Abstract
Microbial infections are leading causes of death and morbidity all over the world due to the development of the resistance to antibiotics by certain microorganisms. In this study, the chemical exploration of the ethanol (EtOH) extract of the aerial part of Dracaena stedneuri (Dracaenaceae) led to the isolation of one previously unreported chalcone derivative, i.e., 2′,4′-dihydroxy-2,3′-dimethoxychalcone (1), together with 12 known compounds: 8-(C)-methylquercetagetin-3,6,3′-trimethyl ether (2), methylgalangine (3), quercetin (4), kaempferol (5), 6,8-dimethylchrysin (6), ombuine-3-O-rutinoside (4ʹ,7-dimethylquercetin-3-O-α-L-rhamnopyranosyl-(1 → 6) -β-D-glucopyranoside) (7), alliospiroside A (8), β-sitosterol 3-O-glucopyranoside (9), ishigoside (10), betulinic acid (11), oleanolic acid (12), and lupeol (13). The structures were determined by spectroscopic and spectrometric analysis including 1- and 2-Dimensional Nuclear Magnetic Resonance (1D- and 2D-NMR), High-Resolution Electrospray Ionization Mass Spectrometry (HRESIMS), and comparison with literature data. The isolated secondary metabolites and crude extract displayed antibacterial activity against some multidrug-resistant strains with minimal inhibitory concentration (MIC) values ranging from 32 to 256 μg/mL. The antibacterial activity of compound 13 against Enterobacter aerogenes ATCC13048 (MIC value: 32 μg/mL) was higher than that of chloramphenicol used as the reference drug (MIC = 64 μg/mL).
1. Introduction
Antibiotics are used in the treatment of infectious diseases, which still represent an important source of mortality in the world. However, the broad and incorrect uses of antimicrobial agents led to the development of microbial resistance [1]. It is therefore necessary to find new effective solutions to combat these multidrug-resistant (MDR) microorganisms. Plant extracts and isolated compounds could constitute an alternative solution to this problem because some of them are used in traditional medicine to treat several ailments, including microbial infections. The genus Dracaena (Dracaenaceae) contains approximately 100 species of shrubs and trees spread in tropical and subtropical regions of the world [2,3]. Dracaena steudneri Engl. is distributed in the DR Congo, Ethiopia, and East to southern African countries. It is an evergreen tree of about 5 m in height with pale white-yellow-green flowers and green fruits [4,5]. The extract from this plant is used in traditional medicine in Tanzania to cure splenomegaly, hernias, asthma, and chest problems [6], and in Rwanda to treat liver diseases [7]. In Kenya, the extract from the stem is used to manage hepatic liver ailments, is a cure for measles, and reduces pain during childbirth [8,9]. The previous phytochemical study of its leaves led to the discovery of six new flavonoids together with thirteen known congeners [9]. As part of our research program based on the exploration of bioactive secondary metabolites from Dracaenaceae [10,11,12,13], we describe in the present paper the isolation and structure elucidation of thirteen compounds (Figure 1) including a new chalcone derivative from the EtOH extract of D. steudneri. Furthermore, the EtOH extract, the ethyl acetate, n-butanol soluble fractions, and some isolated metabolites were screened for their antibacterial activity against a panel of MDR bacteria.
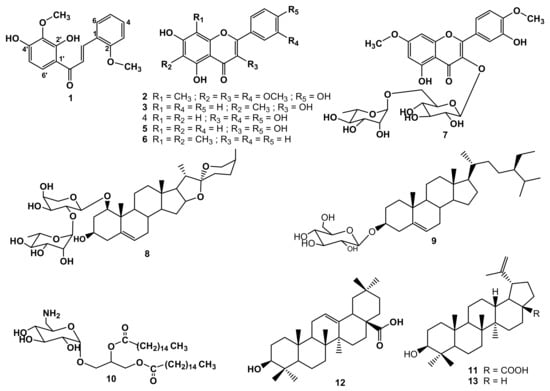
Figure 1.
Structures of secondary metabolites obtained from D. steudneri (1–13).
2. Results and Discussion
The phytochemical investigation of D. steudneri led to the discovery of a new chalcone derivative: 2′,4′-dihydroxy-2,3′-dimethoxychalcone (1), together with twelve known compounds. The known compounds were identified as: 8-C-methylquercetagetin-3,6,3′-trimethyl ether (2) [14], methylgalangine (3) [15], quercetin (4) [16], kaempferol (5) [17], 6,8-dimethylchrysin (6) [18], ombuine-3-O-rutinoside (4′,7-dimethylquercetin-3-O-α-L-rhamnopyranosyl-(1 → 6)-β-D -glucopyranoside) (7) [19], alliospiroside A (8) [20], β-sitosterol 3-O-glucopyranoside (9) [21], ishigoside (1,2-(dipalmitoyl)-3-O-α-D-(6-desoxy-6-amino-glucopyranosyl)glycerol) (10) [22], betulinic acid (11) [23], oleanolic acid (12) [24], and lupeol (13) [24] (Figure 1).
The HRESIMS of compound 1 obtained as a yellow powder, presented a sodium adduct at m/z 323.0887 [M+Na]+ (calcd. for C17H16O5Na+: 323.0895). Its 1H-NMR spectrum revealed two aromatic proton resonances at δH 7.55 (d, 1H, J = 8.8 Hz, H-6′) and 6.48 (d, 1H, J = 8.9 Hz, H-5′). Resonances of four aromatic protons were also depicted at δH 7.55 (brd, 1H, J = 8.8 Hz, H-6); 7.32 (ddd, 1H, J = 8.7, 7.4, 1.7 Hz, H-4); 6.93 (td, 1H, J = 7.5, 1.0 Hz, H-5); and 6.88 (dd, 1H, J = 8.4, 1.0 Hz, H-3) suggesting the presence of one ortho disubstituted aromatic ring [9]. It also exhibited signals of two trans-oriented olefinic protons at δH 8.12 (d, 1H, J = 15.6 Hz, H-β) and 7.62 (d, 1H, J = 15.6 Hz, H-α) characteristic of chalcones [25,26]. The proton resonances observed at δH 3.95 (s, 3H, 3′-OMe) and δH 3.86 (s, 3H, 2-OMe) evidenced the existence of two methoxyl groups (Table 1 and Figure S1,Figure S2,Figure S3,Figure S4,Figure S5,Figure S6).

Table 1.
13C- and 1H-NMR spectroscopic data of compound 1 (100 and 400 MHz, resp.; CDCl3; δ in ppm).
The 13C-NMR spectrum displayed seventeen resonances including a carbonyl at δC 193.2 and an α,β-unsaturated system with resonances depicted at δC 120.9 (C-α) and 140.3 (C-β), characteristic of a chalcone skeleton [25,27]. The signals of two methoxyl groups were observed at δC 60.8 (3′-OCH3) and 55.6 (2-OCH3). The 1H and 13C signals were totally assigned by inspection of the Correlation Spectroscopy (1H-1H COSY) spectrum combined with data from Heteronuclear Single Quantum Coherence (HSQC) and Heteronuclear Multiple Bond Correlation (HMBC) spectra (Table 1). The location of the methoxyl groups was deduced from the strong HMBC correlations observed between the protons at δH 3.95 and 3.86, and the aromatic carbons at δC 134.3 (C-3′) and δC 159.0 (C-2), respectively. This was further confirmed by the Nuclear Overhauser Effect Spectroscopy (NOESY) correlation depicted between the protons at δH 3.86 (2-OCH3) and the proton at δH 6.88 (H-3). The substitution pattern of the B ring was previously identified in some flavonoids, namely (2R)-7-hydroxy-2′,8-dimethoxyflavanone [9] as well as irisones A and B [28]. Hence, compound 1 was characterized as a previously unreported chalcone named 2′,4′-dihydroxy-2,3′-dimethoxychalcone.
The crude extract, fractions, and some isolated compounds were evaluated for their antibacterial activity using the broth microdilution method. The cut-off values of the minimum inhibitory concentrations (MIC) classification scale, indicating the antibacterial activity of extracts and secondary metabolites derived from plants, are well known [29]. According to this scale, the antimicrobial activity of plant extracts can be classified as significant (MIC vakue < 100 µg/mL), moderate (100 < MIC value ≤ 625 µg/mL), and weak (MIC value > 625 µg/mL), while that of pure compounds can be classified as significant (MIC < 10 µg/mL), moderate (10 < MIC value ≤ 100 µg/mL), and weak (MIC value > 100 µg/mL). Consequently, a significant antibacterial activity (MIC < 100 μg/mL) was observed for the crude EtOH extract of D. steudneri against Enterobacter aerogenes ATCC13048 (Table 2). However, a moderate inhibition (10 < MIC value ≤ 100 μg/mL) was observed for methylgalangine (3), quercetin (4), kaempferol (5), 6,8-dimethylchrysin (6), β-sitosterol 3-O-glucopyranoside (9), and lupeol (13) against different bacterial strains used (Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Providencia stuartii, and Pseudomonas aeruginosa). The results obtained are in agreement with the literature since flavonoids, saponins, and triterpenes are known to exhibit potent antibacterial activity [29,30,31]. Among the tested compounds, lupeol (13) was the most active with an MIC value of 32 μg/mL against Enterobacter aerogenes. The reference antibiotic chloramphenicol inhibited the growth of all studied bacteria and its activity against some microbial strains which were as good as those of lupeol (13). Although the most active compounds exhibited moderate antibacterial activity, it should be noted that the recorded MIC values were close to those of the reference drug on the corresponding efflux pump-expressing MDR bacteria. This clearly suggests that a possible combination with other antibiotics or efflux pump inhibitors should be envisaged to improve their potency. The lack of inhibition by some of the tested compounds could be explained by the multi-resistant patterns of the bacterial strains used. Unlike Gram-positive bacteria, the Gram-negative bacteria used in this work are characterised by the multi-drug resistance phenotype, with the main mechanism of resistance being the extracellular expulsion of drugs via active efflux. In these types of bacteria, efflux pumps play many roles, among which are the reduction of the intracellular concentration of exogenous substances such as antibiotics [32,33]. Furthermore, it was reported by Epang et al. (2016) that Gram-negative bacteria are surrounded by two membranes: the cytoplasmic cell membrane and the outer membrane containing lipopolysaccharides. Gram-positive bacteria instead do not have the additional outer membrane layer but share the commonality of having a cell wall surrounding the cytoplasmic membrane of the cell wall consisting of peptidoglycan. Gram-negative bacteria tend to be more resistant to antimicrobial agents than Gram-positive bacteria, because of the presence of the additional protection afforded by the outer membrane [34].

Table 2.
Antimicrobial activities (MIC and MBC values in μg/mL) of the extract, fractions, isolated compounds, and chloramphenicol.
The antimicrobial activity of quercetin (4), β-sitosterol 3-O-glucopyranoside (9), oleanolic acid (12), and lupeol (13) have already been evaluated. Quercetin exhibited a moderate activity against Candida albicans with an MIC value of 32 µg/mL [35], while it was shown that oleanolic acid possesses a broad range of antibacterial activity, mainly against Gram-positive bacteria [30]. Lupeol was reported to display significant zones of inhibition in the cultures of 18 hospital strains of the Gram-negative bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae at a concentration of 30 μg/100 μL [36]. Some of the isolated compounds were also tested against three strains of Staphylococcus aureus (Gram-positive bacteria). As shown in Table S1 (Supplementary data), methylgalangine (3), quercetin (4), kaempferol (5), β-sitosterol 3-O-glucopyranoside (9), and betulinic acid (11) exhibited significant activity against S. aureus ATCC25923 with MIC values ranging from 4 to 8 µg/mL. A significant activity was also observed for compounds 3, 5, and 6 against S. aureus MRSA3 as well as for compounds 3, 5, and 9 against S. aureus MRSA6. Our results further confirmed the fact that Gram-negative bacteria are more resistant to antimicrobial agents than Gram-positive bacteria [34].
3. Materials and Methods
3.1. General Experimental Procedures
HRESIMS spectra were recorded on a Bruker Daltonics Compact Quadrupole Time of Flight (QToF) Mass Spectrometer using an electrospray ionisation probe, using direct injection in the positive mode. A Bruker Advanced III 400 MHz (400.13 MHz; 100.62 MHz) spectrometer at 25 °C was used to record 1H-, 13C-NMR, and 2D NMR spectra. All chemical shifts (δ) are given in ppm with reference to the residual solvent signal and coupling constants (J) are in Hz. Column chromatography was performed on Sephadex LH-20 and silica gel 60 (0.040–0.063 mm, Merck). TLC was performed on percolated silica gel 60 F254 (Merck) plates developed with Hexane-CH2Cl2, Hexane-EtOAc, CH2Cl2, CH2Cl2-MeOH, and EtOAc-MeOH. Thin Layer Chromatography (TLC) spots were visualized under UV light (254 and 365 nm) and by spraying with 10% aqueous or methanolic H2SO4 followed by heating at 90 °C.
3.2. Plant Material
The leaves of D. steudneri were collected in Sud-Kivu, an East region of the DR Congo in September 2019. The specimen was identified at the Research Centre in Natural Sciences Lwiro (CRSN/Lwiro), where a voucher specimen (No. 375) was deposited.
3.3. Extraction and Isolation
The air-dried and ground leaves of D. steudneri (4 kg) were extracted three times (each time for 24 h) with EtOH (96%, 18 L) by maceration. The solvent was evaporated to yield 554.3 g of crude extract. An amount of 544.3 g of this extract was suspended in distilled water (600 mL) and consecutively extracted with EtOAc and n-BuOH to give, after evaporation of the solvent, 102.8 g and 114.5 of EtOAc and n-BuOH fractions, respectively. A portion of the EtOAc fraction (98 g) was subjected to silica gel column chromatography eluting with gradients of n-hexane/EtOAc (90:10 → 10:90) and EtOAc/MeOH (95:5 → 70:30) as mobile phases to afford eight main sub-fractions (Fr.1–Fr.8). Fr.3 (6.3 g) was subjected to Sephadex LH-20 column chromatography using MeOH as the eluent to give four sub-fractions (Fr.3-1–Fr.3-4). Betulinic acid (11) (15 mg) was obtained from the sub-fraction Fr.3-1 (1.4 g) by recrystallization in n-hexane/EtOAc (80:20) followed by simple filtration. To remove chlorophylls, sub-fractions Fr.3-2, Fr.3-3, and Fr.3-4 were separately submitted to Sephadex LH-20 column chromatography eluted with n-hexane/CH2Cl2/MeOH (7:4:0.5) to afford sub-fractions Fr.3-2-1 (620.2 mg), Fr.3-3-1 (350 mg), and Fr.3-4-1 (28.5 mg), respectively. Fr.3-2-1 was then submitted to silica gel column chromatography, eluted with n-hexane/EtOAc (90:10) to yield compounds 6 (25.2 mg) and 12 (62.1 mg), while Fr.3-3-1 and Fr.3-4-1 were purified by silica gel column chromatography using n-hexane/CH2Cl2 (1:1) to give compounds 1 (7 mg) and 3 (8 mg), respectively. The sub-fraction Fr.4 (14 g) was chromatographed on Sephadex LH-20 column eluted with MeOH to yield a mixture that was further separated on a silica gel column using n-hexane/EtOAc (80:20) as the eluent to yield two sub-fractions: Fr.4-1 (103.4 mg) and Fr.4-2 (630 mg). The sub-fraction (Fr.4-2) was first submitted to Sephadex LH-20 column chromatography eluted with n-hexane/CH2Cl2/MeOH (7:4:0.5), then to a silica gel column chromatography eluted with n-hexane/EtOAc (80:20) to give compounds 2 (15 mg) and 5 (12 mg). Fr.5 (61.6 g) was subjected to Sephadex LH-20 column chromatography eluted with MeOH to give three the sub-fractions Fr.5-1–5-3. The purification of Fr.5-3 (122.4 mg) by column chromatography using silica gel eluting with a mixture of n-hexane/EtOAc (70:30) gave compounds 4 (11 mg) and 5 (5 mg). The recrystallization of Fr.2 (12.6 g) in n-hexane/EtOAc, 90:10 yielded compound 13 (60 mg). Part of the n-BuOH extract (108 g) was fractionated by silica gel column chromatography eluted with EtOAc with increasing amounts of MeOH to give five main sub-fractions A-E. Sub-fraction D (34.2 g) was chromatographed on a silica gel column using EtOAc/MeOH/H2O (95:5:2) as the eluent to afford four sub-fractions (D1-D4). Recrystallization and filtration of the sub-fraction D2 (255 mg) yielded β-sitosterol 3-O-glucopyranoside (9) (12 mg) while sub-fraction D4 (22.4 g) was repeatedly purified using silica gel column chromatography eluted with EtOAc/MeOH/H2O (90:10:5) and EtOAc/MeOH/H2O (95:5:2) to give compounds 7 (58.5 mg), 8 (13.5 mg), and 10 (8 mg).
2′,4′-dihydroxy-2,3′-dimethoxychalcone (1): Yellow amorphous powder; HRESIMS: m/z 323.0887 [M+Na]+ (Calcd. for C17H16O5Na+: 323.0895). 1H-NMR (CDCl3, 400 MHz and 13C-NMR (CDCl3, 100 MHz) data: see Table 1.
3.4. Antimicrobial Activity
The MIC and MBC of the tested bacteria were determined by the broth microdilution INT colorimetric assay as previously described [37,38]. The tested samples (plant extract, fractions, some of the isolated compounds, and chloramphenicol) were dissolved in DMSO/MHB. The final concentration of DMSO in the sample solution was less than 2.5%, a concentration innocuous to bacterial growth [39,40]. The solution obtained was then added to MHB and a series of two-fold dilutions were performed. Afterward, prepared inoculum (1.5 × 106 CFU/mL) was added. The microplates were sealed and incubated aerobically for 18 h at 37 °C. Wells containing DMSO and inoculum were used as negative controls, whereas those containing chloramphenicol were used as positive controls, for Gram-negative bacteria, including Escherichia coli ATTC10536 and AG102, Enterobacter aerogenes ATCC13048, Klebsiella pneumoniae ATCC11296 and KP55, Providencia stuartii PS2636, and Pseudomonas aeruginosa PA01. After the incubation period, 40 mL of INT (0.2 mg/mL) was added to each well and re-incubated for 30 min. The MIC value was defined as the lowest sample concentration that did not induce a colour change of the medium, thereby exhibiting complete inhibition of bacterial growth. The MBC was determined by adding 50 µL aliquots of the preparations, which did not show any bacterial growth after incubation during the MIC assays, to 150 µL of MHB. These preparations were incubated at 37 °C for 48 h. The MBC was considered as the lowest sample concentration that did not produce any colour change of the medium after the addition of INT as mentioned above [41]. All assays were performed in triplicate and repeated thrice.
4. Conclusions
The chemical investigation of the EtOH extract of the leaves of D. steudneri led to the isolation, characterization, and identification of a new chalcone derivative together with twelve other known compounds including six flavonoids, two saponins, one glyceride glycoside, and three triterpenes. A panel of spectroscopic and spectrometric methods as well as the comparison with published data were used to characterize the isolated compounds. The extract, fractions, and some pure compounds were screened for their antimicrobial activity against several multidrug-resistant bacteria, the results of which are very promising since the MIC values recorded were in many cases close to those of the reference drug chloramphenicol. The study also evidenced the antibacterial potential of flavonoids, saponins, and triterpenes, although a study of their mechanism of action remains to be explored.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15060725/s1, Figure S1: HRESI-MS of compound 1; Figure S2: 1H-NMR spectrum of compound 1; Figure S3: 13C NMR spectrum of compound 1; Figure S4: 1H-1H COSY spectrum of compound 1; Figure S5: HSQC spectrum of compound 1; Figure S6: HMBC spectrum of compound 1; Table S1: MIC and MBC (in μg/mL) of isolated compounds and chloramphenicol against gram-positive bacterial strains; Table S2: Characteristics of microorganisms used; References [42,43,44,45,46] are cited in the supplementary materials.
Author Contributions
C.M.M. contributed to the isolation, structure elucidation, and manuscript writing; M.-G.F.G. contributed to biological assays and manuscript writing; B.Y.K. contributed to structure elucidation and manuscript writing; H.U.S. contributed to plant collection and extraction; B.K.P. contributed to structure elucidation; X.S.-N. did the spectroscopic analysis, structure elucidation, and manuscript preparation, R.B.T. supervised the isolation, structure elucidation, and manuscript preparation; R.W.M.K., V.K. and L.A.T. supervised and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Alexander von Humboldt Foundation (AvH) (Bonn, Germany, Ref 3.4—1157152—CMR—GFHERMES-E).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the University of Dschang for financing some consumables used in this work.
Conflicts of Interest
The data presented in this study are available in article and supplementary material.
Sample Availability
Samples of some of the isolated compounds are not available from the authors.
References
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-L.; Morden, C. Phylogenetics of the plant genera Dracaena and Pleomele (Asparagaceae). Bot. Orient. J. Plant Sci. 2010, 7, 64–72. [Google Scholar] [CrossRef]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Armijos, C.; Vidari, G. Flavonoids and stilbenoids of the genera Dracaena and Sansevieria: Structures and bioactivities. Molecules 2020, 25, 2608. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.D.; Taye, M.; Chaudhary, B. Extraction and characterization of cellulose single fiber from native Ethiopian Serte (Dracaena steudneri Egler) plant leaf. J. Macromol. Sci. Part A 2019, 56, 837–844. [Google Scholar] [CrossRef]
- Damen, T.H.; Van der Burg, W.; Wiland-Szymańska, J.; Sosef, M. Taxonomic novelties in African Dracaena (Dracaenaceae). Blumea 2018, 63, 31–53. [Google Scholar] [CrossRef]
- Moshi, M.J.; Otieno, D.F.; Weisheit, A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: Plants used in traditional medicine in Kikuku village, Muleba District. J. Ethnobiol. Ethnomed. 2012, 8, 14. [Google Scholar] [CrossRef]
- Mukazayire, M.-J.; Minani, V.; Ruffo, C.K.; Bizuru, E.; Stévigny, C.; Duez, P. Traditional phytotherapy remedies used in Southern Rwanda for the treatment of liver diseases. J. Ethnopharm. 2011, 138, 415–431. [Google Scholar] [CrossRef]
- Kokwaro, J.O. Medicinal Plants of East. Africa, 3rd ed.; University of Nairobi Press: Nairobi, Kenya, 2009; p. 534. [Google Scholar]
- Nchiozem-Ngnintedem, V.-A.; Omosa, L.K.; Bedane, K.G.; Derese, S.; Spiteller, M. Inhibition of proinflammatory cytokine release by flavones and flavanones from the leaves of Dracaena steudneri Engl. Planta Med. 2020, 87, 209–217. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Ponou, K.B.; Teponno, R.B.; Mbiantcha, M.; Djoukeng, J.D.; Nguelefack, T.B.; Watcho, P.; Cadenas, A.G.; Park, H.-J. In vivo anti-inflammatory effect of a new steroidal saponin, mannioside A, and its derivatives isolated from Dracaena mannii. Arch. Pharm. Res. 2008, 31, 653–658. [Google Scholar] [CrossRef]
- Teponno, R.B.; Tanaka, C.; Jie, B.; Tapondjou, L.A.; Miyamoto, T.; Trifasciatosides, A.-J. Steroidal saponins from Sansevieria trifasciata Prain. Chem. Pharm. Bull. 2016, 64, 1347–1355. [Google Scholar] [CrossRef]
- Teponno, R.B.; Dzoyem, J.P.; Nono, R.N.; Kauhl, U.; Sandjo, L.P.; Tapondjou, L.A.; Bakowsky, U.; Opatz, T. Cytotoxicity of secondary metabolites from Dracaena viridiflora Engl & Krause and their semisynthetic analogues. Rec. Nat. Prod. 2017, 11, 421–430. [Google Scholar]
- Mouzié, M.C.; Ponou, K.B.; Fouedjou, T.R.; Teponno, B.R.; Tapondjou, L.A. Steroidal saponins from Dracaena humilis (Dracaenaceae) and their chemotaxonomic significance. Nat. Prod. Sci. 2021, 27, 122–127. [Google Scholar]
- Harborne, J.B.; Greenham, J.; Williams, C.A.; Eagle, J.; Markham, K.R. Ten isoprenylated and C-methylated flavonoids from the leaves of three Vellozia species. Phytochemistry 2013, 34, 219–226. [Google Scholar] [CrossRef]
- Koley, K.T.; Khan, Z.; Oulkar, D.; Singh, B.; Bhatt, P.B.; Banerjee, K. Profiling of polyphenols in phalsa (Grewia asiatica L) fruits based on liquid chromatography high resolution mass spectrometry. J. Food Sci. Tech. 2019, 57, 606–616. [Google Scholar] [CrossRef]
- Petrus, A.J.A.; Hemalatha, S.S.; Suguna, G. Isolation and Characterisation of the antioxidant phenolic metabolites of Boerhaavia erecta L. leaves. J. Pharm. Sci. Res. 2012, 4, 1856–1861. [Google Scholar]
- Xiao, Z.P.; Wu, H.K.; Wu, T.; Shi, H.; Hang, B.; Aisa, H.A. Kaempferol and quercetin flavonoids from Rosa rugosa. Chem. Nat. Comp. 2006, 42, 736–737. [Google Scholar] [CrossRef]
- Basnet, P.; Kadota, S.; Hase, K.; Namba, T. Five new C-methyl flavonoids, the potent aldose reductase inhibitors from Matteuccia orientalis Trev. Chem. Pharm. Bull. 1995, 43, 1558–1564. [Google Scholar] [CrossRef][Green Version]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. 2002, 50, 788–795. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lin, M.-K.; Hwang, S.-Y.; Hwang, T.-L.; Kuo, Y.-H.; Chang, C.-I.; Ou, C.-Y.; Kuo, Y.-H. Two anti-inflammatory steroidal saponins from Dracaena angustifolia Roxb. Molecules 2013, 18, 8752–8763. [Google Scholar] [CrossRef]
- Kianfé, Y.B.; Kühlborn, J.; Tchuenguem, T.R.; Tchegnitegni, T.B.; Ponou, K.B.; Groß, J.; Teponno, R.B.; Dzoyem, J.P.; Opatz, T.; Tapondjou, L.A. Antimicrobial secondary metabolites from the medicinal plant Crinum glaucum A. Chev. (Amaryllidaceae). S. Afr. J. Bot. 2020, 133, 161–166. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Ishigoside, a new glyceroglycolipid isolated from the brown alga Ishige okamurae. Biotechnol. Bioprocess. Eng. 2009, 14, 20–26. [Google Scholar] [CrossRef]
- Egbubine, C.O.; Adeyemi, M.M.; Habila, J.D. Isolation and characterization of betulinic acid from the stem bark of Feretia canthioides Hiern and its antimalarial potential. Bull. Nat. Res. Cent. 2020, 44, 49–55. [Google Scholar] [CrossRef]
- Kianfé, Y.B.; Teponno, R.B.; Kühlborn, J.; Tchuenguem, T.R.; Ponou, B.K.; Helaly, S.E.; Dzoyem, J.P.; Opatz, T.; Tapondjou, L.A. Flavans and other chemical constituents of Crinum biflorum (Amaryllidaceae). Biochem. Syst. Ecol. 2019, 87, 103953. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, M.; Singh, A.; Singh, U.; Pandey, V. A new chalcone glycoside from Rhamnus nipalensis. Nat. Prod. Res. 2008, 22, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Adesanwo, J.K.; Shode, F.O.; Aiyelaagbe, O.; Oyede, R.T.; Baijnath, H. Isolation and characterization of a new chalcone from the leaves of Heteropyxis natalensis. Int. J. Med. Sci. 2009, 1, 28–32. [Google Scholar]
- Pelter, A.; Ward, R.S.; Gray, I.T. The carbon-13 nuclear magnetic resonance spectra of flavonoids and related compounds. J. Chem. Soc. Perk. Trans. I 1976, 223, 2475–2483. [Google Scholar] [CrossRef]
- Wong, S.-M.; Konno, C.; Oshima, Y.; Pezzuto, J.M.; Fong, H.H.S.; Worth, N.R.F. Irisones A and B: Two new isoflavones from Iris missouriensis. J. Nat. Prod. 1987, 50, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V. Potential of Cameroonian plants and derived-products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Demgne, F.O.M.; Damen, F.; Fankam, A.G.; Guefack, F.M.-G.; Wamba, N.E.B.; Nayim, P.; Mbaveng, T.A.; Bitchagno, G.T.M.; Tapondjou, A.L.; Penlap, B.V.; et al. Botanicals and phytochemicals from the bark of Hypericum roeperianum (Hypericaceae) had strong antibacterial activity and showed synergistic effects with antibiotics against multidrug-resistant bacteria expressing active efflux pumps. J. Ethnopharmacol. 2021, 277, 114257. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Nikaido, H. Multidrug resistance mechanisms: Drug efflux across two membranes. Mol. Microbiol. 2000, 37, 219–225. [Google Scholar] [CrossRef]
- Pool, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys Acta Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Tsakem, B.; Eckhardt, P.; Tchuenguem, R.T.; Ponou, K.B.; Dzoyem, J.P.; Teponno, R.B.; Opatz, T.; Barboni, L.; Tapondjou, A.L. Muellerilactone and other bioactive constituents of Phyllanthus muellerianus (kuntze) exell. Biochem. Systemat. Ecol. 2022, 101, 104397. [Google Scholar] [CrossRef]
- Ahamed, B.K.M.; Krishna, V.; Gowdru, H.B.; Rajanaika, H.; Kumaraswamy, H.M.; Rajshekarappa, S.; Dandin, C.J.; Mahadevan, K.M. Isolation of bactericidal constituents from the stem bark extract of Grewia tiliaefolia Vahl. Res. J. Med. Plant 2007, 1, 72–82. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Guefack, M.G.F.; Tankeo, S.B.; Ngaffo, C.M.N.; Nayim, P.; Wamba, B.E.N.; Bonsou, I.N.; Kuete, V.; Mbaveng, A.T. Antibiotic-potentiation activities of three animal species extracts, Bitis arietans, Helix aspersa, and Aristaeomorpha foliacea and mode of action against MDR Gram-negative bacteria phenotypes. Investig. Med. Chem. Pharmacol. 2021, 4, 48–62. [Google Scholar] [CrossRef]
- Nono, E.C.; Mkounga, P.; Kuete, V.; Marat, K.; Hultin, P.G.; Nkengfack, A.E. Pycnanthulignenes A-D, antimicrobial cyclolignene derivatives from the roots of Pycnanthus angolensis. J. Nat. Prod. 2010, 73, 213–216. [Google Scholar] [CrossRef]
- Nguemeving, J.R.; Azebaze, A.G.; Kuete, V.; Nono, N.E.C.; Beng, V.P.; Meyer, M.; Blond, A.; Bodo, B.; Nkengfack, A.E. Laurentixanthones A and B, antimicrobial xanthones from Vismia laurentii. Phytochemistry 2006, 67, 1341–1346. [Google Scholar] [CrossRef]
- Fankam, A.G.; Kuiate, J.R.; Kuete, V. Antibacterial activities of Beilschmiedia obscura and six other Cameroonian medicinal plants against multi-drug resistant Gram-negative phenotypes. BMC Complement. Altern. Med. 2014, 14, 241–249. [Google Scholar] [CrossRef]
- Chevalier, J.; Pages, J.M.; Eyraud, A.; Mallea, M. Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem. Biophys. Res. Commun. 2000, 274, 496–499. [Google Scholar]
- Dzoyem, J.P.; Hamamoto, H.; Ngameni, B.; Ngadjui, B.T.; Sekimizu, K. Antimicrobial action mechanism of flavonoids from Dorstenia species. Drug Discov. Ther. 2013, 7, 66–72. [Google Scholar]
- Kuete, V.; Ngameni, B.; Tangmouo, G.J.; Bola, J.M.; Alibert-Franco, S.; Ngadjui, T.B.; Pages, J.M. Efflux pumps are involved in the defence of gram-negative bacteria against the natural products isobavachalcone and diospyrone. Antimicrob. Agents Chemother. 2010, 54, 1749–1752. [Google Scholar]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pages, J.M.; Amaral, L.; Bolla, J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar]
- Paudel, A.; Hamamoto, H.; Kobayashi, Y.; Yokoshima, S.; Fukuyama, T.; Sekimizu, K. Identification of novel deoxyribofuranosyl indole antimicrobial agents. J. Antibiot. 2012, 65, 53–57. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).