Abstract
Many lakes worldwide, including in China’s Yangtze River Basin, face eutrophication, which reduces phytoplankton diversity and increases bloom risk. Following severe pollution, these Chinese lakes have undergone substantial control and regulation. However, the efficacy of these measures is still unclear. Focusing on Lake Chaohu as a representative case, this study investigated the seasonal phytoplankton dynamics (2022–2023) under concurrent nutrient reduction and a fishing ban. The annual mean concentrations of total nitrogen, total phosphorus, and chlorophyll a were 1.57 mg/L, 0.184 mg/L, and 21.21 μg/L, respectively. The phytoplankton community was dominated by Cyanobacteria, which constituted approximately 75% of the total biomass. Co-occurrence network analysis revealed lower community stability during these warm, Cyanobacteria-dominated periods. Statistical analyses identified total phosphorus and temperature as key drivers, confirming bottom-up control via nutrient limitation as the fundamental mechanism. However, extreme heat events may have partly offset the benefits of nutrient reduction by promoting cyanobacterial dominance, which can decrease phytoplankton diversity. A recorded decrease in phytoplankton phosphorus use efficiency after the fishing ban suggests a potential strengthening of top-down control. These findings highlight that sustained nutrient load reduction is essential to reduce cyanobacterial bloom risk, while continued enforcement of the fishing ban may enhance the regulatory effect of top-down control on cyanobacterial blooms, thereby improving the stability and diversity of phytoplankton communities.
1. Introduction
Phytoplankton is one of the most crucial primary producers in lake ecosystems [1,2], fixing CO2 through photosynthesis and providing material and energy to the ecosystem via the food web [3,4]. A healthy phytoplankton community contributes to maintaining ecosystem stability and function; however, abnormal proliferation or extensive aggregation can lead to algal blooms, posing severe threats to aquatic ecological health [5,6].
Pollution control and ecological regulation are vital ways for controlling algal blooms. As primary producers, phytoplankton are directly influenced by environmental conditions such as temperature, light, and nutrients [7,8,9]. When conditions are favorable, phytoplankton can rapidly proliferate in large numbers, potentially forming blooms. Different phytoplankton taxa have distinct optimal temperature ranges. For instance, Cyanobacteria, with an optimal growth temperature between 25 °C and 35 °C, often dominate in summer [10,11,12,13], whereas Bacillariophyta can adapt to colder winter conditions [14]. Concurrently, besides environmental factors, phytoplankton are also controlled by top-down effects from various organisms within the aquatic ecosystem, including zooplankton, fish, and benthic fauna [15,16,17].
Numerous lakes in economically advanced regions have suffered from long-standing contamination, with restoration initiatives being implemented in recent years. Among these, Lake Chaohu stands out as a representative case study. Lake Chaohu, located in an economically developed and densely populated plain in eastern China, plays an ecologically and economically vital role in the lower Yangtze River basin by sustaining regional biodiversity, water resources, and fisheries [18]. Nevertheless, the ecosystem faces severe pressure; decades of substantial pollutant input have led to severe water quality deterioration and recurrent harmful cyanobacterial blooms [19,20,21], while intensive fishing practices further compound these ecological risks [22]. Compounding these challenges, the increasing frequency of extreme climate events under global change poses an additional threat to the Lake Chaohu ecosystem [23,24]. In recent years, China has made great efforts to mitigate this water pollution. For example, China’s Water Pollution Control Program (2006–2020) regards Chaohu as one of the three most important watersheds for eutrophication control [25]. Consequently, a combination of hard engineering solutions (e.g., sludge dredging, sewage interception, and wastewater treatment plant upgrades) and ecological remediation projects has been implemented to reduce nutrient pollution in the basin [21]. Furthermore, to halt biodiversity decline and restore fishery resources, the government officially implemented the “ten-year fishing ban” across the entire basin in January 2021 [23,26,27].
This study conducted surveys of phytoplankton and water quality from 2022 to 2023, analyzing influencing factors and comparing them with historical data. Specifically, it aims to evaluate the impacts of long-term pollution control and the decade-long fishing ban on the lake’s ecosystem by addressing the following key questions: How do long-term nutrient reduction and the fishing ban collectively reshape the phytoplankton community structure and its stability? Does the implementation of the fishing ban enhance top-down control?
2. Materials and Methods
2.1. Study Area
Lake Chaohu (117°17′–117°52′ E, 31°25′–31°43′ N), situated in Hefei City, Anhui Province, belongs to the river system on the left bank of the lower reaches of the Yangtze River. It covers an area of approximately 770 km2 [28], stretching about 55 km from east to west with an average width of 15 km from north to south. The lake is roughly bird’s nest-shaped, with an average water depth of 2.69 m and a maximum depth of 3.77 m, making it the fifth largest freshwater lake in China [29,30]. The western part of the lake is shallow, while the eastern part is deeper [31]. The shallow depth of Lake Chaohu limits the development of stable water column stratification [32]. More than 30 rivers flow into and out of Lake Chaohu, with major inflows including the Nanfei River, the Shiwuli River, the Pai River, the Hangbu River and other rivers [33]. The Yuxi River is the only outflow river linking to the Yangtze River [34]. Several of these, such as the Nanfei River, receive urban runoff and industrial wastewater, while the Hangbu River primarily receives agricultural runoff. These inputs contribute to nutrient loading in the lake [35]. Due to long-term high population density and intense economic activity within the basin, substantial external pollution loads have maintained Lake Chaohu in a eutrophic state, resulting in persistently high risk of algal bloom occurrence [36,37].
2.2. Sample Collection and Data Acquisition
A two-year field sampling campaign was conducted in Lake Chaohu from 2022 to 2023. Six sampling sites were established across the eastern, central, and western lake regions, as shown in Figure 1. Sampling was performed every two months, amounting to a total of 10 sampling events. Each campaign was completed within a single day. In situ measurements of water temperature (WT, °C), dissolved oxygen (DO, mg/L), and turbidity (Turb, NTU) were obtained using a multi-parameter water quality sonde (EXO2). The water transparency (SD, cm) was measured using the Secchi disk.
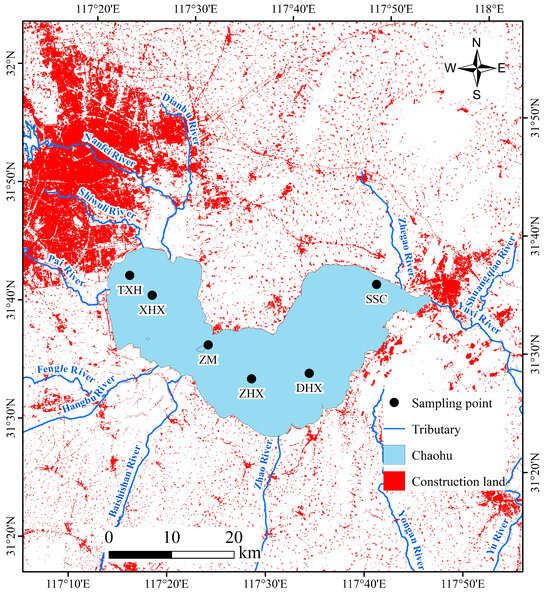
Figure 1.
Location of sampling points in Lake Chaohu. From east to west, site codes and their corresponding mean water depths are TXH (2.72 m), XHX (3.02 m), ZM (3.20 m), ZHX (3.32 m), DHX (3.28 m), and SSC (3.05 m).
Surface water (defined as water from the top 0.5 m depth) was collected using a 1 L plastic bottle and immediately preserved with 15 mL of Lugol’s solution. In the laboratory, the samples were settled for 48 h and then concentrated to a final volume of 30 mL. Phytoplankton cells were counted using an Olympus biomicroscope (CX41). A counting frame of 0.1 mL was used, which contained 100 horizons at 10 × 40 magnification [38]. Taxa were classified and identified to the genus level according to Hu and Wei [39]. Phytoplankton biomass was estimated based on cell volume measurements [40]. The dominant phytoplankton genera were ascertained using a dominance index (Y):
In this formula, ni denotes the cell abundance of genus i in a given phytoplankton community, N represents the total cell abundance across all sampling sites, and fi indicates the occurrence frequency of phytoplankton genus i at all sampling sites. A phytoplankton genus is identified as a dominant genus when its Y value exceeds 0.02 [41].
Data Sources for Long-Term Trend Analysis
The long-term trends of phytoplankton biomass and composition were analyzed using a combined dataset spanning from 2003 to 2021. Data were obtained from published studies on Lake Choahu, which followed analytical protocols.
2.3. Sample Processing and Analysis
Water samples were processed immediately upon return to the laboratory. Filtration was performed using GF/F filters (Whatman, 0.45 μm pore size). The filtrate was collected for the determination of dissolved nutrients, while the filters were retained for subsequent analysis of chlorophyll a (Chla) and suspended solids (SS). All analyses were conducted after sample processing, following Chinese National Standard methods. Total nitrogen (TN) and total dissolved nitrogen (TDN) were determined by the alkaline potassium persulfate digestion–UV spectrophotometric method [42]. Total phosphorus (TP) and total dissolved phosphorus (TDP) were analyzed using the ammonium molybdate spectrophotometric method. Chla was measured via acetone extraction spectrophotometry [43]. SS was calculated by the weight difference method.
2.4. Data Processing and Analysis
2.4.1. Trophic State Assessment
The modified Trophic State Index (TSIm) was employed to characterize the water trophic level [44], calculated using the following formulae:
2.4.2. Phytoplankton Diversity Indices Calculation
Alpha diversity: Four alpha diversity indices were employed to assess phytoplankton biodiversity in Lake Chaohu, namely the Shannon–Wiener diversity index (H), Pielou evenness index (J), Margalef richness index (R) and Simpson diversity index (D) (Table 1).

Table 1.
Evaluation Criteria for Alpha Diversity Indices [45].
Beta diversity: Non-metric multidimensional scaling (NMDS) was applied to reduce dimensionality and visualize differences in phytoplankton community composition between the two years. Permutational multivariate analysis of variance (PERMANOVA) was used to test the statistical significance of differences among groups.
2.4.3. Mathematical Statistics and Analysis of Data
Correlation analysis between the phytoplankton community and water quality parameters was conducted using Canoco 5.0 software. First, detrended correspondence analysis (DCA) was performed on the phytoplankton species data to determine the appropriate ordination model. When the length of the first axis was less than 3, indicating a linear species response, redundancy analysis (RDA) was applied. When the first axis length exceeded 4, indicating a unimodal species response, canonical correspondence analysis (CCA) was used. If the first axis length fell between 3 and 4, either RDA or CCA could be employed. Additionally, Pearson correlation analysis between phytoplankton alpha diversity indices and water quality parameters was performed using SPSS 27.0 software.
In this study, a co-occurrence network was constructed to elucidate interspecies associations. Phytoplankton species with a relative abundance greater than 0.01 in each month were included as nodes. Edges between nodes were established based on strong and significant Spearman’s rank correlations (|r| > 0.6) with a statistical threshold of p < 0.05. Only correlations meeting both criteria were retained to define the network topology. Additionally, Permutational Multivariate Analysis of Variance (PERMANOVA) with 9999 permutations was performed to assess the significance of differences in community structure. Correlation coefficients between nodes were calculated using the “Hmisc” package in R v4.2.0. Co-occurrence networks were then constructed using Gephi 0.9.2 software, and graph density (D) and average clustering coefficient (T) were computed. The structural characteristics of the co-occurrence networks reflect the stability of the phytoplankton community. The D/T ratio was further used to assess changes in community stability across different seasons: a lower D/T value indicates a more stable community structure, while a higher D/T value reflects lower structural stability [46,47]. Apart from network analysis, all other data analyses and graphing were performed using Origin 2021.
3. Results
3.1. Water Quality Status
During 2022–2023, the trophic level of Lake Chaohu exhibited distinct spatial and temporal patterns; it was generally higher in summer than in winter and higher in the western region compared to the eastern region. Furthermore, the overall trophic status was higher in 2022 than in 2023 (Figure 2d). The lake-wide averages over the two years were 1.57 mg/L for TN, 0.184 mg/L for TP and 21.21 μg/L for Chla. The mean TSIm value was 69, indicating that Lake Chaohu was in a state of moderate eutrophication overall.
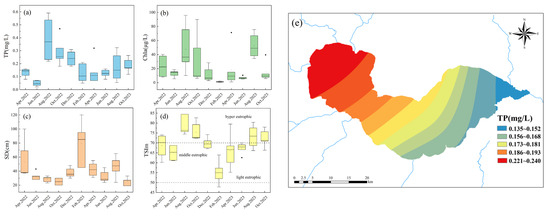
Figure 2.
Temporal variations in water quality parameters and trophic status and spatial distribution of TP in Lake Chaohu from 2022 to 2023 (a) TP; (b) Chla; (c) SD; (d) TSIm; (e) spatial distribution of TP.
Temporally, TP and Chla concentrations exhibited significant variation between summer and other seasons (p < 0.05). TP and Chla were elevated during summer, accompanied by lower water transparency; the opposite pattern was observed in winter. In February 2022, the lowest annual mean values for TP, Chla and the TSIm were recorded at 0.123 mg/L, 1.21 μg/L and 55, respectively. In contrast, these parameters peaked in August of the same year at 0.279 mg/L, 49.70 μg/L and 76. Meanwhile, water transparency reached its highest level of 79 cm in February and dropped to the lowest level of 25 cm in October.
Spatially, the TSIm of Lake Chaohu exhibited a distinct decreasing gradient from west to east. A significant difference was observed in Chla and TP between the western and eastern lake regions (p < 0.05). The western sites, TXH and XHX, showed the higher TP at 0.234 mg/L and 0.224 mg/L (Figure 2e), respectively, alongside the higher Chla levels of 38.48 μg/L and 37.21 μg/L. In contrast, the eastern site SSC recorded the lower TP at 0.145 mg/L and the lower Chla at 10.24 μg/L. Based on the TSIm, the western site TXH was severely eutrophic with a value of 74. The TSIm decreased eastward to 67 at the central site ZHX and to 67 and 66 at the eastern sites DHX and SSC, indicating a transition from severe eutrophication in the west to moderate eutrophication in the central and eastern lake regions.
3.2. Phytoplankton Community Composition and Biomass
A total of 58 genera of phytoplankton belonging to 6 phyla were identified in Lake Chaohu from 2022 to 2023. The community was primarily composed of Chlorophyta (42%), Cyanobacteria (28%), and Ochrophyta (16%). Phytoplankton abundance across the lake varied considerably, ranging from 15.33 to 14,891.58 × 104 cells/L.
Based on the dominance index (Y) (Table 2), 17 genera from four phyla were identified as dominant. Notably, the number of dominant genera was lower in summer than in other seasons. During the summer months across both years, the community was overwhelmingly dominated by Cyanobacteria. Microcystis and Dolichospermum were the most dominant genera, with dominance values ranging from 0.622 to 0.978 and from 0.028 to 0.267. In contrast, the winter community showed higher diversity, being dominated by Chlorophyta, Ochrophyta, and Cryptophyta. Representative winter-dominant genera included Cyclotella (dominance: 0.147) and Ankistrodesmus (dominance: 0.21), among others. This pattern indicates that species richness and evenness were lower in the summer and higher in the winter.

Table 2.
Dominant phytoplankton genera and their dominance indices (Y) in Lake Chaohu.
During the survey period, the phytoplankton biomass in Lake Chaohu ranged from 0.06 to 18.60 mg/L. Cyanobacteria constituted the majority, accounting for 75.32% of the total biomass. This was followed by Cryptophyta (12.52%), Chlorophyta (6.03%), and Ochrophyta (5.72%).
Temporally, Cyanobacteria and Cryptophyta biomass showed significant seasonal fluctuations (Figure 3), whereas Chlorophyta and Ochrophyta remained relatively stable. Cyanobacteria biomass peaked at 16.98 mg/L in August 2022 but dropped below 1.00 mg/L in December 2022 and February 2023. Cryptophyta biomass displayed an opposite seasonal trend; it recorded lows of 0.33 mg/L in August 2023, in contrast to a high of 1.47 mg/L in December 2022. Moderately high Cryptophyta biomass was also observed in April and June 2023. Chlorophyta biomass remained around 0.30 mg/L throughout most months. Ochrophyta biomass was generally stable at approximately 0.25 mg/L.
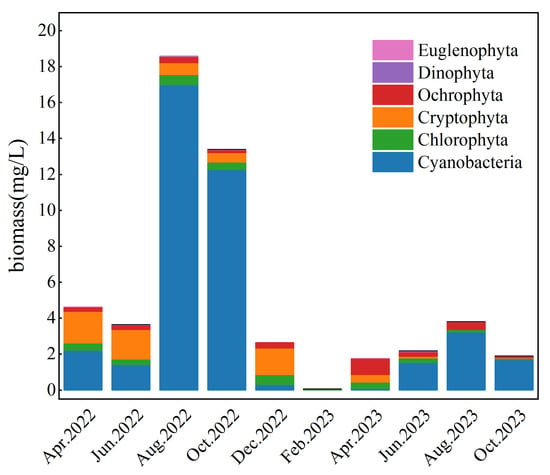
Figure 3.
Temporal Dynamics of Phytoplankton Biomass in Lake Chaohu. Data are presented as the mean of six monitoring stations.
Spatially, during the summer when Cyanobacteria dominate, there is a significant difference in Cyanobacteria biomass between the western and eastern parts of the lake (p < 0.01). Specifically, the Cyanobacteria biomass was highest at the western sampling sites (TXH and XHX), reaching 32.83 mg/L and 43.31 mg/L, respectively, while all sampling sites in the central and eastern regions had biomass levels below 2.00 mg/L, showing a clear pattern of higher biomass in the west and lower in the east.
3.3. Dynamics of Phytoplankton Community Structure
Phytoplankton diversity indices serve as important indicators for assessing community characteristics and aquatic ecosystem health (Table 1) [48,49]. Alpha diversity (Figure 4) analysis of the phytoplankton community in Lake Chaohu revealed that the Shannon–Wiener index ranged from 0.16 to 2.45, with a mean value of 1.25, indicating an overall moderate level. This index reached its highest value in December 2022, with a lake-wide average of 2.01, classified as “Good”. In contrast, it was lowest in August 2022 at only 0.5, categorized as “Poor”. The Simpson index followed a trend largely consistent with the Shannon–Wiener index, ranging from 0.23 to 0.80 with a mean of 0.53, also indicating an overall moderate level. It peaked in December 2022 at 0.8 and was lowest in August 2022 at 0.23. Simpson index values between 0.5 and 0.8, classified as “Good”, were observed in December 2022, February 2023, April 2022, June 2022 and April 2023. Values between 0.0 and 0.3, classified as “Poor”, were recorded in August 2022, October 2022, August 2023 and October 2023. The Pielou evenness index ranged from 0.06 to 0.60 with a mean of 0.24, indicating generally poor evenness overall. It reached 0.6, classified as “Good”, only in February 2023, while other seasons remained “Poor”. The Margalef richness index ranged from 1.70 to 2.60, suggesting a mildly eutrophic state in Lake Chaohu during the study period.
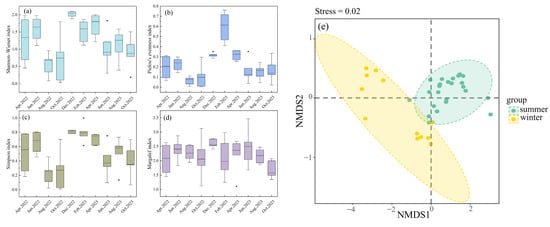
Figure 4.
Temporal Dynamics of Alpha Diversity (a) Shannon–Wiener index; (b) Pielou’s evenness index; (c) Simpson index; (d) Margalef index; (e) Non-metric Multidimensional Scaling Analysis based on Bray–Curtis distance.
The Shannon diversity index, Pielou evenness index, and Simpson diversity index were all higher in winter and spring than in summer and autumn, with highly significant differences between seasons (p < 0.01). The Margalef richness index showed significant differences only between spring and autumn and between winter and autumn (p < 0.05). The phytoplankton species composition in Lake Chaohu showed distinct seasonal differences between summer and winter, which was confirmed by PERMANOVA analysis of Bray–Curtis distances (R2 = 0.204, p < 0.01). The NMDS ordination (Figure 4e) clearly illustrates this separation.
3.4. The Influence of Environmental Conditions on Phytoplankton Community Structure
Water temperature and total phosphorus were the dominant environmental factors controlling the phytoplankton community structure in Lake Chaohu. Pearson correlation analysis revealed significant relationships between key phytoplankton indicators and these factors (Figure 5a). Chla exhibited highly significant positive correlations with both WT and TP (p < 0.01). Similarly, total phytoplankton cell density showed a significant positive correlation with WT (p < 0.05) and a highly significant positive correlation with TP (p < 0.01). Furthermore, TP and WT were the most significant factors negatively impacting phytoplankton alpha diversity, with the Shannon, Pielou, and Simpson indices all showing highly significant negative correlations (p < 0.01). RDA further elucidated the regulatory roles of these environmental factors (Figure 5b). The first two RDA axes collectively explained 33.25% of the total community variation. TP was the primary driver, explaining 18.6% of the variance (p < 0.01), followed by WT (3.0%, p < 0.05). In contrast, the contribution of TN (1.5%) was not statistically significant (p > 0.05). At the phylum level, both TP and WT showed significant positive correlations with Cyanobacteria, Chlorophyta, Chrysophyta, and Euglenophyta.
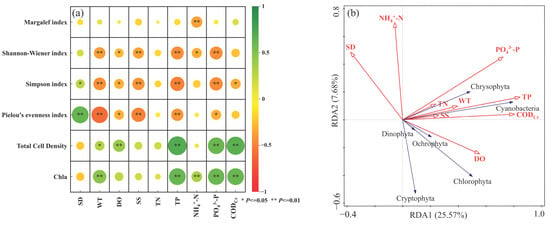
Figure 5.
(a) Pearson correlations between Alpha Diversity and environmental factors, larger circle sizes correspond to stronger correlations; (b) Redundancy Analysis of phytoplankton at the phylum level and environmental factors in Lake Chaohu.
The strong influence of WT and TP was reflected in pronounced seasonal succession. NMDS and PERMANOVA tests confirmed that phytoplankton community composition differed significantly between summer and winter, with the seasonal factor exhibiting the highest explanatory power. The lowest phytoplankton diversity in summer is primarily attributed to the synergistic effect of high WT and high TP inputs, which favored the proliferation of thermophilic Cyanobacteria (e.g., Microcystis). This allowed Microcystis to establish near-monopolistic dominance, suppressing the growth of other algal species and reducing the community’s resilience to environmental disturbances. In contrast, lower winter temperatures limited the growth of Cyanobacteria, reducing their competitive advantage. This facilitated enhanced niche partitioning and coexistence among a wider range of species, thereby maintaining higher community diversity and stability.
4. Discussion
4.1. High Temperatures Partially Offset the Effects of Algal Bloom Control
The elevated temperatures from 2022 to 2023 resulted in increased phytoplankton biomass, partially counteracting the effectiveness of bloom control measures. The Lake Chaohu basin experienced a severe drought in 2022–2023, characterized by high temperatures and low precipitation, particularly in summer. This climatic condition increased the accumulated temperature in the basin, which created favorable conditions for rapid algal proliferation [50,51,52]. Elevated water temperature is a key driver in intensifying blooms of Microcystis and other Cyanobacteria [53,54]. This study aligns with this established understanding, as we observed a significant positive correlation between Chla and WT (r = 0.47, p < 0.01) during the 2022–2023 drought period in Lake Chaohu. The sustained high temperatures not only promoted higher phytoplankton biomass but also directly drove the seasonal succession of the dominant community, facilitating the absolute dominance of the thermophilic genus Microcystis in summer. During low-temperature periods in December 2022 and February and April 2023, the lake was dominated by Chlorophyta and Ochrophyta. In contrast, the thermophilic genus Microcystis achieved absolute dominance during the warmer months of June, August, and October, consistent with the typical optimal growth temperature range of 25–35 °C for Cyanobacteria [55]. Previous studies have indicated clear differences in temperature adaptation among algal groups; Chlorophyta prefer moderate temperatures [56], whereas Bacillariophyta thrive in cooler environments [57]. However, in Lake Chaohu, Chlorophyta were more dominant than Bacillariophyta in winter. This is primarily attributed to the lake’s long-term eutrophic state. Under such conditions, Chlorophyta exhibit higher nutrient utilization efficiency, which enables rapid nutrient uptake and utilization for growth and reproduction. Furthermore, Bacillariophyta growth requires silicate for cell wall construction, whereas Chlorophyta do not share this limitation, granting the latter a competitive advantage for nutrients [58]. In eutrophic Lake Chaohu, elevated temperatures frequently promote monospecific dominance and reduce phytoplankton community diversity [59,60], thereby compromising community stability [24,60,61]. The high-temperature conditions during 2022–2023 had pronounced negative impacts on the lake’s ecosystem health.
Previous studies have demonstrated the utility of microbial co-occurrence networks for investigating associations among key microbial groups [46,47,62]. In this study, we assessed phytoplankton community stability using co-occurrence network analysis based on the D/T ratio [63]. Density (D) is defined as the ratio of the actual number of edges to the possible number of edges in the network. Transitivity (T) represents the probability that two neighboring nodes of a given node are also connected, reflecting the local clustering and structural complexity of the network [47]. The ratio of D to T (D/T) serves as an indicator of network stability, where a lower D/T value denotes higher stability [46]. Monthly D/T values differed significantly (Figure 6). The lowest D/T values occurred in February 2023 and April 2023, with values of 0.337 and 0.314, respectively, indicating significantly greater stability in these months. Conversely, October 2022 exhibited the highest D/T value of 0.824, reflecting the poorest stability. Summer D/T values were 0.624 in 2022 and 0.612 in 2023, suggesting marginally improved stability in summer 2023 compared to summer 2022.
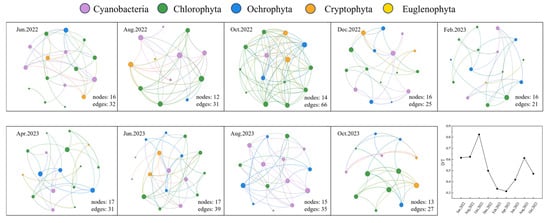
Figure 6.
Co-occurrence Network Analysis based on phytoplankton abundance (the node color represents different phytoplankton taxa, node size is proportional to species abundance, and edges indicate potential interactions between taxa).
4.2. Improved Water Quality Has Reduced the Intensity of Algal Blooms
Long-term monitoring data demonstrate that comprehensive pollution control, including upgraded wastewater treatment and stringent regulation of point sources in the inflowing rivers, has led to a significant reduction in nutrient levels in Lake Chaohu [64]. From 2003 to 2023, TP concentration decreased from 0.229 mg/L to 0.186 mg/L (Figure 7a), and TN concentration decreased from 2.83 mg/L to 1.55 mg/L (Figure 7b). This improvement has been most pronounced in the historically hypereutrophic western region. Following the integrated remediation of major polluting tributaries such as the Shiwuli River, the frequency and spatial extent of cyanobacterial blooms have substantially declined [21,65]. The mechanism behind this trend is primarily bottom-up control. In our study, statistical analyses consistently identified TP as the primary driver of both algal biomass and community shifts. This finding aligns with conclusions from previous studies emphasizing the role of phosphorus in controlling phytoplankton density and community composition in this lake [66,67,68]. The critical role of phosphorus control is further corroborated by evidence from other lakes undergoing similar restoration efforts. For instance, in Lake Dianchi, a reduction in nutrient levels has also been associated with an overall decrease in chlorophyll a concentration and a shorter duration of algal blooms [69]. However, interannual fluctuations in bloom intensity persist, likely modulated by hydrological conditions and extreme climate events, underscoring the need for sustained management.
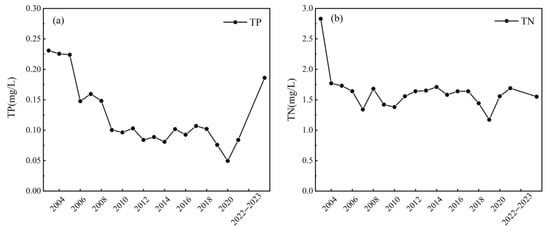
Figure 7.
Changes in nutrient concentrations TP concentration (a) and TN concentration (b) in Lake Chaohu from 2003 to 2023. Data for the period 2003–2021 were obtained from [70,71,72].
4.3. Potential Influence of the Fishing Ban on Top-Down Control
The Yangtze River “Ten-Year Fishing Ban” policy, implemented since 1 January 2021, designates Lake Chaohu as a key water body [27,73,74]. Although not directly targeting eutrophication, this policy may indirectly mitigate cyanobacterial blooms by facilitating fish population recovery. It is expected to bolster filter feeders like silver carp (Hypophtalmichthys molitrix) and bighead carp (Aristichthys nobilis), whose increased abundance can exert top-down control on Cyanobacteria [17,75,76,77]. Preliminary observations also suggest a recovery of higher-trophic-level carnivores (e.g., Culter alburnus), indicating a positive restructuring of the food web towards greater complexity and stability, which could enhance the ecosystem’s intrinsic regulatory capacity [27]. Satellite remote sensing data corroborate a positive trend, showing an 86.95% decrease in the annual average cyanobacterial bloom area from 30.25 km2 (2019) to 3.95 km2 (2022) [78].
However, during the initial phase of the ban (2022–2023), which coincided with basin-wide drought, a significant reduction in total phytoplankton biomass was not observed, and fish species richness increased only modestly by 4 species. This limited immediate response is expected, as most fish species require approximately four years to reach sexual maturity [79,80]. Despite the absence of a biomass decline, a key indirect signal of changing top-down pressure has emerged: a distinct post-ban decrease in phosphorus utilization efficiency (Figure 8). We quantified this efficiency using the chlorophyll a to total phosphorus ratio (Chla/TP), which reflects the conversion rate of phosphorus into algal biomass [81]. A lower Chla/TP ratio suggests that factors other than phosphorus availability—such as enhanced grazing pressure (top-down control)—are becoming increasingly important in limiting phytoplankton productivity [82,83]. Therefore, the observed reduction in phosphorus utilization efficiency suggests that top-down grazing pressure may already be exerting a regulatory influence on the phytoplankton population, even before causing a net reduction in its biomass [17,76,77].
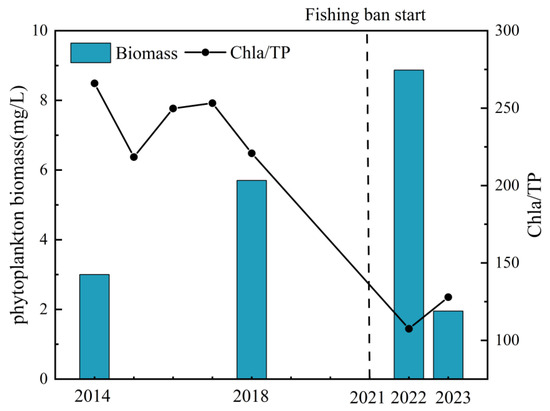
Figure 8.
Phytoplankton biomass and phosphorus use efficiency in the pre-ban and initial ban periods. Data for the period 2003–2021 were obtained from [70,71,72].
In this study, while the lack of zooplankton data prevents direct quantification of the top-down effect, this gap has limited impact on our core conclusions, according to existing research. In Lake Chaohu, the low biomass of zooplankton, coupled with the inhibition of their reproduction by the toxicity of the dominant Microcystis, results in an overall weak top-down control on algal blooms [84,85]. Additionally, our conclusions regarding the effects of the fishing ban on fish populations are based on indirect evidence and inference, highlighting that long-term monitoring of the fishing ban’s effects should be prioritized in the future.
5. Conclusions
This study reveals the persistent dominance of Cyanobacteria in Lake Chaohu’s phytoplankton community (2022–2023), with community structure and alpha diversity exhibiting significant seasonal dynamics. The lowest diversity and stability in summer are linked to highly eutrophic conditions, favoring thermophilic and pollution-tolerant taxa. Critically, our data indicate a decrease in phytoplankton phosphorus utilization efficiency concurrent with the fishing ban period, supporting the hypothesis of increased top-down control pressure. These findings have two key implications: (1) Nutrient reduction remains the foundational strategy for long-term bloom risk control, as eutrophication continues to drive summer community instability. (2) Given the potential of the fishing ban to suppress Cyanobacteria, continuous monitoring is recommended to evaluate its long-term efficacy.
Author Contributions
Conceptualization, T.W. and F.Z.; Formal analysis, F.Z. and R.Y.; Investigation, C.D., Z.H. and H.L.; Supervision, T.W.; Writing—original draft, F.Z., C.D. and H.L.; Writing—review and editing, T.W., Z.C. and R.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly supported by the Open Research Fund of Key Laboratory of Drinking Water Source Protection of the Ministry of Ecology and Environment, Chinese Research Academy of Environmental Sciences (2024YYSYKFZD04), and the Fundamental Research Funds for the Central Public Interest Scientific Institution (2024YSKY-02).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leibold, M.A.; Hall, S.R.; Smith, V.H.; Lytle, D.A. Herbivory enhances the diversity of primary producers in pond ecosystems. Ecology 2017, 98, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Bigalke, A.; Kaulfuß, A.; Pohnert, G. Strategies and ecological roles of algicidal bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.S.; Zhang, Y.; Häder, D.P. Individual and interactive effects of ocean acidification, global warming, and UV radiation on phytoplankton. J. Appl. Phycol. 2018, 30, 743–759. [Google Scholar] [CrossRef]
- Nakamoto, S. Why Does the Biosphere Interact with Earth’s Climate, and How Do the Two-Way Interactions Work? Biology 2024, 13, 1040. [Google Scholar] [CrossRef]
- Dai, Y.H.; Yang, S.B.; Zhao, D.; Hu, C.M.; XU, W.; Anderson, D.M.; Li, Y.; Song, X.P.; Boyce, D.G.; Gibson, L.; et al. Coastal phytoplankton blooms expand and intensify in the 21st century. Nature 2023, 615, 280–284. [Google Scholar] [CrossRef]
- Chun, S.-J.; Cui, Y.; Kim, J.; Roh, D.; Kim, J.; Park, S.; Lee, J.-W.; Nam, K.-H. Freshwater shrimp (Neocaridina denticulata) as a nature-based restoration tool for macrophyte recovery and improved water quality in eutrophic ponds. J. Environ. Manag. 2025, 394, 127372. [Google Scholar] [CrossRef]
- Zeng, L.; Wen, J.; Huang, B.; Yang, Y.; Huang, Z.; Zeng, F.; Fang, H.; Du, H. Environmental DNA metabarcoding reveals the effect of environmental selection on phytoplankton community structure along a subtropical river. Environ. Res. 2023, 243, 117708. [Google Scholar] [CrossRef]
- Pan, S.P.; Li, Q.H.; Meng, C.L.; Han, M.S.; Ma, Y.M.; Brancelj, A. Using a structural equation model to assess the spatiotemporal dynamics and driving factors of phytoplankton in the plateau Hongfeng Reservoir in southwest China. Aquat. Ecol. 2022, 56, 1297–1313. [Google Scholar] [CrossRef]
- Li, Z.X.; Gao, Y.; Wang, S.Y.; Jia, J.J.; Ha, X.R.; Lu, Y. Floodplain lake response to climate-nutrient-hydrological pressure revealed through phytoplankton community succession over the past century. J. Hydrol. 2023, 623, 129838. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2012, 58, 552–559. [Google Scholar] [CrossRef]
- Zapomelová, E.; Reháková, K.; Jezberová, J.; Komárková, J. Polyphasic characterization of eight planktonic Anabaena strains (Cyanobacteria) with reference to the variability of 61 Anabaena populations observed in the field. Hydrobiologia 2010, 639, 99–113. [Google Scholar] [CrossRef]
- Foysal, M.J.; Timms, V.; Neilan, B.A. Dynamics of the benthic and planktic microbiomes in a Planktothrix-dominated toxic cyanobacterial bloom in Australia. Water Res. 2023, 249, 120980. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Wang, H.; Na, X.Y.; Liu, Y.; Zhang, N.N.; Lu, X.X.; Fan, Y.W. Temperate urban wetland plankton community stability driven by environmental variables, biodiversity, and resource use efficiency: A case of Hulanhe Wetland. Front. Ecol. Evol. 2023, 11, 1148580. [Google Scholar] [CrossRef]
- Karpowicz, M.; Feniova, I.Y.; Sakharova, E.G.; Gorelysheva, Z.I.; Więcko, A.; Górniak, A.; Dzialowski, A.R. Top-down and bottom-up control of phytoplankton communities by zebra mussels Dreissena polymorpha (Pallas, 1771). Sci. Total Environ. 2023, 877, 162899. [Google Scholar] [CrossRef]
- Gusha, M.N.C.; Dalu, T.; Wasserman, R.J.; McQuaid, C.D. Zooplankton grazing pressure is insufficient for primary producer control under elevated warming and nutrient levels. Sci. Total Environ. 2019, 651, 410–418. [Google Scholar] [CrossRef]
- Guo, C.; Li, S.; Ke, J.; Liao, C.; Hansen, A.G.; Jeppesen, E.; Zhang, T.; Li, W.; Liu, J. The feeding habits of small-bodied fishes mediate the strength of top-down effects on plankton and water quality in shallow subtropical lakes. Water Res. 2023, 233, 119705. [Google Scholar] [CrossRef]
- Chen, P.; Luo, J.; Xiong, Z.; Wan, N.; Ma, J.; Yuan, J.; Duan, H. Can the establishment of a protected area improve the lacustrine environment? A case study of Lake Chaohu, China. J. Environ. Manag. 2023, 342, 118–152. [Google Scholar] [CrossRef]
- Zheng, L.; Jiang, C.; Chen, X.; Li, Y.; Li, C.; Zheng, L. Combining hydrochemistry and hydrogen and oxygen stable isotopes to reveal the influence of human activities on surface water quality in Chaohu Lake Basin. J. Environ. Manag. 2022, 312, 114933. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Tolonen, K.E.; Yin, H.; Gao, J.; Zhang, Z.; Li, K.; Cai, Y. Substrate degradation and nutrient enrichment structuring macroinvertebrate assemblages in agriculturally dominated Lake Chaohu Basins, China. Sci. Total Environ. 2018, 627, 57–66. [Google Scholar] [CrossRef]
- Zhong, F.; Wu, J.; Dai, Y.; Xiang, D.; Deng, Z.; Cheng, S. Responses of water quality and phytoplankton assemblages to remediation projects in two hypereutrophic tributaries of Chaohu Lake. J. Environ. Manag. 2019, 248, 109276. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Wang, P.; Jeppesen, E.; Xie, P. How to manage fish within and after the 10-year fishing ban. Innovation 2024, 5, 100694. [Google Scholar] [CrossRef] [PubMed]
- O’Beirne, M.D.; Werne, J.P.; Hecky, R.E.; Johnson, T.C.; Katsev, S.; Reavie, E.D. Anthropogenic climate change has altered primary productivity in Lake Superior. Nat. Commun. 2017, 8, 15713. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Qin, B.; Zhang, Q.; Paerl, H.W.; Van Dam, B.; Jeppesen, E.; Zeng, C. Global lake phytoplankton proliferation intensifies climate warming. Nat. Commun. 2024, 15, 10572. [Google Scholar] [CrossRef]
- Wu, H.; Yang, T.; Liu, X.; Li, H.; Gao, L.; Yang, J.; Li, X.; Zhang, L.; Jiang, S. Towards an integrated nutrient management in crop species to improve nitrogen and phosphorus use efficiencies of Chaohu Watershed. J. Clean. Prod. 2020, 272, 122765. [Google Scholar] [CrossRef]
- Yang, L.; Pan, M.; Sun, J.R.; Cui, Y.D.; Dong, J.Y.; Yang, J.J.; Ji, S.H.; Tao, J.; Ding, C.Z. Short-term responses of macroinvertebrate assemblages to the “ten-year fishing ban” in the largest highland lake of the Yangtze basin. J. Environ. Manag. 2023, 343, 118160. [Google Scholar] [CrossRef]
- Han, S.P.; Xia, W.L.; He, J.; Wu, Q.H.; Xu, W.L.; Yu, J.; Chen, J.; Xie, P. Spatiotemporal dynamics of microcystin contamination in fish across the Lake Chaohu basin under the Yangtze River ten-year fishing ban: Ecological and human health implications. Ecotoxicol. Environ. Saf. 2025, 296, 118185. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Shi, K.; Zhang, M.; Han, T.; Lai, L.; Zhan, P. Sensitivity of phytoplankton to climatic factors in a large shallow lake revealed by column-integrated algal biomass from long-term satellite observations. Water Res. 2021, 207, 117786. [Google Scholar] [CrossRef]
- Wang, X.; Xi, B.; Huo, S.; Deng, L.; Pan, H.; Xia, X.; Zhang, J.; Ren, Y.; Liu, H. Polybrominated diphenyl ethers occurrence in major inflowing rivers of Lake Chaohu (China): Characteristics, potential sources and inputs to lake. Chemosphere 2013, 93, 1624–1631. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.; Duan, Z.; Yuan, S. Characterization of phosphorus behavior and microbial response in the river-lake confluence area of Nanfei River and Chaohu Lake. J. Environ. Chem. Eng. 2025, 13, 117478. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Meng, R.X. Preliminary Study of the Planktonic Blue Algae in the Chaohu Lake, Anhui. Trans. Oceanol. Limnol. 1989, 2, 35–41. (In Chinese) [Google Scholar] [CrossRef]
- Xu, Q.W. Analysis About Driving Process for Nutritional Status Change and Impacts of Lake Warming on Internal Nutrient Cycling in Lake Chaohu; Tongfang CNKI Technology Co., Ltd.: Beijing, China, 2020; (In Chinese). [Google Scholar] [CrossRef]
- Fang, T.; Lu, W.; Cui, K.; Li, J.; Yang, K.; Zhao, X.; Liang, Y.; Li, H. Distribution, bioaccumulation and trophic transfer of trace metals in the food web of Chaohu Lake, Anhui, China. Chemosphere 2019, 218, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gong, W.; Lv, J.; Xiong, X.; Wu, C. Accumulation of floating microplastics behind the Three Gorges Dam. Environ. Pollut. 2015, 204, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xie, P.; Xu, J.; Liu, B.; Yang, H. Spatiotemporal variations of internal P-loading and the related mechanisms in the large shallow Lake Chaohu. Sci. China Earth Sci. 2006, 49, 72–81. [Google Scholar] [CrossRef]
- Zeng, S.; Qin, Z.H.; Ruan, B.Z.; Lei, S.H.; Yang, J.; Song, W.W.; Sun, Q. Long-term dynamics and drivers of particulate phosphorus concentration in eutrophic lake Chaohu, China. Environ. Res. 2023, 221, 115219. [Google Scholar] [CrossRef]
- Shao, K.Q.; Yao, X.; Wu, Z.S.; Jiang, X.Y.; Hu, Y.; Tang, X.M.; Xu, Q.J.; Gao, G. The bacterial community composition and its environmental drivers in the rivers around eutrophic Chaohu Lake, China. BMC Microbiol. 2021, 21, 179. [Google Scholar] [CrossRef]
- Yu, X.; Li, Z.; Zhao, S.; Li, K. Methods for the Monitoring and Analysis of Water and Wastewater, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Zhang, Y.; Ma, X.F.; Guo, F.F.; Li, J.Z.; Xiong, B.X. Community structures of phytoplankton and their relationships with environmental factors in the Jinshahe Reservoir, Hubei Province. J. Lake Sci. 2015, 27, 902–910. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Huang, X.; Liu, Y.; Guan, M.; Tian, Y. Hydrological conditions can change the effects of major nutrients and dissolved organic matter on phytoplankton community dynamics in a eutrophic river. J. Hydrol. 2023, 628, 130503. [Google Scholar] [CrossRef]
- D’Elia, C.F.; Steudler, P.A.; Corwin, N. Determination of total nitrogen in aqueous samples using persulfate digestion. Limnol. Oceanogr. 1977, 22, 760–764. [Google Scholar] [CrossRef]
- Lin, K.N.; Huang, S.Y.; Wei, C.J.; Jiang, R.G.; Xu, J.; Zhang, Y.B.; Zhang, P. Spectrophotometric determination of phosphate using sodium molybdate and its field application to the simultaneous measurement with ammonium in seawater. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2024, 330, 125683. (In Chinese) [Google Scholar] [CrossRef]
- Aizaki, M.; Otsuki, A.; Fukushima, T.; Kawai, T.; Muraoka, K. Application of modified Carlson’s trophic state index to a Japanese lake and its relationship to other parameters related to the trophic state. Res. Rep. Natl. Inst. Environ. Stud. 1981, 23, 13–31. [Google Scholar]
- Qu, Y.L.; Lin, Y.Q.; Liu, J.J.; Tang, L.; Guan, T.S.; Chen, Q.W.; Chen, K.; Wang, L. The biodiversity assessment of phytoplankton community in summer within main stream and tributary of Huaihe River. Acta Sci. Circumst. 2018, 38, 1665–1672. (In Chinese) [Google Scholar] [CrossRef]
- Douterelo, I.; Dutilh, B.E.; Arkhipova, K.; Calero, C.; Husband, S. Microbial diversity, ecological networks and functional traits associated to materials used in drinking water distribution systems. Water Res. 2020, 173, 115586. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, H.; Kim, J.J.; Myeong, N.R.; Kim, T.; Park, T.; Kim, E.; Choi, J.-y.; Lee, J.; An, S.; et al. Fragile skin microbiomes in megacities are assembled by a predominantly niche-based process. Sci. Adv. 2018, 4, e1701581. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Wan, X.; Wang, Y.; Wang, W. Community characteristics of benthic macroinvertebrates and identification of environmental driving factors in rivers in semi-arid areas—A case study of Wei River Basin, China. Ecol. Indic. 2021, 121, 107153. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Y.; Peng, C.R.; Li, G.B.; He, F.; Huang, L.C.; Sun, X.Q.; Wu, S.R. Integrated evaluation of the impact of water diversion on water quality index and phytoplankton assemblages of eutrophic lake: A case study of Yilong Lake. J. Environ. Manag. 2024, 357, 120707. [Google Scholar] [CrossRef]
- Ma, Q.; Lv, M.Q.; Wang, X.P.; Wang, Y.; Xing, Q. CO2 and CH4 emission characteristics of eutrophic ponds and reservoirs in hilly and mountainous areas. J. Lake Sci. 2025, 37, 1237–1248. (In Chinese) [Google Scholar] [CrossRef]
- Xu, G.; Wu, Y.; Liu, S.; Cheng, S.; Zhang, Y.; Pan, Y.; Wang, L.; Dokuchits, E.Y.; Nkwazema, O.C. How 2022 extreme drought influences the spatiotemporal variations of terrestrial water storage in the Yangtze River Catchment: Insights from GRACE-based drought severity index and in-situ measurements. J. Hydrol. 2023, 626, 130245. [Google Scholar] [CrossRef]
- Duan, A.; Zhong, Y.; Xu, G.; Yang, K.; Tian, B.; Wu, Y.; Bai, H.; Hu, E. Quantifying the 2022 extreme drought in the Yangtze River Basin using GRACE-FO. J. Hydrol. 2024, 630, 130680. [Google Scholar] [CrossRef]
- Lehman, P.W.; Kurobe, T.; Lesmeister, S.; Baxa, D.; Tung, A.; Teh, S.J. Impacts of the 2014 severe drought on the Microcystis bloom in San Francisco Estuary. Harmful Algae 2017, 63, 94–108. [Google Scholar] [CrossRef]
- Lehman, P.W.; Marr, K.; Boyer, G.L.; Acuna, S.; Teh, S.J. Long-term trends and causal factors associated with Microcystis abundance and toxicity in San Francisco Estuary and implications for climate change impacts. Hydrobiologia 2013, 718, 141–158. [Google Scholar] [CrossRef]
- Jöhnk, K.D.; Huisman, J.E.F.; Sharples, J.; Sommeijer, B.E.N.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Change Biol. 2007, 14, 495–512. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Shang, G.X.; Zhang, J.Y.; Wang, L.Q.; Wei, H. Succession Characteristics of Phytoplankton Functional Groups and Their Relationships with Environmental Factors in Dianshan Lake, Shanghai. Environ. Sci. 2018, 39, 3158–3167. (In Chinese) [Google Scholar] [CrossRef]
- Gongyuan, D.; Jie, L.; Lin, L.; Lirong, S. Spatio-temporal Patterns of Phytoplankton and Related Environmental Factors in the Northern Lake Area of Dianchi Lake. Acta Hydrobiol. Sin. 2012, 36, 946–956. (In Chinese) [Google Scholar] [CrossRef]
- Paerl, H.W.; Plaas, H.E.; Nelson, L.M.; Korbobo, A.S.; Cheshire, J.H.; Yue, L.; Preece, E.P. Dual nitrogen and phosphorus reductions are needed for long-term mitigation of eutrophication and harmful cyanobacterial blooms in the hydrologically-variable San Francisco Bay Delta, CA. Sci. Total Environ. 2024, 957, 177499. [Google Scholar] [CrossRef]
- Strandberg, U.; Hiltunen, M.; Syväranta, J.; Levi, E.E.; Davidson, T.A.; Jeppesen, E.; Brett, M.T. Combined effects of eutrophication and warming on polyunsaturated fatty acids in complex phytoplankton communities: A mesocosm experiment. Sci. Total Environ. 2022, 843, 157001. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.; Kong, W.; Gu, S.; Wang, S. Seasonal instability of phytoplankton community in extreme arid lake basin during interval period of large-scale ecological water transport. Sci. Total Environ. 2024, 958, 178119. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, H.J.; Lee, K.; Franks, P.J.S.; Seong, K.A.; Lee, S.Y.; Lee, M.J.; Hyeon Jang, S.; Potvin, E.; Suk Lim, A.; et al. Effects of warming and eutrophication on coastal phytoplankton production. Harmful Algae 2019, 81, 106–118. [Google Scholar] [CrossRef]
- Ruan, Q.; Dutta, D.; Schwalbach, M.S.; Steele, J.A.; Fuhrman, J.A.; Sun, F. Local similarity analysis reveals unique associations among marine bacterioplankton species and environmental factors. Bioinformatics 2006, 22, 2532–2538. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Xu, Z.W.; Gong, L.Q.; Luo, C.Q.; Yu, D.K.; Sun, M.F.; Shang, J.W.; Qin, H.M. Seasonal succession characteristics of zooplankton community in shallow dished sublakes of Lake Poyang and influencing factors during early Fish Banning period. J. Lake Sci. 2025, 37, 1367–1380. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Wan, J.; Chen, Z.; Wang, N.; Guo, Y.; Wang, Y. Water quality variation and driving factors quantitatively evaluation of urban lakes during quick socioeconomic development. J. Environ. Manag. 2023, 344, 118615. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cao, Z.; Ma, J.; Li, Y.; Qiu, Y.; Duan, H. Influence of climate extremes on long-term changes in cyanobacterial blooms in a eutrophic and shallow lake. Sci. Total Environ. 2024, 939, 173601. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhang, M.; Yin, J. Composition and influential factors of phytoplankton function groups in Lake Chaohu. J. Lake Sci. 2018, 30, 431–440. (In Chinese) [Google Scholar] [CrossRef]
- Guo, N.C.; Ma, Y.H.; Li, K.; Hu, J.X.; Zhang, Z.; He, J.L. Phytoplankton community in the ecological interception ditch approach to the eastern Lake Chaohu. J. Lake Sci. 2014, 26, 277–287. (In Chinese) [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Duan, L.; Liu, Q.; Li, D.; Huang, J.; Fu, J.; Zi, L.; Xu, T. Spatiotemporal variations in water quality parameters and assessment of the current status and challenges of eutrophication in Lake Dian. Ecol. Indic. 2025, 177, 113821. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Cai, H.; Zhang, S. Spatio-temporal heterogeneities in water quality and their potential drivers in Lake Chaohu (China) from 2001 to 2017. Ecohydrology 2021, 14, e2333. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Zhu, C.; Tang, P.; Yang, X.R.; Wang, H.; Zhang, F.H. Correlation analysis of phytoplankton community and water quality factors in Chaohu Lake. J. Biol. 2020, 40, 79–84. (In Chinese) [Google Scholar] [CrossRef]
- Cui, W.J.; Guo, N.C.; Li, J. Distribution and influencing factors of phytoplankton functional groups in the estuary and pelagic area of Chaohu Lake. J. Anhui Agric. Univ. 2022, 40, 979–989. [Google Scholar] [CrossRef]
- Xie, C.; Dai, B.; Wu, J.; Liu, Y.; Jiang, Z. Initial recovery of fish faunas following the implementation of pen-culture and fishing bans in floodplain lakes along the Yangtze River. J. Environ. Manag. 2022, 319, 115743. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Xu, C.; Sun, Y.; Shi, L.; Zhou, L.; Jeppesen, E.; Chen, J.; Xie, P. Can the “10-year fishing ban” rescue biodiversity of the Yangtze River? The Innovation 2022, 3, 100235. [Google Scholar] [CrossRef]
- Yi, C.; Guo, L.; Ni, L.; Luo, C. Silver carp exhibited an enhanced ability of biomanipulation to control cyanobacteria bloom compared to bighead carp in hypereutrophic Lake Taihu mesocosms. Ecol. Eng. 2016, 89, 7–13. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F. Are zooplankton useful indicators of water quality in subtropical lakes with high human impacts? Ecol. Indic. 2020, 113, 106167. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Q.; Wang, J.; Wei, H.; Qian, J.; Zhang, Y.; Feng, K.; Chen, Q.; Yuan, J.; Liu, J.; et al. Evaluation of the control effect of bighead carp and silver carp on cyanobacterial blooms based on the analysis of differences in algal digestion processes. J. Clean. Prod. 2022, 375, 134106. [Google Scholar] [CrossRef]
- Song, T.; Liu, G.; Zhang, H.; Yan, F.; Fu, Y.; Zhang, J. Lake Cyanobacterial Bloom Color Recognition and Spatiotemporal Monitoring with Google Earth Engine and the Forel-Ule Index. Remote Sens. 2023, 15, 3541. [Google Scholar] [CrossRef]
- Zhang, T.S.; Liu, L.G.; Luo, C.Q.; Xie, X.; Sun, Y.D. Overview of fish resource studies in the Yangtze River Basin before and at the beginning of the ten-year fishing ban. J. Hunan Univ. Arts Sci. (Sci. Technol.) 2025, 37, 64–72. (In Chinese) [Google Scholar] [CrossRef]
- Fang, D.-a.; Sun, H.; Peng, Y.; Kuang, Z.; Zhou, Y.; Xu, D. Living Status and Perspective of the Silver Carp (Hypophthalmichthys molitrix) in the Lower Reach of the Yangtze River: Insights from Population Distribution, Age Structure, and Habitat Preference Analyses. Fishes 2022, 7, 254. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Wang, J.; Wang, J.X.L. Seasonal dependency of controlling factors on the phytoplankton production in Taihu Lake, China. J. Environ. Sci. 2019, 76, 278–288. [Google Scholar] [CrossRef]
- Spears, B.M.; Carvalho, L.; Dudley, B.; May, L. Variation in chlorophyll a to total phosphorus ratio across 94 UK and Irish lakes: Implications for lake management. J. Environ. Manag. 2013, 115, 287–294. [Google Scholar] [CrossRef]
- Huo, S.; Ma, C.; Xi, B.; Gao, R.; Deng, X.; Jiang, T.; He, Z.; Su, J.; Wu, F.; Liu, H. Lake ecoregions and nutrient criteria development in China. Ecol. Indic. 2014, 46, 1–10. [Google Scholar] [CrossRef]
- Shan, K.; Song, L.R.; Chen, W.; Li, L.; Liu, L.M.; Wu, Y.L.; Jia, Y.L.; Zhou, Q.C.; Peng, L. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef]
- Li, J.; Chen, F.Z.; Liu, Z.W.; Zhao, X.X.; Yang, K.; Lu, W.X.; Cui, K. Bottom-up versus top-down effects on ciliate community composition in four eutrophic lakes (China). Eur. J. Protistol. 2016, 53, 20–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.