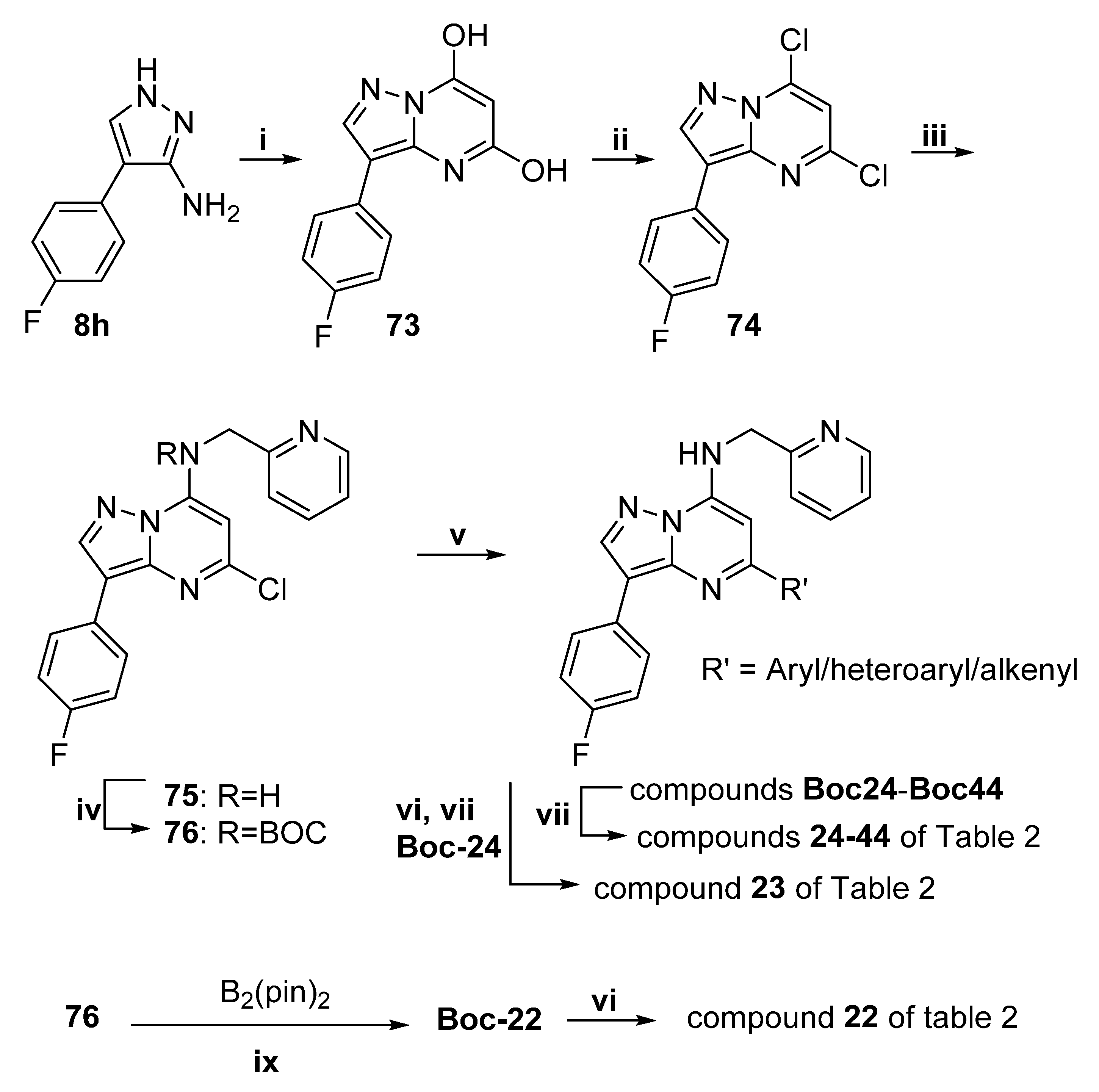
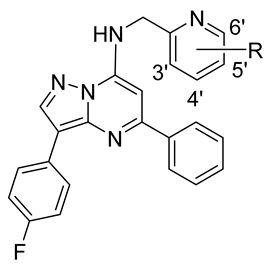
4.5. General Deprotection Procedure
A solution of the Boc-protected product (0.12 mmol) in TFA (10 mL) and DCM (10 mL) was refluxed for 3 h. The solvent was evaporated, and the residue was partitioned between EtOAc, water and saturated aqueous NaHCO3 (until basic). The organic fraction was dried (MgSO4) and evaporated onto silica gel. Column chromatography on silica gel gave the product, which was recrystallised from DCM/heptane by evaporation of DCM to give compounds 22–44.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (22)
A mixture of 76 (0.500 g, 1.10 mmol), bis(pinacolato)diboron (0.310 g, 1.21 mmol) and KOAc (0.650 g, 6.62 mmol) in DMSO (8 mL, anhydrous) was purged with nitrogen in a sealable tube. PdCl2(dppf) (45 mg, 0.055 mmol) was added, and the mixture was heated to 80 °C under nitrogen for 2 h. The mixture was partitioned between EtOAc and water, and the organic fraction was dried and evaporated. Chromatography on silica (33%–50% EtOAc:hexanes) gave Boc-22 (0.126 g, 27%) as a yellow solid. 1H NMR (CDCl3) δ 8.52 (ddd, J = 4.8, 1.7, 0.9 Hz, 1H), 8.49 (d, J = 4.5 Hz, 1H), 8.41 (s, 1H), 8.01 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.65 (td, J = 7.7, 1.8 Hz, 1H), 7.46 (d, J = 7.8 Hz, 1H), 7.09–7.19 (m, 3H), 6.93 (d, J = 4.4 Hz, 1H), 5.14 (s, 2H), 1.36 (s, 9H). LRMS [M + H] = 420.2.
Deprotection of Boc-22 (0.122 g, 0.29 mmol) using the general deprotection procedure gave 22 (0.075 g, 81%) as yellow microcrystals. Purity (HPLC) 99.9%; mp 171–173 °C. 1H NMR (CDCl3) δ 8.67 (bdd, J = 3.5, 0.9 Hz, 1H), 8.33 (s, 1H), 8.32 d, J = 5.1 Hz, 1H), 8.01 (ap dd, J = 8.8, 5.4 Hz, 2H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.60 (bt, J = 4.8 Hz, 1H), 7.35 (d, J = 7.8 Hz, 1H), 7.28 (dd, J = 7.4, 5.7 Hz, 1H), 7.13 (ap t, J = 8.8 Hz, 2H), 6.03 (d, J = 5.1 Hz, 1H), 4.74 (d, J = 5.4 Hz, 2H). HRMS calcd. for C18H15FN5 (M + H+) m/z 320.1306, found 320.1307.
5-Ethyl-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (23)
A solution of Boc-24 (0.100 g, 0.225 mmol) in MeOH (30 mL) was purged with nitrogen in a hydrogenation bottle. 10% Pd/C (10 mg) was added, and the mixture was hydrogenated at 50 psi for 18 h. The mixture was filtered and evaporated to give crude Boc-23. Deprotection of crude Boc-23 using the general deprotection procedure gave 23 (0.030 g, 38%) as cream crystals. Purity (HPLC) 97.4%; mp 137–138 °C. 1H NMR (CDCl3) δ 8.67 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.29 (s, 1H), 8.08 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.41 (bt, J = 4.9 Hz, 1H), 7.35 (d, J = 7.9 Hz, 1H), 7.27 (m, 1H), 7.11 (ap t, J = 8.9 Hz, 2H), 6.58 (s, 1H), 4.72 (d, J = 5.4 Hz, 2H), 2.81 (q, J = 7.6 Hz, 2H), 1.36 (t, J = 7.6 Hz, 3H). HRMS calcd. for C20H19FN5 (M + H+) m/z 348.1619, found 348.1620.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-vinylpyrazolo[1,5-a]pyrimidin-7-amine (24)
A biphasic solution of 76 (1.00 g, 2.20 mmol), potassium vinyltrifluoroborate (1.175 g, 8.77 mmol) and Na2CO3 (1.40 g, 13.2 mmol) in toluene (50 mL) and water (15 mL) was purged with nitrogen in a sealable tube. Pd(PPh3)4 (0.51 g, 0.44 mmol) was added, and the mixture was heated to reflux under nitrogen for 3 h. The mixture was partitioned between EtOAc and water, and the organic fraction was dried and evaporated. Chromatography on silica (2:1 hexanes:EtOAc) gave Boc-24 (0.922 g, 94%) as an orange foam. 1H NMR (CDCl3) δ 8.53 (bd, J = 4.2 Hz, 1H), 8.38 (s, 1H), 8.07 (ap dd, J = 8.8, 5.4 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 2H), 7.49 (d, J = 7.9 Hz, 1H), 7.10–7.18 (m, 2H), 7.05 (s, 1H), 6.86 (dd, J = 17.6, 10.8 Hz, 1H), 6.24 (d, J = 17.6 Hz, 1H), 5.69 (d, J = 10.8 Hz, 1H), 5.13 (s, 2H), 1.36 (s, 9H). LRMS [M + H] = 446.2.
Deprotection of Boc-24 (0.100 g, 0.225 mmol) using the general deprotection procedure gave 24 (0.026 g, 34%) as yellow microcrystals. Purity (HPLC) 97.8%; mp 131–133 °C. 1H NMR (CDCl3) δ 8.68 (dd, J = 3.3, 0.9 Hz, 1H), 8.31 (s, 1H), 8.10 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (bt, J = 5.0 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.28 (dd, J = 7.4, 5.8 Hz, 1H), 7.12 (ap t, J = 8.8 Hz, 2H), 6.80 (dd, J = 17.5, 10.7 Hz, 1H), 6.30 (dd, J = 17.5, 1.0 Hz, 1H), 6.12 (s, 1H), 5.61 (dd, J = 10.7, 1.0 Hz, 1H), 4.76 (d, J = 5.3 Hz, 2H). HRMS calcd. for C20H17FN5 (M + H+) m/z 346.1463, found 346.1459.
5-Cyclopropyl-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (25)
Reaction of 76 (0.100 g, 0.22 mmol) and cyclopropylboronic acid (0.076 g, 0.88 mmol) using the general Suzuki procedure gave Boc-25 (0.090 g, 89%) as a yellow solid. 1H NMR (CDCl3) δ 8.53 (ddd, J = 4.8, 1.7, 0.9 Hz, 1H), 8.33 (s, 1H), 8.02 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.18 (ddd, J = 7.5, 4.9, 1.0 Hz, 1H), 7.11 (ap t, J = 8.8 Hz, 2H), 6.78 (s, 1H), 5.11 (s, 2H), 2.03–2.10 (m, 1H), 1.36 (s, 9H), 1.19–1.22 (m, 2H), 1.08–1.13 (m, 2H). LRMS [M + H] = 460.3.
Deprotection of Boc-25 (0.085 g, 0.19 mmol) using the general deprotection procedure gave 25 (0.054 g, 81%) as cream needles. Purity (HPLC) 100%; mp 149–151 °C. 1H NMR (CDCl3) δ 8.66 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.27 (s, 1H), 8.05 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.72 (dt, J = 7.7, 1.8 Hz, 1H), 7.35 (d, J = 7.9 Hz, 2H), 7.28 (dd, J = 7.4, 5.7 Hz, 1H), 7.10 (ap t, J = 8.9 Hz, 2H), 5.99 (s, 1H), 4.73 (d, J = 5.4 Hz, 2H), 1.96–2.04 (m, 1H), 1.18–1.23 (m, 2H), 1.00–1.05 (m, 2H). 13C NMR (CDCl3) δ 164.80, 162.29, 159.87, 155.34, 149.74, 146.08, 145.56, 141.35, 137.19, 129.56, 129.53, 127.20, 127.13, 123.02, 121.51, 115.61, 115.40, 107.22, 85.00, 46.96, 18.02, 10.74. HRMS calcd. for C21H19FN5 (M + H+) m/z 360.1624, found 360.1628.
5-(Cyclopent-1-en-1-yl)-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (26)
Reaction of 76 (0.100g, 0.22 mmol) and cyclopent-1-en-1-ylboronic acid (0.099 g, 0.88 mmol) using the general Suzuki procedure gave Boc-26 (0.084 g, 79%) as an orange solid. 1H NMR (CDCl3) δ 8.53 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.36 (s, 1H), 8.11 (ap dd, J = 9.0, 5.4 Hz, 2H), 7.65 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.18 (ddd, J = 7.4, 4.9, 1.0 Hz, 1H), 7.13 (ap t, J = 8.9 Hz, 2H), 7.03 (s, 1H), 6.61–6.64 (m, 1H), 5.12 (s, 2H), 2.89–2.94 (m, 2H), 2.61–2.66 (m, 2H), 2.02–2.11 (m, 2H), 1.36 (s, 9H). LRMS [M + H] = 486.3.
Deprotection of Boc-26 (0.025 g, 0.052 mmol) using the general deprotection procedure gave 26 (0.014 g, 71%) as yellow microcrystals. Purity (HPLC) 98.8%; mp 177–179 °C. 1H NMR (CDCl3) δ 8.66 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.31 (s, 1H), 8.13 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.39 (t, J = 5.2 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.24–7.29 (m, 1H), 7.11 (ap t, J = 8.9 Hz, 2H), 6.64–6.68 (m, 1H), 6.17 (s, 1H), 4.75 (d, J = 5.3 Hz, 2H), 2.87–2.93 (m, 2H), 2.58–2.64 (m, 2H), 2.03–2.10 (m, 2H). 13C NMR (CDCl3) δ 162.35, 159.93, 155.39, 155.24, 149.67, 146.24, 145.23, 144.07, 141.31, 137.12, 133.82, 129.4, 129.37, 127.30, 127.22, 122.96, 127.22, 122.96, 121.50, 155.59, 115.38, 108.47, 83.14, 46.91, 33.90, 32.67, 23.45. HRMS calcd. for C23H21FN5 (M + H+) m/z 386.1776, found 386.1777.
5-(Cyclohex-1-en-1-yl)-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (27)
Reaction of 76 (0.100g, 0.22 mmol) and cyclohex-1-en-1-ylboronic acid (0.111 g, 0.88 mmol) using the general Suzuki procedure gave Boc-27 (0.076 g, 69%) as an orange solid. 1H NMR (CDCl3) δ 8.53 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.35 (s, 1H), 8.10 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.65 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.17 (ddd, J = 7.6, 4.9, 1.0 Hz, 1H), 7.14 (ap t, J = 8.8 Hz, 2H), 7.10 (s, 1H), 6.72–6.77 (m, 1H), 5.12 (s, 2H), 2.62–2.67 (m, 2H), 2.27–2.33 (m, 2H), 1.78–1.83 (m, 2H), 1.66–1.73 (m, 2H), 1.36 (s, 9H). LRMS [M + H] = 500.3.
Deprotection of Boc-27 (0.030 g, 0.060 mmol) using the general deprotection procedure gave 27 (0.022 g, 92%) as yellow microcrystals. Purity (HPLC) 97.2%; mp 199–201 °C. 1H NMR (CDCl3) δ 8.67 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.30 (s, 1H), 8.12 (ap dd, J = 9.0, 5.5 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.33–7.39 (m, 2H), 7.27 (dd, J = 7.6, 5.5 Hz, 1H), 7.12 (ap t, J = 8.9 Hz, 2H), 6.74–6.80 (m, 1H), 6.16 (s, 1H), 4.75 (d, J = 5.3 Hz, 2H), 2.61–2.64 (m, 2H), 2.25–2.33 (m, 2H), 1.79–1.85 (m, 2H), 1.67–1.73 (m, 2H). 13C NMR (CDCl3) δ 162.42, 160.00, 159.55, 155.39, 149.77, 146.47, 145.16, 141.44, 137.21, 137.13, 131.04, 129.53, 129.50, 127.40, 127.32, 123.04, 121.56, 115.69, 115.48, 108.46, 82.17, 47.01, 26.35, 25.85, 22.98, 22.29. HRMS calcd. for C24H23FN5 (M + H+) m/z 400.1932, found 400.1933.
5-(2-Fluorophenyl)-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (28)
Reaction of 76 (0.100 g, 0.22 mmol) and (2-fluorophenyl)boronic acid (0.123 g, 0.88 mmol) using the general Suzuki conditions gave Boc-28 (0.060 g, 53%) as a yellow solid. 1H NMR (CDCl3) δ 8.51 (bd, J = 4.2 Hz, 1H), 8.45 (s, 1H), 8.20 (td, J = 7.8, 1.8 Hz, 1H), 8.12 (ap dd, J = 8.8, 5.4 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.43–7.52 (m, 3H), 7.33 (td, J = 7.8, 1.0 Hz, 1H), 7.13–7.21 (m, 4H), 5.20 (s, 2H), 1.39 (s, 9H). LRMS [M + H] = 514.2.
Deprotection of Boc-28 (0.048 g, 0.94 mmol) using the general deprotection procedure gave 28 (0.045 g, 95%) as orange microcrystals. Purity (HPLC) 97.4%; mp 181–184 °C. 1H NMR (CDCl3) δ 8.69 (dd, J = 4.8, 0.6 Hz, 1H), 8.37 (s, 1H), 8.22 (td, J = 7.8, 1.8 Hz, 1H), 8.14 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.73 (td, J = 7.7 Hz, 1.8 Hz, 1H), 7.61 (t, J = 5.0 Hz, 1H), 7.40–7.46 (m, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.32 (td, J = 7.7, 1.2 Hz, 1H), 7.28 (dd, J = 7.5, 5.7 Hz, 1H), 7.11–7.20 (m, 3H), 6.62 (s, 1H), 4.80 (d, J = 5.2 Hz, 2H). 13C NMR (CDCl3) δ 162.57, 162.32, 160.14, 159.83, 154.92, 153.71, 153.69, 149.77, 146.52, 145.33, 141.76, 137.18, 131.56, 131.53, 131.39, 131.31, 129.26, 129.23, 127.76, 127.63, 127.55, 127.19, 127.08, 124.79, 124.75, 123.08, 121.70, 116.63, 116.39, 115.79, 115.58, 109.13, 87.10, 86.99, 46.85. HRMS calcd. for C28H27FN5 (M + H+) m/z 452.2245, found 452.2244.
3-(4-Fluorophenyl)-5-(2-methoxyphenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (29)
Reaction of 76 (0.100g, 0.22 mmol) and 2-methoxyphenylboronic acid (0.134 g, 0.88 mmol) using the general Suzuki conditions gave Boc-29 (0.090 g, 78%) as a yellow solid. 1H NMR (CDCl3) δ 8.51 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.40 (s, 1H), 8.12 (ap dd, J = 8.9, 5.3, Hz, 2H), 8.06 (dd, J = 7.7, 1.8 Hz, 1H), 7.65 (td, J = 7.7, 1.8 Hz, 1H), 7.56 (s, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.43 (ddd, J = 7.4, 4.9, 1.0 Hz, 1H), 7.09–7.18 (m, 4H), 6.99 (d, J = 8.3 Hz, 1H), 5.17 (s, 2H), 3.81 (s, 3H), 1.37 (s, 9H). LRMS [M + H] = 526.3.
Deprotection of Boc-29 (0.086 g, 0.16 mmol) using the general deprotection procedure gave 29 (0.055 g, 79%) as cream microcrystals. Purity (HPLC) 99.4%; mp 150–153 °C. 1H NMR (CDCl3) δ 8.67 (ddd, J = 4.9, 1.6, 0.7 Hz, 1H), 8.35 (s, 1H), 8.15 (ap dd, J = 8.9, 5.3 Hz, 2H), 8.02 (dd, J = 7.6, 1.8 Hz, 1H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.37–7.44 (m, 3H), 7.28 (ddd, J = 8.2, 2.6, 0.8 Hz, 1H), 7.08–7.15 (m, 3H), 7.00 (dd, J = 8.3, 0.7 Hz, 1H), 6.69 (s, 1H), 4.79 (d, J = 5.5 Hz, 2H), 3.82 (s, 3H). HRMS calcd. for C25H21FN5O (M + H+) m/z 426.1725, found 426.1723.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(m-tolyl)pyrazolo[1,5-a]pyrimidin-7-amine (30)
Reaction of 76 (0.100 g, 0.22 mmol) and m-tolylboronic acid (0.120 g, 0.88 mmol) using the general Suzuki conditions gave Boc-30 (0.069 g, 62%) as a yellow solid. 1H NMR (CDCl3) δ 8.53 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.42 (s, 1H), 8.15 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.89–7.92 (m, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.41 (t, J = 7.7 Hz, 1H), 7.39 (s, 1H), 7.31 (d, J = 7.7 Hz, 1H), 7.14–7.20 (m, 3H), 5.19 (s, 2H), 2.47 (s, 3H), 1.38 (s, 9H). LRMS [M + H] = 510.2.
Deprotection of Boc-30 (0.060 g, 0.12 mmol) using the general deprotection procedure gave 30 (0.042 g, 87%) as a cream solid. Purity (HPLC) 99.6%; mp 151–153 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.35 (s, 1H), 8.17 (ap dd, J = 9.0, 5.4 Hz, 2H), 7.91–7.93 (m, 2H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.55 (t, J = 5.1 Hz, 1H), 7.38–7.42 (m, 2H), 7.27–7.30 (m, 2H), 7.15 (ap t, J = 8.8 Hz, 2H), 6.47 (s, 1H), 4.83 (d, J = 5.3 Hz, 2H), 2.47 (s, 3H). 13C NMR (CDCl3) δ 162.54, 160.11, 157.83, 155.12, 149.77, 146.89, 145.50, 141.80, 138.92, 138.49, 137.24, 130.83, 129.37, 129.34, 128.80, 128.19, 127.62, 127.55, 124.79, 123.10, 121.61, 115.77, 115.56, 108.99, 83.41, 46.98, 21.84. HRMS calcd. for C25H21FN5 (M + H+) m/z 410.1776, found 410.1775.
3-(4-Fluorophenyl)-5-(3-methoxyphenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (31)
Reaction of 76 (0.100 g, 0.22 mmol) and (3-methoxyphenyl)boronic acid (0.134 g, 0.88 mmol) using the general Suzuki conditions gave Boc-31 (0.109 g, 94%) as a yellow solid. 1H NMR (CDCl3) δ 8.54 (ddd, J = 4.9, 1.7, 0.9 Hz, 1H), 8.42 (s, 1H), 8.15 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.70 (t, J = 1.7 Hz, 1H), 7.66 (td, J = 7.6, 1.8 Hz, 2H), 7.50 (d, J = 7.8 Hz, 1H), 7.43 (t, J = 8.0 Hz, 1H), 7.40 (s, 1H), 7.14–7.20 (m, 3H), 7.04 (ddd, J = 8.2, 2.6, 0.8 Hz, 1H), 5.18 (s, 2H), 3.92 (s, 3H), 1.38 (s, 9H). LRMS [M + H] = 526.3.
Deprotection of Boc-31 (0.105 g, 0.20 mmol) using the general deprotection procedure gave 31 (0.068 g, 80%) as pale yellow microcrystals. Purity (HPLC) 99.6%; mp 147–149 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.7 Hz, 1H), 8.36 (s, 1H), 8.18 (ap dd, J = 8.9 5.3 Hz, 2H), 7.71–7.76 (m, 2H), 7.68 (ddd, J = 7.7, 1.6, 1.0 Hz, 1H), 7.56 (t, J = 5.2 Hz, 1H), 7.42 (t, J = 8.0 Hz, 1H), 7.39 (d, J = 7.8 Hz, 1H), 7.28 (ddd, J = 7.5, 5.0, 1.0 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 7.02 (ddd, J = 8.2, 2.6, 0.8 Hz, 1H), 6.46 (s, 1H), 4.82 (d, J = 5.3 Hz, 2H), 3.92 (s, 3H). HRMS calcd. for C25H21FN5O (M + H+) m/z 426.1725, found 426.1725.
3,5-Bis(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (32)
Reaction of 76 (0.100g, 0.22 mmol) and (4-fluorophenyl)boronic acid (0.123 g, 0.88 mmol) using the general Suzuki procedure gave Boc-32 (0.105 g, 93%) as a yellow solid. 1H NMR (CDCl3) δ 8.54 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.42 (s, 1H), 8.09–8.15 (m, 4H), 7.67 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.40 (s, 1H), 7.15–7.23 (m, 5H), 5.17 (s, 2H), 1.38 (s, 9H). LRMS [M + H] = 514.1.
Deprotection of Boc-32 (0.097 g, 0.19 mmol) using the general deprotection procedure gave 32 (0.067 g, 86%) as pale yellow microcrystals. Purity (HPLC) 99.6%; mp 181–183 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.35 (s, 1H), 8.09–8.18 (m, 4H), 7.74 (td, J = 7.7, 1.8 Hz, 1H), 7.58 (t, J = 5.1 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.29 (dd, J = 7.5, 5.8 Hz, 1H), 7.12–7.22 (m, 4H), 6.42 (s, 1H), 4.82 (d, J = 5.3 Hz, 2H). HRMS calcd. for C24H18F2N5 (M + H+) m/z 414.1530, found 414.1535.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(p-tolyl)pyrazolo[1,5-a]pyrimidin-7-amine (33)
Reaction of 76 (0.100 g, 0.22 mmol) and p-tolylboronic acid (0.120 g, 0.88 mmol) using the general Suzuki procedure gave Boc-33 (0.094 g, 84%) as an orange solid. 1H NMR (CDCl3) δ 8.53 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.41 (s, 1H), 8.14 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.01 (d, J = 8.2 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.38 (s, 1H), 7.32 (d, J = 8.0 Hz, 2H), 7.14–7.20 (m, 3H), 5.18 (s, 2H), 2.45 (s, 3H), 1.38 (s, 9H). LRMS [M + H] = 510.2.
Deprotection of Boc-33 (0.080 g, 0.16 mmol) using the general deprotection procedure gave 33 (0.052 g, 81%) as orange microcrystals. Purity (HPLC) 99.5%; mp 180–182 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.8 Hz, 1H), 8.34 (s, 1H), 8.17 (ap dd, J = 8.9, 5.2 Hz, 2H), 8.02 (d, J = 8.2 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.52 (t, J = 5.2 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.31 (d, J = 7.9 Hz, 2H), 7.28 (obscured, 1H), 7.15 (ap d, J = 8.8 Hz, 2H), 6.46 (s, 1H), 4.82 (d, J = 5.3 Hz, 2H), 2.43 (s, 3H). HRMS calcd. for C25H21FN5 (M + H+) m/z 410.1776, found 410.1775.
3-(4-Fluorophenyl)-5-(4-methoxyphenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (34)
Reaction of 76 (0.100g, 0.22 mmol) and (4-methoxyphenyl)boronic acid (0.134 g, 0.88 mmol) using the general Suzuki procedure gave Boc-34 (0.092 g, 80%) as a yellow solid. 1H NMR (CDCl3) δ 8.54 (ddd, J = 4.8, 1.7, 0.9 Hz, 1H), 8.39 (s, 1H), 8.14 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.08 (ap d, J = 8.9 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.51 (d, J = 8.5 Hz, 1H), 7.37 (s, 1H), 7.14–7.19 (m, 3H), 7.03 (ap d, J = 8.9 Hz, 2H), 5.17 (s, 2H), 3.90 (s, 3H), 1.37 (s, 9H). LRMS [M + H] = 526.3.
Deprotection of Boc-34 (0.088 g, 0.17 mmol) using the general deprotection procedure gave 34 (0.058 g, 81%) as cream microcrystals. Purity (HPLC) 99.8%; mp 179–181 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.33 (s, 1H), 8.17 (ap dd, J = 9.0, 5.4 Hz, 2H), 8.10 (ap d, J = 8.9 Hz, 2H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (t, J = 5.1 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.28 (dd, J = 7.4, 5.7 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 7.03 (ap d, J = 8.9 Hz, 2H), 6.42 (s, 1H), 4.82 (d, J = 5.3 Hz, 2H), 3.89 (s, 3H). 13C NMR (CDCl3) δ 162.47, 161.39, 160.05, 157.15, 155.21, 149.76, 146.84, 145.51, 141.70, 137.23, 131.36, 129.47, 129.44, 128.96, 127.51, 127.44, 123.08, 121.59, 115.73, 115.51, 114.21, 108.65, 82.64, 55.62, 47.01. HRMS calcd. for C25H21FN5O (M + H+) m/z 426.1730, found 426.1733.
3-(4-Fluorophenyl)-5-(4-isopropylphenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (35)
Reaction of 76 (0.100 g, 0.22 mmol) and (4-isopropylphenyl)boronic acid (0.145 g, 0.88 mmol) using the general Suzuki procedure gave Boc-35 (0.101 g, 86%) as a yellow solid. 1H NMR (CDCl3) δ 8.54 (ddd, J = 4.8, 1.7, 0.9 Hz, 1H), 8.41 (s, 1H), 8.15 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.03 (d, J = 8.4 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.36–7.40 (m, 3H), 7.13–7.20 (m, 3H), 5.18 (s, 2H), 2.99 (sept, J = 6.9 Hz, 1H), 1.38 (s, 9H), 1.30 (d, J = 6.9 Hz, 6H). LRMS [M + H] = 538.2.
Deprotection of Boc-35 (0.096 g, 0.18 mmol) using the general deprotection procedure gave 35 (0.078 g, 99%) as pale yellow microcrystals. Purity (HPLC) 99.5%; mp 88–90 °C. 1H NMR (CDCl3) δ 8.68 (dd, J = 4.9, 0.6 Hz, 1H), 8.35 (s, 1H), 8.18 (ap dd, J = 8.9, 5.5 Hz, 2H), 8.05 (d, J = 8.3 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.51 (t, J = 5.3 Hz, 1H), 7.35–7.40 (m, 3H), 7.28 (dd, J = 6.8, 5.0 Hz, 1H), 7.14 (ap t, J = 8.8 Hz, 2H), 6.45 (s, 1H), 4.83 (d, J = 5.4 Hz, 2H), 2.99 (sept, J = 7.0 Hz, 1H), 1.31 (d, J = 7.0 Hz, 6H). 13C NMR (CDCl3) δ 162.50, 160.07, 157.66, 155.22, 151.17, 149.78, 146.89, 145.54, 141.71, 137.23, 136.54, 129.42, 129.39, 127.61, 127.56, 127.48, 127.00, 123.09, 121.58, 115.73, 115.51, 108.83, 83.16, 47.04, 34.24, 32.09, 29.23, 24.12, 22.90, 14.33. HRMS calcd. for C27H25FN5 (M + H+) m/z 438.2094, found 438.2103.
5-(4-(Tert-butyl)phenyl)-3-(4-fluorophenyl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (36)
Reaction of 76 (0.100 g, 0.22 mmol) and (4-tert-butylphenyl)boronic acid (0.157 g, 0.88 mmol) using the general Suzuki procedure gave Boc-36 (0.100 g, 82%) as an orange solid. 1H NMR (CDCl3) δ 8.54 (ddd, J = 4.8, 1.7, 0.9 Hz, 1H), 8.41 (s, 1H), 8.16 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.04 (ap d, J = 8.6 Hz, 2H), 7.66 (td, J = 7.7, 1.8 Hz, 1H), 7.55 (ap d, J = 8.6 Hz, 2H), 7.49 (d, J = 7.9 Hz, 1H), 7.38 (s, 1H), 7.13–7.19 (m, 3H), 5.18 (s, 2H), 1.38 (s, 18H). LRMS [M + H] = 552.3.
Deprotection of Boc-36 (0.070 g, 0.17 mmol) using the general deprotection procedure gave 36 (0.070 g, 90%) as orange microcrystals. Purity (HPLC) 99.5%; mp 150–152 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.35 (s, 1H), 8.19 (ap dd, J = 8.9, 5.5 Hz, 2H), 8.06 (ap d, J = 8.6 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (ap d, J = 8.6 Hz, 2H), 7.51 (t, J = 5.3 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.28 (ddd, J = 7.5, 4.7, 0.8 Hz, 1H), 7.14 (ap t (J = 8.8 Hz, 2H), 6.46 (s, 1H), 4.82 (d, J = 5.3 Hz, 2H), 1.38 (s, 9H). 13C NMR (CDCl3) δ 162.50, 160.07, 157.59, 155.24, 153.40, 149.80, 146.90, 145.56, 141.71, 137.24, 136.13, 129.42, 129.39, 127.55, 127.48, 127.33, 125.86, 123.09, 121.57, 115.73, 115.52, 108.84, 83.18, 47.06, 35.01, 31.49. HRMS calcd. for C28H27FN5 (M + H+) m/z 452.2245, found 452.2244.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(4-(trifluoromethyl)phenyl)pyrazolo[1,5-a]pyrimidin-7-amine (37)
Reaction of 76 (0.100g, 0.22 mmol) and (4-(trifluoromethyl)phenyl)boronic acid (0.063 g, 0.33 mmol) using the general Suzuki procedure gave Boc-37 (0.092 g, 74%) as a yellow solid. 1H NMR (CDCl3) δ 8.55 (ddd, J = 4.9, 1.6, 0.8 Hz, 1H), 8.45 (s, 1H), 8.23 (d, J = 8.1 Hz, 1H), 8.13 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.78 (d, J = 8.2 Hz, 2H), 7.68 (td, J = 7.7, 1.8 Hz, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.49 (s, 1H), 7.15–7.22 (m, 3H), 5.19 (s, 2H), 1.38 (s, 9H). LRMS [M + H] = 564.2.
Deprotection of Boc-37 (0.082 g, 0.15 mmol) using the general deprotection procedure gave 37 (0.066 g, 98%) as a yellow solid. Purity (HPLC) 98.6%; mp 170–171 °C. 1H NMR (CDCl3) δ 8.70 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.38 (s, 1H), 8.23 (d, J = 8.1 Hz, 2H), 8.15 (ap dd, J = 8.9, 5.2 Hz, 2H), 7.71–7.79 (m, 3H), 7.67 (t, J = 5.2 Hz, 1H), 7.39 (d, J = 7.8 Hz, 1H), 7.30 (dd, J = 7.5, 4.8 Hz, 1H), 7.16 (ap t, J = 8.7 Hz, 2H), 6.49 (s, 1H), 4.83 (d, J = 5.2 Hz, 2H). HRMS calcd. for C25H18F4N5 (M + H+) m/z 464.1498, found 464.1498.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(4-(trifluoromethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-7-amine (38)
Reaction of 76 (0.100g, 0.22 mmol) and (4-(trifluoromethoxy)phenyl)boronic acid (0.181 g, 0.88 mmol) using the general Suzuki procedure gave Boc-38 (0.107 g, 84%) as a yellow solid. 1H NMR (CDCl3) δ 8.55 (ddd, J = 4.8, 1.7, 0.8 Hz, 1H), 8.43 (s, 1H), 8.09–8.18 (m, 4H), 7.67 (J = 7.7, 1.8 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.43 (s, 1H), 7.36 (d, J = 8.0 Hz, 2H), 7.14–7.22 (m, 3H), 5.18 (s, 2H), 1.38 (s, 9H). LRMS [M + H] = 580.2.
Deprotection of Boc-38 (0.093 g, 0.16 mmol) using the general deprotection procedure gave 38 (0.063 g, 82%) as cream microcrystals. Purity (HPLC) 96.2%; mp 181–183 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.36 (s, 1H), 8.12–8.18 (m, 4H), 7.74 (td, J = 7.7, 1.8 Hz, 1H), 7.62 (t, J = 5.2 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.34 (dd, J = 8.9, 0.8 Hz, 2H), 7.29 (dd, J = 7.4, 5.8 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 6.44 (s, 1H), 4.82 (d, J = 5.3 Hz, 2H). 13C NMR (CDCl3) δ 162.62, 160.19, 156.06, 154.91, 150.64, 150.62, 149.81, 147.03, 145.34, 141.98, 137.49, 137.29, 129.17, 129.11, 127.63, 127.56, 124.57, 123.19, 121.97, 121.65, 121.11, 119.41, 116.86, 115.82, 115.61, 109.25, 83.03, 46.98. HRMS calcd. for C25H18F4N5O (M + H+) m/z 480.1442, found 480.1443.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(pyridin-3-yl)pyrazolo[1,5-a]pyrimidin-7-amine (39)
Reaction of 76 (0.100 g, 0.22 mmol) and pyridin-3-ylboronic acid (0.108 g, 0.88 mmol) using the general Suzuki procedure gave Boc-39 (0.060 g, 55%) as a yellow solid. 1H NMR (CDCl3) δ 9.32 (dd, J = 2.2, 0.6 Hz, 1H), 8.73 (dd, J = 4.8, 1.7 Hz, 1H), 8.55 (ddd, J = 4.8, 1.6, 0.8 Hz, 1H), 8.45 (s, 1H), 8.44 (ddd, J = 8.0, 2.2, 1.8 Hz, 1H), 8.14 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.68 (td, J = 7.7, 1.8 Hz, 1H), 7.44–7.51 (m, 3H), 7.15–7.22 (m, 3H), 5.18 (s, 2H), 1.38 (s, 9H). LRMS [M + H] = 497.2.
Deprotection of Boc-39 (0.058 g, 0.12 mmol) using the general deprotection procedure gave 39 (0.038 g, 82%) as yellow microcrystals. Purity (HPLC) 99.9%; mp 198–201 °C. 1H NMR (CDCl3) δ 9.32 (dd, J = 2.2, 0.6 Hz, 1H), 8.69–8.71 (m, 2H), 8.47 (dt, J = 8.0, 1.8 Hz, 1H), 8.37 (s, 1H), 8.15 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.75 (td, J = 7.7, 1.8 Hz, 1H), 7.71 (t, J = 5.7 Hz, 1H), 7.46 (ddd, J = 8.0, 4.8, 0.7 Hz, 1H), 7.40 (d, J = 7.8 Hz, 1H), 7.30 (dd, J = 6.6, 4.9 Hz, 1H), 7.16 (ap t, J = 8.8 Hz, 2H), 6.48 (s, 1H), 4.83 (d, J = 5.2 Hz, 2H). 13C NMR (CDCl3) δ 162.65, 160.22, 154.79, 154.72, 150.77, 149.81, 148.83, 147.11, 145.36, 142.02, 137.29, 135.01, 134.43, 129.04, 129.01, 127.64, 127.57, 123.77, 123.22, 121.71, 115.85, 115.64, 109.40, 82.89, 46.94. HRMS calcd. for C23H18FN6 (M + H+) m/z 396.1577, found 396.1579.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-7-amine (40)
Reaction of 76 (0.100 g, 0.22 mmol) and pyridin-4-ylboronic acid (0.108 g, 0.88 mmol) using the general Suzuki procedure gave Boc-40 (0.066 g, 60%) as a yellow solid. 1H NMR (CDCl3) δ 8.81 (dd, J = 4.6, 1.7 Hz, 2H), 8.55 (bd, J = 4.1 Hz, 1H), 8.47 (s, 1H), 8.13 (ap dd, J = 8.9, 5.4 Hz, 2H), 7.98 (dd, J = 4.5, 1.6 Hz, 2H), 7.68 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (s, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.17–7.23 (m, 3H), 5.19 (s, 2H), 1.38 (s, 9H). LRMS [M + H] = 497.3.
Deprotection of Boc-40 (0.075 g, 0.15 mmol) using the general deprotection procedure gave 40 (0.045 g, 75%) as orange microcrystals. Purity (HPLC) 99.9%; mp 250–253 °C. 1H NMR (CDCl3) δ 8.77 (dd, J = 4.5, 1.6 Hz, 2H), 8.70 (ddd, J = 4.9, 1.5, 0.6 Hz, 1H), 8.39 (s, 1H), 8.15 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.00 (dd, J = 4.5, 1.6 Hz, 2H), 7.71–7.77 (m, 2H), 7.39 (d, J = 7.8 Hz, 1H), 7.30 (dd, J = 7.1, 5.5 Hz, 1H), 7.17 (ap t, J = 8.8 Hz, 2H), 6.51 (s, 1H), 4.84 (d, J = 5.2 Hz, 2H). HRMS calcd. for C23H18FN6 (M + H+) m/z 397.1577, found 397.1586.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(thiophen-2-yl)pyrazolo[1,5-a]pyrimidin-7-amine (41)
Reaction of 76 (0.100 g, 0.22 mmol) and thiophen-2-ylboronic acid (0.452 g, 3.53 mmol; added in 0.113 g and 0.339 g portions) using the general Suzuki procedure gave Boc-41 (0.039 g, 35%) as a yellow solid. 1H NMR (CDCl3) δ 8.55 (ddd, J = 4.8, 1.6, 0.9 Hz, 1H), 8.38 (s, 1H), 8.12 (ap dd, J = 9.0, 5.4 Hz, 2H), 7.67 (td, J = 7.7, 1.8 Hz, 1H), 7.63 (dd, J = 3.7, 1.1 Hz, 1H), 7.52 (dd, J = 5.3, 1.1 Hz, 1H), 7.50 d (J = 7.9 Hz, 1H), 7.28 (s, 1H), 7.13–7.19 (m, 4H), 5.16 (s, 2H), 1.37 (s, 9H). LRMS [M + H] = 502.2.
Deprotection of Boc-41 (0.042 g, 0.084 mmol) using the general deprotection procedure gave 41 (0.032 g, 95%) as a yellow solid. Purity (HPLC) 97.9%; mp 181–183 °C. 1H NMR (CDCl3) δ 8.69 (dd, J = 4.8, 0.6 Hz, 1H), 8.32 (s, 1H), 8.15 (ap dd, J = 9.0, 5.4 Hz, 2H), 7.72 (td, J = 7.7, 1.8 Hz, 1H), 7.66 (dd, J = 3.7, 1.1 Hz, 1H), 7.55 (t, J = 5.1 Hz, 1H), 7.47 (dd, J = 5.0, 1.1 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.28 (dd, J = 7.2, 5.6, 1H), 7.11–7.18 (m, 3H), 6.37 (s, 1H), 4.80 (d, J = 5.3 Hz, 2H). 13C NMR (CDCl3) δ 162.54, 160.11, 154.96, 152.42, 149.75, 146.76, 145.04, 144.80, 141.73, 137.27, 129.22, 129.19, 129.14, 128.15, 127.48, 127.40, 126.32, 123.14, 121.65, 115.75, 115.54, 108.72, 81.86, 77.43, 46.95, 45.78. HRMS calcd. for C22H17FN5S (M + H+) m/z 402.1183, found 402.1189.
3-(4-Fluorophenyl)-N-(pyridin-2-ylmethyl)-5-(thiophen-3-yl)pyrazolo[1,5-a]pyrimidin-7-amine (42)
Reaction of 76 (0.100 g, 0.22 mmol) and thiophen-3-ylboronic acid (0.113 g, 0.88 mmol) using the general Suzuki procedure gave Boc-42 (0.078 g, 71%) as a yellow solid. 1H NMR (CDCl3) δ 8.55 (dd, J = 4.8, 1.7, 0.9 Hz, 1H), 8.38 (s, 1H), 8.10 ( ap dd, J = 8.9, 5.4 Hz, 2H), 8.07 (dd, J = 1.4, 0.8 Hz, 1H), 7.67 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (t, J = 1.7 Hz, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.12–7.20 (m, 3H), 7.09 (s, 1H), 7.02 (dd, J = 1.8, 0.8 Hz, 1H), 5.15 (s, 2H), 1.37 (s, 9H). LRMS [M + H] = 502.2.
Deprotection of Boc-42 (0.073 g, 0.15 mmol) using the general deprotection procedure gave 42 (0.050 g, 86%) as cream microcrystals. Purity (HPLC) 99.9%; mp 155–157 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.33 (s, 1H), 8.15 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.00 (dd, J = 3.0, 1.3 Hz, 1H), 7.79 (dd, J = 5.0, 1.2 Hz, 1H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (t, J = 5.2 Hz, 1H), 7.41 (dd, J = 5.0, 3.0 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.28 (dd, J = 7.4, 5.8 Hz, 1H), 7.14 (ap t, J = 8.8 Hz, 2H), 6.35 (s, 1H), 4.80 (d, J = 5.3 Hz, 2H). 13C NMR (CDCl3) δ 159.98, 154.98, 153.26, 149.66, 145.27, 141.97, 141.64, 137.14, 129.24, 129.21, 127.43, 126.90, 126.28, 125.23, 123.01, 121.51, 115.63, 115.41, 108.67, 83.17, 46.89. HRMS calcd. for C22H17FN5S (M + H+) m/z 402.1183, found 402.1183.
3-(4-Fluorophenyl)-5-(furan-2-yl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (43)
Reaction of 76 (0.100g, 0.22 mmol) and furan-2-ylboronic acid (0.210 g, 1.77 mmol) using the general Suzuki procedure gave Boc-43 (0.045 g, 42%) as an orange solid. 1H NMR (CDCl3) δ 1H NMR (CDCl3) δ 8.54 (ddd, J = 4.9, 1.7, 0.7 Hz, 1H), 8.38 (s, 1H), 8.10 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.07 (dd, J = 1.4, 0.8 Hz, 1H), 7.68 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (t, J = 1.6 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.12–7.20 (m, 3H), 7.09 (s, 1H), 7.02 (dd, J = 1.9, 0.8 Hz, 1H), 5.15 (s, 2H), 1.37 (s, 9H). LRMS [M + H] = 486.2.
Deprotection of Boc-43 (0.038 g, 0.078 mmol) using the general deprotection procedure gave 43 (0.013 g, 43%) as a tan solid. Purity (HPLC) 99.9%; 8.68 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.33 (s, 1H), 8.12 (ap dd, J = 9.0, 5.5 Hz, 2H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.60 (bt, J = 5.0 Hz, 1H), 7.57 (dd, J = 1.7, 0.8 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.27–7.30 (m, 2H), 7.14 (ap t, J = 8.8 Hz, 2H), 6.58 (dd, J = 3.4, 1.8 Hz, 1H), 6.51 (s, 1H), 4.81 (d, J = 5.2 Hz, 2H). HRMS calcd. for C22H17FN5O (M + H+) m/z 386.1412, found 386.1412.
3-(4-Fluorophenyl)-5-(furan-3-yl)-N-(pyridin-2-ylmethyl)pyrazolo[1,5-a]pyrimidin-7-amine (44)
Reaction of 76 (0.300 g, 0.66 mmol) and furan-3-ylboronic acid (0.297 g, 2.66 mmol) using the general Suzuki procedure gave crude Boc-44 (0.075 g, 23%), which was used directly in the subsequent step. LRMS [M + H] = 486.2.
Deprotection of Boc-44 (0.069 g, 0.14 mmol) using the general deprotection procedure gave 44 (0.046 g, 84%) as a yellow solid. Purity (HPLC) 98.5%; mp 151–153 °C. 1H NMR (CDCl3) δ 8.69 (ddd, J = 4.9, 1.6, 0.9 Hz, 1H), 8.32 (s, 1H), 8.13 (ap dd, J = 9.0, 5.4 Hz, 2H), 8.09 (dd, J = 1.4, 0.8 Hz, 1H), 7.73 (td, J = 7.7, 1.8 Hz, 1H), 7.53 (t, J = 5.1 Hz, 1H), 7.52 (dd, J = 3.4, 1.7 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.28 (dd, J = 7.4, 5.7 Hz, 1H), 7.13 (ap t, J = 8.8 Hz, 2H), 7.01 (dd, J = 1.8, 0.8 Hz, 1H), 6.18 (s, 1H), 5.30 (d, J = 4.8 Hz, 2H). 13C NMR (CDCl3) δ 162.54, 160.11, 155.07, 151.98, 149.79, 146.83, 145.44, 144.08, 142.60, 141.72, 137.29, 129.31, 129.28, 127.55, 127.47, 127.28, 123.16, 121.63, 115.74, 115.53, 109.34, 108.59, 83.12, 47.00. HRMS calcd. for C22H17FN5O (M + H+) m/z 386.1412, found 386.1410.
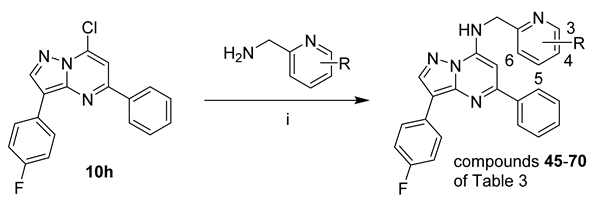
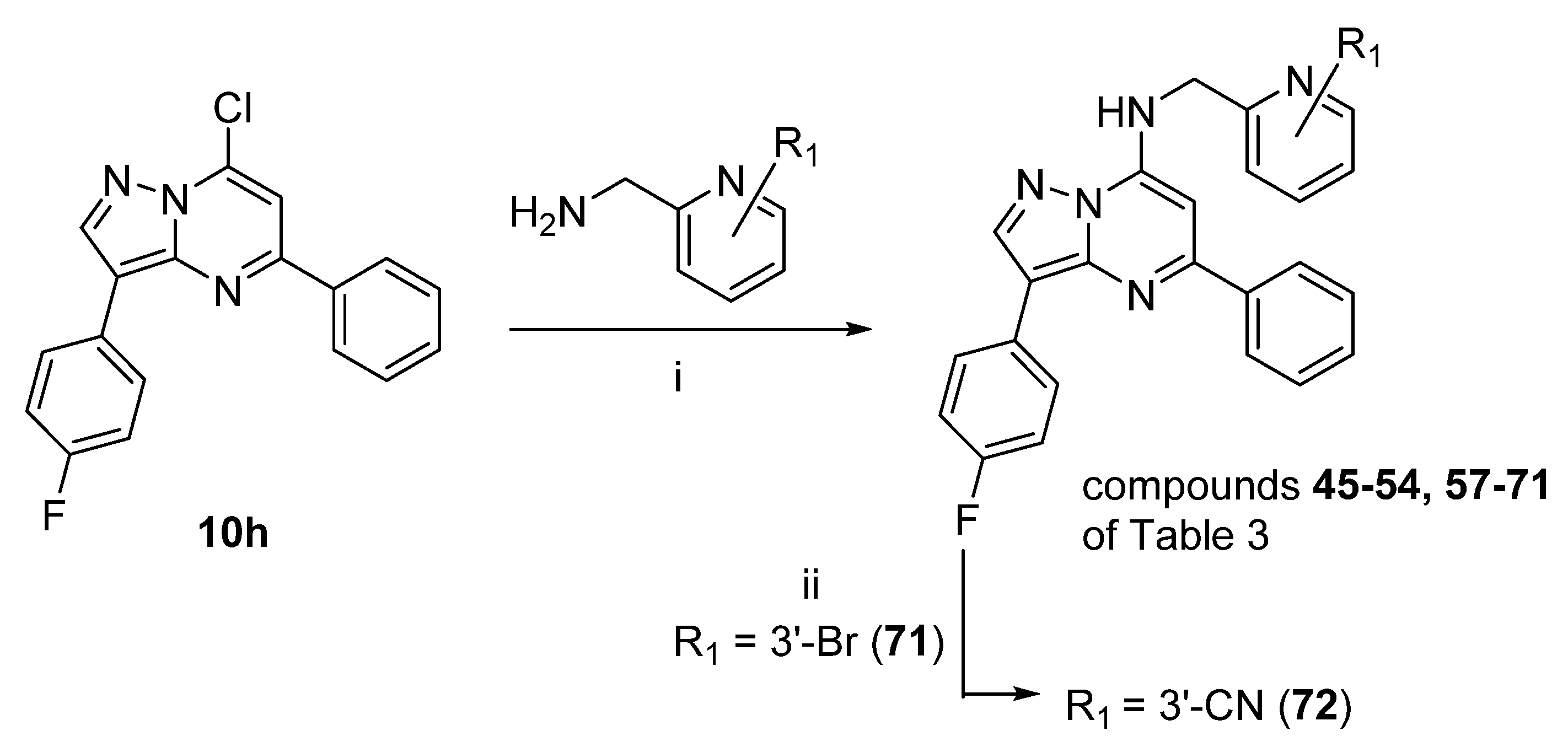
Preparation of compounds
45–
72 of
Table 3:
Reagents and conditions: (i) DIPEA, iPrOH, reflux, 3–15h.
3-(4-Fluorophenyl)-N-((6-fluoropyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (45)
A mixture of 10h (0.046 g, 0.14 mmol), 6-fluoropyridine-2-methanamine hydrochloride (0.0277 mg, 0.170 mmol) and diisopropylethylamine (0.12 mL, 0.71 mmol) was heated to 110 °C for 16 h. The reaction mixture was partitioned between water and ethyl acetate. The aqueous fraction was extracted twice with ethyl acetate, and the organic fraction was combined, dried (MgSO4) and concentrated in vacuo. Column chromatography on silica gel using a gradient of 5:1 hexanes:ethyl acetate to 3:1 hexanes:ethyl acetate gave compound 45 (0.054 g, 92%) as a yellow solid. Purity (HPLC) 99.1%; mp 196–198 °C. 1H NMR (CDCl3) δ 8.34 (s, 1H), 8.20–8.13 (m, 2H), 8.12–8.08 (m, 2H), 7.82 (q, J = 7.9 Hz, 1H), 7.52–7.46 (m, 3H), 7.29 (dd, J = 7.4, 1.7 Hz, 1H), 7.21–7.11 (m, 3H), 6.92 (dd, J = 8.2, 2.2 Hz, 1H), 6.45 (s, 1H), 4.80 (d, J = 6.0 Hz, 2H). HRMS calcd. for C24H18F2N5 (M + H+) m/z 414.1525, found 414.1516.
N-((6-Bromopyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (46)
Similar reaction of 10h (0.0392 g, 0.121 mmol) with (6-bromopyridin-2-yl)methanamine (0.0272 g, 0.145 mmol) gave compound 46 (0.047 g, 82%). Purity (HPLC) 94.9%. mp 160–161 °C. 1H NMR (CDCl3), δ 8.34 (s, 1H), 8.21–8.14 (m, 2H), 8.13–8.08 (m, 2H), 7.60–7.54 (m, 1H), 7.54–7.46 (m, 4H), 7.36 (d, J = 7.5 Hz, 1H), 7.20–7.13 (m, 3H), 6.48 (s, 1H), 4.83 (d, J = 6.0 Hz, 2H). HRMS calcd. for C24H17BrFN5: 473.0651, found 473.0679.
3-(4-Fluorophenyl)-N-((6-methylpyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (47)
Similar reaction of 10h (0.0592 g, 0.183 mmol) with (6-methylpyridin-2-yl)methanamine (0.0268 g, 0.219 mmol) gave compound 47 (0.048 g, 64%). Purity (HPLC) 99.1%; mp 177–180 °C. 1H NMR (CDCl3), δ 8.35 (s, 1H), 8.20–8.11 (m, 4H), 7.60 (t, J = 7.7 Hz, 1H), 7.53–7.44 (m, 4H), 7.19–7.10 (m, 4H), 6.50 (s, 1H), 4.78 (d, J = 5.4 Hz, 2H). 2.64 (s, 3H). HRMS calcd. for C25H20FN5: 409.1703, found 409.1718.
3-(4-Fluorophenyl)-N-((6-methoxypyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (48)
Similar reaction of 10h (0.0440 g, 0.136 mmol) with (6-methoxypyridin-2-yl)methanamine (0.0265 g, 0.163 mmol) gave compound 48 (0.052 g, 90%). Purity (HPLC) 99.6%; mp 163–165 °C. 1H NMR (CDCl3), δ 8.37 (s, 1H), 8.19–8.10 (m, 4H), 7.60–7.56 (m, 1H), 7.53–7.40 (m, 4H), 7.17–7.11 (m, 2H), 6.92 (d, J = 7.2 Hz, 1H), 6.69 (d, J = 8.2 Hz, 1H), 6.51 (s, 1H), 4.71 (d, J = 5.5 Hz, 2H). 4.00 (s, 3H). HRMS calcd. for [M + H]+ = C25H21FN5O: 426.1725, found 426.1711.
N-((6-(Dimethylamino)pyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (49)
Similar reaction of 10h (0.21 g, 0.64 mmol) with 6-(aminomethyl)-N,N-dimethylpyridin-2-amine dihydrochloride (0.17 g, 0.77 mmol) gave compound 49 (0.21 g, 75%). Purity (HPLC) 98.0%; mp 151–153 °C. 1H NMR (CDCl3), δ 8.41 (dd, J = 4.5, 1.4 Hz, 1H), 8.36 (s, 1H), 8.21–8.16 (m, 4H), 8.08 (br t, J = 4.6 Hz, 1H), 7.56–7.48 (m, 4H), 7.27 (m, 1H), 7.15 (t, J = 8.9 Hz, 2H), 6.58 (s, 1H), 4.79 (d, J = 4.7 Hz, 2H), 2.80 (s, 6H). HRMS calcd. for C26H24FN6 (M + H+) m/z 439.2041, found 439.2045.
3-(4-Fluorophenyl)-5-phenyl-N-((6-(piperidin-1-yl)pyridin-2-yl)methyl)pyrazolo[1,5-a]pyrimidin-7-amine (50)
Similar reaction of 10h (0.20 g, 0.62 mmol) with (6-(piperidin-1-yl)pyridin-2-yl)methanamine dihydrochloride (0.19 g, 0.74 mmol) gave compound 50 (0.25 g, 83%). Purity (HPLC) 98.0%; mp 185–187 °C. 1H NMR (CDCl3) δ 8.39 (dd, J = 4.7, 1.4 Hz, 1H), 8.35 (s, 1H), 8.20–8.15 (m, 4H), 8.05 (br t, J = 4.9 Hz, 1H), 7.56–7.48 (m, 4H), 7.27 (m, 1H), 7.14 (t, J = 8.9 Hz, 2H), 6.56 (s, 1H), 4.80 (d, J = 5.0 Hz, 2H), 2.91 (br t, J = 5.2 Hz, 4H), 1.85 (m, 4H), 1.66 (m, 2H). HRMS calcd. for C29H28FN6 (M + H+) m/z 479.2354, found 479.2361.
3-(4-Fluorophenyl)-N-((6-(4-methylpiperidin-1-yl)pyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (51)
Similar reaction of 10h (0.20 g, 0.62 mmol) with (6-(4-methylpiperazin-1-yl)pyridin-2-yl)methanamine dihydrochloride (0.21 g, 0.74 mmol) gave compound 51 (0.14 g, 45%). Purity (HPLC) 98.0%; mp 183–185 °C. 1H NMR (CDCl3) δ 8.43 (dd, J = 4.7, 1.4 Hz, 1H), 8.34 (s, 1H), 8.20–8.15 (m, 4H), 8.04 (br t, J = 4.9 Hz, 1H), 7.59–7.48 (m, 4H), 7.29 (m, 1H), 7.15 (t, J = 8.8 Hz, 2H), 6.55 (s, 1H), 4.80 (d, J = 5.0 Hz, 2H), 3.07 (t, J = 4.9 Hz, 4H), 2.78 (m, 4H), 2.47 (s, 3H). HRMS calcd. for C29H29FN7 (M + H+) m/z 494.2463, found 494.2463.
3-(4-Fluorophenyl)-N-((5-fluoropyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (52)
Similar reaction of 10h (0.0475 g, 0.147 mmol) with (5-fluoropyridin-2-yl)methanamine (0.0222 g, 0.176 mmol) gave compound 52 (0.050 g, 82%). Purity (HPLC) 99.5%; mp 118–120 °C. 1H NMR (CDCl3), δ 8.54 (d, J = 2.7 Hz, 1H), 8.34 (s, 1H), 8.20–8.09 (m, 4H), 7.52–7.38 (m, 6H), 7.19–7.11 (m, 2H), 6.48 (s, 1H), 4.82 (d, J = 5.4 Hz, 2H). 13C NMR (CDCl3) δ 162.59, 160.48, 160.17, 157.93, 157.60, 151.17, 151.13, 146.87, 145.47, 141.85, 138.83, 138.17, 137.93, 130.12, 129.26, 129.22, 128.92, 127.64, 127.57, 124.33, 124.14, 122.55, 122.50, 115.81, 115.59, 109.17, 83.27, 16.57, 16.56, 0.21. HRMS calcd. for C24H17F2N5: 413.1452, found 413.1464.
N-((5-Chloropyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (53)
Similar reaction of 10h (0.0475 g, 0.147 mmol) with (4-chloropyridin-2-yl)methanamine (0.0222 g, 0.176 mmol) gave compound 53 (0.053 g, 86%). Purity (HPLC) 99.5%; mp 139–141 °C. 1H NMR (CDCl3) δ 8.63 (d, J = 2.0 Hz, 1H), 8.37 (s, 1H), 8.20–8.09 (m, 4H), 7.72 (d, J = 8.4, 2.5 Hz, 1H), 7.53–7.47 (m, 3H), 7.41 (t, J = 5.5 Hz, 1H), 7.36 (d, J = 8.3 Hz, 1H), 7.19–7.13 (m, 2H), 6.46 (s, 1H), 4.82 (d, J = 5.5 Hz, 2H). 13C NMR (CDCl3) δ 162.60, 160.17, 157.58, 153.47, 148.73, 146.83, 145.45, 141.86, 138.79, 137.07, 131.61, 130.13, 129.23, 129.20, 128.92, 127.64, 127.57, 122.32, 115.81, 115.59, 109.18, 83.25, 46.60. HRMS calcd. for C24H17ClFN5: 429.1157, found 429.1158.
3-(4-Fluorophenyl)-N-((5-methoxypyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (54)
Similar reaction of 10h (0.0625 g, 0.193 mmol) with (4-methoxypyridin-2-yl)methanamine (0.0320 mg, 0.232 mmol) gave compound 54 (0.028 g, 34%). Purity (HPLC) 98.8%; mp 212–214 °C. 1H NMR (CDCl3) δ 8.36 (d, J = 2.8 Hz, 1H), 8.34 (s, 1H), 8.20–8.11 (m, 4H), 7.54–7.48 (m, 3H), 7.40 (t, J = 5.4Hz, 1H), 7.31 (d, J = 8.6 Hz, 1H), 7.24–7.21 (m, 1H), 7.18–7.12 (m, 2H), 6.49 (s, 1H), 4.77 (d, J = 5.4 Hz, 2H). 13C NMR (CDCl3) δ 162.54, 160.11, 157.54, 155.44, 147.10, 146.98, 145.49, 141.75, 138.92, 137.15, 130.03, 129.36, 129.33, 128.88, 127.58, 127.51, 121.99, 121.93, 115.77, 115.56, 108.99, 83.29, 55.93, 46.60. HRMS calcd. for C25H20FN5O: 425.1652, found 425.1657.
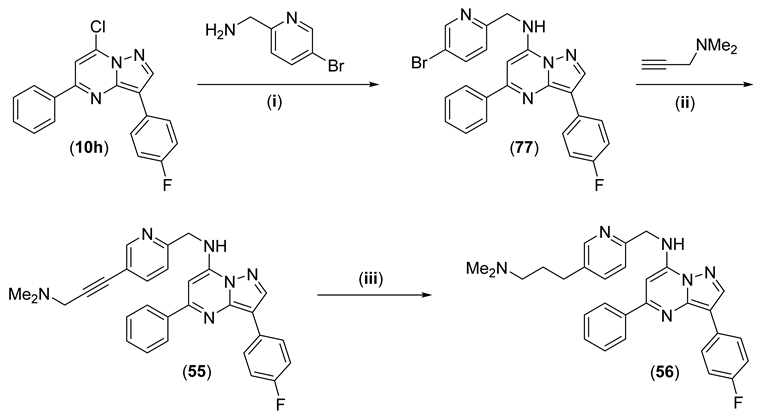
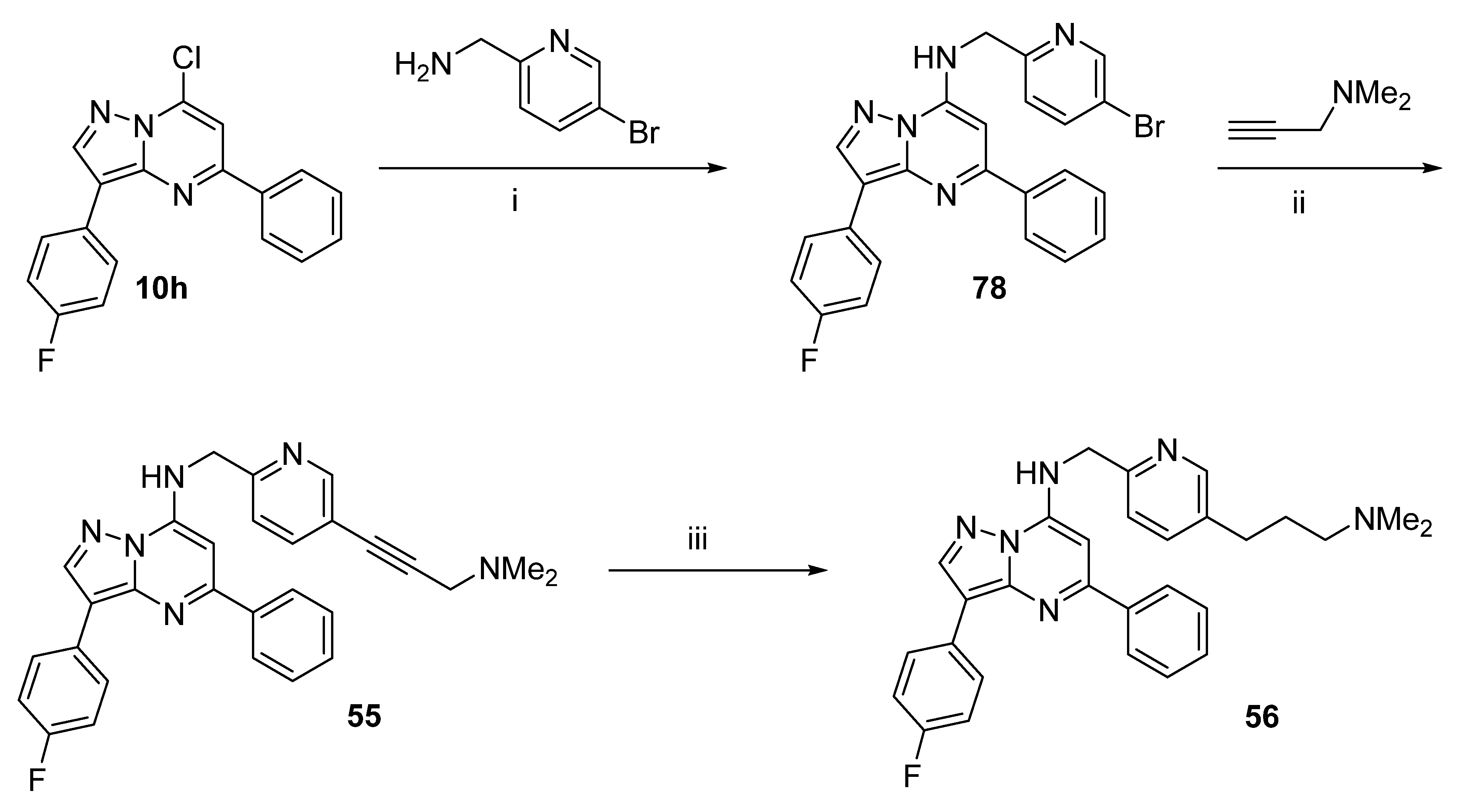
N-((5-(3-(Dimethylamino)prop-1-yn-1-yl)pyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (55) and N-((5-(3-(dimethylamino)propyl)pyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (56)
From 10h (0.803 g, 2.48 mmol) and (5-bromopyridin-2-yl)methanamine (0.635 g, 3.40 mmol), 77 (1.165 g, 99%) was synthesised using the general procedure, 19 h reflux. 1H NMR (CDCl3) δ 8.74 (d, J = 1.9 Hz, 1H), 8.35 (s, 1H), 8.17 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.11 (ap dd, J = 5.9, 1.4 Hz, 2H), 7.85 (dd, J = 8.3, 2.4 Hz, 1H), 7.45–7.55 (m, 3H), 7.40 (t, J = 5.4 Hz, 1H), 7.30 (d, J = 8.3 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 6.45 (s, 1H), 4.79 (d, J = 5.5 Hz, 2H). LRMS [M + H] = 475.2.
A mixture of 77 (0.200 g, 0.422 mmol), PdCl2(PPh3)2 (0.030 g, 0.043 mmol) and CuI (4 mg, 0.021 mmol) in anhydrous DMF (1 mL) and triethylamine (1 mL) was purged with nitrogen in a sealable tube. N,N-Dimethylprop-2-yn-1-amine (0.23 mL, 2.1 mmol) was added, and the mixture was sealed then heated to 65 °C under nitrogen for 15 h. The mixture was partitioned between EtOAc and water, the organic fractions were dried (MgSO4) and evaporated on to silica gel. Column chromatography using a gradient of EtOAc to 95:5 EtOAc:MeOH with 0.75% aq. ammonia gave 55 (0.192 g, 95%) as yellow microcrystals. Purity (HPLC) 98.3%; mp 182–184 °C. 1H NMR (CDCl3) δ 8.72 (d, J = 1.4 Hz, 1H), 8.35 (s, 1H), 8.17 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.12 (ap dd, J = 0.3, 1.7 Hz, 2H), 7.75 (dd, J = 8.1, 2.1 Hz, 1H), 7.44–7.54 (m, 4H), 7.33 (d, J = 8.1 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 6.46 (s, 1H), 4.82 (d, J = 5.4 Hz, 2H), 3.49 (s, 2H), 2.38 (s, 6H). 13C NMR (CDCl3) δ 162.50, 160.07, 157.50, 154.07, 152.26, 146.81, 145.39, 141.77, 139.78, 138.75, 130.12, 129.19, 129.16, 128.83, 127.55, 127.50, 127.48, 120.82, 119.71, 115.71, 115.50, 109.06, 88.75, 83.20, 81.75, 48.66, 46.88, 44.42. HRMS calcd. for C29H26FN6 (M + H+) m/z 477.2197, found 477.2196.
A solution of 55 (0.079 g, 0.166 mmol) in EtOH (20 mL) was purged with nitrogen in a hydrogenation bottle, 5% Pd/C (39 mg) was added, and the mixture was hydrogenated at 60 psi for 18 h. The mixture was filtered through celite and evaporated. Column chromatography using a gradient of EtOAc to 95:5 EtOAc:MeOH with 0.75% aq. ammonia gave 56 (0.020 g, 25%) as pale yellow microcrystals. Purity (HPLC) 96.0%; mp 151–153 °C. 1H NMR (CDCl3) δ 8.52 (d, J = 1.8 Hz, 1H), 8.35 (s, 1H), 8.18 (ap dd, J = 8.9, 5.5 Hz, 2H), 8.13 (ap dd, J = 8.0, 1.3 Hz, 2H), 7.45–7.58 (m, 5H), 7.30 (d, J = 7.8 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 6.49 (s, 1H), 4.79 (d, J = 5.3 Hz, 2H), 2.68 (t, J = 7.6 Hz, 2H), 2.30 (t, J = 7.1 Hz, 2H), 2.23 (s, 6H), 1.76–1.84 (m, 2H). HRMS calcd. for C29H30FN6 (M + H+) m/z 481.2516, found 481.2511.
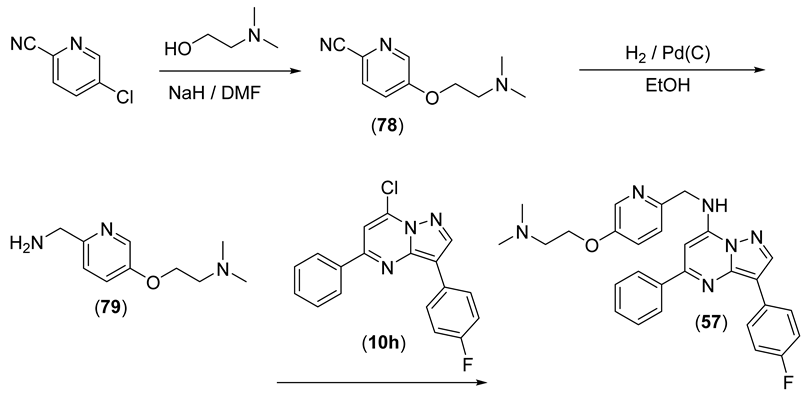
N-((5-(2-(Dimethylamino)ethoxy)pyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (57)
To a slurry of NaH (60% w/w, 1.04 g, 26.0 mmol) in anhydrous DMF (8 mL), 2-(dimethylamino)ethan-1-ol (2.18 mL, 19.4 mmol) was added, and this mixture was stirred for 15 min and then 5-chloropicolinonitrile (2.00 g, 14.4 mmol) and anhydrous DMF (8 mL) were added. The mixture was stirred for 2 h, then partitioned between EtOAc and water, and the organic fractions were dried and evaporated on to silica gel. Column chromatography using a gradient of 5–7.5% MeOH:EtOAc gave 78 (1.333 g, 48%) as a faint pinkish solid. 1H NMR (CDCl3) δ 8.40 (d, J = 2.7 Hz, 1H), 7.64 (dd, J = 8.6, 0.2 Hz, 1H), 7.27 (dd, J = 8.6, 2.9 Hz, 1H), 4.16 (t, J = 5.6 Hz, 2H), 2.77 (t, J = 5.6 Hz, 2H), 2.34 (s, 6H). LRMS [M + H] = 192.2.
A solution of 78 (0.848 g, 4.43 mmol) in MeOH (20 mL) was purged with nitrogen in a hydrogenation bottle, 5% Pd/C (85 mg) was added, and the mixture was hydrogenated at 50 psi for 16 h. The mixture was filtered through celite and evaporated to give crude 79, which was used without further purification. 1H NMR (CDCl3) δ 8.27 (d, J = 2.6 Hz, 1H), 7.26 (d, J = 8.7 Hz, 1H), 7.18 (dd, J = 8.7, 2.9 Hz, 1H), 4.08–4.11 (m, 2H), 3.88 (s, 2H), 2.72–2.75 (m, 2H), 2.34 (s, 6H), 2H exchanged. LRMS [M + H] = 196.2.
Using the general procedure, 57 (0.107 g, 28%) was synthesised as yellow microcrystals from 10h (0.260 g, 0.803 mmol) and 79 (0.283 g, 1.45 mmol). Purity (HPLC) 99.1%; mp 130–132 °C. 1H NMR (CDCl3) δ 8.38 (d, J = 2.4 Hz, 1H), 8.34 (s, 1H), 8.18 (ap, dd, J = 8.9, 5.4 Hz, 2H), 8.13 (ap dd, J = 7.9, 1.2 Hz, 2H), 7.44–7.55 (m, 3H), 7.40 (t, J = 5.2 Hz, 1H), 7.24–7.32 (m, 2H), 7.13 (ap t, J = 8.8 Hz, 2H), 6.49 (s, 1H), 4.76 (d, J = 5.4 Hz, 2H), 4.12 (t, J = 5.6 Hz, 2H), 2.76 (t, J = 5.6 Hz, 2H), 2.35 (s, 6H). HRMS calcd. for C28H27FN6NaO (M + Na+) m/z 505.2123, found 505.2121.
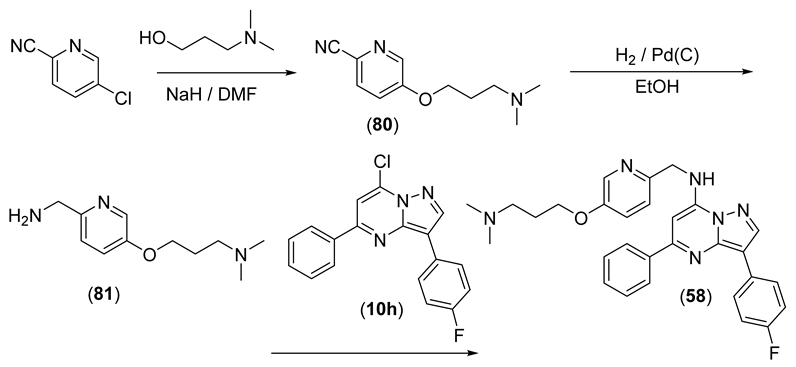
N-((4-(2-(Dimethylamino)ethoxy)pyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (58)
Reaction of 5-chloropicolinonitrile (2.00 g, 1.44 mmol) and 3-(dimethylamino)propan-1-ol (2.6 mL, 2.2 mmol) gave 80 (2.249 g, 76%) as a pinkish solid. 1H NMR (CDCl3) δ 8.37 (d, J = 2.6 Hz, 1H), 7.63 (dd, J = 8.6, 0.4 Hz, 1H), 7.24 (dd, J = 8.6, 2.9 Hz, 1H), 4.13 (t, J = 6.4 Hz, 2H), 2.45 (t, J = 7.0 Hz, 2H), 2.25 (s, 6H), 1.96–2.03 (m, 2H). LRMS [M + H] = 206.2.
Hydrogenation of 80 (2.028 g, 9.88 mmol) gave 81 (1.94 g, 94%), which was used directly without further purification. 1H NMR (CDCl3) δ 8.24 (d, J = 2.6 Hz, 1H), 7.26 (d, J = 8.5 Hz, 1H), 7.16 (d, J = 8.5, 2.9 Hz, 1H), 4.03–4.13 (m, 2H), 3.88 (s, 2H), 2.45 (t, J = 7.0 Hz, 2H), 2.25 (s, 6H), 1.94–2.00 (m, 2H). LRMS [M + H] = 210.2
Using the general procedure, 58 (0.099 g, 32%) was synthesised as yellow microcrystals from 10h (0.200 g, 0.618 mmol) and 81 (0.259 g, 1.24 mmol). Purity (HPLC) 96.0%; mp 151–153 °C. 1H NMR (CDCl3) δ 8.34 (d, J = 3.2 Hz, 1H), 8.34 (s, 1H), 8.18 (ap dd, J = 8.9, 5.4 Hz, 2H), 8.13 (ap dd, J = 7.9, 2.6 Hz, 2H), 7.45–7.55 (m, 3H), 7.39 (t, J = 5.3 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.23 (dd, J = 8.6, 2.8 Hz, 1H), 7.15 (ap t, J = 8.8 Hz, 2H), 6.49 (s, 1H), 4.76 (d, J = 5.4 Hz, 2H), 4.09 (t, J = 6.3 Hz, 2H), 2.52 (t, J = 7.1 Hz, 2H), 2.30 (s, 6H), 1.98–2.05 (m, 2H), 2H exchanged. HRMS calcd. for C29H30FN6O (M + H+) m/z 497.2465, found 497.2470.
3-(4-Fluorophenyl)-5-phenyl-N-((5-(piperidin-1-yl)pyridin-2-yl)methyl)pyrazolo[1,5-a]pyrimidin-7-amine (59)
Similar reaction of 10h (0.0196 g, 0.0605 mmol) with (5-(piperidin-1-yl)pyridin-2-yl)methanamine (0.014 g, 0.073 mmol) gave compound 59 (0.016 g, 55%). HPLC = 98.4%. mp 179–182 °C. 1H NMR (CDCl3, 400 MHz) δ 8.33 (s, 2H), 8.19–8.12 (m, 4H), 7.53–7.47 (m, 3H), 7.37 (t, J = 5.5 Hz, 1H), 7.22–7.20 (m, 2H), 7.18–7.11 (m, 2H), 6.51 (s, 1H), 4.72 (d, J = 5.3 Hz, 2H), 3.21 (t, J = 5.4 Hz, 4H), 1.75–1.69 (m, 4H), 1.63–1.58 (m, 2H). HRMS calcd. for C29H27FN6: 478.2281, found 478.2297.
N-((4-Chloropyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (60)
Similar reaction of 10h (0.0425 g, 0.131 mmol) with (4-chloropyridin-2-yl)methanamine (0.0282, 0.158 mmol) gave compound 60 (0.045 g, 80%). Purity (HPLC = 99.1%); mp 220–222 °C. 1H NMR (CDCl3, 400 MHz) δ 8.58 (d, J = 5.3 Hz, 1H), 8.36 (s, 1H), 8.20–8.15 (m, 2H), 8.14–8.10 (m, 2H), 7.55–7.44 (m, 4H), 7.41 (d, J = 1.4 Hz, 1H), 7.30 (dd, J = 5.4, 1.9 Hz, 1H), 7.18–7.12 (m, 2H), 6.46 (s, 1H), 4.81 (d, J = 5.5 Hz, 2H). HRMS calcd. for C24H17ClFN5: 429.1157, found 429.1168.
2-(((3-(4-Fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-yl)amino)methyl)isonicotinonitrile (61)
Similar reaction of 10h (0.0663 g, 0.205 mmol) with 2-(aminomethyl)isonicotinonitrile (0.0417 g, 0.246 mmol) gave compound 61 (0.032 g, 38%). Purity (HPLC = 96.0%); mp 233–236 °C. 1H NMR (CDCl3, 400 MHz) δ 8.89 (dd, J = 5.0, 0.8 Hz, 1H), 8.37 (s, 1H), 8.20–8.14 (m, 2H), 8.13–8.09 (m, 2H), 7.62 (s, 1H), 7.53–7.42 (m, 5H), 7.20–7.11 (m, 2H), 6.44 (s, 1H), 4.90 (d, J = 5.5 Hz, 2H). HRMS calcd. for C25H17FN6: 420.1499, found 420.1508.
3-(4-Fluorophenyl)-N-((4-methylpyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (62)
Similar reaction of 10h (0.236 g, 0.730 mmol) with (4-methylpyridin-2-yl)methanamine (0.107 g, 0.876 mmol) gave 62 (0.260 g, 87%). Purity (HPLC) 99.1%; mp 157–159 °C. 1H NMR (CDCl3) δ 8.52 (d, J = 5.0 Hz, 1H), 8.35 (s, 1H), 8.20–8.10 (m, 4H), 7.53–7.46 (m, 4H), 7.20–7.11 (m, 3H), 7.09 (d, J = 5.0 Hz, 1H), 6.48 (s, 1H), 4.78 (d, J = 5.3 Hz, 2H), 2.37 (s, 3H). 13C NMR (CDCl3) δ 162.55, 160.12, 157.57, 149.47, 148.60, 146.99, 145.50, 141.80, 138.92, 130.04, 129.36, 129.33, 128.89, 127.59, 127.53, 124.18, 122.36, 115.78, 115.56, 109.01, 83.26, 46.99, 21.32. HRMS calcd. for C23H19FN8: 409.1703, found 409.1723.
3-(4-Fluorophenyl)-N-((4-methoxypyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (63)
Similar reaction of 10h (0.144 g, 0.445 mmol) with 4-methoxypyridin-2-yl)methanamine (0.0933 g, 0.534 mmol) gave 63 (0.166 g, 88%). Purity (HPLC) 98.8%; mp 205–208 °C. 1H NMR (CDCl3, 400 MHz) δ 8.50 (d, J = 5.8 Hz, 1H), 8.35 (s, 1H), 8.20–8.10 (m, 4H), 7.52–7.45 (m, 4H), 7.18–7.11 (m, 2H), 6.89 (d, J = 2.4 Hz, 1H), 6.80 (dd, J = 5.8, 2.5 Hz, 1H), 6.48 (s, 1H), 4.78 (d, J = 5.4 Hz, 2H), 3.85 (s, 3H). 13C NMR (CDCl3) δ 166.78, 162.54, 160.12, 157.55, 156.89, 151.03, 146.96, 145.49, 141.80, 138.89, 133.28, 132.36, 132.26, 132.16, 132.13, 130.04, 129.34, 129.31, 129.08, 128.88, 128.77, 128.65, 127.60, 127.58, 127.53, 115.77, 115.56, 109.21, 109.02, 107.67, 83.26, 55.50, 47.12. HRMS calcd. for C25H20FN5O: 425.1652, found 425.1659.
3-(4-Fluorophenyl)-5-phenyl-N-((4-(pyrrolidin-1-yl)pyridin-2-yl)methyl)pyrazolo[1,5-a]pyrimidin-7-amine (64)
Similar reaction of 10h (0.20 g, 0.62 mmol) with (4-(pyrrolidin-1-yl)pyridin-2-yl)methanamine hydrochloride (0.16 g, 0.74 mmol) gave 64 (0.10 g, 35%). Purity (HPLC) 96.5%; mp 221–223 °C. 1H NMR (CDCl3) δ 8.34 (s, 1H), 8.23 (d, J = 6.0 Hz, 1H), 8.21–8.11 (m, 4H), 7.54–7.43 (m, 4H), 7.14 (br t, J = 7.0 Hz, 2H), 6.49 (s, 1H), 6.42 (d, J = 2.5 Hz, 1H), 6.34 (dd, J = 6.0, 2.5 Hz, 1H), 4.68 (d, J = 5.1 Hz, 2H), 3.29 (m, 4H), 2.01 (m, 4H). HRMS calcd. for C28H26FN6 (M + H+) m/z 465.2197, found 465.2206.
3-(4-Fluorophenyl)-N-((4-(4-methylpiperazin-1-yl)pyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (65)
Similar reaction of 10h (0.20 g, 0.62 mmol) with (4-(4-methylpiperazin-1-yl)pyridin-2-yl)methanamine (0.21 g, 0.74 mmol) gave 65 (0.18 g, 59%). Purity (HPLC) 100%; mp 203–205 °C. 1H NMR (CDCl3) δ 8.35 (s, 1H), 8.32 (d, J = 6.0 Hz, 1H), 8.20–8.11 (m, 4H), 7.53–7.44 (m, 4H), 7.15 (br t, J = 7.0 Hz, 2H), 6.73 (d, J = 2.5 Hz, 1H), 6.64 (dd, J = 6.0, 2.5 Hz, 1H), 6.49 (s, 1H), 4.70 (d, J = 5.0 Hz, 2H), 3.36 (t, J = 5.1 Hz, 4H), 2.51 (t, J = 5.1 Hz, 4H), 2.33 (s, 3H). HRMS calcd. for C29H29FN7 (M + H+) m/z 494.2463, found 494.2466.
3-(4-Fluorophenyl)-N-((4-morpholinopyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (66)
Similar reaction of 10h (0.20 g, 0.62 mmol) with (4-morpholinopyridin-2-yl)methanamine (0.20 g, 0.74 mmol) gave 66 (0.19 g, 64%). Purity (HPLC) 99%; mp 208 °C. 1H NMR (CDCl3) δ 8.35 (s, 1H), 8.35 (d, J = 6.0 Hz, 1H), 8.20–8.11 (m, 4H), 7.53–7.44 (m, 4H), 7.16 (br t, J = 7.0 Hz, 2H), 6.73 (d, J = 2.5 Hz, 1H), 6.64 (dd, J = 6.0, 2.5 Hz, 1H), 6.48 (s, 1H), 4.71 (d, J = 5.0 Hz, 2H), 3.82 (t, J = 4.9 Hz, 4H), 3.28 (t, J = 5.1 Hz, 4H). HRMS calcd. for C28H26FN6O (M + H+) m/z 481.2147, found 481.2153.
N1-(2-(((3-(4-Fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-yl)amino)methyl)pyridin-4-yl)-N1,N2,N2-trimethylethane-1,2-diamine (67)
Similar reaction of 10h (0.20 g, 0.62 mmol) with N1-(2-(aminomethyl)pyridin-4-yl)-N1,N2,N2-trimethylethane-1,2-diamine dihydrochloride (0.21 g, 0.74 mmol) gave 67 (0.21 g, 70%). Purity (HPLC) 96.5%; mp 158–160 °C. 1H NMR (CDCl3) δ 8.34 (s, 1H), 8.26 (d, J = 6.0 Hz, 1H), 8.20–8.11 (m, 4H), 7.53–7.44 (m, 4H), 7.16 (br t, J = 6.9 Hz, 2H), 6.55 (d, J = 2.5 Hz, 1H), 6.49 (s, 1H), 6.47 (dd, J = 6.0, 2.5 Hz, 1H), 4.69 (d, J = 5.3 Hz, 2H), 3.46 (t, J = 7.3 Hz, 2H), 2.99 (s, 3H), 2.40 (t, J = 7.3 Hz, 2H), 2.21 (s, 6H). HRMS calcd. for C29H31FN7 (M + H+) m/z 496.2631, found 496.2628.
3-(4-Fluorophenyl)-5-phenyl-N-((4-(piperidin-1-yl)pyridin-2-yl)methyl)pyrazolo[1,5-a]pyrimidin-7-amine (68)
Similar reaction of 10h (0.0542 mg, 0.167 mmol) with (4-(piperidin-1-yl)pyridin-2-yl)methanamine (0.064 g, 0.34 mmol) gave 68 (0.023 g, 29%). Purity (HPLC) 98.4%; mp 189–191 °C. 1H NMR (CDCl3) δ 8.34 (s, 1H), 8.28 (d, J = 6.1 Hz, 1H), 8.20–8.12 (m, 4H), 7.53–7.44 (m, 4H), 7.18–7.11 (m, 2H), 6.71 (d, J = 2.4 Hz, 1H), 6.62 (dd, J = 6.1, 2.6 Hz, 1H), 6.49 (s, 1H), 4.68 (d, J = 5.2 Hz, 2H), 3.36–3.31 (m, 4H), 1.66–1.60 (m, 6H). 13C NMR (CDCl3) δ 162.51, 160.08, 157.54, 155.88, 155.64, 149.98, 147.10, 145.52, 141.73, 138.97, 129.99, 129.80, 129.42, 129.39, 129.05, 128.86, 127.79, 127.59, 127.56, 127.49, 124.93, 120.64, 120.59, 115.76, 115.54, 108.87, 107.78, 105.43, 83.32, 47.53, 47.46, 25.28, 24.45. HRMS calcd. for C29H27FN6: 478.2281, found 478.2300.
N-((3-(Dimethylamino)pyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (69)
Similar reaction of 10h (0.0696 g, 0.215 mmol) with 2-(aminomethyl)-N,N-dimethylpyridin-3-amine (0.039 g, 0.258 mmol) gave 69 (0.058 g, 62%). Purity (HPLC) 98.2%; mp 167–170 °C. 1H NMR (CDCl3) δ 8.42 (dd, J = 4.7, 1.4 Hz, 1H), 8.36 (s, 1H), 8.22–8.16 (m, 4H), 8.08 (t, J = 4.6 Hz, 1H), 7.56–7.48 (m, 4H), 7.29–7.27 (m, 1H), 7.16–7.12 (m, 2H), 6.59 (s, 1H), 4.81 (d, J = 4.7 Hz, 2H), 2.81 (s, 6H). HRMS calcd. for C26H23FN6: 438.1968, found 438.1985.
3-(4-Fluorophenyl)-N-((3-fluoropyridin-2-yl)methyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (70)
Similar reaction of 10h (0.0815 g, 0.252 mmol) with (3-fluoropyridin-2-yl)methanamine (0.0491 g, 0.302 mmol) gave 70 (0.080 g, 77%). Purity (HPLC) 99.0%; mp 165–168 °C. 1H NMR (CDCl3,) δ 8.53–8.50 (m, 1H), 8.38 (s, 1H), 8.21–8.15 (m, 4H), 7.75 (t, J = 4.8 Hz, 1H), 7.57–7.47 (m, 4H), 7.35 (qui, J = 4.4 Hz, 1H), 7.18–7.12 (m, 2H), 6.62 (s, 1H), 4.87 (dd, J = 5.0, 1.5 Hz, 2H). HRMS calcd. for C24H17F2N5: 413.1452, found 413.1452.
N-((3-Bromopyridin-2-yl)methyl)-3-(4-fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-amine (71)
Similar reaction of 10h (0.0652, 0.201 mmol) with (3-bromopyridin-2-yl)methanamine (0.054 g, 0.24 mmol) gave 71 (0.048 g, 50%). Purity (HPLC) 98.9%; mp 189–191 °C. 1H NMR (CDCl3) δ 8.68 (dd, J = 4.7, 1.4 Hz, 1H), 8.38 (s, 1H), 8.23–8.16 (m, 4H), 8.07 (t, J = 4.4 Hz, 1H), 7.96 (dd, J = 8.0, 1.4 Hz, 1H), 7.58–7.47 (m, 3H), 7.25–7.21 (m, 1H), 7.19–7.12 (m, 2H), 6.60 (s, 1H), 4.82 (d, J = 4.6 Hz, 2H). HRMS calcd. for C24H17BrFN5: 473.0651, found 473.0660.
2-(((3-(4-Fluorophenyl)-5-phenylpyrazolo[1,5-a]pyrimidin-7-yl)amino)methyl)nicotinonitrile (72)
In DMF (2 mL), 71 (0.051 g, 0.108 mmol), zinc cyanide (0.038 g, 0.323 mmol) and Pd(PPh3)4 (0.031 g, 0.027 mmol) were purged with N2 for 10 min. The reaction was sealed in a sealed tube and heated at 115 °C for 4 h. The reaction mixture was added to water, extracted with EtOAc and was dried over anhydrous sodium sulfate. The solvent was removed to give the crude product, which was purified by silica column chromatography using hexanes:EA (2:1) as eluent to give 72 (0.09 g, 20%). Purity (HPLC) 98.8%; mp 241–244 °C. 1H NMR (DMSO) δ 8.79 (dd, J = 4.9, 1.6 Hz, 1H), 8.69 (s, 1H), 8.52 (t, J = 6.1 Hz, 1H), 8.38 (dd, J = 7.8, 1.6 Hz, 1H), 8.33–8.28 (m, 2H), 8.24–8.20 (m, 2H), 7.57–7.50 (m, 4H), 7.32–7.25 (m, 2H), 6.94 (s, 1H), 5.18 (d, J = 6.0 Hz, 2H). HRMS calcd. for C25H17FN6: 420.1499, found 420.1528.