Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact
Abstract
1. Introduction
2. Methodology
3. Plant Sources and Bioactives
4. Prevalence and Patterns of Use
5. Legal Status
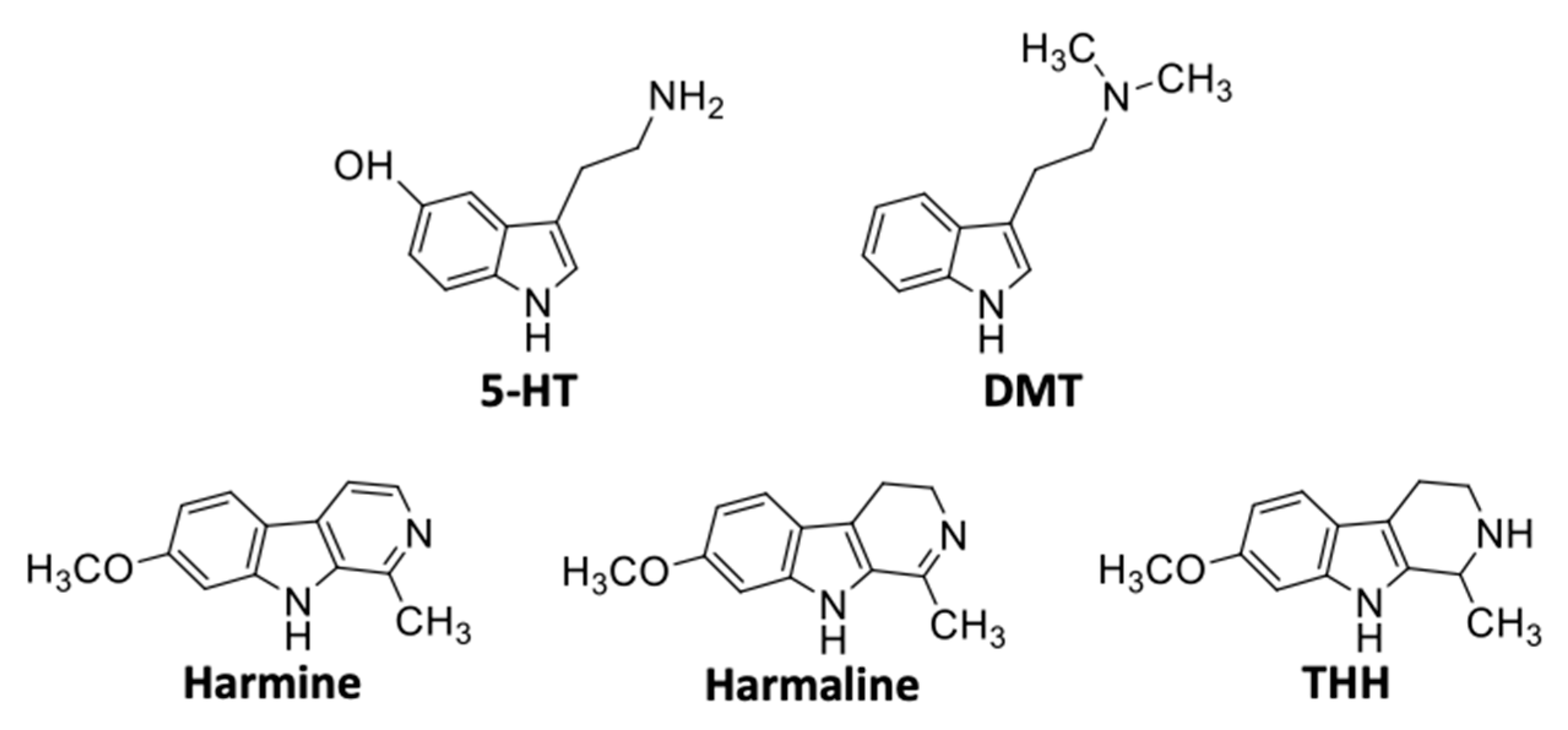
6. Structure and Physicochemical Properties
7. Pharmacokinetics
7.1. Routes of Administration and Absorption
7.2. Distribution
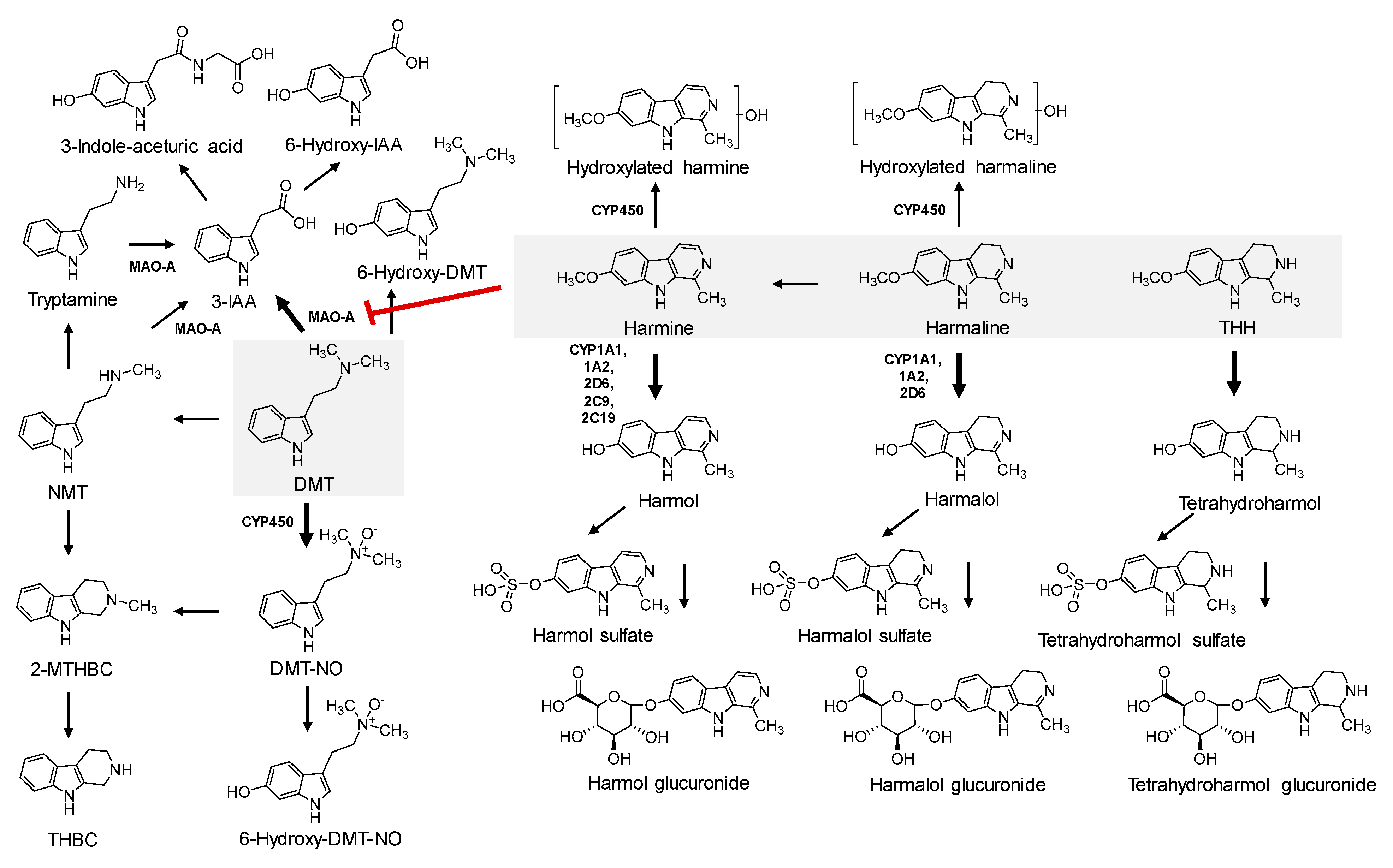
7.3. Metabolism
7.4. Excretion
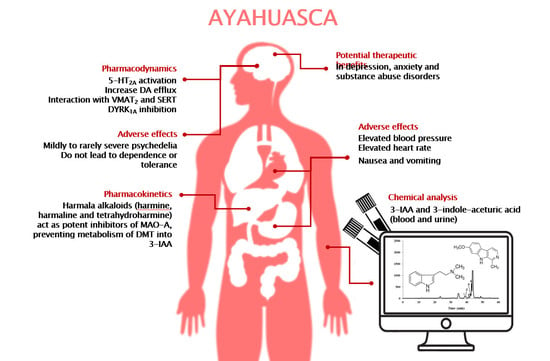
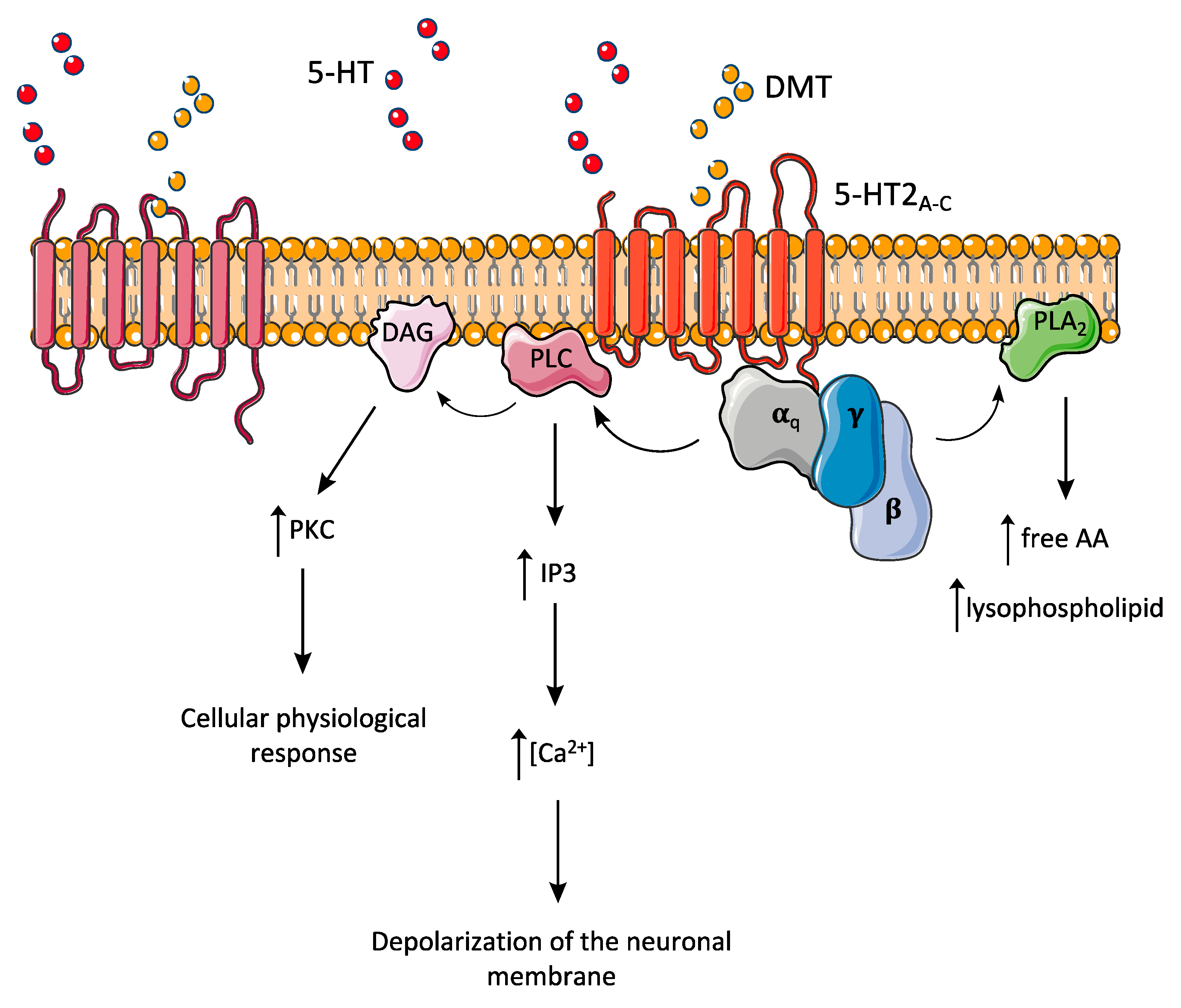
8. Pharmacodynamics
9. Psychological and Physiological Effects
10. Toxicological Effects
11. Substance Dependence and Tolerance
12. Potential Therapeutic Benefits
13. Ayahuasca Metabolomic Aspects
14. Toxicological Analysis and Forensic Relevance
15. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J. DMT: The Spirit Molecule: A Doctor’s Revolutionary Research into the Biology of Near-Death and Mystical Experiences; Park Street Press: Rochester, NY, USA, 2001. [Google Scholar]
- Cunha-Oliveira, T. Alucinogénios. In Toxicologia Forense; Dinis-Oliveira, R.J., Carvalho, F.D., Bastos, M.D.L., Eds.; PACTOR: Lisboa, Portugal, 2015; pp. 233–247. [Google Scholar]
- Araujo, A.M.; Carvalho, F.; Bastos Mde, L.; de Pinho, G.P.; Carvalho, M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, J.H. Drug addiction and drug abuse. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Rall, T.W., Nies, A.S., Taylor, P., Eds.; McGraw-Hill: New York, NY, USA, 1990; pp. 522–573. [Google Scholar]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.P. Drug Use Disorders and Addiction. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W., Gilman, A.G., Eds.; McGraw-Hill: New York, NY, USA, 2018; pp. 607–627. [Google Scholar]
- Chilcoat, H.D.; Schutz, C.G. Age-specific patterns of hallucinogen use in the US population: An analysis using generalized additive models. Drug Alcohol. Depend. 1996, 43, 143–153. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Pereira, C.L.; da Silva, D.D. Pharmacokinetic and pharmacodynamic aspects of peyote and mescaline: Clinical and forensic repercussions. Curr. Mol. Pharmacol. 2019, 12, 184–194. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Hallak, J.E.C. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci. Biobehav. Rev. 2020, 108, 423–434. [Google Scholar] [CrossRef]
- McKenna, D.J. Clinical investigations of the therapeutic potential of ayahuasca: Rationale and regulatory challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef]
- Callaway, J.C.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Raymon, L.P.; Poland, R.E.; Andrade, E.N.; Andrade, E.O.; Mash, D.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- Schultes, R.E. The identity of malpighiaceous narcotics of South America. Bot. Mus. Leafl. Harv. Univ. 1957, 18, 1–56. [Google Scholar]
- Zhao, T.; Zheng, S.S.; Zhang, B.F.; Li, Y.Y.; Bligh, S.W.; Wang, C.H.; Wang, Z.T. Metabolic pathways of the psychotropic-carboline alkaloids, harmaline and harmine, by liquid chromatography/mass spectrometry and NMR spectroscopy. Food Chem. 2012, 134, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J.; Towers, G.H.; Abbott, F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and beta-carboline constituents of ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. [Google Scholar] [CrossRef]
- Szara, S. Dimethyltryptamin: Its metabolism in man; the relation to its psychotic effect to the serotonin metabolism. Experientia 1956, 12, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A. LSD—My Problem Child; McGraw-Hill: New York, NY, USA, 1976; p. 209. [Google Scholar]
- Santos, R.G.; Landeira-Fernandez, J.; Strassman, R.J.; Motta, V.; Cruz, A.P. Effects of ayahuasca on psychometric measures of anxiety, panic-like and hopelessness in Santo Daime members. J. Ethnopharmacol. 2007, 112, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Labate, B.C.; Cavnar, C. Ayahuasca Shamanism in the Amazon and Beyond; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Yritia, M.; Riba, J.; Ortuno, J.; Ramirez, A.; Castillo, A.; Alfaro, Y.; de la Torre, R.; Barbanoj, M.J. Determination of N,N-dimethyltryptamine and beta-carboline alkaloids in human plasma following oral administration of Ayahuasca. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 779, 271–281. [Google Scholar] [CrossRef]
- Hamill, J.; Hallak, J.; Dursun, S.M.; Baker, G. Ayahuasca: Psychological and physiologic effects, pharmacology and potential uses in addiction and mental illness. Curr. Neuropharmacol. 2019, 17, 108–128. [Google Scholar] [CrossRef]
- Winstock, A.R.; Kaar, S.; Borschmann, R. Dimethyltryptamine (DMT): Prevalence, user characteristics and abuse liability in a large global sample. J. Psychopharmacol. 2014, 28, 49–54. [Google Scholar] [CrossRef]
- Ott, J. Pharmacotheon: Entheogenic Drugs Their Plant Sources and History; Natural Products Company: Kennewick, WA, USA, 1993. [Google Scholar]
- Schultes, R.E. Ethnotoxocological significance of additives to New World hallucinogens. Plant Sci. Bull. 1972, 18, 34–41. [Google Scholar]
- Rivier, L.; Lindgren, J. Ayahuasca, the South American hallucinogenic drink: Ethnobotanical and chemical investigations. Econ. Bot. 1972, 29, 101–129. [Google Scholar] [CrossRef]
- McIlhenny, E.H.; Riba, J.; Barbanoj, M.J.; Strassman, R.; Barker, S.A. Methodology for and the determination of the major constituents and metabolites of the Amazonian botanical medicine ayahuasca in human urine. Biomed. Chromatogr. 2011, 25, 970–984. [Google Scholar] [CrossRef]
- Callaway, J.C.; Raymon, L.P.; Hearn, W.L.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Mash, D.C. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J. Anal. Toxicol. 1996, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Samoylenko, V.; Tekwani, B.L.; Khan, I.A.; Miller, L.S.; Chaurasiya, N.D.; Rahman, M.M.; Tripathi, L.M.; Khan, S.I.; Joshi, V.C.; et al. Composition, standardization and chemical profiling of Banisteriopsis caapi, a plant for the treatment of neurodegenerative disorders relevant to Parkinson’s disease. J. Ethnopharmacol. 2010, 128, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.H. Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol. Ther. 2004, 102, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shulgin, A.T.; Shulgin, A. TIHKAL: The Continuation; Transform: Berkeley, CA, USA, 1997. [Google Scholar]
- Barker, S.A.; McIlhenny, E.H.; Strassman, R. A critical review of reports of endogenous psychedelic N,N-dimethyltryptamines in humans: 1955–2010. Drug Test Anal. 2012, 4, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Franzen, F.; Gross, H. Tryptamine, N,N-dimethyltryptamine, N,N-dimethyl-5-hydroxytryptamine and 5-methoxytryptamine in human blood and urine. Nature 1965, 206, 1052. [Google Scholar] [CrossRef] [PubMed]
- Christian, S.T.; Harrison, R.; Quayle, E.; Pagel, J.; Monti, J. The in vitro identification of dimethyltryptamine (DMT) in mammalian brain and its characterization as a possible endogenous neuroregulatory agent. Biochem. Med. 1977, 18, 164–183. [Google Scholar] [CrossRef]
- Dominguez-Clave, E.; Soler, J.; Elices, M.; Pascual, J.C.; Alvarez, E.; de la Revenga, F.M.; Friedlander, P.; Feilding, A.; Riba, J. Ayahuasca: Pharmacology, neuroscience and therapeutic potential. Brain Res. Bull. 2016, 126, 89–101. [Google Scholar] [CrossRef]
- McKenna, D.J.; Towers, G.H. Biochemistry and pharmacology of tryptamines and beta-carbolines. A minireview. J. Psychoact. Drugs 1984, 16, 347–358. [Google Scholar] [CrossRef]
- Gable, R.S. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007, 102, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Simao, A.Y.; Goncalves, J.; Duarte, A.P.; Barroso, M.; Cristovao, A.C.; Gallardo, E. Toxicological aspects and determination of the main components of ayahuasca: A critical review. Medicines 2019, 6, 106. [Google Scholar] [CrossRef]
- De Rios, D.M. Ayahuasca—The healing vine. Int. J. Soc. Psychiatry 1971, 17, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Labate, B.C. Consumption of ayahuasca by children and pregnant women: Medical controversies and religious perspectives. J. Psychoact. Drugs 2011, 43, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Tupper, K.W. The globalization of ayahuasca: Harm reduction or benefit maximization? Int. J. Drug Policy 2008, 19, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, A.I.E. Personality traits in former spanish substance users recovered with ayahuasca. J. Psychoact. Drugs 2020, 1–9. [Google Scholar] [CrossRef]
- Barbosa, P.C.; Mizumoto, S.; Bogenschutz, M.P.; Strassman, R.J. Health status of ayahuasca users. Drug Test Anal. 2012, 4, 601–609. [Google Scholar] [CrossRef]
- Dalgarno, P. Buying ayahuasca and other entheogens online: A word of caution. Addict. Res. Theory 2008, 16, 1–4. [Google Scholar] [CrossRef]
- Cakic, V.; Potkonyak, J.; Marshall, A. Dimethyltryptamine (DMT): Subjective effects and patterns of use among Australian recreational users. Drug Alcohol. Depend. 2010, 111, 30–37. [Google Scholar] [CrossRef]
- Kaasik, H.; Kreegipuu, K. Ayahuasca users in estonia: Ceremonial practices, subjective long-term effects, mental health, and quality of life. J. Psychoact. Drugs 2020, 1–9. [Google Scholar] [CrossRef]
- Bullis, R.K. The “vine of the soul” vs. the controlled substances act: Implications of the hoasca case. J. Psychoact. Drugs 2008, 40, 193–199. [Google Scholar] [CrossRef]
- International Center for Ethnobotanical Education Research & Service. Ayahuasca’s Legal Status. Available online: https://www.iceers.org/ayahuasca-legal-status/ (accessed on 30 July 2020).
- Lanaro, R.; Calemi, D.B.; Togni, L.R.; Costa, J.L.; Yonamine, M.; Cazenave Sde, O.; Linardi, A. Ritualistic use of ayahuasca versus street use of similar substances seized by the police: A key factor involved in the potential for intoxications and overdose? J. Psychoact. Drugs 2015, 47, 132–139. [Google Scholar] [CrossRef]
- Arunotayanun, W.; Gibbons, S. Natural product ‘legal highs’. Nat. Prod. Rep. 2012, 29, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Stiffler, J.D. Ayahuasca: From the Amazon to a city near you. Am. J. Addict. 2018, 27, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Kikura-Hanajiri, R.; Hayashi, M.; Saisho, K.; Goda, Y. Simultaneous determination of nineteen hallucinogenic tryptamines/beta-calbolines and phenethylamines using gas chromatography-mass spectrometry and liquid chromatography-electrospray ionisation-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 825, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Aixalà, M.; Bouso, J.C. Technical Report on Psychoactove Ethnobotanicals; International Center for Ethnobotanical Education Research & Service: Barcelona, Spain, 2018. [Google Scholar]
- Lin, R.L.; Sargeant, S.; Narasimhachari, N. Indolethylamine-N-methyltransferase in developing rabbit lung. Dev. Psychobiol. 1974, 7, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A. N,N-dimethyltryptamine (DMT), an endogenous hallucinogen: Past, present, and future research to determine its role and function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef]
- Cameron, L.P.; Olson, D.E. Dark classics in chemical neuroscience: N,N-dimethyltryptamine (DMT). ACS Chem. Neurosci. 2018, 9, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Kelly, S.; Hsieh, S.C.; Murphy, M.; Chen, L.; Kotb, A.; Peterson, J.; Coyle, D.; Skidmore, B.; Gomes, T.; et al. Triptans in the acute treatment of migraine: A systematic review and network meta-analysis. Headache 2015, 55 (Suppl. 4), 221–235. [Google Scholar] [CrossRef]
- Moffat, A.C.; Osselton, M.D.; Widdop, B.; Watts, J. Clarke’s Analysis of Drugs and Poisons, 4th ed.; Pharmaceutical Press: London, UK, 2011; p. 2736. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6089, N,N-Dimethyltryptamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/N_N-Dimethyltryptamine (accessed on 29 July 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3564, Harmaline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Harmaline (accessed on 29 July 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 159809, Tetrahydroharmine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tetrahydroharmine (accessed on 29 July 2020).
- Ott, J. Pharmahuasca: Human pharmacology of oral DMT plus harmine. J. Psychoact. Drugs 1999, 31, 171–177. [Google Scholar] [CrossRef]
- Turner, W.J.; Merlis, S. Effect of some indolealkylamines on man. AMA Arch. Neurol. Psychiatry 1959, 81, 121–129. [Google Scholar] [CrossRef]
- Strassman, R.J.; Qualls, C.R.; Berg, L.M. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol. Psychiatry 1996, 39, 784–795. [Google Scholar] [CrossRef]
- Strassman, R.J.; Qualls, C.R. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch. Gen. Psychiatry 1994, 51, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J.; Qualls, C.R.; Uhlenhuth, E.H.; Kellner, R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch. Gen. Psychiatry 1994, 51, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, A.R.; Strassman, R.J. A model for the application of target-controlled intravenous infusion for a prolonged immersive DMT psychedelic experience. Front. Pharmacol. 2016, 7, 211. [Google Scholar] [CrossRef]
- Naranjo, P. Estudio comparativo de la harmina, la dietilamida del ácido lisérgico (LSD-25) y la mescalina. Rev. Confed. Médica Panam. 1959, 6, 1–8. [Google Scholar]
- Wang, Y.; Wang, H.; Zhang, L.; Zhang, Y.; Sheng, Y.; Deng, G.; Li, S.; Cao, N.; Guan, H.; Cheng, X.; et al. Subchronic toxicity and concomitant toxicokinetics of long-term oral administration of total alkaloid extracts from seeds of Peganum harmala Linn: A 28-day study in rats. J. Ethnopharmacol. 2019, 238, 111866. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Deng, G.; Wang, Y.; Qi, S.; Cheng, X.; Ma, Y.; Xie, Y.; Wang, C. Exposure characteristics of the analogous β-carboline alkaloids harmaline and harmine based on the efflux transporter of multidrug resistance protein 2. Front. Pharmacol. 2017, 8, 541. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Balthazar, F.M.; Bouso, J.C.; Hallak, J.E. The current state of research on ayahuasca: A systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J. Psychopharmacol. 2016, 30, 1230–1247. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Valle, M.; Bouso, J.C.; Nomdedeu, J.F.; Rodriguez-Espinosa, J.; McIlhenny, E.H.; Barker, S.A.; Barbanoj, M.J.; Riba, J. Autonomic, neuroendocrine, and immunological effects of ayahuasca: A comparative study with d-amphetamine. J. Clin. Psychopharmacol. 2011, 31, 717–726. [Google Scholar] [CrossRef]
- Riba, J.; Valle, M.; Urbano, G.; Yritia, M.; Morte, A.; Barbanoj, M.J. Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J. Pharmacol. Exp. Ther. 2003, 306, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, B.R.; Lockett, L.; Talomsin, R.; Blackman, G.L.; McLeod, W.R. In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem. Pharmacol. 1987, 36, 1509–1512. [Google Scholar] [CrossRef]
- Cohen, I.; Vogel, W.H. Determination and physiological disposition of dimethyltryptamine and diethyltryptamine in rat brain, liver and plasma. Biochem. Pharmacol. 1972, 21, 1214–1216. [Google Scholar] [CrossRef]
- Yanai, K.; Ido, T.; Ishiwata, K.; Hatazawa, J.; Takahashi, T.; Iwata, R.; Matsuzawa, T. In vivo kinetics and displacement study of a carbon-11-labeled hallucinogen, N,N-[11C]dimethyltryptamine. Eur. J. Nucl. Med. 1986, 12, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sangiah, S.; Gomez, M.V.; Domino, E.F. Accumulation of N,N-dimethyltryptamine in rat brain cortical slices. Biol. Psychiatry 1979, 14, 925–936. [Google Scholar] [PubMed]
- Kaplan, J.; Mandel, L.R.; Stillman, R.; Walker, R.W.; VandenHeuvel, W.J.; Gillin, J.C.; Wyatt, R.J. Blood and urine levels of N,N-dimethyltryptamine following administration of psychoactive dosages to human subjects. Psychopharmacologia 1974, 38, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Hedden, M.P. Behavioral effects and metabolic fate of N,N-dimethyltryptamine in mice pretreated with beta-diethylaminoethyl-diphenylpropylacetate (SKF 525-A), improniazid and chlorpromazine. Pharmacol. Biochem. Behav. 1978, 8, 351–356. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Valle, M.; Bouso, J.C.; Barker, S.A. Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012, 4, 610–616. [Google Scholar] [CrossRef]
- Erspamer, V. Observations on the fate of indolalkylamines in the organism. J. Physiol. 1955, 127, 118–133. [Google Scholar] [CrossRef]
- Orlefors, H.; Sundin, A.; Fasth, K.J.; Oberg, K.; Langstrom, B.; Eriksson, B.; Bergstrom, M. Demonstration of high monoaminoxidase-A levels in neuroendocrine gastroenteropancreatic tumors in vitro and in vivo-tumor visualization using positron emission tomography with 11C-harmine. Nucl. Med. Biol. 2003, 30, 669–679. [Google Scholar] [CrossRef]
- Liester, M.B.; Prickett, J.I. Hypotheses regarding the mechanisms of ayahuasca in the treatment of addictions. J. Psychoact. Drugs 2012, 44, 200–208. [Google Scholar] [CrossRef]
- Gaweska, H.; Fitzpatrick, P.F. Structures and mechanism of the monoamine oxidase family. Biomol. Concepts 2011, 2, 365–377. [Google Scholar] [CrossRef]
- McEwen, C.M., Jr.; Sober, A.J. Rabbit serum monoamine oxidase. II. Determinants of substrate specificity. J. Biol. Chem. 1967, 242, 3068–3078. [Google Scholar] [PubMed]
- Barker, S.A.; Monti, J.A.; Christian, S.T. N, N-dimethyltryptamine: An endogenous hallucinogen. Int. Rev. Neurobiol. 1981, 22, 83–110. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Monti, J.A.; Christian, S.T. Metabolism of the hallucinogen N,N-dimethyltryptamine in rat brain homogenates. Biochem. Pharmacol. 1980, 29, 1049–1057. [Google Scholar] [CrossRef]
- Fish, M.S.; Johnson, N.M.; Lawrence, E.P.; Horning, E.C. Oxidative N-dealkylation. Biochem. Biophys. Acta 1955, 18, 564–565. [Google Scholar] [CrossRef]
- McIlhenny, E.H.; Riba, J.; Barbanoj, M.J.; Strassman, R.; Barker, S.A. Methodology for determining major constituents of ayahuasca and their metabolites in blood. Biomed. Chromatogr. 2012, 26, 301–313. [Google Scholar] [CrossRef]
- Sitaram, B.R.; Lockett, L.; Blackman, G.L.; McLeod, W.R. Urinary excretion of 5-methoxy-N,N-dimethyltryptamine, N,N-dimethyltryptamine and their N-oxides in the rat. Biochem. Pharmacol. 1987, 36, 2235–2237. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Bouso, J.C.; Barker, S.A. Metabolism and urinary disposition of N,N-dimethyltryptamine after oral and smoked administration: A comparative study. Drug Test Anal. 2015, 7, 401–406. [Google Scholar] [CrossRef]
- Szara, S.; Axelrod, J. Hydroxylation and N-demethylation of N,N-dimethyltryptamine. Experientia 1959, 15, 216–217. [Google Scholar] [CrossRef]
- Miksys, S.; Rao, Y.; Hoffmann, E.; Mash, D.C.; Tyndale, R.F. Regional and cellular expression of CYP2D6 in human brain: Higher levels in alcoholics. J. Neurochem. 2002, 82, 1376–1387. [Google Scholar] [CrossRef]
- Yu, A.M.; Idle, J.R.; Krausz, K.W.; Kupfer, A.; Gonzalez, F.J. Contribution of individual cytochrome P450 isozymes to the O-demethylation of the psychotropic beta-carboline alkaloids harmaline and harmine. J. Pharmacol. Exp. Ther. 2003, 305, 315–322. [Google Scholar] [CrossRef]
- Yu, A.M. Indolealkylamines: Biotransformations and potential drug-drug interactions. AAPS J. 2008, 10, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Randic, M.; Padjen, A. Effect of N,N-dimethyltryptamine and D-lysergic acid diethylamide on the release of 5-hydroxyindoles in rat forebrain. Nature 1971, 230, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.X.; Gottleib, R.; Lovell, R.A. Psychotomimetic drugs and brain 5-hydroxytryptamine metabolism. Biochem. Pharmacol. 1970, 19, 1181–1188. [Google Scholar] [CrossRef]
- Anden, N.E.; Corrodi, H.; Fuxe, K. Hallucinogenic drugs of the indolealkylamine type and central monoamine neurons. J. Pharmacol. Exp. Ther. 1971, 179, 236–249. [Google Scholar] [PubMed]
- Glennon, R.A.; Young, R.; Rosecrans, J.A. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur. J. Pharmacol. 1983, 91, 189–196. [Google Scholar] [CrossRef]
- Huot, P.; Johnston, T.H.; Darr, T.; Hazrati, L.N.; Visanji, N.P.; Pires, D.; Brotchie, J.M.; Fox, S.H. Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov. Disord. 2010, 25, 1399–1408. [Google Scholar] [CrossRef]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Smith, R.L.; Canton, H.; Barrett, R.J.; Sanders-Bush, E. Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol. Biochem. Behav. 1998, 61, 323–330. [Google Scholar] [CrossRef]
- Strassman, R.J. Human psychopharmacology of N,N-dimethyltryptamine. Behav. Brain Res. 1996, 73, 121–124. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994, 23, 163–178. [Google Scholar] [CrossRef]
- McKenna, D.J.; Repke, D.B.; Lo, L.; Peroutka, S.J. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 1990, 29, 193–198. [Google Scholar] [CrossRef]
- Descarries, L.; Cornea-Hebert, V.; Riad, M. Cellular and subcellular localization of serotonin receptors in the central nervous system. In The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics, 1st ed.; Roth, B.L., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 277–317. [Google Scholar]
- Gonzalez-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Frankel, P.S.; Cunningham, K.A. The hallucinogen d-lysergic acid diethylamide (d-LSD) induces the immediate-early gene c-Fos in rat forebrain. Brain Res. 2002, 958, 251–260. [Google Scholar] [CrossRef]
- O’Donovan, K.J.; Tourtellotte, W.G.; Millbrandt, J.; Baraban, J.M. The EGR family of transcription-regulatory factors: Progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999, 22, 167–173. [Google Scholar] [CrossRef]
- DeSteno, D.A.; Schmauss, C. Induction of early growth response gene 2 expression in the forebrain of mice performing an attention-set-shifting task. Neuroscience 2008, 152, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.W.; Errington, M.L.; French, P.J.; Fine, A.; Bliss, T.V.; Garel, S.; Charnay, P.; Bozon, B.; Laroche, S.; Davis, S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001, 4, 289–296. [Google Scholar] [CrossRef]
- Bouso, J.C.; Palhano-Fontes, F.; Rodriguez-Fornells, A.; Ribeiro, S.; Sanches, R.; Crippa, J.A.; Hallak, J.E.; de Araujo, D.B.; Riba, J. Long-term use of psychedelic drugs is associated with differences in brain structure and personality in humans. Eur. Neuropsychopharmacol. 2015, 25, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Osorio, F.L.; Crippa, J.A.; Hallak, J.E. Antidepressive and anxiolytic effects of ayahuasca: A systematic literature review of animal and human studies. Braz. J. Psychiatry 2016, 38, 65–72. [Google Scholar] [CrossRef]
- Fde, O.L.; Sanches, R.F.; Macedo, L.R.; Santos, R.G.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Braz. J. Psychiatry 2015, 37, 13–20. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Eshleman, A.J.; Forster, M.J.; Cheng, K.; Rice, K.C.; Gatch, M.B. The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology 2015, 232, 275–284. [Google Scholar] [CrossRef]
- Sotelo, C.; Cholley, B.; El Mestikawy, S.; Gozlan, H.; Hamon, M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur. J. Neurosci. 1990, 2, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; de Montigny, C. Electrophysiological investigation of the adaptive response of the 5-HT system to the administration of 5-HT1A receptor agonists. J. Cardiovasc. Pharmacol. 1990, 15 (Suppl. 7), S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology 1999, 21, 99s–105s. [Google Scholar] [CrossRef]
- Karila, D.; Freret, T.; Bouet, V.; Boulouard, M.; Dallemagne, P.; Rochais, C. Therapeutic potential of 5-HT6 receptor agonists. J. Med. Chem. 2015, 58, 7901–7912. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Labus, J.; Volpicelli, F.; Guseva, D.; Lacivita, E.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; Bijata, M.; Perrone-Capano, C.; et al. Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J. Neurochem. 2017, 141, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Fone, K.C. An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function. Neuropharmacology 2008, 55, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, F.; Speranza, L.; di Porzio, U.; Crispino, M.; Perrone-Capano, C. The serotonin receptor 7 and the structural plasticity of brain circuits. Front Behav. Neurosci. 2014, 8, 318. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Gopalakrishnan, A.; Anderson, L.L.; Feih, J.T.; Shulgin, A.T.; Daley, P.F.; Ruoho, A.E. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J. Neural. Transm. 2009, 116, 1591–1599. [Google Scholar] [CrossRef]
- Nagai, F.; Nonaka, R.; Kamimura, S.H.K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007, 559, 132–137. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-dimethyltryptamine. Brain Res. Bull. 2016, 126, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Fulop, L.; Szucs, M.; Frecska, E. The role of sigma-1 receptor, an intracellular chaperone in neurodegenerative diseases. Curr. Neuropharmacol. 2018, 16, 97–116. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef]
- Hayashi, T. Sigma-1 receptor: The novel intracellular target of neuropsychotherapeutic drugs. J. Pharmacol. Sci. 2015, 127, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Cobos, E.J.; Entrena, J.M.; Nieto, F.R.; Cendán, C.M.; Del Pozo, E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr. Neuropharmacol. 2008, 6, 344–366. [Google Scholar] [CrossRef]
- Schenberg, E.E. Ayahuasca and cancer treatment. SAGE Open Med. 2013, 1, 2050312113508389. [Google Scholar] [CrossRef]
- Sanches, R.F.; de Osorio, L.F.; Dos Santos, R.G.; Macedo, L.R.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A SPECT study. J. Clin. Psychopharmacol. 2016, 36, 77–81. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Premont, R.T.; Gainetdinov, R.R.; Caron, M.G. Following the trace of elusive amines. Proc. Natl. Acad. Sci. USA 2001, 98, 9474–9475. [Google Scholar] [CrossRef]
- Grammenos, D.; Barker, S.A. On the transmethylation hypothesis: Stress, N,N-dimethyltryptamine, and positive symptoms of psychosis. J. Neural. Transm. 2015, 122, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Freedland, C.S.; Mansbach, R.S. Behavioral profile of constituents in ayahuasca, an Amazonian psychoactive plant mixture. Drug Alcohol. Depend. 1999, 54, 183–194. [Google Scholar] [CrossRef]
- Frison, G.; Favretto, D.; Zancanaro, F.; Fazzin, G.; Ferrara, S.D. A case of beta-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Sci. Int. 2008, 179, e37–e43. [Google Scholar] [CrossRef] [PubMed]
- Iurlo, M.; Leone, G.; Schilstrom, B.; Linner, L.; Nomikos, G.; Hertel, P.; Silvestrini, B.; Svensson, H. Effects of harmine on dopamine output and metabolism in rat striatum: Role of monoamine oxidase-A inhibition. Psychopharmacology 2001, 159, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Buckholtz, N.S.; Boggan, W.O. Inhibition by beta-carbolines of monoamine uptake into a synaptosomal preparation: Structure-activity relationships. Life Sci. 1977, 20, 2093–2099. [Google Scholar] [CrossRef]
- Glennon, R.A.; Dukat, M.; Grella, B.; Hong, S.; Costantino, L.; Teitler, M.; Smith, C.; Egan, C.; Davis, K.; Mattson, M.V. Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors. Drug Alcohol. Depend. 2000, 60, 121–132. [Google Scholar] [CrossRef]
- Brierley, D.I.; Davidson, C. Developments in harmine pharmacology—Implications for ayahuasca use and drug-dependence treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Drucker, G.; Raikoff, K.; Neafsey, E.J.; Collins, M.A. Dopamine uptake inhibitory capacities of beta-carboline and 3,4-dihydro-beta-carboline analogs of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) oxidation products. Brain Res. 1990, 509, 125–133. [Google Scholar] [CrossRef]
- Schmitt, K.C.; Reith, M.E. Regulation of the dopamine transporter: Aspects relevant to psychostimulant drugs of abuse. Ann. N. Y. Acad. Sci. 2010, 1187, 316–340. [Google Scholar] [CrossRef]
- De Castro-Neto, E.F.; da Cunha, R.H.; da Silveira, D.X.; Yonamine, M.; Gouveia, T.L.; Cavalheiro, E.A.; Amado, D.; Mda, N.M.G. Changes in aminoacidergic and monoaminergic neurotransmission in the hippocampus and amygdala of rats after ayahuasca ingestion. World J. Biol. Chem. 2013, 4, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Kjellgren, A.; Eriksson, A.; Norlander, T. Experiences of encounters with ayahuasca—“The vine of the soul”. J. Psychoact. Drugs 2009, 41, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Checkley, S.A.; Murray, R.M.; Oon, M.C.; Rodnight, R.; Birley, J.L. A longitudinal study of urinary excretion of N,N-dimethyltryptamine in psychotic patients. Br. J. Psychiatry 1980, 137, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, J.F.; Mandel, L.R.; Ahn, H.S.; Vanden Heuvel, W.J.; Walker, R.W. Blood dimethyltryptamine concentrations in psychotic disorders. Biol. Psychiatry 1974, 9, 89–91. [Google Scholar] [PubMed]
- Gillin, J.C.; Kaplan, J.; Stillman, R.; Wyatt, R.J. The psychedelic model of schizophrenia: The case of N,N-dimethyltryptamine. Am. J. Psychiatry 1976, 133, 203–208. [Google Scholar] [CrossRef]
- De Araujo, D.B.; Ribeiro, S.; Cecchi, G.A.; Carvalho, F.M.; Sanchez, T.A.; Pinto, J.P.; de Martinis, B.S.; Crippa, J.A.; Hallak, J.E.; Santos, A.C. Seeing with the eyes shut: Neural basis of enhanced imagery following Ayahuasca ingestion. Hum. Brain Mapp. 2012, 33, 2550–2560. [Google Scholar] [CrossRef]
- Bouso, J.C.; Gonzalez, D.; Fondevila, S.; Cutchet, M.; Fernandez, X.; Ribeiro Barbosa, P.C.; Alcazar-Corcoles, M.A.; Araujo, W.S.; Barbanoj, M.J.; Fabregas, J.M.; et al. Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of Ayahuasca: A longitudinal study. PLoS ONE 2012, 7, e42421. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bouso, J.C.; Hallak, J.E.C. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Ther. Adv. Psychopharmacol. 2017, 7, 141–157. [Google Scholar] [CrossRef]
- De Rios, M.D. Interview with Guillermo Arrevalo, a Shipibo urban shaman, by Roger Rumrrill. J. Psychoact. Drugs 2005, 37, 203–207. [Google Scholar] [CrossRef]
- Airaksinen, M.M.; Lecklin, A.; Saano, V.; Tuomisto, L.; Gynther, J. Tremorigenic effect and inhibition of tryptamine and serotonin receptor binding by beta-carbolines. Pharmacol. Toxicol. 1987, 60, 5–8. [Google Scholar] [CrossRef]
- Louis, E.D.; Zheng, W.; Jurewicz, E.C.; Watner, D.; Chen, J.; Factor-Litvak, P.; Parides, M. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology 2002, 59, 1940–1944. [Google Scholar] [CrossRef]
- Davydova, S.M.; Cheido, M.A.; Gevorgyan, M.M.; Idova, G.V. Effects of 5-HT2A receptor stimulation and blocking on immune response. Bull. Exp. Biol. Med. 2010, 150, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G. Immunological effects of ayahuasca in humans. J. Psychoact. Drugs 2014, 46, 383–388. [Google Scholar] [CrossRef] [PubMed]
- House, R.V.; Thomas, P.T.; Bhargava, H.N. Comparison of the hallucinogenic indole alkaloids ibogaine and harmaline for potential immunomodulatory activity. Pharmacology 1995, 51, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J. Adverse reactions to psychedelic drugs. A review of the literature. J. Nerv. Ment. Dis. 1984, 172, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Halpern, J.H.; Pope, H.G., Jr. Do hallucinogens cause residual neuropsychological toxicity? Drug Alcohol. Depend. 1999, 53, 247–256. [Google Scholar] [CrossRef]
- Reynolds, P.C.; Jindrich, E.J. A mescaline associated fatality. J. Anal. Toxicol. 1985, 9, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Keeler, M.H.; Reifler, C.B. Suicide during an LSD reaction. Am. J. Psychiatry 1967, 123, 884–885. [Google Scholar] [CrossRef]
- Schatz, H.; Mendelblatt, F. Solar retinopathy from sun-gazing under the influence of LSD. Br. J. Ophthalmol. 1973, 57, 270–273. [Google Scholar] [CrossRef]
- Fuller, D.G. Severe solar maculopathy associated with the use of lysergic acid diethylamide (LSD). Am. J. Ophthalmol. 1976, 81, 413–416. [Google Scholar] [CrossRef]
- Ikeda, A.; Sekiguchi, K.; Fujita, K.; Yamadera, H.; Koga, Y. 5-methoxy-N,N-diisopropyltryptamine-induced flashbacks. Am. J. Psychiatry 2005, 162, 815. [Google Scholar] [CrossRef]
- Callaway, J.C.; Grob, C.S. Ayahuasca preparations and serotonin reuptake inhibitors: A potential combination for severe adverse interactions. J. Psychoact. Drugs 1998, 30, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, B.J.; Lee, K.C. Ayahuasca: An ancient sacrament for treatment of contemporary psychiatric illness? Ment. Health Clin. 2017, 7, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Volpi-Abadie, J.; Kaye, A.M.; Kaye, A.D. Serotonin syndrome. Ochsner. J. 2013, 13, 533–540. [Google Scholar] [PubMed]
- Halpern, J.H.; Sherwood, A.R.; Passie, T.; Blackwell, K.C.; Ruttenber, A.J. Evidence of health and safety in American members of a religion who use a hallucinogenic sacrament. Med. Sci. Monit. 2008, 14, Sr15–Sr22. [Google Scholar]
- Pic-Taylor, A.; da Motta, L.G.; de Morais, J.A.; Junior, W.M.; Santos Ade, F.; Campos, L.A.; Mortari, M.R.; von Zuben, M.V.; Caldas, E.D. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav. Process. 2015, 118, 102–110. [Google Scholar] [CrossRef]
- Sklerov, J.; Levine, B.; Moore, K.A.; King, T.; Fowler, D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J. Anal. Toxicol. 2005, 29, 838–841. [Google Scholar] [CrossRef]
- Bauer, I.L. Ayahuasca: A risk for travellers? Travel Med. Infect. Dis. 2018, 21, 74–76. [Google Scholar] [CrossRef]
- Warren, R.J. Fatal nicotine intoxication resulting from the ingestion of “ayahuasca”. J. Anal. Toxicol. 2004, 28, 287. [Google Scholar]
- Colaco, C.S.; Alves, S.S.; Nolli, L.M.; Pinheiro, W.O.; de Oliveira, D.G.R.; Santos, B.W.L.; Pic-Taylor, A.; Mortari, M.R.; Caldas, E.D. Toxicity of ayahuasca after 28 days daily exposure and effects on monoamines and brain-derived neurotrophic factor (BDNF) in brain of Wistar rats. Metab. Brain Dis. 2020. [Google Scholar] [CrossRef]
- Halpern, J.H.; Sherwood, A.R.; Hudson, J.I.; Yurgelun-Todd, D.; Pope, H.G., Jr. Psychological and cognitive effects of long-term peyote use among Native Americans. Biological. Psychiatry 2005, 58, 624–631. [Google Scholar] [CrossRef]
- Krebs, T.S.; Johansen, P.O. Psychedelics and mental health: A population study. PLoS ONE 2013, 8, e63972. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, M.; Etemad, L.; Javidi, S.; Alizadeh, A. Peganum harmala intoxication, a case report. Avicenna. J. Phytomed. 2013, 3, 288–292. [Google Scholar] [PubMed]
- Ben Salah, N.; Amamou, M.; Jerbi, Z.; Ben Salah, F.; Yacoub, M. A case of overdose with Peganum harmala L. J. Toxicol. Clin. Exp. 1986, 6, 319–322. [Google Scholar]
- Yuruktumen, A.; Karaduman, S.; Bengi, F.; Fowler, J. Syrian rue tea: A recipe for disaster. Clin. Toxicol. 2008, 46, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G. Toxicity of chronic ayahuasca administration to the pregnant rat: How relevant it is regarding the human, ritual use of ayahuasca? Birth. Defects. Res. B Dev. Reprod. Toxicol. 2010, 89, 533–535, author reply 531–532. [Google Scholar] [CrossRef]
- Kamel, S.H.; Ibrahim, T.M.; Hamza, S.M. Effect of harmine and harmaline hydrochloride on pregnancy in white rats. Zent. Vet. A 1971, 18, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Poulson, E.; Robson, J.M. The effect of amine oxidase inhibitors on pregnancy. J. Endocrinol. 1963, 27, 147–155. [Google Scholar] [CrossRef]
- Oliveira, C.D.; Moreira, C.Q.; de Sa, L.R.; Spinosa Hde, S.; Yonamine, M. Maternal and developmental toxicity of ayahuasca in Wistar rats. Birth. Defects. Res. B Dev. Reprod. Toxicol. 2010, 89, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bouso, J.C.; Fabregas, J.M.; Antonijoan, R.M.; Rodriguez-Fornells, A.; Riba, J. Acute effects of ayahuasca on neuropsychological performance: Differences in executive function between experienced and occasional users. Psychopharmacology 2013, 230, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Grasa, E.; Valle, M.; Ballester, M.R.; Bouso, J.C.; Nomdedeu, J.F.; Homs, R.; Barbanoj, M.J.; Riba, J. Pharmacology of ayahuasca administered in two repeated doses. Psychopharmacology 2012, 219, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.M.; Pieper, W.A. The effects of N,N-dimethyltryptamine on operant behavior in squirrel monkeys. Psychopharmacologia 1973, 29, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Gillin, J.C.; Cannon, E.; Magyar, R.; Schwartz, M.; Wyatt, R.J. Failure of N,N-dimethyltryptamine to evoke tolerance in cats. Biol. Psychiatry 1973, 7, 213–220. [Google Scholar] [PubMed]
- Rosenberg, D.E.; Isbell, H.; Miner, E.J.; Logan, C.R. The effect of N,N-dimethyltryptamine in human subjects tolerant to lysergic acid diethylamide. Psychopharmacologia 1964, 5, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, A.; Fontanari, D. Acquired and crossed tolerance to mescaline, LSD-25, and BOL-148. AMA Arch. Gen. Psychiatry 1959, 1, 279–282. [Google Scholar] [CrossRef]
- Appel, J.B.; Freedman, D.X. Tolerance and cross-tolerance among psychotomimetic drugs. Psychopharmacologia 1968, 13, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.; Campos, M.G.; Orfão, J.M.; Ribeiro, C.A. Plants with neurobiological activity as potential targets for drug discovery. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1372–1389. [Google Scholar] [CrossRef]
- Frecska, E.; Bokor, P.; Winkelman, M. The therapeutic potentials of ayahuasca: Possible effects against various diseases of civilization. Front. Pharmacol. 2016, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, M.J. Therapeutic applications of Ayahuasca and other sacred medicines. In The Therapeutic Use of Ayahuasca; Labate, B.C., Cavnar, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–21. [Google Scholar]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osorio, F.L.; Sanches, R.; Dos Santos, R.G.; et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.G.; Osorio, F.L.; Crippa, J.A.S.; Hallak, J.E.C. Classical hallucinogens and neuroimaging: A systematic review of human studies: Hallucinogens and neuroimaging. Neurosci. Biobehav. Rev. 2016, 71, 715–728. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S.; McKenna, D.J.; Callaway, J.C.; Brito, G.S.; Neves, E.S.; Oberlaender, G.; Saide, O.L.; Labigalini, E.; Tacla, C.; Miranda, C.T.; et al. Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J. Nerv. Ment. Dis. 1996, 184, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Grob, C.S. The psychology of ayahuasca. In Ayahuasca: Hallucinogens, Consciousness, and the Spirit of Nature; Metzner, R., Ed.; Thunder’s Mouth Press: New York, NY, USA, 1999. [Google Scholar]
- Da Silveira, D.X.; Grob, C.S.; de Rios, M.D.; Lopez, E.; Alonso, L.K.; Tacla, C.; Doering-Silveira, E. Ayahuasca in adolescence: A preliminary psychiatric assessment. J. Psychoact. Drugs 2005, 37, 129–133. [Google Scholar] [CrossRef]
- Doering-Silveira, E.; Lopez, E.; Grob, C.S.; de Rios, M.D.; Alonso, L.K.; Tacla, C.; Shirakawa, I.; Bertolucci, P.H.; Da Silveira, D.X. Ayahuasca in adolescence: A neuropsychological assessment. J. Psychoact. Drugs 2005, 37, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, P.S.; Thorne, C.B.; Clark, C.B.; Coombs, D.W.; Johnson, M.W. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol. 2015, 29, 280–288. [Google Scholar] [CrossRef]
- Lawn, W.; Hallak, J.E.; Crippa, J.A.; Dos Santos, R.; Porffy, L.; Barratt, M.J.; Ferris, J.A.; Winstock, A.R.; Morgan, C.J.A. Well-being, problematic alcohol consumption and acute subjective drug effects in past-year ayahuasca users: A large, international, self-selecting online survey. Sci. Rep. 2017, 7, 15201. [Google Scholar] [CrossRef]
- Frecska, E.; Szabo, A.; Winkelman, M.J.; Luna, L.E.; McKenna, D.J. A possibly sigma-1 receptor mediated role of dimethyltryptamine in tissue protection, regeneration, and immunity. J. Neural. Transm. 2013, 120, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, K.; Wieloch, T. The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration. J. Pharmacol. Sci. 2015, 127, 30–35. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test. Eur. Neuropsychopharmacol. 2006, 16, 324–328. [Google Scholar] [CrossRef]
- Dai, F.; Chen, Y.; Song, Y.; Huang, L.; Zhai, D.; Dong, Y.; Lai, L.; Zhang, T.; Li, D.; Pang, X.; et al. A natural small molecule harmine inhibits angiogenesis and suppresses tumour growth through activation of p53 in endothelial cells. PLoS ONE 2012, 7, e52162. [Google Scholar] [CrossRef]
- Reus, G.Z.; Stringari, R.B.; de Souza, B.; Petronilho, F.; Dal-Pizzol, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Harmine and imipramine promote antioxidant activities in prefrontal cortex and hippocampus. Oxid. Med. Cell. Longev. 2010, 3, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Katchborian-Neto, A.; Santos, W.T.; Nicacio, K.J.; Correa, J.O.A.; Murgu, M.; Martins, T.M.M.; Gomes, D.A.; Goes, A.M.; Soares, M.G.; Dias, D.F.; et al. Neuroprotective potential of Ayahuasca and untargeted metabolomics analyses: Applicability to Parkinson’s disease. J. Ethnopharmacol. 2020, 255, 112743. [Google Scholar] [CrossRef] [PubMed]
- Lo Faro, A.F.; Di Trana, A.; La Maida, N.; Tagliabracci, A.; Giorgetti, R.; Busardò, F.P. Biomedical analysis of New Psychoactive Substances (NPS) of natural origin. J. Pharm. Biomed. Anal. 2020, 179, 112945. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.D.; Choy, K.W.; Drummer, O.H.; Schneider, H.G. Harmala alkaloids identify ayahausca intoxication in a urine drug screen. J. Anal. Toxicol. 2019, 43, e23–e27. [Google Scholar] [CrossRef]
- Oliveira, C.D.; Okai, G.G.; da Costa, J.L.; de Almeida, R.M.; Oliveira-Silva, D.; Yonamine, M. Determination of dimethyltryptamine and β-carbolines (ayahuasca alkaloids) in plasma samples by LC-MS/MS. Bioanalysis 2012, 4, 1731–1738. [Google Scholar] [CrossRef]
- Tavares, L.; Monedeiro, F.; Bordin, D.M.; De Martinis, B.S. Investigation of ayahuasca beta-carboline alkaloids and tryptamine in sweat samples from religious community participants by GC-MS. J. Anal. Toxicol. 2020. [Google Scholar] [CrossRef]
- Pichini, S.; Marchei, E.; Garcia-Algar, O.; Gomez, A.; Di Giovannandrea, R.; Pacifici, R. Ultra-high-pressure liquid chromatography tandem mass spectrometry determination of hallucinogenic drugs in hair of psychedelic plants and mushrooms consumers. J. Pharm. Biomed. Anal. 2014, 100, 284–289. [Google Scholar] [CrossRef]
- Regester, L.E.; Chmiel, J.D.; Holler, J.M.; Vorce, S.P.; Levine, B.; Bosy, T.Z. Determination of designer drug cross-reactivity on five commercial immunoassay screening kits. J. Anal. Toxicol. 2015, 39, 144–151. [Google Scholar] [CrossRef]
- Liu, C.H.; Chu, W.L.; Liao, S.C.; Yang, C.C.; Lin, C.C. Syrian rue seeds interacted with acacia tree bark in an herbal stew resulted in N,N-dimethyltryptamine poisoning. Clin. Toxicol. 2019, 57, 867–869. [Google Scholar] [CrossRef]
- Souza, R.C.Z.; Zandonadi, F.S.; Freitas, D.P.; Tofoli, L.F.F.; Sussulini, A. Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1124, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.P.; De Oliveira, C.D.; Moura, S.; Dörr, F.A.; Silva, W.A.; Yonamine, M. Gas chromatographic analysis of dimethyltryptamine and beta-carboline alkaloids in ayahuasca, an Amazonian psychoactive plant beverage. Phytochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, C.; Aroni, K.; Rossi, R.; Moretti, L.; Bacci, M. Identification of N,N-dimethyltryptamine and beta-carbolines in psychotropic ayahuasca beverage. Biomed. Chromatogr. 2008, 22, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Gaujac, A.; Dempster, N.; Navickiene, S.; Brandt, S.D.; de Andrade, J.B. Determination of N,N-dimethyltryptamine in beverages consumed in religious practices by headspace solid-phase microextraction followed by gas chromatography ion trap mass spectrometry. Talanta 2013, 106, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Eller, S.; Borges, G.R.; Ossanes, D.S.; Birk, L.; Scheid, C.; Yonamine, M.; Grossi, P.; Merib, J.O.; Oliveira, T.F. A rapid analytical strategy for the determination of ayahuasca alkaloids in non-ritualistic approaches by UHPLC-MS/MS. Forensic Sci. Int. 2020, 312, 110298. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.M.; Musah, R.A. Detection of diagnostic plant-derived psychoactive biomarkers in fingerprints by MALDI-SPIRALTOF-mass spectrometry imaging. Methods Mol. Biol. 2018, 1810, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.C. Fast and slow metabolizers of Hoasca. J. Psychoact. Drugs 2005, 37, 157–161. [Google Scholar] [CrossRef]
- Lesiak, A.D.; Musah, R.A. Application of ambient ionization high resolution mass spectrometry to determination of the botanical provenance of the constituents of psychoactive drug mixtures. Forensic Sci. Int. 2016, 266, 271–280. [Google Scholar] [CrossRef]
- Gaujac, A.; Navickiene, S.; Collins, M.I.; Brandt, S.D.; de Andrade, J.B. Analytical techniques for the determination of tryptamines and beta-carbolines in plant matrices and in psychoactive beverages consumed during religious ceremonies and neo-shamanic urban practices. Drug Test Anal. 2012, 4, 636–648. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Hallak, J.E.C. Ayahuasca, an ancient substance with traditional and contemporary use in neuropsychiatry and neuroscience. Epilepsy Behav. 2019, 106300. [Google Scholar] [CrossRef]
- Johansen, P.O.; Krebs, T.S. Psychedelics not linked to mental health problems or suicidal behavior: A population study. J. Psychopharmacol. 2015, 29, 270–279. [Google Scholar] [CrossRef]
- Johnson, M.; Richards, W.; Griffiths, R. Human hallucinogen research: Guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar] [CrossRef] [PubMed]

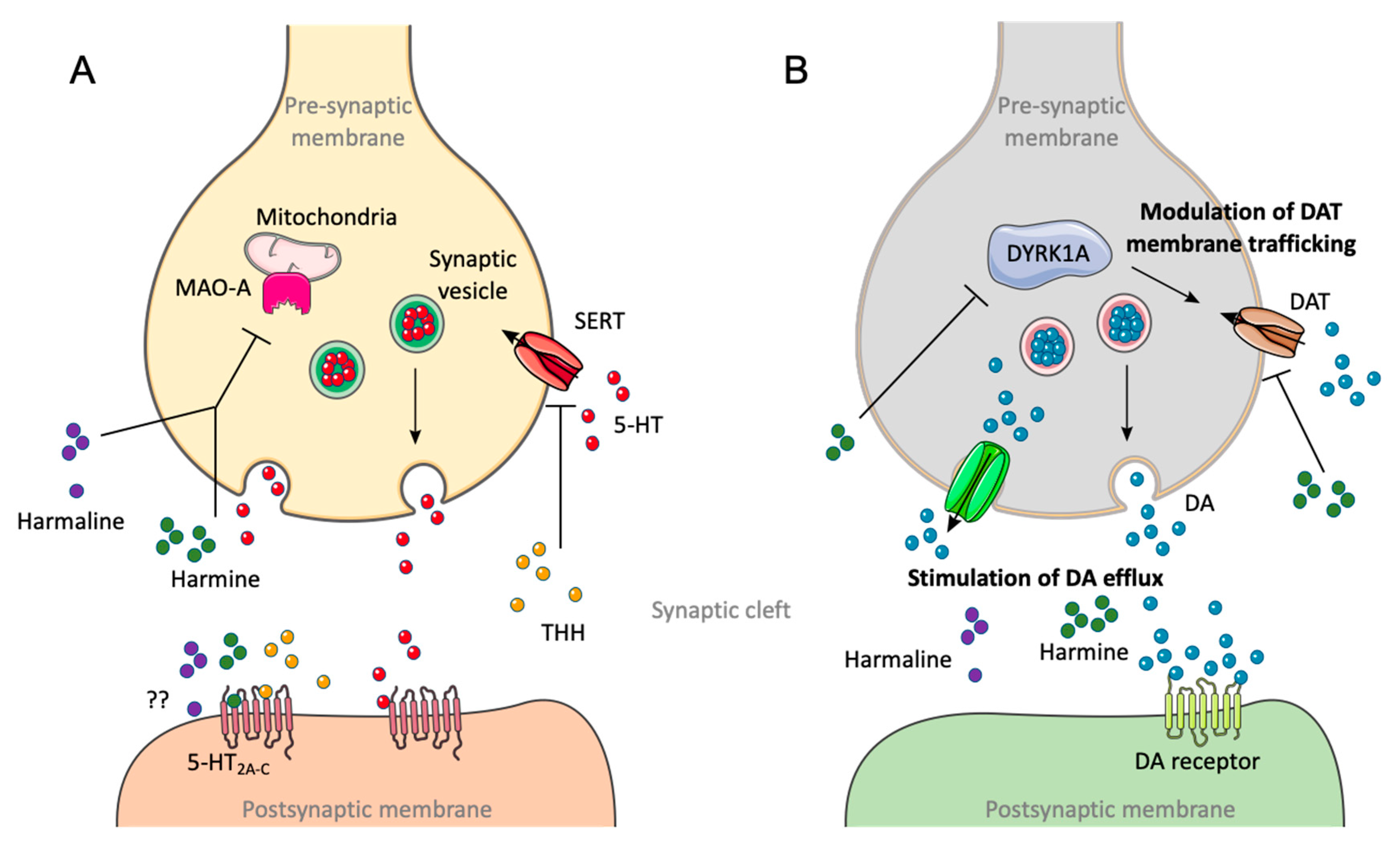
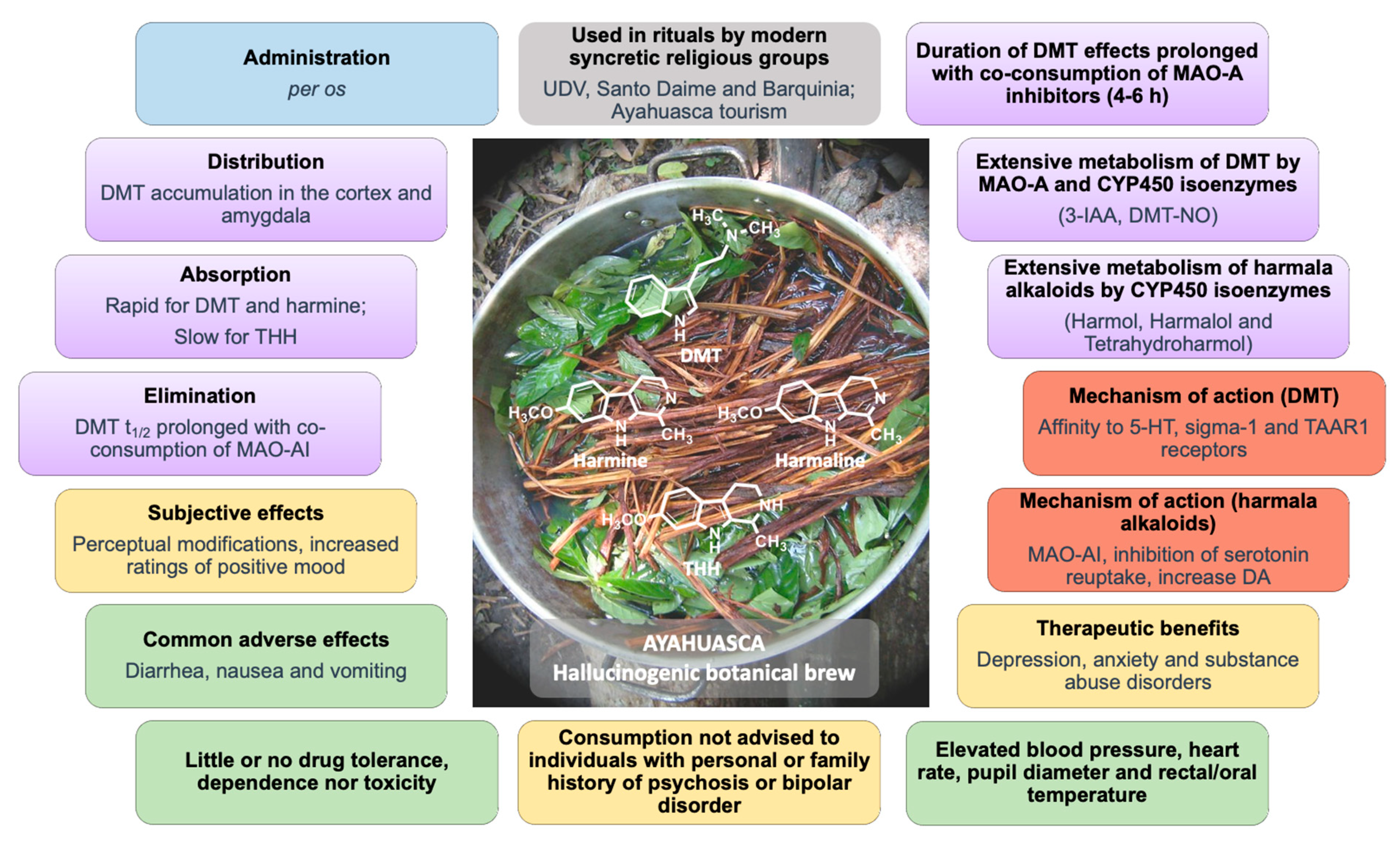
| Ayahuasca Preparations | DMT (mg/mL) | Harmine (mg/mL) | Harmaline (mg/mL) | THH (mg/mL) | Total Alkaloids (mg/mL) | Reference |
|---|---|---|---|---|---|---|
| Rio Purús 1 | 0.13 | 0.15 | n.a. | 0.05 | 0.33 | [26] |
| UDV | 0.24 | 1.70 | 0.20 | 1.07 | 3.21 | [13] |
| Pucallpa 2 | 0.60 | 4.67 | 0.41 | 1.60 | 7.28 | [16] |
| Ayahuasca Alkaloids | LogP | MW (g/mol) | Mp (°C) | pKa |
|---|---|---|---|---|
| DMT | 2.573 | 188.27 | 44.6–46.8 | 8.68 |
| Harmine | 3.56 | 212.25 | 261 | 7.6 |
| Harmaline | 2.1 | 214.3 | 249–250 | n.a. |
| THH | 1.9 | 216.28 | n.a. | n.a. |
| Ayahuasca Alkaloids | Route of Administration, Doses and Onset/Duration of Effects | Absorption | Distribution | Excretion | ||||
|---|---|---|---|---|---|---|---|---|
| Individual | Ayahuasca | Individual | Ayahuasca | Individual | Ayahuasca | Individual | Ayahuasca | |
| DMT | Smoked (40–50 mg): onset of effects at 1–15 s, peak at 5 min, and duration of 1 h; Injected: similar effects | Oral: peak of effects at 1.5–2 h; duration of effects 4–6 h | Extensive first pass metabolism (no bioavailability) | Tmax 1 = 1.5–1.8 h | Rapid distribution through the liver and kidney (5–10 min), and brain (10–15 min) | - | t1/2 2 = 5.7 min for brain, 9.6 min for liver, 17.2 min for kidney, and 15.8 min for blood Oral: 3-IAA (97%), DMT-NO (3%) Smoked: 3-IAA (63%), DMT-NO (28%), DMT (10%) | t1/2 2 = 1.07–4 h Recovery in urine: 3-IAA (50%), DMT-NO (10%), DMT (1%), 2-MTHBC and NMT (0.2%) |
| Harmine | Oral (20–50 mg): onset of effects at 20–30 min, peak at 30 min–1 h, and duration of 6–8 h; i.m. injection: onset of effects at 5–10 min, peak at 30 min, and duration of 3–5 h | Oral | Tmax 1 = 0.56–2.7 h Bioavailability: 17.11 % | Tmax 1 = 1.7 h | High concentrations in the liver, kidney, spleen, and lung | - | - | t1/2 2 = undetectable-2 h |
| Harmaline | - | Oral | Tmax 1 = 0.73–4 h Bioavailability: 1.09 % | Tmax 1 = 2.4 h | High concentrations in the liver, kidney, spleen, and lung Found in the brain | - | - | t1/2 2 = 1.95–2.1 h |
| THH | - | Oral | - | Tmax 1 = 2.5–3 h | - | - | - | t1/2 2 = 4.68–8.9 h |
| Analytes | Analytical Method | Matrix | Recovery (%) | Linearity (Mean R2) | LOD and LOQ | Reference |
|---|---|---|---|---|---|---|
| DMT; harmine, harmaline, THH, harmol and harmalol | GC-NPD; HPLC-FLD | Plasma | 74 (DMT); >87 (harmine, harmaline, THH, harmol and harmalol) | 0.9946 (DMT); >0.9916 (harmine, harmaline, THH, harmol and harmalol) | 0.5 ng/mL and 1.6 ng/mL for DMT; 0.1 ng/mL and 0.5 ng/mL for harmine; 0.1 ng/mL and 0.3 ng/mL for harmaline, harmol and harmalol; 0.3 ng/mL and 1.0 ng/mL for THH | [21] |
| DMT; harmine, harmaline, and THH | GC-NPD; HPLC-FLD | Plasma | Not available | 0.994 (DMT) | 0.5 ng/mL and 5 ng/mL for DMT; 0.1 ng/mL and 2.0 ng/mL for harmine; 0.05 ng/mL and 1.0 ng/mL for harmaline; 0.1 ng/mL and 1.9 ng/mL for THH | [28] |
| DMT, harmine, harmaline, THH, harmol, harmalol, and other metabolites | LC-MS/MS (ESI) | Urine | Not available | 0.9997 (DMT), 0.9996 (harmine), 0.9992 (harmaline), 0.9990 (THH), 0.9995 (harmol), 0.9986 (harmalol), 0.9995 (NMT), 0.9991 (2-MTHBC), 0.9995 (DMT-NO) | 0.12 ng/mL and 5 ng/mL for DMT; 0.18 ng/mL and 5 ng/mL for harmine; 0.07 ng/mL and 5 ng/mL for harmaline; 0.21 ng/mL and 5 ng/mL for THH; 0.57 ng/mL and 5 ng/mL for harmol; 0.18 ng/mL and 5 ng/mL for harmalol; 0.04 ng/mL and 5 ng/mL for NMT; 0.14 ng/mL and 5 ng/mL for 2-MTHBC; 0.07 ng/mL and 5 ng/mL for DMT-NO | [27] |
| DMT, harmine, harmaline, THH, harmol, harmalol, and other metabolites | LC-MS/MS (HESI) | Blood | 60.28–76.31 | 0.9995 (DMT), 0.9990 (harmine), 0.9986 (harmaline), 0.9992 (THH), 0.9992 (harmol), 0.9994 (harmalol), 0.9993 (NMT), 0.9986 (2-MTHBC), 0.9990 (DMT-NO) | 0.45 ng/mL and 1 ng/mL for DMT; 0.25 ng/mL and 1 ng/mL for harmine; 0.22 ng/mL and 1 ng/mL for harmaline; 0.36 ng/mL and 1 ng/mL for THH; 0.30 ng/mL and 1 ng/mL for harmol; 0.38 ng/mL and 1 ng/mL for harmalol; 0.32 ng/mL and 1 ng/mL for NMT; 0.33 ng/mL and 1 ng/mL for 2-MTHBC; 0.25 ng/mL and 1 ng/mL for DMT-NO | [89] |
| DMT, harmine, harmaline, and THH | LC-MS/MS (HESI) | Plasma | 89.4–107.7 | 0.9984 (DMT), 0.9934 (harmine), 0.9972 (harmaline), 0.9908 (THH) | 0.1 ng/mL and 0.2 ng/mL for DMT; 0.1 ng/mL and 0.3 ng/mL for harmine; 0.1 ng/mL and 0.4 ng/mL for harmaline; 0.1 ng/mL and 0.4 ng/mL for THH | [212] |
| DMT, harmine, and harmaline | GC-MS | Sweat | 72.1–90.1 | 0.9922 (DMT), 0.9943 (harmine), 0.9931 (harmaline) | 10 ng/patch and 20 ng/patch for DMT; 15 ng/ patch and 20 ng/patch for harmine; 15 ng/ patch and 20 ng/patch for harmaline | [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito-da-Costa, A.M.; Dias-da-Silva, D.; Gomes, N.G.M.; Dinis-Oliveira, R.J.; Madureira-Carvalho, Á. Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact. Pharmaceuticals 2020, 13, 334. https://doi.org/10.3390/ph13110334
Brito-da-Costa AM, Dias-da-Silva D, Gomes NGM, Dinis-Oliveira RJ, Madureira-Carvalho Á. Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact. Pharmaceuticals. 2020; 13(11):334. https://doi.org/10.3390/ph13110334
Chicago/Turabian StyleBrito-da-Costa, Andreia Machado, Diana Dias-da-Silva, Nelson G. M. Gomes, Ricardo Jorge Dinis-Oliveira, and Áurea Madureira-Carvalho. 2020. "Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact" Pharmaceuticals 13, no. 11: 334. https://doi.org/10.3390/ph13110334
APA StyleBrito-da-Costa, A. M., Dias-da-Silva, D., Gomes, N. G. M., Dinis-Oliveira, R. J., & Madureira-Carvalho, Á. (2020). Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact. Pharmaceuticals, 13(11), 334. https://doi.org/10.3390/ph13110334