Abstract
The use of bioinoculants aligns with ecological intensification in agriculture, but their effects on crop performance and soil microbiota under different fertilization regimes remain unclear. This study evaluated the impact of a bioinoculant containing two phosphate-solubilizing bacterial strains (Priestia megaterium and Bacillus subtilis) on maize yield, root architecture, and rhizosphere microbial communities via seed inoculation in a clayey soil. Maize was cultivated for two consecutive seasons under treatments combining inoculation, phosphorus sources (triple superphosphate or reactive rock phosphate), and P doses (0 or 120 kg ha−1 of P2O5). Root traits, phosphatase activities, and microbial diversity were assessed at flowering, while agronomic parameters and nutrient content were measured at harvest. In the first season, microbial alpha diversity was higher, accompanied by a 31.5% increase in root surface area and a 46.2% increase in P-resin availability. In contrast, the second season showed greater phosphatase activity and higher grain P and K concentrations, by 42.3% and 38.2%, respectively. Grain yield did not differ significantly between inoculated and non-inoculated treatments; however, root, plant, and microbial traits varied markedly across seasons. Principal component analysis revealed that productivity was primarily driven by seasonal variation rather than by fertilization or inoculation. These findings emphasize that the effectiveness of bioinoculants and P fertilization, as well as their influence on the microbiota, are highly context-dependent, being shaped by environmental conditions, soil nutrient availability, and crop genotype.
1. Introduction
Maize is a globally important cereal crop [], with its demand rising due to its diverse applications []. However, one of the main challenges in expanding maize production, particularly in tropical regions such as Brazil, is the reliance on synthetic fertilizers to sustain yields, as phosphorus (P) availability remains a key limiting factor []. Modern agriculture relies heavily on more soluble synthetic P sources such as superphosphates and ammonium phosphates, which promote rapid uptake but often bind to soil particles, reducing long-term availability [,].
A more sustainable alternative involves using reactive natural phosphate sources, which are low in solubility but may become more available over time through microbial activity in the soil [,,,]. Microbial inoculants, including those containing phosphate-solubilizing bacteria (PSB), can enhance P availability and improve nutrient use efficiency in cropping systems [,,]. PSBs increase P availability by converting insoluble inorganic forms into plant-accessible forms via the production of organic acids and by mineralizing organic P through enzymes like phosphatases, phosphohydrolases, and phytases [,,,]. These microbes can associate with plants early in root development, acting in the rhizosphere to release P bound to iron and aluminum oxides, especially in acidic, clay-rich soils [,,]. Various P inoculants containing Bacillus and other PSBs are available on the global market; however, in Brazil, commercial inoculants formulated with native PSBs were not available until 2019, when BiomaPhos® (Simbiose Agro Cruz Alta, Brazil) was introduced. BiomaPhos® is a phosphate-solubilizing microbial consortium composed of two Bacillus strains: Priestia megaterium (former Bacillus megaterium) CNPMS B119 and Bacillus subtilis CNPMS B2084 []. Both strains are part of the Collection of Multifunctional Microorganisms and Phytopathogens (CMMF) of Embrapa Maize and Sorghum. P. megaterium was initially isolated from the rhizosphere, whereas B. subtilis was obtained from the leaf endosphere of a phosphorus-efficient tropical maize genotype [,]. These strains are capable of producing siderophores, acid and alkaline phosphatases, and mineralizing phytate. Additionally, they can produce extracellular polymeric substances (EPS), form biofilms, and possess limited biological nitrogen fixation capabilities. For both Bacillus strains, total organic acids for calcium phosphate solubilization were higher than for iron phosphate solubilization, and P. megaterium produced greater total organic acids than B. subtilis [,].
Despite over a century of use, the widespread adoption of PSB-based inoculants remains limited due to inconsistent field performance []. In tropical environments, adoption rates often stay below 10%, mainly reflecting variability in environmental responses and limited consistency across seasons [,]. Their effectiveness can vary depending on soil type, plant genotype, microbial strain, and a range of biotic and abiotic factors that affect microbial survival [,,,]. Additionally, interactions with other agricultural inputs may reduce their efficacy. To improve the success of microbial inoculants under field conditions, it is essential to identify beneficial microbial traits, assess compatibility with other inputs, evaluate their effects on native microbiota, and understand their persistence in the soil. Moreover, microbial diversity is increasingly recognized not only as a key driver of nutrient cycling but also as a reliable indicator of agroecosystem resilience and sustainability [,].
Nevertheless, the impact of PSB inoculation on the native rhizosphere microbial community and its associated ecological functions remains poorly understood. This knowledge gap is particularly critical in high-fertility soils, where excess legacy phosphorus may reduce plant responsiveness to microbial inoculants and influence microbial recruitment patterns. In this context, the present study aimed to investigate the effects of PSB inoculation with P. megaterium CNPMS B119 and B. subtilis CNPMS B2084 on rhizosphere microbiota diversity and composition, as well as on root system architecture, in maize cultivated under different phosphorus fertilization regimes in a clayey soil with high P availability. Additionally, temporal dynamics of the microbial community were assessed across consecutive seasons. By elucidating context-dependent microbial responses, this study advances the understanding of PSB performance under tropical field conditions. It contributes to the development of targeted, sustainable phosphorus management strategies within precision agriculture frameworks.
2. Materials and Methods
2.1. Experimental Site, Design, and Cultivation Conditions
Field experiments were conducted at the Embrapa Maize and Sorghum Research Station, Sete Lagoas, Minas Gerais, Brazil (19°28′ S, 44°15′ W; 732 m elevation). The site features a dystrophic Red Latosol-Oxisol (clayey texture: 66% clay, 11% sand, 23% silt) under native Cerrado vegetation. Before maize planting in 2019, the area was fallow but retained high fertility from previous experiments, characterizing it as a site with constructed fertility.
Soil samples (0–20 cm) were collected in December 2019 and analyzed by the Soil Laboratory of the Federal University of Viçosa (Viçosa, Brazil). The physico-chemical analyses included pH measured in water (H2O), potassium chloride (KCl), and calcium chloride (CaCl2) solution (1:2.5); potential acidity (H + Al) in Ca-acetate soil extract (0.5 mol L−1, pH 7.0); phosphorus (P), potassium (K), and iron (Fe) by Mehlich−1; calcium (Ca) and magnesium (Mg) by KCl extraction (1 mol L−1); and organic matter by the Walkley–Black method. The baseline values were pH = 6.02; H + Al = 5.8 cmolc dm−3; Ca = 5.51 cmolc dm−3; Mg = 1.24 cmolc dm−3; Fe = 33 mg dm−3; P = 21.7 mg dm−3; K = 177 mg dm−3; and organic matter (OM) = 44.8 g dm−3.
Experiments were conducted over two seasons, 2019/2020 (Season 1) and 2020/2021 (Season 2), using the commercial maize hybrid DKB390 (Dekalb®, Bayer CropScience, São Paulo, Brazil). The experimental design was a randomized complete block design (RCBD) in a 2 × 2 × 2 factorial arrangement, consisting of: (i) presence or absence of the BiomaPhos® inoculant (Simbiose Company, Cruz Alta, Brazil), applied as a seed inoculation at 100 mL ha−1; (ii) phosphate source, either triple superphosphate (TSP; a conventional synthetic fertilizer widely used in Brazilian agriculture, containing 41% total P2O5, granulated) or reactive rock phosphate (RockP; powder form, supplied by the Office Chérifien des Phosphates (OCP), Morocco, containing 32% total P2O5, of which 3.7% is soluble in citric acid); and (iii) two doses (0 or 120 kg ha−1 of P2O5), applied in-furrow. RockP was not reapplied in Season 2 to assess its residual effect. Each plot contained six 5 m long rows, spaced 0.5 m apart, with irrigation applied as needed.
Agronomic measurements included grain yield, plant height, ear diameter, resin-extractable P (P-resin), total soil P, leaf P, and grain concentrations of N, P, and K. Grain yield was measured from the central four rows and adjusted to a moisture content of 13%. Leaf P and grain NPK were determined by Brazilian Laboratory of Environmental and Agricultural Analyses (LABRAS—Monte Carmelo, Brazil) following []: sulfuric digestion (N; LOQ = 0.6 g kg−1) and nitroperchloric digestion (P, K; LOQ = 0.01 and 0.3 g kg−1, respectively) using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES).
2.2. Root System Architecture (RSA)
During flowering, three maize plants per plot (in the outer rows) were sampled for root system analysis using the Shovelomics method []. Root crowns were excavated to a depth of 20 cm, washed, and photographed. RSA parameters were quantified using DIRT 5.2 [,] with modifications proposed by [].
RSA evaluation focused on treatments with 0 and 120 kg ha−1 of P2O5 from both TSP and RockP. Only traits with coefficients of variation (CV) ≤ 20% were retained (Table 1).

Table 1.
Description of maize root traits according to [].
2.3. Soil DNA Extraction and Microbial Profiling
Maize rhizosphere samples (fine roots with adhered soil) were collected during the flowering stage (BBCH 60–69) [], a period of enhanced root exudation, increased phosphorus demand, and high microbial activity. It was collected samples from three plants, with three replicates for each selected treatment (0 and 120 kg ha−1 of P2O5 applied as TSP or RockP). A total of 5 g of fine roots with adhered soil were suspended in 35 mL of 0.1% sodium pyrophosphate and shaken for 30 min. After root removal and centrifugation (10,000× g, 30 min), 0.45 g of soil was frozen in liquid N2 and stored at −80 °C.
DNA samples were extracted from rhizosphere and bulk soil using the PowerSoil® DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA), according to the manufacturer’s recommendations. DNA quantification was performed using a Nanodrop® spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and concentrations were adjusted to 5 ng μL−1.
The bacterial community was assessed by amplification of the 16S rRNA gene using primers 8F-FAM (5′[FAM]-AGAGTTGATCCTGGCTCAG-3′) [] and 1492R (5′-TACGGTACCTTGTACGACTT-3′) []. PCR reactions were performed in a ProFlex PCR thermocycler (Applied Biosystems, Foster City, CA, USA) with 15 ng of DNA, 0.2 μM of each primer, 0.1 mM of each dNTP, 1x reaction buffer, 2.5 mM MgCl2, and 2.5 U of Taq DNA Polymerase (Promega, Madison, WI, USA), in a final volume of 50 μL. Cycling conditions were: 95 °C for 2 min; 30 cycles of 95 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min; and a final extension at 72 °C for 5 min.
The community of total fungi was assessed by amplification of the 18S rRNA gene using primers Euk1A-HEX (5′[HEX]-CTGGTTGATCCTGCCAG-3′) and Euk516r (5′-ACCAGACTTGCCCTCC-3′). Reactions contained 15 μL of DNA, 0.3 μM of each primer, 0.25 mM of each dNTP, 1× reaction buffer, MgCl2 2.5 mM, and 2.5 U of Taq DNA Polymerase (Invitrogen, Carlsbad, CA, USA) in a total volume of 50 μL. Amplification consisted of 95 °C for 2 min and 10 s; 35 cycles of 94 °C for 50 s, 60 °C for 45 s, and 72 °C for 2 min and 10 s; and a final extension at 72 °C for 7 min.
The community of arbuscular mycorrhizal fungi (AMF) was assessed by nested PCR targeting the 28S rRNA gene. The first round used primers LR1 (5′-GCATATCAATAAGCGGAGGA-3′) [] and FLR2 (5′-GTCGTTTAAAGCCATTACGTC-3′) []. For the second PCR, 2.5 µL of the first reaction product was used as template with primers FLR3-FAM (5′[FAM]-TTGAAAGGGAAACGATTGAAGT-3′) and FLR4-HEX (5′[HEX]-TACGTCAACATCCTTAACGAA-3′) []. Reaction mixture and cycling conditions followed those used for bacterial amplification.
PCR products from all reactions were verified by electrophoresis in 1% (w/v) agarose gels and visualized under UV light after staining with GelRed (Biotium, Fremont, CA, USA).
2.3.1. Terminal Restriction Fragment Length Polymorphism (T-RFLP)
Amplified DNA from the 16 S rRNA (bacteria) and 28 S rRNA (AMF) genes was digested with the restriction enzymes AluI, HaeIII, and HhaI for both bacteria and AMF (Invitrogen, USA). Digested products were mixed with HiDi formamide and ROX 500 size standard (Applied Biosystems, USA) and analyzed on a 3500XL Genetic Analyzer (Applied Biosystems, USA). Terminal restriction fragments (T-RFs; 30–500 bp, >40 fluorescence units) were filtered with T-REX []. For bacteria and AMF, Bray–Curtis similarity matrices were calculated in Past v3.25 [] for NMDS analyses.
In addition, 18 S rRNA (for total fungi) was digested with HhaI, MspI, and BsuRI. The digested products were mixed with HiDi formamide and ROX 500 size standard (Applied Biosystems, USA) and analyzed using a 3500XL Genetic Analyzer (Applied Biosystems, USA). T-RFs (30–500 bp, >40 fluorescence units) were filtered with T-REX [], and the resulting fragments were obtained exclusively for subsequent taxonomic classification.
2.3.2. Taxonomic Classification
For bacteria, T-RFs obtained from 16 S rRNA were compared with predicted fragment sizes generated with MICA3 (http://mica.ibest.uidaho.edu/) (accessed on 8 October 2021) against the RDP database (r12u10). For total fungi, T-RFs obtained from 18 S rRNA were assigned by comparison with the PHYMYCO-DB fungal phylogenetic database. All results were cross-validated through the NCBI Taxonomy Browser. Diversity indices (Shannon, Simpson, and Chao) were calculated from bacterial family-level abundances using Past [].
2.4. Phosphatase Enzyme Activity
Rhizosphere soil was sieved (2 mm) to remove root and organic debris. For each sample, 0.150 g was analyzed for acid and alkaline phosphatase activities as described in []. Soil was incubated at 37 °C for 1 h with p-nitrophenyl phosphate in a universal buffer at pH 6.5 (acid) or 11.0 (alkaline). Reactions were stopped with CaCl2 and NaOH, centrifuged (8000× g, 5 min), and absorbance was measured at 400 nm. Enzyme activity was expressed as µg p-nitrophenol h−1 g−1 soil. Weather data (minimum and maximum temperatures, and precipitation) were plotted alongside enzyme activity using the ggplot2 package in R software version 2024.12.1 [].
2.5. Statistical Analyses
Agronomic and RSA data were subjected to ANOVA and Tukey’s test (p ≤ 0.05) using ExpDes.pt [] and agricolae [] in R software []. A subset of RSA traits (AREA, WIDTH_MAX, D90, SKL_WIDTH, ANG_TOP; Table 1), P-resin, diversity indices, and grain yield were analyzed by principal component analysis (PCA) using factoextra [].
Microbial beta diversity (β-diversity) for both bacterial and AMF communities was assessed using non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarity, followed by analysis of similarities (ANOSIM) in Past []. A similarity percentage (SIMPER) analysis was applied to Hellinger-transformed data [] to identify the taxa contributing most to treatment dissimilarities. Relative abundances (>3%) were visualized using the ggplot2 and reshape2 packages in R software [].
The effects of treatments on microbial abundance, diversity indices, and enzyme activities were also tested using ANOVA followed by Tukey’s test (p ≤ 0.05) in R software.
Microbial co-occurrence networks were constructed from Pearson correlation matrices (p ≤ 0.05) based on the relative abundances of bacterial and fungal families. Only taxa with a relative abundance >3% in at least one sample were included to reduce noise from rare taxa. Network construction and visualization were performed using the qgraph [] and igraph [] packages in R software. Nodes were arranged using the Fruchterman–Reingold spring-embedded layout [].
3. Results
3.1. Inoculation Altered Bacterial and AMF Community Composition and Phosphatase Activity in Season 2
Genetic diversity analysis revealed no significant differences in AMF communities between seasons. For bacterial communities, no significant differences were detected during Season 1 (Figure 1a,b). However, inoculation significantly influenced bacterial community composition in Season 2, with important differences observed between inoculated and non-inoculated samples in treatments without phosphate fertilizer (p = 0.0032, R = 0.5056; Figure 1c) and in those with 120 kg ha−1 of P2O5 from TSP (p = 0.098, R = 0.5926; Figure 1d).
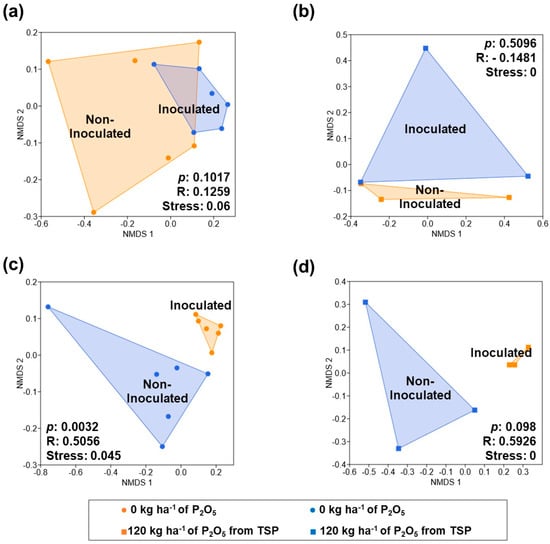
Figure 1.
Non-metric multidimensional scaling (NMDS) plots based on Bray–Curtis distance matrices, showing the bacterial community structure in the maize rhizosphere under different phosphorus fertilization and inoculation treatments during Season 1 (a,b) and 2 (c,d).
In both seasons, the most abundant bacterial families included Streptomycetaceae, Micrococcaceae, Methylobacteriaceae, and Bradyrhizobiaceae (Figure 2a,b), which belong to the Actinobacteria and Proteobacteria phyla, respectively. Pseudomonadaceae abundance increased 4.22% with TSP in Season 1, while Rhizobiaceae increased 3.44% with TSP treatments during Season 2 (Table A1 and Table A2). Methylobacteriaceae showed 8.54% higher abundance in inoculated plots in Season 2. No consistent increases in other microbial families were observed as a result of inoculation (Table A1 and Table A2). Micrococcaceae was 2.82% more abundant in Season 1, while Rhizobiaceae and Sphingomonadaceae were more prevalent in Season 2, being 2.44 and 2.47% higher, respectively. Clostridiaceae, Geobacteraceae, and Pseudomonadaceae were exclusive to Season 1, while Brucellaceae appeared only in Season 2 (Table 2).
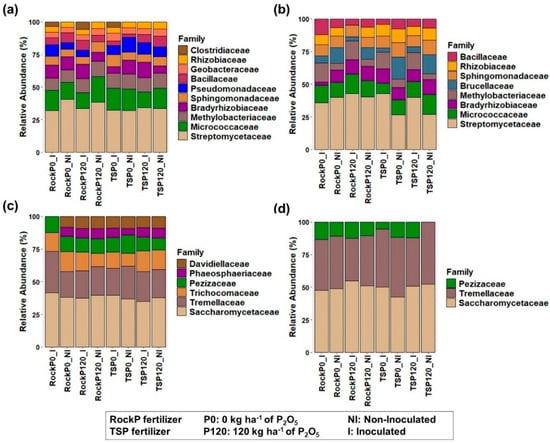
Figure 2.
Relative abundance of microbial families (>3%) in the maize rhizosphere during Season 1 (a,c) and 2 (b,d), presented separately for bacterial (a,b) and fungal (c,d) communities.

Table 2.
Comparison of the relative abundance (RA) of bacterial families between Season 1 and Season 2.
Streptomycetaceae was the primary contributor to bacterial dissimilarity in treatments without phosphate fertilizer (40.00%) and with 120 kg ha−1 of P2O5 from TSP (48.23%). In treatments with RockP and unfertilized control, Bradyrhizobiaceae (19.79%) and Streptomycetaceae (16.15%) contributed most to differences.
Saccharomycetaceae and Tremellaceae were the most abundant fungal families (Figure 2c,d), which belong to the phyla Basidiomycota and Ascomycota. Pezizaceae was 1.83% more prevalent in unfertilized plots in comparison with a 120 kg ha−1 of P2O5 dose in Season 1, while Tremellaceae increased 6% with TSP in comparison with RockP in Season 2 (Table A3). Seasonal differences in fungal composition were observed: Saccharomycetaceae and Tremellaceae showed higher abundance in Season 2, whereas Phaeosphaeriaceae, Trichocomaceae, and Davidiellaceae were exclusively detected in Season 1 (Table 3).

Table 3.
Comparison of the relative abundance (RA) of fungal families between Season 1 and Season 2.
Saccharomycetaceae drove 62.48% of the dissimilarity in RockP treatments and 40.71% in TSP treatments. Pezizaceae accounted for 55.62% of the TSP treatment differences.
In Season 1, the bacterial network included 10 nodes and 27 edges (Figure 3a), while the fungal network comprised six nodes and three edges (Figure 3c).
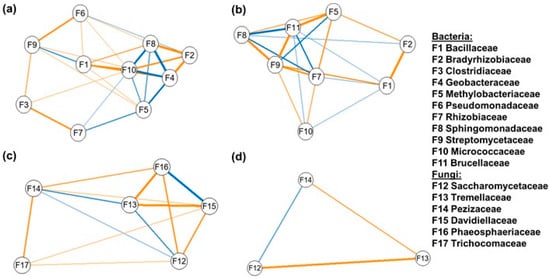
Figure 3.
Microbial co-occurrence networks based on Pearson’s correlation for bacterial (a,b) and fungal (c,d) families in maize rhizosphere soil during Season 1 (a,c) and Season 2 (b,d). Only correlations with |r| ≥ 0.2 and p ≤ 0.05 were included. Each node represents a bacterial family. Blue edges indicate positive correlations, while orange edges indicate negative correlations. The spatial arrangement of nodes reflects the strength of their relationships, with nodes that are closer together showing stronger associations.
The family Sphingomonadaceae (F8) emerged as a central hub in the bacterial network, showing positive correlations with Geobacteraceae (F4), Methylobacteriaceae (F5), and Micrococcaceae (F10). Rhizobiaceae (F7) also displayed positive correlations with Methylobacteriaceae (F5) and Micrococcaceae (F10), and a positive correlation was observed between Bacillaceae (F1) and Streptomycetaceae (F9). In contrast, several negative correlations were identified: Clostridiaceae (F3) was negatively correlated with Streptomycetaceae (F9) and Rhizobiaceae (F7); Bacillaceae (F1) with Micrococcaceae (F10); and Bradyrhizobiaceae (F2) with both Sphingomonadaceae (F8) and Geobacteraceae (F4). Pseudomonadaceae (F6) showed negative correlations with Streptomycetaceae (F9) and Bacillaceae (F1). These patterns suggest potential co-occurrence and exclusion relationships among bacterial taxa, reflecting possible ecological or functional interactions. In the fungal network of the same season (Figure 3c), Saccharomycetaceae (F12) displayed a negative correlation with Davidiellaceae (F15) and Phaeosphaeriaceae (F16), and positive correlations with Tremellaceae (F13) and Pezizaceae (F14). Trichocomaceae (F17) showed only negative correlations with the other fungal families.
In Season 2, eight nodes and 28 edges were found in the bacterial network (Figure 3b), and three nodes and three edges in the fungal network (Figure 3d). Sphingomonadaceae (F8) showed positive correlations with Brucellaceae (F11) and Rhizobiaceae (F7), while Methylobacteriaceae (F5) and Streptomycetaceae (F9) were also positively correlated. However, both families displayed several negative correlations with other taxa. In the fungal network (Figure 3d), Tremellaceae (F13) had negative correlations with Saccharomycetaceae (F12) and Pezizaceae (F14), while Pezizaceae (F14) was positively correlated with Saccharomycetaceae (F12).
In general, Shannon, Simpson, and Chao bacterial diversity indices were higher in non-inoculated samples and RockP treatments during Season 1 than in inoculated and TSP treatments, respectively (Table 4).

Table 4.
Shannon, Simpson, and Chao diversity indices of bacterial families in the maize rhizosphere under inoculated (I) and non-inoculated (NI) conditions, with two phosphorus doses (0 and 120 kg ha−1 of P2O5) and two fertilizer types (rock phosphate—RockP and triple superphosphate—TSP), during Seasons 1 and 2.
In Season 2, bacterial diversity was greater in the unfertilized control compared with 120 kg ha−1 of P2O5 of phosphate fertilizer, and higher under TSP treatments than under RockP. Overall, bacterial diversity indices were higher in Season 1 (Figure 4).
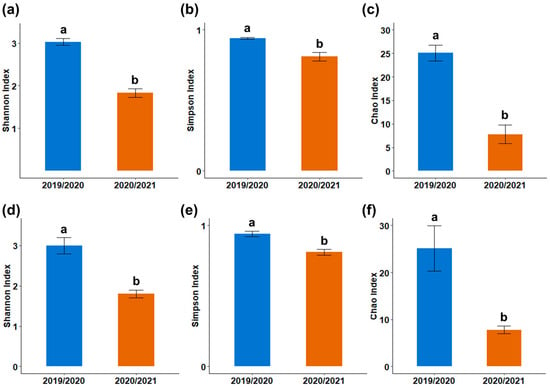
Figure 4.
Mean values of diversity indices in the maize rhizosphere during seasons 1 and 2. (a) Shannon diversity index, (b) Simpson diversity index, and (c) Chao richness estimator for bacterial communities; and (d) Shannon diversity index, (e) Simpson diversity index, and (f) Chao richness estimator for fungal communities.
Fungal diversity indices (Shannon, Simpson, and Chao) were higher in Season 1 (Figure 4), particularly in treatments with 120 kg ha−1 of P2O5 of fertilizer compared to the treatments without the addition of phosphate fertilizer (Table 5).

Table 5.
Shannon, Simpson, and Chao diversity indices of fungal families in the maize rhizosphere under inoculated (I) and non-inoculated (NI) conditions, with two phosphorus doses (0 and 120 kg ha−1 of P2O5) and two fertilizer types (rock phosphate—RockP and triple superphosphate—TSP), during Seasons 1 and 2.
Alkaline phosphatase activity was higher in RockP compared to TSP treatments during Season 1 (Table A4). Both acid and alkaline phosphatase activities were generally higher in Season 2 (Table A5), possibly due to increased rainfall (13.2 mm vs. 9.3 mm) (Figure 5).
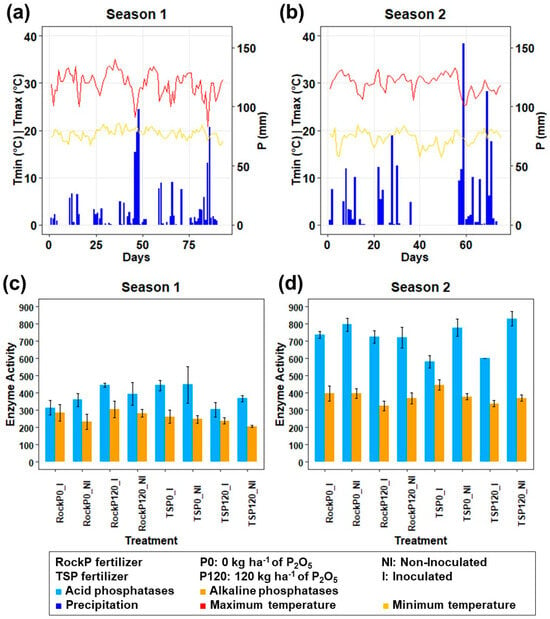
Figure 5.
Climate variables and enzymatic activity in the maize rhizosphere during Season 1 (a,c) and 2 (b,d). (a,b) Minimum temperature (Tmin), maximum temperature (Tmax), and precipitation (P). (c,d) Enzymatic activity of acid and alkaline phosphatases.
3.2. Agronomic and Root Architecture Responses
Phosphorus availability (P-resin and total soil P) was consistently higher with 120 kg ha−1 of P2O5 of phosphate fertilizer in both seasons (Table A6 and Table A7). In Season 2, total P was also 347.4 mg dm−3 higher in TSP and inoculated treatments (Table A7). Grain yield remained similar between seasons. P-resin, total soil P, leaf P, grain N, and plant height were higher in Season 1, while grain P, K, and ear diameter were higher in Season 2 (Table 6).

Table 6.
Average values of maize agronomic traits measured during Seasons 1 and 2.
Most root architecture traits did not differ significantly among treatments (Table A8). In Season 1, hypocotyl diameter (HYP_DIA) was greater in unfertilized plots, and soil–tissue angle (STA_MAX) was higher in plots with 120 kg ha−1 of P2O5 fertilizer. In Season 2, D90 (depth at 90% root density) was higher with TSP (Table A9). Root traits such as root area (AREA), maximum root system width (WIDTH_MAX), and skeletal width calculated from the medial axis (SKL_WIDTH) were greater in Season 1. In contrast, root angle (ANG_TOP) was higher in Season 2 (Table 7).

Table 7.
Average values of maize root system architecture (RSA) traits measured during Season 1 and 2. Traits identification is in Table 1.
Principal Component Analysis (PCA) explained 73.2% and 72.4% of the variability in Seasons 1 and 2, respectively (Figure 6).
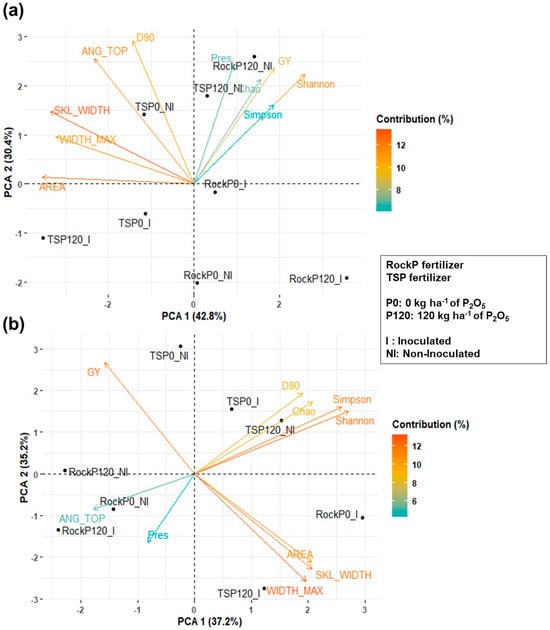
Figure 6.
Principal component analysis (PCA) of plant, microbial, and productivity traits in the maize rhizosphere during the (a) Season 1 and (b) Season 2. Variables included: maximum root system width (WIDTH_MAX), skeletal width (SKL_WIDTH), root top angle (ANG_TOP), projected root area (AREA), depth at 90% root width accumulation (D90), Shannon diversity index (Shannon), Simpson diversity index (Simpson), Chao richness estimator (Chao), and grain yield (GY).
In Season 1, PC1 was mainly influenced by AREA (12.5%) and SKL_WIDTH (13.4%) (Table 8).

Table 8.
Contribution of root system architecture (RSA) traits, microbial genetic diversity, and grain yield during seasons 1 and 2. Traits identification is in Table 1.
In Season 1, non-inoculated (NI) and 120 kg ha−1 of P2O5 from rock phosphate (RockP120) treatments were associated with higher diversity indices, while grain yield was also located in this quadrant. Non-inoculated treatments with 0 kg ha−1 of P2O5 from triple superphosphate (TSP0_NI) clustered with root traits, although these traits did not differ significantly (Figure 6a). In Season 2, WIDTH_MAX (13.2%) and Shannon index (12.15%) contributed most to the variance (Table 8). In Season 2, inoculated treatments with 120 kg ha−1 of P2O5 from triple superphosphate (TSP120_I) clustered with larger root traits (AREA, WIDTH_MAX, SKL_WIDTH), while non-inoculated treatments with 120 kg ha−1 of P2O5 from triple superphosphate (TSP120_NI) were associated with higher diversity and D90. RockP treatments clustered with ANG_TOP and P-resin. Grain yield was higher in TSP0_NI, suggesting a negative correlation with root traits and diversity indices in Season 2 (Figure 6b).
4. Discussion
The bacterial family Streptomycetaceae was the most abundant in both seasons, while Saccharomycetaceae dominated the fungal community (Figure 2). Both families include species carrying genes for phosphatase enzymes [,,]. Streptomyces spp. are notable for producing bioactive metabolites that enhance soil fertility, stimulate plant growth, protect against phytopathogens, and confer tolerance to environmental stress []. They also synthesize antimicrobial compounds, form resistant spores, and contribute to plant resilience through hormone production and other biochemical activities [,]. Among fungi, Saccharomyces cerevisiae is recognized as a growth-promoting yeast due to its cytokinin and tryptophan content (a precursor of indole-3-acetic acid), which stimulates cell division and vegetative growth. It can also improve phosphorus and manganese uptake and act as a biocontrol agent [].
In Season 2, the fungal families Saccharomycetaceae and Tremellaceae showed significantly higher relative abundances compared with Season 1 (Table 3). Saccharomycetaceae, Pezizaceae, and Tremellaceae persisted into Season 2, representing distinct phyla: Saccharomycetaceae and Pezizaceae (Ascomycota) and Tremellaceae (Basidiomycota). All reproduce via spore formation, which likely favored dispersal and survival under changing conditions. The increased abundance of Streptomycetaceae and Saccharomycetaceae in Season 2 paralleled higher acid and alkaline phosphatase activity relative to Season 1 (Table A5). Phosphatases, produced by bacteria, fungi, and plant roots, hydrolyze organic phosphate compounds into inorganic forms available for uptake. Their production is driven by plant and microbial P demand, organic P availability, soil P limitation, pH, and water availability, with drought conditions strongly reducing microbial biomass and enzyme activity [,].
Microbial co-occurrence networks revealed season-specific interaction patterns, reflecting shifts in the dynamics of bacterial and fungal communities between seasons (Figure 3). The bacterial network was more complex than the fungal network in both seasons. In Season 1 (Figure 3a,c), the bacterial network displayed higher modularity and connectivity (10 nodes and 27 edges), suggesting a more structured, more resilient ecological community and potentially an interactive microbial community, compared to Season 2 (Figure 3b,d). Microbial network analysis is increasingly employed to evaluate how microbial communities respond to environmental disturbances []. Although more complex networks, characterized by a greater number of interconnections and cooperative associations, as observed in Season 1, are often associated with higher resistance to ecological perturbations, this is not a universal pattern []. The simplified network structure in Season 2 may therefore reflect a community more sensitive to environmental fluctuations, such as climate variability or input use.
In Season 1, Sphingomonadaceae showed positive correlations with key bacterial families, including Geobacteraceae, Methylobacteriaceae, and Micrococcaceae, suggesting a potential role in promoting microbial cooperation or occupying shared ecological niches. This family has also been identified in other agricultural systems as a biocontrol agent against fungal diseases in maize and as a contributor to soil health and nutrient cycling []. Furthermore, Sphingomonas contributes to plant growth and health [] and produces exopolysaccharides that improve soil aggregation []. Negative correlations were detected with Clostridiaceae, Bacillaceae, and Bradyrhizobiaceae. Negative interactions within a network may result from mechanisms such as parasitism, antagonism, predation, or competition for shared resources []. The fungal network in Season 1 was simpler, with Saccharomycetaceae showing both positive and negative correlations, highlighting possible antagonistic and synergistic interactions.
In Season 2, although the number of nodes in the bacterial (Figure 3b) and fungal (Figure 3d) networks decreased, indicating reduced network complexity, Sphingomonadaceae again played a central role, reaffirming its ecological relevance across seasons. The recurring positive and negative associations of Methylobacteriaceae and Streptomycetaceae with other families in Season 2 suggest a context-dependent dual function, acting as both facilitators and competitors. These interactions are essential for shaping microbial community structure and influencing their capacity to promote plant growth, enhance nutrient uptake, and improve stress tolerance [,].
The fungal network remained relatively simple, likely due to fundamental differences between soil fungi and bacteria in their growth environments, dispersal abilities, and interactions with plants. In general, fungi have lower dispersal ability compared to bacteria []. Tremellaceae showed negative correlations with Pezizaceae and Saccharomycetaceae, potentially acting as a suppressive fungal family against other fungal taxa (Figure 3d). These findings are consistent with increasing evidence that fungal networks, although less connected, play key regulatory roles in shaping rhizosphere dynamics. However, future research should consider the role of fungal guilds (saprotrophic vs. mycorrhizal) to better understand community assembly under varying moisture conditions [].
The T-RFLP analysis effectively captured these microbial dynamics. Bacterial community composition responded to inoculation only in Season 2, whereas AMF communities remained stable across treatments in both seasons. As a cost-efficient approach, T-RFLP enables the assessment of rhizosphere community structure under varying conditions, including phosphate fertilization and inoculants. Its scalability allowed for the evaluation of numerous samples across two seasons, making it well-suited for detecting community-level shifts in response to inoculation under field conditions [].
Acid phosphatase activity was consistently higher than alkaline phosphatase activity in both seasons (Figure 5), as expected for the acidic soil of the experimental field []. While both plants and microbes produce acid phosphatase, alkaline phosphatase is exclusively of microbial origin []. The higher phosphatase activities in Season 2 also coincided with greater rainfall (Figure 5). Climate variability affects microbial communities and enzymatic activities, as enzymes are susceptible to changes in temperature, moisture, and substrate interactions. Soil water availability, for instance, directly influences microbial activity and the diffusion of soluble nutrients; low water content may restrict nutrient diffusion and enzyme-substrate interactions []. These results align with those of [], who reported increased phosphatase activity during seasons with higher precipitation. Margalef et al. [] also found that higher temperature and rainfall were positively associated with phosphatase activity.
Alpha diversity, based on bacterial and fungal family composition, was lower in Season 2. This reduction may be linked to higher precipitation levels, as rainfall is a key environmental factor shaping rhizosphere microbial communities. Soil microorganisms are sensitive to changes in moisture, and shifts in rainfall can alter microbial abundance and composition. However, there is still no clear consensus on how microbial diversity responds to changes in precipitation [,]. It is also important to note that greater microbial diversity does not necessarily lead to improved plant performance, such as increased productivity. Instead, plant species, land-use history, and climate factors play critical roles in shaping microbial community structure and diversity, highlighting that the influence of rhizosphere microbiota on plant community dynamics is highly context-dependent []. Moreover, Simpson’s diversity index values for bacterial and fungal communities were close to 1, and Shannon index values were moderate, indicating low diversity and high dominance in both seasons []. The dominance of a few microbial families may be more related to their adaptation to climatic fluctuations than to the fertilization or inoculation treatments.
The experimental field used in this study has a history of successive fertilizer applications. Consequently, the initial soil analysis indicated a high-P concentration in the area, likely due to the cumulative residual effect of previous experiments, which contributed to its high fertility. As a result, the average maize grain yield was statistically similar across most treatments (Table 3 and Table 4). Nevertheless, the productivity levels observed were comparable to those reported in other studies [,,], suggesting that the hybrid may have reached its yield potential under the region’s conditions.
There is growing evidence that plants respond more positively to microbial inoculation under low-fertility or stress conditions. For instance, in low-P soils, inoculation with B. subtilis (CNPMS B2084) and P. megaterium (CNPMS B119) significantly increased grain yield. In high-P soils, strain B119 was more effective than B2084 in promoting yield, particularly when triple superphosphate (TSP) was applied []. Similarly, [] found that inoculation with Penicillium bilaiae was more effective in enhancing maize yield in soils with low or very low-P levels. Inoculation with Pseudomonas fluorescens S3X and Cupriavidus necator 1C2 improved maize growth and nutrient use efficiency under water deficit [], while strains such as Sinorhizobium sp. A15, Bacillus sp. A28, Sphingomonas sp. A55, and Enterobacter sp. P24 enhanced maize yield under low nitrogen and phosphorus conditions [].
Neither fertilization nor inoculation significantly affected the relative abundance of bacterial (Table A1 and Table A2) or fungal (Table A3) families, indicating microbial community stability under the experimental conditions. These findings are consistent with [], who reported no significant effect of bacterial inoculants on root-associated bacterial communities in the same maize genotype. Although some taxonomic shifts were observed between seasons, the most abundant families remained unchanged, and maize yield was not significantly affected. This stability suggests that the dominant microbial families play key functional roles, such as phosphorus solubilization, pathogen suppression, and stress tolerance, thereby creating functional redundancy within the system. Consequently, even under different seasons, the microbial community maintained its capacity to support plant growth without major impacts on yield.
Some phenotypic differences in maize plants were observed between the two cropping seasons; however, these did not significantly affect yield. The rock phosphate (RockP) fertilizer demonstrated agronomic efficiency similar to TSP in Season 1 and outperformed TSP in terms of residual effects in Season 2. Notably, RockP was not reapplied in Season 2; thus, no phosphorus was replenished for that treatment, allowing evaluation of its residual effect. RockP can contribute to crop fertilization, and its availability may be enhanced through bioinoculants containing phosphate-solubilizing bacteria or through the action of native soil microbiota. Therefore, combining reactive phosphate rock fertilizers with microbial inoculants and selecting more efficient and responsive plant genotypes may allow for a reduction in annual P fertilizer inputs in agricultural systems.
Root architectural traits were not significantly affected by the biological inoculant or different phosphorus fertilization doses (Table 5). In a controlled-environment study, inoculation with the same Bacillus strains increased the total root surface area of maize seedlings [,]. Root architecture can vary according to P availability, soil type, sampling time, plant species, and genotype [,,]. Although root growth is essential for nutrient uptake, it demands high energy expenditure []. Therefore, the high soil P availability in our field may have limited the plant’s need to invest in root development or respond to inoculation and/or fertilization.
Together, these findings reinforce the notion that the success of microbial inoculants is influenced by the dynamics of native microbial communities. No significant changes in microbial community structure and agronomic performance were observed in treatments with inoculation under such conditions. This suggests that the native microbiota may already exhibit functional redundancy, as different bacterial strains can perform the same functions [].
The effectiveness of microbial inoculants depends on a balance between their competitive traits and ecological compatibility. Three main outcomes can be expected following inoculant application: first, the microorganisms in the inoculant may successfully establish themselves alongside the native microflora and induce changes in the microbial community composition; second, due to soil resilience, the introduced microorganisms may be eliminated, leading to restoration of the original condition and maintaining the microbial community as it was before inoculation; and third, the inoculant microorganisms may establish themselves and induce temporary changes in the community structure, followed by restoration of the initial conditions [,].
Native microbial communities, already well-adapted to local environmental conditions, often outcompete introduced strains, limiting their establishment and functional activity. As a result, the use of microbial consortia does not necessarily lead to additive or synergistic effects, but may instead trigger competitive interactions []. This underscores the importance of rigorous pre-screening and the selection of plant growth-promoting bacteria that are not only effective but also ecologically compatible with the resident microbiota. Such precautions are essential to safeguard soil health and prevent disruptions to the very processes that microbial inoculation is intended to enhance [].
Finally, plant development is influenced by multiple factors, with successful plant–microbe interactions depending on plant genotype, microbial strain, soil management, and environmental conditions []. Our results indicate that when a cultivar is well adapted to the local climate and cultivated in nutrient-rich soils, enhanced root growth becomes unnecessary. Consequently, once the crop’s yield potential is reached, neither fertilizer application nor inoculation significantly improves productivity. These findings underscore the importance of precise soil fertility monitoring in promoting more sustainable agricultural practices, both economically and environmentally. Furthermore, they emphasize the importance of site-specific evaluation of bioinoculant strategies and underscore the role of soil diagnostics as a decision-support tool in sustainable agriculture.
5. Conclusions
This study demonstrates that rhizosphere microbial communities associated with maize remain stable under inoculation and phosphorus fertilization in high-fertility soils. Streptomycetaceae and Saccharomycetaceae were consistently dominant, underscoring their functional role in phosphorus cycling and stress resilience. Seasonal variation, particularly in fungal taxa and network complexity, reflected climatic influences rather than management inputs, while maize yield remained unaffected. These findings suggest that functional redundancy within native microbial communities buffers external interventions, maintaining ecological stability and crop performance.
The results emphasize that the effectiveness of microbial inoculants is strongly context-dependent, with greater benefits expected under nutrient limitation or stress. Recognizing the ecological compatibility between inoculants and resident microbiota is therefore essential to avoid disruption of established community dynamics. By integrating soil fertility diagnostics with biodiversity-aware management, strategies that combine microbial inoculants, locally adapted cultivars, and low-input fertilization can support sustainable agriculture while conserving soil microbial diversity and ecosystem function.
Author Contributions
Conceptualization, S.M.d.S.T.; methodology, R.G.d.O. and M.L.C.; software, T.T.S.; validation, U.G.d.P.L. and S.M.d.S.T.; formal analysis, R.G.d.O.; investigation, R.G.d.O., U.G.d.P.L., J.V.S.A.d.A., T.T.S. and F.C.d.S.; resources, S.M.d.S.T., C.A.d.O.-P. and F.C.d.S.; data curation, R.G.d.O.; writing—original draft preparation, R.G.d.O. and S.M.d.S.T.; writing—review and editing, U.G.d.P.L., C.A.d.O.-P., M.L.C., E.A.G. and F.C.d.S.; visualization, R.G.d.O. and S.M.d.S.T.; supervision, S.M.d.S.T. and F.C.d.S.; project administration, S.M.d.S.T.; funding acquisition, S.M.d.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Empresa Brasileira de Pesquisa Agropecuária—Embrapa (Grant No. 20.20.03.024.00.00) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais—Fapemig (Grant No. APQ-00606-21). This work was partially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq/INCT—‘Plant-Growth Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility’ (Grant No. 465133/2014–2, Fundação Araucaria-STI, Capes), Finep/CT AGRO/FNDCT (Acordo de Cooperação No. 01.22.0080.00, Ref. 1219/21), Fertilize 4 Life, a multi-national scientific collaboration between University of Florida, Institute of Food and Agricultural Sciences (UF|IFAS), the Brazilian Agricultural Research Corporation (EMBRAPA), USDA Agricultural Research Service (ARS), the International Fertilizer Development Center (IFDC), and the USDA Foreign Agricultural Service (FAS) (Grant No. 10.25.00.117.00.00). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (Capes/Proex) (Process number: 88881.844786/2023-01). R.G.O. and J.V.S.A.A. were recipients of a research fellowship from Capes.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Appendix A. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors thank the technicians and students from Embrapa Maize and Sorghum for their support in planting, harvesting, and conducting agronomic measurements, as well as in collecting, washing, and processing roots.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Appendix A
Appendix A.1

Table A1.
Most abundant bacterial families in the rhizosphere of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Season 1.
Table A1.
Most abundant bacterial families in the rhizosphere of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Season 1.
| Treatment | Sphingnomonadaceae | Pseudomonadaceae | Bacilaceae | Geobacteraceae | |
| Inoculation | NI | 6.77 a | 8.01 a | 5.76 a | 4.86 a |
| I | 6.88 a | 7.45 a | 5.70 a | 4.66 a | |
| Fertilization | TSP | 7.04 a | 8.84 a | 5.05 a | 5.13 a |
| RockP | 6.60 a | 4.62 b | 6.42 a | 4.40 a | |
| Dose (kg ha−1 of P2O5) | 0 | 6.95 a | 8.05 a | 4.69 a | 4.85 a |
| 120 | 6.70 a | 5.42 a | 6.77 a | 4.68 a | |
| Treatment | Streptomycetaceae | Micrococcaceae | Methylobacteriaceae | Bradyrhizobiaceae | |
| Inoculation | NI | 36.16 a | 16.16 a | 11.11 a | 8.32 a |
| I | 33.05 a | 14.12 a | 10.67 a | 9.54 a | |
| Fertilization | TSP | 33.01 a | 15.24 a | 11.39 a | 8.32 a |
| RockP | 36.21 a | 15.03 a | 10.38 a | 9.52 a | |
| Dose (kg ha−1 of P2O5) | 0 | 34.30 a | 15.42 a | 10.48 a | 8.08 a |
| 120 | 34.91 a | 14.85 a | 11.20 a | 8.88 a | |
| Treatment | Rhizobiaceae | Clostridiaceae | |||
| Inoculation | NI | 4.85 a | 0.00 a | ||
| I | 4.54 a | 3.37 a | |||
| Fertilization | TSP | 4.51 a | 1.08 a | ||
| RockP | 4.88 a | 2.29 a | |||
| Dose (kg ha−1 of P2O5) | 0 | 4.36 a | 1.93 a | ||
| 120 | 5.04 a | 1.44 a | |||
Note: Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
Appendix A.2

Table A2.
Most abundant bacterial families in the rhizosphere of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Season 2.
Table A2.
Most abundant bacterial families in the rhizosphere of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Season 2.
| Treatment | Sphingnomonadaceae | Rhizobiaceae | Bacilaceae | Brucellaceae | |
| Inoculation | NI | 9.72 a | 8.41 a | 7.02 a | 5.16 a |
| I | 8.87 a | 5.85 a | 6.74 a | 4.01 a | |
| Fertilization | TSP | 10.01 a | 8.85 a | 6.26 a | 4.47 a |
| RockP | 8.57 a | 5.41 b | 7.50 a | 4.62 a | |
| Dose (kg ha−1 of P2O5) | 0 | 9.51 a | 8.36 a | 7.63 a | 4.93 a |
| 120 | 9.07 a | 5.90 a | 6.12 a | 4.24 a | |
| Treatment | Streptomycetaceae | Micrococcaceae | Methylobacteriaceae | Bradyrhizobiaceae | |
| Inoculation | NI | 33.46 a | 12.63 a | 05.16 b | 10.47 a |
| I | 40.36 a | 12.01 a | 13.70 a | 08.46 a | |
| Fertilization | TSP | 34.10 a | 14.34 a | 09.10 a | 10.30 a |
| RockP | 39.72 a | 12.21 a | 09.78 a | 08.63 a | |
| Dose (kg ha−1 of P2O5) | 0 | 36.31 a | 14.00 a | 09.59 a | 08.21 a |
| 120 | 37.52 a | 12.83 a | 09.27 a | 10.72 a | |
Note: Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
Appendix A.3

Table A3.
Most abundant fungal families in the rhizosphere of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
Table A3.
Most abundant fungal families in the rhizosphere of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
| Season | Treatment | Saccharomycetaceae | Tremellaceae | Trichocomaceae | Pezizaceae | |
| 1 | Inoculation | NI | 38.12 a | 22.02 a | 12.52 a | 11.59 a |
| I | 38.32 a | 24.23 a | 14.11 a | 11.24 a | ||
| Fertilization | TSP | 37.24 a | 22.66 a | 13.17 a | 11.23 a | |
| RockP | 39.30 a | 23.59 a | 13.45 a | 11.59 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 39.02 a | 24.36 a | 12.87 a | 12.33 a | |
| 120 | 37.42 a | 21.89 a | 13.75 a | 10.50 b | ||
| Phaeosphaeriaceae | Davidiellaceae | |||||
| Inoculation | NI | 11.59 a | 8.93 a | |||
| I | 11.24 a | 6.70 a | ||||
| Fertilization | TSP | 11.23 a | 8.89 a | |||
| RockP | 11.60 a | 6.73 a | ||||
| Dose (kg ha−1 of P2O5) | 0 | 12.33 a | 6.56 a | |||
| 120 | 10.50 a | 9.07 a | ||||
| Season | Treatment | Saccharomycetaceae | Tremellaceae | Pezizaceae | ||
| 2 | Inoculation | NI | 48.78 a | 42.97 a | 08.25 a | |
| I | 50.81 a | 38.38 a | 10.81 a | |||
| Fertilization | TSP | 49.02 a | 43.70 a | 07.28 a | ||
| RockP | 60.57 a | 37.66 b | 11.77 a | |||
| Dose (kg ha−1 of P2O5) | 0 | 47.30 a | 42.38 a | 10.31 a | ||
| 120 | 52.28 a | 38.98 a | 08.74 a | |||
Note: Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
Appendix A.4

Table A4.
Enzymatic activity of acid and alkaline phosphatases in the rhizosphere soil of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
Table A4.
Enzymatic activity of acid and alkaline phosphatases in the rhizosphere soil of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
| Season | Treatment | Phosphatase Activity (µg p-Nitrofenol h−1 g−1 Soil) | ||
|---|---|---|---|---|
| Acid | Alkaline | |||
| 1 | Inoculation | NI | 376.3 a | 260.8 a |
| I | 392.3 a | 272.4 a | ||
| Fertilization | TSP | 390.3 a | 238.2 b | |
| RockP | 378.3 a | 295.2 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 391.2 a | 275.3 a | |
| 120 | 377.2 a | 257.9 a | ||
| 2 | Inoculation | NI | 711.9 a | 403.4 a |
| I | 690.2 a | 406.0 a | ||
| Fertilization | TSP | 691.9 a | 406.5 a | |
| RockP | 710.3 a | 402.9 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 698.8 a | 429.2 a | |
| 120 | 703.4 a | 380.3 a | ||
Note: Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
Appendix A.5

Table A5.
Comparison between seasons of acid and alkaline phosphatase enzymatic activity across the different treatments evaluated.
Table A5.
Comparison between seasons of acid and alkaline phosphatase enzymatic activity across the different treatments evaluated.
| Phosphatase Activity (µg p-Nitrofenol h−1 g−1 Soil) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Acid | Alkaline | |||||||
| Treatment | Season 1 | Season 2 | Season 1 | Season 2 | ||||
| RockP0_I | 314.6 | b | 737.2 | a | 284.9 | b | 395.6 | a |
| RockP120_I | 444.3 | b | 671.5 | a | 304.9 | a | 401.8 | a |
| TSP0_I | 443.6 | a | 667.9 | a | 261.3 | b | 446.5 | a |
| TSP120_I | 302.7 | b | 684.3 | a | 238.5 | b | 380.1 | a |
| RockP0_NI | 359.6 | b | 710.6 | a | 308.2 | a | 445.4 | a |
| RockP120_NI | 393.9 | b | 722.4 | a | 282.3 | b | 368.9 | a |
| TSP0_NI | 446.9 | a | 680.1 | a | 246.8 | b | 429.2 | a |
| TSP120_NI | 367.9 | b | 735.3 | a | 206.1 | b | 370.4 | a |
RockP0_NI: RockP fertilizer, 0 kg ha−1 of P2O5 dose, without bioinoculant; RockP120_NI: RockP fertilizer, 120 kg ha−1 of P2O5, without bioinoculant; RockP0_I: RockP fertilizer, 0 kg ha−1 of P2O5, with bioinoculant; RockP120_I: RockP fertilizer, 120 kg ha−1 of P2O5, with bioinoculant; TSP0_NI: TSP fertilizer, 0 kg ha−1 of P2O5, without bioinoculant; TSP120_NI: TSP fertilizer, 120 kg ha−1 of P2O5 without bioinoculant; TSP0_I: TSP fertilizer, 0 kg ha−1 of P2O5, with bioinoculant; TSP120_I: TSP fertilizer, 120 kg ha−1 of P2O5, with bioinoculant. Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
Appendix A.6

Table A6.
ANOVA of soil and agronomic data during Seasons 1 and 2.
Table A6.
ANOVA of soil and agronomic data during Seasons 1 and 2.
| Mean Square | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | DF | Yield | K Grain | N Grain | P Grain | Plant Height | Ear Diameter | P Shoot | P-Resin | Soil Total P | |
| 1 | Block | 2 | 931,275.1 | 39.74 | 530.1 | 26.20 | 0.01078 * | 2.76 | 2 × 10−4 | 269.2 | 563,201.2 * |
| Fertilization | 1 | 633,025.5 | 3.95 | 61.7 | 0.72 | 0.00482 | 0.73 | 0.00082 | 241.3 | 103,451.3 | |
| Dose | 1 | 152,201.8 | 9.60 | 6.32 | 2.05 | 0.00202 | 0.31 | 0.00082 | 8314.2 * | 723,808.0 * | |
| Inoculation | 1 | 917,268.0 | 5.30 | 528.84 | 8.01 | 6 × 10−4 | 0.004 | 0.00107 | 1474.2 | 101,647.1 | |
| Fertilization * Dose | 1 | 335,321.7 | 29.48 | 5.43 | 30.98 | 0.00015 | 0.64 | 2 × 10−5 | 0.22 | 4269.3 | |
| Fertilization * Inoculation | 1 | 481,354.1 | 0.01 | 537.33 | 4.34 | 0.00107 | 0.24 | 0 | 23.8 | 26,553.4 | |
| Dose * Inoculation | 1 | 34,817.0 | 6.34 | 167.16 | 3.72 | 0.0054 | 2.47 | 0 | 1547.2 | 12,802.0 | |
| Fertilization * Dose * Inoculation | 1 | 124,283.6 | 9.52 | 78.91 | 2.32 | 0.0024 | 0.77 | 7 × 10−5 | 153.5 | 27,479.4 | |
| Error | 14 | 367,032.5 | 17.84 | 355.99 | 7.59 | 0.00238 | 1.15 | 0.00039 | 702.1 | 77,822.7 | |
| Total | 21 | ||||||||||
| Coefficient of variation (%) | 6.7 | 19.2 | 13.22 | 18.45 | 2.08 | 2.28 | 5.87 | 37.5 | 26.15 | ||
| Season | DF | Yield | K grain | N grain | P grain | Plant height | Ear Diameter | P shoot | P-resin | Soil total P | |
| 2 | Block | 2 | 3,860,390.62 | 21.98 | 2115.58 | 24.17 | 0.012 | 0.36 | 0.00615 | 454.65 | 252,362.51 |
| Fertilization | 1 | 7704.1 | 6.35 | 23.44 | 9.02 | 0.00522 | 4.95 | 0 | 551.04 | 28,132.95 | |
| Dose | 1 | 1,288,066.66 * | 14.99 | 29.43 | 14.01 | 0.00191 | 5.51 | 7 × 10−4 | 1267.30 * | 57,849.62 * | |
| Inoculation | 1 | 948,037.5 | 0.38 | 898.17 * | 0.004 | 0.00213 | 1.26 | 4 × 10−5 | 334.50 | 35,581.70 | |
| Fertilization * Dose | 1 | 429,337.5 | 49.91 * | 296.94 | 42.88 * | 0.04735 * | 2.87 | 1 × 10−4 | 598.00 | 48,447.12 | |
| Fertilization * Inoculation | 1 | 72,600 | 14.12 | 4.42 | 6.44 | 0.01571 | 1.35 | 0 | 415.00 | 36,449.42 | |
| Dose * Inoculation | 1 | 9,204.16 | 7.88 | 654.79 | 7.64 | 0.00313 | 0.18 | 0 | 436.90 | 503.25 | |
| Fertilization * Dose * Inoculation | 1 | 14,016.66 | 6.15 | 124.03 | 3.65 | 0.01739 | 0.51 | 0 | 110.08 | 137,849.88 | |
| Error | 14 | 387,989.43 | 5.34 | 222.56 | 3.95 | 0.00835 | 1.95 | 0.00032 | 213.38 | 33,905.89 | |
| Total | 21 | ||||||||||
| Coefficient of variation (%) | 6.84 | 7.6 | 11.62 | 9.38 | 4.37 | 2.77 | 7.47 | 30.24 | 28.98 | ||
* significant at p ≤ 0.05; DF: degrees of freedom.
Appendix A.7

Table A7.
Soil and agronomic data of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
Table A7.
Soil and agronomic data of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
| Agronomic Trait | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | Treatment | Yield (kg ha−1) | N Grain (g kg−1) | P Grain (g kg−1) | K Grain (g kg−1) | Plant Height (m) | Ear Diameter (mm) | P Shoot (g kg−1) | P-Resin (mg dm−3) | Soil Total P (mg dm−3) | |
| 1 | Inoculation | I | 8846.0 a | 138.1 a | 14.4 a | 21.5 a | 2.34 a | 47.2 a | 0.33 a | 62.8 a | 1131.9 a |
| NI | 9237.0 a | 147.4 a | 15.5 a | 22.5 a | 2.35 a | 47.2 a | 0.34 a | 78.5 a | 1001.7 a | ||
| Fertilization | RockP | 9203.9 a | 144.3 a | 14.8 a | 21.6 a | 2.36 a | 47.3 a | 0.33 a | 67.6 a | 1001.1 a | |
| TSP | 8879.1 a | 141.1 a | 15.1 a | 22.4 a | 2.33 a | 46.9 a | 0.34 a | 73.8 a | 1132.5 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 9121.2 a | 142.2 a | 15.2 a | 22.6 a | 2.35 a | 47.1 a | 0.33 a | 52.0 b | 893.1 b | |
| 120 | 8961.9 a | 143.3 a | 14.6 a | 21.4 a | 2.34 a | 47.3 a | 0.34 a | 89.3 a | 1240.5 a | ||
| 2 | Inoculation | I | 8911.2 a | 122.3 b | 21.2 a | 30.3 a | 2.1 a | 50.7 a | 0.24 a | 52.0 a | 673.9 a |
| NI | 9308.7 a | 134.5 a | 21.2 a | 30.6 a | 2.1 a | 50.3 a | 0.24 a | 44.6 a | 596.9 b | ||
| Fertilization | RockP | 9127.9 a | 127.4 a | 20.6 a | 29.9 a | 2.1 a | 50.9 a | 0.24 a | 43.5 a | 601.1 b | |
| TSP | 9092.1 a | 129.4 a | 21.8 a | 30.9 a | 2.1 a | 50.0 a | 0.24 a | 53.1 a | 669.6 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 9341.7 a | 129.5 a | 21.9 a | 31.2 a | 2.1 a | 50.9 a | 0.24 a | 41.0 b | 586.3 b | |
| 120 | 8878.3 b | 127.3 a | 20.4 a | 29.7 a | 2.1 a | 50.0 a | 0.23 a | 55.6 a | 684.5 a | ||
Note: Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
Appendix A.8

Table A8.
ANOVA of root traits during Seasons 1 and 2.
Table A8.
ANOVA of root traits during Seasons 1 and 2.
| Mean Square | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Season | DF | AREA | WIDTH_MAX | SKL_WIDTH | ANG_TOP | STA_MAX | HYP_DIA | D90 | |
| 1 | Block | 2 | 19,790,645.59 | 598.09 | 690.00 | 7.90 | 0.12216 | 0.0094 | 0.00072 |
| Fertilization | 1 | 15,212,123.22 | 266.77 | 914.66 | 21.81 | 0.00681 | 0.06526 | 0.0012 | |
| Dose | 1 | 4,717,015.95 | 9.62 | 8.79 | 0.087 | 0.48187 * | 0.3688 * | 0.0001 | |
| Inoculation | 1 | 53,217.47 | 8.54 | 67.50 | 10.54 | 0.00884 | 0.03314 | 0.0009 | |
| Fertilization * Dose | 1 | 877,072.21 | 222.26 | 148.32 | 5.46 | 0.02581 | 0.00053 | 0.00029 | |
| Fertilization * Inoculation | 1 | 3,704,619.69 | 46.32 | 100.94 | 2.22 | 0.02636 | 0.0559 | 0.00039 | |
| Dose * Inoculation | 1 | 1,524,640.48 | 33.91 | 0.32 | 3.59 | 0.02793 | 0.01208 | 0.00216 | |
| Fertilization * Dose *Inoculation | 1 | 8,113,911.67 | 805.83 | 627.27 | 12.65 | 0.03051 | 0.11379 | 0.00049 * | |
| Error | 14 | 5,287,099.25 | 378.25 | 413.83 | 14.91 | 0.08724 | 0.06143 | 5 × 10−4 | |
| Total | 21 | ||||||||
| Coefficient of variation (%) | 10.2 | 9.82 | 9.67 | 7.2 | 0.33 | 10.9 | 2.56 | ||
| Season | DF | AREA | WIDTH_MAX | SKL_WIDTH | ANG_TOP | STA_MAX | HYP_DIA | D90 | |
| 2 | Block | 2 | 17,465,084.24 | 167.32 | 124.69 | 60.58 | 0.08799 | 0.14658 | 0.00055 |
| Fertilization | 1 | 1,552,861.78 | 23.86 | 21.97 | 2.20 | 0.1053 | 0.16307 | 0.00647 * | |
| Dose | 1 | 28,471.80 | 2.01 | 66.90 | 107.03 | 0.0951 | 0.00396 | 1 × 10−5 | |
| Inoculation | 1 | 5,107,313.15 | 798.58 | 275.40 | 106.89 | 0.06243 | 0.01 | 1 × 10−5 | |
| Fertilization * Dose | 1 | 6,739,093.71 | 1109.10 * | 1091.38 | 0.80 | 0.0352 | 0.07162 | 4 × 10−5 | |
| Fertilization * Inoculation | 1 | 31,313.71 | 0.22 | 125.39 | 93.15 | 0.02543 | 0.33523 * | 0.00175 | |
| Dose * Inoculation | 1 | 2,623,802.97 | 12.88 | 6.00 | 124.12 | 0.06563 | 0.00339 | 0.00103 | |
| Fertilization * Dose * Inoculation | 1 | 2,715,829.23 | 22.93 | 126.27 | 93.29 | 0.12605 | 0.00092 | 2 × 10−5 | |
| Error | 14 | 4,780,079.35 | 239.37 | 338.63 | 75.27 | 0.1263 | 0.02912 | 0.00066 | |
| Total | 21 | ||||||||
| Coefficient of variation (%) | 12.76 | 9.45 | 10.35 | 14.7 | 0.4 | 9.81 | 2.98 | ||
* significant at p ≤ 0.05; DF: degrees of freedom.
Appendix A.9

Table A9.
Root traits of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
Table A9.
Root traits of inoculated (I) and non-inoculated (NI) maize grown with different phosphate doses (0 and 120 kg ha−1 of P2O5) and fertilizer types (rock phosphate—RockP and triple superphosphate—TSP) during Seasons 1 and 2.
| Root Trait | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Season | Treatment | AREA (cm2) | WIDTH_MAX (cm) | SKL_WIDTH (cm) | ANG_TOP (o) | STA_MAX (cm) | HYP_DIA (cm) | D90 (cm) | |
| 1 | Inoculation | I | 22,585.27 a | 197.55 a | 208.78 a | 52.98 a | 89.59 a | 2.31 a | 0.871 a |
| NI | 22,491.09 a | 198.74 a | 212.14 a | 54.31 a | 89.55 a | 2.24 a | 0.883 a | ||
| Fertilization | RockP | 21,742.04 a | 194.81 a | 204.29 a | 52.69 a | 89.59 a | 2.22 a | 0.870 a | |
| TSP | 23,334.32 a | 201.48 a | 216.63 a | 54.60 a | 89.55 a | 2.33 a | 0.884 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 22,981.51 a | 197.51 a | 209.85 a | 53.58 a | 89.43 b | 2.40 a | 0.879 a | |
| 120 | 22,094.85 a | 198.78 a | 211.06 a | 53.70 a | 89.71 a | 2.15 b | 0.875 a | ||
| 2 | Inoculation | I | 17,600.28 a | 169.45 a | 181.26 a | 56.90 a | 89.72 a | 1.72 a | 0.867 a |
| NI | 16,676.28 a | 157.69 a | 174.40 a | 61.05 a | 89.61 a | 1.75 a | 0.865 a | ||
| Fertilization | RockP | 17,393.34 a | 164.67 a | 176.91 a | 59.31 a | 89.60 a | 1.75 a | 0.850 b | |
| TSP | 16,889.14 a | 162.58 a | 178.87 a | 58.56 a | 89.73 a | 1.72 a | 0.882 a | ||
| Dose (kg ha−1 of P2O5) | 0 | 17,104.53 a | 163.39 a | 176.20 a | 56.90 a | 89.60 a | 1.75 a | 0.866 a | |
| 120 | 17,186.20 a | 163.87 a | 179.60 a | 61.05 a | 89.73 a | 1.72 a | 0.866 a | ||
AREA—Projected root area, WIDTH_MAX—Maximum width of the root system measured horizontally, SKL_WIDTH—Skeletal width calculated from the medial axis, ANG_TOP—Root Top Angle measured between the Random Sample Consensus fit line at the depth of D10 value and the horizontal soil line, STA_MAX—Maximum ground fabric angle measured over all RTPs, HYP_DIA—Hypocotyl diameter estimated over the hypocotyl region detected as the average of the medial circle diameters, D90—Width accumulation percentage at 90% depth. Means followed by the same letter do not differ significantly according to Tukey’s test at 5% probability level.
References
- Olanrewaju, O.; Olubukola, O.B. Bacterial consortium for improved maize (Zea mays L.) production. Microorganisms 2019, 7, 519. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Cherubin, M.R.; Soltangheisi, A.; Rocha, G.C.; Chadwick, D.R.; Jones, D.L. Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci. Rep. 2020, 10, 15615. [Google Scholar] [CrossRef]
- Muraoka, T.; Boaretto, A.E.; Scivittaro, W.B.; Brasil, E.C. Availability of P from phosphate rock, thermophosphate, and triple superphosphate after different incubation periods. In Assessment of Soil Phosphorus Status and Management of Phosphatic Fertilisers to Optimise Crop Production; IAEA: Vienna, Austria, 2002; Volume 473, Available online: http://www-pub.iaea.org/MTCD/Publications/PDF/te_1272_prn.pdf (accessed on 20 March 2024).
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of phosphate-solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Neal, A.L.; McLaren, T.; Campolino, M.L.; Hughes, D.; Coelho, A.M.; Lana, U.G.P.; Gomes, E.A.; Sousa, S.M. Crop type exerts greater influence upon rhizosphere phosphohydrolase gene abundance and phylogenetic diversity than phosphorus fertilization. FEMS Microbiol. Ecol. 2021, 97, fiab033. [Google Scholar] [CrossRef] [PubMed]
- Campolino, M.L.; Santos, T.T.; Lana, U.G.P.; Gomes, E.A.; Guilhen, J.H.S.; Pastina, M.M.; Coelho, A.M.; Sousa, S.M. Crop type determines the relation between root system architecture and microbial diversity indices in different phosphate fertilization conditions. Field Crops Res. 2023, 295, 108893. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.X.; Dong, D.F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbio. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Velloso, C.C.V.; Ribeiro, V.P.; Carvalho, C.; Oliveira, C.A.; Lana, U.G.P.; Marriel, I.E.; Sousa, S.M. Tropical Endophytic Bacillus Species Enhance Plant Growth and Nutrient Uptake in Cereals. Endophytes Miner. Nutr. Manag. 2021, 3, 157–180. [Google Scholar] [CrossRef]
- Oliveira-Paiva, C.A.; Bini, D.; Sousa, S.M.; Ribeiro, V.P.; Santos, F.C.; Lana, U.G.P.; Gomes, E.A.; Marriel, I.E. Inoculation with Bacillus megaterium CNPMS B119 and Bacillus subtilis CNPMS B2084 improves P-acquisition and maize yield in Brazil. Front. Microbiol. 2024, 15, 1426166. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth-promoting rhizobacteria (PGPRs) with multiple plant growth-promoting traits in stress agriculture: Action mechanisms and prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Qarni, A.; Billah, M.; Hussain, K.; Shah, S.H.; Ahmed, W.; Alam, S.; Sheikh, A.A.; Jfri, L.; Munir, A.; Malik, K.M.; et al. Isolation and characterization of phosphate-solubilizing microbes from rock phosphate mines and their potential effect for sustainable agriculture. Sustainability 2021, 13, 2151. [Google Scholar] [CrossRef]
- Velloso, C.C.V.; Oliveira, C.A.; Gomes, E.A.; Lana, U.P.G.; Carvalho, C.G.; Guimarães, L.J.M.; Pastina, M.M.; Sousa, S.M. Genome-guided insights of tropical Bacillus strains efficient in maize growth promotion. FEMS Microbiol. Ecol. 2020, 9, 96. [Google Scholar] [CrossRef]
- Sousa, S.M.; Oliveira, C.A.; Andrade, D.L.; Carvalho, C.G.; Ribeiro, V.P.; Pastina, M.M.; Marriel, I.E.; Lana, U.G.P.; Gomes, E.A. Tropical Bacillus strains inoculation enhances maize root surface area, dry weight, nutrient uptake, and grain yield. J. Plant Growth Regul. 2021, 40, 867–877. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Alves, V.M.C.; Marriel, I.E.; Gomes, E.A.; Scotti, M.R.; Portilho, N.; Guimaraes, C.T.; Schaffert, R.E.; SA, N.M.H. Phosphate-solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil. Biol. Bioch. 2009, 41, 1782–1787. [Google Scholar] [CrossRef]
- Abreu, C.S.; Figueiredo, J.E.F.; Oliveira, C.A.; Santos, V.L.; Gomes, E.A.; Ribeiro, V.P.; Barros, B.A.; Lana, U.G.P.; Marriel, I.E. Maize endophytic bacteria as mineral phosphate solubilizers. Genet. Mol. Res. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H. The dual nature of plant growth-promoting bacteria: Benefits, risks, and pathways to sustainable deployment. Curr. Res. Microb. Sci. 2025, 9, 100421. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Khan, M.S.I.; Shahid, N.; Aaliya, K. Bottlenecks in commercialization and future prospects of PGPR. Appl. Soil. Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- French, E.; Kaplan, I.; Iyer-Pascuzzi, A.; Nakatsu, C.H.; Enders, L. Emerging strategies for precision microbiome management in diverse agroecosystems. Nat. Plants 2021, 7, 256–267. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137. [Google Scholar] [CrossRef]
- Ferrarezi, J.A.; Carvalho-Estrada, P.A.; Batista, B.D.; Aniceto, R.M.; Tschoeke, B.A.P.; Andrade, P.A.M.; Lopes, B.D.M.; Bonatelli, M.L.; Odisi, E.J.; Azevedo, J.L.; et al. Effects of inoculation with plant growth-promoting rhizobacteria from the Brazilian Amazon on the bacterial community associated with maize in the field. Appl. Soil. Ecol. 2022, 170, 104297. [Google Scholar] [CrossRef]
- Ferrarezi, J.A.; Defant, H.; Souza, L.F.; Azevedo, J.L.; Hungria, M.; Quecine, M.C. Meta-omics integration approach reveals the effect of soil native microbiome diversity in the performance of inoculant Azospirillum brasilense. Front. Plant Sci. 2023, 14, 1172839. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Asuming-Brempong, S.; Nüsslein, K. Opening the black box of soil microbial diversity. Appl. Soil. Ecol. 1999, 13, 109–122. [Google Scholar] [CrossRef]
- Burlakoti, S.; Devkota, A.R.; Poudyal, S.; Kaundal, A. Beneficial plant–microbe interactions and stress tolerance in maize. Appl. Microbiol. 2024, 4, 1000–1015. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 2nd ed.; Potafos: Piracicaba, Brazil, 1997; 319p. [Google Scholar]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil. 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Bucksch, A.; Burridge, J.; York, L.M.; Das, A.; Nord, E.; Weitz, J.S. Image-based high-throughput field phenotyping of crop roots. Plant Physiol. 2014, 166, 470–486. [Google Scholar] [CrossRef]
- Das, A.; Schneider, H.; Burridge, J.; Ascanio, A.K.M.; Wojciechowski, T.; Topp, C.N.; Bucksch, A. Digital imaging of root traits (DIRT): A high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 2015, 11, 51. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin/Braunschweig, Germany, 2001.
- LaMontagne, M.G.; Holden, P.A. Comparison of free-living and particle-associated bacterial communities in a coastal lagoon. Microb. Ecol. 2003, 46, 228–237. [Google Scholar] [CrossRef]
- Liu, W.T.; Marsh, T.L.; Cheng, H.; Forney, L.J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 4516–4522. [Google Scholar] [CrossRef]
- Tuinen, D.; Zhao, B.; Gianinazzi-Pearson, V. PCR in studies of AM fungi: From primers to application. In Mycorrhiza Manual; Varma, A.K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 387–400. [Google Scholar] [CrossRef]
- Gollotte, A.; Van Tuinen, D.; Atkinson, D. Diversity of arbuscular mycorrhizal fungi colonizing roots of the grass species Agrosystem cappilaris and Lolium perenne in a field experiment. Mycorrhiza 2004, 14, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Culman, S.W.; Bukowski, R.; Gauch, H.G.; Cadillo-Quiroz, H.; Buckley, D.H. T-REX: Software for the processing and analysis of T-RFLP data. BMC Bioinform. 2009, 10, 171. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2. Microbiological and Biochemical Properties, 2nd ed.; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America/American Society of Agronomy: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes.pt: Experimental Designs Package (Portuguese), R package version 1.2.0.; 2021. Available online: https://cran.r-project.org/web/packages/ExpDes.pt/ExpDes.pt.pdf (accessed on 10 July 2025).
- Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research, R package version 1.4; 2020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 July 2025).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 10 July 2025).
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJournal 2006, Complex Systems, 1695. Available online: https://igraph.org (accessed on 20 February 2025).
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Nahas, J.V.; Iosue, C.L.; Shaik, N.F.; Selhorst, K.; He, B.Z.; Wykoff, D.D. Dynamic changes in yeast phosphatase families allow for specialization in phosphate and thiamine starvation. G3 Genes Genomes Genet. 2018, 8, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Bousselham, M.; Lemriss, S.; Dhiba, D.; Aallam, Y.; Souiri, A.; Abbas, Y.; Saidi, N.; Boukcim, H.; Hamdali, H. Streptomycetaceae and Promicromonosporaceae: Two Actinomycete Families from Moroccan Oat Soils Enhancing Solubilization of Natural Phosphate. Microorganisms 2022, 10, 1116. [Google Scholar] [CrossRef]
- Mine, A.; Berens, M.L.; Nobori, T.; Anver, S.; Fukumoto, K.; Winkelmüller, T.M.; Takeda, A.; Becker, D.; Tsuda, K. Pathogen exploitation of an abscisic acid- and jasmonate-inducible MAPK phosphatase and its interception by Arabidopsis immunity. Proc. Natl. Acad. Sci. USA 2017, 114, 7456–7461. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Ho, L.; Rees, C.A.; Hill, J.E.; Nodwell, J.R.; Elliot, M.A. Streptomyces exploration is triggered by fungal interactions and volatile signals. eLife 2017, 6, e21738. [Google Scholar] [CrossRef] [PubMed]
- Karajeh, M.R. Efficacy of Saccharomyces cerevisiae on controlling the root-knot nematode (Meloidogyne javanica) infection and promoting cucumber growth and yield under laboratory and field conditions. Arch. Phytopathol. Plant Prot. 2013, 46, 2492–2500. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of Soil Microbiota Enzymes in Soil Health and Activity Changes Depending on Climate Change and the Type of Soil Ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Philippot, L.; Griffiths, B.S.; Langenheder, S. Microbial community resilience across ecosystems and multiple disturbances. Microbiol. Mol. Biol. Rev. 2021, 85, 10–1128. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.A.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Wang, L.; Jia, J.; Su, Q.; Cao, H.; Jia, S.; Si, H.; Cao, Z.; Ma, S.; Xing, J.; Zhang, K.; et al. Root-associated microbial diversity and metabolomics in maize resistance to stalk rot. Front. Microbiol. 2024, 15, 1468627. [Google Scholar] [CrossRef]
- Oberholster, T.; Vikram, S.; Cowan, D.; Valverde, A. Key microbial taxa in the rhizosphere of sorghum and sunflower grown in crop rotation. Sci. Total Environ. 2018, 624, 530–539. [Google Scholar] [CrossRef]
- Miljenka, V.; Barbara, C.; Cordula, V.; Susanne, K.; Michael, S.; Stefanie, S. Shifts in reclamation management strategies shape the role of exopolysaccharide and lipopolysaccharide producing bacteria during soil formation. Microb. Biotechnol. 2020, 13, 584–598. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Chepsergon, J.; Moleleki, L.N. Rhizosphere bacterial interactions and impact on plant health. Curr. Opin. Microbiol. 2023, 73, 102297. [Google Scholar] [CrossRef]
- Song, S.; Yang, X.; Tang, R.; Zhang, Y.; Tang, Z. Soil properties and plant functional traits have different importance in shaping rhizosphere soil bacterial and fungal communities in a meadow steppe. mSystems 2025, 10, e00570-25. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Campolino, M.L.; Paula, U.G.L.; Gomes, E.A.; Coelho, A.M.; de Sousa, S.M. Phosphate fertilization affects rhizosphere microbiome of maize and sorghum genotypes. Braz. J. Microbiol. 2022, 53, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.J.; Carcedo, S.G.; Mateos, M.P. Distribution and characterization of phosphatase and organic phosphorus in soil fractions. Soil. Biol. Biochem. 1990, 22, 169–174. [Google Scholar] [CrossRef]
- Oberson, A.; Besson, J.M.; Maire, N.; Sticher, H. Microbiological processes in soil organic phosphorus transformations in conventional and biological cropping systems. Biol. Fertil. Soils 1996, 21, 138–148. [Google Scholar] [CrossRef]
- Siddique, M.B.A.; Khalid, A.; Ditta, A.; Mahmood, S.; Alataway, A.; Dewidar, A.Z.; Mattar, M.A. Climate change variables modify microbial community structure and soil enzymes involved in nitrogen and phosphorus metabolism. Rhizosphere 2023, 28, 100793. [Google Scholar] [CrossRef]
- Huang, W.; Liu, J.; Zhou, G.; Zhang, D.; Deng, Q. Effects of precipitation on soil acid phosphatase activity in three successional forests in southern China. Biogeosciences 2011, 8, 1901–1910. [Google Scholar] [CrossRef]
- Yang, X.C.; Zhu, K.; Loik, M.E.; Sun, W. Differential responses of soil bacteria and fungi to altered precipitation in a meadow steppe. Geoderma 2021, 384, 114812. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.X.; Li, S.; Yue, L.X.; Ali, M.; Han, J.R.; Lian, W.H.; Hu, C.J.; Lin, Z.L.; Shi, G.Y.; et al. Aridity drives the variability of desert soil microbiomes across north-western China. Sci. Total Environ. 2024, 907, 168048. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- He, F.; Hu, X. Hubbell’s fundamental biodiversity parameter and the Simpson diversity index. Ecol. Lett. 2005, 8, 386–390. [Google Scholar] [CrossRef]
- Carvalho, R.P.; von Pinho, R.G.; Davide, L.M.C. Desempenho de cultivares de milho quanto à eficiência de utilização de nitrogênio. Rev. Bras. Milho Sorgo 2011, 10, 108–120. [Google Scholar] [CrossRef]
- Leggett, M.; Newlands, N.K.; Greenshields, D.; West, L.; Inman, S.; Koivunen, M.E. Maize yield response to a phosphorus-solubilizing microbial inoculant in field trials. J. Agric. Sci. 2015, 153, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, K.; Shang, J.; Wu, Y.; Chen, T.; Wanyan, Y.; Wang, E.T.; Tian, C.F.; Chen, W.; Chen, W.; et al. Plant growth–promoting bacteria improve maize growth through reshaping the rhizobacterial community in low-nitrogen and low-phosphorus soil. Biol. Fertil. Soils 2021, 57, 1075–1088. [Google Scholar] [CrossRef]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef]
- Herrmann, A.M.; Colombi, T. Energy use efficiency of root growth–a theoretical bioenergetics framework. Plant Signal. Behav. 2019, 14, 1685147. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mhamdi, R. Microbial inoculants and their impact on soil microbial communities: A review. BioMed Res. Int. 2013, 2013, 863240. [Google Scholar] [CrossRef]
- Vuolo, F.; Novello, G.; Bona, E.; Gorrasi, S.; Gamalero, E. Impact of plant-beneficial bacterial inocula on the resident bacteriome: Current knowledge and future perspectives. Microorganisms 2022, 10, 2462. [Google Scholar] [CrossRef]
- Santos, F.C.; Melkani, S.; Oliveira-Paiva, C.A.; Bini, D.; Pavuluri, K.; Gatiboni, L.; Mahmud, A.; Torres, M.; Mclamore, E.; Bhadha, J.H. Biofertilizer use in the United States: Definition, regulation, and prospects. App. Microbiol. Biotechnol. 2024, 108, 511. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).