Abstract
Climate change and global warming are deeply impacting natural foraging dependent upon rain fall. To understand how xerophytes cope with these dramatic changes, comparative transcriptomic profiling of Atriplex halimus and Atriplex leucoclada was investigated under drought stress. The data revealed both shared and species-specific adaptive mechanisms. Differentially expressed genes (DEGs) clustered into major conserved gene families, including stress signaling, transcriptional regulation, antioxidant defense, metabolism, transport, and hormone signaling. In A. halimus, drought tolerance was characterized by strong transcriptional regulation, redox balance, and energy homeostasis, highlighted by the up-regulation of WRKY, MYB, and SET-domain transcription factors, calcium transporters, SnRK1 kinases, and stress-protective proteins such as HSPs and LEAs. On the other hand, A. leucoclada exhibited broader signaling flexibility and structural reinforcement through enrichment of MAPKs, CDPKs, 14-3-3 proteins, and cell wall-modifying enzymes (XTHs, expansins, chitinase-like proteins), as well as high expression of transporters and hormone-responsive genes. Such patterns indicated distinct drought adaptation strategies: A. halimus relied on rapid transcriptional and redox adjustments suited for fluctuating moisture regimes, while A. leucoclada employed multi-layered, constitutive defenses for persistent arid conditions. Together, these results elucidate complementary molecular strategies enabling ecological divergence and drought resilience among closely related halophytes.
1. Introduction
According to the 2020 FAO report, 11% and 14% of worldwide rainfed cropland and pastureland (1.3 × 106 ha and 6.6 × 106 ha), respectively, are negatively affected by drought [1], with climate change as the major contributing factor. Drought is a major limitation to the development and production of many crop plants, such as tomato [2], wheat [3], and olive [4]. Plant species exhibit a wide range of drought tolerance and can be classified into five broad categories: sensitive, low, moderate, high, and extreme (xerophytes). Xerophytes are among the most drought-tolerant plants and include halophytic species such as Atriplex, e.g., A. leucoclada [5] and A. canescens [6], which can grow with as little as 100 mm of annual rainfall [7].
Differentially expressed genes (DEGs) have been characterized in several crops [2,3,4] in order to understand their unique and shared strategies for drought mitigation. The activation of stress-responsive genes initiates signaling cascades that lead to biochemical, physiological, and morphological adjustments involved in adaptation [8,9]. Transcriptome profiling of key regulatory genes has become an important tool for dissecting plant responses to abiotic stress [4]. These genes include transcription factors, stress sensors, and protein kinases that regulate pathways involved in osmotic adjustment, antioxidant defense against reactive oxygen species (ROS), hormonal signaling (ABA, JA, and SA), bZIP and AP2/ERF transcription factors, LEA proteins, heat shock proteins, Ca2+ signaling, membrane stabilization, and C/N ratio regulation [2,3,4,10,11,12,13].
Mediterranean saltbush (A. halimus L.) and orache (A. leucoclada Boiss.) are two important xerophytic fodder plants traditionally used in arid and semi-arid regions due to their high protein content, nutritional value, and ability to thrive under drought and salinity stress [5,8,14,15,16,17]. Both species are perennial C4 shrubs; however, A. halimus is widely distributed around the Mediterranean basin and is valued for its higher biomass and protein yield, whereas A. leucoclada is native to the Fertile Crescent and Arabian Peninsula and is considered more drought adapted, although it remains less widely studied [5,15,18,19].
Despite their ecological and agricultural importance as stress-tolerant fodder plants, molecular-level studies on drought-response regulation in these species remain scarce. Therefore, the aim of the present study was to conduct a comparative transcriptomic analysis of A. halimus and A. leucoclada under short-term drought stress. This approach is expected to identify novel DEGs and regulatory components that may contribute to drought adaptation. While the primary purpose is to understand drought-responsive genes in Atriplex species, the resulting data are expected to provide novel genetic resources that could inform breeding strategies for improving stress resilience in related cultivated crops.
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Stress Treatment and Physiological Parameters
Two Atriplex species, Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.), were used in this study, conducted under greenhouse conditions at the Agricultural Research Station in Jubaiha, University of Jordan. The seeds of both species were sown in small pots (100 mL) with “peat moss” under greenhouse conditions, with diurnal temperatures of 25 ± 1 °C (day) and 29 ± 1 °C (night). One-month-old seedlings were transplanted into soil in larger pots (10 L) with 1:1:1 soil:peat moss:sand in the same greenhouse. The soil EC was around 2 dS m−1. Fertilization was added after one week of transplanting (NPK 20:20:20). Seedlings were irrigated every other day with 500 mL tap water (EC around 0.5 dS m−1).
Four-month-old plants were used for the onset of the experiment. Drought stress treatment was applied to stress plants for both species (5 pots, each a replicate) by withholding irrigation for two consecutive weeks, while control plants (another 5 pots, each a replicate) remained under irrigation as previously indicated.
For physiological parameters, relative water content (RWC), leaf dry weight percentage, shoot dry weight percentage and root dry weight percentage were measured for all five replicates for drought and for control plants. RWC was calculated for leaves immersed overnight in distilled water for turgid weight, dried at 65 °C for 3 days, as fresh weight–dry weight/turgid weight–dry weight. Dry weight percentage was calculated as (dry weight/fresh weight) after oven drying at 65 °C for 3 days.
2.2. RNA Isolation and cDNA Preparation
After completion of the drought stress treatment, leaves from the two species were collected from both control and stressed plants. For each treatment and each species, three biological replicates were taken randomly from the five replicates used for the physiological parameters above. Total RNA from leaves was isolated using the RNeasy Plant Midi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. All materials were treated with RNase Away (RNase Away, Molecular Bio Products, San Diego, CA, USA) to avoid the degradation of RNA by RNase. The RNA (5 μg) was used for the subsequent preparation of cDNA for each sample using the SMARTer cDNA Synthesis kit (Clontech, San Jose, CA, USA). The reaction was performed in 0.2 mL nuclease-free PCR tubes (Axgen, Stanford, CA, USA) according to the manufacturer’s instructions. The tubes were placed in a thermal cycler (Veriti 96-well; Applied Biosystems, Singapore, Singapore) according to the manufacturer’s protocol.
2.3. RNA Sequencing and Data Analysis of Transcriptome
High-throughput RNA sequencing was essential for obtaining a comprehensive, unbiased profile of transcript abundance in both Atriplex species under drought and control conditions. NGS allows detection of thousands of differentially expressed genes, including novel transcripts and low-abundance regulatory genes, which cannot be reliably captured using targeted methods, such as qPCR or microarrays.
The RNA sequencing was performed at Macrogen (Seoul, Republic of Korea). All samples were bar-coded using dedicated primer adapters (Illumina, San Diego, CA, USA). The amplified and coded libraries were run as paired-end reads with 101 cycles in GAIIx (Illumina, San Diego, CA, USA). The RNA sequencing was analyzed using CLC Genomics Workbench v. 9.1. The RNA-seq analysis was performed in several steps.
First, all A. halimus reads were de novo assembled (200 cut off for contig lengths). Assembled contigs for the new ESTs were annotated using Blast2Go software [20]. The annotated file was used as our reference sequence for Atriplex spp. Second, all reads of RNA sequencing and the reference cDNA, with their annotation, were imported into the program. The 92 bp reads were mapped with the reference Atriplex spp. cDS with the mapping set, as described; up to three mismatches were allowed. The minimum length of the fraction was 0.9, the minimum similarity fraction was 0.8, and the maximum number of hits for a read was 10.
The expression levels based on the number of mapped reads for the A. leucoclada (A.l.) transcriptome under drought stress and control conditions (A.l. d/c) were compared with each other, as were those of the A. halimus (A.h.) transcriptome under drought stress and control conditions (A.h. d/c). In addition, the expression levels of A.l. and A.h. transcriptomes under the drought condition were also compared (A.l._d/A.h._d). The majority of reads could be mapped uniquely to one location within the Atriplex spp. reference (cDNA) sequence. To normalize for sequencing depth and gene length, reads per kilobase of exon model per million mapped reads (RPKM) were calculated. DEGs with a p-value ≤ 0.05 were selected. CLC Genomics Workbench (Version 9.5) uses Student’s t-test as a statistical model to calculate the p-values for each DEG for two or more conditions. In addition, the software automatically adjusts the p-values using the Benjamini–Hochberg method to control the false discovery rate (FDR).
2.4. Estimation of Gene Expression Level Using qPCR
Following transcriptome analysis, several DEGs were randomly selected for independent validation. Gene-specific qPCR primers were designed for each selected transcript using Vector NTI 10 (Invitrogen, Carlsbad, CA, USA). Primer parameters were set as follows: length 22–28 bp, Tm 58–60 °C, GC content ~60%, amplicon size 150–200 bp, and a CG clamp at the 3′ end (Supplementary Table S1).
Total RNA from control and drought-treated samples was diluted to a final concentration of 10 ng μL−1 and used for first-strand cDNA synthesis with the GoScript Reverse Transcription System (Promega, Madison, WI, USA), following the manufacturer’s protocol. cDNA was synthesized in multiple reactions to produce a sufficient template for all qPCR assays.
Quantitative PCR was performed in 96-well plates (Applied Biosystems) using the QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany). All reactions were run in triplicate (technical replicates) for each of the three biological replicates. Melting-curve analysis was conducted after each run to verify specificity and to exclude primer dimer formation.
Relative expression levels were calculated using the 2−ΔΔCt method [21]. For each sample, the Ct of the target gene was normalized to the Ct of the internal reference gene actin (ΔCt = Ct_target − Ct_actin). Actin was selected because it displayed stable Ct values across all treatments (variation < 0.5 cycles). Fold changes were obtained by comparing ΔCt values of drought-treated samples with their corresponding controls (ΔΔCt).
3. Results
The assessed physiological parameters showed a dramatic effect of drought stress over both species as compared to the control (Figure 1). RWC dramatically dropped for both A. halimus and A. leucoclada after drought stress (Figure 1A). On the other hand, this was reflected by increased dry weight percentage for leaves (Figure 1B), shoots (Figure 1C) and roots (Figure 1D).
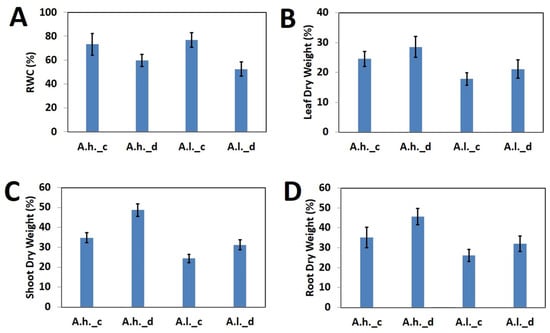
Figure 1.
Physiological parameters for Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) under drought stress (_d) and the control (_c). (A) RWC. (B) Leaf dry weight percentage. (C) Shoot dry weight percentage. (D) Root dry weight percentage. Bars represent SD.
RNA-seq libraries were generated for both A. leucoclada (A.l.) and A. halimus (A.h.) under control (_c) and drought (_d) conditions. Across three biological replicates per treatment, each library produced approximately 60 million reads. Following quality filtering, approximately 5% of reads required trimming to remove low-quality ends, and ambiguous nucleotides represented <1% of all bases, indicating high sequencing quality.
Principal component analysis (PCA) showed tight clustering of biological replicates within each treatment group (Supplementary Figure S1).Differentially expressed genes (DEGs) were identified using a significance threshold of p ≤ 0.05 (Figure 2), enabling the characterization of transcriptional responses to drought in both Atriplex species.
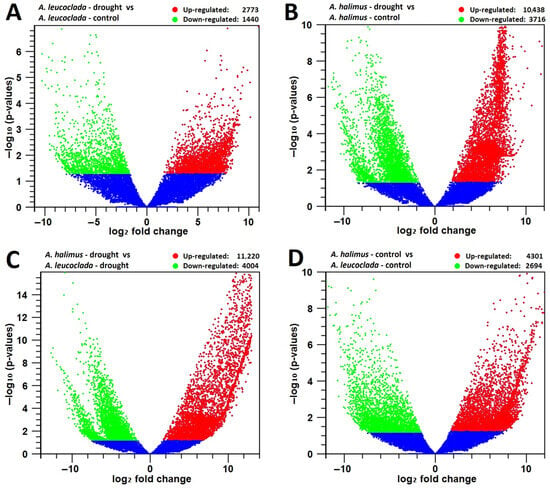
Figure 2.
Global analysis of differentially expressed genes (DEGs) with fold change ≥ 2 in transcriptome. (A) Volcano plot of DEGs for A. leucoclada (drought) and A. leucoclada (control). (B) Volcano plot of DEGs for A. halimus (drought) and A. halimus (control). (C) Volcano plot of DEGs for A. halimus (drought) and A. leucoclada (drought). (D) Volcano plot of DEGs for A. halimus (control) and A. leucoclada (control). The abscissa shows the fold change difference in the expression of genes in different comparison groups, and the vertical coordinates indicate the adjusted p-values for the differences in expression. Genes without significant differences (p value ≤ 0.05) are indicated by blue dots below the threshold value (1.3). The up-regulated genes are represented by red dots, and the down-regulated genes are represented by green dots.
In the case of the A.l._d vs. A.l._c pair comparison and with a threshold of Log2FC ≥ 2, a total of 4213 DEGs were identified: 2773 with up-regulated expression and 1440 with down-regulated expression (Figure 2A). On the other hand, for the A.h._d vs. A.h._c pair comparison and with a threshold of Log2FC ≥ 2, a total of 14,154 DEGs were identified; 10,438 with up-regulated expression and 3716 with down-regulated expression (Figure 2B). However, when comparing the two species under drought stress, for the A.h._d vs. A.l._d pair comparison and with a threshold of Log2FC ≥ 2, a total of 15,224 DEGs were identified: 11,220 with up-regulated expression and 4004 with down-regulated expression (Figure 2C). With similar comparison of the two species under control conditions, for the A.h._c vs. A.l._c pair comparison and with a threshold of Log2FC ≥ 2, a total of 6995 DEGs were identified: 4301 with up-regulated expression and 2694 with down-regulated expression (Figure 2D). qPCR data confirm the RNAseq data, as they showed similar trends in gene expression (Supplementary Figure S2).
A representative group of Venn diagrams were compiled for major possible combinations of Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) under drought stress relative to the control (as baseline) at a cutoff of fold changes ≥ 2, 4, 6 and 8 for up-regulated DEGs (Figure 3A–D). A.l. showed an up-regulation of 6328, 5115, 2727 and 482 unique DEGs with fold changes ≥ 2, 4, 6 and 8, respectively. With the same baseline (control), unique DEGs were also evident for A.h., showing an up-regulation of 17,583, 8228, 733 and 24 unique DEGs with fold changes ≥ 2, 4, 6 and 8, respectively.
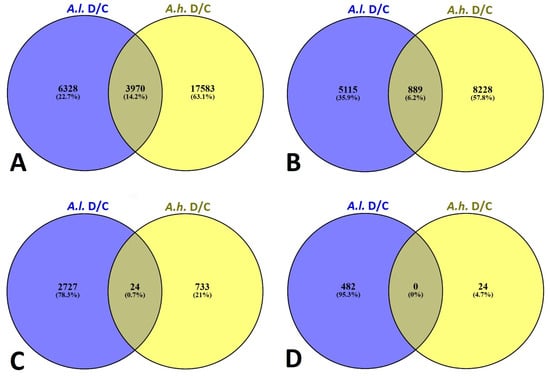
Figure 3.
Venn diagrams showing the overlap of differentially expressed genes (DEGs) between Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) under drought stress relative to the control (D/C). Four thresholds of fold-change are shown: (A) ≥2-fold, (B) ≥4-fold, (C) ≥6-fold, and (D) ≥8-fold. Numbers inside circles: the count of DEGs unique to each species or shared between species. Numbers in parentheses: percentage of DEGs relative to the total DEGs in each species. Intersection (overlap) area: DEGs that are common to both species at the indicated fold-change threshold.
Because A.l. is more tolerant to drought compared with A.h., the number of drought-responsive genes are much higher in A.h. to mitigate the drought stress. Nonetheless, major common drought responsive genes were also evident in the overlap section between the two species (Figure 3A–D), showing 3970, 889, 24 and 0 common DEGs between the two species under drought stress with fold changes ≥ 2, 4, 6 and 8, respectively.
Several thousand DEGs during drought stress showed significant up-regulation or down-regulation (p-value < 0.01, fold change > 2 or <0.5). On the basis of similar kinetic patterns of expression, all DEGs were classified into unique gene expression patterns (clusters) (Figure 4). In our dataset, the optimal number of distinct expression profiles produced by the algorithm was 15. Even if some patterns appear visually close (such as clusters 11 and 12), their underlying expression values differ sufficiently for the algorithm to classify them as unique groups.
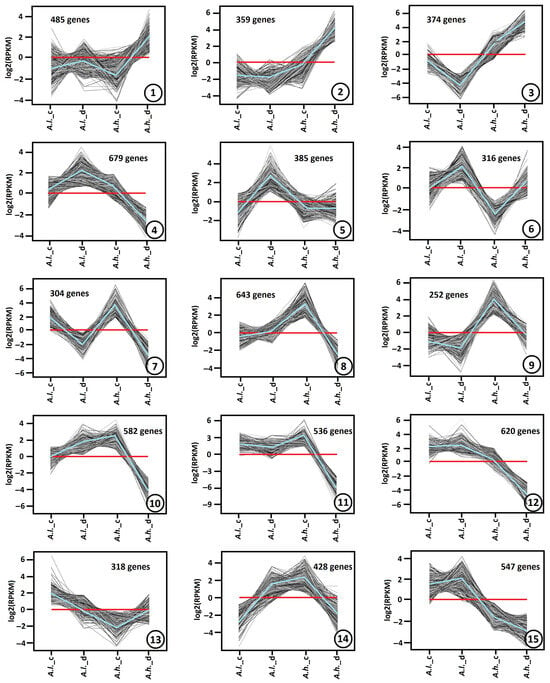
Figure 4.
Gene expression patterns obtained by hierarchical clustering. Differentially expressed genes (DEGs) in Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) were categorized into 15 clusters (cluster number is depicted within a circle inside each panel). Gray lines show the relative expression levels of DEGs in the cluster under both control (_c) and drought (_d) conditions. Blue lines show the average values for each relative expression cluster. Red lines represent the baseline. Expression levels were represented along the y axis as log2(RPKM), and species were represented along the x axis.
Differentially expressed genes (DEGs) of Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) species under both control (_c) and drought stress (_d) conditions were used to reveal these 15 clusters. Each cluster was built from a large number of DEGs, ranging from 252 up to 679 genes.
The gene expression patterns of cluster 1 (485 DEGs), cluster 2 (359 DEGs) and cluster 3 (374 DEGs) exhibit similar changes, where DEGs are up-regulated mainly in A.h. (under drought stress). The major difference between them is the fold change, which is minimal in cluster 1, moderate in cluster 2 and prominent in cluster 3 (Figure 3). In addition, it was deeply down-regulated in A.l. (under drought stress) in cluster 3. On the contrary, cluster 4 (679 DEGs), cluster 5 (385 DEGs) and cluster 6 (316 DEGs) show the opposite pattern for A.l., where DEGs are up-regulated in A.l. compared to A.h. (under drought stress). However, the major difference between them is the expression level in A.h. (under drought stress), which is down-regulated in cluster 4, flat in cluster 5 and slightly up-regulated in cluster 6.
All DEGs in the first six clusters (Figure 5) were retrieved from the RNA-seq data along with their annotation. Their expression (FRKM calibration) were subjected to hierarchal clustering for Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) under both control (_c) and drought (_d) conditions, and their expression levels are presented as a heat map (Figure 5A–F). It was clear from the heat maps for clusters 1, 2 and 3 (Figure 5A–C) that most DEGs were more up-regulated in A.h. (under drought stress), indicating unique gene expression mainly under drought stress in A. halimus in comparison to its control counterpart and to A. leucoclada.
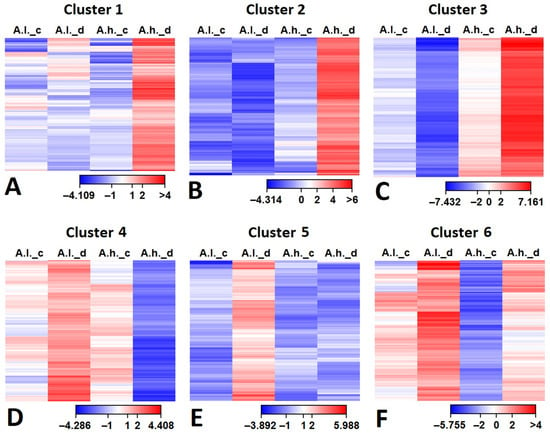
Figure 5.
Hierarchical clustering of differentially expressed genes (DEGs) of clusters 1–6 (Figure 3) in Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) under both control (_c) and drought (_d) conditions: (A) Cluster 1, (B) Cluster 2, (C) Cluster 3, (D) Cluster 4, (E) Cluster 5, (F) Cluster 6.
Extensive tables covering major gene families were compiled (Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6). They contain essential information generated from the RNA-seq analysis, including differential expression results, annotations, and pathway details. These datasets are crucial for transparency and reproducibility in transcriptomics research.

Table 1.
Major genes families along with gene description and their expression level (log2(RPKM)) in Atriplex halimus (A.h.) under drought stress condition for cluster 1 DEGs (Figure 3).

Table 2.
Major gene families along with gene description and their expression level (log2(RPKM)) in Atriplex halimus (A.h.) under drought stress condition for cluster 2 DEGs (Figure 3).

Table 3.
Major gene families along with gene description and their expression level (log2(RPKM)) in Atriplex halimus (A.h.) under drought stress condition for cluster 3 DEGs (Figure 3).

Table 4.
Major gene families along with gene description and their expression level (log2(RPKM)) in Atriplex leucoclada (A.l.) under drought stress condition for cluster 4 DEGs (Figure 3).

Table 5.
Major gene families along with gene description and their expression level (log2(RPKM)) in Atriplex leucoclada (A.l.) under drought stress condition for cluster 5 DEGs (Figure 3).

Table 6.
Major gene families along with gene description and their expression level (log2(RPKM)) in Atriplex leucoclada (A.l.) under drought stress condition for cluster 6 DEGs (Figure 3).
When comparing DEGs between the three clusters (1, 2 and 3) in Atriplex halimus (A.h.) (Table 1, Table 2 and Table 3), four common gene families were prominent: transcriptional regulation, protective proteins and enzymes, metabolism and osmotic adjustment, and finally hormone signaling. While transporters were common between clusters 1 and 2 (Table 1 and Table 2), stress perception and signaling were common between clusters 1 and 3 (Table 1 and Table 3), while photosynthesis and energy balance were common between clusters 2 and 3 (Table 2 and Table 3). Major DEGs with high expression in cluster 1 included F-box protein (2.83 fold) and WRKY TF (3.47 fold) (Table 1). In cluster 2, highly expressed genes included zinc knuckle family protein (3.89 fold) and cation calcium exchanger 4 (CAX4) (4.59 fold) (Table 2). In cluster 3, top up-regulated genes were recorded for calcineurin-like phosphoesterase-like protein (5.85 fold) and ARM repeat protein interacting with ABF2 (ARIA) (6.21) (Table 3).
On the other hand, when comparing DEGs between the three clusters (4, 5 and 6) in Atriplex leucoclada (A.l.) (Table 4, Table 5 and Table 6), six common gene families were prominent: stress perception and signaling, transcriptional regulation, protective proteins and enzymes, metabolism and osmotic adjustment and finally transporters and hormone signaling, while cell wall structural proteins were common between clusters 4 and 5 (Table 4 and Table 5). Major DEGs with high expression in cluster 4 included casein kinase I (4.41 fold) and JAZ-like protein (2.49 fold) (Table 4). In cluster 5, highly expressed genes included Leucine-rich repeat receptor-like kinase (LRR-RLK) (3.55 fold) and Sodium bile acid cotransporter 7-like (BASS, SLC10-like) (2.26 fold) (Table 5). In cluster 6, top up-regulated genes were recorded for calmodulin-binding (4.12 fold) and protein Clp1 homolog (chaperone) (4.07 fold) (Table 6).
4. Discussion
Comparative transcriptomic analysis was performed for Atriplex halimus and Atriplex leucoclada under drought stress. The physiological parameters clearly indicate the onset of the drought stress, revealed by reduced RWC and increased dry weight percentage of all plant organs. The data revealed distinct yet overlapping molecular strategies underpinning drought tolerance. The DEGs across six major co-expression clusters (Figure 5A–F) fell into major conserved functional gene families: stress perception and signaling, transcriptional regulation, protective proteins and enzymes, metabolism and osmotic adjustment, transporters, and hormone signaling. These are consistent with drought adaptation mechanisms reported in other xerophytes [22,23,24,25,26].
4.1. Atriplex Halimus: Transcriptional Regulation and Redox Balance
Clusters 1–3 of A. halimus showed a strong enrichment for transcriptional regulators (WRKY, MYB, ARID, and ARM-repeat TFs), ubiquitin ligases, and protective enzymes (HSPs, LEAs, aldose reductase, and SOD). These genes are central to reprogramming stress-responsive gene expression and mitigating oxidative stress, similar to responses seen in Suaeda salsa and Salicornia europaea under drought or salt stress [27]. The up-regulation of WRKY and SET-domain proteins suggests chromatin remodeling and activation of downstream defense pathways [28,29,30].
The presence of CAX4 and other Ca2+ transporters in cluster 2 supports calcium-mediated signaling, crucial for osmotic adjustment and activation of stress kinases [31,32]. Concurrently, calcineurin-like phosphoesterases and SnRK1 kinases in cluster 3 indicate strong regulation of energy homeostasis and metabolic reprogramming under limited water availability [33]. The co-expression of Jasmonate-induced proteins (JIP) and ethylene-related ETO1 suggests hormone cross-talk controlling adaptive growth suppression and antioxidant defense [34,35].
4.2. Atriplex Leucoclada: Signaling Flexibility and Cell Wall Remodeling
In A. leucoclada, clusters 4–6 demonstrated a broader integration of signaling, transport, and cell wall remodeling genes, aligning this species with known higher drought resilience [36,37,38]. The presence of casein kinase I, CDPKs, and MAPKs reflects a dynamic phosphorylation-based signaling network enabling rapid perception and transmission of drought signals [39,40]. Enrichment of 14-3-3 proteins, CBL–CIPK modules, and SnRK1 kinases would suggest regulation of ion homeostasis and carbon metabolism, reflecting tangible drought-acclimation networks [41].
Cell wall structural proteins (XTHs, expansins, chitinase-like proteins) were specific to clusters 4 and 5, supporting enhanced cell wall flexibility and growth recovery post-stress [42], which is consistent with findings in rice [43] and sugarcane [44]. Leucine-rich receptor-like kinases (LRR-RLKs) and BASS transporters imply intercellular communication and metabolite transport critical for osmotic regulation. The presence of ABA-, JA-, and auxin-related genes across clusters further highlights hormonal integration in coordinating defense, stomatal regulation, and developmental plasticity [45].
The high expression of heat shock proteins, thioredoxin systems, and aldehyde dehydrogenases in cluster 6 points to a sustained antioxidant and detoxification capacity, which are essential in desert-adapted xero-halophytes [46]. Similar findings were also recorded in other crops, such as pepper [47] and rye grass [48]. Furthermore, enhanced sugar transporters, sucrose phosphate synthase, and ABC transporters would reinforce metabolic adjustment through osmolyte accumulation and vacuolar sequestration [49].
4.3. Comparative Insights
Comparative transcriptomic analysis between A. halimus and A. leucoclada highlights divergent yet complementary drought-adaptation strategies. The two species, though phylogenetically close, exhibited key differences in the balance between transcriptional control and structural or signaling plasticity. A. halimus showed a stronger enrichment of transcriptional regulators and chromatin modifiers such as WRKY, MYB, SET-domain proteins, and DNA methyltransferases, which are central in early stress perception and long-term transcriptional reprogramming [50,51,52]. This pattern indicates a “regulatory-centric” drought response, where rapid activation and repression of downstream defense pathways allow energy-efficient modulation of stress responses. Similar transcriptional prioritization has been reported in other Mediterranean xerohalophytes such as Haloxylon ammodendron [53] and Salsola spp. [54], where early expression of transcriptional and epigenetic modulators fine-tunes drought tolerance without extensive metabolic remodeling.
In contrast, A. leucoclada exhibited a broader transcriptional activation across cell wall structural proteins, transporters, and signaling kinases, suggesting a “multi-layered adaptive” mechanism. The presence of expansins, XTHs, and chitinase-like proteins (clusters 4 and 5) would indicate active remodeling of the cell wall architecture, an essential process in maintaining elasticity and prevent cellular collapse under water deficit [55]. This structural reinforcement is complemented by up-regulated expression of MAPKs, CDPKs, and SnRK1 kinases, which regulate cellular homeostasis and energy sensing [33,56]. Moreover, A. leucoclada plants under drought stress showed up-regulation of transporters (ABC, CAX, and aquaporins) and hormone signaling genes (ABA- and JA-responsive), supporting their exceptional ability to regulate ion flux and osmotic balance, traits that have been associated with enhanced water use efficiency in other xerophytes and halophytes, like Salicornia and Suaeda salsa [46].
These interspecific differences presumably reflect ecological adaptation to their native habitats. A. halimus, which typically grows in saline regions, appears to rely on transcriptional flexibility and redox protection. This strategy would be suitable for environments with periodic drought and recovery cycles. In contrast, A. leucoclada, which is native to harsher arid zones with persistent water deficit, employs a constitutive structural and signaling defense network, enabling it to maintain growth and osmotic balance over longer stress durations. This aligns with reported physiological studies [55,56], where resilient physiological indicators were recorded under drought stress. The observed expression of cell wall and kinase networks in A. leucoclada may represent evolutionary modification for sustained drought resilience, whereas A. halimus exhibits greater plasticity, optimizing gene regulation for fluctuating conditions. Together, these complementary strategies demonstrate how closely related halophytes partition their molecular responses to cope with contrasting drought intensities.
5. Conclusions
Comparative transcriptome analysis between Atriplex halimus and Atriplex leucoclada revealed distinct molecular responses to drought stress. A. halimus showed greater transcriptional flexibility and redox modulation, whereas A. leucoclada emphasized cell-wall remodeling, kinase signaling, and osmotic regulation. These contrasting patterns may be consistent with species-specific divergence in drought-response strategies, but further phylogenetic or population-level analyses would be required to infer evolutionary mechanisms. Overall, the results highlight transcriptional regulation and structural adaptability as complementary drought-tolerance paradigms in xerophytic species and provide candidate genes for improving stress resilience in related crops
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d18010010/s1. Table S1: Sequence of primers for selected drought stress responsive genes in Atriplex spp. Figure S1: PCA for the biological replicates of RNA-seq data in Atriplex leucoclada (A.l.) and Atriplex halimus (A.h.) under control (_c) or under drought stress (_d) conditions. Figure S2: RNA-seq validation by quantitative real-time PCR (qRT-PCR). Each graph contains the relative expression level of selected genes in (A) Atriplex leucoclada. (B) Atriplex halimus. Bars represent data from qRT-PCR, while lines indicate data from RNA-seq.
Author Contributions
Conceptualization, M.B. and M.A.A.; methodology, A.K.K. and S.D.A.; software, B.A.; validation, H.R.H.; formal analysis, F.A.B.K.; investigation, H.A.-S.; resources, M.M.A.-Z.; data curation, A.M.; writing—original draft preparation, all authors; writing—review and editing, M.T.S.; visualization, supervision, M.K.A.-R.; project administration, M.T.S.; funding acquisition, S.A.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Scientific Research and Innovation Support Fund, Jordan (AGR/1/24/2018).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the University of Jordan for support in conducting this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DEGs | Differentially expressed genes |
| A.h. | Atriplex halimus |
| A.l. | Atriplex leucoclada |
| ABA | Abscisic Acid |
| JA | Jasmonic Acid |
| ROS | reactive oxygen species |
| RWC | Relative Water Content |
References
- FAO. The State of Food and Agriculture 2020; Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Sadder, M.T.; Alsadon, A.; Wahb-Allah, M. Transcriptomic analysis of tomato lines reveals putative stress-specific biomarkers. Turk. J. Agric. For. 2014, 38, 700–715. [Google Scholar] [CrossRef]
- Sadder, M.T.; Musallam, A.; Allouzi, M.; Duwayri, M.A. Dehydration stress memory genes in Triticum turgidum L. ssp. durum (Desf.). BioTech 2022, 11, 43. [Google Scholar] [CrossRef]
- Alkhatatbeh, H.A.; Sadder, M.T.; Haddad, N.; Al-Amad, I.; Brake, M.; Alsakarneh, N.A.; Alnajjar, A.M. Transcriptome analysis of historic olives reveals stress-specific biomarkers. Front. Plant Sci. 2025, 16, 1549305. [Google Scholar] [CrossRef]
- Sadeghi, H.; Delaviz, M. Response of three new Atriplex species (Atriplex spp.) to drought and its recovery. Acta Ecol. Sin. 2016, 36, 212–217. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; He, Z.; Ma, D.; Sun, W.; Xu, X.; Tian, Q. Effects of drought stress on leaf functional traits and biomass characteristics of Atriplex canescens. Plants 2024, 13, 2006. [Google Scholar] [CrossRef] [PubMed]
- FAO. EcoCrop Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996–2025. Available online: https://gaez.fao.org/ (accessed on 14 September 2025).
- Sadder, M.T. Gene expression and physiological analysis of Atriplex halimus (L.) under salt stress. Aust. J. Crop Sci. 2013, 7, 112–118. [Google Scholar]
- Musallam, A.; Abu-Romman, S.; Sadder, M.T. Molecular Characterization of Dehydrin in Azraq Saltbush among Related Atriplex Species. BioTech 2023, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018; pp. 1–25. [Google Scholar]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Pamungkas, S.S.T.; Farid, N. Drought stress: Responses and mechanism in plants. Rev. Agric. Sci. 2022, 10, 168–185. [Google Scholar] [CrossRef]
- Nemat Alla, M.M.; Khedr, A.H.A.; Serag, M.M.; Abu-Alnaga, A.Z.; Nada, R.M. Physiological aspects of tolerance in Atriplex halimus L. to NaCl and drought. Acta Physiol. Plant. 2011, 33, 547–557. [Google Scholar] [CrossRef]
- Osmond, C.B.; Björkman, O.; Anderson, D.J. Physiological Processes in Plant Ecology: Toward a Synthesis with Atriplex; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 36. [Google Scholar]
- Walker, D.J.; Lutts, S.; Sánchez-García, M.; Correal, E. Atriplex halimus L.: Its biology and uses. J. Arid Environ. 2014, 100, 111–121. [Google Scholar] [CrossRef]
- Benzarti, M.; Ben Rejeb, K.; Debez, A.; Abdelly, C. Environmental and economical opportunities for the valorisation of the genus Atriplex: New insights. In Crop Improvement: New Approaches and Modern Techniques; Springer: Boston, MA, USA, 2013; pp. 441–457. [Google Scholar]
- Goodin, J.R. Atriplex as a forage crop for arid lands. In New Agricultural Crops; CRC Press: Boca Raton, FL, USA, 2019; pp. 133–148. [Google Scholar]
- Le Houérou, H.N. The role of saltbushes (Atriplex spp.) in arid land rehabilitation in the Mediterranean Basin: A review. Agrofor. Syst. 1992, 18, 107–148. [Google Scholar] [CrossRef]
- Alotibi, M.M.; AL-Huqail, A.A.; Ghoneim, A.M.; Eissa, M.A. Seasonal Variations in Yield and Biochemical Composition of the Mediterranean Saltbush (Atriplex halimus L.) Under Saline Agriculture in Semi-Arid Regions. J. Soil Sci. Plant Nutr. 2023, 23, 3834–3844. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Life Sci. 2018, 13, 149–154. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, Z.; Bao, J.; Wang, Y.; Li, J.; Wang, Q.; Tian, X. Transcriptomic study of Suaeda salsa in response to salt and drought stress. Funct. Plant Biol. 2023, 50, 765–776. [Google Scholar] [CrossRef]
- Song, Z.T.; Liu, J.X.; Han, J.J. Chromatin remodeling factors regulate environmental stress responses in plants. J. Integr. Plant Biol. 2021, 63, 438–450. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, M. Delineating the epigenetic regulation of heat and drought response in plants. Crit. Rev. Biotechnol. 2022, 42, 548–561. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Majee, M.; Datta, A. Chromatin-based transcriptional reprogramming in plants under abiotic stresses. Plants 2022, 11, 1449. [Google Scholar] [CrossRef]
- Ketehouli, T.; Nguyen Quoc, V.H.; Dong, J.; Do, H.; Li, X.; Wang, F. Overview of the roles of calcium sensors in plants’ response to osmotic stress signalling. Funct. Plant Biol. 2022, 49, 589–599. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and other regulators assists plants in responding to abiotic stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kong, F.; Yang, W. SnRK1 as the Core Node Integrating Energy Homoeostasis, Stress Adaptation and Hormonal Crosstalk in Plants. Plant Cell Environ. 2025, 48, 7830–7847. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, H.; Ahmed, W. Role of Plant Hormones Under Abiotic Stress Conditions. J. Adv. Nutr. Sci. Technol. 2022, 2, 14–24. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Burchardt, S.; Karwaszewski, J. Ethylene: A Powerful Coordinator of Drought Responses. In Ethylene in Plant Biology; Singh, S., Husain, T., Singh, V.P., Tripathi, D.K., Prasad, S.M., Dubey, N.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 82–108. [Google Scholar] [CrossRef]
- Nada, R.M.; Khedr, A.H.A.; Serag, M.S.; El-Qashlan, N.R.; Abogadallah, G.M. Molecular and physiological responses of naturally grown Atriplex halimus L. to drought-stress recovery in the absence or presence of Na+ ions under natural conditions. J. Plant Growth Regul. 2022, 41, 1578–1593. [Google Scholar] [CrossRef]
- Alam, H.; Zamin, M.; Adnan, M.; Ahmad, N.; Nawaz, T.; Saud, S.; Basir, A.; Liu, K.; Harrison, M.T.; Hassan, S.; et al. Evaluating the resistance mechanism of Atriplex leucoclada (Orache) to salt and water stress; A potential crop for biosaline agriculture. Front. Plant Sci. 2022, 13, 948736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, T.; Yin, B.; Wang, Z.; Li, R.; Li, S. The influence of sodium salt on growth, photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens under drought stress. Agronomy 2023, 13, 2434. [Google Scholar] [CrossRef]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef]
- Dekomah, S.D.; Bi, Z.; Dormatey, R.; Wang, Y.; Haider, F.U.; Sun, C.; Yao, P.; Bai, J. The role of CDPKs in plant development, nutrient and stress signaling. Front. Genet. 2022, 13, 996203. [Google Scholar] [CrossRef]
- Batistič, O.; Kudla, J. Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1283–1293. [Google Scholar] [CrossRef]
- Li, X.; Bao, J.; Wang, J.; Blackman, C.; Tissue, D. Antecedent drought condition affects responses of plant physiology and growth to drought and post-drought recovery. Front. For. Glob. Change 2021, 4, 704470. [Google Scholar] [CrossRef]
- Ganie, S.A.; Ahammed, G.J. Dynamics of cell wall structure and related genomic resources for drought tolerance in rice. Plant Cell Rep. 2021, 40, 437–459. [Google Scholar] [CrossRef]
- Narayan, J.A.; Chakravarthi, M.; Nerkar, G.; Manoj, V.M.; Dharshini, S.; Subramonian, N.; Premachandran, M.N.; Kumar, R.A.; Surendar, K.K.; Hemaprabha, G.; et al. Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind. Crops Prod. 2021, 159, 113035. [Google Scholar] [CrossRef]
- Mei, S.; Zhang, M.; Ye, J.; Du, J.; Jiang, Y.; Hu, Y. Auxin contributes to jasmonate-mediated regulation of abscisic acid signaling during seed germination in Arabidopsis. Plant Cell 2023, 35, 1110–1133. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Yang, B.; Shang, Y.; Wen, L.; Cui, Y.; Li, Z.; Cheng, Y.; Liu, C. Genome-wide identification of aldehyde dhydrogenase (ALDH) family genes in pepper (Capsicum annuum) reveals involvement of CaALDH7B1 in heat and drought stress tolerance. Plant Gene 2025, 43, 100524. [Google Scholar] [CrossRef]
- Rahman, M.A.; Woo, J.H.; Song, Y.; Lee, S.H.; Hasan, M.M.; Azad, M.A.K.; Lee, K.W. Heat shock proteins and antioxidant genes involved in heat combined with drought stress responses in perennial rye grass. Life 2022, 12, 1426. [Google Scholar] [CrossRef]
- Thomas, A.; Beena, R. Sucrose Metabolism in Plants under Drought Stress Condition: A Review. Indian J. Agric. Res. 2024, 58, 943. [Google Scholar] [CrossRef]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and mechanism of WRKY transcription factors in abiotic stress responses of plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Cruz De Carvalho, M.H.; Torres-Jerez, I.; Kang, Y.U.N.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; Udvardi, M.K. Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar]
- Sadhukhan, A.; Prasad, S.S.; Mitra, J.; Siddiqui, N.; Sahoo, L.; Kobayashi, Y.; Koyama, H. How do plants remember drought? Planta 2022, 256, 7. [Google Scholar] [CrossRef]
- Yang, F.; Lv, G. Combined analysis of transcriptome and metabolome reveals the molecular mechanism and candidate genes of Haloxylon drought tolerance. Front. Plant Sci. 2022, 13, 1020367. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gao, S.; Zhang, X.; Liu, G.; Chen, P.; Gao, X.; Yuan, L.; Tian, Y.; Li, D.; Zhang, X.; et al. Comparative Transcriptome Analysis Reveals Mechanisms of Differential Salinity Tolerance Between Suaeda glauca and Suaeda salsa. Genes 2024, 15, 1628. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-activated protein kinase cascades in plant hormone signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.