Xanthone-1,2,4-triazine and Acridone-1,2,4-triazine Conjugates: Synthesis and Anticancer Activity
Abstract
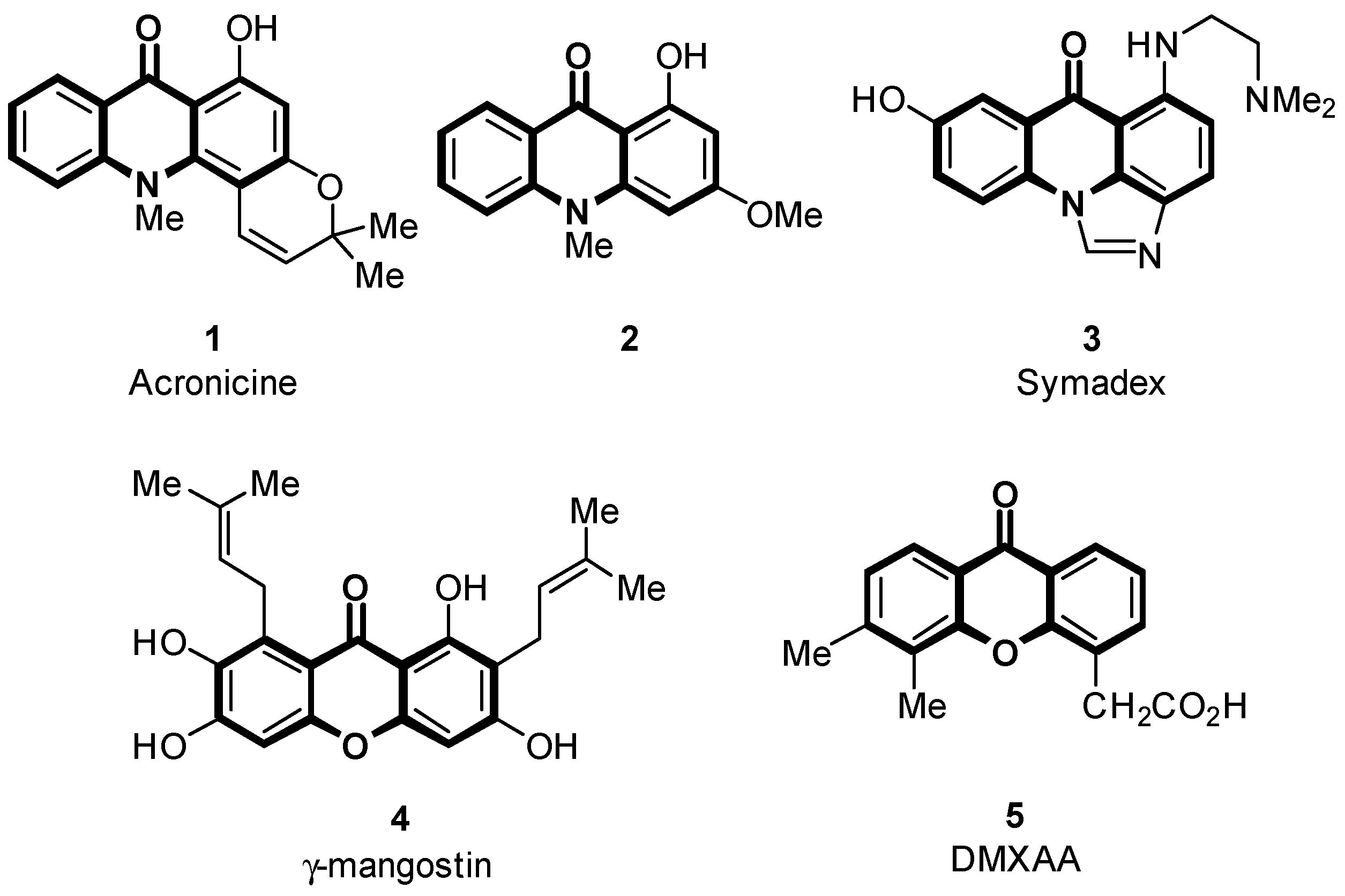
1. Introduction
2. Results and Discussion
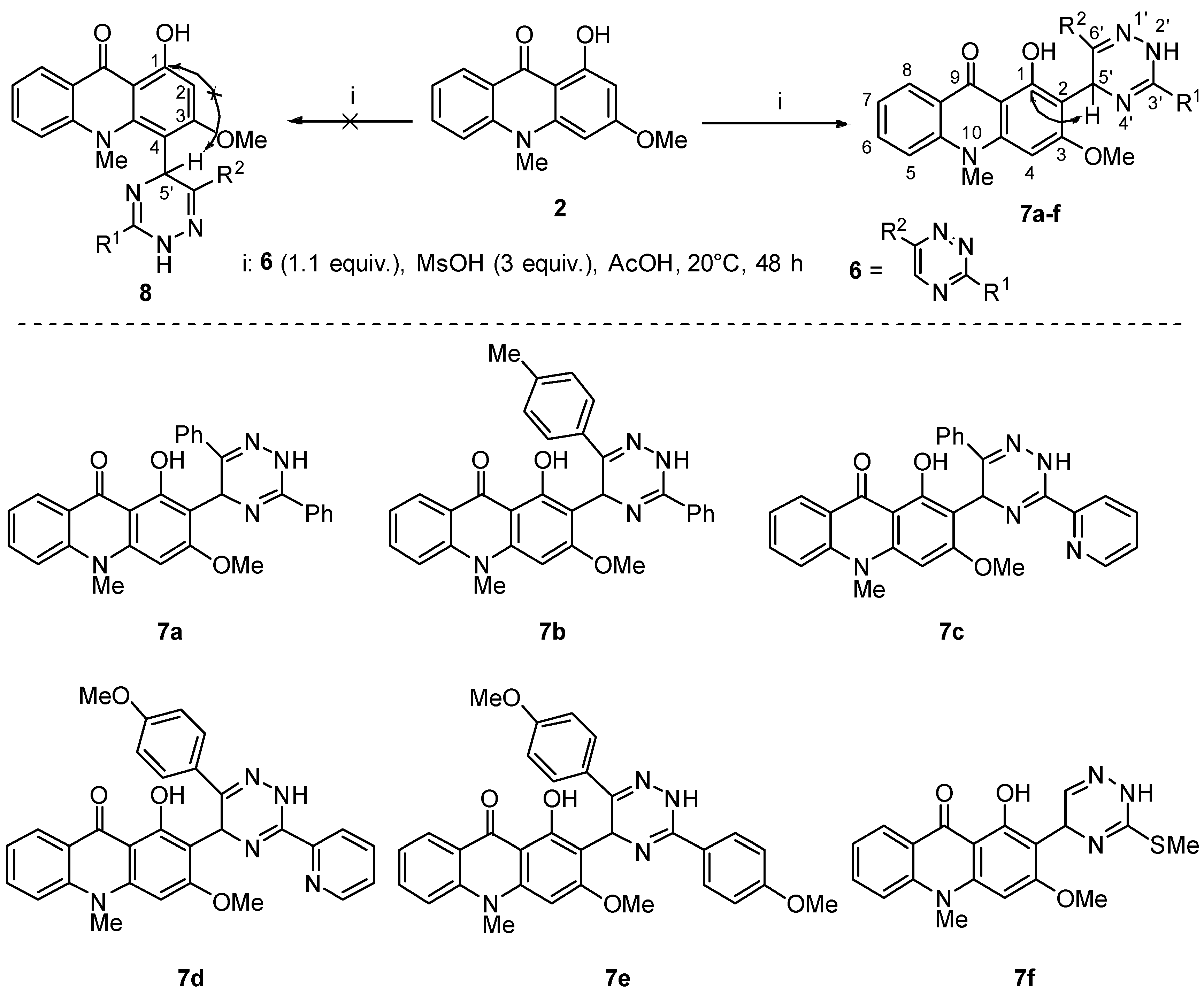
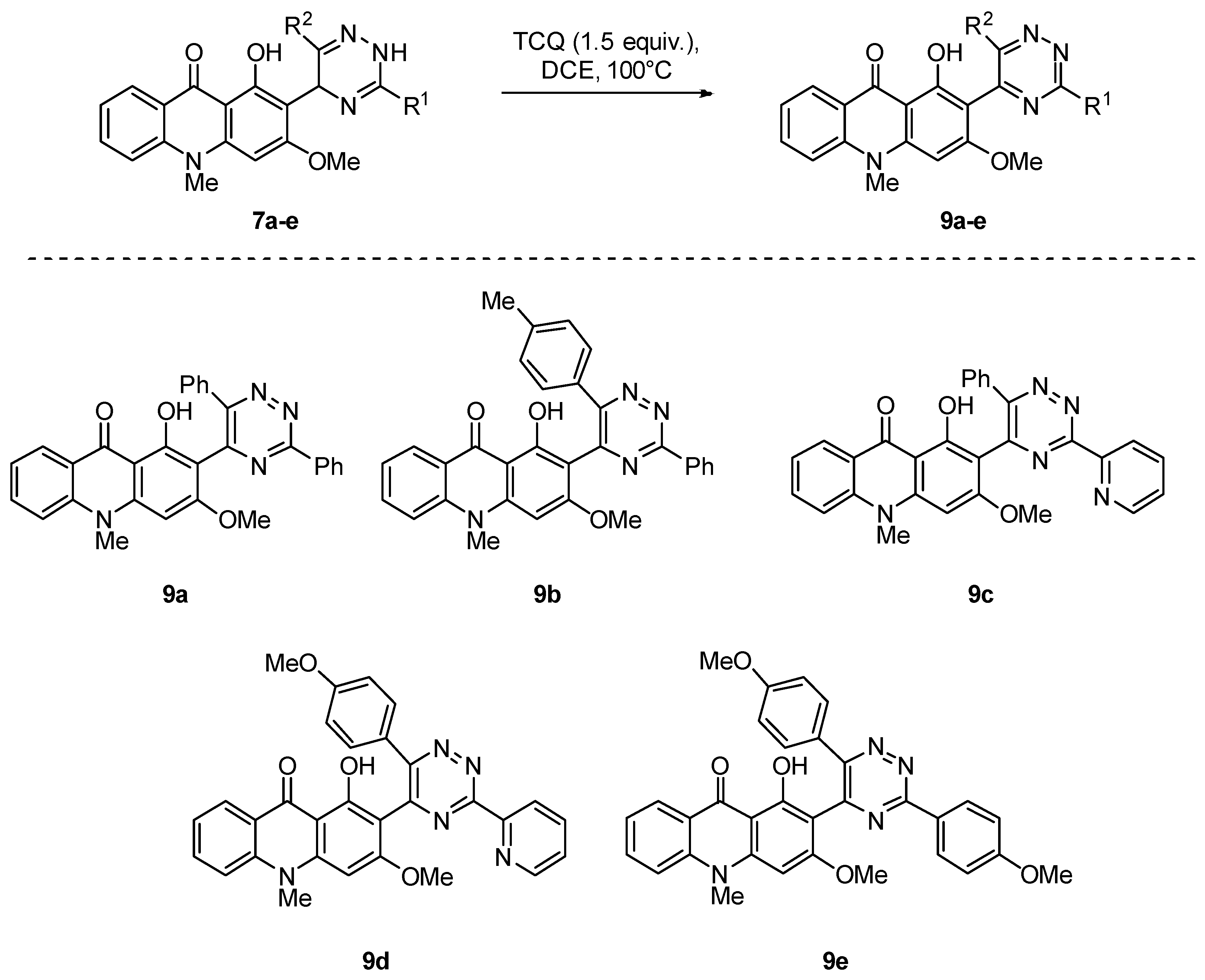
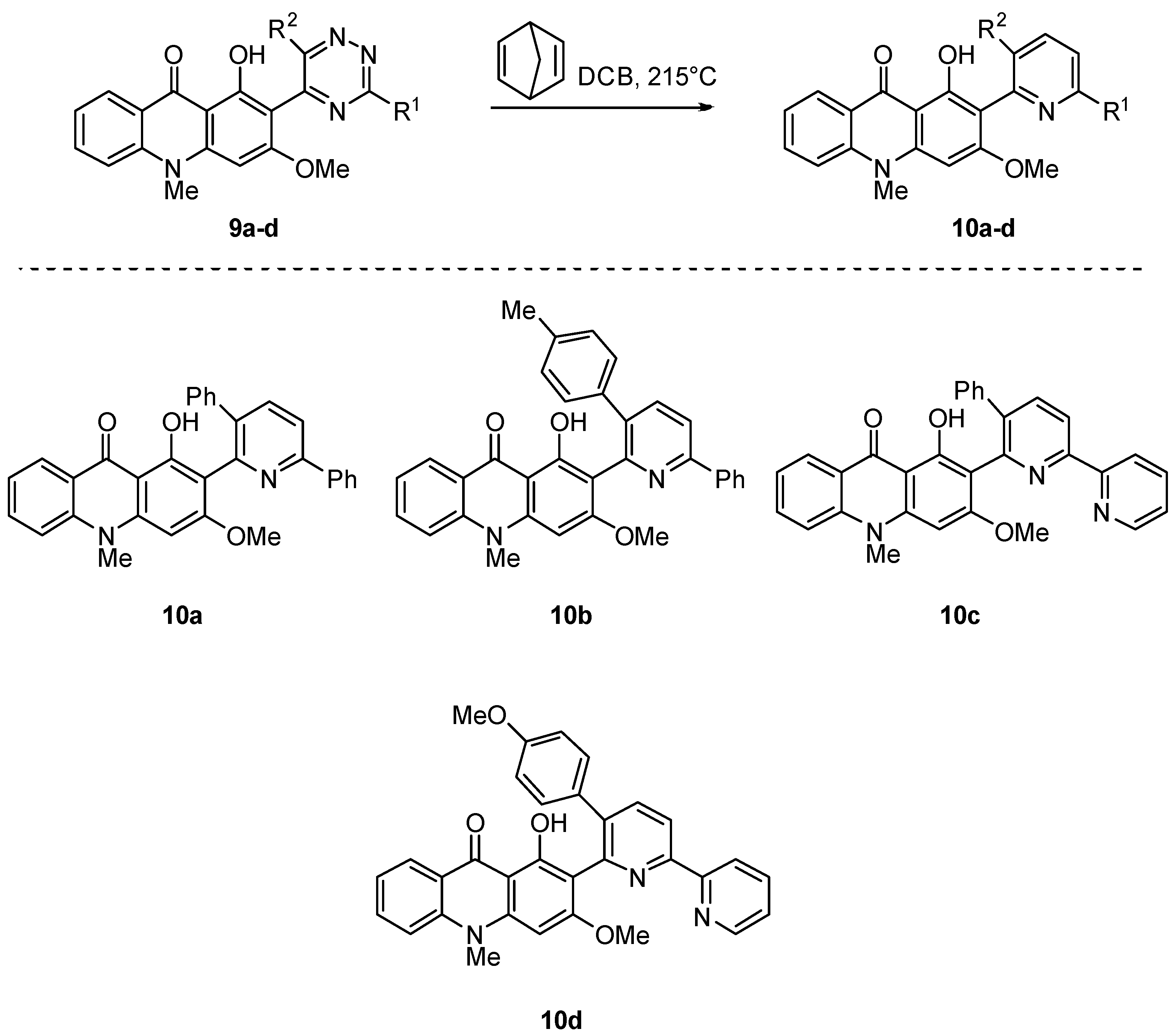
2.1. Synthesis of Novel Acridone and Xanthone Derivatives
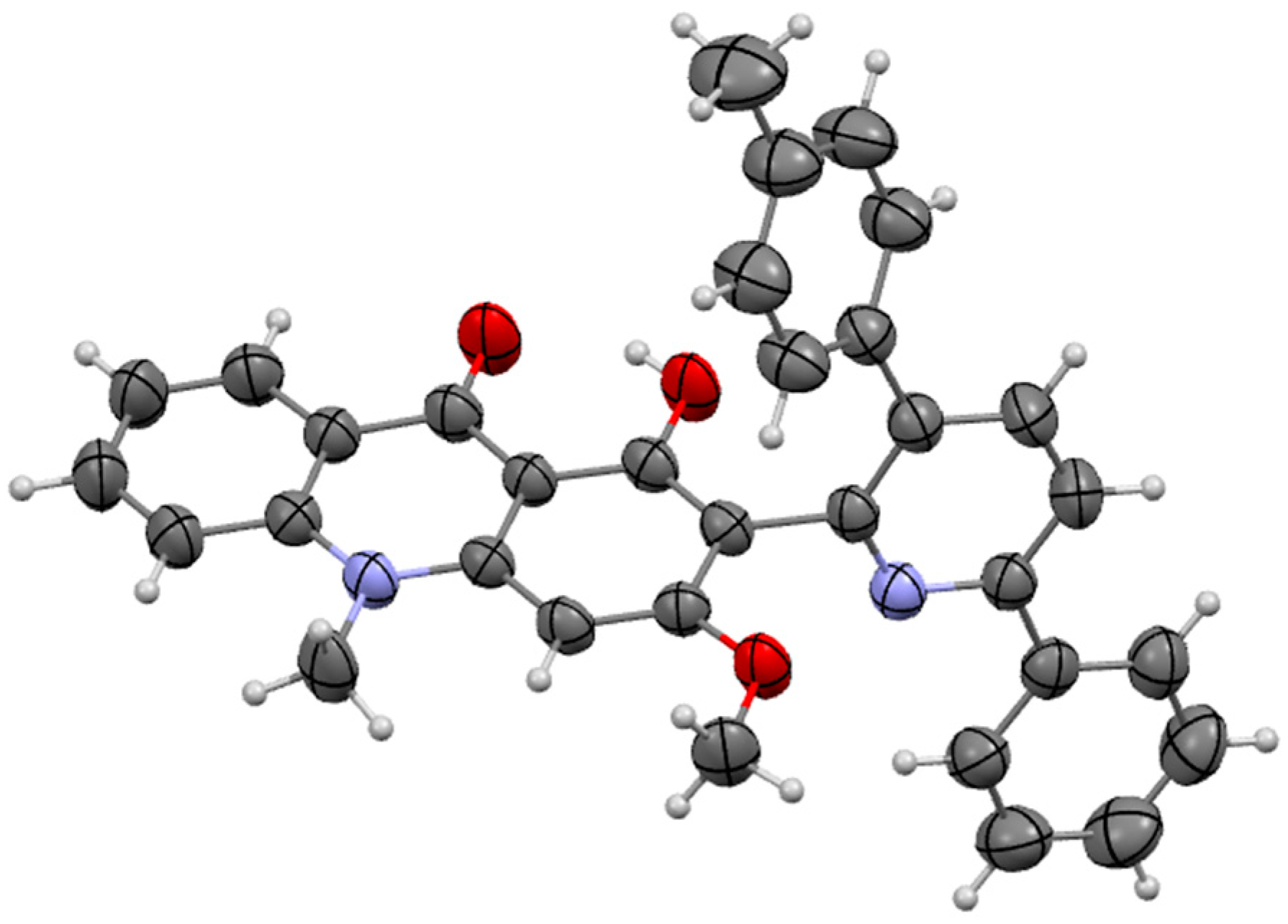
2.1.1. Synthesis of Acridone Derivatives
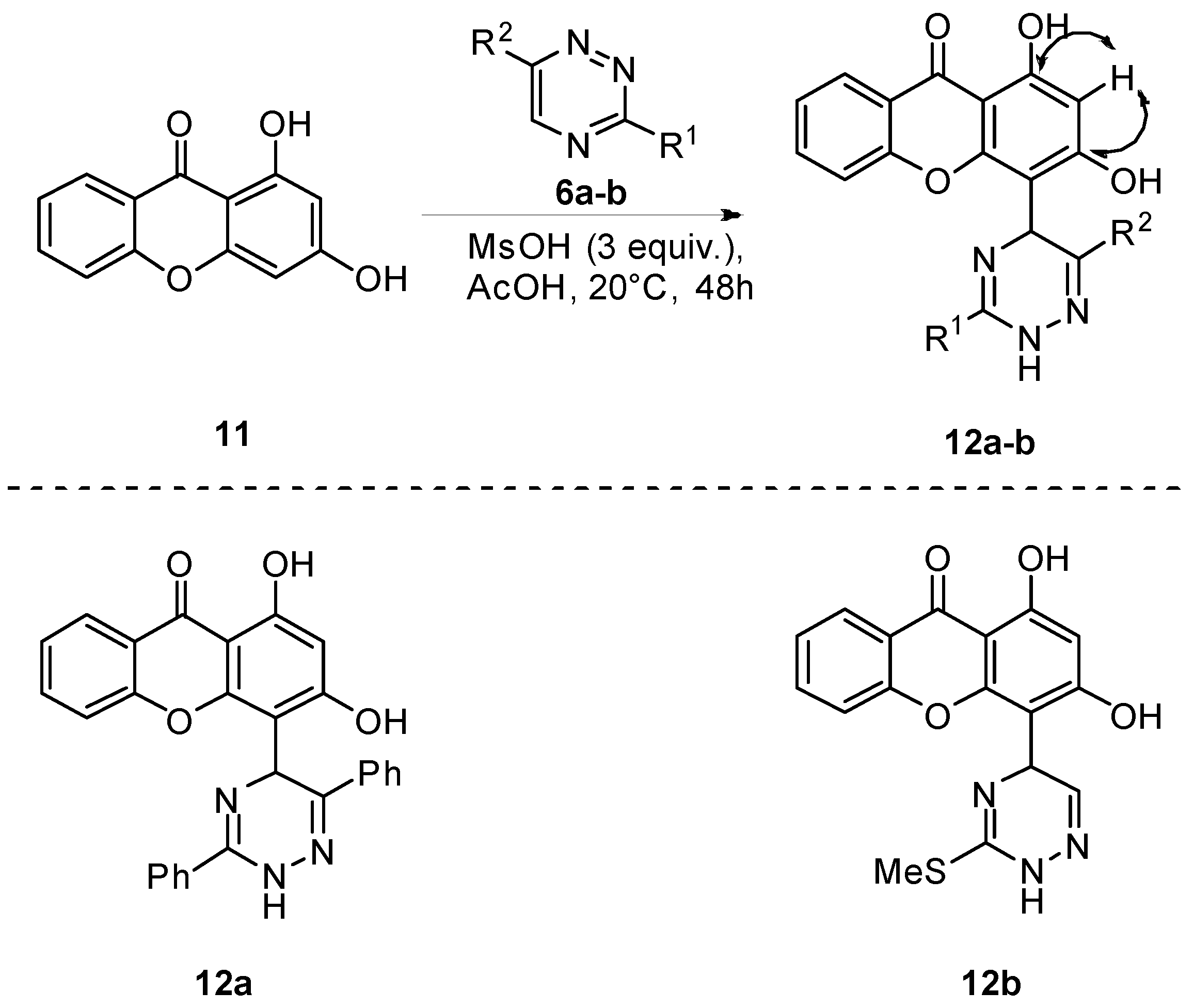
2.1.2. Synthesis of Xanthone Derivatives
2.2. Anticancer Activity of the Obtained Acridone and Xanthone Compounds
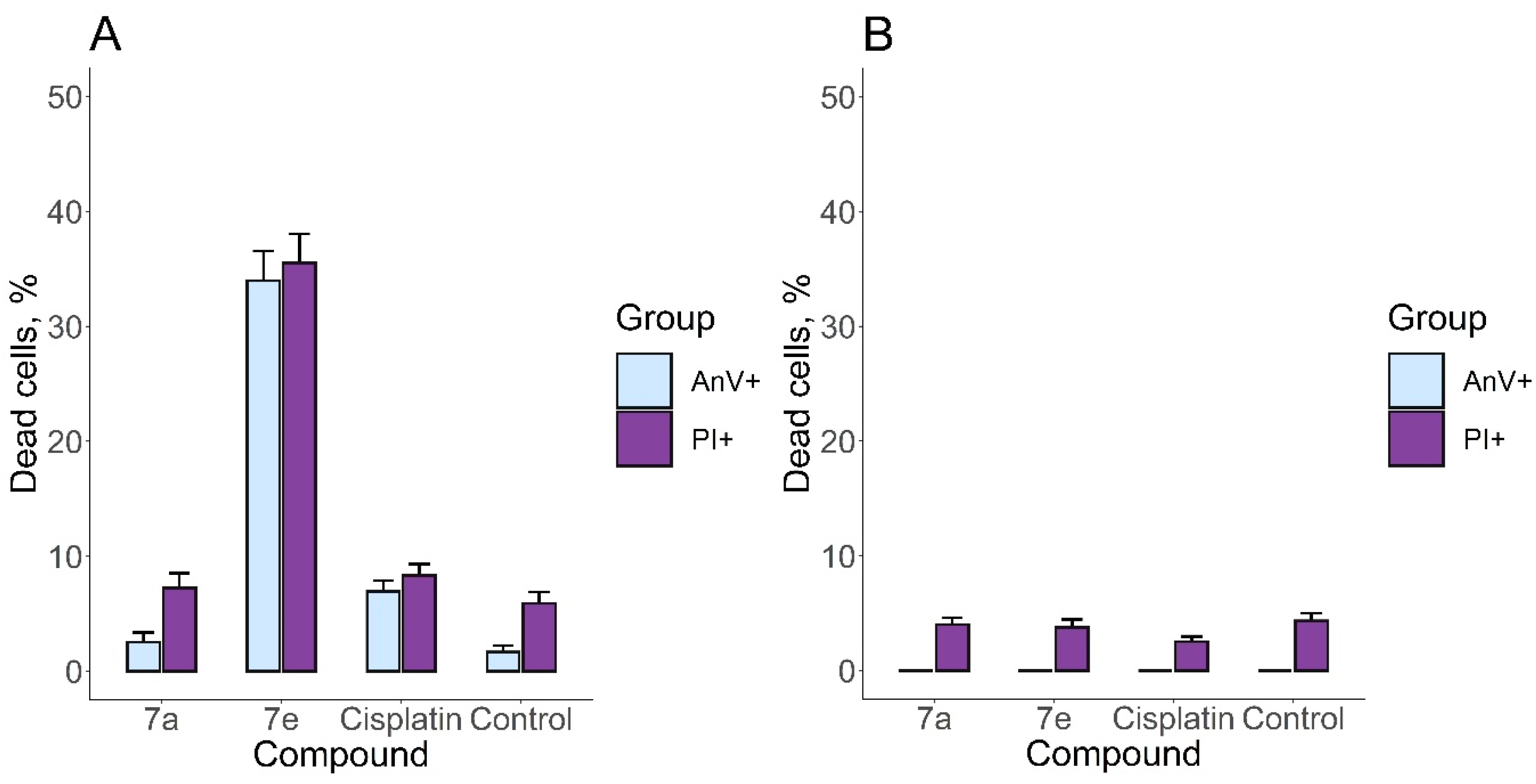
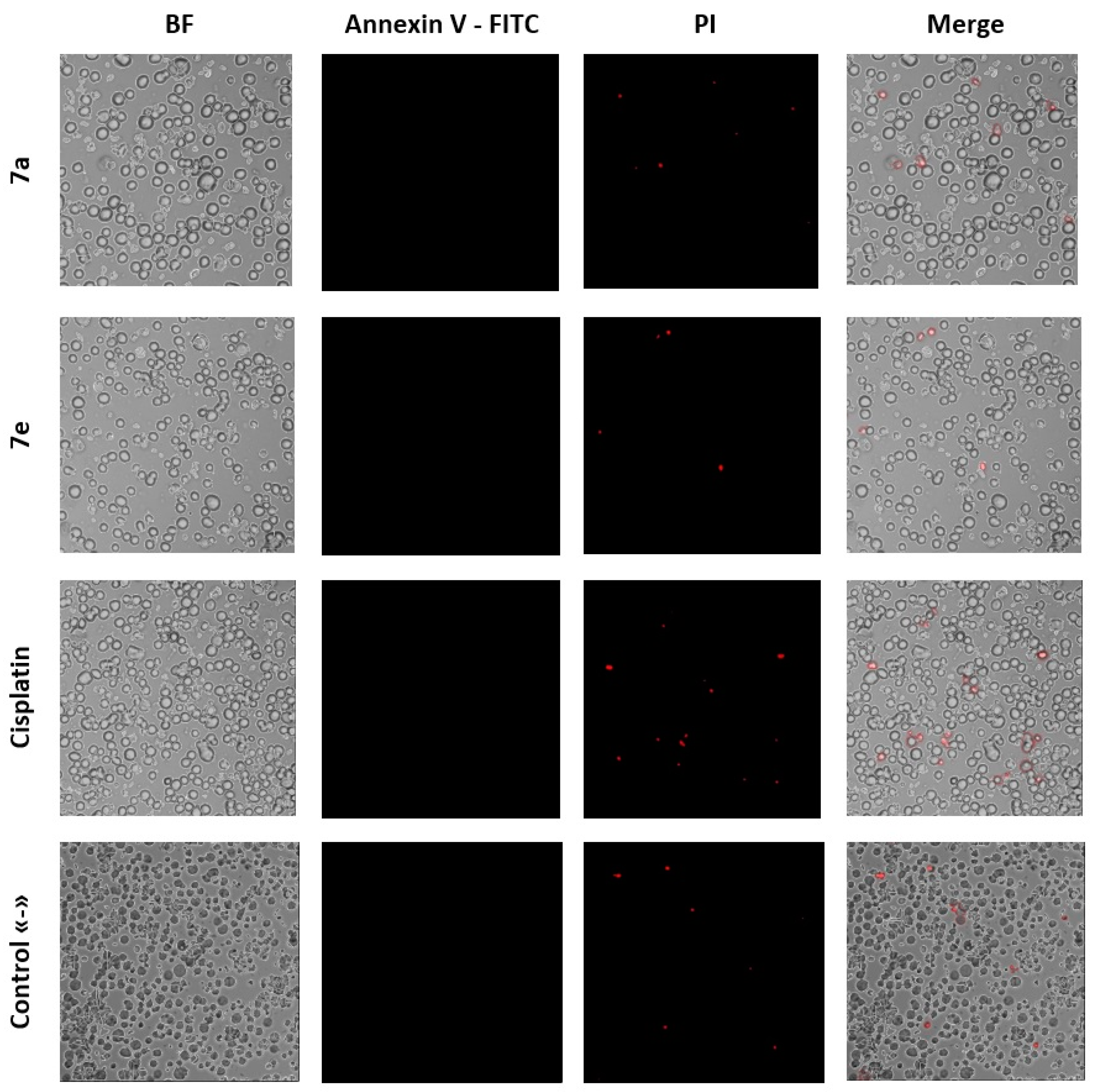
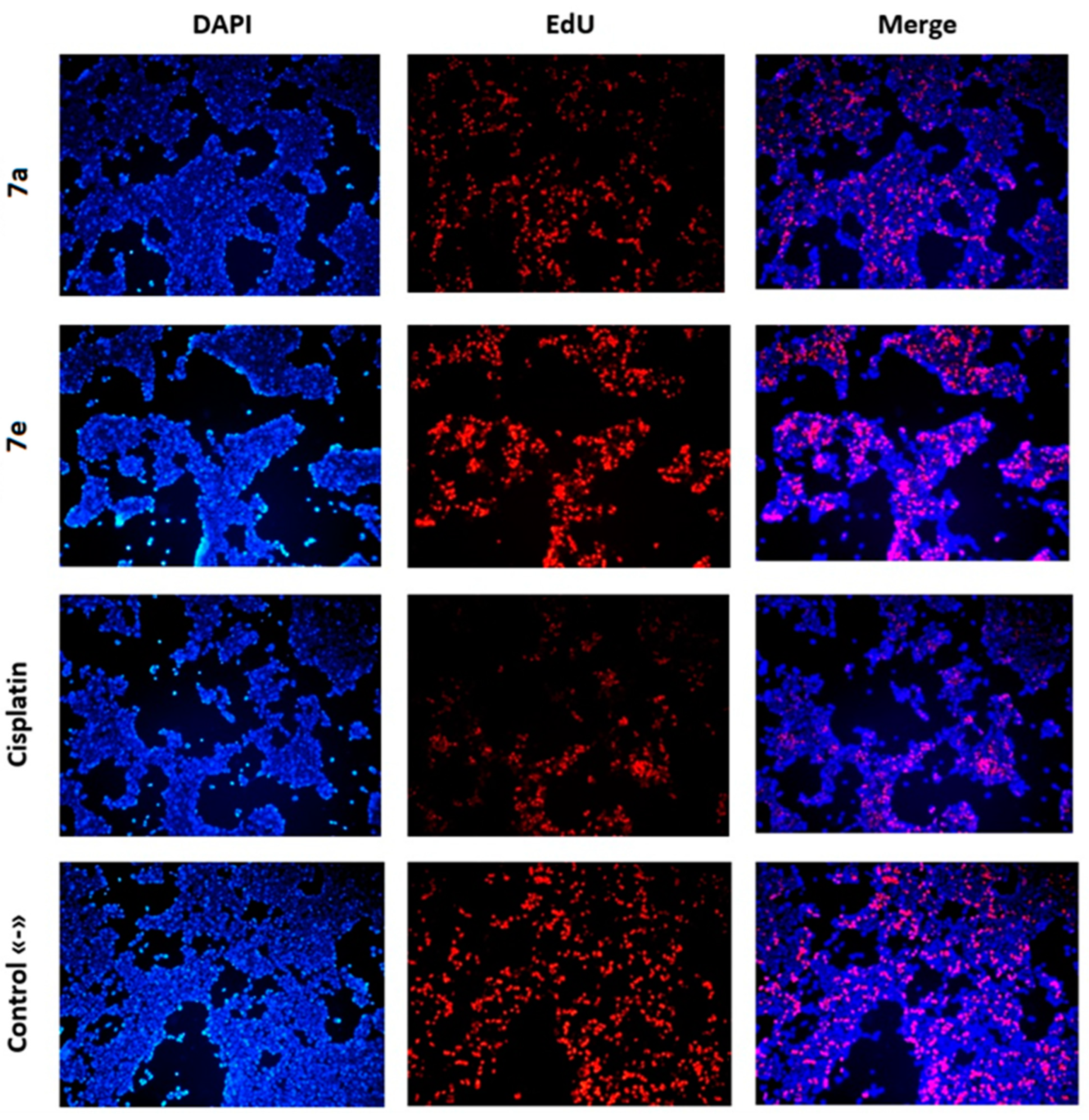
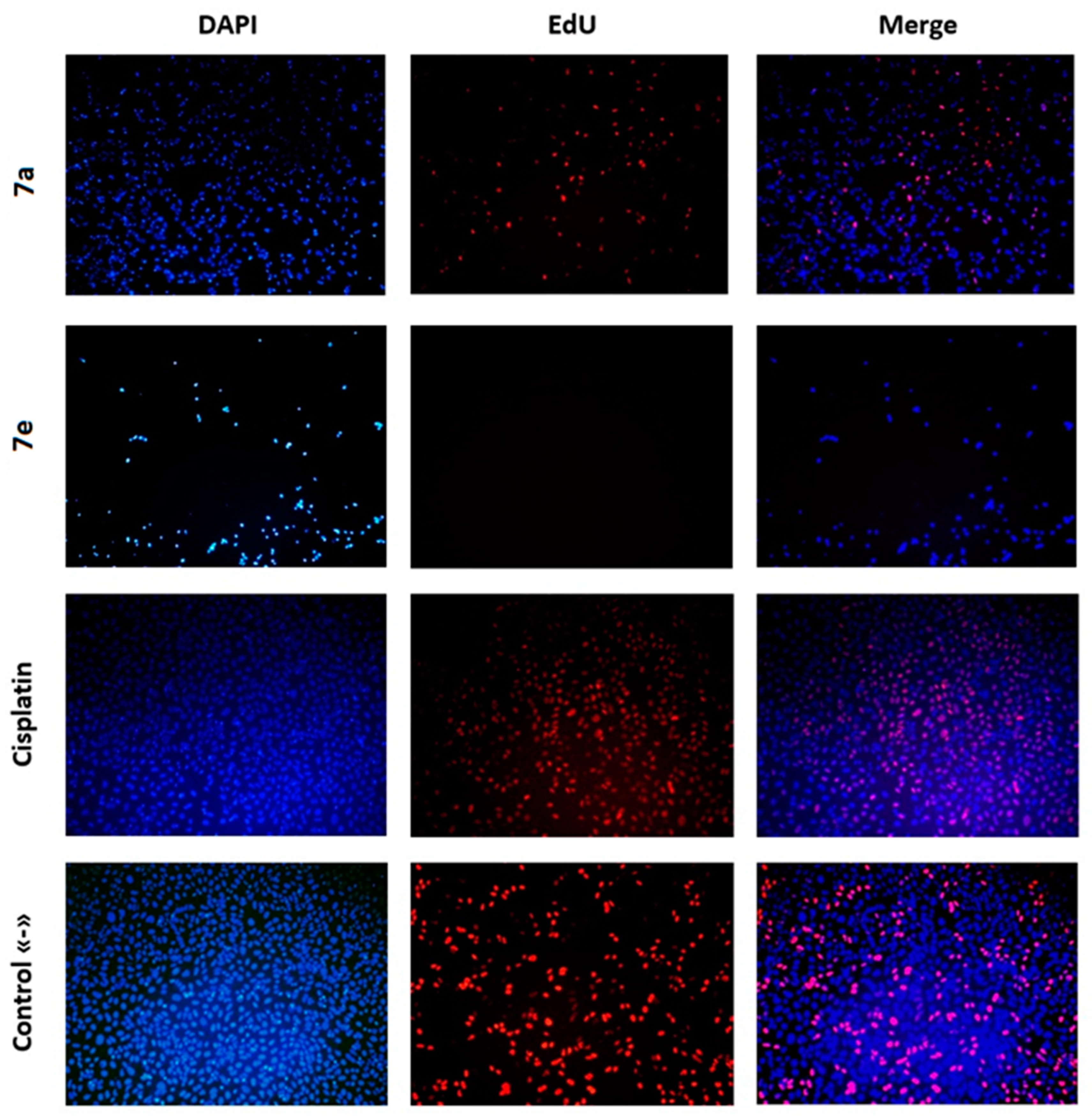
2.3. Investigation of the Anticancer Mechanism of Action of Acridone Compounds
3. Materials and Methods
3.1. Synthesis of Novel Acridone and Xanthone Compounds
3.1.1. Synthesis of Compounds 7a–7f
3.1.2. Synthesis of Compounds 9a–e
3.1.3. Synthesis of Compounds 10a–d
3.1.4. Synthesis of Compounds 12a and 12b
3.1.5. Synthesis of Compounds 14a and 14b
3.1.6. Synthesis of Compounds 15a and 15b
3.2. Anticancer Activity Evaluating
3.2.1. Cell Culture
3.2.2. Viability Assessment
3.2.3. Statistical Analysis
3.2.4. Apoptosis
3.2.5. DNA Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Rahman, A.F.M.M.; Liang, J.L.; Lee, S.H.; Son, J.K.; Jung, M.-J.; Kwon, Y.; Jahng, Y. 2,2-Dimethyl-2H-pyran-derived alkaloids I. Practical synthesis of acronycine and benzo[b]acronycine and their biological properties. Arch. Pharm. Res. 2008, 31, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Sittisombut, C.; Boutefnouchet, S.; Lallemand, M.-C.; Michel, S.; Koch, M.; Tillequin, F.; Mazinghien, R.; Lansiaux, A.; David-Cordonnier, M.-H.; et al. Synthesis, Antitumor Activity, and Mechanism of Action of Benzo[a]pyrano[3,2-h]acridin-7-one Analogues of Acronycine. J. Med. Chem. 2006, 49, 3383–3394. [Google Scholar] [CrossRef]
- Tian, W.; Yougnia, R.; Depauw, S.; Lansiaux, A.; David-Cordonnier, M.-H.; Pfeiffer, B.; Kraus-Berthier, L.; Léonce, S.; Pierré, A.; Dufat, H.; et al. Synthesis, Antitumor Activity, and Mechanism of Action of Benzo[b]chromeno[6,5-g][1,8]naphthyridin-7-one Analogs of Acronycine. J. Med. Chem. 2014, 57, 10329–10342. [Google Scholar] [CrossRef]
- Gaslonde, T.; Léonce, S.; Pierré, A.; Pfeiffer, B.; Michel, S.; Tillequin, F. Tröger’s bases in the acronycine, benzo[a]acronycine, and benzo[b]acronycine series. Tetrahedron Lett. 2011, 52, 4426–4429. [Google Scholar] [CrossRef]
- Sittisombut, C.; Boutefnouchet, S.; Trinh, V.-D.H.; Tian, W.; Michel, S.; Koch, M.; Tillequin, F.; Pfeiffer, B.; Pierré, A. Synthesis and Cytotoxic Activity of Benzo[a]pyrano[3,2-h] and [2,3-i]xanthone Analogues of Psorospermine, Acronycine, and Benzo[a]acronycine. Chem. Pharm. Bull. 2006, 54, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Gaslonde, T.; Michel, S.; Koch, M.; Pfeiffer, B.; Léonce, S.; Pierré, A.; Tillequin, F. Synthesis and Cytotoxic Activity of Dimeric Analogs of Acronycine in the Benzo[b]pyrano[3,2-h]acridin-7-one Series. Chem. Pharm. Bull. 2007, 55, 734–738. [Google Scholar] [CrossRef][Green Version]
- Skaltsounis, A.L.; Mitaku, S.; Tillequin, F. Acridone Alkaloids: In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: London, UK, 2000; Volume 54, pp. 259–377. [Google Scholar]
- Bellete, B.S.; Gil de Sá, I.C.; Mafezoli, J.; Cerqueira, C.d.N.; da Silva, M.F.D.G.F.; Fernandes, J.B.; Vieira, P.C.; Zukerman-Schpector, J.; Pirani, J.R. Phytochemical and chemosystematics studies of Conchocarpus marginatus and C. inopinatus (Rutaceae). Quím. Nova 2012, 35, 2132–2138. [Google Scholar] [CrossRef]
- Demirkol, O.; Ersatir, M.; Giray, E.S.; Kirici, S. Comparison of the effects of green and sustainable extraction methods on the extraction yield and chemical composition of Ruta chalepensis roots. Sustain. Chem. Pharm. 2022, 29, 100750. [Google Scholar] [CrossRef]
- Bunalema, L.; Fotso, G.W.; Waako, P.; Tabuti, J.; Yeboah, S.O. Potential of Zanthoxylum leprieurii as a source of active compounds against drug resistant Mycobacterium tuberculosis. BMC Complement. Altern. Med. 2017, 17, 89/1–89/6. [Google Scholar] [CrossRef]
- Hussain, M.A.; Nathar, V.N.; Mir, J.I. Gas chromatography-Mass Spectrometry (GC-MS) analysis in callus extracts of Ruta graveolens L. World J. Pharm. Res. 2017, 6, 1195–1210. [Google Scholar]
- Wouatsa, V.N.A.; Misra, L.; Kumar, S.; Prakash, O.; Khan, F.; Tchoumbougnang, F.; Venkatesh, R.K. Aromatase and glycosyl transferase inhibiting acridone alkaloids from fruits of Cameroonian Zanthoxylum species. Chem. Cent. J. 2013, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Eze, F.I.; Siwe-Noundou, X.; Isaacs, M.; Patnala, S.; Osadebe, P.O.; Krause, R.W.M. Anti-cancer and anti-trypanosomal properties of alkaloids from the root bark of Zanthoxylum leprieurii Guill and Perr. Trop. J. Pharm. Res. 2020, 19, 2377–2383. [Google Scholar]
- Kuete, V.; Fouotsa, H.; Mbaveng, A.T.; Wiench, B.; Nkengfack, A.E.; Efferth, T. Cytotoxicity of a naturally occurring furoquinoline alkaloid and four acridone alkaloids toward smulti-factorial drug-resistant cancer cells. Phytomedicine 2015, 22, 946–951. [Google Scholar] [CrossRef]
- Ngoumfo, R.M.; Jouda, J.-B.; Mouafo, F.T.; Komguem, J.; Mbazoa, C.D.; Shiao, T.C.; Choudhary, M.I.; Laatsch, H.; Legault, J.; Pichette, A.; et al. In vitro cytotoxic activity of isolated acridones alkaloids from Zanthoxylum leprieurii Guill. et Perr. Bioorg. Med. Chem. 2010, 18, 3601–3605. [Google Scholar] [CrossRef]
- Wiśniewska, A.; Chrapkowska, A.; Kot-Wasik, A.; Konopa, J.; Mazerska, Z. Metabolic transformations of antitumor imidazoacridinone, C-1311, with microsomal fractions of rat and human liver 2007. Acta Biochim. Pol. 2007, 54, 831–838. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An Update on the Anticancer Activity of Xanthone Derivatives: A Review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef]
- Jameson, M.B.; Thompson, P.I.; Baguley, B.C.; Evans, B.D.; Harvey, V.J.; Porter, D.J.; McCrystal, M.R.; Small, M.; Bellenger, K.; Gumbrell, L.; et al. Clinical aspects of a phase I trial of 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a novel antivascular agent. Br. J. Cancer 2003, 88, 1844–1850. [Google Scholar] [CrossRef]
- Svoboda, G.H.; Poore, G.A.; Simpson, P.J.; Boder, G.B. Alkaloids of Acronychia Baueri Schott I: Isolation of the Alkaloids and a Study of the Antitumor and Other Biological Properties of Acronycine. J. Pharm. Sci. 1966, 55, 758–768. [Google Scholar] [CrossRef]
- Guilbaud, N.; Léonce, S.; Tillequin, F.; Koch, M.; Hickman, J.A.; Pierré, A. Acronycine derivatives as promising antitumor agents. Anti-Cancer Drugs 2002, 13, 445–449. [Google Scholar] [CrossRef]
- Fatykhov, R.F.; Khalymbadzha, I.A.; Rusinov, V.L.; Chupakhin, O.N. Modification of 1-Hydroxy-3-Methoxy-10-Methylacridone by Quinazoline and Quinoxalone. AIP Conf. Proc. 2020, 2280, 040015. [Google Scholar]
- Reisch, J.; Herath, H.M.T.B.; Kumar, N.S. Natural product chemisrty, 143. Convenient synthesis of isoacronycine and some other new acridone derivatives. Justus Liebigs Ann. Chem. 1991, 1991, 685–689. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Shtaitz, Y.K.; Aluru, R.; Butorin, I.I.; Savchuk, M.I.; Khalymbadzha, I.A.; Kopchuk, D.S.; Slepukhin, P.A.; Melekhin, V.V.; Shcheglova, A.V.; et al. 1H-Pyrazole-Appended Pyridines and Their 1,2,4-Triazine Precursors: A Rational Synthesis and in silico and in vitro Evaluation of Anti-Cancer Activity. Eur. J. Med. Chem. 2022, 2022, e202200227. [Google Scholar]
- Fatykhov, R.F.; Sharapov, A.D.; Starnovskaya, E.S.; Shtaitz, Y.K.; Savchuk, M.I.; Kopchuk, D.S.; Nikonov, I.L.; Zyryanov, G.V.; Khalymbadzha, I.A.; Chupakhin, O.N. Coumarin-Pyridine Push-Pull Fluorophores: Synthesis and Photophysical Studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Das, A.; Shaikh, M.M.; Jana, S. Design, synthesis, and in vitro antibacterial screening of some novel 3-pentyloxy-1-hydroxyxanthone derivatives. Med. Chem. Res. 2014, 23, 436–444. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Hackett, A.J.S.; Smith, H.; Springer, E.L.; Owens, R.B.; Nelson-Rees, W.A.; Riggs, J.L.; Gardner, M.B. Two syngeneic cell lines from human breast tissue: The aneuploid mammary epithelial (Hs 578T) and the diploid myoepithelial (Hs 578Bst) cell lines. J. Natl. Cancer Inst. 1977, 58, 1795–1806. [Google Scholar] [CrossRef]
- Graham, F.L.; Smiley, J.; Russell, W.C.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]


| Compound | HCT116 | A-172 | Hs-578T | HEK-293 |
|---|---|---|---|---|
| 2 | 43.45 ± 8.54 | |||
| 7a | 14.56 ± 3.81 | 6.92 ± 2.29 | 7.13 ± 2.83 | 115.15 ± 15.56 |
| 7b | >100 | |||
| 7c | 1.37 ± 0.48 | |||
| 7d | >100 | |||
| 7e | 6.1 ± 1.74 | 3.56 ± 0.36 | 1.74 ± 0.08 | 47.43 ± 3.99 |
| 9e | 7.15 ± 1.02 | |||
| 10a | >128 | >128 | >128 | |
| 10c | >128 | >128 | >128 | |
| 12a | >100 | |||
| 12b | >100 | |||
| 14a | 28.01 ± 5.64 | |||
| 14b | 17.99 ± 1.63 | |||
| 15b | >100 | |||
| Etoposide | 1.08 ± 0.34 | |||
| Cisplatin | 39.5 ± 6.4 | 122.0 ± 13.0 | 59.9 ± 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santra, S.; Sharapov, A.D.; Fatykhov, R.F.; Potapova, A.P.; Khalymbadzha, I.A.; Valieva, M.I.; Kopchuk, D.S.; Zyryanov, G.V.; Bunev, A.S.; Melekhin, V.V.; et al. Xanthone-1,2,4-triazine and Acridone-1,2,4-triazine Conjugates: Synthesis and Anticancer Activity. Pharmaceuticals 2023, 16, 403. https://doi.org/10.3390/ph16030403
Santra S, Sharapov AD, Fatykhov RF, Potapova AP, Khalymbadzha IA, Valieva MI, Kopchuk DS, Zyryanov GV, Bunev AS, Melekhin VV, et al. Xanthone-1,2,4-triazine and Acridone-1,2,4-triazine Conjugates: Synthesis and Anticancer Activity. Pharmaceuticals. 2023; 16(3):403. https://doi.org/10.3390/ph16030403
Chicago/Turabian StyleSantra, Sougata, Ainur D. Sharapov, Ramil F. Fatykhov, Anastasya P. Potapova, Igor A. Khalymbadzha, Maria I. Valieva, Dmitry S. Kopchuk, Grigory V. Zyryanov, Alexander S. Bunev, Vsevolod V. Melekhin, and et al. 2023. "Xanthone-1,2,4-triazine and Acridone-1,2,4-triazine Conjugates: Synthesis and Anticancer Activity" Pharmaceuticals 16, no. 3: 403. https://doi.org/10.3390/ph16030403
APA StyleSantra, S., Sharapov, A. D., Fatykhov, R. F., Potapova, A. P., Khalymbadzha, I. A., Valieva, M. I., Kopchuk, D. S., Zyryanov, G. V., Bunev, A. S., Melekhin, V. V., Gaviko, V. S., & Zonov, A. A. (2023). Xanthone-1,2,4-triazine and Acridone-1,2,4-triazine Conjugates: Synthesis and Anticancer Activity. Pharmaceuticals, 16(3), 403. https://doi.org/10.3390/ph16030403









