Unlocking Therapeutic Potential of Novel Thieno-Oxazepine Hybrids as Multi-Target Inhibitors of AChE/BChE and Evaluation Against Alzheimer’s Disease: In Vivo, In Vitro, Histopathological, and Docking Studies
Abstract
1. Introduction
2. Results and Discussion
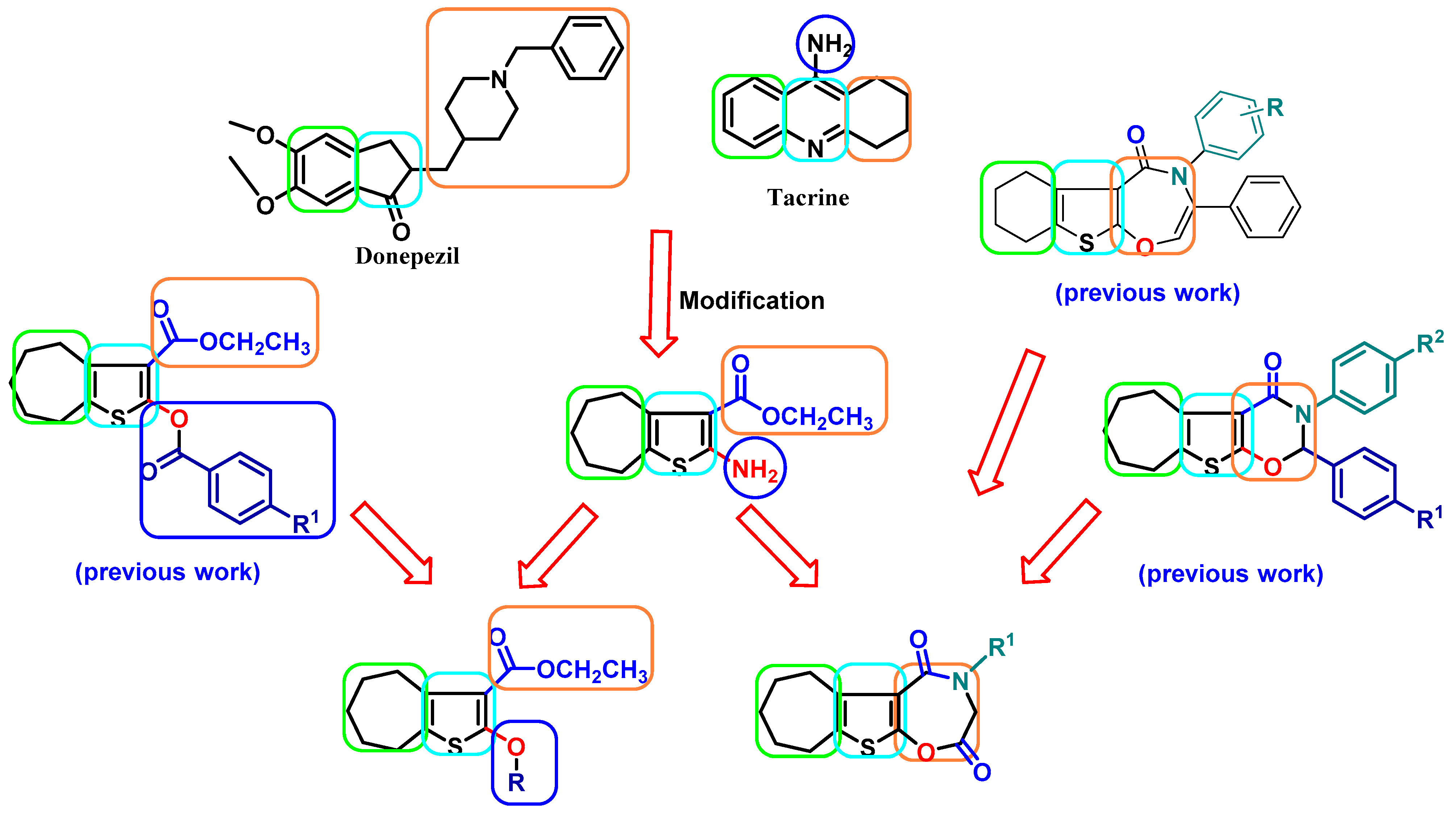
2.1. Chemistry
2.2. Biology
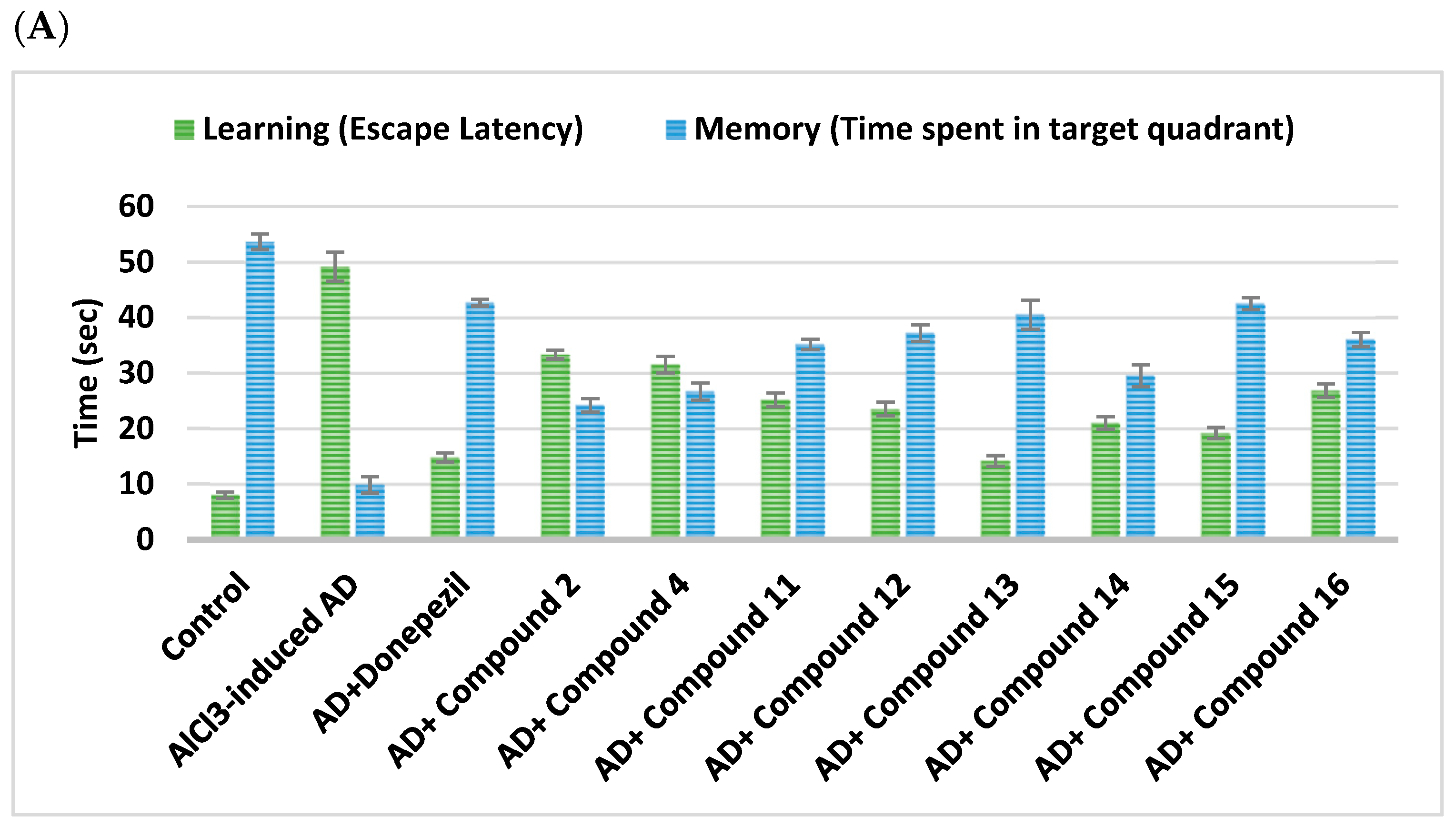
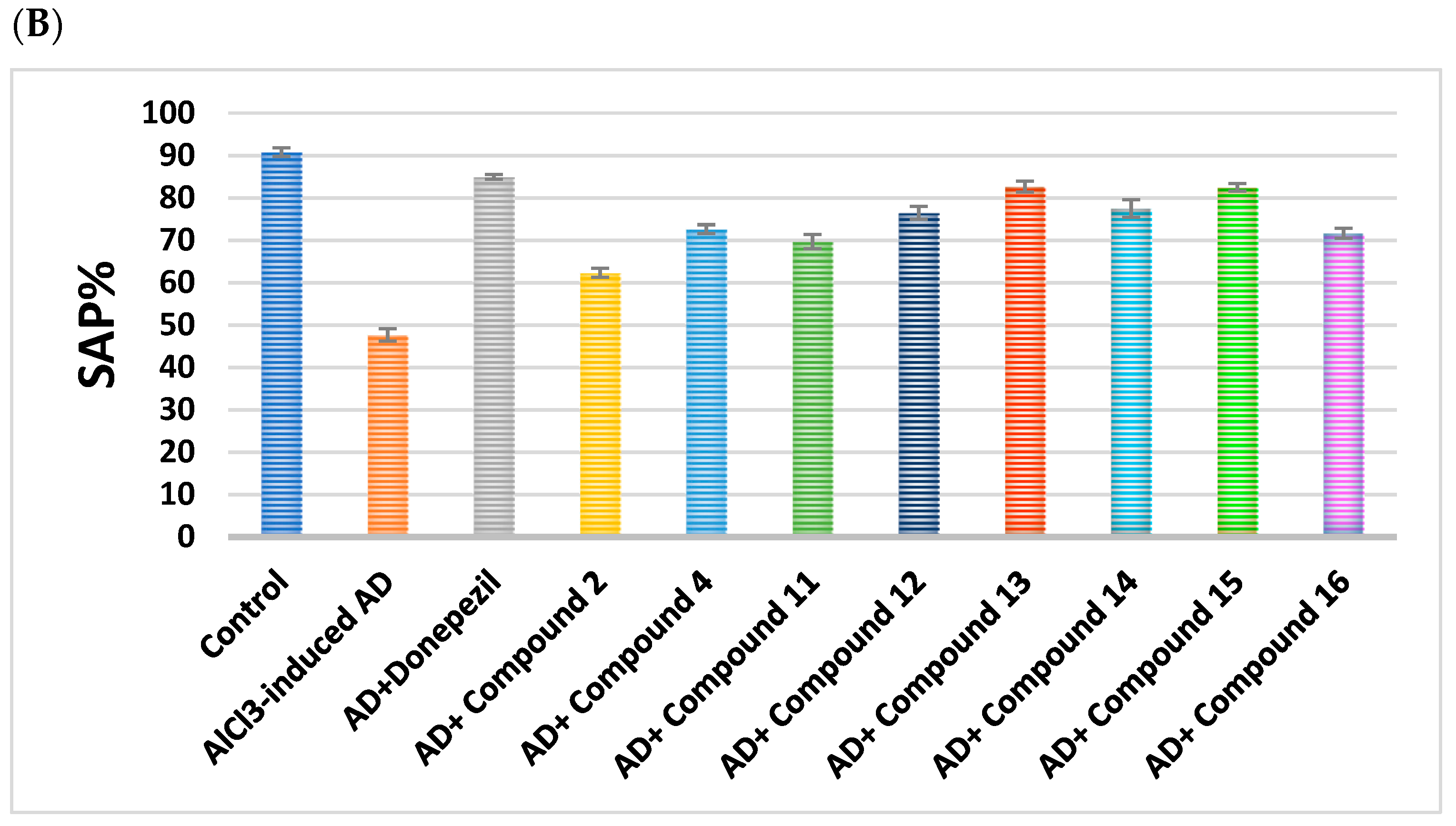
2.2.1. Impact on Behavioral Assessments in AlCl3-Induced AD
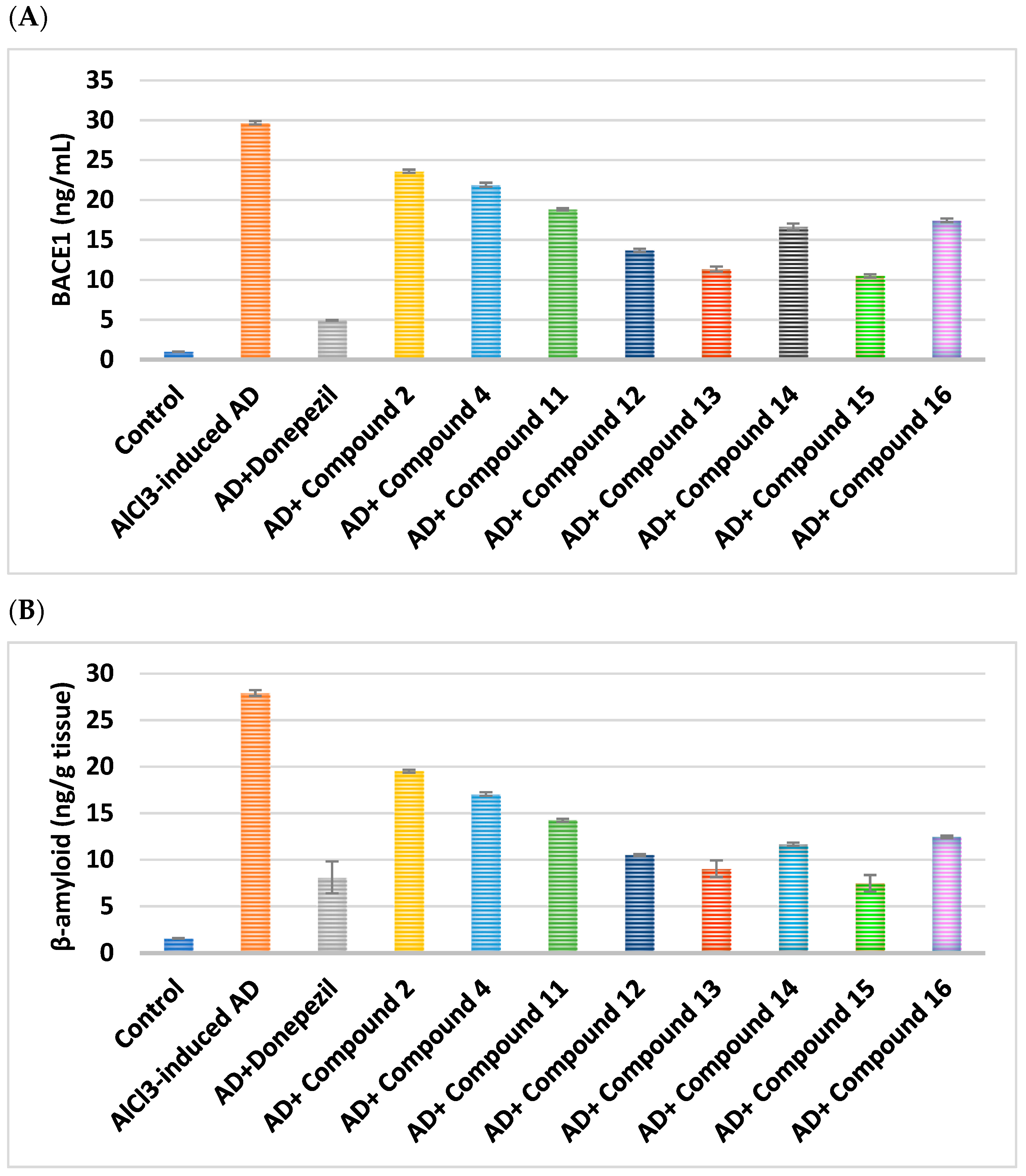
2.2.2. Effect on BACE1 and Amyloid-β
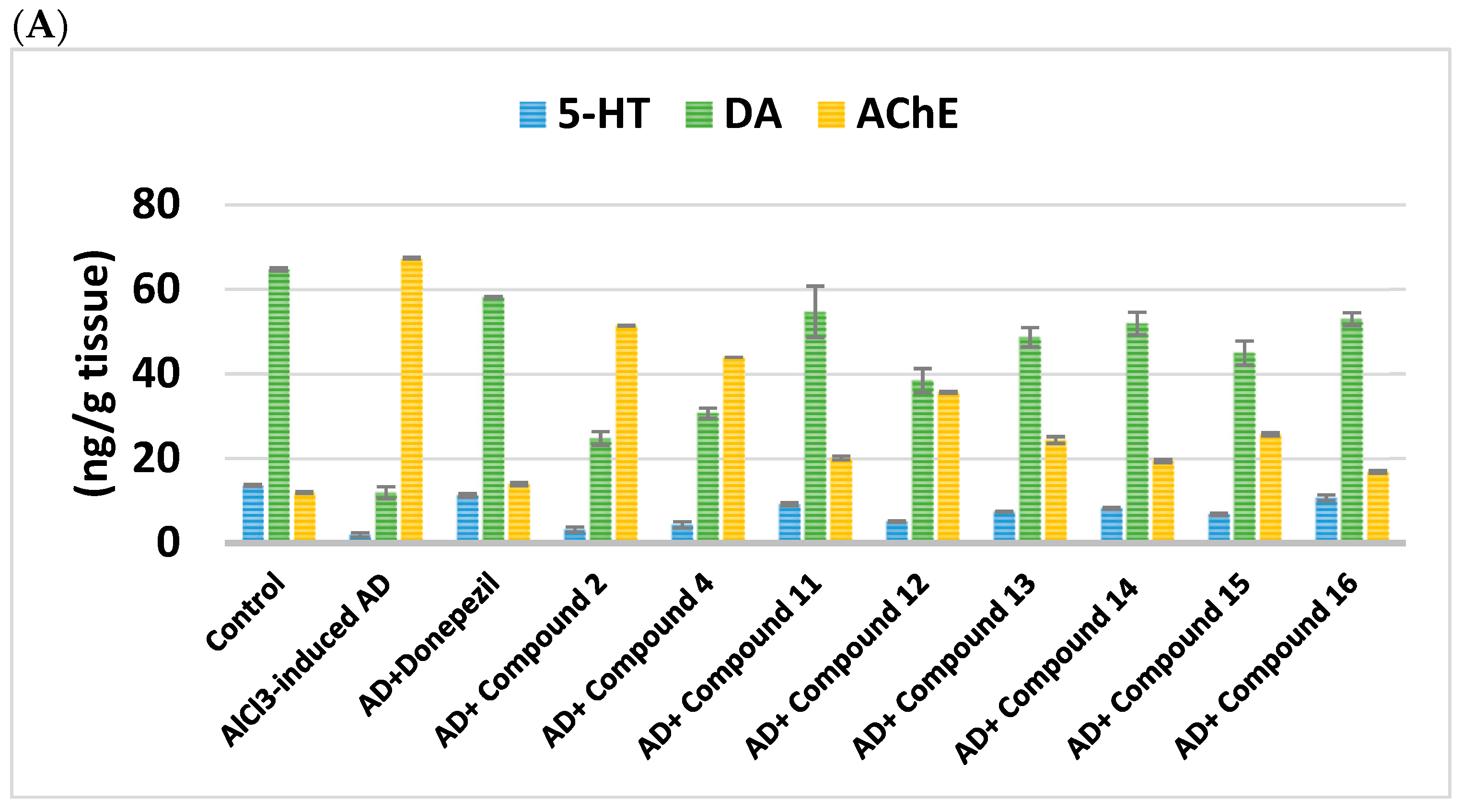
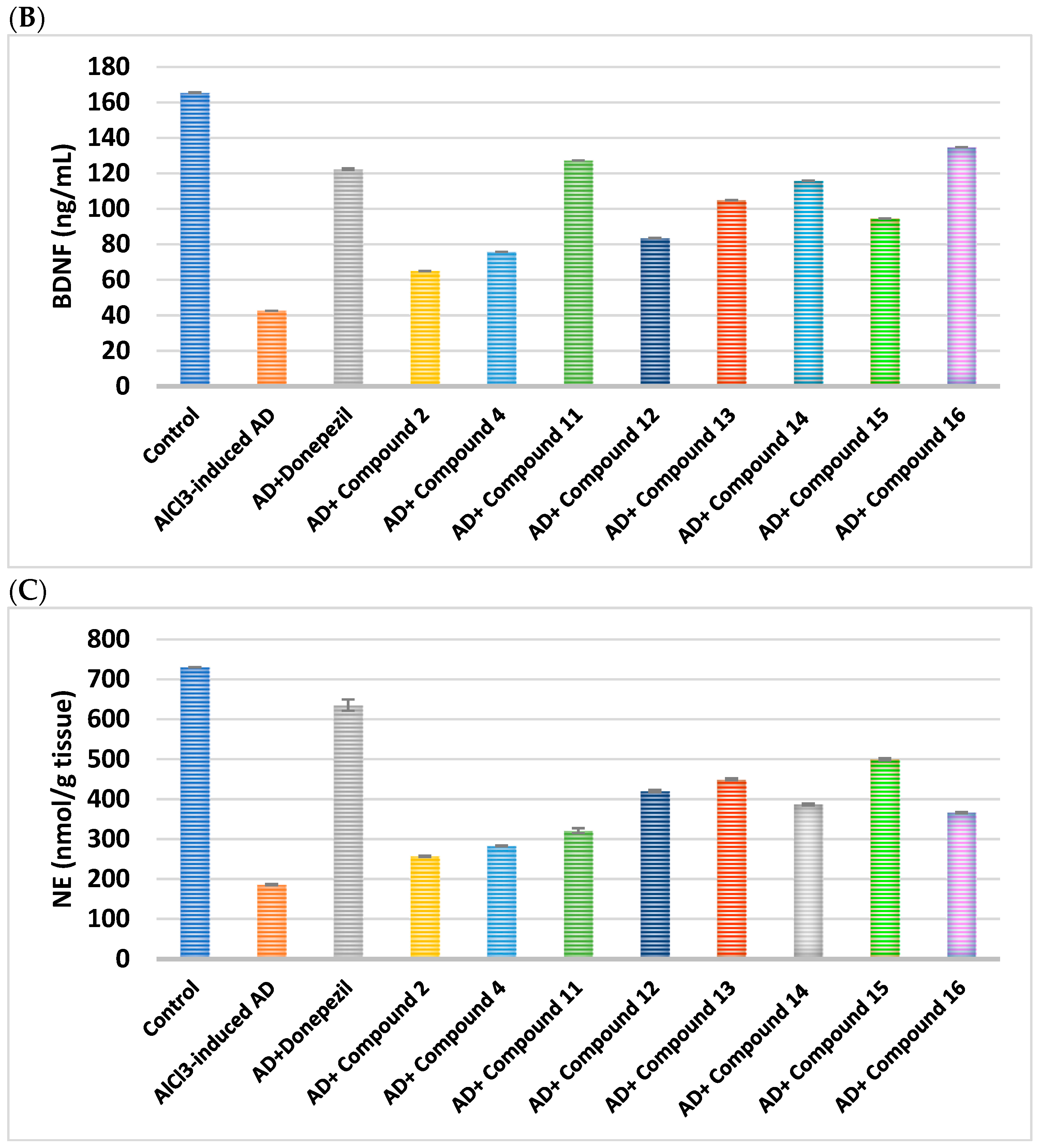
2.2.3. Impact on Brain Neurotransmitters
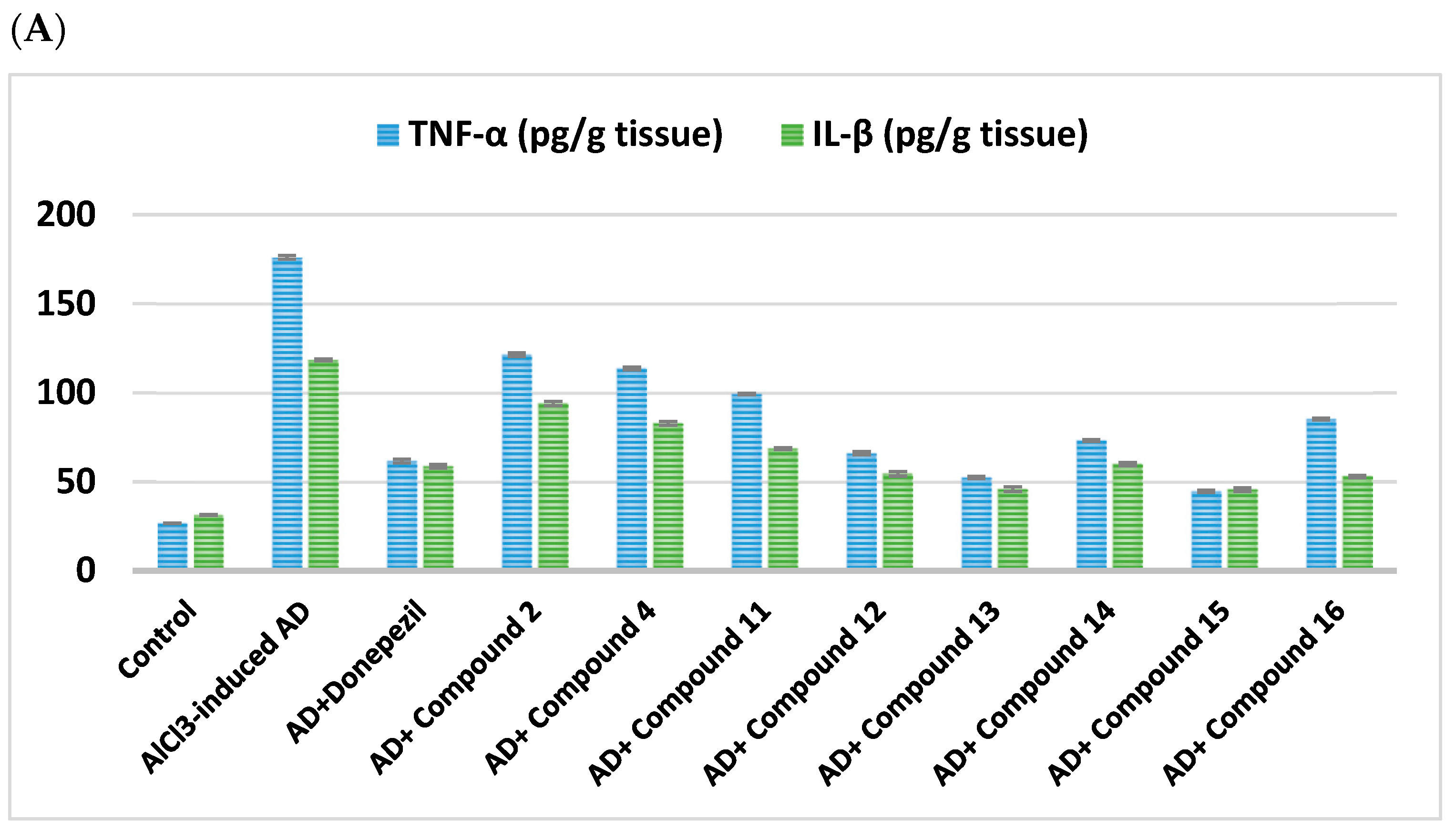
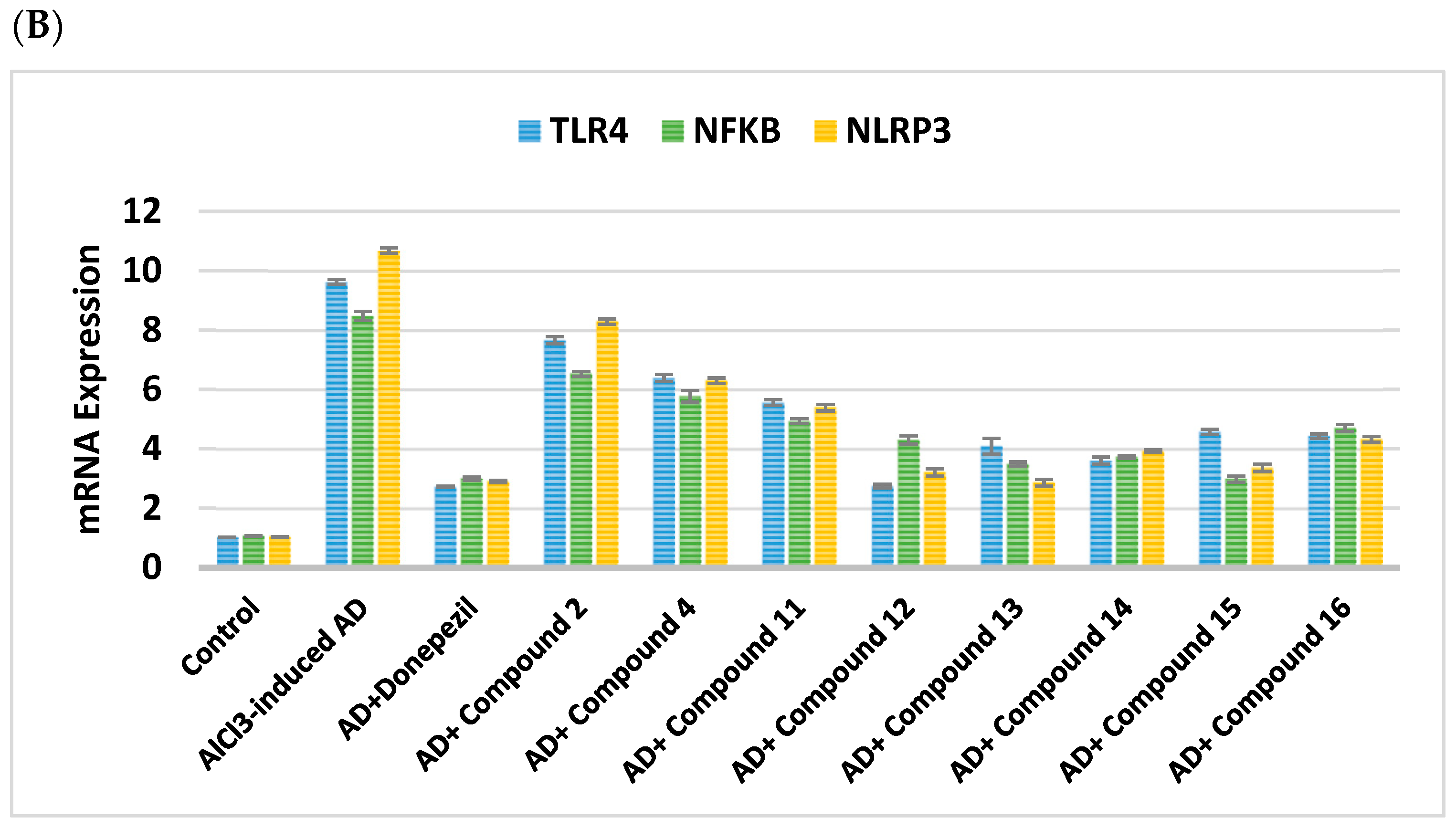
2.2.4. Impact on Neuroinflammatory Biomarkers
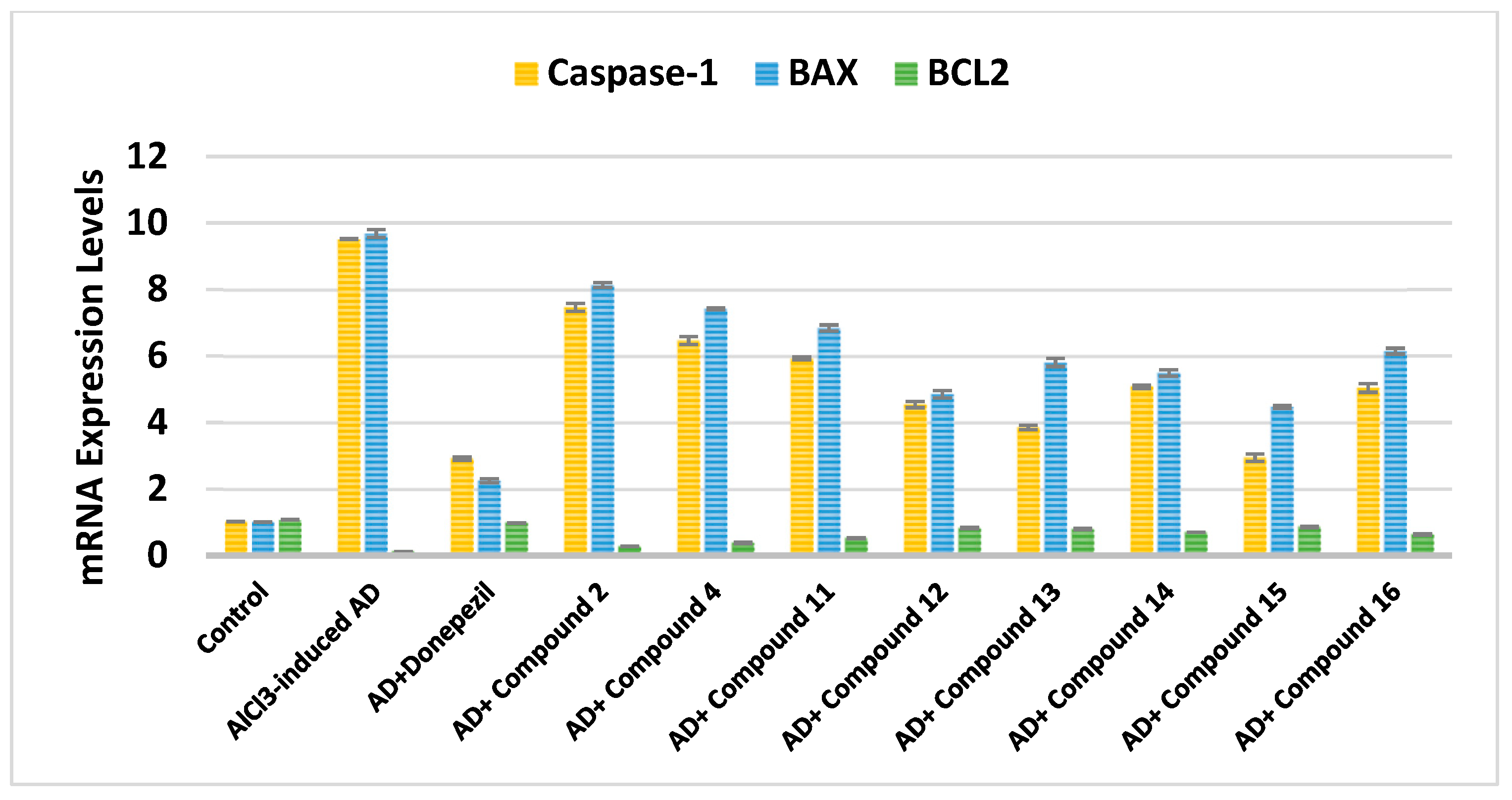
2.2.5. Effect on Caspase-1, BAX, and Bcl-2
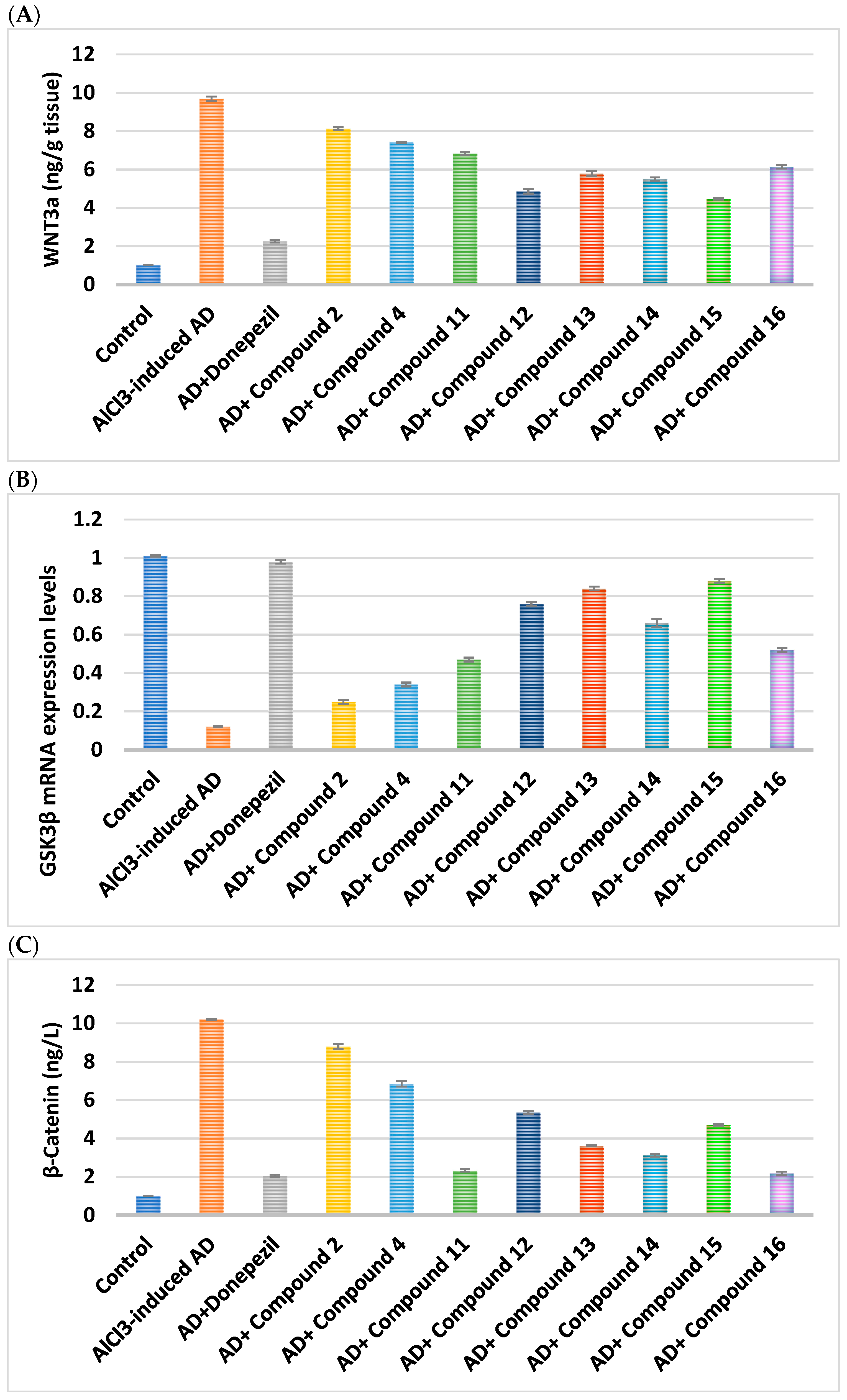
2.2.6. Effect on WNT Signaling Pathway
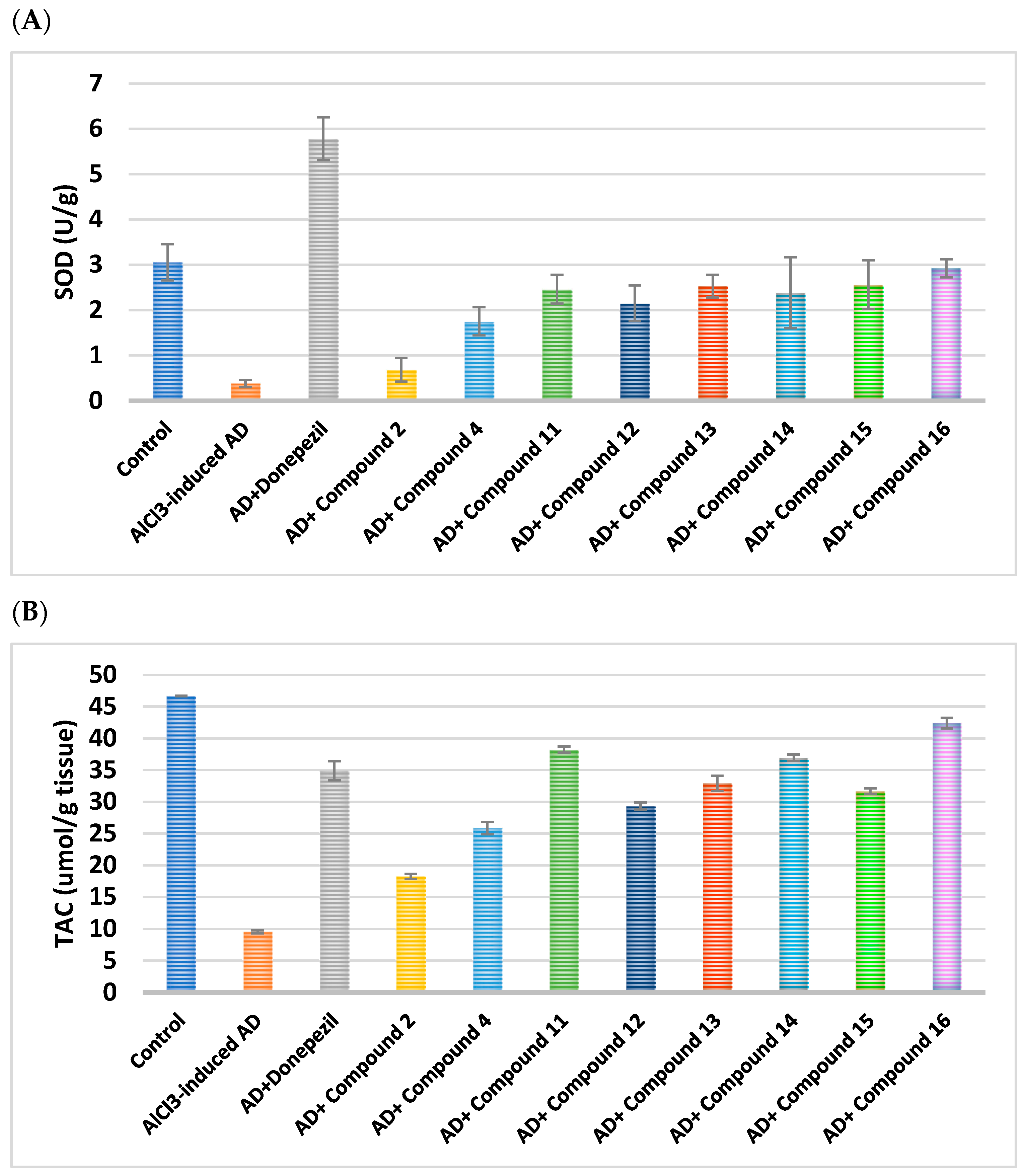
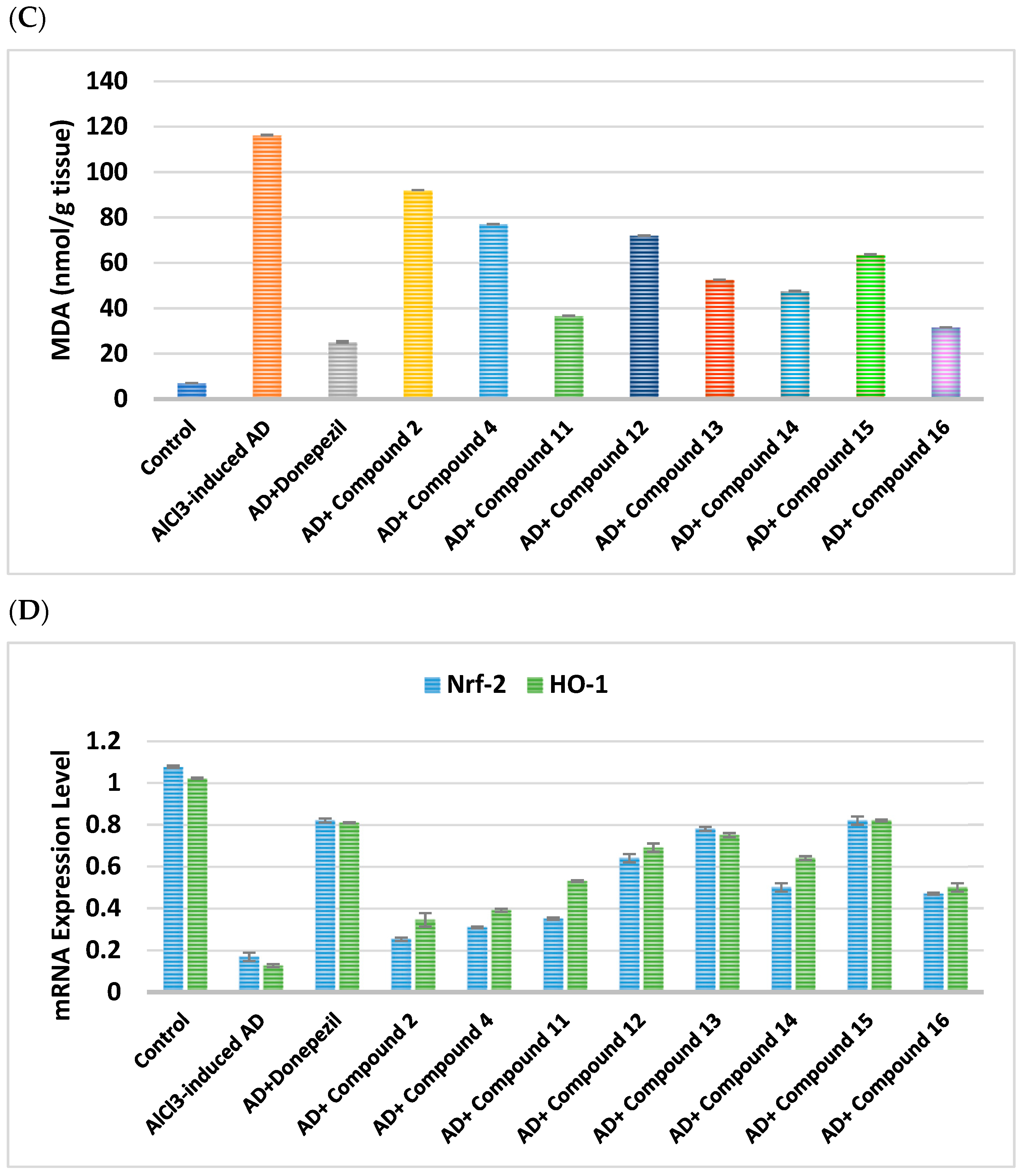
2.2.7. Impacts on Brain Oxidative Stress
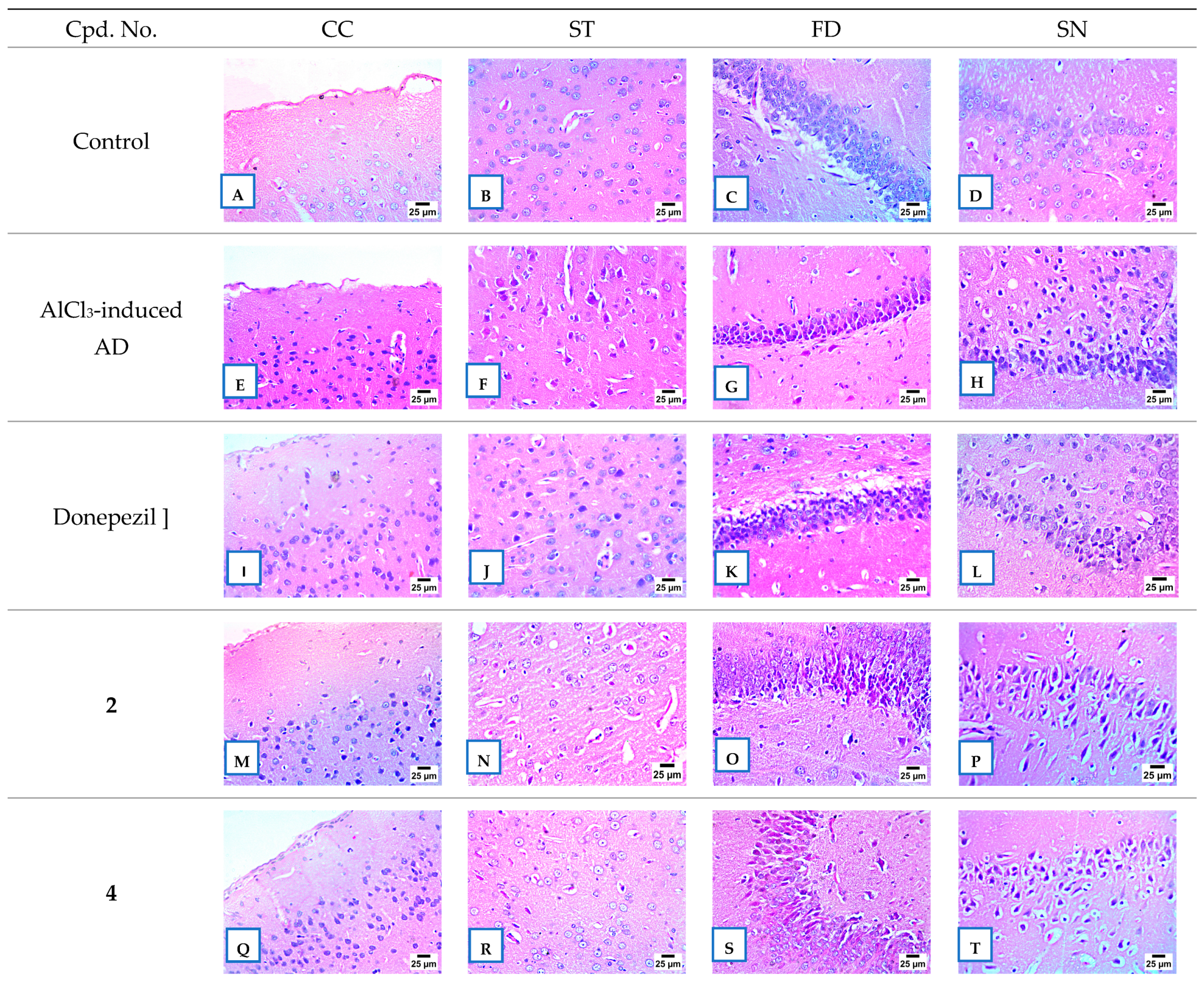
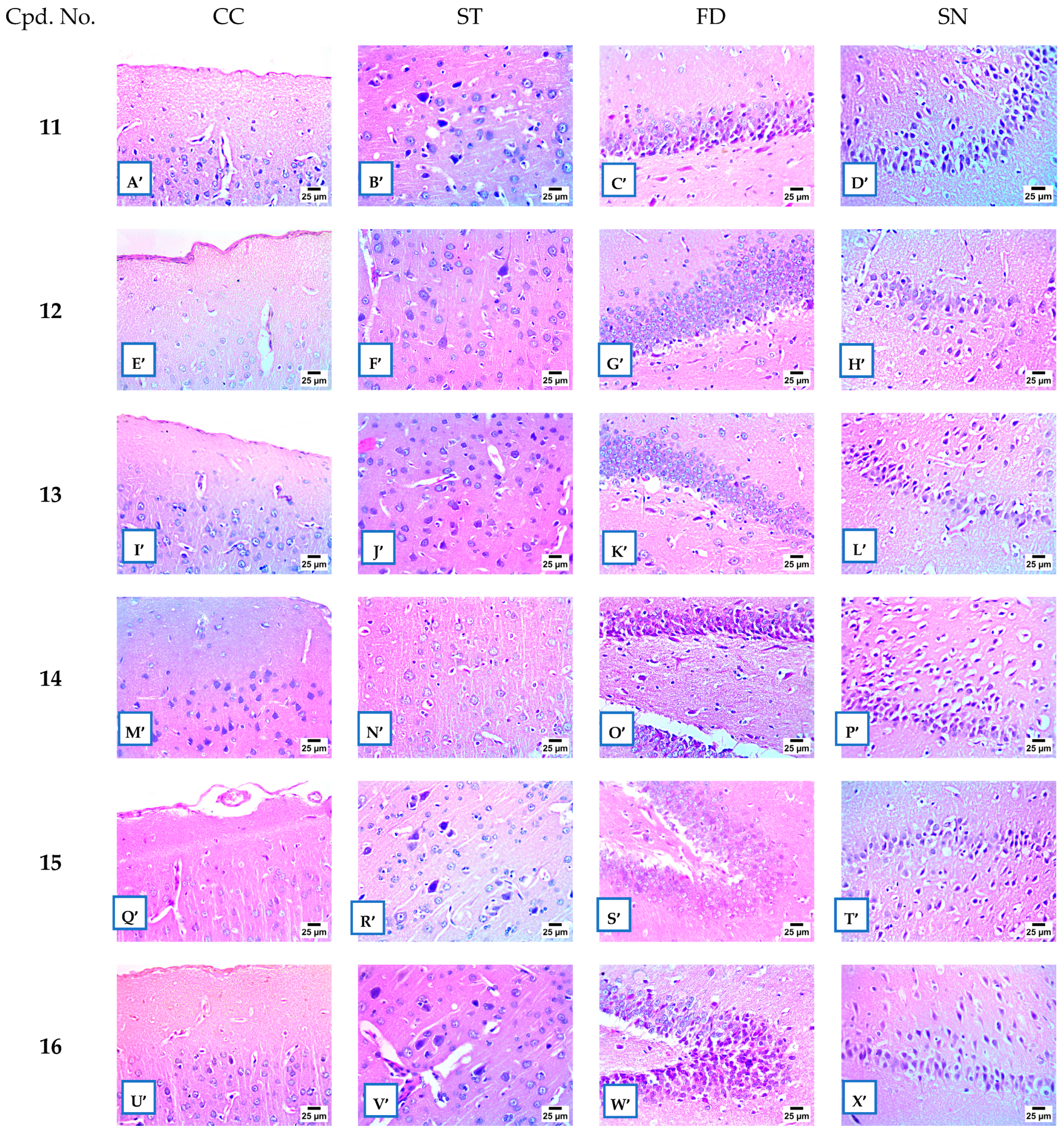
2.2.8. Histopathological Examination
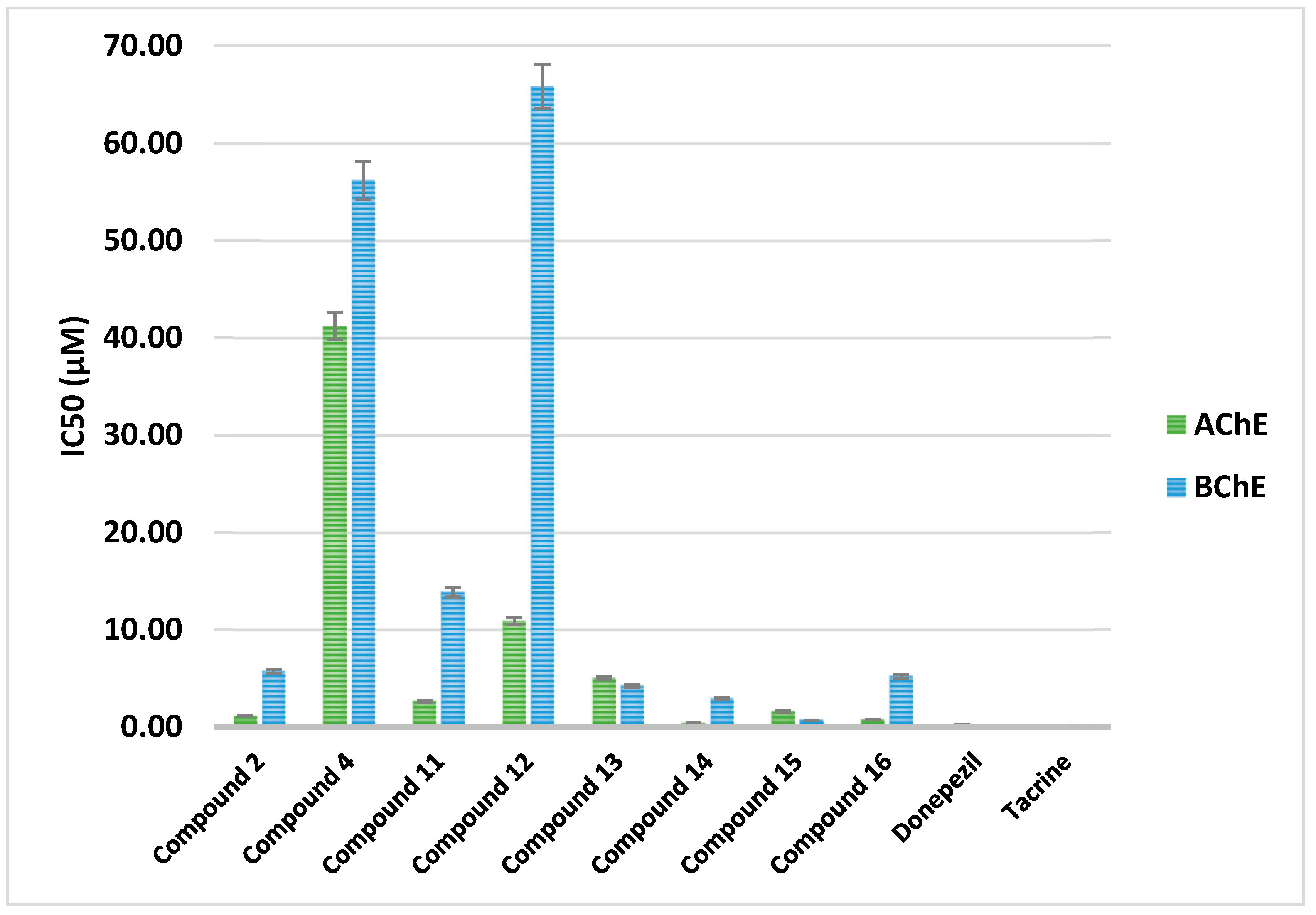
2.2.9. AChE and BChE Inhibition
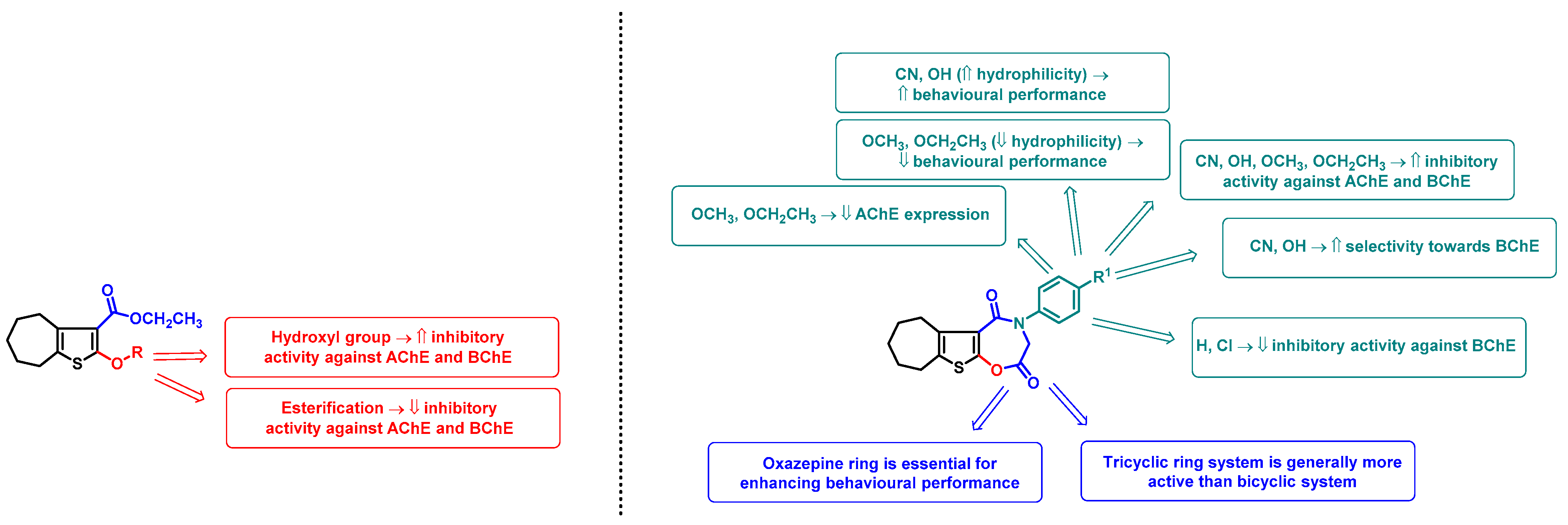
2.3. SAR Analysis
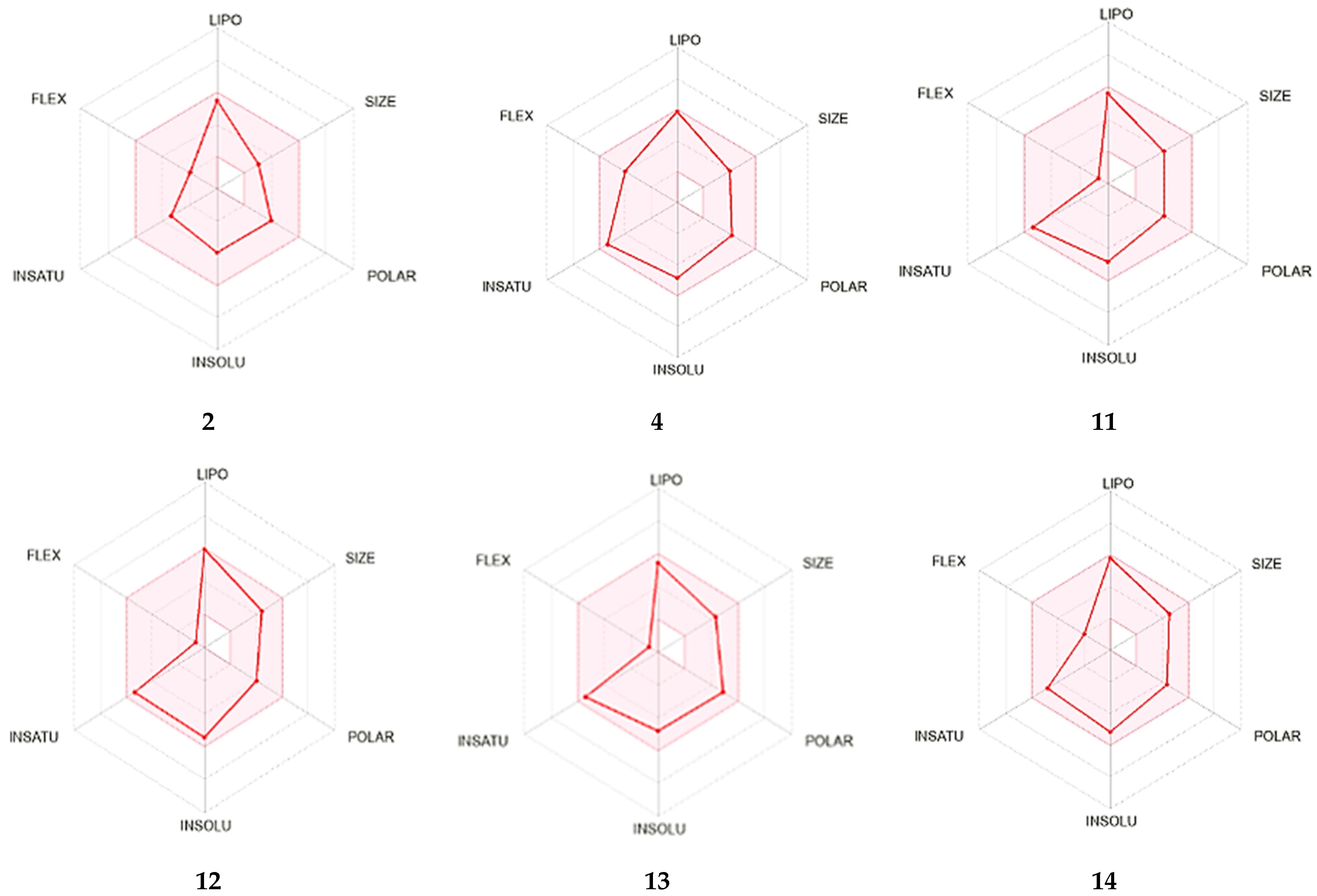
2.4. In Silico ADME Evaluation
2.5. In Silico Toxicity Evaluation
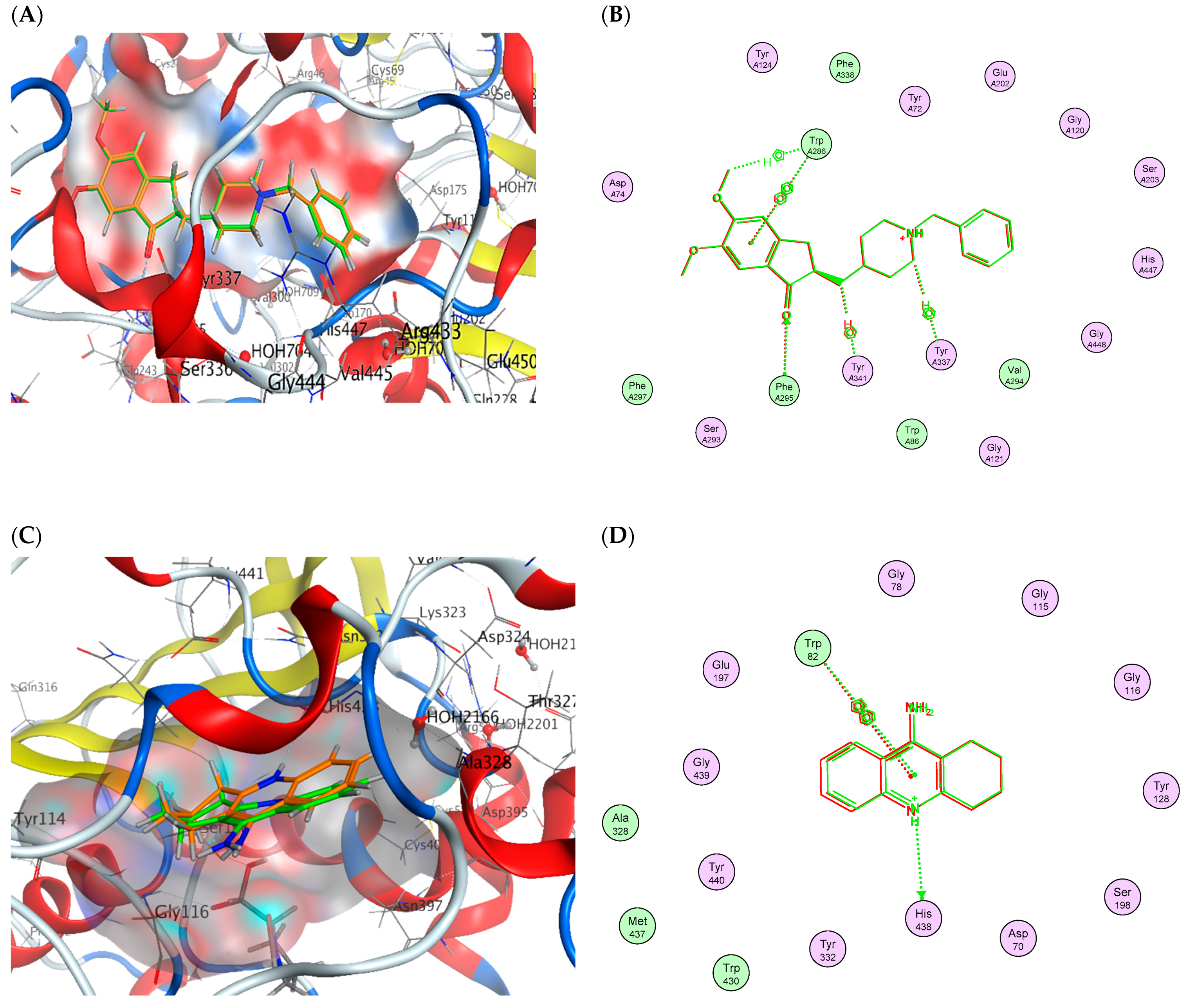
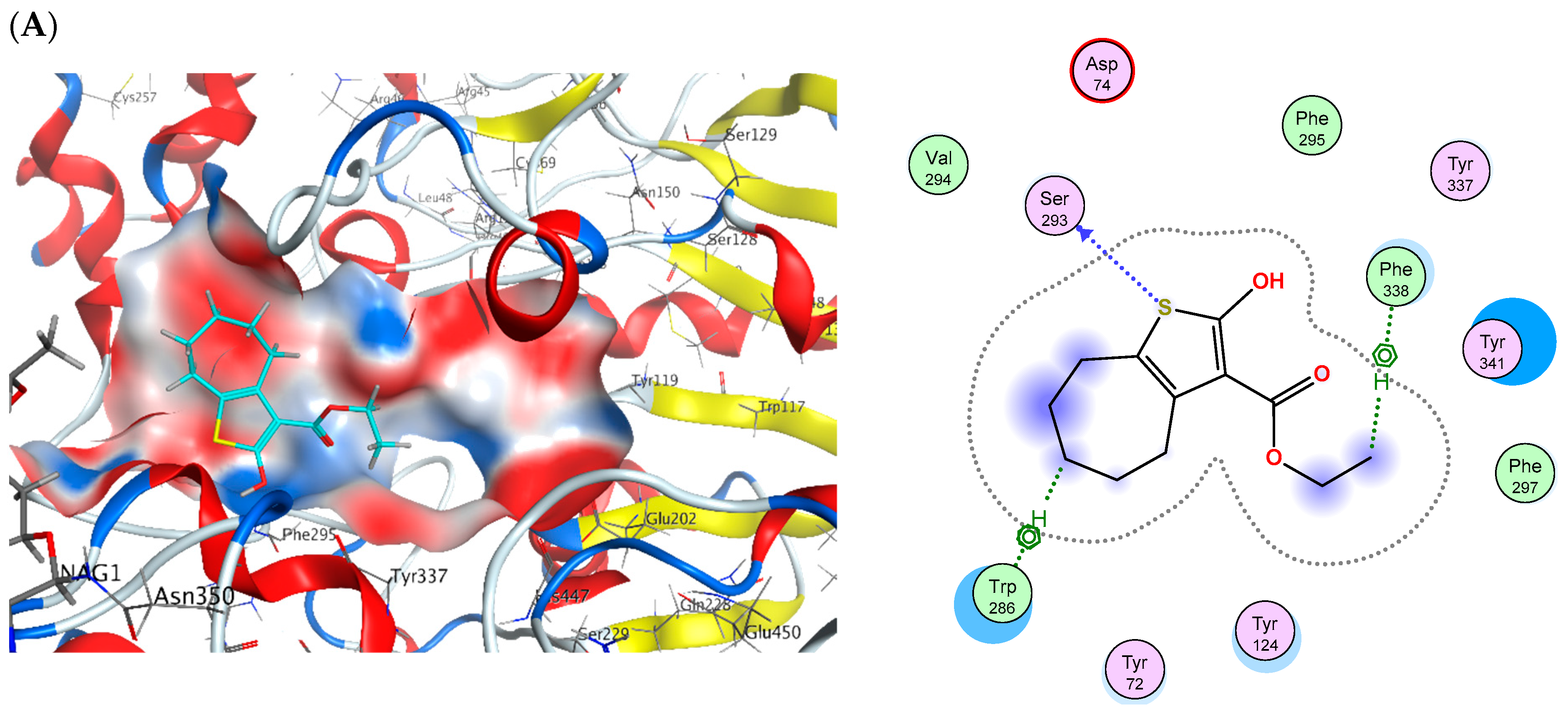
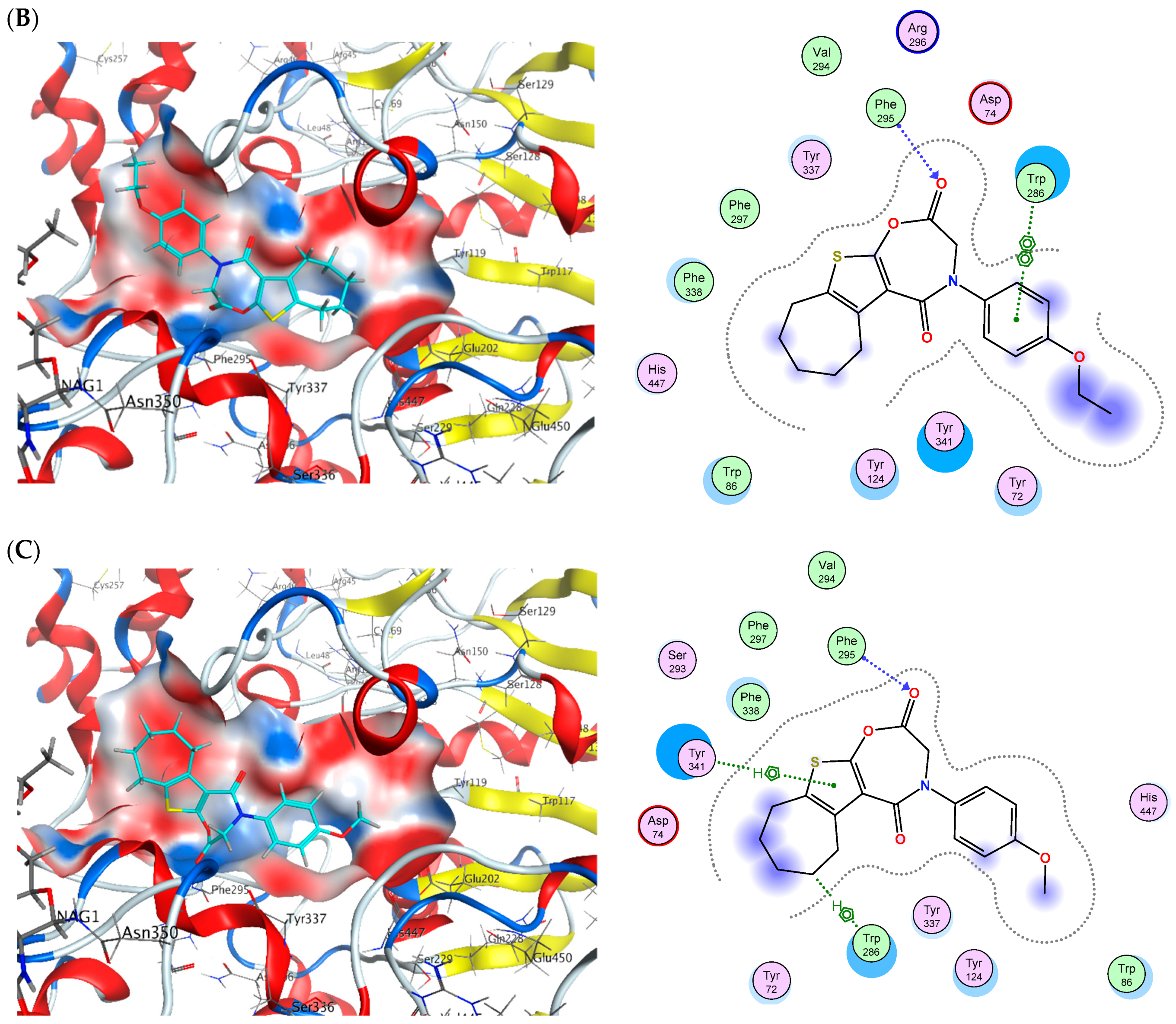
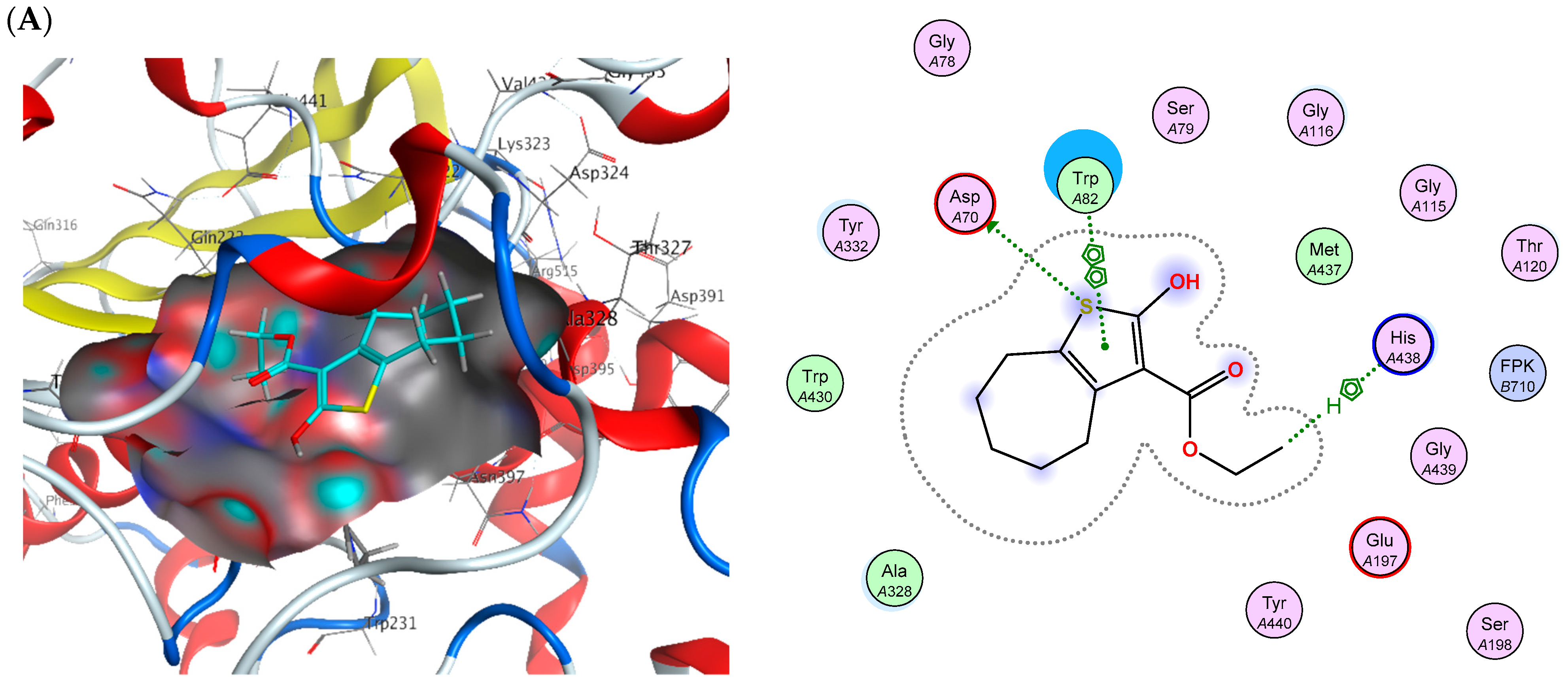
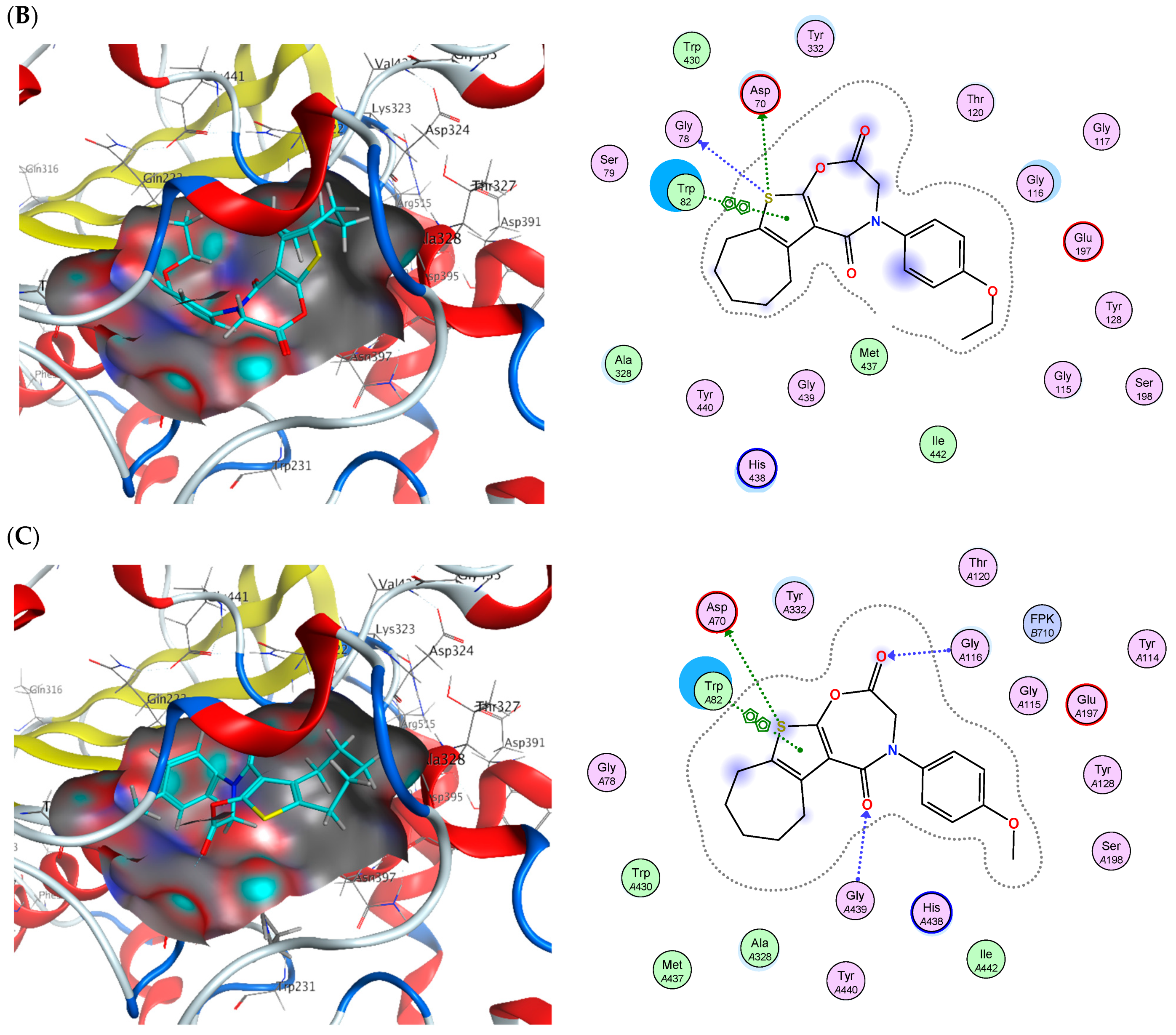
2.6. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. General Details
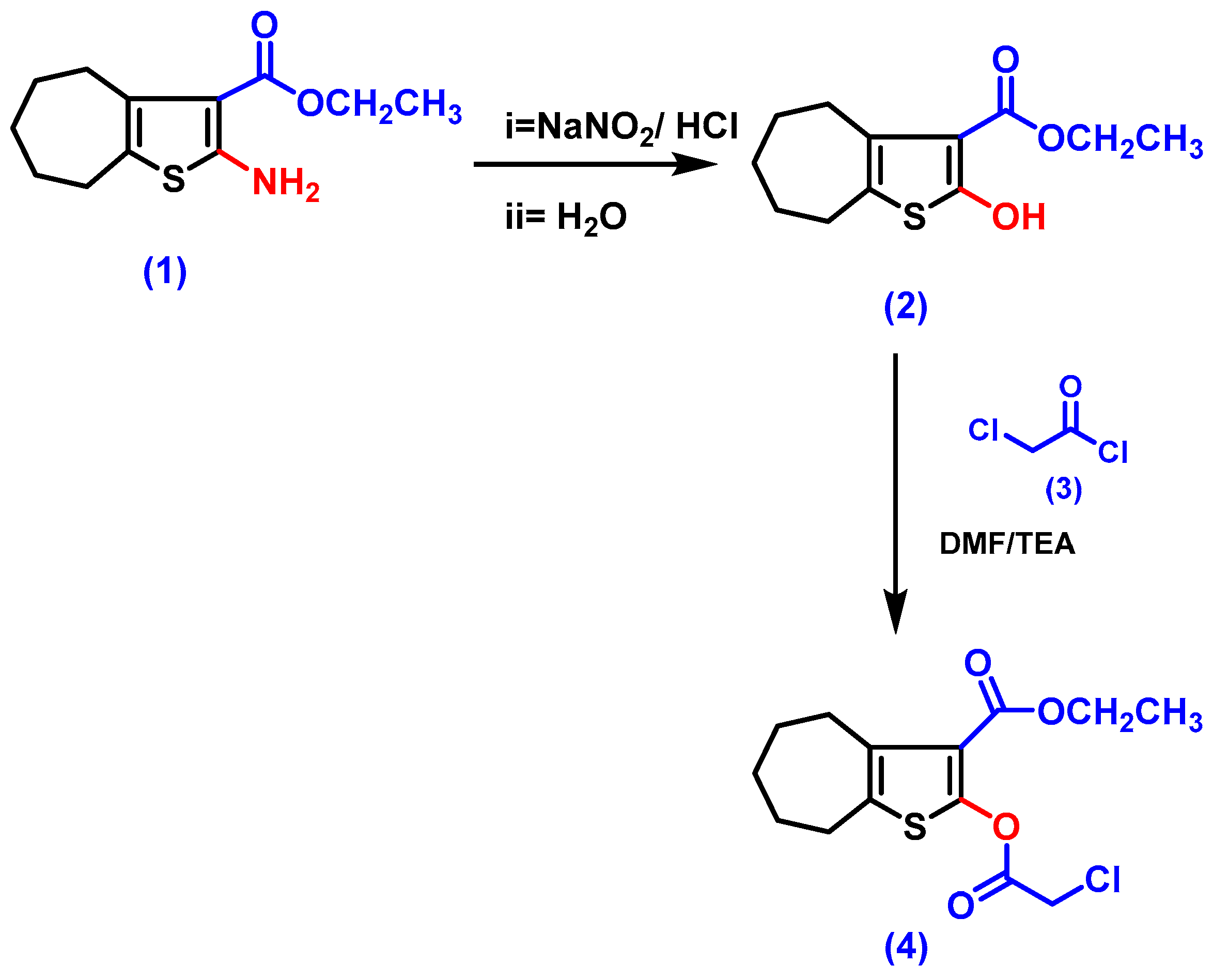
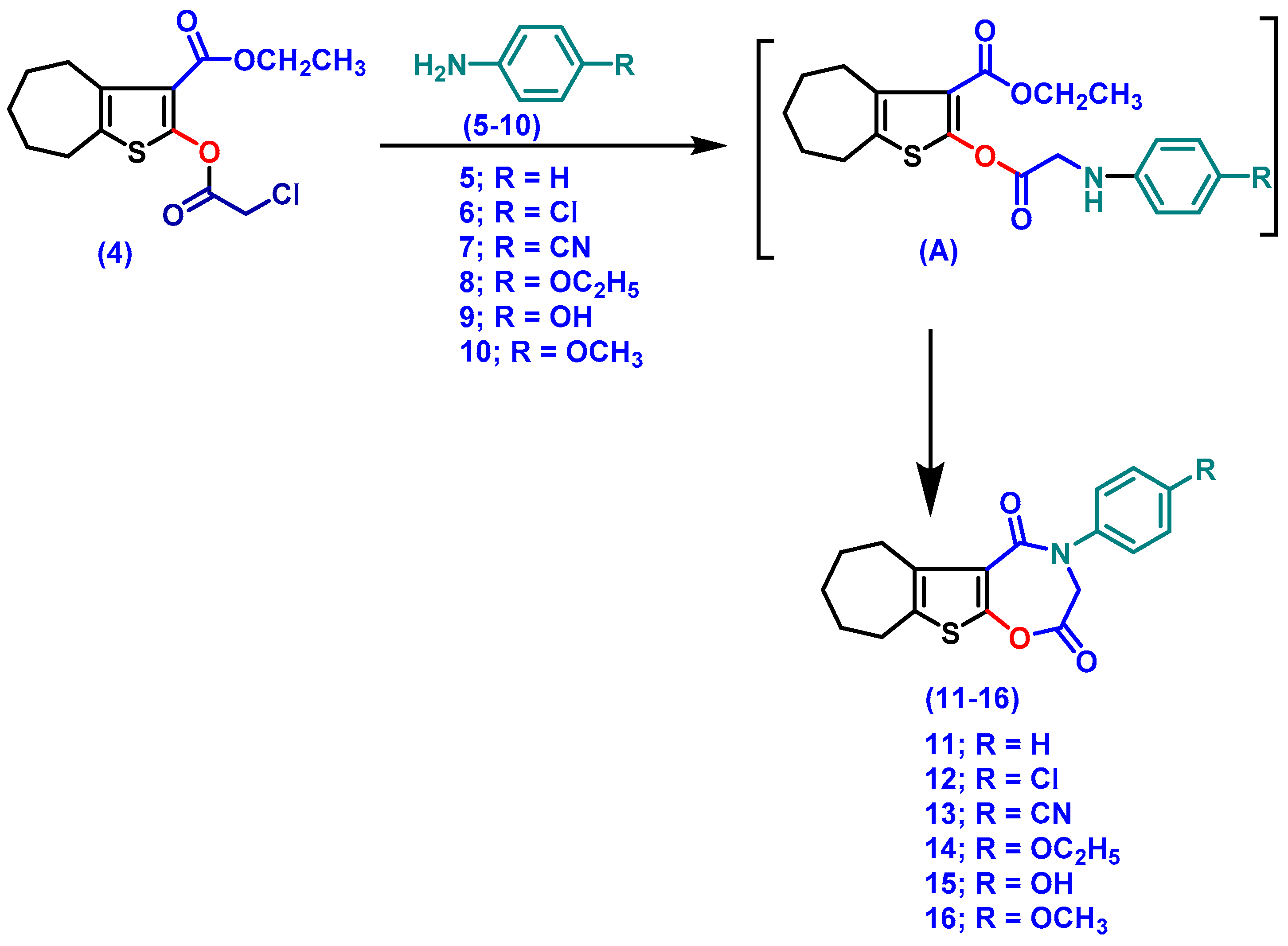
3.1.2. Synthesis
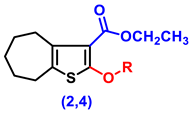
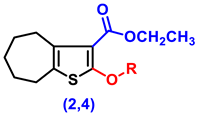
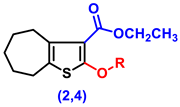
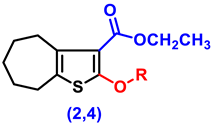
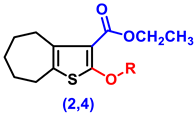
- Ethyl 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate (1)
- Ethyl 2-hydroxy-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate (2)
- Ethyl 2-(2-chloroacetoxy)-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate, (4)
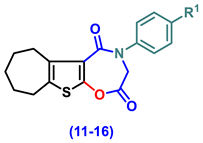
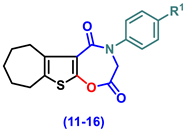
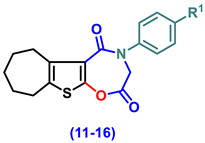
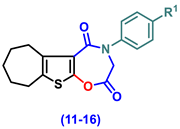
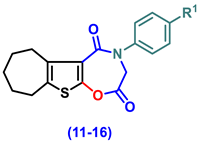
- 4-(Aryl)-3,4,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxazepine-2,5-dione (11–16)
- 4-Phenyl-3,4,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxazepine-2,5-dione, (11)
- 4-(4-Chlorophenyl)-3,4,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxazepine-2,5-dione (12)
- 4-(2,5-Dioxo-2,3,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxazepin-4(5H)-yl)benzonitrile (13)
- 4-(4-Ethoxyphenyl)-3,4,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxazepine-2,5-dione (14)
- 4-(4-Hydroxyphenyl)-3,4,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxa-zepine-2,5-dione (15)
- 4-(4-Methoxyphenyl)-3,4,7,8,9,10-hexahydro-6H-cyclohepta[4,5]thieno[3,2-f][1,4]oxa-zepine-2,5-dione (16)
3.2. Biology
3.2.1. Induction of AD Rat Models
3.2.2. Experimental Design
3.2.3. Behavioral Tests
MWM Test
Y-Maze SAP Test
3.2.4. Tissue Sampling and Preparation
3.2.5. Biochemical Measurements
Fluorometric Technique
Colorimetric Technique
ELISA Technique
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
AChE Inhibition Assay
BChE Inhibition Assay
3.2.6. Statistical Analysis
3.2.7. Histopathology of Brain Tissue
3.3. In Silico ADME and Toxicity Prediction
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef]
- Heilman, K.M.; Nadeau, S.E. Emotional and Neuropsychiatric Disorders Associated with Alzheimer’s Disease. Neurotherapeutics 2022, 19, 99–116. [Google Scholar] [CrossRef]
- Kung, H.F. The β-Amyloid Hypothesis in Alzheimer’s Disease: Seeing Is Believing. ACS Med. Chem. Lett. 2012, 3, 265–267. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Pavlov, P.F.; Petersen, C.H.; Glaser, E.; Ankarcrona, M. Mitochondrial accumulation of APP and Aβ: Significance for Alzheimer disease pathogenesis. J. Cell. Mol. Med. 2009, 13, 4137–4145. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Haam, J.; Yakel, J.L. Cholinergic modulation of the hippocampal region and memory function. J. Neurochem. 2017, 142, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, D.; Wallace, T.L. A Review of the Cholinergic System and Therapeutic Approaches to Treat Brain Disorders. In Behavioral Pharmacology of the Cholinergic System; Shoaib, M., Wallace, T.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mitić, M.; Lazarević-Pašti, T. Does the application of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease lead to depression? Expert Opin. Drug Metab. Toxicol. 2021, 17, 841–856. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, Y.; Wang, L. Neurotransmitters in Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3841. [Google Scholar] [CrossRef]
- Jakob-Roetne, R.; Jacobsen, H. Alzheimer’s Disease: From Pathology to Therapeutic Approaches. Angew. Chem. Int. Ed. 2009, 48, 3030–3059. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, G.; Soreq, H. Termination and beyond: Acetylcholinesterase as a modulator of synaptic transmission. Cell Tissue Res. 2006, 326, 655–669. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Thapa, S.; Lv, M.; Xu, H. Acetylcholinesterase: A Primary Target for Drugs and Insecticides. Mini-Rev. Med. Chem. 2017, 17, 1665–1676. [Google Scholar] [CrossRef]
- Akıncıoğlu, H.; Gülçin, İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini Rev. Med. Chem. 2020, 20, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.B.; Rauter, A.P.; Serralheiro, M.L.M. Polyphenols as acetylcholinesterase inhibitors: Structural specificity and impact on human disease. Nutr. Aging 2012, 1, 99–111. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shimohama, S.; Kamoshima, W.; Ota, T.; Matsuoka, Y.; Nomura, Y.; Smith, M.A.; Perry, G.; Whitehouse, P.J.; Taniguchi, T. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 1998, 780, 260–269. [Google Scholar] [CrossRef]
- Huang, J.; Fairbrother, W.; Reed, J.C. Therapeutic targeting of Bcl-2 family for treatment of B-cell malignancies. Expert Rev. Hematol. 2015, 8, 283–297. [Google Scholar] [CrossRef]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef]
- Thomas, S.; Quinn, B.A.; Das, S.K.; Dash, R.; Emdad, L.; Dasgupta, S.; Wang, X.-Y.; Dent, P.; Reed, J.C.; Pellecchia, M.; et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin. Ther. Targets 2013, 17, 61–75. [Google Scholar] [CrossRef]
- Zhou, J.-D.; Zhang, T.-J.; Xu, Z.-J.; Gu, Y.; Ma, J.-C.; Li, X.-X.; Guo, H.; Wen, X.-M.; Zhang, W.; Yang, L.; et al. BCL2 overexpression: Clinical implication and biological insights in acute myeloid leukemia. Diagn. Pathol. 2019, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Clementi, M.E.; Pezzotti, M.; Orsini, F.; Sampaolese, B.; Mezzogori, D.; Grassi, C.; Giardina, B.; Misiti, F. Alzheimer’s amyloid β-peptide (1–42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: An intriguing role for methionine 35. Biochem. Biophys. Res. Commun. 2006, 342, 206–213. [Google Scholar] [CrossRef]
- Liu, L.-S.; Bai, X.-Q.; Gao, Y.; Wu, Q.; Ren, Z.; Li, Q.; Pan, L.-H.; He, N.-Y.; Peng, J.; Tang, Z.-H. PCSK9 Promotes oxLDL-Induced PC12 Cell Apoptosis Through the Bcl-2/Bax-Caspase 9/3 Signaling Pathway. J. Alzheimer’s Dis. 2017, 57, 723–734. [Google Scholar] [CrossRef]
- Keskin-Aktan, A.; Akbulut, K.G.; Yazici-Mutlu, Ç.; Sonugur, G.; Ocal, M.; Akbulut, H. The effects of melatonin and curcumin on the expression of SIRT2, Bcl-2 and Bax in the hippocampus of adult rats. Brain Res. Bull. 2018, 137, 306–310. [Google Scholar] [CrossRef]
- Abd-Elbaset, M.; Mansour, A.M.; Ahmed, O.M.; Abo-Youssef, A.M. The potential chemotherapeutic effect of β-ionone and/or sorafenib against hepatocellular carcinoma via its antioxidant effect, PPAR-γ, FOXO-1, Ki-67, Bax, and Bcl-2 signaling pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1611–1624. [Google Scholar] [CrossRef]
- Fathy, U.; Abd El Salam, H.A.; Fayed, E.A.; Elgamal, A.M.; Gouda, A. Facile synthesis and in vitro anticancer evaluation of a new series of tetrahydroquinoline. Heliyon 2021, 7, e08117. [Google Scholar] [CrossRef]
- Arab, H.H.; Khames, A.; Mohammad, M.K.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Gad, A.M. Meloxicam Targets COX-2/NOX1/NOX4/Nrf2 Axis to Ameliorate the Depression-like Neuropathology Induced by Chronic Restraint Stress in Rats. Pharmaceuticals 2023, 16, 848. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Khames, A.; Alsufyani, S.E.; El-Sheikh, A.A.K.; Gad, A.M. Targeting the Endoplasmic Reticulum Stress-Linked PERK/GRP78/CHOP Pathway with Magnesium Sulfate Attenuates Chronic-Restraint-Stress-Induced Depression-like Neuropathology in Rats. Pharmaceuticals 2023, 16, 300. [Google Scholar] [CrossRef] [PubMed]
- Ammar, Y.; Fayed, E.; Bayoumi, A.; Saleh, M. Synthesis and biological evaluation of new amides pro-drugs containing naproxen moiety as anti-inflammatory and antimicrobial agents. Pharma Chem. 2016, 8, 495–508. [Google Scholar]
- Liu, Y.; Dai, Y.; Li, Q.; Chen, C.; Chen, H.; Song, Y.; Hua, F.; Zhang, Z. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci. Lett. 2020, 736, 135279. [Google Scholar] [CrossRef]
- Hassan, M.-A.M.; Gad, A.M.; Menze, E.T.; Badary, O.A.; El-Naga, R.N. Protective effects of morin against depressive-like behavior prompted by chronic unpredictable mild stress in rats: Possible role of inflammasome-related pathways. Biochem. Pharmacol. 2020, 180, 114140. [Google Scholar] [CrossRef]
- Huang, J.; Huang, N.; Xu, S.; Luo, Y.; Li, Y.; Jin, H.; Yu, C.; Shi, J.; Jin, F. Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J. Nutr. Biochem. 2021, 88, 108552. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Q. Activation of NLRP3 Inflammasome and Onset of Alzheimer’s Disease. Front. Immunol. 2021, 12, 701282. [Google Scholar] [CrossRef]
- Van Zeller, M.; Dias, D.; Sebastião, A.M.; Valente, C.A.; Wu, Z. NLRP3 Inflammasome: A Starring Role in Amyloid-β- and Tau-Driven Pathological Events in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 83, 939–961. [Google Scholar] [CrossRef]
- Hulse, J.; Bhaskar, K. Crosstalk Between the NLRP3 Inflammasome/ASC Speck and Amyloid Protein Aggregates Drives Disease Progression in Alzheimer’s and Parkinson’s Disease. Front. Mol. Neurosci. 2022, 15, 805169. [Google Scholar] [CrossRef]
- Anwar, H.M.; Georgy, G.S.; Hamad, S.R.; Badr, W.K.; El Raey, M.A.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. A Leaf Extract of Harrisonia abyssinica Ameliorates Neurobehavioral, Histological and Biochemical Changes in the Hippocampus of Rats with Aluminum Chloride-Induced Alzheimer’s Disease. Antioxidants 2021, 10, 947. [Google Scholar] [CrossRef]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 2019, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Shen, Y. Interruption of β-Catenin Signaling Reduces Neurogenesis in Alzheimer’s Disease. J. Neurosci. 2009, 29, 6545–6557. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Giovannini, D.; Rossi, S.; Cozzolino, M. Targeting the Wnt/β-catenin pathway in neurodegenerative diseases: Recent approaches and current challenges. Expert Opin. Drug Discov. 2020, 15, 803–822. [Google Scholar] [CrossRef]
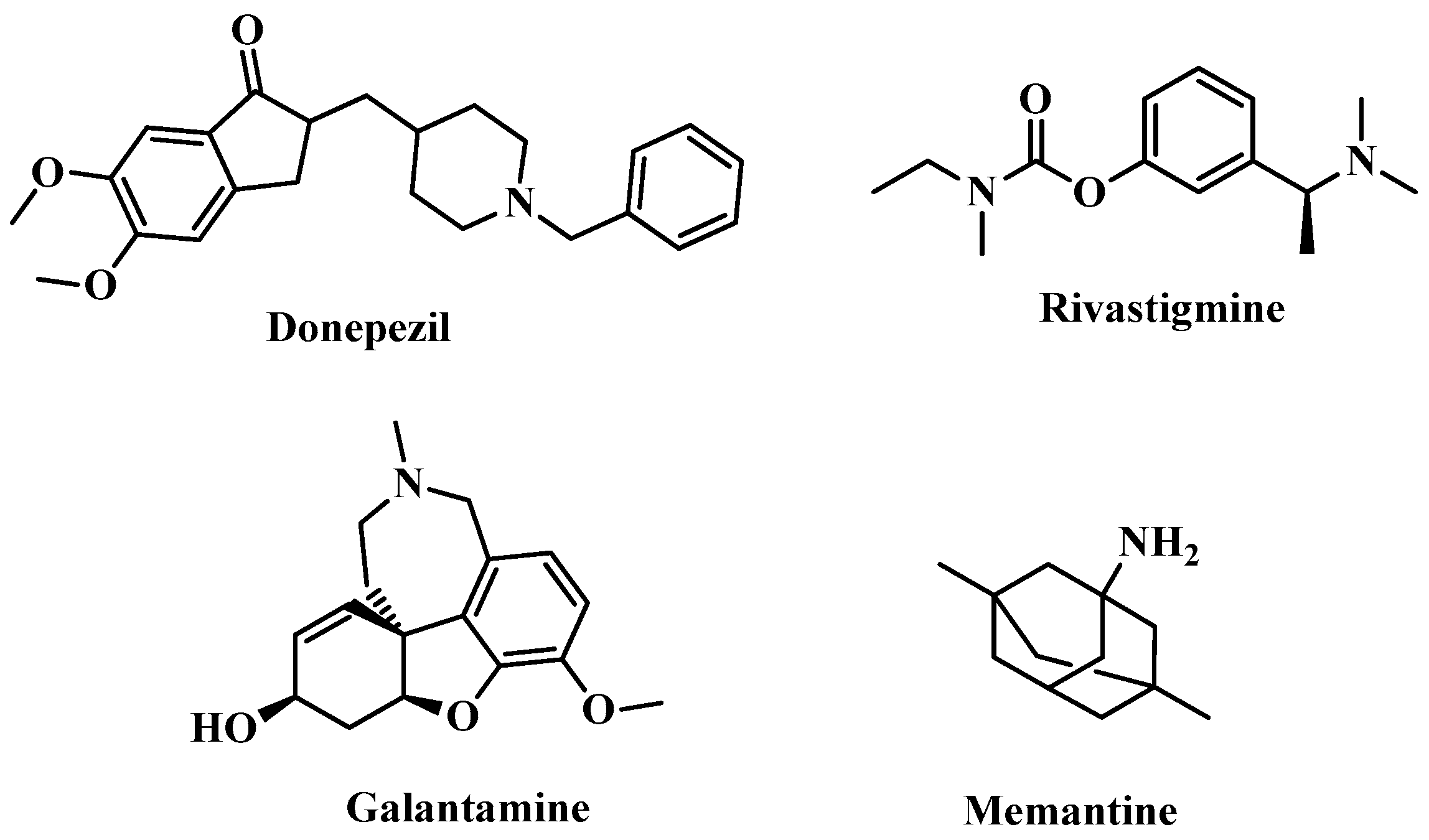
- Shigeta, M.; Homma, A. Donepezil for Alzheimer’s Disease: Pharmacodynamic, Pharmacokinetic, and Clinical Profiles. CNS Drug Rev. 2001, 7, 353–368. [Google Scholar] [CrossRef]
- Birks, J.S.; Evans, J.G. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 2015, CD001191. [Google Scholar] [CrossRef]
- Robinson, D.M.; Keating, G.M. Memantine. Drugs 2006, 66, 1515–1534. [Google Scholar] [CrossRef] [PubMed]
- Prvulovic, D.; Hampel, H.; Pantel, J. Galantamine for Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2010, 6, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-C.; Yu, J.-T.; Wang, H.-F.; Tan, M.-S.; Meng, X.-F.; Wang, C.; Jiang, T.; Zhu, X.-C.; Tan, L. Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2014, 41, 615–631. [Google Scholar] [CrossRef]
- Shen, Z.X. Brain cholinesterases: II. The molecular and cellular basis of Alzheimer’s disease. Med. Hypotheses 2004, 63, 308–321. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterases: New Roles in Brain Function and in Alzheimer’s Disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef]
- Jasiecki, J.; Targońska, M.; Wasąg, B. The Role of Butyrylcholinesterase and Iron in the Regulation of Cholinergic Network and Cognitive Dysfunction in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 2033. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Yang, H.; Chen, Y.; Wang, F.; Li, J.; Tang, Z.; Cheng, X.; Yang, Y.; Xu, L.; et al. Discovery of Selective Butyrylcholinesterase (BChE) Inhibitors through a Combination of Computational Studies and Biological Evaluations. Molecules 2019, 24, 4217. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Sun, T.; Dong, Z.; Malugulu, P.M.; Zhen, T.; Wang, L.; Chen, Y.; Sun, H. Advances in design strategies and imaging applications of specific butyrylcholinesterase probes. Chin. Chem. Lett. 2024, 36, 110451. [Google Scholar] [CrossRef]
- Stanciu, G.D.; Luca, A.; Rusu, R.N.; Bild, V.; Beschea Chiriac, S.I.; Solcan, C.; Bild, W.; Ababei, D.C. Alzheimer’s Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi-Mohammareh, S.; Shiri, A.; Maleki, E.H.; Matin, M.M.; Beyzaei, H.; Baranipour, P.; Oroojalian, F.; Memariani, T. Synthesis of Various Derivatives of [1,3]Selenazolo[4,5-d]pyrimidine and Exploitation of These Heterocyclic Systems as Antibacterial, Antifungal, and Anticancer Agents. ChemistrySelect 2020, 5, 10060–10066. [Google Scholar] [CrossRef]
- Fayed, E.A.; Al-Arab, E.M.E.; Saleh, A.S.; Bayoumi, A.H.; Ammar, Y.A. Design, synthesis, in silico studies, in vivo and in vitro assessment of pyridones and thiazolidinones as anti-inflammatory, antipyretic and ulcerogenic hits. J. Mol. Struct. 2022, 1260, 132839. [Google Scholar] [CrossRef]
- Ramsis, T.; Refat, M.; Selim, H.M.; Elseedy, H.; Fayed, E.A. The role of current synthetic and possible plant and marine phytochemical compounds in the treatment of acne. RSC Adv. 2024, 14, 24287–24321. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Palumbo Piccionello, A. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Kassab, A.E.; Gedawy, E.M.; Sayed, A.S. Fused thiophene as a privileged scaffold: A review on anti-Alzheimer’s disease potentials via targeting cholinesterases, monoamine oxidases, glycogen synthase kinase-3, and Aβ aggregation. Int. J. Biol. Macromol. 2024, 265, 131018. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Heterocyclic Compounds: Importance in Anticancer Drug Discovery. Anticancer Agents Med. Chem. 2022, 22, 3196–3207. [Google Scholar] [CrossRef]
- Azzam, R.A.; Gad, N.M.; Elgemeie, G.H. Novel Thiophene Thioglycosides Substituted with the Benzothiazole Moiety: Synthesis, Characterization, Antiviral and Anticancer Evaluations, and NS3/4A and USP7 Enzyme Inhibitions. ACS Omega 2022, 7, 35656–35667. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, R.M.D.; Mendonça-Junior, F.J.B.; de Mélo, N.B.; Scotti, L.; de Araújo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-Based Compounds with Potential Anti-Inflammatory Activity. Pharmaceuticals 2021, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Fathy, U.; Yousif, M.N.M.; Mohi El-Deen, E.M.; Fayed, E. Design, Synthesis, and biological evaluation of a novel series of thiazole derivatives based on pyrazoline as anticancer agents. Egypt. J. Chem. 2022, 65, 1241–1252. [Google Scholar] [CrossRef]
- Shah, R.; Verma, P.K. Therapeutic importance of synthetic thiophene. Chem. Cent. J. 2018, 12, 137. [Google Scholar] [CrossRef]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef]
- Cale, A.D., Jr.; Gero, T.W.; Walker, K.R.; Lo, Y.S.; Welstead, W.J., Jr.; Jaques, L.W.; Johnson, A.F.; Leonard, C.A.; Nolan, J.C.; Johnson, D.N. Benzo- and pyrido-1,4-oxazepin-5-ones and -thiones: Synthesis and structure-activity relationships of a new series of H1-antihistamines. J. Med. Chem. 1989, 32, 2178–2199. [Google Scholar] [CrossRef]
- Ramajayam, R.; Girdhar, R.; Yadav, M.R. Current Scenario of 1,4-Diazepines as Potent Biomolecules-A Mini Review. Mini Rev. Med. Chem. 2007, 7, 793–812. [Google Scholar] [CrossRef]
- Fayed, E.A.; El-Sebaey, S.A.; Ebrahim, M.A.; Abu-Elfotuh, K.; El-Sayed Mansour, R.; Mohamed, E.K.; Hamdan, A.M.E.; Al-subaie, F.T.; Albalawi, G.S.; Albalawi, T.M.; et al. Discovery of novel bicyclic and tricyclic cyclohepta[b]thiophene derivatives as multipotent AChE and BChE inhibitors, In-Vivo and In-Vitro assays, ADMET and molecular docking simulation. Eur. J. Med. Chem. 2025, 284, 117201. [Google Scholar] [CrossRef]
- Stanisheva, D.V.; Gerova, M.S.; Petrov, O.I. Synthesis of a new polycyclic heterocyclic ring system. Part III. Benzo[b]imidazo[1,5-d][1,4]oxazepine-1,4(2H,5H)-diones. Heterocycl. Commun. 2017, 23, 23–27. [Google Scholar] [CrossRef]
- dos Santos, G.C.; Martins, L.M.; Bregadiolli, B.A.; Moreno, V.F.; da Silva-Filho, L.C.; da Silva, B.H.S.T. Heterocyclic compounds as antiviral drugs: Synthesis, structure–activity relationship and traditional applications. J. Heterocycl. Chem. 2021, 58, 2226–2260. [Google Scholar] [CrossRef]
- Nirmala, K.A.; Vasu; Chopra, D.; Mohan, S.; Prasad, M.R. Ethyl 2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carboxylate. Acta Crystallogr. Sect. E 2005, 61, o1541–o1543. [Google Scholar] [CrossRef]
- Fayed, E.; Ahmed, H. Synthesis, characterization and pharmacological evaluation of some new 1, 4-diazepine derivatives as anticancer agents. Pharma Chem. 2016, 8, 77–90. [Google Scholar]
- Durán-Peña, M.J.; Botubol-Ares, J.M.; Hanson, J.R.; Hernández-Galán, R.; Collado, I.G. Efficient O-Acylation of Alcohols and Phenol Using Cp2TiCl as a Reaction Promoter. Eur. J. Org. Chem. 2016, 2016, 3584–3591. [Google Scholar] [CrossRef]
- Anbu, N.; Nagarjun, N.; Jacob, M.; Kalaiarasi, J.M.V.K.; Dhakshinamoorthy, A. Acetylation of Alcohols, Amines, Phenols, Thiols under Catalyst and Solvent-Free Conditions. Chemistry 2019, 1, 69–79. [Google Scholar] [CrossRef]
- Gavin, J.T.; Annor-Gyamfi, J.K.; Bunce, R.A. Quinazolin-4(3H)-ones and 5,6-Dihydropyrimidin-4(3H)-ones from β-Aminoamides and Orthoesters. Molecules 2018, 23, 2925. [Google Scholar] [CrossRef]
- Annor-Gyamfi, J.K.; Bunce, R.A. 4H-Benzo[d][1,3]oxazin-4-ones and Dihydro Analogs from Substituted Anthranilic Acids and Orthoesters. Molecules 2019, 24, 3555. [Google Scholar] [CrossRef]
- Fayed, E.A.; Gohar, N.A.; Farrag, A.M.; Ammar, Y.A. Upregulation of BAX and caspase-3, as well as downregulation of Bcl-2 during treatment with indeno[1,2-b]quinoxalin derivatives, mediated apoptosis in human cancer cells. Arch. Pharm. 2022, 355, 2100454. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, S.A.; Pennington, L.D. Structure−Brain Exposure Relationships. J. Med. Chem. 2006, 49, 7559–7583. [Google Scholar] [CrossRef]
- Prinz, M.; Parlar, S.; Bayraktar, G.; Alptüzün, V.; Erciyas, E.; Fallarero, A.; Karlsson, D.; Vuorela, P.; Burek, M.; Förster, C.; et al. 1,4-Substituted 4-(1H)-pyridylene-hydrazone-type inhibitors of AChE, BuChE, and amyloid-β aggregation crossing the blood–brain barrier. Eur. J. Pharm. Sci. 2013, 49, 603–613. [Google Scholar] [CrossRef]
- Capuzzi, S.J.; Muratov, E.N.; Tropsha, A. Phantom PAINS: Problems with the Utility of Alerts for Pan-Assay Interference Compounds. J. Chem Inf. Model. 2017, 57, 417–427. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef]
- AbdelFattah, B.A.; Khalifa, M.M.; El-Sehrawi, H.; Fayed, E.; Bayoumi, A.; Said, M. Synthesis and Anxiolytic Activity of Some Novel 5-oxo-1, 4-oxazepine Derivatives. Lett. Drug Des. Discov. 2011, 8, 330–338. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Skalicka-Woźniak, K.; Budzyńska, B.; El Sayed, N.S. NLRP3 inflammasome inhibition and M1-to-M2 microglial polarization shifting via scoparone-inhibited TLR4 axis in ovariectomy/D-galactose Alzheimer’s disease rat model. Int. Immunopharmacol. 2023, 119, 110239. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models: Techniques and Protocols; Guest, P.C., Ed.; Springer: New York, NY, USA, 2019; pp. 105–111. [Google Scholar]
- Ciarlone, A.E. Further modification of a fluorometric method for analyzing brain amines. Microchem. J. 1978, 23, 9–12. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356. [Google Scholar] [CrossRef]
- Maczynska, I.; Millo, B.; Ratajczak-Stefańska, V.; Maleszka, R.; Szych, Z.; Kurpisz, M.; Giedrys-Kalemba, S. Proinflammatory cytokine (IL-1β, IL-6, IL-12, IL-18 and TNF-α) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE). Immunol. Lett. 2006, 102, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.F.; Sabry, M.A.; Abdelwahab, G. Araucaria heterophylla oleogum resin essential oil is a novel aldose reductase and butyryl choline esterase enzymes inhibitor: In vitro and in silico evidence. Sci. Rep. 2023, 13, 11446. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.T.; Brogi, S. MicroRNA-Based Multitarget Approach for Alzheimer’s Disease: Discovery of the First-In-Class Dual Inhibitor of Acetylcholinesterase and MicroRNA-15b Biogenesis. J. Med. Chem. 2020, 63, 9695–9704. [Google Scholar] [CrossRef]
- Beigom Hejaziyan, L.; Hosseini, S.M.; Taravati, A.; Asadi, M.; Bakhshi, M.; Moshaei Nezhad, P.; Gol, M.; Mououdi, M. Effect of Rosa damascena Extract on Rat Model Alzheimer’s Disease: A Histopathological, Behavioral, Enzyme Activities, and Oxidative Stress Study. Evid.-Based Complement. Altern. Med. 2023, 2023, 4926151. [Google Scholar] [CrossRef]
- Knezovic, A.; Osmanovic-Barilar, J.; Curlin, M.; Hof, P.R.; Simic, G.; Riederer, P.; Salkovic-Petrisic, M. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J. Neural Transm. 2015, 122, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.A.; Ebrahim, M.A.; Fathy, U.; Elawady, A.M.; Khalaf, W.S.; Ramsis, T.M. Pyrano-coumarin hybrids as potential antimicrobial agents against MRSA strains: Design, synthesis, ADMET, molecular docking studies, as DNA gyrase inhibitors. J. Mol. Struct. 2024, 1295, 136663. [Google Scholar] [CrossRef]
- Fayed, E.A.; Thabet, A.; El-Gilil, S.M.A.; Elsanhory, H.M.A.; Ammar, Y.A. Fluorinated thiazole–thiosemicarbazones hybrids as potential PPAR-γ agonist and α-amylase, α-glucosidase antagonists: Design, synthesis, in silico ADMET and docking studies and hypoglycemic evaluation. J. Mol. Struct. 2024, 1301, 137374. [Google Scholar] [CrossRef]
- Gohar, N.A.; Fayed, E.A.; Ammar, Y.A.; Abu Ali, O.A.; Ragab, A.; Mahfoz, A.M.; Abusaif, M.S. Fluorinated indeno-quinoxaline bearing thiazole moieties as hypoglycaemic agents targeting α-amylase, and α-glucosidase: Synthesis, molecular docking, and ADMET studies. J. Enzym. Inhib. Med. Chem. 2024, 39, 2367128. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.-N.; Yao, Z.-J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.-P.; Cao, D.-S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE). 2014.09 Chemical Computing Group ULC, 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7. 2014. Available online: https://www.chemcomp.com/ (accessed on 1 February 2025).
- Refai, M.Y.; Elazzazy, A.M.; Desouky, S.E.; Abu-Elghait, M.; Fayed, E.A.; Alajel, S.M.; Alajlan, A.A.; Albureikan, M.O.; Nakayama, J. Interception of Epoxide ring to quorum sensing system in Enterococcus faecalis and Staphylococcus aureus. AMB Express 2023, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.A.; Gohar, N.A.; Bayoumi, A.H.; Ammar, Y.A. Novel fluorinated pyrazole-based heterocycles scaffold: Cytotoxicity, in silico studies and molecular modelling targeting double mutant EGFR L858R/T790M as antiproliferative and apoptotic agents. Med. Chem. Res. 2023, 32, 369–388. [Google Scholar] [CrossRef]
- Abusaif, M.S.; Ragab, A.; Fayed, E.A.; Ammar, Y.A.; Gowifel, A.M.H.; Hassanin, S.O.; Ahmed, G.E.; Gohar, N.A. Exploring a novel thiazole derivatives hybrid with fluorinated-indenoquinoxaline as dual inhibitors targeting VEGFR2/AKT and apoptosis inducers against hepatocellular carcinoma with docking simulation. Bioorg. Chem. 2025, 154, 108023. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Jagadeshwar, V.; Narsihmulu, C.; Sarangapani, M.; Krishna, D.R.; Vidyasagar, J.; Vijay, D.; Sastry, G.N. Design, synthesis and cytotoxic studies on the simplified oxy analog of eleutherobin. Bioorg. Med. Chem. Lett. 2004, 14, 3687–3689. [Google Scholar] [CrossRef] [PubMed]
- Desouky, S.E.; Abu-Elghait, M.; Fayed, E.A.; Selim, S.; Yousuf, B.; Igarashi, Y.; Abdel-Wahab, B.A.; Mohammed Alsuhaibani, A.; Sonomoto, K.; Nakayama, J. Secondary Metabolites of Actinomycetales as Potent Quorum Sensing Inhibitors Targeting Gram-Positive Pathogens: In Vitro and In Silico Study. Metabolites 2022, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, M.A.; Ramsis, T.M.; Gohar, N.A.; metwally, S.A.; Rushdi, A.; Fayed, E.A. Novel Pyrrolidine-bearing quinoxaline inhibitors of DNA Gyrase, RNA polymerase and spike glycoprotein. Bioorg. Chem. 2025, 156, 108218. [Google Scholar] [CrossRef] [PubMed]
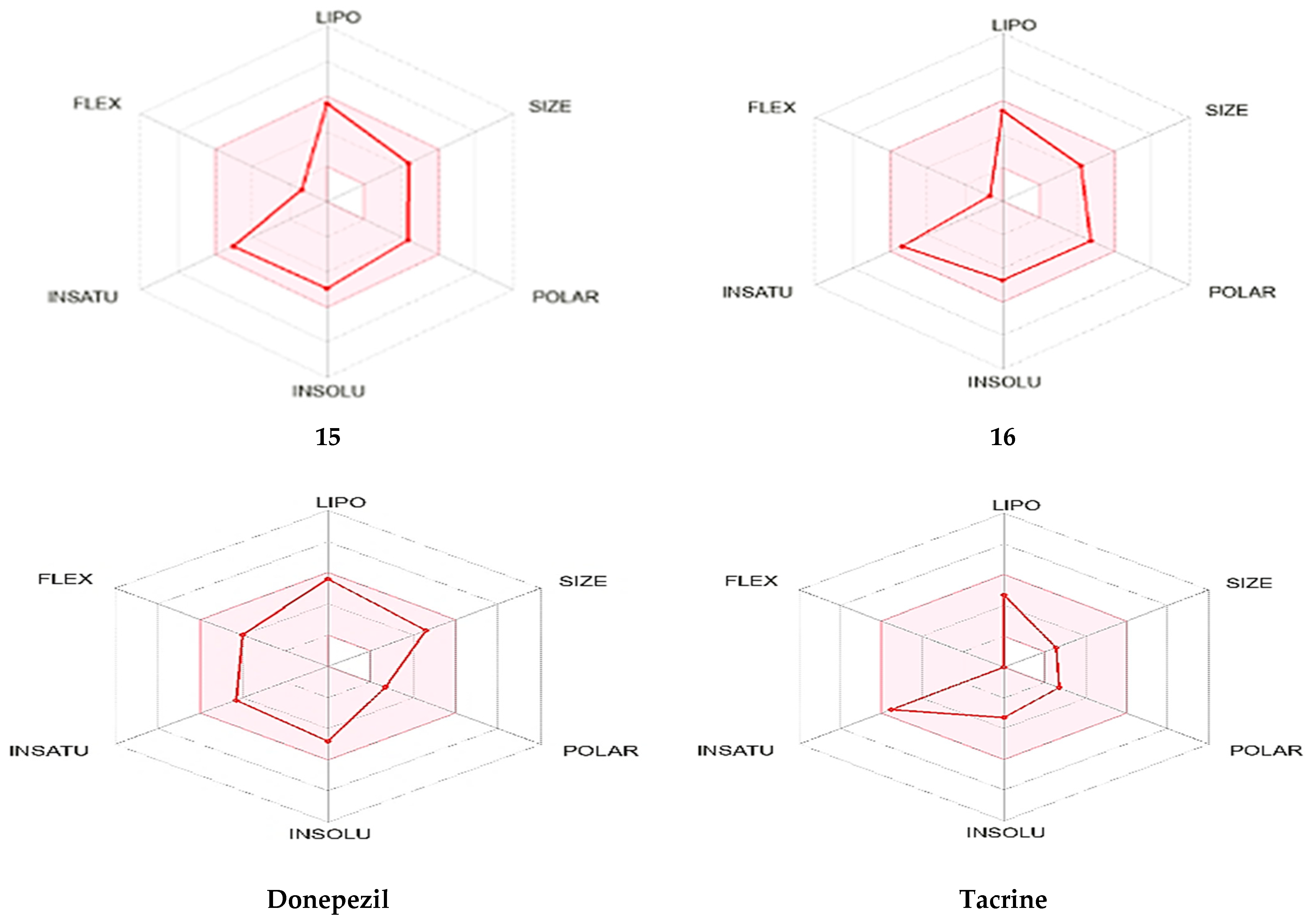
 | ||||
|---|---|---|---|---|
| Cpd. No. | R | Memory (s) | Learning (s) | SAP% |
| Control | - | 53.67 ± 1.41 | 8 ± 0.58 | 90.83 ± 1.01 |
| AlCl3-induced AD | - | 9.83 ± 1.49 a | 49.17 ± 2.63 a | 47.67 ± 1.48 a |
| Donepezil | - | 42.67 ± 0.61 ab | 14.78 ± 0.83 ab | 85 ± 0.58 ab |
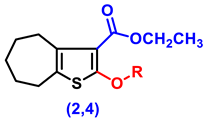
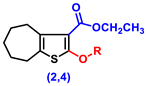
| 2 | H | 24.17 ± 1.22 abc | 33.33 ± 0.8 abc | 62.33 ± 1.05 abc |
| 4 | -CO-CH2-Cl | 26.67 ± 1.54 abc | 31.5 ± 1.48 abc | 72.67 ± 1.02 abc |
 | ||||
|---|---|---|---|---|
| Cpd. No. | R1 | Memory (s) | Learning (s) | SAP% |
| Control | - | 53.67 ± 1.41 | 8 ± 0.58 | 90.83 ± 1.01 |
| AlCl3-induced AD | - | 9.83 ± 1.49 a | 49.17 ± 2.63 a | 47.67 ± 1.48 a |
| Donepezil | - | 42.67 ± 0.61 ab | 14.78 ± 0.83 ab | 85 ± 0.58 ab |
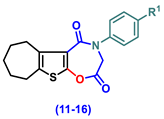
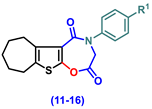
| 11 | H | 35.17 ± 0.95 abc | 25.17 ± 1.25 abc | 69.67 ± 1.69 abc |
| 12 | Cl | 37.17 ± 1.52 abc | 23.5 ± 1.23 abc | 76.5 ± 1.52 abc |
| 13 | CN | 40.50 ± 2.62 abc | 14.17 ± 0.95 abc | 82.67 ± 1.28 abc |
| 14 | -OCH2CH3 | 29.50 ± 2 abc | 21 ± 1.13 abc | 77.5 ± 2.11 abc |
| 15 | OH | 42.50 ± 1.06 abc | 19.17 ± 1.01 abc | 82.5 ± 0.89 abc |
| 16 | -OCH3 | 36.00 ± 1.27 abc | 26.83 ± 1.2 abc | 71.67 ± 1.15 abc |
 | |||
|---|---|---|---|
| Cpd. No. | R | BACE1 (ng/mL) | Amyloid-β (ng/g Tissue) |
| Control | - | 1.01 ± 0.01 | 1.59 ± 0.03 |
| AlCl3-induced AD | - | 29.68 ± 0.2 a | 27.92 ± 0.32 a |
| Donepezil | - | 4.93 ± 0.04 ab | 8.10 ± 1.71 ab |
| 2 | H | 23.60 ± 0.2 abc | 19.52 ± 0.15 abc |
| 4 | -CO-CH2-Cl | 21.89 ± 0.27 abc | 17.05 ± 0.2 abc |
 | |||
|---|---|---|---|
| Cpd. No. | R1 | BACE1 (ng/mL) | Amyloid-β (ng/g Tissue) |
| Control | - | 1.01 ± 0.01 | 1.59 ± 0.03 |
| AlCl3-induced AD | - | 29.68 ± 0.2 a | 27.92 ± 0.32 a |
| Donepezil | - | 4.93 ± 0.04 ab | 8.10 ± 1.71 ab |
| 11 | H | 18.83 ± 0.13 abc | 14.25 ± 0.16 abc |
| 12 | Cl | 13.68 ± 0.2 abc | 10.51 ± 0.1 abc |
| 13 | CN | 11.35 ± 0.31 abc | 9.04 ± 0.9 abc |
| 14 | -OCH2CH3 | 16.66 ± 0.38 abc | 11.66 ± 0.17 abc |
| 15 | OH | 10.50 ± 0.2 abc | 7.46 ± 0.9 abc |
| 16 | -OCH3 | 17.44 ± 0.23 abc | 12.44 ± 0.14 abc |
 | ||||||
|---|---|---|---|---|---|---|
| Cpd. No. | R | 5-HT (ng/g Tissue) | DA (ng/g Tissue) | NE (nmol/g Tissue) | BDNF (ng/mL) | AChE (ng/g Tissue) |
| Control | - | 13.59 ± 0.22 | 64.73 ± 0.36 | 730.6 ± 0.36 | 165.5 ± 0.14 | 11.93 ± 0.26 |
| AlCl3-induced AD | - | 1.887 ± 0.49 a | 11.84 ± 1.42 a | 185.7 ± 1.42 a | 42.56 ± 0.04 a | 67.34 ± 0.22 a |
| Donepezil | - | 11.23 ± 0.41 ab | 58.12 ± 14.01 ab | 635.4 ± 14.01 ab | 122.4 ± 0.48 ab | 13.91 ± 0.42 b |
| 2 | H | 3.16 ± 0.63 abc | 24.71 ± 1.62 abc | 256.9 ± 1.62 abc | 64.95 ± 0.01 abc | 51.41 ± 0.13 abc |
| 4 | -CO-CH2-Cl | 4.29 ± 0.76 abc | 30.69 ± 1.2 abc | 282.6 ± 1.2 abc | 75.75 ± 0.01 abc | 43.89 ± 0.19 abc |
 | ||||||
|---|---|---|---|---|---|---|
| Cpd. No. | R1 | 5-H (ng/g Tissue) | DA (ng/g Tissue) | NE (nmol/g Tissue) | BDNF (ng/mL) | AChE (ng/g Tissue) |
| Control | - | 13.59 ± 0.22 | 64.73 ± 0.36 | 730.6 ± 0.36 | 165.5 ± 0.14 | 11.93 ± 0.26 |
| AlCl3-induced AD | - | 1.887 ± 0.49 a | 11.84 ± 1.42 a | 185.7 ± 1.42 a | 42.56 ± 0.04 a | 67.34 ± 0.22 a |
| Donepezil | - | 11.23 ± 0.41 ab | 58.12 ± 0.14 ab | 635.4 ± 14.01 ab | 122.4 ± 0.48 ab | 13.91 ± 0.42 ab |
| 11 | H | 9.13 ± 0.36 abc | 54.63 ± 6.11 abc | 321.2 ± 6.11 abc | 127.3 ± 0.01 abc | 20.09 ± 0.45 abc |
| 12 | Cl | 5.07 ± 0.18 abc | 38.37 ± 2.86 abc | 420.5 ± 2.86 abc | 83.62 ± 0.06 abc | 35.66 ± 0.19 abc |
| 13 | CN | 7.39 ± 0.1 abc | 48.65 ± 2.3 abc | 449.6 ± 2.3 abc | 104.9 ± 0.07 abc | 24.35 ± 0.8 abc |
| 14 | -OCH2CH3 | 8.22 ± 0.19 abc | 51.88 ± 2.66 abc | 386.7 ± 2.66 abc | 115.8 ± 0.07 abc | 19.4 ± 0.39 abc |
| 15 | OH | 6.77 ± 0.22 abc | 44.92 ± 2.85 abc | 499.8 ± 2.85 abc | 94.48 ± 0.05 abc | 25.73 ± 0.37 abc |
| 16 | -OCH3 | 10.65 ± 0.71 abc | 52.96 ± 1.52 abc | 366.4 ± 1.52 abc | 134.8 ± 0.01 abc | 16.83 ± 0.29 abc |
 | ||||||
|---|---|---|---|---|---|---|
| Cpd. No. | R | TNF-α (pg/g Tissue) | IL-β (pg/g Tissue) | TLR4 | NFκβ | NLRP3 |
| Control | - | 26.75 ± 0.16 | 31.3 ± 0.28 | 1.02 ± 0.01 | 1.07 ± 0.01 | 1.04 ± 0.004 |
| AlCl3-induced AD | - | 176 ± 1.05 a | 118.4 ± 0.5 a | 9.63 ± 0.08 a | 8.48 ± 0.15 a | 10.68 ± 0.09 a |
| Donepezil | - | 61.7 ± 1.12 ab | 58.75 ± 0.99 ab | 2.72 ± 0.02 ab | 3.0 ± 0.05 ab | 2.90 ± 0.04 ab |
| 2 | H | 121.4 ± 0.97 abc | 93.93 ± 1.26 abc | 7.66 ± 0.12 abc | 6.53 ± 0.08 abc | 8.29 ± 0.09 abc |
| 4 | -CO-CH2-Cl | 113.6 ± 0.83 abc | 82.82 ± 1.1 abc | 6.39 ± 0.12 abc | 5.77 ± 0.19 abc | 6.30 ± 0.09 abc |
 | ||||||
|---|---|---|---|---|---|---|
| Cpd. No. | R1 | TNF-α (pg/g Tissue) | IL-β (pg/g Tissue) | TLR4 | NFκβ | NLRP3 |
| Control | - | 26.75 ± 0.16 | 31.3 ± 0.28 | 1.02 ± 0.01 | 1.07 ± 0.01 | 1.04 ± 0.004 |
| AlCl3-induced AD | - | 176 ± 1.05 a | 118.4 ± 0.5 a | 9.63 ± 0.08 a | 8.48 ± 0.15 a | 10.68 ± 0.09 a |
| Donepezil | - | 61.7 ± 1.12 ab | 58.75 ± 0.99 ab | 2.72 ± 0.02 ab | 3.0 ± 0.05 ab | 2.90 ± 0.04 ab |
| 11 | H | 99.23 ± 0.42 abc | 68.56 ± 0.54 abc | 5.56 ± 0.1 abc | 4.93 ± 0.08 abc | 5.39 ± 0.11 abc |
| 12 | Cl | 65.98 ± 0.91 abc | 54.51 ± 1.3 abc | 2.75 ± 0.06 abc | 4.30 ± 0.14 abc | 3.21 ± 0.12 abc |
| 13 | CN | 52.38 ± 0.59 abc | 45.79 ± 1.34 abc | 4.09 ± 0.27 abc | 3.49 ± 0.07 abc | 2.86 ± 0.11 abc |
| 14 | -OCH2CH3 | 73.21 ± 0.58 abc | 59.92 ± 0.98 abc | 3.60 ± 0.12 abc | 3.73 ± 0.04 abc | 3.92 ± 0.04 abc |
| 15 | OH | 44.6 ± 0.6 abc | 45.66 ± 1.03 abc | 4.57 ± 0.09 abc | 2.98 ± 0.10 abc | 3.36 ± 0.12 abc |
| 16 | -OCH3 | 85.17 ± 0.46 abc | 53.04 ± 0.61 abc | 4.43 ± 0.08 abc | 4.70 ± 0.12 abc | 4.32 ± 0.10 abc |
 | |||||
|---|---|---|---|---|---|
| Cpd. No. | R | Caspase-1 | BAX | Bcl-2 | BAX/Bcl-2 Ratio |
| Control | - | 1.02 ± 0.003 | 1.01 ± 0.004 | 1.08 ± 0.006 | 0.94 |
| AlCl3-induced AD | - | 9.52 ± 0.009 a | 9.68 ± 0.160 a | 0.12 ± 0.002 a | 80.67 |
| Donepezil | - | 2.92 ± 0.050 ab | 2.25 ± 0.058 ab | 0.98 ± 0.002 ab | 2.30 |
| 2 | H | 7.46 ± 0.120 abc | 8.13 ± 0.070 abc | 0.27 ± 0.007 abc | 30.11 |
| 4 | -CO-CH2-Cl | 6.46 ± 0.120 abc | 7.42 ± 0.030 abc | 0.38 ± 0.020 abc | 19.53 |
 | |||||
|---|---|---|---|---|---|
| Cpd. No. | R1 | Caspase-1 | BAX | Bcl-2 | BAX/Bcl-2 Ratio |
| Control | - | 1.02 ± 0.003 | 1.01 ± 0.004 | 1.08 ± 0.006 | 0.94 |
| AlCl3-induced AD | - | 9.52 ± 0.009 a | 9.68 ± 0.160 a | 0.12 ± 0.002 a | 80.67 |
| Donepezil | - | 2.92 ± 0.050 ab | 2.25 ± 0.0580 ab | 0.98 ± 0.002 ab | 2.30 |
| 11 | H | 5.92 ± 0.040 abc | 6.84 ± 0.090 abc | 0.52 ± 0.020 abc | 13.15 |
| 12 | Cl | 4.54 ± 0.090 abc | 4.85 ± 0.110 abc | 0.82 ± 0.020 abc | 5.91 |
| 13 | CN | 3.85 ± 0.070 abc | 5.80 ± 0.120 abc | 0.79 ± 0.020 abc | 7.34 |
| 14 | -OCH2CH3 | 5.08 ± 0.050 abc | 5.49 ± 0.100 abc | 0.70 ± 0.004 abc | 7.84 |
| 15 | OH | 2.94 ± 0.110 abc | 4.47 ± 0.040 abc | 0.86 ± 0.020 abc | 5.20 |
| 16 | -OCH3 | 5.04 ± 0.130 abc | 6.14 ± 0.090 abc | 0.63 ± 0.020 abc | 9.75 |
 | ||||
|---|---|---|---|---|
| Cpd. No. | R | Wnt3a (ng/g Tissue) | GSK3β | β-Catenin (ng/L) |
| Control | - | 1.01 ± 0.003 | 1.01 ± 0.004 | 16.94 ± 0.14 |
| AlCl3-induced AD | - | 0.12 ± 0.003 a | 10.20 ± 0.03 a | 1.578 ± 0.09 a |
| Donepezil | - | 0.98 ± 0.01 ab | 2.05 ± 0.07 ab | 12.92 ± 0.48 ab |
| 2 | H | 0.25 ± 0.01 abc | 8.80 ± 0.12 abc | 4.262 ± 0.12 abc |
| 4 | -CO-CH2-Cl | 0.34 ± 0.01 abc | 6.86 ± 0.15 abc | 6.17 ± 0.07 abc |
 | ||||
|---|---|---|---|---|
| Cpd. No. | R1 | Wnt3a (ng/g Tissue) | GSK3β | β-Catenin (ng/L) |
| Control | - | 1.01 ± 0.003 | 1.01 ± 0.004 | 16.94 ± 0.14 |
| AlCl3-induced AD | - | 0.12 ± 0.003 a | 10.20 ± 0.03 a | 1.578 ± 0.09 a |
| Donepezil | - | 0.98 ± 0.01 ab | 2.05 ± 0.07 ab | 12.92 ± 0.48 ab |
| 11 | H | 0.47 ± 0.01 abc | 2.34 ± 0.06 abc | 7.25 ± 0.04 abc |
| 12 | Cl | 0.76 ± 0.01 abc | 5.36 ± 0.07 abc | 9.91 ± 0.15 abc |
| 13 | CN | 0.84 ± 0.01 abc | 3.63 ± 0.04 abc | 11.67 ± 0.13 abc |
| 14 | -OCH2CH3 | 0.66 ± 0.02 abc | 3.13 ± 0.07 abc | 8.4 ± 0.09 abc |
| 15 | OH | 0.88 ± 0.01 abc | 4.72 ± 0.05 abc | 13.86 ± 0.11 abc |
| 16 | -OCH3 | 0.52 ± 0.01 abc | 2.18 ± 0.10 abc | 7.34 ± 0.12 abc |
 | ||||||
|---|---|---|---|---|---|---|
| Cpd. No. | R | SOD(U/g) | TAC (µmol/g Tissue) | MDA (nmol/g Tissue) | Nrf-2 | HO-1 |
| Control | - | 3.052 ± 0.4 | 46.68 ± 0.05 | 7.105 ± 0.03 | 1.07 ± 0.006 | 1.02 ± 0.003 |
| AlCl3-induced AD | - | 0.38 ± 0.38 a | 9.55 ± 0.250 a | 116.3 ± 0.07 a | 0.16 ± 0.020 a | 0.12 ± 0.007 a |
| Donepezil | - | 5.78 ± 0.97 ab | 34.88 ± 1.510 ab | 25.25 ± 0.35 ab | 0.82 ± 0.001 ab | 0.81 ± 0.002 ab |
| 2 | H | 0.68 ± 0.26 abc | 18.26 ± 0.395 abc | 92.07 ± 0.04 abc | 0.25 ± 0.008 abc | 0.34 ± 0.032 abc |
| 4 | -CO-CH2-Cl | 1.75 ± 0.31 abc | 25.87 ± 0.965 abc | 77.14 ± 0.06 abc | 0.31 ± 0.004 abc | 0.39 ± 0.007 abc |
 | ||||||
|---|---|---|---|---|---|---|
| Cpd. No. | R1 | SOD(U/g) | TAC (µmol/g Tissue) | MDA (nmol/g Tissue) | Nrf-2 | HO-1 |
| Control | - | 3.05 ± 0.4 | 46.68 ± 0.05 | 7.10 ± 0.03 | 1.07 ± 0.006 | 1.02 ± 0.003 |
| AlCl3-induced AD | - | 0.38 ± 0.38 a | 9.55 ± 0.25 a | 116.3 ± 0.07 a | 0.16 ± 0.02 a | 0.12 ± 0.007 a |
| Donepezil | - | 5.78 ± 0.97 ab | 34.88 ± 1.51 ab | 25.25 ± 0.35 ab | 0.82 ± 0.01 ab | 0.81 ± 0.002 ab |
| 11 | H | 2.46 ± 0.32 abc | 38.23 ± 0.50 abc | 36.75 ± 0.09 abc | 0.35 ± 0.006 abc | 0.53 ± 0.004 abc |
| 12 | Cl | 2.15 ± 0.39 abc | 29.33 ± 0.57 abc | 72.02 ± 0.05 abc | 0.64 ± 0.020 abc | 0.69 ± 0.020 abc |
| 13 | CN | 2.53 ± 0.25 abc | 32.9 ± 1.22 abc | 52.58 ± 0.08 abc | 0.78 ± 0.010 abc | 0.75 ± 0.010 abc |
| 14 | OCH2CH3 | 2.38 ± 0.78 abc | 36.96 ± 0.51 abc | 47.6 ± 0.12 abc | 0.50 ± 0.020 abc | 0.64 ± 0.009 abc |
| 15 | OH | 2.56 ± 0.54 abc | 31.67 ± 0.42 abc | 63.68 ± 0.12 abc | 0.82 ± 0.020 abc | 0.82 ± 0.005 abc |
| 16 | OCH3 | 2.92 ± 0.20 abc | 42.42 ± 0.84 abc | 31.63 ± 0.05 abc | 0.47 ± 0.005 abc | 0.50 ± 0.020 abc |
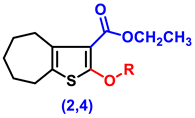 | ||||
|---|---|---|---|---|
| Cpd. No. | R | IC50(µM) | Selectivity Index BChE/AChE | |
| AChE | BChE | |||
| 2 | H | 1.10 ± 0.04 | 5.72 ± 0.20 | 5.21 |
| 4 | -CO-CH2-Cl | 41.22 ± 1.42 | 56.19 ± 1.93 | 1.36 |
| Donepezil | - | 0.22 ± 0.01 | - | - |
| Tacrine | - | - | 0.18 ± 0.01 | - |
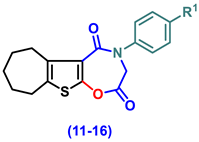 | ||||
|---|---|---|---|---|
| Cpd. No. | R1 | IC50 (µM) | Selectivity Index BChE/AChE | |
| AChE | BChE | |||
| 11 | H | 2.67 ± 0.09 | 13.85 ± 0.48 | 5.18 |
| 12 | Cl | 10.90 ± 0.38 | 65.86 ± 2.27 | 6.04 |
| 13 | CN | 5.04 ± 0.17 | 4.22 ± 0.14 | 0.84 |
| 14 | -OCH2CH3 | 0.39 ± 0.01 | 2.93 ± 0.10 | 7.50 |
| 15 | OH | 1.59 ± 0.06 | 0.70 ± 0.02 | 0.44 |
| 16 | -OCH3 | 0.76 ± 0.03 | 5.23 ± 0.18 | 6.89 |
| Donepezil | - | 0.22 ± 0.01 | - | - |
| Tacrine | - | - | 0.18 ± 0.01 | - |
| Molecule | #H-Bond Donors | #H-Bond Acceptors | MLogP | MW | PAINS Alerts | Synthetic Accessibility |
|---|---|---|---|---|---|---|
| 2 | 1 | 3 | 2.17 | 240.32 | 0 | 3.14 |
| 4 | 0 | 4 | 2.83 | 316.80 | 0 | 3.48 |
| 11 | 0 | 3 | 3.18 | 327.40 | 0 | 3.34 |
| 12 | 0 | 3 | 3.68 | 361.84 | 0 | 3.33 |
| 13 | 0 | 4 | 2.51 | 352.41 | 0 | 3.38 |
| 14 | 0 | 4 | 3.08 | 371.45 | 0 | 3.58 |
| 15 | 1 | 4 | 2.62 | 343.40 | 0 | 3.37 |
| 16 | 0 | 4 | 2.85 | 357.42 | 0 | 3.47 |
| Donepezil | 0 | 4 | 3.06 | 379.49 | 0 | 3.36 |
| Tacrine | 1 | 1 | 2.33 | 198.26 | 0 | 2.08 |
| Cpd. No. | TPSA (Å2) | %ABS |
|---|---|---|
| 2 | 80.840 | 81.110 |
| 4 | 80.840 | 81.110 |
| 11 | 57.780 | 89.060 |
| 12 | 57.780 | 89.060 |
| 13 | 67.010 | 85.880 |
| 14 | 67.010 | 85.880 |
| 15 | 57.780 | 89.060 |
| 16 | 57.780 | 89.060 |
| Donepezil | 38.770 | 95.620 |
| Tacrine | 38.910 | 95.580 |
| Cpd. No | BBB Perm-eant | GI Abs | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Log Kp |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 0.199 | High | − | + | + | + | − | − | −4.84 |
| 4 | 0.112 | High | − | + | + | + | − | − | −5.18 |
| 11 | 0.382 | High | − | + | + | + | − | + | −5.26 |
| 12 | 0.824 | High | − | + | + | + | − | − | −5.02 |
| 13 | 0.017 | High | − | + | + | + | − | + | −5.61 |
| 14 | 0.002 | High | − | + | + | + | + | + | −5.29 |
| 15 | 0.027 | High | − | + | + | + | + | + | −5.60 |
| 16 | 0.027 | High | − | + | + | + | + | + | −5.46 |
| Donepezil | 0.353 | High | + | − | − | − | + | + | −5.58 |
| Tacrine | 0.977 | High | + | + | − | − | − | + | −5.59 |
| Cpd. No. | Lipinski | Veber | Ghose | Muegge | Egan | Bioavailability Score |
|---|---|---|---|---|---|---|
| 2 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 4 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 11 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 12 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 13 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 14 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 15 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 16 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| Donepezil | Yes | Yes | Yes | Yes | Yes | 0.55 |
| Tacrine | Yes | Yes | Yes | No | Yes | 0.55 |
| pkCSM | |||||
|---|---|---|---|---|---|
| Test | 2 | 14 | 16 | Donepezil | Tacrine |
| AMES toxicity | No | No | Yes | No | Yes |
| Max. tolerated dose (human) | 0.668 | −0.116 | −0.18 | −0.217 | 0.55 |
| hERG I inhibition | No | No | No | No | No |
| hERG II inhibition | No | No | No | Yes | No |
| LD50 | 2.201 | 2.666 | 2.762 | 2.753 | 2.33 |
| LOAEL | 1.884 | 2.048 | 2.113 | 0.991 | 1.204 |
| Hepatotoxicity | No | No | No | Yes | Yes |
| Skin Sensitization | Yes | No | No | No | No |
| T. Pyriformis toxicity | 0.98 | 1.052 | 0.981 | 0.804 | 0.642 |
| Minnow toxicity | 1.138 | −0.041 | 0.05 | −2.011 | 0.206 |
| ProTox-II prediction | |||||
| LD50 (mg/kg) | 1500 | 2000 | 2000 | 505 | 40 |
| Toxicity Class | IV | IV | IV | IV | II |
| Immunotoxicity | (−) (0.99) | (−) (0.91) | (−) (0.89) | (+) (0.95) | (−) (0.98) |
| Mutagenicity | (−) (0.74) | (−) (0.67) | (−) (0.66) | (−) (0.53) | (+) (0.91) |
| Cytotoxicity | (−) (0.76) | (+) (0.50) | (−) (0.50) | (+) (0.63) | (−) (0.72) |
| (Tumor Suppressor) p53 | (−) (0.85) | (−) (0.80) | (−) (0.79) | (−) (0.94) | (−) (0.95) |
| Drug | (S) (kCal/mol) | Receptor Amino Acid | Interaction | Distance (Å) |
|---|---|---|---|---|
| Donepezil | −17.710 | Phe295 | H-acceptor | 2.98 |
| Tyr341 | H–arene | 3.67 | ||
| Tyr337 | H–arene | 3.71 | ||
| Trp286 | H–arene | 3.87 | ||
| Trp286 | arene-arene | 3.88 | ||
| Tacrine | −10.080 | His438 | H-donor | 3.32 |
| His438 | H–arene | 4.54 | ||
| Trp82 | arene-arene | 3.89 |
| Cpd. No. | (S) (kCal/mol) | Receptor Amino Acid | Interaction | Distance (Å) | (S) (kCal/mol) | Receptor Amino Acid | Interaction | Distance (Å) |
|---|---|---|---|---|---|---|---|---|
| AChE | BChE | |||||||
| 2 | −14.45 | Ser293 Trp286 Trp286 Phe338 | H-donor H-pi H-pi H-pi | 3.8 4.07 4.1 4.53 | −14.97 | Asp70 His438 Trp82 | H-donor H-pi pi-pi | 4.01 4.68 3.85 |
| 14 | −14.74 | Phe295 Trp286 | H-acceptor pi-pi | 3.1 3.6 | −18.10 | Asp70 Gly78 Trp82 | H-donor H-donor pi-pi | 4.22 3.68 3.89 |
| 16 | −15.09 | Phe295 Trp286 Tyr341 | H-acceptor H-pi pi-H | 2.77 4.01 4.21 | −16.41 | Asp70 Gly439 Gly116 Trp82 | H-donor H-acceptor H-acceptor pi-pi | 4.4 3.34 3.08 3.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudah, K.H.; Najm, M.A.A.; Ramsis, T.M.; Ebrahim, M.A.; Gohar, N.A.; Abu-Elfotuh, K.; Mohamed, E.K.; Hamdan, A.M.E.; Hamdan, A.M.; Almotairi, R.; et al. Unlocking Therapeutic Potential of Novel Thieno-Oxazepine Hybrids as Multi-Target Inhibitors of AChE/BChE and Evaluation Against Alzheimer’s Disease: In Vivo, In Vitro, Histopathological, and Docking Studies. Pharmaceuticals 2025, 18, 1214. https://doi.org/10.3390/ph18081214
Oudah KH, Najm MAA, Ramsis TM, Ebrahim MA, Gohar NA, Abu-Elfotuh K, Mohamed EK, Hamdan AME, Hamdan AM, Almotairi R, et al. Unlocking Therapeutic Potential of Novel Thieno-Oxazepine Hybrids as Multi-Target Inhibitors of AChE/BChE and Evaluation Against Alzheimer’s Disease: In Vivo, In Vitro, Histopathological, and Docking Studies. Pharmaceuticals. 2025; 18(8):1214. https://doi.org/10.3390/ph18081214
Chicago/Turabian StyleOudah, Khulood H., Mazin A. A. Najm, Triveena M. Ramsis, Maha A. Ebrahim, Nirvana A. Gohar, Karema Abu-Elfotuh, Ehsan Khedre Mohamed, Ahmed M. E. Hamdan, Amira M. Hamdan, Reema Almotairi, and et al. 2025. "Unlocking Therapeutic Potential of Novel Thieno-Oxazepine Hybrids as Multi-Target Inhibitors of AChE/BChE and Evaluation Against Alzheimer’s Disease: In Vivo, In Vitro, Histopathological, and Docking Studies" Pharmaceuticals 18, no. 8: 1214. https://doi.org/10.3390/ph18081214
APA StyleOudah, K. H., Najm, M. A. A., Ramsis, T. M., Ebrahim, M. A., Gohar, N. A., Abu-Elfotuh, K., Mohamed, E. K., Hamdan, A. M. E., Hamdan, A. M., Almotairi, R., Abdelmohsen, S. R., Abdelhakim, K. R., Elsharkawy, A. M. A., & Fayed, E. A. (2025). Unlocking Therapeutic Potential of Novel Thieno-Oxazepine Hybrids as Multi-Target Inhibitors of AChE/BChE and Evaluation Against Alzheimer’s Disease: In Vivo, In Vitro, Histopathological, and Docking Studies. Pharmaceuticals, 18(8), 1214. https://doi.org/10.3390/ph18081214