Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets
Abstract
:1. Introduction
2. Results and Discussion
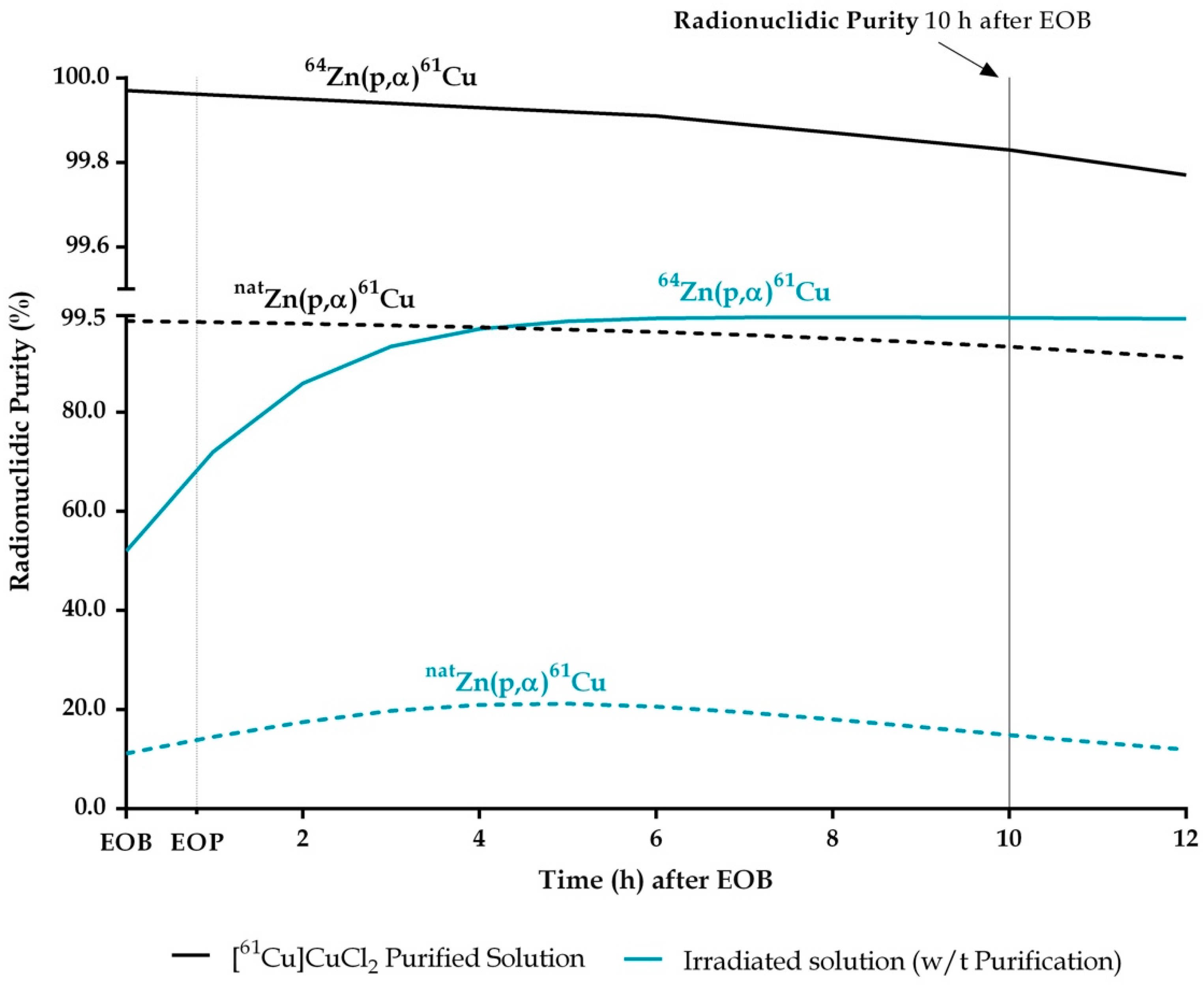
2.1. [61Cu]CuCl2 Production
2.2. Recovery and Recycling of 64Zn
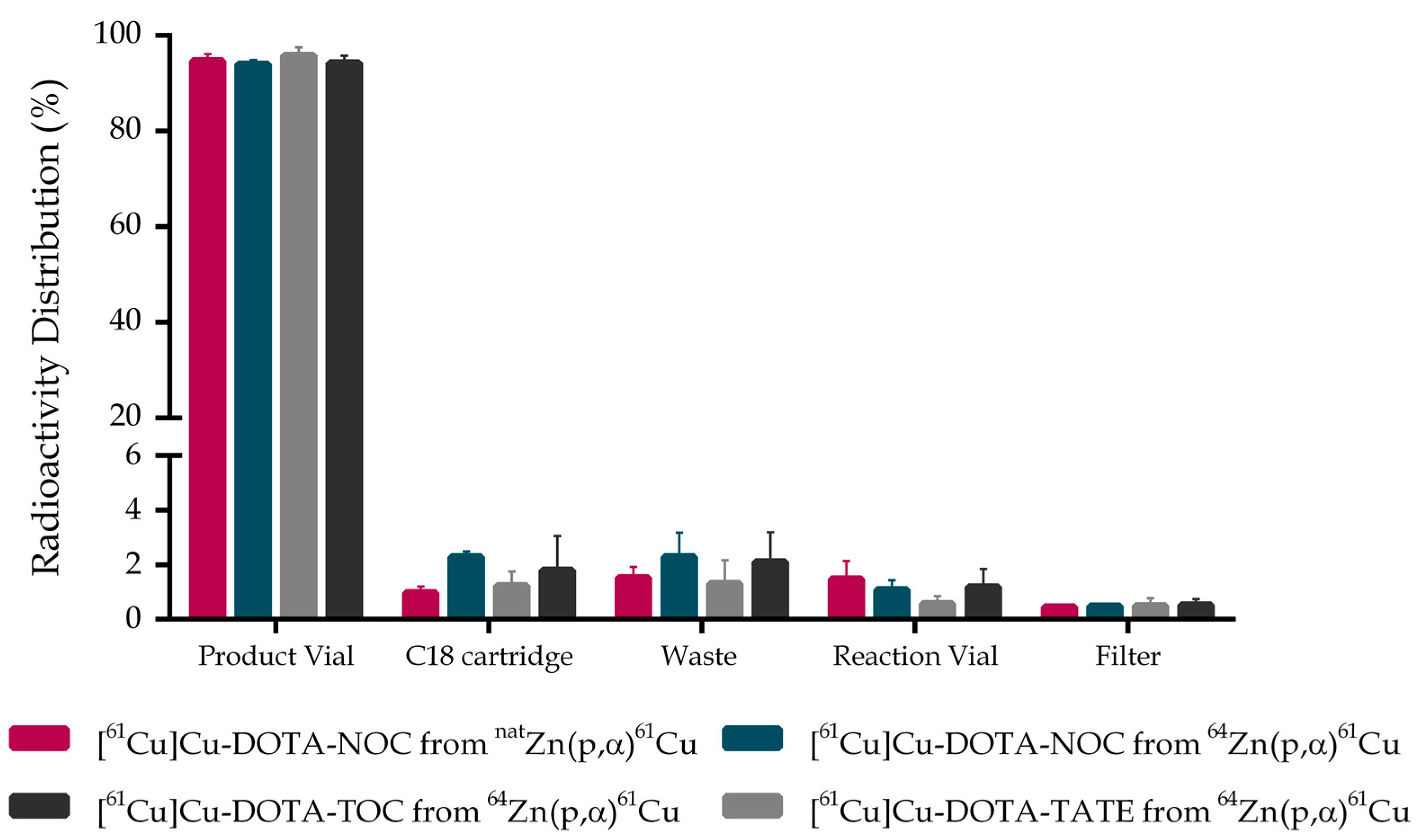
2.3. [61Cu]Cu-DOTA-NOC, [61Cu]Cu-DOTA-TATE and [61Cu]Cu-DOTA-TOC Production Activity Distribution
2.4. Quality Control
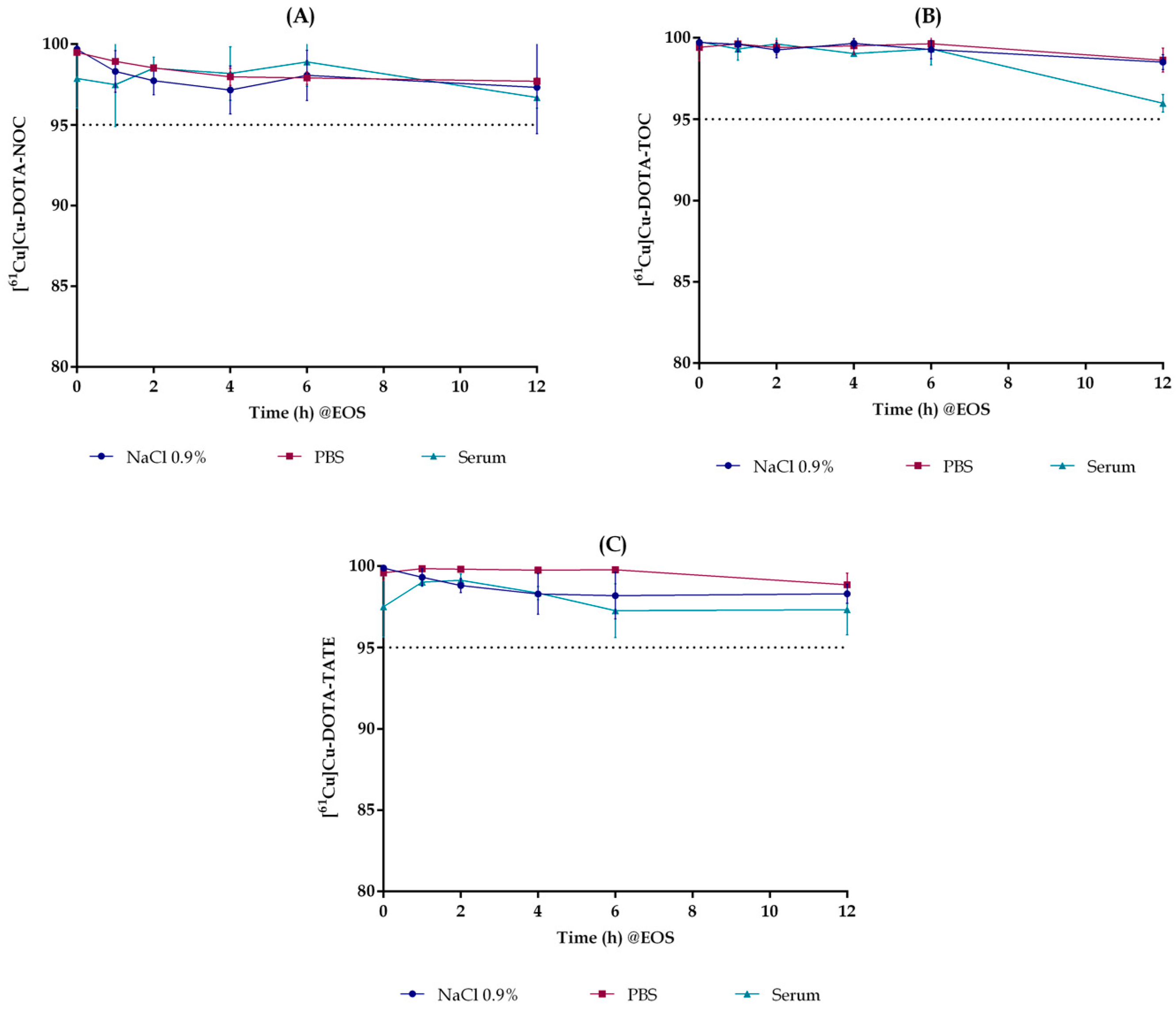
2.5. In Vitro Stability
3. Materials and Methods
3.1. Irradiation and Purification of [61Cu]CuCl2
3.2. EtOH as Radiolytic Scavenger
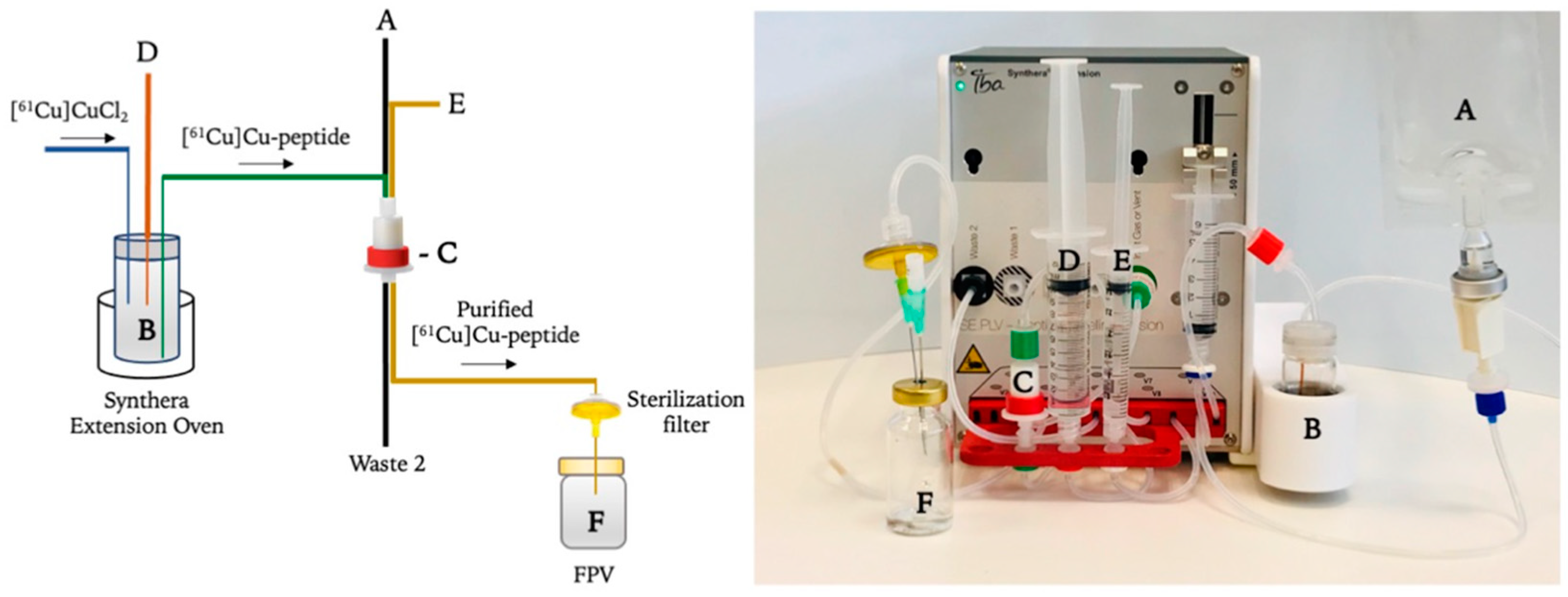
3.3. Synthesis of [61Cu]Cu-Conjugated Peptides on the IBA Synthera® Extension Module
- The C18 cartridge (C) is preconditioned with ethanol (10 mL) followed by water (10 mL) prior to use;
- Purified [61Cu]CuCl2 (3 mL, 0.5 M HCl) is transferred to the reaction vial (B);
- Peptide (50 μg), previously diluted in 2.5 M sodium acetate (3 mL) and EtOH (200–300 μL) (D) to prevent radiolysis, is transferred to the reaction vial (B) and mixed with [61Cu]CuCl2 for 10 s;
- Radiolabelling reaction is conducted for 10 min, with variable temperature (85–100 °C) and pH fixed between 4 and 5;
- Reaction mixture is cooled down with water (12 mL) (A) and passed through a C18 cartridge at 3 mL/min flow to the waste container (Waste 2);
- C18 cartridge is then rinsed with water (10 mL) (A), which rinses the column at a 3 mL/min flow;
- [61Cu]Cu-labelled peptide is finally eluted from the C18 column with a solution of water/EtOH (50/50%) (E) to the final product vial (F) with a 3 mL/min flow.
3.4. Quality Control
3.4.1. Radionuclidic Purity (HPGe)
3.4.2. Radiochemical Purity (Radio-HPLC)
3.4.3. Stability Experiments
3.4.4. Stability in Aqueous Solvents
3.4.5. Stability in Mice Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eder, M.; Hennrich, U. [68Ga]Ga-PSMA-11: The first FDA-Approved 68Ga-Radiopharmaceutical for pET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. [Google Scholar] [CrossRef]
- Suzuki, H.; Kise, S.; Kaizuka, Y.; Watanabe, R.; Sugawa, T.; Furukawa, T.; Fujii, H.; Uehara, T. Copper-64-Labeled Antibody Fragments for Immuno-PET/ Radioimmunotherapy with Low Renal Radioactivity Levels and Amplified Tumor-Kidney Ratios. ACS Omega 2021, 6, 21556–21562. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.; Sreekumar, S.; Mpoy, C.; Rogers, B.E. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharmaceuticals 2021, 14, 229. [Google Scholar] [CrossRef]
- Merkx, R.I.J.; Rijpkema, M.; Franssen, G.M.; Kip, A.; Smeets, B.; Morgenstern, A.; Bruchertseifer, F.; Yan, E.; Wheatcroft, M.P.; Oosterwijk, E.; et al. Carbonic Anhydrase IX-Targeted α-Radionuclide Therapy with 225Ac Inhibits Tumor Growth in a Renal Cell Carcinoma Model. Pharmaceuticals 2022, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Reardon, D.A.; Pozzi, O.R. Targeted alpha-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl. Med. Biol. 2007, 34, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Krasikova, R.N.; Aliev, R.A.; Kalmykov, S.N. The next generation of positron emission tomography radiopharmaceuticals labeled with non-conventional radionuclides. Mendeleev Commun. 2016, 26, 85–94. [Google Scholar] [CrossRef]
- Boros, E.; Packard, A.B. Radioactive Transition Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar] [CrossRef]
- Dash, A.; Chakravarty, R. Radionuclide generators: The prospect of availing PET radiotracers to meet current clinical needs and future research demands. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 30–66. [Google Scholar]
- Thisgaard, H.; Kumlin, J.; Langkjær, N.; Chua, J.; Hook, B.; Jensen, M.; Kassaian, A.; Zeisler, S.; Borjian, S.; Cross, M.; et al. Multi-curie production of gallium-68 on a biomedical cyclotron and automated radiolabelling of PSMA-11 and DOTATATE. EJNMMI Radiopharm. Chem. 2021, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. AIEA-TECDOC-1863: Gallium-68 Cyclotron Production; AIEA: Vienna, Austria, 2019. [Google Scholar]
- Gallium (68Ga) Chloride (Accelerator-Produced) Solution for Radiolabelling. Ph Eur. PA/PH/Exp. 14/T (18) 13 ANP(Monograph 3109). 2017.
- International Atomic Energy Agency. AIEA-TECDOC-1955: Production of Emerging Radionuclides towards Theranostic Applications: Copper-61, Scandium-43 and -44, and Yttrium-86; AIEA: Vienna, Austria, 2021. [Google Scholar]
- Chaple, I.F.; Lapi, S.E. Production and Use of the First-Row Transition Metal PET Radionuclides 43,44Sc, 52Mn, and 45Ti. J. Nucl. Med. 2018, 59, 1655–1659. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, D.W.; A Bass, L.; Cutler, P.; E Shefer, R.; E Klinkowstein, R.; Herrero, P.; Lewis, J.; Cutler, C.S.; Anderson, C.; Welch, M.J. High purity production and potential applications of copper-60 and copper-61. Nucl. Med. Biol. 1999, 26, 351–358. [Google Scholar] [CrossRef]
- Das, S.S.; Chattopadhyay, S.; Barua, L.; Das, M.K. Production of 61Cu using natural cobalt target and its separation using ascorbic acid and common anion exchange resin. Appl. Radiat. Isot. 2012, 70, 365–368. [Google Scholar] [CrossRef]
- Asad, A.H.; Smith, S.V.; Chan, S.; Jeffery, C.M.; Morandeau, L.; Price, R.I. Cyclotron production of 61Cu using natural Zn & enriched 64Zn targets. AIP Conf. Proc. 2012, 1509, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Asad, A.H.; Smith, S.V.; Morandeau, L.M.; Chan, S.; Jeffery, C.M.; Price, R.I. Production of 61Cu by the natZn(p,α) reaction: Improved separation and specific activity determination by titration with three chelators. J. Radioanal. Nucl. Chem. 2015, 305, 899–906. [Google Scholar] [CrossRef]
- Thieme, S.; Walther, M.; Preusche, S.; Rajander, J.; Pietzsch, H.-J.; Lill, J.-O.; Kaden, M.; Solin, O.; Steinbach, J. High specific activity 61Cu via 64Zn(p,α)61Cu reaction at low proton energies. Appl. Radiat. Isot. 2013, 72, 169–176. [Google Scholar] [CrossRef]
- Do Carmo, S.J.C.; Alves, V.H.; Alves, F.; Abrunhosa, A.J. Fast and cost-effective cyclotron production of 61Cu using a natZn liquid target: An opportunity for radiopharmaceutical production and R&D. Dalton Trans. 2017, 46, 14556–14560. [Google Scholar] [CrossRef]
- Fonseca, A.I.; Alves, V.H.; Carmo, S.J.C.D.; Falcão, A.; Abrunhosa, A.J.; Alves, F. GMP automated purification of copper-61 produced in cyclotron liquid targets: Methodological aspects. Curr. Radiopharm. 2021, 14, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Svedjehed, J.; Kutyreff, C.J.; Engle, J.W.; Gagnon, K. Automated, cassette-based isolation and formulation of high-purity [61Cu]CuCl2 from solid Ni targets. EJNMMI Radiopharm. Chem. 2020, 5, 21. [Google Scholar] [CrossRef]
- Jalilian, A.; Rowshanfarzad, P.; Sabet, M.; Shafiee, A. Preparation of [61Cu]-2-acetylpyridine thiosemicarbazone complex as a possible PET tracer for malignancies. Appl. Radiat. Isot. 2006, 64, 337–341. [Google Scholar] [CrossRef]
- Jalilian, A.; Rowshanfarzad, P.; Sabet, M. Preparation of [61Cu]pyruvaldehyde-bis (N4-methylthiosemicarbazone) complex as a possible PET radiopharmaceutical. Radiochim. Acta 2006, 94, 113–117. [Google Scholar] [CrossRef]
- Fukumura, T.; Okada, K.; Szelecsényi, F.; Kovács, Z.; Suzuki, K. Practical production of 61Cu using natural Co target and its simple purification with a chelating resin for 61Cu-ATSM. Radiochim. Acta 2004, 92, 209–214. [Google Scholar] [CrossRef]
- Jalilian, A.; Rostampour, N.; Rowshanfarzad, P.; Shafaii, K.; Kamali-Dehghan, M.; Akhlaghi, M. Preclinical studies of [61Cu]ATSM as a PET radiopharmaceutical for fibrosarcoma imaging. Acta Pharm. 2009, 59, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, A.R.; Nikzad, M.; Zandi, H. Preparation and evaluation of [61Cu]-thiophene-2-aldehyde thiosemicarbazone for PET studies. Nucl. Med. Rev. 2008, 11, 41–47. [Google Scholar]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C.; et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA- conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Krenning, E.; Breeman, W.; Kooij, P.; Lameris, J.; Bakker, W.; Koper, J.; Ausema, L.; Reubi, J.; Lamberts, S. Localisation of Endocrine-Related Tumours With Radioiodinated Analogue of Somatostatin. Lancet 1989, 333, 242–244. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Krenning, E.P.; Scheidhauer, K.; Lewington, V.; Lebtahi, R.; Grossman, A.; Vitek, P.; Sundin, A.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Somatostatin Receptor Imaging with 111In-Pentetreotide. Neuroendocrinology 2009, 90, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Krenning, E.P.; Bakker, W.H.; Kooij, P.P.M.; Breeman, W.A.P.; Oei, H.Y.; de Jong, M.; Reubi, J.C.; Visser, T.J.; Bruns, C.; Kwekkeboom, D.J.; et al. Somatostatin Receptor Scintigraphy with Indium 111-DTPA-D-Phe-1-Octreotide in Man: Metabolism, Dosimetry and Comparison with Iodine- 123-Tyr-3-Octreotide. J. Nucl. Med. 1992, 33, 652–658. [Google Scholar]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine and Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.B.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Peptide Receptor Radionuclide Therapy with Radiolabelled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef]
- Seemann, J.; Waldron, B.; Parker, D.; Roesch, F. DATATOC: A novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature. EJNMMI Radiopharm. Chem. 2016, 1, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, A.; Knigge, U.; Mortensen, J.; Oturai, P.; Berthelsen, A.K.; Loft, A.; Binderup, T.; Rasmussen, P.; Elema, D.; Klausen, T.L.; et al. Clinical PET of Neuroendocrine Tumors Using 64Cu-DOTATATE: First-in-Humans Study. J. Nucl. Med. 2012, 53, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, A.; Knigge, U.; Binderup, T.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; Rasmussen, P.; Elema, D.; et al. 64Cu-DOTATATE PET for Neuroendocrine Tumors: A Prospective Head-to-Head Comparison with 111In-DTPA-Octreotide in 112 Patients. J. Nucl. Med. 2015, 56, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, S.; Revheim, M.-E.; Raynor, W.; Zehetner, W.; Knoll, P.; Zandieh, S.; Alavi, A. 64Cu-DOTATOC PET-CT in Patients with Neuroendocrine Tumors. Oncol. Ther. 2020, 8, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loft, M.; Carlsen, E.A.; Johnbeck, C.B.; Johannesen, H.H.; Binderup, T.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; et al. 64Cu-DOTATATE PET in patients with neuroendocrine neoplasms: Prospective, head-to-head comparison of imaging at 1 hour and 3 hours after injection. J. Nucl. Med. 2021, 62, 62–68. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Ranganathan, D.; Wagh, N.; Shafie, A.; Gaber, A.; Abbasi, A.; Kjaer, A.; Tworowska, I.; Núñez, R. 64Cu-DOTATATE PET/CT for imaging patients with known or suspected somatostatin receptor-Positive neuroendocrine tumors: Results of the first U.S. prospective, reader-masked clinical trial. J. Nucl. Med. 2020, 61, 890–896. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Johnbeck, C.B.; Binderup, T.; Loft, M.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; Langer, S.W.; et al. 64Cu-DOTATATE PET/CT and prediction of overall and progression-free survival in patients with neuroendocrine neoplasms. J. Nucl. Med. 2020, 61, 1491–1497. [Google Scholar] [CrossRef]
- Hicks, R.J.; Jackson, P.; Kong, G.; Ware, R.E.; Hofman, M.S.; Pattison, D.A.; Akhurst, T.A.; Drummond, E.; Roselt, P.; Callahan, J.; et al. 64Cu-SARTATE PET imaging of patients with neuroendocrine tumors demonstrates high tumor uptake and retention, potentially allowing prospective dosimetry for peptide receptor radionuclide therapy. J. Nucl. Med. 2019, 60, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Do Carmo, S.J.C.; Alves, V.H.; Alves, F.; Abrunhosa, A.J. Oral presentation at the 17th Workshop on Targetry and Target Chemistry (WTTC17). 2018. Available online: https://slideslive.com/38910264/production-of-cu61-in-liquid-targets (accessed on 21 April 2022).
- Do Carmo, S.J.C.; Scott, P.J.H.; Alves, F. Production of radiometals in liquid targets. EJNMMI Radiopharm. Chem. 2020, 5, 21. [Google Scholar] [CrossRef]
- Fonseca, A.I.; Alves, V.H.; do Carmo, S.J.C.; Alves, F.; Abrunhosa, A.J. Copper-61 from liquid targets: Optimized purification for GMP labelling. Nucl. Med. Biol. 2021, 96, 96–97. [Google Scholar] [CrossRef]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Consensus nomenclature rules for radiopharmaceutical chemistry—Setting the record straight. Nucl. Med. Biol. 2017, 55, i-xi. [Google Scholar] [CrossRef] [Green Version]
- Decristoforo, C.; Neels, O.; Patt, M. Emerging Radionuclides in a Regulatory Framework for Medicinal Products – How Do They Fit? Front. Med. 2021, 8, 769. [Google Scholar] [CrossRef]
- Alves, V.H.; Carmo, S.J.C.D.; Alves, F.; Abrunhosa, A.J. Automated Purification of Radiometals Produced by Liquid Targets. Instruments 2018, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Alves, F.; Alves, V.H.P.; Do Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Garrison, W.M. Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev. 1987, 87, 381–398. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Ellars, C.E.; Edwards, D.S. Ascorbic acid: Useful as a buffer agent and radiolytic stabilizer for metalloradiopharmaceuticals. Bioconjug. Chem. 2003, 14, 1052–1056. [Google Scholar] [CrossRef]
- Liu, S.; Edwards, D.S. Bifunctional chelators for therapeutic lanthanide radiopharmaceuticals. Bioconjug. Chem. 2001, 12, 7–34. [Google Scholar] [CrossRef]
- Meisenheimer, M.; Kürpig, S.; Essler, M.; Eppard, E. Manual vs. automated 68Ga-radiolabelling—A comparison of optimized processes. J. Label. Compd. Radiopharm. 2020, 63, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Eppard, E.; Pèrez-Malo, M.; Rösch, F. Improved radiolabeling of DOTATOC with trivalent radiometals for clinical application by addition of ethanol. EJNMMI Radiopharm. Chem. 2017, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Eppard, E.; Wuttke, M.; Nicodemus, P.L.; Rösch, F. Ethanol-based post-processing of generator-derived 68Ga Toward kit-type preparation of 68Ga-radiopharmaceuticals. J. Nucl. Med. 2014, 55, 1023–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domnanich, K.A.; Müller, C.; Farkas, R.; Schmid, R.M.; Ponsard, B.; Schibli, R.; Türler, A.; Van Der Meulen, N.P. 44Sc for labeling of DOTA- and NODAGA-functionalized peptides: Preclinical in vitro and in vivo investigations. EJNMMI Radiopharm. Chem. 2016, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Target | n | [HNO3] (M) | [Zn] (mg/mL) | I (μAh) | Irrad. Time (min) | Act. Produced (GBq) |
|---|---|---|---|---|---|---|
| natZn(p,α)61Cu | 20 | 0.01 | 200 | 70.1 ± 0.3 | 180 | 1.84 ± 0.24 |
| 64Zn(p,α)61Cu | 32 | 0.01 | 2001 | 67.4 ± 2.9 | 180 | 3.28 ± 0.41 |
| Radiopharmaceutical | Target | Process Duration (min) | Activity @EOS (GBq) | Labelling Yield (%) | RCY (%) |
|---|---|---|---|---|---|
| [61Cu]Cu-DOTA-NOC N = 5 | Natural Zinc | 32 ± 4 | 0.99 ± 0.16 | 98.48 ± 0.89 | 94.73 ± 3.03 |
| [61Cu]Cu-DOTA-NOC N = 5 | Zinc-64 | 38 ± 2 | 1.95 ± 0.21 | 97.72 ± 2.01 | 94.03 ± 1.84 |
| [61Cu]Cu-DOTA-TATE N = 3 | Zinc-64 | 37 ± 6 | 2.06 ± 0.08 | 98.61 ± 0.84 | 95.91 ± 1.50 |
| [61Cu]Cu-DOTA-TOC N = 3 | Zinc-64 | 38 ± 4 | 1.77 ± 0.12 | 97.87 ± 1.10 | 94.67 ± 1.19 |
| Production Route | natZn(p,α)61Cu | 64Zn(p,α)61Cu | ||
|---|---|---|---|---|
| TEST | [61Cu]Cu-DOTA-NOC | [61Cu]Cu-DOTA-NOC | [61Cu]Cu-DOTA-TATE | [61Cu]Cu-DOTA-TOC |
| MA (MBq/nmol) | 28.93 ± 4.58 | 56.82 ± 6.25 | 52.31 ± 9.83 | 50.27 ± 3.40 |
| Activity at EOS (GBq) | 0.99 ± 0.16 | 1.95 ± 0.21 | 2.06 ± 0.08 | 1.77 ± 0.12 |
| RCP (%) | 99.48 ± 0.51 | 98.71 ± 0.57 | 99.90 ± 0.03 | 99.77 ± 0.16 |
| RNP (%) | 98.49 ± 0.07 | 99.97 ± 0.03 | ||
| Radionuclidic identity (h) | 3.33 ± 0.04 | 3.33 ± 0.04 | 3.33 ± 0.04 | 3.33 ± 0.04 |
| pH | 3–5 | 3–5 | 3–5 | 3–5 |
| Visual Inspection | Clear, Colourless | Clear, Colourless | Clear, Colourless | Clear, Colourless |
| Volume (mL) | 5–10 | 5–10 | 5–10 | 5–10 |
| Time (min) | Mobile Phase A (Per Cent v/v) | Mobile Phase B (Per Cent v/v) | |
|---|---|---|---|
| Solvents | Water/0.1% TFA | ACN/0.1% TFA | |
| Method A | 0–11 | 74 → 60 | 26 → 40 |
| 11–12 | 60 → 40 | 40 → 60 | |
| 12–14 | 40 | 60 | |
| Method B | 0–8 | 78 | 22 |
| 8–9 | 78 → 40 | 22 → 60 | |
| 9–14 | 40 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, A.I.; Alves, V.H.; do Carmo, S.J.C.; Silva, M.; Hrynchak, I.; Alves, F.; Falcão, A.; Abrunhosa, A.J. Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets. Pharmaceuticals 2022, 15, 723. https://doi.org/10.3390/ph15060723
Fonseca AI, Alves VH, do Carmo SJC, Silva M, Hrynchak I, Alves F, Falcão A, Abrunhosa AJ. Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets. Pharmaceuticals. 2022; 15(6):723. https://doi.org/10.3390/ph15060723
Chicago/Turabian StyleFonseca, Alexandra I., Vítor H. Alves, Sérgio J. C. do Carmo, Magda Silva, Ivanna Hrynchak, Francisco Alves, Amílcar Falcão, and Antero J. Abrunhosa. 2022. "Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets" Pharmaceuticals 15, no. 6: 723. https://doi.org/10.3390/ph15060723
APA StyleFonseca, A. I., Alves, V. H., do Carmo, S. J. C., Silva, M., Hrynchak, I., Alves, F., Falcão, A., & Abrunhosa, A. J. (2022). Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets. Pharmaceuticals, 15(6), 723. https://doi.org/10.3390/ph15060723