Abstract
Objectives: Research on the antimicrobial effect of Hypericum plant constituents is very rarely accompanied by studies of the cytotoxic effect on cell lines. In the current study, besides microbiological tests, an investigation of the cytotoxicity of Hypericum active ingredients on five non-tumorigenic cell lines, as well as research into the effect on other factors of host homeostasis, was performed. Methods: The main methods applied included an MTT assay, the broth microdilution method (BMD), real-time PCR, live cell imaging with Hoechst dye, Western blot, an enzyme-linked immunosorbent assay (ELISA), and skin irritation test on rabbits. Results: The mean inhibitory concentrations (IC50) of six selected agents—previously phytochemically characterized extracts and compounds—ranged from 0.63 to 48 µg/mL. Due to their strong antimicrobial effect and favorable cytotoxic profile, the extract RochC from Hypericum rochelii and the compound olympiforin B from Hypericum olympicum were selected for subsequent studies at their previously determined minimum inhibitory concentrations (MICs) against Staphylococcus aureus—0.625 and 1 µg/mL, respectively. These doses were lower than their IC50 values and the maximum tolerated concentrations (MTCs), according to ISO 10993-5, Annex C, for fibroblast cells, including a human gingival line. The MIC values of RochC and Olympiforin B against the cariogenic Streptococcus mutans were 6 and 3 µg/mL, respectively, values lower than the IC50 values of the gingival cells. Olympiforin B inhibited the gene expression of the staphylococcal biofilm-related genes icaA and icaD, while RochC induced icaA and had a versatile effect on icaD. The MIC values for lactobacilli strains were higher than for S. aureus. The phytoconstituents did not cause cytopathic effects or apoptosis in CCL-1 fibroblasts at 2 × MIC. However, the agents at 1 × MIC significantly induced Atg5 and Atg7, proteins related to autophagy. Cytochrome P450 was not induced in liver cells, with the exception of a dose of 2 × MIC of RochC. The agents did not irritate rabbit skin in vivo at a dose of even 10 × MIC. Conclusions: The extract and compound have potential for further pharmacological development.
1. Introduction
Antimicrobial resistance (AMR) is reported as a top priority health threat by the World Health Organization (WHO), the G7, and G20 [1] and there is an urgent need for the development of novel antimicrobial agents [2]. Nevertheless, the best efforts of synthetic library chemistry have not led to an increase in the rate of new chemical entities reaching the market [3]. Thus, natural products remain an important source of complex structures for antimicrobial drug leads [4,5].
Interestingly, reports state that while in the West medicinal plant use is steadily rising [6], in Eastern Europe the rate of increase is higher. It has been stated that in Eastern Europe more than 60% of prescriptions contain plant-based drugs; thus, traditional medicine seems to have amalgamated with modern medicine [7]. That tradition drives and necessitates scientific research on native flora for potential medicinal plants, and lesser known species from the genus Hypericum L. are potential candidates.
Plants from the Hypericum L. genus (Hypericaceae) have traditionally been used for wounds, burns, and ulcers, largely because of their antimicrobial effects [8]. The most popular one, H. perforatum L. (St. John’s Wort), has one more distinct property. It has been approved in some European countries for the treatment of depression [9]. Notably, its antidepressant effect seems partly to be connected to restoration of gut microbiota [10].
The antimicrobial effect of Hypericum representatives, e.g., H. perforatum [11], H. androsaemum L., [12], H. tetrapterum Fr., [13], H. roeperianum Schimp. ex A. Rich. [14], etc., has been well-documented, including in reviews [15,16]. An extract from H. perforatum has been patented as a food preservative in the United States [17]. A preparation from St. John’s Wort by the name novoimanine has even been used as a clinical drug in Russia to treat infections [4,18]. The genus and its phytoconstituents have other bioactivities, such as antitumor [19,20,21,22,23,24], antioxidant [12,15,19,20,25], anti-inflamatory [19,26], etc. Hyperforin (HF) is the main antidepressant and antibacterial compound in H. perforatum [4]. It is a polycyclic polyprenylated acylphloroglucinol (PPAP) and numerous PPAPs with antimicrobial, cytotoxic, and other activities have been isolated so far from Hypericum plants [27,28,29,30,31]. Examples of reviews describing their effect can be found in [16,32,33].
Recently, a number of constituents from perennial Hypericum species growing in Bulgaria were isolated, and the antibacterial activity of several of them turned out to be remarkable. This applies to PPAPs from Hypericum olympicum L., for extracts and fractions from Hypericum hirsutum L., and for extracts from Hypericum rochelii Griseb. ex Schenk. The most active principles were phytochemically characterized through ultra-high-performance liquid chromatography–high-resolution mass spectrometry (UHPLC–HRMS) and found to contain polyprenylated phloroglucinols [4]. Most notably, the RochC extract, obtained by supercritical CO2 extraction from H. rochelii, showed antibacterial activity against Staphylococcus aureus as great as the best extract from a Hypericum plant so far—an extract from H. perforatum with a minimum inhibitory concentration (MIC) of 0.625 µg/mL against a caries-causing Lactobacillus sp. [34]. The agents also turned out to have anti-biofilm effects against methicillin-resistant S. aureus (MRSA).
These findings are promising, as in the last two decades S. aureus and MRSA have been among the main human pathogens and their infections have become more dangerous and expensive to treat due to their increasing AMR [35,36]. Pathogenic Gram-positive bacteria such as S. aureus, Corynebacterium, etc., cause a diverse range of infections, from mild tonsillitis and pharyngitis to endocarditis, meningitis, septicemia, and toxic shock syndrome [37].
Biofilms are notorious for causing chronic infections in the sites of colonization (wounds, urinary tracts, etc.) [38]. As discussed before, bacterial biofilms contribute to >80% of all infections and 65% of infections in developed countries [35,39]. MRSA, together with Pseudomonas aeruginosa, is the most ubiquitous pathogen in nosocomial biofilms [40]. Staphylococci, particularly S. aureus and Staphylococcus epidermidis, are the most frequent causes of device-associated infections and biofilms [41]. MRSA biofilm colonizes catheters, prosthetic joints, pacemakers, and other devices [38]. This process may lead to implant failure, localized tissue destruction, chronic infection, e.g., bacteremia, or other tissue colonization [41]. MRSA is more frequently associated with mortality than other bacterial infections, especially in patients with wounds and necrotic tissue [35,36,39,42].
The mechanical biofilm barrier between drugs, host immune factors, and the inner biofilm bacterial cells, as well as the dormant cells within, are other mechanisms of AMR, which increases several hundred times in the biofilms of many bacterial species [43]. In addition, the direct bactericidal action of antimicrobials causes more AMR than the bacteriostatic action, or the property of targeting some virulence factor such as biofilm formation [44]. All this makes S. aureus and MRSA biofilms promising targets for antimicrobial therapy.
While numerous studies are devoted to the in vitro cytotoxic and anticancer activity of active ingredients from Hypericum [19,20,21,22,23,24], they are not always accompanied by examination of the direct toxic effect on normal cell lines. Research into their other effects, such as antimicrobial, antioxidant, antiviral, etc. [11,12,15,19,20,25], almost always lacks such studies. Examples of works focused on the effect on normal cell lines are [20,24,45,46,47,48]. In addition, despite its promising antimicrobial properties, the data on the safety profile of Hypericum with respect to beneficial microbiota are also limited and rather underexplored. This is particularly relevant for lactobacilli, which are commonly used probiotic bacteria and key components of the human microbiota [49]. Established antibiotics, e.g., penicillin and tetracycline, often collaterally damage the favorable human microbiota, including the lactobacilli [50,51]. Thus, an important consideration for natural product formulations is to be as harmless as possible to both the patient and their normal microbiota functions.
A complex evaluation of different aspects of the biological activity of Hypericum constituents was performed. It aimed to complement their proven effect against dysbiotic pathogens in direct relation to other factors responsible for the body’s healthy homeostasis. Additional antimicrobial research, including the cariogenic S. mutans and gene expression related to staphylococcal biofilm formation, turned out to be promising. A cytotoxic study of selected Hypericum agents on non-tumorigenic cell lines led to a consistent and building assessment where just two agents, an extract with known phytochemical content and a compound, with an optimal antibacterial and cytotoxic profile, were selected to be tested further. They were studied for cytopathic effect and the induction of apoptosis and autophagy in normal cells at relevant therapeutic antistaphylococcal concentrations, but only autophagy was detected. Other factors in the host system were examined. The agents did not harm beneficial Lactobacillales representatives of human and dairy origin, nor did they induce the cytochrome P450 system (with one exception) at those concentrations. The extract and the compound did not irritate rabbit skin in vivo, even at concentrations ten times higher. The two phytoconstituents have potential for further pharmacological development to treat infections caused by bacterial strains sensitive to them, but more research is warranted to show their full therapeutic potential.
2. Results
2.1. Experiments with a Broad Panel of Active Ingredients from Hypericum Species
2.1.1. Antibacterial Activity Against Pathogenic Bacteria—Supplementing the Existing Data
In order to broaden and enrich the published research involving several extracts, fractions, and pure compounds from a number of Hypericum species growing in Bulgaria [4,29], several experiments were performed. While the MIC values of the PPAPs hyperpolyphyllirin/hyperibine J, olympiforin A, and olympiforin B against several bacteria had been published [29], their minimal bactericidal concentrations (MBCs) were unknown. These values are presented in Table 1.

Table 1.
Minimal bactericidal concentrations (MBC) of pure compounds from Hypericum species against four pathogenic bacteria (µg/mL and or µM).
The Hypericum constituents are often not very effective against Gram-negative bacteria, possibly due to their outer membrane [14,52,53]. For that reason, only two agents that were in large enough amounts were tested against P. aeruginosa PAO1. RochD is a dichloromethane extract from H. rochelii, containing polyprenylated phloroglucinols. The results are presented in Table 2 and are another confirmation that polyprenylated phloroglucinols from Hypericum often do not have a significant effect against Gram-negative bacteria.

Table 2.
Minimum inhibitory concentrations (MICs) and MBCs of the extract RochD and the compound Olympiforin B against Pseudomonas aeruginosa PAO1 (µg/mL).
Filling some gaps in the existing knowledge, the MIC values against Staphylococcus aureus of the agents RochD and HirDM100 were refined to be 4.9 and 9.8 µg/mL (mg/L). The MIC of RochD against Enterococcus faecalis was refined to 4.9 µg/mL.
2.1.2. The MBC/MIC Ratio Points to Bacteriostatic Activity for Most of the Agents
According to Hlima et al. [54], an agent is considered bactericidal if the MBC-to-MIC ratio is below or equal to four (MBC/MIC ≤ 4) and bacteriostatic if MBC/MIC > 4. Following these instructions, the ratio was calculated for the agents from [4,29], where possible, including some results for the caries-causing bacteria Streptococcus mutans [55] (Table 3).

Table 3.
MBC/MIC ratio of the agents isolated from Hypericum species against pathogenic bacterial species.
Except for RochCM for S. aureus and olympiforin B for S. mutans, all other agents were bacteriostatic for the tested bacteria. Being bacteriostatic can be a beneficial quality, as bactericidal antibiotics and antimicrobials are more prone to elicit resistance [44].
2.1.3. Total Activity of the Tested Extracts and Fractions According to Eloff [mL/g]
Table 4 shows the calculated total activity of the extracts and fractions according to the method proposed by Eloff (2000) [6], which takes into account how much can be extracted from a given plant or natural source. The amount extracted from the plant [expressed as mg/g of dried plant material] was divided by the MIC value [mg/mL]. The unit [mL/g] indicates the degree to which one gram of plant material can be diluted and still inhibit the growth of the tested microorganism.

Table 4.
Total activity of the tested extracts and fractions [mL/g].
The table shows that RochC, which has previously been found to have an exceptionally high antibacterial effect, again leads in total activity, followed by HirDM90 and RochD. In some cases, the MIC values were similar, but the quantity of material extracted from the plants differed. Thus, H. rochelii and H. hirsutum are the most promising plants for ethnobotanical use, and finding such plants is one of the main goals of calculating total activity.
2.1.4. Cytotoxicity on Normal Cell Lines—Mean Inhibitory (IC50) and Maximum Tolerated Concentrations (MTCs)
The half-maximum inhibitory concentrations, also known as the mean or median inhibitory concentrations (IC50), of six agents selected for their strong antibacterial activity were determined. A 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay was used. The results are presented in Table 5 and Table 6.

Table 5.
Mean growth-inhibitory concentrations (IC50) of selected agents on five non-tumorigenic cell lines.

Table 6.
IC50 of only the tested substances converted to µM.
The results showed that the human embryonic kidney-293 (HEK-293) cell line was the most sensitive, followed by HaCaT, CCL-1, and HEPG2. The human gingival fibroblast (HGF) cell line was the most resilient one.
Table 7 shows the maximum tolerated concentrations (MTCs) of the agents. According to ISO 10993-5 [56], this is the concentration at which at least 70% of the tested cells are viable. An agent has therapeutic potential if its MIC value for a certain bacterial species is lower than its MTC value for a certain cell line.

Table 7.
Maximum tolerated concentration (MTC) of the tested active ingredients compared with the antibacterial MIC values [µg/mL].
For RochC, that was the case for S. aureus and all the cell lines except HaCaT, and for E. faecalis for HGF and HEPG2. RochD was promising for HGF and HEPG2 against S. aureus and E. faecalis. For HirDM90, that was valid only for S. aureus for HGF and HEPG2. Hyperpolyphyllirin/hyperibine J was promising for HGF against S. aureus, MRSA, and E. faecalis, and for CCL-1 and HEPG2 against S. aureus. Olympiforin A showed promise for HGF against all bacteria, and for CCL-1 and HEPG2 against S. aureus. Olympiforin B was promising for HGF against all bacteria and for CCL-1 and HEPG2 against S. aureus and MRSA.
2.1.5. Results from the Colony-Forming Unit Assay
Three of the least cytotoxic in the MTT assay and most promising agents were selected for each cell line with concentrations of 1/2 × IC50, IC50, and 2 × IC50. The results are presented in Table 8.

Table 8.
Colony-forming unit (CFU) assay of selected Hypericum agents on three cell lines after 48 h exposure to the IC50 dose of 72 h and then seeding the cells which formed colonies. The untreated control is normalized as 100% colonies.
Most concentrations of 1/2 × IC50 stimulated clonogenicity (except for RochD for HaCaT and olympiforin B and hyperpolyphyllirin/hyperibine J for HEK-293). In three out of ten cases, there was stimulation even at the IC50 concentrations (hyperibine J and RochC for HaCaT and again RochC for CCL-1). For two agents, stimulation was also observed even at the 2 × IC50 dose (hyperpolyphyllirin/hyperibine J for HaCaT and RochC for CCL-1). In some cases, the 2 × IC50 concentrations, despite producing fewer colonies than in the untreated control, had almost no difference in colony number compared to the IC50 dose or even slightly exceeded it (RochD for HaCaT and HEK-293 and olympiforin B and hyperpolyphyllirin/hyperibine J). Therefore, the results show that hyperpolyphyllirin/hyperibine J and RochC do not impair the clonogenic ability of the HaCaT and CCL-1 lines, respectively, but stimulate it, even at doses of 2 × IC50. RochC does not harm the clonogenicity of HaCaT cells at the IC50 dose, only at 2 × IC50. Most of the tested agents, selected as the least cytotoxic, stimulate the clonogenicity of HaCaT, HEK-293, and CCL-1 lines at doses of 1/2 × IC50.
Two of the cell lines in this study, HEPG2 and HGF, were not suitable for the CFU test, and this is elaborated on in the Discussion.
2.2. Selection of Agents with Optimal Activity Profiles and of Cell Lines for Further Experiments
Based on the antibacterial activity, the IC50 and the MTC values, in addition to the CFU assay, we selected the extract RochC and the compound olympiforin B for further studies. They are the agents with an optimal activity profile and ratio of antibacterial activity to cytotoxicity. They have outstanding antibacterial activity [4] according to Eloff [57] and relatively low and satisfactory toxicity to eukaryotic cells. In the further experiments, RochC and the compound olympiforin B were most often applied at their MIC concentrations for S. aureus—0.625 and 1 µg/mL (mg/L), respectively.
The IC50 values of the two agents were higher for all five cell lines than these MIC values (except for olympiforin B in HEK-293). Therefore, at therapeutically relevant concentrations for staphylococcal infections, they would likely be safe for eukaryotic cells in the human or in a veterinary patient. The MTC values were higher for these agents, mostly for the CCL-1 and HGF cell lines. That proved useful, because CCL-1 is the recommended line in ISO 10993-5 [56]. Therefore, this cell line was used for live-cell imaging and Western blot (HGF was also used for the Western blot in addition).
RochC is an extract obtained by supercritical CO2 extraction from H. rochelii, as mentioned, and has been found to contain some prenylated phloroglucinols, which generally have high antibacterial activity [58], such as olympiforin A [29], hyperpolyphyllirin/hyperibine J, and maculatoquiones A–D [4]. The species that they are found in are listed in the Discussion.
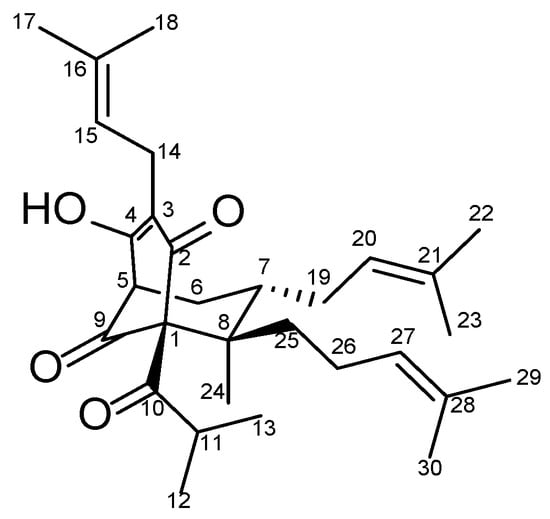
Figure 1.
Structure of olympiforin B.
2.3. Experiments with the Selected Agents RochC and Olympiforin B
2.3.1. Results from the Time Kill Assay
In order to study the dynamics of the bacteriostatic and bactericidal action of the agents, a time kill assay with S. aureus was performed. Figure 2 shows that after a peak in bacterial growth in the first hour after treatment, a decline in their number was observed, most sharply between the first and third hours. By 24 h, the MBC concentrations killed all bacteria, while 1/2 × MBC doses reduced the bacteria to 124,000 CFU/mL (RochC) and 302,000 CFU/mL (olympiforin B). The MBC concentrations did not always have fewer bacteria than the 1/2 × MBC doses.
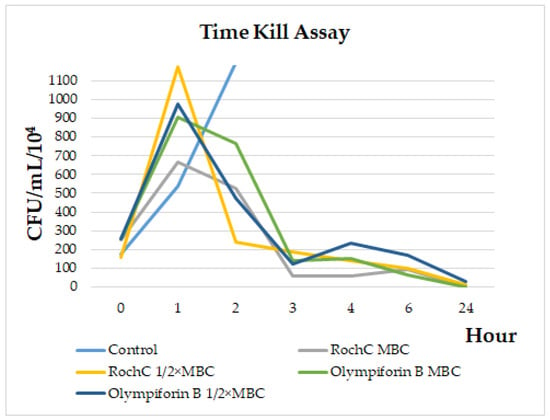
Figure 2.
Time kill assay for RochC and olympiforin B on S. aureus.
2.3.2. RochC and Olympiforin B Had Very Low MIC and MBC Values Against the Cariogenic S. mutans
Because of the promising results and relatively gentle effect of the two agents on gingival fibroblasts (the least cytotoxic in this study), a test against the cariogenic S. mutans was performed. Table 9 shows that the two agents inhibited one of the main culprits for dental cavities with very low concentrations for MICs and MBCs. The MIC value of RochC was below 20 μg/mL, once again nominating this extract with an outstanding activity against a pathogen, according to Eloff’s criteria [57]. Olympiforin B, in this case, turned out to be one of the few agents in this study with a bactericidal mode of action, but not bacteriostatic. It can also be noted that their MIC values and even the MBC value of olympiforin B are lower than their IC50 and MTC values for the human gingival fibroblasts HGF. The MIC of the compound was five times lower than the MTC and almost six times lower than the IC50 for the gingival cells. Therefore, the agents are promising for inhibitory treatment of that cariogenic bacterium and have the potential to be developed as oral care products.

Table 9.
MIC and MBC of RochC and olympiforin B against the cariogenic S. mutans, compared to the IC50 values for the gingival fibroblasts HGF (µg/mL).
2.3.3. The Antibiofilm Test Against Staphylococci Showed Stronger Activity Against MRSA
The antibiofilm activity of RochC against MRSA and S. aureus and the antibiofilm activity of olympiforin B against S. aureus (it has already been tested against MRSA [29]) were assessed. Table 10 shows that the MRSA biofilm was more sensitive to the agents, with minimum biofilm inhibitory concentration (MBIC)—resulting in no visible biofilm—lower than their MIC values. The MBIC of RochC was even one-eighth of the MIC value. The median biofilm inhibitory concentrations (MBIC50)—or the dose resulting in a reduction in the biofilm by half—were much below the corresponding MIC values for both agents and both bacteria.

Table 10.
Biofilm inhibition activity of the chosen agents against S. aureus and MRSA.
The photo-documented effect on the biofilm is presented in Figure 3 and Figure 4. The sigmoidal graphs associated with the MBIC50 calculation are shown in Figure 5.
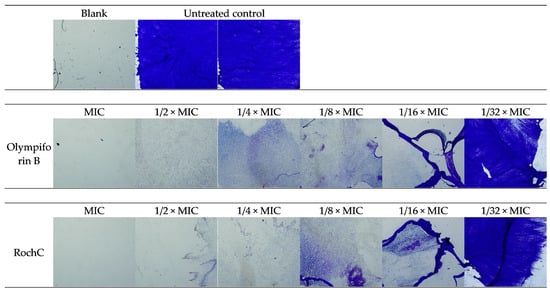
Figure 3.
Microscopic images of S. aureus biofilm inhibition—40× magnitude.
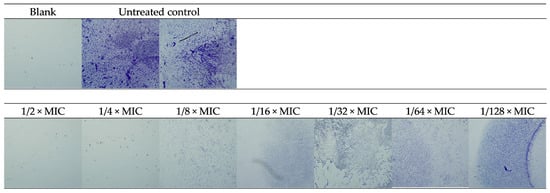
Figure 4.
Microscopic images of biofilm inhibition of RochC on MRSA—40× magnitude.
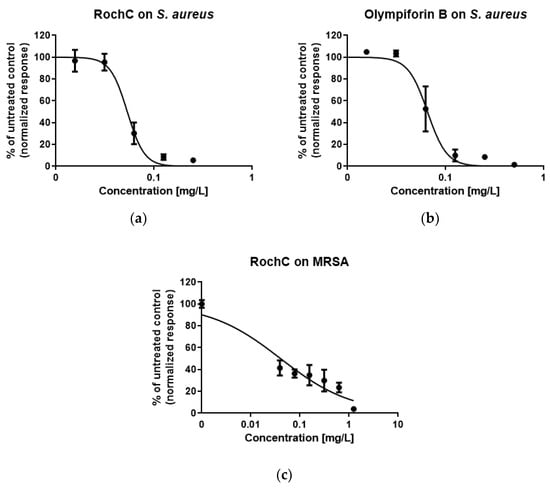
Figure 5.
Sigmoidal graphs for biofilm inhibition of (a) S. aureus after exposure to RochC; (b) S. aureus after exposure to olympiforin B; (c) MRSA after exposure to RochC.
2.3.4. Olympiforin B Inhibited the Expression of Biofilm-Related Adhesion Genes in MRSA
The more sensitive bacterial strain, MRSA, was chosen for subsequent assessment of the expression of genes related to biofilm formation—icaA and icaD. The real-time PCR (Table 11) showed that olympiforin B inhibited the relative gene expression of both genes. RochC induced icaA, while icaD was inhibited by a dose of 1/16 MIC and induced by the lower concentration of 1/32 MIC.

Table 11.
Relative gene expression of MRSA after exposure to the selected agents.
The visible MRSA biofilm was suppressed by a lower dose of olympiforin B (MBIC 0.5 µg/mL) compared to RochC (MBIC 1.25 µg/mL). However, for the compound, that corresponds to 1/2 × MIC in comparison with 1/8 × MIC for the extract RochC. Nevertheless, the biofilm-inducing genes icaA and icaD were more suppressed by the compound, despite its MBIC not being that much lower than its MIC value, in comparison to RochC.
2.3.5. Effect on Candidate Probiotic Lactobacilli
The bacterial viability of six pre-selected candidate probiotic gut and skin-associated Lactobacilalles strains was estimated in the presence of the two agents.
Table 12 shows that the two agents had MIC values for Limosilactobacillus fermentum and Lactiplantibacillus plantarum higher than the MIC values for S. aureus, implying that they are not a threat to the normal and beneficial microbiota if applied at the anti-staphylococcal therapeutic concentration. The MICs were 2 and 4 µg/mL and even higher than 4 µg/mL for Lf53. Therefore, the MIC of RochC for that strain was a concentration greater than six times its MIC for S. aureus. For the other strains, the MICs were two, three, four, and six times greater than the MIC for S. aureus of the agents. Although these values are not particularly high, they show that the agents are gentle to lactic acid bacteria in a narrow interval of therapeutic concentrations. Figure 6 shows the dehydrogenase activity of the tested strains treated with the two agents, which can also be considered metabolic activity.

Table 12.
Antimicrobial activity (MIC values, µg/mL) of the tested extracts and fractions on lactobacilli strains.
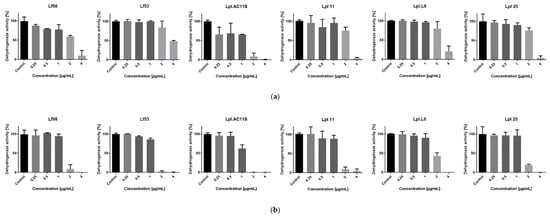
Figure 6.
Metabolic (dehydrogenase) activity of lactic acid bacteria treated with the chosen agents: (a) RochC; (b) olympiforin B.
2.3.6. No Cytopathic or Apoptogenic Effect in CCL-1 Was Caused by the MIC and 2 × MIC Doses of the Agents, as Observed with the Help of Live-Cell Imaging
The cells were treated with the low concentrations, therapeutically relevant for S. aureus, of RochC and olympiforin B (MIC and 2 × MIC), as well as with a higher concentration of HirDM90 for comparison (IC50 and 2 × IC50 for the respective cell line), as well as with daunorubicin and hypertonic buffer as positive controls.
Notably, there was no cytopathic effect with RochC and olympiforin B for all time points applied at concentrations of MIC and 2 × MIC for S. aureus. There was a cytopathic effect only associated with the Hypericum agent applied at concentrations of IC50 and 2 × IC50 (HirDM90) and with the hypertonic solution (Figure 7).
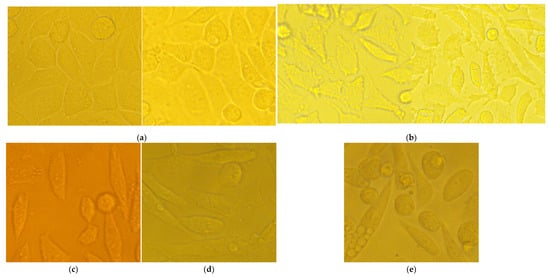
Figure 7.
Cytopathic effect of chosen samples with magnification of 400×: (a) untreated control; (b) only cells treated with hypertonic buffer show increased number of apoptotic blebs on the cell surface; (c) HirDM90, dose of 2 × IC50, 24 h’ exposure; (d) HirDM90, dose of 2 × IC50, 48 h exposure; (e) HirDM90, dose of 2 × IC50, 72 h exposure.
Remarkably, apoptotic blebs (membrane bulges typical for apoptotic cells), seen with the higher 400× magnification, were associated only with the hypertonic solution and not with the high dose of HirDM90 at any time point of its exposure.
Summarized, the cytopathic effect was expressed as:
- Apoptotic blebs—in CCL-1 at the hypertonic solution application only.
- Antiproliferative effect (fewer cells in comparison with the untreated control)—at the HirDM90 treatment only; however, the protocol for hypertonic buffer did not allow long enough exposure to check for this effect.
- A rise in the fraction of round, unattached cells—seemed more pronounced with the Hypericum agent.
- Shrinkage of the cells and their separation from each other, so that they no longer bordered each other, and the cells, probably as a result, acquired a more irregular and polygonal shape compared to their previous more spindle-like shape—at the hypertonic buffer treatment.
The cytopathic effect of HirDM90 was concentration- and time-dependent. At 24 h after seeding, there was already a cell monolayer, and a cytopathic antiproliferative effect was observed only for HirDM90 at the 2 × IC50 dose, in the form of fewer cells in some fields of view. At 48 h and even more so at 72 h, the untreated control was characterized by a second layer of rounded cells growing on the lower cell monolayer (in many places at 48 h and everywhere at 72 h). At hour 48, only the treatment with HirDM90 2 × IC50 resulted in a clearly distinguishable effect—very sparse, rounded cells, growing on the single-cell monolayer. At hour 72, there was already a clear cytopathic effect with HirDM90 IC50 with the same manifestation (Figure 8). Therefore, a cytopathic effect was observed only at doses of IC50 and 2 × IC50 of HirDM90, with the former at hour 72 and conditionally at hour 48, and with the latter from hour 24 onwards.
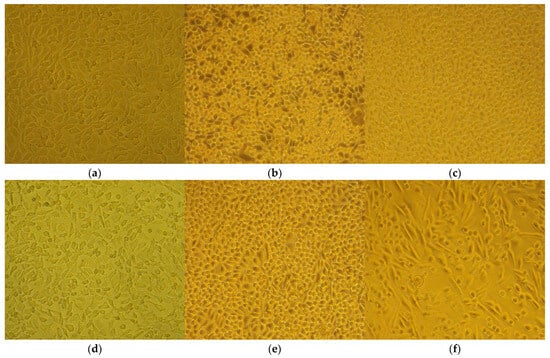
Figure 8.
Cytopathic effect after exposure to hypertonic buffer (2 h) or to Hypericum agents (72 h) with 100× or 200× magnification. The MIC values are for S. aureus. The different concentrations of seeding required two separate controls for the buffer and for the Hypericum agents. The panels are the following: (a) untreated control for the hypertonic buffer test; (b) untreated control for Hypericum testing; (c) RochC, dose of 2 × MIC; (d) hypertonic buffer; (e) olympiforin B, dose of 2 × MIC; (f) HirDM90, dose of 2 × IC50.
Hoechst is a dye that binds to DNA, hence making cell nuclei fluoresce blue. In the CCL-1 line, at 5 h after seeding, there was no monolayer yet. Mitotic spindles were dyed by Hoechst; therefore, active mitosis was noticed in all samples, and no cytopathic effect was observed (Figure S3). At 24 h, there was already a monolayer; mitosis was observed both in the untreated control and in all treated samples, including HirDM90 2 × IC50. Highly condensed and shrunken nuclei, a marker of apoptosis, were observed only when the cells were treated with hypertonic buffer before staining, but not in samples treated with daunorubicin or Hypericum agents at all time exposures (5 to 72 h) (Figure 9 and Figures S1–S6).
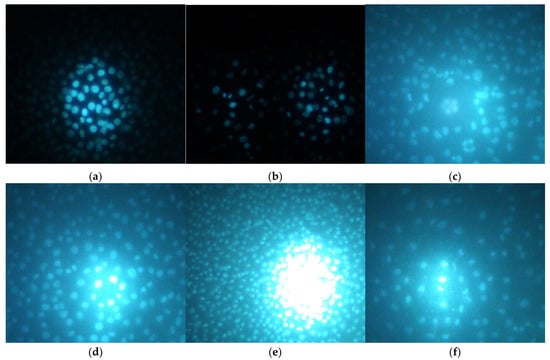
Figure 9.
Hoechst staining of chosen samples after exposure to hypertonic buffer (2 h) or to Hypericum agents (48 h) with 100× and 200× magnification. The MIC values are for S. aureus. The panels are the following: (a) untreated control for the hypertonic buffer test; (b) hypertonic buffer; (c) untreated control for the Hypericum test; (d) RochC, dose of 2 × MIC; (e) olympiforin B, dose of 2 × MIC; (f) HirDM90, dose of 2 × IC50.
As a summary, there was no cytopathic effect from RochC and olympiforin B treatment with concentrations of MIC and 2 × MIC for S. aureus for all time points applied (Figure 8). Also, there were no signs of apoptosis—condensed nuclei and blebs—associated with any of the Hypericum agents at any of the tested time points. This is valid not only at low concentrations multiples of the MIC against S. aureus but also at high concentrations multiples of the IC50 (of HirDM90) (Figure 7, Figure 8 and Figure 9). The effect was antiproliferative, and further studies are needed to elucidate the underlying mechanism of action.
All the photographed images can be seen in Supplementary Figures S1–S6.
2.3.7. Activation of Autophagy Was Detected with Western Blot
Autophagy is an adaptive mechanism to stress factors such as starvation, cytotoxic agents, etc. Treatment with Hypericum agents induced (increased the expression of) proteins related to autophagosome formation (Figure 10). Atg5 and Atg7 were induced, indicating the activation of autophagy in all samples, while LC3A/B was converted to the lower migrating form visibly—also an indicator of autophagy—only in the positive control (30 µM erufosine) [59] and the HirDM90 IC50 treatment. Significant induction of Atg5 and Atg7 was observed. The proteins were increased two, three, and even four times (for RochC at HGF cells), even at the low MIC concentrations of the agents. Only olympiforin B led to a smaller increase in HGF cells (to 106–135%).
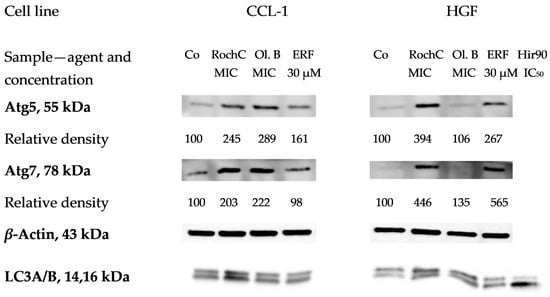
Figure 10.
Expression of Atg5, Atg7 and LC3A/B proteins related to autophagy in CCL-1 and HGF cells treated with Hypericum agents. Legend: Co—untreated control, Ol. B—olympiforin B, Hir90—HirDM90. The MIC values are the concentrations for S. aureus.
Further experiments may include antibodies for other proteins from the autophagic cascade, such as Beclin-1, Atg3, Atg16L1, etc.
2.3.8. Cytochrome P450 (CYP450) Was Not Induced in HEPG2 Liver Cells with a Few Exceptions
CYP450 enzymes are responsible for the metabolism and inactivation of medications. The expression of CYP450 (quantity of protein) in HEPG2 liver cells was tested by enzyme-linked immunosorbent assay (ELISA). One of the main differences between the HEPG2 cell line and normal hepatocytes is the weak or absent expression of the cytochrome P450 (CYP) superfamily, e.g., CYP3A4, CYP1A2, etc. [60]. However, exactly that low basal activity of CYP proteins makes the cell line suitable for studies of CYP inducers [61].
It can be observed that at therapeutic concentrations appropriate for S. aureus, the selected Hypericum agents hardly induce the cytochrome system. Therefore, the risk that they would contribute to drug inactivation and the reduction in their bioavailability is low. This was valid for the total CYP450 and its main isoform, 3A4, which metabolizes approximately half of the drugs metabolized by CYP450 enzymes (Figure 11). The only exception was the dose of 2 × MIC of RochC, which induced CYP450 3A4. HirDM90 was deliberately applied at higher concentrations (2 × IC50), and it had a higher effect on both total CYP450 (inducing it) and 3A4 (inhibiting it).
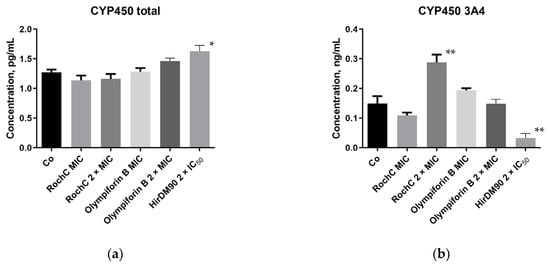
Figure 11.
Effect of selected active substances from Hypericum on (a) human total cytochrome P450 (CYP450); (b) human CYP450 3A4 isoform in HEPG2 cells. The MIC values refer to S. aureus. The number of asterisks indicates the degree of significant difference according to One-way ANOVA (Tables S1 and S2): *—p ≤ 0.05; **—p ≤ 0.01.
2.3.9. The Chosen Agents Did Not Irritate Rabbit Skin In Vivo at a Dose of Ten Times MIC
There was no skin irritation on rabbits, and the results indicate that RochC and olympiforin B do not cause skin toxicity at the MIC concentration for S. aureus and even at ten times the tested concentration (10 × MIC). Irritation was observed only in the positive control field with 10–20% SDS, in the upper left corner, as seen in Figure 12.
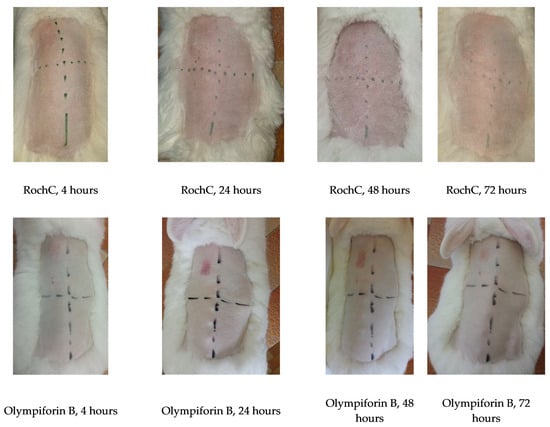
Figure 12.
Lack of skin irritation on rabbits. The agents RochC and olympiforin B were applied at concentrations of MIC and 10 × MIC for S. aureus. Hours indicate exposure time. The positive SDS control is in the upper left panel, except for the last rabbit, where the exchange is indicated by arrows. One rabbit per agent, from a total of three rabbits per tested agent, is shown in the figure.
3. Discussion
3.1. Antibacterial Activity
In some cases, the naphtodianthrones [62], benzopyrans, xanthones [4], etc., in the Hypericum plants also exert an antibacterial effect. However, the compounds found in RochC were polyprenylated phloroglucinols, highlighting their strong antimicrobial action. They are typical of the Hypericum genus and found in other species. Olympiforin A has been found in H. olympicum [29]. Maculatoquiones A–D have been found in H. maculatum Crantz [63]. Hyperpolyphyllirin/hyperibine J has been found in H. perforatum, H. androsaemum, H. tetrapterum [64], H. maculatum [63], H. triquetrifolium Turra [65], H. empetrifolium Willd. [66], etc. HirDM90, an abundant fraction of an H. hirsutum extract that was used in higher quantities in the live-cell imaging and Western blot—in order to compare the effect of low and high doses of a cytostatic agent—had been found to contain other PPAPs such as hyperfirin or secohyperforin and adhyperfirin or adsecohyperforin [4].
Besides finding the most promising plants among those used in folk medicine, another main goal of calculating the total activity of a plant with the method of Eloff [6] is reversing, at least partially, the one-way benefit and flow of information. Scientists, with very little additional effort, could return to the local inhabitants of rural and less developed areas the favor of sharing ethnobotanical information about medicinal plants. H. olympicum and H. hirsutum are confirmed to be used in traditional medicine. The former is native to the Balkan Peninsula and northwestern Turkey and is used in Turkish folk medicine for stomachache, inflamed wounds and cuts [67]. H. hirsutum L., or Hairy St. John’s Wort, is a Eurasian herb with a large range from western Europe to western China [68]. It is used in traditional Asian medicine for hematochezia, irregular menstrual periods, and hematemesis [69]. There are unconfirmed data also for H. rochelii, or Rochel’s St. John’s Wort—with an area of the Balkan Peninsula [70]—that it is used in folk medicine to treat various ailments such as fever, headache, and stomachache, as well as an ornamental and fragrant plant [71]. Therefore, revealing that these plants have high total activity, i.e., strong antimicrobial activity and good exctractability, could be of help for the local communities where these species grow.
As discussed, biofilms of pathogenic bacteria may cause chronic infections and inflammation, which damage the surrounding tissues. Examples include chronic wounds, urinary tract infections, and endocarditis [38]. P. aeruginosa biofilms may lead to respiratory failure in cystic fibrosis patients [72]. Biofilms of foodborne pathogens in food production plants are also a threat to public health, as detached biofilm can spread infections and AMR to the environment, humans, and their microbiota [73].
In regard to staphylococcal biofilm on medical devices, such as catheters, mechanical heart valves, ventilators, and contact lenses, etc., the source can be the skin or other colonized body sites of patients or health care workers. Immunocompromised patients have an increased risk of developing infections when receiving a medical implant [41]. The polysaccharide adhesin (poly-N-acetylglucosamine polymer) in the staphylococcal biofilm is synthesized with the help of N-acetylglucosamine transferase, a product of the icaA gene. The product of icaD is required for maximum expression and optimal enzymatic activity of this transferase [74], which is associated with the formation of slime and biofilm and the phenotypic expression of capsular polysaccharide [75].
At 1/16 × MIC, Roch C induced icaA expression and decreased the icaD expression. This may be due to the stress factors in the media [2] or to the expression of icaR, the locus opposite of the icaABCD [76], as in the study with extracts from Ginkgo biloba L. [77]. In addition, there are data showing that with increasing glucose concentration, the biofilm increases, while the expression of the icaA and icaD genes decreases [78]; therefore, there is not always a straight correlation between the rise of the expression of the two genes and biofilm formation.
The constant mutations of MRSA may make the antibiotics that it is susceptible to soon ineffective [79], and new agents to combat this strain are needed. As mentioned, the direct bactericidal action of antimicrobials causes more AMR than the bacteriostatic action or the property of targeting some virulence factor, such as biofilm formation [44]. In addition, the MBIC and/or MBIC50 values being below the corresponding MIC values of an agent is beneficial. Low concentrations of the antimicrobial would achieve the aim of biofilm prevention, i.e., abolishing the phenotypic pathogenic expression, without influencing the microbial growth, including that of the normal human microbiota. Both active ingredients that this study focuses on, especially RochC, are promising for the mitigation of AMR in MRSA because of their sub-MIC MBIC50 concentrations for both tested bacteria and their sub-MIC MBIC concentrations for MRSA.
Considering the biofilm formation of S. mutans, St. John’s Wort extracts at a concentration of 20 µg/mL [80] and oil at a concentration of 512 μg/mL have inhibited it (including inhibition of E. faecalis biofilm) [81]. Given that the activity of RochC against S. mutans with an MIC of 6.25 µg/mL surpasses that of the St. John’s Wort oil with an MIC of 128 μg/mL, future investigation of the antibiofilm properties specifically against that bacterium deserves consideration.
3.2. Cytotoxic Activity on Normal Cell Lines
Research on the direct cytotoxic effect of active ingredients from Hypericum on normal and non-tumorigenic cell lines is a supporting component of broader studies focused on anticancer activity and thus does not always accompany such research. This is expected, as the aim to identify therapeutic potential is prioritized, and cytotoxicity in normal cells, although crucial, is often briefly addressed unless the agent shows promise. Limited funding resources often reserve such studies for follow-up investigations. If safe for normal cell lines in vitro, the final research step for a phytoconstituent would be mechanistic studies, animal models, and/or formulation development.
In the studies of the cytotoxic effect on both tumor and normal cells, the selectivity index (SI) is the ratio of the IC50 for a normal cell line to the IC50 for a cancer cell line. It is considered high and promising if it is a value greater than or equal to two, according to some authors [82,83], or greater than three, according to others [84]. Literature shows that the same extract can be more cytotoxic in vitro to a certain cancer cell line in comparison to a normal cell line, i.e., it can be a selective agent, but it can be less cytotoxic to another tumor cell line than to the normal type of cells [85].
A comparison between the IC50 values of the compounds in this study with their average IC50 values against tumor cell lines from another study [29] shows that olympiforin A is not very selective, as only its IC50 for HGF was higher than those for the tumor cell lines. Hyperpolyphyllirin/hyperibine J has a higher IC50 for HGF, CCL-1, and HEPG2. Olympiforin B is greatly selective, having a higher IC50 value for all cell lines in this study than its average IC50 for the tumor cell lines (1.3 µM), which further justifies its selection for additional experiments in the present work. The IC50 of the compounds and of other phloroglucinols from H. olympicum towards HEK-293 and the normal vascular endothelial cells EA.hy926 in the previous study varied from 0.9 to 34 µM. The SI for two normal cell lines varied between 0.35 and 7.14, meaning that the compounds in some cases were quite selective towards certain tumor cell lines.
The best selectivity, according to our knowledge, is so far demonstrated by a methanol extract of H. hookerianum Wight ex Arn., with an SI reaching 10–50 for tumor lines with respect to the normal monkey Vero kidney epithelial cell line. The IC50 values against Vero were between 100 and 271 µg/mL [45].
The methanol extract and its fractions, as well as the xanthones and phloroglucinols isolated from H. roeperianum Schimp. ex A.Rich, had IC50 values against AML12 normal hepatocytes in the range of 42 to >80 µg/mL and 21 to >165 µM, respectively. They also possessed very good SI within the range of 1.2 to 4.6 and 0.9 to >3.74, respectively [24].
There are cases where the pro-apoptotic action of a PPAP, e.g., HF, is selectively observed in cancer cells, rather than in healthy cells. For instance, the same concentration of HPF that induces distinct damage in B leukemic cells does not harm the viability of human B lymphocytes from healthy donors at all [86]. The current hypothesis explaining this is related to the fact that HPF is a protonophore. In both tumor and normal cells, the negative cytosolic side of the plasma membrane facilitates proton influx, but in cancer cell lines, the pH gradient (∆pH) across the plasma membrane is greater because of the higher activity of proton channels that extrude protons from the cells. Thus, in the presence of a protonophore, only a faint proton entry is made in normal cells, but a significant and persistent cytosolic proton influx occurs in cancer cells, restoring cytosolic acidity and allowing apoptosis. At the same time, HF makes the extracellular tumor microenvironment less acidic, thus impeding tumor cell migration and invasiveness and extracellular matrix digestion. In addition, cancer cells have a hyperpolarized inner mitochondria membrane and a higher concentration of HF could collapse this membrane, thus leading to cell death.
This hypothesis is yet to be verified in cancer cells but is already confirmed by Sell et al. [87] in both the plasma membranes of normal cells and a synthetic lipid bilayer without channel proteins. HF caused proton entry into the cell and cytosol acidification directly dependent on the proton gradient between the two sides of the membrane. Interestingly, the authors also demonstrated that HF accumulates in the membrane, which might explain the effects of even low doses of HF and the extracts containing it. Other PPAPs from Hypericum, such as olympiforins A and B, being also lipophilic and protonophores, likely have the same mechanism of action [88].
An H. perforatum extract had an IC50 against seven tumor cell lines in the interval of 6.7 to 45 µg/mL. The IC50 for the healthy human lung fibroblasts CCD-34Lu was 13.6 µg/mL, and the IC50 for HEK293 cells was higher than in the current study—28.3 µg/mL [89]. The IC50 values of an olive oil extract from that plant were high and not very different for SW-480 and bone marrow-derived mesenchymal stem cells—4800 and 4900 mg/mL, respectively. The cell migration and colony formation were significantly reduced at the IC50 values for both cell lines [90]. Different organic solvent extracts from H. perforatum aerial parts had an average IC50 of 28 µg/mL on three cancer cell lines and an average IC50 of 24 µg/mL on the normal fibroblasts MRC-5 [85]. The cell viability of the non-tumorigenic brain endothelial cell line hCMEC/D3—a blood–brain barrier model—was moderately affected by an H. perforatum decoction with an IC50 of 732 μg/mL, but NSC-34—a hybrid cell line of neuroblastoma and mouse motoneurons—was even less affected with an IC50 of >1000 μg/mL [91]. The effect of a methanol extract from that species on PC-3 human prostate cancer cells and the normal human chondrocyte cell line C28/I2 was compared. Upon 48 h treatment at 2.720 mg/mL (about twice the dose of IC50), 82% of PC-3 cells underwent death, while C28/I2 cells remained viable up to 65% under similar conditions. The CD82 protein has the function of inhibiting tumor metastasis and thus is a therapeutic target in prostate cancer cells. A two-fold increase in the relative gene expression of CD82 in PC-3 cells was revealed in comparison to the untreated control. The increase in the normal cells was much less impressive, suggesting again a selective action of the plant only upon malignant tissues [46]. H. perforatum extract encapsulated in poly(lactic-co-glycolic acid) nanoparticles for 24 h at a dose of 5 mg/mL inhibited KYSE30 cancer cells by approximately 70% and normal squamous cells by up to only 29%. Cyclin D1 is a therapeutic target for esophageal cancer, and the nanoparticles exerted selectivity, causing a significant decrease in cyclin D1 expression in the KYSE30 cells, while in the normal cells it was at least 2-fold higher [92].
Although the IC50 for a breast cancer cell line of hypericin was significantly lower when compared to cisplatin—5 vs. 20 μg/mL for 24 h—the two compounds did not have a significant effect on the cell survival of unspecified fibroblasts at concentrations up to 30 μg/mL for 24 h [93]. Hypericin is a naphtodianthrone and another major secondary metabolite of H. perforatum. It has a phototoxic effect since it is able to produce reactive oxygen species (ROS) as a result of adequate photoexcitation, so numerous works have revealed that it has no cytotoxicity in the dark. It also accumulates much more in neoplastic tissue than in normal tissue [94], and likely for that reason, it induced apoptosis in a gastric cancer cell line but not in normal human fibroblasts [95].
The aqueous and organic solvent extracts from H. empetrifolium and H. lydium Boiss. had IC50 values against HEK-293 cells varying from 63 to 304 μg/mL. Unfortunately, they were more cytotoxic to HEK-293 cells than to the three tumor cell lines DU-145, A549, and MCF-7. The only exception was an acetone/water extract from H. empetrifolium with an IC50 of 189 μg/mL against DU-145 prostate cancer cells and 304 μg/mL against HEK-293 [47]. None of the phenolic substances (flavonoids, acids, etc.) isolated from H. cerastioides (Spach) N.Robson had toxicity to a normal fibroblast cell line, L929 (IC50 > 200 µM). Cerastioside A, a normonoterpene, and I3-II8-biapigenin displayed selectivity, as they had weak cytotoxic activity against a panel of cancer cell lines with IC50 values in the interval 107–198 μM [48]. The ethanol extracts prepared in the beginning of flowering and full flowering periods from H. heterophyllum Vent. again did not have a significant cytotoxic effect in L929 cells, while being cytotoxic to MDA-MB-231 breast cancer cells [20].
One of the few works comparing the antimicrobial effect and the toxicity on normal cell lines of Hypericum ingredients [96] reported that methanol, petroleum ether, and ethyl acetate extracts from H. triquetrifolium and H. scabrum L. aerial parts had an antimicrobial effect, with MICs against S. aureus and S. epidermidis between 90 and 5000 µg/mL. The extracts did not show toxicity in a normal cell line (L-929) at a concentration of 100 µg/mL (cell viability over 70% and up to 91%, no exposure time mentioned). In another study, the methanol leaf extract of H. triquetrifolium had an average IC50 for four cancer cell lines of 142 μg/mL. The average IC50 (n = 4) for the normal cell line WRL-68 (HeLa derivative) was 314 μg/mL (24 h exposure for all cell lines). Therefore, the SI was over two, and the extract was selective. In addition, the extract did not have any cytogenetic effect, because it raised the metaphase index of the bone marrow cells in healthy mice from 5.2% to 5.5, 6.7, and 7.8% at 50, 100, and 200 mg/kg, respectively, while cyclophosphamide reduced it to 3.6% [97].
An ethyl acetate extract from H. japonicum was more cytotoxic to human lung epithelial tumor cells (A549) than to normal human lung fibroblast cells (WI-26VA4) [98]. Balikci et al. found the major components of the aerial parts of a methanol extract from H. olympicum to be volatile compounds such as eicosane, heptacosane, 2-propen-1-ol, etc. This extract had an IC50 against breast cancer cell lines of approximately 25–40 μg/mL. In human lymphocytes, the concentrations up to 1750 µg/mL induced genotoxic activity without decreasing the mitotic index. The extract caused significant DNA damage at selected doses (250–750 μg/mL), as shown by a comet assay, while chromosomal damage, i.e., genotoxic effect, was observed at relatively high doses (≥500 µg/mL) by employing sister chromatid exchange and micronucleus methods. The extract did not act on the mechanisms pertaining to the proliferation of the cells [99]. Fifteen substances from H. longistylum Oliv. (sesquiterpenes and flavanones) did not harm normal mouse lung fibroblasts [100].
An interesting study sought potential side effects of H. connatum Lam. and H. caprifoliatum Boiss. on the placental development and function. The plants could be used as drug alternatives to mild depression or viral infections by pregnant women. The non-fusogenic JEG-3 cells and the fusogenic BeWo cells were used. They both originate from tumor cell lines, but are used as an in vitro model for studies of the normal placental trophoblast. The differentiation of this trophoblast yields the syncytiotrophoblast cell, whose successful formation and expansion are crucial for the functioning of the placenta. The main functions of syncytiotrophoblasts are absorption, exchanges, specific hormonal secretion, and fetal Ca2+ homeostasis regulation. The studies on the viability of the trophoblast-like cells showed that the methanol extract of both plants, as well as the hexane extract from H. connatum (HCo), did not harm the BeWo cells up to 30 μg/mL. However, these extracts were cytotoxic to JEG-3 cells, as was the hexane extract from H. caprifoliatum (HCa) to the BeWo cells. In doses as low as 5 μg/mL, they significantly decreased the cell viability. Still, in the case of 5 and 15 μg/mL of HCa, cells were morphologically unchanged as observed by microscopy. All concentrations and plant extracts exerted a significant decrease in the biochemical cell differentiation (hormone production). However, the inhibition of the morphological cell differentiation (cell fusion) was significant only for the HCa at 15 μg/mL. In addition, H. connatum was revealed to interfere with the Ca2+ transport system. Both methanol extracts contained phenolic acid and flavonoids. The hexane extracts from both plants presented dimeric acylphloroglucinols, and there was a tautomeric mixture of unresolved acylphloroglucinols in the HCa. The results indicated that the two Hypericum species extracts can interfere with trophoblast viability, differentiation, and Ca2+ influx. Their intake by pregnant women should be cautious, although more in vivo research is necessary for assessing the full extent of their effect [101].
The phototoxic effect of the ethanolic extracts of eleven Hypericum species was studied on NIH/3T3 normal murine fibroblasts. The IC50 values in the dark (light-independent cytotoxicity) were between 101 and 267 µg/mL. Those values under light were 31–256 µg/mL. The ratio of two (a higher ratio corresponds to higher photosensitizing and phototoxic activity) varied from 0.9 to 4.3. As expected, under light exposure the light-dependent cytotoxicity was higher for those species with a higher content of naphthodianthrones. The lowest cytotoxicity, under both dark and light conditions, was observed for H. hirsutum, which lacked hypericins. The high toxicity of that species in the current study highlights that fractions of less polar solvents could have different activity. The three most active species, H. perfoliatum L., H. perforatum, and H. tetrapterum, had the highest ratio of dark:light toxicity and high amounts not only of naphthodianthrones but also of HF [25].
While the direct cytotoxic effect on normal cell lines has not been often elucidated, Hypericum constituents are reported to have in vivo wound healing properties, possibly attributed to their anti-inflammatory, antimicrobial, and antioxidant activity. For example, oily extract of H. perforatum was found to effectively reduce scar heights in human tissue [102].
Hong et al. [103] reported that H. hookerianum ingredients strongly protect HT-22 murine hippocampal cells from glutamate-induced cell death and SH-SY5Y cells from 6-hydroxydopamine (6-OHDA)-mediated neurotoxicity. Cell viability was reduced to approximately 50% after exposure to 5 mM glutamate and to 36% after exposure to 6-OHDA. The plant ingredients, e.g., the dichloromethane and ethyl acetate fractions with concentrations of 6, 17, and 50 μg/mL, were able to restore the viability almost completely. The effective concentration 50 (EC50)—in this case, the concentration where the viability was restored to about 75%—of 4-hydroxy-2,6,4′-trimethoxydihydrochalcone and sesamine, out of several compounds isolated from that plant, was 1.48 µM and 2.85 μM, respectively. Therefore, extracts and compounds from H. hookerianum are capable of significant neuroprotective effects in HT-22 and SH-SY5Y cells. Only the n-butanol fraction at 50 μg/mL showed cytotoxicity.
Hypericum ingredients have also been frequently reported to have protective and anti-inflammatory activity in non-tumorigenic cell lines, tissues, and animal models of acute and chronic inflammation.
For example, HF and an HF-containing H. perforatum extract both markedly inhibited interferon-elicited signaling pathways in pancreatic beta cells and in rat and human pancreatic islets, leading to prevention of inducible nitric oxide synthase (iNOS) gene expression and protection against cell damage. HF also influenced the pro-inflammatory and immunological responses of mouse microglia and macrophages which are involved in the progression of neuropathological disorders [104]. Pretreatment with H. perforatum extract protected the PC12 cell line—an immortalized cell line derived from a rat pheochromocytoma—from H2O2-induced ROS generation and damage in a concentration-dependent manner (1–100 µg/mL) [105]. In zymosan-injected mice, pretreatment with H. perforatum extract led to an increase in the intracellular amounts of antioxidant enzymes associated with the reduction in ROS levels and iNOS expression and to a decrease in the interleukin 1β production, as compared to untreated controls [106]. In summary, Menegazzi et al. give a lot of instances in their review [88] that HF-containing H. perforatum extract or HF can attenuate inflammatory response and subsequent tissue injury triggered by injuring stimuli in several cell types and animal models, mainly by lowering ROS production and downregulating the expression or activity of inflammatory mediators.
In regard to the results of the current study, all the tested agents had cytotoxicity comparable to clinically used drugs such as cisplatin [85,107,108,109]. They were least toxic to HGF. For comparison, the other Hypericum agent besides RochC that has presented an antibacterial MIC value of only 0.625 µg/mL, a hydroalcoholic extract of H. perforatum containing 0.1 mg/mL hypericin, had an IC50 value of only 0.604 μg/mL against the human gingival fibroblasts HGF1-PI1 [34]. The IC50 of RochC against HGF in the current study is 9.22 μg/mL, i.e., it is much more selective and spares the gingival cells.
It is noteworthy that 1 µM of the PPAP HF was found to trigger differentiation in primary cultures of human keratinocytes and in derived HaCaT cell lines and to inhibit their proliferation [110]. If we compare our results, where the IC50 of the PPAPs used in this study was between 2.7 and 4.7 µM, it appears that a small dose can have a differentiating effect, while a higher dose can exert cytotoxic activity on skin fibroblasts.
A literature inquiry showed that the CFU assay from ISO 10993-5 has not been applied to HEPG2 cells before, to the best of our knowledge. Nevertheless, CFU tests with different protocols have been applied to this line [111,112], including one with a semi-solid medium and a seeding concentration similar to ours [113].
However, that protocol did not include an approximate counting of the cells in a colony in order to start the detection of results after they reach 20–50 cells per colony. In our case, HEPG2, after reaching about 20 cells in a colony, formed a kind of plaque covered with a substance similar to fat droplets, and in this aggregate, individual cells could not be distinguished and counted (Figure S8a). Generally, this cell line yielded poor results for the CFU assay in our case, with treatment dramatically reducing their clonogenic potential and/or them being poorly clonogenic in the untreated control in the first place.
For fibroblast cells, such as HGF and CCL-1, a different protocol could be used, named CFU assay for fibroblasts (CFU-F), but it demands different reagents [114,115], so we tried the CFU assay from ISO 10993-5 as for the other cell lines. Unfortunately, HGF did not form colonies but a network (Figure S8b,c).
Some synthetic or natural cytotoxic agents produce distinct features of apoptosis, necrosis, or other types of cell death in the cell. For example, Hoechst staining after podophyllotoxin exposure produced clear nuclear condensation and shrinkage in HaCaT cells, a sign of apoptosis [116]. However, cytotoxic agents may not directly kill cells but inhibit their division and multiplication, i.e., be cytostatic. In the current study, not only RochC and olympiforin B in the low MIC doses, but the undoubtedly cytotoxic doses of 2 × IC50 of HirDM90 did not cause any nuclear and DNA condensation, the distinct signs of apoptosis, even after 24–48 h exposure in the few surviving cells. The concentration of 2 × IC50 of HirDM90 certainly resulted in a much less dense layer of cells and fewer cells, but their nuclei appeared normal and intact. Therefore, the tested Hypericum agents are most likely cytostatic. Nevertheless, olympiforin A [29], HF [117] and other PPAPs [108] have been reported to either induce apoptosis or activate caspase 9 in tumor cell lines. We know that the Hypericum ingredients are selective and target and kill mostly cancer cells. Therefore, it is worth elucidating the mechanism of action for their cytotoxic effect on normal cells when applied in high enough concentrations.
Autophagy is a process of autophagosomal–lysosomal degradation of cytoplasmic components. This process is activated by nutrient deficiency, infection, or other stress factors and is associated with neurodegenerative and other diseases but is also observed in physiological processes such as development, differentiation, etc. Autophagy is an adaptive and pro-survival mechanism of the cell to metabolize its components that are not vital and immediately important for survival. If starvation or other stress factors continue, autophagy is not sufficient as a compensation, and excessive autophagy may lead to cell death, a process morphologically distinct from apoptosis [118].
The Atg genes control autophagosome formation through Atg12-Atg5 and LC3B (Atg8-II) complexes [119]. During autophagy, LC3A is converted to LC3B through lipidation by a ubiquitin-like system involving Atg7 and Atg3 that allows LC3B (the lipidated form of LC3) to become associated with autophagic vesicles and attached to the autophagosome membrane. The presence of LC3 in autophagosomes and the conversion of LC3 to the lower migrating form, LC3B, are used as indicators of autophagy [120].
The results of this study showed that Atg5 and Atg7 were induced significantly in most samples, indicating the activation of autophagy, while LC3A/B was converted to the lower migrating form visibly—also an indicator of autophagy—only in the positive control (30 µM erufosine) and the HirDM90 IC50 treatment. Further experiments may include antibodies for other proteins from the autophagic cascade, such as Beclin-1, Atg3, Atg16L1, etc.
3.3. Effect on Other Factors of the Host Homeostasis—CYP450 and Beneficial Lactobacilli
H. perforatum is a notorious CYP450 inducer, which reduces the efficacy of drugs such as digoxin, indinavir, warfarin, oral contraceptives, etc. The degree of CYP3A4 induction correlates significantly with the content of HF, a PPAP [121]. Testing for effects on hepatic enzyme systems’ activity of the PPAP olympiforin B and the extract RochC rich in PPAPs—agents selected for their optimal cytotoxic profile—showed that at concentrations inhibiting S. aureus, they hardly induce total CYP450 and its main isoform 3A4. The only exception was the dose of 2 × MIC of RochC, which induced CYP450 3A4, and this has to be taken into account for possible future application.
As mentioned, the data on the direct in vitro effects of Hypericum on beneficial lactobacilli is limited and a niche field. However, lactobacilli can have a dual role. For instance, L. plantarum has a beneficial and probiotic role when in fermented foods, but on our teeth, although a part of the normal oral microbiota and not a cavity initiator like S. mutans, it can be a secondary caries invader [80]. Lactobacillus acidophilus is an essential member of the natural vaginal microbiota but can also be cariogenic. Thus, the research is mainly focused on oral lactobacilli involved in tooth decay. For example, an H. perforatum extract had an MIC value over 300 μg/mL against L. acidophilus [122]. The aqueous fraction of an ethanolic extract of H. perforatum had a MIC of 8 μg/mL against L. plantarum, and its alcoholic extract suppressed L. acidophilus, thus allowing us to consider the plant for potential oral disinfectant formulations [34,80].
In regard to the beneficial role of lactobacilli, topical preparations of H. perforatum oil did not affect Lactobacillus acidophilus, therefore highlighting the possible selectivity of such formulations [123], since they inhibited some pathogenic bacteria such as S. pyogenes, Moraxella catarrhalis, etc. The study suggests that application of the ointments will not distort the normal vaginal microbiota. Milutinović et al. examined the effect of two extracts of H. perforatum on probiotic L. rhamnosus and L. plantarum. The first extract was made according to the standard procedures of the European Medicines Agency (EMA) with 50% ethanol, and the second one was an ethanol extract produced under optimized microwave-assisted extraction (MAE). The EMA extract inhibited the growth of two L. rhamnosus strains with MICs of 10 and 20 mg/mL and did not suppress L. plantarum. The MAE extract did not suppress L. rhamnosus and stimulated the growth of L. plantarum [124].
There is also interesting research focusing on the in vivo administration of H. perforatum in animals and following the changes in their gut lactobacilli and other microbiota members. The Lactobacillus population increased significantly, and the Escherichia coli population decreased in the gut of broilers whose drinking water had been supplemented with 150 to 250 mg/L of a hydroalcoholic extract from the plant [125]. In broilers again, the tested olive oil extract at doses of 3 to 4.5 mL/kg increased the total lactic acid bacteria count and decreased the total Enterococcus spp. counts. Interestingly, the 1.5 mL/kg dose and powdered H. perforatum added to the basal diet decreased the total lactic acid bacteria count [126]. A St. John’s Wort extract significantly elevated the abundance of Lactobacillus and other bacterial genera in the rat gut, which could contribute to the attenuation of hypercholesterolemia indirectly by modulating metabolic pathways in the host–microbiota system [127]. This could be related to the fact that the antidepressant effect of St. John’s Wort seems to be partly connected to the restoration of gut microbial composition by enriching the Akkermansia muciniphila intestinal symbiont bacterium, which leads to a reduction of microbiota-derived kynurenine levels, an increase in 5-hydroxytryptophan levels, and regulation of the NFκB-NLRP2-Caspase1-IL1β pathway [10].
Since Hypericum plants are rich in polyphenols, it is important to notice that they and their metabolites have a positive impact on the gut microbiota. They increase the levels of Lactobacillus and Bifidobacterium both in vitro [128] and in vivo. Regular consumption of polyphenol-rich foods increases the levels of these probiotic bacteria while decreasing pathogens such as Clostridium, S. aureus, etc. [129]. In addition, the effect of polyphenols on the human host is typically mediated through interaction with the gut microbiota because of their poor absorption in the digestive tract. The microorganisms catabolize them to metabolites with antioxidant and beneficial effects that could be transported within the host and take part in preventing chronic diseases such as cancer, diabetes, etc. Or the catabolites could in turn affect the gut microbiota, thus contributing to health promotion, for example, through the intestinal immune function [130]. For that reason, polyphenols can be named a novel group of prebiotics [129,131].
All the contrasting findings underline the importance of evaluating the specific effects of Hypericum active ingredients on beneficial lactobacilli when considering therapeutic applications.
For the purposes of this work, Lactobacillaceae species from the list of the European Food Safety Authority (EFSA) with qualified presumption of safety (QPS) or generally recognized as safe (GRAS) status (according to the Food and Drug Administration (FDA)) were selected. As part of a laboratory collection, they were characterized as candidate probiotics, fulfilling EFSA’s criteria [132]. Thus, selected L. fermentum from human origin and L. plantarum from dairy origin were included. In the current study, the MIC values for the tested candidate-probiotic strains were higher than those obtained for S. aureus, implying that both agents targeted the pathogen and could be used at anti-staphylococcal doses without significantly compromising beneficial lactic acid bacteria (LAB).
Many antibiotics used against staphylococci, especially if they have broad-spectrum Gram-positive activity, such as clindamycin [133] or vancomycin [134], are also active against Gram-positive LAB, which may lead to overgrowth of opportunistic Candida and other pathogens as well as gastrointestinal disturbances [50]. This is consistent with the successful in vitro experiments for the use of prebiotics and probiotics to prevent infection [135].
The higher MICs against candidate probiotic lactobacilli of RochC and Olympiforin B reinforce their safety and selectivity, important features for topical or systemic anti-staphylococcal treatments. Whether they would be less affecting and more unlikely to harm the normal microbiota than broad-spectrum Gram-positive antimicrobials is a question for future research.
There are observations of market trends suggesting that plant extracts and possibly their compounds can be used as food additives in flavored fermented dairy products containing lactic acid bacteria [136]. Further in vivo research to establish the effect of Hypericum phenolics specifically on the complex host–microbiota–pathogens system is needed. This is to be considered for possible applications and formulations of these phenolics, such as PPAPs, flavonoids, phenolic acids, hypericins, etc., in the direction of functional foods, rather than pharmaceuticals.
3.4. Limitations of the Study
Undoubtedly, the main limitation of the study is that it is in vitro research, which does not include an in vivo infection model. Indeed, it includes cytotoxicity tests on normal mammalian cell lines, but they are a supplement and not yet a replacement when developing an antimicrobial candidate drug. This limitation is not valid for the lactic acid bacteria, because according to the criteria of EFSA, in vitro tests on commercial or candidate probiotic strains are sufficient [132].
In the present, the pathway before human clinical trials must include an infection model, for instance, a model of biofilm on a wound, dental caries, skin, soft tissue infection, pneumonia, etc. The requirements for even superficial infections, such as cutaneous or dental cavities, often include the sacrifice of the test animals for histopathological examination. Although in vitro tests have their limitations in predicting the pharmacokinetics, host immune interactions, and tissue penetration of the pre-drug, they are mandatory to be pioneering at the preclinical stage. Therefore, from an ethical point of view, the authors find that the main limitation of the work may be considered an advantage.
The limitation of the lack of systemic toxicity elucidation of the agents can be partly overcome by in silico tests, cytotoxic tests on 3D cell culture models and organ-on-chip systems, which are one step further than 2D cell cultures and are a good plan for future research.
The MTT assay and live-cell imaging performed in the current study have their limitations of being unable to determine the mechanism of the cytotoxic and antiproliferative effect of PPAPs and PPAP-rich extracts on normal cell lines when in high enough concentrations. The low number of antibodies for autophagy detection tested is another limitation of the work. Last but not least, the limited quantity of compounds, e.g., hyperpolyphyllirin/hyperibine J, due to the unpredictable yield of plant samples, is another limitation of the study, and overcoming it would need time in order to wait for the new flowering season. The listed limitations warrant further studies.
4. Materials and Methods
4.1. MIC and MBC Evaluation
The MIC and MBC assessment on the pathogenic bacteria and the candidate probiotic lactic acid bacteria was carried out by the broth microdilution assay (BMD) and the agar plate assay, respectively, as described in [4]. The metabolic (dehydrogenase) activity of the lactic acid bacteria was assessed as described in [4].
4.1.1. MIC and MBC Evaluation on Pathogenic Bacteria
P. aeruginosa PAO1 ATCC 15692 was purchased from the American Type Cell Culture Collection (ATCC, Manassas, VA, USA). S. mutans DSM 20523 was bought from the German Collection of Microorganisms and Cell Cultures (DSMZ GmbH, Braunschweig, Germany). All other bacteria and strains were purchased as described in [4].
4.1.2. MIC Evaluation on Lactobacilli
Lactobacilli of different origins from the collection of Laboratory “Lactic Acid Bacteria and Probiotics”, the Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences (Sofia, Bulgaria), were selected (Table 13). The strains had been assessed previously, as candidate probiotics, according to the in vitro criteria recommended by the EFSA and the WHO guidelines [132]. They were stored at −20 °C in MRS broth (Merck Group, Darmstadt, Germany) supplemented with 20% v/v sterile glycerol. Prior to the experiments, lactobacilli were subcultured twice in MRS broth (GM369, HiMedia, Mumbai, India) at 37 °C. An overnight exponential-phase culture (5% v/v inoculum) was used for the tests.

Table 13.
Lactobacilli strains used in the present study.
4.2. Time Kill Assay
A time kill assay was performed according to the modified protocol of Sendi and Ruppen, 2015 [137]. A bacterial suspension of S. aureus was treated with two concentrations of the selected test agents (MBC and 1/2 MBC), as in the broth microdilution assay, and three decimal dilutions were immediately made from it. From these dilutions, 25 µL was seeded in brain heart infusion agar in a well of a 12-well plate. An untreated control was plated in the same way. Then, the bacterial suspension (treated and untreated) was placed in an incubator (MLW Kombinat, Leipzig, Germany) at 37 °C. Again, 25 µL of it was taken for seeding after 1, 2, 3, 4, 6, and 24 h, which equals a total of 7 time points. After 16–24 h of incubation, the colonies in the seeded plates from all dilutions were counted, and the number of colony-forming units (CFU) per mL, or in other words, bacteria per mL, was calculated.
4.3. Biofilm Assay
The biofilm assay was performed according to the protocol of Stepanović [138], as already described [4].
4.4. MTT Assay for Cytotoxicity
The MTT assay was performed according to Annex C, ISO 10993-5 [56] as in [116]. The panel of non-tumorigenic cell lines consisted of one murine (CCL-1 fibroblasts, which are recommended as a standard for cytotoxicity evaluation in Annex C of ISO 10993-5 [56]) and four human cell lines—HaCaT (immortalized epidermal keratinocytes), HGF (gingival fibroblasts), HEK-293 (embryonic cells from kidney epithelium), and HEPG2 (hepatoblastoma). Although technically a cancer cell line, HEPG2 is non-tumorigenic and retains many hepatocyte-related features in its proteome and a high degree of morphological and functional differentiation, also being a stage one hepatoblastoma [139,140,141,142,143].
CCL-1 was purchased from ATCC and HaCaT and HGF were bought from CLS Cell Lines Service (GmbH, Eppelheim, Germany). HEK-293 and HEPG2 were obtained from DSMZ. The cell culture medium for HaCaT and HEPG2 was Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/L glucose (DMEM-HA). For HGF it was DMEM/F-12 (DMEM-12-A, a 1:1 mixture of DMEM and Ham’s F-12 medium). The cell medium for CCL-1 and HEK-293 was Eagle’s Minimum Essential Medium (MEM) (MEM-A). Ten percent heat-inactivated fetal bovine serum (FBS) was added to all media but to the one for the CCL-1 cells, to which 10% heat-inactivated horse serum was added. All media were supplemented with 2 mM L-glutamine and Penicillin/Streptomycin (Pen/Strep, 100×). The cell culture media, sera, and additives were from Capricorn Scientific (Düsseldorf, Germany).
4.5. Colony-Forming Unit Assay
The CFU was also performed according to ISO 10993-5 [56]. Briefly, the cell lines were seeded at a concentration like for the MTT assay in 6-well plates, treated with the chosen agents and left for a 48 h exposure. Then, the cells were trypsinized, and pre-calculated 20 to 100 µL from the cell suspension was added to a sterile Roswell Park Memorial Institute (RPMI) medium (RPMI-A, Capricorn Scientific), containing 20% of the respective cell culture medium for each cell line but with a final concentration of FBS and L-glutamine of 40–42% and 0.8–1.2 mM, respectively, 0.2% NaHCO3 (S6297, Merck Sigma-Aldrich, Saint Louis, MI, USA), and 0.8% methylcellulose (M0512, Merck Sigma-Aldrich) for viscosity. The viscous cell solution was vortexed for 10 s and dispensed in an equal number of cells per well in 6-well plates (1.2 mL per well) in triplicate for each concentration of treatment. HaCaT and HEK-293 were seeded at a concentration of 2500 cells per mL, CCL-1 and HEPG2—2000 cells per mL, and HGF—1000 cells per mL. The plates were incubated for 6 to 10 days, and when the colonies reached 20 to 50 cells per colony, 100 µL MTT salt solution was added to each well. After half an hour, the stained colonies were counted manually using a microscope.
4.6. Gene Expression of Adhesion Genes
4.6.1. Isolation of RNA and cDNA Synthesis
The preparation of the samples for the RNA isolation was carried out as for the biofilm assay. RNA from the treated samples and the untreated positive MRSA control was isolated with the help of the Environmental DNA & RNA Purification Kit (E3572, EURx Ltd., Gdańsk, Poland). The chosen treating concentrations were sub-MICs—1/8 × to 1/32 × MIC, or 1.225, 0.613, and 0.306 for RochC and 0.125, 0.0625, and 0.031 µg/mL for olympiforin B. The protocol of the manufacturer was followed with some optimization. A total of 50 µL lysozyme (20 mg/mL) from chicken egg white (L6876, Merck Sigma-Aldrich) and 10 µL lysostaphin (L4402, Merck Sigma-Aldrich) from Staphylococcus staphylolyticus (200 U/mL) were added per sample. The isolated quantity of RNA was measured on a NanoDrop Lite Spectrophotometer (Thermo Scientific, Waltham, MA, USA) and standardized to equal concentrations of RNA (1 μg in 20 μL) according to the protocol of iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., 170-8896, Hercules, CA, USA) for reverse-transcription PCR (RT-PCR) on C1000 TouchTM PCR Thermal Cycler (Bio-Rad Laboratories, Inc., Singapore, Southeast Asia). The samples, being bacterial, were subjected to cDNA synthesis using random hexamer primers and reverse transcriptase enzymes. The obtained cDNA samples were stored at −80 °C and used for real-time PCR.
4.6.2. Real-Time PCR
Quantitative real-time PCR was prepared using Sso Advanced Universal SYBR Green Supermix (Bio-Rad Bio-Rad Laboratories, Inc., 1725270, Hercules, CA, USA) on a CFX96 TouchTM Real-Time Detection System (Bio-Rad, Singapore, Southeast Asia) in order to determine the expression of the icaA and icaD genes. The rho gene (coding a gene for transcription termination factor [144]) was used for reference and normalization (Table 14).

Table 14.
Primers and their melting temperatures (Tm) used for the gene expression analysis.
4.6.3. Gene Expression Analysis
The quantity of gene expression of the icaA and icaD expression levels in MRSA was determined by the 2−∆∆Ct method [145]. For normalization of the data, the rho gene was used as a housekeeping gene.
4.7. Live-Cell Imaging for Cytopathic Effect in Unstained Cells and Cells Stained with Hoechst for Apoptosis Detection
The cytopathic effect assay was performed in 24-well plates using the CCL-1 cell line, as recommended in ISO 10993-5 [56]. The seeding concentration was the same as in the MTT assay. Treated cells were viewed under an inverted microscope without fluorescence filters and documented microscopically.
The Hoechst 33342 dye (B2261, Merck & Co., Inc., Rahway, NJ, USA) was used to monitor apoptosis in the CCL-1 cell line, too. Cell suspension was seeded in the same manner as for MTT, but 50 µL per well in 96-well clear bottom plates. Overnight culture was treated with the Hypericum agents, and after the defined exposure time, the recommended amount of dyes was added, and after 20–30 min in the dark, the cells were observed under an inverted fluorescence microscope (BOE 5000.930, mode BIB100, BOECO, Hamburg, Germany) and photodocumented microscopically (B-CAM16, BOE 1900.16000, BOECO, Hamburg, Germany) with the application software B-View (BOECO, version x64, 4.7.14531.20190425). Daunorubicin (30450, Merck Sigma-Aldrich) and hypertonic buffer were used as positive controls, and the treatment protocol and recipe of the latter were taken and adapted from the kit Cell Death Detection ELISA (11544675001, Roche, Basel, Switzerland). For the hypertonic buffer, the exposure time was 2 h, and the cells were plated at twice the concentration as for the MTT and cytopathic tests in order to comply with the kit protocol.
At least two wells were seeded as replicates, and at least two photographs were taken for all samples and treatments, stained with dyes or not. The assessment of the cytopathic effect was performed visually.
4.8. Western Blot
For Western blot, CCL-1 and HGF cell lines were chosen, and they were seeded in either 6-well plates or 55 mm Petri dishes suitable for cell culture. They were treated with an exposure time of 24 h in an incubator (Panasonic MCO-18AC, Panasonic Healthcare Ltd., Oizumi-Machi, Japan). The treatment was with the selected agents RochC and olympiforin B, and a positive control erufosine (30 µM), a substance proven to cause autophagy [59], kindly provided by Prof. Hans-Jörg Eibl [146]. In addition, a higher concentration of an agent (HirDM90) was added to one of the tests for comparison. The samples were trypsinized, collected, washed with PBS (TS1101, HiMedia, Mumbai, India), centrifuged, and frozen at −80 °C if storage was necessary. Then they were lysed on ice with a buffer containing 100 mM Tris–HCl (648313, Sigma-Aldrich, St. Louis, MO, USA) at pH 8.0, 4% sodium dodecyl sulfate (SDS, L5750, Sigma-Aldrich), 20% glycerol (49779, Sigma-Aldrich), 1% Na3VO4 (450243, Sigma-Aldrich), 200 mM dithiothreitol (DTT, 3483-12-3, Sigma-Aldrich, Burlington, MA, USA) supplemented with protease inhibitor (05892970001, Roche). The cell lysate was heated to boiling for 15 min, then centrifuged at 13,000 rpm for 10 min at 4 °C. Lysates were subjected to denaturing SDS polyacrylamide gel electrophoresis on acrylamide 4–20% gradient Mini-PROTEAN® TGX™ Stain-Free gels (4568094, Bio-Rad Laboratories, Hercules, CA, USA). The total protein content of the gels of one of the two replicates was photodocumented. The separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, a part of the Trans-Blot Turbo Transfer Pack (1704156, Bio-Rad Laboratories), using the Trans-Blot® Turbo™ Transfer System (Bio-Rad Laboratories) according to the manufacturer’s instructions. The blots were then fixed with a blocking solution of 1% casein in PBS (1610783, Bio-Rad Laboratories) and incubated for 2.5 h with specific primary antibodies—rabbit antibodies Atg5, Atg7, and LC3A/B from the Autophagy Antibody Sampler kit (4445, Cell signaling Technology, Danvers, MA, USA) and mouse antibody β-actin (8H10D10, Cell signaling Technology). This was followed by washing and 1 h incubation with a secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody from the aforementioned kit or horse anti-mouse (7076P2, Cell signaling Technology) for the respective primary antibodies and washing again. Visualization was performed by chemiluminescent reaction with a chemiluminescent substrate (170-5061, Bio-Rad Laboratories). The Western blot was performed in two independent duplicates. Control over the uniformity of sample loading was performed by comparing the protein content of the individual samples with the total protein content for one of the replicates and the actin content, a product of a house keeping gene, for the other replicate. After that optimization, which showed the same tendency in the semi-quantitative results, the results with actin were presented in this work because of their better visual appeal. Densitometric calculation and analysis was performed with Quantity One software version 4.6.9 (Bio-Rad Laboratories).
4.9. ELISA for Cytochrome P450
The effect on CYP450 was tested using the ELISA method on the HEPG2 liver cell line. As mentioned, it has weak or absent expression of the CYP450 superfamily, e.g., CYP3A4, CYP1A2, etc. [60], and that low basal activity makes it a good model for studies of CYP inducers [61]. In addition, these cells have the advantage of immortalization due to their cancerous nature. The standard curves after the two ELISA tests (Figure S7) were prepared as a standard dose–response curve, also called a four-parameter logistic (4-PL) curve.
4.9.1. Total Cytochrome P450
Human total cytochrome P450 was examined using the Human Cytochrome P450 ELISA Kit (MBS705506, MyBioSource, San Diego, CA, USA) according to the manufacturer’s instructions. HEPG2 cells were seeded at a concentration of 100,000 cells/mL in a 6-well plate, and the overnight culture was treated with selected Hypericum agents. After 24 h of exposure, the protocol was performed according to the kit instructions.
The test principle uses the sandwich quantitative enzyme immunoassay technique. A microplate was pre-coated with an antibody specific for cytochrome P450. Standards and samples were pipetted into the wells, and if cytochrome P450 molecules were present, they were bound by the immobilized antibody. After removing any unbound material, a biotin-conjugated antibody specific for cytochrome P450 was added to the wells. After washing, avidin-conjugated horseradish peroxidase was added to the wells. After washing to remove any unbound avidin-enzyme reagent, a substrate solution was added to the wells, and a color proportional to the amount of cytochrome P450 bound in the initial step developed. The color development was stopped, and its intensity was measured on a microplate reader ELx800 (BioTek Instruments, Inc., Winooski, VT, USA).
4.9.2. Cytochrome P450 3A4
The effect on human cytochrome P450 3A4, the major member of the CYP450 superfamily of enzymes, which is predominantly found in the liver and metabolizes approximately half of the drugs metabolized by CYP450 enzymes, was assayed using the CYP3A4 ELISA Kit (DL-CYP3A4-Hu, DLdevelop, Kelowna, BC, Canada). Sample selection and initial preparation were as for the total cytochrome ELISA. The experiment was performed according to the manufacturer’s instructions. This assay is a sandwich enzyme immunoassay based on the binding of CYP3A4 in standards and samples to a biotin-conjugated antibody specific for CYP3A4. The binding of biotin to avidin conjugated to horseradish peroxidase ensures that after the addition of TMB substrate solution, only those microtiter wells of the plate that contain CYP3A4, biotin-conjugated antibody, and enzyme-bound avidin show a color change. The reaction was stopped by adding sulfuric acid solution, and the plate was read at 450 nm on a microplate reader ELx800 (BioTek Instruments, Inc.). The CYP3A4 concentration was determined by comparing the O.D. of the samples with the standard curve.
4.10. In Vivo Skin Irritation Test on Rabbits
Dermal toxicological screening of the chosen two non-toxic active substances was performed on animals. This was carried out according to the European standard ISO 10993-10: 2010 (E): Biological evaluation of medical devices, Part 10 Skin irritation and sensitization tests (6. Irritation tests, 6.3 Animal irritation test) (withdrawn; superseded by ISO 10993-10:2021) [147]. The animal study protocol was approved by the opinion No. 148 of 09.04.2019 of the Ethics Committee of the Bulgarian Food Safety Agency of the Ministry of Agriculture, Food, and Forestry. The Permit to work with experimental rabbits No. 232 was valid until 11.04.2024 and issued on the basis of Article 155, paragraph 7, of the Veterinary Medical Activity Act. The albino rabbits were cared for as specified in ISO 10993-2 (withdrawn; superseded by ISO 10993-2:2022) [148] and Ordinance No. 20 (State Gazette of Bulgaria, No. 87, 9 November 2012). Four square test sites were made on the back of the rabbit by clipping the fur. The samples were applied to 2 of them, while the other 2 were used as a positive control (10–20% SDS) and a negative control (the buffer in which the agents were diluted, i.e., PBS). The exposure time was 4 h, and at the end of it the appearance was checked for erythema (redness) and edema. Additional inspections were performed at hours 24, 48, and 72. The first rabbit per both applied agents did not show any signs of skin irritation, so two more animals per agent were tested, as specified by the ISO standard.
4.11. Data Processing and Statistics
At least two wells per concentration (duplicate) were used for the BMD, as well as for the ELISA, live-cell imaging, and Western blot. The CFU and skin irritation test were performed in triplicate—final samples seeded in 3 wells per concentration or 3 animals per agent, respectively. The biofilm assay had 3 to 5 replicates, and the MTT assay had 4 replicates (3–5 or 4 wells per concentration, respectively). The replicates for the time kill assay were provided by the three decimal dilutions of each sample at each hour, from which one replicate was seeded.
The total activity [mL/g] in terms of antimicrobial activity was calculated according to Eloff (2000) [6]. The amount extracted from the plant [expressed as mg/g of dried plant material] was divided by the MIC value [mg/mL].
GraphPad Prism software version 9 (GraphPad Software Inc., San Diego, CA, USA) was used to calculate the IC50 values from the MTT assays as in [116], as well as the MTC values, and the ELISA standard curves. The MTC values are, according to ISO 10993-5, the maximum concentration at which at least 70% of the cells are viable (nonlin fit, range) [56]. The 95% confidence interval (CI) of the IC50 values is derived from the dose–response curves with a regression coefficient (R2) in the range of 0.87–0.99.
5. Conclusions
With a view to characterizing them as potential therapeutic agents, a screening of antimicrobial phytoconstituents from Hypericum plants with known phytochemical content was performed on selected cell lines, proven models for non-tumorigenic cells, representing different body tissues. All the tested agents had cytotoxicity compared to clinically used drugs such as cisplatin. Most of the ingredients selected as least cytotoxic stimulated the clonogenicity of HaCaT, HEK-293, and CCL-1 lines at doses of 1/2 × IC50. A consistent and building assessment selected just two agents—the extract RochC and the compound olympiforin B—to be tested further, as they had outstanding antibacterial activity and satisfactory cytotoxic effect on eukaryotic cells. The concentrations chosen for further research were their MIC values against S. aureus—0.625 and 1 µg/mL, respectively. Their IC50 values were higher for all five cell lines than these MIC values (except for olympiforin B in HEK-293) and their MTC values were higher for these agents, mostly for the CCL-1 and HGF cell lines. Therefore, at antistaphylococcal doses the agents would be relatively safe for the normal human cells.
RochC and Olympiforin B had MIC values against the cariogenic S. mutans of 6 and 3 µg/mL, respectively, values lower than their IC50 and even their MTC values for the gingival cells, and have the potential to be developed as oral care products. The MRSA biofilm was more sensitive to the agents, with MBIC values lower than their MIC values. The MBIC of RochC was even one-eighth of the MIC value. The MBIC50 was much below the corresponding MIC values for both agents and both bacteria. Olympiforin B at a dose of 1/8 to 1/32 MIC inhibited the relative gene expression of both genes. RochC induced icaA, while icaD was inhibited by a dose of 1/16 MIC and induced by the lower concentration of 1/32 MIC, showing that the phenotypic inhibition of biofilm formation is not always accompanied by genotypic inhibition of all its related genes. Given that almost all ingredients from the panel of agents initially tested in this study turned out to be bacteriostatic, that quality, in addition to targeting some virulence factor, such as biofilm formation, is a contributing factor for AMR mitigation.
The MIC values for the tested QPS candidate-probiotic lactobacilli were higher than those obtained for S. aureus, implying that both agents could be used at anti-staphylococcal doses without significantly compromising the beneficial lactobacilli. These safety and selectivity features are important for topical or systemic anti-staphylococcal treatments, but additional in vivo research to establish their effect on the complex system of host–microbiota–pathogens is to be considered.
There was no cytopathic effect in CCL-1 cells from RochC and olympiforin B treatment, even from doses of 2 × MIC for S. aureus. High concentrations of 2 × IC50 of another Hypericum ingredient also did not cause condensed nuclei, blebs, or other signs of apoptosis, implying that the effect was antiproliferative and cytostatic. Given that other PPAPs cause apoptosis in cancer cells, it is worth elucidating their mechanism of action for their cytotoxic effect on normal cells when applied in high enough concentrations. However, the agents significantly induced Atg5 and Atg7 proteins related to autophagosome formation, while LC3A/B was converted to the lower migrating form—an indicator of autophagy—only by a high concentration of another Hypericum constituent and the positive control erufosine.
CYP450 was not induced in HEPG2 liver cells by the agents, with the exception of 3A4 induced by RochC at a dose of 2 × MIC. The selected agents did not irritate rabbit skin in vivo at a dose of even ten times MIC.
The complex evaluation of different aspects of biological activity of the agents shows that RochC and olympiforin B are highly antimicrobial but with a satisfactory gentle effect on the host cells. Therefore, they have potential for further pharmacological development to treat infections caused by certain bacterial strains that are sensitive to them, or as oral care products, or functional foods. However, more research is needed to demonstrate their full therapeutic potential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18101591/s1, Figure S1: CCL-1 cell line treated with hypertonic buffer. Cells are unstained and stained with Hoechst. Images are at 100× and 200× magnification; Figure S2: Increased number of apoptotic blebs are observed on the cell surface only of CCL-1 cells treated with hypertonic buffer, but not on cells treated with Hypericum agents. The magnification is 400×; Figure S3: Cytopathic effect after 5 h of exposure to Hypericum agents in CCL-1 cells. The MIC values are for S. aureus. The cells are unstained and stained with Hoechst. Images are at 100× and 200× magnification; Figure S4: Cytopathic effect after 24 h of exposure to Hypericum agents in CCL-1. The MIC values are for S. aureus. The cells are unstained and stained with Hoechst. The images are at 100×, 200×, and 400× magnification; Figure S5: Cytopathic effect after 48 h of exposure to Hypericum agents in CCL-1. The MIC values are for S. aureus. The cells are unstained and stained with Hoechst. The images are at 100×, 200×, and 400× magnification; Figure S6: Cytopathic effect after 72 h of exposure to Hypericum agents in CCL-1. The MIC values are for S. aureus. The cells are unstained and stained with Hoechst. The images are at 100×, 200×, and 400× magnification; Figure S7: Standard curves with linear (a) and logarithmic (b) representation of concentration for human total CYP450 and CYP450 3A4 obtained in the present study; Figure S8: Cell lines not very suitable for colony-forming unit assay. (a) HEPG2 cells, which form a plaque-like colony with difficult-to-count cells; (b) HGF cells, which form a network rather than colonies; (c) HGF cells after MTT salt staining; Table S1: One-way ANOVA analysis for total CYP450; Table S2: One-way ANOVA analysis for CYP450 3A4.
Author Contributions
Conceptualization, M.M.Z., H.N. and P.N.; methodology, M.M.Z., I.T., L.D., S.D. and P.N.; software, not applicable.; validation, Y.I., M.M.Z., L.D. and P.N.; formal analysis, M.M.Z., Y.I., L.D. and P.N.; investigation, Y.I., M.M.Z., L.D., M.D.K., T.C.K., L.I.D., S.T., T.M., Z.K.-N., S.S.B. and P.N.; resources, Y.I., M.M.Z., S.D., P.N. and. H.N.; data curation, Y.I., M.M.Z., L.D. and P.N.; writing—original draft preparation, Y.I., M.M.Z., L.D., L.I.D. and S.D.; writing—review and editing Y.I., M.M.Z., L.D. and S.D.; visualization, Y.I. and M.M.Z.; supervision, Y.I., M.M.Z., I.T. and S.D.; project administration, Y.I.; funding acquisition, Y.I., M.M.Z. and H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bulgarian National Science Fund, contract KΠ-06-M51/4 from 12 November 2021 from “Competition for financial support for projects of junior basic researchers and postdocs—2021”.
Institutional Review Board Statement
The animal study protocol was approved by the opinion No. 148 of 09.04.2019 of the Ethics Committee of the Bulgarian Food Safety Agency of the Ministry of Agriculture, Food, and Forestry. The Permit to work with experimental rabbits No. 232 was valid until 11.04.2024 and issued on the basis of Article 155, paragraph 7, of the Veterinary Medical Activity Act.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
The culturing of cell lines, the reading of O.D. for the ELISA, and the MTT assay were performed on equipment donated to Maya Zaharieva by the Alexander von Humboldt Foundation in the frame of the Program “Equipment Subsidies”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Antimicrobial Resistance (AMR)|Fighting Antibiotic Resistant Germs. Antimicrobial Resistance (AMR), Tuberculosis. Available online: https://www.bioversys.com/medical-needs/antimicrobial-resistance/ (accessed on 28 July 2025).
- Krome, A.K.; Becker, T.; Kehraus, S.; Schiefer, A.; Gütschow, M.; Chaverra-Muñoz, L.; Hüttel, S.; Jansen, R.; Stadler, M.; Ehrens, A.; et al. Corallopyronin A: Antimicrobial Discovery to Preclinical Development. Nat. Prod. Rep. 2022, 39, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Menakha, M. Pharmaceutical Applications of Cyanobacteria—A Review. J. Acute Med. 2015, 5, 15–23. [Google Scholar] [CrossRef]
- Ilieva, Y.; Marinov, T.; Trayanov, I.; Kaleva, M.; Zaharieva, M.M.; Yocheva, L.; Kokanova-Nedialkova, Z.; Najdenski, H.; Nedialkov, P. Outstanding Antibacterial Activity of Hypericum rochelii—Comparison of the Antimicrobial Effects of Extracts and Fractions from Four Hypericum Species Growing in Bulgaria with a Focus on Prenylated Phloroglucinols. Life 2023, 13, 274. [Google Scholar] [CrossRef]
- Ilieva, Y.; Zaharieva, M.M.; Najdenski, H.; Kroumov, A.D. Antimicrobial Activity of Arthrospira (Former Spirulina) and Dunaliella Related to Recognized Antimicrobial Bioactive Compounds. Int. J. Mol. Sci. 2024, 25, 5548. [Google Scholar] [CrossRef]
- Eloff, J. A Proposal on Expressing the Antibacterial Activity of Plant Extracts—A Small First Step in Applying Scientific Knowledge to Rural Primary Health Care in South Africa. S. Afr. J. Sci. 2000, 96, 116–118. [Google Scholar]
- Akerele, O.; Heywood, V.; Synge, H. (Eds.) Conservation of Medicinal Plants; Cambridge University Press: Cambridge, UK, 1991; ISBN 978-0-521-11202-4. [Google Scholar]
- Crockett, S.L.; Robson, N.K.B. Taxonomy and Chemotaxonomy of the Genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar] [PubMed]
- Bilia, A.R.; Gallori, S.; Vincieri, F.F. St. John’s Wort and Depression: Efficacy, Safety and Tolerability—An Update. Life Sci. 2002, 70, 3077–3096. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Wang, F.-F.; Zhao, Y.-Y.; Lu, L.-F.; Jiang, X.-Y.; Huang, T.-Q.; Lin, Y.; Guo, L.; Weng, Z.-B.; Liu, E.-H. Hypericum perforatum L. Attenuates Depression by Regulating Akkermansia muciniphila, Tryptophan Metabolism and NFκB-NLRP2-Caspase1-IL1β Pathway. Phytomed. Int. J. Phytother. Phytopharm. 2024, 132, 155847. [Google Scholar] [CrossRef]
- Sotirova, Y.; Ivanova, N.; Ermenlieva, N.; Vilhelmova-Ilieva, N.; Simeonova, L.; Metodiev, M.; Gugleva, V.; Andonova, V. Antimicrobial and Antiherpetic Properties of Nanoencapsulated Hypericum perforatum Extract. Pharmaceuticals 2025, 18, 366. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Hellio, C.; Patel, A.V.; Abbas, G. Phytochemical Profile, Antioxidant and Antibacterial Activity of Four Hypericum Species from the UK. S. Afr. J. Bot. 2020, 133, 45–53. [Google Scholar] [CrossRef]
- Kladar, N.; Božin, B.; Bijelić, K.; Bogavac, M.; Karaman, M.; Srđenović Čonić, B.; Rat, M.; Anačkov, G. Biological Activity of Genus Hypericum Sect. Hypericum Species—H. tetrapterum, H. maculatum subsp. immaculatum, H. triquetrifolium. Molecules 2023, 28, 6218. [Google Scholar] [CrossRef]
- Guefack, M.-G.F.; Kuete, V. Hypericum roeperianum as a Source of Antibacterial Agents. In Advances in Botanical Research; Kuete, V., Ed.; African Flora to Fight Bacterial Resistance, Part II: The Best Source of Herbal Drugs and Pharmaceuticals; Academic Press: Cambridge, MA, USA, 2023; Volume 107, pp. 193–211. [Google Scholar]
- El-Chaghaby, G.A.; Mohammed, F.S.; Rashad, S.; Uysal, I.; Koçer, O.; Lekesiz, Ö.; Doğan, M.; Şabik, A.E.; Sevindik, M. Genus Hypericum: General Properties, Chemical Contents and Biological Activities. Egypt. J. Bot. 2024, 64, 1–26. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.; Conforti, F. Hypericum spp.: An Update on the Biological Activities and Metabolic Profiles. Mini Rev. Med. Chem. 2020, 20, 66–87. [Google Scholar] [CrossRef]
- Brondz, I.; Greibrokk, T.; Groth, P.A.; Aasen, A.J. The Relative Stereochemistry of Hyperforin—An Antibiotic from Hypericum perforatum L. Tetrahedron Lett. 1982, 23, 1299–1300. [Google Scholar] [CrossRef]
- Reichling, J.; Weseler, A.; Saller, R. A Current Review of the Antimicrobial Activity of Hypericum perforatum L. Pharmacopsychiatry 2001, 34 (Suppl. S1), S116–S118. [Google Scholar] [CrossRef]
- Barciela, P.; Rodrigues, D.B.; Perez-Vazquez, A.; da Silveira, T.F.F.; Pires, T.C.S.P.; Mandim, F.; Carpena, M.; Pereira, C.; Ferreira, I.C.F.R.; Barros, L.; et al. Phytochemical Diversity and Biological Activities of Hypericum japonicum and Hypericum sampsonii: Potential for Natural Product-Based Food Applications. Food Chem. 2025, 484, 144355. [Google Scholar] [CrossRef]
- Eruygur, N.; Uçar, E.; Tüzün, B.; Ataş, M.; İnanır, M.; Demirbaş, A.; Bal, H.; Şenkal, B.C.; Uskutoğlu, T. Evaluation of Antioxidant, Antimicrobial, Enzyme Inhibition Activity, and Cell Viability Capacity of Hypericum Heterophyllum Vent., an Endemic Species in Turkey’s Flora. J. Mol. Struct. 2024, 1307, 137908. [Google Scholar] [CrossRef]
- Javrushyan, H.; Ginovyan, M.; Harutyunyan, T.; Gevorgyan, S.; Karabekian, Z.; Maloyan, A.; Avtandilyan, N. Elucidating the Impact of Hypericum Alpestre Extract and L-NAME on the PI3K/Akt Signaling Pathway in A549 Lung Adenocarcinoma and MDA-MB-231 Triple-Negative Breast Cancer Cells. PLoS ONE 2025, 20, e0303736. [Google Scholar] [CrossRef]
- Meenakshi, G.; Rajendiran, N.; Sivaraj, C.; Saraswathi, K.; Arumugam, P. Anticancer Activity of Leaves Extract of Hypericum mysorense L. J. Pharmacogn. Phytochem. 2020, 9, 172–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.Q.; Yu, M.J.; Ren, C.Y.; Yu, X.H.; Yan, W.; Tang, J. Ten Flavonoids of Hypericum Przewalskii and Selective Cytotoxicity Against Six Human Tumor Cell Lines. Chem. Nat. Compd. 2024, 60, 500–502. [Google Scholar] [CrossRef]
- Guefack, M.-G.F.; Damen, F.; Mbaveng, A.T.; Tankeo, S.B.; Bitchagno, G.T.M.; Çelik, İ.; Simo Mpetga, J.D.; Kuete, V. Cytotoxic Constituents of the Bark of Hypericum roeperianum Towards Multidrug-Resistant Cancer Cells. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 4314807. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical Profiles, Phototoxic and Antioxidant Properties of Eleven Hypericum Species—A Comparative Study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef]
- Ma, W.; Ren, F.; Yan, X.; Wang, X.; Wu, T.; Li, N. Cytotoxic and Anti-Inflammatory Constituents from Roots of Hypericum beanii and the Antitumor Potential Under the View of Cancer-Related Inflammation. Fitoterapia 2024, 172, 105745. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, N.; Yang, X.; Xu, G. Polycyclic Polyprenylated Acylphloroglucinol with an Unprecedented Spirocyclic Core from Hypericum patulum. Chin. Chem. Lett. 2020, 31, 2433–2436. [Google Scholar] [CrossRef]
- Tian, W.-J.; Zhou, M.; Qiu, D.-R.; Chen, J.-J.; Liu, X.-Z.; Li, J.-D.; Lin, T.; Wang, G.-H.; Chen, H. Seco-Polyprenylated Acylphloroglucinols from Hypericum elodeoides Induced Cell Cycle Arrest and Apoptosis in MCF-7 Cells via Oxidative DNA Damage. Bioorg. Chem. 2022, 128, 106088. [Google Scholar] [CrossRef]
- Ilieva, Y.; Momekov, G.; Zaharieva, M.M.; Marinov, T.; Kokanova-Nedialkova, Z.; Najdenski, H.; Nedialkov, P.T. Cytotoxic and Antibacterial Prenylated Acylphloroglucinols from Hypericum olympicum L. Plants 2023, 12, 1500. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Yue, G.G.-L.; Li, X.-R.; Xu, G.; Lau, C.B.-S. Structurally Diverse Spirocyclic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum ascyron Linn. and Their Anti-Tumor Activity. Phytochemistry 2023, 212, 113727. [Google Scholar] [CrossRef]
- Traeger, A.; Voelker, S.; Shkodra-Pula, B.; Kretzer, C.; Schubert, S.; Gottschaldt, M.; Schubert, U.S.; Werz, O. Improved Bioactivity of the Natural Product 5-Lipoxygenase Inhibitor Hyperforin by Encapsulation into Polymeric Nanoparticles. Mol. Pharm. 2020, 17, 810–816. [Google Scholar] [CrossRef]
- Bridi, H.; Meirelles, G.d.C.; von Poser, G.L. Structural Diversity and Biological Activities of Phloroglucinol Derivatives from Hypericum Species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-Y.; Mu, Q.; Gibbons, S. The Phytochemistry and Pharmacology of Hypericum. Prog. Chem. Org. Nat. Prod. 2020, 112, 85–182. [Google Scholar] [CrossRef]
- Khadem Nezhad, S.; Taghavi Zenouz, A.; Aghazadeh, M.; Samadi Kafil, H. Strong Antimicrobial Activity of Hypericum perforatum L. Against Oral Isolates of Lactobacillus spp. Cell. Mol. Biol. Noisy Gd. Fr. 2017, 63, 58–62. [Google Scholar] [CrossRef]
- Piechota, M.; Kot, B.; Frankowska-Maciejewska, A.; Grużewska, A.; Woźniak-Kosek, A. Biofilm Formation by Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Strains from Hospitalized Patients in Poland. BioMed Res. Int. 2018, 2018, 4657396. [Google Scholar] [CrossRef]
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory Effects of Polysorbate 80 on MRSA Biofilm Formed on Different Substrates Including Dermal Tissue. Sci. Rep. 2019, 9, 3128. [Google Scholar] [CrossRef]
- Adhoni, S.; Sharana, S.; Kaliwal, B. Phytochemical Analysis and Antimicrobial Activity of Chorella Vulgaris Isolated from Unkal Lake. J. Coast. Life Med. 2016, 4, 368–373. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and Their Role on Diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Gayatri, K.; Soundhari, C.; Pavithra, B. Biofilm Inhibitory Effect of Chlorella Extracts on Pseudomonas Aeruginosa. Int. J. Pharm. Sci. Res. 2019, 23, 927. [Google Scholar]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Pietrocola, G.; Campoccia, D.; Motta, C.; Montanaro, L.; Arciola, C.R.; Speziale, P. Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants. Int. J. Mol. Sci. 2022, 23, 5958. [Google Scholar] [CrossRef] [PubMed]
- Neopane, P.; Nepal, H.P.; Shrestha, R.; Uehara, O.; Abiko, Y. In Vitro Biofilm Formation by Staphylococcus Aureus Isolated from Wounds of Hospital-Admitted Patients and Their Association with Antimicrobial Resistance. Int. J. Gen. Med. 2018, 11, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of Antibiotic Resistance in Bacterial Biofilms. Int. J. Med. Microbiol. IJMM 2002, 292, 107–113. [Google Scholar] [CrossRef]
- LewisOscar, F.; Nithya, C.; Bakkiyaraj, D.; Arunkumar, M.; Alharbi, N.S.; Thajuddin, N. Biofilm Inhibitory Effect of Spirulina platensis Extracts on Bacteria of Clinical Significance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 537–544. [Google Scholar] [CrossRef]
- Vijayan, P.; Vinod Kumar, S.; Shrishailappa, B.; Mukherjee, P.K.; Dhanaraj, S.A.; Suresh, B. Selective in Vitro Cytotoxicity of Hypericum Hookerianum towards Cancer Cell Lines. Adv. Tradit. Med. 2003, 3, 141–146. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Hosseini, F.; Ahmadi, A.; Mozafari, M.; Amjadi, I. Antiproliferative Activity of Hypericum perforatum, Achillea millefolium, and Aloe Vera in Interaction with the Prostatic Activity of CD82. Rep. Biochem. Mol. Biol. 2019, 8, 260–268. [Google Scholar]
- Sut, S.; Dall’Acqua, S.; Flores, G.A.; Cusumano, G.; Koyuncu, İ.; Yuksekdag, O.; Emiliani, C.; Venanzoni, R.; Angelini, P.; Selvi, S.; et al. Hypericum empetrifolium and H. lydium as Health Promoting Nutraceuticals: Assessing Their Role Combining In Vitro In Silico and Chemical Approaches. Food Sci. Nutr. 2025, 13, e70053. [Google Scholar] [CrossRef]
- Konya Konuk, R.; Aru, B.; Öztürk, C.; Kırmızıbekmez, H. A Novel Normonoterpene Glycoside and a New Benzophenone Derivative from Hypericum cerastioides and Their In Vitro Cytotoxic Activities. Fitoterapia 2024, 179, 106276. [Google Scholar] [CrossRef]
- Aggarwal, J.; Swami, G.; Kumar, M. Probiotics and Their Effects on Metabolic Diseases: An Update. J. Clin. Diagn. Res. JCDR 2013, 7, 173–177. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Stefańska, I.; Kwiecień, E.; Jóźwiak-Piasecka, K.; Garbowska, M.; Binek, M.; Rzewuska, M. Antimicrobial Susceptibility of Lactic Acid Bacteria Strains of Potential Use as Feed Additives—The Basic Safety and Usefulness Criterion. Front. Vet. Sci. 2021, 8, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Daferera, D.; Trigas, P.; Charalambous, D.; Pantelidou, M.; Tarantilis, P.A.; Kanakis, C.D. Comparative Study of the Antibacterial Activity, Total Phenolic and Total Flavonoid Content of Nine Hypericum Species Grown in Greece. Appl. Sci. 2023, 13, 3305. [Google Scholar] [CrossRef]
- Nürk, N.M.; Crockett, S.L. Morphological and Phytochemical Diversity among Hypericum Species of the Mediterranean Basin. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 14–28. [Google Scholar] [PubMed]
- Hlima, H.B.; Bohli, T.; Kraiem, M.; Ouederni, A.; Mellouli, L.; Michaud, P.; Abdelkafi, S.; Smaoui, S. Combined Effect of Spirulina Platensis and Punica Granatum Peel Extacts: Phytochemical Content and Antiphytophatogenic Activity. Appl. Sci. 2019, 9, 5475. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/36406.html (accessed on 15 April 2025).
- Eloff, J.N. A Proposal Towards a Rational Classification of the Antimicrobial Activity of Acetone Tree Leaf Extracts in a Search for New Antimicrobials. Planta Med. 2021, 87, 836–840. [Google Scholar] [CrossRef]
- Ammanath, A.V.; Matsuo, M.; Wang, H.; Kraus, F.; Bleisch, A.; Peslalz, P.; Mohammad, M.; Deshmukh, M.; Grießhammer, A.; Purkayastha, M.; et al. Antimicrobial Evaluation of Two Polycyclic Polyprenylated Acylphloroglucinol Compounds: PPAP23 and PPAP53. Int. J. Mol. Sci. 2024, 25, 8023. [Google Scholar] [CrossRef]
- Kapoor, V.; Zaharieva, M.M.; Das, S.N.; Berger, M.R. Erufosine Simultaneously Induces Apoptosis and Autophagy by Modulating the Akt-mTOR Signaling Pathway in Oral Squamous Cell Carcinoma. Cancer Lett. 2012, 319, 39–48. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 Research and The Journal of Biological Chemistry. J. Biol. Chem. 2019, 294, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Takemura, A.; Gong, S.; Sato, T.; Kawaguchi, M.; Sekine, S.; Kazuki, Y.; Horie, T.; Ito, K. Evaluation of Parent- and Metabolite-Induced Mitochondrial Toxicities Using CYP-Introduced HepG2 Cells. J. Pharm. Sci. 2021, 110, 3306–3312. [Google Scholar] [CrossRef]
- Avato, P.; Raffo, F.; Guglielmi, G.; Vitali, C.; Rosato, A. Extracts from St John’s Wort and Their Antimicrobial Activity. Phytother. Res. 2004, 18, 230–232. [Google Scholar] [CrossRef]
- Nedialkov, P.T.; Momekov, G.; Kokanova-Nedialkova, Z.K.; Heilmann, J. Polyprenylated Phloroglucinols from Hypericum Maculatum. Nat. Prod. Commun. 2015, 10, 1231–1235. [Google Scholar] [CrossRef]
- Porzel, A.; Farag, M.A.; Mülbradt, J.; Wessjohann, L.A. Metabolite Profiling and Fingerprinting of Hypericum Species: A Comparison of MS and NMR Metabolomics. Metabolomics 2014, 10, 574–588. [Google Scholar] [CrossRef]
- Alali, F.Q.; Tawaha, K. Dereplication of Bioactive Constituents of the Genus Hypericum Using LC-(+,−)-ESI-MS and LC-PDA Techniques: Hypericum triquterifolium as a Case Study. Saudi Pharm. J. 2009, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Alali, F.Q.; Tawaha, K.; Gharaibeh, M. LC-MS and LC-PDA Analysis of Hypericum empetrifolium and Hypericum sinaicum. Z. Naturforsch. C J. Biosci. 2009, 64, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Tuzlacı, E.; Aymaz, P.E. Turkish Folk Medicinal Plants, Part IV: Gönen (Balikesir). Fitoterapia 2001, 72, 323–343. [Google Scholar] [CrossRef]
- Heydon, P.A.; Miller, G.C.; Oldham, M.J. Hairy St. John’s-Wort (Hypericum hirsutum L.) in the Toronto Area, New to North America. Can. Field-Nat. 2011, 125, 248–251. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum Species in China: A Comprehensive Review on Ethnobotany, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef]
- Hypericum Rochelii Griseb & Schenk|Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:433809-1 (accessed on 7 August 2025).
- Hypericum Rochelii (Hypericum Rochelii, St. John’s Wort, Tutsan)—Uses, Benefits & Common Names. Available online: https://www.selinawamucii.com/plants/hypericaceae/hypericum-rochelii/ (accessed on 16 May 2025).
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas Aeruginosa Biofilms in Cystic Fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Chitlapilly Dass, S.; Bosilevac, J.M.; Weinroth, M.; Elowsky, C.G.; Zhou, Y.; Anandappa, A.; Wang, R. Impact of Mixed Biofilm Formation with Environmental Microorganisms on E. Coli O157:H7 Survival Against Sanitization. Npj Sci. Food 2020, 4, 16. [Google Scholar] [CrossRef]
- Bekir, K. Molecular Detection of Adhesins Genes and Biofilm Formation in Methicillin Resistant Staphylococcus Aureus. Afr. J. Microbiol. Res. 2012, 6, 4908–4917. [Google Scholar] [CrossRef]
- Gerke, C.; Kraft, A.; Süssmuth, R.; Schweitzer, O.; Götz, F. Characterization of the N-Acetylglucosaminyltransferase Activity Involved in the Biosynthesis of the Staphylococcus Epidermidis Polysaccharide Intercellular Adhesin. J. Biol. Chem. 1998, 273, 18586–18593. [Google Scholar] [CrossRef]
- Dimitrova, L.; Zaharieva, M.M.; Tserovska, L.; Popova, M.; Bankova, V.; Najdenski, H. Inhibition of the MRSA Biofilm Formation and Skin Antineoplastic Activity of Ethyl Acetate Roots and Aerial Parts Extracts from Geum urbanum L. Antibiotics 2025, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, P.-W.; Wan, S.; Yao, Y.; Song, C.-R.; Song, P.-P.; Xu, G.-B.; Hu, Z.-Q.; Zeng, Z.; Wang, C.; et al. Ginkgo Biloba Exocarp Extracts Inhibit S. Aureus and MRSA by Disrupting Biofilms and Affecting Gene Expression. J. Ethnopharmacol. 2021, 271, 113895. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.A.; Al-Mathkhury, H.J.F. Estimation the Expression of Glucose-Dependent Biofilm-Encoding icaA and icaD Genes in Methicillin Resistant Staphylococcus aureus Isolates. Iraqi J. Biotechnol. 2025, 24, 270–282. [Google Scholar]
- Dorcheh, F.A.; Balmeh, N.; Sanjari, S. In-Silico Investigation of Antibacterial Herbal Compounds in Order to Find New Antibiotic Against Staphylococcus aureus and Its Resistant Subtypes. Inform. Med. Unlocked 2022, 28, 100843. [Google Scholar] [CrossRef]
- Süntar, I.; Oyardı, O.; Akkol, E.K.; Ozçelik, B. Antimicrobial Effect of the Extracts from Hypericum Perforatum Against Oral Bacteria and Biofilm Formation. Pharm. Biol. 2016, 54, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Bohlouli, S.; Maleki Dizaj, S.; Shahi, S.; Memar, M.Y.; Salatin, S. The Antimicrobial and Anti-Biofilm Effects of Hypericum perforatum Oil on Common Pathogens of Periodontitis: An In Vitro Study. Clin. Pract. 2022, 12, 1009–1019. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays Related to Cancer Drug Discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Tugba Artun, F.; Karagoz, A.; Ozcan, G.; Melikoglu, G.; Anil, S.; Kultur, S.; Sutlupinar, N. In Vitro Anticancer and Cytotoxic Activities of Some Plant Extracts on HeLa and Vero Cell Lines. J. BUON Off. J. Balk. Union Oncol. 2016, 21, 720–725. [Google Scholar]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic Activity of Some Lichen Extracts on Murine and Human Cancer Cell Lines. Phytomed. Int. J. Phytother. Phytopharm. 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Matić, I.Z.; Ergün, S.; Đorđić Crnogorac, M.; Misir, S.; Aliyazicioğlu, Y.; Damjanović, A.; Džudžević-Čančar, H.; Stanojković, T.; Konanç, K.; Petrović, N. Cytotoxic Activities of Hypericum perforatum L. Extracts Against 2D and 3D Cancer Cell Models. Cytotechnology 2021, 73, 373–389. [Google Scholar] [CrossRef]
- Quiney, C.; Billard, C.; Faussat, A.M.; Salanoubat, C.; Ensaf, A.; Naït-Si, Y.; Fourneron, J.D.; Kolb, J.-P. Pro-Apoptotic Properties of Hyperforin in Leukemic Cells from Patients with B-Cell Chronic Lymphocytic Leukemia. Leukemia 2006, 20, 491–497. [Google Scholar] [CrossRef]
- Sell, T.S.; Belkacemi, T.; Flockerzi, V.; Beck, A. Protonophore Properties of Hyperforin Are Essential for Its Pharmacological Activity. Sci. Rep. 2014, 4, 7500. [Google Scholar] [CrossRef]
- Menegazzi, M.; Masiello, P.; Novelli, M. Anti-Tumor Activity of Hypericum perforatum L. and Hyperforin Through Modulation of Inflammatory Signaling, ROS Generation and Proton Dynamics. Antioxidants 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Çöven, F.O.; Gür, S.; Uyar, E.; Alsakini, K.A.M.H.; Karabey, F.; Çöven, F.; Çaliş, İ.; Nalbantsoy, A. Assessment of In-Vitro Cytotoxicity and In-Ovo Virucidal Antiviral Efficacy of Various Plant Extracts and Bioactive Molecules. Kafkas Univ. Vet. Fakültesi Derg. 2024, 30, 171–178. [Google Scholar] [CrossRef]
- Yildirim, H.; Daşkan, B.E. Cytotoxic and Anti-Metastatic Effects of Hypericum perforatum Olive Oil Extract in Colorectal Cancer Cells and Human Bone Marrow Derived Stem Cells. Nat. Prod. Res. 2024, 38, 4166–4174. [Google Scholar] [CrossRef]
- Fernandes, F.; Barroso, M.F.; De Simone, A.; Emriková, E.; Dias-Teixeira, M.; Pereira, J.P.; Chlebek, J.; Fernandes, V.C.; Rodrigues, F.; Andrisano, V.; et al. Multi-Target Neuroprotective Effects of Herbal Medicines for Alzheimer’s Disease. J. Ethnopharmacol. 2022, 290, 115107. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, I.; Mohajeri, M.; Borisov, A.; Hosseini, M.-S. Antiproliferative Effects of Free and Encapsulated Hypericum perforatum L. Extract and Its Potential Interaction with Doxorubicin for Esophageal Squamous Cell Carcinoma. J. Pharmacopunct. 2019, 22, 102–108. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Ghasemzadeh kolagar, H.; Alizadeh-Navaei, R. Cytotoxic and Apoptogenic Effect of Hypericin, the Bioactive Component of Hypericum Perforatum on the MCF-7 Human Breast Cancer Cell Line. Cancer Cell Int. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Jendželovská, Z.; Jendželovský, R.; Kuchárová, B.; Fedoročko, P. Hypericin in the Light and in the Dark: Two Sides of the Same Coin. Front. Plant Sci. 2016, 7, 560. [Google Scholar] [CrossRef]
- Naderi, M.; Rahmani Cherati, M.; Mohammadian, A.; Baghery Bidhendy, M.; Ghiasvand, S.; Zare Marzouni, H.; Aryan, H.; Jangholi, E.; Javidi, M.A. Hypericin Induces Apoptosis in AGS Cell Line with No Significant Effect on Normal Cells. Iran. J. Pharm. Res. IJPR 2020, 19, 349–357. [Google Scholar] [CrossRef]
- Taşkın, T.; Ermanoğlu, M.; Rayaman, E.; Taşkın, D.; Atici, C.E.; Acar, A.G.; Tatar, E.; Gürdal, B.; Doğan, A. Chemical Composition, In Vitro Bioactivity Evaluation, In Silico Molecular Docking and ADMET Study of Hypericum scabrum and Hypericum triquetrifolium. Braz. Arch. Biol. Technol. 2024, 67, e24240063. [Google Scholar] [CrossRef]
- Al-Anee, R.S.; Al-Ani, E.H.; Mousa, Z.S. Cytotoxic Activity of Hypericum Triquetrifolium Turra Methanolic Extract Against Cancer Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 3599–3603. [Google Scholar] [CrossRef]
- Hu, H.; Yang, Y.; Aissa, A.; Tekin, V.; Li, J.; Panda, S.K.; Huang, H.; Luyten, W. Ethnobotanical Study of Hakka Traditional Medicine in Ganzhou, China and Their Antibacterial, Antifungal, and Cytotoxic Assessments. BMC Complement. Med. Ther. 2022, 22, 244. [Google Scholar] [CrossRef]
- Balikci, N.; Sarimahmut, M.; Ari, F.; Aztopal, N.; Zafer Özel, M.; Ulukaya, E.; Celikler, S. Toxicity Assessment of Hypericum olympicum Subsp. Olympicum L. on Human Lymphocytes and Breast Cancer Cell Lines. J. Appl. Biomed. 2020, 18, 18–25. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Zhai, Y.; Cao, X.; Gao, S.; Huang, M.; Guo, Y.; Xie, C.; Zhou, H. In Vitro Screening for Compounds from Hypericum Longistylum with Anti-Pulmonary Fibrosis Activity. Bioorg. Med. Chem. Lett. 2019, 29, 126695. [Google Scholar] [CrossRef]
- da Conceição, A.O.; von Poser, G.L.; Barbeau, B.; Lafond, J. Hypericum Caprifoliatum and Hypericum Connatum Affect Human Trophoblast-like Cells Differentiation and Ca2+ Influx. Asian Pac. J. Trop. Biomed. 2014, 4, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Khadivzadeh, T.; Emami, A.; Moosavi, N.S.; Tafaghodi, M.; Behnam, H.R. The Effect of Hypericum Perforatum on the Wound Healing and Scar of Cesarean. J. Altern. Complement. Med. 2010, 16, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.D.; Vu, V.T.; Selvaraj, B.; Tuyen, N.M.; Khoi, N.M.; Thuan, N.D.; Thuong, P.T.; Lee, J.W. Chemical Constituents and Neuroprotective Activity of Hypericum Hookerianum. Nat. Prod. Commun. 2023, 18, 1934578X231164818. [Google Scholar] [CrossRef]
- Kraus, B.; Wolff, H.; Elstner, E.F.; Heilmann, J. Hyperforin Is a Modulator of Inducible Nitric Oxide Synthase and Phagocytosis in Microglia and Macrophages. Naunyn. Schmiedebergs Arch. Pharmacol. 2010, 381, 541–553. [Google Scholar] [CrossRef]
- Benedí, J.; Arroyo, R.; Romero, C.; Martín-Aragón, S.; Villar, A.M. Antioxidant Properties and Protective Effects of a Standardized Extract of Hypericum perforatum on Hydrogen Peroxide-Induced Oxidative Damage in PC12 Cells. Life Sci. 2004, 75, 1263–1276. [Google Scholar] [CrossRef]
- Di Paola, R.; Mazzon, E.; Muià, C.; Crisafulli, C.; Genovese, T.; Di Bella, P.; Esposito, E.; Menegazzi, M.; Meli, R.; Suzuki, H.; et al. Protective Effect of Hypericum Perforatum in Zymosan-Induced Multiple Organ Dysfunction Syndrome: Relationship to Its Inhibitory Effect on Nitric Oxide Production and Its Peroxynitrite Scavenging Activity. Nitric Oxide Biol. Chem. 2007, 16, 118–130. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Chen, C.; Huang, J.; Li, X.-N.; Liu, J.; Zhu, H.; Tong, Q.; Zhang, J.; Luo, Z.; et al. Tricyclic Polyprenylated Acylphloroglucinols from St John’s Wort, Hypericum Perforatum. J. Nat. Prod. 2017, 80, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Tong, Q.; Chen, X.; Yang, J.; Liu, J.; Sun, B.; Wang, J.; Yao, G.; Luo, Z.; et al. Hyperisampsins H-M, Cytotoxic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum sampsonii. Sci. Rep. 2015, 5, 14772. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Yang, J.; Li, D.; Zhang, J.; Guo, Y.; Wang, J.; Luo, Z.; Xue, Y.; Zhang, Y. Hyperhexanone A, a Crucial Intermediate from Bicyclo [3.3.1]- to Cyclohexanone Monocyclic-Polycyclic Polyprenylated Acylphloroglucinols. Tetrahedron 2016, 72, 4655–4659. [Google Scholar] [CrossRef]
- Novelli, M.; Beffy, P.; Menegazzi, M.; De Tata, V.; Martino, L.; Sgarbossa, A.; Porozov, S.; Pippa, A.; Masini, M.; Marchetti, P.; et al. St. John’s Wort Extract and Hyperforin Protect Rat and Human Pancreatic Islets against Cytokine Toxicity. Acta Diabetol. 2014, 51, 113–121. [Google Scholar] [CrossRef]
- Saygıdeğer Demir, B.; Sezan, A.; Derinöz, E.; Nas, E.; Özerten, M.; Mahmoudi, G.; Saygideger, Y. Antitumoral Properties of a Pincer-Type Isonicotinohydrazone-Hg(II) Complex. Eur. J. Biol. 2021, 80, 129–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, J.; Yang, J. Genistein-Triggered Anticancer Activity Against Liver Cancer Cell Line HepG2 Involves ROS Generation, Mitochondrial Apoptosis, G2/M Cell Cycle Arrest and Inhibition of Cell Migrationand Inhibition of Cell Migration. Arch. Med. Sci. 2019, 15, 1001–1009. [Google Scholar] [CrossRef]
- Lai, X.; Nie, Y.; Huang, H.; Li, Y.; Cao, C.; Yang, H.; Shen, B.; Feng, Z. NIMA-related Kinase 2 Regulates Hepatocellular Carcinoma Cell Growth and Proliferation. Oncol. Lett. 2017, 13, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.A.; Pham, T.A.V. Effects of Platelet-Rich Plasma on Human Gingival Fibroblast Proliferation and Migration In Vitro. J. Appl. Oral Sci. Rev. FOB 2018, 26, e20180077. [Google Scholar] [CrossRef] [PubMed]
- Ar, N.; Maiti, S.; Kumar, S.; P, S.; Kritaniya, D.; Gupta, S.; Saxena, A.; Kumar, N. Isolation, Proliferation, Characterization and In Vivo Osteogenic Potential of Bone-Marrow Derived Mesenchymal Stem Cells (rBMSC) in Rabbit Model. Indian J. Exp. Biol. 2017, 55, 79–87. [Google Scholar]
- Kalinova, R.G.; Dimitrov, I.V.; Ilieva, Y.; Iliev, D.B.; Miloshev, G.A.; Staneva, D.N.; Zaharieva, M.M.; Nesheva, A.; Staneva, G.; Ivanova, D.I.; et al. Antineoplastic Activity of Podophyllotoxin and Juniper Extracts Encapsulated in MPEG-b-PLA Diblock Copolymer Micelles in Cutaneous Squamous Carcinoma Cells. Int. J. Mol. Sci. 2025, 26, 5167. [Google Scholar] [CrossRef]
- Schempp, C.M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kiss, J.; Termeer, C.C.; Gilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J.P.; et al. Inhibition of Tumour Cell Growth by Hyperforin, a Novel Anticancer Drug from St. John’s Wort That Acts by Induction of Apoptosis. Oncogene 2002, 21, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Codogno, P.; Mehrpour, M.; Proikas-Cezanne, T. Canonical and Non-Canonical Autophagy: Variations on a Common Theme of Self-Eating? Nat. Rev. Mol. Cell Biol. 2011, 13, 7–12. [Google Scholar] [CrossRef]
- Autophagy Signaling|Cell Signaling Technology. Available online: https://www.cellsignal.com/pathways/autophagy-signaling-pathway?srsltid=AfmBOorXMUy8UXC6mXoyX_O4W1Mkkdgnbex5iTur_o0HBK1uO6g1RgkP (accessed on 30 July 2025).
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. LC3, GABARAP and GATE16 Localize to Autophagosomal Membrane Depending on Form-II Formation. J. Cell Sci. 2004, 117, 2805–2812. [Google Scholar] [CrossRef]
- Nicolussi, S.; Drewe, J.; Butterweck, V.; Meyer zu Schwabedissen, H.E. Clinical Relevance of St. John’s Wort Drug Interactions Revisited. Br. J. Pharmacol. 2020, 177, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Tambur, Z.; Miljković-Selimović, B.; Opačić, D.; Aleksic, E.; Ivančajić, L.; Jovičić, B.; Vuković, B. Inhibitory Effects of Different Medicinal Plants on Growth of Some Oral Microbiome Member. Med. Weter. 2020, 76, 476–479. [Google Scholar] [CrossRef]
- Peeva-Naumovska, V.; Panovski, N.; Grdanovska, T.; Fredro-Kumbaradzi, E. Formulations of St. John’s Wort Oil Ointment and Evaluation of Its Antibacterial Effect. In Proceedings of the first CMAPSEEC, Arandjelovac, Serbia, 29 May–3 June 2000. [Google Scholar]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Davoodi, S.; Kheiri, F.; Rahimian, Y. Effect of poultry feed supplemented with Hypericum perforatum extract and Virginiamycine on growth performance, some immune responses and intestinal microbial population of broilers. Russ. J. Agric. Socio-Econ. Sci. 2014, 36, 27–33. [Google Scholar] [CrossRef]
- İlhan, Z.; Zengin, M.; Bacaksız, O.K.; Demir, E.; Ekin, İ.H.; Azman, M.A. Hypericum perforatum L. (St. John’s Wort) in Broilers Diet Improve Growth Performance, Intestinal Microflora and Immunity. Poult. Sci. 2024, 103, 104419. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Tang, Z.; Shi, X.; Song, Z.; Cao, F.; Wei, P.; Li, M.; Li, X.; Jiang, D.; et al. Improvements in Estrogen Deficiency-Induced Hypercholesterolemia by Hypericum perforatum L. Extract Are Associated with Gut Microbiota and Related Metabolites in Ovariectomized (OVX) Rats. Biomed. Pharmacother. 2021, 135, 111131. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry Polyphenols Metabolism and Impact on Human Gut Microbiota and Health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-Based Prebiotics and Synbiotics: Potential for Cancer Chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization and Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Aroutcheva, A.; Simoes, J.A.; Shott, S.; Faro, S. The Inhibitory Effect of Clindamycin on Lactobacillus in Vitro. Infect. Dis. Obstet. Gynecol. 2001, 9, 239–244. [Google Scholar] [CrossRef]
- Aggarwal, H.; Pathak, P.; Singh, V.; Kumar, Y.; Shankar, M.; Das, B.; Jagavelu, K.; Dikshit, M. Vancomycin-Induced Modulation of Gram-Positive Gut Bacteria and Metabolites Remediates Insulin Resistance in iNOS Knockout Mice. Front. Cell. Infect. Microbiol. 2022, 11, 795333. [Google Scholar] [CrossRef]
- Fooks, L.J.; Gibson, G.R. In Vitro Investigations of the Effect of Probiotics and Prebiotics on Selected Human Intestinal Pathogens. FEMS Microbiol. Ecol. 2002, 39, 67–75. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Ścibisz, I.; Kowalczyk, M.; Pawelec, S.; Stochmal, A.; Szleszyński, B. The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity. Appl. Sci. 2021, 11, 3898. [Google Scholar] [CrossRef]
- Dobreva, L.; Borisova, D.; Paunova-Krasteva, T.; Dimitrova, P.D.; Hubenov, V.; Atanasova, N.; Ivanov, I.; Danova, S. From Traditional Dairy Product “Katak” to Beneficial Lactiplantibacillus Plantarum Strains. Microorganisms 2023, 11, 2847. [Google Scholar] [CrossRef]
- Time Kill Assays for Streptococcus Agalactiae and Synergy Testing. Available online: https://www.protocols.io/view/time-kill-assays-for-em-streptococcus-agalactiae-e-4r3l29j9pv1y/v1 (accessed on 12 May 2025).
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Hep G2 [HEPG2]—HB-8065|ATCC. Available online: https://www.atcc.org/products/hb-8065 (accessed on 15 April 2025).
- Slany, A.; Haudek, V.J.; Zwickl, H.; Gundacker, N.C.; Grusch, M.; Weiss, T.S.; Seir, K.; Rodgarkia-Dara, C.; Hellerbrand, C.; Gerner, C. Cell Characterization by Proteome Profiling Applied to Primary Hepatocytes and Hepatocyte Cell Lines Hep-G2 and Hep-3B. J. Proteome Res. 2010, 9, 6–21. [Google Scholar] [CrossRef]
- Qiu, G.-H.; Xie, X.; Xu, F.; Shi, X.; Wang, Y.; Deng, L. Distinctive Pharmacological Differences Between Liver Cancer Cell Lines HepG2 and Hep3B. Cytotechnology 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moscato, S.; Ronca, F.; Campani, D.; Danti, S. Poly(Vinyl Alcohol)/Gelatin Hydrogels Cultured with HepG2 Cells as a 3D Model of Hepatocellular Carcinoma: A Morphological Study. J. Funct. Biomater. 2015, 6, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. Clifton NJ 2015, 1250, 77–93. [Google Scholar] [CrossRef]
- Theis, T.; Skurray, R.A.; Brown, M.H. Identification of Suitable Internal Controls to Study Expression of a Staphylococcus Aureus Multidrug Resistance System by Quantitative Real-Time PCR. J. Microbiol. Methods 2007, 70, 355–362. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-10:2010; Biological Evaluation of Medical Devices. Part 10: Tests for Irritation and Skin Sensitization. ISO: Geneva, Switzerland, 2010; (withdrawn; superseded by ISO 10993-10:2021). Available online: https://www.iso.org/standard/40884.html (accessed on 31 July 2025).
- ISO 10993-2:2006; Biological Evaluation of Medical Devices. Part 2: Animal Welfare Requirements. ISO: Geneva, Switzerland, 2006; (withdrawn; superseded by ISO 10993-2:2022). Available online: https://www.iso.org/standard/36405.html (accessed on 31 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).