Targeted Alpha Therapy: Exploring the Clinical Insights into [225Ac]Ac-PSMA and Its Relevance Compared with [177Lu]Lu-PSMA in Advanced Prostate Cancer Management
Abstract
1. Introduction
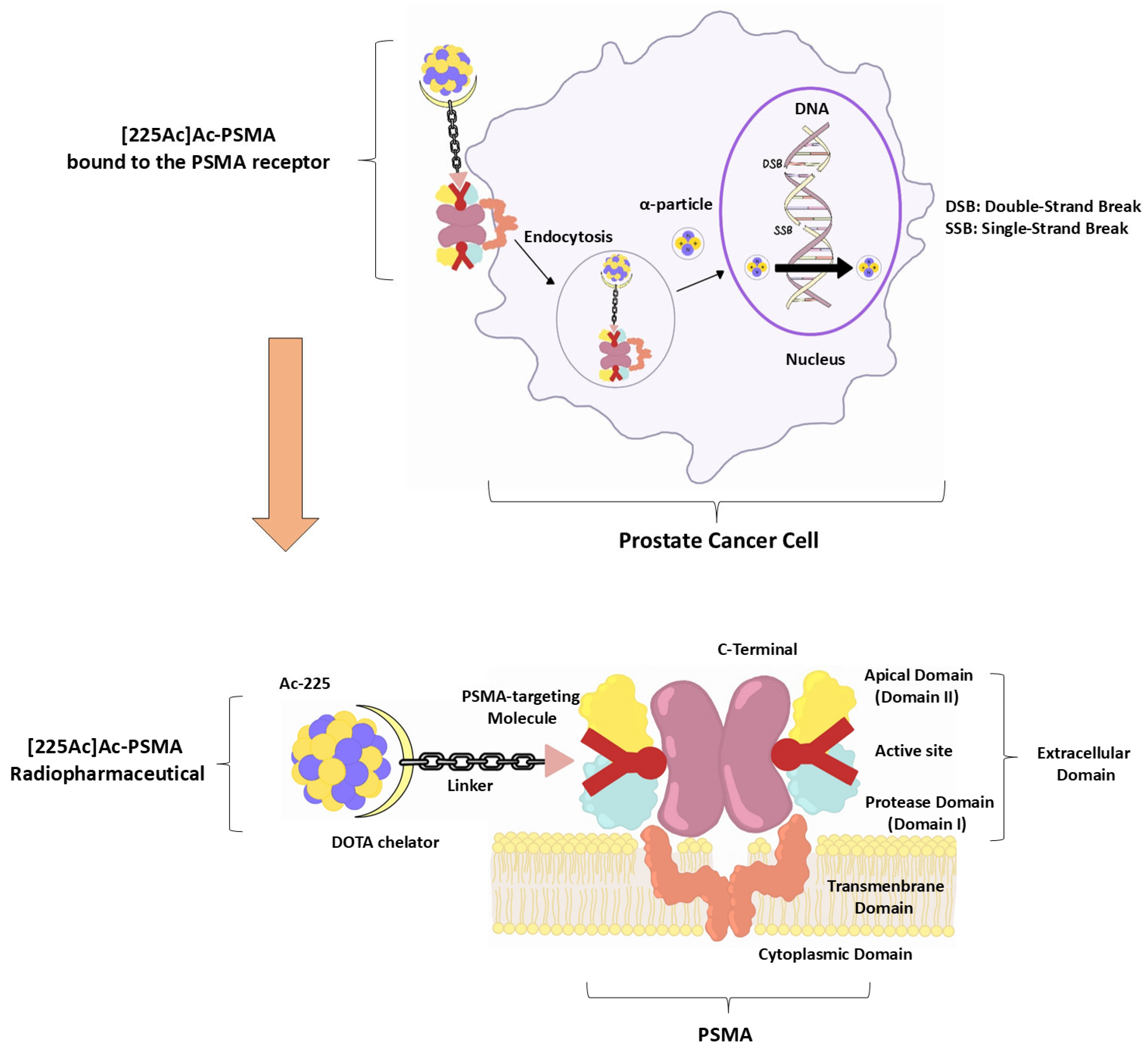
2. Mechanism of Action of [225Ac]Ac-PSMA RLT
2.1. PSMA Targeting and Internalisation
2.2. Alpha-Particle Emission and DNA Damage
2.3. Dosimetric Advantage in Micrometastatic Disease
2.4. Chelation Stability and Biodistribution
3. Clinical Indications and Patient Selection
3.1. Eligibility Criteria Typically Include the Following:
- ❖
- Histologically confirmed mCRPC.
- ❖
- Progressive disease despite androgen receptor signalling inhibitors (e.g., enzalutamide, abiraterone) and/or taxane-based chemotherapy.
- ❖
- Adequate organ function (e.g., bone marrow, renal, hepatic reserve).
- ❖
- PSMA expression on PET/CT using [68Ga]Ga-PSMA or [18F]F-PSMA ligands (i.e., tumour uptake ≥ liver background).
- ❖
- ECOG performance status ≤ 2.
3.2. Exclusion Criteria Often Include the Following:
- ❖
- Diffuse bone marrow infiltration.
- ❖
- Poor PSMA expression on imaging.
- ❖
- Prior to myelotoxic therapy <6 weeks before RLT.
- ❖
- Active uncontrolled infections or autoimmune diseases.
4. Administration Protocols and Dosimetry
5. Response Evaluation and Outcomes
5.1. Biochemical Response—PSA
5.2. Imaging-Based Assessment
- ❖
- SUVₘₐₓ reduction: often used as a robust semiquantitative parameter with high reproducibility.
- ❖
- Whole-body tumour volume (PSMA TV): the total volume of PSMA-avid lesions; higher baseline PSMA TV is a statistically significant negative predictor of overall and PFS.
- ❖
- Total lesion PSMA (TL PSMA): the product of PSMA TV by SUVmean, analogous to total lesion glycolysis (TLG) in [18F]F-FDG PET; elevated TL PSMA correlates with worse prognosis.
- ❖
- Complete metabolic response: no detectable PSMA uptake.
- ❖
- Partial response: decrease in the number or size of PSMA-avid lesions.
- ❖
- Stable disease: no significant change in lesion burden; that is, neither new nor regressing lesions.
5.3. Survival Outcomes
6. Side Effects and Toxicity
Mitigation Strategies and Long-Term Safety Considerations in Xerostomia
7. Comparison with [177Lu]Lu-PSMA
7.1. Clinical Efficacy
7.2. Dosimetry Considerations
7.3. Toxicity Profile
7.4. Tandem and Sequential Strategies
8. Challenges and Future Perspectives
8.1. Isotope Availability
8.2. Toxicity Management
8.3. Lack of Randomised Controlled Trials (RCTs)
- ❖
- AcTION trial (NCT04597411): Phase I dose-escalation study of [225Ac]Ac-PSMA617 in PSMA-positive mCRPC [26].
- ❖
- TATCIST trial (NCT05219500): Phase II study of [225Ac]Ac-PSMAI&T (FPI2265) in patients pre-treated with [177Lu]Lu-PSMA [66].
- ❖
- NCT06402331: Ongoing Phase II/III randomised study of FPI2265 ([225Ac]Ac-PSMA-I&T) in patients previously treated with [177Lu]Lu-PSMA [67].
- ❖
- NCT03276572, NCT04506567, NCT04886986: Studies using antibody-targeted alpha therapy (Ac225 J591), either alone or in tandem with [177Lu]Lu-PSMA or immunotherapy [68].
8.4. Optimal Sequencing and Combination Strategies
8.5. Long-Term Safety and Secondary Malignancy Risk
9. Regulatory and Ethical Considerations
9.1. Regulatory Status
9.2. Ethical Considerations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAT | Targeted alpha therapy |
| mCRPC | metastatic castration-resistant prostate cancer |
| [225Ac]Ac-PSMA | actinium-225-labelled prostate-specific membrane antigen |
| LET | linear energy transfer |
| RLT | radioligand therapy |
| Lu-177 | lutetium-177 |
| PSA | prostate-specific antigen |
| ADT | androgen deprivation therapy |
| PFS | progression-free survival |
| RBE | relative biological effectiveness |
| ECOG | Eastern Cooperative Oncology Group |
| PCWG3 | Prostate Cancer Clinical Trials Working Group 3 |
| mHSPC | hormone-sensitive metastatic prostate cancer |
| TV | tumour volume |
| TL-PSMA | Total lesion PSMA |
| TLG | total lesion glycolysis |
| OS | overall survival |
| Th-229 | Thorium-229 |
| RCTs | Randomised Controlled Trials |
| t-MDS | therapy-related myelodysplastic syndromes |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| IAEA | International Atomic Energy Agency |
| PARP | poly (ADP-ribose) polymerase |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Mier, W.; Haufe, S.; Debus, N.; Eder, M.; Eisenhut, M.; Schäfer, M.; et al. Diagnostic Performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in Patients with Recurrent Prostate Cancer: Evaluation in 1007 Patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1258–1268. [Google Scholar] [CrossRef]
- Sheehan, B.; Guo, C.; Neeb, A.; Paschalis, A.; Sandhu, S.; de Bono, J.S. Prostate-Specific Membrane Antigen Biology in Lethal Prostate Cancer and Its Therapeutic Implications. Eur. Urol. Focus. 2022, 8, 1157–1168. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and Safety of 225Ac-PSMA-617 Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in Chemotherapy-Naive Patients with Advanced Prostate Cancer: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.C.; Malhotra, M.; Cryan, J.F.; O’Driscoll, C.M. The Therapeutic and Diagnostic Potential of the Prostate Specific Membrane Antigen/Glutamate Carboxypeptidase II (PSMA/GCPII) in Cancer and Neurological Disease. Br. J. Pharmacol. 2016, 173, 3041–3079. [Google Scholar] [CrossRef]
- van der Gaag, S.; Bartelink, I.H.; Vis, A.N.; Burchell, G.L.; Oprea-Lager, D.E.; Hendrikse, H. Pharmacological Optimization of PSMA-Based Radioligand Therapy. Biomedicines 2022, 10, 3020. [Google Scholar] [CrossRef]
- Benesova, M.; Kratochwil, C.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. PSMA-617—A Novel Theranostic PSMA Inhibitor for Both Diagnosis and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 63. [Google Scholar]
- Zhao, Y.; Culman, J.; Cascorbi, I.; Nithack, N.; Marx, M.; Zuhayra, M.; Lützen, U. PSMA-617 Inhibits Proliferation and Potentiates the 177Lu-PSMA-617-Induced Death of Human Prostate Cancer Cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3315–3326. [Google Scholar] [CrossRef]
- Hooijman, E.L.; Radchenko, V.; Ling, S.W.; Konijnenberg, M.; Brabander, T.; Koolen, S.L.W.; de Blois, E. Implementing Ac-225 Labelled Radiopharmaceuticals: Practical Considerations and (Pre-)Clinical Perspectives. EJNMMI Radiopharm. Chem. 2024, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Jalloul, W.; Ghizdovat, V.; Stolniceanu, C.R.; Ionescu, T.; Grierosu, I.C.; Pavaleanu, I.; Moscalu, M.; Stefanescu, C. Targeted Alpha Therapy: All. We Need to Know about 225Ac’s Physical Characteristics and Production as a Potential Theranostic Radionuclide. Pharmaceuticals 2023, 16, 1679. [Google Scholar] [CrossRef]
- Lee, H. Relative Efficacy of 225Ac-PSMA-617 and 177Lu-PSMA-617 in Prostate Cancer Based on Subcellular Dosimetry. Mol. Imaging Radionucl. Ther. 2022, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bidkar, A.P.; Zerefa, L.; Yadav, S.; VanBrocklin, H.F.; Flavell, R.R. Actinium-225 Targeted Alpha Particle Therapy for Prostate Cancer. Theranostics 2024, 14, 2969–2992. [Google Scholar] [CrossRef]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Larsen, R.H. Preclinical and Clinical Status of PSMA-Targeted Alpha Therapy for Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 779. [Google Scholar] [CrossRef]
- Murce, E.; Beekman, S.; Spaan, E.; Handula, M.; Stuurman, D.; de Ridder, C.; Seimbille, Y. Preclinical Evaluation of a PSMA-Targeting Homodimer with an Optimized Linker for Imaging of Prostate Cancer. Molecules 2023, 28, 4022. [Google Scholar] [CrossRef]
- Feuerecker, B.; Kratochwil, C.; Ahmadzadehfar, H.; Morgenstern, A.; Eiber, M.; Herrmann, K.; Pomykala, K.L. Clinical Translation of Targeted α-Therapy: An Evolution or a Revolution? J. Nucl. Med. 2023, 64, 685–692. [Google Scholar] [CrossRef]
- Sallam, M.; Nguyen, N.-T.; Sainsbury, F.; Kimizuka, N.; Muyldermans, S.; Benešová-Schäfer, M. PSMA-Targeted Radiotheranostics in Modern Nuclear Medicine: Then, Now, and What of the Future? Theranostics 2024, 14, 3043–3079. [Google Scholar] [CrossRef]
- Nikfarjam, Z.; Zargari, F.; Nowroozi, A.; Bavi, O. Metamorphosis of Prostate Specific Membrane Antigen (PSMA) Inhibitors. Biophys. Rev. 2022, 14, 303–315. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Bruchertseifer, F.; Vorster, M.; Morgenstern, A.; Lawal, I.O. Global Experience with PSMA-Based Alpha Therapy in Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 30–46. [Google Scholar] [CrossRef]
- Laarhuis, B.I.; Janssen, M.J.R.; Simons, M.; van Kalmthout, L.W.M.; van der Doelen, M.J.; Peters, S.M.B.; Westdorp, H.; van Oort, I.M.; Litjens, G.; Gotthardt, M.; et al. Tumoral Ki67 and PSMA Expression in Fresh Pre-PSMA-RLT Biopsies and Its Relation With PSMA-PET Imaging and Outcomes of PSMA-RLT in Patients With mCRPC. Clin. Genitourin. Cancer 2023, 21, e352–e361. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Ricci, M.; Cimini, A.; Filippi, L.; Conte, M.; De Feo, M.S.; De Vincentis, G. The Role of PET and SPECT Imaging in Prostate Cancer Targeted Alpha Therapy: When and How? Appl. Sci. 2023, 13, 1890. [Google Scholar] [CrossRef]
- Endocyte AcTION: A Phase I Study of [225Ac]Ac-PSMA-617 in Men With PSMA-Positive Prostate Cancer With or Without Prior [177Lu]Lu-PSMA-617 Radioligand Therapy. 2025. Available online: https://clinicaltrials.gov/study/NCT04597411 (accessed on 19 July 2025).
- Ling, S.W.; van der Veldt, A.A.M.; Konijnenberg, M.; Segbers, M.; Hooijman, E.; Bruchertseifer, F.; Morgenstern, A.; de Blois, E.; Brabander, T. Evaluation of the Tolerability and Safety of [225Ac]Ac-PSMA-I&T in Patients with Metastatic Prostate Cancer: A Phase I Dose Escalation Study. BMC Cancer 2024, 24, 146. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Singh, S.K.; Mavuduru, R.S.; Bora, G.S. Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer. Indian. J. Nucl. Med. 2020, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.M.; Lawal, I.O.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer (WARMTH Act): A Multicentre, Retrospective Study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Mdlophane, A.; Maes, A.; Mokoala, K.; et al. mCRPC Patients Receiving 225Ac-PSMA-617 Therapy in the Post-Androgen Deprivation Therapy Setting: Response to Treatment and Survival Analysis. J. Nucl. Med. 2022, 63, 1496–1502. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Mokoala, K.; Reed, J.; Maseremule, L.; Ndlovu, H.; Hlongwa, K.; Maes, A.; et al. 225Ac-PSMA-617 Radioligand Therapy of de Novo Metastatic Hormone-Sensitive Prostate Carcinoma (mHSPC): Preliminary Clinical Findings. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2210–2218. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals SatisfACtion: Phase I/II, Open-Label, Multi-Center Study of [225Ac]Ac-PSMA-R2 in Men With mHSPC and Heavily Pre-Treated PSMA-Positive mCRPC, With/Without Prior 177Lu-Labelled PSMA-Targeted Radioligand Therapy. 2025. Available online: https://clinicaltrials.gov/study/NCT05983198 (accessed on 19 July 2025).
- Lee, D.Y.; Kim, Y.-I. Effects of 225Ac-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis. J. Nucl. Med. 2022, 63, 840–846. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C.; et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J. Nucl. Med. 2020, 61, 62–69. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-Resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Rosar, F.; Krause, J.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and Safety of [225Ac]Ac-PSMA-617 Augmented [177Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Thakral, P.; Tiwari, P.; Pant, V.; Das, S.S.; Manda, D.; Raina, V. Therapeutic Efficacy of 225Ac-PSMA-617 Targeted Alpha Therapy in Patients of Metastatic Castrate Resistant Prostate Cancer after Taxane-Based Chemotherapy. Ann. Nucl. Med. 2021, 35, 794–810. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 Tandem Therapy of Metastatic Castration-Resistant Prostate Cancer: Pilot Experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Shah, S.; Efstathiou, E.; Smith, M.R.; Taplin, M.-E.; Bubley, G.J.; Logothetis, C.J.; Kheoh, T.; Kilian, C.; Haqq, C.M.; et al. Phase II Study of Abiraterone Acetate in Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer Displaying Bone Flare Discordant with Serologic Response. Clin. Cancer Res. 2011, 17, 4854–4861. [Google Scholar] [CrossRef] [PubMed]
- Siebinga, H.; de Wit-van der Veen, B.J.; de Vries-Huizing, D.M.V.; Vogel, W.V.; Hendrikx, J.J.M.A.; Huitema, A.D.R. Quantification of Biochemical PSA Dynamics after Radioligand Therapy with [177Lu]Lu-PSMA-I&T Using a Population Pharmacokinetic/Pharmacodynamic Model. EJNMMI Phys. 2024, 11, 39. [Google Scholar] [CrossRef]
- Conteduca, V.; Oromendia, C.; Eng, K.W.; Bareja, R.; Sigouros, M.; Molina, A.; Faltas, B.M.; Sboner, A.; Mosquera, J.M.; Elemento, O.; et al. Clinical Features of Neuroendocrine Prostate Cancer. Eur. J. Cancer 2019, 121, 7–18. [Google Scholar] [CrossRef]
- Gili, R.; Miceli, A.; Giannubilo, I.; Fornarini, G.; Bauckneht, M.; Tomasello, L.; Zanardi, E. Discrepancy between Biochemical and Radiological Response in Metastatic Hormone- Sensitive Prostate Cancer: A Case Report and Literature Review. Ann. Case Rep. 2023, 8, 1185. [Google Scholar] [CrossRef]
- Yamada, Y.; Beltran, H. Clinical and Biological Features of Neuroendocrine Prostate Cancer. Curr. Oncol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Francis, R.J.; Hassan, G.M.; Rowshanfarzad, P.; Ong, J.S.L.; Barry, N.; Rusanov, B.; Ebert, M.A. Quantitative [68Ga]Ga-PSMA-11 PET Biomarkers for the Analysis of Lesion-Level Progression in Biochemically Recurrent Prostate Cancer: A Multicentre Study. Sci. Rep. 2023, 13, 17673. [Google Scholar] [CrossRef]
- Kudura, K.; Schaulin, Y.; Templeton, A.J.; Zellweger, T.; Harms, W.; Georis, R.; Kreissl, M.C.; Foerster, R. Tumor Segmentation on PSMA PET/CT Predicts Survival in Biochemical Recurrence of Prostate Cancer: A Retrospective Study Using [68Ga]Ga-PSMA-11 and [18F]-PSMA-1007. Cancers 2025, 17, 2249. [Google Scholar] [CrossRef]
- Lawal, I.O.; Ndlovu, H.; Kgatle, M.; Mokoala, K.M.G.; Sathekge, M.M. Prognostic Value of PSMA PET/CT in Prostate Cancer. Semin. Nucl. Med. 2024, 54, 46–59. [Google Scholar] [CrossRef]
- Murthy, V.; Appiah-Kubi, E.; Nguyen, K.; Thin, P.; Hotta, M.; Shen, J.; Drakaki, A.; Rettig, M.; Gafita, A.; Calais, J.; et al. Associations of Quantitative Whole-Body PSMA-PET Metrics with PSA Progression Status under Long-Term Androgen Deprivation Therapy in Prostate Cancer Patients: A Retrospective Single-Center Study. Eur. J. Hybrid. Imaging 2023, 7, 18. [Google Scholar] [CrossRef]
- Seifert, R.; Sandach, P.; Kersting, D.; Fendler, W.P.; Hadaschik, B.; Herrmann, K.; Sunderland, J.J.; Pollard, J.H. Repeatability of 68Ga-PSMA-HBED-CC PET/CT–Derived Total Molecular Tumor Volume. J. Nucl. Med. 2022, 63, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, L.M.; Beyer, L.; Zacherl, M.J.; Gildehaus, F.J.; Todica, A.; Kunte, S.C.; Holzgreve, A.; Sheikh, G.T.; Herlemann, A.; Casuscelli, J.; et al. Total Tumor Volume on 18F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with 225Ac-PSMA-I&T. Biomedicines 2022, 10, 946. [Google Scholar] [CrossRef]
- Gafita, A.; Calais, J.; Grogan, T.R.; Hadaschik, B.; Wang, H.; Weber, M.; Sandhu, S.; Kratochwil, C.; Esfandiari, R.; Tauber, R.; et al. Nomograms to Predict Outcomes after 177Lu-PSMA Therapy in Men with Metastatic Castration-Resistant Prostate Cancer: An International, Multicentre, Retrospective Study. Lancet Oncol. 2021, 22, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Perrone, E.; Giordano, A.; Calcagni, M.L.; Leccisotti, L.; Moretti, R.; Eismant, A.; Ghai, K.; Parkar, T.; Mishra, A.; Heidenreich, A.; et al. Long-Term Safety and Survival Outcomes of [225Ac]Ac-PSMA (Prostate-Specific Membrane Antigen) and [225Ac]Ac-/[177Lu]Lu-PSMA (TANDEM) Radioligand Therapy (PRLT) in Metastatic Castration-Resistant Prostate Cancer. Cancers 2025, 17, 405. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657. [Google Scholar] [CrossRef]
- Ling, S.W.; de Blois, E.; Hooijman, E.; van der Veldt, A.; Brabander, T. Advances in 177Lu-PSMA and 225Ac-PSMA Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Pharmaceutics 2022, 14, 2166. [Google Scholar] [CrossRef]
- Alan-Selcuk, N.; Beydagi, G.; Demirci, E.; Ocak, M.; Celik, S.; Oven, B.B.; Toklu, T.; Karaaslan, I.; Akcay, K.; Sonmez, O.; et al. Clinical Experience with [225Ac]Ac-PSMA Treatment in Patients with [177Lu]Lu-PSMA-Refractory Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2023, 64, 1574–1580. [Google Scholar] [CrossRef]
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium 177 PSMA Radionuclide Therapy for Men with Prostate Cancer: A Review of the Current Literature and Discussion of Practical Aspects of Therapy. J. Med. Radiat. Sci. 2017, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Kgatle, M.; Bruchertseifer, F.; Morgernstern, A.; Sathekge, M.M. Design of 225Ac-PSMA for Targeted Alpha Therapy in Prostate Cancer. Ann. Transl. Med. 2024, 12, 67. [Google Scholar] [CrossRef]
- Stangl-Kremser, J.; Ricaurte-Fajardo, A.; Subramanian, K.; Osborne, J.R.; Sun, M.; Tagawa, S.T.; Bander, N.H. Response to RL-225Ac in Prostate Cancer: Effect of Prior Treatment with RL-177Lu: A Systematic Review of the Literature. Prostate 2023, 83, 901–911. [Google Scholar] [CrossRef]
- Delker, A.; Schleske, M.; Liubchenko, G.; Berg, I.; Zacherl, M.J.; Brendel, M.; Gildehaus, F.J.; Rumiantcev, M.; Resch, S.; Hürkamp, K.; et al. Biodistribution and Dosimetry for Combined [177Lu]Lu-PSMA-I&T/[225Ac]Ac-PSMA-I&T Therapy Using Multi-Isotope Quantitative SPECT Imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Tulik, M.; Kuliński, R.; Tabor, Z.; Brzozowska, B.; Łaba, P.; Bruchertseifer, F.; Morgenstern, A.; Królicki, L.; Kunikowska, J. Quantitative SPECT/CT Imaging of Actinium-225 for Targeted Alpha Therapy of Glioblastomas. EJNMMI Phys. 2024, 11, 41. [Google Scholar] [CrossRef]
- Herrmann, K.; Rahbar, K.; Eiber, M.; Sparks, R.; Baca, N.; Krause, B.J.; Lassmann, M.; Jentzen, W.; Tang, J.; Chicco, D.; et al. Renal and Multiorgan Safety of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer in the VISION Dosimetry Substudy. J. Nucl. Med. 2024, 65, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Langbein, T.; Kulkarni, H.R.; Schuchardt, C.; Mueller, D.; Volk, G.F.; Baum, R.P. Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation. Diagnostics 2022, 12, 1926. [Google Scholar] [CrossRef]
- Muniz, M.; Loprinzi, C.L.; Orme, J.J.; Koch, R.M.; Mahmoud, A.M.; Kase, A.M.; Riaz, I.B.; Andrews, J.R.; Thorpe, M.P.; Johnson, G.B.; et al. Salivary Toxicity from PSMA-Targeted Radiopharmaceuticals: What We Have Learned and Where We Are Going. Cancer Treat. Rev. 2024, 127, 102748. [Google Scholar] [CrossRef] [PubMed]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stuparu, A.; Lueckerath, K.; Calais, J.; Czernin, J.; Slavik, R.; Dahlbom, M. Tandem Isotope Therapy with 225Ac- and 177Lu-PSMA-617 in a Murine Model of Prostate Cancer. J. Nucl. Med. 2023, 64, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Fusion Pharmaceuticals Inc. PSMA-Directed Targeted Alpha Therapy With FPI-2265 (225Ac-PSMA-I&T) for the Treatment of Metastatic Castration-resISTant Prostate Cancer (TATCIST). A Phase II Clinical Trial. 2025. Available online: https://www.clinicaltrials.gov/study/NCT05219500 (accessed on 19 July 2025).
- Fusion Pharmaceuticals Inc. A Phase 2/3, Randomized, Open-Label, Multicenter Study to Evaluate the Safety and Efficacy of FPI-2265 (225Ac-PSMA-I&T) in Patients With PSMA-Positive Metastatic Castration-Resistant Prostate Cancer (mCRPC), Previously Treated With 177Lu-PSMA Radioligand Therapy (RLT). 2025. Available online: https://www.clinicaltrials.gov/study/NCT06402331 (accessed on 19 July 2025).
- Ramnaraign, B.; Sartor, O. PSMA-Targeted Radiopharmaceuticals in Prostate Cancer: Current Data and New Trials. Oncologist 2023, 28, 392–401. [Google Scholar] [CrossRef]
- Clarke, H. FDA Grants Fast Track Designation to 225Ac-FL-020 in mCRPC. Urol. Times J. 2024, 52, 10. [Google Scholar]
- Korde, A.; Patt, M.; Selivanova, S.V.; Scott, A.M.; Hesselmann, R.; Kiss, O.; Ramamoorthy, N.; Todde, S.; Rubow, S.M.; Gwaza, L.; et al. Position Paper to Facilitate Patient Access to Radiopharmaceuticals: Considerations for a Suitable Pharmaceutical Regulatory Framework. EJNMMI Radiopharm. Chem. 2024, 9, 2. [Google Scholar] [CrossRef]
- Agency, I.A.E. Production and Quality Control of Actinium-225 Radiopharmaceuticals; International Atomic Energy Agency: Vienna, Austria, 2024; pp. 1–54. [Google Scholar]

| References | Study Type | Doses | Administration Protocol | Patients | Treatment Response Based on PSA Value |
|---|---|---|---|---|---|
| M. Sathekge et al. (2019) [8] | Prospective | Initial dose 8 MBq; de-escalated to 7, 6, or 4 MBq with good response; one patient escalated to 13 MBq in cycle 3 | At 2-month intervals, 14 patients received 3 cycles; treatment stopped after 2 cycles in 3 patients due to good response; 6 patients received additional treatment after cycle 3 | 17 heavily pre-treated mCRPC patients | ≥90% PSA decline in 82% of patients; 41% had undetectable PSA and remained in remission at 12 months; Radiological Response > 50% in 88% |
| Yadav et al. (2020) [7] | Prospective | 100 kBq/kg body weight | 2 cycles given 8 weeks apart; at 6-week assessment: stop if disease progresses, continue if stable or responsive | 28 mCRPC patients refractory to or without prior [177Lu]Lu-PSMA-617 therapy | 50% PSA decline in 25% (initial follow-up) and 39% (end); molecular response in 22/28 patients: 9% complete, 45.4% partial, 9% stable, 36% progressive; disease control: 82% (biochemical), 63.6% (molecular). |
| D. Y. Lee & Kim. (2022) [33] | Retrospective | 1.5–13 MBq/cycle | 1–8 cycles | 263 patients (9 studies) | Pooled > 50% PSA decline: 61%; any PSA decline: 83.6%. |
| Kratochwil et al. (2018) [9] | Prospective | 100 kBq/kg per cycle | At 8-week intervals, 3–5 cycles per patient | 40 mCRPC patients | 63% had >50% PSA drop; 87% had any PSA response |
| M. Sathekge et al. (2020) [34] | Prospective | Initial dose: 8 MBq; reduced to 7, 6, or 4 MBq based on response; given every 8 weeks | Median 3 cycles (range 1–8) | 73 mCRPC patients | 70% had >50% PSA drop; 82% had any PSA response |
| Zacherl et al. (2021) [35] | Retrospective | Median/mean dose 7.8 MBq | 34 cycles given; most received 1–2 cycles | 14 mCRPC patients | 50% had >50% PSA drop; 79% had any PSA response |
| Feuerecker et al. (2021) [36] | Prospective | Median/mean dose 9 MBq (range, 4–13 MBq), every 2 months | Median of 2 (range 1–6) | 26 late-stage mCRPC patients | PSA decline of ≥50% was achieved in 17/26 patients |
| Rosar et al. (2021) [37] | Retrospective | Median/mean of 2.7 ± 1.1 MBq (corresponding to 33 ± 15 kBq/kg) | Median of 2 (range 1–6) | 15 mCRPC patients received [225Ac]Ac-PSMA-617 during initial [177Lu]Lu-PSMA-617 RLT | >50% of PSA decline, any PSA decline |
| Sen et al. (2021) [38] | Retrospective | At 2-month intervals, median/mean of 100 kBq/kg | Median of 2 (range 2–5) | 38 mCRPC patients with progressive disease following at least one taxane-based chemotherapy | 66% composite response (≥50% PSA decline and/or radiological) |
| Khreish et al. (2020) [39] | Retrospective | Ac-225: median 5.3 MBq (1.5–7.9) + Lu-177: median 6.9 GBq (5.0–11.6) | 1 cycle of tandem therapy; responders continued [177Lu]Lu-PSMA (0–5 cycles) | 20 mCRPC patients post-[177Lu]Lu-PSMA-617 monotherapy failure | 65% had PSA decline >50% |
| Satapathy et al. (2020) [28] | Retrospective | Median/mean of 100 kBq/kg/cycle Median cumulative dose: 8.3 MBq | 1–4 cycles, at 8–12 week intervals | 11 mCRPC patients | 46% had ≥50% PSA decline; 27% stable PSA |
| References | Side Effects | Frequency | Patients |
|---|---|---|---|
| Perrone et al. (2025) [52] | Flare pain Grade 3 Anaemia Grade 3 Leukocytopenia Grade 3/4 Thrombocytopenia Grade 3 Nephrotoxicity Grade 3 Hepatotoxicity Xerostomia (G1/G2) | 16 patients (22.5%) 15 patients (21.1%) 6 patients (8.4%) 14 patients (19.7%) 1 patient (1.4%) 6 patients (8.4%) 9 patients (12.7%) | 71 |
| M. Sathekge et al. (2019) [8] | Grade 1/2 xerostomia Grade 3 anaemia Grade 4 renal toxicity | 17 patients (100%) 1 patient (5.9%) 1 patient (5.9%) | 17 |
| Ma et al. (2022) [53] Meta-analysis (6 retrospective studies) | Xerostomia (any grade) Xerostomia (grade III) Anaemia (any grade) Anaemia (grade III) Leukopenia (any grade) Leukopenia (grade III) Thrombocytopenia (any grade) Thrombocytopenia (grade III) Nephrotoxicity (grade III) Weight loss Fatigue Anorexia Nausea Constipation Xerophthalmia Hearing loss | 155/201 (77.1%) 6/201 (3.0%) 61/201 (30.3%) 15/201 (7.5%) 30/201 (14.9%) 9/201 (4.5%) 30/201 (14.9%) 11/201 (5.5%) 6/201 (3.0%) 54/201 (26.9%) 52/201 (25.9%) 34/201 (16.9%) 26/201 (12.9%) 21/201 (10.4%) 4 cases (2%) 2 cases (1%) | 201 |
| Yadav et al. (2020) [7] | Fatigue (grade I/II) Fatigue (grade III) Xerostomia (grade I) Xerostomia (grade II) Xerostomia (any grade) Anaemia (grade III) | 14/28 (50%) 1/28 (3.6%) 3/28 (10.7%) 5/28 (17.9%) 8/28 (28.6%) 1/28 (3.6%) | 28 |
| D. Y. Lee & Kim. (2022) [33] Meta-analysis of 9 studies | Xerostomia (any grade) Anaemia (grade 3–4) Leukocytopenia (grade 3–4) Thrombocytopenia (grade 3–4) | 165/263 (63%) 37/263 (14%) 11/263 (4%) 18/263 (7%) | 263 |
| Ling et al. (2022) [54] Meta-analysis (10 studies) | Xerostomia (any grade) Xerostomia (Grade ≥ 3) Xerophthalmia Dysgeusia Fatigue (any grade) Fatigue (Grade ≥ 3) Anorexia Diarrhoea Constipation Nausea Vomiting Anaemia (any grade) Anaemia (Grade ≥ 3) Leukopenia (any grade) Leukopenia (Grade ≥ 3) Thrombocytopenia (any grade) Thrombocytopenia (Grade ≥ 3) Nephrotoxicity (any grade) Nephrotoxicity (Grade ≥ 3) | 161/210 (77%) 2/12 (17%) 5/87 (6%) 10/87 (11%) 73/166 (44%) 1/28 (4%) 36/124 (29%) 1/11 (9%) 21/84 (25%) 23/112 (21%) 5/84 (6%) 52/170 (31%) 18/169 (11%) 26/139 (19%) 10/113 (9%) 22/151 (15%) 9/122 (7%) 27/126 (21%) 6/84 (7%) | 210 12 87 87 166 28 124 11 84 112 84 170 169 139 113 151 122 126 84 |
| Alan-Selcuk et al. (2023) [55] | Grade 1–3 Haematologic Toxicity Grade 1–3 Nephrotoxicity Xerostomia | 4 (17.4%) 1 (4.3%) 23 (100%) | 23 |
| Property | [225Ac]Ac-PSMA | [177Lu]Lu-PSMA |
|---|---|---|
| Radiation type | Alpha (α) particles (4 α particles with energies between 5.8 and 8.4 MeV) | Beta (β) particles |
| Path length | ~47–85 μm | ~0.7–2.1 mm |
| LET | High LET (~100 keV/μm) | Low LET (~0.2 keV/μm) |
| Physical half-life | ~9.9 days | ~6.7 days |
| Cytotoxicity | Highly potent and localised cellular destruction due to short range and high LET | More diffuse cytotoxicity due to extended path and lower LET |
| Toxicity | [225Ac]Ac-PSMA | [177Lu]Lu-PSMA |
|---|---|---|
| Xerostomia | Common (70–84%), mainly mild–moderate (G1–2); severe rare (~1–2%) | Occurs in ~8%, generally mild–moderate |
| Haematologic | Anaemia 10–12%, leukopenia ~6–8%, thrombocytopenia ~5–6%; higher in heavily pre-treated or marrow involved patients | Anaemia ~7%, leukopenia ~3–4%, thrombocytopenia ~4–5%; mostly reversible |
| Renal toxicity | Rare (~3–4%); isolated deterioration, esp. in patients with baseline renal issues | Uncommon; no grade 3–4 events in some series; mild creatinine elevation seen (~25%) |
| Long-term effects | Still under evaluation; rare grade 3 toxicities reported (anaemia ~21%, thrombocytopenia ~20%, nephrotoxicity ~1–2%) | Better characterised; toxicities are mostly transient and manageable over time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalloul, W.; Ghizdovat, V.; Saviuc, A.; Jalloul, D.; Grierosu, I.C.; Stefanescu, C. Targeted Alpha Therapy: Exploring the Clinical Insights into [225Ac]Ac-PSMA and Its Relevance Compared with [177Lu]Lu-PSMA in Advanced Prostate Cancer Management. Pharmaceuticals 2025, 18, 1215. https://doi.org/10.3390/ph18081215
Jalloul W, Ghizdovat V, Saviuc A, Jalloul D, Grierosu IC, Stefanescu C. Targeted Alpha Therapy: Exploring the Clinical Insights into [225Ac]Ac-PSMA and Its Relevance Compared with [177Lu]Lu-PSMA in Advanced Prostate Cancer Management. Pharmaceuticals. 2025; 18(8):1215. https://doi.org/10.3390/ph18081215
Chicago/Turabian StyleJalloul, Wael, Vlad Ghizdovat, Alexandra Saviuc, Despina Jalloul, Irena Cristina Grierosu, and Cipriana Stefanescu. 2025. "Targeted Alpha Therapy: Exploring the Clinical Insights into [225Ac]Ac-PSMA and Its Relevance Compared with [177Lu]Lu-PSMA in Advanced Prostate Cancer Management" Pharmaceuticals 18, no. 8: 1215. https://doi.org/10.3390/ph18081215
APA StyleJalloul, W., Ghizdovat, V., Saviuc, A., Jalloul, D., Grierosu, I. C., & Stefanescu, C. (2025). Targeted Alpha Therapy: Exploring the Clinical Insights into [225Ac]Ac-PSMA and Its Relevance Compared with [177Lu]Lu-PSMA in Advanced Prostate Cancer Management. Pharmaceuticals, 18(8), 1215. https://doi.org/10.3390/ph18081215






