Abstract
Nature presents a wide range of biomolecules with pharmacological potential, including venomous animal proteins. Among the protein components from snake venoms, phospholipases (PLA2) are of great importance for the development of new anticancer compounds. Thus, we aimed to evaluate the PLA2 anticancer properties from Bothrops moojeni venom. The crude venom was purified through three chromatographic steps, monitored by enzymatic activity and SDS-PAGE (12%). The purified PLA2 denominated BmPLA2 had its molecular mass and N-terminal sequence identified by mass spectrometry and Edman degradation, respectively. BmPLA2 was assayed against human epithelial colorectal adenocarcinoma cells (Caco-2), human rhabdomyosarcoma cells (RD) and mucoepidermoid carcinoma of the lung (NCI-H292), using human fibroblast cells (MRC-5) and microglia cells (BV-2) as a cytotoxicity control. BmPLA2 presented 13,836 Da and a 24 amino acid-residue homologue with snake PLA2, which showed a 90% similarity with other Bothrops moojeni PLA2. BmPLA2 displayed an IC50 of 0.6 µM against Caco-2, and demonstrated a selectivity index of 1.85 (compared to MRC-5) and 6.33 (compared to BV-2), supporting its selectivity for cancer cells. In conclusion, we describe a new acidic phospholipase, which showed antitumor activity and is a potential candidate in the development of new biotechnological tools.
1. Introduction
Cancer is a generic term for several diseases characterized by abnormal cell growth, with the potential to invade and spread to other tissues, thus becoming one of the most important health problems and responsible for the third largest number of deaths worldwide. The high incidence of patients’ morbidity and mortality is triggered by several molecular and anatomical subtypes of cells, requiring specific treatment strategies [1].
In 2020, 19.3 million cases and 10 million cancer deaths were estimated worldwide, where the most diagnosed were: prostate (7.3% of new cases), colorectal (10%), lung (11.4%) and breast (11.7%) cancer. Lung and colorectal cancer are the deadliest types of cancer, accounting for 1.8 million and 800,000 deaths, respectively, representing nearly 28% of all cancer deaths [2].
In addition to the high mortality rates, anticancer therapies involve invasive procedures, such as catheter-administered chemotherapy, surgical removal procedures and the use of non-selective cytotoxic drugs. Thus, bioprospection for new active drugs for cancer therapy is one of the goals of biotechnology, which depends directly on the extraction and purification of toxins and secondary metabolites from microorganisms, plants and animals [3,4].
Snake venom toxin’s ability to cause toxicity is associated with its high number of different molecules acting on the cells and tissues. Despite their toxicological effects, these biological samples possess a complex mixture of different components, including peptides, proteins, enzymes, carbohydrates and minerals. However, isolated compounds, such as proteins and peptides, have the potential application as pharmaceutical agents. The enzyme groups frequently found are L-amino acid oxidases, serine proteases, metalloproteases and phospholipases A2 (PLA2) [5,6,7].
Due to their role in a large number of human inflammatory diseases, PLA2 presents a medical–scientific interest. The classification of this protein group occurs according to the site of hydrolysis, where PLA2 are enzymes that catalyze the hydrolysis of phospholipids at the sn-2 position, releasing free fatty acids, arachidonic acid and lysophospholipids. By consequence, they play important roles in the metabolism of dietary and structural lipids in cell membranes. Hydrolysis of the lipids in cell membranes leads to a loss of their structure, impairing their selective permeability. As a consequence of this activity, svPLA2 has a diverse antimicrobial activity, being able to act on cultures of pathogenic bacteria, fungi, protozoa, viruses and tumor lineages [8].
Previous reports demonstrated that Bothrops svPLA2 from snake venom presents antitumor activity. BthA-I-PLA2 from B. jararacussu showed antitumor activity in leukemia (Jurkat), human breast tumor (BR-3) and Ehrlich ascites (EAT) lines; BmooTX-I (B. moojeni) and MTX-I (B. brazili) indicating activity against leukemic lineage (Jurkat); and Myotoxin III (B. asper) demonstrating cytotoxicity in adrenal tumors. In addition, other molecules of svPLA2 presented antitumor activity, such as PLA2 RVV-7 (Daboia russeli) showing activity against melanoma (B16F10), MVL-PLA2 (Macrovipera lebetina) with activity against fibrosarcoma, melanoma, adenocarcinoma and leukemia, and F1 CTX (Crotalus durissus terrificus) indicating cytotoxicity against cervical and esophagus cancer cell lines [9,10,11,12,13].
In an attempt to seek new alternative therapies, nature is an inexhaustible reservoir of compounds with the potential to treat a wide spectrum of diseases. In this context, we report, for the first time, a purified phospholipase A2 isoform from B. moojeni venom (BmPLA2), which displayed anticancer activity against colorectal adenocarcinoma (Caco-2) and human rhabdomyosarcoma (RD) cell lines.
2. Results
2.1. Purification and Biochemical Characterization
B. moojeni crude venom was fractionated by molecular exclusion chromatography on a Sephacryl S-100 column, resulting in five peaks, named F1 to F5 (Figure 1A). Peak F3 was selected for the next chromatographic step, based on enzymatic assay and its protein profile on SDS-PAGE. When F3 was separated in a C18 column, four peaks were collected, named F3.1 to F3.4 (Figure 1B). The peak F3.4 was chosen according to the above-mentioned parameters. As the last purification step, F3.4 was resubmitted to the RP-HPLC C18 column (Figure 1C), confirming the presence of a single peak, named BmPLA2. The BmPLA2 was eluted with 52.4% of solvent B.
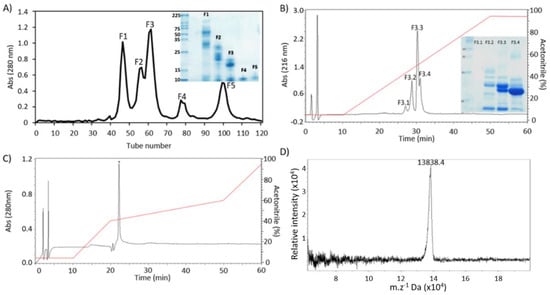
Figure 1.
Purification steps of BmPLA2 from B. moojeni venom. (A) Separation profile of B. moojeni venom on Sephacryl S-100 column using 50 mM Ambic, pH 7.8. Inset: 12% SDS-PAGE showing peaks F1 to F5. (B) Profile of F3 peak on a RP-HPLC C18 column. Elution was performed using a linear gradient 5–95% of solvent B, for 60 min, at a flow rate of 1 mL·min−1. Inset, 12% SDS-PAGE of peaks F3.1 to F3.4. (C) Profile of F3.4 on a RP-HPLC C18 column: Elution was performed using a stepwise gradient: 5% of solvent B to 10 min, 5–40% to 10–20 min, 40–60% to 20–50 min, 60–95% to 50–60 min at a flow rate of 1 mL·min−1. (D) Molecular mass of BmPLA2, 13,838.4 Da, determined by MALDI-ToF.
To determine the BmPLA2 intact mass, a sample was subjected to MALDI-ToF analysis, which showed a protein with 13,838.4 Da (Figure 1D). Furthermore, the combination of HPLC and MALDI-ToF confirmed the molecular homogeneity of BmPLA2, since a single peak was noticed in both analyses. Through Edman degradation, the first 24 amino acid residues from the BmPLA2 N-terminal sequence were sequenced. The obtained sequence was NH2-FKWQFEMLIMKIAKTSGFMFYSSY-COOH. This sequence was submitted to the NCBI Protein BLAST and three PLA2 sequences with high sequential similarity were found. The PLA2 BmooPLA2 (B. moojeni), Tgc-E6 (Tantilla gracilis) and D1E6b (Cerrophidion godmani), showed a 95, 77 and 77% similarity, respectively, (Table 1). The presence of 15 identical residues and five conserved substitutions suggests that BmPLA2 belongs to the PLA2 family. The most diverse region in the PLA2 was in the first three amino acids. However, for PLA2 from the Bothrops genus, Trp-3 occupied an identical position.

Table 1.
Multiple alignment of PLAs N-terminal demonstrated region of similarity with BmPLA. PLA2 from B. moojeni venom; Tgc-E6 from Tantilla gracilis venom and D1E6b from Cerrophidion godmani venom, with a 77% identity. Positions with fully conserved residue (*); One of the high-scoring groups is conserved (:).
2.2. Enzymatic Activity
Enzymatic activity assays were carried out to monitor PLA2 activity in the fractions collected in the chromatographic steps (Figure 2). The substrate used, 4N3OBA, is specific for PLA2 activity. BmPLA2 showed the highest PLA2 activity when compared to the positive control and the fractions obtained in the chromatographic steps, presenting an activity that was 34% higher than the commercial PLA2.
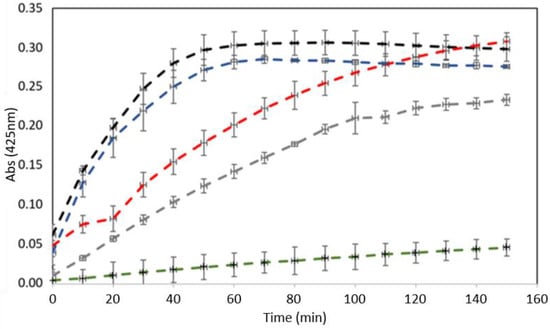
Figure 2.
Enzymatic activity of BmPLA2 incubated with specific substrate (4N3OBA). Legend color: BSA, negative control (green); commercial PLA2 (gray); B. moojeni crude venom (black); peak F3 from Sephacryl S-100 (blue); and BmPLA2 (red).
2.3. Hemolytic Activity
The hemolytic assay indicated that BmPLA2 displayed a low activity against mouse erythrocytes, when compared to the Triton X-100, indicating less than 3% of hemolysis in a high concentration (37 µM).
2.4. Anticancer Activity
Cytotoxicity against MRC-5, BV-2, Caco-2, NCI and RD cells was determined by cellular viability assay using MTT. The CACO-2 and RD cells’ viability was significantly inhibited by BmPLA2, while the NCI lineage did not undergo a significant reduction in the evaluated concentrations. At the highest concentration used (9.25 µM), 70% of the inhibition was noticed for the Caco-2 cell viability (Figure 3). In addition, the RD strain showed an approximately 60% reduction in cell viability at all concentrations evaluated. In comparison, for MRC-5 cells, at 9.25 µM, BmPLA2 prompted a reduction in cellular viability of 70% and the BV-2 cells showed a reduction in cell viability of approximately 40% (at 9.25 µM). Against the NCI tumor lineage, it was observed that cell viability did not show any differences between the concentrations evaluated, reducing approximately 30% of the cell viability.
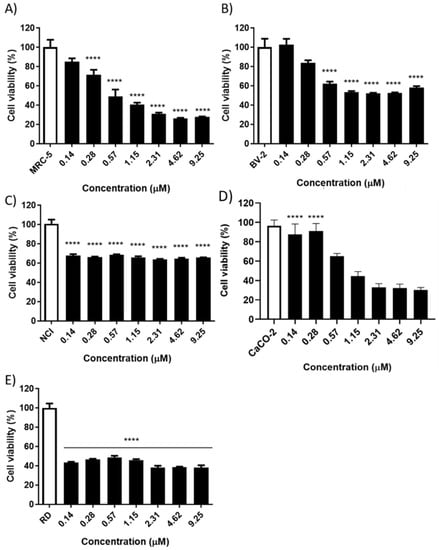
Figure 3.
Assay of cellular viability investigating the effects of BmPLA2 on cancer and healthy cells. (A) Healthy human fibroblast cell line (MRC-5); (B) Microglia cell line; (C) Mucoepidermoid carcinoma of the lung (NCI-H292); (D) Human colorectal cancer cell line (Caco-2); (E) Human rhabdomyosarcoma cells (RD). All cell lines were treated with BmPLA2 (9.25, 4.62, 2.31, 1.15, 0.57 and 0.28 µM) for 24 h. The viability was determined using MTT reagent. All data were expressed as mean ± S.E.M and procedures were carried out in duplicate. **** means differences between the groups were considered statistically significant.
Based on the IC50 obtained for cancer and healthy cells, we calculated the selectivity index (SI) (Table 2). Using MRC-5 as a comparison, in the Caco-2 strains, the SI values were 1.85, while when comparing with the BV2, the SI values were 6.33, suggesting that BmPLA2 possesses selectivity for cancer cell membranes in comparison with healthy membranes. The SI values for the NCI and RD strains were not calculated because the IC50 of these strains could not be obtained. To obtain this value in the RD strain, it would be necessary to repeat the evaluation, using lower concentrations of the BmPLA.

Table 2.
IC50 and selectivity index of MRC-5, BV-2, Caco-2 and RD for BmPLA2.
3. Discussion
Snake venoms are a rich source of new potential therapeutic compounds, presenting a wide range of pharmacological activities. All venoms showing biotechnological potential should be investigated through protein purification methods. Thus, the isolation and functional characterization of components will provide a basis for understanding the venom mechanisms of action and/or future molecular design [14].
PLA2 has been successfully isolated from snake venom using different chromatographic techniques. A general consensus is held that the PLA2 purification strategies involve the combination of types of gel filtration reverse phase chromatography, such as for venoms from the Bothrops genus (BA SpII RP4 from B. alternatus, BaPLA2-I and BaPLA2- III from B. atrox), and PLA2 from another genus (Pgo K49 from C. goodmani, Cdcum6 from Crotallus durissus cumanensis, LmTX-I, LmTX-II from Lachesis muta). Beyond the classic strategy used here, other strategies combining gel filtration and ion-exchange, anion-exchange followed by cation-exchange, and ion-exchange followed by a reverse phase have been reported for PLA2 purification [15,16,17,18,19,20].
The acetonitrile percentage required for the elution of BmPLA2 was 52.2%, with a similar value for BaTX and BmTX-I, and a PLA2 from B. alternatus and B. moojeni was eluted with about 50% acetonitrile in RP-HPLC. The svPLA2 purification investigations, and svPLA2 using RP-HPLC with a C18 column, demonstrated that the percentage of acetonitrile needed to elute the molecule is close to 60%, for example, Bmaj-9 (B. marajoensis) and Cdr-13 (C. durissus ruruima). This reflects the similarity of the overall folding of the amino acids into the primary sequence, shared by snake PLA2 [21,22,23,24].
BmPLA2 showed a molecular mass of 13,838.4 Da, determined by mass spectrometry. The pattern of molecular mass of further PLA2 can be highlighted, such as PrTX-III, from B. pirajai (13,867 Da); Mtx-I, from B. brazili (13,870 Da); Myotoxin-IV, from B. asper (13,886 Da); BmooPLA2, from B. moojeni (13,601 Da); and a PLA isoform from B. erythromelas (13,656 Da). Furthermore, BmooPLA2 showed a 95% identity with BmPLA2 through an amino-terminal sequence (Figure 3). The amino-terminal sequencing of BmPLA2 showed homology with other snake PLA2, sharing the highest identity with BmooPLA2. These two types of PLA2 differed only by three amino acids: Phe1, Lys2 and Met19, which in BmPLA2 corresponded to Asn1, Leu2 and Leu19 [25,26,27,28].
Enzymatic activity with the specific substrate was performed to track the PLA2 through the purification process. Regarding PLA2 activity, the tracking might be performed by direct and indirect methods. First, the direct detection uses chromogenic substrates, such as 4N3OBA, which, when the hydrolysate releases a colorimetric product, is detected at 425 nm. As described in our results, BmPLA2 and the fractions assayed showed catalytic activity when compared to the positive control (bovine pancreas PLA2), which indicates that the specific substrate used was consumed by BmPLA2. Observing Figure 2, we noted that BmPLA2 presented catalytic activity superior to commercial PLA2, showing 34% greater activity, at the same concentrations.
The enzymatic activity of svPLA2 has been reported in previous works. Normally, PLA2 Asp49 has catalytic activity, as seen in sPLA2 from C. durissus collilineatus and PrTX-IIIB from B. pirajai. In contrast, the replacement of position 49 by a Lys49 indicates a reduction or even absence of catalytic activity of svPLA2, such as Basp-III (B. asper), BthTXI (B. jararacussu) or BnSP6 (B. matogrossensis). Thus, the enzymatic activity observed for BmPLA2 supports the hypothesis that position 49 contains an Asp residue (Asp49), due to its catalytic activity [29,30,31,32,33].
Low hemolytic activity observed for BmPLA2 (~3%) at the highest concentration evaluated (37 µM) is a common feature among svPLA2. BE-I-PLA2, a PLA2 from B. erythromelas, did not show hemolysis at the highest concentration tested (37.5 µM) and presented a greater catalytic activity than the evaluated control (bovine PLA2), at the concentration of 1 mg·mL−1. Three PLA2 (BdTX-I, BdTX-II and BdTX-III) found in B. diporus, also did not show hemolytic activity at the concentrations evaluated (10 µg·mL−1/ 0.8 μM). From the B. jararacussu venom, four PLA2 were obtained (SIIISPIIA, SIIISPIIB, SIIISPIIIA and SIIISPIIIB), and only SIIISPIIIB showed hemolytic activity at 13.3 µM (95.4%) [34].
Therefore, the high catalytic activity of PLA2 is not correlated with hemolytic activity, which is an important finding that supports its safe use as a model for bioprospection for new drugs. On the other hand, VRV-PL-VIIIa, from Daboia russeli, showed 100% hemolysis at 5 µg. A further study demonstrated that BmooPLA2, from B. moojeni, showed hemolytic activity at 0.07 µM. Indirect hemolytic activity was found in sheep blood, when incubated with 47.26 µM of a PLA2 from the venom of B. alternatus. This information confirms that the pharmacological activities present in PLA2 do not depend only on the catalytic activity [16,34,35,36,37,38].
The present study demonstrated that BmPLA2 has significant anticancer activity against Caco-2 (IC50 of 0.6 µM) and RD cells (no IC50), showing lower activity against the healthy strains MRC-5 and BV-2 (IC50 of 1.1 and 3.8 µM, respectively). The first investigation into the anticancer activity of snake venom dates from the 1970s, suggesting that Ancrod, a polypeptide from Agkistrodon rhodostoma, can generate defibrination, reducing the size of a tumor by fibrinolysis [39].
The svPLA2 strains MTX-I and MTX-II, from B. brazili, showed anticancer activity against Jurkat cells (leukemia cells), inhibiting 40% of the antitumor activity with 7.2 µM. BthA-I-PLA2, obtained from the venom of B. jararacussu, demonstrated antitumor activity against three different strains, showing 60% cytotoxicity against EAT (Erlich ascitic tumor), 50% against Jurkat (T-cell leukemia) and 30% against SKBr3 (human breast cancer), using a 7.4 µM (100 µg·mL−1) sample. BthTX-I, a PLA2 from B. jararacussu, presented an IC50 of 6.8 and 8 µM (81.2 and 104.35 µg·mL−1) against SK-BR-3 (human breast cancer cells) and MCF-7 (human breast cancer cells), respectively. In addition, in vivo experiments demonstrated that BthTX-I showed activity against S180 (sarcoma), reducing the tumor size by 76% after 60 days of treatment, when compared to the control [25,36,40,41].
From Daboia russelii venom, Drs-PLA2 was isolated and showed to be able to reduce the cell viability of human skin melanoma (SK-MEL-28) in a dose-dependent manner, with N IC50 of 0.02 µM. Another PLA2 with therapeutic potential against tumor cells, BthA-I- PLA2, from B. jararacussu, showed anticancer activity against human breast SK- BR-3 (20%), T-cell leukemia Jurkat (50%) and Erlich ascitic tumor EAT (70%), at 7.7 µM (100 µg·mL−1). From B. mattogrossensis venom, two PLA2, BmatTX-I and BmatTX- II, were obtained, showing activities against T-cell leukemia (Jurkat) (40 and 50% cytotoxic activity, respectively) and against human breast adenocarcinoma (SK-BR-3) (20% cytotoxic activity), at a concentration of 7.7 µM (100 µg·mL−1). From the B. moojeni toxin, BmooPLA2 showed cytotoxic activity against EAT and Jurkat strains, indicating 60 and 50% cellular growth reduction activity, respectively [10,36,42,43].
The svPLA2 have more than one possible mechanism suggesting the observed antitumor effect. The most observed mechanism, cytotoxicity, among others, occurs through the action of arachidonic acid (generated after the cleavage of phospholipids), which is cytotoxic both as a detergent and as an inducer of mitochondria permeability, as well as causing an uncoupling of oxidative phosphorylation. In addition, svPLA2 can affect cellular adhesion. Tumor growth can be facilitated by the expression of adhesion molecules (e.g., ICAM-1), considered a signal molecule. In contact with a svPLA2, a reduction in the expression and invasion of ICAM-1 in lung cancer cells was observed [44,45].
Another mechanism of PLA2 antitumor action is by acting on growth factors. For example, a svPLA2 from the venom of Vipera ammodytes, named as RVV-7, is able to bind to vascular endothelial growth factor (VEGF-A165), inhibiting the proliferative effect of VEGF on the endothelium. Treatment of melanoma cells (B16F10) with RVV-7 reduced tumor growth in guinea pigs [46].
PLA2 antitumor activities, as well as other antimicrobial activities, may be related to the interactions of the C-terminal region of the molecule with the cell membranes, usually described as capable of disrupting the hydrophilic matrix of membranes. Furthermore, PLA2 can act on the cell cycles of tumor lineages. BthTX-I was able to delay the G0/G1 phase of the cell cycle of PC-12 (pheochromocytoma) and B16F10 (melanoma) tumor cells, indicating that PLA2 interrupts mitotic progression, resulting in a reduction in cell duplication [25,40,41,47].
The SI results showed that BmPLA2 seems to be more selective in tumor cells, with values dependent on the non-tumorigenic lineages to be evaluated. When compared to MRC-5, BmPLA2 presents an SI of 1.35 for the Caco-2 strains. In addition, when compared to BV-2, the SI value is 6.33. The SI value of the NCI and RD lineage was not calculated as it did not have an IC50. This difference in SI values is observed because the healthy cells used in the experiment come from different tissues. The MRC-5 lineage comes from lung tissue composed of fibroblasts, while the BV-2 lineage comes from brain tissue, more specifically, microglial cells.
4. Materials and Methods
4.1. Bothrops Moojeni Venom, Cell Lines and Ethics Committee Guidelines
B. moojeni crude venom was collected from four adult specimens from the Serpentarium at the Universidade Católica Dom Bosco, Campo Grande, Mato Grosso do Sul, Brazil, by direct pressure on the snake venom glands. The cell lines used were as follows: the healthy human fibroblast cell line (MRC-5), the human colorectal cancer cell line (Caco-2), human rhabdomyosarcoma cells (RD) and mucoepidermoid carcinoma of the lung (NCI- H292), which were obtained from the Adolf Lutz Institute, and microglia cells (BV-2) were acquired from the Cell Bank of Rio de Janeiro. Mice (Mus musculus) erythrocytes were also used for assays, approved by the animal ethics committee from the Universidade Católica Dom Bosco under registration number 014/2018.
4.2. B. moojeni Venom Purification
Initially, B. moojeni crude venom (10 mg) was suspended in 1 mL of 50 mM ammonium bicarbonate buffer (Ambic), pH 7.8. Next, the sample was applied onto a molecular exclusion Sephacryl S-100 column (30 × 450 mm, Cytiva, Telangana, India) previously equilibrated with the same buffer, at room temperature. Fractions of 2.5 mL were collected at a flow rate of 0.625 mL·min−1. All fractions were monitored in a Biodrop (Biodrop Touch Duo Spectrophotometer) at 280 nm. The fractions corresponding to five different peaks were pooled and named F1 to F5.
Next, the peak 3 (F3) was applied onto a C18 column (Xterra MS 5 µm—4.6 × 250 mm) in HPLC (Waters e2695). The sample was dissolved with 0.1% TFA:water (v:v) and the elution was performed using a linear concentration gradient of 5–95% of solvent B (acetonitrile + 0.1% TFA) for 60 min at a flow rate of 1 mL·min−1. The protein profile was monitored at 216 nm. The four peaks obtained from F3 separated in Xterra MS 5 column were named F3.1 to F3.4.
As the final chromatographic step, the F3.4 peak was applied onto a C18 column (Xterra MS 5 µm—4.6 × 250 mm column) and separated in a stepwise gradient of solvent B (5% during 10 min, 5–40% 10–20 min, 40–60% 20–50 min and 60–95% 50–60 min). After this step, the sample purified was denominated BmPLA2.
4.3. SDS-PAGE Electrophoresis
Chromatographic fractions and purified BmPLA2 were analyzed by SDS-PAGE 12%, performed in Mini BioRad Gel Electrophoresis (Bio-Rad, Hercules, CA, USA), according to Laemmli (1970). The samples were incubated with a reducing sample buffer, containing β-mercaptoethanol. The gels were separated at 85 V and were stained with Coomassie brilliant blue R250. The PROMEGA® Molecular weight marker (MW) was used (Laemmli, 1970).
4.4. Quantification of Proteins
The protein content from the B. moojeni crude venom, and in all fractions obtained through the chromatography steps, was determined using the Bradford method (Bradford, 1976). Bovine serum albumin (BSA) was used as the standard. The assays were carried out in triplicate.
4.5. Mass Spectrometry
BmPLA2 was analyzed using AutoFlex III matrix-assisted laser desorption ionization time-of-flight (MALDI-ToF) mass spectrometry equipment (Bruker Daltonics, Bremen, Germany) controlled by Flex Control 3.0 software (Bruker Daltonics, Bremen, Germany). The sample (15 μg·μL−1) was mixed with a sinapinic acid matrix solution (1:1, v:v), directly placed onto a target plate and dried at room temperature. The accurate mass of BmPLA2 was obtained in a positive linear mode, after external calibration, using the Protein Calibration Standard (Bruker Daltonics, Bremen, Germany). MALDI spectra were processed with Flex Analysis 3.0 software (Bruker Daltonics, Bremen, Germany).
4.6. Amino-Terminal Sequencing
The N-terminal primary sequence was determined using a Shimadzu PPSQ-31B/33B automated protein sequencer, performing Edman degradation. The equipment was previously calibrated with a phenylthiohydantoin (PTH) amino acids mixture standard and a sample from the lyophilized BmPLA2 was dissolved in 37% acetonitrile and applied on the PVDF membrane, dried under nitrogen flow and then submitted for analysis. The PTH amino acids were detected after separation on a RP-HPLC C18 column (4.6 × 250 mm), according to the manufacturer’s instructions. The resulting sequence was applied to NCBI protein BLAST search (BLASTP 2.8.0+) and the sequences that produced significant were aligned using CLUSTAL Omega (1.2.4).
4.7. Phospholipase Activity
The protocol used for the measurement of phospholipase activity was described by Holzer and Mackessy, with changes proposed by Serino-Silva and co-workers [48,49]. The BmPLA2 and fractions obtained during the purification were evaluated on a 96-well plate using 4-nitro-3-octanoyloxybenzoic acid (4N3OBA, Enzy Life Science, Oyster Bay, New York, NY, USA). Each well received 200 µL of 10 mM Tris-HCl buffer, containing 10 mM CaCl2 and 100 mM NaCl, pH 7.8. Then, 20 µL of 4N3OBA substrate (1 mg·mL−1, dissolved in acetonitrile), 20 µL of water and 20 µL of the source of enzyme (crude venom, F3 and BmPLA2) were added. As a positive control, a commercial PLA2 (Phospholipase A2 from bovine pancreas—P9913 Sigma) was used, and the negative control was performed with BSA, a non-enzymatic protein. The reaction was developed at 37 °C, with readings at 10 min intervals, with a total of 150 min, determined at 425 nm. The assay was carried out in triplicate.
4.8. Hemolytic Assay
Mouse erythrocyte blood was collected and stored at 4 °C until use. The cells were washed three times with a 50 μM phosphate buffer, pH 7.4. The erythrocyte suspension was incubated with B. moojeni venom fractions in serial dilution (512 to 16 μg·mL−1). From the molecular mass of BmPLA2, these concentrations are equivalent to 37 to 1.2 μM in 100 mL final volume. The samples were incubated at room temperature for 60 min. After centrifugation at 3000 rpm, hemoglobin release was monitored by reading the supernatant at 425 nm in a Spectramax microplate reader. The negative control of hemolysis was performed with erythrocytes suspended in a 50 mM phosphate buffer, pH 7.4. The positive control for hemolysis was carried out with 1% triton X-100 solution dissolved in distilled water. The assays were performed in triplicate.
4.9. Anticancer Activity
4.9.1. Cell Culture
Cell lines were cultivated at the Immunology Laboratory (UCDB). MRC-5 and RD cells were maintained in DMEM high glucose medium, BV-2 and NCI-H292 cells were maintained in RPMI-1640 medium and the Caco-2 lineage was maintained in 199 medium. All media were supplemented with 10% fetal bovine serum (FBS) and for the MRC-5, RD, BV-2, NCI strains were added 100 µg·mL−1 penicillin and 100 µg·mL−1 streptomycin (Gibco, São Paulo, Brazil) at 37 °C in an oven with 5% CO2.
4.9.2. Cytotoxicity by MTT Assay
To verify the anticancer activity, the viability of cancer cells for NCIH292, RD and Caco-2, and normal cells MRC-5 and BV-2, were evaluated according to the method adapted from Mosmann (1983), based on the enzymatic reduction of the 3- (4,5-demethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, São Paulo, Brazil) to formazan crystals. Cells were plated at 1 × 104 cells/well-1 in 96-well microplates and treated with 100 µL of different concentrations of BmPLA2 (9.25, 4.62, 2.31, 1.15, 0.57, 0.28 and 0.14 µM) for 24 h. As a negative control, culture medium was used. After the incubation period, the supernatant was removed and 100 µL of MTT solution (1 mg·mL−1 diluted in culture medium) was added to the cells. After 4 h of incubation, the formazan crystals were resuspended with 100 µL of dimethyl sulfoxide (DMSO) and read at 570 nm in a Thermo scientific reader (Model MultiSkan Go). Three independent experiments were carried out in triplicate. Cell viability was calculated from the following formula:
Cell viability (%) = (Abssample ÷ Absnegative control) × 100
4.9.3. Statistics
All assays were carried out in triplicate and the results were expressed as the mean ± S.E.M. Differences between treatments and controls were analyzed by One-Way ANOVA followed by the Dunnet and Bonferroni post-test, using the software GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA).
5. Conclusions
PLA2 isolated from snake venoms is a multifunctional set of proteins displaying great potential for biotechnological application in the medical field. Purification of BmPLA2 from Bothrops moojeni venom resulted in a new protein of 13,868 Da, sharing a high sequential identity with other types of PLA2. In addition to the enzymatic activity, very low hemolytic activity was observed against murine erythrocytes, which makes this novel PLA2 safe to use as a potential pharmacological compound for cancer treatment. Finally, anticancer activity against Caco-2 and RD cells was observed, presenting an SI that indicates the promising application of BmPLA2 for this purpose, supporting further studies and future structural modifications of the molecule.
Author Contributions
Conceptualization, B.E.F.F. and P.H.S.R.; methodology, B.E.F.F., S.C.S., C.F.R.d.O., P.H.d.O.C. and N.V.; software, B.E.F.F., L.M. and C.F.R.d.O.; validation, M.L.R.M., C.F.R.d.O. and N.V.; formal analysis, B.E.F.F., P.H.d.O.C. and A.P.d.A.B.; investigation, B.E.F.F., A.P.d.A.B., N.V. and L.M.; resources, C.M.E.C. and L.M.; data curation, M.L.R.M.; writing—original draft preparation, B.E.F.F. and P.H.S.R.; writing—review and editing, B.E.F.F., L.M., A.P.d.A.B., C.F.R.d.O. and S.C.S.; supervision, L.M.; project administration, B.E.F.F. and L.M.; funding acquisition, C.M.E.C. and M.L.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, grant number 001. This research also was funded by National Council for Scientific and Technological Development, grant number 04/2021. Additionally, this research was funded by FUNDECT, grant number 03/2016.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Universidade Católica Dom Bosco (protocol code 014/2018 and 15 November 2018 of approval).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Redig, A.J.; Mcallister, S.S. Breast Cancer as a Systemic Disease: A View of Metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-Saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic Toxins from Snake Venom: Structural Characterization and Mechanism of Catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake Venom Peptides: Tools of Biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake Venom PLA2, a Promising Target for Broad-Spectrum Antivenom Drug Development. Biomed Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef]
- Quach, N.D.; Arnold, R.D.; Cummings, B.S. Secretory Phospholipase A2 Enzymes as Pharmacological Targets for Treatment of Disease. Biochem. Pharmacol. 2014, 90, 338–348. [Google Scholar] [CrossRef]
- Azevedo, F.V.P.V.; Lopes, D.S.; Cirilo Gimenes, S.N.; Achê, D.C.; Vecchi, L.; Alves, P.T.; Guimarães, D.D.O.; Rodrigues, R.S.; Goulart, L.R.; Rodrigues, V.D.M.; et al. Human Breast Cancer Cell Death Induced by BnSP-6, a Lys-49 PLA2 Homologue from Bothrops Pauloensis Venom. Int. J. Biol. Macromol. 2016, 82, 671–677. [Google Scholar] [CrossRef]
- Cedro, R.C.A.; Menaldo, D.L.; Costa, T.R.; Zoccal, K.F.; Sartim, M.A.; Santos-Filho, N.A.; Faccioli, L.H.; Sampaio, S.V. Cytotoxic and Inflammatory Potential of a Phospholipase A2 from Bothrops Jararaca Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Daniele, J.J.; Bianco, I.D.; Delgado, C.; Carrillo, D.B.; Fidelio, G.D. A New Phospholipase A2 Isoform Isolated from Bothrops Neuwiedii (Yarara Chica) Venom with Novel Kinetic and Chromatographic Properties. Toxicon 1997, 35, 1205–1215. [Google Scholar] [CrossRef]
- De Vasconcelos Azevedo, F.V.P.; Zóia, M.A.P.; Lopes, D.S.; Gimenes, S.N.; Vecchi, L.; Alves, P.T.; Rodrigues, R.S.; Silva, A.C.A.; Yoneyama, K.A.G.; Goulart, L.R.; et al. Antitumor and Antimetastatic Effects of PLA2-BthTX-II from Bothrops Jararacussu Venom on Human Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 135, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Stábeli, R.G.; Amui, S.F.; Sant’Ana, C.D.; Pires, M.G.; Nomizo, A.; Monteiro, M.C.; Romão, P.R.T.; Guerra-Sá, R.; Vieira, C.A.; Giglio, J.R.; et al. Bothrops Moojeni Myotoxin-II, a Lys49-Phospholipase A2 Homologue: An Example of Function Versatility of Snake Venom Proteins. Comp. Biochem. Physiol.-C Toxicol. Pharmacol. 2006, 142, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.C.; Zuliani, J.P.; et al. Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy. Biomed Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Damico, D.C.S.; Lilla, S.; de Nucci, G.; Ponce-Soto, L.A.; Winck, F.V.; Novello, J.C.; Marangoni, S. Biochemical and Enzymatic Characterization of Two Basic Asp49 Phospholipase A2 Isoforms from Lachesis Muta Muta (Surucucu) Venom. Biochim. Biophys. Acta 2005, 1726, 75–86. [Google Scholar] [CrossRef]
- Denegri, M.E.G.; Acosta, O.C.; Huancahuire-Vega, S.; Martins-de-Souza, D.; Marangoni, S.; Maruñak, S.L.; Teibler, G.P.; Leiva, L.C.; Ponce-Soto, L.A. Isolation and Functional Characterization of a New Acidic PLA2 Ba SpII RP4 of the Bothrops Alternatus Snake Venom from Argentina. Toxicon 2010, 56, 64–74. [Google Scholar] [CrossRef]
- Menaldo, D.L.; Jacob-Ferreira, A.L.; Bernardes, C.P.; Cintra, A.C.O.; Sampaio, S.V. Purification Procedure for the Isolation of a P-I Metalloprotease and an Acidic Phospholipase A2 from Bothrops Atrox Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 28. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and Biological Characterization of a PLA2 from Crotoxin Complex of Crotalus Durissus Cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef]
- Stábeli, R.G.; Simões-Silva, R.; Kayano, A.M.; Gimenez, G.S.; Moura, A.A.; Caldeira, C.A.S.; Coutinho-Neto, A.; Zaqueo, K.D.; Zuliani, J.P.; Calderon, L.A. Purification of Phospholipases A2 from American Snake Venoms. In Chromatography—The Most Versatile Method of Chemical Analysis; Calderón, L.A., Ed.; Intechopen: London, UK, 2012; pp. 1–34. [Google Scholar]
- Tsai, I.H.; Chen, Y.H.; Wang, Y.M.; Tu, M.C.; Tu, A.T. Purification, Sequencing, and Phylogenetic Analyses of Novel Lys-49 Phospholipases A(2) from the Venoms of Rattlesnakes and Other Pit Vipers. Arch. Biochem. Biophys. 2001, 394, 236–244. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; Damico, D.C.S.; Ponce-Soto, L.A.; Baldasso, P.A.; Da Silva, S.L.; Souza, G.H.M.F.; Eberlin, M.N.; Marangoni, S. Biological and Biochemical Characterization of New Basic Phospholipase A2 BmTX-I Isolated from Bothrops Moojeni Snake Venom. Toxicon 2008, 51, 1509–1519. [Google Scholar] [CrossRef]
- Galbiatti, C.; Rocha, T.; Randazzo-Moura, P.; Ponce-Soto, L.A.; Marangoni, S.; Cruz-Höfling, M.A.; Rodrigues-Simioni, L. Pharmacological and Partial Biochemical Characterization of Bmaj-9 Isolated from Bothrops Marajoensis Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 62–72. [Google Scholar] [CrossRef][Green Version]
- Ponce-Soto, L.A.; Baldasso, P.A.; Romero-Vargas, F.F.; Winck, F.V.; Novello, J.C.; Marangoni, S. Biochemical, Pharmacological and Structural Characterization of Two PLA2 Isoforms Cdr-12 and Cdr-13 from Crotalus Durissus Ruruima Snake Venom. Protein J. 2007, 26, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Soto, L.A.; Lomonte, B.; Gutiérrez, J.M.; Rodrigues-Simioni, L.; Novello, J.C.; Marangoni, S. Structural and Functional Properties of BaTX, a New Lys49 Phospholipase A2 Homologue Isolated from the Venom of the Snake Bothrops Alternatus. Biochim. Biophys. Acta-Gen. Subj. 2007, 1770, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Menaldo, D.L.; Oliveira, C.Z.; Santos-Filho, N.A.; Teixeira, S.S.; Nomizo, A.; Fuly, A.L.; Monteiro, M.C.; de Souza, B.M.; Palma, M.S. Myotoxic Phospholipases A2 Isolated from Bothrops Brazili Snake Venom and Synthetic Peptides Derived from Their C-Terminal Region: Cytotoxic Effect on Microorganism and Tumor Cells. Peptides 2008, 29, 1645–1656. [Google Scholar] [CrossRef]
- Díaz Oreiro, C.; Lomonte, B.; Zamudio, F.; Gutiérrez, J.M. Purification and Characterization of Myotoxin IV, a Phospholipase A2 Variant, from Bothrops Asper Snake Venom. Nat. Toxins 1995, 3, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L.; et al. Antibiofilm Activity of Acidic Phospholipase Isoform Isolated from Bothrops Erythromelas Snake Venom. Toxins 2013, 12, 35–43. [Google Scholar] [CrossRef]
- Soares, A.M.; Andrião-Escarso, S.H.; Bortoleto, R.K.; Rodrigues-Simioni, L.; Arni, R.K.; Ward, R.J.; Gutiérrez, J.M.; Giglio, J.R. Dissociation of Enzymatic and Pharmacological Properties of Piratoxins-I and-III, Two Myotoxic Phospholipases A2 from Bothrops Pirajai Snake Venom. Arch. Biochem. Biophys. 2001, 387, 188–196. [Google Scholar] [CrossRef]
- Borges, I.P.; Castanheira, L.E.; Barbosa, B.F.; de Souza, D.L.N.; da Silva, R.J.; Mineo, J.R.; Tudini, K.A.Y.; Rodrigues, R.S.; Ferro, E.A.V.; de Melo Rodrigues, V. Anti-Parasitic Effect on Toxoplasma Gondii Induced by BnSP-7, a Lys49-Phospholipase A2 Homologue from Bothrops Pauloensis Venom. Toxicon 2016, 119, 84–91. [Google Scholar] [CrossRef]
- Moreira, V.; Gutiérrez, J.M.; Soares, A.M.; Zamunér, S.R.; Purgatto, E.; Teixeira, C.D.F.P. Secretory Phospholipases A2 Isolated from Bothrops Asper and from Crotalus Durissus Terrificus Snake Venoms Induce Distinct Mechanisms for Biosynthesis of Prostaglandins E2 and D2 and Expression of Cyclooxygenases. Toxicon 2008, 52, 428–439. [Google Scholar] [CrossRef]
- Trento, E.P.; Garcia, O.S.; Rucavado, A.; França, S.C.; Batalini, C.; Arantes, E.C.; Giglio, J.R.; Soares, A.M. Inhibitory Properties of the Anti-Bothropic Complex from Didelphis Albiventris Serum on Toxic and Pharmacological Actions of Metalloproteases and Myotoxins from Bothrops Asper Venom. Biochem. Pharmacol. 2001, 62, 1521–1529. [Google Scholar] [CrossRef]
- Veronese, E.L.G.; Esmeraldino, L.E.; Trombone, A.P.F.; Santana, A.E.; Bechara, G.H.; Kettelhut, I.; Cintra, A.C.O.; Giglio, J.R.; Sampaio, S.V. Inhibition of the Myotoxic Activity of Bothrops Jararacussu Venom and Its Two Major Myotoxins, BthTX-I and BthTX-II, by the Aqueous Extract of Tabernaemontana Catharinensis A. DC.(Apocynaceae). Phytomedicine 2005, 12, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, R.M.; Rabello, M.M.; Araújo, R.M.; Silveira, E.R.; Fagundes, F.H.R.; Diz-Filho, E.; Buzzo, S.C.; Soares, V.C.G.; Toyama, D.D.O.; Gaeta, H.H. Inhibition of Neurotoxic Secretory Phospholipases A2 Enzymatic, Edematogenic, and Myotoxic Activities by Harpalycin 2, an Isoflavone Isolated from Harpalyce Brasiliana Benth. Evid.-Based Complement. Altern. Med. 2012, 2012, 987517. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque Modesto, J.C.; Spencer, P.J.; Fritzen, M.; Valenca, R.C.; Oliva, M.L.V.; Bezerra da Silva, M.; Chudzinski-Tavassi, A.M.; Camargo Guarnieri, M. BE-I-PLA2, a Novel Acidic Phospholipase A (2) from Bothrops Erythromelas Venom: Isolation, Cloning and Characterization as Potent Anti-Platelet and Inductor of Prostaglandin I-2 Release by Endothelial Cells. Biochem. Pharmacol. 2006, 72, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ketelhut, D.F.J.; de Mello, M.H.; Veronese, E.L.G.; Esmeraldino, L.E.; Murakami, M.T.; Arni, R.M.; Giglio, J.R.; Cintra, A.C.O.; Sampaio, S.V. Isolation, Caharacterization and Biological Activity of Acidic Phospholipase A2 Isoforms from Bothrops Jararacussu Snake Venom. Biochimie 2003, 85, 983–991. [Google Scholar] [CrossRef]
- Silveira, L.B.; Marchi-Salvador, D.P.; Santos-Filho, N.A.; Silva Jr, F.P.; Marcussi, S.; Fuly, A.L.; Nomizo, A.; da Silva, S.L.; Stábeli, R.G.; Arantes, E.C. Isolation and Expression of a Hypotensive and Anti-Platelet Acidic Phospholipase A2 from Bothrops Moojeni Snake Venom. J. Pharm. Biomed. Anal. 2013, 73, 35–43. [Google Scholar] [CrossRef]
- Sudharshan, S.; Dhananjaya, B.L. Antibacterial Potential of a Basic Phospholipase A2 (VRV-PL-VIIIa) from Daboia Russelii Pulchella (Russell’s Viper) Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 17. [Google Scholar] [CrossRef][Green Version]
- Vitorino, K.A.; Alfonso, J.J.; Gómez, A.F.; Santos, A.P.A.; Antunes, Y.R.; Caldeira, C.A.D.S.; Gómez, C.V.; Teles, C.B.G.; Soares, A.M.; Calderon, L.A. Antimalarial Activity of Basic Phospholipases A2 Isolated from Paraguayan Bothrops Diporus Venom against Plasmodium Falciparum. Toxicon X 2020, 8, 100056. [Google Scholar] [CrossRef]
- Pizzo, S.V.; Schwartz, M.L.; Hill, R.L.; McKee, P.A. Mechanism of Ancrod Anticoagulation. A Direct Proteolytic Effect on Fibrin. J. Clin. Investig. 1972, 51, 2841–2850. [Google Scholar] [CrossRef]
- Gebrim, L.C.; Marcussi, S.; Menaldo, D.L.; de Menezes, C.S.R.; Nomizo, A.; Hamaguchi, A.; Silveira-Lacerda, E.P.; Homsi-Brandeburgo, M.I.; Sampaio, S.V.; Soares, A.M. Antitumor Effects of Snake Venom Chemically Modified Lys49 Phospholipase A2-like BthTX-I and a Synthetic Peptide Derived from Its C-Terminal Region. Biologicals 2009, 37, 222–229. [Google Scholar] [CrossRef]
- Da Silva, C.P.; Costa, T.R.; Paiva, R.M.A.; Cintra, A.C.O.; Menaldo, D.L.; Antunes, L.M.G.; Sampaio, S.V. Antitumor Potential of the Myotoxin BthTX-I from Bothrops Jararacussu Snake Venom: Evaluation of Cell Cycle Alterations and Death Mechanisms Induced in Tumor Cell Lines. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 44. [Google Scholar]
- Moura, A.A.D.; Kayano, A.M.; Oliveira, G.A.; Setúbal, S.S.; Ribeiro, J.G.; Barros, N.B.; Nicolete, R.; Moura, L.A.; Fuly, A.L.; Nomizo, A.; et al. Purification and Biochemical Characterization of Three Myotoxins from Bothrops Mattogrossensis Snake Venom with Toxicity against Leishmania and Tumor Cells. Biomed Res. Int. 2014, 2014, 195356. [Google Scholar] [CrossRef] [PubMed]
- Khunsap, S.; Pakmanee, N.; Khow, O.; Chanhome, L.; Sitprija, V.; Suntravat, M.; Lucena, S.E.; Perez, J.C.; Sánchez, E.E. Purification of a Phospholipase A2 from Daboia Russelii Siamensis Venom with Anticancer Effects. J. Venom Res. 2011, 2, 42. [Google Scholar] [PubMed]
- Cummings, B.S. Phospholipase A2 as Targets for Anti-Cancer Drugs. Biochem. Pharmacol. 2007, 74, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.A.; Kalatardi, S.; Dohse, J.; Sadaria, M.R.; Meng, X.; Fullerton, D.A.; Weyant, M.J. Group IIa SPLA2 Inhibition Attenuates NF-ΚB Activity and Promotes Apoptosis of Lung Cancer Cells. Anticancer Res. 2012, 32, 3601–3607. [Google Scholar] [PubMed]
- Tokunaga, Y.; Yamazaki, Y.; Morita, T. Identification and Localization of Heparin-Binding Region of Snake Venom VEGF and Its Blocking of VEGF-A165. Pathophysiol. Haemost. Thromb. 2005, 34, 194–196. [Google Scholar]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular Pathology Induced by Snake Venom Phospholipase A2 Myotoxins and Neurotoxins: Common Aspects of Their Mechanisms of Action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef]
- Serino-Silva, C.; Morais-Zani, K.; Hikari Toyama, M.; Toyama, D.D.O.; Gaeta, H.H.; Rodrigues, C.F.B.; Aguiar, W.D.S.; Tashima, A.K.; Grego, K.F.; Tanaka-Azevedo, A.M. Purification and Characterization of the First γ-Phospholipase Inhibitor (ΓPLI) from Bothrops Jararaca Snake Serum. PLoS ONE 2018, 13, e0193105. [Google Scholar] [CrossRef]
- Holzer, M.; Mackessy, S.P. An Aqueous Endpoint Assay of Snake Venom Phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).