacles in the exploitation of T. catappa. Recent studies have highlighted significant variations in the concentration of bioactive compounds, with fluctuations of up to 25–40% depending on geographical region and harvesting conditions. This variability poses considerable challenges for the standardization of extracts and their use in commercial formulations. The lack of standardized extraction protocols also represents a major constraint, with efficiency rates varying considerably between 45 and 85% depending on the methods used [127].
In economic terms, the current market structure presents a number of limitations. Production costs absorb a significant proportion of revenues, representing between 30 and 40% of total sales. Access to international markets remains restricted for many producers, particularly in developing countries. Price volatility, characterized by variations of up to 25% over twelve months, creates additional uncertainty for industry players [9].
Environmental issues also represent a major challenge. Growing pressure on natural resources, exacerbated by increasing global demand, raises questions about the long-term sustainability of T. catappa farming. The impacts of climate change on production are beginning to show, affecting growth cycles and the quality of bioactive compounds. The need to adopt more sustainable practices is therefore becoming imperative to ensure the sustainability of the resource.
8.2. Prospects
Recent technological advances open up promising prospects for the development of new pharmaceutical formulations. Ongoing research into innovative extraction methods, notably the use of ultrasound and microwaves, holds out the prospect of significant improvements in extract yields and quality. Developments in biotechnology, particularly in the field of personalized medicine, offer new opportunities for exploiting the therapeutic properties of T. catappa [128]. Commercial expansion is a major development focus. Current research into by-product valorization is paving the way for new industrial applications. The development of high-value-added products, combined with certification and labeling strategies, could lead to greater penetration of international markets. This diversification of commercial applications is accompanied by innovations in processing and packaging, aimed at improving the stability and bioavailability of active compounds.
The socio-economic dimension also offers encouraging prospects. The development of the T. catappa sector represents a significant opportunity for job creation along the entire value chain. The emergence of local entrepreneurial models, supported by training programs and technical support, could help to structure the sector. The expected increase in exports, combined with a better valuation of products on international markets, suggests a positive impact on local economies.
Environmental sustainability is becoming a key strategic focus. Initiatives for the conservation of genetic resources and the implementation of sustainable agricultural practices bear witness to a growing awareness of environmental issues. The development of more environmentally friendly production methods, including the optimization of extraction processes and the reduction of carbon footprints, is becoming a priority to ensure the long-term viability of the sector. An integrated framework for the sustainable development of T. catappa should be explored (Figure 5).
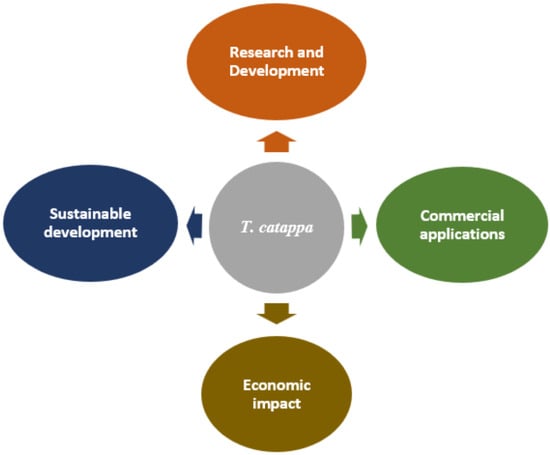
Figure 5.
Integrated framework for the development of T. catappa.
9. Conclusions
An in-depth analysis of T. catappa reveals its significant potential as a valuable natural resource with diverse applications. Scientific evidence confirms its traditional uses while identifying new development opportunities. The different parts of T. catappa are rich sources of nutrients and bioactive compounds. Furthermore, they displayed tremendous biological activities, including antioxidant, antidiabetic, anti-atherosclerosis, antitumor, antimicrobial, anthelmintic, antimalaria, hepatoprotective, insecticidal, anti-inflammatory and antihyperlipidemic activities. Integrating sustainable practices with modern scientific approaches offers a promising route to maximizing the benefits of this versatile species. However, success in realizing these opportunities will require continued investment in research, improved standardization processes and effective collaboration between stakeholders. Future research directions should focus on resolving current challenges while exploring innovative applications that can contribute to both economic development and environmental sustainability.
Funding
This work was carried out with the aid of a grant in the UNESCO-TWAS programme, “Seed Grant for African Principal Investigators” financed by the German Ministry of Education and Research, (BMBF).
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
Conflicts of Interest
There is no conflict of interest.
References
- Singh, M.; Parmar, F.; Solanki, H. Tropical Almond: The Valuable Tree. Vidya-A J. Gujarat Univ. 2023, 2, 348–353. [Google Scholar] [CrossRef]
- Ramanan S, S.; Arunachalam, A.; Singh, R.; Verdiya, A. Tropical Almond (Terminalia catappa): A Holistic Review. Heliyon 2025, 11, e41115. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.; Soares, S.D.; Dias, P.C.S.; Santos, M.P.L.; Nascimento, F.C.A.; Duarte, S.P.A.; Teixeira-Costa, B.E. Chemical-Functional Composition of Terminalia catappa Oils from Different Varieties. Grasas y Aceites 2022, 73, e454. [Google Scholar] [CrossRef]
- Nugroho, A.; Pambudi, N.A.; Harjanto, B.; Febryanto, A.; Firdaus, R.A.; Setyawan, N.D.; Syamsiro, M.; Gandidi, I.M. Production of Solid Fuel by Hydrothermal Treatment Using Terminalia catappa Peels Waste as Renewable Energy Sources. J. Phys. Conf. Ser. 2019, 1153, 012083. [Google Scholar] [CrossRef]
- Namadi, S.; Musa, A.O.; Gana, U.M. Physico-Mechanical Properties of Fuel Briquette Made from Raw and Torrefied Neem and Tropical Almond Tree Branches Using Different Organic Binders. Asian J. Res. Rev. Phys. 2025, 9, 20–35. [Google Scholar] [CrossRef]
- Oliveira, D.; Cristina, A.; Martins, S.; Andr, J.; Maria, L.; Dutra, G.; Ferreira, E.; Mangueira, Y.; Sobral, M.; Lima, S.; et al. Industrial Crops & Products Exploring the Potential of the Tropical Almond (Terminalia catappa L.): Analysis of Bioactive Compounds, Morphology and Metabolites. Ind. Crops Prod. 2024, 221, 119378. [Google Scholar] [CrossRef]
- Kaneria, M.J.; Rakholiya, K.D.; Marsonia, L.R.; Dave, R.A.; Golakiya, B.A. Nontargeted Metabolomics Approach to Determine Metabolites Profile and Antioxidant Study of Tropical Almond (Terminalia catappa L.) Fruit Peels Using GC-QTOF-MS and LC-QTOF-MS. J. Pharm. Biomed. Anal. 2018, 160, 415–427. [Google Scholar] [CrossRef]
- Chukwuma, I.F.; Ossai, E.C.; Nworah, F.N.; Apeh, V.O.; Abiaziem, E.O.; Iheagwam, F.N.; Skendrović, H.; Juchniewicz, S.; Leicht, K.; Okpala, C.O.R.; et al. Changes in Nutritional, Health Benefits, and Pharmaceutical Potential of Raw and Roasted Tropical Almond (Terminalia catappa Linn.) Nuts from Nigeria. PLoS ONE 2024, 19, e0287840. [Google Scholar] [CrossRef]
- Silalahi, M. Ketapang (Terminalia catappa L.): Potential Utilization as Foodstuffs and Traditional Medicine. Open Access Res. J. Life Sci. 2022, 3, 035–041. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Rodríguez-Nogales, A.; Algieri, F.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Antiinflammatory and Immunomodulatory Activity of an Ethanolic Extract from the Stem Bark of Terminalia catappa L. (Combretaceae): In Vitro and in Vivo Evidences. J. Ethnopharmacol. 2016, 192, 309–319. [Google Scholar] [CrossRef]
- Punniyakotti, P.; Rengarajan, R.L.; Velayuthaprabhu, S.; Vijayakumar, K.; Manikandan, R.; Anand, A.V. Protective Effect of Terminalia catappa Leaves and Terminalia Chebula Fruits on the Enzymatic and Non-Enzymatic Anti-Oxidant Levels in the Doxorubicin Induced Toxicity Rats. Pharmacogn. J. 2019, 11, 346–349. [Google Scholar] [CrossRef]
- Hussain, M.M. A Short Review on the Bioactive Constituents from Six Terminalia Species. Bangladesh Pharm. J. 2021, 24, 76–82. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Oyeleye, S.I.; Ejakpovi, I. Erectogenic, Antihypertensive, Antidiabetic, Anti-Oxidative Properties and Phenolic Compositions of Almond Fruit (Terminalia catappa L.) Parts (Hull and Drupe)–In Vitro. J. Food Biochem. 2017, 41, 1–12. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Adeline, K.B.; Norazlina, M.R.; Islam, S.; Shihabul, A.; Zaidul, I.S.M. Characterization and Nutritional Content of Terminalia catappa Kernel and Its Oil from Sabah, Malaysia. Appl. Food Res. 2022, 2, 100088. [Google Scholar] [CrossRef]
- Udotong, J.I.R.; Bassey, M.I. Evaluation of the Chemical Composition, Nutritive Value and Antinutrients of Terminalia catappa L. Fruit (Tropical Almond). Int. J. Eng. Tech. Res. 2015, 3, 96–99. [Google Scholar]
- ParsodkarLawal, A.R.; Olayinka, B.U.; Abdulkareem, K.A.; Abdulra’uf, L.B.; Murthadah, R.A.; Kayode, O.V. Mineral Analysis, Terminalia catappa. Biosci. J. 2022, 10, 191–203. [Google Scholar]
- Iyekowa, O.; Ukpebor, J.E.; Ehigimetor, E. Physicochemical Analysis of Terminalia catappa (Almond) Seed Nuts Grown in Benin City, Nigeria. Mediterr. J. Pharm. Pharm. Sci. 2023, 3, 6–10. [Google Scholar]
- Moussa, A.Y.; Siddiqui, S.A.; Elhawary, E.A.; Guo, K.; Anwar, S.; Xu, B. Phytochemical Constituents, Bioactivities, and Applications of Custard Apple (Annona squamosa L.): A Narrative Review. Food Chem. 2024, 459, 140363. [Google Scholar] [CrossRef]
- Baldé, A.O.; Baldé, E.S.; Bah, F.; Camara, A.; Baldé, M.A.; Dramé, A.; Dembélé, F.; Barry, H.; Traoré, M.S.; Baldé, A.M. Ethnobotanical and Antiplasmodial Investigation on Guinean Terminalia Species. S. Afr. J. Bot. 2020, 131, 443–447. [Google Scholar] [CrossRef]
- de Araújo, S.A.; Silva, C.M.P.; Costa, C.S.; Ferreira, C.S.C.; Ribeiro, H.S.; da Silva Lima, A.; Quintino da Rocha, C.; Calabrese, K.d.S.; Abreu-Silva, A.L.; Almeida-Souza, F. Leishmanicidal and Immunomodulatory Activity of Terminalia catappa in Leishmania Amazonensis in Vitro Infection. Heliyon 2024, 10, e24622. [Google Scholar] [CrossRef]
- Anand, A.V.; Divya, N.; Kotti, P.P. An Updated Review of Terminalia catappa. Pharmacogn. Rev. 2015, 9, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kalkar, S.A.; Parsodkar, V.J. Traditional Uses of Terminalia catappa and Its Validation by Phytochemical Screening. Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 631–635. [Google Scholar] [CrossRef]
- Venkatalakshmi, P.; Vadivel, V.; Brindha, P. Phytopharmacological Significance of Terminalia catappa L.: An Updated Review. Int. J. Res. Ayurveda Pharm. 2016, 7, 130–137. [Google Scholar] [CrossRef]
- Hung, H.D.; Tien, D.D.; Ngoan, N.T.; Duong, B.T.; Viet, D.Q.; Dien, P.G.; Anh, B.K. Chemical Constituents From the Leaves of Terminalia catappa L. (Combretaceae). Vietnam J. Sci. Technol. 2022, 60, 625–630. [Google Scholar] [CrossRef]
- Vibha, B. Nutritional Potential of Fruit Bark and Leaves of Terminalia catappa. J. Clin. Med. Images, Case Reports 2023, 3, 6–9. [Google Scholar] [CrossRef]
- Terças, A.G.; Monteiro, A.d.S.; Moffa, E.B.; dos Santos, J.R.A.; de Sousa, E.M.; Pinto, A.R.B.; Costa, P.C.d.S.; Borges, A.C.R.; Torres, L.M.B.; Barros Filho, A.K.D.; et al. Phytochemical Characterization of Terminalia catappa Linn. Extracts and Their Antifungal Activities against Candida spp. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Jawad Yousaf Zai, M.; James Cheesman, M.; Edwin Cock, I. A Review of the Ethnobotany, Phytochemistry AndMedicinal Properties of Australian Terminalia Species. Pharmacogn. Commun. 2024, 14, 13–23. [Google Scholar] [CrossRef]
- Cock, I.E. The Medicinal Properties and Phytochemistry of Plants of the Genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef]
- Kloucek, P.; Polesny, Z.; Svobodova, B.; Vlkova, E.; Kokoska, L. Antibacterial Screening of Some Peruvian Medicinal Plants Used in Callería District. J. Ethnopharmacol. 2005, 99, 309–312. [Google Scholar] [CrossRef]
- Silva, L.P.; De Angelis, C.D.; Bonamin, F.; Kushima, H.; José Mininel, F.; Dos Santos, L.C.; Delella, F.K.; Felisbino, S.L.; Vilegas, W.; MacHado Da Rocha, L.R.; et al. Terminalia catappa L.: A Medicinal Plant from the Caribbean Pharmacopeia with Anti-Helicobacter Pylori and Antiulcer Action in Experimental Rodent Models. J. Ethnopharmacol. 2015, 159, 285–295. [Google Scholar] [CrossRef]
- Sasu, P.; Attoh-Kotoku, V.; Akorli, D.E.; Adjei-Mensah, B.; Tankouano, R.A.; Kwaku, M. Nutritional Evaluation of the Leaves of Oxytenanthera Abyssinica, Bambusa Balcooa, Moringa Oleifera, Terminalia catappa, Blighia sapida, and Mangifera indica as Non-Conventional Green Roughages for Ruminants. J. Agric. Food Res. 2023, 11, 100466. [Google Scholar] [CrossRef]
- dos Santos, O.V.; Lorenzo, N.D.; Lannes, S.C.d.S. Chemical, Morphological, and Thermogravimetric of Terminalia catappa Linn. Food Sci. Technol. 2016, 36, 151–158. [Google Scholar] [CrossRef]
- Ng, S.; Lasekan, O.; Muhammad, K.S.; Hussain, N.; Sulaiman, R. Physicochemical Properties of Malaysian-Grown Tropical Almond Nuts (Terminalia catappa). J. Food Sci. Technol. 2015, 52, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Anuforo, P.C. Proximate Analysis and Determination of Some Selected Vitamins and Minerals Contents of Terminalia catappa Endocarp Flour. J. Nutr. Health Food Sci. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Wijesekera, M.M.T.; Fahmidha, H.F.; Ulpathakumbura, B.S.K.; Jayasinghe, L.; Marikkar, J.M.N. Nutritional Composition, Anti-Oxidative and Anti-Hyperglycemic Potential of the Kernels of Two Varieties of Terminalia catappa L. J. Agric. Sci.–Sri Lanka 2024, 19, 507–516. [Google Scholar] [CrossRef]
- Chakradhari, S.; Rajhans, K.P.; Patel, K.S.; Towett, E.K.; Martín-Gil, J.; Martín-Ramos, P. Nutritional and Spectral Characteristics of Terminalia Plants. Eur. J. Med. Plants 2019, 27, 1–13. [Google Scholar] [CrossRef]
- Katiki, L.M.; Gomes, A.C.P.; Barbieri, A.M.E.; Pacheco, P.A.; Rodrigues, L.; Veríssimo, C.J.; Gutmanis, G.; Piza, A.M.; Louvandini, H.; Ferreira, J.F.S. Terminalia catappa: Chemical Composition, in Vitro and in Vivo Effects on Haemonchus Contortus. Vet. Parasitol. 2017, 246, 118–123. [Google Scholar] [CrossRef]
- Agu, C.M.; Menkiti, M.C.; Agulanna, A.C.; Okolo, B.I.; Nwosu-Obieogu, K. Modeling of Methyl Ester Yield from Terminalia catappa L. Kernel Oil by Artificial Neural Network and Response Surface Methodology for Possible Industrial Application. Clean. Eng. Technol. 2022, 6, 100360. [Google Scholar] [CrossRef]
- El Qarnifa, S.; El Antari, A.; Hafidi, A. Effect of Maturity and Environmental Conditions on Chemical Composition of Olive Oils of Introduced Cultivars in Morocco. J. Food Qual. 2019, 2019, 1854539. [Google Scholar] [CrossRef]
- Devadiga, A.; Vidya Shetty, K.; Saidutta, M.B. Highly Stable Silver Nanoparticles Synthesized Using Terminalia catappa Leaves as Antibacterial Agent and Colorimetric Mercury Sensor. Mater. Lett. 2017, 207, 66–71. [Google Scholar] [CrossRef]
- Mwangi, W.C.; Waudo, W.; Shigwenya, M.E.; Gichuki, J. Phytochemical Characterization, Antimicrobial and Antioxidant Activities of Terminalia catappa Methanol and Aqueous Extracts. BMC Complement. Med. Ther. 2024, 24, 1–11. [Google Scholar] [CrossRef]
- Warnasih, S.; Mulyati, A.H.; Widiastuti, D.; Zahra, A.C.; Sugita, P.; Ambarsari, L. Anticancer Potency of Methanol Extract from Terminalia catappa Leaves Using In Vitro and In Silico Methods. Trends Sci. 2024, 21, 8057. [Google Scholar] [CrossRef]
- Uchida, V.H.; de Araújo Padilha, C.E.; Rios, N.S.; dos Santos, E.S. Enzymatic Inhibition of α-Amylase and Encapsulation of Bioactive Compounds by Nanoemulsion from Pulp Extract Terminalia catappa Linn Fruit. Results Chem. 2023, 5, 100736. [Google Scholar] [CrossRef]
- Raphaël, B.; Akué Rony, M.; Loumpangou Célestine, N.; Engonga Louis-Clément, O.; Jacques, L.; Bikanga Raphaël, C.; Jean-Maurille, O. Phytochemical Study and Antioxidant Activities of Terminalia catappa L. and Mitragyna Ciliata Aubrev and Pellegr Medicinal Plants of Gabon. J. Med. Plants Stud. 2019, 7, 33–38. [Google Scholar]
- Pandya, N.B.; Tigari, P.; Dupadahalli, K.; Kamurthy, H.; Nadendla, R.R. Antitumor and Antioxidant Status of Terminalia catappa against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Indian J. Pharmacol. 2013, 45, 464–469. [Google Scholar] [CrossRef]
- Meneses, J.O.; dos Santos Cunha, F.; Dias, J.A.R.; da Cunha, A.F.S.; dos Santos, F.J.; da Costa Sousa, N.; do Couto, M.V.S.; Paixão, P.E.G.; Abe, H.A.; dos Santos Lima, B.; et al. Acute Toxicity of Hot Aqueous Extract from Leaves of the Terminalia catappa in Juvenile Fish Colossoma Macropomum. Aquac. Int. 2020, 28, 2379–2396. [Google Scholar] [CrossRef]
- Shodehinde, S.A.; Bello, L.; Awojulu, O.V.; Ayejuni, V.O. Phenolics, Antioxidants and Minerals in Fermented vs. Unfermented Terminalia catappa. Res. J. Med. Plants 2025, 7924, 1–11. [Google Scholar]
- Odutayo, O.E.; Omonigbehin, A.E.; Ogunlana, O.O.; Afolabi, I.S. Biochemical Effects of Fermentation on Selected Phytochemicals, Enzymes and Antioxidant Activities in The Under-Utilized Seeds of Chrysophyllum albidum Linn and Terminalia catappa Linn. Trop. J. Nat. Prod. Res. 2023, 7, 4945–4953. [Google Scholar]
- Chen, P.S.; Li, J.H.; Liu, T.Y.; Lin, T.C. Folk Medicine Terminalia catappa and Its Major Tannin Component, Punicalagin, Are Effective against Bleomycin-Induced Genotoxicity in Chinese Hamster Ovary Cells. Cancer Lett. 2000, 152, 115–122. [Google Scholar] [CrossRef]
- Dwevedi, A.; Dwivedi, R.; Sharma, Y. Exploration of Phytochemicals Found in Terminalia Sp. and Their Antiretroviral Activities. Pharmacogn. Rev. 2016, 10, 73–83. [Google Scholar] [CrossRef]
- Ikele, C.B.; Okwuonu, E.; Ijem, A.N. Further Studies on Evaluation of the Toxicity Potential of Terminalia catappa Lin. Combretaceae Leaf Extract: Effects on the Histology, Liver Enzymes, and Haematology Profile of Albino Rats. Jordan J. Biol. Sci. 2021, 14, 889–897. [Google Scholar] [CrossRef]
- Martínez, M.; Vera, A.; Parra, J.; Beltrán, O. Physicochemical Parameters of the Gum of Terminalia catappa L. (Almendrón). Ciencia 2012, 20, 25–31. [Google Scholar]
- Olatunji, L.K.; Jimoh, A.O.; Tukur, U.M.; Imam, M.U. A Review of the Effects of Policosanol on Metabolic Syndrome. Clin. Complement. Med. Pharmacol. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Daram, P.; Jitta, S.R.; Shreedhara, C.S.; Misra, C.S.; Gourishetti, K.; Lobo, R. Investigation of Anti-Inflammatory and Anti-Arthritic Potentials of Terminalia catappa Bark Using in Vitro Assays and Carrageenan-Induced Inflammation, Complete Freund’s Adjuvant Induced Arthritis Model in Rats. S. Afr. J. Bot. 2021, 141, 313–321. [Google Scholar] [CrossRef]
- Salawu, A.R.; Onyegbula, A.F.; Lawal, I.O.; Akande, S.A.; Oladipo, A.K. Comparative Study of the Nutritional, Phytochemical and Mineral Compositions of the Nuts of Tropical Almond (Terminalia catappa) and Sweet Almond (Prunus Amygdalus). Ruhuna J. Sci. 2018, 9, 70. [Google Scholar] [CrossRef]
- Oduro, I.; Larbie, C.; Amoako, T.; Antwi-Boasiako, A. Proximate Composition and Basic Phytochemical Assessment of Two Common Varieties of Terminalia catappa (Indian Almond). J. Sci. Technol. 2009, 29, 1–6. [Google Scholar] [CrossRef]
- Muhammad, A.; Mudi, S.Y.; Muhammad, A.; Mudi, S.Y. Phytochemical Screening and Antimicrobial Activities of Terminalia catappa, Leaf Extracts Phytochemical Screening and Antimicrobial Activities of Terminalia catappa, Leaf Extracts. Niger. Soc. Exp. Biol. 2011, 23, 35–39. [Google Scholar]
- Odongo, E.A.; Mutai, P.C.; Amugune, B.K.; Mungai, N.N.; Akinyi, M.O.; Kimondo, J. Evaluation of the Antibacterial Activity of Selected Kenyan Medicinal Plant Extract Combinations against Clinically Important Bacteria. BMC Complement. Med. Ther. 2023, 23, 100. [Google Scholar] [CrossRef]
- Iha, O.K.; Alves, F.C.S.C.; Suarez, P.A.Z.; Silva, C.R.P.; Meneghetti, M.R.; Meneghetti, S.M.P. Potential Application of Terminalia catappa L. and Carapa Guianensis Oils for Biofuel Production: Physical-Chemical Properties of Neat Vegetable Oils, Their Methyl-Esters and Bio-Oils (Hydrocarbons). Ind. Crops Prod. 2014, 52, 95–98. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Vasconcelos, I.M.; Bezerra, L.C.N.M.; Silveira, S.B.; Monteiro, A.C.O.; Moreira, R.A. Composition and Nutritional Properties of Seeds from Pachira Aquatica Aubl, Sterculia Striata St Hil et Naud and Terminalia catappa Linn. Food Chem. 2000, 70, 185–191. [Google Scholar] [CrossRef]
- Janporn, S.; Ho, C.T.; Chavasit, V.; Pan, M.H.; Chittrakorn, S.; Ruttarattanamongkol, K.; Weerawatanakorn, M. Physicochemical Properties of Terminalia catappa Seed Oil as a Novel Dietary Lipid Source. J. Food Drug Anal. 2015, 23, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Mbah, B.O.; Eme, P.E.; Eze, C.N. Nutrient Potential of Almond Seed (Terminalia catappa) Sourced from Three States of Eastern Nigeria. Afr. J. Agric. Res. 2013, 8, 629–633. [Google Scholar]
- dos Santos, I.C.F.; de Carvalho, S.H.V.; Solleti, J.I.; Ferreira de La Salles, W.; Teixeira da Silva de La Salles, K.; Meneghetti, S.M.P. Studies of Terminalia catappa L. Oil: Characterization and Biodiesel Production. Bioresour. Technol. 2008, 99, 6545–6549. [Google Scholar] [CrossRef]
- Yapo Monnet, T.; Patrice, K.; Thierry Monnet, Y.; Gbogouri, A.; Kouadio Bony Koffi, P.; Patrice Kouamé, L.; Patrice, L. Chemical Characterization of Seeds and Seed Oils from Mature Terminalia catappa Fruits Harvested in Côte d’Ivoire. Int. J. Biosci. 2012, 2012, 110–124. [Google Scholar]
- Ajayi, I.A.; Oderinde, R.A.; Taiwo, V.O.; Agbedana, E.O. Short-Term Toxicological Evaluation of Terminalia catappa, Pentaclethra Macrophylla and Calophyllum Inophyllum Seed Oils in Rats. Food Chem. 2008, 106, 458–465. [Google Scholar] [CrossRef]
- Pereira, H.; Simões, R.; Miranda, I. Cuticular Waxes and Cutin in Terminalia catappa Leaves from the Equatorial São Tomé and Príncipe Islands. Molecules 2023, 28, 6365. [Google Scholar] [CrossRef]
- Batubo, N.P.; Ogbu, O.S.; Victor, D.D. Chemical Profiles and Proximate Analysis of N-Hexane Extract of Terminalia catappa Kernel from Nigeria. Int. J. Res. Med. Sci. 2023, 12, 17–25. [Google Scholar] [CrossRef]
- Samarasinghe, S.P.A.K.; Chandimala, U.R.; Gunathilake, D.M.C.C. Production of Plant-Based Milk from Local Almond Nuts (Terminalia catappa L.) and Evaluation of Its Sensory and Nutritional Properties. Trop. Agric. Res. Ext. 2023, 26, 104–110. [Google Scholar] [CrossRef]
- Ogunmoye, A.O.; Olubomehin, O.; Ogundare, S.; Yussuf, S.T. GC-MS Analysis of The Volatile Constituents from The Air-Died Leaves of Terminalia catappa (LINNAEUS). FUW Trends Sci. Technol. J. 2020, 5, 948–951. [Google Scholar]
- Owolabi, M.; Lawal, O.; Ogunwande, I.A.; Hauser, R.M.; Setzer, W.N. Chemical Composition of the Leaf Essential Oil of Terminalia catappa L. Growing in Southwestern Nigeria. Am. J. Essent. Oils Nat. Prod. 2013, 1, 51–54. [Google Scholar]
- Moronkola, D.O.; Ekundayo, O. Chemical Constituents in the Fruit Essential Oil of Terminalia catappa Linn (Almond Fruits). J. For. Trop. Resour. 2000, 16, 72–79. [Google Scholar]
- Momoh, J.O.; Kumar, S.; Olelaye, O.N.; Adekunle, O.M.; Aiyelero, T.S. Green synthesis of Characterized Bio-functionalized ZnO Nanoparticles from Terminalia catappa (Almond) Methanol Leaf Extract and their Potential Antioxidant and Antibacterial Properties. Trop. J. Nat. Prod. Res. 2024, 8, 9296–9309. [Google Scholar]
- Madhavan, K.; Rukayadi, Y.; Mutalib, N.A.A. Phytochemical Constituents and Toxicity Analysis of Ethanolic Ketapang (Terminalia catappa L.) Leaf Extract. Malays. Appl. Biol. 2023, 52, 105–114. [Google Scholar] [CrossRef]
- Yakubu, Y.; Lee, S.Y.; Shaari, K. Chemical Profiles of Terminalia catappa LINN Nut and Terminalia Subspathulata KING Fruit. Pertanika J. Trop. Agric. Sci. 2021, 44, 795–823. [Google Scholar] [CrossRef]
- Ogbeide, O.K.; Eze, O.F.; Akaeze, D.A.; Akhigbe, I.U.; Omoruyi, U.; Iyekowa, O.; Owolabi, B.J. Physico-Chemical Properties, Chemical Composition, Biodiesel Production and Antibacterial Potential of Terminalia CatapaSeed Oil. ChemSearch J. 2021, 12, 70–80. [Google Scholar]
- Vinturelle, R.; Cabral, T.d.S.; Oliveira, P.C.O.d.; Salles, J.P.; Faria, J.V.; Teixeira, G.P.; Faria, R.X.; Veloso, M.C.C.; Romeiro, G.A.; Chagas, E.F. das Slow Pyrolysis of Terminalia catappa L. Municipal Solid Waste and the Use of the Aqueous Fraction Produced for Bovine Mastitis Control. Biochem. Biophys. Rep. 2024, 38, 101704. [Google Scholar] [CrossRef]
- Du, J.R.; Long, F.Y.; Chen, C. Research Progress on Natural Triterpenoid Saponins in the Chemoprevention and Chemotherapy of Cancer, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 36, ISBN 9780128022153. [Google Scholar]
- Gao, J.; Tang, X.; Dou, H.; Fan, Y.; Zhao, X.; Xu, Q. Hepatoprotective Activity of Terminalia catappa L. Leaves and Its Two Triterpenoids. J. Pharm. Pharmacol. 2010, 56, 1449–1455. [Google Scholar] [CrossRef]
- Tabansi, D.; Dahiru, D.; Patrick, A.T.; Jahng, W.J. Anti-Atherosclerosis and Anti-Hyperlipidemia Functions of Terminalia catappa Fruit. ACS Omega 2023, 8, 35571–35579. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Proposed Mechanisms of Terminalia catappa in Hyperglycaemia and Associated Diabetic Complications. J. Pharm. Pharmacol. 2017, 69, 123–134. [Google Scholar] [CrossRef]
- Sakib, S.A.; Rahman, T.; Mahmud, Z.; Haque, M.E. Secondary Metabolites from Terminalia catappa and Evaluation of Its Bioactivities. Bioresearch Commun. 2023, 9, 1310–1319. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 5482. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, T.N.; Raveesha, K.A. Polyphenol-Rich Purified Bioactive Fraction Isolated from Terminalia catappa L.: UHPLC-MS/MS-Based Metabolite Identification and Evaluation of Their Antimicrobial Potential. Coatings 2021, 11, 1210. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, H.T.; Zhang, M.; Wang, D.; Yang, C.R.; Zhang, Y.J. Termitomenins F and G, Two New Lignan Glucosides from Terminalia Chebula Var. Tomentella (Kurz) C. B. Clarke. Nat. Prod. Bioprospect. 2021, 11, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Muhit, M.A.; Umehara, K.; Mori-Yasumoto, K.; Noguchi, H. Furofuran Lignan Glucosides with Estrogen-Inhibitory Properties from the Bangladeshi Medicinal Plant Terminalia Citrina. J. Nat. Prod. 2016, 79, 1298–1307. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. Phytochem.-Isol. Characterisation Role Hum. Health 2015, 25, 533–538. [Google Scholar] [CrossRef]
- Patel, S.S.; Savjani, J.K. Systematic Review of Plant Steroids as Potential Antiinflammatory Agents: Current Status and Future Perspectives. J. Phytopharm. 2015, 4, 121–125. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Gana Suganda, A. Nawawi, ari Phytochemical Study of Ketapang Bark (Terminalia catappa L.). In Proceedings of the International Conference on Medicinal Plants (ICOMP), Surabaya, Indonesia, 21–22 July 2010; pp. 21–22. [Google Scholar]
- Dembitsky, V.M. Biological Activity and Structural Diversity of Steroids Containing Aromatic Rings, Phosphate Groups, or Halogen Atoms. Molecules 2023, 28, 5549. [Google Scholar] [CrossRef]
- Boulis, A.G.; El Zalabani, S.M.; Ghaly, N.S.; Sabry, O.M.; El-Manawaty, M.A.; Afifi, A.H.; Melek, F.R. Secondary Metabolites from the Leaves of Terminalia Myriocarpa and Their α-Glucosidase Inhibitory Potential. J. Appl. Pharm. Sci. 2024, 14, 244–251. [Google Scholar] [CrossRef]
- Houngbédji, M.; Johansen, P.; Padonou, S.W.; Akissoé, N.; Arneborg, N.; Nielsen, D.S.; Hounhouigan, D.J.; Jespersen, L. Occurrence of Lactic Acid Bacteria and Yeasts at Species and Strain Level during Spontaneous Fermentation of Mawè, a Cereal Dough Produced in West Africa. Food Microbiol. 2018, 76, 267–278. [Google Scholar] [CrossRef]
- Colendres, R.J.; Pradera, C.L. In Vitro Activity of Indian Almond (Terminalia catappa) Leaf Crude Extracts against Selected Dermatophytes. Ann. Trop. Res. 2021, 43, 55–66. [Google Scholar] [CrossRef]
- Bushra, H.; Panezai, M.A.; Kakar, M.A.; Khan, J.; Kakar, A.M.; Yousaf, N.; Tareen, A.K.; Khan, A.; Zahoor, S.; Tareen, S.; et al. Biological Studies on Leaves of Tropical Almond (Terminalia catappa) (A Review). Eur. Acad. Res. 2023, 11, 135–158. [Google Scholar]
- Divya, N.; Vijaya Anand, A. In Vitro Antioxidant Activity of Ethanolic Extract of Terminalia catappa Leaves. Asian J. Pharm. Clin. Res. 2015, 8, 269–271. [Google Scholar]
- Zheng, M.; Lu, S.; Xing, J. Enhanced Antioxidant, Anti-Inflammatory and α-Glucosidase Inhibitory Activities of Citrus Hesperidin by Acid-Catalyzed Hydrolysis. Food Chem. 2021, 336, 127539. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Wang, Y.; Gao, H.; Li, X.; Huang, X.; Huang, T. Effects of Rutin and Its Derivatives on Citrinin Production by Monascus Aurantiacus Li AS3.4384 in Liquid Fermentation Using Different Types of Media. Food Chem. 2019, 284, 205–212. [Google Scholar] [CrossRef]
- Liu, S.; Loo, Y.T.; Li, Z.; Ng, K. Alginate-Inulin-Chitosan Based Microspheres Alter Metabolic Fate of Encapsulated Quercetin, Promote Short Chain Fatty Acid Production, and Modulate Pig Gut Microbiota. Food Chem. 2023, 418, 135802. [Google Scholar] [CrossRef]
- Nga, N.T.T.; Bac, N.X.; Hanh, V.T.; Ha, L.T.N. Polyphenols from Tropical Almond Leaves (Terminalia catappa L.): Optimized Extraction Conditions and α-Glucosidase Inhibitory Activity. Vietnam J. Agric. Sci. 2024, 7, 2064–2075. [Google Scholar] [CrossRef]
- Chakkalakal, M.; Pan, A.; Nadora, D.; Gahoonia, N.; Chaudhuri, R.K.; Burney, W.; Thacker, S.; Shakhbazova, A.; Subramanyam, C.; Chambers, C.J.; et al. Randomized Double-Blind Placebo-Controlled Supplementation with Standardized Terminalia Chebula Fruit Extracts Reduces Facial Sebum Excretion, Erythema, and Wrinkle Severity. J. Clin. Med. 2023, 12, 1591. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Mohammed, F.; Shaikh, R.; Uzgare, A.S. Study of Lectin-like Protein from Terminalia catappa (TC) Seeds for Its Physicochemical and Antimicrobial Properties. Chem. Proc. 2024, 16, 75. [Google Scholar] [CrossRef]
- Nguy, L.H.; Tran, L.B.H.; Dong, T.A.D. Effects of Edible Terminalia catappa L. Seed Oil on Physiological Parameters of Mus Musculus L Mice. J. Agric. Food Res. 2023, 12, 100587. [Google Scholar] [CrossRef]
- Vingadassalon, A.; Pejcz, E.; Wojciechowicz-Budzisz, A.; Olędzki, R.; Groton, K.; Aurore, G.; Harasym, J. Terminalia catappa Kernel Flour Characterization as a Functional and Bioactive Ingredient for Cookies Formulation. Appl. Sci. 2024, 14, 11201. [Google Scholar] [CrossRef]
- Iheagwam, F.N.; Garuba, P.A.; Ogunlana, O.O.; Chinedu, S.N. Counteractive Role of Terminalia catappa Leaf Extract on Hematological and Coagulation Disturbance in Type 2 Diabetic Rats. Vet. World 2023, 16, 1593–1599. [Google Scholar] [CrossRef]
- Iheagwam, F.N.; Batiha, G.E.S.; Ogunlana, O.O.; Chinedu, S.N. Terminalia catappa Extract Palliates Redox Imbalance and Inflammation in Diabetic Rats by Upregulating Nrf-2 Gene. Int. J. Inflam. 2021, 2021, 9778486. [Google Scholar] [CrossRef]
- Chinaka, C.N.; Ezealisiji, K.M.; Akpofure, R.E. Phyto-Chemical Characterization of the Leaf Extracts of Terminalia catappa L. (Combretaceae) Using Ultra Violet-Visible, Fourier Transform Infrared and Gas Chromatography-Mass Spectroscopic Techniques. J. Pharmacogn. Phytochem. 2018, 7, 2017–2023. [Google Scholar]
- Issahaku, A.; Adebayo, L. Gas Chromatography-Mass Spectrometry and Fourier Transform Infrared Spectroscopy Analysis of Potential α -Glucosidase Inhibitors from Terminalia catappa Leaf Extracts. J. Complement. Altern. Med. Res. 2024, 25, 95–111. [Google Scholar] [CrossRef]
- Ben, E.E.; Ben, E.E.; Asuquo, A.E.; George, U.A. Sperm Cells and Reproductive Hormones Abnormalities Associated with Terminalia catappa (Indian Almond) Leaf Extract Administration in Streptozotocin- Induced Diabetic Male Wistar Rats. Drug Discov. 2024, 14, e14dd1987. [Google Scholar]
- Jahid, M.; Khan, K.U.; Haq, R.U.; Ahmed, R.S. Overview of Rheumatoid Arthritis and Scientific Understanding of the Disease. Mediterr. J. Rheumatol. 2023, 34, 284. [Google Scholar] [CrossRef]
- Kumar, V.D.; Kokila, G.S.; Sarvatha, A.D.; Pradeepa, D. Phytochemical Profiles, In Vitro Antioxidant, Anti Inflammatory and Antibacterial Activities of Aqueous Extract of Terminalia catappa L. Leaves-ProQuest. J. Pharm. Sci. Res. 2021, 13, 340–346. [Google Scholar]
- Lucena, F.; McDougall, J.J. Protease Activated Receptors and Arthritis. Int. J. Mol. Sci. 2021, 22, 9352. [Google Scholar] [CrossRef] [PubMed]
- Balling, M.; Afzal, S.; Davey Smith, G.; Varbo, A.; Langsted, A.; Kamstrup, P.R.; Nordestgaard, B.G. Elevated LDL Triglycerides and Atherosclerotic Risk. J. Am. Coll. Cardiol. 2023, 81, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, S.A.; Arca, M.; Scicchitano, P.; Alonzo, A.; Perone, F.; Gulizia, M.M.; Gabrielli, D.; Oliva, F.; Imperoli, G.; Colivicchi, F. Lipoprotein(a): A Risk Factor for Atherosclerosis and an Emerging Therapeutic Target. Heart 2022, 109, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, P.; Xu, S.; Koroleva, M.; Zhang, S.; Si, S.; Jin, Z.G. Tannic Acid as a Plant-Derived Polyphenol Exerts Vasoprotection via Enhancing KLF2 Expression in Endothelial Cells. Sci. Rep. 2017, 7, 6686. [Google Scholar] [CrossRef]
- Gong, G.; Qin, Y.; Huang, W.; Zhou, S.; Wu, X.; Yang, X.; Zhao, Y.; Li, D. Protective Effects of Diosgenin in the Hyperlipidemic Rat Model and in Human Vascular Endothelial Cells against Hydrogen Peroxide-Induced Apoptosis. Chem. Biol. Interact. 2010, 184, 366–375. [Google Scholar] [CrossRef]
- Feitosa, I.B.; Mori, B.; Teles, C.B.G.; Costa, A.G.d. What Are the Immune Responses during the Growth of Ehrlich’s Tumor in Ascitic and Solid Form? Life Sci. 2021, 264, 118578. [Google Scholar] [CrossRef]
- Minsakorn, S.; Watthanadirek, A.; Poolsawat, N.; Puttarak, P.; Chawengkirttikul, R.; Anuracpreeda, P. The Anthelmintic Potentials of Medicinal Plant Extracts and an Isolated Compound (Rutin, C27H30O16) from Terminalia catappa L. against Gastrothylax Crumenifer. Vet. Parasitol. 2021, 291, 109385. [Google Scholar] [CrossRef]
- Samuel, B.; Adekunle, Y.A. Isolation and Structure Elucidation of Anti-Malarial Principles from Terminalia Mantaly H. Perrier Stem Bark. Int. J. Biol. Chem. Sci. 2021, 15, 282–292. [Google Scholar] [CrossRef]
- World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 24 January 2025).
- Patrick, A.O.; Ozioma, O.E.; Shine, G.K.; Michael, A.T.; Nwosu, S.N.; Bassey, E.B.; Favour, N.C.; Damilola, A.M.; Tochi, N.S. Proximate Analysis, Extraction, and Characterization of Oil from Terminalia catappa Fruit in Anambra State, Nigeria. Asian J. Res. Biochem. 2024, 14, 126–137. [Google Scholar] [CrossRef]
- Polytechnic, F.; Ekiti, A. Chemical Properties of Kunu Beverage Enriched with Almond (Terminalia catappa) Nut Flour. Appl. Trop. Agric. 2024, 29, 48–54. [Google Scholar]
- Oyeniran, O.H.; Ademiluyi, A.O.; Oboh, G. African Mistletoe (Tapinanthus Bangwensis Lor.) Infestation Improves the Phenolic Constituents, Antioxidative and Antidiabetic Effects of Almond (Terminalia catappa Linn.) Host Leaf in Sucrose-Rich Diet-Induced Diabetic-like Phenotypes in Fruit Fly (Drosophila melanogaster Meigen). Food Front. 2021, 2, 77–90. [Google Scholar] [CrossRef]
- Luka, C.; Istifanus, G.; Olatunde, A.; Eli, Z. Effect of Aqueous Seed Extract of Terminalia catappa Linn on Some Biochemical Parameters in Alloxan-Induced Diabetic Rats. J. Appl. Life Sci. Int. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Nagappa, A.N.; Thakurdesai, P.A.; Rao, N.V.; Singh, J. Antidiabetic Activity of Terminalia catappa Linn Fruits. J. Ethnopharmacol. 2003, 88, 45–50. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Bai, R.; Xiong, F.; Jin, Y.; Liu, H.; Wang, Z.; Yang, C.; Yu, Y.; Chowdhury, A.; et al. Geographic Variation in Secondary Metabolites Contents and Their Relationship with Soil Mineral Elements in Pleuropterus Multiflorum Thunb. from Different Regions. Sci. Tradit. Chin. Med. 2024, 2, 214–223. [Google Scholar] [CrossRef]
- Chihomvu, P.; Ganesan, A.; Gibbons, S.; Woollard, K.; Hayes, M.A. Phytochemicals in Drug Discovery—A Confluence of Tradition and Innovation. Int. J. Mol. Sci. 2024, 25, 8792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.