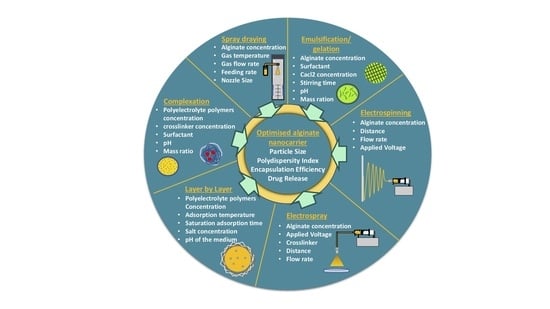
Alginate Nanoformulation: Influence of Process and Selected Variables
Abstract
1. Introduction
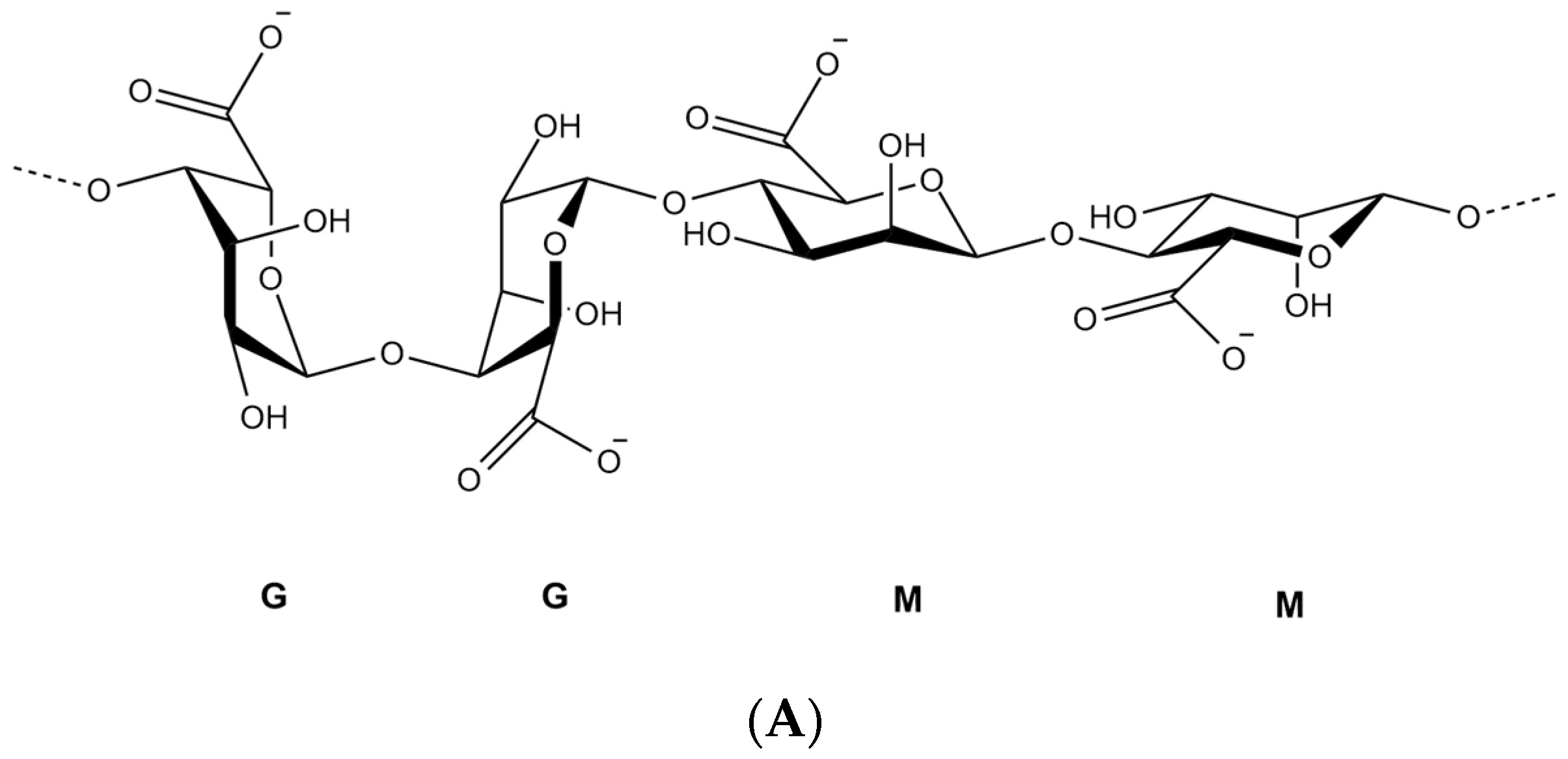
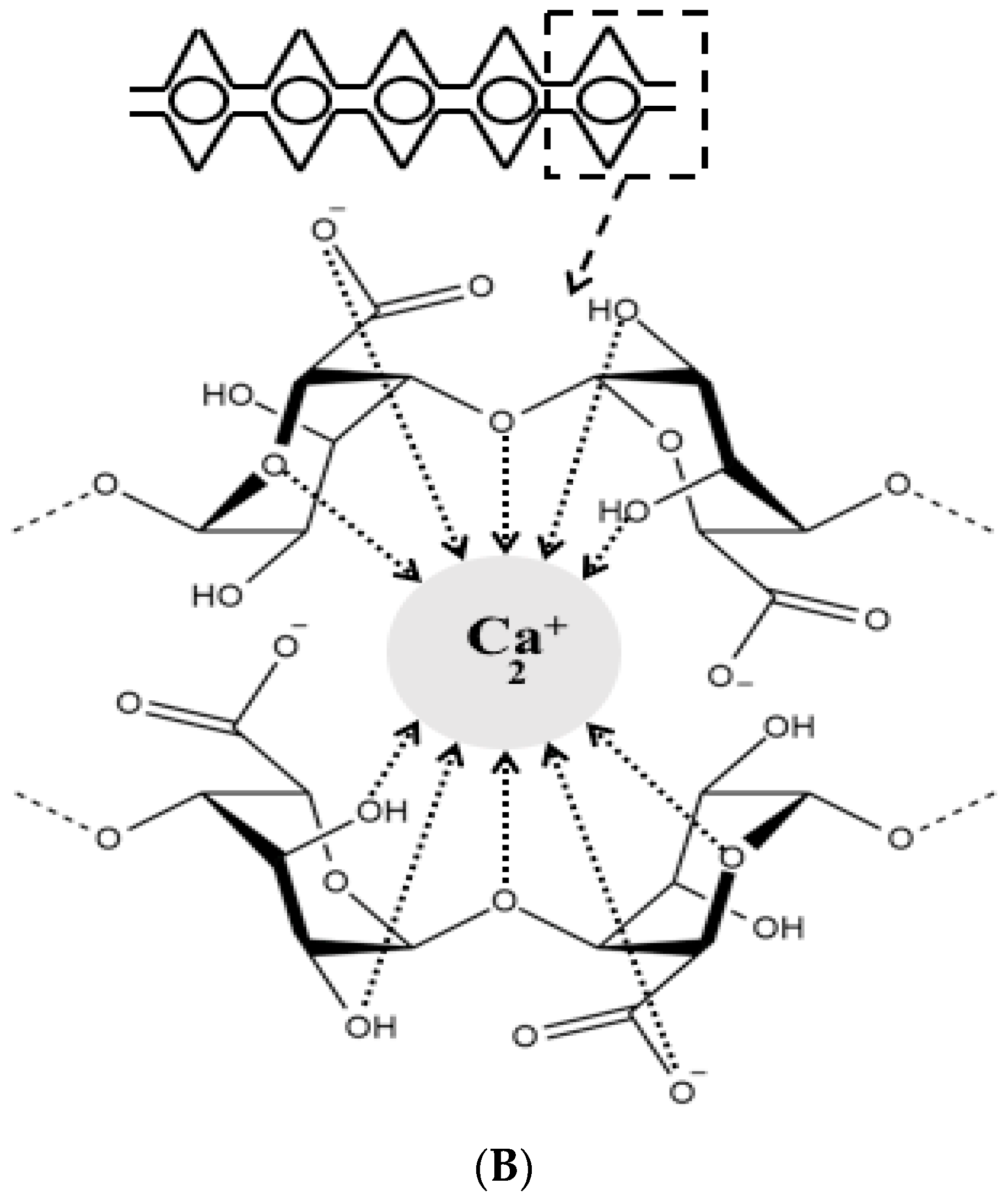
2. Alginate Polymer
3. Sources of Alginates, Extraction, and Purification Methods
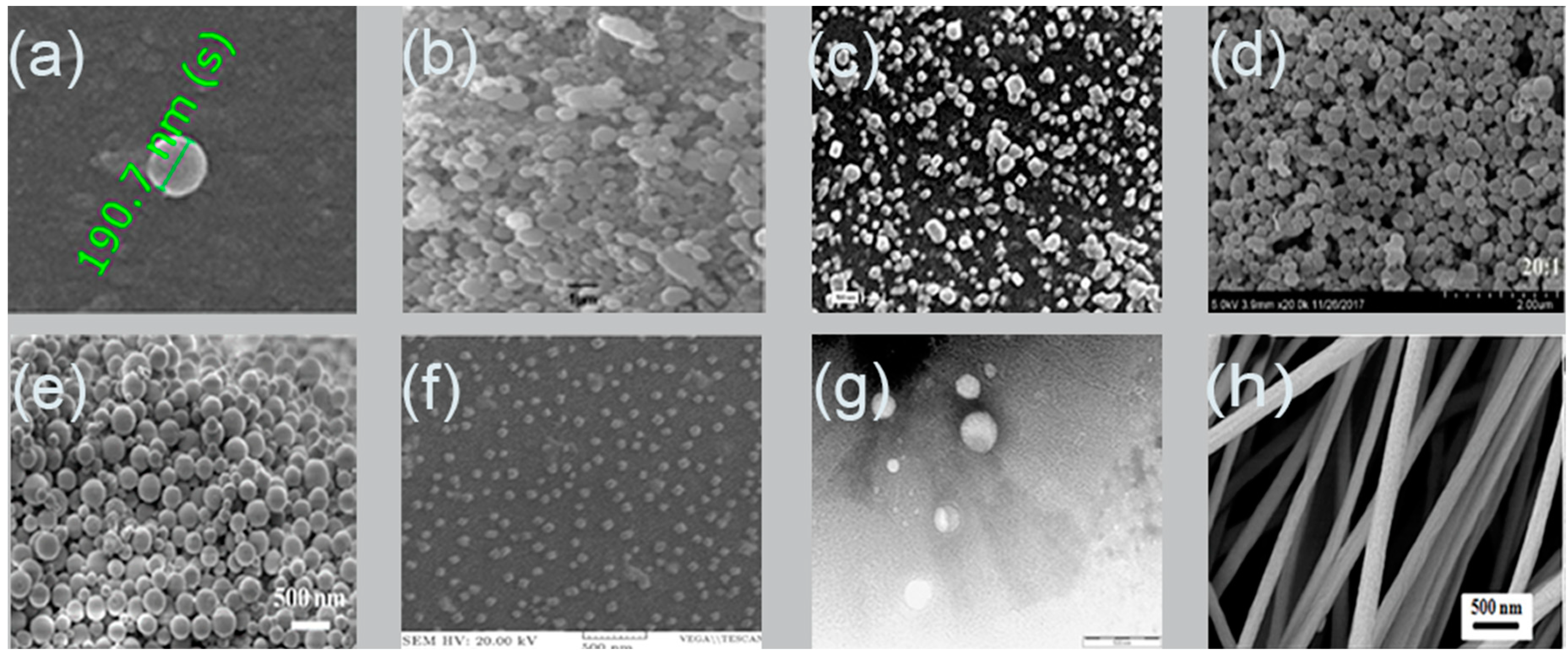
4. Alginate Nanoparticles
5. Alginate Nanoparticles Preparation Methods
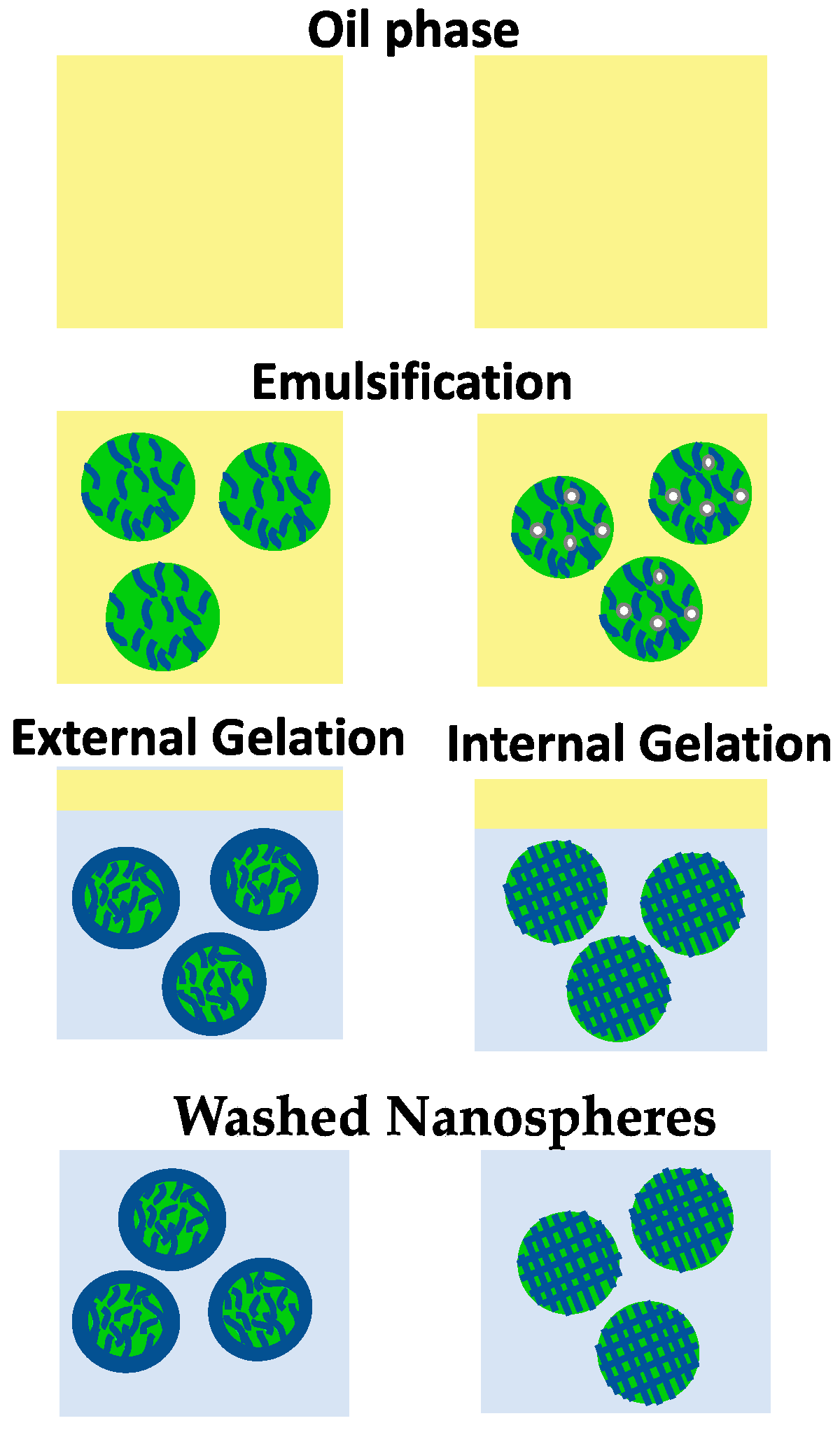
5.1. Emulsification/Gelation
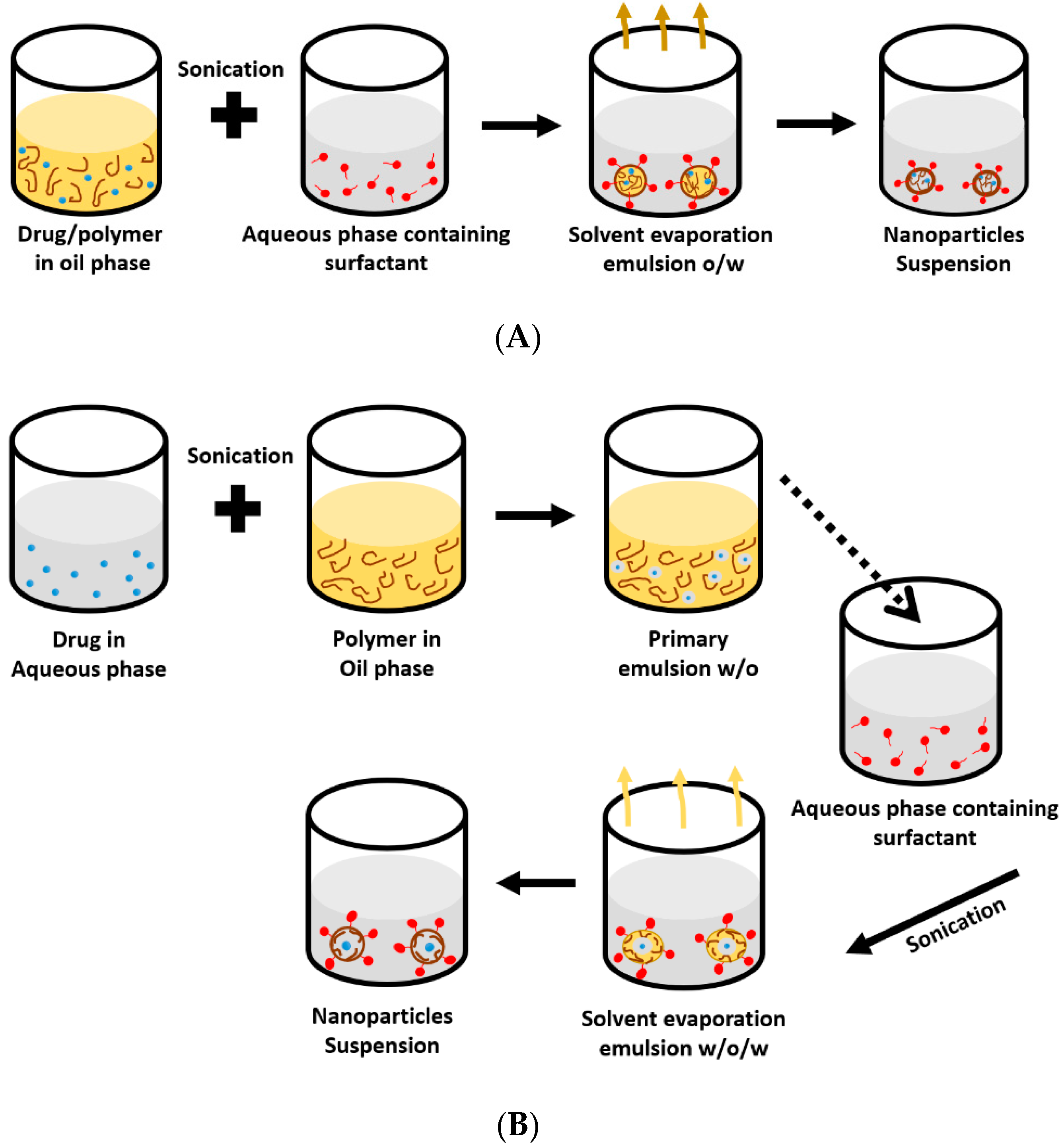
5.2. Emulsification-Solvent Displacement Technique
5.3. Solvent Evaporation Technique
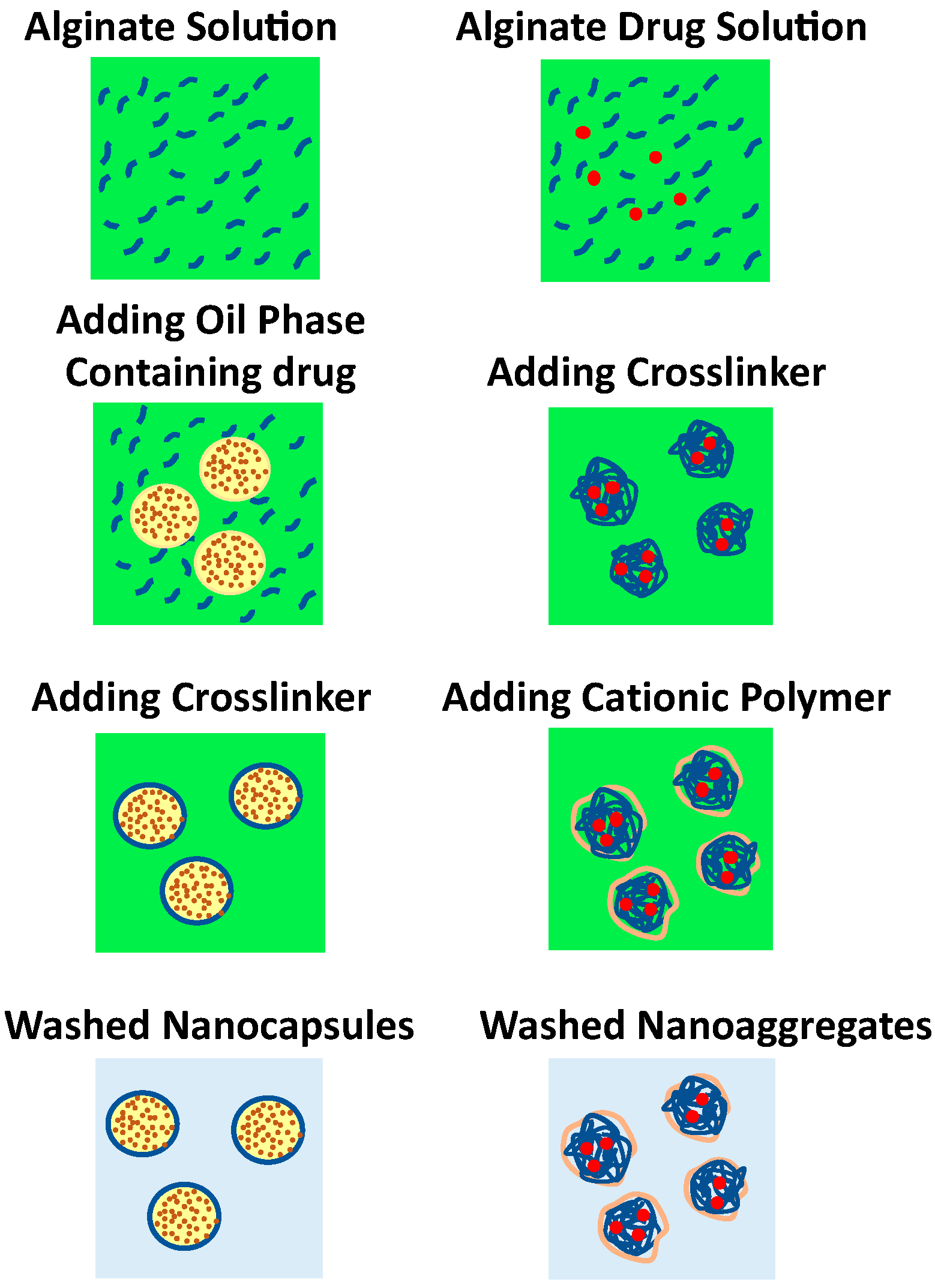
5.4. Complexation
5.5. Alginate as a Coating Material for Nanocarrier (Layer-by-Layer Approach)
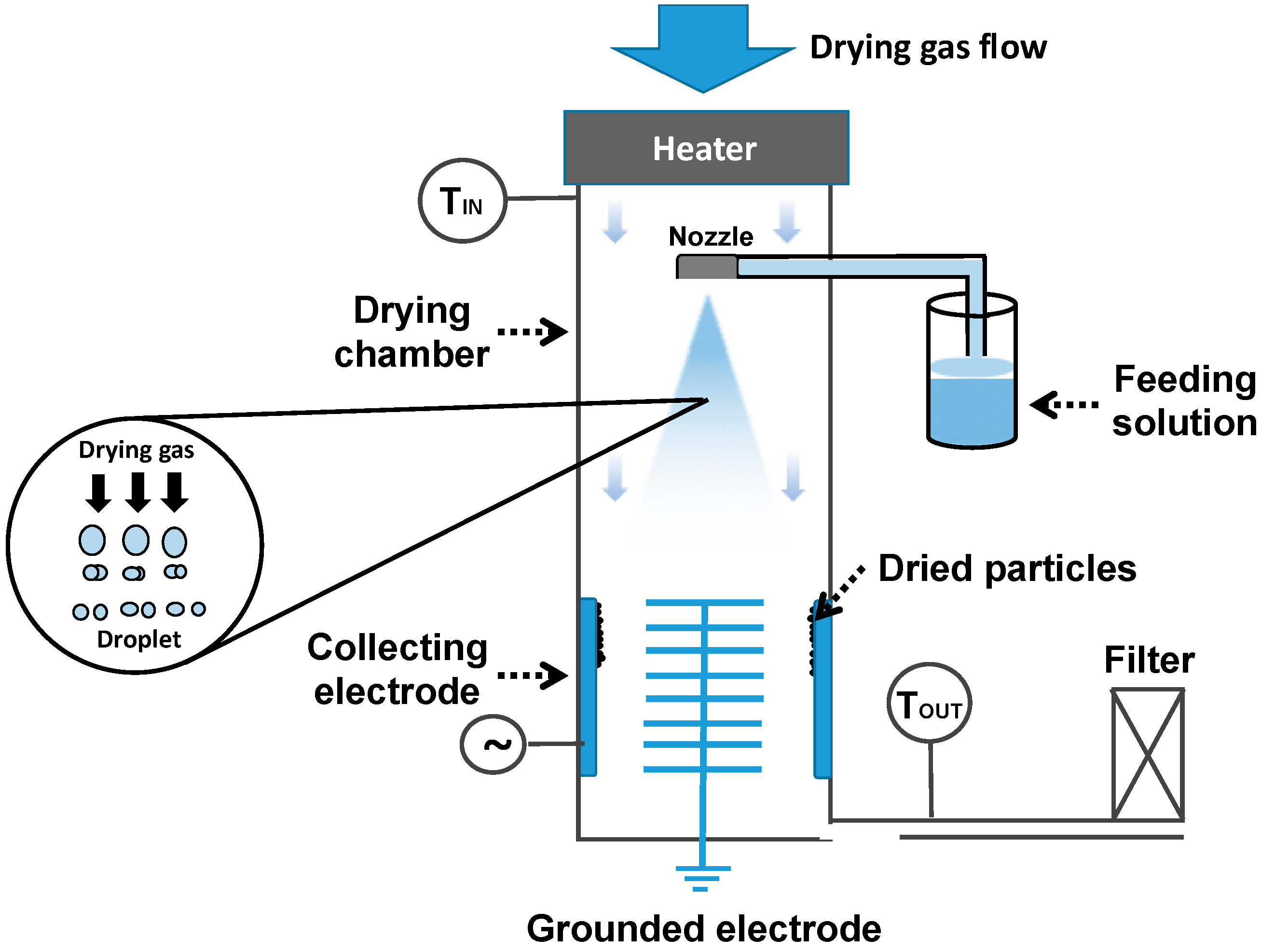
5.6. Spray Drying
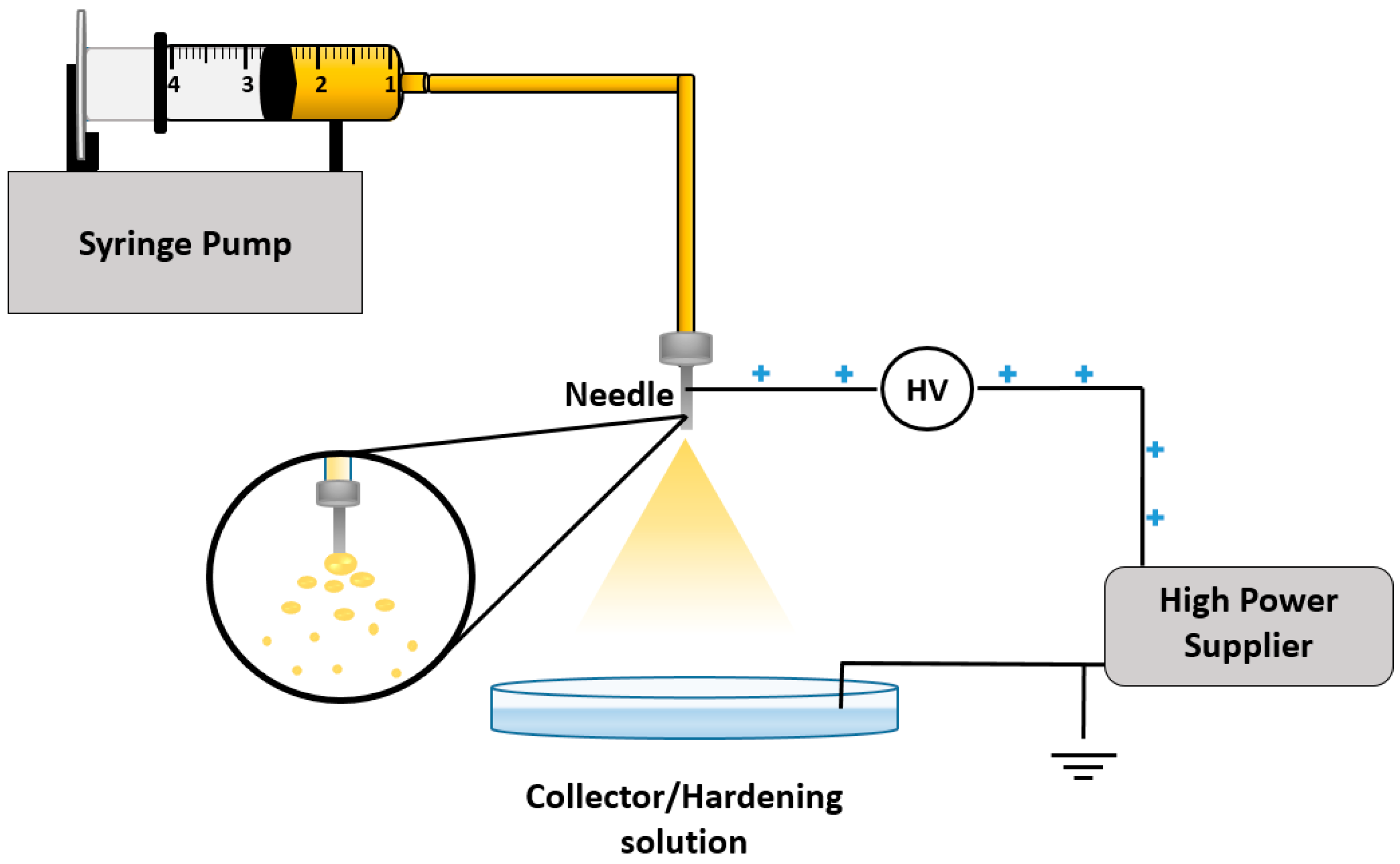
5.7. Electrospray
5.8. Electrospinning
6. Limitations of Alginate Nanofabrication
7. Factors Influencing Alginate Nanoparticles’ Characteristics: Particle Size, Size Distribution, Encapsulation Efficiency and Drug Release
7.1. The Influence of Alginate Concentration
7.2. The Influence of Surfactant
7.3. The Influence of CaCl2 Concentration
7.4. The Influence of Crosslinking Time
7.5. The Influence of Stirring Rate
7.6. The Influence of pH
7.7. The Influence of Alginate: Chitosan Mass Ratio
8. Comparison of Alginate Nanoparticles’ Synthesis Methods
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jawahar, N.; Meyyanathan, S. Polymeric Nanoparticles for Drug Delivery and Targeting: A Comprehensive Review. Int. J. Health Allied Sci. 2012, 1, 217. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Witzigmann, D.; Kulkarni, J.A.; Leung, J.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticle Technology for Therapeutic Gene Regulation in the Liver. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Poovi, G.; Damodharan, N. Lipid Nanoparticles: A Challenging Approach for Oral Delivery of BCS Class-II Drugs. Future J. Pharm. Sci. 2018, 4, 191–205. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Pansieri, J.; Gerstenmayer, M.; Lux, F.; Mériaux, S.; Tillement, O.; Forge, V.; Larrat, B.; Marquette, C. Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review. Nanomaterials 2018, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Payne, L.G. Polymeric Carriers for Oral Uptake of Microparticulates. Adv. Drug Deliv. Rev. 1998, 34, 155–170. [Google Scholar] [CrossRef]
- Rinaudo, M. Main Properties and Current Applications of Some Polysaccharides as Biomaterials. Polym. Int. 2007, 57, 397–430. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Salleh, S.N.; Fairus, A.A.H.; Zahary, M.N.; Raj, N.B.; Jalil, A.M.M. Unravelling the Effects of Soluble Dietary Fibre Supplementation on Energy Intake and Perceived Satiety in Healthy Adults: Evidence from Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Foods 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Lim, F.; Sun, A.M. Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef]
- Ismail, I.; Fauzi, N.H.M.; Baki, M.Z.; Hoon, H.L. Effects of Different Drying Methods and Hydrocolloids on Quality Properties of Semi-Dried Catfish Jerky. Malays. J. Appl. Sci. 2016, 2, 11–18. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles—A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Loquercio, A.; Castell-Perez, E.; Gomes, C.; Moreira, R.G. Preparation of Chitosan-Alginate Nanoparticles for Trans-Cinnamaldehyde Entrapment. J. Food Sci. 2015, 80, N2305–N2315. [Google Scholar] [CrossRef]
- Spadari, C.D.C.; Lopes, L.B.; Ishida, K. Potential Use of Alginate-Based Carriers as Antifungal Delivery System. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, N.; Muhammad, N.; Hashim, H.; Huda, N. Improving the Texture of Sardine Surimi Using Duck Feet Gelatin. J. Agrobiotechnol. 2017, 8, 25–32. [Google Scholar]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey Loaded Alginate/PVA Nanofibrous Membrane as Potential Bioactive Wound Dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Laisak, E.; Gao, M.; Tang, Y. AIEgen Quantitatively Monitoring the Release of Ca2+ during Swelling and Degradation Process in Alginate Hydrogels. Mater. Sci. Eng. 2019, 104, 109951. [Google Scholar] [CrossRef] [PubMed]
- Al-kafaween, M.A.; Mohd Hilmi, A.B.; Jaffar, N.; Nagi Al-Jamal, H.A.; Zahri, M.K.; Amonov, M.; Mabrouka, B.; Elsahoryi, N.A. Effects of Trigona Honey on the Gene Expression Profile of Pseudomonas Aeruginosa ATCC 10145 and Streptococcus Pyogenes ATCC 19615. Jordan J. Biol. Sci. 2020, 13, 133–138. [Google Scholar]
- Rehm, B.H.A.; Valla, S. Bacterial Alginates: Biosynthesis and Applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. Biol. Chem. Biotechnol. Appl. 2005, 6. [Google Scholar] [CrossRef]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolye Complex Formation between Alginate and Chitosan as a Function of PH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Murano, E.; Paoletti, S. Alginate as Immobilization Material. II: Determination of Polyphenol Contaminants by Fluorescence Spectroscopy, and Evaluation of Methods for Their Removal. Biotechnol. Bioeng. 1989, 33, 90–94. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Enzyme-Assistant Extraction (EAE) of Bioactive Components: A Useful Approach for Recovery of Industrially Important Metabolites from Seaweeds: A Review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, M.N. Three Phase Partitioning of Carbohydrate Polymers: Separation and Purification of Alginates. Carbohydr. Polym. 2002, 48, 391–395. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Wei, Q.; Tao, D.; Xu, Y. Functional Nanofibers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F. Processing of Composite Functional Nanofibers. In Functional Nanofibers and their Applications; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Zagho, M.M.; Elzatahry, A.A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 259. [Google Scholar] [CrossRef]
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of Lutein-Loaded PVA/Sodium Alginate Nanofibers and Investigation of Its Release Behavior. Pharmaceutics 2019, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2001, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Paques, J.P.; Sagis, L.M.C.; van Rijn, C.J.M.; van der Linden, E. Nanospheres of Alginate Prepared through w/o Emulsification and Internal Gelation with Nanoparticles of CaCO3. Food Hydrocoll. 2014, 40, 182–188. [Google Scholar] [CrossRef]
- Shehata, T.M.; Ibrahima, M.M. BÜCHI Nano Spray Dryer B-90: A Promising Technology for the Production of Metformin Hydrochloride-Loaded Alginate–Gelatin Nanoparticles. Drug Dev. Ind. Pharm. 2019, 45, 1907–1914. [Google Scholar] [CrossRef]
- Zohri, M.; Alavidjeh, M.S.; Haririan, I.; Ardestani, M.S.; Ebrahimi, S.E.S.; Sani, H.T.; Sadjadi, S.K. A Comparative Study Between the Antibacterial Effect of Nisin and Nisin-Loaded Chitosan/Alginate Nanoparticles on the Growth of Staphylococcus Aureus in Raw and Pasteurized Milk Samples. Probiotics Antimicrob. Proteins 2010, 2, 258–266. [Google Scholar] [CrossRef]
- Dai, L.; Zhan, X.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Composite Zein—Propylene Glycol Alginate Particles Prepared Using Solvent Evaporation: Characterization and Application as Pickering Emulsion Stabilizers. Food Hydrocoll. 2018, 85, 281–290. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, J.; Zhang, Q.; Yang, S.; Jiang, S.; Huang, C. PTX-Loaded Three-Layer PLGA/CS/ALG Nanoparticle Based on Layer-by-Layer Method for Cancer Therapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1566–1578. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Alallam, B.; Altahhan, S.; Taher, M.; Mohd Nasir, M.H.; Doolaanea, A.A. Electrosprayed Alginate Nanoparticles as CRISPR Plasmid DNA Delivery Carrier: Preparation, Optimization, and Characterization. Pharmaceuticals 2020, 13, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.X.; Zhang, B.; Ramakrishna, S.; Yu, M.; Ma, J.W.; Long, Y.Z. In Situ Assembly of Well-Dispersed Ag Nanoparticles throughout Electrospun Alginate Nanofibers for Monitoring Human Breath—Smart Fabrics. ACS Appl. Mater. Interfaces 2018, 10, 19863–19870. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.H.D.; Paul, J.B. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of Drugs: Effect on Dissolution Rate. Res. Pharm. Sci. 2015, 10, 95–108. [Google Scholar]
- Alshora, D.H.; Ibrahim, M.A.; Alanazi, F.K. Nanotechnology from Particle Size Reduction to Enhancing Aqueous Solubility; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, Characterization and Biological Activity Screening of Sodium Alginate-Gum Arabic Nanoparticles Loaded with Curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef]
- Ortega, E.; Blanco, S.; Ruiz, A.; Peinado, M.Á.; Peralta, S.; Morales, M.E. Lipid nanoparticles for the transport of drugs like dopamine through the blood–brain barrier. Beilstein J. Nanotechnol. 2020, 1, 79. [Google Scholar] [CrossRef]
- Thomas, D.; KurienThomas, K.; Latha, M.S. Preparation and Evaluation of Alginate Nanoparticles Prepared by Green Method for Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 154, 888–895. [Google Scholar] [CrossRef]
- Roces, C.B.; Christensen, D.; Perrie, Y. Translating the Fabrication of Protein-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles from Bench to Scale-Independent Production Using Microfluidics. Drug Deliv. Transl. Res. 2020, 10, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Dario, P. Alginate and Chitosan Particles as Drug Delivery System for Cell Therapy. Biomed. Microdevices 2008, 10, 131–140. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Ma, C.; Hua, Y.; Zhang, L.; Shen, J. Design and Investigation of Penetrating Mechanism of Octaarginine-Modified Alginate Nanoparticles for Improving Intestinal Insulin Delivery. J. Pharm. Sci. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Alfatama, M.; Lim, L.Y.; Wong, T.W. Alginate-C18 Conjugate Nanoparticles Loaded in Tripolyphosphate-Cross-Linked Chitosan-Oleic Acid Conjugate-Coated Calcium Alginate Beads as Oral Insulin Carrier. Mol. Pharm. 2018, 15, 3369–3382. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/Alginate Nanoparticles as a Promising Approach for Oral Delivery of Curcumin Diglutaric Acid for Cancer Treatment. Mater. Sci. Eng. 2018, 93, 178–190. [Google Scholar] [CrossRef]
- Markeb, A.A.; El-Maali, N.A.; Sayed, D.M.; Osama, A.; Abdel-Malek, M.A.Y.; Zaki, A.H.; Elwanis, M.E.A.; Driscoll, J.J. Synthesis, Structural Characterization, and Preclinical Efficacy of a Novel Paclitaxel-Loaded Alginate Nanoparticle for Breast Cancer Treatment. Int. J. Breast Cancer 2016, 2016. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Toborek, M. Treatment of Antibiotic-Resistant Bacteria by Encapsulation of ZnO Nanoparticles in an Alginate Biopolymer: Insights into Treatment Mechanisms. J. Hazard. Mater. 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Scolari, I.R.; Páez, P.L.; Musri, M.M.; Petiti, J.P.; Torres, A.; Granero, G.E. Rifampicin Loaded in Alginate/Chitosan Nanoparticles as a Promising Pulmonary Carrier against Staphylococcus Aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M. Nanoarchitectonics for Smart Delivery and Drug Targeting; William Andrew: Oxford, UK, 2016. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of Alginate Microspheres for Drug Delivery: A Review. Int. J. Biol. Macromol. 2019, 153, 1035–1046. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Mechanisms of External and Internal Gelation and Their Impact on the Functions of Alginate as a Coat and Delivery System. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in Fabricating Spherical Alginate Hydrogels with Controlled Particle Designs by Ionotropic Gelation as Encapsulation Systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Synthesis of Alginate Microparticles and Nanoparticles. Drug Des. Intellect. Prop. Int. J. 2019, 3, 293–327. [Google Scholar] [CrossRef]
- Reis, C.P.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.J.; Veiga, F. Review and Current Status of Emulsion/Dispersion Technology Using an Internal Gelation Process for the Design of Alginate Particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef]
- Liu, X.D.; Yu, W.Y.; Zhang, Y.; Xue, W.M.; Yu, W.T.; Xiong, Y.; Ma, X.J.; Chen, Y.; Yuan, Q. Characterization of Structure and Diffusion Behaviour of Ca-Alginate Beads Prepared with External or Internal Calcium Sources. J. Microencapsul. 2002, 19, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.G.; Brown, A.L.; Duross, A.N.; Duross, E.L.; Sahay, G.; Sun, C. Nanoalginates via Inverse-Micelle Synthesis: Doxorubicin-Encapsulation and Breast Cancer Cytotoxicity. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Spadari, C.C.; de Bastiani, F.W.M.D.S.; Lopes, L.B.; Ishida, K. Alginate Nanoparticles as Non-Toxic Delivery System for Miltefosine in the Treatment of Candidiasis and Cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E. Pseudolatex Preparation Using a Novel Emulsion-Diffusion Process Involving Direct Displacement of Partially Water-Miscible Solvents by Distillation. Int. J. Pharm. 1999, 188, 155–164. [Google Scholar] [CrossRef]
- Néstor Mendoza-MuñozSergio Alcalá-AlcaláDavid Quintanar-Guerrero. Preparation of Polymer Nanoparticles by the Emulsification-Solvent Evaporation Method: From Vanderhoff’s Pioneer Approach to Recent Adaptations. In Polymer Nanoparticles for Nanomedicines; Springer: Cham, Switzerland, 2016; pp. 87–121. [Google Scholar] [CrossRef]
- Muhaimin; Bodmeier, R. Effect of Solvent Type on Preparation of Ethyl Cellulose Microparticles by Solvent Evaporation Method with Double Emulsion System Using Focused Beam Reflectance Measurement. Polym. Int. 2017, 66, 1448–1455. [Google Scholar] [CrossRef]
- Lemoine, D.; Préat, V. Polymeric Nanoparticles as Delivery System for Influenza Virus Glycoproteins. J. Control. Release 1998, 54, 15–27. [Google Scholar] [CrossRef]
- Urbaniak, T.; Musiał, W. Influence of Solvent Evaporation Technique Parameters on Diameter of Submicron Lamivudine-Poly-ε-Caprolactone Conjugate Particles. Nanomaterials 2019, 9, 1240. [Google Scholar] [CrossRef]
- Subedi, G.; Shrestha, A.K.; Shakya, S. Study of Effect of Different Factors in Formulation of Micro and Nanospheres with Solvent Evaporation Technique. Open Pharm. Sci. J. 2016, 3, 182–195. [Google Scholar] [CrossRef]
- Joshy, K.S.; George, A.; Jose, J.; Kalarikkal, N.; Pothen, L.A.; Thomas, S. Novel Dendritic Structure of Alginate Hybrid Nanoparticles for Effective Anti-Viral Drug Delivery. Int. J. Biol. Macromol. 2017, 103, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Seyam, S.; Nordin, N.A.; Alfatama, M. Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals 2020, 13, 307. [Google Scholar] [CrossRef]
- Sepúlveda-Rivas, S.; Fritz, H.F.; Valenzuela, C.; Santiviago, C.A.; Morales, J.O. Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and in Vitro Safety. Pharmaceutics 2019, 11, 103. [Google Scholar] [CrossRef]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a New Drug Carrier Made from Alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef]
- Sæther, H.V.; Holme, H.K.; Maurstad, G.; Smidsrød, O.; Stokke, B.T. Polyelectrolyte Complex Formation Using Alginate and Chitosan. Carbohydr. Polym. 2008, 74, 813–821. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of Turmeric Oil-Loaded Chitosan-Alginate Biopolymeric Nanocapsules. Mater. Sci. Eng. 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Grebinişan, D.; Holban, M.; Şunel, V.; Popa, M.; Desbrieres, J.; Lionte, C. Novel Acyl Derivatives of N-(p-Aminobenzoyl)-l-Glutamine Encapsulated in Polymeric Nanocapsules with Potential Antitumoral Activity. Cellul. Chem. Technol. 2011, 45, 571–577. [Google Scholar]
- Khalid, A.; Bashir, S.; Sohail, M.; Amirzada, M.I. Characterization of Doxorubicin Nanoparticles Prepared by Ionic Gelation. Trop. J. Pharm. Res. 2018, 17, 2329–2334. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Perera, A.D.L.C.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J.; Karunaratne, D.N. Alginate Nanoparticles Protect Ferrous from Oxidation: Potential Iron Delivery System. Int. J. Pharm. 2016, 513, 404–409. [Google Scholar] [CrossRef]
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles Based on Crocin Loaded Chitosan-Alginate Biopolymers: Antioxidant Activities, Bioavailability and Anticancer Properties. Int. J. Biol. Macromol. 2017, 99, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, J.J.; Dhanaraj, S. Exemestane Loaded Alginate Nanoparticles for Cancer Treatment: Formulation and in Vitro Evaluation. Int. J. Biol. Macromol. 2017, 105, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Saralkar, P.; Dash, A.K. Alginate Nanoparticles Containing Curcumin and Resveratrol: Preparation, Characterization, and In Vitro Evaluation Against DU145 Prostate Cancer Cell Line. AAPS PharmSciTech 2017, 18, 2814–2823. [Google Scholar] [CrossRef]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.T.; Petkov, P.S.; Vayssilov, G.N.; Garrido, M.J. Optimization and In-Vitro/in-Vivo Evaluation of Doxorubicin-Loaded Chitosan-Alginate Nanoparticles Using a Melanoma Mouse Model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, J.; Li, F.; Shi, Y.; Li, D.; Huang, Q. Chitosan-Sodium Alginate Nanoparticle as a Delivery System for ε-Polylysine: Preparation, Characterization and Antimicrobial Activity. Food Control 2018, 91, 302–310. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Preparation of Polyurethane–Alginate/Chitosan Core Shell Nanoparticles for the Purpose of Oral Insulin Delivery. Eur. Polym. J. 2017, 92, 294–313. [Google Scholar] [CrossRef]
- Jardim, K.V.; Palomec-Garfias, A.F.; Andrade, B.Y.G.; Chaker, J.A.; Báo, S.N.; Márquez-Beltrán, C.; Moya, S.E.; Parize, A.L.; Sousa, M.H. Novel Magneto-Responsive Nanoplatforms Based on MnFe2O4 Nanoparticles Layer-by-Layer Functionalized with Chitosan and Sodium Alginate for Magnetic Controlled Release of Curcumin. Mater. Sci. Eng. 2018, 92, 184–195. [Google Scholar] [CrossRef]
- Ye, S.; Wang, C.; Liu, X.; Tong, Z. Multilayer Nanocapsules of Polysaccharide Chitosan and Alginate through Layer-by-Layer Assembly Directly on PS Nanoparticles for Release. J. Biomater. Sci. Polym. Ed. 2005, 16, 909–923. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Li, T.; Liu, C.; Liu, W. Improved Physical and in Vitro Digestion Stability of a Polyelectrolyte Delivery System Based on Layer-by-Layer Self-Assembly Alginate-Chitosan-Coated Nanoliposomes. J. Agric. Food Chem. 2013, 61, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Webster, T.J. Doxorubicin-Loaded Poly (Lactic-Co-Glycolic Acid) Nanoparticles Coated with Chitosan/Alginate by Layer by Layer Technology for Antitumor Applications. Int. J. Nanomed. 2017, 12, 1791–1802. [Google Scholar]
- Khan, M.A.; Yue, C.; Fang, Z.; Hu, S.; Cheng, H.; Bakry, A.M.; Liang, L. Alginate/Chitosan-Coated Zein Nanoparticles for the Delivery of Resveratrol. J. Food Eng. 2019, 258, 45–53. [Google Scholar] [CrossRef]
- Poozesh, S.; Bilgili, E. Scale-up of Pharmaceutical Spray Drying Using Scale-up Rules: A Review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.J.; Norwood, E.A.; O’Mahony, J.A.; Kelly, A.L. Atomisation Technologies Used in Spray Drying in the Dairy Industry: A Review. J. Food Eng. 2019, 243, 57–69. [Google Scholar] [CrossRef]
- Arpagaus, C.; Collenberg, A.; Rütti, D.; Assadpour, E.; Jafari, S.M. Nano Spray Drying for Encapsulation of Pharmaceuticals. Int. J. Pharm. 2018, 546, 194–214. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A.; Sedki, M.; Ali, S.M.; El-Sherbiny, I.M. Nanoformulated Ellagic Acid Ameliorates Pentylenetetrazol-Induced Experimental Epileptic Seizures by Modulating Oxidative Stress, Inflammatory Cytokines and Apoptosis in the Brains of Male Mice. Metab. Brain Dis. 2020, 35, 385–399. [Google Scholar] [CrossRef]
- De Cicco, F.; Porta, A.; Sansone, F.; Aquino, R.P.; Del Gaudio, P. Nanospray Technology for an in Situ Gelling Nanoparticulate Powder as a Wound Dressing. Int. J. Pharm. 2014, 473, 30–37. [Google Scholar] [CrossRef]
- Arpagaus, C. Pharmaceutical Particle Engineering via Nano Spray Drying—Process Parameters and Application Examples on the Laboratory-Scale. Int. J. Med. Nano Res. 2018, 5. [Google Scholar] [CrossRef]
- Correâ-Filho, L.C.; Lourenço, M.M.; Moldaõ-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Su, C.Y.; Wang, J.C.; Chen, C.Y.; Chu, K.; Lin, C.K. Spherical Composite Powder by Coupling Polymethyl Methacrylate and Boron Nitride via Spray Drying for Cosmetic Application. Materials 2019, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fu, A.; Wang, Y.; Guo, P.; Wu, G. Colloids and Surfaces A: Physicochemical and Engineering Aspects Spray Drying Assisted Assembly of ZnO Nanocrystals Using Cellulose as Sacrificial Template and Studies on Their Photoluminescent and Photocatalytic Properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 173–182. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Majidi, R.F.; Faramarzi, M.A.; Baharifar, H.; Amani, A. Preparation, Optimization and Activity Evaluation of PLGA/Streptokinase Nanoparticles Using Electrospray. Adv. Pharm. Bull. 2017, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Si, T.; Gai, M.; Frueh, J.; He, Q. Hydrodynamic Electrospray Ionization Jetting of Calcium Alginate Particles: Effect of Spray-Mode, Spraying Distance and Concentration. RSC Adv. 2018, 8, 24243–24249. [Google Scholar] [CrossRef]
- Mehregan Nikoo, A.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Controlling the Morphology and Material Characteristics of Electrospray Generated Calcium Alginate Microhydrogels. J. Microencapsul. 2016, 33, 605–612. [Google Scholar] [CrossRef]
- Correia, C.R.; Ghasemzadeh-Hasankolaei, M.; Mano, J.F. Cell Encapsulation in Liquified Compartments: Protocol Optimization and Challenges. PLoS ONE 2019, 14, e218045. [Google Scholar] [CrossRef]
- Suksamran, T.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T.; Ruktanonchai, U.; Supaphol, P. Biodegradable Alginate Microparticles Developed by Electrohydrodynamic Spraying Techniques for Oral Delivery of Protein. J. Microencapsul. 2009, 26, 563–570. [Google Scholar] [CrossRef]
- Naim, M.N.; Mokhtar, M.N.; Rahmam, S.; Bakar, N.F.A.; Ng, E. Encapsulation of Bioactive Compound from Extracted Jasmine Flower Using β-Cyclodextrin via Electrospray. IOP Conf. Ser. Earth Environ. Sci. 2016, 36, 012054. [Google Scholar] [CrossRef]
- Torres-Chávez, P.I.; Ramírez-Wong, B.; Rangel-Vázquez, N.A.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A.; Plascencia-Jatomea, M.; Rodríguez-Félix, F.; Rascón-Chu, A. Micro- and Nanoparticles by Electrospray: Advances and Applications in Foods. J. Agric. Food Chem. 2015, 63, 4699–4707. [Google Scholar] [CrossRef]
- Tsai, S.; Ting, Y. Synthesize of Alginate/Chitosan Bilayer Nanocarrier by CCD-RSM Guided Co-Axial Electrospray: A Novel and Versatile Approach. Food Res. Int. 2019, 116, 1163–1172. [Google Scholar] [CrossRef]
- Xu, Y.; Skotak, M.; Hanna, M. Electrospray Encapsulation of Water-Soluble Protein with Polylactide. I. Effects of Formulations and Process on Morphology and Particle Size. J. Microencapsul. 2006, 23, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [PubMed]
- Ignatious, F.; Sun, L.; Lee, C.P.; Baldoni, J. Electrospun Nanofibers in Oral Drug Delivery. Pharm. Res. 2010, 27, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An Enabling Nanotechnology Platform for Drug Delivery and Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Tuan, R.S. Fabrication and Application of Nanofibrous Scaffolds in Tissue Engineering. Curr. Protoc. Cell Biol. 2009, 42, 2–25. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Development of Multifunctional Nano/Ultrafiltration Membrane Based on a Chitosan Thin Film on Alginate Electrospun Nanofibres. J. Clean. Prod. 2017, 156, 470–479. [Google Scholar] [CrossRef]
- Ni, P.; Bi, H.; Zhao, G.; Han, Y.; Wickramaratne, M.N.; Dai, H.; Wang, X. Electrospun Preparation and Biological Properties in Vitro of Polyvinyl Alcohol/Sodium Alginate/Nano-Hydroxyapatite Composite Fiber Membrane. Colloids Surf. B Biointerfaces 2019, 173, 171–177. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and Dye Filtration Property of Electrospun Polyhydroxybutyrate–Calcium Alginate/Carbon Nanotubes Composite Nanofibrous Filtration Membrane. Sep. Purif. Technol. 2016, 161, 69–79. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G.H. Nano/Microscale Topographically Designed Alginate/PCL Scaffolds for Inducing Myoblast Alignment and Myogenic Differentiation. Carbohydr. Polym. 2019, 223, 115041. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Goh, K.L.; Ratnayake, S.P.; Amaratunga, G.A.J.; De Silva, K.M.N. Magnesium Oxide Nanoparticles Reinforced Electrospun Alginate-Based Nanofibrous Scaffolds with Improved Physical Properties. Int. J. Biomater. 2017, 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Abrahim, B.; Veiga, F.; Seiça, R.; Cabral, M.; Arnaud, P.; Andrade, J.C.; Ribeiro, A.J.; Lopes, M.; Abrahim, B.; et al. Preparation Methods and Applications behind Alginate-Based Particles. Expert Opin. Drug Deliv. 2016, 14, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.O.; Umoru, L.E.; Aribo, S. Natural Products: A Minefield of Biomaterials. ISRN Mater. Sci. 2012, 2012, 20. [Google Scholar] [CrossRef]
- Orive, G.; Ponce, S.; Hernández, R.M.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Biocompatibility of Microcapsules for Cell Immobilization Elaborated with Different Type of Alginates. Biomaterials 2002, 23, 3825–3831. [Google Scholar] [CrossRef]
- Dusseault, J.; Tam, S.K.; Ménard, M.; Polizu, S.; Jourdan, G.; Yahia, L.; Hallé, J.-P. Evaluation of Alginate Purification Methods: Effect on Polyphenol, Endotoxin, and Protein Contamination. J. Biomed. Mater. Res. Part A 2006, 76A, 243–251. [Google Scholar] [CrossRef]
- Flo, T.H.; Ryan, L.; Latz, E.; Takeuchi, O.; Monks, B.G.; Lien, E.; Halaas, O.; Akira, S.; Skjåk-Bræk, G.; Golenbock, D.T.; et al. Involvement of Toll-like Receptor (TLR) 2 and TLR4 in Cell Activation by Mannuronic Acid Polymers. J. Biol. Chem. 2002, 277, 35489–35495. [Google Scholar] [CrossRef]
- Rahaiee, S.; Shojaosadati, S.A.; Hashemi, M.; Moini, S.; Razavi, S.H. Improvement of Crocin Stability by Biodegradeble Nanoparticles of Chitosan-Alginate. Int. J. Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Alginates: Versatile Polymers in Biomedical Applications and Therapeutics; Apple Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Pawar, S.N. Chemical Modification of Alginate. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 111–155. [Google Scholar] [CrossRef]
- Banks, S.R.; Enck, K.; Wright, M.; Opara, E.C.; Welker, M.E. Chemical Modification of Alginate for Controlled Oral Drug Delivery. J. Agric. Food Chem. 2019, 67, 10481–10488. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, N. Chemical modifications of alginate and its derivatives. Int. J. Chem. Res. 2019, 4, 1–17. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Chemical Modification of Alginates in Organic Solvent Systems. Biomacromolecules 2011, 12, 4095–4103. [Google Scholar] [CrossRef]
- Yasmin, F.; Chen, X.; Eames, B.F. Effect of Process Parameters on the Initial Burst Release of Protein-Loaded Alginate Nanospheres. J. Funct. Biomater. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Sarei, F.; Dounighi, N.; Zolfagharian, H.; Khaki, P.; Bidhendi, S. Alginate Nanoparticles as a Promising Adjuvant and Vaccine Delivery System. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Assadpour, E. Development of a Nutraceutical Nano-Delivery System through Emulsification/Internal Gelation of Alginate. Food Chem. 2017, 229, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, A.B.; Mishra, S.; Kulkarni, R.D.; Naik, J.B. Influence of Different Viscosity Grade Ethylcellulose Polymers on Encapsulation and in Vitro Release Study of Drug Loaded Nanoparticles. J. Pharm. Res. 2013, 7, 414–420. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ahdyani, R.; Novitasari, L.; Martien, R.; Danarti, R. Formulation and Characterization of Timolol Maleate-Loaded Nanoparticles Gel by Ionic Gelation Method Using Chitosan and Sodium Alginate. Int. J. Appl. Pharm. 2019, 11, 48–54. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Faccio, R.; Brugnini, G.; Miraballes, I.; Rufo, C.; Pardo, H. Optimization and Characterization of Nisin-Loaded Alginate-Chitosan Nanoparticles with Antimicrobial Activity in Lean Beef. LWT Food Sci. Technol. 2018, 91, 107–116. [Google Scholar] [CrossRef]
- Mansourpour, M.; Mahjub, R.; Amini, M.; Ostad, S.N.; Shamsa, E.S.; Rafiee-Tehrani, M.; Dorkoosh, F.A. Development of Acid-Resistant Alginate/Trimethyl Chitosan Nanoparticles Containing Cationic β-Cyclodextrin Polymers for Insulin Oral Delivery. AAPS PharmSciTech 2015, 16, 952–962. [Google Scholar] [CrossRef]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.K.; Joshi, H.K.; Divakar, G. Enhanced Water Dispersibility of Curcumin Encapsulated in Alginate-Polysorbate 80 Nano Particles and Bioavailability in Healthy Human Volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef]
- Baghbani, F.; Moztarzadeh, F.; Mohandesi, J.A.; Yazdian, F.; Mokhtari-Dizaji, M.; Hamedi, S. Formulation Design, Preparation and Characterization of Multifunctional Alginate Stabilized Nanodroplets. Int. J. Biol. Macromol. 2016, 89, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Elgegren, M.; Kim, S.; Cordova, D.; Silva, C.; Noro, J.; Cavaco-Paulo, A.; Nakamatsu, J. Ultrasound-Assisted Encapsulation of Sacha Inchi (Plukenetia Volubilis Linneo.) Oil in Alginate-Chitosan Nanoparticles. Polymers 2019, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Sánchez-Borzone, M.E.; Granero, G.E. Promising Chitosan-Coated Alginate-Tween 80 Nanoparticles as Rifampicin Coadministered Ascorbic Acid Delivery Carrier Against Mycobacterium Tuberculosis. AAPS PharmSciTech 2019, 20, 67. [Google Scholar] [CrossRef]
- Rosa, G.D.; Iommelli, R.; La Rotonda, M.I.; Miro, A.; Quaglia, F. Influence of the Co-Encapsulation of Different Non-Ionic Surfactants on the Properties of PLGA Insulin-Loaded Microspheres. J. Control. Release 2000, 69, 283–295. [Google Scholar] [CrossRef]
- Sabbagh, H.A.K.; Hussein-Al-Ali, S.H.; Hussein, M.Z.; Abudayeh, Z.; Ayoub, R.; Abudoleh, S.M. A Statistical Study on the Development of Metronidazole-Chitosan-Alginate Nanocomposite Formulation Using the Full Factorial Design. Polymers 2020, 12, 772. [Google Scholar] [CrossRef]
- Cafaggi, S.; Russo, E.; Stefani, R.; Leardi, R.; Caviglioli, G.; Parodi, B.; Bignardi, G.; De Totero, D.; Aiello, C.; Viale, M. Preparation and Evaluation of Nanoparticles Made of Chitosan or N-Trimethyl Chitosan and a Cisplatin-Alginate Complex. J. Control. Release 2007, 121, 110–123. [Google Scholar] [CrossRef]
- Mane, S.; Ponrathnam, S.; Chavan, N. Effect of Chemical Crosslinking on Properties of Polymer Microbeads: A Review. Can. Chem. Trans. 2016, 3, 473–485. [Google Scholar] [CrossRef]
- Güncüm, E.; Işıklan, N.; Anlaş, C.; Ünal, N.; Bulut, E.; Bakırel, T. Development and Characterization of Polymeric-Based Nanoparticles for Sustained Release of Amoxicillin—An Antimicrobial Drug. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–973. [Google Scholar] [CrossRef]
- Jin, M.; Zheng, Y.; Hu, Q. Preparation and Characterization of Bovine Serum Albumin Alginate/Chitosan Microspheres for Oral Administration. Asian J. Pharm. Sci. 2009, 4, 215–220. [Google Scholar]
- Patil, S.B.; Sawant, K.K. Development, Optimization and in Vitro Evaluation of Alginate Mucoadhesive Microspheres of Carvedilol for Nasal Delivery. J. Microencapsul. 2009, 26, 432–443. [Google Scholar] [CrossRef]
- Peretz, S.; Florea-Spiroiu, M.; Anghel, D.-F.; Bala, D.; Stoian, C.; Zgherea, G. Preparation of Porous Calcium Alginate Beads and Their Use for Adsorption of O-Nitrophenol from Aqueous Solutions. Micro Nanoeng. 2013, 22, 123–136. [Google Scholar]
- Rastogi, R.; Sultana, Y.; Aqil, M.; Ali, A.; Kumar, S.; Chuttani, K.; Mishra, A.K. Alginate Microspheres of Isoniazid for Oral Sustained Drug Delivery. Int. J. Pharm. 2007, 334, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Synthesis And Optimization of Streptomycin Loaded Chitosan-Alginate Nanoparticles. Int. J. Sci. Technol. Res. 2012, 1, 31–34. [Google Scholar]
- Lopes, M.A.; Abrahim-Vieira, B.; Oliveira, C.; Fonte, P.; Souza, A.M.; Lira, T.; Sequeira, J.A.; Rodrigues, C.R.; Cabral, L.M.; Sarmento, B.; et al. Probing Insulin Bioactivity in Oral Nanoparticles Produced by Ultrasonication-Assisted Emulsification/Internal Gelation. Int. J. Nanomedicine 2015, 10, 5865–5880. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of Sonication on Particle Dispersion, Administered Dose and Metal Release of Non-Functionalized, Non-Inert Metal Nanoparticles. J. Nanoparticle Res. 2016, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of Insulin-Loaded Alginate Nanoparticles Produced by Ionotropic Pre-Gelation through DSC and FTIR Studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-Alginate Biocomposite Containing Fucoidan for Bone Tissue Engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Shetab Boushehri, M.S.; Varshosaz, J. Preparation, Characterization and Optimization of Glipizide Controlled Release Nanoparticles. Res. Pharm. Sci. 2014, 9, 301–314. [Google Scholar]
- Gupta, V.K.; Karar, P.K. Optimization of Process Variables for the Preparation of Chitosanalginate Nanoparticles. Int. J. Pharm. Pharm. Sci. 2011, 3, 78–80. [Google Scholar]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of Alpha Mangostin-Loaded Chitosan/Alginate Controlled-Release Nanoparticles Using Genipin as Crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Nait Mohamed, F.A.; Laraba-Djebari, F. Development and Characterization of a New Carrier for Vaccine Delivery Based on Calcium-Alginate Nanoparticles: Safe Immunoprotective Approach against Scorpion Envenoming. Vaccine 2016, 34, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Sansdrap, P.; Moës, A.J. Influence of Manufacturing Parameters on the Size Characteristics and the Release Profiles of Nifedipine from Poly(DL-Lactide-Co-Glycolide) Microspheres. Int. J. Pharm. 1993, 98, 157–164. [Google Scholar] [CrossRef]
- Mateovic, T.; Kriznar, B.; Bogataj, M.; Mrhar, A. The Influence of Stirring Rate on Biopharmaceutical Properties of Eudragit RS Microspheres. J. Microencapsul. 2002, 19, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Denkbas, E.B.; Kilic, E.; Birlikseven, C.; Ozturk, E. Magnetic Chitosan Microspheres: Preparation and Characterization. React. Funct. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Denkbaş, E.B.; Odabaşi, M. Chitosan Microspheres and Sponges: Preparation and Characterization. J. Appl. Polym. Sci. 2000, 76, 1637–1643. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, B.; Kang, T.W.; Noh, J.H.; Kim, M.J.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. Electrostatically Interactive Injectable Hydrogels for Drug Delivery. Tissue Eng. Regen. Med. 2018, 15, 513–520. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Chrzanowska, E. PH-Responsive Chitosan/Alginate Polyelectrolyte Complex Membranes Reinforced by Tripolyphosphate. Eur. Polym. J. 2018, 101, 282–290. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zha, L.S.; Zhou, M.H.; Ma, J.H.; Liang, B.R. Preparation and Characterization of PH- and Temperature-Responsive Semi-Interpenetrating Polymer Network Hydrogels Based on Linear Sodium Alginate and Crosslinked Poly(N-Isopropylacrylamide). J. Appl. Polym. Sci. 2005, 97, 1931–1940. [Google Scholar] [CrossRef]
- Wang, A.; Li, P.; Dai, Y.; Zhang, J.; Wang, A.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Sorasitthiyanukarn, F.N.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/Alginate Nanoparticles as a Promising Carrier of Novel Curcumin Diethyl Diglutarate. Int. J. Biol. Macromol. 2015, 131, 1125–1136. [Google Scholar] [CrossRef]
- Dupuy, B.; Arien, A.; Perrot Minnot, A. FT-IR of Membranes Made with Alginate/Polylysine Complexes. Variations with the Mannuronic or Guluronic Content of the Polysaccharides. Artif. Cells Blood Substitutes Biotechnol. 1994, 22, 71–82. [Google Scholar] [CrossRef]
- Gazori, T.; Khoshayand, M.R.; Azizi, E.; Yazdizade, P.; Nomani, A.; Haririan, I. Evaluation of Alginate/Chitosan Nanoparticles as Antisense Delivery Vector: Formulation, Optimization and in Vitro Characterization. Carbohydr. Polym. 2009, 77, 599–606. [Google Scholar] [CrossRef]
- Bhunchu, S.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Curcumin Diethyl Disuccinate Encapsulated in Chitosan/Alginate Nanoparticles for Improvement of Its in Vitro Cytotoxicity against MDA-MB-231 Human Breast Cancer Cells. Pharmazie 2016, 71, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of Chitosan/Alginate/Lovastatin Nanoparticles and Investigation of Their Toxic Effects in Vitro and in Vivo. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Duong, A.; Lee, L.J.; Wyslouzil, B.E. Electrospray Production of Nanoparticles for Drug/Nucleic Acid Delivery. In The Delivery of Nanoparticles; Intech Europe: Rijeka, Croatia, 2012; p. 223. [Google Scholar]
- Arthanari, S.; Mani, G.; Jang, J.H.; Choi, J.O.; Cho, Y.H.; Lee, J.H.; Cha, S.E.; Oh, H.S.; Kwon, D.H.; Jang, H.T. Preparation and Characterization of Gatifloxacin-Loaded Alginate/Poly (Vinyl Alcohol) Electrospun Nanofibers. Artif. Cells Nanomed. Biotechnol. 2014, 44, 1–6. [Google Scholar] [CrossRef]

| Alginate Concentration (% w/v) | Components | Drug | Aims | Mass Ratio | pH | Mean Particle Size (nm) | Polydispersity Index (%) | Zeta Potential (mV) | Encapsulation Efficiency (%) | Drug Release | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | CaCl2% w/v | Doxorubicin | Site-targeting and controlled release | - | - | - | - | [84] | |||
| 0.04 | 0.39 | 350 | 0.481 | 65.0 | |||||||
| 0.05 | 0.39 | 479 | 0.139 | 68.0 | |||||||
| 0.06 | 0.39 | 490 | 0.273 | 71.0 | |||||||
| 0.08 | 0.19 | 3997 | 1 | 77.0 | |||||||
| 0.10 | 0.19 | 6638 | 1 | 84.0 | |||||||
| 0.30 | CaCl2 0.10% w/v Span 80 Iron (1%) | Ferrous sulphate | To protect ferrous from oxidation oral iron therapy | - | ~5.0 | 20 ± 6 | - | −38.0 ± 4 | 95.0 ± 4 | 20% at pH 2.0 for 100 h 65% at pH 6.0 for 100 h 70% at pH 7.4 for 100 h | [85] |
| 0.10 | Chitosan (Cs) 0.08% w/v | Crocin | To improve bioavailability, anticancer, and antioxidant activity | - | 4.70 | 236 | 0.476 | - | 38.16 | 30% at pH 1.2 for 48 h 50% at 6.8 for 48 h | [86] |
| 0.50 | CaCl2 2% w/v | Exemestane | To reduce and control the release of exemestane | - | - | 197 | - | −18.3 | 98.0 | Maximum release within 7 h at pH 7.4 | [87] |
| 0.06 | CaCl2 0.05% w/v Tween 80 | Curcumin and resveratrol | Site-targeting | - | - | 12.53 ± 1.06 | - | −22.0 ± 2.17 | 49.30 ± 4.3 | Curcumin 16.35 ± 3.8% for 24 h | [88] |
| 60.23 ± 15 | 70.99 ± 6.1 | Resveratrol 87 ± 7% for 24 h | |||||||||
| 0.30 | Chitosan 0.08% w/v CaCl2 | Doxorubicin | Site-targeting and controlled release | Alg:Cs 10:1 | Alginate (Alg) 5.30 | ~300 | 0.2 | −22.5 to −25.0 | ~97 | 52% at pH 5.5 for 6 h 35% at pH 7.4 for 6 h | [89] |
| Cs 4.50 | |||||||||||
| 0.1 | CaCl2 0.1% w/v Chitosan 0.1% w/v | ε-polylysine (ε-PL) | Evaluating the possibility of Cs/Alg nanoparticles as carriers of ε-polylysine | Alg:Cs 4.93:1 | Alg 5.14 | Alg-Cs 276.38 | 0.24 | −33.7 | 53.37 | 17.5% at pH 6.6 for 2 h 80% at pH 6.6 for 10 h 90% at pH6.6 for 25 h | [90] |
| Alg:ε-PL 100:8.55 | - | ε-PL-Alg-Cs 372.05 | 0.29 | −30.3 | |||||||
| - | Polyurethane-alginate (PU:Alg) CaCl2 0.5% w/v Chitosan 1% w/v | Insulin | Enhancing potential of oral insulin delivery | PU:Alg 7:3 | 5.10 | 90–110 | - | +38.5 | 90.0 | 15% at pH 1.2 for 2 h 50% at pH 6.8 for 10 h 100% at pH 7.4 for 20 h | [91] |
| Formulation | Voltage (kV) | Needle Size (Gauge) | Flow Rate (mL/h) | Distance (cm) | Fibers Diameter (nm) | Reference |
|---|---|---|---|---|---|---|
| Sodium alginate 3% w/w Polyethylene oxide 3% w/w Triton X-100 0.5% w/w Dimethylsulphoxide 5% w/w | 25 | - | 0.7 | 18 | 97.4 | [123] |
| Sodium alginate 2% w/v Polyvinyl alcohol 14% w/v Nano-hydroxyapatite | 11 | 21 | 0.32 | 17 | 270 | [124] |
| Sodium alginate 1.5% w/w Polyethylene oxide 1.5% w/w | 18 | 23 | 2 | 6 | 288 | [125] |
| Sodium alginate 1.5% w/w Polyethylene oxide 1.5% w/w Carboxyl multi walled carbon nanotubes | 280 | |||||
| Polyethylene oxide 3% w/w Sodium alginate 2% w/w | 10.5 | 180 μm | 0.25 | 14 | 300 | [126] |
| Sodium alginate 1.74% w/w Triton X-100 1.1% w/w Polyethylene oxide 0.43% w/w Dimethyl sulfoxide 5.43% w/w | 12 | 27 | 0.6 | 12 | 240 | [47] |
| Sodium alginate 2% w/v olyvinyl alcohol 10% w/w | 26 | 25 | 0.48 0.6 | 10 | 62 180 | [127] |
| Alginate Concentration (% w/v) | Preparation Method | Drug | Mean Particle Size (nm) | Polydispersity Index (%) | Zeta Potential (mV) | Encapsulation Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| 3 | Emulsification/external gelation | Protein | 700 | - | - | 47 | [139] |
| 5 | 900 | 51 | |||||
| 0.50 | Emulsification/internal gelation | Peppermint | 512 | - | - | Increase | [141] |
| 1 | 4303 | ||||||
| 0.05 | Ionic gelation/complexation | Crocin | Increase | - | - | Increase | [133] |
| 0.30 | |||||||
| 0.1 | Ionic gelation/complexation | Timolol Maleate | 473.1 | 0.37 ± 0.05 | - | 33.71 ± 4.7 | [144] |
| 0.5 | 489.3 | 0.51 ± 0.1 | 39.01 ± 2.8 | ||||
| 0.03 | Ionic gelation/complexation | Nisaplin® | 86 | - | −33.2 | 35.6 | [145] |
| 0.07 | 204 | −38.7 | 30.5 | ||||
| 0.1 | Emulsification/external gelation | Doxorubicin | 39.2 | 0.19 | - | 92.2 | [148] |
| 0.2 | 149.6 | 0.38 | 98.4 | ||||
| 0.01 | Ionotropic pre-gelation/complexation | Insulin | Increase | Increase | - | - | [146] |
| 0.1 | |||||||
| 0.6 | Ionotropic gelation | Curcumin | 105 | - | 150 ± 1.15 | 94 ± 4.2 | [147] |
| 0.8 | 107 | 200 ± 2.15 | 92 ± 3.6 | ||||
| 1.0 | Electrospray | - | 315.9 ± 37.5 | 0.24 ± 0.10 | - | - | [115] |
| 2.0 | 348.2 ± 63.9 | 0.28 ± 0.03 |
| Surfactant | Surfactant Concentration | Method of Nanoparticle Preparation | Drug Loaded | Mean Particle Size (nm) | Polydispersity Index (%) | Encapsulation Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| Tween 20 | 0.20% v/v | Emulsification/external gelation | Doxorubicin | 102.4 | 0.25 | 87.2 | [148] |
| 0.30% v/v | 39.2 | 0.19 | 92.2 | ||||
| 0.40% v/v | 93.5 | 0.26 | 85.4 | ||||
| Span 60 co-surfactant | 0% w/v | 51.8 | 0.23 | 93.5 | [148] | ||
| 0.15% w/v | 42.3 | 0.26 | 84.6 | ||||
| 0.30% w/v | 95.1 | 0.24 | 76.2 | ||||
| Poloxamer 188 co-surfactant | 0% w/v | 51.8 | 0.23 | 93.5 | [148] | ||
| 0.15% w/v | 35.6 | 0.29 | 92.1 | ||||
| 0.30% w/v | 48.4 | 0.30 | 90.8 | ||||
| Poloxamer 407 | 0.10% w/v | Emulsification/external gelation complexation | Sacha inchi oil | 900 | - | Decrease 0.1 to 0.3% w/v Increase 0.5 to 1% w/v | [149] |
| 0.20% w/v | 1050 | ||||||
| 0.30% w/v | 1000 | ||||||
| 0.50% w/v | 700 | ||||||
| 1% w/v | 800 | ||||||
| Polysorbate 80 | 10% v/v | Ionotropic gelation | Curcumin | Increase | - | 92 | [147] |
| 20% v/v | 94 | ||||||
| 40% v/v | 91 | ||||||
| Tween 80 | 0% w/v | Ionic gelation | Rifampicin Ascorbic Acid | 450 | - | - | [150] |
| 0.20% w/v | 250 | ||||||
| 0.40% w/v | 700 |
| CaCl2 Concentration (% w/v) | Preparation Method | Drug | Particle Size (nm) | Polydispersity Index | Zeta Potential (mV) | Encapsulation Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| 0.05 | Emulsification/internal gelation | Vegetable Oils | 361 | - | - | 5.10 | [141] |
| 0.15 | 140 | 6.66 | |||||
| 0.05 | Ionic gelation/complexation | Timolol Maleate | 473 | 0.37 | - | 33.71 | [144] |
| 0.25 | 200 | 0.27 | 35.23 | ||||
| 3.0 | Ionotropic pre-gelation/complexation | Metronidazole | Decrease | - | Less negative | - | [152] |
| 6.0 | |||||||
| 0.5 | Ionotropic gelation/complexation | Insulin | Increase | Increase | - | - | [146] |
| 3.0 |
| Drug | Preparation Method | Formulation Composition | pH | Particle Size (nm) | Encapsulation Efficiency (%) | Zeta Potential (mV) | Reference |
|---|---|---|---|---|---|---|---|
| Crocin | Ionic gelation | Alginate 0.025% w/v Chitosan 0.04% w/v | 5.16 4.74 | 341 268 | 30.7 33.1 | - | [133] |
| Insulin | Ionotropic pre-gelation | Alginate Chitosan Cationic β-cyclodextrin | 4.90 | 150.82 | 93.2 | - | [146] |
| Blank/ε-polylysine | Ionic gelation | Alginate 0.1% w/v Chitosan 0.1% w/v | 5.14 | 276.38 | 54.18 | −33.7 | [90] |
| Blank/doxorubicin | Ionic gelation | Alginate 0.3% w/v Chitosan 0.075% w/v | 5.30 | 352 | 90 | −32 | [89] |
| Curcum | Layer-by-layer | Chitosan layer 200 mL Alginate layer 150 mL | 3.0 5.0 7.0 | - | - | +1 −30 −27 | [92] |
| Curcumin diethyl diglutarate | o/w Emulsification and ionotropic gelation | Alginate 0.6 mg/mL/pH = 4.9 Chitosan/pH = 4.6 Pluronic®F-127 (surfactant) | - | 215 | 85 | −24.1 | [177] |
| Drug | Preparation Method | Mass Ratio | Particle Size (nm) | Encapsulation Efficiency (%) | Zeta Potential (mV) | Reference |
|---|---|---|---|---|---|---|
| Nisin | Ionic gelation | 8:2 | 40 | 15.9 | −45.6 | [145] |
| complexation | 472 | 15.1 | −29.8 | |||
| Curcumin diethyl disuccinate | Emulsification ionotropic gelation | 1:0.05 | 279 ± 71 | 38.7 ± 2.8 | −27.8 ± 0.3 | [180] |
| 1:0.15 | 434 ± 17 | 17.1 ± 2.3 | −19.8 ± 1.4 | |||
| Insulin | Alginate/chitosan core shell nanoformation | 3:1 | - | 63.0 | - | [91] |
| 3:2 | 71.0 | |||||
| 3:3 | 77.0 | |||||
| lovastatin | Ionic gelation | 6:3 | 900 ± 101 | - | - | [181] |
| 6.5:3 | 86 ± 3.7 | |||||
| 7:3 | 220 ± 17.5 | |||||
| - | Electrospray | 11:1 | 112 ± 35 | 0.42 ± 0.06 | - | [115] |
| 7:1 | 259 ± 68 | 0.34 ± 0.12 |
| Preparation Method | Nanoparticles Size Range (nm) | Polydispersity Index | Encapsulation Efficiency (%) | Formulation and Processing Factors | Reference |
|---|---|---|---|---|---|
| Complexation | 20 ± 6 nm to 372.05 nm | 0.2 to 0.476 | 38.16% to 98.0% | Alginate concentration CaCl2 concentration pH value Alginate: chitosan mass ratio | [85,86,87,89,90] |
| Emulsification/gelation | 39.2 nm to 407.67 ± 19.18 nm | 0.204 to 0.42 ± 0.15 | 81.70 ± 6.64% to 92.2% | Alginate concentration Type/concentration of surfactant/cosurfactant CaCl2 concentration Stirring time | [69,70,77,148] |
| Spray dryer | 350 nm to 670 nm | 0.54 to 0.74 | 44.4% to 80% | Alginate concentration Air flow rate Solution feed rate Inlet temperature Outlet temperature Nozzle spray mesh size | [41,101,102] |
| Electrospray | 112.1 nm to 228 nm | 0.17 to 0.43 | ~99% | Alginate concentration CaCl2 concentration Nozzle size Flow rate Distance between needle tip and collector Applied voltage | [46,115] |
| Electrospinning | 62 nm to 300 nm | - | - | Alginate concentration Nozzle size Flow rate Distance Applied voltage | [126,127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. https://doi.org/10.3390/ph13110335
Choukaife H, Doolaanea AA, Alfatama M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals. 2020; 13(11):335. https://doi.org/10.3390/ph13110335
Chicago/Turabian StyleChoukaife, Hazem, Abd Almonem Doolaanea, and Mulham Alfatama. 2020. "Alginate Nanoformulation: Influence of Process and Selected Variables" Pharmaceuticals 13, no. 11: 335. https://doi.org/10.3390/ph13110335
APA StyleChoukaife, H., Doolaanea, A. A., & Alfatama, M. (2020). Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals, 13(11), 335. https://doi.org/10.3390/ph13110335