Analytical Biomarkers for Inflammation Status Monitoring of Psychotropic and Antiepileptic Drugs
Abstract
1. Introduction
2. Analytical Biomarkers of Inflammation—Characteristics and Biological Functions
2.1. C-Reactive Protein
2.2. Interleukin 6
2.3. Interleukin 1β
2.4. Interleukin 4 and 10
2.5. Tumour Necrosis Factor Alpha
3. Analytical Determination of Inflammatory Biomarkers
4. Psychotropic and Antiepileptic Drugs and Inflammatory Biomarkers: An Immunological Perspective of the Mutual Relationship
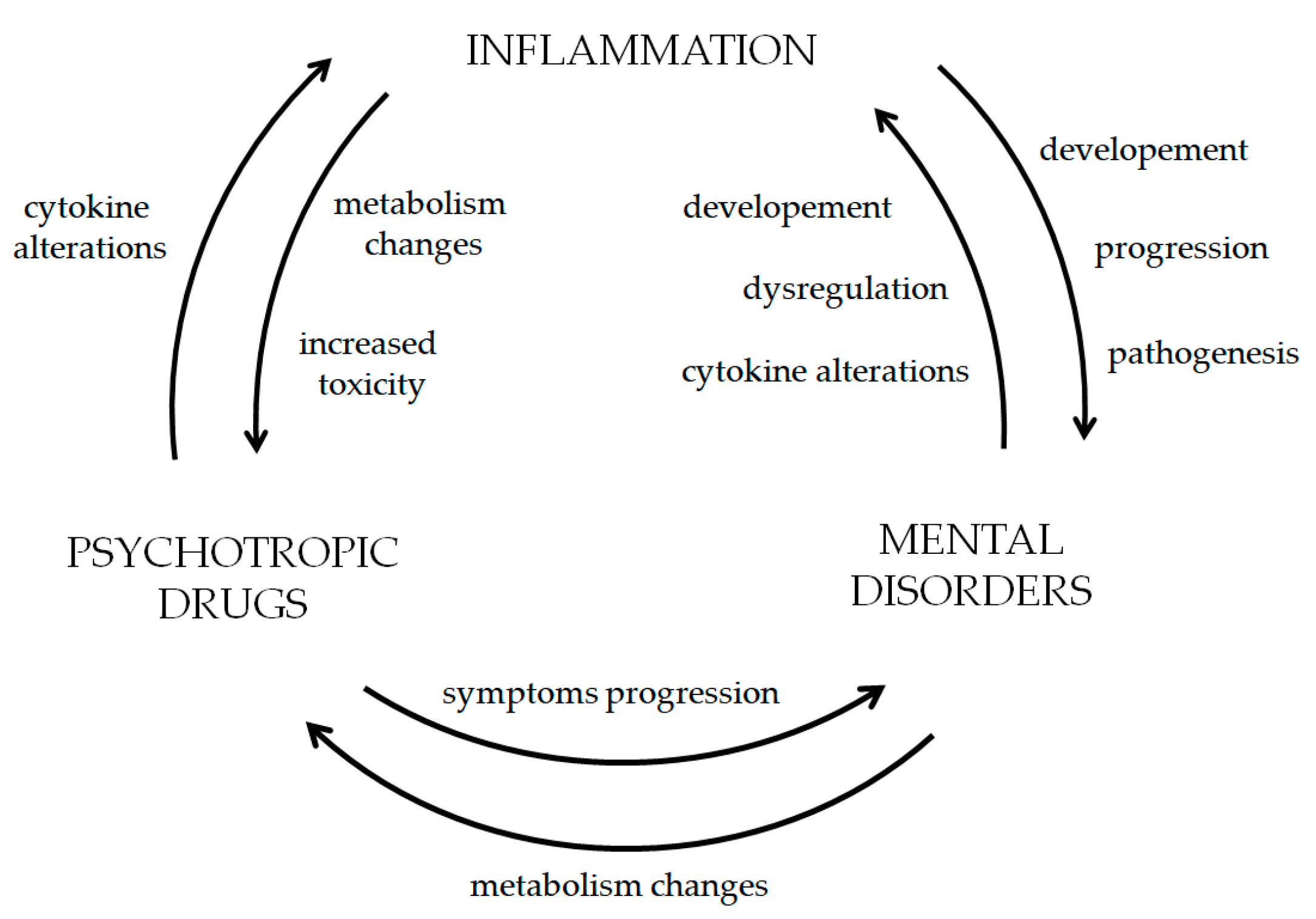
5. Correlation Cycle—Inflammation, Psychiatric and Neurological Disorders with Psychotropics and Antiepileptics
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef]
- Müller, N.; Weidinger, E.; Leitner, B.; Schwarz, M.J. The Role of Inflammation in Schizophrenia. Front. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The Role of Inflammation in the Development of Epilepsy. J. Neuroinflammation 2018, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Lorigados Pedre, L.; Morales Chacón, L.M.; Orozco Suárez, S.; Pavón Fuentes, N.; Estupiñán Díaz, B.; Serrano Sánchez, T.; García Maeso, I.; Rocha Arrieta, L. Inflammatory Mediators in Epilepsy. Curr. Pharm. Des. 2013, 19, 6766–6772. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The Spectrum of Inflammatory Responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Antonelli, M.; Kushner, I. It’s Time to Redefine Inflammation. FASEB J. 2017, 31, 1787–1791. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological Control of Inflammation in Wound Healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef]
- Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 31 January 2025).
- Herrera-Imbroda, J.; Flores-López, M.; Ruiz-Sastre, P.; Gómez-Sánchez-Lafuente, C.; Bordallo-Aragón, A.; Rodríguez de Fonseca, F.; Mayoral-Cleríes, F. The Inflammatory Signals Associated with Psychosis: Impact of Comorbid Drug Abuse. Biomedicines 2023, 11, 454. [Google Scholar] [CrossRef]
- Borsini, A.; Zunszain, P.A.; Thuret, S.; Pariante, C.M. The Role of Inflammatory Cytokines as Key Modulators of Neurogenesis. Trends Neurosci. 2015, 38, 145–157. [Google Scholar] [CrossRef]
- Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 30 July 2025).
- Garés-Caballer, M.; Sánchez-Ortí, J.V.; Correa-Ghisays, P.; Balanzá-Martínez, V.; Selva-Vera, G.; Vila-Francés, J.; Magdalena-Benedito, R.; San-Martin, C.; Victor, V.M.; Escribano-Lopez, I.; et al. Immune–Inflammatory Biomarkers Predict Cognition and Social Functioning in Patients With Type 2 Diabetes Mellitus, Major Depressive Disorder, Bipolar Disorder, and Schizophrenia: A 1-Year Follow-Up Study. Front. Neurol. 2022, 13, 883927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, B.; Yang, W.; Ge, T.; Cui, R. Brain-Immune Interaction Mechanisms: Implications for Cognitive Dysfunction in Psychiatric Disorders. Cell Prolif. 2022, 55, e13295. [Google Scholar] [CrossRef] [PubMed]
- Bhikram, T.; Sandor, P. Neutrophil-Lymphocyte Ratios as Inflammatory Biomarkers in Psychiatric Patients. Brain Behav. Immun. 2022, 105, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Keating, B.A.; Dale, R.C. Anti-Inflammatory Properties of Commonly Used Psychiatric Drugs. Front. Neurosci. 2022, 16, 1039379. [Google Scholar] [CrossRef] [PubMed]
- Fitton, R.; Sweetman, J.; Heseltine-Carp, W.; van der Feltz-Cornelis, C. Anti-Inflammatory Medications for the Treatment of Mental Disorders: A Scoping Review. Brain Behav. Immun. Health 2022, 26, 100518. [Google Scholar] [CrossRef]
- Baumeister, D.; Ciufolini, S.; Mondelli, V. Effects of Psychotropic Drugs on Inflammation: Consequence or Mediator of Therapeutic Effects in Psychiatric Treatment? Psychopharmacology 2016, 233, 1575–1589. [Google Scholar] [CrossRef]
- Radtke, F.A.; Chapman, G.; Hall, J.; Syed, Y.A. Modulating Neuroinflammation to Treat Neuropsychiatric Disorders. Biomed. Res. Int. 2017, 2017, 5071786. [Google Scholar] [CrossRef]
- Ting, E.Y.-C.; Yang, A.C.; Tsai, S.-J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. Anti-Inflammatory Effects of 2nd Generation Antipsychotics in Patients with Schizophrenia: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2023, 160, 126–136. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why They Are More than Inflammatory Biomarkers, and Why It Matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Baysak, E.; Guden, D.S.; Aricioglu, F.; Halaris, A. C-Reactive Protein as a Potential Biomarker in Psychiatric Practice: Are We There Yet? World J. Biol. Psychiatry 2022, 23, 243–256. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Steiner, J.; Bernstein, H.-G.; Dodd, S.; Pasco, J.A.; Dean, O.M.; Nardin, P.; Gonçalves, C.-A.; Berk, M. C-Reactive Protein Is Increased in Schizophrenia but Is Not Altered by Antipsychotics: Meta-Analysis and Implications. Mol. Psychiatry 2016, 21, 554–564. [Google Scholar] [CrossRef]
- Borovcanin, M.M.; Jovanovic, I.; Radosavljevic, G.; Pantic, J.; Minic Janicijevic, S.; Arsenijevic, N.; Lukic, M.L. Interleukin-6 in Schizophrenia-Is There a Therapeutic Relevance? Front. Psychiatry 2017, 8, 221. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative Meta-Analysis of Interleukins 6 and 1β, Tumour Necrosis Factor α and C-Reactive Protein in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-Analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Remnitz, A.D.; Hadad, R.; Keane, R.W.; Dietrich, W.D.; de Rivero Vaccari, J.P. Comparison of Methods of Detecting IL-1β in the Blood of Alzheimer’s Disease Subjects. Int. J. Mol. Sci. 2025, 26, 831. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Şimşek, Ş.; Yıldırım, V.; Çim, A.; Kaya, S. Serum IL-4 and IL-10 Levels Correlate with the Symptoms of the Drug-Naive Adolescents with First Episode, Early Onset Schizophrenia. J. Child. Adolesc. Psychopharmacol. 2016, 26, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Worthen, R.J.; Garzon Zighelboim, S.S.; Torres Jaramillo, C.S.; Beurel, E. Anti-Inflammatory IL-10 Administration Rescues Depression-Associated Learning and Memory Deficits in Mice. J. Neuroinflamm. 2020, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Ronström, J.W.; Williams, S.B.; Payne, A.; Obray, D.J.; Hafen, C.; Burris, M.; Scott Weber, K.; Steffensen, S.C.; Yorgason, J.T. Interleukin-10 Enhances Activity of Ventral Tegmental Area Dopamine Neurons Resulting in Increased Dopamine Release. Brain Behav. Immun. 2023, 113, 145–155. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R. Biomarkers: Potential Uses and Limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Aziz, N.; Detels, R.; Quint, J.J.; Li, Q.; Gjertson, D.; Butch, A.W. Stability of Cytokines, Chemokines and Soluble Activation Markers in Unprocessed Blood Stored under Different Conditions. Cytokine 2016, 84, 17–24. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kim, Y.-K. Immunomodulatory Effects of Antipsychotic Drugs in Whole Blood Cell Cultures from Healthy Subjects. Curr. Psychiatry Res. Rev. 2019, 15, 261–266. [Google Scholar] [CrossRef]
- Sommerfeld, K.; Łukasik-Głębocka, M.; Krawczak, E.; Stodolska, A.; Zielińska-Psuja, B. Participation of Quetiapine in Oxidative Stress and Inflammation Status in the Treatment of Drug Overdose. Acta Pol. Pharm.-Drug Res. 2023, 80, 327–333. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Wang, N.; Zhou, H.; Huang, H.-L.; Shan, S.-C. Effect of Oxcarbazepine on Immune Function, Thyroid Function and Related Factors in Epilepsy Patients. J. Hainan Med. Univ. 2019, 3, 205–208. [Google Scholar]
- Shiah, I.-S.; Yatham, L.N.; Yeh, C.-B.; Ravindran, A.V. Effect of Valproate on Plasma Levels of Interleukin-6 in Healthy Male Humans. Int. Clin. Psychopharmacol. 2005, 20, 295–298. [Google Scholar] [CrossRef]
- Sonmez, F.M.; Serin, H.M.; Alver, A.; Aliyazicioglu, R.; Cansu, A.; Can, G.; Zaman, D. Blood Levels of Cytokines in Children with Idiopathic Partial and Generalized Epilepsy. Seizure 2013, 22, 517–521. [Google Scholar] [CrossRef]
- Guenther, S.; Bauer, S.; Hagge, M.; Knake, S.; Olmes, D.G.; Tackenberg, B.; Rosenow, F.; Hamer, H.M. Chronic Valproate or Levetiracetam Treatment Does Not Influence Cytokine Levels in Humans. Seizure 2014, 23, 666–669. [Google Scholar] [CrossRef]
- Azizi, E.; Zavaran Hosseini, A.; Soudi, S.; Noorbala, A.A. Alteration of Serum Levels of Cytokines in Schizophrenic Patients before and after Treatment with Risperidone. Iran. J. Allergy Asthma Immunol. 2019, 18, 262–268. [Google Scholar] [CrossRef]
- Mathieu, O.; Picot, M.-C.; Gelisse, P.; Breton, H.; Demoly, P.; Hillaire-Buys, D. Effects of Carbamazepine and Metabolites on IL-2, IL-5, IL-6, IL-10 and IFN-γ Secretion in Epileptic Patients: The Influence of Co-Medication. Pharmacol. Rep. 2011, 63, 86–94. [Google Scholar] [CrossRef]
- Himmerich, H.; Bartsch, S.; Hamer, H.; Mergl, R.; Schönherr, J.; Petersein, C.; Munzer, A.; Kirkby, K.C.; Bauer, K.; Sack, U. Impact of Mood Stabilizers and Antiepileptic Drugs on Cytokine Production In-Vitro. J. Psychiatr. Res. 2013, 47, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, B.; Wang, H.; Li, Q.; Li, X.; Hu, P.; Lai, Q.; Fan, H. Is the Regulation of Lamotrigine on Depression in Patients with Epilepsy Related to Cytokines? Heliyon 2024, 10, e33129. [Google Scholar] [CrossRef] [PubMed]
- Kalmady, S.V.; Shivakumar, V.; Jose, D.; Ravi, V.; Keshavan, M.S.; Gangadhar, B.N.; Venkatasubramanian, G. Plasma Cytokines in Minimally Treated Schizophrenia. Schizophr. Res. 2018, 199, 292–296. [Google Scholar] [CrossRef]
- Noto, C.; Ota, V.K.; Gouvea, E.S.; Rizzo, L.B.; Spindola, L.M.N.; Honda, P.H.S.; Cordeiro, Q.; Belangero, S.I.; Bressan, R.A.; Gadelha, A.; et al. Effects of Risperidone on Cytokine Profile in Drug-Naïve First-Episode Psychosis. Int. J. Neuropsychopharmacol. 2014, 18, pyu042. [Google Scholar] [CrossRef]
- Labh, R.; Gupta, R.; Narang, M.; Halder, S.; Kar, R. Effect of Valproate and Add-on Levetiracetam on Inflammatory Biomarkers in Children with Epilepsy. Epilepsy Behav. 2021, 125, 108358. [Google Scholar] [CrossRef]
- Gulcebi, M.I.; Kendirli, T.; Turgan, Z.A.; Patsalos, P.N.; Onat Yilmaz, F. The Effect of Serum Levetiracetam Concentrations on Therapeutic Response and IL1-Beta Concentration in Patients with Epilepsy. Epilepsy Res. 2018, 148, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczak, D. Padaczka a Cytokiny Prozapalne. Immunomodulujące Właściwości Leków Przeciwpadaczkowych. Neurol. I Neurochir. Pol. 2011, 45, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Schirmer, B. A Simple Mechanistic Terminology of Psychoactive Drugs: A Proposal. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, S.N. A New Nomenclature for Psychotropic Drugs. J. Clin. Psychopharmacol. 2015, 35, 428–433. [Google Scholar] [CrossRef]
- Sankaraneni, R.; Lachhwani, D. Antiepileptic Drugs—A Review. Pediatr. Ann. 2015, 44, e36–e42. [Google Scholar] [CrossRef]
- Rahman, M.; Awosika, A.O.; Nguyen, H. Valproic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ximenes, J.C.M.; Neves, K.R.T.; Leal, L.K.A.M.; do Carmo, M.R.S.; Brito, G.A.d.C.; Naffah-Mazzacoratti, M.d.G.; Cavalheiro, É.A.; Viana, G.S.d.B. Valproic Acid Neuroprotection in the 6-OHDA Model of Parkinson’s Disease Is Possibly Related to Its Anti-Inflammatory and HDAC Inhibitory Properties. J. Neurodegener. Dis. 2015, 2015, 313702. [Google Scholar] [CrossRef]
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef]
- Du, Y.; Dou, Y.; Wang, M.; Wang, Y.; Yan, Y.; Fan, H.; Fan, N.; Yang, X.; Ma, X. Efficacy and Acceptability of Anti-Inflammatory Agents in Major Depressive Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2024, 15, 1407529. [Google Scholar] [CrossRef]
- Schmidt, D.; Elger, C.E. What Is the Evidence That Oxcarbazepine and Carbamazepine Are Distinctly Different Antiepileptic Drugs? Epilepsy Behav. 2004, 5, 627–635. [Google Scholar] [CrossRef]
- Beydoun, A.; DuPont, S.; Zhou, D.; Matta, M.; Nagire, V.; Lagae, L. Current Role of Carbamazepine and Oxcarbazepine in the Management of Epilepsy. Seizure 2020, 83, 251–263. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Salahuddin, M.; Khan, F.A.; Al Dhamen, M.A.; Alsaeed, W.J.; Gomaa, M.S.; Vatte, C.; Alomary, M.N. Combinatorial Regimen of Carbamazepine and Imipramine Exhibits Synergism against Grandmal Epilepsy in Rats: Inhibition of Pro-Inflammatory Cytokines and PI3K/Akt/mTOR Signaling Pathway. Pharmaceuticals 2021, 14, 1204. [Google Scholar] [CrossRef]
- Meltzer, H.Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef]
- Marcinowicz, P.; Więdłocha, M.; Zborowska, N.; Dębowska, W.; Podwalski, P.; Misiak, B.; Tyburski, E.; Szulc, A. A Meta-Analysis of the Influence of Antipsychotics on Cytokines Levels in First Episode Psychosis. J. Clin. Med. 2021, 10, 2488. [Google Scholar] [CrossRef]
- Moots, R.J.; Al-Saffar, Z.; Hutchinson, D.; Golding, S.P.; Young, S.P.; Bacon, P.A.; McLaughlin, P.J. Old Drug, New Tricks: Haloperidol Inhibits Secretion of Proinflammatory Cytokines. Ann. Rheum. Dis. 1999, 58, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Handley, R.; Mondelli, V.; Zelaya, F.; Marques, T.; Taylor, H.; Reinders, A.A.T.S.; Chaddock, C.; McQueen, G.; Hubbard, K.; Papadopoulos, A.; et al. Effects of Antipsychotics on Cortisol, Interleukin-6 and Hippocampal Perfusion in Healthy Volunteers. Schizophr. Res. 2016, 174, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cheremnykh, E.G.; Ivanov, P.A.; Sokolov, O.Y.; Prokhorova, T.A.; Tereshkina, E.B.; Baymeeva, N.V.; Miroshnichenko, I.I.; Kost, N.V. Haloperidol Reduces the Activity of Complement and Induces the Anti-Inflammatory Transformation of Peritoneal Macrophages in Rats. J. Neuroimmune Pharmacol. 2019, 14, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Obuchowicz, E.; Bielecka-Wajdman, A.M.; Paul-Samojedny, M.; Nowacka, M. Different Influence of Antipsychotics on the Balance between Pro- and Anti-Inflammatory Cytokines Depends on Glia Activation: An in Vitro Study. Cytokine 2017, 94, 37–44. [Google Scholar] [CrossRef]
- Himmerich, H.; Schönherr, J.; Fulda, S.; Sheldrick, A.J.; Bauer, K.; Sack, U. Impact of Antipsychotics on Cytokine Production In-Vitro. J. Psychiatr. Res. 2011, 45, 1358–1365. [Google Scholar] [CrossRef]
- Haddad, P.M.; Dursun, S.M. Neurological Complications of Psychiatric Drugs: Clinical Features and Management. Hum. Psychopharmacol. 2008, 23 (Suppl. S1), 15–26. [Google Scholar] [CrossRef]
- Divac, N.; Prostran, M.; Jakovcevski, I.; Cerovac, N. Second-Generation Antipsychotics and Extrapyramidal Adverse Effects. Biomed. Res. Int. 2014, 2014, 656370. [Google Scholar] [CrossRef] [PubMed]
- Szota, A.M.; Radajewska, I.; Araszkiewicz, A.S. Atypical Neuroleptic Malignant Syndrome: Case Reports and Diagnostic Challenges. J. Psychoact. Drugs 2022, 54, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Juncal-Ruiz, M.; Riesco-Dávila, L.; Ortiz-García de la Foz, V.; Martínez-Garcia, O.; Ramírez-Bonilla, M.; Ocejo-Viñals, J.G.; Leza, J.C.; López-Hoyos, M.; Crespo-Facorro, B. Comparison of the Anti-Inflammatory Effect of Aripiprazole and Risperidone in 75 Drug-Naïve First Episode Psychosis Individuals: A 3 months Randomized Study. Schizophr. Res. 2018, 202, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Méndez, M.A.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; Villar-Soto, M.; Genis-Mendoza, A.D.; González-Castro, T.B.; López-Narváez, M.L.; Martínez-Magaña, J.J.; Castillo-Avila, R.G.; Villar-Juárez, G.E. Effect of Risperidone on Serum IL-6 Levels in Individuals with Schizophrenia: A Systematic Review and Meta-Analysis. Int. J. Psychiatry Clin. Pract. 2023, 27, 171–178. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, S.; Shi, Y.; Yang, Y.; Zhang, Y.; Xia, L.; Zhang, K.; Liu, H. Pro-Inflammatory Cytokine Levels Are Elevated in Female Patients with Schizophrenia Treated with Clozapine. Psychopharmacology 2022, 239, 765–771. [Google Scholar] [CrossRef]
- Su, W.-J.; Hu, T.; Jiang, C.-L. Cool the Inflamed Brain: A Novel Anti-Inflammatory Strategy for the Treatment of Major Depressive Disorder. Curr. Neuropharmacol. 2024, 22, 810–842. [Google Scholar] [CrossRef]
- Köhler-Forsberg, O.; Lydholm, C.N.; Hjorthøj, C.; Nordentoft, M.; Mors, O.; Benros, M.E. Efficacy of Anti-Inflammatory Treatment on Major Depressive Disorder or Depressive Symptoms: Meta-Analysis of Clinical Trials. Acta Psychiatr. Scand. 2019, 139, 404–419. [Google Scholar] [CrossRef]
- Müller, N. Immunological Aspects of the Treatment of Depression and Schizophrenia. Dialogues Clin. Neurosci. 2017, 19, 55–63. [Google Scholar] [CrossRef]
- Arabzadeh, S.; Ameli, N.; Zeinoddini, A.; Rezaei, F.; Farokhnia, M.; Mohammadinejad, P.; Ghaleiha, A.; Akhondzadeh, S. Celecoxib Adjunctive Therapy for Acute Bipolar Mania: A Randomized, Double-Blind, Placebo-Controlled Trial. Bipolar Disord. 2015, 17, 606–614. [Google Scholar] [CrossRef]
- Shalbafan, M.; Mohammadinejad, P.; Shariat, S.-V.; Alavi, K.; Zeinoddini, A.; Salehi, M.; Askari, N.; Akhondzadeh, S. Celecoxib as an Adjuvant to Fluvoxamine in Moderate to Severe Obsessive-Compulsive Disorder: A Double-Blind, Placebo-Controlled, Randomized Trial. Pharmacopsychiatry 2015, 48, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Cai, D.-B.; Yang, X.-H.; Ungvari, G.S.; Ng, C.H.; Müller, N.; Ning, Y.-P.; Xiang, Y.-T. Adjunctive Celecoxib for Schizophrenia: A Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Trials. J. Psychiatr. Res. 2017, 92, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Adzic, M.; Brkic, Z.; Mitic, M.; Francija, E.; Jovicic, M.J.; Radulovic, J.; Maric, N.P. Therapeutic Strategies for Treatment of Inflammation-Related Depression. Curr. Neuropharmacol. 2018, 16, 176–209. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Lizcano, L.; Arenas-Villamizar, V.V.; Jaimes-Duarte, E.B.; García-Pacheco, H.; Paredes, C.S.; Bermúdez, V.; Rivera-Porras, D. Metabolic Adverse Effects of Psychotropic Drug Therapy: A Systematic Review. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 1505–1520. [Google Scholar] [CrossRef]
- Moschny, N.; Hefner, G.; Grohmann, R.; Eckermann, G.; Maier, H.B.; Seifert, J.; Heck, J.; Francis, F.; Bleich, S.; Toto, S.; et al. Therapeutic Drug Monitoring of Second- and Third-Generation Antipsychotic Drugs—Influence of Smoking Behavior and Inflammation on Pharmacokinetics. Pharmaceuticals 2021, 14, 514. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-Related Biomarkers in Major Psychiatric Disorders: A Cross-Disorder Assessment of Reproducibility and Specificity in 43 Meta-Analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Bishop, J.R.; Zhang, L.; Lizano, P. Inflammation Subtypes and Translating Inflammation-Related Genetic Findings in Schizophrenia and Related Psychoses: A Perspective on Pathways for Treatment Stratification and Novel Therapies. Harv. Rev. Psychiatry 2022, 30, 59. [Google Scholar] [CrossRef]
- Byrne, J.F.; Healy, C.; Mongan, D.; Susai, S.R.; Zammit, S.; Fӧcking, M.; Cannon, M.; Cotter, D.R. Transdiagnostic Inflammatory Subgroups among Psychiatric Disorders and Their Relevance to Role Functioning: A Nested Case-Control Study of the ALSPAC Cohort. Transl. Psychiatry 2022, 12, 377. [Google Scholar] [CrossRef]
- Simon, M.S.; Burger, B.; Weidinger, E.; Arteaga-Henríquez, G.; Zill, P.; Musil, R.; Drexhage, H.A.; Müller, N. Efficacy of Sertraline Plus Placebo or Add-On Celecoxib in Major Depressive Disorder: Macrophage Migration Inhibitory Factor as a Promising Biomarker for Remission After Sertraline—Results From a Randomized Controlled Clinical Trial. Front. Psychiatry 2021, 12, 615261. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Y.Z.; Slavish, D.C. Measuring Salivary Markers of Inflammation in Health Research: A Review of Methodological Considerations and Best Practices. Psychoneuroendocrinology 2021, 124, 105069. [Google Scholar] [CrossRef] [PubMed]
- Gigase, F.A.J.; Smith, E.; Collins, B.; Moore, K.; Snijders, G.J.L.J.; Katz, D.; Bergink, V.; Perez-Rodriquez, M.M.; De Witte, L.D. The Association between Inflammatory Markers in Blood and Cerebrospinal Fluid: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2023, 28, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
| Protein Designation | Analytical Methods | Biological Material | Patients/Evaluated Drug | Reference |
|---|---|---|---|---|
| CRP | ELISA | Whole blood | Healthy volunteers | [43] |
| Immunoturbidimetry | Serum | Quetiapine overdose | [44] | |
| Serum/plasma | Epileptic patients; OXC | [45] | ||
| IL-6 | ELISA | Plasma | Healthy male volunteers; VPA | [46] |
| Serum | Epileptic children; VPA | [47] | ||
| Epileptic patients; VPA, LEV | [48] | |||
| Schizophrenic patients; RIS | [49] | |||
| Flow cytometry | Whole blood | Epileptic patients; CBZ, LTG, VPA | [50] | |
| Healthy female volunteers; a.o LTG, CBZ, VPA, OXC, LEV | [51] | |||
| Plasma | Epileptic patients; LTG, VPA | [52] | ||
| Schizophrenic patients | [53] | |||
| Serum | First-episode psychosis; RIS | [54] | ||
| IL-1β | ELISA | Serum | Epileptic children; VPA | [47] |
| Schizophrenic patients; RIS | [49] | |||
| Epileptic patients; VPA, LEV | [48] | |||
| Epileptic children; VPA, LEV | [55] | |||
| CLIA | Serum | Epileptic patients; LEV | [56] | |
| Flow cytometry | Whole blood | Healthy female volunteers; a.o LTG, CBZ, VPA, OXC, LEV | [51] | |
| Plasma | Epileptic patients; LTG, VPA | [52] | ||
| IL-4 | Flow cytometry | Whole blood | Healthy female volunteers; a.o LTG, CBZ, VPA, OXC, LEV | [51] |
| Serum | First-episode psychosis; RIS | [54] | ||
| Plasma | Schizophrenic patients | [53] | ||
| IL-10 | ELISA | Serum | Epileptic children; VPA | [47] |
| Flow cytometry | Whole blood | Epileptic patients; CBZ, LTG, VPA | [50] | |
| Serum | First-episode psychosis; RIS | [54] | ||
| Plasma | Schizophrenic patients | [53] | ||
| TNF-α | ELISA | Serum | Epileptic children; VPA | [47] |
| Epileptic patients; VPA, LEV | [48] | |||
| Schizophrenic patients; RIS | [49] | |||
| Flow cytometry | Whole blood | Healthy female volunteers; a.o LTG, CBZ, VPA, OXC, LEV | [51] | |
| Serum | First-episode psychosis; RIS | [54] | ||
| Plasma | Epileptic patients; LTG, VPA | [52] |
| Antiepileptic Drugs | Psychotropic Drugs | |||||
|---|---|---|---|---|---|---|
| Drug | Cytokine Changes | Reference | Drug | Cytokine Changes | Reference | |
| First generation | VPA | ↑ IL-6 −IL-6, IL-1β ↓ IL-22 | [8] [47,48,55] [51] | HLP | ↓ IL-6, TNF-α ↓ IL-6 ↓ IL-10 ↑ IL-1β, TNF-α | [71] [72] [74] [74] |
| CBZ | ↓ IL-22 ↓ IL-1, IL-6 | [51] [68] | CHPZ | ↑ TNF-α, IL-2 ↑ IL-4 | [75] [75] | |
| Second generation | LTG | ↓ IL-6, IL-1β, IL-2, TNF-α −IL-22 | [52] [51] | RIS | ↓ IL-10 ↑ IL-1β, TNF-α ↓ TNF-α, IL-10 ↑ IL-4 ↑ IL-6 | [74] [74] [54] [54] [80] |
| LEV | −IL-6, IL-1β, TNF-α ↓ IL-1β ↑ IL-22 | [48,55] [56] [51] | CLO | ↑ IL-2, IL-6, TNF-α | [81] | |
| Drug Category | Drug | Molecular Mechanism Used in Therapy |
|---|---|---|
| Cytokine inhibitors | Adalimumab | TNF-α inhibition |
| Infliximab | ||
| Etanercept | ||
| NSAIDs | Celecoxib | Non-selective COX-inhibition/Selective COX-2 inhibition |
| Aspirin | ||
| Ibuprofen | ||
| Naproxen | ||
| Antibiotics | Minocycline | Anti-inflammatory, antioxidant and neuroprotective effects on CNS |
| Rapamycin | ||
| Antidiabetic drugs | Pioglitazone | Anti-inflammatory, neuroprotective, and anti-excitotoxic effects |
| Metformin | ||
| Statins | Simvastatin, | Anti-inflammatory and antioxidant effects (NMDA receptor modulation and inhibition of NO) |
| Atorvastatin | ||
| Lovastatin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiers, W.; Sommerfeld-Klatta, K.; Gumustas, M.; Mozdziak, P.; Łukasik-Głębocka, M.; Teżyk, A.; Żaba, Z.; Żaba, C.; Piotrowska-Kempisty, H. Analytical Biomarkers for Inflammation Status Monitoring of Psychotropic and Antiepileptic Drugs. Pharmaceuticals 2025, 18, 1213. https://doi.org/10.3390/ph18081213
Jiers W, Sommerfeld-Klatta K, Gumustas M, Mozdziak P, Łukasik-Głębocka M, Teżyk A, Żaba Z, Żaba C, Piotrowska-Kempisty H. Analytical Biomarkers for Inflammation Status Monitoring of Psychotropic and Antiepileptic Drugs. Pharmaceuticals. 2025; 18(8):1213. https://doi.org/10.3390/ph18081213
Chicago/Turabian StyleJiers, Wiktoria, Karina Sommerfeld-Klatta, Mehmet Gumustas, Paul Mozdziak, Magdalena Łukasik-Głębocka, Artur Teżyk, Zbigniew Żaba, Czesław Żaba, and Hanna Piotrowska-Kempisty. 2025. "Analytical Biomarkers for Inflammation Status Monitoring of Psychotropic and Antiepileptic Drugs" Pharmaceuticals 18, no. 8: 1213. https://doi.org/10.3390/ph18081213
APA StyleJiers, W., Sommerfeld-Klatta, K., Gumustas, M., Mozdziak, P., Łukasik-Głębocka, M., Teżyk, A., Żaba, Z., Żaba, C., & Piotrowska-Kempisty, H. (2025). Analytical Biomarkers for Inflammation Status Monitoring of Psychotropic and Antiepileptic Drugs. Pharmaceuticals, 18(8), 1213. https://doi.org/10.3390/ph18081213